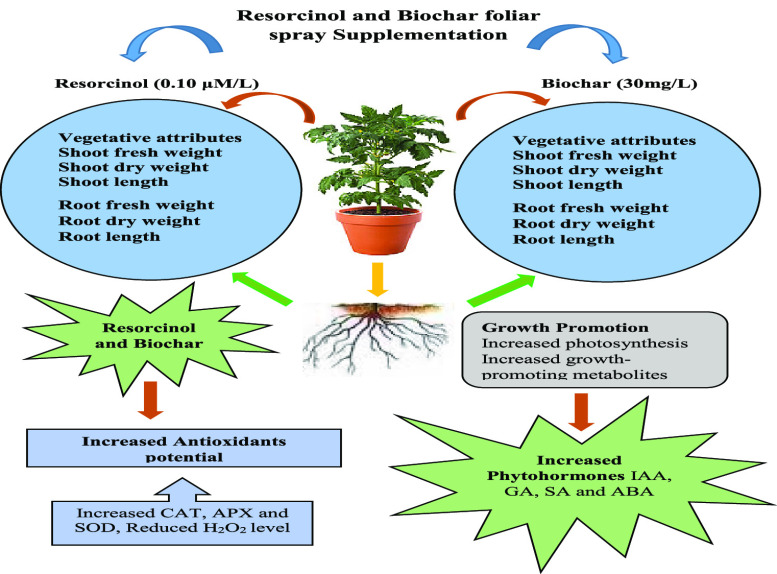
Abstract
Climate variability has been a catalytic factor in inducing both biotic and abiotic stresses, exerting detrimental impacts on crop yields. This, in turn, leads to the manifestation of biochemical and physiological impairments within plant systems. This study aimed to evaluate the effects of different concentrations of resorcinol and biochar on tomato (Lycopersicon esculentum Mill.) growth, primary and secondary metabolites, and antioxidant enzyme content levels. Biochar was synthesized from Cedrus deodara (Roxb. ex D. Don) G. Don, sawmill shavings using pyrolysis and subjected to comprehensive characterization employing contemporary techniques including scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDX), X-ray diffraction (XRD) analysis, Fourier transform infrared (FTIR) spectroscopy, and UV–vis spectroscopy (UV). Both resorcinol at 0.1 μM/L and biochar at 30 mg/L significantly enhanced tomato seed germination and plant growth, promoting increased shoot/root length and fresh/dry weights in tomato plants compared to controls. This supplementation also amplifies tomato chlorophyll contents, growth metabolites, and antioxidant enzyme activities, contributing to robust plant development. Resorcinol at 0.1 μM/L concentration significantly enhanced total protein (79.9 μg/g), total phenol (58.8 μg/g), total proline (0.03 μg/g), total lipid (3.8 μg/g), total soluble sugar (42.5 μg/g), and flavonoid (0.09 μg/g) as compared to control. Biochar at 30 mg/L concentration showed maximum values of total protein (92.1 μg/g), total phenol (61.3 μg/g), total proline (0.03 μg/g), total lipid (5.5 μg/g), total soluble sugar (48.9 μg/g), and flavonoid (0.08 μg/g). This research indicated that foliar application of these specific concentrations of resorcinol and biochar has the ability to improve tomato plant growth, osmolytes, and antioxidant activity.
Research Highlights
-
(1)
Resorcinol (0.1 μM/L) and biochar (30 mg/L) significantly enhance tomato germination and growth, leading to higher shoot/root length and fresh/dry weights than controls
-
(2)
Resorcinol (0.1 μM/L) and biochar (30 mg/L) significantly enhance phytohormone levels, primary and secondary metabolite concentrations, and antioxidant enzyme activities in tomato plants.
1. Introduction
It is essential to highlight that intensive agricultural practices have resulted in the depletion of vital mineral nutrients, thereby constraining the growth, development, and yield of crops. Agricultural waste, wood, sewage sludge, and municipal solid waste are frequently employed as feedstock materials for the production of biochar (BC).1 Biochar technology has the potential to mitigate climate change, eliminate pollutants, and enhance plant growth and soil health, all through its carbon-rich properties that effectively sequester greenhouse gases.2 Moreover, BC serves as a potential alternative to chemical fertilizers, supplying plant nutrients and enriching soil organic carbon. Additionally, it enhances beneficial microorganisms for improved plant growth3 and lessens nutritional losses from rainfall-induced erosion.3−5 Earlier studies extensively reported the positive effect of BC on plant growth and yield6 through improvements in soil properties such as soil fertility,7−9 soil porosity,10,11 water-holding capacity,12,13 cation exchange capacity,14 and bulk density.15,16 In recent times, the utilization of BC has garnered significant attention from scientists worldwide.17,18
Key physiological pathways crucial for plant growth, reproduction, and metabolism, including glycolysis, Krebs cycle, and Calvin cycle, are regulated by primary plant metabolites like carbohydrates, amino acids, organic acids, and enzymes.17,19 Conversely, secondary metabolites, including phenols, alkaloids, flavonoids, and terpenoids, assume a substantial role in the plant’s stress response and defense mechanisms8,20 and are not directly associated with plant growth.21−23 Primary metabolites are essential for plant metabolism and are vital for the production of secondary metabolites.24,25 While the absence of primary metabolites may not severely affect plant survival, their presence is crucial for synthesizing secondary metabolites. Secondary metabolites serve critical roles in plants, including adaptation to the environment, defense mechanisms, metal transport, and symbiotic relationships, acting as hormones, and promoting cellular differentiation.26,27 Phenolic compounds, with hydroxyl groups on aromatic rings, are crucial secondary metabolites, categorized into four main types as flavonoids, phenolic acids, stilbenes, and lignans.28,29
Resorcinol, a phenolic chemical (1,3-isomer of benzenediol) with the formula C6H4(OH)2, was utilized in this investigation. Topal describes resorcinol as an aromatic hydrocarbon group with a benzene ring structure and a two-hydroxyl functional group (−OH) in the ortho position.30 This chemical and its derivatives are used in a wide range of applications, including anti-inflammatory, antitumor, anticonvulsant, and antioxidant properties.31−34 From various plants and microorganisms, different derivatives of resorcinol, like 2-hexyl,5-propyl resorcinol (HPR), ethyl (6′R)-2,4-dihydroxy-6-(6′-hydroxyheptyl) benzoate, isobutyl (6′R)-2,4-dihydroxy-6-(6′-hydroxyheptyl) benzoate, 2-methyl-5-(Z-heptadec-8-enyl) resorcinol, 5-(Z-heptadec-8-enyl), and so many had been isolated various authors.35−39 Numerous authors have studied the effects of other phenolic benzenediol compounds like catechol (1,2-benzenediol) and hydroquinone (1,4-benzenediol) on the growth, osmolytes, and antioxidant activity of lemongrass, soybean, alfalfa, and lupine plants.40−43 Limited research is available regarding the effects of resorcinol on plant vegetative and reproductive parameters in soybean, tobacco, and sunflower. However, no information on its osmolytic and antioxidant actions is available.44−46
Tomato (Lycopersicon esculentum Mill.) is a globally produced annual vegetable crop in the Solanaceae family.47 It contributes significantly, accounting for around 14% of global annual vegetable production.48 Tomatoes are versatile and used in various dishes like sauces, soups, juices, and ketchup, offering rich nutrients including vitamins and minerals. They are also valued for their natural antioxidants, thanks to their high levels of lycopene, carotene, and anthocyanins.49 This study aimed to improve tomato growth and metabolite production through various concentrations of resorcinol and biochar. Additionally, this research aimed to optimize the best concentrations for promoting plant growth. It explored their impact on germination and growth regulation, potentially leading to their use as growth stimulants and nutrient sources for plants. These findings could enhance plant growth and the nutrient supply.
2. Methodology
2.1. Site Description
Field experiments were conducted within the natural habitat at the Department of Botany, University of Peshawar, Pakistan (coordinates 34° 10′ 33.301200″ N, 71° 33′ 36.486000″ E), specifically during the rapeseed log phase in the year 2023.
2.2. Biochar Preparation and Its Characterization
Biomass was derived from Cedrus deodara (Roxb. ex D. Don) G. Don as sawmill shavings were employed to produce biochar. The pyrolysis process was conducted at the temperature range of 340–350 °C until the biomass turned black.50 Characterization procedures encompassed UV–vis spectrophotometry, FT-IR spectroscopy, X-ray diffraction analysis (XRD), and microscopy techniques such as scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDX) and zeta potential (ZP) and dynamic light scattering (DLS) spectroscopy.7
2.3. Seed Sterilization and Priming
Tomato seeds were sourced from the National Agricultural Research Center (NARC) Islamabad, Pakistan. Healthy seeds were surface sterilized by washing with tap water for 25 min and then soaking in a 2% sodium hypochlorite solution for 20 min.51 The sterilized tomato seeds were subsequently primed for 30 min with different concentrations of resorcinol (Sigma-Aldrich, molecular weight: 110.11 and CAS number: 108-46-3) as control, 0.1, 1, 10, and 100 μM/L and biochar as control, 10, 30, 50, and 100 mg/L. Primed seeds were placed on filter paper in Petri dishes and incubated at 25 °C.
2.4. Preparation of Pot and Foliar Spray for Experiment
Pots were filled with well-composted agricultural manure and soil and sand in 3:1 ratio, respectively.52 The study used a completely randomized design with nine treatments, each replicated five times. Tomato seedlings were transplanted into pots with soil composition: 71–74% sand, 11–13% silt, and 11–16% clay. The soil had a pH range of 7.3–7.8 and an electrical conductivity of 3–5%. It contained 1.5% organic matter, 1.32 mequiv/L carbonate, 2.8 mequiv/L bicarbonate, and 15 mequiv/L Cl–1. With a minor modification, Hussain et al. approach was used to produce the solutions of precise concentrations for the foliar application of each treatment.53 These solutions were made by stirring the predicted solute amounts in 1 L of distilled water in individual flasks for approximately 30 min before application. Resorcinol concentrations (control, 0.10, 1.0, 10, and 100 μM/L) and biochar concentrations (control, 10, 30, 50, and 100 mg/L) were prepared for foliar sprays. Tomato plant leaves were sprayed at weekly intervals until harvest with a fresh solution prepared for each spray. Control plants received an equal amount of distilled water during the same period.
