Key Points
Question
Will a single psychedelic dose of psilocybin with psychotherapy demonstrate evidence for efficacy and/or safety in drug-free, treatment-resistant participants with bipolar II depression?
Findings
In this nonrandomized open-label trial of 15 individuals with bipolar II depression, most participants met remission criteria on the Montgomery-Åsberg Depression Rating Scale 3 weeks after a single 25-mg psilocybin dose, and most remained in remission 12 weeks postdose with no increase in mania/hypomania symptoms or suicidality.
Meaning
The findings suggest efficacy and safety of psilocybin in bipolar II depression and support further study of psychedelics in this population.
This open-label nonrandomized open-label trial evaluates the safety and efficacy of single-dose psilocybin with psychotherapy for depressive episodes in individuals with bipolar disorder.
Abstract
Importance
Bipolar II disorder (BDII) is a debilitating condition frequently associated with difficult-to-treat depressive episodes. Psilocybin has evidence for rapid-acting antidepressant effects but has not been investigated in bipolar depression.
Objective
To establish the safety and efficacy of psilocybin in patients with BDII in a current depressive episode.
Design, Setting, and Participants
This was a 12-week, open-label nonrandomized open-label trial conducted at Sheppard Pratt Hospital. Participants aged 18 to 65 years with BDII, a current depressive episode longer than 3 months, and documented insufficient benefit with at least 2 pharmacologic treatments during the current episode were invited to participate. Of 70 approached, 19 met inclusion criteria and were enrolled. The trial was conducted between April 14, 2021, and January 5, 2023.
Interventions
A single dose of synthetic psilocybin, 25 mg, was administered. Psychotropic medications were discontinued at least 2 weeks prior to dosing. Therapists met with patients for 3 sessions during pretreatment, during the 8-hour dosing day, and for 3 integration sessions posttreatment.
Main Outcomes and Measures
The primary outcome measure was change in Montgomery-Åsberg Depression Rating scale (MADRS) at 3 weeks posttreatment. Secondary measures included MADRS scores 12 weeks posttreatment, the self-rated Quick Inventory of Depression Symptoms-Self Rating (QIDS-SR), and the self-rated Quality of Life Enjoyment and Satisfaction Questionnaire-Short Form (Q-LES-Q-SF), each completed at baseline and all subsequent visits. Safety measures included the Columbia Suicide Severity Rating Scale (CSSRS) and the Young Mania Rating Scale (YMRS) completed at each visit.
Results
Of the 15 participants in this study (6 male and 9 female; mean [SD] age, 37.8 [11.6] years), all had lower scores at week 3, with a mean (SD) change of −24.00 (9.23) points on the MADRS, (Cohen d = 4.08; 95% CI, −29.11 to −18.89; P < .001). Repeat measures analysis of variance showed lower MADRS scores at all tested posttreatment time points, including the end point (Cohen d = 3.39; 95% CI, −33.19 to −16.95; adjusted P < .001). At week 3, 12 participants met the response criterion (50% decrease in MADRS), and 11 met remission criterion (MADRS score ≤10). At the study end point, 12 patients met both response and remission criteria. QIDS-SR scores and Q-LES-Q-SF scores demonstrated similar improvements. YMRS and CSSRS scores did not change significantly at posttreatment compared to baseline.
Conclusions and Relevance
The findings in this open-label nonrandomized open-label trial suggest efficacy and safety of psilocybin with psychotherapy in BDII depression and supports further study of psychedelics in this population.
Introduction
Bipolar II disorder (BDII) is a lifelong condition characterized by recurrent hypomanic and depressive episodes with a lifetime prevalence of at least 0.4% among adults.1 It causes a level of functional impairment and disability comparable to bipolar I disorder (BDI).2,3,4 Despite treatment, patients with BDII are typically symptomatic most of the time, primarily experiencing protracted and difficult-to-treat periods of depression.5 Bipolar disorder has high mortality, as 30% of affected individuals attempt and 5% to 15% commit suicide.4 Historically, BDII was viewed as the lesser of the bipolar disorders due to the absence of florid mania.6 However, recent studies document that functional impact and risk of suicide are similar in BDI and BDII.1,2,7
Treatment options are limited for patients with BDII depression.8 Until recently, treatment guidelines did not distinguish between BDI and BDII.9 The recent Canadian Network for Mood and Anxiety Treatments and International Society for Bipolar Disorders 2018 guidelines for management of bipolar disorder devoted a separate discussion to BDII.10 The primary conclusion was that the extant literature is inadequate to make evidence-based recommendations for patients with BDII.10 This is largely because most randomized trials of pharmacological treatments for bipolar depression do not include patients with BDII in sufficient number to independently test efficacy.11 In general, antidepressant medications have not demonstrated reliable benefit for this population,12 with ongoing debate as to whether their potential harms outweigh benefits in BDII.13 There is compelling need for novel agents in BDII depression for patients who have insufficiently benefited with or not tolerated existing therapies.14
Psilocybin is a naturally occurring plant alkaloid derived from psilocybin mushrooms. When ingested, it creates a mind-altering effect mediated in part through its action as a 5HT2a receptor agonist.15 Psilocybin appears to increase associative learning, cognitive flexibility, and neuroplasticity.16,17,18 Several recent studies suggest that this agent has therapeutic properties in patients with major depressive disorder.18,19 The largest study compared 1-mg, 10-mg, and 25-mg dosages of psilocybin accompanied by psychological support in 233 patients with treatment-resistant major depressive disorder. The higher doses showed a marked antidepressant effect with evidence of durability at 3 months following the single exposure to psilocybin.19 These trials were not without adverse effects, with headache, nausea, fatigue, and insomnia being the most common.20,21
While there is considerable interest in using psilocybin in major depressive disorder, we are not aware of any reports on the clinical impact of psilocybin in patients with BDI or BDII depression. We report on an open prospective pilot study of 15 participants with BDII depression treated with a single administration of psilocybin accompanied with psychological support. The primary outcome was change in depression severity scores 3 weeks after psilocybin administration. This study also assessed safety by looking for emergent mixed or manic symptoms, such as agitation or insomnia, psychotic symptoms, and change in suicidal ideation.
