Abstract
To safeguard proteomic integrity, cells rely on the proteasome to degrade aberrant polypeptides, but it is unclear how cells remove defective proteins that have escaped degradation owing to proteasome insufficiency or dysfunction. Here we report a pathway termed misfolding-associated protein secretion, which uses the endoplasmic reticulum (ER)-associated deubiquitylase USP19 to preferentially export aberrant cytosolic proteins. Intriguingly, the catalytic domain of USP19 possesses an unprecedented chaperone activity, allowing recruitment of misfolded proteins to the ER surface for deubiquitylation. Deubiquitylated cargos are encapsulated into ER-associated late endosomes and secreted to the cell exterior. USP19-deficient cells cannot efficiently secrete unwanted proteins, and grow more slowly than wild-type cells following exposure to a proteasome inhibitor. Together, our findings delineate a protein quality control (PQC) pathway that, unlike degradation-based PQC mechanisms, promotes protein homeostasis by exporting misfolded proteins through an unconventional protein secretion process.
Misfolded proteins pose a major threat to cell homeostasis because they not only form non-functional aggregates, but also recruit and inactivate other essential proteins. To cope with protein-misfolding crisis, cells have crafted an extensive protein quality control (PQC) network1. Known PQC strategies include chaperone-dependent disassembly of protein aggregates, chaperone-assisted folding, and sequestration of misfolded proteins into spatially distinct PQC compartments2–5, but degradation by the ubiquitin proteasome system is the most significant PQC mechanism1. A key regulator of the ubiquitin system is the family of deubiquitylases, which modulate substrate stability by disassembling ubiquitin chains6,7. The human genome encodes ~100 deubiquitylases. Among them, only the ubiquitin-specific protease 19 (USP19) possesses a carboxy-terminal transmembrane (TM) domain that localizes it to the ER (Fig. 1a)8. It is also the only deubiquitylase known to interact with Hsp909, but the identified USP19 substrates do not reveal a precise PQC function for this unique deubiquitylase8–13.
Figure 1.
USP19 promotes secretion of a cytosolic protein. (a) The domain architecture of USP19. TM, transmembrane; UBL, ubiquitin-like; ZnF, zinc finger; CS, CHORD-containing proteins and SGT1; USP, ubiquitin-specific protease. (b) Overexpression of USP19 induces GFP secretion from HEK293T cells. Cells (0.8×105) in a 12-well plate were transfected with 250ng pEGFP together with 250ng pcDNA3 or FLAG–USP19-encoding plasmid. At 24h post-transfection, the medium was replaced with fresh medium. Cells were incubated for 16h. The conditioned media and cell lysates were collected and directly analysed by immunoblotting (IB) (lanes 1 and 2). A fraction of the media was subjected to immunoprecipitation (IP) by GFP antibodies to enrich GFP (lanes 3 and 4). Asterisk indicates a nonspecific IgG band. Left panels show two different exposures (long and short). (c) USP19 but not USP7 induces GFP secretion from HEK293T cells. Conditioned media (16h) and lysates prepared from cells co-transfected with GFP together with the indicated constructs were analysed by immunoblotting. (d) Overexpression of WT USP19 or USP19C506S (the catalytically inactive mutant) does not affect PM permeability. HEK293T cells transfected with the indicated plasmids for 48h were stained with trypan blue and counted (mean ± s.e.m., n=3 independent experiments). (e) USP19 induces GFP secretion in a dose-dependent manner in HEK293T cells. The arrowhead indicates endogenous USP19. (f) WT USP19 but not the catalytically inactive mutant (C506S) induces GFP secretion from HeLa cells. (g) GFP secretion from HEK293T cells requires both the catalytic activity and the TM domain of USP19. HEK293T cells transfected as indicated were analysed as in c. (h) GFP secretion is not inhibited by brefeldin A (BFA). Media collected at the indicated time points from HEK293T cells stably expressing GFP were analysed by immunoblotting. A fraction of the samples was subjected to immunoprecipitation with GFP antibodies before immunoblotting. Where indicated, dimethylsulfoxide (DMSO; control) or BFA (10μgml−1) was added at the beginning of the chase. Asterisk, IgG band. Statistics source data for d can be found in Supplementary Table 2. Unprocessed original scans of blots are shown in Supplementary Fig. 8.
Dysfunction in PQC has been linked to human diseases such as Alzheimer’s, Parkinson’s and Huntington’s diseases14–16. The pathology of these diseases is often associated with protein aggregates both within and out of the cell14. In addition, cell-to-cell transmission of aggregation-prone polypeptides is thought to contribute to the progression of neurodegeneration17. How misfolded proteins are released into the extracellular space has been elusive. A few reports suggested that some misfolded proteins (exemplified by Tau) might ‘piggyback’ an exosome pathway to exit the cell18–20. However, the amount of misfolded proteins secreted by exosomes seems inconsequential because the exosome is one of a myriad of unconventional protein secretion (UPS) mechanisms that export mainly folded proteins lacking signal sequences21,22. Over the years, numerous UPS cargos were identified, but no unified mechanism could account for their secretion23,24. The term UPS now merely reflects the only shared feature among these processes—the avoidance of the canonical ER–Golgi network. Besides exosomes, UPS cargos may use vesicles such as autophagosomes or lysosomes to exit the cell25,26. In the case of FGF-2, direct translocation across the plasma membrane (PM) was proposed27.
Here we establish a UPS pathway termed misfolding-associated protein secretion (MAPS) that preferentially targets aberrant cytosolic proteins for secretion and USP19 as a pivotal regulator. Our study suggests that MAPS, while offeringshort-termprotectiontoindividual cells under proteasome dysfunctional conditions, might contribute to the prion-like behaviours of aggregation-prone proteins that are detrimental to a multicellular organism in the long term.
RESULTS
USP19 promotes unconventional secretion of misfolded cytosolic proteins
The ER localization of USP19 prompted us to investigate whether it regulates protein secretion. Immunoblotting analysis of conditioned medium from control and USP19-overexpressing HEK293T cells showed that USP19 did not affect the secretion of clusterin, a conventional ER cargo (Fig. 1b). Surprisingly, USP19 but not USP7 significantly increased the secretion of green fluorescent protein (GFP) (expressed as a cytosolic control protein) with no detectable effect on cell permeability (Fig. 1b–d). A titration experiment showed that even a twofold increase over the endogenous USP19 level was sufficient to upregulate GFP secretion (Fig. 1e). USP19-mediated secretion was observed in different cell lines (Fig. 1e,f), and required both the catalytic activity and membrane localization of USP19 (Fig. 1g). As expected, GFP secretion was insensitive to brefeldin A (BFA; Fig. 1h), which blocks the export of signal sequence-bearing proteins such as clusterin28.
As the previously established Hsp90 connection hinted at a potential function for USP19 in handling misfolded proteins9, and because under overexpression conditions, even wild-type proteins may become misfolded to some extent29, we postulated that secreted GFP might be a misfolded species exported through a PQC pathway regulated by USP19. Consistent with this idea, when compared with GFP in the cell, most GFP molecules in conditioned medium did not have green fluorescence (Fig. 2a and Supplementary Fig. 1a). To further test our model, we created a GFP mutant lacking the last β-strand (GFP1–10). This mutant was more prone to misfolding, as indicated by reduced solubility in the non-ionic detergent NP-40 (Supplementary Fig. 1b). In accordance with our model, this variant was secreted more efficiently than GFP (Fig. 2b, lane 5 versus 1; Fig. 2c and Supplementary Fig. 1c lane 2 versus 1). Furthermore, when we co-expressed the deleted β-strand (GFP11) together with GFP1–10, GFP1–10 folding was partially rescued, as indicated by a small but consistent gain in fluorescence (Supplementary Fig. 1d). Improvement in GFP1–10 folding correlated with reduced secretion (Fig. 2c and Supplementary Fig. 1c). Like GFP, GFP1–10 secretion was enhanced by wild-type (WT) USP19, but not by the catalytically inactive USP19C506S mutant or a mutant lacking the TM segment (USP191–1290) (Fig. 2b,d), and it was not sensitive to BFA (Supplementary Fig. 1e). The USP19-stimulated secretion could not be attributed to perturbed ubiquitin homeostasis due to deubiquitylase overexpression because overexpression of USP7–TM that had the USP19 TM domain fused to USP7 did not affect GFP1–10 secretion (Fig. 2e).
Figure 2.