2.5. Growth Parameters
After 3 weeks of growth, tomato plants were harvested, and their yield was assessed by measuring shoot and root lengths as well as fresh and dry biomasses. The plant samples were divided in half, with one-half air-dried at 65 ± 05 °C for 3 days and weighed. The other half was stored at −20 °C for subsequent physiological analysis.54
2.6. Analysis of Physiological and Biochemical Attributes
Chlorophyll and carotenoid levels were determined by crushing fresh leaves (0.2 g) into acetone (80%) and centrifuged after 24 h of dark incubation. Chlorophyll a contents at 649 nm, chlorophyll b contents at 663 nm, and carotenoid contents at 430 nm were measured using a UV-vis spectrophotometer.19 To determine total flavonoid contents, dry shoot samples (0.5 g) were soaked in 0.5 mL of methanol (1:10 mL/L). A mixture of 1.0 M potassium acetate (0.1 mL), methanol (1.5 mL), deionized water (2.8 mL), and 10% aluminum chloride (0.1 mL) was added, and later absorption at 415 nm was measured using UV-vis spectrophotometry.22 To assess the phenolic contents, dried shoot samples (0.25 g) were mixed with 10 mL of 90% methanol and stirred for an hour. After centrifugation, 1 mL of the diluted Folin–Ciocalteu reagent (4:1 v/v) was added. After the addition of 1 mL of 10% Na2CO3, the final optical density was measured at 760 nm.54 For proline content analysis, 0.5 g of leaves was ground in 10 mL of 3% sulfosalicylic acid. The filtered solution of 10 mL was mixed with 2 mL of acid ninhydrin in glacial acetic acid, and 4 mL of toluene was added. Absorption was measured at 520 nm using a UV-vis spectrophotometer.55 To determine the total soluble sugars, 0.5 g of fresh plant material (leaves) was crushed in 10 mL of distilled water and then centrifuged. The absorbance of each sample was measured at 420 nm after mixing 0.1 mL of filtrate and 1 mL of 80% phenol (w/v).56 To extract total lipids, we mixed finely ground plant material with 0.5 mL of chloroform–methanol solution. After adding 0.2 mL of conc. sulfuric acid and 5 mL of vanillin reagent, absorbance at 525 nm was measured using UV-vis spectrophotometry.57
2.7. Estimation of Phytohormone Contents
For auxin (IAA) measurement in plant samples, plant material (0.5 g) in distilled water was crushed, centrifuged, and mixed with 2 mL of Salkowski reagent. Optical density was measured at 540 nm using a UV-vis spectrophotometer.58 To quantify gibberellic acid (GA) and abscisic acid (ABA), 0.5 g of plant material was crushed in a mixture of 60 mL of 2N ammonium hydroxide, chloroform, and methanol (3:5:12 v/v/v). After extraction and incubation, GA and ABA were quantified by recording the optical densities using a UV-vis spectrophotometer at 254 and 263 nm, respectively.59 To determine the salicylic acid (SA) content, plant tissue (0.5 g) was mixed and centrifuged in 0.1% aqueous FeCl3 solution. The absorbance at 540 nm was measured using a UV–vis spectrophotometer.18
2.8. Determination of Antioxidant Potential
For hydrogen peroxide (H2O2) estimation, fresh foliar material was crushed and centrifuged in trichloroacetic acid (5 mL). Then, potassium iodide reagent and phosphate buffer were mixed, with optical density measured at 390 nm.60 For catalase (CAT) activity determination, shoot material (0.5 g) was mixed with buffer solution. After centrifugation, H2O2 (1 mL) and phosphate buffer (1.9 mL) were added. Optical density was measured at 240 nm, with initial and final readings taken 60 s apart.61 To determine superoxide dismutase activity (SOD), shoot materials were crushed in phosphate buffer and centrifuged. The residue (0.1 mL) was mixed with riboflavin (150 μL), nitro-blue-tetrazolium (24 μL), and methionine (5 mL). Optical density was measured at 560 nm, with initial and final readings taken 3 min apart.62 To determine ascorbic acid peroxidase (APX) activity, shoot samples (0.5 g) were crushed in 5 mL of phosphate buffer and water and centrifuged. The final filtrate contained 0.6 mM ascorbic acid, 0.1 mM H2O2, and 0.1 mM EDTA. Optical density was measured at 290 nm, with initial and final readings taken 60 s apart.63
2.9. Statistical Analysis
The experiment was conducted in quintuplicate, ensuring robustness of the results. Subsequently, the collected data were subjected to analysis of variance (ANOVA) utilizing SPSS-20. For cases where means exhibited significant differences, further investigation was conducted by using the Duncan multiple range test at a significance level of 0.05 (5%). This analysis was executed with the assistance of SPSS, Inc., headquartered in Chicago, IL, USA.
3. Results
3.1. Characterization of Biochar
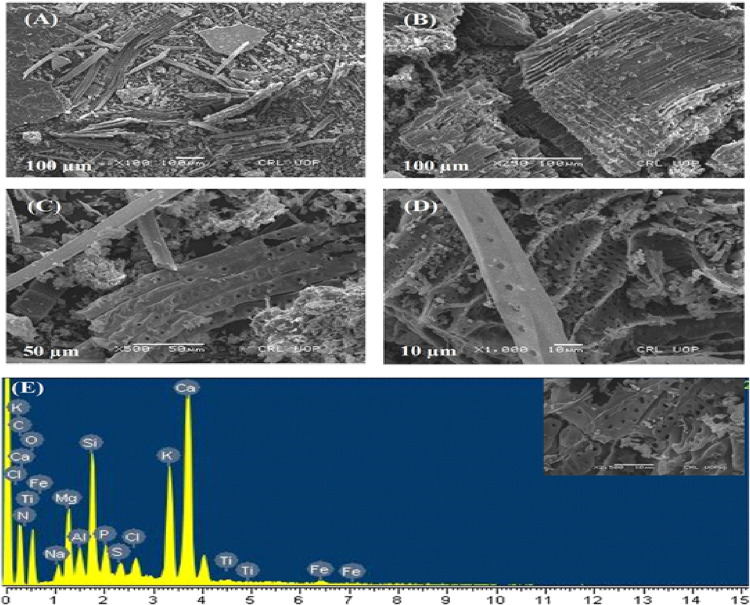
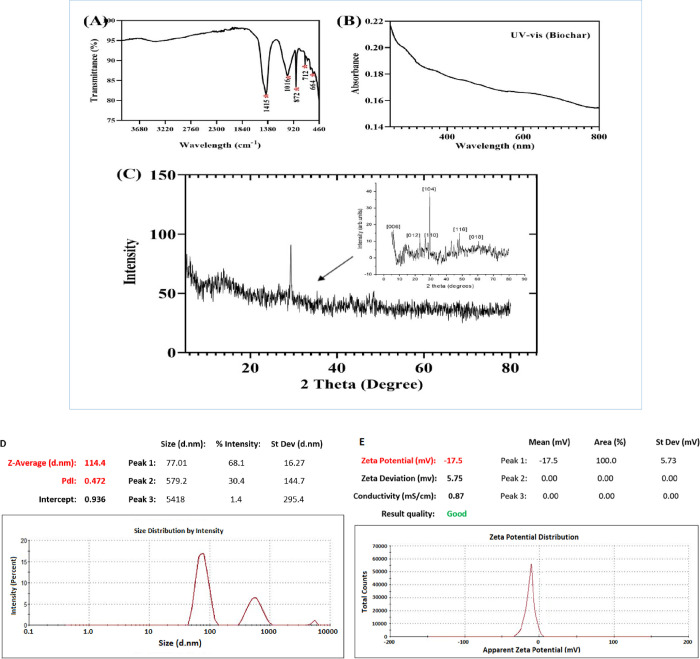
The distinctive morphological characteristics of biochar were elucidated by using scanning electron microscopy (SEM). In Figure 1, SEM micrographs of biochar were presented (Figure 1A–D). The figures depicted a surface with a smooth area characterized by a porous structure and polygonal shape, as evidenced. Notably, the SEM micrographs provided clear visual evidence of the alterations in the surface morphology observed in biomass samples following the pyrolysis process. The elemental composition of biochar was analyzed by EDX (Figure 1E), indicating the presence of elements as detailed in Table 1. The surface functional groups present in all carbon-based samples were examined via FT-IR spectroscopy, as illustrated in Figure 2A. The FTIR spectra were captured in transmission mode within the range of 4000 to 460, 868–875, 710–714, 660–669, and 616–623 cm–1. These peaks correspond to the out-of-plane bending of ring C–H bonds in heteroatomic and aromatic compounds. The most prominent peaks detected at 1415, 1016, and 872 cm–1 within the biochar spectrum indicate a higher content of C=C and C–H aromatic bonds. UV–visible spectroscopy is recognized for surface characteristics of biochar that were notably discernible at a wavelength of 420 nm (Figure 2B). The XRD distinct peaks at specific 2θ values (0.12, 104, 110, 116, and 018°) revealed crystal planes within the biochar’s face-centered cubic structure. Notably, the XRD pattern (Figure 2D) showed a peak at 104°, indicating both crystalline and amorphous features within the biochar extract’s organic phase. The DLS spectrogram of biochar shows a trimodel size distribution with an average size of 114.4 nm, and the size is ranging from 43 nm to 6.2 μm. The peak 1 occupies the maximal percent intensity of 68.1% with a mean size of 77.01 nm (Figure 2D). The zeta potential chromatogram shows a unimodel potential distribution with a mean zeta potential of −17.5 mV and 100% area of occupancy (Figure 2E).
Figure 1.
Characterization of biochar; SEM shows scanning electron micrographs of biochar at (A) 100×, (B) 250×, (C) 500×, and (D) 1000×; (E) EDX spectrum of biochar.
Table 1. Elemental Composition of Cedrus deodara-Derived Biochar by EDX Analysis.
| elements | weight (%) | atomic (%) |
|---|---|---|
| C | 20.09 | 32.39 |
| N | 4.32 | 5.97 |
| O | 28.84 | 34.89 |
| Na | 1.37 | 1.15 |
| Mg | 4.54 | 3.62 |
| Si | 6.74 | 4.65 |
| P | 2.11 | 1.32 |
| S | 0.85 | 0.51 |
| Cl | 1.32 | 0.72 |
| K | 8.79 | 4.35 |
| Ca | 18.74 | 9.05 |
| Ti | 0.28 | 0.11 |
| Fe | 0.50 | 0.17 |
Figure 2.
