This cohort study investigates the association between removal of race stratification from the atherosclerotic cardiovascular pooled cohort risk equations and model performance.
Key Points
Question
Is the removal of race stratification from the atherosclerotic cardiovascular disease pooled cohort risk equations (PCEs) associated with a change model performance?
Findings
In this cohort study of 11 638 participants, removal of race-specific and race-stratified PCEs and the addition of social determinants of health (SDOH) did not change model performance or calibration compared with the original PCE.
Meaning
Results suggest that removal of race or the addition of SDOH did not improve model performance in any subgroup.
Abstract
Importance
Use of race-specific risk prediction in clinical medicine is being questioned. Yet, the most commonly used prediction tool for atherosclerotic cardiovascular disease (ASCVD)—pooled cohort risk equations (PCEs)—uses race stratification.
Objective
To quantify the incremental value of race-specific PCEs and determine whether adding social determinants of health (SDOH) instead of race improves model performance.
Design, Setting, and Participants
Included in this analysis were participants from the biracial Reasons for Geographic and Racial Differences in Stroke (REGARDS) prospective cohort study. Participants were aged 45 to 79 years, without ASCVD, and with low-density lipoprotein cholesterol level of 70 to 189 mg/dL or non–high-density lipoprotein cholesterol level of 100 to 219 mg/dL at baseline during the period of 2003 to 2007. Participants were followed up to 10 years for incident ASCVD, including myocardial infarction, coronary heart disease death, and fatal and nonfatal stroke. Study data were analyzed from July 2022 to February 2023.
Main outcome/measures
Discrimination (C statistic, Net Reclassification Index [NRI]), and calibration (plots, Nam D’Agostino test statistic comparing observed to predicted events) were assessed for the original PCE, then for a set of best-fit, race-stratified equations including the same variables as in the PCE (model C), best-fit equations without race stratification (model D), and best-fit equations without race stratification but including SDOH as covariates (model E).
Results
This study included 11 638 participants (mean [SD] age, 61.8 [8.3] years; 6764 female [58.1%]) from the REGARDS cohort. Across all strata (Black female, Black male, White female, and White male participants), C statistics did not change substantively compared with model C (Black female, 0.71; 95% CI, 0.68-0.75; Black male, 0.68; 95% CI, 0.64-0.73; White female, 0.77; 95% CI, 0.74-0.81; White male, 0.68; 95% CI, 0.64-0.71), in model D (Black female, 0.71; 95% CI, 0.67-0.75; Black male, 0.68; 95% CI, 0.63-0.72; White female, 0.76; 95% CI, 0.73-0.80; White male, 0.68; 95% CI, 0.65-0.71), or in model E (Black female, 0.72; 95% CI, 0.68-0.76; Black male, 0.68; 95% CI, 0.64-0.72; White female, 0.77; 95% CI, 0.74-0.80; White male, 0.68; 95% CI, 0.65-0.71). Comparing model D with E using the NRI showed a net percentage decline in the correct assignment to higher risk for male but not female individuals. The Nam D’Agostino test was not significant for all race-sex strata in each model series, indicating good calibration in all groups.
Conclusions
Results of this cohort study suggest that PCE performed well overall but had poorer performance in both BM and WM participants compared with female participants regardless of race in the REGARDS cohort. Removal of race or the addition of SDOH did not improve model performance in any subgroup.
Introduction
In recent times, race-based clinical measures have come under increased scrutiny, underscoring the specious relationship between race and the biology of disease.1,2 A growing body of evidence suggests that race-based clinical measures may be systematically biased.3 Given that race is a social construct and race-based clinical measures can reinforce systemic inequities, many have called for removal of race-based estimating equations.2,4 Given recent efforts to remove race stratification from other risk prediction models such as lung spirometry5 and estimated glomerular filtration rate for kidney function,6 we examined the association between race and social determinants of health (SDOH) and atherosclerotic cardiovascular disease (ASCVD) risk prediction.
The 2018 American Heart Association/American College of Cardiology multisociety guideline on the clinical management of blood cholesterol recommends using the ASCVD pooled cohort risk equations (PCEs) for 10-year ASCVD risk prediction to guide initiation of statin therapy and risk factor management for primary prevention of ASCVD.7 The PCEs are sex- and race-stratified equations.7 To evaluate the discrimination and calibration of revised risk prediction equations without race-specific strata (and addition of SDOH), we examined data from the biracial Reasons for Geographic and Racial Differences in Stroke (REGARDS) cohort, which has been used to examine performance of the PCEs previously8 and notably includes participants with data on several SDOH systematically measured at baseline across the 48 continuous US.9
Methods
Study Population
The REGARDS study10 is a National Institutes of Health–sponsored biracial cohort study designed to understand the reasons underlying the Black vs White racial differences in stroke rates as well as geographic differences in stroke mortality comparing the southeastern US to other US regions by oversampling Black participants and participants residing in the southeastern US. Coronary heart disease events continue to be identified and adjudicated in an ancillary study, REGARDS–Myocardial Infarction (REGARDS-MI). A total of 30 239 participants 45 years and older from all 48 contiguous US states and the District of Columbia were enrolled between January 2003 and October 2007. Race was self-identified by REGARDS participants. In the current study, concordant with Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines, we studied adults between the ages of 45 years (inclusive) and 79 years without a history of ASCVD who were not taking statins, had a low-density lipoprotein cholesterol (LDL-C) level between 70 and 189 mg/dL or non–high-density lipoprotein cholesterol (non–HDL-C) level between 100 and 219 mg/dL (to convert LDL-C and HDL-C to millimoles per liter, multiply by 0.0259), and for whom follow-up data existed.
