Abstract
Sucrose-inducible secretory sucrose:sucrose 1-fructosyltransferase (1-SST) from Aspergillus foetidus has been purified and subjected to N-terminal amino acid sequence determination. The enzyme is extensively glycosylated, and the active form is probably represented by a dimer of identical subunits with an apparent molecular mass of 180 kDa as judged from mobility in seminative acrylamide gels. The enzyme catalyzes fructosyl transfer from sucrose to sucrose producing glucose and 1-kestose. Oligosaccharides with a higher degree of polymerization are not obtained with sucrose as the substrate. The cDNA encoding the A. foetidus 1-SST has been cloned and sequenced. Sequence homology was found to be highest to levanases, but no hydrolytic activity was observed when levan was incubated with the enzyme. Expression of the cloned gene in an invertase-deficient mutant of Saccharomyces cerevisiae resulted in 1-kestose production, with 6-kestose and neokestose being side products of the reaction. Products were well distinguishable from those formed by yeast transformants expressing a cytosolic invertase.
Fructans offer various benefits for human health as nutrients and are also of industrial interest. Thus there is considerable interest in the isolation and characterization of enzymatic activities capable of fructosyl polymerization and also in the genes encoding them. To date, genes of bacterial (9, 29, 32, 34) and plant (31, 38) origin have been described, and there is also evidence for fructan production by fungi (13). Whereas fructan polymerization in bacteria is performed by a single enzyme starting from sucrose, a widely accepted model proposes that sucrose:sucrose fructosyltransferase (SST) (EC 2.4.1.99) and 1,2-β-fructan 1-fructosyltransferase (FFT) (EC 2.4.1.100) are the key enzymes of fructan biosynthesis in plants (6).
In vivo characterization of fructan-synthesizing enzymes is often complicated by competitive reactions of fructan hydrolase and invertases (EC 3.2.1.26), the latter of which is able to catalyze the formation of all three kestose isomers when it is incubated with sufficiently high concentrations of sucrose (3, 4). Whether fructosyl transfer to water, which results in cleavage of sucrose, is a side reaction catalyzed by SST or is due to possible contamination of enzyme preparations by invertase can be answered only by expression of sucrose:sucrose 1-fructosyltransferase (1-SST) genes in an invertase-free background. Up to now, however, no successful heterologous expression of SST in Saccharomyces cerevisiae has been described.
In this report we describe the purification, cloning, and characterization of a 1-SST-like enzyme from the fungus Aspergillus foetidus and its heterologous expression in S. cerevisiae. The corresponding enzyme converts sucrose to 1-kestose with high efficiency but does not give rise to fructans with higher degrees of polymerization. Therefore, it seems to resemble plant 1-SST enzymes rather than bacterial levansucrases or inulinsucrases.
MATERIALS AND METHODS
Strains and plasmids.
A. foetidus (NRRL 337) was obtained from the American Type Culture Collection (Rockville, Md.). suc2 (invertase)-deficient S. cerevisiae YSH 2.64-1A (10) was used for expression of A. foetidus 1-SST cDNA. Escherichia coli XL1blue (Stratagene, Heidelberg, Germany) was used for molecular methods.
Vectors pBluescript SK (Stratagene), pUC 19 (New England Biolabs, Schwalbach, Germany), and pCR II (Invitrogen, Leek, The Netherlands) were used for transformation of E. coli. p112A1NE (24) was used as a yeast expression vector; the construct 128A1-INV (24) was used to obtain cytosolic invertase expression in YSH 2.64. Both vectors contain a promoter and a terminator of an alcohol dehydrogenase (ADH) gene (1) as an expression cassette.
Phage vector λ ZAP II (Stratagene) was used for construction of the cDNA library, and EMBL3 was used for construction of a genomic library.
Standard procedures.
Standard molecular cloning techniques were performed as described by Sambrook et al. (28). Protein concentrations were determined with the Bio-Rad (Munich, Germany) protein assay kit. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed with 12% polyacrylamide gels according to Laemmli (18), and staining was done with Coomassie brilliant blue R250. Rainbow high-molecular-weight markers (Amersham, Braunschweig, Germany) were used as standards.
Purification of 1-SST.