Characterization of biochar. (A) Fourier transform infrared spectroscopy (FTIR) spectra, (B) UV–visible spectra, (C) XRD patterns, (D) dynamic light scattering spectroscopy, and (E) zeta potential.
3.2. Agronomic Attribute of Tomato
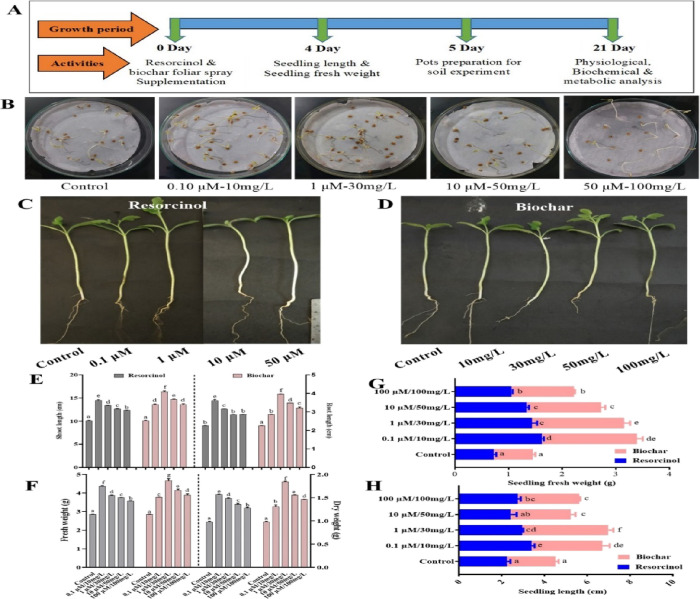
To evaluate the seed germination performance, different concentrations of resorcinol and biochar were used for seed priming. Among the different concentrations, the concentration of resorcinol, 0.1 μM/L, and biochar concentration of 30 mg/L exhibited a notable enhancement in tomato seed germination compared to the control group (Figure 3B). This improvement was further reflected in growth parameters, wherein seedlings treated with 0.1 μM/L resorcinol displayed increased seedling length (3.4 cm) and fresh weight (1.62 g) and those treated with 30 mg/L of biochar exhibited elevated seedling length (4.0 cm) and fresh weight (1.72 g), as opposed to control seedlings with a seedling length of 2.25 cm and fresh weight of 0.72 g (Figure 3G,H). Additionally, in a field experiment, the results demonstrated significant improvements in vegetative parameters following the foliar application of resorcinol (0.1 μM/L) and biochar (30 mg/L). Specifically, the application of resorcinol led to an increased shoot length (14.5 cm) and root length (3.6 cm), along with enhanced fresh weight (4.37 g) and dry weight (1.57 g). Similarly, the biochar-treated plants exhibited heightened shoot length (16.3 cm) and root length (3.97 cm) as well as elevated fresh weight (4.65 g) and dry weight (1.84 g). These findings collectively underscore the efficacy of the application of resorcinol and biochar, particularly at concentrations of 0.1 μM/L and 30 mg/L, respectively, in significantly improving tomato seed germination and subsequent growth parameters (Figure 3E,F).
Figure 3.
The evaluation of the impact of resorcinol and biochar on tomato seed germination and plant growth regulation was conducted by using a bioassay approach. The different facets of this assessment are presented as follows: (A) a schematic representation illustrating the experimental setup of the tomato plant bioassay, (B) the germination response of seeds subjected to varying concentrations of resorcinol and biochar, (C) the phenotypic evaluation of tomato plants following treatment with different concentrations of resorcinol, (D) the phenotypic assessment of tomato plants upon treatment with varying concentrations of biochar, (E) representation of shoot length (right panel) and root length (left panel) measurements, (F) presentation of total fresh weight (right panel) and total dry weight (left panel) measurements, and (G) depiction of seedling fresh weight and seedling length measurements. The quantitative data are depicted as means ± SE, derived from five distinct biological replicates. (H) These data are visually represented with different letters, indicating statistical differences at a significance level of p ≤ 0.05, as determined using Duncan’s multiple range test (DMRT).
3.3. Impact of Resorcinol and Biochar on Physiological and Biochemical Attributes
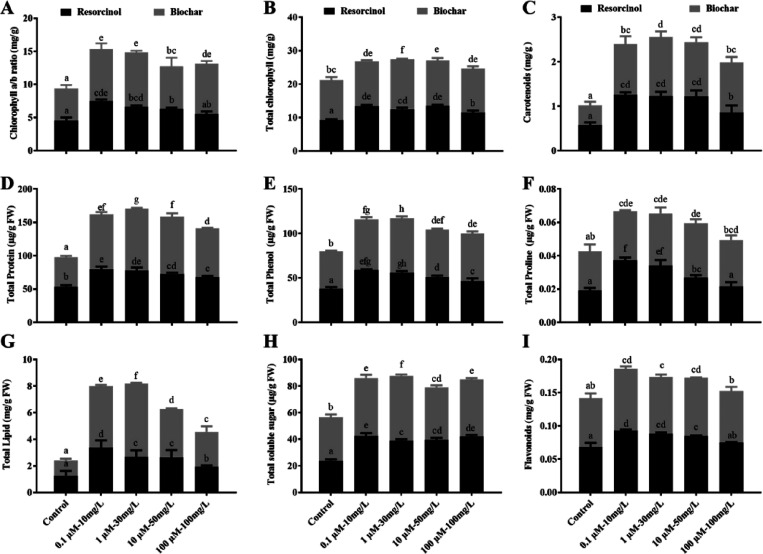
Likewise, individual applications of both resorcinol and biochar led to significant enhancements in chlorophyll and carotenoid contents. Remarkably, resorcinol at 0.1 μM/L exhibited significant effects, notably enhancing the accumulation of total chlorophyll contents (13.3 mg/g), carotenoids (1.2 mg/g), and chlorophyll a/b ratio (7.4 mg/g) compared to the control. Similarly, biochar at 30 mg/L showed significant effects on total chlorophyll contents (14.8 mg/g), carotenoids (1.3 mg/g), and chlorophyll a/b ratio (8.2 mg/g) in comparison with the control. Respectively, the resorcinol concentration of 0.1 μM/L substantially increased tomato growth-related metabolites, including total protein (79.9 μg/g), total phenol (58.8 μg/g), total proline (0.03 μg/g), total lipid (3.8 μg/g), total soluble sugar (42.5 μg/g), and flavonoid (0.09 μg/g) content. Similarly, the biochar concentration of 30 mg/L in foliar spray also increased the total protein (92.1 μg/g), total phenol (61.3 μg/g), total proline (0.03 μg/g), total lipid (5.5 μg/g), total soluble sugar (48.9 μg/g), and flavonoid (0.08 μg/g) contents in tomato compared to the control condition (Figure 4A–I).
Figure 4.
The impact of various concentrations of resorcinol and biochar foliar spray on the enhancement of growth-promoting metabolites in tomato was evaluated. The results are depicted in the figure, where the different letters signify statistically significant differences (p ≤ 0.05) based on Duncan’s multiple range test (DMRT). The analysis encompassed several key metabolites, including (A) the chlorophyll a/b ratio, (B) total chlorophyll contents, (C) carotenoid contents, (D) total soluble proteins, (E) total phenols, (F) proline levels, (G) total lipids, (H) total soluble sugars, and (I) flavonoid contents. The quantitative data presented in the figure are derived from five independent biological replicates.
3.4. Impact of Resorcinol and Biochar on Phytohormonal Contents
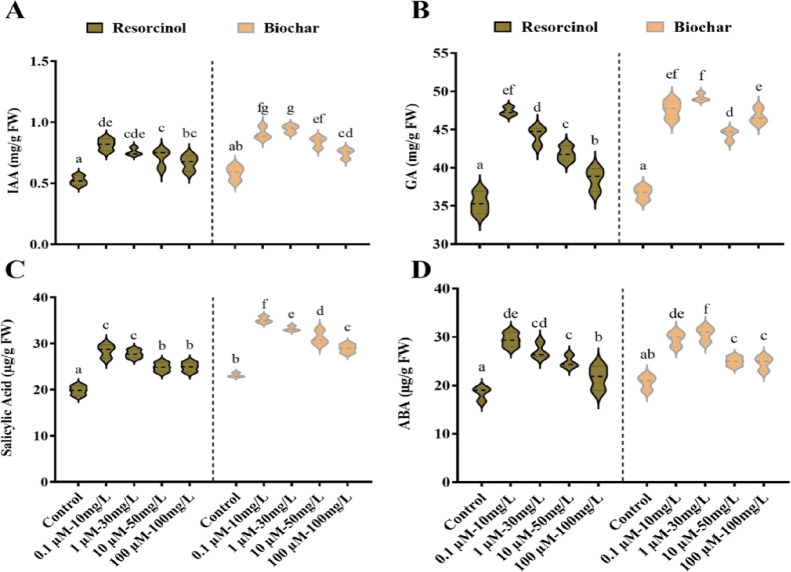
The foliar application of resorcinol at a concentration of 0.10 μM/L and biochar at 30 mg/L resulted in a substantial increase in phytohormone contents compared to the control. Specifically, this treatment led to enhanced synthesis of key phytohormones such as IAA (0.81 μg/g), GA (47.3 μg/g), SA (28.4 mg/g), and ABA (29.4 mg/g) levels with a concentration of 0.10 μM/L on tomato plants. Similarly, the biochar 30 mg/L spray increased the synthesis of IAA (0.94 μg/g), GA (49.1 μg/g), SA (33.2 mg/g), and ABA (30.6 mg/g) levels at 30 mg/L on tomato plants. These results show that resorcinol at 0.10 μM/L and biochar at 30 mg/L play an important role in controlling hormone synthesis in tomato plants (Figure 5).
Figure 5.
Impact of resorcinol concentrations and biochar foliar spray on the tomato phytohormonal contents. (A) IAA, (B) GA, (C) SA, and (D) ABA contents. The quantitative data presented in the study represent means ± standard error (SE) of five independent biological replicates. Variations between the means are denoted by different letters, indicating significant differences at the level of significance p ≤ 0.05, as determined using Duncan’s multiple range test (DMRT).
