This cohort study evaluates patterns of telemedicine use and their association with glycemic control among adults receiving endocrinology care for type 2 diabetes.
Key Points
Question
What is the association between telemedicine use and glycemic outcomes among adults with varying clinical complexity receiving endocrinology care for type 2 diabetes from 2020 to 2022?
Findings
In this cohort study including 3778 adults, there was no significant change in estimated hemoglobin A1c (HbA1c) over 12 months (−0.06%) among patients using telemedicine alone, while patients who used in-person (−0.37%) and mixed care (−0.22%) had significant HbA1c improvements.
Meaning
These findings suggest that patients with type 2 diabetes who rely on telemedicine alone to access endocrinology care may require additional support to achieve glycemic goals.
Abstract
Importance
Telemedicine can increase access to endocrinology care for people with type 2 diabetes (T2D), but patterns of use and outcomes of telemedicine specialty care for adults with T2D beyond initial uptake in 2020 are not known.
Objective
To evaluate patterns of telemedicine use and their association with glycemic control among adults with varying clinical complexity receiving endocrinology care for T2D.
Design, Setting, and Participants
Retrospective cohort study in a single large integrated US health system. Participants were adults who had a telemedicine endocrinology visit for T2D from May to October 2020. Data were analyzed from June 2022 to October 2023.
Exposure
Patients were followed up through May 2022 and assigned to telemedicine-only, in-person, or mixed care (both telemedicine and in-person) cohorts according to visit modality.
Main Outcomes and Measures
Multivariable regression models were used to estimate hemoglobin A1c (HbA1c) change at 12 months within each cohort and the association of factors indicating clinical complexity (insulin regimen and cardiovascular and psychological comorbidities) with HbA1c change across cohorts. Subgroup analysis was performed for patients with baseline HbA1c of 8% or higher.
Results
Of 11 498 potentially eligible patients, 3778 were included in the final cohort (81 Asian participants [2%], 300 Black participants [8%], and 3332 White participants [88%]); 1182 used telemedicine only (mean [SD] age 57.4 [12.9] years; 743 female participants [63%]), 1049 used in-person care (mean [SD] age 63.0 [12.2] years; 577 female participants [55%]), and 1547 used mixed care (mean [SD] age 60.7 [12.5] years; 881 female participants [57%]). Among telemedicine-only patients, there was no significant change in adjusted HbA1c at 12 months (−0.06%; 95% CI, −0.26% to 0.14%; P = .55) while in-person and mixed cohorts had improvements of 0.37% (95% CI, 0.15% to 0.59%; P < .001) and 0.22% (95% CI, 0.07% to 0.38%; P = .004), respectively. Patients with a baseline HbA1c of 8% or higher had a similar pattern of glycemic outcomes. For patients prescribed multiple daily injections vs no insulin, the 12-month estimated change in HbA1c was 0.25% higher (95% CI, 0.02% to 0.47%; P = .03) for telemedicine vs in-person care. Comorbidities were not associated with HbA1c change in any cohort.
Conclusions and Relevance
In this cohort study of adults with T2D receiving endocrinology care, patients using telemedicine alone had inferior glycemic outcomes compared with patients who used in-person or mixed care. Additional strategies may be needed to support adults with T2D who rely on telemedicine alone to access endocrinology care, especially for those with complex treatment or elevated HbA1c.
Introduction
Use of telemedicine to deliver endocrinology care for type 2 diabetes (T2D) increased dramatically in 2020 and is likely to continue given a shortage of endocrinologists and persistent barriers to in-person visits.1,2,3,4 Prior randomized clinical trials have demonstrated the efficacy of telemedicine, defined as synchronous audio or video communication between patients and practitioners, for improving glycemic outcomes for adults with T2D.5,6,7,8,9 However, data are lacking on utilization patterns and outcomes of routine telemedicine care for T2D since 2020, especially in the endocrinology setting.10,11,12,13 Examination of clinical outcomes is critical, as patients with T2D may be more diverse, clinically complex, and face additional barriers to accessing care than clinical trial participants.14,15 Moreover, telemedicine interventions for diabetes that have been tested in clinical trials frequently include intensive care components that are not routinely implemented in current diabetes telemedicine care, such as remote monitoring of blood glucose, multidisciplinary team care, and patient engagement between visits.5,16,17,18,19
An understanding of which patients with T2D have continued to use telemedicine and how their glycemic outcomes vary across different clinical scenarios is necessary to identify which patients can successfully manage their diabetes with telemedicine alone and which may need additional support or in-person care to reach treatment goals. Endocrinology practitioners have expressed concern that patients with increased medical complexity may be less well served by telemedicine care.19,20 Although guidelines recommend use of telemedicine to increase access to diabetes care, additional evidence is needed on short- and long-term clinical outcomes across distinct patient populations to guide best practices.21,22,23 In this study, we aimed to address this evidence gap by evaluating (1) the characteristics of adults with T2D who persisted in using telemedicine-only vs those who switched to in-person or mixed endocrinology care after initial telemedicine use early in the COVID-19 pandemic, (2) the association of these care modalities with glycemic outcomes, and (3) how factors that contribute to clinical complexity, including insulin regimen and comorbidities, are associated with glycemic outcomes across these different care modalities.
Methods
Study Design and Clinical Setting
This retrospective cohort study included adults with T2D who were seen via telemedicine for either an initial or follow-up visit between May 1 and October 31, 2020, in the endocrinology division of a large health system, which includes more than 30 practitioners across 8 clinics in rural and urban counties. Similar to other care settings, most patients with T2D in this health system are managed by their primary care practitioners. However, patients may receive care from an endocrinologist through referral by a health care practitioner or self-referral, with associated costs varying according to their specific heath insurance plan. In this clinical setting, use of telemedicine vs in-person care was based on individual patient and practitioner preference and availability; there were no blanket policies determining visit modality. To reduce bias in assessment of ongoing telemedicine use and glycemic outcomes, we focused on patients with capability to use telemedicine at baseline. This study was approved by the University of Pittsburgh institutional review board and determined to be exempt from informed consent as it involves secondary research on data collected as part of routine care. The results are reported in accordance with Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.24
Study Cohort
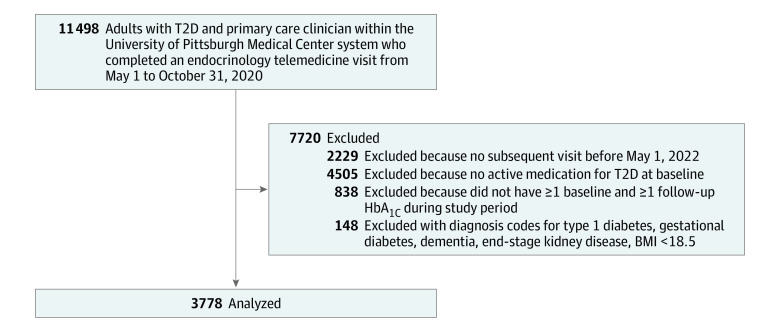
Patients were included if they had at least 2 hemoglobin A1c (to convert from percentage to proportion of total hemoglobin, multiply by 0.01) values and 1 subsequent encounter in the division of endocrinology during the follow-up period, which extended through May 1, 2022. A diagnosis of T2D was based on encounter diagnosis codes (International Statistical Classification of Diseases and Related Health Problems, Tenth Revision [ICD-10] code E11.X). Patients had to be over age 18 years, have a primary care practitioner within the health system to ensure adequate capture of comorbidities, and at least 1 prescription for an antihyperglycemic medication at baseline, to exclude patients seen in endocrinology for another condition who also have T2D controlled without medication. Patients with type 1 diabetes, gestational diabetes, end-stage kidney disease, and dementia were excluded. Details on cohort creation and inclusion and exclusion criteria can be found in the cohort flowchart in Figure 1.