Unless otherwise indicated, all purification steps were performed at 4°C. A. foetidus cultures were grown for 60 h at room temperature in sodium phosphate (200 mM, pH 7.5)-buffered medium containing 6% sucrose, 0.5% yeast nitrogen base (Difco, Detroit, Mich.), and M9 salts. Mycelia were separated from the supernatant by filtration through Miracloth. Proteins of the filtrate were precipitated with ammonium sulfate (90% saturation). The precipitate was recovered by centrifugation at 15,000 × g for 45 min, resuspended in 50 mM sodium acetate buffer (pH 5.6), and dialyzed against 50 mM Tris-HCl (pH 7.5)–500 mM NaCl. The dialyzed fraction had a final volume of about 50 ml per 3 liters of culture.
Adsorption to concanavalin A (ConA)-Sepharose was performed according to the supplier’s instruction (Pharmacia Biotech, Uppsala, Sweden). ConA was equilibrated in 50 mM Tris-HCl (pH 7.5)–500 mM NaCl. Adsorbed protein was eluted with 0.25 M α-d-methylmannoside in 50 mM Tris-HCl (pH 7.5). The ConA eluate was loaded onto a Mono Q anion-exchange column (Pharmacia HR 5/5) equilibrated with 50 mM Tris-HCl (pH 7.5). Proteins were eluted with a linear KCl gradient (0 to 1 M) at a flow rate of 1 ml/min. Fractions of 1 ml were collected and stored at −20°C. Mono Q fractions showing sucrolytic activity were applied to a Superdex 200 HR 10/30 column (bed volume, 24 ml [Pharmacia]) equilibrated with 50 mM Tris-HCl (pH 7.5) at a concentration of 8.3 μg of protein per ml and eluted with the same buffer at a flow rate of 0.25 ml/min. Fractions containing the active protein were pooled and concentrated in Centricon 30 concentrators (Amicon, Beverley, Mass.).
Activity staining of seminative polyacrylamide gels.
Seminative PAGE gels were prepared according to Laemmli (18) containing 0.1% SDS, but samples were loaded in a buffer containing the same amount of SDS but omitting β-mercaptoethanol and the heat step. After the electrophoresis, the gel was washed extensively with 50 mM sodium acetate (pH 5.6) containing 0.5% (vol/vol) Triton X-100 for 15 min to remove the SDS. Afterwards, the gel was incubated in 1 M sucrose–50 mM sodium acetate (pH 5.6) for 30 min at room temperature. After being washed repeatedly with water, reducing sugars were stained with 1% (wt/vol) 2,3,5-triphenyltetrazolium chloride (TTC) in 0.25 M NaOH, which forms red insoluble formazan. To localize glucose release, TTC was heated to 100°C and then poured onto the gel. After a few minutes the TTC solution was discarded, and the staining was stopped with 5% (vol/vol) acetic acid.
Product identification by TLC.
Soluble sugars were analyzed by thin-layer chromatography (TLC) according to the method of Wagner and Wiemken (39). F 1500 TLC-Ready-Foils (Schleicher & Schüll, Dassel, Germany) were used and developed at least two times with acetone-H2O (87:13 [vol/vol]). Sugars were visualized by applying urea phosphoric acid spray (25).
Photometric determination of glucose and fructose.
Enzyme (1 μg) was incubated with sucrose for 1 h at 37°C. An aliquot of the incubation solution was added to 100 mM imidazol-HCl (pH 6.9)–5 mM MgCl2–2 mM NADP–1 mM ATP–2 U of glucose-6-phosphate-dehydrogenase (from yeast) per ml and used for photometric determination of glucose and fructose. Absorption differences at 334 nm were determined by sequential addition of 0.5 U of hexokinase (from yeast) and 2 U of phosphoglucoisomerase (from yeast) (33).
Construction of cDNA and genomic libraries of A. foetidus.
Mycelia of A. foetidus cultivated for 48 h were isolated by filtration through Miracloth. RNA was isolated following the method of Logemann et al. (20). Isolation of mRNA was done with a poly(A)Tract poly(A)-RNA isolation kit (Promega, Madison, Wis.). cDNA was synthesized by using a λ ZAP-cDNA synthesis kit and Gigapack II gold packaging extract (Stratagene).
Genomic DNA was isolated according to standard methods and partially digested with 0.2 U of Sau3A for 4 min. After agarose gel electrophoresis and elution with a GeneClean kit (Bio 101 Inc., La Jolla, Calif.), fragments were ligated into a BamHI-predigested lambda EMBL3 vector and packaged by using Gigapack II gold packaging extract (Stratagene).
