Abstract
The limitations of current cancer therapies, including the increasing prevalence of multidrug resistance, underscore the urgency for more effective treatments. One promising avenue lies in the repurposing of existing drugs. This review explores the impact of phenothiazines, primarily used as antipsychotic agents, on key mechanisms driving tumor growth and metastasis. The cationic and amphiphilic nature of phenothiazines allows interaction with the lipid bilayer of cellular membranes, resulting in alterations in lipid composition, modulation of calcium channels, fluidity, thinning, and integrity of the plasma membrane. This is especially significant in the setting of increased metabolic activity, a higher proliferative rate, and the invasiveness of cancer cells, which often rely on plasma membrane repair. Therefore, properties of phenothiazines such as compromising plasma membrane integrity and repair, disturbing calcium regulation, inducing cytosolic K-RAS accumulation, and sphingomyelin accumulation in the plasma membrane might counteract multidrug resistance by sensitizing cancer cells to membrane damage and chemotherapy. This review outlines a comprehensive overview of the mechanisms driving the anticancer activities of phenothiazines derivates such as trifluoperazine, prochlorperazine, chlorpromazine, promethazine, thioridazine, and fluphenazine. The repurposing potential of phenothiazines paves the way for novel approaches to improve future cancer treatment.
Keywords: phenothiazines, repurposing, annexins, membrane biophysical properties, membrane integrity, cancer treatment, membrane repair, plasma membrane
1. Introduction
Cancer remains a complex and heterogeneous disease that poses a significant global health challenge. Drug resistance and side effects restrict the effectiveness of existing therapies, emphasizing the need for new and effective treatments (1). In recent years, drug repurposing has emerged as a promising strategy for identifying new anticancer agents, given its potential to rapidly develop drugs with established safety profiles and known pharmacokinetic properties (2).
Phenothiazines belong to important antipsychotic drugs used for schizophrenia and bipolar disorder treatment (3, 4). They demonstrate a broad spectrum of biological activities in mammalian cancer cells, as well as pathogenic bacteria and fungi with antipsychotic, antiemetic, antihistaminic, and anti-inflammatory properties (5, 6). Beyond psychiatric use, phenothiazines may act as potential anticancer agents, targeting processes involved in tumor growth and metastasis (7, 8).
Cancer cells are exposed to membrane stress due to their enhanced metabolic activity (9), making them more reliant on an effective plasma membrane repair mechanism to restore membrane integrity and avoid cell death (10). Annexins, a group of essential plasma membrane repair proteins, are often overexpressed in cancer cells (11, 12). They are characterized by their calcium-dependent binding to anionic phospholipids and the ability to aggregate vesicles and fuse membranes (13, 14). Despite excessive research on annexin-mediated membrane repair and annexins’ ability to accumulate and fuse with membranes (15–17), pharmacological approaches to impair membrane repair in cancer cells need to be elucidated. Compromising plasma membrane repair makes cancer cells more susceptible to membrane damage and cell death (18, 19).
Phenothiazine derivatives interfere with plasma membrane junctions, induce lipid phase separation (20, 21), and, as amphiphilic drugs, modify cell membrane properties. They achieve this by altering lipid composition, disrupting lipid rafts (22), thinning the plasma membrane (23), and modulating calcium channels (24). These properties are important aspects in cancer therapy, as phenothiazines have been shown to counteract multidrug resistance in various types of cancer cells and sensitize them to chemotherapy (25).
This review aims to provide a comprehensive overview of the molecular mechanisms underlying the anticancer activity of phenothiazines by influencing the biophysical properties of the plasma membrane. We will summarize current advances in understanding the therapeutic potential of established phenothiazines and their effects on plasma membrane integrity, while discussing the prospects of repurposing these drugs for cancer therapy.
2. Phenothiazines: from antipsychotics to anticancer agents
2.1. Structure and mechanism of actions
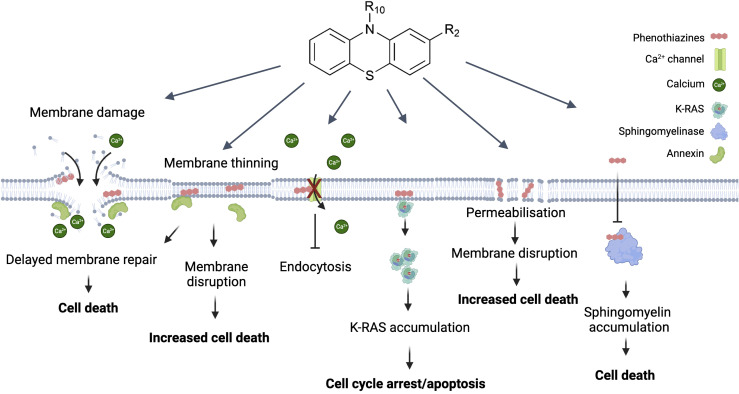
Phenothiazines represent a class of cationic and amphiphilic compounds characterized by the presence of two phenyl rings and thiazine ring containing sulfur and nitrogen atoms ( Figure 1 ). An alkyl bridge is linked to the nitrogen atom within the thiazine ring (26). Phenothiazines are a group of heterocyclic neuroleptic agents known as dopamine receptor blockers that also affect GABA-mediated inhibitory synaptic transmission in cultured hippocampal neurons (27). Additionally, they demonstrated the capacity to inhibit voltage-gated Kv1.3 channels in T lymphocytes (28).
Figure 1.
Direct effects of phenothiazine derivates on the plasma membrane and their potential as anti-cancer drugs. The most direct effect caused by phenothiazines is disruption of the plasma membrane. This causes a rapid influx of Ca2+ which causes depolarization of the actin filaments and activates the membrane repair machinery. The presence of phenothiazines can delay membrane resealing and thus lead to cell death. Similarly, phenothiazines can primarily induce membrane thinning and increased membrane permeabilization which can then also lead to membrane disruption. In contrast to the increased Ca2+ influx associated with membrane disruption phenothiazines can also inactivate the Ca2+ channels causing Ca2+ dysregulation affecting multiple cellular functions including growth. A more specific effect on growth is the interaction between phenothiazine and K-RAS, causing K-RAS to dissociate from the plasma membrane and accumulate in the cytosol, leading to cell cycle arrest and/or apoptosis. Finally, phenothiazines can inhibit the sphingomyelinase leading to sphingomyelin accumulation in the plasma membrane and subsequent cell death. Created with Biorender.
Phenothiazines demonstrate a broad spectrum of biological activities in mammalian cancer cells, as well as pathogenic microorganisms, which include bacteria (29), fungi, and protozoa. These compounds exhibit antipsychotic, antiemetic, antihistaminic, and anti-inflammatory properties and have been used in the treatment of a wide range of diseases (6).
Phenothiazines exert their anticancer effects through multiple mechanisms (30). They inhibit cell proliferation by targeting different stages of the cell cycle (8), including DNA repair (31) and microtubule dynamics (32). In addition, they also modulate signaling pathways such as, PDK1/Akt and MAPK/ERK1/2, which are involved in cancer progression and survival (7, 33). Other studies support the induction of apoptosis by phenothiazines through inhibiting the Akt/mTOR pathway, leading to decreased cell proliferation (33, 34).