USP19 preferentially targets misfolded cytosolic proteins for secretion. (a) Secreted GFP molecules are mostly unfolded. HEK293T cells (0.5×106) were transfected with 1μg GFP together with 1μg USP19. Conditioned medium collected between 36 and 52h post-transfection and cell lysate were examined for green fluorescence. Shown is GFP intensity normalized by protein level determined by immunoblotting (Supplementary Fig. 1a). (b) USP19-induced secretion requires its deubiquitylating activity and the TM domain. Conditioned media (16h) from HEK293T cells transfected with the indicated plasmids were analysed together with lysates by immunoblotting. (c) Secretion of GFP1–10 is affected by its folding state. Quantification of four independent experiments represented by Supplementary Fig. 1c. The level of GFP1–10 secretion was normalized by the GFP1–10 level in lysate (mean ± s.e.m., n = 4 independent experiments, ∗∗P < 0.01 as determined by paired Student’s t-test). (d) Kinetic analysis of GFP1–10 secretion using HEK293T cells co-transfected with GFP1–10 and the indicated plasmids. Media collected at the indicated time points (top panel) and lysates prepared at the end of the chase (bottom panels) were analysed by immunoblotting. (e) Overexpression of ER-tethered USP7 does not promote GFP1–10 secretion. The same as in b, except that the indicated deubiquitylases were transfected. (f) USP19 does not induce secretion of endogenous cytosolic proteins. Conditioned media and lysates from HEK293T cells transfected with control or USP19 were analysed by immunoblotting. (g) USP19 promotes secretion of overexpressed Ubl4A. HEK293T cells co-transfected with Ubl4A–FLAG together with the indicated USP19 variants were analysed as in b. (h) USP19 promotes α-synuclein secretion. As in g, except that Ubl4A–FLAG was replaced by α-synuclein–FLAG in transfection. (i) USP19 promotes the secretion of Parkinson’s disease-associated α-synuclein mutants. The graph shows the relative secretion efficiency (mean ± s.e.m., n = 3 independent experiments). (j) USP19 does not promote Tau secretion. Note that Tau was not detected in media in any of the tested conditions. Statistics source data for c,i can be found in Supplementary Table 2. Unprocessed original scans of blots are shown in Supplementary Fig. 8.
To see whether USP19 can stimulate secretion of mammalian proteins, we tested several abundant endogenous proteins including ubiquitin-activating enzyme (UBE1), p97, Hsp90, Bag6 and Ubl4A. Immunoblotting detected a small amount of UBE1 and p97 in conditioned medium independent of USP19 overexpression, but not Hsp90, Bag6 or Ubl4A (Fig. 2f). This further confirmed the PM integrity. Interestingly, although endogenous Ubl4A was not secreted, a fraction of overexpressed Ubl4A was secreted, which was enhanced by USP19, but not by USP19C506S or USP191–1290 (Fig. 2g). As Ubl4A normally forms a complex with two partners30, overexpressed Ubl4A should be mostly in an unassembled form. Thus, USP19 seems to mediate a PQC process that preferentially targets misfolded or unassembled cytosolic proteins for secretion. We term this pathway misfolding-associated protein secretion (MAPS).
To investigate whether MAPS is involved in cell-to-cell transmission of misfolded proteins observed in neurodegenerative diseases17, we tested whether USP19 promotes the secretion of α-synuclein, a natively unfolded cytosolic protein known to be released from cells through exosomes as well as an undefined mechanism31,32. Interestingly, WT USP19 but not USP191–1290 or USP19C506S significantly stimulated α-synuclein secretion (Fig. 2h). USP19 also promoted the secretion of several Parkinson’s disease-associated α-synuclein mutants (Fig. 2i). As USP19 did not promote exosome-dependent secretion of Tau (Fig. 2j), MAPS seems to be the long-sought mechanism that accounts for exosome-independent α-synuclein secretion.
As short interfering RNA (siRNA)-mediated downregulation of USP19 reduced GFP1–10 secretion (Fig. 3a,b), endogenous USP19 seemed to play a critical role in MAPS. To further explore the function of endogenous USP19 in MAPS, we generated USP19-null CRISPR cells33 (Fig. 3c). Consistent with the siRNA study, knock-out of USP19 reduced GFP1–10 secretion (Fig. 3d–f). As expected, overexpression of USP19 in both USP19-deficient and control cells increased GFP1–10 secretion to a similar level (Fig. 3f). The USP19-null cells are also defective in secretion of Ubl4A–FLAG (Fig. 3g,h) and α-synuclein–FLAG (Fig. 3i,j). These results confirmed USP19 as a rate-limiting factor in MAPS, but also suggested that redundant regulators may exist to mediate the reduced secretion in USP19-null cells.
Figure 3.
Endogenous USP19 is required for MAPS. (a) Knockdown of endogenous USP19 reduces GFP1–10 secretion. HEK293T cells were transfected with GFP1–10 together with the indicated siRNAs. The conditioned media (16h) and lysates were analysed by immunoblotting. (b) Quantification of GFP1–10 secretion from HEK293T cells transfected with control and USP19 siRNA-2 (mean ± s.e.m., n = 4 independent experiments, ∗∗P < 0.01). (c) The design of the CRISPR guide RNAs that target the USP19 gene. The predicted nicking sites are labelled. (d) USP19 deficiency reduces GFP1–10 secretion. GFP1–10 secretion was analysed using a control CRISPR clone and two USP19 knockout (KO) clones as well as the parental HEK293T cells. Asterisk, a nonspecific band. (e) Quantification of GFP1–10 secretion from control and USP19-null cells (mean ± s.e.m., n = 4 independent experiments, ∗∗∗P< 0.001, ∗∗P<0.01). (f) Overexpression of USP19 rescues GFP1–10 secretion in USP19-null cells. (g,h) Knockout of USP19 impairs the secretion of overexpressed Ubl4A–FLAG. (g) The same as in d, except that control and USP19-null cells were transfected with Ubl4A–FLAG. (h) Quantification of the experiments represented in g (mean ± s.e.m., n = 3 independent experiments, ∗∗P<0.01). (i,j) Knockout of USP19 reduces the secretion of overexpressed α-synuclein–FLAG (mean ± s.e.m., n = 3 independent experiments, ∗∗∗P <0.001). Statistics source data for b,e,h,j can be found in Supplementary Table 2. Unprocessed original scans of blots are shown in Supplementary Fig. 8.
The functional interplay between MAPS and proteasomal degradation
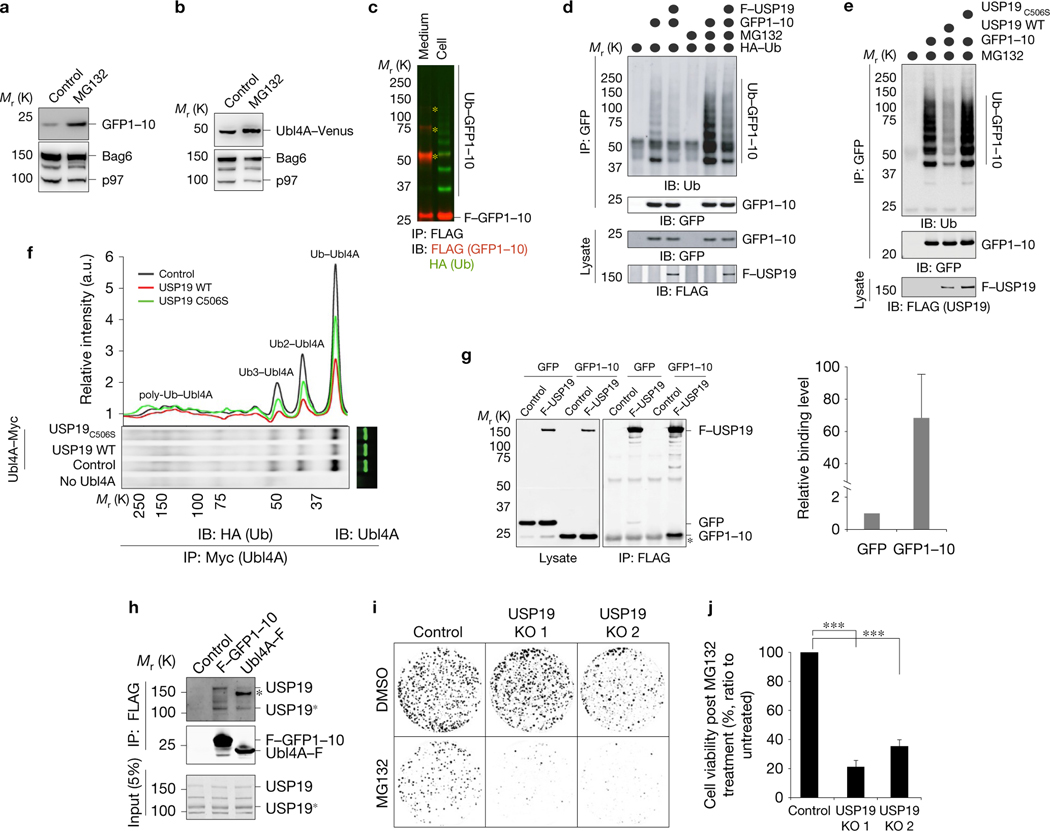
To understand the functional interplay between MAPS and proteasome-mediated PQC, we treated cells transfected with either GFP1–10 or Ubl4A–Venus with the proteasome inhibitor MG132. Immunoblotting showed that MG132 treatment increased the steady-state level of these proteins (Fig. 4a,b). A translation shutdown experiment further confirmed that GFP1–10 was degraded by the proteasome with a relatively long half-life (~4 h) (Supplementary Fig. 2a). As α-synuclein is also degraded slowly by the proteasome34, MAPS seems to constitute an alternative path for elimination of misfolded substrates that are inefficiently processed by the proteasome (Supplementary Fig. 2b).