Cloning of A. foetidus 1-SST.
Two degenerated, nested PCR primers were designed by using the N-terminal amino acid sequence. Primer AF1 (5′-GGAATTCAAYTAYGAYCARCCNTAYMGNGGNCARTAYCA), derived from the N terminus, was used in combination with an M13 universal primer (Stratagene) for PCR with the whole cDNA library as the template. The fragment amplified by the AF1-universal primer was eluted after agarose gel electrophoresis with the GeneClean kit (Bio 101 Inc.) and used as the template for the second PCR by using AF2 (5′-ATAGGATCCNCARAARAATGGATGAAYGA) in combination with the universal primer (Stratagene). The amplified PCR fragment was cloned into the pCRII vector (Invitrogen, Inc.). The insert, recovered by NotI-BamHI digestion, was used as the probe to screen cDNA and genomic libraries. The full-length cDNA clone obtained was named pCK14.
Transformation of S. cerevisiae.
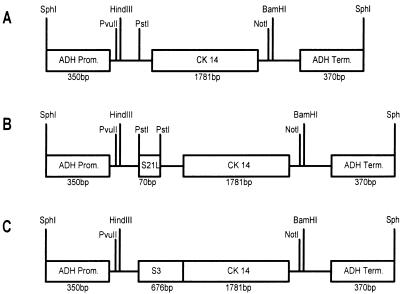
Yeast cells were transformed according to the method of Dohmen et al. (5). Construct 112CK14 contains the BamHI-Asp718 fragment of pCK14, which was blunt ended and cloned into the SmaI site of p112A1NE (Fig. 1A). Construct 112CK14L contains the 5′ untranslated leader sequence of the spinach sucrose transporter (nucleotides 1 to 69 [24]) as a spacer between transcriptional and translational initiation sites, cloned into the PstI site of 112CK14 (Fig. 1B). Construct 112CK14S contains the vacuole-targeting sequence of the patatin gene B33 of Solanum tuberosum (nucleotides 724 to 1399 [28]), which was cloned into the blunted PstI site of 112CK14 (Fig. 1C).
FIG. 1.
Schematic drawing of constructs used to transform S. cerevisiae YSH 2.64-1A. (A) Construct 112CK14 contains the cDNA insert of A. foetidus 1-SST without modifications. (B) Construct 112CK14L contains a 70-bp 5′ untranslated leader sequence of the spinach sucrose transporter cloned into the PstI site. (C) Construct 112CK14S carries the vacuole-targeting sequence of the potato patatin gene B33 as a translational fusion to the CK14 coding region. Prom., promoter; Term., terminator.
Nucleotide sequence accession number.
The sequence of the open reading frame (ORF) of 1,611 bp encoding a 537-amino-acid protein (1-SST) with a deduced molecular mass of 59.1 kDa has been assigned EMBL accession no. AJ000493.
RESULTS
A. foetidus 1-SST production is dependent on growth conditions.
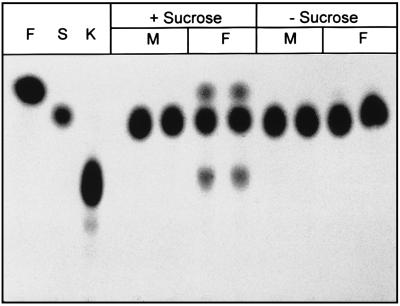
When A. foetidus NRRL 337 was cultivated in rich liquid medium (2% glucose, 2% malt extract, 0.1% peptone), fructan-producing activity (degree of polymerization = 3) was secreted by the fungus, as demonstrated by TLC analysis of the culture supernatant incubated with sucrose. As expected, protein extracts of homogenized mycelia did not contain 1-kestose-producing activity. Furthermore, no other sucrolytic activities were detectable in the medium or in enzyme preparations of mycelia (data not shown). Different minimal media containing yeast nitrogen base and either glucose or sucrose as carbon source were tested. With both sugars, fungal growth was detectable within 2 days, but 1-SST activity was found only when sucrose was present in the culture medium (Fig. 2). Sucrose (0.2% [wt/vol]) added to a medium based on glucose as the main carbon source was sufficient to induce 1-SST production.
FIG. 2.
