Abstract
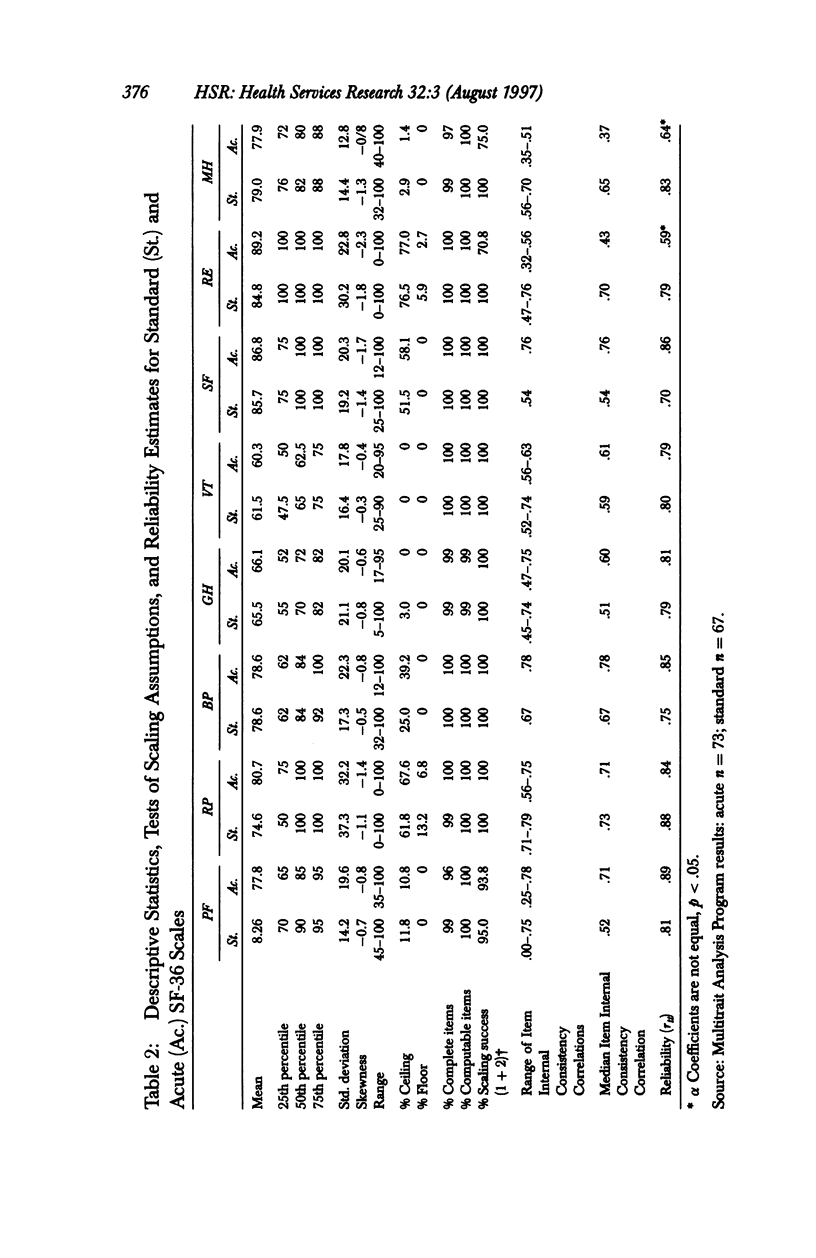
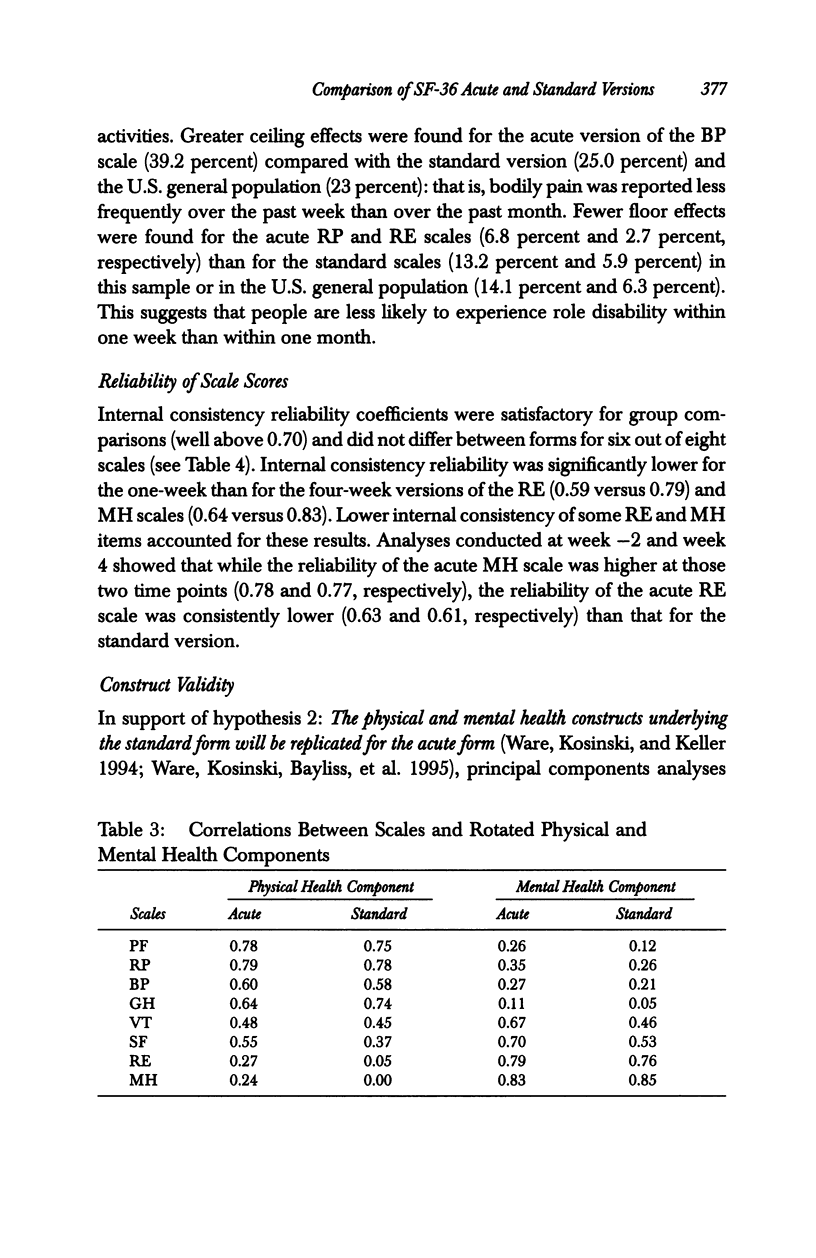
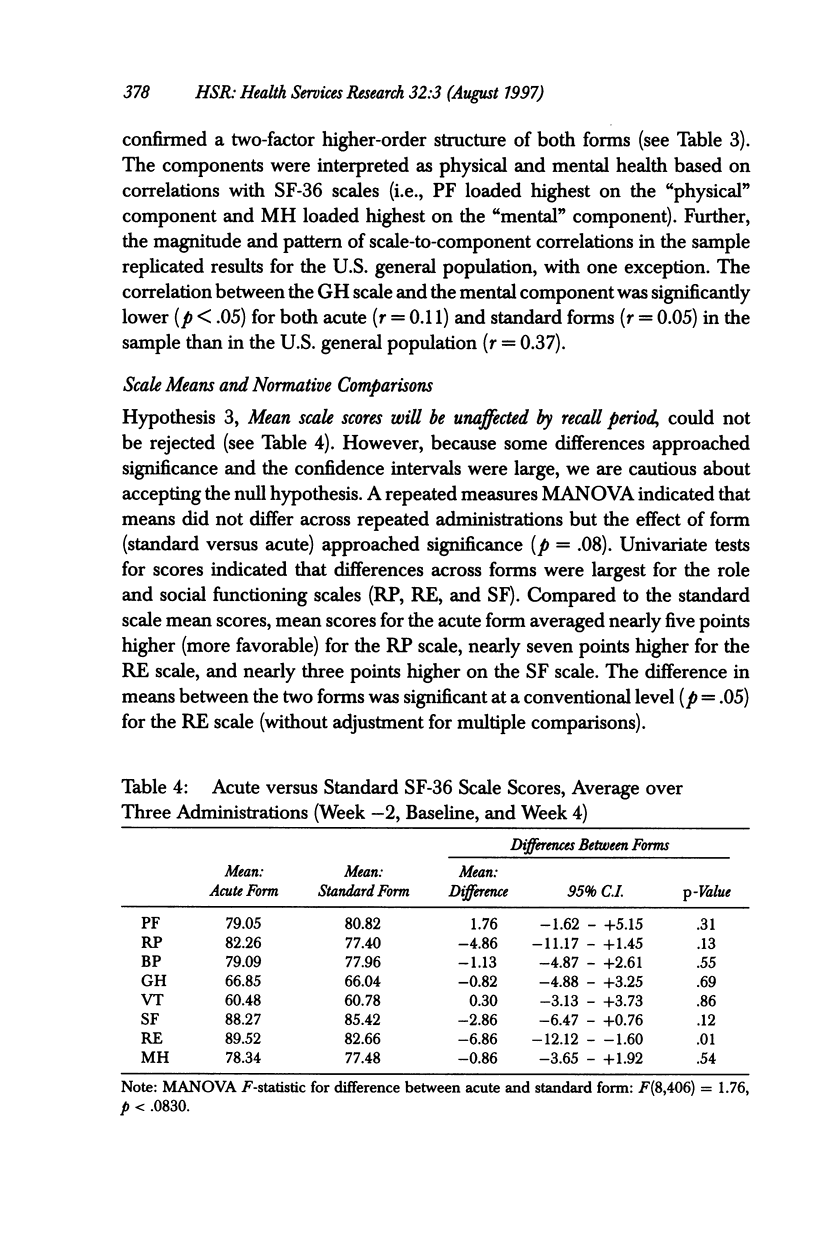
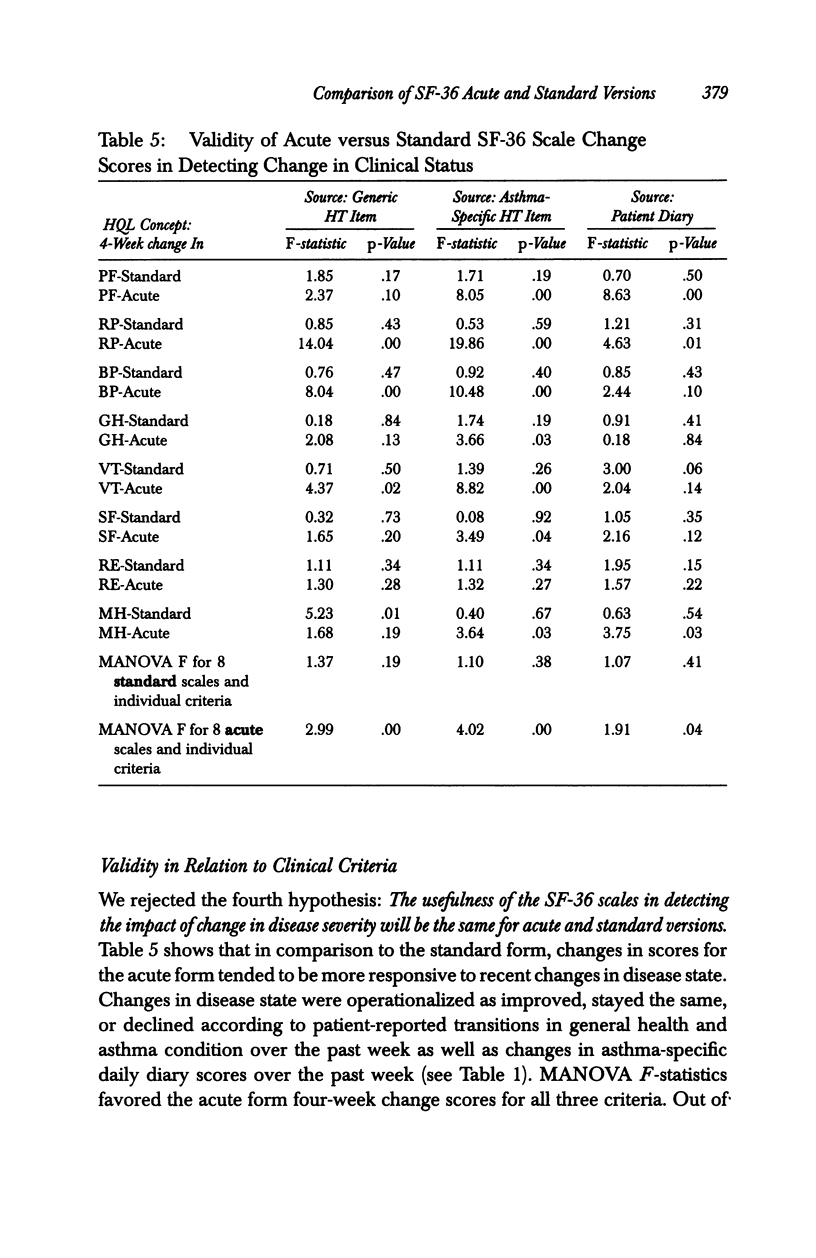
OBJECTIVE: To compare the measurement properties of acute (one-week recall) and standard (four-week recall) versions of SF-36 Health Survey (SF-36) scale scores. DATA SOURCES: SF-36 data collected from 142 participants (60% female, average age 39) in a clinical trial of an asthma medication: 74 patients randomized to the acute form and 68 to the standard. DATA COLLECTION: The SF-36 was self-administered at the time of a clinic visit (before clinical examination) to synchronize with clinical measures of disease severity at three different time points during the clinical trial: -2 weeks (two weeks before randomization to treatment), baseline (week 0 or randomization), and +4 weeks (four weeks after