As this study was designed to understand the association of removing race stratification from and/or adding SDOH with 10-year ASCVD risk (and not necessarily guide statin initiation), participants with a history of diabetes were included, but we excluded patients with heart failure and atrial fibrillation. We also excluded participants with missing baseline survey details from the analysis. Characteristics of the baseline assessment and ASCVD adjudication have been reported elsewhere.9 The REGARDS study protocol was approved by the institutional review boards of the participating institutions, and all participants provided written informed consent.
Inclusion of Social Determinants of Health Variables
Our selection of SDOH variables used in this analysis was informed by the World Health Organization Commission on the SDOH,11 and the Centers for Disease Control and Prevention’s Healthy People 2020 framework.12 These variables included the following: (1) measures of socioeconomic status including self-reported annual income, dichotomized to low (≤$35 000 per annum, unadjusted) vs high (>$35 000),13 residence in a zip code in poverty (defined as zip code where >25% of residents’ income was below the Federal Poverty Level according to the 2000 US Census), low educational status defined as a participant having less than high school education, and 2015 Area Deprivation Index (ADI)—a multivariable measure of census block group disadvantage in national percentile rankings, where lower ADI represents less social deprivation14; (2) a measure of rural vs urban residence, defined as Rural Urban Community Area Codes of 9 or greater; and (3) a measure of social support (which has been shown to be associated with increased ASCVD risk and is based on Lubben Social Network Scale),15,16,17 defined as the proportion of participants who reported having no social visits by friends or family at least once per month.
Furthermore, health-related individual-level SDOH variables included absence of health insurance (vs full or partial health insurance), and area-level variables used were (1) whether or not the participant resided in a Health Professional Shortage Area, a Health Resources and Services Administration designation of county-level health professional shortage and (2) the presence of poor public health infrastructure, defined at the state level as those states in the lowest quintiles in at least 80% of decade before REGARDS study enrollment as per America’s Health Ranking.18
We also included a measure of racial segregation, the Dissimilarity Index.19 The Dissimilarity Index is a measure of residential racial segregation at the state-level based on the 2000 US Census. It is defined as the population of Black residents who need to change counties within the state to have a similar proportion of White and Black residents in each county as the state-level proportion.
Statistical Analysis
We assessed the discrimination and calibration of the PCEs in their original form as defined in Goff et al7 (model series A) and using the PCE model specifications with REGARDS study–specific model coefficients to ensure best fit (model series B). Then, we created 3 risk prediction models derived from the PCE:
Best-fit race-sex–specific versions including main effects for all covariates included in the original PCE (model series C).
Best-fit sex-specific versions (ie, without race stratification), including main effects for all covariates included in the original PCE (model series D).
Best-fit sex-specific versions (ie, without race stratification), including main effects for all covariates included in the original PCE but adding SDOH measures (model series E).
For each model series, Cox proportional hazard models were fit to the data, and C statistics with 95% CIs were computed among Black and White participants separately. To further assess model discrimination, we calculated the category-free Net Reclassification Indices (NRIs) for the event, nonevent, and overall for model series D to model series E.20 Then, calibration for the model series C, D, and E were assessed using bar plots comparing predicted 10-year ASCVD risk to observed 10-year ASCVD risk by grouping REGARDS participants into quintiles of predicted probability of ASCVD at 10 years. Observed risk was estimated by fitting Kaplan-Meier survival curves to the censored outcome data. The Nam D’Agostino test statistic21 was calculated for the calibration curves for each race-stratum within model series C, D, and E.
In model series C, we simplified the 4 original race- and sex-stratified PCEs by removing all interaction terms except the interaction between systolic blood pressure and an indicator variable of blood pressure treatment (to account for treatment for hypertension). Therefore, in model series C, each model was race- and sex-stratified and had the same model specification that included the following covariates: age, HDL-C level, total cholesterol level, presence or absence of smoking, and presence or absence of diabetes, and an interaction term between systolic blood pressure and blood pressure treatment.
In model series D, we removed the race stratification, leaving 2 sex-specific risk equations. Then, in model series E, we included SDOH variables to the 2 sex-specific risk equations in model series D. SDOH variable selection was undertaken using age- and sex-stratified univariate Cox proportional hazard models on the REGARDS cohort. SDOH variables whose hazard ratio (HR) was greater than 1 or whose 2-sided P value was significant at a prespecified α level of significance = .05 were included in model series E. In the final analysis, low income, residence in zip code in poverty, low educational status, ADI, absence of health insurance, presence of poor public health infrastructure, and Dissimilarity Index were included in model series E. All C statistics and 95% CIs were cross-validated.
Proportional hazards assumptions were examined by plotting the estimated hazard function over time and testing for whether it has a nonzero slope. We used random forest imputation to account for missingness in all covariates (eTable in Supplement 1), and these models with imputed values were used to select SDOH variables and undertake modeling across all series.
In model series D and E, race as a variable was not included in the model specification; although, C statistics were calculated for each race-sex stratum. As a further analysis, race as a variable itself was added to the models for statistical adjustment to examine for model performance improvements. All analyses were conducted in R, version 4.2.0 (R Project for Statistical Computing), and RStudio, version 2022.02.3 (Posit, PBC). Study data were analyzed from July 2022 to February 2023.
