Abstract
BACKGROUND
Exoscopy in neurosurgery offers various advantages, including increased freedom of the viewing axis while the surgeon maintains a comfortable upright position. However, the optimal monitor positioning to avoid interference with surgical manipulation remains unresolved. Herein, the authors describe two cases in which a three-dimensional head-mounted display (3D-HMD) was introduced into a transcranial neurosurgical procedure using an exoscope.
OBSERVATIONS
Case 1 was a 50-year-old man who presented with recurrent epistaxis and was diagnosed with an olfactory neuroblastoma that extended from the nasal cavity to the anterior cranial base and infiltrated the right anterior cranial fossa. Case 2 was a 65-year-old man who presented with epistaxis and was diagnosed with a left-sided olfactory neuroblastoma. In both cases, en bloc tumor resection was successfully performed via a simultaneous exoscopic transcranial approach using a 3D-HMD and an endoscopic endonasal approach, eliminating the need to watch a large monitor beside the patient.
LESSONS
This is the first report of using a 3D-HMD in transcranial surgery. The 3D-HMD effectively addressed issues with the field of vision and concentration while preserving the effectiveness of traditional microscopic and exoscopic procedures when observed on a 3D monitor. Combining the 3D-HMD with an exoscope holds the potential to become a next-generation surgical approach.
Keywords: 3D head-mounted display, ORBEYE, neuroblastoma
ABBREVIATIONS: 3D-HMD = three-dimensional head-mounted display, CT = computed tomography, MRI = magnetic resonance imaging
Exoscopy in neurosurgery reportedly offers various advantages, including increased freedom of the viewing axis while the surgeon maintains a comfortable upright position.1,2 However, optimal positioning of the monitor remains an unresolved matter. The monitor should be positioned to avoid causing interference of other staff with the surgeon and the screen and interrupting the surgeon’s concentration. Herein, we report our novel experience with the use of a specific three-dimensional head-mounted display (3D-HMD), the 3D View Vision (FASE Co., Ltd.), as a potential solution to overcome these challenges in cranial surgery.
Illustrative Cases
Case 1
Presentation and Preoperative Imaging
A 50-year-old man presented with a chief complaint of recurrent epistaxis. Initial examination revealed a protruding nasal cavity tumor, and the patient was subsequently diagnosed with an olfactory neuroblastoma via biopsy. The patient’s medical history included diabetes mellitus with a hemoglobin A1c concentration of 8.0%. He reported normosmia but presented with right-sided epiphora and persistent episodes of epistaxis. Magnetic resonance imaging (MRI) revealed that the tumor extended from the nasal cavity to the anterior cranial base, infiltrating the right anterior cranial fossa and displaying contrast enhancement. We performed en bloc tumor resection with a simultaneous exoscopic transcranial approach using a 3D-HMD and an endoscopic endonasal approach.
Operation and Postoperative Course
The head and neck surgeon performed dissection of the tumor from the inner aspect of the orbit, followed by endonasal endoscopic dissection for the nasal cavity procedures. We simultaneously performed bilateral frontal craniotomies through bilateral coronal skin incisions. After the craniotomy, the ORBEYE system (Olympus America) was introduced, and instead of watching a large monitor, we wore the 3D-HMD for surgical visualization (Fig. 1). We carefully removed the tumor from the normal cranial base boundaries, separated it from the affected right anterior cranial lobe, and simultaneously conducted intranasal procedures to successfully achieve complete en bloc resection. The dura mater was reconstructed in a watertight fashion using the free temporalis muscle fascia, and a pericranial flap was appressed over it. The operation time was 9 hours 36 minutes, including use of the 3D-HMD for 6 hours 53 minutes. No new neurological deficits were observed postoperatively, and head computed tomography (CT) revealed no concerning findings. Postoperative contrast-enhanced MRI showed complete removal of all enhanced lesions (Fig. 2). The patient had an uneventful recovery with no cerebrospinal fluid leakage. Thereafter, he received a course of intensity-modulated radiation therapy totaling 66 Gy delivered over two fractions.