Methods
This was a 12-week open-label trial of a single 25-mg dose of a synthetic psilocybin formulation provided by COMPASS Pathways administered to 15 participants with BDII depression. The trial was conducted between April 14, 2021, and January 5, 2023, at the Sheppard Pratt Health System. The study was approved by the institutional review board of the Sheppard Pratt Health System (protocol #49345) and the US Food and Drug Administration (FDA) (IND 13788). A Certificate of Confidentiality was obtained. All participants provided written informed consent. The ClinicalTrials.gov registration number was NCT04433845. The trial protocol can be found in Supplement 1.
Participants
Recruitment was via word of mouth and web listings on the Sheppard Pratt website and clinicaltrials.gov. Participants were aged 18 to 65 years, with a primary diagnosis of BDII disorder according to DSM-5 criteria and supported by medical records, clinical assessment, and completion of the Mini International Neuropsychiatric Interview version 7.0.2 by an experienced clinician.22 Participants had documented insufficient benefit with at least 2 pharmacologic treatments during their current depressive episode, administered at adequate dose and duration by Massachusetts General Hospital–Antidepressant Treatment Response Questionnaire criteria (expanded to include FDA-approved mood stabilizing agents).23 Patients were required to have a current episode duration greater than 3 months. At both screening and baseline visits, participants had a Hamilton Rating Scale for Depression 17 (HRSD) score 18 or higher and a Young Mania Rating Scale (YMRS) score less than 10.24,25 They also had normal screening bloodwork and electrocardiograph.
Exclusion criteria included history of BDI disorder, schizophrenia, psychosis (including substance-induced or due to a medical disorder), delusions, paranoid, schizoaffective, or borderline personality disorder, or any substance use disorder within the past 12 months.
Trial Design and Procedures
Eligible participants completed a run-in period of 3 to 6 weeks during which antidepressants and other medications (including mood stabilizers, stimulants, and benzodiazepines) were discontinued at least 2 weeks before the baseline visit, which was 1 day before the psilocybin administration visit. To build therapeutic rapport, receive psychoeducation, and prepare for the psychedelic experience, participants met for at least 3 hours during the run-in period with a specially trained lead therapist from among a team of doctoral-level psychologists. The lead therapist and an assistant therapist were in attendance during the 8- to 9-hour psilocybin dosing day. The dosing room was designed to provide a nonclinical, calming, and supportive environment. Participants were encouraged to listen to a specially designed playlist and wear eyeshades to direct their focus inward. The therapists provided support as needed. The study physician was on site for the dosing day, meeting with the participant at the end of the day when psychedelic effects had dissipated to confirm the participant was safe to return home in the care of a support person they had designated in advance.
Participants returned 1 day postdosing for assessment by the study physician and to participate in the first of 3 one-hour integration sessions with the lead therapist. Integration sessions occurred during the day after and first and second week of follow-up. These sessions were nondirective and were meant to support the participant in consolidating insights from their psychedelic experience. Participants were administered rating scales by a study psychiatrist at 1, 2, 3, 6, 9, and 12 weeks’ follow-up. As clinically feasible, participants were requested to refrain from taking psychotropic medication for the first 3 weeks after dosing. Visits the day after dosing and at 1, 3, and 12 weeks’ follow-up were in person. Other visits could be conducted remotely.
Efficacy End Points
The primary outcome was change in clinician-rated Montgomery-Åsberg Depression Rating Scale (MADRS)26 total score from baseline visit to 3 weeks’ follow-up. Secondary end points included MADRS score at each follow-up visit, percentage of participants meeting remission criteria (MADRS score ≤10) at each visit, and percentage of participants meeting response criteria at each visit (decrease in MADRS score of 50% or more from baseline).
Additional outcome measures included the self-rated Quick Inventory of Depression Symptoms-Self Rating (QIDS-SR)27 and the self-rated Quality of Life Enjoyment and Satisfaction Questionnaire-Short Form (Q-LES-Q-SF).28 These were completed at baseline and all subsequent visits. The QIDS-SR was completed at baseline and all subsequent visits. The Q-LES-Q-SF was completed at baseline and posttreatment weeks 3 and 12.
Safety End Points
A concern with using psychedelics in patients with BD is induction of a manic, hypomanic, or mixed state or increased suicidal ideation. Participants were assessed with the Columbia Suicide Severity Rating Scale (CSSRS)29 and the Young Mania Rating Scale (YMRS) by a study psychiatrist at each visit.
Exploratory End Point
The Five Dimension Altered State of Consciousness Questionnaire (5D-ASC) measures the short-term effects of a drug using 5 primary dimensions and respective subdimensions to assess alterations in mood, perception, and experience of self, and thought disorder.30 The 5 dimensions include oceanic boundlessness, anxious ego dissolution, visionary restructuralization, auditory alterations, and reduction of vigilance. This scale was administered after psilocybin administration as part of the postdosing assessment, providing a measure of the nature and intensity of the psychedelic experience.