Synthesis of 1-kestose by an enzyme secreted from A. foetidus at different growth conditions. F, fructose; S, sucrose; K, 1-kestose; +Sucrose, liquid medium containing 2% (wt/vol) sucrose; −Sucrose, liquid medium containing 2% (wt/vol) glucose as carbon source; M, protein extracts of mycelia were incubated with 500 mM sucrose; F (under sucrose), culture filtrate was incubated with 500 mM sucrose.
When sucrose was used as sole carbon source, 1-SST production increased during the first 60 h of cultivation. Extended cultivation led to a decrease in 1-SST activity and a drop to pH 2. At pH 2, A. foetidus 1-SST was still active, but sucrose turnover was reduced.
Purification of 1-SST.
A culture filtrate of a 60-h culture was used for the purification of the secreted protein. Protein in the culture filtrate was collected by ammonium sulfate precipitation at 90% saturation. About 3 mg of protein could be precipitated per liter of culture. After dialysis, protein from the ammonium sulfate precipitation was incubated with ConA-lectin at a concentration of 1 mg of protein per ml of ConA-Sepharose, as described in Materials and Methods.
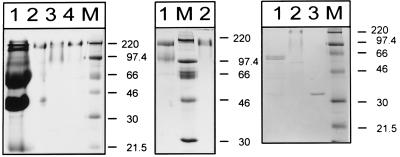
Mono Q anion-exchange chromatography allowed enrichment of A. foetidus 1-SST by a factor of 28. Subsequent size exclusion chromatography on Superdex 200 yielded a single protein band with a size of around 180 kDa in seminative SDS-PAGE (Fig. 3, left panel, lane 4). This protein fraction, showing an 87.5-fold increase of specific activity of A. foetidus 1-SST (expressed as micromoles of hexose released from sucrose per minute and milligrams of protein at a temperature of 37°C [Table 1]), was used to determine the N-terminal amino acid sequence of A. foetidus 1-SST and also to characterize its enzymatic properties.
FIG. 3.
(Left panel) Seminative SDS-PAGE analysis of protein preparations from A. foetidus. Lanes: 1, ammonium sulfate precipitate of culture filtrate; 2, ConA eluate with 0.25 M α-d-methylmannoside; 3, Mono Q chromatography with 150 to 205 mM KCl; 4, Superdex 200 fraction at 48 to 49 min; M, molecular mass markers with sizes in kilodaltons indicated on the right. (Middle panel) Denaturing (lane 1) versus seminative (lane 2) SDS-PAGE analyses of A. foetidus 1-SST. The Superdex 200 fraction (5 μg) at 48 to 49 min was loaded per lane. M, molecular mass markers, with sizes in kilodaltons indicated on the right. (Right panel) Deglycosylation of A. foetidus 1-SST with N-glycosidase F. Lanes: 1, deglycosylated purified A. foetidus 1-SST; 2, untreated control; 3, N-glycosidase F; M, molecular mass markers, with sizes in kilodaltons indicated on the right.
TABLE 1.
Purification of A. foetidus 1-SST
| Purification step | Total activitya | Protein concn (mg) | Sp actb | Purification factor |
|---|---|---|---|---|
| Culture medium | 85.2 | 29.38 | 2.9 | |
| (NH4)2SO4 pellet | 64 | 20.03 | 3.2 | 1.1 |
| ConA | 31.3 | 2.27 | 13.8 | 4.69 |
| Mono Q | 27.9 | 0.34 | 82.1 | 27.93 |
| Superdex 200 | 23.6 | 0.092 | 257.2 | 87.5 |
Total activity is expressed in micromoles of glucose min−1 at 37°C.
Specific activity is expressed in micromoles of glucose μg of protein−1 min−1 at 37°C.
The molecular mass of the A. foetidus 1-SST protein was estimated to be approximately 90 kDa, as judged by SDS-PAGE run under fully denaturing conditions (Fig. 3, middle panel). Under these conditions, the intensity of the 180-kDa band seen in seminative PAGE was greatly reduced and a putative degradation product with a molecular mass of around 60 kDa could be detected by SDS-PAGE. As the 180-kDa complex resulted in only one N-terminal peptide sequence, it is reasonable to assume that the active enzyme is a homodimer.
Cloning of the 1-SST gene.