Figure 4.
USP19 interacts with MAPS cargos and alters their ubiquitylation state. (a,b) Both GFP1–10 and Ubl4A are proteasome substrates. (a) HEK293T cells transfected with GFP1–10 were treated with MG132 (10 μM) for 16h. Lysates were subjected to immunoblot analysis. (b) The same as in a, except that cells transfected with Ubl4A–Venus were analysed. (c) GFP1–10 is ubiquitylated in cells, but secreted GFP1–10 is mostly unmodified. GFP1–10 was immunoprecipitated under denaturing conditions from lysates or conditioned media using HEK293T cells co-transfected with GFP1–10 and haemagglutinin (HA)-tagged ubiquitin. Asterisks, nonspecific bands. (d) USP19 promotes deubiquitylation of GFP1–10 in cells. HEK293T cells transfected with the indicated constructs were treated with DMSO as a control or MG132 (20 μM) for 4h. GFP1–10 immunoprecipitated from cell extracts was analysed by immunoblotting (top panels). A fraction of the lysates was analysed directly by immunoblotting (bottom panels). (e) WT USP19 but not USP19C506S reduced ubiquitylated GFP1–10. The same as in d, except that cells were transfected and treated as indicated. (f) USP19 promotes deubiquitylation of Ubl4A–FLAG. Cells transfected with the indicated constructs were treated with MG132 (20 μM) for 4h. Ubl4A–FLAG was immunoprecipitated and analysed by immunoblotting. The graph shows the intensity of ubiquitylated Ubl4A bands quantified from the gel below. (g) USP19 binds GFP1–10 more strongly than GFP. Co-immunoprecipitation using cells co-transfected with either control or FLAG–USP19 together with the indicated MAPS cargos. Asterisk, IgG bands. The graph shows the quantification result (mean ± s.e.m., n = 3 independent experiments). (h) Endogenous USP19 interacts with FLAG–GFP1–10 and Ubl4A–FLAG. Cells transfected with the indicated constructs were treated with formaldehyde. Cell lysates were subjected to immunoprecipitation by FLAG antibodies. Asterisk, nonspecific band; USP19*, a truncated USP19 product. (i,j) USP19-null cells have a growth defect after treatment with a proteasome inhibitor. (i) Control and USP19 knockout cells treated with MG132 (5 μM, 15h) or DMSO were incubated in inhibitor-free medium for 14 days and stained. (j) Quantification of relative cell viability of MG132-treated cells (mean ± s.e.m., n = 3 independent experiments). ∗∗∗P < 0.001. Statistics source data for g,j can be found in Supplementary Table 2. Unprocessed original scans of blots are shown in Supplementary Fig. 8.
As immunoprecipitation under denaturing conditions showed that GFP1–10 and Ubl4A–FLAG both contained ubiquitin conjugates, but the same proteins once secreted were largely ubiquitin free (Fig. 4c and Supplementary Fig. 2c), and because USP19-mediated deubiquitylation promotes MAPS, we proposed that USP19 removes ubiquitin conjugates from a subset of ubiquitylated substrates to promote their secretion. Several lines of evidence further support this model. First, overexpression of WT USP19 but not USP19C506S or USP7 significantly reduced ubiquitylated GFP1–10 and Ubl4A–FLAG in cells (Fig. 4d–f and Supplementary Fig. 2d). Second, coimmunoprecipitation showed that USP19 interacted with GFP1–10 and GFP with different affinity that mirrored their secretion efficiency (Fig. 4g). Last, crosslinking experiments using the reversible crosslinker formaldehyde showed that FLAG–GFP1–10 and Ubl4A–FLAG both interacted with endogenous USP19 (Fig. 4h and Supplementary Fig. 2e).
The notion that MAPS acts in parallel to the proteasome is further supported by the observation that cells treated with MG132 divert more GFP1–10 to the MAPS pathway (Supplementary Fig. 2f). Quantitative immunoblotting showed that the amount of GFP1–10 and Ubl4A–FLAG secreted within 16 h was approximately 3–5% of that in the cell (Supplementary Fig. 2g,h), suggesting that MAPS is a supplementary PQC mechanism that assists the proteasome in removing unwanted proteins. Nevertheless, this mechanism might become essential when the proteasome function is impaired under stress or disease conditions. To test this idea, we treated control and USP19-null cells with MG132, and then grew these cells in inhibitor-free medium. Although, under normal conditions, USP19-null cells grew similarly to WT cells, after MG132 treatment, they grew significantly more slowly (Fig. 4i,j). In contrast, no difference in growth rate was observed between WT and USP19-null cells treated with the ER stress inducers tunicamycin and dithiothreitol (Supplementary Fig. 2i). These results suggest that USP19 promotes cell adaptation to proteasome dysfunction.
USP19 has a chaperone activity
The differential interaction of USP19 with GFP and GFP1–10 prompted us to investigate whether USP19 could directly recognize a model misfolded protein in vitro. As USP19 purified from Escherichia coli is not folded properly, we purified USP19 and USP19C506S from mammalian cells (Fig. 5a). The lack of deubiquitylase activity for USP19C506S was confirmed by an in vitro deubiquitylating assay (Fig. 5b). We then incubated these proteins with luciferase at 42 °C. Heat treatment caused luciferase to unfold and reduced its solubility, but the presence of either USP19 or USP19C506S resulted in a dose-dependent increase in soluble luciferase (Fig. 5c). Co-immunoprecipitation showed that USP19 bound to denatured but not native luciferase (Fig. 5d, lane 8 versus 6). Deletion studies showed that the amino-terminal CS (CHORD-containing proteins and SGT1) domains are not required for luciferase binding (Fig. 5e). Coincidentally, a USP19 mutant lacking the CS domains also induced GFP1–10 and Ubl4A–FLAG secretion (Fig. 5f,g). Thus, USP19 has a chaperoning activity associated with the USP domain.
Figure 5.
USP19 has a chaperone activity. (a) Purified FLAG-tagged WT USP19 and the USP19C506S mutant. (b) An in vitro deubiquitylation assay using ubiquitin-AFC as the substrate confirms the activity of WT USP19. (c) USP19 inhibits luciferase aggregation in vitro. Luciferase incubated with an increased concentration of either WT USP19 or USP19C506S at 42 °C (15min) was subjected to centrifugation. The resulting soluble fractions and a fraction of the samples not exposed to the heat treatment were analysed by immunoblotting with FLAG (USP19) and luciferase antibodies. The numbers indicate band intensity. (d) USP19 preferentially recognizes unfolded luciferase. Luciferase incubated with WT USP19 either on ice or at 42 °C was subjected to centrifugation. The resulting soluble fractions were used for immunoprecipitation by FLAG beads. (e) The N-terminal CS domains are dispensable for recognition of unfolded luciferase. The same as in d, except that all samples were heat-treated and that purified USP19 Δ1–493 was tested together with WT USP19. (f,g) The USP19 N-terminal domain is dispensable for MAPS. Where indicated, different amounts of USP19 WT and USP19 Δ1–493 plasmids were transfected together with either GFP1–10 (f) or Ubl4A–FLAG (g). The numbers show band intensity. Unprocessed original scans of blots are shown in Supplementary Fig. 8.
As the USP domain of USP19 binds Hsp90 (Supplementary Fig. 3a)9, the detected chaperoning activity could be attributed to either Hsp90 or the USP domain itself. To distinguish between these possibilities, we attempted to remove Hsp90 from the purified USP19 USP domain by treating cell extract with nucleotides or high salt before purification. We found that either ADP or salt treatment reduced the interaction of USP19 USP with Hsp90 (Supplementary Fig. 3b), but not with denatured luciferase (Supplementary Fig. 3c). Moreover, the chaperone activity of USP19 could not be abolished by pre-treatment with the Hsp90-specific inhibitor ganetespib (Supplementary Fig. 3d). We therefore concluded that the USP domain of USP19 has an intrinsic chaperone activity capable of sensing the folding state of its client proteins.
USP19 recruits misfolded protein to the ER and an ER-associated domain
Given its ER localization, USP19 might initiate MAPS by first recruiting misfolded proteins to the ER membranes using its chaperone activity. We therefore performed biochemical fractionation using cells expressing GFP and GFP1–10 either alone or together with FLAG–USP19. Indeed, a fraction of the MAPS cargos was detected in the membrane fractions (Fig. 6a, lanes 5–8). Importantly, the level of membrane association correlates with the secretion efficiency: more GFP1–10 bound to the membranes than GFP in both control and USP19-overexpressing cells, and USP19 overexpression enhanced membrane interaction for both GFP and GFP1–10. Moreover, USP19 depletion consistently reduced GFP1–10 in membrane fractions (Fig. 6b). These results establish a USP19-dependent interaction between misfolded polypeptides and the membranes. The remaining association of GFP1–10 with the membranes in USP19-null cells might be mediated by chaperones irrelevant to MAPS.