3.5. Estimation of Antioxidant Contents
In response to varying concentrations of resorcinol and biochar, the study investigated oxidative damage in tomato plants by assessing the production of reactive oxygen species (ROS). The highest concentration of resorcinol (100 μM/L) and biochar (100 mg/L) led to increased H2O2 accumulation (171.4 and 163.5 μM/g) compared to the control. Conversely, a reduced level of H2O2 accumulation was observed in tomato plants treated with resorcinol (0.1 μM/L) and biochar (30 mg/L) compared with the control. These results highlight the potential role of resorcinol (0.1 μM/L) and biochar (30 mg/L) in mitigating oxidative stress and ROS production in tomato plants. The level of CAT was significantly increased (p ≤ 0.05) under the influence of resorcinol (0.1 μM/L) and biochar (30 mg/L) (3.7 and 4.0 units/g) compared to the control (1.9 units/g). Specifically, the SOD content displayed a significant increase with the application of resorcinol (0.1 μM/L) and biochar (30 mg/L) (9.6 and 10.8 units/g, respectively) compared to the respective control samples (6.7 units/g). The activity of APX exhibited a significant increase (p < 0.05) with resorcinol (0.1 μM/L) and biochar (30 mg/L) (42.3 and 43.2 units/g, respectively) compared to the corresponding control (34.1 units/g) (Figure 6A–D). These results underscore the positive impact of resorcinol and biochar on CAT, SOD, and APX enzymatic activities, highlighting their potential to enhanced antioxidant defense mechanisms in tomato plants.
Figure 6.
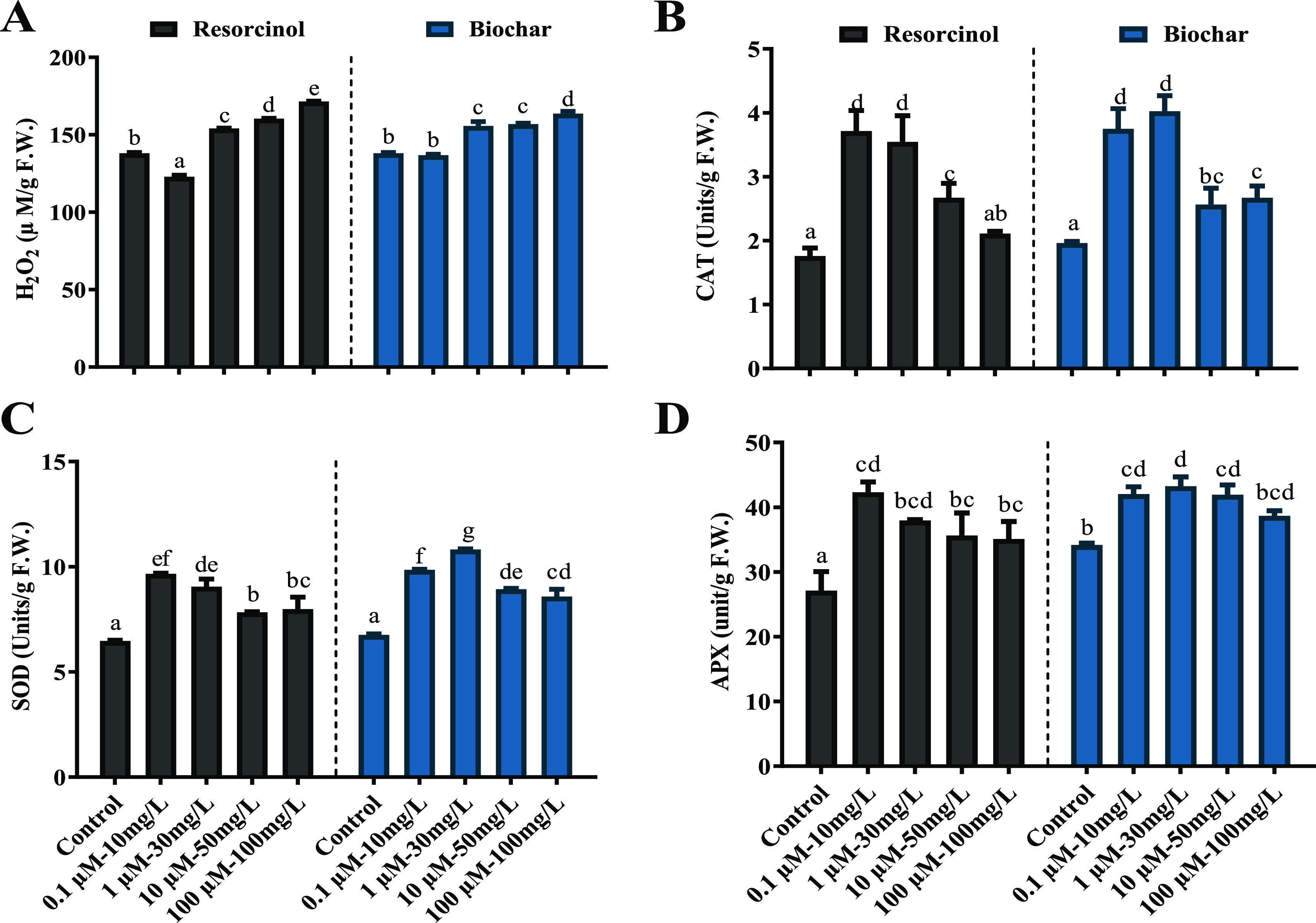
Impact of resorcinol concentrations (0, 0.1, 1.0, 10, and 100 μM/L) and biochar foliar spray (0, 10, 30, 50, and 100 mg/L) on tomato antioxidant system. (A) H2O2 level, (B) CAT, (C) SOD, and (D) APX contents. The quantitative data presented in this study represent means ± SE of five independent biological replicates, and the differences between means were determined using Duncan’s multiple range test (DMRT) at a significance level of p ≤ 0.05.
4. Discussion
The present study has elucidated that resorcinol and biochar foliar application in tomatoes could potentially influence various physiological processes, including the accumulation of antioxidant capacity, shifts in phytohormone balance, and fluctuations in protein contents, as indicated by previous research.11,52 The efficacy of foliar biochar application in enhancing plant growth and development has garnered significant attention among researchers due to its advantages like its fast and easy preparation, eco-friendly nature, reusability, and cost-effectiveness.64,65 The properties of biochar are greatly influenced by the composition and type of biomass as well as the specific conditions under which the biomass is carbonized. The chemical and physical characterizations are essential for determining the fundamental structure and properties of biochar as well as for assessing its potential in diverse applications. Biochar is considered a highly promising alternative owing to its elemental composition, charged surface, and presence of functional groups, including carboxyl, hydroxyl, phenolic hydroxyl, and carbonyl groups.66,67Our observations of the SEM structure of biochar samples revealed an internal porous structure with the presence of tubes, in contrast to the less common porous framework observed in biomass samples. This characteristic suggests the potential utility of biochar samples as a source of carbon biomass for the production of various carbon materials, including carbon nanotubes and activated carbon.49 The present design work through SEM revealed a porous and smooth structure of biochar.50 The EDX results obtained in the previous investigation are consistent with our study, as both studies revealed elemental peaks in powdered biochar that closely match our findings.17 The biochar’s UV-visible spectra were notably discernible at a wavelength of 420 nm and FTIR spectra, recorded using transmission mode between 4000 and 400 cm–1, exhibited significant peaks at 1410–1420, 1008–1024, 868–875, 710–714, 660–669, and 616–623 wavenumbers cm–1 .70 These peaks indicate the out-of-plane bending of C–H bonds in both heteroatomic and aromatic compounds, as demonstrated in the study.51 The XRD pattern exhibits pronounced peaks at 2θ values of (31), (45), (33), (39), and (27)°, corresponding to the crystal planes 012, 104, 110, 116, and 018 of the face-centered cubic structure of biochar. Moreover, a peak is evident at 104° in Figure 2C, indicating the coexistence of both crystalline and amorphous characteristics within the organic phase of the nanoparticle extract.50
Furthermore, the study aims to assess the impact of foliar application of resorcinol and biochar on tomato growth and optimize the best concentration.69,71 Notably, resorcinol at concentration (0.1 μM/L) boosted shoot length (14.5 cm), root length (3.6 cm), fresh weight (4.37 g), and dry weight (1.57 g) compared to control plants, which had shorter shoot (10.09 cm) and root (2.26 cm) lengths and lower fresh (2.85 g) and dry (0.98 g) weights. Conversely, the higher concentration (100 μM/L) had negative impacts on seedling length, seedling fresh weight, and both fresh and dry biomass, in contrast to the lowest resorcinol concentration (0.1 μM/L). Our results are consistent with those of Khan et al., who conducted substantial research on the stimulation of growth and yield in lemongrass by foliar application of phenolic substances such as salicylic acid and catechol. When compared to the control group, the treated group increased fresh and dry weights by 26.1 and 43.4%, respectively.40 Khaleda et al. and Bhardwaj et al. confirmed similar results with seedlings of alfalfa and wheat. The root exhibited a similar pattern of elongation.42,72 However, these findings contradict those of Noel et al., who studied the influence of phenolic chemicals in common sunflowers.46 Catechol and resorcinol treatments reduced the taproot length by 7.7-fold and 3.3-fold, respectively, although hydroquinone treatment had no impact on root attributes. Plants treated with 30 mg/L of biochar showed enhanced shoot length (16.3 cm) and root length (3.97 cm) and increased fresh (4.65 g) and dry (1.84 g) weights compared to the control group, which had shorter shoot and root lengths (10.09 and 2.26 cm, respectively) and lower fresh (2.86 g) and dry (0.98 g) weights. Arshad et al. investigated the influence of three different biochars (wheat straw, rice husk, and sugar cane) at 2, 3, and 5% (w/w) on soil characteristics and tomato CV “Money Maker″. In comparison to the control, rice husk at a concentration of 3% improved plant height, number of leaves, fresh and dry shoot weight, fresh and dry root weight, and root length significantly.73 Li et al. found that the application of biochar (0, 0.5, 1, and 2% w/w) improved the morphophysiology of sugar beet. This improvement might be due to biochar’s ability to function as a nutrient supply (P, K, Ca, Na, Mg, Fe, Mn, and Zn) to directly promote plant development. In our investigation, the biochar contains substantial quantities of phosphorus (2.11%), calcium (18.74%), potassium (8.79%), magnesium (4.54%), carbon (20.09%), and other elements as shown in elemental analysis (Table 1). Such readily accessible nutrients may play an important role in seedling development promotion.74,75
Remarkably, significant effects were obtained when 0.1 μM/L resorcinol was used. In comparison to the control group, this led to an increase in the levels of total chlorophyll (13.3 mg/g), carotenoids (1.2 mg/g), and the chlorophyll a/b ratio (7.4 mg/g). Our results are consistent with those of Khan et al., who found that using the phenolic component salicylic acid increased chlorophyll and carotenoid levels by 52.6 and 62.8%, respectively.40 Elblasy et al. and Munsif et al. discovered similar patterns in chlorophyll content augmentation in soybean and wheat plants, respectively.40,76 The chlorophyll contents steadily reduced as the resorcinol concentration increased. At a concentration of 100 μM/L, total chlorophyll contents, carotenoids, and the chlorophyll a/b ratio reduced to 11.53 0.86, and 5.55 mg/g, respectively. Our results are consistent with those of Jagetiya and Kaur, who examined the influence of different resorcinol foliar spray concentrations on soybeans. At varied doses, chlorophyll contents decreased throughout different development stages.44 Foliar treatment with resorcinol at a lower concentration improves growth and yield. While higher resorcinol concentrations were proven to be harmful. The decrease in chlorophyll content at higher concentrations of resorcinol (100 μM/L), observed in this study, might be ascribed to the fact that higher concentrations of phenolic chemicals promote peroxidase-mediated chlorophyll breakdown. Peroxidase oxidizes phenolic compounds with H2O2 to generate phenoxy radicals, which oxidize chlorophyll.77 In comparison to the control, biochar at 30 mg/L had a significant influence on the total chlorophyll contents (14.8 mg/g), carotenoids (1.3 mg/g), and chlorophyll a/b ratio (8.2 mg/g). The administration of BC significantly increased antioxidant activities, which prevents oxidative damage to photosynthetic pigments and photosynthetic machinery in plants.78 Mg2+ absorption is also stimulated by BC supplementation, which is a building component in the production of chlorophyll In contrast, the study conducted by Rehman et al. did not establish a correlation between the application of biochar and the chlorophyll levels in wheat and rice crops. The impact of biochar on photosynthetic pigments may vary based on factors such as the type of biochar, application rate, soil conditions, and plant species.79,80