On the basis of a 22-amino-acid N-terminal peptide sequence obtained by protein sequencing, two nested oligonucleotides were designed and used as primers for consecutive PCRs, each in combination with the M13 universal primer, which hybridized to a vector sequence that was ligated to cDNA of A. foetidus mycelia during construction of a cDNA library in the lambda ZapII phage vector. The amplified fragment was approximately 1.6 kb in length and was cloned into the pCRII vector. The 1.6-kb BamHI-NotI fragment was prepared from the recombinant plasmid and used to probe the A. foetidus cDNA library to screen for full-length cDNA clones.
Phage particles corresponding to 23 hybridizing plaques were isolated, and plasmids were rescued by in vivo excision. Sequence analysis of three clones containing the largest inserts showed that they contained the same cDNA insertion. One of the clones, termed CK14, was completely sequenced on both strands. The sequence comprises an ORF of 1,611 bp encoding a protein of 537 amino acids with a deduced molecular mass of 59.1 kDa. Part of the ORF is a putative signal sequence encoding 19 amino acids. These amino acids were not present at the N terminus of the mature peptide that was purified from culture filtrate, indicating that the signal peptide for extracellular location is cleaved during secretion.
A genomic clone, GK1, isolated by using the 1.6-kb PCR fragment as a probe, was used for further investigation of the 5′ end of the A. foetidus 1-SST gene. An in-frame stop codon 75 bp upstream of the putative ATG start codon of CK14 was identified in GK1, indicating that nucleotide 682 most probably represents the translational start of the A. foetidus 1-SST gene.
Comparison of A. foetidus 1-SST with other β-fructofuranosidases reveals that the six domains identified by Gunasekaran et al. (12), which are well conserved among bacterial sucrases and yeast invertase, are also present in A. foetidus 1-SST and other fructosyltransferases of different origins. We compared sucrolytic enzymes, e.g., cell wall invertase of Nicotiana tabacum (11), suc2 invertase of S. cerevisiae (36), and levanase of Bacillus subtilis (22) with transfructosylases such as 1-SST from artichoke (14) and Jerusalem artichoke (PCT/NL961/00012) and sucrose:fructan 6-fructosyltransferase from barley (31). Plant enzymes show an insertion of a threonine in box 3, whereas they miss the less-conserved amino acid 4 in box 6 (Fig. 4). An asparagine-to-serine exchange in box 1 seems to be characteristic for the plant fructosyltransferases shown but not for fructosyltransferases in general as it is not present in the ftf gene of Streptococcus mutans. In A. foetidus 1-SST, it is also missing.
FIG. 4.
The deduced peptide sequence of A. foetidus 1-SST was compared with conserved domains of invertases and fructosyltransferases (12). Sequences are presented in order of descending identity. Insertions in domains 3 and 6 that clearly discriminate plant sequences from microbial sequences and the N-to-S exchange typical for plant fructosyltransferases are shown in boxes. The numbers at the bottom indicate the amino acid positions of the sacA sucrase of Zymomonas mobilis (12). Cs_Sst, C. scolymus sucrose:sucrose 1-fructosyltransferase; Ht_Sst, H. tuberosus sucrose:sucrose 1-fructosyltransferase; 6_Sft, H. vulgare sucrose:fructan 6-fructosyltransferase; Nt_C winv, N. tabacum cell wall invertase; Bs_Leva, B. subtilis levanase; Af_sst, A. foetidus SST; Sc_Inv1, S. cerevisiae invertase; SM_Sucr, S. mutans fructosyltransferase.
The high apparent molecular mass of the mature 1-SST protein of 90 kDa by SDS-PAGE probably results from posttranslational modification, e.g., glycosylation. Eight possible N glycosylation sites are present in the A. foetidus 1-SST peptide sequence. To analyze the effect of N glycosylation on the molecular mass of A. foetidus 1-SST, the Superdex 200 protein fraction was deglycosylated with N-glycosidase F. In Fig. 3 (right panel), two bands of around 60 kDa after deglycosylation of A. foetidus 1-SST with N-glycosidase F are shown (lane 1). A molecular mass of 60 kDa is in good agreement with the calculated molecular mass. The occurrence of a double band and some minor bands of lower molecular weights might be due to degradation during the deglycosylation reaction.
Expression of A. foetidus 1-SST in S. cerevisiae.
