Abstract
Background.
Density notification laws require notifying women of dense breasts with dense breast prevalence varying by race/ethnicity. We evaluated whether differences in body mass index (BMI) account for differences in dense breasts prevalence by race/ethnicity.
Methods.
Prevalence of dense breasts (heterogeneously or extremely dense) according to Breast Imaging Reporting and Data System and obesity (BMI >30 kg/m2) were estimated from 2,667,207 mammography examinations among 866,033 women in the Breast Cancer Surveillance Consortium from January 2005 through April 2021. Prevalence ratios (PR) for dense breasts relative to overall prevalence by race/ethnicity were estimated by standardizing race/ethnicity prevalence in the BCSC to the 2020 U.S. population, and adjusting for age, menopausal status, and BMI using logistic regression.
Results.
Dense breasts were most prevalent among Asian women (66.0%) followed by non-Hispanic/Latina (NH) White (45.5%), Hispanic/Latina (45.3%), and NH Black (37.0%) women. Obesity was most prevalent in Black women (58.4%) followed by Hispanic/Latina (39.3%), NH White (30.6%) and Asian (8.5%) women. The adjusted prevalence of dense breasts was 19% higher (PR=1.19, 95%CI=1.19–1.20) in Asian women, 8% higher (PR=1.08, 95%CI=1.07–1.08) in Black women, the same in Hispanic/Latina women (PR=1.00, 95%CI=0.99–1.01), and 4% lower (PR=0.96, 95%CI=0.96–0.97) in NH White women relative to the overall prevalence.
Conclusion.
Clinically important differences in breast density prevalence are present across racial/ethnic groups after accounting for age, menopausal status, and BMI.
Impact.
If breast density is the sole criterion used to notify women of dense breasts and discuss supplemental screening it may result in implementing inequitable screening strategies across racial/ethnic groups.
Keywords: Breast density, body mass index, race/ethnicity
Introduction
Having dense breasts (heterogeneously or extremely dense) is a strong, well-established risk factor that increases invasive (1,2), interval (3–5), and advanced (6,7) breast cancer risk, and reduces mammography sensitivity (8,9). Breast density notification laws have been enacted in at least 38 states and a national density notification law will be enacted September 10, 2024 that will inform all women if their breasts are nondense or dense and that dense breasts can mask tumors and increase breast cancer risk (10). Dense breasts are common (11), and studies have shown that the distribution of breast density categories varies across racial and ethnic groups (12,13). For example, Asian women are more likely to have dense breasts than non-Hispanic/Latina (NH) White women (1), and some studies show that Black and Hispanic/Latina women are less likely to have dense breasts than NH White women (14–17).
Body mass index (BMI) is inversely associated with breast density (11); accounting for BMI strengthens the association between breast density and breast cancer risk (18). Racial/ethnic differences in breast density may be confounded by BMI since obesity varies widely across groups with more than 50% of Black women being obese compared with less than 10% of Asian women (13,19). Additionally, being overweight/obese is a risk factor for breast cancer and women who are both overweight/obese and have dense breasts are at highest breast cancer risk (15,18,20).
Prior studies reporting breast density by race/ethnicity have been limited by small sample sizes, reporting results only for limited number of racial/ethnic groups, and reporting results for breast density and BMI separately, not jointly (14,16,21–31). Whether differences in BMI account for racial/ethnic differences in the prevalence of dense breasts is not well understood. We used the large, racially, ethnically, and geographically diverse Breast Cancer Surveillance Consortium (BCSC) cohort to estimate and compare prevalence of dense breasts by race/ethnicity adjusting for age, menopausal status, and BMI.
Materials and Methods
Study Setting, Data Sources, and Participants
This report follows STROBE reporting guidelines for cohort studies. Prospective data were collected at 7 U.S.-based BCSC registries (www.bcsc-research.org): Carolina Mammography Registry, Kaiser Permanente Washington, Metro Chicago Breast Cancer Registry, New Hampshire Mammography Network, Sacramento Area Breast Imaging Registry, San Francisco Mammography Registry, and Vermont Breast Cancer Surveillance System. Registries collect individual-level characteristics and clinical information from community radiology facilities and link to state or regional cancer registries and pathology databases for complete capture of breast cancer diagnoses. We included all digital mammography and digital breast tomosynthesis screening examinations from 140 BCSC facilities performed from January 2005 to April 2021 among women aged 40–74 years without a personal history of breast cancer; non-missing information on Breast Imaging Reporting and Data System (BI-RADS) breast density and BMI; and who self-identified as Hispanic/Latina (of any race) or non-Hispanic/Latina Asian, Black, or White. We excluded women who self-identified as another race or as having multiple races. The final study population included 2,667,207 BI-RADS breast density measurements from 866,033 women interpreted.
BCSC registries and the Statistical Coordinating Center received Institutional Review Board approval for active or passive consenting processes or a waiver of consent to enroll participants, link, and pool data, and perform analysis. Procedures were Health Insurance Portability and Accountability Act compliant, and a Federal Certificate of Confidentiality protects the identities of participants.
Measures, Definitions, and Outcomes
Age, height, weight, menopausal status, race, and ethnicity were collected from self-administered health history questionnaires or extracted from electronic health records at the time of each mammography examination. BMI was calculated as weight in kilograms divided by height in meters squared (kg/m2) and categorized as underweight (<18.5), normal weight (18.5–24.9), overweight (25.0–29.9), obesity class I (30.0–34.4), and obesity class II/III (≥35.0) (32). Postmenopausal status included women who reported periods that stopped naturally, had both ovaries removed, were age 60 years or older, or had no period for ≥365 days. Pre/perimenopausal status included women who reported continued menstrual periods, were not sure if periods had stopped, had their last menstrual period <365 days ago, or used hormonal birth control. Women who could not be classified using these definitions were considered postmenopausal if age ≥52 years or pre/perimenopausal if age <52 years.