Figure 6.
USP19 recruits misfolded proteins to the ER and ER-associated late endosomes. (a) USP19 preferentially recruits GFP1–10 to membranes. The cytosol and membrane fractions from HEK293T cells co-transfected with the indicated constructs were analysed by immunoblotting. (b) Knockout of USP19 reduces membrane-associated GFP1–10. Control and USP19 CRISPR (KO) cells were transfected with GFP1–10. After fractionation, GFP1–10 in the membrane and cytosol fractions was analysed by immunoblotting. The graph shows the level of membrane-associated GFP1–10 relative to cytosolic GFP1–10 (mean ± s.e.m., n = 3 independent experiments, ∗∗P < 0.01). (c) USP19 recruits GFP1–10 to the ER membrane. COS7 cells co-transfected with GFP1–10 and FLAG-USP19 were permeabilized, stained with GFP and FLAG antibodies and analysed by confocal microscopy. Bottom panels show the outline of GFP1–10 and USP19 signal in the outlined area. Scale bar, 5 μm. (d,e) A photobleaching-based assay reveals vesicles containing MAPS cargos. (d) A schematic illustration of the photobleaching experiment shown in e. The marked area in d and e was photobleached with a 568nm laser to remove cytosolic and ER-associated mCh–GFP1–10 background. Arrows indicate a few examples of vesicles revealed after photobleaching. Scale bar, 5 μm. (f) MAPS vesicles were labelled with Rab9. Shown are two frames from a live-cell imaging experiment using cells transfected with mCe–USP19, mCi–Rab9, and mCh–GFP1–10 after photobleaching. Panels 4–6 are enlarged views of the indicated area in panel 1 after 20s. (g) Structure-illuminated microscopy analysis of MAPS vesicles. Cells transfected with mCe–USP19, Ubl4A–Venus and mCh–Rab9 were permeabilized, fixed and imaged. Note that Ubl4A–Venus is detected on the membranes of intraluminal vesicles, but not in the lumen of these vesicles. (h) Transmission electron microscopy analyses of MAPS vesicles. COS7 cells expressing FLAG-tagged GFP1–10 were permeabilized, fixed, and stained with anti-FLAG antibodies and immunogold-labelled secondary antibodies. Blue arrows show examples of luminal GFP1–10 signals. Arrowheads show PM-associated GFP1–10. The inset shows an example of GFP1–10 association with the limiting membrane on the luminal side (yellow arrows). Statistics source data for b can be found in Supplementary Table 2. Unprocessed original scans of blots are shown in Supplementary Fig. 8.
To better define the localization of membrane-associated MAPS cargos, we performed immunostaining in COS7 cells using GFP1–10 as a model substrate. To detect membrane-associated GFP1–10, we developed a permeabilization-based staining procedure, which removed cytosolic but not membrane-associated GFP1–10 (Supplementary Video 1). A small fraction of GFP1–10 was also retained in the nucleus owing to the nuclear envelope barrier. Consequently, immunostaining with GFP antibodies showed that the nuclear and cytoplasmic GFP1–10 ratio was reversed after permeabilization (Supplementary Fig. 4a). In cells transfected with GFP1–10, we observed membrane-associated GFP1–10 in ~27% cells, whereas in USP19-co-expressing cells, membrane-associated GFP1–10 was detected in more than 60% cells (Supplementary Fig. 4b). Moreover, membrane-associated GFP1–10 signal was more pronounced in USP19-overexpressing cells than in control cells (Supplementary Fig. 4c,d). Confocal microscopy demonstrated that GFP1–10 co-localized significantly with USP19 and the ER marker PDI (Fig. 6c and Supplementary Fig. 4e–k). Noticeably, in addition to the ER, GFP1–10 was also detected in punctae juxtaposed to the ER (Supplementary Fig. 4e–k).
Encapsulation of MAPS cargos by late endosomes
To characterize the ER-associated domain bearing MAPS cargos, we developed a photobleaching-based live-cell imaging assay (Fig. 6d). As proteins on the ER surface or in the cytosol diffuse freely throughout the cell, if we repeatedly photobleach a fluorescence-labelled MAPS substrate in a large cytoplasmic area, we should deplete cytosolic and ER-associated fluorescence signal throughout the cell. However, for MAPS cargos trapped in the punctae, the signal should remain unaffected as long as these structures stay out of the bleached area. To this end, we tagged USP19 and GFP1–10 with monomeric green fluorescence variants to avoid aggregation caused by GFP dimerization. Immunoblotting showed that mCherry–GFP1–10 (mCh–GFP1–10) was secreted similarly to GFP1–10 (Supplementary Fig. 5a). We then treated cells co-transfected with mCitrine–USP19 (mCi–USP19) and mCh–GFP1–10 with six rounds of photobleaching. Interestingly, as the cytoplasmic mCh–GFP1–10 signal became diminished, punctate structures containing mCh–GFP1–10 became visible (Fig. 6e and Supplementary Video 2). Similar results were observed for Ubl4A bearing a yellow fluorescent tag (Ubl4A–Venus) (see below).
Live confocal microscopy showed that MAPS-associated punctae could undergo fission and tubulation (Supplementary Fig. 5b and Supplementary Videos 3 and 4), revealing them as vesicles (hereafter referred to as MAPS vesicles) rather than protein aggregates. Two-colour confocal microscopy showed that MAPS vesicles exhibited a specific mobility pattern; they seemed to be ‘tied’ to an ER domain for a period of time, and then ‘hopped’ to another region to form contact with another ER domain (Supplementary Video 5). The mobility pattern and the morphology of these vesicles are consistent with the previously reported ER-associated early or late endosomes (LEs)35.
To determine the identity of MAPS vesicles, we imaged cells expressing mCh–GFP1–10 and USP19 together with fluorescence-tagged Rab GTPases that labelled either early (Rab5) or LEs (Rab9). The results showed that mCh–GFP1–10 vesicles co-localized extensively with Rab9-labelled LEs (Fig. 6f), but not with Rab5 (Supplementary Fig. 5c); among 393 mCh–GFP1–10 vesicles (13 cells) counted, 85% were labelled with Rab9. Time-lapse microscopy showed that mCh–GFP1–10 co-migrated with Rab9-containing LEs (Fig. 6f and Supplementary Video 6). A similar observation was made with Ubl4A–Venus (Supplementary Fig. 5d and Supplementary Video 7). When we stained photobleached cells with a LysoTracker dye, only 29% of GFP1–10 vesicles were labelled with LysoTracker (Supplementary Fig. 5e,f), which probably represent acidified LEs. As pre-treatment with lysosome inhibitors did not significantly increase LysoTracker-labelled GFP1–10 vesicles (Supplementary Fig. 5f), it seems that most MAPS vesicles are not bound to lysosomes for degradation.
To determine the topology of MAPS cargos in LEs, we performed super-resolution microscopy, which showed that GFP1–10 was localized either in the lumen or associated with the limiting membranes of LEs (Supplementary Fig. 5g). Occasionally, a MAPS cargo might appear on the membrane of intraluminal vesicles (ILVs), but not in the lumen of these vesicles (Fig. 6g). Transmission electron microscopy analysis confirmed that GFP1–10 was either associated with the limiting membrane from the luminal side or in the LE lumen (Fig. 6h and Supplementary Fig. 5h). These results suggest a translocation mechanism for MAPS cargos to reach the LE lumen.
MAPS does not involve exosomes or autophagosomes
The proposed translocation mechanism sets an important distinction between MAPS and exosome-mediated secretion because the latter involves encapsulation of cargos into ILVs by inward vesicle budding24,25 (Fig. 7a). Accordingly, in exosome-mediated secretion, cargos reside in small extracellular vesicles that can be purified by ultracentrifugation. In contrast, secreted GFP1–10 could not be sedimented by ultracentrifugation (Fig. 7b), further confirming that MAPS is not mediated by exosomes.
Figure 7.
Secretion of misfolded proteins does not involve autophagosomes or exosomes. (a) Two established UPS pathways that involve an intracellular vesicle carrier. (b) GFP1–10 is not secreted by exosomes. Conditioned media (16h) collected from cells transfected with GFP1–10 together with the indicated USP19 variants were subjected to differential centrifugation and immunoblot analysis. S, supernatant; P, pellet. The faint bands in the outlined areas are caused by serum proteins crossreacting with the antibodies. (c,d) Most MAPS vesicles are not autophagosomes. COS7 cells transfected with mCh–GFP1–10 and mCe–USP19 were photobleached, methanol-fixed, and stained with LC3 antibodies. Arrowheads in c,d show examples of MAPS vesicles not labelled by LC3 antibodies. Scale bar, 5 μm. (d) A 3D reconstructed image after z-section confocal analysis. (e) Secretion of Ubl4A–FLAG is not affected by starvation. Cells transfected with the indicated plasmids were either incubated in complete medium or an EBSS starvation medium. Media collected at the indicated time points and lysates prepared at the end of the chase were analysed by immunoblotting. (f,g) GFP1–10 secretion does not require GRASPs. (f) Secretion of GFP1–10 was analysed in cells transfected with GFP1–10 and FLAG–USP19 together with the indicated siRNAs. Knockdown of GRASP65 was confirmed by immunoblotting. (h) The same as in g, except that GRASP55 and Tsg101 were included and that gene knockdown was confirmed by quantitative PCR with reverse transcription (indicated in green labels). Unprocessed original scans of blots are shown in Supplementary Fig. 8.