Phenothiazines also inhibit angiogenesis, the formation of new blood vessels necessary for tumor growth, by inhibiting the production of VEGF (vascular endothelial growth factor) and VEGF-mediated signaling. Additionally, phenothiazines modulate other molecular pathways involved in angiogenesis, such as the MAPK signaling pathway (6, 35).
Furthermore, phenothiazines can induce oxidative stress by generating reactive oxygen species (ROS) or inhibiting antioxidant enzymes. This oxidative stress leads to DNA damage, mitochondrial dysfunction, and cell death. Cancer cells, which often have higher levels of oxidative stress, are particularly susceptible to this cytotoxicity (36).
2.2. Disruption of membrane integrity
Phenothiazines disrupt the integrity of cell membranes via their intercalation with the lipid bilayer (22). These compounds accumulate selectively within the lipid membrane and have profound effects on its biophysical properties (20, 37). By influencing membrane fluidity and organization, phenothiazines can impact crucial membrane-dependent processes such as signal transduction, ion channel activity, and membrane repair mechanisms (23). The study of the complex interaction between phenothiazines and membrane dynamics gives significant insight into their multiple pharmacological activities and highlights their potential as therapeutic agents in various contexts of disease.
3. Plasma membrane integrity is essential for cell life
3.1. Membrane integrity in maintaining cellular homeostasis
The plasma membrane is a vital component of all living cells, serving as a selective barrier between the intracellular and extracellular environments. Maintaining membrane integrity is essential for cellular homeostasis, since membrane disturbances may impair function and result in cell death (38, 39). The plasma membrane is composed of a phospholipid bilayer containing various proteins and molecules. Integral membrane proteins, like receptors, transporters, and channels, are pivotal for specific cellular functions and external interactions. The plasma membrane is involved in cell signaling through surface receptors that sense external signals like hormones or neurotransmitters. These signals are conveyed into the cell, triggering specific responses crucial for communication, growth, differentiation, and survival (40).
Furthermore, the plasma membrane contributes to maintaining cellular homeostasis by regulating the balance of ions, nutrients, and waste products. This regulation ensures that the intracellular environment remains stable and suitable for cellular function. Additionally, the membrane facilitates cellular adhesion, allowing cells to interact with neighboring cells and form tissues and organs (41).
Beyond its structural and functional roles, the plasma membrane is dynamic and capable of remodeling and reorganizing in response to various stimuli (41). It can change its shape, form specialized structures such as microvilli or pseudopodia, and undergo processes such as endocytosis and exocytosis, allowing an internalization or release of substances (42).
3.2. Perturbations in membrane integrity associated with cancer development and progression
Compromised membrane integrity is closely associated with the induction of cell death pathways (38). Understanding repair mechanisms is crucial for unraveling the complex relationship between membrane integrity and cellular homeostasis, offering therapeutic opportunities in conditions like cancer. A notable characteristic of metastatic cancer cell membranes is that lipid content may change over time. For example, cells undergoing metastasis reduce their cholesterol levels and increase their fluidity and plasticity to facilitate penetration into blood arteries (43). Additionally, reduced cholesterol levels disrupt lipid raft formation and can affect the localization and activity of membrane-associated proteins, influencing important cellular processes such as proliferation, apoptosis, and invasion (44, 45). Calcium ions (Ca2+) are crucial molecules involved in intracellular signaling, which is important for cell proliferation and survival (46). Repair of plasma membrane wounds is initiated by the influx of Ca2+ and the recruitment of Ca2+-regulated proteins, particularly annexins (13, 47). Annexin protein family members (ANXA) (in mammals: ANXA1-11 and ANXA13) play a crucial role in membrane fusion and wound healing (14). They are recruited to the damaged plasma membrane by binding to negatively charged phospholipids, facilitating membrane reshaping and fusion, thus promoting effective resealing. Annexins have diverse properties that contribute to membrane shaping and enable customized responses for efficient repair (15, 16, 48, 49).
Understanding membrane repair mechanisms opens novel avenues to target these processes and develop novel potential therapeutic strategies.
4. Changes in the structure of the cell membrane in response to phenothiazines
Multiple studies support the notion that phenothiazines exert therapeutic effects by modulating membrane function (72, 73). Derivatives of phenothiazine have demonstrated the ability to induce a range of alterations in the structure of cell membranes through molecular interactions with lipid bilayers in cancer cells (25). In this context, we have investigated the impact of various well-known phenothiazines on the plasma membrane of cancer cells and their ability to inhibit repair upon membrane damage ( Table 1 ).
Table 1.
Phenothiazines effecting cell membrane integrity and their respective anti-tumor activities.
| Phenothiazines | Anti- tumor activity | Cancer Types | Effects on Membrane Integrity | In Vivo/Vitro Efficacy |
|---|---|---|---|---|
| Chlorpromazine | Induces cytotoxic autophagy in glioblastoma cells via ER stress and the unfolded protein response, causes mitotic arrest through KSP/Eg5 inhibition (50), affects CcO, complex IV in chemo resistant cells in GBM (51). | Leukemia (52), GBM (51), EC (53) | Reduces the association of K-Ras with the plasma membrane and increases its exchange between membrane and cytoplasmic pools leading to apoptosis (54). |
In vitro: CPZ suppresses in vitro wound healing of PANC-1 GFP-K-Ras (G12V) cells and inhibits colony formation in soft agar (54). In vivo: cell-cycle arrest at the G2/M phase in rat C6 glioma cells, selectively inhibits growth and proliferation of chemo resistant glioma cells expressing COX4-1 (51). |
| Fluphenazine | Inhibits sphingomyelinase and causes cellular sphingomyelin accumulation (55), targets the Akt and Wnt signaling, induces DNA alterations and affects migration (8, 36, 56). | liver (36), oral and ovarian cancer (36), LC (8, 56), TNBC (56). | Alters membrane integrity by perturbing lipid bilayer structure and affecting membrane dynamics (23). Potentially, affects membrane repair processes (36). |
In vitro: Induced G0/G1 cell cycle arrest and mitochondria mediated intrinsic apoptosis (8). In vivo: induced cancer cell apoptosis in a TNBC subcutaneous xenograft mouse model (56). |
| Prochlorperazine | Inhibits the P2X7 receptor on plasma membrane (57), enhance the efficacy of anti-tumor mAbs (58), Blocks D2 dopamine receptors (57). | TNBC (58), LC (59) GBM (57). | Calcium channel blockade (57), disrupts the structural organization between lipids and proteins in microsomal membranes (59). |
In vitro: PCZ exhibits a synergistic effect on cancer cell death, both in vitro and in xenograft models, and improves the overall survival of mice (59). In vivo: alters EGFR distribution, reversibly inhibit the endocytosis of membrane proteins targeted by therapeutic monoclonal antibodies (58). |