Results
This study included 11 638 participants (mean [SD] age, 61.8 [8.3] years; 6764 female [58.1%]) from the REGARDS cohort (eFigure in Supplement 1). Table 1 describes the baseline characteristics of the REGARDS cohort by each race-sex stratum. At baseline, participants belonged to the following race and sex categories: 2238 Black female (26.9%), 1331 Black male (15.4%), 2665 White female (30.8%), and 2368 White male (26.9%). The mean (SD) age was 61.1 (8.3) years for Black female participants, 62.1 (8.0) years for Black male participants, 61.5 (8.5) years for White female participants, and 62.8 (8.1) years for White male participants. Compared with White participants, more Black participants had lower income (Black female, 1278 [57.1%] and Black male, 611 [45.9%] vs White female, 1007 [37.8%] and White male, 585 [24.7%]) and a greater burden of chronic diseases (eg, diabetes: Black female, 376 [16.8%] and Black male, 242 [18.2%] vs White female, 157 [5.9%] and White male, 215 [9.1%]) and ASCVD risk factors (eg, smoking: Black female, 374 [16.7%] and Black male, 291 [21.9%] vs White female, 362 [13.6%] and White male, 301 [12.9%]).
Table 1. Baseline Characteristics of Reasons for Geographic and Racial Differences in Stroke–Myocardial Infarction (REGARDS-MI) Participants By Race and Sex Strataa.
| Characteristics | Race-sex strata | |||
|---|---|---|---|---|
| Black | White | |||
| Female | Male | Female | Male | |
| Observations, No. | 2238 | 1331 | 2665 | 2368 |
| Age, mean (SD), y | 60.91 (8.3) | 61.8 (7.9) | 61.2 (8.4) | 62.5 (8.0) |
| High-density lipoprotein, mg/dL | 57.9 (15.5) | 48.9 (14.3) | 59.7 (16.7) | 45.1 (13.2) |
| Systolic blood pressure, mean (SD), mm Hgb | 128.1 (17.3) | 130.6 (16.7) | 120.6 (14.8) | 125.8 (14.6) |
| Proportion taking antihypertensive medications, No. (%) | 1229 (54.9) | 607 (45.6) | 797 (29.9) | 677 (28.6) |
| Proportion of smokers, No. (%)c | 374 (16.7) | 291 (21.9) | 362 (13.6) | 301 (12.7) |
| Proportion with diabetes, No. (%)d | 376 (16.8) | 242 (18.2) | 157 (5.9) | 215 (9.1) |
| Proportion of low income, No. (%)e | 1278 (57.1) | 611 (45.9) | 1007 (37.8) | 585 (24.7) |
| Proportion with low educational status, No. (%)f | 289 (12.9) | 185 (13.9) | 104 (3.9) | 116 (4.9) |
| Proportion with zip code in poverty, No. (%)g | 696 (31.1) | 395 (29.7) | 205 (7.7) | 225 (9.5) |
| Proportion without health insurance, No. (%)h | 275 (12.3) | 150 (11.3) | 168 (6.3) | 116 (4.9) |
| Proportion of participants with no social visits, No. (%)i | 94 (4.2) | 63 (4.7) | 96 (3.6) | 123 (5.2) |
SI conversion factor: To convert high-density lipoprotein to millimoles per liter, multiply by 0.0259.
Complete case analysis.
Includes patients who report taking antihypertensive agents.
Defined by self-report.
Defined as diabetic by self-report or fasting glucose greater than or equal to 126 mg/dL or nonfasting glucose greater than or equal to 200 mg/dL or taking insulin or other antiglycemic medications.
Low income defined as participant’s annual income of $35 000 or less in non–inflation-adjusted dollars.
Low educational status defined as participant has less than high school education.
Zip code in poverty defined as zip code where greater than 25% of residents’ income in that zip code is below the Federal Poverty Level.
Participants without health insurance defined as having neither full nor partial health insurance.
Social visits are defined as participants do not see family nor friends at least once per month.
In Table 2, we report the HR for the SDOH variables derived from age- and sex-stratified Cox models estimating 10-year ASCVD risk under consideration for inclusion in our sequential modeling strategy. Of the variables under consideration, low income (HR, 1.57; 95% CI, 1.38-1.78; P < .001), zip code in poverty (HR, 1.19; 95% CI, 1.02-1.39; P = .02), low education status (HR, 1.40; 95% CI, 1.16-1.69; P < .001), ADI (HR, 1.01; 95% CI, 1.00-1.01; P < .001), the absence of health insurance (HR, 1.81; 95% CI, 1.44-2.28; P < .001), and residing in a state with poor public health infrastructure (HR, 1.16; 95% CI, 1.02-1.32; P = .02) were included in the final model series (model series E).
Table 2. Hazard Ratios, 95% CIs, and P values From Age-, and Sex-Stratified Cox Proportional Hazard Modelsa.
| Variable | Hazard ratio (95% CI) | P value |
|---|---|---|
| ADIb | 1.01 (1.00-1.01) | <.001 |
| DIc | 1.19 (0.79-1.80) | .4 |
| Low educational statusd | 1.40 (1.14-1.73) | .001 |
| Low incomee | 1.57 (1.36-1.81) | <.001 |
| Zip code in povertyf | 1.21 (1.02-1.44) | .03 |
| Health Professional Shortage Area categoryg | 0.94 (0.81-1.07) | .36 |
| Absence of health insuranceh | 1.79 (1.39-2.31) | <.001 |
| Rurali | 0.94 (0.73-1.23) | .66 |
| Poor public health infrastructurej | 1.16 (1.00-1.33) | .05 |
| Proportion of participants with no social visitsk | 1.19 (0.90-1.56) | .22 |
Abbreviations: ADI, Area Deprivation Index; DI, Dissimilarity Index; RUCA, rural-urban commuting area codes; UA, urban area; UC, urban center.