Statistical Analyses
Statistical analyses were performed using SPSS version 27 (IBM). General linear models were used to determine the effect of psilocybin treatment on the primary (MADRS) and each of the secondary (QIDS-SR, YMRS, CSSRS, and Q-LES-Q-SF) measures. Raw MADRS scores at baseline and weeks 1, 3, and 12 were submitted to a 1-way repeated-measures analysis of variance (ANOVA) to assess the impact of time point on MADRS scores. Bonferroni-corrected post hoc comparisons identified the pairwise differences among the 4 time points in MADRS scores. Similar repeated-measures ANOVA was conducted on the secondary outcomes of QIDS-SR, YMRS, and Q-LES-Q-SF. Pearson correlation coefficients were computed to explore the correlation between psychedelic experience as captured by the 5D-ASC and antidepressant effects. One patient withdrew from the study after week 6. The last observation carried forward was used for subsequent missing data for this patient.
Results
Fifteen participants (9 female and 6 male; mean [SD] age, 37.8 [11.6] years) completed the baseline assessment and were administered psilocybin (eFigure in Supplement 2). One screened participant withdrew from the study prior to psilocybin administration due to intolerance of the medication withdrawal and is not included in the analyzed cohort. Demographic and clinical characteristics of the sample are presented in the Table and eTable 1 in Supplement 2. Many participants had a prolonged current episode with a mean (SD) length of 2.5 (2.3) years. The depression rating scales data indicated that the participants had moderate to severe levels of symptom severity.
Table. Demographic and Clinical Characteristics of the Study Population.
| Characteristic | Mean (SD) |
|---|---|
| Age, y | 37.8 (11.6) |
| Female, No. (%) | 9 (60) |
| Male, No. (%) | 6 (40) |
| Ethnicity, No. (%)a | |
| Non-White (amalgamated to prevent identifiability)b | 3 (20) |
| White | 12 (80) |
| Age at BDII onset, y | 29.1 (10.4) |
| Duration of illness, y | 9.4 (8.6) |
| Lifetime suicide attempts, No. (%) | 5 (33.3) |
| No. of attempts among patients with suicide attempts | 2.4 (2.2) |
| Lifetime psychiatric hospitalizations, No. (%) | 7 (46.7) |
| No. of admissions among patients hospitalized | 2.4 (2.1) |
| History of alternative therapies (ECT/TMS/ketamine), No. (%) | 3 (20) |
| Currently in psychotherapy, No. (%) | 11 (73) |
| Currently in CBT, No. (%) | 3 (20) |
| Lifetime treatment failures (medications + interventional trials) | 4.27 (1.5) |
| Duration of current episode, mo | 30.2 (27.9) |
| Lifetime prior psychedelic experience, No. (%) | 5 (33.3) |
| No. of psychedelic experiences among patients with prior psilocybin use | 1.2 (0.45) |
| HRSD score at baseline | 21.9 (4.0) |
| MADRS score at baseline | 31.3 (5.2) |
| QIDS-SR score at baseline | 18.6 (4.9) |
| YMRS score at baseline | 2.1 (1.7) |
| Q-LES-Q-SF score at baseline | 42.9 (7.6) |
Abbreviations: BDII, bipolar II disorder; CBT, cognitive behavior therapy; ECT, electroconvulsive therapy; HRSD, Hamilton Rating Scale for Depression; MADRS, Montgomery-Åsberg Depression Rating Scale; QIDS-SR, Quick Inventory of Depression Symptoms-Self Rating; Q-LES-Q-SF, Quality of Life Enjoyment and Satisfaction Questionnaire-Short Form; TMS, transcranial magnetic stimulation; YMRS, Young Mania Rating Scale.
Ethnicity data were gathered by self-report and included to evaluate the diversity of the sample.
Other ethnicity groups represented are not listed to protect participants’ identity.
Primary Outcome Measure: Change in MADRS Score
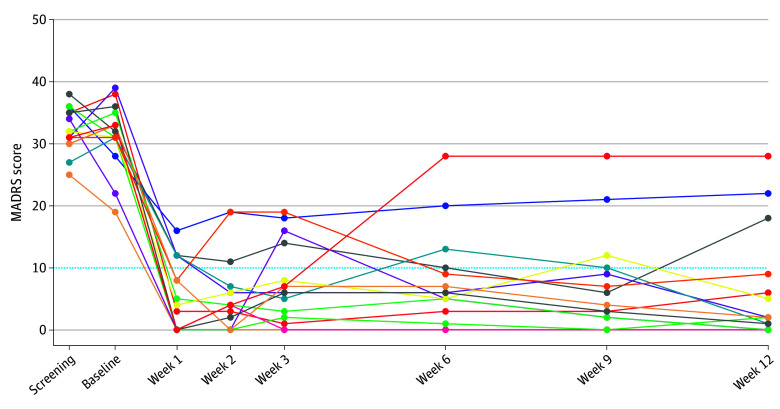
Figure 1 presents the raw MADRS scores for each participant at each assessment time point. The primary outcome was change in MADRS score from baseline to week 3. All participants had lower scores at week 3, with a mean (SD) change of −24.00 (9.23) points (paired t14 = 10.07; Cohen d = 4.08; 95% CI, −29.11 to −18.89; P < .001), corresponding to a mean (SD) 76.3% (29.3) reduction from baseline. The 1-way repeated-measures ANOVA on the raw scores at baseline, week 1, week 3, and week 12 yielded a robust effect of time point (F3,42 = 67.05; P < .001) (Figure 2A). MADRS scores at each posttreatment time point were significantly decreased from baseline (week 1: Cohen d = 4.78; 95% CI, −31.65 to −20.62; adjusted P < .001; week 3: Cohen d = 4.08; 95% CI, −31.32 to −16.68; adjusted P < .001; week 12: Cohen d = 3.39; 95% CI, −33.19 to −16.95; adjusted P < .001). Mean MADRS scores did not significantly differ among weeks 1, 3, and 12, suggesting stability of posttreatment effects.