Fresh leaves of tomatoes had higher levels of total protein (79.9 μg/g), total phenol (58.8 μg/g), total proline (0.03 μg/g), total lipid (3.8 μg/g), total soluble sugar (42.5 μg/g), and flavonoid (0.09 μg/g) when exposed to a 0.1 μM/L dose of resorcinol. In soybean plants, Elblasy et al. found that hydroquinone (HQ) considerably raised total protein, total sugar, total oil contents, and total phenols in all treatments as compared to the untreated control treatment.41 Munsif et al. and Singh et al. used phenolic compounds (salicylic acid) on wheat plants and discovered a significant increase in proline and soluble sugar content in the leaves of the respective plants.76,81 In this research, a biochar concentration of 30 mg/L in foliar spray substantially improved tomato fresh leaf total protein (92.1 g/g), total phenol (61.3 g/g), total proline (0.03 g/g), total lipid (5.5 g/g), total soluble sugar (48.9 g/g), and flavonoid (0.08 g/g). As a consequence of these findings, biochar treatment altered the osmo-regulators in tomato leaves, allowing them to respond to changes in soil environmental conditions. This is similar to the findings of earlier investigations by Li et al.,74 who found that applying biochar enhanced the soluble sugar content of cotton leaves. Cong et al. also examined that spraying 31.50 t ha–1 of biochar increased the soluble sugar and protein contents of maize plants.82
It is well recognized that plants can activate their antioxidant systems, in which the enzymes SOD, POD, APX, and CAT play crucial roles in defending the plants against the oxidative stress that comes from reactive oxygen species (ROS).75,83 These enzymatic biomarkers play critical roles in the plant’s defense mechanism.84 Superoxide ions are converted by SOD into hydrogen peroxide (H2O2) and oxygen (O2). Following that, CAT and POD degrade H2O2 into H2O and O2.85 During our research, the levels of CAT (3.7 units/g), SOD (9.6 units/g), and APX (42.3 units/g) increased significantly under the influence of resorcinol (0.1 μmol/L) compared to the control (1.9 6.7, and 34.1 units/g, respectively). The work of Li et al. on fragrant rice cultivars (Yuxiangyouzhan and Xiangyaxian) validates our findings. Catechol (phenolic compound) boosted superoxide dismutase (SOD) and peroxidase (POD) activity while lowering malondialdehyde (MDA) contents at a concentration of 20 μmol/L.86 The ability of phenolic acids to scavenge free radicals, donate H atoms or electrons, or bind metal ions makes them more stable and less easily accessible to promote autoxidation.87 The antioxidant free radical may also disrupt the chain-propagation processes. However, the influence of antioxidant concentration on autoxidation rates is determined by a variety of parameters, including the structure of the antioxidant, oxidation circumstances, and the type of the sample being oxidized.88 Biochar (30 mg/L) treatment increased the CAT (4.0 units/g), APX (43.2 units/g), and SOD (10.8 units/g) activity, resulting in significant improvements in tomato plant performance. Previous research has indicated that using BC enhances plant nutrition, antioxidant activity, and osmolyte accumulation89,90by triggering the synthesis of antioxidant enzymes, biochar may scavenge ROS.91,92
Hydrogen peroxide (H2O2) is regarded as a reliable indicator for assessing the generation of reactive oxygen species (ROS) in response to environmental stressors. Hydrogen peroxide has a multifaceted nature, functioning as a signaling molecule under normal conditions, while triggering oxidative stress in the presence of aberrant levels of biotic or abiotic stress.93 The tomato plants exhibited elevated levels of H2O2 accumulation (171.4 and 163.5 μM/g) when subjected to foliar application of resorcinol and biochar at concentrations of 100 μM/L and 100 mg/L. This increase in H2O2 concentration suggests that the plants experienced a state of stress, as compared to the control group. Nevertheless, the application of resorcinol at a concentration of 0.1 μM/L and biochar at a concentration of 30 mg/L led to a decrease in the level of hydrogen peroxide (H2O2) in tomato plants. The results presented align with the findings of Khan et al. research, which also demonstrated a reduction in H2O2 levels after the application of biochar.94 The findings of our study are consistent with the research conducted by Abdelaal et al., who investigated the effects of applying 0.5 mM salicylic acid on barley (Hordeum vulgare L. Giza126). They observed a decrease in lipid peroxidation and hydrogen peroxide levels.95
In plants, phytohormones can control a wide range of cellular functions. They operate as chemical messengers in higher plants, communicating cellular activity.98 Phytohormones perform critical functions in the abiotic stress response, coordinating numerous signal transduction pathways. According to previous study, external as well as internal stimuli are regulated by them.96 The tomato plants exhibited the most elevated concentrations of essential phytohormones, including indole-3-acetic acid (IAA) at 0.81 μg/g, gibberellic acid (GA) at 47.3 μg/g, salicylic acid (SA) at 28.4 mg/g, and abscisic acid (ABA) at 29.4 mg/g, when treated with a resorcinol quantity of 0.10 μM/L. Fahad et al. and Voß et al. also observed similar improvements in phytohormones in their respective studies. Application of biochar spray led to an increase in the quantities of IAA (0.94 μg/g), GA (49.1 μg/g), SA (33.2 mg/g), and ABA (30.6 mg/g) in tomato plants when applied at a dosage of 30 mg/L.97,98 Racioppi et al. observed a significant increase in the concentrations of indole-3-acetic acid (IAA) and gibberellic acid (GA) as a result of the application of biochar.68 The concentrations of ABA were found to be substantially lower compared to the concentrations of IAA and GA. In contrast, Farhangi-Abriz and Torabian find no evidence that biochar significantly alters the levels of phytohormones.99 The substantial accumulation of these growth-related compounds consistently supports the beneficial impact of resorcinol 0.1 μM/L and biochar 30 mg/L on enhancing the growth of tomato plants.23 Additionally, this study showed a strong connection between the balance of ABA and H2O2 and different physiological and biochemical factors. These factors include proline, photosynthetic pigments, enzymes like APX and CAT, SOD levels, phenols, flavonoids, carotenoids, lipids, carbohydrates, proteins, and phytohormones like GA, IAA, and SA. Our study further demonstrates that applying resorcinol at 0.1 μM/L and biochar at 30 mg/L stimulates the production of primary and secondary metabolites as well as hormones and antioxidants. This supports the sustainable growth and overall development of the tomato plants.
5. Conclusions
This study demonstrates the conversion of biomass into porous biochar through pyrolysis, with graphite-like structures for versatile carbon synthesis. Using 0.1 μM/L resorcinol and 30 mg/L biochar significantly improves tomato seed germination and plant growth. Field trials show increased shoot/root length and higher fresh/dry weights compared to controls. Resorcinol and biochar boost the chlorophyll content, growth metabolites, and antioxidant enzyme activity, enhancing overall plant development. These specific concentrations also elevate protein, phenol, sugar, flavonoid, lipid, and proline levels. Future research should explore combined treatments of resorcinol and biochar under various stresses to understand their synergistic potential for crop growth in changing climates.
Acknowledgments
The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP2023R301), King Saud University, Riyadh, Saudi Arabia.
Data Availability Statement
All data sets presented in this article have been included in the manuscript.
The authors declare no competing financial interest.