| Promethazine | Initiating of autophagy-associated apoptosis through AMPK activation and PI3K/AKT/mTOR inhibition (60), promotes apoptosis by suppressing the PI3K/AKT signaling pathway (61), hinders proliferation and induces autophagy by increasing LC3II and p62 levels in cancer cell lines (62). | CML (60), CRC (61), SCLC (63), PDAC (62). | Indicates an early phosphatidylserine externalization followed by later plasma membrane permeabilization (60). |
In vitro: Exhibits potent and specific cytotoxicity against various leukemia cell types through the activation of AMPK and the inhibition of the PI3K/AKT/mTOR pathway (60), impedes cell proliferation and triggers autophagy by elevating the levels of LC3II and p62 in human pancreatic ductal adenocarcinoma (PDAC) cell lines (62). In vivo: Reduces the growth of both mouse and human SCLC by inducing cell death (63). |
| Thioridazine | Induces eryptosis (64), targeting and inhibiting the PI3K/Akt/mTOR/p70S6K signaling pathway, leading to cell cycle arrest, apoptosis, and cytotoxic effects (65, 66), modulates endothelial cells and impedes angiogenesis via the VEGFR-2/PI3K/mTOR pathway, triggers autophagy by upregulating AMPK activity (67). | TNBC (65), cervical and endometrial cancer (34), OC (35), GBM (67) | Membrane permeabilization (66); triggering of cell membrane scrambling with increase of phosphatidylserine abundance at the cell surface, Thioridazine is partially effective by activation of p38 kinase and by increase of cytosolic Ca2+ concentration (64). |
In vitro: induces autophagy in glioblastoma multiforme (GBM) cell lines and upregulates AMPK activity (67), inhibited the viability and migration of TNBC cells (65). In vivo: Strong antiproliferative effects on B16 melanoma cells, inducing DNA fragmentation and increasing the expression of Caspase-3, a key mediator of apoptosis (68), TZ reduces growth and angiogenesis in ovarian cancer by reducing the phosphorylation of VEGFR-2 and inhibiting PI3K/mTOR signaling in xenografts (35). |
| Trifluoperazine | Disrupts ANXA-mediated plasma membrane repair (23), induces G0/G1 cell cycle arrest and inhibit proliferation and apoptosis of tumor cells (69), suppress tumor cell growth (70, 71). | Metastatic melanoma (69), TNBC (70), GBM (71) | disrupts ANXA-mediated plasma membrane repair (23), reduces plasma membrane fluidity by intercalating into the lipid bilayer, thins the membrane bilayer and making it more fragile (23, 69). |
In vitro: Induced G0/G1 cell cycle arrest via decreasing the expression of both cyclinD1/CDK4 and cyclin E/CDK2 in TNBC (70), decreased cell viability and proliferation, colony formation and spheroid growth on metastatic melanoma (69). In vivo: Increased the radiosensitivity of GBM, resulting in increased tumor cell death and prolonged animal survival (71), cytotoxic effects on melanoma brain metastases (69) |
4.1. Trifluoperazine (TFP)
TFP has been shown to induce lysosomal membrane permeabilization (69) and conformational alterations in membrane organization, caused by a reorganization of the surrounding lipids (74).
Moreover, TFP offers great potential as an inhibitor of plasma membrane repair that sensitizes cancer cells to plasma membrane damage (23). The findings of our study demonstrate that TFP intercalation in the plasma membrane induces membrane thinning and sensitizes cells to membrane injury and cell death. Moreover, the cationic properties of TFP compromise ANXA2 binding to the membrane, delaying the recruitment of ANXA proteins and weakening their attachment to the membrane. This further reduces their ability to induce ANXA4 and ANXA6-mediated membrane curvature around the damaged areas of the membrane (49, 75). This cascade of events initiated by TFP compromises the overall membrane repair response, leaving ruptures unrepaired and sensitizing cells to potential spontaneous injury and death (23).
Other in vitro experiments have shown that TFP induces cell cycle arrest and apoptosis in different cancer cell lines, including triple-negative breast cancer (TNBC) and brain metastases (70). Both in vitro and in vivo xenograft models demonstrated TFP binding to calmodulin (CaM), inhibiting glioblastoma proliferation and invasion by targeting Ca2+ signals (76). This interaction may have a significant impact on the inositol 1,4,5-triphosphate receptor (IP3R), a Ca2+ release channel located in intracellular Ca2+ stores, and IP3R-mediated Ca2+ release (24, 77, 78). Moreover, TFP has demonstrated the ability to enhance the radiosensitivity of glioblastoma multiforme (GBM), resulting in increased tumor cell mortality and extended survival (71). These findings highlight the potential of TFP as an anticancer agent with the ability to sensitize cancer cells to plasma membrane damage and target Ca2+ signals in glioblastoma, offering new possibilities for therapeutic interventions in cancer treatment.
4.2. Prochlorperazine (PCZ)
PCZ, as primarly an antipsychotic and antiemetic medication, shows promise in cancer therapy by targeting specific cancer-related molecules, including KRAS mutants. PCZ binds to KRAS mutants’ GTP-binding sites, inhibiting their continuous activation. Additionally, the combination of PCZ and irradiation treatment synergistically increases the radiosensitivity of xenografted mice by downregulating the Ras/Raf/MEK/ERK signaling pathway and reducing the clonogenic survival of KRAS-mutant NSCLC. This combination treatment activates p-ATM, p53, and p21 proteins, leading to cell cycle arrest (59). PCZ also modulates plasma membrane P2X7 receptors, leading to the inhibition of P2X7-mediated Ca2+ entry, and potential impacts on cellular processes such as proliferation and apoptosis (57). PCZ disrupts the structural organization between lipids and proteins in microsomal membranes, thereby altering the activity and regulation of integral membrane proteins (79). Moreover, studies have shown that PCZ can reversibly inhibit the in vivo endocytosis of membrane proteins (58).
4.3. Chlorpromazine (CPZ)
CPZ is known for its evident interactions with biological membranes. It accumulates in membranes and modulates their permeability and fluidity, contributing to the biochemical and pharmacological effects of phenothiazines (73, 80). As an antipsychotic drug, CPZ antagonizes the CNS dopamine D2 receptor (DRD2) and reduces the postsynaptic effect of dopamine (81). CPZ has also demonstrated potential as an anticancer agent through interactions with key cancer-related proteins, including p53, YAP, Ras protein, ion channels, and MAPKs, influencing cell cycle regulation, cancer growth, metastasis, resistance to chemotherapy, and stemness (50, 82). CPZ has shown a suppression of cell growth in chemoresistant glioma cells and glioma stem cells. In terms of its mechanism of action, CPZ inhibited the activity of cytochrome c oxidase (CcO, complex IV) in chemoresistant cells while leaving chemosensitive cells unaffected, and it had no impact on other mitochondrial complexes (51). CPZ also disrupts Ca2+ signaling, raising intracellular Ca2+, altering Ca2+ homeostasis, and causing cytotoxicity in glioblastoma cells (83, 84). Furthermore, CPZ induces endoplasmic reticulum (ER) stress and unfolded protein response (UPR), influencing cell fate through autophagy (50). The interaction between CPZ and negatively charged phospholipids has demonstrated a reduction of the link between oncogenic K-Ras and the plasma membrane, hence causing an increase in the cytosolic pool of K-Ras, followed by cell cycle arrest and apoptosis in cancer cells (53, 54).