Figure 1. Individual Montgomery-Åsberg Depression Rating Scale (MADRS) Scores Over the Study Period.
The dotted line indicates the threshold for remission from bipolar depression. Each color represents an individual patient.
Figure 2. Mood Symptom Assessments Following Psilocybin Treatment.
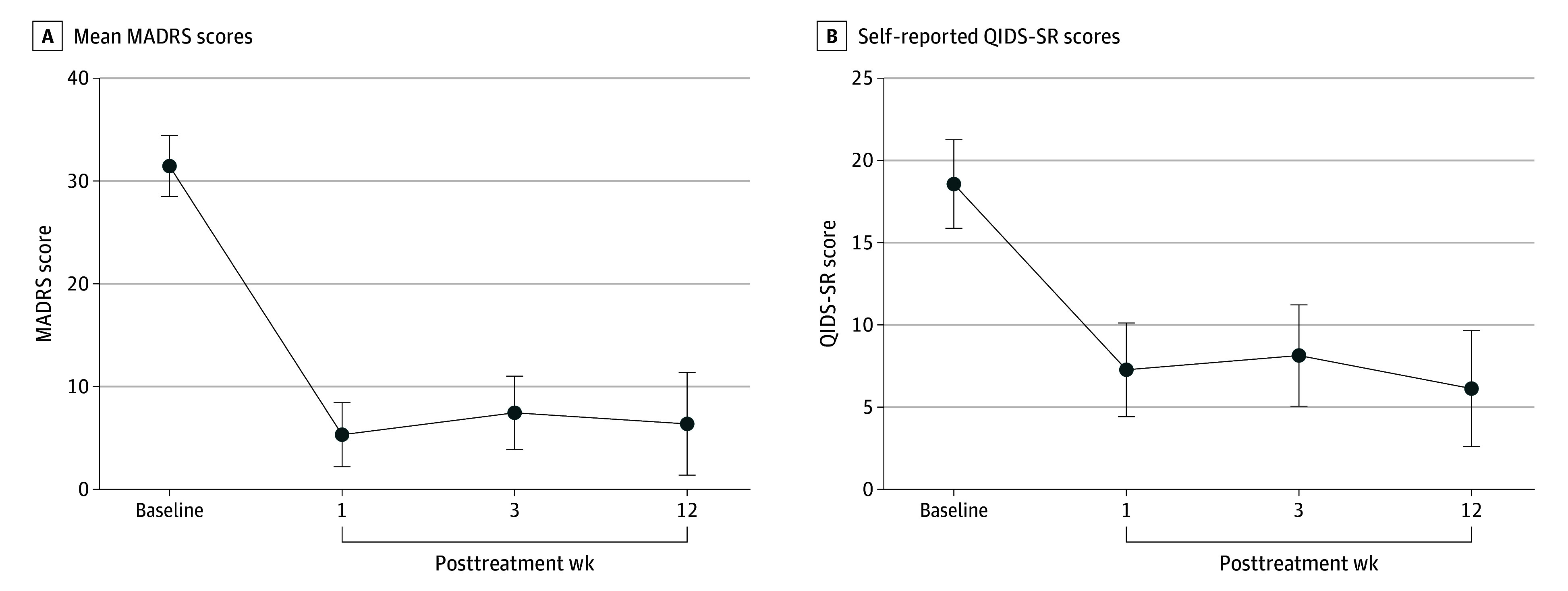
MADRS indicates Montgomery-Åsberg Depression Rating Scale; QIDS-SR, Quick Inventory of Depression Symptoms-Self Rating.
At week 3 (primary outcome), 12 participants met the response criterion and 11 met the remission criterion. At week 12 (study end point) 12 patients met both response and remission criteria.
Secondary Outcome Measure: Change in QIDS-SR Scores
A 1-way repeated-measures ANOVA was conducted on self-report QIDS-SR scores at baseline and weeks 1, 3, and 12 (Figure 2B). The ANOVA yielded a robust effect of time point (F3,42 = 21.78; P < .001), with significant reductions from baseline at week 1 (Cohen d = 2.26; 95% CI, −15.58 to −6.95; adjusted P < .001), week 3 (Cohen d = 2.00; 95% CI, −16.77 to −4.03; adjusted P = .001), week 12 (Cohen d = 2.19; 95% CI, −19.13 to −5.67; adjusted P < .001). None of the follow-up weeks differed from each other.
Additional Secondary Outcome Measures: Mania Symptoms, Suicidal Ideation, Quality of Life
The repeated-measures ANOVA on YMRS scores at the 4 time points suggested an overall trend for a reduced mania symptom severity over time (F3,42 = 2.85; P = .049). However, post hoc comparison did not reveal significant differences between any time points.
Patients were monitored for suicidality via the CSSRS. No patients attempted or committed suicide during the study. Suicidal ideation severity, a subscore, was compared at posttreatment time points vs baseline. The 1-way, repeated-measures ANOVA was conducted on suicidal ideation severity scores at the 4 time points (Figure 3A). The main effect of time point was not significant (F3,42 = 2.29; P = .09).