FIG. 1.
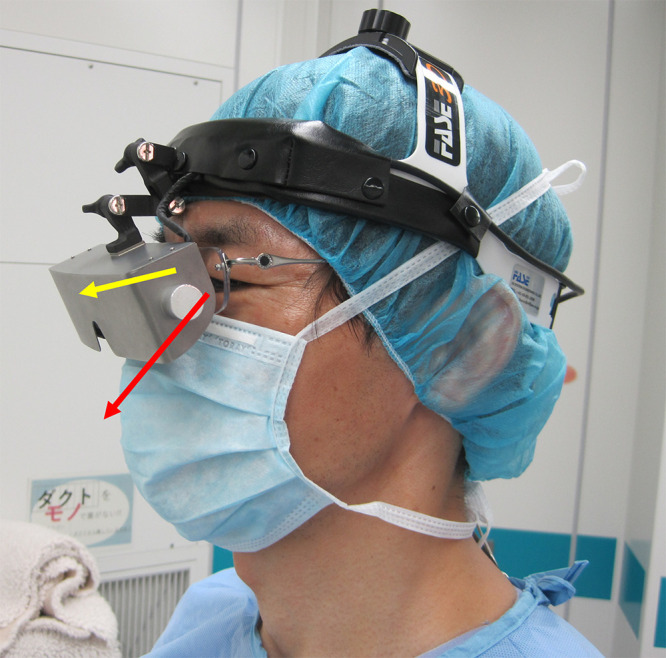
Operator wearing the 3D View Vision with a side view. The yellow arrow indicates the line of sight directed toward the surgical field as observed through the 3D-HMD. The red arrow indicates the line of sight used to directly watch the surgical field.
FIG. 2.
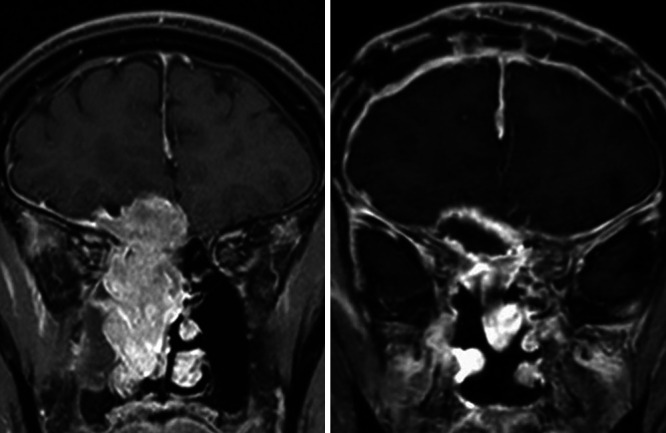
Case 1. Preoperative (left) and postoperative (right) coronal gadolinium-enhanced T1-weighted MRI. The contrast-enhancing lesion was completely removed.
Case 2
Presentation and Preoperative Imaging
A 65-year-old man initially presented to a local otolaryngologist for evaluation of recurrent epistaxis. A biopsy confirmed the diagnosis of olfactory neuroblastoma, and the patient was referred to our institution for further management with surgical intent. Preoperative T2-weighted MRI showed that the tumor filled the left nasal cavity and extended into the left ethmoid sinus, with contrast-enhancing lesions infiltrating the anterior cranial fossa.
Operation and Postoperative Course
We performed a frontoethmoidal tumor resection, similar to the first case, using a combination of craniotomy and endoscopic transnasal approaches with the assistance of the 3D-HMD. The operation time was 5 hours 47 minutes, including the use of the 3D-HMD for 5 hours 33 minutes. Postoperatively, no new neurological deficits were observed, and head CT revealed no concerning findings. Postoperative contrast-enhanced MRI showed complete removal of all enhanced lesions (Fig. 3). The patient had an uneventful recovery without cerebrospinal fluid leakage. At the time of this writing, postoperative radiation therapy was planned.