4.4. Promethazine (PMTZ)
PMTZ, as an initial-generation antihistamine, antipsychotic, sedative, and antiemetic drug, has shown a wide range of effects on several cancer types. PMTZ induces cell death in leukemia by activating AMPK and inhibiting the PI3K/AKT/mTOR pathway, leading to autophagy-associated apoptosis (60, 61). In chronic myeloid leukemia (CML), increasing concentrations of PMTZ have been associated with early phosphatidylserine externalization, followed by subsequent plasma membrane permeabilization (60). In colorectal cancer (CRC), PMTZ not only suppresses the proliferation of cancer cells but also initiates mitochondrial apoptosis through the PI3K/AKT pathway (61). Additionally, research has illuminated PMTZ’s capacity to induce autophagy in pancreatic ductal adenocarcinoma (PDAC), where it functions as an antagonist of proliferation (62). Furthermore, PMTZ has demonstrated a potent inhibitory impact on the proliferation of both human and murine small cell lung cancer (SCLC). Its ability to inhibit the growth of human H82 SCLC xenografts demonstrates its potential as a diverse and effective anticancer treatment (63).
4.5. Thioridazine (TZ)
TZ shows promise as a multifaceted anticancer agent with the ability to induce apoptosis, inhibit tumor growth, modulate angiogenesis, and target key signaling pathways involved in cancer progression. Earlier studies have demonstrated that TZ triggers eryptosis, the programmed death of red blood cells. This process is marked by disruption of the cell membrane, resulting in heightened binding of Annexin V to red blood cells situated on the cell surface, along with an elevation in cytosolic Ca2+ concentration and the activating p38 kinase (64). TZ exhibited inhibitory effects on TNBC cells, both in vitro and in vivo, by targeting the PI3K/AKT signaling pathway, resulting in G0/G1 cell cycle arrest, apoptosis, and mitochondrial dysfunction. This led to tumor growth suppression and the prevention of lung metastasis in TNBC models (65). TZ possesses the capability to suppress the PI3K/Akt/mTOR/p70S6K signaling pathway and exhibits cytotoxic effects on cervical and endometrial cancer cells through the induction of cell cycle arrest and apoptosis (34, 66). Moreover, TZ was found to disrupt signaling pathways downstream of PI3K, including Akt, PDK1, and mTOR, in ovarian tumor progression via vascular endothelial growth factor receptor 2 (VEGFR-2). This suggests that TZ can modulate endothelial cell function and inhibit angiogenesis through the VEGFR-2/PI3K/mTOR pathway, making it a potential anti-angiogenic agent in ovarian cancer (OC) treatment (35). Furthermore, TZ induces autophagy in GBM cell lines and upregulates AMPK activity (67). TZ has shown a strong antiproliferative effect on melanoma by inducing DNA fragmentation and increasing the expression of caspase-3 (68). These findings highlight the potential of TZ as a therapeutic agent against cancer.
4.6. Fluphenazine
Fluphenazine shows promising potential as a repurposed drug for cancer treatment, effectively reducing the viability of various types of cancers such as lung, TNBC, colon, liver, brain, leukemia, oral, ovarian, and skin (36). Fluphenazine shows anticancer properties, and its antitumor activity is mainly mediated by an effect on the cell cycle, proliferation, or apoptosis. This effect is partly mediated by the inhibition of the lysosomal enzyme sphingomyelinase which leads to increased cellular levels of sphingomyelin (55). It should also be noted that this mechanism differs from other known lysosomal-disrupting agents (85, 86). Furthermore, fluphenazine’s interaction with dipalmitoyl phosphatidylcholine (DPPC) bilayers, the main component of pulmonary surfactants, leads to the disruption of the lipid bilayer and the formation of an isotropic phase at higher concentrations. These interactions contribute to its multidrug-resistant (MDR) activity, which offers a potential strategy for cancer chemoprevention (87). In the context of TNBC and brain metastases, fluphenazine hydrochloride (Flu) was investigated. Flu effectively inhibited the survival of metastatic TNBC cells, inducing arrest of the G0/G1 cell cycle and mitochondrial-mediated intrinsic apoptosis in vitro. Pharmacokinetic studies in mice demonstrated favorable brain bioavailability of Flu for at least 24 hours. In particular, Flu exhibited strong antimetastatic effects in a mouse model of brain metastasis, achieving an impressive 85% inhibition rate. Furthermore, Flu showed a significant inhibition of spontaneous lung metastasis without severe side effects (56). These promising findings urge further research to evaluate Flu’s potential as a treatment option for metastatic TNBC and address the urgent need for novel therapeutic approaches.
5. Conclusions and prospects
Repurposing drugs offers innovative solutions that can exceed standard cancer treatments in effectiveness and safety. Phenothiazines show promise against drug resistance and cancer due to their unique properties, including hydrophobicity and specific structure (2, 6, 26). They exhibit diverse effects on cancer cells, including inhibiting proliferation, disrupting cell cycles, preventing metastasis, inducing apoptosis, and enhancing chemotherapy sensitivity (61, 65, 70, 82).
Maintaining cell membrane integrity is vital for survival. Cancer cells, much like normal cells, reprogram themselves to repair damaged membranes and avoid apoptosis (38). Phenothiazines are gaining scientific attention for their impact on membrane dynamics. They interact with the lipid bilayer and profoundly disturb the biophysical properties of cell membranes, such as fluidity and lipid organization, affecting downstream signal transduction and ion channel activity (20, 37). These compounds also inhibit annexin-mediated plasma membrane repair, which induces membrane thinning and reduces annexin-mediated membrane curvature (23). Disturbances in membrane repair machinery sensitize cells to membrane ruptures, ultimately triggering a cascade of cellular responses that culminate in cell death (15, 23, 39, 49). In addition, they may influence Ca2+ regulation by modifying the activation of Ca2+ receptors such as PMCA and IP3R, hence influencing downstream signaling cascades (24, 83, 84). Furthermore, phenothiazines suppress the PI3K/AKT (7, 34, 61, 65) pathway and interfere with critical cancer-related proteins like K-RAS (54), directing cellular outcomes toward cycle arrest, apoptosis, and reduced proliferation and survival. Their involvement in disturbing membrane permeability and sphingomyelin accumulation provides insights into the complex mechanisms driving cytotoxicity (21, 55, 86, 87).
The anticancer properties of phenothiazines may vary depending on their dosage, since it has been shown that clinically significant levels (~ 1-2 µM) might promote tumor growth (88, 89). However, the membrane-compromising actions of phenothiazines seem to need greater concentrations (~ 7-15 µM) (23). Consequently, the use of higher dosages may elevate the risk of potential side effects, particularly when taken in combination with chemotherapeutic agents. The inconsistent findings regarding these antipsychotic drugs in cancer cells underscore their concentration-dependent characteristics. The role of phenothiazines in cancer treatment may not only vary in relation to concentration but also in accordance with the cancer type. Hence, it is important to evaluate both aspects, when assessing the therapeutic potential of phenothiazines.
In summary, the multifaceted effects of phenothiazines on cellular membranes present significant potential for their repurposing in cancer therapy. Their ability to disrupt membrane integrity, inhibit repair processes, and modify critical cellular pathways positions them as intriguing options for the targeted therapy of cancer. A comprehensive understanding of their interaction with membrane dynamics introduces a fresh perspective for developing innovative therapeutic approaches to combat cancer and address various pathological conditions.