Figure 3. Assessment of Suicidal Ideation Severity and Quality of Life.
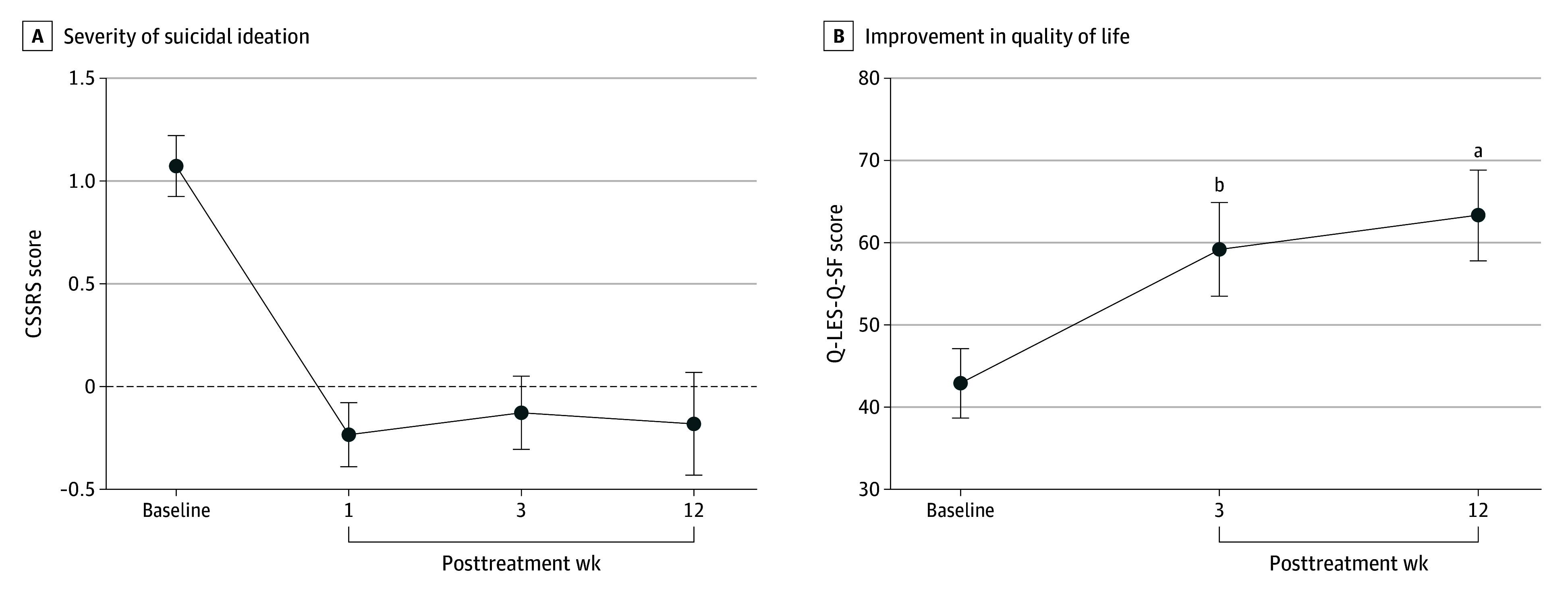
CSSRS indicates Columbia Suicide Severity Rating Scale; Q-LES-Q-SF, Quality of Life Enjoyment and Satisfaction Questionnaire-Short Form.
A repeated-measures ANOVA was conducted on Q-LES-Q-SF scores at baseline, week 3, and week 12 (Figure 3B). There was a significant effect of time point (F2,28 = 28.29; P < .001), with significant increases over baseline at both postdosing time points (week 3: Cohen d = 1.80; 95% CI, 8.14 to 24.39; adjusted P < .001; week 12: Cohen d = 2.30; 95% CI, 11.11 to 29.69; adjusted P < .001).
Correlation of Acute Psychedelic Experience With Longer-Term Antidepressant Efficacy
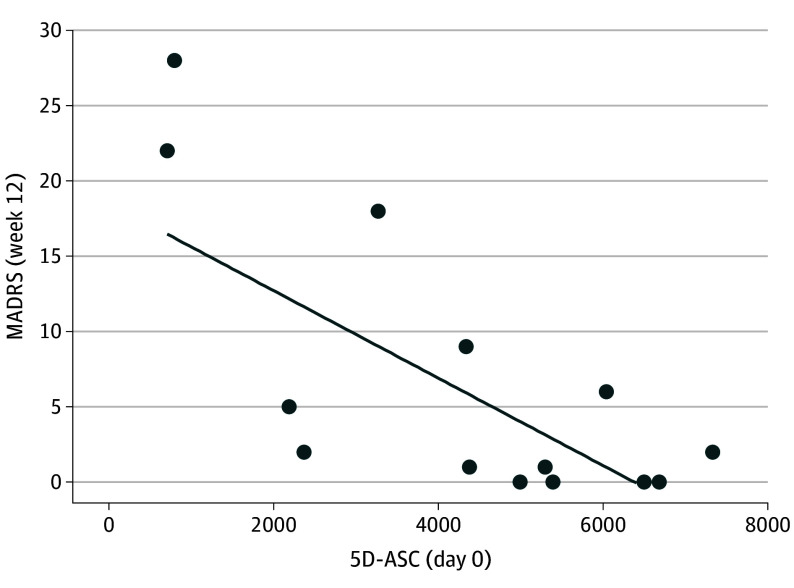
The 5 dimensional subscales of the 5D-ASC were correlated with one another in this sample (oceanic boundlessness [OB]–anxious ego dissolution [AED]: r = 0.73; 95% CI, 0.32 to 0.90; P = .002; OB–visionary restructuralization [VR]: r = 0.93; 95% CI, 0.80 to 0.98; P < .001; OB–auditory alterations [AA]: r = 0.89; 95% CI, 0.67 to 0.96; P < .001; OB–reduction of vigilance [RV]: r = 0.79; 95% CI, 0.45 to 0.93; P < .001; AED–VR: r = 0.65; 95% CI, 0.18 to 0.87; P = .009; AED–AA: r = 0.68; 95% CI, 0.24 to 0.88; P = .005; AED–RV: r = 0.82; 95% CI, 0.50 to 0.93; P < .001; VR–AA: r = 0.78; 95% CI, 0.42 to 0.92; P < .001; VR–RV: r = 0.75; 95% CI, 0.37 to 0.91; P = .001; AA–RV: r = 0.79; 95% CI, 0.44 to 0.92; P < .001) (eTable 2 in Supplement 2). All 5 dimensions, with the exception of AA, were found to significantly correlate with the MADRS end point (OB: r = −0.66; 95% CI; −0.87 to −0.20; P = .008; AED: r = −0.55, 95% CI; −0.82 to −0.03; P = .03; VR: r = −0.79; 95% CI, −0.92 to −0.44; P < .001; RV: r = −0.59; 95% CI, −0.84 to −0.09; P = .02; AA: r = −0.51; 95% CI, −0.80 to 0.03; P = 0.06) (eTable 2 in Supplement 2). When a global 5D-ASC score summing across all subscales was taken as a measure of overall intensity of the psychedelic experience, there was significant correlation with MADRS scores at week 12 (Pearson r = −0.69; 95% CI, −0,88 to −0.25; P = .005) (Figure 4). This suggests that the intensity of psychedelic experience is predictive of longer-term antidepressant effects.
Figure 4. Association Between Psychedelic Experience and Sustained Antidepressant Effect.
5D-ASC indicates Five Dimension Altered State of Consciousness Questionnaire; MADRS, Montgomery-Åsberg Depression Rating Scale.
Safety
There were no significant adverse events related to the psilocybin dosing. The most common adverse event was headache in 4 of 15 patients on the day of dosing, with symptoms resolving within 24 hours.
Medication Taper and Restart
All 15 participants were fully withdrawn from all antidepressant and mood stabilizing medications at least 2 weeks prior to dosing. Pretreatment medication regimens and medications restarted during the study are shown in eTable 1 in Supplement 2. Nine of the 15 participants did not recommence antidepressant or mood stabilizing medications for the duration of the study period. Six restarted at least 1 agent from their prior regimen. Of these 6, 1 restarted at week 2, before the primary outcome visit at week 3, and was considered a nonresponder throughout the study. One participant remitted at week 3, relapsed at week 6, dropped out of the study, and restarted medication and was coded as a nonresponder for weeks 9 and 12. Another participant was nonresponder at weeks 3 and 6, recommenced an antidepressant at week 6, and remitted at week 12. The other 3 participants resumed medications at week 6 or 9 for prophylaxis, and were considered responders or remitters at all visits.