Figure 1. Flowchart of Study Cohort.
BMI indicates body mass index (calculated as weight in kilograms divided by height in meters squared); HbA1c, hemoglobin A1c; T2D, type 2 diabetes.
Measures
All data were extracted from electronic medical records. Telemedicine encounters were defined as completed synchronous visits with an endocrinology practitioner including audio-visual or audio-only communication. Encounters designated as phone calls, which are used to provide unscheduled support between visits, were excluded. Baseline factors including patient demographics and clinical characteristics were extracted as close to the initial qualifying visit date as possible starting May 1, 2020. The first HbA1c value recorded in the study period was defined as the baseline value; follow-up HbA1c values were collected through May 1, 2022, and had to be at least 10 weeks apart. Body mass index (BMI) was categorized into standard levels as shown in Table 1. Comorbidities of interest included cardiovascular disease and psychological conditions documented at least twice in outpatient encounters during the study period (see eTable 1 in Supplement 1 for ICD-10 codes). These were chosen to include 1 category of concordant comorbidities, which have management strategies similar to T2D (eg, coronary artery disease), and 1 category of discordant comorbidities with management unrelated to T2D (eg, bipolar disorder) to assess whether these had distinct associations with glycemic outcomes.25,26 Insulin prescription was based on active medication orders at baseline and categorized as no insulin, basal insulin only, or multiple daily injections (MDI; ie, both basal and prandial insulin). Social Deprivation Index (SDI), a composite measure of local area deprivation linked to health outcomes, was based on 5-digit zip code.27,28 Rural-urban commuting area (RUCA) codes were used to assess rurality, which also impacts diabetes care and outcomes.29,30,31,32 Race was extracted from the electronic medical record, based on patient self-report on clinical intake forms, and was included in this study to evaluate racial and ethnic variation in patterns of telemedicine use because race and ethnicity have previously been associated with factors, such as health insurance and physical environment, that affect both patterns of health care use and glycemic outcomes. Patients were separated into 3 categories: telemedicine only, in which all visits in the study period were conducted via telemedicine; in-person only, in which all visits after the initial telemedicine visit were in-person; or mixed follow-up, in which patients had both telemedicine and in-person visits following the initial visit in the study period.
Table 1. Baseline Characteristics of Patient Cohort.
| Characteristic | Participants, No. (%) | ||||
|---|---|---|---|---|---|
| Total (N = 3778) | Telemedicine only (n = 1182) | In-person only (n = 1049) | Mixed follow-up (n = 1547) | P valuea | |
| Age, mean (SD), y | 60.3 (12.7) | 57.4 (12.9) | 63.0 (12.2) | 60.7 (12.5) | <.001 |
| Sex | |||||
| Female | 2201 (58) | 743 (63) | 577 (55) | 881 (57) | <.001 |
| Male | 1577 (42) | 439 (37) | 472 (45) | 666 (43) | |
| Race | |||||
| Asian | 81 (2) | 23 (2) | 14 (1) | 44 (3) | <.001 |
| Black | 300 (8) | 114 (10) | 77 (7) | 109 (7) | |
| White | 3332 (88) | 1024 (87) | 944 (90) | 1364 (88) | |
| Other or missingb | 65 (1) | 21 (2) | 14 (1) | 30 (2) | |
| Hispanic or Latino | 66 (2) | 18 (2) | 5 (0) | 43 (3) | <.001 |
| Not specified or missing | 158 (4) | 63 (5) | 45 (4) | 50 (3) | |
| SDI Score, mean (SD) | 40.5 (23.9) | 38.3 (25.3) | 41.4 (22.0) | 41.7 (23.9) | <.001 |
| RUCA | |||||
| Urban | 2645 (70) | 926 (78) | 669 (64) | 1050 (68) | <.001 |
| Suburban | 836 (22) | 204 (17) | 260 (25) | 372 (24) | |
| Rural | 297 (8) | 52 (4) | 120 (11) | 125 (8) | |
| HbA1c, mean (SD)% | 7.6 (1.7) | 7.6 (1.8) | 7.4 (1.6) | 7.7 (1.7) | <.001 |
| Insulin | |||||
| No insulin | 1476 (39) | 528 (45) | 435 (41) | 513 (33) | <.001 |
| Basal only | 652 (17) | 199 (17) | 180 (17) | 273 (18) | |
| MDI | 1650 (44) | 455 (38) | 434 (41) | 761 (49) | |
| No. of noninsulin medications, mean (SD) | 1.9 (1.1) | 1.8 (1.1) | 1.9 (1.1) | 1.9 (1.1) | .05 |
| Body mass indexc | |||||
| 18.5-24.9 | 196 (5) | 53 (4) | 69 (7) | 74 (5) | <.001 |
| 25-29.9 | 639 (17) | 169 (14) | 228 (22) | 242 (16) | |
| 30-34.9 | 880 (23) | 211 (18) | 291 (28) | 378 (24) | |
| 35-39.9 | 688 (18) | 207 (18) | 198 (19) | 283 (18) | |
| >40 | 770 (20) | 247 (21) | 195 (19) | 328 (21) | |
| Missing | 605 (16) | 295 (25) | 68 (6) | 242 (16) | |
| Comorbid conditions | |||||
| Cardiovascular | 1393 (37) | 401 (34) | 418 (40) | 574 (37) | .02 |
| Psychological | 1246 (33) | 454 (38) | 277 (26) | 515 (33) | <.001 |
| Appointments per 12 mo, mean (SD), No. | 2.6 (1.0) | 2.1 (0.8) | 2.5 (1.1) | 2.9 (0.9) | <.001 |
| No. of follow-up HbA1c test results per 12 mo, mean (SD) | 1.7 (0.8) | 1.4 (0.8) | 1.8 (0.8) | 1.8 (0.8) | <.001 |
Abbreviations: HbA1c, hemoglobin A1c; MDI, multiple daily injections; RUCA, rural-urban commuting area; SDI, social deprivation index.