Author contributions
SM: Conceptualization, Writing – original draft, Writing – review & editing. SE: Writing – review & editing. JN: Conceptualization, Writing – original draft, Writing – review & editing.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Work discussed here was supported by the Novo Nordisk Foundation (NNF18OC0034936), The Danish Cancer Society Scientific Committee (Knæk Cancer, R343-A19644), and NEYE-Fonden.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Debela DT, Muzazu SG, Heraro KD, Ndalama MT, Mesele BW, Haile DC, et al. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Med (2021) 9:20503121211034366. doi: 10.1177/20503121211034366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Singhal S, Maheshwari P, Krishnamurthy PT, Patil VM. Drug repurposing strategies for non-cancer to cancer therapeutics. Anticancer Agents Med Chem (2022) 22(15):2726–56. doi: 10.2174/1871520622666220317140557 [DOI] [PubMed] [Google Scholar]
- 3. Ohlow MJ, Moosmann B. Phenothiazine: the seven lives of pharmacology's first lead structure. Drug Discovery Today (2011) 16(3-4):119–31. doi: 10.1016/j.drudis.2011.01.001 [DOI] [PubMed] [Google Scholar]
- 4. Edinoff AN, Armistead G, Rosa CA, Anderson A, Patil R, Cornett EM, et al. Phenothiazines and their evolving roles in clinical practice: A narrative review. Health Psychol Res (2022) 10(4):38930. doi: 10.52965/001c.38930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mosnaim AD, Ranade VV, Wolf ME, Puente J, Antonieta Valenzuela M. Phenothiazine molecule provides the basic chemical structure for various classes of pharmacotherapeutic agents. Am J Ther (2006) 13(3):261–73. doi: 10.1097/01.mjt.0000212897.20458.63 [DOI] [PubMed] [Google Scholar]
- 6. Varga B, Csonka A, Csonka A, Molnar J, Amaral L, Spengler G. Possible biological and clinical applications of phenothiazines. Anticancer Res (2017) 37(11):5983–93. doi: 10.21873/anticanres.12045 [DOI] [PubMed] [Google Scholar]
- 7. Choi JH, Yang YR, Lee SK, Kim SH, Kim YH, Cha JY, et al. Potential inhibition of PDK1/Akt signaling by phenothiazines suppresses cancer cell proliferation and survival. Ann New York Acad Sci (2008) 1138(1):393–403. doi: 10.1196/annals.1414.041 [DOI] [PubMed] [Google Scholar]
- 8. Xi H, Wu M, Ma H, Li S, Huang Q, Zhang Y, et al. Repurposing fluphenazine to suppress melanoma brain, lung and bone metastasis by inducing G0/G1 cell cycle arrest and apoptosis and disrupting autophagic flux. Clin Exp Metastasis (2023) 40(2):161–75. doi: 10.1007/s10585-023-10202-0 [DOI] [PubMed] [Google Scholar]
- 9. Pavlova NN, Thompson CB. The emerging hallmarks of cancer metabolism. Cell Metab (2016) 23(1):27–47. doi: 10.1016/j.cmet.2015.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jaiswal JK, Nylandsted J. S100 and annexin proteins identify cell membrane damage as the Achilles heel of metastatic cancer cells. Cell Cycle (2015) 14(4):502–9. doi: 10.1080/15384101.2014.995495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu X, Ma D, Jing X, Wang B, Yang W, Qiu W. Overexpression of ANXA2 predicts adverse outcomes of patients with Malignant tumors: a systematic review and meta-analysis. Med Oncol (2015) 32(1):392. doi: 10.1007/s12032-014-0392-y [DOI] [PubMed] [Google Scholar]
- 12. Qi H, Liu S, Guo C, Wang J, Greenaway FT, Sun MZ. Role of annexin A6 in cancer. Oncol Lett (2015) 10(4):1947–52. doi: 10.3892/ol.2015.3498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gerke V, Creutz CE, Moss SE. Annexins: linking Ca2+ signalling to membrane dynamics. Nat Rev Mol Cell Biol (2005) 6(6):449–61. doi: 10.1038/nrm1661 [DOI] [PubMed] [Google Scholar]
- 14. Gerke V, Moss SE. Annexins: from structure to function. Physiol Rev (2002) 82(2):331–71. doi: 10.1152/physrev.00030.2001 [DOI] [PubMed] [Google Scholar]
- 15. Lauritzen SP, Boye TL, Nylandsted J. Annexins are instrumental for efficient plasma membrane repair in cancer cells. Semin Cell Dev Biol (2015) 45:32–8. doi: 10.1016/j.semcdb.2015.10.028 [DOI] [PubMed] [Google Scholar]
- 16. Boye TL, Nylandsted J. Annexins in plasma membrane repair. Biol Chem (2016) 397(10):961–9. doi: 10.1515/hsz-2016-0171 [DOI] [PubMed] [Google Scholar]
- 17. Berg Klenow M, Iversen C, Wendelboe Lund F, Mularski A, Busk Heitmann AS, Dias C, et al. Annexins A1 and A2 accumulate and are immobilized at cross-linked membrane-membrane interfaces. Biochemistry (2021) 60(16):1248–59. doi: 10.1021/acs.biochem.1c00126 [DOI] [PubMed] [Google Scholar]
- 18. Frandsen SK, McNeil AK, Novak I, McNeil PL, Gehl J. Difference in membrane repair capacity between cancer cell lines and a normal cell line. J Membr Biol (2016) 249(4):569–76. doi: 10.1007/s00232-016-9910-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jaiswal JK, Lauritzen SP, Scheffer L, Sakaguchi M, Bunkenborg J, Simon SM, et al. S100A11 is required for efficient plasma membrane repair and survival of invasive cancer cells. Nat Commun (2014) 5:3795. doi: 10.1038/ncomms4795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hendrich AB, Wesołowska O, Michalak K. Trifluoperazine induces domain formation in zwitterionic phosphatidylcholine but not in charged phosphatidylglycerol bilayers. Biochim Biophys Acta (BBA) - Biomembranes (2001) 1510(1-2):414–25. doi: 10.1016/S0005-2736(00)00373-4 [DOI] [PubMed] [Google Scholar]
- 21. Hendrich AB, Michalak K, Wesolowska O. Phase separation is induced by phenothiazine derivatives in phospholipid/sphingomyelin/cholesterol mixtures containing low levels of cholesterol and sphingomyelin. Biophys Chem (2007) 130(1-2):32–40. doi: 10.1016/j.bpc.2007.07.001 [DOI] [PubMed] [Google Scholar]
- 22. Wesołowska O, Michalak K, Hendrich AB. Direct visualization of phase separation induced by phenothiazine-type antipsychotic drugs in model lipid membranes. Mol Membrane Biol (2011) 28(2):103–14. doi: 10.3109/09687688.2010.533706 [DOI] [PubMed] [Google Scholar]