Discussion
To our knowledge, this nonrandomized open-label trial is the first prospective and systematic, albeit noncomparator, study reporting clinical experience with psilocybin dosing and psychotherapy in a cohort of individuals with BDII currently experiencing a major depressive episode. The 15 participants in this trial had well-documented treatment-resistant BDII depression of marked severity and a lengthy duration of the current depressive episode. Individuals in this study displayed strong and persistent antidepressant effects, with no signal of worsening mood instability or increased suicidality. As a first open-label foray into this underserved and treatment-resistant population, care should be taken not to overinterpret the findings. Administration of a psychedelic agent under carefully controlled and supportive conditions may yield distinct effects compared to self-report surveys on recreational use of psychedelics by people with BD.
A survey-based report of anecdotal use of psychedelics in patients who reported having BD showed one-third of respondents reporting manic symptoms, insomnia, or anxiety after psilocybin exposure,31 along with some indications of mental health benefit.32 These surveys report both positive and negative effects from psychedelics in individuals with putative BD. The positive effects from anecdotal reports include decreased depression, increased emotional processing, and development of new perspectives, which were seen in most of our study participants. The negative outcomes from anecdotal reports include poor sleep, mania, and hospitalization, none of which were seen in this study. Concomitant medication or substances, inadequate diagnostic information, or unreliable reporting may confound these survey results.
Given the lack of information on the risks and benefits of psilocybin administration in this population, in addition to documenting efficacy, this study specifically focused on the emergence of mixed or manic symptoms, such as agitation or insomnia, psychotic symptoms, and worsening suicidal ideation. These were not observed. The low baseline CSSRS and YMRS scores gave little room to observe significant improvement.
In this study, most participants remitted rapidly (ie, within 1 week of dosing), and in most participants, remission persisted for the 12-week study duration. The 3 participants who restarted medication due to lack of benefit or relapse following improvement generally had poorer response throughout the trial. Thus, in a sample of patients with treatment-resistant cyclical mood disorder, achieving persistent remission over a 3-month period is notable, especially given the single dosing of psilocybin. Further follow-up is warranted.
Despite the high remission rate, there was an association between general intensity of the psychedelic experience and clinical benefit. In particular, individuals in whom psilocybin administration had little subjective impact showed little clinical benefit. The necessity of a distinct psychedelic experience for response remains a point of debate within the field,33 and the findings of this study suggest that the degree of psychedelic experience is predictive of longer-term antidepressant effects.