FIG. 3.
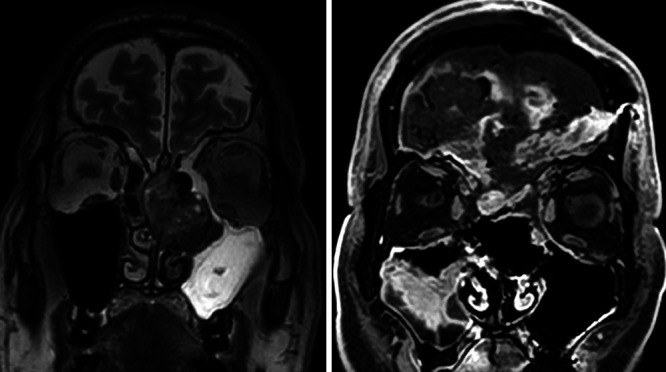
Case 2. Coronal preoperative T2-weighted head MRI (left) and postoperative gadolinium-enhanced T1-weighted MRI (right). The tumor was completely removed.
Patient Informed Consent
The necessary patient informed consent was obtained in this study.
Discussion
Observations
Herein, we have reported two surgical cases in which a 3D-HMD was introduced for transcranial surgery in combination with an exoscope. No other reports on the use of a 3D-HMD for cranial surgery have been published to date. We previously reported that the use of simultaneous transcranial exoscopic and endoscopic endonasal approaches is an effective surgical strategy for en bloc resection of anterior skull base malignancies.3 An important advantage of simultaneous transcranial exoscopic and endoscopic endonasal approaches in anterior skull base surgery is the reduction in surgical time.3 Furthermore, it is worth emphasizing that both transnasal and transcranial surgeons can collaborate to confirm the skull base bone-cutting lines in real time during the surgery, leading to more precise incisions. This coordinated approach also allows the use of both pericranial and nasoseptal flaps, which are highly effective in preventing postoperative cerebrospinal fluid leakage.4 However, one unresolved issue with this coordinated approach is the positioning of the large exoscope monitor. Depending on the monitor’s placement, other staff members may obstruct the view between the surgeon and the monitor, leading the surgeon to tilt their head to clearly see the monitor. The distance between the monitor and the surgeon is greater when using a large monitor than when using a conventional binocular microscope.5 Under these conditions, dissecting around deeply located sites is challenging. Additionally, positioning an assistant with the ORBEYE is more complex than with a traditional binocular microscope.5 For the present cases, we were able to create a more comfortable surgical environment by leveraging the effectiveness of the ORBEYE and addressing challenges through the use of the 3D-HMD.
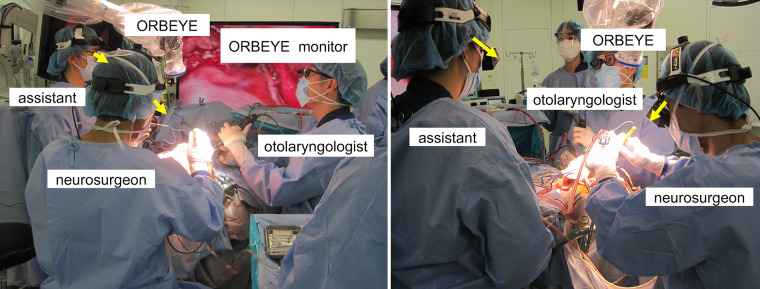
The advantage of using an exoscope lies in the ability to perform surgery without changing the surgeon’s position, even in procedures requiring various viewing angles.2,6,7 Additionally, in combined surgeries performed with a head and neck surgeon, the spatial expansion above the surgical field allows concurrent endonasal procedures.3 However, challenges still exist: The monitor is frequently distant, obstacles can be present in the surgeon’s field of view because of other staff members positioned between the surgeon and the screen, and there may be difficulties in staying focused. Furthermore, the ORBEYE can make the procedure more challenging for the assistant because the screen may not be correctly oriented toward the operative field. The assistant is required to bend their neck at a 90° angle and to perform manual operations in an uncomfortable posture to view the monitor (Fig. 4).5
FIG. 4.