Uses imputed values based on multiple imputation.
The ADI is a continuous, multivariable measure of census block group disadvantage in national percentile rankings (ranging from 1 to 100), where 1 is lowest ADI representing most disadvantaged census block groups nationally, and 100 represents the highest ADI, representing least disadvantaged census block groups nationally.
DI is a measure of residential racial segregation at the state level from 2000 Census ranging from 0 to 1, defined as the population of Black residents that need to change counties to have an equal proportion of White and Black residents in each county. DI = 0 implies fully Black-White integrated counties (ie, no proportion of Black residents in the county would have to exchange geographic locations with White residents to achieve equal county-level distribution), whereas DI = 1 suggests full segregation.
Low educational status defined as participant has less than high school education vs greater than high school education.
Low income defined as participant’s annual income $35 000 or less vs more than $35 000.
Zip code in poverty defined as zip code where greater than 25% of residents’ income in that zip code is below the Federal Poverty Level.
Defined as whether participant resides in county defined by the health resources and services administration as having health professional shortage.
Defined as participants without health insurance defined as having neither full nor partial health insurance.
Defined by RUCA from 2000 US Census. Rural defined as RUCA greater than or equal to 9 (where RUCA 9 = small town low community: primary flow 10% to 30% to a small UC; RUCA 10 = rural areas; primary flow to a tract outside a UA or UC).
Defined as states in lowest quintiles in at least 80% of decade prior to the Reasons for Geographic and Racial Differences in Stroke study enrollment as per America’s Health Ranking.
Defined as participants do not have social visits (ie, see family nor friends) at least once per month.
Table 3 describes the C statistics and associated 95% CIs across all the model series (model series A to E).7 Model discrimination was numerically the same in model series C (race-sex–stratified model) vs model series A and B, after removing race stratification (model series D vs C), and finally after the addition of SDOH variables (model series E vs D). Furthermore, model series C to E had lower discrimination among Black and White male participants compared with Black and White female participants (eg, model C: Black male, 0.68; 95% CI, 0.64-0.73 and White male, 0.68; 95% CI, 0.65-0.71 vs Black female, 0.71; 95% CI, 0.68-0.75 and White female, 0.77; 95% CI, 0.74-0.80; model D: Black male, 0.68; 95% CI, 0.63-0.72 and White male, 0.68; 95% CI, 0.65-0.71 vs Black female, 0.71; 95% CI, 0.67-0.75 and White female, 0.76; 95% CI, 0.73-0.80; model E: Black male, 0.68; 95% CI, 0.64-0.72 and White male, 0.68; 95% CI, 0.65-0.71 vs Black female, 0.72; 95% CI, 0.68-0.76 and White female, 0.77; 95% CI, 0.74-0.80). There was minimal difference between Black and White male participants, but there were big differences between male and female participants, regardless of race.
Table 3. C Statistics and 95% CIs for Each Model and Number of Observations and Atherosclerotic Cardiovascular Disease (ASCVD) Events, by Race and Sex Strataa.
| Model | C statistic (95% CI) | |||
|---|---|---|---|---|
| Black | White | |||
| Female | Male | Female | Male | |
| Observations by each race and sex stratum | ||||
| No. of participants/No. of ASCVD events | 2934/166 | 1709/153 | 3830/195 | 3165/297 |
| Model A: original pooled cohort risk equationb | 0.69 (0.66-0.73) | 0.66 (0.61-0.70) | 0.75 (0.72-0.79) | 0.66 (0.63-0.69) |
| Model B: original pooled cohort risk equation with REGARDS-specific coefficientsc | 0.71 (0.68-0.75) | 0.69 (0.64-0.73) | 0.77 (0.74-0.81) | 0.68 (0.64-0.71) |
| Model C: simplified REGARDS-specific pooled cohort risk equationc | 0.71 (0.68-0.75) | 0.68 (0.64-0.73) | 0.77 (0.74-0.80) | 0.68 (0.65-0.71) |
| Model D: model C without raced | 0.71 (0.67-0.75) | 0.68 (0.63-0.72) | 0.76 (0.73-0.80) | 0.68 (0.65-0.71) |
| Model E: model D plus SDOH variablese | 0.72 (0.68-0.76) | 0.68 (0.64-0.72) | 0.77 (0.74-0.80) | 0.68 (0.65-0.71) |
Abbreviations: REGARDS, Reasons for Geographic and Racial Differences in Stroke; SDOH, social determinants of health.
All models employed the entire study population (n = 11 138) with imputation for missing values.
Defined in Goff et al.7
Simplified Pooled Cohort Risk Equation includes age, high-density lipoprotein, systolic blood pressure interaction with treatment, current smoking status, and diabetes status.
Model fitted on each sex stratum (male and female) and C statistics and associated 95% CIs were computed for each race stratum using bootstrapping.
The following SDOH variables were included in this model: Area Deprivation Index (continuous variable), low education, low income, zip code in poverty, no insurance, health professional shortage area category (indicator variables as defined in Table 1 and 2).