This study supports further study of the utility of psychedelics in the BDII population. Consideration should be given to whether psilocybin administration alters (increases or decreases) the high risk of substance use disorders in the population,34,35 whether the novel changes in perspective and emotional processing seen with psychedelic administration can be reliably assessed in this population, and whether such cognitive alterations are necessary or sufficient for therapeutic effects. In light of this study, the safety and efficacy of psilocybin administration should be explored in a randomized clinical trial enrolling patients with BDII. Care should be taken in expanding this paradigm to BDI disorder given the higher potential risk.
Limitations
As an uncontrolled, open-label study, the efficacy findings could be influenced by multiple factors in addition to the effects of psilocybin, including selection biases in study participation, intensity of therapeutic contact, and expectation effects. The small sample size also limits results. The selected nature of the study population and high levels of motivation prevent extrapolation to the general population of people with bipolar illness. Participants were only followed up with for 12 weeks postdosing, providing limited information on the durability of response. Follow-up studies are needed to provide longer-term assessment. The single dose protocol of this study does not capture benefits or harms that could occur with multiple dosings. Also, these findings cannot be extrapolated to patients with BDI or BDII in a mixed or hypomanic phase of their illness.
Conclusions
The findings support further study of psychedelics in the BDII population. Consideration should be given as to whether administration of psilocybin affects the high risk of substance use disorders in the BD population. It is premature to extrapolate these data to the BDI population, who are at higher risk of mania and psychosis.
Trial protocol
eTable 1. Participant Medication History and MADRS Outcomes
eTable 2. Correlations Across 5 Dimensions of the 5D-ASC, and with Endpoint MADRS
eFigure. Consort Diagram
Data sharing statement
References
- 1.Merikangas KR, Lamers F. The ‘true’ prevalence of bipolar II disorder. Curr Opin Psychiatry. 2012;25(1):19-23. doi: 10.1097/YCO.0b013e32834de3de [DOI] [PubMed] [Google Scholar]
- 2.Rosa AR, Bonnín CM, Vázquez GH, et al. Functional impairment in bipolar II disorder: is it as disabling as bipolar I? J Affect Disord. 2010;127(1-3):71-76. doi: 10.1016/j.jad.2010.05.014 [DOI] [PubMed] [Google Scholar]
- 3.Ruggero CJ, Chelminski I, Young D, Zimmerman M. Psychosocial impairment associated with bipolar II disorder. J Affect Disord. 2007;104(1-3):53-60. doi: 10.1016/j.jad.2007.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karanti A, Kardell M, Joas E, Runeson B, Pålsson E, Landén M. Characteristics of bipolar I and II disorder: a study of 8766 individuals. Bipolar Disord. 2020;22(4):392-400. doi: 10.1111/bdi.12867 [DOI] [PubMed] [Google Scholar]
- 5.Mignogna KM, Goes FS. Characterizing the longitudinal course of symptoms and functioning in bipolar disorder. Psychol Med. Published online June 4, 2022. doi: 10.1017/S0033291722001489 [DOI] [PubMed] [Google Scholar]
- 6.Gitlin M, Malhi GS. The existential crisis of bipolar II disorder. Int J Bipolar Disord. 2020;8(1):5. doi: 10.1186/s40345-019-0175-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goffin KC, Dell’Osso B, Miller S, et al. Different characteristics associated with suicide attempts among bipolar I versus bipolar II disorder patients. J Psychiatr Res. 2016;76:94-100. doi: 10.1016/j.jpsychires.2016.02.006 [DOI] [PubMed] [Google Scholar]
- 8.McIntyre RS, Calabrese JR. Bipolar depression: the clinical characteristics and unmet needs of a complex disorder. Curr Med Res Opin. 2019;35(11):1993-2005. doi: 10.1080/03007995.2019.1636017 [DOI] [PubMed] [Google Scholar]
- 9.Paterniti S, Bisserbe JC. Pharmacotherapy for bipolar disorder and concordance with treatment guidelines: survey of a general population sample referred to a tertiary care service. BMC Psychiatry. 2013;13:211. doi: 10.1186/1471-244X-13-211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97-170. doi: 10.1111/bdi.12609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grunze H, Vieta E, Goodwin GM, et al. ; Members of the WFSBP Task Force on Bipolar Affective Disorders Working on this topic . The World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for the Biological Treatment of Bipolar Disorders: acute and long-term treatment of mixed states in bipolar disorder. World J Biol Psychiatry. 2018;19(1):2-58. doi: 10.1080/15622975.2017.1384850 [DOI] [PubMed] [Google Scholar]
- 12.Sachs GS, Dupuy JM, Wittmann CW. The pharmacologic treatment of bipolar disorder. J Clin Psychiatry. 2011;72(5):704-715. doi: 10.4088/JCP.10m06523 [DOI] [PubMed] [Google Scholar]
- 13.Goldberg JF, Nierenberg AA, Iosifescu DV. Wrestling with antidepressant use in bipolar disorder: the ongoing debate. J Clin Psychiatry. 2021;82(1):19ac13181. doi: 10.4088/JCP.19ac13181 [DOI] [PubMed] [Google Scholar]