References
- Oleszczuk P.; Ćwikła-Bundyra W.; Bogusz A.; Skwarek E.; Ok Y. S. Characterization of nanoparticles of biochars from different biomass. Journal of Analytical and Applied Pyrolysis. 2016, 121, 165–172. 10.1016/j.jaap.2016.07.017. [DOI] [Google Scholar]
- Zhao J.; Shen X. J.; Domene X.; Alcañiz J. M.; Liao X.; Palet C. Comparison of biochars derived from different types of feedstock and their potential for heavy metal removal in multiple-metal solutions. Sci. Rep. 2019, 9 (1), 9869. 10.1038/s41598-019-46234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W.; Li Y.; Feng Y.; Qiao J.; Zhao H.; Xie J.; Fang Y.; Shen S.; Liang S. The effectiveness of nanobiochar for reducing phytotoxicity and improving soil remediation in cadmium-contaminated soil. Sci. Rep. 2020, 10 (1), 858. 10.1038/s41598-020-57954-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. H.; Kim J. Y.; Cho T. S.; Choi J. W. Influence of pyrolysis temperature on physicochemical properties of biochar obtained from the fast pyrolysis of pitch pine (Pinus rigida). Bioresource technology. 2012, 118, 158–162. 10.1016/j.biortech.2012.04.094. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Wei Y.; Sun J. Biochar application promotes growth parameters of soybean and reduces the growth difference. Commun. Soil Sci. Plant Anal. 2016, 47 (12), 1493–1502. 10.1080/00103624.2016.1194988. [DOI] [Google Scholar]
- Singh R.; Singh P.; Singh H.; Raghubanshi A. S. Impact of sole and combined application of biochar, organic and chemical fertilizers on wheat crop yield and water productivity in a dry tropical agro-ecosystem. Biochar. 2019, 1, 229–235. 10.1007/s42773-019-00013-6. [DOI] [Google Scholar]
- Rawat J.; Saxena J.; Sanwal P.; Abrol V.; Sharma P. Biochar: a sustainable approach for improving plant growth and soil properties. Biochar-an imperative amendment for soil and the environment. 2019, 8, 1–7. 10.5772/intechopen.82151. [DOI] [Google Scholar]
- Jin Z.; Chen C.; Chen X.; Hopkins I.; Zhang X.; Han Z.; Jiang F.; Billy G. The crucial factors of soil fertility and rapeseed yield-A five year field trial with biochar addition in upland red soil, China. Sci. Total Environ. 2019, 649, 1467–1480. 10.1016/j.scitotenv.2018.08.412. [DOI] [PubMed] [Google Scholar]
- Lebrun M.; Miard F.; Nandillon R.; Morabito D.; Bourgerie S. Biochar application rate: Improving soil fertility and Linum usitatissimum growth on an arsenic and lead contaminated technosol. International Journal of Environmental Research. 2021, 15, 125–134. 10.1007/s41742-020-00302-0. [DOI] [Google Scholar]
- Chan K. Y.; Van Zwieten L.; Meszaros I.; Downie A.; Joseph S. Using poultry litter biochars as soil amendments. Soil Research. 2008, 46 (5), 437–444. 10.1071/SR08036. [DOI] [Google Scholar]
- Liu Z.; Dugan B.; Masiello C. A.; Gonnermann H. M. Biochar particle size, shape, and porosity act together to influence soil water properties. Plos one. 2017, 12 (6), e0179079 10.1371/journal.pone.0179079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werdin J.; Fletcher T. D.; Rayner J. P.; Williams N. S.; Farrell C. Biochar made from low density wood has greater plant available water than biochar made from high density wood. Sci. Total Environ. 2020, 705, 135856 10.1016/j.scitotenv.2019.135856. [DOI] [PubMed] [Google Scholar]
- Hien T. T.; Tsubota T.; Taniguchi T.; Shinogi Y. Enhancing soil water holding capacity and provision of a potassium source via optimization of the pyrolysis of bamboo biochar. Biochar. 2021, 3, 51–61. 10.1007/s42773-020-00071-1. [DOI] [Google Scholar]
- Munera-Echeverri J. L.; Martinsen V.; Strand L. T.; Zivanovic V.; Cornelissen G.; Mulder J. Cation exchange capacity of biochar: An urgent method modification. Science of the total environment. 2018, 642, 190–197. 10.1016/j.scitotenv.2018.06.017. [DOI] [PubMed] [Google Scholar]
- Verheijen F. G.; Zhuravel A.; Silva F. C.; Amaro A.; Ben-Hur M.; Keizer J. J. The influence of biochar particle size and concentration on bulk density and maximum water holding capacity of sandy vs sandy loam soil in a column experiment. Geoderma. 2019, 347, 194–202. 10.1016/j.geoderma.2019.03.044. [DOI] [Google Scholar]
- Khaliq H.; Anwar S.; Shafiq F.; Ashraf M.; Zhang L.; Haider I.; Khan S. Interactive effects of soil and foliar-applied nanobiochar on growth, metabolites, and nutrient composition in Daucus carota. Journal of Plant Growth Regulation. 2023, 42 (6), 3715–3729. 10.1007/s00344-022-10832-w. [DOI] [Google Scholar]
- Lalay G.; Ullah S.; Ahmed I. Physiological and biochemical responses of Brassica napus L. to drought-induced stress by the application of biochar and Plant Growth Promoting Rhizobacteria. Microscopy research and technique. 2022, 85 (4), 1267–1281. 10.1002/jemt.23993. [DOI] [PubMed] [Google Scholar]
- Warrier R. R.; Paul M.; Vineetha M. V. Estimation of salicylic acid in Eucalyptus leaves using spectrophotometric methods. Genet. Plant Physiol. 2013, 3 (1–2), 90–97. [Google Scholar]
- Maclachlan S.; Zalik S. Plastid structure, chlorophyll concentration, and free amino acid composition of a chlorophyll mutant of barley. Canadian Journal of Botany. 1963, 41 (7), 1053–1062. 10.1139/b63-088. [DOI] [Google Scholar]
- de Ascensao A. R.; Dubery I. A. Soluble and wall-bound phenolics and phenolic polymers in Musa acuminata roots exposed to elicitors from Fusarium oxysporum f. sp. cubense. Phytochemistry. 2003, 63 (6), 679–686. 10.1016/S0031-9422(03)00286-3. [DOI] [PubMed] [Google Scholar]
- Hounsome N.; Hounsome B.; Tomos D.; Edwards-Jones G. Plant metabolites and nutritional quality of vegetables. Journal of food science. 2008, 73 (4), R48–65. 10.1111/j.1750-3841.2008.00716.x. [DOI] [PubMed] [Google Scholar]
- Javed J.; Rauf M.; Arif M.; Hamayun M.; Gul H.; Ud-Din A.; Ud-Din J.; Sohail M.; Rahman M. M.; Lee I. J. Endophytic fungal consortia enhance basal drought-tolerance in Moringa oleifera by upregulating the antioxidant enzyme (APX) through Heat shock factors. Antioxidants. 2022, 11 (9), 1669. 10.3390/antiox11091669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naz F.; Hamayun M.; Rauf M.; Arif M.; Afzal Khan S.; Ud-Din J.; Gul H.; Hussain A.; Iqbal A.; Kim H. Y.; Lee I. J. Molecular mechanism of Cu metal and drought stress resistance triggered by Porostereum spadiceum AGH786 in Solanum lycopersicum L. Front. Plant Sci. 2022, 10 (13), 1029836 10.3389/fpls.2022.1029836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghuveer I.; Anurag K.; Anumalik Y.; Nitika G.; Swadesh K.; Nikhil G.; Santosh K.; Vinay Y.; Anuj P.; Himanshu G. Metabolites in plants and its classification. World J. Pharm. Pharm. Sci. 2015, 4 (1), 287–305. [Google Scholar]
- Mohammadkhani N.; Heidari R. Drought-induced accumulation of soluble sugars and proline in two maize varieties. World Appl. Sci. J. 2008, 3 (3), 448–453. [Google Scholar]
- Misra D.; Dutta W.; Jha G.; Ray P. Interactions and regulatory functions of phenolics in soil-plant-climate nexus. Agronomy. 2023, 13 (2), 280. 10.3390/agronomy13020280. [DOI] [Google Scholar]
- Hossain M. F.; Piash M. I.; Parveen Z. Effect of biochar and fertilizer application on the growth and nutrient accumulation of rice and vegetable in two contrast soils. Acta Sci. Agric. 2019, 3 (3), 74–83. [Google Scholar]
- Uysal A.; Zengin G.; Durak Y.; Aktumsek A. Screening for antioxidant and antimutagenic properties of extracts from Centaurea pterocaula as well as theirs enzyme inhibitory potentials. Marmara Pharmaceutical Journal. 2016, 20 (3), 232–242. 10.12991/mpj.20162094922. [DOI] [Google Scholar]
- Tian F.; Li H.; Li M.; Li C.; Lei Y.; Yang B. Synthesis of one-dimensional poly (3, 4-ethylenedioxythiophene)-graphene composites for the simultaneous detection of hydroquinone, catechol, resorcinol, and nitrite. Synth. Met. 2017, 226, 148–156. 10.1016/j.synthmet.2017.02.016. [DOI] [Google Scholar]
- Topal F. Antidiabetic Potential: Effect of Resorcinol on α-Glycosidase and α-Amylase Enzymes. Cumhuriyet Science Journal. 2018, 39 (4), 828–832. 10.17776/csj.452514. [DOI] [Google Scholar]
- Enache T. A.; Oliveira-Brett A. M. Phenol and para-substituted phenols electrochemical oxidation pathways. J. Electroanal. Chem. 2011, 655 (1), 9–16. 10.1016/j.jelechem.2011.02.022. [DOI] [Google Scholar]
- Salazar R.; Vidal J.; Martínez-Cifuentes M.; Araya-Maturana R.; Ramírez-Rodríguez O. Electrochemical characterization of hydroquinone derivatives with different substituents in acetonitrile. New J. Chem. 2015, 39 (2), 1237–1246. 10.1039/C4NJ01657B. [DOI] [Google Scholar]
- Dunlap T.; Chandrasena R. E.; Wang Z.; Sinha V.; Wang Z.; Thatcher G. R. Quinone formation as a chemoprevention strategy for hybrid drugs: balancing cytotoxicity and cytoprotection. Chemical research in toxicology. 2007, 20 (12), 1903–1912. 10.1021/tx7002257. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Lian X. Y.; Li S.; Stringer J. L. Characterization of chemical ingredients and anticonvulsant activity of American skullcap (Scutellaria lateriflora). Phytomedicine. 2009, 16 (5), 485–493. 10.1016/j.phymed.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Sun Y. G.; Cui H.; Li Y. H.; Lin X. Q. Determination of some catechol derivatives by a flow injection electrochemiluminescent inhibition method. Talanta. 2000, 53 (3), 661–666. 10.1016/S0039-9140(00)00550-6. [DOI] [PubMed] [Google Scholar]