Primary outcomes were a dichotomized density as dense (heterogeneously dense or extremely dense) versus nondense (almost entirely fatty or scattered fibroglandular densities) breasts, which is typically utilized for breast density reporting laws, and the four BI-RADS breast density categories recorded by the radiologist as part of clinical practice as almost entirely fatty, scattered fibroglandular densities, heterogeneously dense, or extremely dense (33).
Statistical Analysis
We summarized the study cohort characteristics by race/ethnicity, inversely weighting frequencies by the total number of observations each woman contributed to the study, giving an effective sample size equal to the number of women. We plotted the age-standardized joint distribution of BI-RADS breast density and BMI by race/ethnicity, inversely weighing by the total number of observations each woman contributed. We used age-standardized pairwise Mantel-Haenszel (MH) and Cochran-Mantel-Haenszel (CMH) tests of significance to test if the distribution of breast density within BMI strata differed by race/ethnicity and to test if the joint distribution of breast density and BMI differed by race/ethnicity.
We used logistic regression to model the probability of having dense vs. nondense breasts and polytomous logistic regression with a generalized-logit link to estimate the distribution of the 4 breast density categories to model the probabilities of having dense vs. nondense breasts and the 4 breast density categories as a function of race/ethnicity, fitting 3 sequential models: unadjusted; adjusted for age (linear and quadratic) and menopausal status; and additionally adjusted for BMI (linear and quadratic). We used predictive margins to compute race/ethnicity-specific estimates of the density probabilities, standardizing to the overall cohort distribution for the adjustment variables (34). We estimated the race and ethnicity-specific distribution in the U.S. from the 2020 census (https://data.census.gov/cedsci/table?q=Race%20and%20Ethnicity&g=0100000US&tid=DECENNIALPL2020.P2) and used model-predicted probabilities for each density category and racial/ethnic group in the BCSC to calculate prevalence ratios (PR) of each breast density category for each racial/ethnic group relative to the estimated overall prevalence for the U.S. population; 95% confidence intervals (CI) were computed using 10,000 non-parametric bootstrap (35) iterations clustered at the woman level and selecting one random observation per woman, which showed good convergence for the mean prevalence ratio estimates.
Statistical analyses were performed using SAS/STAT version 14.2 (Cary, NC), R version 4.1.2, and RStudio version 2021.9.1.372. Tests of statistical significance used a two-sided alpha of 0.05.
Data availability
Study protocol and statistical code available on request, please contact kpwa.scc@kp.org with specific queries. Data set available after study aims of funded grants through 2027 are addressed and with appropriate approval from the Breast Cancer Surveillance Consortium Steering Committee. The data underlying this article will be shared on reasonable request to the corresponding author and BCSC with appropriate regulatory approvals.
Results
NH White women constituted 67.8% of the cohort, followed by Asian (12.8%), NH Black (11.8%), and Hispanic/Latina (7.5%) women (Table 1). Age distributions were generally similar across racial/ethnic groups, except a larger proportion of Hispanic/Latina women were age 40–49. Asian women had the largest prevalence of dense breasts (65.9%) followed by NH White (45.5%), Hispanic/Latina (45.2%), and NH Black (36.9%) women. Obesity was most prevalent in NH Black women (58.4%) followed by Hispanic/Latina (39.3%), NH White (30.6%), and Asian (8.5%) women.
Table 1. Characteristics of 2,667,207 observations from 866,033 women in the study cohort by race/ethnicity.
| NH Asian N = 111,209 |
NH Black N = 102,388 |
Hispanic/Latina N = 65,117 |
NH White N = 587,320 |
|
|---|---|---|---|---|
| % | % | % | % | |
| Age Group (years) | ||||
| 40–49 | 32.1 | 28.2 | 40.2 | 28.6 |
| 50–59 | 34.4 | 33.8 | 33.1 | 32.7 |
| 60–69 | 26.4 | 27.3 | 20.2 | 28.2 |
| 70–74 | 7.0 | 10.7 | 6.4 | 10.6 |
| Menopausal status | ||||
| Pre/perimenopausal | 40.6 | 38.5 | 48.2 | 36.6 |
| Postmenopausal | 59.4 | 61.5 | 51.8 | 63.4 |
| BI-RADS breast density | ||||
| Almost entirely fatty | 4.2 | 12.4 | 10.7 | 11.3 |
| Scattered fibroglandular densities | 29.9 | 50.7 | 44.1 | 43.2 |
| Heterogeneously dense | 49.9 | 33.3 | 39.4 | 37.6 |
| Extremely dense | 16.1 | 3.7 | 5.9 | 7.9 |
| Body mass index (kg/m2) | ||||
| Underweight (<18.5) | 3.5 | 0.6 | 0.7 | 1.5 |
| Normal (18.5–24.9) | 61.9 | 13.5 | 25.6 | 39.3 |
| Overweight (25.0–29.9) | 26.1 | 27.5 | 34.4 | 28.7 |
| Obese I (30.0–34.9) | 6.5 | 26.1 | 22.8 | 16.6 |
| Obese II/III (≥35.0) | 2.0 | 32.3 | 16.5 | 14.0 |
| BI-RADS breast density measures per woman | ||||
| 1 | 40.5 | 36.6 | 41.0 | 32.3 |
| 2 | 23.5 | 22.6 | 23.2 | 19.7 |
| 3 | 13.3 | 13.5 | 13.1 | 12.7 |
| 4 | 8.4 | 9.3 | 8.3 | 9.6 |
| 5 or more | 14.2 | 17.9 | 14.3 | 25.6 |
Abbreviations: NH, non-Hispanic/Latina; kg, kilograms; m, meters; BI-RADS, Breast Imaging Reporting and Data System.