In Table 4, we computed the respective NRIs for each race-sex stratum comparing models D and E. Notably, negative event NRIs (defined as the net percentage of persons with an event of interest being corrected assigned a higher predicted risk) are reported for both Black male participants (NRI event = −15%; 95% CI, −39% to 8%) and White male participants (NRI event = −7%; 95% CI, −32% to 17%) compared with both Black female participants (NRI event = 39%; 95% CI, 15%-63%) and White female participants (NRI event = 6%; 95% CI, −12% to 25%), further underscoring sex-based differences in model discrimination
Table 4. Net Reclassification Index (NRI) Values and 95% CIs Comparing Model D and Model E by Race and Sex Strata.
| Race-sex strata | NRI event (95% CI) | NRI nonevent (95% CI) |
|---|---|---|
| Black female | 39% (15% to 63%) | −1% (−14% to 51%) |
| Black male | −15% (−39% to 8%) | 36% (13% to 44%) |
| White female | 6% (−12% to 25%) | 19% (1% to 43%) |
| White male | −7% (−32% to 17%) | 25% (3% to 39%) |
In the bar plots (Figure), calibration across all the simplified PCE model series (model series C to E) by each race-sex stratum showed good calibration across all quintiles of predicted 10-year ASCVD risk, as demonstrated by similar predicted-to-observed risk calculated across all quintiles of predicted risk and nonsignificance of the Nam D’Agostino test statistic for each model series.
Figure. Calibration Bar Plots of Observed and Predicted Atherosclerotic Cardiovascular Disease (ASCVD) Risk Among Participants in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study by Race-Sex Strata, Model Series C,a,b D,c,b and E,d,b and Associated Nam-D’Agostino (N-D) Statistic.
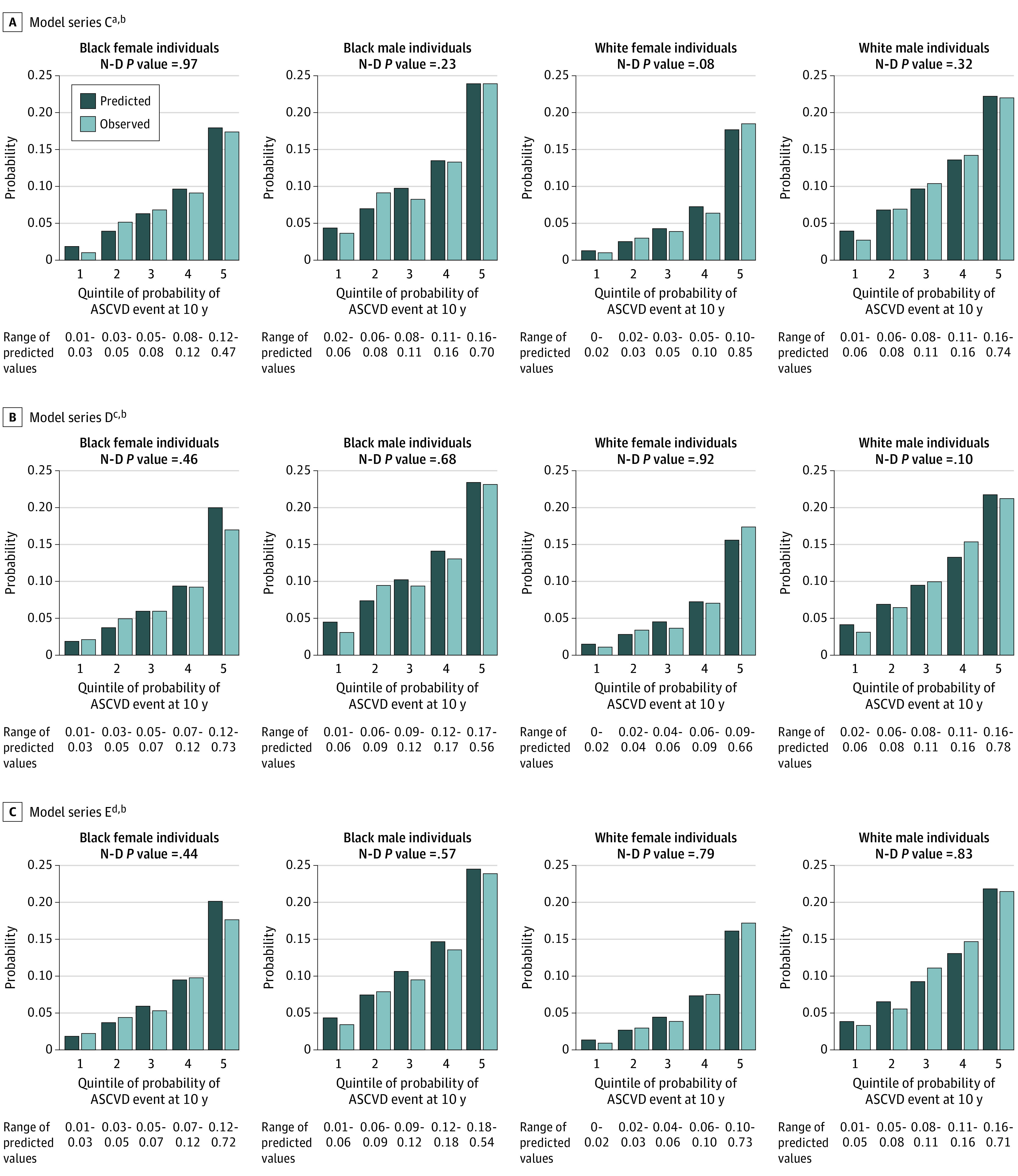
aModel C: simplified REGARDS-specific pooled cohort risk equation includes age, high-density lipoprotein, systolic blood pressure interaction with treatment, current smoking status, and diabetes status.
bRange of predicted values to 2 decimal places.
cModel D: model C without race stratification.
dModel E: model D plus social determinants of health variables (Area Deprivation Index [continuous variable], low education, low income, zip code in poverty, no insurance, health professional shortage area category).