As it was recently reported that IL1β secretion involves translocation of IL1β into autophagosomes36, we wished to determine whether MAPS vesicles are truly LEs or Rab9-positive autophagosomes. If the second scenario was true, MAPS might use an autophagy-mediated UPS mechanism to export misfolded cargos. However, several lines of evidence argue against this model. First, confocal microscopy showed that most MAPS vesicles are not positive for the autophagosome marker LC3 (Fig. 7c,d). Second, unlike IL1β whose secretion is stimulated by autophagy induction36, secretion of GFP1–10 and Ubl4A–FLAG was not significantly affected by starvation (Fig. 7e and Supplementary Fig. 6a). USP19-induced MAPS was also not inhibited by the autophagy inhibitor 3-methyladenine (Supplementary Fig. 6b,c). Last, autophagosome-dependent secretion requires the Golgi protein GRASP and the ESCRT-I component Tsg10136–38, but knockdown of GRASP65 or GRASP55, the two mammalian GRASP homologues, or of Tsg101 did not affect GFP1–10 secretion (Fig. 7f,g). These results collectively establish MAPS as a previously unknown UPS pathway.
Misfolded proteins are secreted through late endosomes
To examine whether LEs are important for MAPS, we quantified the number of MAPS vesicles in cells expressing mCherry or mCh–GFP1–10. More mCh–GFP1–10 vesicles were found in cells than mCherry vesicles (Fig. 8a,c). We also analysed GFP1–10-containing vesicles in cells expressing different USP19 variants: WT USP19, but not USP19C506S or USP191–1290, significantly increased the number of GFP1–10 vesicles (Fig. 8b,c). The correlation between the number of MAPS vesicles and the secretion efficiency suggests that these vesicles play a significant role in MAPS.
Figure 8.
Secretion of misfolded proteins through late endosomes. (a–c) The number of MAPS vesicles in cells correlates with the secretion efficiency. (a) Representative cells transfected with mCherry or mCh–GFP1–10 before and after photobleaching. (b) Representative cells transfected with mCh–GFP1–10 together with either mCi–USP19 WT or the mCi–USP19C506S (CS) mutant. (c) Quantification of MAPS vesicles in photobleached COS7 cells that were transfected as indicated. (d) Confocal analysis of v-SNAREs in live cells transfected with mCh–Rab9 and the indicated EGFP-tagged v-SNAREs. (e) Expression of late endosome-localized v-SNAREs promotes GFP1–10 secretion. Secretion of GFP1–10 was analysed in cells transfected with GFP1–10 and the indicated EGFP–v-SNAREs. (f) The graph shows the quantification result from experiments represented in e (mean ± s.e.m., n=3 independent experiments, ∗P <0.05). (g) A schematic illustration of the MAPS pathway. Statistics source data for f can be found in Supplementary Table 2. Unprocessed original scans of blots are shown in Supplementary Fig. 8.
If MAPS is caused by fusion of MAPS vesicles with the PM, increasing the concentration of a fusion machinery factor such as v-SNAREs on these vesicles might promote secretion. We therefore screened a collection of v-SNAREs using confocal microscopy. The results confirmed that VAMP7 and VAMP8 are LE-localized v-SNAREs whereas VAMP2, 3, 4 are localized to other vesicles (Fig. 8d and Supplementary Fig. 7a). Consistent with our model, overexpression of either VAMP7 or VAMP8 but not other VAMPs induced GFP1–10 secretion by ~5-fold (Fig. 8e,f). Additionally, the LE–PM fusion model predicted that cargos associated with the limiting membranes of LEs should diffuse to the cell surface before their release into the medium (Supplementary Fig. 7b). Indeed, live-cell staining detected cell surface-bound GFP1–10, which was increased by USP19 but not by USP19C506S (Supplementary Fig. 7c). Altogether, these results strongly indicate that MAPS is achieved by a unique UPS mechanism that involves LEs as a carrier.
DISCUSSION
Our study uncovers a UPS pathway that targets misfolded proteins for secretion. In this process, USP19 recruits aberrant polypeptides to the ER, which presumably facilitates proximity-based transfer of cargos to LEs because >99% of LEs form tight association with the ER35. Misfolded proteins are secreted to the extracellular milieu when LEs fuse with the PM (Fig. 7g). This pathway is distinct from exosome-mediated secretion because the latter involves inward vesicle budding to form cargo-bearing ILVs, which later become exosomes. The MAPS pathway is also different from autophagy-mediated secretion because secretory LEs do not contain LC3, and because MAPS is not affected by changes in autophagic flow.
In previously established UPS pathways, the mechanism of cargo sorting is unclear. Although a few misfolded proteins were reported to enter exosomes, most exosome cargos are folded polypeptides. Thus, this pathway cannot distinguish misfolded proteins from native ones.
In contrast, MAPS uses a chaperone activity in USP19 to initiate secretion, illustrating a straightforward cargo sorting mechanism. This not only explains the substrate specificity of MAPS, but also establishes MAPS as an unprecedented PQC mechanism.
The precise fate of secreted MAPS substrates remains unclear. We speculate that in a multicellular organism, they may be internalized by neighbouring cells and ultimately degraded. The establishment of α-synuclein as a MAPS substrate supports this notion because it is known that α-synuclein can be released from one area of the brain and taken up by other cells through clathrin-mediated endocytosis, and this prion-like behaviour is thought to contribute to α-synucleinopathies39–41. Our finding that USP19 promotes α-synuclein secretion not only suggests a physiological link between MAPS and protein-misfolding disease, but also provides a potential target for therapeutic intervention in α-synuclein propagation and α-synucleinopathies.
Why cells need a mechanism besides proteasomal degradation to remove misfolded proteins is unclear, particularly as the contribution from MAPS is limited compared with the proteasome. One possible explanation is that building long ubiquitin chains required for proteasome degradation may not be efficient for misfolded proteins given the anticipated transient interactions between PQC ubiquitin ligases and misfolded clients and also because of promiscuous deubiquitylation in cells housing many deubiquitylases. In this regard, the MAPS pathway might be required to remove substrates with defective degradation signals. Alternatively, the proteasome function is often overwhelmed by acute protein-misfolding stress42–44, necessitating additional stress-relieving pathways. Sequestration of misfolded proteins in aggregates for autophagic clearance might be an alternative mechanism to minimize damage by protein misfolding, but this strategy could lead to co-depletion of essential cellular factors trapped in aggregates. In contrast, MAPS is highly selective for misfolded proteins. The observation that MAPS becomes critically essential only when the proteasome function is impaired is analogous to that during adaptation to ER stress—the ER PQC mechanism endoplasmic-reticulum-associated protein degradation is dispensable unless ER homeostasis is overwhelmingly disrupted45. Thus, MAPS might be an unprecedented stress-relieving mechanism that subsidizes the proteasome to minimize protein misfolding in mammalian cells.
METHODS
Cell lines, siRNAs, and plasmids.
The HEK293T, HeLa and COS7 cells were purchased from ATCC. These cells were not authenticated or tested for mycoplasma contamination recently. No cell lines used in this study were found in the database of commonly misidentified cell lines that is maintained by ICLAC and NCBI Biosample. The cells were maintained in DMEM medium (Corning cellgro) containing 10% fetal bovine serum and penicillin–streptomycin (10 units ml−1). Cells recovered from liquid nitrogen freezing were maintained for at least 2 weeks before being used in the secretion experiments. Mammalian expression constructs for USP7, wild-type USP19, USP19C506S, USP191–493 and USP191–1290 were described previously9. The USP19494–1318 construct was generated by ligating the PCR-amplified cDNA fragment encoding residues 494 to 1318 of human USP19 isoform 1 into the SalI and NotI sites of the pRK vector. pCMV6–Ubl4A–DDK and pCMV6–α-synuclein–DDK were purchased from Origene. pEGFP–C1 and pCMV–mGFP1–10 plasmids were purchased from Clontech and Sandia Biotech, respectively. The mCerulean–C1 (mCe–C1) (Addgene 54592), mCitrine–C1 (mCi–C1) (Addgene 54587), mCherry–C1 (mCh–C1) (Addgene 54563), mCe–Rab5 (Addgene 55388) and mCe–Rab7 (Addgene 55389) were a gift from M. Davidson. The pEGFP–VAMP2 (Addgene 42308), pEGFP–VAMP3 (Addgene 42310), pEGFP–VAMP4 (Addgene 42313), pEGFP–VAMP7 (Addgene 42316) and pEGFP–VAMP8 (Addgene 42311) plasmids were a gift from T. Galli. mCerulean–C1, mCitrine–C1 and mCherry–C1 were used for tagging monomeric fluorescence proteins to wild-type USP19, USP19C506S, USP19 1–1290, GFP1–10 and Rab9. Rab9 cDNA was PCR amplified from GFP–Rab9 WT (Addgene 12663, gift from R. Pagano) and inserted into these vectors. FLAG–GFP1–10 plasmid was generated by ligating the PCR fragment from pCMV–mGFP1–10 plasmids into the pRK vector. pX330–U6–Chimeric_ BB–CBh–hSpCas9 was a gift from F. Zhang (Addgene 42230). The D10A mutation in CAS9 was introduced by site-directed mutagenesis. siRNAs for gene knockdown and corresponding control siRNA were purchased from Invitrogen. The targeting sequences for USP19 are:
siRNA-1: 5′-GGCGUGACAAGAUCAAUGA-3′;
siRNA-2: 5′-CCAGAGUUGUUGCUCGAUU-3′;
siRNA-3: 5′-CCAUCACUUUUGACCCGUU-3′.