- 23. Heitmann ASB, Zanjani AAH, Klenow MB, Mularski A, Sønder SL, Lund FW, et al. Phenothiazines alter plasma membrane properties and sensitize cancer cells to injury by inhibiting annexin-mediated repair. J Biol Chem (2021) 297(2):101012. doi: 10.1016/j.jbc.2021.101012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kang S, Hong J, Lee JM, Moon HE, Jeon B, Choi J, et al. Trifluoperazine, a well-known antipsychotic, inhibits glioblastoma invasion by binding to calmodulin and disinhibiting calcium release channel IP3R. Mol Cancer Ther (2017) 16(1):217–27. doi: 10.1158/1535-7163.MCT-16-0169-T [DOI] [PubMed] [Google Scholar]
- 25. Michalak K, Wesolowska O, Motohashi N, Molnar J, Hendrich A. Interactions of phenothiazines with lipid bilayer and their role in multidrug resistance reversal. Curr Drug Targets (2006) 7(9):1095–105. doi: 10.2174/138945006778226570 [DOI] [PubMed] [Google Scholar]
- 26. Jaszczyszyn A, Gasiorowski K, Swiatek P, Malinka W, Cieslik-Boczula K, Petrus J, et al. Chemical structure of phenothiazines and their biological activity. Pharmacol Rep (2012) 64(1):16–23. doi: 10.1016/S1734-1140(12)70726-0 [DOI] [PubMed] [Google Scholar]
- 27. Mozrzymas JW, Barberis A, Michalak K, Cherubini E. Chlorpromazine inhibits miniature GABAergic currents by reducing the binding and by increasing the unbinding rate of GABAA receptors. J Neurosci (1999) 19(7):2474–88. doi: 10.1523/JNEUROSCI.19-07-02474.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Teisseyre A, Michalak K. The voltage- and time-dependent blocking effect of trifluoperazine on T lymphocyte Kv1. 3 channels Biochem Pharmacol (2003) 65(4):551–61. doi: 10.1016/S0006-2952(02)01561-7 [DOI] [PubMed] [Google Scholar]
- 29. Dastidar SG, Kristiansen JE, Molnar J, Amaral L. Role of phenothiazines and structurally similar compounds of plant origin in the fight against infections by drug resistant bacteria. Antibiotics (Basel) (2013) 2(1):58–72. doi: 10.3390/antibiotics2010058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sroda-Pomianek K, Michalak K, Palko-Labuz A, Uryga A, Swiatek P, Majkowski M, et al. The combined use of phenothiazines and statins strongly affects doxorubicin-resistance, apoptosis, and Cox-2 activity in colon cancer cells. Int J Mol Sci (2019) 20(4):955. doi: 10.3390/ijms20040955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gangopadhyay S, Karmakar P, Dasgupta U, Chakraborty A. Trifluoperazine stimulates ionizing radiation induced cell killing through inhibition of DNA repair. Mutat Res (2007) 633(2):117–25. doi: 10.1016/j.mrgentox.2007.05.011 [DOI] [PubMed] [Google Scholar]
- 32. Ghinet A, Moise IM, Rigo B, Homerin G, Farce A, Dubois J, et al. Studies on phenothiazines: New microtubule-interacting compounds with phenothiazine A-ring as potent antineoplastic agents. Bioorg Med Chem (2016) 24(10):2307–17. doi: 10.1016/j.bmc.2016.04.001 [DOI] [PubMed] [Google Scholar]
- 33. Colturato-Kido C, Lopes RM, Medeiros HCD, Costa CA, Prado-Souza LFL, Ferraz LS, et al. Inhibition of autophagy enhances the antitumor effect of thioridazine in acute lymphoblastic leukemia cells. Life (2021) 11(4):365. doi: 10.3390/life11040365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kang S, Dong SM, Kim B-R, Park MS, Trink B, Byun H-J, et al. Thioridazine induces apoptosis by targeting the PI3K/Akt/mTOR pathway in cervical and endometrial cancer cells. Apoptosis (2012) 17(9):989–97. doi: 10.1007/s10495-012-0717-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Park MS, Dong SM, Kim B-R, Seo SH, Kang S, Lee E-J, et al. Thioridazine inhibits angiogenesis and tumor growth by targeting the VEGFR-2/PI3K/mTOR pathway in ovarian cancer xenografts. Oncotarget (2014) 5(13):4929–34. doi: 10.18632/oncotarget.2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Duarte D, Vale N. Antipsychotic drug fluphenazine against human cancer cells. Biomolecules (2022) 12(10):1360. doi: 10.3390/biom12101360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hidalgo AA, Caetano W, Tabak M, Oliveira ON, Jr. Interaction of two phenothiazine derivatives with phospholipid monolayers. Biophys Chem (2004) 109(1):85–104. doi: 10.1016/j.bpc.2003.10.020 [DOI] [PubMed] [Google Scholar]
- 38. Dias C, Nylandsted J. Plasma membrane integrity in health and disease: significance and therapeutic potential. Cell Discovery (2021) 7(1):4. doi: 10.1038/s41421-020-00233-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Andrews NW, Corrotte M. Plasma membrane repair. Curr Biol (2018) 28(8):R392–R7. doi: 10.1016/j.cub.2017.12.034 [DOI] [PubMed] [Google Scholar]
- 40. Nicolson GL. Cell membrane fluid-mosaic structure and cancer metastasis. Cancer Res (2015) 75(7):1169–76. doi: 10.1158/0008-5472.CAN-14-3216 [DOI] [PubMed] [Google Scholar]
- 41. Blazek AD, Paleo BJ, Weisleder N. Plasma membrane repair: A central process for maintaining cellular homeostasis. Physiol (Bethesda) (2015) 30(6):438–48. doi: 10.1152/physiol.00019.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P, et al. Transport into the cell from the plasma membrane: endocytosis. In: Molecular Biology of the Cell, 4th. New York: Garland Science; (2002). [Google Scholar]
- 43. Szlasa W, Zendran I, Zalesinska A, Tarek M, Kulbacka J. Lipid composition of the cancer cell membrane. J Bioenerg Biomembr (2020) 52(5):321–42. doi: 10.1007/s10863-020-09846-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Buschiazzo J, Ialy-Radio C, Auer J, Wolf JP, Serres C, Lefevre B, et al. Cholesterol depletion disorganizes oocyte membrane rafts altering mouse fertilization. PLoS One (2013) 8(4):e62919. doi: 10.1371/journal.pone.0062919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhuang L, Kim J, Adam RM, Solomon KR, Freeman MR. Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. J Clin Invest (2005) 115(4):959–68. doi: 10.1172/JCI200519935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kang SS, Han KS, Ku BM, Lee YK, Hong J, Shin HY, et al. Caffeine-mediated inhibition of calcium release channel inositol 1,4,5-trisphosphate receptor subtype 3 blocks glioblastoma invasion and extends survival. Cancer Res (2010) 70(3):1173–83. doi: 10.1158/0008-5472.CAN-09-2886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bendix PM, Simonsen AC, Florentsen CD, Hager SC, Mularski A, Zanjani AAH, et al. Interdisciplinary synergy to reveal mechanisms of annexin-mediated plasma membrane shaping and repair. Cells (2020) 9(4):1029. doi: 10.3390/cells9041029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Koerdt SN, Ashraf APK, Gerke V. Annexins and plasma membrane repair. Curr Top Membr (2019) 84:43–65. doi: 10.1016/bs.ctm.2019.07.006 [DOI] [PubMed] [Google Scholar]