In sensitivity analyses, race was not significantly associated with ASCVD risk, and the adjustment for race did not significantly change performance for any race- and sex-stratum in model series D and E.
Discussion
This cohort study has 3 important findings. First, risk prediction equations performed similarly by race but performed worse in men compared with women despite sex stratification. Second, race-free sex-specific PCEs yield similar discrimination as race- and sex-specific equations across all groups. Third, the addition of SDOHs was not associated with improved discrimination or calibration in race-free, sex-specific PCEs.
To date, several studies have examined the limitations of the original PCEs9,22,23,24,25 and shown that alternative methods can improve model performance. Moreover, although the a priori conceptualization of race adjustment or stratification in the equations has been questioned before,26 this is the first study, to our knowledge, to provide empirical evidence. Our findings support the possibility of removing race from the PCE in the background of the growing call to remove race-based stratification for specific clinical measures.
Race is a social construct and may serve as a surrogate for SDOH in risk models. We, therefore, examined race-free risk prediction equations with the addition of SDOH. However, once again, performance was not substantively changed. Rather than suggesting that SDOH are unimportant in CVD risk prediction, our findings likely underscore the strength of proximate clinical risk factors in predicting the risk of a CVD event.27
The findings here do not suggest that the experience of being a Black person in the US is a trivial contributor to outcomes. Indeed, access to health care, trust in the health care system, engagement in the health care system, and health outcomes themselves are deeply intertwined with the experience of one’s race,28,29,30 and indeed influence the diagnosis, management, and control of important cardiovascular risk factors such as hypertension31,32 and hypercholesterolemia.33 However, our findings do suggest that drawbacks to the continued use of race as a covariate in one of the most widely used risk predictors in the country, including a misconceived conception of race as a biological construct, likely outweigh any incremental benefits in terms of predictive value. Our findings also suggest the need for novel models and methodologies for predicting risk, particularly among individuals, where both biological and social factors may uniquely confer added ASCVD risk. These include modeling ASCVD outcomes using more sophisticated methods such as machine learning and expanding beyond ASCVD risk factors and minimal demographics in the variables incorporated. Furthermore, ASCVD risk prediction performance may be better improved through the incorporation of the duration of exposure to clinical risk factors to account for the cumulative exposure risk over the individual’s life course.34,35
A noteworthy finding was also the significant, sex-based differences in PCE performance across all the model series, in keeping with previous studies of a contemporary, national cohort of adult patients in the Veterans Affairs health care system using both the 2013 PCE and cohort-derived β coefficients.36 These findings deserve further investigation given the well-known differences in sex-based ASCVD outcomes with higher rates among male individuals compared with female individuals.37,38
Limitations
There are several limitations to our findings. First, the REGARDS cohort is a geographically diverse population with participants from all 48 contiguous states and includes a substantial proportion of participants from rural areas. However, it is also a cohort study that required informed consent for inclusion. Consequently, there is a degree of selection bias in terms of people who agreed to participate in the study. This underscores the need for additional studies across other data sets including nationally representative cohorts. Second, participants recruited for the REGARDS study had to have a dwelling and a telephone and be willing to allow a health professional into their home, which may introduce selection bias.9 It is possible that these recruitment and measurement protocols may have selected participants who differ from those whose ASCVD risk may be conferred earlier in life, thus biasing the role of race stratification or addition of SDOH toward a null effect. Third, several variables were self-reported, with known biases. Fourth, the available SDOH (collected at baseline) in the REGARDS cohort were unlikely to capture all social and socioeconomic (including race-specific) influences on ASCVD outcomes; many were calculated at the area-level rather than the individual-level and were not collected over time. These include the influence of various neighborhood factors known to be associated with ASCVD risk,39,40,41,42,43 which, although important to consider, need to be weighed against the clinical application of the PCE and validation using other cohorts.
Conclusions
In summary, results of this cohort study suggest the limited role of race-specific models in predicting ASCVD outcomes using the PCEs. Our findings suggest reconsideration of the role of race stratification in the PCE as currently constructed. Adding SDOH to the PCEs did not clinically meaningfully improve model performance. If confirmed in other cohorts, our work suggests a need for a broader and more nuanced discussion of the role of race in the assessment of ASCVD.
eFigure. Exclusion Cascade of REGARDS Participants
eTable. Number of Missing Observations in Baseline Characteristics of REGARDS-MI Participants by Race-Sex Strata
Data Sharing Statement.