Frequencies are inversely weighted by the number of observations per woman and rounded.
The age-adjusted distribution of breast density within each BMI category (Figure 1) and the joint distribution of breast density and BMI were significantly different across racial/ethnic groups (Supplemental Figure S1) (all p-values <0.0001). Asian women had the highest prevalence of dense breasts within each BMI category. NH Black and Hispanic/Latina women had lower prevalence of dense breasts within the underweight and normal BMI categories compared to White women, but higher prevalence within overweight and obese I/II/III categories. There was an inverse relationship between BMI and breast density for all racial/ethnic groups.
Figure 1:
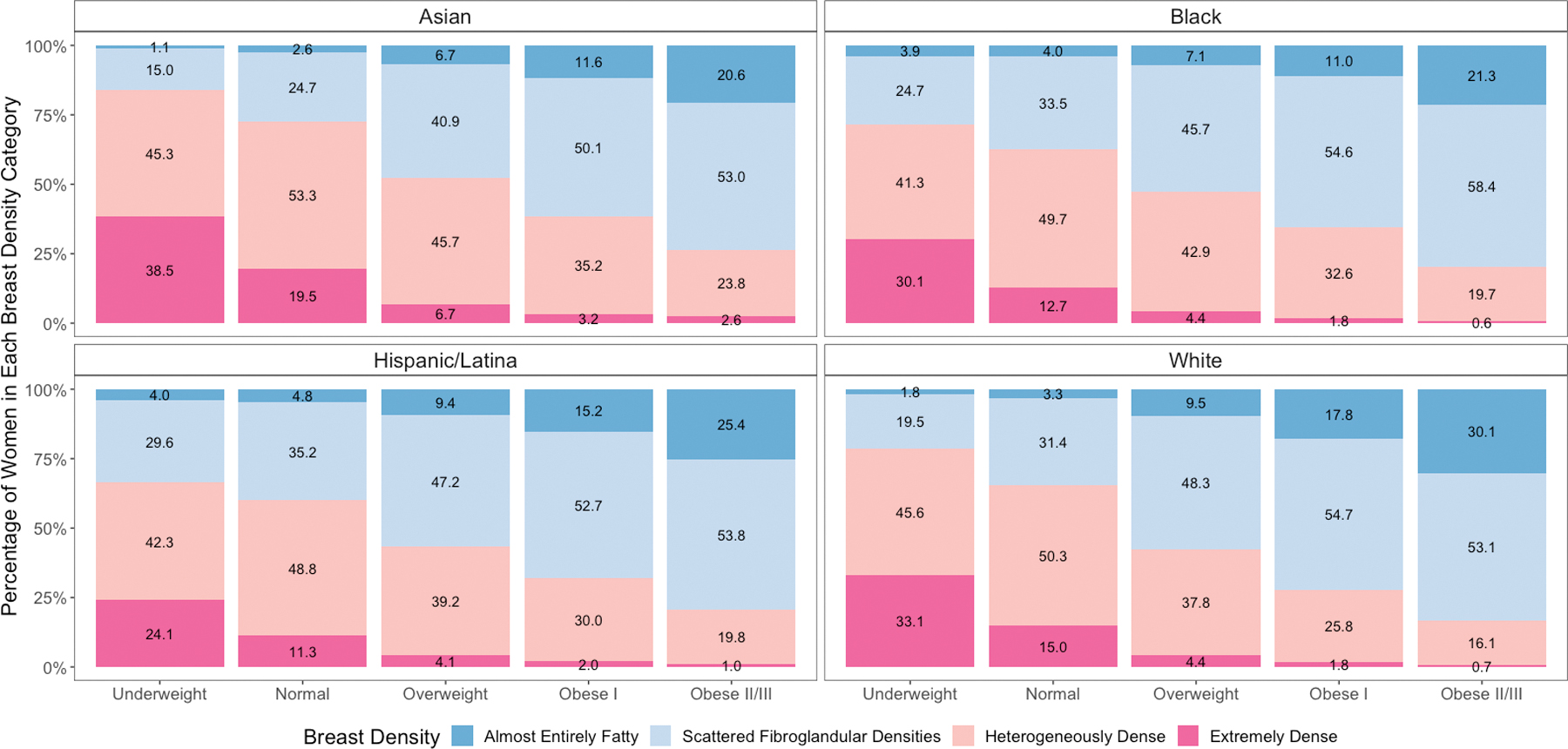
Distribution of Breast Imaging Reporting and Data System breast density categories within body mass index categories by race and ethnicity age-adjusted and inversely weighted for the number of mammograms per woman. Panels according to race and ethnicity.
Results were largely unchanged with partial adjustment for age and menopausal status, with larger changes after additional adjustment for BMI (Table 2). Relative to the overall population, Asian women had a higher prevalence of dense breasts in both unadjusted and adjusted models. The fully adjusted prevalence of dense breasts was 19% higher (PR=1.19, 95%CI=1.19–1.20) in Asian women, 8% higher (PR=1.08, 95%CI=1.07–1.08) in NH Black women, (PR=1.00, 95%CI=0.99–1.01) the same in Hispanic/Latina women, and 4% lower (PR=0.96, 95%CI=0.96–0.97) in White women relative to the overall prevalence.