- 49. Mularski A, Sonder SL, Heitmann ASB, Pandey MP, Khandelia H, Nylandsted J, et al. Interplay of membrane crosslinking and curvature induction by annexins. Sci Rep (2022) 12(1):22568. doi: 10.1038/s41598-022-26633-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Matteoni S, Matarrese P, Ascione B, Ricci-Vitiani L, Pallini R, Villani V, et al. Chlorpromazine induces cytotoxic autophagy in glioblastoma cells via endoplasmic reticulum stress and unfolded protein response. J Exp Clin Cancer Res (2021) 40(1):347. doi: 10.1186/s13046-021-02144-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Oliva CR, Zhang W, Langford C, Suto MJ, Griguer CE. Repositioning chlorpromazine for treating chemoresistant glioma through the inhibition of cytochrome c oxidase bearing the COX4-1 regulatory subunit. Oncotarget (2017) 8(23):37568–83. doi: 10.18632/oncotarget.17247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhelev Z, Ohba H, Bakalova R, Hadjimitova V, Ishikawa M, Shinohara Y, et al. Phenothiazines suppress proliferation and induce apoptosis in cultured leukemic cells without any influence on the viability of normal lymphocytes. Cancer Chemother Pharmacol (2004) 53(3):267–75. doi: 10.1007/s00280-003-0738-1 [DOI] [PubMed] [Google Scholar]
- 53. Cui Y, Wu H, Yang L, Huang T, Li J, Gong X, et al. Chlorpromazine sensitizes progestin-resistant endometrial cancer cells to MPA by upregulating PRB. Front Oncol (2021) 11:665832. doi: 10.3389/fonc.2021.665832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Eisenberg S, Giehl K, Henis YI, Ehrlich M. Differential interference of chlorpromazine with the membrane interactions of oncogenic K-Ras and its effects on cell growth. J Biol Chem (2008) 283(40):27279–88. doi: 10.1074/jbc.M804589200 [DOI] [PubMed] [Google Scholar]
- 55. Klutzny S, Lesche R, Keck M, Kaulfuss S, Schlicker A, Christian S, et al. Functional inhibition of acid sphingomyelinase by Fluphenazine triggers hypoxia-specific tumor cell death. Cell Death Disease (2017) 8(3):e2709–e. doi: 10.1038/cddis.2017.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Xu F, Xia Y, Feng Z, Lin W, Xue Q, Jiang J, et al. Repositioning antipsychotic fluphenazine hydrochloride for treating triple negative breast cancer with brain metastases and lung metastases. Am J Cancer Res (2019) 9(3):459–78. [PMC free article] [PubMed] [Google Scholar]
- 57. Hempel C, Norenberg W, Sobottka H, Urban N, Nicke A, Fischer W, et al. The phenothiazine-class antipsychotic drugs prochlorperazine and trifluoperazine are potent allosteric modulators of the human P2X7 receptor. Neuropharmacology (2013) 75:365–79. doi: 10.1016/j.neuropharm.2013.07.027 [DOI] [PubMed] [Google Scholar]
- 58. Chew HY, De Lima PO, Gonzalez Cruz JL, Banushi B, Echejoh G, Hu L, et al. Endocytosis inhibition in humans to improve responses to ADCC-mediating antibodies. Cell (2020) 180(5):895–914.e27. doi: 10.1016/j.cell.2020.02.019 [DOI] [PubMed] [Google Scholar]
- 59. Sad K, Parashar P, Tripathi P, Hungyo H, Sistla R, Soni R, et al. Prochlorperazine enhances radiosensitivity of non-small cell lung carcinoma by stabilizing GDP-bound mutant KRAS conformation. Free Radic Biol Med (2021) 177:299–312. doi: 10.1016/j.freeradbiomed.2021.11.001 [DOI] [PubMed] [Google Scholar]
- 60. Medeiros HCD, Colturato-Kido C, Ferraz LS, Costa CA, Moraes VWR, Paredes-Gamero EJ, et al. AMPK activation induced by promethazine increases NOXA expression and Beclin-1 phosphorylation and drives autophagy-associated apoptosis in chronic myeloid leukemia. Chemico-Biological Interactions (2020) 315:108888. doi: 10.1016/j.cbi.2019.108888 [DOI] [PubMed] [Google Scholar]
- 61. Tan X, Gong L, Li X, Zhang X, Sun J, Luo X, et al. Promethazine inhibits proliferation and promotes apoptosis in colorectal cancer cells by suppressing the PI3K/AKT pathway. Biomed Pharmacother (2021) 143:112174. doi: 10.1016/j.biopha.2021.112174 [DOI] [PubMed] [Google Scholar]
- 62. Avendano-Felix M, Aguilar-Medina M, Bermudez M, Lizarraga-Verdugo E, Lopez-Camarillo C, Ramos-Payan R. Refocusing the use of psychiatric drugs for treatment of gastrointestinal cancers. Front Oncol (2020) 10:1452. doi: 10.3389/fonc.2020.01452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jahchan NS, Dudley JT, Mazur PK, Flores N, Yang D, Palmerton A, et al. A drug repositioning approach identifies tricyclic antidepressants as inhibitors of small cell lung cancer and other neuroendocrine tumors. Cancer Discovery (2013) 3(12):1364–77. doi: 10.1158/2159-8290.CD-13-0183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lang E, Modicano P, Arnold M, Bissinger R, Faggio C, Abed M, et al. Effect of thioridazine on erythrocytes. Toxins (2013) 5(10):1918–31. doi: 10.3390/toxins5101918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Song Y, Li L, Chen J, Chen H, Cui B, Feng Y, et al. Thioridazine hydrochloride: an antipsychotic agent that inhibits tumor growth and lung metastasis in triple-negative breast cancer via inducing G0/G1 arrest and apoptosis. Cell Cycle (2020) 19(24):3521–33. doi: 10.1080/15384101.2020.1850969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Spengler G, Csonka A, Molnar J, Amaral L. The anticancer activity of the old neuroleptic phenothiazine-type drug thioridazine. Anticancer Res (2016) 36(11):5701–6. doi: 10.21873/anticanres.11153 [DOI] [PubMed] [Google Scholar]
- 67. Cheng H-W, Liang Y-H, Kuo Y-L, Chuu C-P, Lin C-Y, Lee M-H, et al. Identification of thioridazine, an antipsychotic drug, as an antiglioblastoma and anticancer stem cell agent using public gene expression data. Cell Death Disease (2015) 6(5):e1753–e. doi: 10.1038/cddis.2015.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gil-Ad I, Shtaif B, Levkovitz Y, Nordenberg J, Taler M, Korov I, et al. Phenothiazines induce apoptosis in a B16 mouse melanoma cell line and attenuate in vivo melanoma tumor growth. Oncol Rep (2006) 15(1):107–12. doi: 10.3892/or.15.1.107 [DOI] [PubMed] [Google Scholar]