SI conversion factor: to convert to proportion of total hemoglobin, multiply by 0.01.
For continuous variables, Kruskal-Wallis test was used; for categorical variables, χ2 test was used.
Other includes Indigenous American, Alaska Native, Samoan, Other Pacific Islanders, and those who indicated their race was unknown, not specified, declined to answer, or for whom race data was missing.
Body mass index is calculated as weight in kilograms divided by height in meters squared.
Statistical Analysis
We summarized baseline patient characteristics using frequency (percentage) for categorical variables and mean (SD) or median (IQR) for continuous variables. Baseline characteristics were compared among those who used telemedicine only, in-person only follow-up, and mixed follow-up using χ2 tests for categorical variables and Kruskal-Wallis tests for continuous variables. To examine the outcomes of excluding patients without follow-up HbA1c, we also compared characteristics of excluded patients with the modeled cohort. Each demographic variable that differed between the modeled cohort and excluded patients was included as a covariate in final model.
Our primary outcome was 12-month HbA1c change, with a secondary outcome of HbA1c change at 24 months to explore longer-term glycemic outcomes. We used a linear mixed model fitted via maximum likelihood estimation to assess HbA1c change by follow-up care modality and clinical factors. Random effects for patient and practitioner were included, with the endocrinology practitioner of the initial encounter serving as the unit of the random practitioner effect. Variables of main interest were treated as fixed effects and included follow-up care modality, insulin treatment regimen, and composite binary indicators for presence of cardiovascular disease or psychological conditions. To allow HbA1c response to vary over the follow-up period, we included a quadratic function of time, defined as number of months since baseline HbA1c. To capture between-patient variability in trajectories of HbA1c response, a random slope for time was also incorporated. We adjusted for patient age, sex, race, ethnicity, SDI, RUCA category (urban, suburban, rural), baseline HbA1c, and BMI (with patients with missing BMI included in a discrete level) to control for potential confounding. To quantify HbA1c change over time within follow-up care modalities and test whether HbA1c change differed significantly between modalities, we included 2-way interactions between follow-up care modality and fixed effects for time. Least-squares means (LS means) estimation quantified HbA1c change over time within follow-up modalities, and contrasts of LS means estimated differences in HbA1c change over time between modalities.
We conducted 2 additional analyses. First, a subgroup analysis limited to patients with baseline HbA1c 8% or higher was performed to further explore outcomes specifically for patients with elevated HbA1c. Second, difference-in-difference analyses contrasted LS means estimates of HbA1c change for patients with insulin use and comorbidities vs those without between care modalities and between patients who used telemedicine vs mixed or in-person care between levels of insulin use. Two- and 3-way interactions between time, care modality, and insulin regimen, as well as interactions between time, care modality, and comorbidities, were included in the mixed models. All analyses assumed a type 1 error rate of .05 and were performed using SAS software, version 9.4 (SAS Institute Inc). Data were analyzed from June 2022 to October 2023.
Results
Characteristics of Patients by Care Modality
There were 3778 patients in the final cohort, with a mean (SD) age of 60.3 (12.7) years, 58% female (2201 participants), 2% Asian (81 participants), 8% Black (300 participants), and 88% White (3332 participants) (Table 1). The plurality of patients (1547 participants [41%]) used mixed modalities after the initial telemedicine visit, with similar proportions of patients (1182 participants [31%]) using telemedicine only and in-person only (1049 participants [28%]) over the study period. Patients who used telemedicine only were younger and more likely to be women and Black than patients in the in-person and mixed follow-up groups. In addition, patients who used telemedicine only had less local area deprivation (SDI) and were more likely to be urban dwelling. The in-person follow-up cohort had lower baseline HbA1c compared with the other cohorts. Patients in the telemedicine-only cohort were more likely to have a psychological comorbidity and not be prescribed insulin at baseline than patients in the other 2 cohorts.
Comparison of patients included in final models with those excluded due to lack of follow-up HbA1c demonstrated significant differences in demographics (eTable 2 in Supplement 1). Excluded patients were more likely to be younger, women, Black, urban dwelling, not prescribed insulin, have lower baseline HbA1c, and have fewer visits per year than included patients.
Patterns of Care Utilization
The proportion of endocrinology visits for T2D that were conducted via telemedicine was highest from May to October 2020 at 84% (10 987 of 13 031 visits), dropped to 63% from November 2020 to April 2021 (4923 of 7783 visits), then to 42% from May 2021 to October 2021 (3373 of 8053 visits), and stabilized at 41% from November 2021 to May 2022 (2310 of 5618 visits). Patients in the telemedicine-only group had fewer mean (SD) appointments per year (2.1 [0.8] appointments per 12 months) than those in the in-person and mixed follow-up groups (2.5 [1.1] appointments per 12 months and 2.9 [0.9] appointments per 12 months, respectively; rate ratio of appointments per 12 months comparing telemedicine to in-person, 0.803; 95% CI, 0.771-0.836; rate ratio comparing telemedicine to mixed follow-up groups, 0.699; 95% CI, 0.674-0.725; P < .001 for comparison of rate of appointments for in-person and mixed follow-up groups with telemedicine) (Table 1). Patients who used telemedicine only also had fewer follow-up HbA1c measurements per 12 months than those who used in-person and mixed follow-up.
Glycemic Outcomes by Care Modality
There was no significant change in HbA1c from baseline to 12 months in the telemedicine-only group (−0.06%; 95% CI, −0.26 to 0.14), while the in-person and mixed follow-up groups demonstrated HbA1c improvement (estimated change for in-person group, −0.37; 95% CI, −0.59 to −0.15; estimated change for mixed group, −0.22; 95% CI, −0.38 to −0.07) (Table 2). There was no significant change in the secondary outcome of HbA1c change from baseline to 24 months across any care modality (Table 2). Model-derived trajectories of HbA1c over time differed within care modalities based on insulin regimen (Figure 2). In the telemedicine group, there was minimal estimated HbA1c change over time among patients not on insulin and those on MDI, while HbA1c increased steadily from baseline to 24 months for patients on basal insulin. In contrast, among the in-person and mixed follow-up cohorts, adjusted HbA1c declined from baseline to 12 months with subsequent increase from 12 to 24 months for patients in all 3 insulin groups.