References
- 1.Roberts DE. Abolish race correction. Lancet. 2021;397(10268):17-18. doi: 10.1016/S0140-6736(20)32716-1 [DOI] [PubMed] [Google Scholar]
- 2.Vyas DA, Eisenstein LG, Jones DS. Hidden in plain sight—reconsidering the use of race correction in clinical algorithms. N Engl J Med. 2020;383(9):874-882. doi: 10.1056/NEJMms2004740 [DOI] [PubMed] [Google Scholar]
- 3.Gutiérrez OM, Sang Y, Grams ME, et al. ; Chronic Kidney Disease Prognosis Consortium . Association of estimated GFR calculated using race-free equations with kidney failure and mortality by Black vs non-Black race. JAMA. 2022;327(23):2306-2316. doi: 10.1001/jama.2022.8801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Organ Procurement and Transplantation Network . OPTN Board approves elimination of race-based calculation for transplant candidate listing. Accessed November 18, 2022. https://optn.transplant.hrsa.gov/news/optn-board-approves-elimination-of-race-based-calculation-for-transplant-candidate-listing/
- 5.Baugh AD, Shiboski S, Hansel NN, et al. Reconsidering the utility of race-specific lung function prediction equations. Am J Respir Crit Care Med. 2022;205(7):819-829. doi: 10.1164/rccm.202105-1246OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsu CY, Yang W, Parikh RV, et al. ; CRIC Study Investigators . Race, genetic ancestry, and estimating kidney function in CKD. N Engl J Med. 2021;385(19):1750-1760. doi: 10.1056/NEJMoa2103753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2935-2959. doi: 10.1016/j.jacc.2013.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muntner P, Colantonio LD, Cushman M, et al. Validation of the atherosclerotic cardiovascular disease pooled cohort risk equations. JAMA. 2014;311(14):1406-1415. doi: 10.1001/jama.2014.2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colantonio LD, Richman JS, Carson AP, et al. Performance of the atherosclerotic cardiovascular disease pooled cohort risk equations by social deprivation status. J Am Heart Assoc. 2017;6(3):e005676. doi: 10.1161/JAHA.117.005676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howard VJ, Cushman M, Pulley L, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25(3):135-143. doi: 10.1159/000086678 [DOI] [PubMed] [Google Scholar]
- 11.Solar O, Irwin A. A conceptual framework for action on the social determinants of health. Accessed November 30, 2022. https://www.afro.who.int/sites/default/files/2017-06/SDH_conceptual_framework_for_action.pdf
- 12.US Centers for Disease Control and Prevention . Healthy People 2020 leading health indicators. Accessed December 2, 2022. https://www.cdc.gov/nchs/healthy_people/hp2020/hp2020_indicators.htm
- 13.Redmond N, Richman J, Gamboa CM, et al. Perceived stress is associated with incident coronary heart disease and all-cause mortality in low—but not high—income participants in the Reasons for Geographic And Racial Differences in Stroke study. J Am Heart Assoc. 2013;2(6):e000447. doi: 10.1161/JAHA.113.000447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kind AJH, Buckingham WR. Making neighborhood-disadvantage metrics accessible—the neighborhood atlas. N Engl J Med. 2018;378(26):2456-2458. doi: 10.1056/NEJMp1802313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Golaszewski NM, LaCroix AZ, Godino JG, et al. Evaluation of social isolation, loneliness, and cardiovascular disease among older women in the US. JAMA Netw Open. 2022;5(2):e2146461. doi: 10.1001/jamanetworkopen.2021.46461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valtorta NK, Kanaan M, Gilbody S, Ronzi S, Hanratty B. Loneliness and social isolation as risk factors for coronary heart disease and stroke: systematic review and meta-analysis of longitudinal observational studies. Heart. 2016;102(13):1009-1016. doi: 10.1136/heartjnl-2015-308790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lubben J, Blozik E, Gillmann G, et al. Performance of an abbreviated version of the Lubben Social Network Scale among 3 European community-dwelling older adult populations. Gerontologist. 2006;46(4):503-513. doi: 10.1093/geront/46.4.503 [DOI] [PubMed] [Google Scholar]
- 18.Reshetnyak E, Ntamatungiro M, Pinheiro LC, et al. Impact of multiple social determinants of health on incident stroke. Stroke. 2020;51(8):2445-2453. doi: 10.1161/STROKEAHA.120.028530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duncan OD, Duncan B. A methodological analysis of segregation indexes. Am Sociol Rev. 1955;20(2):210-217. doi: 10.2307/2088328 [DOI] [Google Scholar]
- 20.Leening MJG, Vedder MM, Witteman JCM, Pencina MJ, Steyerberg EW. Net reclassification improvement: computation, interpretation, and controversies: a literature review and clinician’s guide. Ann Intern Med. 2014;160(2):122-131. doi: 10.7326/M13-1522 [DOI] [PubMed] [Google Scholar]
- 21.Demler OV, Paynter NP, Cook NR. Tests of calibration and goodness-of-fit in the survival setting. Stat Med. 2015;34(10):1659-1680. doi: 10.1002/sim.6428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vasan RS, van den Heuvel E. Differences in estimates for 10-year risk of cardiovascular disease in Black vs White individuals with identical risk factor profiles using pooled cohort equations: an in silico cohort study. Lancet Digit Health. 2022;4(1):e55-e63. doi: 10.1016/S2589-7500(21)00236-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yadlowsky S, Hayward RA, Sussman JB, McClelland RL, Min YI, Basu S. Clinical implications of revised pooled cohort equations for estimating atherosclerotic cardiovascular disease risk. Ann Intern Med. 2018;169(1):20-29. doi: 10.7326/M17-3011 [DOI] [PubMed] [Google Scholar]