- 14.Hidalgo-Mazzei D, Berk M, Cipriani A, et al. Treatment-resistant and multi-therapy-resistant criteria for bipolar depression: consensus definition. Br J Psychiatry. 2019;214(1):27-35. doi: 10.1192/bjp.2018.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halberstadt AL, Geyer MA. Multiple receptors contribute to the behavioral effects of indoleamine hallucinogens. Neuropharmacology. 2011;61(3):364-381. doi: 10.1016/j.neuropharm.2011.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jefsen OH, Elfving B, Wegener G, Müller HK. Transcriptional regulation in the rat prefrontal cortex and hippocampus after a single administration of psilocybin. J Psychopharmacol. 2021;35(4):483-493. doi: 10.1177/0269881120959614 [DOI] [PubMed] [Google Scholar]
- 17.Magaraggia I, Kuiperes Z, Schreiber R. Improving cognitive functioning in major depressive disorder with psychedelics: a dimensional approach. Neurobiol Learn Mem. 2021;183:107467. doi: 10.1016/j.nlm.2021.107467 [DOI] [PubMed] [Google Scholar]
- 18.Meccia J, Lopez J, Bagot RC. Probing the antidepressant potential of psilocybin: integrating insight from human research and animal models towards an understanding of neural circuit mechanisms. Psychopharmacology (Berl). 2023;240(1):27-40. doi: 10.1007/s00213-022-06297-0 [DOI] [PubMed] [Google Scholar]
- 19.Goodwin GM, Aaronson ST, Alvarez O, et al. Single-dose psilocybin for a treatment-resistant episode of major depression. N Engl J Med. 2022;387(18):1637-1648. doi: 10.1056/NEJMoa2206443 [DOI] [PubMed] [Google Scholar]
- 20.Carhart-Harris R, Giribaldi B, Watts R, et al. Trial of psilocybin versus escitalopram for depression. N Engl J Med. 2021;384(15):1402-1411. doi: 10.1056/NEJMoa2032994 [DOI] [PubMed] [Google Scholar]
- 21.Rucker JJ, Marwood L, Ajantaival RJ, et al. The effects of psilocybin on cognitive and emotional functions in healthy participants: results from a phase 1, randomised, placebo-controlled trial involving simultaneous psilocybin administration and preparation. J Psychopharmacol. 2022;36(1):114-125. doi: 10.1177/02698811211064720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheehan D, Janavs J, Baker R, Sheehan K, Knapp E, Sheehan M. The Mini International Neuropsychiatric Interview (Version 7.0. 2) for DSM-5. Harm Research Institute; 2016. [Google Scholar]
- 23.Chandler GM, Iosifescu DV, Pollack MH, Targum SD, Fava M. RESEARCH: validation of the Massachusetts General Hospital Antidepressant Treatment History Questionnaire (ATRQ). CNS Neurosci Ther. 2010;16(5):322-325. doi: 10.1111/j.1755-5949.2009.00102.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zimmerman M, Martinez JH, Young D, Chelminski I, Dalrymple K. Severity classification on the Hamilton Depression Rating Scale. J Affect Disord. 2013;150(2):384-388. doi: 10.1016/j.jad.2013.04.028 [DOI] [PubMed] [Google Scholar]
- 25.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429-435. doi: 10.1192/bjp.133.5.429 [DOI] [PubMed] [Google Scholar]
- 26.Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382-389. doi: 10.1192/bjp.134.4.382 [DOI] [PubMed] [Google Scholar]
- 27.Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54(5):573-583. doi: 10.1016/S0006-3223(02)01866-8 [DOI] [PubMed] [Google Scholar]
- 28.Endicott J, Nee J, Harrison W, Blumenthal R. Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacol Bull. 1993;29(2):321-326. [PubMed] [Google Scholar]
- 29.Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266-1277. doi: 10.1176/appi.ajp.2011.10111704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hasler F, Grimberg U, Benz MA, Huber T, Vollenweider FX. Acute psychological and physiological effects of psilocybin in healthy humans: a double-blind, placebo-controlled dose-effect study. Psychopharmacology (Berl). 2004;172(2):145-156. doi: 10.1007/s00213-003-1640-6 [DOI] [PubMed] [Google Scholar]
- 31.Morton E, Sakai K, Ashtari A, Pleet M, Michalak EE, Woolley J. Risks and benefits of psilocybin use in people with bipolar disorder: an international web-based survey on experiences of ‘magic mushroom’ consumption. J Psychopharmacol. 2023;37(1):49-60. doi: 10.1177/02698811221131997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DellaCrosse M, Pleet M, Morton E, et al. “A sense of the bigger picture:” a qualitative analysis of follow-up interviews with people with bipolar disorder who self-reported psilocybin use. PLoS One. 2022;17(12):e0279073. doi: 10.1371/journal.pone.0279073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nautiyal KM, Yaden DB. Does the trip matter? investigating the role of the subjective effects of psychedelics in persisting therapeutic effects. Neuropsychopharmacology. 2023;48(1):215-216. doi: 10.1038/s41386-022-01424-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hunt GE, Malhi GS, Cleary M, Lai HM, Sitharthan T. Prevalence of comorbid bipolar and substance use disorders in clinical settings, 1990-2015: systematic review and meta-analysis. J Affect Disord. 2016;206:331-349. doi: 10.1016/j.jad.2016.07.011 [DOI] [PubMed] [Google Scholar]
- 35.Grunze H, Schaefer M, Scherk H, Born C, Preuss UW. Comorbid bipolar and alcohol use disorder-a therapeutic challenge. Front Psychiatry. 2021;12:660432. doi: 10.3389/fpsyt.2021.660432 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eTable 1. Participant Medication History and MADRS Outcomes
eTable 2. Correlations Across 5 Dimensions of the 5D-ASC, and with Endpoint MADRS
eFigure. Consort Diagram
Data sharing statement