Table 2. Adjusted HbA1c Change From Baseline for Each Follow-Up Care Modality.
| Care modality | Primary outcome: adjusted 12-mo HbA1c change from baseline (95% CI), percentage pointa | P value | Secondary outcome: adjusted 24-mo HbA1c change from baseline (95% CI), percentage pointa | P value |
|---|---|---|---|---|
| Entire cohort (N = 3778) | ||||
| Telemedicine only (n = 1182) | −0.06 (−0.26 to 0.14) | .55 | 0.02 (−0.24 to 0.29) | .86 |
| In-person only (n = 1049) | −0.37 (−0.59 to −0.15) | <.001 | −0.19 (−0.46 to 0.07) | .15 |
| Mixed follow-up (n = 1547) | −0.22 (−0.38 to −0.07) | .004 | −0.11 (−0.31 to 0.09) | .27 |
| Baseline HbA1c ≥8% subgroup (n = 1198) | ||||
| Telemedicine only (n = 381) | −0.43 (−0.97 to 0.10) | .11 | −0.32 (−1.00 to 0.36) | .35 |
| In-person only (n = 280) | −1.80 (−2.44 to −1.15) | <.001 | −1.81 (−2.57 to −1.04) | <.001 |
| Mixed follow-up (n = 537) | −1.10 (−1.44 to −0.77) | <.001 | −0.95 (−1.42 to −0.49) | <.001 |
Abbreviation: HbA1c, hemoglobin A1c.
SI Conversion: to convert to proportion of total hemoglobin, multiply by 0.01.
Adjusted 12-month HbA1c changes are model-based estimates derived from linear mixed modeling of repeated measures of HbA1c adjusted for patient age, sex, race, ethnicity, social deprivation index, rurality, baseline HbA1c, and body mass index; patients were nested within practitioners.
Figure 2. Adjusted Hemoglobin A1c (HbA1c) Levels by Follow-Up Care Modality and Baseline Insulin Regimen.
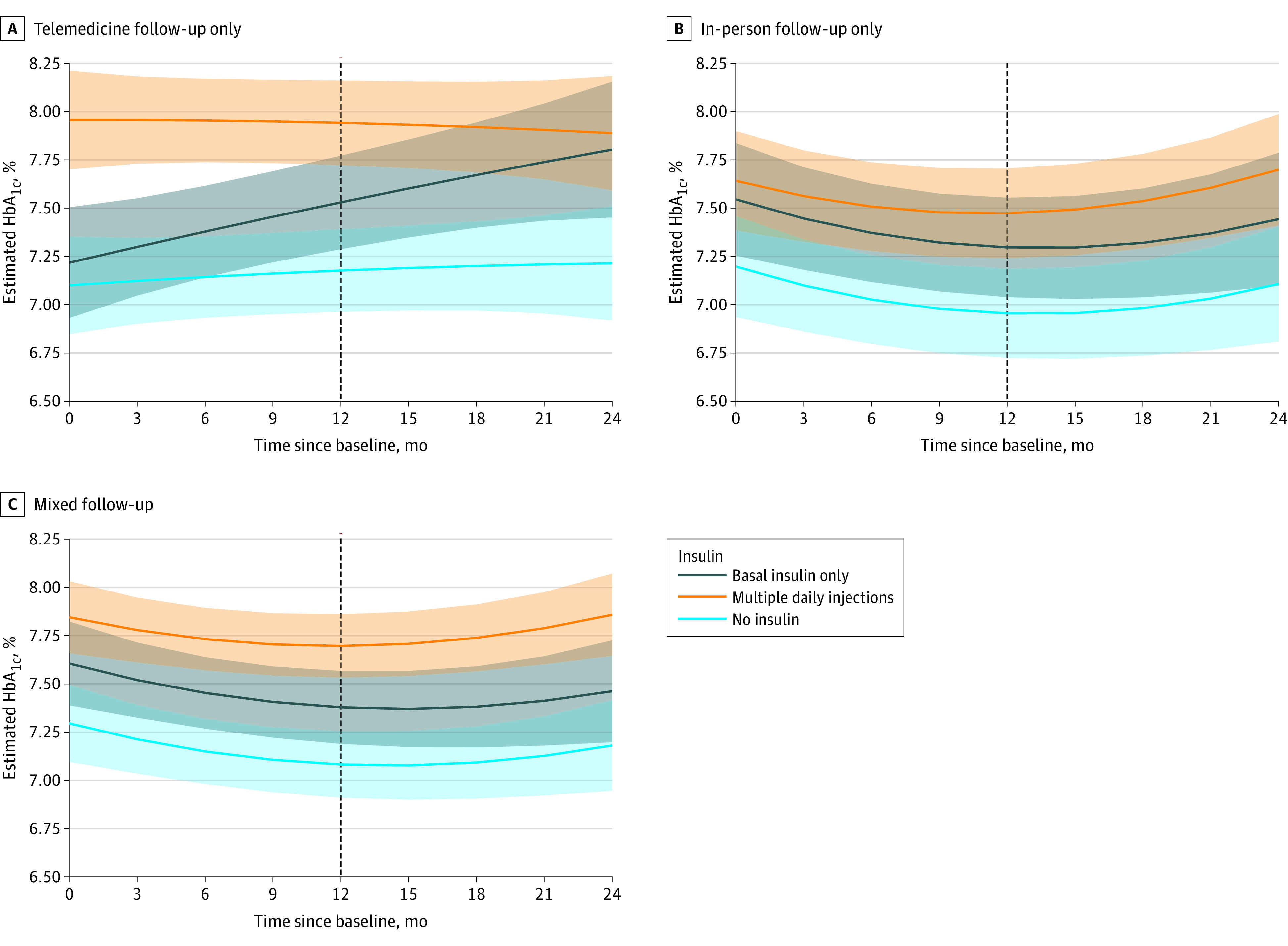
Adjusted 12-month HbA1c changes are model-based estimates derived from linear mixed modeling of repeated measures of HbA1c adjusted for patient age, sex, race, ethnicity, social deprivation index, rurality, baseline HbA1c, and body mass index; patients were nested within practitioners. To convert to proportion of total hemoglobin, multiply by 0.01.