Table 2. Prevalence ratios and 95% bootstrap confidence intervals for BI-RADS dense versus nondense breasts by race and ethnicity.
| Model | Race/ethnicity | Nondense PR |
Dense PR |
|---|---|---|---|
| Unadjusteda | NH Asian | 0.62 (0.62, 0.63) |
1.45 (1.44, 1.46) |
| NH Black | 1.16 (1.15, 1.16) |
0.81 (0.81, 0.82) |
|
| Hispanic/Latina | 1.00 (1.00, 1.01) |
0.99 (0.99, 1.00) |
|
| NH White | 1.00 (1.00, 1.00) |
1.00 (1.00, 1.00) |
|
| Partially adjustedb |
NH Asian | 0.62 (0.62, 0.63) |
1.48 (1.47, 1.49) |
| NH Black | 1.15 (1.15, 1.16) |
0.82 (0.81, 0.82) |
|
| Hispanic/Latina | 1.06 (1.05, 1.07) |
0.93 (0.92, 0.93) |
|
| NH White | 0.98 (0.98, 0.99) |
1.02 (1.02, 1.02) |
|
| Fully adjustedc |
NH Asian | 0.84 (0.83, 0.85) |
1.19 (1.19, 1.20) |
| NH Black | 0.93 (0.93, 0.94) |
1.08 (1.07, 1.08) |
|
| Hispanic/Latina | 1.00 (0.99, 1.01) |
1.00 (0.99, 1.01) |
|
| NH White | 1.03 (1.03, 1.03) |
0.96 (0.96, 0.97) |
Abbreviations: NH, non-Hispanic/Latina; BI-RADS, Breast Imaging Reporting and Data System. PR, prevalence ratio=prevalence of racial/ethnic and density group relative to overall Breast Cancer Surveillance Consortium study population standardized to the US population
Unadjusted race/ethnicity.
Additionally adjusted for age (linear and quadratic) and menopausal status.
Additionally adjusted for body mass index (linear and quadratic).
When examining results by the four density categories, unadjusted PRs for heterogeneously dense (PR=1.31, 95%CI=1.30–1.32) and extremely dense (PR=2.17, 95%CI=2.14–2.20) breasts in Asian women increased minimally to PR=1.35 (95%CI=1.34–1.35) and PR=2.25 (95%CI=2.22–2.29) after adjustment for age and menopause, but decreased substantially to PR=1.16 (95%CI=1.15–1.16) and PR=1.48 (95%CI=1.45–1.50) after additional adjustment for BMI (Figure 2 and Supplemental Table S1). NH Black women had lower unadjusted prevalence of heterogeneously dense (PR=0.87, 95%CI=0.87–0.88) and extremely dense (PR=0.50, 95%CI=0.48–0.51) breasts than the overall population, but following full adjustment for age, menopausal status, and BMI, they had higher prevalence of heterogeneously dense (PR=1.08, 95%CI=1.08–1.09) and extremely dense breasts (PR=1.02, 95%CI=0.99–1.05) than the overall population (Figure 2 and Supplemental Table S1). Hispanic/Latina women had a similar prevalence of dense breasts as the overall population in both unadjusted and adjusted models, but their lower prevalence of extremely dense breasts attenuated somewhat from unadjusted (PR=0.79, 95%CI=0.77–0.81) to fully adjusted (PR=0.89, 95%CI=0.86–0.91) models. NH White women were significantly more likely to have almost entirely fatty and significantly less likely to have heterogeneously or extremely dense breasts after adjustment for age, menopausal status, and BMI, though absolute differences were small.
Figure 2:
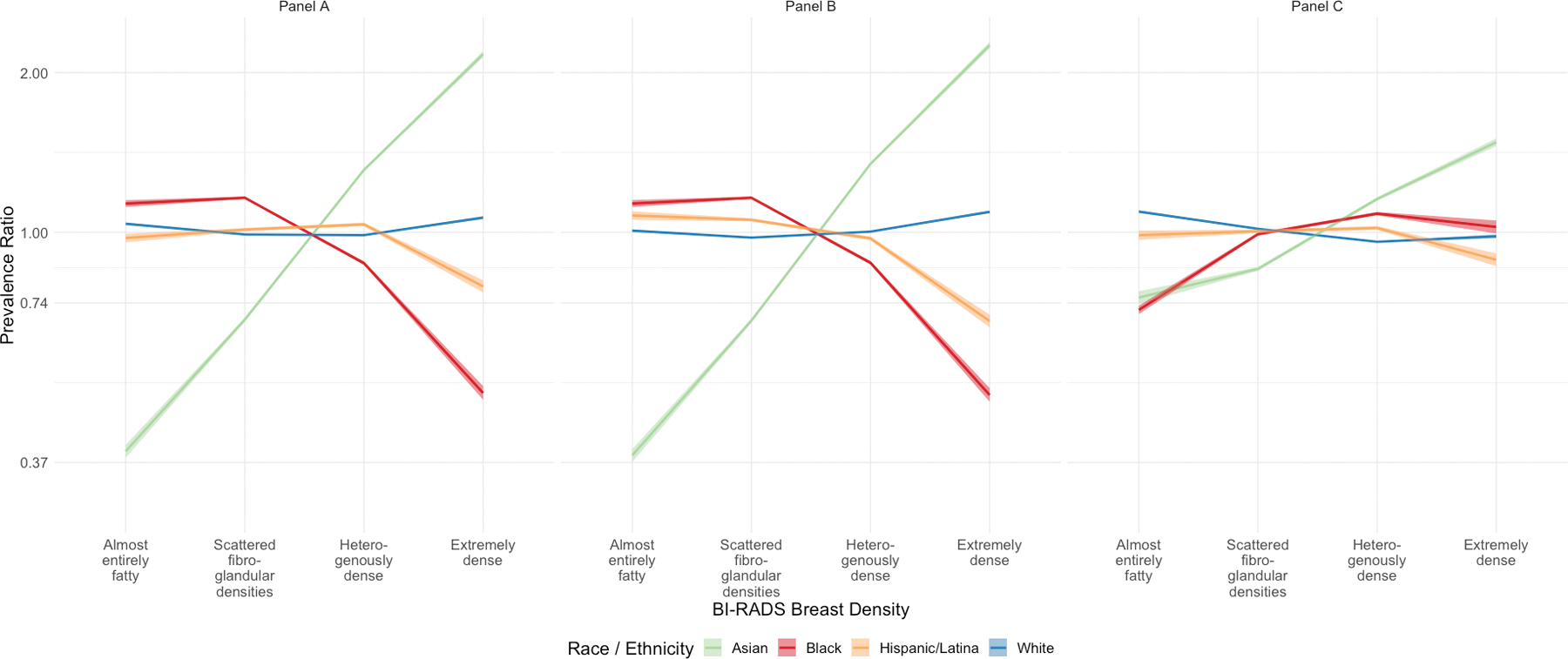
Prevalence ratios and 95% bootstrap confidence intervals for polytomous BI-RADS breast density category by race and ethnicity. Graph A is unadjusted, Graph B is adjusted for age (linear and quadratic) and menopausal status, and Graph C is adjusted for age (linear and quadratic) and menopausal status, and body mass index (linear and quadratic).