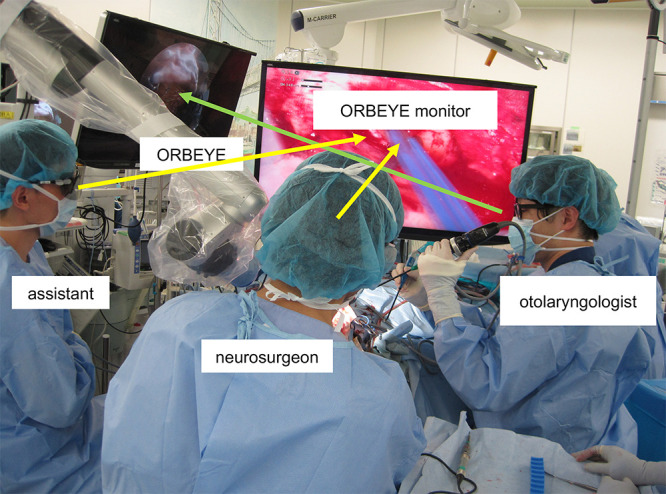
Conventional intraoperative scene of simultaneous transcranial and endonasal surgery using the ORBEYE. The monitor was often too far away, and other staff members sometimes blocked the surgeon’s view of the screen (yellow arrows), making it difficult for the surgeon to stay fully focused. The assistant also had to deal with uncomfortable positions to view the monitor. The green arrow indicates the head and neck surgeon’s and assistant’s line of sight.
During the surgery described herein, the positions of the surgeon and the assistant, as well as the machine settings, remain unchanged. Occasionally, doctors and other staff members may enter the space between the screens at the foot of the operating table. However, neither the surgeon nor the assistant looks at the screens at their feet; instead, they view the screens reflected inside their goggles, ensuring that the procedure is not disrupted (Fig. 5). The HMD used in this case not only preserved the effectiveness of conventional microscopic and endoscopic surgeries under the observation of a 3D-HMD but also resolved issues related to visibility and concentration (Video 1). Thus, it is considered a potential next-generation surgical modality.
FIG. 5.
Intraoperative scene of simultaneous transcranial and endonasal surgery using the 3D-HMD. Using this display, neither the surgeon nor the assistant needs to look at the screens at the patient’s feet. The yellow arrows indicate the neurosurgeon’s line of sight (left). The surgeon and the assistant view the screens reflected inside their goggles, ensuring an uninterrupted procedure. The yellow arrows indicate the neurosurgeon’s and assistant’s line of sight (right).
VIDEO 1. Clip showing an intraoperative scene of simultaneous transcranial and endonasal surgery using a 3D-HMD. Wearing a 3D-HMD, the neurosurgeon and the assistant can perform the surgery without the need to continually watch a distant exoscope monitor located at the patient’s feet. Click here to view.
The 3D View Vision, which has a total weight of 280 g and provides a viewing angle equivalent to 57 inches, offers the capability to survey the environment from the lower part of the HMD. The exoscopic images are transmitted to a 3D-HMD via either wired or wireless connections, allowing the surgeon and assistant to visualize the surgical field. Furthermore, the HMD delivers separate images to each eye, ensuring a lifelike stereoscopic vision experience. Importantly, there is minimal to no discernible time lag between the live surgical procedure and the corresponding images displayed on the monitor. Nevertheless, use of the 3D-HMD presents several issues. First, although the surgeon can directly view the surgical field from below the 3D-HMD, achieving precise manual manipulation can be challenging, especially during intricate procedures such as spatula replacement. Second, visual alignment of the 3D-HMD can frequently be disturbed by movement of the device, necessitating frequent readjustments during surgery. Third, the monitor has a resolution of 3840 × 1080 pixels, which is lower than that of 4K monitors, indicating a need for improvement. Moreover, there is unused space within the field of view that could benefit from enhancement. In this series, the average time of wearing the 3D-HMD in the two cases was 5 hours 40 minutes. Although the weight was not a major concern, it is important to consider that improvements may be needed to support extended use of this device in future.