To generate USP19 CRISPR knockout cell lines, we constructed two RNA-CAS9 guide constructs on the basis of a published protocol33. Briefly, oligonucleotides targeting two sequences near the translation starting site of the USP19 gene were designed. The primer sequences are:
Target 1, forward primer: 5′-caccgAGAGCAAGGATGGAGATCCT-3′;
Target 1, reverse primer: 5′-aaacAGGATCTCCATCCTTGCTCTc-3′;
Target 2, forward primer: 5′-caccgCTTCTGCTTCTTCTTACTAG-3′;
Target 2, reverse primer: 5′-aaacCTAGTAAGAAGAAGCAGAAGc-3′.
Each pair of oligonucleotides (10 mM) in distilled water was heated at 95 °C for 5 min and annealed by ramping down the temperature from 95 °C to 25 °C at 5 °C min−1. The annealed oligonucleotides were ligated into pX330–hSpCas9n containing the D10A mutation using the BbsI ligation sites. The two USP19 targeting constructs were co-transfected into HEK293T cells. At 24 h post-transfection, cells were diluted and seeded into in a 96-well plate at <1 cell per well. Clones derived from single cells were obtained and screened for USP19 deficiency by immunoblotting. Transfection was performed with TransIT-293 (Mirus) for HEK293T cells, and with Lipofectamine2000 (Invitrogen) for COS7 and HeLa cells. Lipofectamine2000 was also used according to the manufacturer’s protocol in all gene silencing experiments.
Antibodies, chemicals and proteins.
The antibody for USP19 was raised against recombinant GST–USP19 1–493 protein and affinity-purified GFP, Bag6, Ubl4A and H2A antibodies were described previously46. Commercial antibodies used in this study are listed below. MG132, luciferase and brefeldin A were purchased from EMD Bioscience, Sigma and Cell Signaling, respectively. 3-Methyladenine was purchased from Sigma and reconstituted in pre-warmed pure water at 100 mM before use. Earle’s balanced salt solution (EBSS) was purchased from Invitrogen. Commercial antibodies are listed in Supplementary Table 1.
Protein expression and purification.
The purification of recombinant USP19 and various USP19 mutants was described previously47. Briefly, HEK293T cells (3 × 106) were transfected with 5 μg plasmids expressing the indicated proteins. Cells were grown for 72 h and then collected. Cell lysates were prepared in the buffer LNP (50 mM Tris-HCl pH 7.4, 150 mM sodium chloride, 2 mM magnesium chloride, 0.5% NP40) containing 1 mM dithiothreitol (DTT) and a protease inhibitor cocktail. Following centrifugation at ×16,100g for 10 min, the cleared cell extract was incubated with FLAG M2 beads (Sigma) and the bound materials were extensively washed with buffer WNP (50 mM Tris-HCl pH 7.4, 150 mM sodium chloride, 2 mM magnesium chloride, 0.5% NP40) and then once with buffer PB (25 mM Hepes pH 7.2, 115 mM potassium acetate, 5 mM sodium acetate, 2.5 mM magnesium acetate, 1 mM DTT). The bound materials were eluted using 0.2 mg ml−1 3 × FLAG peptide (Sigma) in the buffer PB.
Immunoblotting, protein level measurements and statistical analyses.
Immunoblottings were performed using the standard protocols. To quantify GFP or GFP1–10 secreted into the media without immunoprecipitation, HRP-conjugated secondary antibodies were used. Immunoblotting signal was detected by the enhanced chemiluminescence method (ECL) and recorded by a Fuji LAS-4000 imager. The intensity of the detected protein bands was quantified by ImageGauge v3.0. Protein secretion efficiency was determined by normalizing the level of secreted proteins by the amount of expressed proteins in cell lysates. For other immunoblotting quantifications, fluorescent-labelled secondary antibodies were used. Immunoblots were scanned by a LI-COR Odyssey scanner, and the intensity of protein bands was determined by the Odyssey software. Green fluorescence intensity in cells was measured in phosphate-buffered saline (PBS) using a Cytomics FC500 FACS instrument (Beckman Coulter).
Crosslinking, immunoprecipitation under denaturing and native conditions.
To detect ubiquitin conjugates on misfolded GFP1–10, HEK293T cells (~0.6 × 106) were seeded and grown for 24 h, and then transfected with 400 ng pCMV–HA–Ub, and 300 ng pCMV–GFP1–10 together with either WT USP19 or the catalytically inactive USP19 mutant or control plasmids (400 ng). Cells were collected 24 h post-transfection and lysed in 150 μl buffer D (1% SDS, 5 mM DTT). Cells were immediately heated at 95 °C for 10 min to disrupt protein complexes. Cell extract was diluted tenfold with the buffer LNP containing a protease inhibitor cocktail. After centrifugation at ×16,100g for 10 min to remove insoluble materials, cleared cell lysates were subjected to immunoprecipitation with anti-GFP antibodies.
To detect the interaction of USP19 with MAPS substrates in cells, HEK293T cells (~1.5 ×105) were seeded and grown for 24 h, and then transfected with 500 ng plasmid expressing the misfolded proteins indicated in the figure legends together with 500 ng plasmid expressing either WT or mutant USP19 proteins as specified in the figure legends. Cells were collected 24 h post-transfection and lysed in 350 μl buffer LCHAPS containing 1% CHAPS, 30 mM Tris/HCl pH 7.4, 150 mM potassium acetate, 4 mM magnesium acetate, 1 mM DTT, and a protease inhibitor cocktail. Cleared cell lysates were incubated with FLAG M2 beads to precipitate FLAG-tagged proteins. Immunoprecipitated protein complexes were washed two times with the buffer WCHAPS (0.1% CHAPS, 30 mM Tris/HCl pH 7.4, 150 mM potassium acetate, 4 mM magnesium acetate), and then eluted with the Laemmli buffer for immunoblotting.
To detect the interaction of MAPS substrates with endogenous USP19, cells (1.5 × 106) transfected with 6 μg of GFP1–10 or Ubl4A–FLAG were washed with PBS and then treated with 0.05% formaldehyde at 4 °C for 20 min. Cells were washed with PBS twice and then lysed in 500 μl of the buffer WNP. The cell lysates were subjected to immunoprecipitation with FLAG M2 beads (Sigma). The precipitated proteins were washed with the buffer WNP two times and then eluted with the Laemmli buffer. Samples were heated at 95 °C for 30 min to break the crosslinker before SDS–PAGE analysis.
To analyse the interaction of purified USP19 with luciferase in vitro, luciferase 200 nM was incubated with 400 nM wild-type USP19 or the indicated USP19 mutants in buffer PB either on ice or at 42 °C for 12 min. The samples were subjected to centrifugation at ×20,000g for 10 min to remove insoluble materials. The soluble supernatant fractions were diluted threefold with the buffer LNP and then subjected to immunoprecipitation with FLAG M2 beads. The precipitated protein complexes were washed with the buffer WNP two times and analysed by SDS–PAGE.
Proteinsecretionexperiments.
To measure the secretion of misfolded GFP1–10 and other model substrates, cells (0.8 × 105) were seeded and grown for 24 h, and then transfected with 250 ng plasmids expressing the indicated MAPS substrates together with 250 ng plasmids expressing the indicated deubiquitylases or control. At 24 h post-transfection, we replaced the medium with 2.5 ml fresh DMEM medium. Cells were grown for another 16 h before the conditioned medium was collected. The medium was subjected to sequential centrifugation, first at ×1,000g for 5 min to remove contaminated cells, and then at ×10,000g for 30 min to remove cell debris. The cells were lysed in 150 μl buffer LNP. A fraction of the cell lysates and medium was then analysed directly by SDS–PAGE and immunoblotting. To knock down USP19, cells (0.2 × 106) were seeded and grown for 24 h. Cells were transfected with plasmid expressing GFP1–10 together with 100 pmol siRNA in the presence of 1.5 ml medium. Cells were maintained for 60 h to deplete endogenous USP19. Cells were then incubated with fresh medium for another 16 h before the medium was collected for analysis of protein secretion. Secreted proteins in the medium were detected by immunoblotting.