Discussion
Among more than 2.6 million breast density measurements from 140 US-based radiology facilities in the BCSC, we found clinically important differences in the prevalence of dense breasts across racial/ethnic groups that remained after adjusting for age, menopausal status, and BMI. Given BMI and breast density are both risk factors for breast cancer (13,36) and the strength of their association with breast cancer varies by racial/ethnic groups (1), considering these prevalence differences may be important for estimating breast cancer risk in racial/ethnic groups and reporting breast density results to women.
The goal of breast density notification laws is to identify women at increased risk of breast cancer and masked tumors that may benefit from supplemental imaging. State laws do not consider other breast cancer risk factors, such as BMI, and the Food & Drug Administration (FDA) Mammography Quality and Standards Act Final Rule has proposed to only report if women have dense or nondense breasts (10). Some have suggested that under current state notification laws, the use of BI-RADS breast density alone may result in suboptimal risk stratification and recommendations for supplemental imaging (5,37–39,40). Our study suggests that Asian women are most likely to have dense breasts with associated density notification letters suggesting consideration of supplemental imaging, whereas Black women are less likely to have dense breasts which do not trigger letters suggesting consideration of supplemental imaging. Specifically, Asian women are most likely to be notified of dense breasts irrespective of adjustment for BMI, whereas Black women are less likely to be notified of dense breasts if density is not adjusted for BMI. Studies indicate disparities already exist such that non-Hispanic White women are more likely to have supplemental imaging for dense breasts than non-Hispanic Black or Asian women which may lead to disparities in cancer outcomes (39, 41). Additionally, advanced cancer rates after mammography are higher in Black women (7) and interval rates are lower in Asian women (42). Reporting breast cancer risk that incorporates breast density and BMI would better identify women at highest risk of a missed or advanced cancer for discussion of supplemental imaging rather than using breast density alone as the sole criterion (5,7,38) with the result of potentially more equitable receipt of supplemental imaging.
BI-RADS breast density is a qualitative measure of the relative proportion of fibroglandular and fat tissue on mammograms that is inversely associated with BMI. Dense volume is a quantitative measure of the volume of fibroglandular tissue that can be measured with commercial software and is minimally influenced by BMI or increased among overweight and obese women (18,27,43). Volpara and Quantra are FDA approved commercial software used in clinical practice to assess volumetric breast density. Although Asian women have the highest prevalence of BI-RADS dense breasts, they have the lowest dense volume (43). Thus, many Asian women, in whom a large proportion have dense BI-RADS breast density and normal BMI, may be inappropriately notified, and recommended for supplemental imaging recommendations under current notification laws based on BI-RADS breast density alone. Dense volume is a strong predictor of interval and advanced cancers, and, if used for density notification, would more accurately identify women at risk for a missed cancer than BI-RADS breast density unadjusted for BMI (44,45).
Five studies have examined BI-RADS breast density by race/ethnicity considering age, menopausal status, and BMI. McCarthy et al. reported that Black women had higher breast density than White women across all quantitative measures of breast density and no difference in the qualitative BI-RADS density distribution by race (27). Moore et al. compared Black and White women and found Black women had a higher odds of extremely dense breasts than White women (46). Oppong et al. compared BI-RADS breast density in non-Hispanic/Latina White, Black, and Hispanic/Latina women and found Hispanic/Latina women have the highest density followed by Black and non-Hispanic/Latina White women (28). One study examined Asian, Black, non-Hispanic White, and Hispanic/Latina women and compared dense breasts to almost entirely fatty breasts and found Asian and Black women have higher odds of dense breasts than Hispanic/Latina and NH White women, which have similar odds of dense breasts (12). A recent study observed the lowest prevalence of extremely dense breasts among American Indian and Alaska native women and the greatest prevalence of extremely dense breasts among Chinese women (47). Our results extend the literature by assessing BI-RADS breast density in the BCSC cohort, a large representative sample of U.S. women, and report that the adjusted prevalence of dense breasts is highest in Asian and Black women compared to Hispanic and White women after adjusting for BMI. These results are consistent with a study that measured the same four racial/ethnic groups but assessed odds of extremely dense breasts rather than prevalence of the four density categories (12).