baseline). PRINCIPAL FINDINGS: The acute form yielded high-quality data; scales conformed to the assumptions of the summated ratings method used to score the standard SF-36; and scales had good distributional properties, were reliable, and had a factor content similar to the standard. The data indicated that while the acute form was more sensitive than the standard to change in health status associated with changes in acute symptoms, acute scale scores may not be comparable to national norms based on the standard, particularly for those scales that assess frequency of health events during a specified time period. CONCLUSIONS: Results support the use of the acute form in its intended applications; however, further research is required to document the generalizability of greater sensitivity of the acute form to recent changes in health and to explore whether norms based on the standard can be used to interpret the acute scale scores.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergner M., Bobbitt R. A., Kressel S., Pollard W. E., Gilson B. S., Morris J. R. The sickness impact profile: conceptual formulation and methodology for the development of a health status measure. Int J Health Serv. 1976;6(3):393–415. doi: 10.2190/RHE0-GGH4-410W-LA17. [DOI] [PubMed] [Google Scholar]
- McHorney C. A., Kosinski M., Ware J. E., Jr Comparisons of the costs and quality of norms for the SF-36 health survey collected by mail versus telephone interview: results from a national survey. Med Care. 1994 Jun;32(6):551–567. doi: 10.1097/00005650-199406000-00002. [DOI] [PubMed] [Google Scholar]
- McHorney C. A., Ware J. E., Jr, Lu J. F., Sherbourne C. D. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994 Jan;32(1):40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- McHorney C. A., Ware J. E., Jr, Raczek A. E. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993 Mar;31(3):247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- Spitzer W. O., Dobson A. J., Hall J., Chesterman E., Levi J., Shepherd R., Battista R. N., Catchlove B. R. Measuring the quality of life of cancer patients: a concise QL-index for use by physicians. J Chronic Dis. 1981;34(12):585–597. doi: 10.1016/0021-9681(81)90058-8. [DOI] [PubMed] [Google Scholar]
- Ware J. E., Jr, Kosinski M., Bayliss M. S., McHorney C. A., Rogers W. H., Raczek A. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the Medical Outcomes Study. Med Care. 1995 Apr;33(4 Suppl):AS264–AS279. [PubMed] [Google Scholar]
- Ware J. E., Jr, Sherbourne C. D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992 Jun;30(6):473–483. [PubMed] [Google Scholar]