To measure the kinetics of protein secretion, cells were seeded in poly-lysine D-coated 6-well plates at 0.4 × 106 per well. After 24 h, cells were transfected with 1 μg plasmid encoding a MAPS cargo together with 1 μg of another plasmid as indicated in the figure legends. At 36 h post-transfection, the cells were washed two times with complete DMEM medium, and then incubated with 2.0 ml complete medium at 37 °C. Medium collected at the different time points was subjected to sequential centrifugation, first at ×1,000g for 5 min to remove contaminated cells, and then at ×10,000g for 30 min to remove cell debris. The resulting supernatant fractions were analysed directly by immunoblotting. For drug treatment experiments, chemical inhibitors were added at the beginning of the chase at the concentrations indicated in the figure legends.
Biochemical fractionation experiments.
HEK293T cells (1 × 107) were collected and washed with ice-cold PBS. Cells were then treated with 800 μl buffer LHP (10 mM Tris-HCl pH 7.4, 10 mM potassium chloride, 2 mM magnesium chloride, 1 mM DTT) containing a protease inhibitor cocktail on ice for 10 min before being homogenized by a dounce homogenizer. Sucrose was added to 250 mM to prevent damage of subcellular organelles and membrane vesicles. Homogenized cells were subjected to centrifugation at ×1,000g for 5 min to remove unbroken cells and nuclei. The supernatant fractions were further centrifuged at ×100,000g for 30 min to sediment total microsome membrane vesicles. The membranes were washed with the PB buffer containing 250 mM sucrose before further fractionation or protease treatment analyses.
Cell permeabilization and immunostaining.
Cells expressing GFP1–10 either alone or together with USP19 were washed with ice-cold PBS and then treated with the PB buffer containing 0.055% digitonin and 1 mM DTT plus a proteasome inhibitor cocktail for 30 s. Cells were washed with PBS extensively and then fixed with 2% paraformaldehyde. Fixed cells were washed and stained with the antibodies as indicated in the figure legends in PBS containing 5% fetal bovine serum and 0.2% saponin. Cells were imaged on an LSM 880 confocal microscope equipped with an Airyscan detector array and running ZEN2 software (Carl Zeiss Microscopy). The 32-channel array of GaAsP detectors was configured so that 0.2 Airy units covered each detector in the array. The objective lens used was the Zeiss Plan-Apo 63×/1.4 Oil DIC. Z-stacks were acquired sequentially for each fluorescence colour, while sharing the MBS 488/561/633 dichroic. For Alexa 488 fluorescence: excited by the 488 nm argon laser line at 0.9%; emission filtered by BP 495–550. For Alexa 594 fluorescence: excited by the 561 nm DPSS laser at 1%; emission filtered by LP 570. Z-stacks were acquired with the following settings: pixel size = 0.043 μm; dwell time per pixel = 1.09 μs; line ave. = 1; distance between slices = 0.185 μm. Post-processing with ZEN2 software provided online reassignment of pixel data (Sheppard Sum) followed by linear deconvolution to enhance SNR and resolution.
To quantify cells with membrane-associated GFP1–10 (Supplementary Fig. 5b), COS7 cells were transfected with either GFP1–10 alone or GFP1–10 together with FLAG–USP19 for 24 h. After permeabilization, cells were fixed, stained by GFP and FLAG antibodies, and then imaged by a Zeiss Axiovert fluorescence microscope using a 63 × oil immersion Plan-Apochromat objective (NA 1.4). For the experiment shown in Supplementary Fig. 5i–k, cells transfected with mCh–GFP1–10 and mCi–USP19 were permeabilized and directly imaged without fixation by a Zeiss LSM780 confocal microscope.
Forcellsurfacestaining,cellswereincubatedwithaffinity-purifiedGFPantibody in PBS at 4 °C for 90 min. After three washes with PBS, cells were incubated with fluorescence-conjugated secondary antibodies in the same buffer for 90 min at 4 °C. After three washes, cells were immediately imaged by a Zeiss LSM780 confocal microscope.
Live confocal microscopy and SIM imaging.
To characterize the ER-associated vesicles that contain MAPS cargos, COS7 cells were seeded at 1.5 × 105 per well in a 35 mm u-dish coated with fibronectin (ibidi GmbH, Germany). A total of 1 μg plasmid (700 ng MAPS cargos, 200 ng USP19 variants, 10 ng Rabs) was transfected into cells using Lipofectamine2000 following the manufacturer’s instructions.
Photobleaching and live confocal microscopy were performed 24 h post-transfection. For live-cell imaging, cells were incubated with phenol red-free MEM medium at 37 °C in a live-cell incubation chamber. Cells were illuminated with a 568 nm laser at maximum intensity for 6 rounds. Photobleached area is approximately 1/4 or 1/3 of the total cytoplasmic region, usually on the side where few endosome vesicles are present. Live-cell imaging was performed using an LSM 780 confocal microscope. The pin hole was set so the section thickness is about 1 μm, allowing continuous focus on cell peripheral regions where the cytoplasm is thin. To inhibit lysosomal degradation, cells were pre-treated with leupeptin 125 μM (Sigma L5793) together with a lysosomal protease inhibitor cocktail(SigmaP8340,1:100dilution)for30 minbeforeimaging.Tostainlysosomes, LysoTracker blue (Invitrogen, L-7525) was used to stain the cells for 5 min according to the instructions from the manufacturer. Images were acquired using Zen (Zeiss) and processed using Photoshop (Adobe). Supplementary videos were generated using ImageJ. For SIM microscopy, COS7 cells were permeabilized with digitonin and fixed with 2% paraformaldehyde, and stained with anti-Rab9 antibodies (Cell Signaling). Images were acquired with a DeltaVision OMX SIM system (GE).
To examine co-localization of MAPS cargos with the autophagosome marker LC3, live cells (20) were first photobleached and then fixed with methanol/acetone at 1:1 ratio at −20 °C for 10 min. The cells were then stained with LC3 antibodies at 1:300 in PBS containing 5% fetal bovine serum and 0.2% saponin.
Immunogold labelling and electron microscopy analysis.
Cells transfected with GFP1–10–FLAG together with Myc–USP19-WT were permeabilized with digitonin and fixed with PBS containing 2% paraformaldehyde and 0.1% glutaraldehyde for 1 h at 25 °C. After extensive washing, cells were treated with a PBS-based staining solution containing 5% fetal bovine serum and 0.2% saponin for 1 h at 25 °C, and then stained with anti-FLAG M2 monoclonal antibodies (Sigma) at 1:400 dilution in the same solution overnight at 4 °C. Cells were washed, and stained with immunogold-labelled secondary antibodies (Nanoprobes) at 1:50 dilution overnight at 4 °C. After rinsing with PBS, samples were fixed with 2% glutaraldehyde for 20 min at 25 °C. The samples were washed with PBS first and then with ultrapure water. Silver enhancement was then performed using a kit (Nanoprobes) for 7 min following the instructions from the manufacturer. The reactions were stopped by washing with water. The samples were dehydrated, embedded, and processed following a standard electron microscopy protocol.
The stress recovery assay.
CRISPR cells of different genotypes were seeded at 0.5×106 ml−1 in a 12-well plate and treated with MG132 (5 μM) for 15 h, or tunicamycin (1 μg ml−1) for 18 h, or DTT (5 mM) for 8 h. Cells were collected and washed with a drug-free medium once. Approximately 20,000 inhibitor-treated or 1,000 DMSO-treated control cells were seeded in a poly-lysine D-coated plate. After incubation in a tissue culture incubator for two weeks, cells were fixed in 10% formalin for 5 min at 25 °C and then stained with 0.05% crystal violet in distilled water for 30 min. Cells were gently washed with distilled water twice and dried. The plates were scanned by a LI-COR Odyssey scanner. The cell clone number was determined by measuring the total stained signal using the Odyssey software.
Statistics and reproducibility.
All experiments were repeated at least twice with a representative gel being shown. Where indicated, the n values in the figure legend indicate the number of independent experiments conducted for each experiment shown, error bars show mean ± s.e.m., and P values were calculated using paired Student’s t-test.
Supplementary Material
ACKNOWLEDGEMENTS
We thank X. Wu (NHLBI) and J. Reece (NIDDK) for assistance with microscopy analyses, M. Davidson (Florida State University, USA), R. Pagano (Mayo Clinic, USA), T. Galli (Institut Jacques Monod, France) and F. Zhang (Broad Institute, USA) for plasmids, and W. Prinz, H. Bernstein and M. Gellert (NIDDK) for critical reading of the manuscript. This research is supported by the Intramural Research Program of the National Institute of Diabetes, Digestive & Kidney Diseases and of the National Eye Institute in the National Institutes of Health.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
METHODS
Methods, including statements of data availability and any associated accession codes and references, are available in the online version of this paper.
Supplementary Information is available in the online version of the paper
Data availability.