Glycemic Outcomes by Clinical Complexity
In all 3 cohorts, patients prescribed basal insulin had worse adjusted HbA1c changes at 12 months compared with those not prescribed insulin (Table 3). Similar trends were seen at 24 months, but the estimated difference in HbA1c change between patients prescribed basal insulin and those not prescribed insulin was significant only for the telemedicine group. For patients prescribed MDI compared with those not prescribed insulin, estimated HbA1c change was worse at both 12 and 24 months across all modalities (Table 3). In difference-in-difference analysis, HbA1c change from baseline to 12 months for patients prescribed MDI vs no insulin was significantly worse in the telemedicine group than the in-person group (estimated difference in HbA1c change, 0.25% higher; 95% CI, 0.02 to 0.47; P = .03) but was not significantly different between the mixed and telemedicine groups (estimated difference in HbA1c change, 0.15% higher for telemedicine only; 95% CI, −0.36 to 0.05; P = .05). Similarly, outcomes of telemedicine vs in-person care among patient cohorts defined according to insulin use demonstrate significantly worse HbA1c changes at 12 months for telemedicine vs in-person care among patients on MDI (estimated difference in HbA1c change, −0.47%; 95% CI, −0.78 to −0.15) for in-person vs telemedicine only (eTable 3 in Supplement 1). There was no significant association of cardiovascular or psychological comorbidities with HbA1c changes at 12 or 24 months in any care modality cohort (Table 3).
Table 3. Adjusted Difference in HbA1c Change for Those With Insulin Use or Comorbidities vs Without for Each Follow-Up Care Modality.
| Clinical characteristic | Primary outcome: difference in adjusted HbA1c change at 12 mo (95% CI), percentage pointa | P value | Secondary outcome: difference in adjusted HbA1c change at 24 mo (95% CI, percentage point)a | P value |
|---|---|---|---|---|
| Telemedicine only follow-up, entire cohort (n = 1182) | ||||
| Basal insulin vs no insulin | 0.35 (0.15 to 0.56) | <.001 | 0.59 (0.22 to 0.96) | <.001 |
| MDI vs no insulin | 0.77 (0.59 to 0.94) | <.001 | 0.67 (0.37 to 0.98) | <.001 |
| Cardiovascular vs none | 0.04 (−0.12 to 0.19) | >.99 | −0.01 (−0.27 to 0.26)) | >.99 |
| Psychological vs none | −0.001 (−0.15 to 0.15) | >.99 | 0.02 (−0.24 to 0.28 | >.99 |
| In-person only follow-up, entire cohort (n = 1049) | ||||
| Basal insulin vs no insulin | 0.34 (0.13 to 0.55) | <.001 | 0.34 (−0.01 to 0.68) | .06 |
| MDI vs no insulin | 0.52 (0.34 to 0.70) | <.001 | 0.59 (0.30 to 0.88) | <.001 |
| Cardiovascular vs none | −0.01 (−0.16 to 0.15) | .99 | 0.02 (−0.23 to 0.26) | >.99 |
| Psychological vs none | 0.05 (−0.11 to 0.21) | .99 | 0.04 (−0.23 to 0.31) | >.99 |
| Mixed follow-up, entire cohort (n = 1547) | ||||
| Basal insulin vs no insulin | 0.30 (0.12 to 0.47) | <.001 | 0.28 (−0.00 to 0.57) | .06 |
| MDI vs no insulin | 0.61 (0.46 to 0.76) | <.001 | 0.68 (0.43 to 0.92) | <.001 |
| Cardiovascular vs none | 0.01 (−0.12 to 0.14) | >.99 | 0.04 (−0.16 to 0.25) | >.99 |
| Psychological vs none | −0.05 (−0.18 to 0.08) | .75 | −0.04 (−0.25 to 0.17) | >.99 |
| Telemedicine only follow-up, subgroup baseline HbA1c ≥8% (n = 381) | ||||
| Basal insulin vs no insulin | 0.44 (−0.10 to 0.99) | .14 | 0.68 (−0.30 to 1.65) | .24 |
| MDI vs no insulin | 0.95 (0.45 to 1.44) | <.001 | 0.67 (−0.17 to 1.51) | .15 |
| Macrovascular vs none | −0.18 (−0.55 to 0.19) | .57 | −0.37 (−1.0 to 0.27) | .39 |
| Psychological vs none | 0.31 (−0.05 to 0.68) | .11 | 0.39 (−0.22 to 1.01) | .31 |
| In-person only follow-up, subgroup baseline HbA1c ≥8% (n = 280) | ||||
| Basal insulin vs no insulin | 0.42 (−0.27 to 1.11) | .34 | 0.09 (−1.05 to 1.23) | >.99 |
| MDI vs no insulin | 0.58 (−0.04 to 1.21) | .07 | 0.35 (−0.64 to 1.33) | .87 |
| Macrovascular vs none | −0.06 (−0.48 to 0.36) | >.99 | −0.28 (−0.95 to 0.40) | .72 |
| Psychological vs none | 0.09 (−0.36 to 0.55) | >.99 | 0.20 (−0.54 to 0.93) | >.99 |
| Mixed follow-up, subgroup baseline HbA1c ≥8% (n = 537) | ||||
| Basal insulin vs no insulin | 0.50 (−0.03 to 1.04) | .07 | 0.28 (−0.61 to 1.16) | .96 |
| MDI vs no insulin | 0.79 (0.31 to 1.26) | <.001 | 0.52 (−0.22 to 1.26) | .23 |
| Macrovascular vs none | 0.13 (−0.17 to 0.43) | .64 | 0.26 (−0.22 to 0.73) | .46 |
| Psychological vs none | −0.00 (−0.30 to 0.29) | >.99 | 0.12 (−0.37 to 0.60) | >.99 |
Abbreviations: HbA1c, hemoglobin A1c; MDI, multiple daily injections.
SI conversion factor: to convert to proportion of total hemoglobin, multiply by 0.01.
Adjusted differences in HbA1c change are model-based estimates of the difference in HbA1c change from baseline between each category compared with the reference group, as indicated. These model-based estimates were obtained from linear mixed modeling of repeated measures of HbA1c adjusted for patient age, sex, race, ethnicity, social deprivation index, rurality, baseline HbA1c, and body mass index; patients were nested within practitioners.
Subgroup Analysis: Baseline HbA1c of 8% or Higher
Among patients whose baseline HbA1c was 8% or higher, those who used telemedicine only had no significant change in adjusted HbA1c at 12 or 24 months (Table 2). However, patients who used in-person or mixed follow-up had significant improvement in adjusted HbA1c at both 12 and 24 months, mirroring patterns observed for the overall cohort. Among patients who used telemedicine or mixed follow-up, those prescribed MDI had a worse estimated change in HbA1c at 12 months compared with those not prescribed insulin; there was no significant difference among patients who used in-person follow-up only (Table 3). The estimated difference in HbA1c change at 24 months for patients prescribed either basal or MDI compared with no insulin was not significant across any care modality in this subgroup. As in the overall cohort, cardiovascular and psychological comorbidities were not significantly associated with glycemic outcomes in any care modality.