Our study strengths include the large, geographically, and racially/ethnically diverse BCSC cohort, which is broadly representative of the U.S. population and has larger sample sizes than many other studies for the four largest U.S. racial and ethnic groups. Our analysis included both digital mammography and digital breast tomosynthesis, which reflect recent clinical practice. We have previously reported that BI-RADS breast density categories were similar on digital breast tomosynthesis exams compared to digital mammography exams (48). We also have demonstrated that density agreement on digital breast tomosynthesis vs. digital mammography is high based on women with both examinations less than 36 months apart (49).
Our study has limitations. Even with a very large study cohort and multiple observations per woman, some estimated CIs were wide due to small sample sizes for NH Black and Hispanic/Latina women in the underweight category or for Asian women in the obese I/II/III categories, but sample sizes were still larger than many other studies. We were unable to evaluate quantitative measures of breast density; however, BI-RADS breast density is the most commonly collected density measure in clinical practice in the U.S., triggers density notification laws, and is used in several major breast cancer risk prediction models (36,50,51). We were not able to analyze other measures of adiposity, such as central adiposity, waist-to-hip ratios, or visceral versus subcutaneous fat distribution, and BMI was primarily self-reported, but there is good overall agreement between self-reported and measured height, weight, and BMI (52). Also, BMI measures in the BCSC cohort have associations with breast cancer consistent with the literature (38), suggesting self-reported height and weight in the BCSC reflect height and weight measures in published studies. We did not consider other factors that are correlated with breast density, such as a family history of breast cancer, but this association is weaker compared to age and BMI, reduced by controlling for age and BMI (11), and does not vary by race/ethnicity (53). However, caution should be used in extrapolating the results of this study to populations that vary markedly in such characteristics.
Conclusions
Clinically important differences in the prevalence of dense breasts across racial/ethnic groups remain after adjusting for BMI. The probability of dense breasts assessed by BI-RADS compared to the overall U.S. population for Asian women was attenuated but remained high following adjustment for BMI. The probability of dense breasts in NH Black women increased after adjustment for BMI. Hispanic/Latina women had significantly lower probability of extremely dense breasts compared to the general population with and without adjustment for BMI. Current density notification laws that only require notification of breast density may intensify racial/ethnic disparities when assessing eligibility for supplemental imaging if they do not consider the joint effects of BMI and breast density on risk of a missed or advanced cancer. Differences in the distribution of breast density by race/ethnicity following adjustment for BMI has important implications for equity in breast density notification, access to supplemental imaging, and estimating population breast cancer risk. Directly calculating risk of mammography failure such as advanced breast cancer risk (7) that incorporates race/ethnicity, breast density, and BMI is warranted and could mitigate potential disparities in recommending supplemental imaging.
Supplementary Material
Acknowledgements
This work was supported with funding from the National Cancer Institute (P01CA154292) and the National Institute of Health Office of Research on Women’s Health (3 P01CA154292 - 07S1) which supports effort for Drs. Kerlikowske, Sprague, Tice, Miglioretti and Erin Bowles (P01CA154292) and Dr. Bissell (3 P01CA154292 - 07S1). Data collection for this research was additionally supported by the Breast Cancer Surveillance Consortium with funding from the National Cancer Institute [P01CA154292 (Kerlikowske, Sprague, Bowles, and Miglioretti), U54CA163303 (Sprague), the Patient-Centered Outcomes Research Institute Program Award (PCS-1504-30370)], the Agency for Healthcare Research and Quality [R01 HS018366-01A1 (Dr. Garth Rauscher)] and the University of Vermont Cancer Center with funds generously awarded by the Lake Champlain Cancer Research Organization (grant #032800). The collection of SABIR data was supported by the UC Davis Comprehensive Cancer Center, the Placer County Breast Cancer Foundation, and the UC Davis Clinical and Translational Science Center. Cancer and vital status data collection was supported by several state public health departments and cancer registries (http://www.bcsc-research.org/work/acknowledgement.html). Dr. Sprague’s effort was also supported by the National Institute of General Medical Sciences (P20GM103644) and the National Cancer Institute (R01CA248068). Erin Bowles’ time was funded by the National Cancer Institute (R50CA211115). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute, its Board of Governors or Methodology Committee, the National Cancer Institute, or the National Institutes of Health.
We thank Yang Vang from the University of California Davis Department of Public Health Sciences who provided data management but did not receive additional compensation besides their regular salary. We thank the participating individuals, mammography facilities, and radiologists for the data they have provided for this study. Information about the BCSC may be found at: http://www.bcsc-research.org/.
Footnotes
Disclosures and Conflicts of Interest
Diana Miglioretti, PhD, has textbook royalties from Elsevier outside the submitted work. All other authors declare no conflicts of interest.