Source data for Fig. 1d, Fig. 2c,i, Fig. 3b,e,h,j, Fig. 4g,j, Fig. 6b and Fig. 8f, and Supplementary Fig. 1d and Supplementary Fig. 6a have been provided as Supplementary Table 2. All other data supporting the findings of this study are available from the corresponding author on request.
References
- 1.Wolff S, Weissman JS & Dillin A. Differential scales of protein quality control. Cell 157, 52–64 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Bukau B, Weissman J. & Horwich A. Molecular chaperones and protein quality control. Cell 125, 443–451 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Sontag EM, Vonk WI & Frydman J. Sorting out the trash: the spatial nature of eukaryotic protein quality control. Curr. Opin. Cell Biol. 26, 139–146 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchberger A, Bukau B. & Sommer T. Protein quality control in the cytosol and the endoplasmic reticulum: brothers in arms. Mol. Cell 40, 238–252 (2010). [DOI] [PubMed] [Google Scholar]
- 5.Chen B, Retzlaff M, Roos T. & Frydman J. Cellular strategies of protein quality control. Cold Spring Harb. Perspect. Biol. 3, a004374 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clague MJ et al. Deubiquitylases from genes to organism. Physiol. Rev. 93, 1289–1315 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Eletr ZM & Wilkinson KD Regulation of proteolysis by human deubiquitinating enzymes. Biochim. Biophys. Acta 1843, 114–128 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hassink GC et al. The ER-resident ubiquitin-specific protease 19 participates in the UPR and rescues ERAD substrates. EMBO Rep. 10, 755–761 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JG, Kim W, Gygi S. & Ye Y. Characterization of the deubiquitinating activity of USP19 and its role in endoplasmic reticulum-associated degradation. J. Biol. Chem. 289, 3510–3517 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiles B. et al. USP19 deubiquitinating enzyme inhibits muscle cell differentiation by suppressing unfolded-protein response signaling. Mol. Biol. Cell 26, 913–923 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mei Y, Hahn AA, Hu S. & Yang X. The USP19 deubiquitinase regulates the stability of c-IAP1 and c-IAP2. J. Biol. Chem. 286, 35380–35387 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu Y. et al. USP19 deubiquitinating enzyme supports cell proliferation by stabilizing KPC1, a ubiquitin ligase for p27Kip1. Mol. Cell Biol. 29, 547–558 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Combaret L. et al. USP19 is a ubiquitin-specific protease regulated in rat skeletal muscle during catabolic states. Am. J. Physiol. Endocrinol. Metab. 288, E693–E700 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Knowles TP, Vendruscolo M. & Dobson CM The amyloid state and its association with protein misfolding diseases. Nat. Rev. Mol. Cell Biol. 15, 384–396 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Ryno LM, Wiseman RL & Kelly JW Targeting unfolded protein response signaling pathways to ameliorate protein misfolding diseases. Curr. Opin. Chem. Biol. 17, 346–352 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hetz C. & Mollereau B. Disturbance of endoplasmic reticulum proteostasis in neurodegenerative diseases. Nat. Rev. Neurosci. 15, 233–249 (2014). [DOI] [PubMed] [Google Scholar]
- 17.Brundin P, Melki R. & Kopito R. Prion-like transmission of protein aggregates in neurodegenerative diseases. Nat. Rev. Mol. Cell Biol. 11, 301–307 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baixauli F, Lopez-Otin C. & Mittelbrunn M. Exosomes and autophagy: coordinated mechanisms for the maintenance of cellular fitness. Front. Immunol. 5, 403 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohamed NV, Herrou T, Plouffe V, Piperno N. & Leclerc N. Spreading of tau pathology in Alzheimer’s disease by cell-to-cell transmission. Eur. J. Neurosci. 37, 1939–1948 (2013). [DOI] [PubMed] [Google Scholar]
- 20.Saman S. et al. Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J. Biol. Chem. 287, 3842–3849 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nickel W. & Rabouille C. Mechanisms of regulated unconventional protein secretion. Nat. Rev. Mol. Cell Biol. 10, 148–155 (2009). [DOI] [PubMed] [Google Scholar]
- 22.Lo Cicero A, Stahl PD & Raposo G. Extracellular vesicles shuffling intercellular messages: for good or for bad. Curr. Opin. Cell Biol. 35, 69–77 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Rabouille C, Malhotra V. & Nickel W. Diversity in unconventional protein secretion. J. Cell Sci. 125, 5251–5255 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Zhang M. & Schekman R. Cell biology. Unconventional secretion, unconventional solutions. Science 340, 559–561 (2013). [DOI] [PubMed] [Google Scholar]
- 25.Nickel W. Pathways of unconventional protein secretion. Curr. Opin. Biotechnol. 21, 621–626 (2010). [DOI] [PubMed] [Google Scholar]
- 26.Malhotra V. Unconventional protein secretion: an evolving mechanism. EMBO J. 32, 1660–1664 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steringer JP, Muller HM & Nickel W. Unconventional secretion of fibroblast growth factor 2—A novel type of protein translocation across membranes? J. Mol. Biol. 427, 1202–1210 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Fujiwara T, Oda K, Yokota S, Takatsuki A. & Ikehara Y. Brefeldin A causes disassembly of the Golgi complex and accumulation of secretory proteins in the endoplasmic reticulum. J. Biol. Chem. 263, 18545–18552 (1988). [PubMed] [Google Scholar]
- 29.Baneyx F. & Mujacic M. Recombinant protein folding and misfolding in Escherichia coli. Nat. Biotechnol. 22, 1399–1408 (2004). [DOI] [PubMed] [Google Scholar]
- 30.Lee JG & Ye Y. Bag6/Bat3/Scythe: a novel chaperone activity with diverse regulatory functions in protein biogenesis and degradation. BioEssays 35, 377–385 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsunemi T, Hamada K. & Krainc D. ATP13A2/PARK9 regulates secretion of exosomes and α-synuclein. J. Neurosci. 34, 15281–15287 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hasegawa T. et al. The AAA-ATPase VPS4 regulates extracellular secretion and lysosomal targeting of α-synuclein. PLoS ONE 6, e29460 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ran FA et al. Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell 154, 1380–1389 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagano Y. et al. Siah-1 facilitates ubiquitination and degradation of synphilin-1. J. Biol. Chem. 278, 51504–51514 (2003). [DOI] [PubMed] [Google Scholar]
- 35.Friedman JR, Dibenedetto JR, West M, Rowland AA & Voeltz GK Endoplasmic reticulum-endosome contact increases as endosomes traffic and mature. Mol. Biol. Cell 24, 1030–1040 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang M, Kenny SJ, Ge L, Xu K. & Schekman R. Translocation of interleukin-1β into a vesicle intermediate in autophagy-mediated secretion. eLife 4, e11205 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kinseth MA et al. The Golgi-associated protein GRASP is required for unconventional protein secretion during development. Cell 130, 524–534 (2007). [DOI] [PubMed] [Google Scholar]
- 38.Duran JM, Anjard C, Stefan C, Loomis WF & Malhotra V. Unconventional secretion of Acb1 is mediated by autophagosomes. J. Cell Biol. 188, 527–536 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hansen C. et al. α-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J. Clin. Invest. 121, 715–725 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oh SH et al. Mesenchymal stem cells inhibit transmission of α-synuclein by modulating clathrin-mediated endocytosis in a Parkinsonian model. Cell Rep. 14, 835–849 (2016). [DOI] [PubMed] [Google Scholar]
- 41.Desplats P. et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of α-synuclein. Proc. Natl Acad. Sci. USA 106, 13010–13015 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vernace VA, Arnaud L, Schmidt-Glenewinkel T. & Figueiredo-Pereira ME Aging perturbs 26S proteasome assembly in Drosophila melanogaster. FASEB J. 21, 2672–2682 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andersson V, Hanzen S, Liu B, Molin M. & Nystrom T. Enhancing protein disaggregation restores proteasome activity in aged cells. Aging 5, 802–812 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bence NF, Sampat RM & Kopito RR Impairment of the ubiquitin-proteasome system by protein aggregation. Science 292, 1552–1555 (2001). [DOI] [PubMed] [Google Scholar]
- 45.Tsai B, Ye Y. & Rapoport TA Retro-translocation of proteins from the endoplasmic reticulum into the cytosol. Nat. Rev. Mol. Cell Biol. 3, 246–255 (2002). [DOI] [PubMed] [Google Scholar]
- 46.Wang Q. et al. A ubiquitin ligase-associated chaperone holdase maintains polypeptides in soluble states for proteasome degradation. Mol. Cell 42, 758–770 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee JG, Baek K, Soetandyo N. & Ye Y. Reversible inactivation of deubiquitinases by reactive oxygen species in vitro and in cells. Nat. Commun. 4, 1568 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Source data for Fig. 1d, Fig. 2c,i, Fig. 3b,e,h,j, Fig. 4g,j, Fig. 6b and Fig. 8f, and Supplementary Fig. 1d and Supplementary Fig. 6a have been provided as Supplementary Table 2. All other data supporting the findings of this study are available from the corresponding author on request.