Discussion
In this retrospective study of adults who received endocrinology care for T2D in a large health system from 2020 to 2022, patients accessing care through telemedicine alone had worse glycemic outcomes compared with patients who transitioned to in-person or mixed care. These findings build on and contrast with prior studies conducted in the primary care setting, which demonstrated similar glycemic outcomes of telemedicine and in-person care for T2D.10,11,13,33 Patients with T2D who receive endocrinology care and have more complex care needs, including those who use insulin or have HbA1c above goal, may not be well served by telemedicine care alone as currently implemented.
Telemedicine emerged as a prominent modality of diabetes care delivery during the COVID-19 pandemic, but utilization patterns have changed over time. Our findings on patient subgroups who rely on telemedicine to access specialty diabetes care are consistent with prior work and identify new characteristics associated with ongoing telemedicine use. We found that younger,34,35,36,37,38 female,10,35,36,37,38 and urban-dwelling39,40,41 patients were more likely to use telemedicine only, similar to previous data in primary care and endocrinology settings. Black patients in our study were more likely to use telemedicine only, while prior evidence on the association of race and ethnicity with telemedicine use is mixed.35,36,37,38,42,43 We found that patients with less complex diabetes were more likely to use telemedicine only. In addition, our findings add new evidence that telemedicine may be particularly important for ensuring access to endocrinology care for patients who have psychological comorbidities, which are known to impact diabetes self-management and outcomes, and may require additional support to achieve treatment goals.44,45
In contrast to our findings, studies conducted earlier in the pandemic in primary care or general diabetes populations found similar glycemic control between patients receiving telemedicine and in-person diabetes care.10,11,12,13,33 There are several potential explanations for the observed lack of HbA1c improvement among patients using telemedicine alone to access endocrinology care in our study. First, patient-level factors that may lead to preferential use of telemedicine care can also impact diabetes self-management and health care access in general. The telemedicine group was more urban dwelling, younger, more likely to be Black and female, and may face unmeasured competing demands to diabetes self-management, such as caregiving responsibilities, transportation barriers, or work schedules.46 Telemedicine-only users also had lower care utilization, including less frequent appointments and HbA1c testing, which may lead to more clinical inertia47 and less intensification of treatment by endocrinology practitioners. Although it is not clear whether lower care utilization was driven by patients, practitioners, or systemic barriers, prior studies of diabetes telemedicine have found similar results.13,48,49 Additionally, differences in patient-practitioner communication via telemedicine, including difficulty building rapport,50,51 may lead to differences in both patient self-management and practitioner treatment decisions.
Another potential explanation for inferior glycemic outcomes in the telemedicine group is that strategies to support glycemic improvement that are available during in-person appointments have not consistently been translated to telemedicine care. Care elements which may be particularly influential for patients with elevated HbA1c or complex insulin regimens, such as self-management education and support, sharing of home blood glucose data through device downloads or written logs, and educational resources for initiation of diabetes technology or new medications, may not currently be routinely delivered through telemedicine or available at the point-of-care during telemedicine visits. In our prior work in this care setting, practitioners described how inferior availability of glucose data limited their ability to intensify treatment through telemedicine.19 Implementation of approaches to overcome these differences, such as team-based virtual care and technological tools to automate blood glucose data sharing, are needed to ensure all patients receive high-quality diabetes care regardless of care modality.52
Strengths and Limitations
There are a number of strengths and limitations to this study. This is the first study, to our knowledge, to examine outcomes of telemedicine care specifically in the endocrinology setting and according to clinical factors that impact treatment complexity. Although demographic variables that differed between groups were included as covariates, cohorts were not balanced on potentially confounding baseline characteristics. Factors including treatment complexity and glycemic control, as well as geographic and transportation barriers, may have impacted whether patients received care via telemedicine or in-person care; thus, findings reflect glycemic outcomes for clinical patients who received care via each modality, and do not indicate causal associations. This study was performed in a single health system, and patients had to use at least 1 medication for diabetes and were predominantly white and urban; thus, results may not generalize to other settings with different infrastructure for telemedicine care, rural areas, or patient populations with more racial and ethnic diversity or who have diet-controlled diabetes. In addition, HbA1c values were not consistently captured for patients who had testing done at facilities that do not communicate with the electronic medical records. However, demographic variables which differed between included patients and those excluded due to lack of follow-up HbA1c value (11.6% of eligible patients) were controlled for in models, limiting the impact of this issue on our results. Finally, with loss to follow-up over time, there were fewer patients who had HbA1c results at 24 months compared with 12 months; thus, these results should be interpreted as exploratory only.
Conclusions
In this cohort study of adults who received endocrinology specialist care for T2D in a large health system from 2020 to 2022, patients who accessed care through telemedicine alone had worse glycemic outcomes compared with patients who transitioned to in-person or mixed care. Since some patients with barriers to in-person endocrinology care will continue to rely on telemedicine to access care, structured approaches to ensure routine delivery of high-quality team-based diabetes care are needed. Translation of successful strategies from clinical trials into routine telemedicine care, especially targeted toward adults with more complex diabetes, is critical to improve clinical outcomes for patients who rely on this care modality.