- Calderón C. E.; Pérez-García A.; de Vicente A.; Cazorla F. M. The dar genes of Pseudomonas chlororaphis PCL1606 are crucial for biocontrol activity via production of the antifungal compound 2-hexyl, 5-propyl resorcinol. Molecular plant-microbe interactions. 2013, 26 (5), 554–565. 10.1094/MPMI-01-13-0012-R. [DOI] [PubMed] [Google Scholar]
- Calderón C. E.; Tienda S.; Heredia-Ponce Z.; Arrebola E.; Cárcamo-Oyarce G.; Eberl L.; Cazorla F. M. The compound 2-hexyl, 5-propyl resorcinol has a key role in biofilm formation by the biocontrol rhizobacterium Pseudomonas chlororaphis PCL1606. Front. Microbiol. 2019, 10, 396. 10.3389/fmicb.2019.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q.; Asai M.; Yoshihara T. Novel resorcinol derivatives from Lasiodiplodia theobromae. Zeitschrift für Naturforschung C 2000, 55 (7–8), 546–551. 10.1515/znc-2000-7-812. [DOI] [Google Scholar]
- Zheng Y.; Wu F. E. Resorcinol derivatives from Ardisia maculosa. Journal of Asian natural products research. 2007, 9 (6), 545–549. 10.1080/10286020600882692. [DOI] [PubMed] [Google Scholar]
- Khan M. M.; Afreen R.; Quasar N.; Khanam N.; Uddin M. Steam-mediated foliar application of catechol and plant growth regulators enhances the growth attributes, photosynthesis, and essential oil production of lemongrass [Cymbopogon flexuosus (Steud.) Wats]. Biocatalysis and Agricultural Biotechnology. 2023, 48, 102638 10.1016/j.bcab.2023.102638. [DOI] [Google Scholar]
- Elblasy S. A.; Shehata H. S.; Ebrahiem A. M.; Hewait H. M. Effectiveness of some Bio-control Agents and Chemical Resistance Inducers Against Brown Stem Rot in Soybean (Glycine max (L.) Merrill). Egyptian Journal of Phytopathology 2023, 51 (1), 103–121. 10.21608/ejp.2023.196335.1087. [DOI] [Google Scholar]
- Khaleda L.; Kim M. G.; Jeon J. R.; Cha J. Y.; Kim W. Y. Foliar application of humic acid or a mixture of catechol and vanillic acid enhanced growth and productivity of alfalfa. J. Korean Soc. Grassl. Forage Sci. 2017, 37 (3), 248–253. 10.5333/KGFS.2017.37.3.248. [DOI] [Google Scholar]
- Zian A. H.; El-Gendy H. M.; Shehata H. S. Enhancing biocontrol agents by hydroquinone and salicylic acid for controlling root-rot and wilt diseases of lupine. Egyptian Journal of Phytopathology 2019, 47 (1), 97–120. 10.21608/ejp.2019.120017. [DOI] [Google Scholar]
- Jagetiya B. L.; Kaur M. J. Effect of foliar application of resorcinol on certain biochemical parameters and yield of soybean. Asian J. Bio Sci. 2006, 1 (2), 129–132. [Google Scholar]
- Wang M.; Schoettner M.; Xu S.; Paetz C.; Wilde J.; Baldwin I. T.; Groten K. Catechol, a major component of smoke, influences primary root growth and root hair elongation through reactive oxygen species-mediated redox signaling. New Phytologist. 2017, 213 (4), 1755–1770. 10.1111/nph.14317. [DOI] [PubMed] [Google Scholar]
- Noel R.; Benoit M.; Wilder S. L.; Waller S.; Schueller M.; Ferrieri R. A. Treatments with Liquid Smoke and Certain Chemical Constituents Prevalent in Smoke Reduce Phloem Vascular Sectoriality in the Sunflower with Improvement to Growth. International Journal of Molecular Sciences. 2022, 23 (20), 12468. 10.3390/ijms232012468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa J. M.; Heuvelink E. P.; The global tomato industry. In Tomatoes; CABI: Wallingford UK: 2018. (pp 1–26). [Google Scholar]
- Kousar B.; Bano A.; Khan N. PGPR modulation of secondary metabolites in tomato infested with Spodoptera litura. Agronomy. 2020, 10 (6), 778. 10.3390/agronomy10060778. [DOI] [Google Scholar]
- GE S.; ZHU Z.; PENG L.; CHEN Q.; JIANG Y. Soil nutrient status and leaf nutrient diagnosis in the main apple producing regions in China. Horticult. Plant J. 2018, 4 (3), 89–93. 10.1016/j.hpj.2018.03.009. [DOI] [Google Scholar]
- Nisar J.; Nasir U.; Ali G.; Shah A.; Farooqi Z. H.; Iqbal M.; Shah M. R. Kinetics of pyrolysis of sugarcane bagasse: effect of catalyst on activation energy and yield of pyrolysis products. Cellulose. 2021, 28, 7593–7607. 10.1007/s10570-021-04015-1. [DOI] [Google Scholar]
- Hamayun M.; Hussain A.; Khan S. A.; Kim H. Y.; Khan A. L.; Waqas M.; Irshad M.; Iqbal A.; Rehman G.; Jan S.; Lee I. J. Gibberellins producing endophytic fungus Porostereum spadiceum AGH786 rescues growth of salt affected soybean. Front. Microbiol. 2017, 8, 686. 10.3389/fmicb.2017.00686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A.; Akhtar S. S.; Li L.; Fu Q.; Li Q.; Naeem M. A.; He X.; Zhang Z.; Jacobsen S. E. Biochar mitigates combined effects of drought and salinity stress in quinoa. Agronomy. 2020, 10 (6), 912. 10.3390/agronomy10060912. [DOI] [Google Scholar]
- Hussain A.; Ali S.; Rizwan M.; ur Rehman M. Z.; Javed M. R.; Imran M.; Chatha S. A.; Nazir R. Zinc oxide nanoparticles alter the wheat physiological response and reduce the cadmium uptake by plants. Environmental Pollution. Environ. Pollut. 2018, 242, 1518–1526. 10.1016/j.envpol.2018.08.036. [DOI] [PubMed] [Google Scholar]
- Abdul-Baki A. A.; Anderson J. D. Vigor determination in soybean seed by multiple criteria 1. Crop science. 1973, 13 (6), 630–633. 10.2135/cropsci1973.0011183X001300060013x. [DOI] [Google Scholar]
- Bates L. S.; Waldren R. P.; Teare I. D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. 10.1007/BF00018060. [DOI] [Google Scholar]
- Husen A. Stock-plant etiolation causes drifts in total soluble sugars and anthraquinones, and promotes adventitious root formation in teak (Tectona grandis L. f.) coppice shoots. Plant Growth Reg. 2007, 54, 13–21. 10.1007/s10725-007-9222-y. [DOI] [Google Scholar]
- Van Handel E. Rapid determination of total lipids in mosquitoes. J. Am. Mosq Control Assoc. 1985, 1 (3), 302–304. [PubMed] [Google Scholar]
- Benizri E.; Ginouves A.; Berra E. The magic of the hypoxia-signaling cascade. Cell. Mol. Life Sci. 2008, 65, 1133–1149. 10.1007/s00018-008-7472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergün N.; TOPCUOĞLU Ş.F.; Yildiz A. Auxin (Indole-3-acetic acid), gibberellic acid (GA_3), abscisic acid (ABA) and cytokinin (Zeatin) production by some species of mosses and lichens. Turkish J. Bot. 2002, 26 (1), 13–18. [Google Scholar]
- Velikova V.; Yordanov I.; Edreva A. J. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci. 2000, 151 (1), 59–66. 10.1016/S0168-9452(99)00197-1. [DOI] [Google Scholar]
- Tyburski J.; Dunajska K.; Mazurek P.; Piotrowska B.; Tretyn A. Exogenous auxin regulates H 2 O 2 metabolism in roots of tomato (Lycopersicon esculentum Mill.) seedlings affecting the expression and activity of CuZn-superoxide dismutase, catalase, and peroxidase. Acta Physiol. Plant. 2009, 31, 249–260. 10.1007/s11738-008-0225-8. [DOI] [Google Scholar]
- Yuan Y.; Qian H.; Yu Y.; Lian F.; Tang D. Thermotolerance and antioxidant response induced by heat acclimation in Freesia seedlings. Acta Physiologiae Plantarum. 2011, 33, 1001–1009. 10.1007/s11738-010-0633-4. [DOI] [Google Scholar]
- Salimi F.; Shekari F.; Hamzei J. Methyl jasmonate improves salinity resistance in German chamomile (Matricaria chamomilla L.) by increasing activity of antioxidant enzymes. Acta Physiol. Plant. 2016, 38, 1–4. 10.1007/s11738-015-2023-4. [DOI] [Google Scholar]
- Hemavathy R. V.; Kumar P. S.; Kanmani K.; Jahnavi N. Adsorptive separation of Cu (II) ions from aqueous medium using thermally/chemically treated Cassia fistula based biochar. Journal of cleaner production. 2020, 249, 119390 10.1016/j.jclepro.2019.119390. [DOI] [Google Scholar]
- Gayathri R.; Gopinath K. P.; Kumar P. S. Adsorptive separation of toxic metals from aquatic environment using agro waste biochar: Application in electroplating industrial wastewater. Chemosphere. 2021, 262, 128031 10.1016/j.chemosphere.2020.128031. [DOI] [PubMed] [Google Scholar]
- Nartey O. D.; Zhao B. Biochar preparation, characterization, and adsorptive capacity and its effect on bioavailability of contaminants: an overview. Adv. Mater. Sci. Eng. 2014, 1, 1. 10.1155/2014/715398. [DOI] [Google Scholar]
- Yaashikaa P. R.; Kumar P. S.; Varjani S.; Saravanan A. A critical review on the biochar production techniques, characterization, stability and applications for circular bioeconomy. Biotechnology Reports. 2020, 28, e00570 10.1016/j.btre.2020.e00570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racioppi M.; Tartaglia M.; De la Rosa J. M.; Marra M.; Lopez-Capel E.; Rocco M. Response of ancient and modern wheat varieties to biochar application: effect on hormone and gene expression involved in germination and growth. Agronomy. 2020, 10 (1), 5. 10.3390/agronomy10010005. [DOI] [Google Scholar]