This report has two main limitations. First, it is based on only two cases of using the 3D-HMD; therefore, further accumulation of cases is needed to fully understand the benefits of this device. Additionally, because this report mainly involves subjective user experiences, there is a need for more objective evaluation and analysis in future studies.
Lessons
The 3D-HMD used in this study successfully addressed the issues associated with the field of vision and concentration while ensuring the effectiveness of conventional microscopic and exoscopic surgeries under 3D monitor observation. The 3D-HMD has the potential to become a next-generation surgical modality.
Author Contributions
Conception and design: Kim, Kimura, Mori, Maeyama. Acquisition of data: Kim. Analysis and interpretation of data: Kim, Kimura, Kajimoto. Drafting the article: Kim, Kimura. Critically revising the article: Kim, Kajimoto. Reviewed submitted version of manuscript: Kim, Kimura, Tanaka. Approved the final version of the manuscript on behalf of all authors: Kim. Statistical analysis: Kim. Administrative/technical/material support: Kim, Kajimoto. Study supervision: Kim, Kajimoto, Tanaka, Sasayama.
Supplemental Information
Videos
Video 1. https://vimeo.com/881718286.
References
- 1. Amoo M, Henry J, Javadpour M. Beyond magnification and illumination: preliminary clinical experience with the 4K 3D ORBEYE™ exoscope and a literature review. Acta Neurochir (Wien) 2021;163(8):2107–2115. doi: 10.1007/s00701-021-04838-8. [DOI] [PubMed] [Google Scholar]
- 2. Iwata T, Toyota S, Kudo A, et al. Microsurgery “under the eaves” using ORBEYE: a case of dural arteriovenous fistula of the anterior cranial fossa. World Neurosurg. 2020;138:178–181. doi: 10.1016/j.wneu.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 3. Shibano A, Kimura H, Tatehara S, et al. Efficacy of a high-definition three-dimensional exoscope in simultaneous transcranial and endoscopic endonasal surgery: a case report. NMC Case Rep J. 2022;9(0):243–247. doi: 10.2176/jns-nmc.2022-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gabriel PJ, Kohli G, Hsueh WD, Eloy JA, Liu JK. Efficacy of simultaneous pericranial and nasoseptal “double flap” reconstruction of anterior skull base defects after combined transbasal and endoscopic endonasal approaches. Acta Neurochir (Wien) 2020;162(3):641–647. doi: 10.1007/s00701-019-04155-1. [DOI] [PubMed] [Google Scholar]
- 5. Takahashi S, Toda M, Nishimoto M, et al. Pros and cons of using ORBEYE™ for microneurosurgery. Clin Neurol Neurosurg. 2018;174:57–62. doi: 10.1016/j.clineuro.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 6. Murai Y, Sato S, Yui K, et al. Preliminary clinical microneurosurgical experience with the 4K3-dimensional microvideoscope (ORBEYE) system for microneurological surgery: observation study. Oper Neurosurg (Hagerstown) 2019;16(6):707–716. doi: 10.1093/ons/opy277. [DOI] [PubMed] [Google Scholar]
- 7. Khalessi AA, Rahme R, Rennert RC, et al. First-in-man clinical experience using a high-definition 3-dimensional exoscope system for microneurosurgery. Oper Neurosurg (Hagerstown) 2019;16(6):717–725. doi: 10.1093/ons/opy320. [DOI] [PubMed] [Google Scholar]