- 24.Khera R, Pandey A, Ayers CR, et al. Performance of the pooled cohort equations to estimate atherosclerotic cardiovascular disease risk by body mass index. JAMA Netw Open. 2020;3(10):e2023242. doi: 10.1001/jamanetworkopen.2020.23242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zinzuwadia AN, Li C, Dashti H, et al. Abstract P021: performance of pooled cohort equations and MESA risk score across race/ethnicity and socioeconomic status to estimate 10-year cardiovascular risk in diverse New England cohort. Circulation. 2022;145(suppl 1):AP021. doi: 10.1161/circ.145.suppl_1.P021 [DOI] [Google Scholar]
- 26.Vyas DA, James A, Kormos W, Essien UR. Revising the atherosclerotic cardiovascular disease calculator without race. Lancet Digit Health. 2022;4(1):e4-e5. doi: 10.1016/S2589-7500(21)00258-2 [DOI] [PubMed] [Google Scholar]
- 27.Pencina MJ, Navar AM, Wojdyla D, et al. Quantifying importance of major risk factors for coronary heart disease. Circulation. 2019;139(13):1603-1611. doi: 10.1161/CIRCULATIONAHA.117.031855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams DR, Collins C. US socioeconomic and racial differences in health: patterns and explanations. Annu Rev Sociol. 1995;21(1):349-386. doi: 10.1146/annurev.so.21.080195.002025 [DOI] [Google Scholar]
- 29.Williams DR, Mohammed SA, Leavell J, Collins C. Race, socioeconomic status, and health: complexities, ongoing challenges, and research opportunities. Ann N Y Acad Sci. 2010;1186(1):69-101. doi: 10.1111/j.1749-6632.2009.05339.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams DR, Priest N, Anderson NB. Understanding associations among race, socioeconomic status, and health: Patterns and prospects. Health Psychol. 2016;35(4):407-411. doi: 10.1037/hea0000242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aggarwal R, Chiu N, Wadhera RK, et al. Racial/ethnic disparities in hypertension prevalence, awareness, treatment, and control in the US, 2013 to 2018. Hypertension. 2021;78(6):1719-1726. doi: 10.1161/HYPERTENSIONAHA.121.17570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gu A, Yue Y, Desai RP, Argulian E. Racial and ethnic differences in antihypertensive medication use and blood pressure control among US adults with hypertension: the National Health and Nutrition Examination Survey, 2003 to 2012. Circ Cardiovasc Qual Outcomes. 2017;10(1):e003166. doi: 10.1161/CIRCOUTCOMES.116.003166 [DOI] [PubMed] [Google Scholar]
- 33.Nelson K, Norris K, Mangione CM. Disparities in the diagnosis and pharmacologic treatment of high serum cholesterol by race and ethnicity: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2002;162(8):929-935. doi: 10.1001/archinte.162.8.929 [DOI] [PubMed] [Google Scholar]
- 34.Gee GC, Hing A, Mohammed S, Tabor DC, Williams DR. Racism and the life course: taking time seriously. Am J Public Health. 2019;109(S1):S43-S47. doi: 10.2105/AJPH.2018.304766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gee GC, Walsemann KM, Brondolo E. A life course perspective on how racism may be related to health inequities. Am J Public Health. 2012;102(5):967-974. doi: 10.2105/AJPH.2012.300666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vassy JL, Lu B, Ho YL, et al. Estimation of atherosclerotic cardiovascular disease risk among patients in the Veterans Affairs health care system. JAMA Netw Open. 2020;3(7):e208236. doi: 10.1001/jamanetworkopen.2020.8236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Safford MM, Brown TM, Muntner PM, et al. ; REGARDS Investigators . Association of race and sex with risk of incident acute coronary heart disease events. JAMA. 2012;308(17):1768-1774. doi: 10.1001/jama.2012.14306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peters SAE, Muntner P, Woodward M. Sex differences in the prevalence of, and trends in, cardiovascular risk factors, treatment, and control in the US, 2001 to 2016. Circulation. 2019;139(8):1025-1035. doi: 10.1161/CIRCULATIONAHA.118.035550 [DOI] [PubMed] [Google Scholar]
- 39.Barber S, Hickson DA, Wang X, Sims M, Nelson C, Diez-Roux AV. Neighborhood disadvantage, poor social conditions, and cardiovascular disease incidence among African American adults in the Jackson Heart Study. Am J Public Health. 2016;106(12):2219-2226. doi: 10.2105/AJPH.2016.303471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borrell LN, Diez Roux AV, Rose K, Catellier D, Clark BL; Atherosclerosis Risk in Communities Study . Neighborhood characteristics and mortality in the Atherosclerosis Risk in Communities Study. Int J Epidemiol. 2004;33(2):398-407. doi: 10.1093/ije/dyh063 [DOI] [PubMed] [Google Scholar]
- 41.Hussein M, Diez Roux AV, Mujahid MS, et al. Unequal exposure or unequal vulnerability? contributions of neighborhood conditions and cardiovascular risk factors to socioeconomic inequality in incident cardiovascular disease in the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2018;187(7):1424-1437. doi: 10.1093/aje/kwx363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kershaw KN, Osypuk TL, Do DP, De Chavez PJ, Diez Roux AV. Neighborhood-level racial/ethnic residential segregation and incident cardiovascular disease: the multiethnic study of atherosclerosis. Circulation. 2015;131(2):141-148. doi: 10.1161/CIRCULATIONAHA.114.011345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nordstrom CK, Diez Roux AV, Jackson SA, Gardin JM; Cardiovascular Health Study . The association of personal and neighborhood socioeconomic indicators with subclinical cardiovascular disease in an elderly cohort: the cardiovascular health study. Soc Sci Med. 2004;59(10):2139-2147. doi: 10.1016/j.socscimed.2004.03.017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Exclusion Cascade of REGARDS Participants
eTable. Number of Missing Observations in Baseline Characteristics of REGARDS-MI Participants by Race-Sex Strata
Data Sharing Statement.