References
- 1.Bissell MCS, Kerlikowske K, Sprague BL, Tice JA, Gard CC, Tossas KY, et al. ; Breast Cancer Surveillance Consortium. Breast cancer population attributable risk proportions associated with body mass index and breast density by race/ethnicity and menopausal status. Cancer Epidemiol, Biomarkers, & Prev. 2020;29(10):2048–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engmann NJ, Golmakani MK, Miglioretti DL, Sprague BL, Kerlikowske K, Breast Cancer Surveillance C. Population-attributable risk proportion of clinical risk factors for breast cancer. JAMA Oncol. 2017;3(9):1228–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henderson LM, Miglioretti DL, Kerlikowske K, Wernli KJ, Sprague BL, Lehman CM. Breast cancer characteristics associated with digital versus screen-film mammography for screen-detected and interval cancers. Am J Roentgenol. 2015;205(3):676–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kerlikowske K, Scott CG, Mahmoudzadeh AP, Ma L, Winham S, Jensen MR, et al. Automated and clinical Breast Imaging Reporting and Data System density measures predict risk for screen-detected and interval cancers: A case-control study. Ann Intern Med. 2018;168(11):757–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kerlikowske K, Zhu W, Tosteson AN, Sprague BL, Tice JA, Lehman CD, et al. Identifying women with dense breasts at high risk for interval cancer: a cohort study. Ann Intern Med. 2015;162(10):673–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCarthy AM, Ehsan S, Appel S, Welch M, He W, Bahl M, et al. Risk factors for an advanced breast cancer diagnosis within 2 years of a negative mammogram. Cancer. 2021;127(18):3334–3342. [DOI] [PubMed] [Google Scholar]
- 7.Kerlikowske K, Chen S, Golmakani MK, Sprague BL, Tice JA, Tosteson ANA, et al. Cumulative advanced breast cancer risk prediction model developed in a screening mammography population. J Natl Cancer Inst. 2022;114(5):676–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carney PA, Miglioretti DL, Yankaskas BC, Kerlikowske K, Rosenberg R, Rutter CM, et al. Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography. Ann Intern Med. 2003;138(3):168–175. [DOI] [PubMed] [Google Scholar]
- 9.Houssami N, Abraham LA, Miglioretti DL, Sickles EA, Kerlikowske K, Buist DS, et al. Accuracy and outcomes of screening mammography in women with a personal history of early-stage breast cancer. JAMA. 2011;305(8):790–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Food and Drug Administration. Mammography Quality Standards Act. HHS2023federalregister.gov/d/2023-04550 Accessed 3/10/23. [Google Scholar]
- 11.Sprague BL, Gangnon RE, Burt V, Trentham-Dietz A, Hampton JM, Wellman RD, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Bastawissi AY, White E, Mandelson MT, Taplin S Variation in mammographic breast density by race. Ann Epidemiol. 2001;11(4):257–263. [DOI] [PubMed] [Google Scholar]
- 13.Tice JA, Cummings SR, Smith-Bindman R, Ichikawa L, Barlow WE, Kerlikowske K. Using clinical factors and mammographic breast density to estimate breast cancer risk: development and validation of a new predictive model. Ann Intern Med. 2008;148(5):337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCormack VA, Perry N, Vinnicombe SJ, Silva Idos S Ethnic variations in mammographic density: a British multiethnic longitudinal study. Am J Epidemiol. 2008;168(4):412–421. [DOI] [PubMed] [Google Scholar]
- 15.Razzaghi H, Troester MA, Gierach GL, Olshan AF, Yankaskas BC, Millikan RC. Mammographic density and breast cancer risk in White and African American women. Breast Cancer Res Treat. 2012;135(2):571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ursin G, Ma H, Wu AH, Bernstein L, Salane M, Parisky YR, et al. Mammographic density and breast cancer in three ethnic groups. Cancer Epidemiol Biomarkers Prev. 2003;12(4):332–338. [PubMed] [Google Scholar]
- 17.Chen Z, Wu AH, Gauderman WJ, Bernstein L, Ma H, Pike MC, et al. Does mammographic density reflect ethnic differences in breast cancer incidence rates? Am J Epidemiol. 2004;159(2):140–147. [DOI] [PubMed] [Google Scholar]
- 18.Engmann NJ, Scott CG, Jensen MR, Winham S, Miglioretti DL, Ma L, et al. Combined effect of volumetric breast density and body mass index on breast cancer risk. Breast Cancer Res Treat. 2019;177(1):165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shieh Y, Scott CG, Jensen MR, Norman AD, Bertrand KA, Pankratz VS, et al. Body mass index, mammographic density, and breast cancer risk by estrogen receptor subtype. Breast Cancer Res. 2019;21(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conroy SM, Woolcott CG, Koga KR, Byrne C, Nagata C, Ursin G, et al. Mammographic density and risk of breast cancer by adiposity: an analysis of four case-control studies. Int J Cancer. 2012;130(8):1915–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duffy SW, Jakes RW, Ng FC, Gao F. Interaction of dense breast patterns with other breast cancer risk factors in a case-control study. Br J Cancer. 2004;91(2):233–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evans DG, Brentnall AR, Harvie M, Astley S, Harkness EF, Stavrinos P, et al. Breast cancer risk in a screening cohort of Asian and White British/Irish women from Manchester UK. BMC Public Health. 2018;18(1):178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim U, Monroe KR, Buchthal S, Fan B, Cheng I, Kristal BS, et al. Propensity for Intra-abdominal and Hepatic Adiposity Varies Among Ethnic Groups. Gastroenterology. 2019;156(4):966–975 e910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maskarinec G, Jacobs S, Park SY, Haiman CA, Setiawan VW, Wilkens LR, et al. Type II diabetes, obesity, and breast cancer risk: The Multiethnic Cohort. Cancer Epidemiol Biomarkers Prev. 2017;26(6):854–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maskarinec G, Meng L, Ursin G. Ethnic differences in mammographic densities. Int J Epidemiol. 2001;30(5):959–965. [DOI] [PubMed] [Google Scholar]
- 26.Maskarinec G, Pagano I, Lurie G, Wilkens LR, Kolonel LN. Mammographic density and breast cancer risk: the multiethnic cohort study. Am J Epidemiol. 2005;162(8):743–752. [DOI] [PubMed] [Google Scholar]