- Eltaweil A. S.; Abdelfatah A. M.; Hosny M.; Fawzy M. Novel biogenic synthesis of a Ag@ Biochar nanocomposite as an antimicrobial agent and photocatalyst for methylene blue degradation. ACS omega. 2022, 7 (9), 8046–8059. 10.1021/acsomega.1c07209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiu L.; Zhang W.; Sun Y.; Wu D.; Meng J.; Chen W. Effects of biochar and straw returning on the key cultivation limitations of Albic soil and soybean growth over 2 years. Catena. 2019, 173, 481–493. 10.1016/j.catena.2018.10.041. [DOI] [Google Scholar]
- Nafees M.; Ullah S.; Ahmed I. Morphological and elemental evaluation of biochar through analytical techniques and its combined effect along with plant growth promoting rhizobacteria on Vicia faba L. under induced drought stress. Microscopy Research and Technique. 2021, 84 (12), 2947–2959. 10.1002/jemt.23854. [DOI] [PubMed] [Google Scholar]
- Bhardwaj R. D.; Kaur L.; Srivastava P. Comparative evaluation of different phenolic acids as priming agents for mitigating drought stress in wheat seedlings. Proceedings of the National Academy of Sciences, India Section B: Biological Sciences. 2017, 87, 1133–1142. 10.1007/s40011-015-0690-y. [DOI] [Google Scholar]
- Arshad U.; Raheel M.; Ashraf W.; Ur Rehman A.; Zahid M. S.; Moustafa M.; Ali M. A. Influence of Biochar Application on morpho-physiological attributes of tomato (Lycopersicon esculentum Mill) and soil Properties. Commun. Soil Sci. Plant Anal. 2023, 54 (4), 515–525. 10.1080/00103624.2022.2118295. [DOI] [Google Scholar]
- LI W.; Wang H. J.; Zhong M. T.; Song J. H.; Shi X. Y.; Tian T.; Wang J. G.; Zhu Y. Q.; Jiang M. H. Effects of straw return and biochar application on soil nutrients and osmotic regulation in cotton under different soil salinity levels. Appl. Ecol. Environ. Res. 2023, 21 (2), 957. 10.15666/aeer/2102_957974. [DOI] [Google Scholar]
- Sun G.; Geng S.; Zhang H.; Jia M.; Wang Z.; Deng Z.; Tao S.; Liao R.; Wang F.; Kong X.; Fu M.; Liu S.; Li A.; Mao L. Matrilineal empowers wheat pollen with haploid induction potency by triggering postmitosis reactive oxygen species activity. New Phytol. 2022, 233 (6), 2405–2414. 10.1111/nph.17963. [DOI] [PubMed] [Google Scholar]
- Munsif F.; Shah T.; Arif M.; Jehangir M.; Afridi M. Z.; Ahmad I.; Jan B. L.; Alansi S. Combined effect of salicylic acid and potassium mitigates drought stress through the modulation of physio-biochemical attributes and key antioxidants in wheat. Saudi J. Biol. Sc. 2022, 29 (6), 103294 10.1016/j.sjbs.2022.103294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi N.; Funamoto Y.; Shigyo M. Peroxidasemediated chlorophyll degradation in horticultural crops. Phytochem. Rev. 2004, 3 (1–2), 221–228. 10.1023/B:PHYT.0000047796.98784.06. [DOI] [Google Scholar]
- Rasheed R.; Parveen A.; Riaz M.; Arif M. S.; Hussain I.; Firdous S.; Iqbal M.; Ashraf M. A. Soil biochar ameliorates salinity stress and improves nutrient uptake, biomass production and physiochemical parameters in sunflower. Inter. J. Agric Biol. 2019, 22 (6), 1663–1674. [Google Scholar]
- Rehman U. M. Z.; Rizwan M.; Khalid H.; Ali S.; Naeem A.; Yousaf B.; Liu G.; Sabir M.; Farooq M. Farmyard manure alone and combined with immobilizing amendments reduced cadmium accumulation in wheat and rice grains grown in field irrigated with raw effluents. Chemosphere. 2018, 199, 468–476. 10.1016/j.chemosphere.2018.02.030. [DOI] [PubMed] [Google Scholar]
- He Y.; Yao Y.; Ji Y.; Deng J.; Zhou G.; Liu R.; Shao J.; Zhou L.; Li N.; Zhou X.; Bai S. H. Biochar amendment boosts photosynthesis and biomass in C3 but not C4 plants: A global synthesis. GCB Bioenergy. 2020, 12 (8), 605–617. 10.1111/gcbb.12720. [DOI] [Google Scholar]
- Singh S.; Prakash P.; Singh A. K. Salicylic acid and hydrogen peroxide improve antioxidant response and compatible osmolytes in wheat (Triticum aestivum L.) under water deficit. Agricultural Research. 2021, 10, 175–186. 10.1007/s40003-020-00490-3. [DOI] [Google Scholar]
- Cong M.; Hu Y.; Sun X.; Yan H.; Yu G.; Tang G.; Chen S.; Xu W.; Jia H. Long-term effects of biochar application on the growth and physiological characteristics of maize. Front. Plant Sci. 2023, 14, 1172425. 10.3389/fpls.2023.1172425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L.; Zhang J.; Wang C.; Liao W. Recent progress in the knowledge on the alleviating effect of nitric oxide on heavy metal stress in plants. Plant Physiology and Biochemistry. 2020, 147, 161–171. 10.1016/j.plaphy.2019.12.021. [DOI] [PubMed] [Google Scholar]
- Pirzadah T. B.; Malik B.; Tahir I.; Rehman R. U.; Hakeem K. R.; Alharby H. F. Aluminium stress modulates the osmolytes and enzyme defense system in Fagopyrum species. Plant Physiology and Biochemistry. 2019, 144, 178–186. 10.1016/j.plaphy.2019.09.033. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Branicky R.; Noë A.; Hekimi S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217 (6), 1915–1928. 10.1083/jcb.201708007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.; Liang L.; Huang S.; Li W.; Ashraf U.; Ma L.; Mo Z. Exogenous melatonin and catechol application modulate physio-biochemical attributes and early growth of fragrant rice under Cd toxicity. Journal of Soil Science and Plant Nutrition. 2021, 21 (3), 2285–2296. 10.1007/s42729-021-00521-0. [DOI] [Google Scholar]
- Kiokias S.; Varzakas T.; Oreopoulou V. In vitro activity of vitamins, flavonoids, and natural phenolic antioxidants against the oxidative deterioration of oil-based systems. Critical reviews in food science and nutrition. 2008, 48 (1), 78–93. 10.1080/10408390601079975. [DOI] [PubMed] [Google Scholar]
- Amarowicz R.; Pegg R. B.; Rahimi-Moghaddam P.; Barl B.; Weil J. A. Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chem. 2004, 84 (4), 551–562. 10.1016/S0308-8146(03)00278-4. [DOI] [Google Scholar]
- Gharred J.; Derbali W.; Derbali I.; Badri M.; Abdelly C.; Slama I.; Koyro H. W. Impact of biochar application at water shortage on biochemical and physiological processes in medicago ciliaris. Plants. 2022, 11 (18), 2411. 10.3390/plants11182411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulfiqar B.; Raza M. A.; Saleem M. F.; Aslam M. U.; Iqbal R.; Muhammad F.; Amin J.; Ibrahim M. A.; Khan I. H. Biochar enhances wheat crop productivity by mitigating the effects of drought: Insights into physiological and antioxidant defense mechanisms. PloS One. 2022, 17 (4), e0267819 10.1371/journal.pone.0267819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider F. U.; Wang X.; Farooq M.; Hussain S.; Cheema S. A.; Ain N. u.; Virk A. L.; Ejaz M.; Janyshova U.; Liqun C. Biochar application for the remediation of trace metals in contaminated soils: Implications for stress tolerance and crop production. Ecotoxicol. Environ. Saf. 2022, 230, 113165 10.1016/j.ecoenv.2022.113165. [DOI] [PubMed] [Google Scholar]
- Mahmoud A. W.; Samy M. M.; Sany H.; Eid R. R.; Rashad H. M.; Abdeldaym E. A. Nanopotassium, nanosilicon, and biochar applications improve potato salt tolerance by modulating photosynthesis, water status, and biochemical constituents. Sustainability. 2022, 14 (2), 723. 10.3390/su14020723. [DOI] [Google Scholar]
- Mittler R.; Zandalinas S. I.; Fichman Y.; Van Breusegem F. Reactive oxygen species signalling in plant stress responses. Nature Reviews Molecular Cell Biology. 2022, 23 (10), 663–679. 10.1038/s41580-022-00499-2. [DOI] [PubMed] [Google Scholar]
- Khan Z.; Khan M. N.; Zhang K.; Luo T.; Zhu K.; Hu L. The application of biochar alleviated the adverse effects of drought on the growth, physiology, yield and quality of rapeseed through regulation of soil status and nutrients availability. Industrial Crops and Products. 2021, 171, 113878 10.1016/j.indcrop.2021.113878. [DOI] [Google Scholar]
- Abdelaal K. A. A.; Attia K. A.; Alamery S. F.; El-Afry M. M.; Ghazy A. I.; Tantawy D. S.; Al-Doss A. A.; El-Shawy E. S. E.; Abu-Elsaoud A. M.; Hafez Y. M. Exogenous application of proline and salicylic acid can mitigate the injurious impacts of drought stress on barley plants associated with physiological and histological characters. Sustainability 2020, 12 (5), 1736. 10.3390/su12051736. [DOI] [Google Scholar]
- Kazan K. Diverse roles of jasmonates and ethylene in abiotic stress tolerance. Trends in plant science. 2015, 20 (4), 219–229. 10.1016/j.tplants.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Fahad S.; Hussain S.; Matloob A.; Khan F. A.; Khaliq A.; Saud S.; Hassan S.; Shan D.; Khan F.; Ullah N.; Faiq M.; Khan M. R.; Tareen A. K.; Khan A.; Ullah A.; Ullah N.; Huang J. Phytohormones and plant responses to salinity stress: a review. Plant Growth Reg. 2015, 75, 391–404. 10.1007/s10725-014-0013-y. [DOI] [Google Scholar]
- Voß U.; Bishopp A.; Farcot E.; Bennett M. J. Modelling hormonal response and development. Trends in plant science. 2014, 19 (5), 311–319. 10.1016/j.tplants.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhangi-Abriz S.; Torabian S. Biochar increased plant growth-promoting hormones and helped to alleviates salt stress in common bean seedlings. Journal of Plant Growth Regulation. 2018, 37, 591–601. 10.1007/s00344-017-9756-9. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data sets presented in this article have been included in the manuscript.