eTable 1. ICD-10 Codes Used to Identify Comorbid Conditions
eTable 2. Characteristics of Modeled vs Excluded Patients According to Presence of Follow-Up HbA1c
eTable 3. Supplemental Analysis: Adjusted Difference HbA1c Change for Each Care Modality Cohort by Insulin Use
Data Sharing Statement
References
- 1.Patel SY, Mehrotra A, Huskamp HA, Uscher-Pines L, Ganguli I, Barnett ML. Variation in telemedicine use and outpatient care during the COVID-19 pandemic in the United States: study examines variation in total US outpatient visits and telemedicine use across patient demographics, specialties, and conditions during the COVID-19 pandemic. Health Aff. 2021;40(2):349-358. doi: 10.1377/hlthaff.2020.01786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doximity . State of telemedicine report, second edition. 2022. Accessed December 8, 2022. https://c8y.doxcdn.com/image/upload/Press%20Blog/Research%20Reports/Doximity-Telemedicine-Report-2022.pdf
- 3.Walker AF, Hood KK, Gurka MJ, et al. Barriers to technology use and endocrinology care for underserved communities with type 1 diabetes. Diabetes Care. 2021;44(7):1480-1490. doi: 10.2337/dc20-2753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu H, Holt JB, Cheng YJ, Zhang X, Onufrak S, Croft JB. Population-based geographic access to endocrinologists in the United States, 2012. BMC Health Serv Res. 2015;15(1):541. doi: 10.1186/s12913-015-1185-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hangaard S, Laursen SH, Andersen JD, et al. The effectiveness of telemedicine solutions for the management of type 2 diabetes: a systematic review, meta-analysis, and meta-regression. J Diabetes Sci Technol. Published online December 26, 2021:19322968211064630. doi: 10.1177/19322968211064633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flodgren G, Rachas A, Farmer AJ, Inzitari M, Shepperd S. Interactive telemedicine: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2015;2015(9):CD002098. doi: 10.1002/14651858.CD002098.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Timpel P, Oswald S, Schwarz PEH, Harst L. Mapping the evidence on the effectiveness of telemedicine interventions in diabetes, dyslipidemia, and hypertension: an umbrella review of systematic reviews and meta-analyses. J Med Internet Res. 2020;22(3):e16791. doi: 10.2196/16791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eberle C, Stichling S. Clinical improvements by telemedicine interventions managing type 1 and type 2 diabetes: systematic meta-review. J Med Internet Res. 2021;23(2):e23244. doi: 10.2196/23244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tchero H, Kangambega P, Briatte C, Brunet-Houdard S, Retali GR, Rusch E. Clinical effectiveness of telemedicine in diabetes mellitus: a meta-analysis of 42 randomized controlled trials. Telemed J E Health. 2019;25(7):569-583. doi: 10.1089/tmj.2018.0128 [DOI] [PubMed] [Google Scholar]
- 10.Quinton JK, Ong MK, Sarkisian C, et al. The impact of telemedicine on quality of care for patients with diabetes after March 2020. J Gen Intern Med. 2022;37(5):1198-1203. Published online January 28, 2022. doi: 10.1007/s11606-021-07367-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kubes JN, Jones L, Hassan S, Franks N, Wiley Z, Kulshreshtha A. Differences in diabetes control in telemedicine vs. in-person only visits in ambulatory care setting. Prev Med Rep. 2022;30:102009. doi: 10.1016/j.pmedr.2022.102009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grauer A, Duran AT, Liyanage-Don NA, et al. Association between telemedicine use and diabetes risk factor assessment and control in a primary care network. J Endocrinol Invest. 2022;45(9):1749-1756. doi: 10.1007/s40618-022-01814-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel SY, McCoy RG, Barnett ML, Shah ND, Mehrotra A. Diabetes care and glycemic control during the COVID-19 pandemic in the United States. JAMA Intern Med. 2021;181(10):1412-1414. doi: 10.1001/jamainternmed.2021.3047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li G, Zhang J, Van Spall HGC, et al. Exploring ethnic representativeness in diabetes clinical trial enrolment from 2000 to 2020: a chronological survey. Diabetologia. 2022;65(9):1461-1472. doi: 10.1007/s00125-022-05736-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sen A, Goldstein A, Chakrabarti S, et al. The representativeness of eligible patients in type 2 diabetes trials: a case study using GIST 2.0. J Am Med Inform Assoc. 2018;25(3):239-247. doi: 10.1093/jamia/ocx091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Groot J, Wu D, Flynn D, Robertson D, Grant G, Sun J. Efficacy of telemedicine on glycaemic control in patients with type 2 diabetes: a meta-analysis. World J Diabetes. 2021;12(2):170-197. doi: 10.4239/wjd.v12.i2.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faruque LI, Wiebe N, Ehteshami-Afshar A, et al. ; Alberta Kidney Disease Network . Effect of telemedicine on glycated hemoglobin in diabetes: a systematic review and meta-analysis of randomized trials. CMAJ. 2017;189(9):E341-E364. doi: 10.1503/cmaj.150885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crowley MJ, Tarkington PE, Bosworth HB, et al. Effect of a comprehensive telehealth intervention vs telemonitoring and care coordination in patients with persistently poor type 2 diabetes control: a randomized clinical trial. JAMA Intern Med. 2022;182(9):943-952. Published online July 25, 2022. doi: 10.1001/jamainternmed.2022.2947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zupa MF, Alexopoulos AS, Esteve L, Rosland AM. Specialist perspectives on delivering high-quality telemedicine for diabetes: a mixed methods survey study. J Endocr Soc. 2023;7(5):bvad039. doi: 10.1210/jendso/bvad039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sitter KE, Wong DH, Bolton RE, Vimalananda VG. Clinical appropriateness of telehealth: a qualitative study of endocrinologists’ perspectives. J Endocr Soc. 2022;6(8):bvac089. doi: 10.1210/jendso/bvac089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ElSayed NA, Aleppo G, Aroda VR, et al. ; on behalf of the American Diabetes Association . 1. Improving care and promoting health in populations: standards of care in diabetes-2023. Diabetes Care. 2023;46(suppl 1):S10-S18. doi: 10.2337/dc23-S001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vimalananda VG, Brito JP, Eiland LA, et al. Appropriate use of telehealth visits in endocrinology: policy perspective of the endocrine society. J Clin Endocrinol Metab. 2022;107(11):2953-2962. doi: 10.1210/clinem/dgac494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Timpel P, Harst L. Research implications for future telemedicine studies and innovations in diabetes and hypertension-a mixed methods study. Nutrients. 2020;12(5):E1340. doi: 10.3390/nu12051340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344-349. doi: 10.1016/j.jclinepi.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 25.Pentakota SR, Rajan M, Fincke BG, et al. Does diabetes care differ by type of chronic comorbidity?: An evaluation of the Piette and Kerr framework. Diabetes Care. 2012;35(6):1285-1292. doi: 10.2337/dc11-1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piette JD, Kerr EA. The impact of comorbid chronic conditions on diabetes care. Diabetes Care. 2006;29(3):725-731. doi: 10.2337/diacare.29.03.06.dc05-2078 [DOI] [PubMed] [Google Scholar]