- 69. Zhang X, Ding K, Ji J, Parajuli H, Aasen SN, Espedal H, et al. Trifluoperazine prolongs the survival of experimental brain metastases by STAT3-dependent lysosomal membrane permeabilization. Am J Cancer Res (2020) 10(2):545–63. [PMC free article] [PubMed] [Google Scholar]
- 70. Feng Z, Xia Y, Gao T, Xu F, Lei Q, Peng C, et al. The antipsychotic agent trifluoperazine hydrochloride suppresses triple-negative breast cancer tumor growth and brain metastasis by inducing G0/G1 arrest and apoptosis. Cell Death Disease (2018) 9(10):1006. doi: 10.1038/s41419-018-1046-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhang X, Xu R, Zhang C, Xu Y, Han M, Huang B, et al. Trifluoperazine, a novel autophagy inhibitor, increases radiosensitivity in glioblastoma by impairing homologous recombination. J Exp Clin Cancer Res (2017) 36(1):118. doi: 10.1186/s13046-017-0588-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Samuels AM, Carey MC. Effects of chlorpromazine hydrochloride and its metabolites on Mg2+- and Na+,K+-ATPase activities of canalicular-enriched rat liver plasma membranes. Gastroenterology (1978) 74(6):1183–90. doi: 10.1016/0016-5085(78)90690-X [DOI] [PubMed] [Google Scholar]
- 73. Bhise SB, Marwadi PR, Mathur SS, Srivastava RC. Liquid membrane phenomena in chlorpromazine action. Biophys Chem (1983) 17(3):187–92. doi: 10.1016/0301-4622(83)87003-3 [DOI] [PubMed] [Google Scholar]
- 74. Ruggiero AC, Meirelles NC. Effects of trifluoperazine on the conformation and dynamics of membrane proteins in human erythrocytes. Mol Genet Metab (1998) 64(2):148–51. doi: 10.1006/mgme.1998.2689 [DOI] [PubMed] [Google Scholar]
- 75. Florentsen CD, Kamp-Sonne A, Moreno-Pescador G, Pezeshkian W, Hakami Zanjani AA, Khandelia H, et al. Annexin A4 trimers are recruited by high membrane curvatures in giant plasma membrane vesicles. Soft Matter (2021) 17(2):308–18. doi: 10.1039/D0SM00241K [DOI] [PubMed] [Google Scholar]
- 76. Vandonselaar M, Hickie RA, Quail JW, Delbaere LT. Trifluoperazine-induced conformational change in Ca(2+)-calmodulin. Nat Struct Biol (1994) 1(11):795–801. doi: 10.1038/nsb1194-795 [DOI] [PubMed] [Google Scholar]
- 77. Yeh CT, Wu AT, Chang PM, Chen KY, Yang CN, Yang SC, et al. Trifluoperazine, an antipsychotic agent, inhibits cancer stem cell growth and overcomes drug resistance of lung cancer. Am J Respir Crit Care Med (2012) 186(11):1180–8. doi: 10.1164/rccm.201207-1180OC [DOI] [PubMed] [Google Scholar]
- 78. Michikawa T, Hirota J, Kawano S, Hiraoka M, Yamada M, Furuichi T, et al. Calmodulin mediates calcium-dependent inactivation of the cerebellar type 1 inositol 1,4,5-trisphosphate receptor. Neuron (1999) 23(4):799–808. doi: 10.1016/S0896-6273(01)80037-4 [DOI] [PubMed] [Google Scholar]
- 79. Dannenberg A, Zakim D. Effects of prochlorperazine on the function of integral membrane proteins. Biochem Pharmacol (1988) 37(7):1259–62. doi: 10.1016/0006-2952(88)90779-4 [DOI] [PubMed] [Google Scholar]
- 80. Maruoka N, Murata T, Omata N, Takashima Y, Tanii H, Yonekura Y, et al. Effects of chlorpromazine on plasma membrane permeability and fluidity in the rat brain: a dynamic positron autoradiography and fluorescence polarization study. Prog Neuropsychopharmacol Biol Psychiatry (2007) 31(1):178–86. doi: 10.1016/j.pnpbp.2006.08.019 [DOI] [PubMed] [Google Scholar]
- 81. Horn AS, Snyder SH. Chlorpromazine and dopamine: conformational similarities that correlate with the antischizophrenic activity of phenothiazine drugs. Proc Natl Acad Sci U S A (1971) 68(10):2325–8. doi: 10.1073/pnas.68.10.2325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yde CW, Clausen MP, Bennetzen MV, Lykkesfeldt AE, Mouritsen OG, Guerra B. The antipsychotic drug chlorpromazine enhances the cytotoxic effect of tamoxifen in tamoxifen-sensitive and tamoxifen-resistant human breast cancer cells. Anticancer Drugs (2009) 20(8):723–35. doi: 10.1097/CAD.0b013e32832ec041 [DOI] [PubMed] [Google Scholar]
- 83. Chu C-S, Lin Y-S, Liang W-Z. The impact of the antipsychotic medication chlorpromazine on cytotoxicity through Ca2+ Signaling pathway in glial cell models. Neurotoxicity Res (2022) 40(3):791–802. doi: 10.1007/s12640-022-00507-5 [DOI] [PubMed] [Google Scholar]
- 84. Plenge-Tellechea F, Dominguez-Solis CA, Diaz-Sanchez AG, Melendez-Martinez D, Vargas-Medrano J, Sierra-Fonseca JA. Chlorpromazine and dimethyl sulfoxide modulate the catalytic activity of the plasma membrane Ca(2+)-ATPase from human erythrocyte. J Bioenerg Biomembr (2018) 50(1):59–69. doi: 10.1007/s10863-017-9741-9 [DOI] [PubMed] [Google Scholar]
- 85. Ellegaard A-M, Groth-Pedersen L, Oorschot V, Klumperman J, Kirkegaard T, Nylandsted J, et al. Sunitinib and SU11652 inhibit acid sphingomyelinase, destabilize lysosomes, and inhibit multidrug resistance. Mol Cancer Ther (2013) 12(10):2018–30. doi: 10.1158/1535-7163.MCT-13-0084 [DOI] [PubMed] [Google Scholar]
- 86. Petersen Nikolaj HT, Olsen Ole D, Groth-Pedersen L, Ellegaard A-M, Bilgin M, Redmer S, et al. Transformation-associated changes in sphingolipid metabolism sensitize cells to lysosomal cell death induced by inhibitors of acid sphingomyelinase. Cancer Cell (2013) 24(3):379–93. doi: 10.1016/j.ccr.2013.08.003 [DOI] [PubMed] [Google Scholar]
- 87. Perez-Isidoro R, Costas M. The effect of neuroleptic drugs on DPPC/sphingomyelin/cholesterol membranes. Chem Phys Lipids (2020) 229:104913. doi: 10.1016/j.chemphyslip.2020.104913 [DOI] [PubMed] [Google Scholar]
- 88. Wen Y, Zhang Y, Li J, Luo F, Huang Z, Liu K. Low concentration trifluoperazine promotes proliferation and reduces calcium-dependent apoptosis in glioma cells. Sci Rep (2018) 8(1):1147. doi: 10.1038/s41598-018-19413-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Souza dos Santos P, Saraiva DF, Ferraz da Costa DC, Scofano HM, de Carvalho-Alves PC. Trifluoperazine protects brain plasma membrane Ca(2+)-ATPase from oxidative damaging. Exp Brain Res (2007) 177(3):347–57. doi: 10.1007/s00221-006-0678-1 [DOI] [PubMed] [Google Scholar]