- 27.McCarthy AM, Keller BM, Pantalone LM, Hsieh MK, Synnestvedt M, Conant EF, et al. Racial differences in quantitative measures of area and volumetric breast density. J Natl Cancer Inst. 2016;108(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oppong BA, Dash C, O’Neill S, Li Y, Makambi K, Pien E, et al. Breast density in multiethnic women presenting for screening mammography. Breast J. 2018;24(3):334–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rajaram N, Mariapun S, Eriksson M, Tapia J, Kwan PY, Ho WK, et al. Differences in mammographic density between Asian and Caucasian populations: a comparative analysis. Breast Cancer Res Treat. 2017;161(2):353–362. [DOI] [PubMed] [Google Scholar]
- 30.White KK, Park SY, Kolonel LN, Henderson BE, Wilkens LR. Body size and breast cancer risk: the Multiethnic Cohort. Int J Cancer. 2012;131(5):E705–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong CS, Lim GH, Gao F, Jakes RW, Offman J, Chia KS, et al. Mammographic density and its interaction with other breast cancer risk factors in an Asian population. Br J Cancer. 2011;104(5):871–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i-xii, 1–253. [PubMed] [Google Scholar]
- 33.D’Orsi CJ. ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology; 2013. [Google Scholar]
- 34.Graubard BI, Korn EL. Predictive margins with survey data. Biometrics. 1999;55(2):652–659. [DOI] [PubMed] [Google Scholar]
- 35.Efron B, Tibshirani R. An Introduction to the Bootstrap. Boca Raton, FL: Chapman & Hall/CRC; 1993. [Google Scholar]
- 36.Tice JA, Miglioretti DL, Li CS, Vachon CM, Gard CC, Kerlikowske K. Breast density and benign breast disease: Risk assessment to identify women at high risk of breast cancer. J Clin Oncol. 2015;33(28):3137–3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kerlikowske K, Sprague BL, Tosteson ANA, Wernli KJ, Rauscher GH, Johnson D, et al. Strategies to identify women at high risk of advanced breast cancer during routine screening for discussion of supplemental imaging. JAMA Intern Med. 2019;179(9):1230–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kerlikowske K, Walker R, Miglioretti DL, Desai A, Ballard-Barbah R, Buist DSM. Obesity, mammography use and accuracy, and advanced breast cancer risk. J Natl Cancer Inst. 2008;100(23):1724–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ezratty C, Vang S, Brown J, Margolies LR, Jandorf L, Lin JJ. Racial/ethnic differences in supplemental imaging for breast cancer screening in women with dense breasts. Breast Cancer Res Treat. 2020;182(1):181–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tossas KY, Winn RA, Seewaldt VL. Mammographic density laws and inclusion-time for change. JAMA Oncol. 2022;8(1):39–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang S, Houssami N, Brennan M, Nickel B. The impact of mandatory mammographic breast density notification on supplemental screening practice in the United States: a systematic review. Breast Cancer Res Treat. 2021;187(1):11–30. [DOI] [PubMed] [Google Scholar]
- 42.Kerlikowske K, Creasman J, Leung J, Smith-Bindman R, Ernster V. Differences in screening mammography outcomes among white and Asian women. Arch Intern Med 2005;165:1862–1868. [DOI] [PubMed] [Google Scholar]
- 43.Brandt KR, Scott CG, Ma L, Mahmoudzadeh AP, Jensen MR, Whaley DH, et al. Comparison of clinical and automated breast density measurements: implications for risk prediction and supplemental screening. Radiology. 2016;279(3):710–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wanders JOP, Holland K, Karssemeijer N, Peeters PHM, Veldhuis WB, Mann RM, et al. The effect of volumetric breast density on the risk of screen-detected and interval breast cancers: a cohort study. Breast cancer research. 2017;19(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vachon C, Scott CG, Norman AD, Khanani SA, Jensen MR, Moritz RH, et al. Impact of AI system and volumetric density on risk prediction of interval, screen-detected, and advanced breast cancer J Clin Oncol; In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moore JX HY, Appleton C, Colditz G, Toriola AT. Determinants of Mammographic Breast Density by Race Among a Large Screening Population. JNCI Cancer Spectr. 2020;4(2):pkaa010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barnard ME, Martheswaran T, Van Meter M, Buys SS, Curtin K, Doherty JA. Body Mass Index and Mammographic Density in a Multiracial and Multiethnic Population-Based Study. Cancer Epidemiol, Biomarkers, & Prev. 2022;31(7):1313–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sprague BL, Kerlikowske K, Bowles EJA, Rauscher GH, Lee CI, Tosteson ANA, et al. Trends in clinical breast density assessment from the Breast Cancer Surveillance Consortium. J Natl Cancer Inst. 2019;111(6):629–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tice JA, Gard CC, Miglioretti DL, Sprague BL, Tosteson ANA, Joe BN, et al. Comparing mammographic density assessed by digital breast tomosynthesis or digital mammography: The Breast Cancer Surveillance Consortium. Radiology. 2022;302(2):286–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee A, Mavaddat N, Wilcox AN, Cunningham AP, Carver T, Hartley S, et al. BOADICEA: a comprehensive breast cancer risk prediction model incorporating genetic and nongenetic risk factors. Genet Med. 2019;21(8):1708–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brentnall AR, Cuzick J, Buist DSM, Bowles EJA. Long-term accuracy of breast cancer risk assessment combining classic risk factors and breast density. JAMA Oncol. 2018;4(9):e180174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hodge JM, Shah R, McCullough ML, Gapstur SM, Patel AV. Validation of self-reported height and weight in a large, nationwide cohort of U.S. adults. PLoS One. 2020;15(4):e0231229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Han Y, Moore JX, Colditz GA, Toriola AT. Family history of breast cancer and mammographic breast density in premenopausal women. JAMA Netw Open. 2022;5(2):e2148983. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Study protocol and statistical code available on request, please contact kpwa.scc@kp.org with specific queries. Data set available after study aims of funded grants through 2027 are addressed and with appropriate approval from the Breast Cancer Surveillance Consortium Steering Committee. The data underlying this article will be shared on reasonable request to the corresponding author and BCSC with appropriate regulatory approvals.