- 27.Butler DC, Petterson S, Phillips RL, Bazemore AW. Measures of social deprivation that predict health care access and need within a rational area of primary care service delivery. Health Serv Res. 2013;48(2 Pt 1):539-559. doi: 10.1111/j.1475-6773.2012.01449.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robert Graham Center - Policy Studies in Family Medicine & Primary Care . Social deprivation index (SDI). Accessed January 10, 2023.https://www.graham-center.org/maps-data-tools/social-deprivation-index.html
- 29.United States Department of Agriculture Economic Research Service . Rural-urban commuting area codes. Accessed March 30, 2022.https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes
- 30.Mercado CI, McKeever Bullard K, Gregg EW, Ali MK, Saydah SH, Imperatore G. Differences in U.S. rural-urban trends in diabetes ABCS, 1999-2018. Diabetes Care. 2021;44(8):1766-1773. doi: 10.2337/dc20-0097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lutfiyya MN, Patel YR, Steele JB, et al. Are there disparities in diabetes care? A comparison of care received by US rural and non-rural adults with diabetes. Prim Health Care. 2009;10(04):320. doi: 10.1017/S146342360999017X [DOI] [Google Scholar]
- 32.Dugani SB, Mielke MM, Vella A. Burden and management of type 2 diabetes in rural United States. Diabetes Metab Res Rev. 2021;37(5):e3410. doi: 10.1002/dmrr.3410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aubert CE, Henderson JB, Kerr EA, Holleman R, Klamerus ML, Hofer TP. Type 2 diabetes management, control and outcomes during the COVID-19 pandemic in older us veterans: an observational study. J Gen Intern Med. 2022;37(4):870-877. doi: 10.1007/s11606-021-07301-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haynes SC, Kompala T, Neinstein A, Rosenthal J, Crossen S. Disparities in telemedicine use for subspecialty diabetes care during COVID-19 shelter-in-place orders. J Diabetes Sci Technol. 2021;15(5):986-992. doi: 10.1177/1932296821997851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kakani P, Sorensen A, Quinton JK, et al. Patient characteristics associated with telemedicine use at a large academic health system before and after COVID-19. J Gen Intern Med. 2021;36(4):1166-1168. doi: 10.1007/s11606-020-06544-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khatana SAM, Yang L, Eberly LA, Julien HM, Adusumalli S, Groeneveld PW. Predictors of telemedicine use during the COVID-19 pandemic in the United States-an analysis of a national electronic medical record database. PLoS One. 2022;17(6):e0269535. doi: 10.1371/journal.pone.0269535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drake C, Lian T, Cameron B, Medynskaya K, Bosworth HB, Shah K. Understanding telemedicine’s “new normal”: variations in telemedicine use by specialty line and patient demographics. Telemed J E Health. 2022;28(1):51-59. doi: 10.1089/tmj.2021.0041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reed ME, Huang J, Graetz I, et al. Patient characteristics associated with choosing a telemedicine visit vs office visit with the same primary care clinicians. JAMA Netw Open. 2020;3(6):e205873. doi: 10.1001/jamanetworkopen.2020.5873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Friedman AB, Gervasi S, Song H, et al. Telemedicine catches on: changes in the utilization of telemedicine services during the COVID-19 pandemic. Am J Manag Care. 2022;28(1):e1-e6. [DOI] [PubMed] [Google Scholar]
- 40.Fear K, Hochreiter C, Hasselberg MJ. Busting three myths about the impact of telemedicine parity. NEJM Catal. 2022;3(10). doi: 10.1056/CAT.22.0086 [DOI] [Google Scholar]
- 41.Ferguson JM, Jacobs J, Yefimova M, Greene L, Heyworth L, Zulman DM. Virtual care expansion in the Veterans Health Administration during the COVID-19 pandemic: clinical services and patient characteristics associated with utilization. J Am Med Inform Assoc. 2021;28(3):453-462. doi: 10.1093/jamia/ocaa284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Molina F, Soulos PR, Brockman A, Oldfield BJ. Clinical and sociodemographic factors associated with telemedicine engagement in an urban community health center cohort during the COVID-19 pandemic. Telemed J E Health. 2023;29(6):875-885. doi: 10.1089/tmj.2022.0389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Campos-Castillo C, Anthony D. Racial and ethnic differences in self-reported telehealth use during the COVID-19 pandemic: a secondary analysis of a US survey of internet users from late March. J Am Med Inform Assoc. 2021;28(1):119-125. doi: 10.1093/jamia/ocaa221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmitt A, Bendig E, Baumeister H, Hermanns N, Kulzer B. Associations of depression and diabetes distress with self-management behavior and glycemic control. Health Psychol. 2021;40(2):113-124. doi: 10.1037/hea0001037 [DOI] [PubMed] [Google Scholar]
- 45.Nouwen A, Adriaanse MC, van Dam K, et al. ; European Depression in Diabetes (EDID) Research Consortium . Longitudinal associations between depression and diabetes complications: a systematic review and meta-analysis. Diabet Med. 2019;36(12):1562-1572. doi: 10.1111/dme.14054 [DOI] [PubMed] [Google Scholar]
- 46.McEwen LN, Kim C, Ettner SL, et al. Competing demands for time and self-care behaviors, processes of care, and intermediate outcomes among people with diabetes: Translating Research Into Action for Diabetes (TRIAD). Diabetes Care. 2011;34(5):1180-1182. doi: 10.2337/dc10-2038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khunti K, Gomes MB, Pocock S, et al. Therapeutic inertia in the treatment of hyperglycaemia in patients with type 2 diabetes: a systematic review. Diabetes Obes Metab. 2018;20(2):427-437. doi: 10.1111/dom.13088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nielsen VM, Song G, Ojamaa LS, Blodgett RP, Rocchio CM, Pennock JN. The COVID-19 pandemic and access to selected ambulatory care services among populations with severely uncontrolled diabetes and hypertension in Massachusetts. Public Health Rep. Published online January 28, 2022:003335492110655. doi: 10.1177/00333549211065515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hooker SA, Truitt A, Anderson JP, Kane S, O’Connor PJ, Sperl-Hillen JM. Impact of the COVID-19 pandemic on type 2 diabetes care delivery. Clin Diabetes. 2022;40(4):442-448. doi: 10.2337/cd21-0116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mog AC, Moldestad M, Kenney R, et al. “I was unsure at first”: a qualitative evaluation of patient perceptions of va clinical video telehealth visits in the V-IMPACT program. J Patient Exp. 2022;9:23743735221107237. doi: 10.1177/23743735221107237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andreadis K, Muellers K, Ancker JS, Horowitz C, Kaushal R, Lin JJ. Telemedicine impact on the patient-provider relationship in primary care during the COVID-19 pandemic. Med Care. 2023;61(suppl 1):S83-S88. doi: 10.1097/MLR.0000000000001808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McDonnell ME. Telemedicine in complex diabetes management. Curr Diab Rep. 2018;18(7):42. doi: 10.1007/s11892-018-1015-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. ICD-10 Codes Used to Identify Comorbid Conditions
eTable 2. Characteristics of Modeled vs Excluded Patients According to Presence of Follow-Up HbA1c
eTable 3. Supplemental Analysis: Adjusted Difference HbA1c Change for Each Care Modality Cohort by Insulin Use
Data Sharing Statement