To prove the identity of CK14 as a cDNA for the A. foetidus 1-SST gene, we expressed it in S. cerevisiae. The yeast system was chosen in preference to bacterial expression systems because of its competence for protein glycosylation.
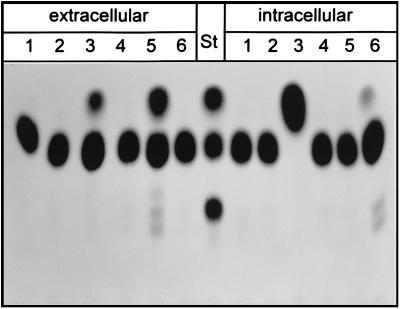
For yeast transformation, the insert of pCK14 and two chimeric gene constructs were cloned in the shuttle vector p112A1NE (24). Transformants containing the cDNA insert of pCK14 without modifications were not able to synthesize 1-kestose (Fig. 5, lane 4).
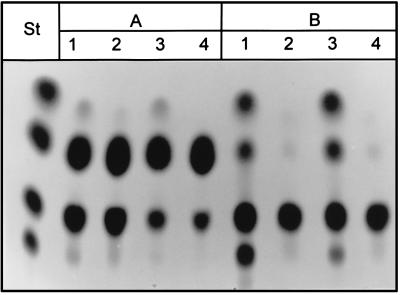
FIG. 5.
TLC analysis of protein preparations from various yeast strains incubated with 500 mM sucrose for 48 h at room temperature. Extracellular, protein preparation from culture filtrate; intracellular, protein preparation from cell extracts. Lanes: 1, YSH; 2, 112A1NE; 3, 128INV; 4, 112CK14; 5, 112CK14L; 6, 112CK14S. Fructose, sucrose, and 1-kestose were used as standards (St).
The first chimeric construct, based on 112CK14, contains 70 bp of the 5′ untranslated region of the spinach sucrose carrier cDNA (24) fused to the coding region of A. foetidus 1-SST. Transformants containing this construct, termed 112CK14L, secreted an active 1-SST that had the same enzymatic specificity as the 1-SST purified from the A. foetidus culture filtrate (Fig. 5, lane 5, extracellular).
With a second strategy, we produced A. foetidus 1-SST as a nonsecreted form by fusing a 675-bp vacuole-targeting sequence of the S. tuberosum patatin gene B33 (26) to the N terminus of CK14. No kestose-producing activity was found in the medium of yeast transformants, but instead 1-SST activity could be detected in cell extracts and could be due to a successful targeting of the CK14 gene product to the yeast vacuole (Fig. 5, lane 6, intracellular). Transformants harboring the vector 128INV with a modified suc2 gene lacking the endogenous signal sequence, and thus leading to an intracellular localization of invertase activity, and a strain containing the empty vector alone were used as controls, demonstrating that the sucrolytic activity encoded by CK14 is clearly distinct from yeast invertase and is not present in the host strain YSH 2.64-1A.
Enzymatic properties of A. foetidus 1-SST are dependent on sucrose concentration.
Purified 1-SST from A. foetidus was used to analyze enzyme specificity. The protein was purified from culture filtrate as described above, and homogeneity was confirmed by SDS-PAGE. The specific activity of the enzyme was 257.2 μmol of hexose μg−1 min−1 when it was incubated with 1 M sucrose.
Interestingly, not only the rate of sucrose turnover but also substrate specificity and the reaction products depended on substrate concentration. At a sucrose concentration of 500 mM, 1-kestose added in a concentration of 300 or 50 mM was not used as the fructosyl acceptor, and the amount of fructose released was only slightly above that in the control (Fig. 6). Small amounts of nystose were already present in the 1-kestose preparation, as shown by the incubations that did not contain protein (lanes with even numbers). In contrast, when no sucrose was present, 1-kestose was used as both a donor and an acceptor of fructosyl moieties, yielding free fructose, sucrose, and nystose (Fig. 6, lanes B1 and B3). Even fructosylnystose (degree of polymerization, 5) was synthesized when the initial 1-kestose concentration was 300 mM (lane B1). This result clearly demonstrates that both synthesis and degradation of 1-kestose can be catalyzed by A. foetidus 1-SST and are triggered by the ambient sucrose concentration.
FIG. 6.
TLC analysis of products formed after 2 h of incubation of 10 ng of purified A. foetidus 1-SST. (A) Initial sucrose concentration, 500 mM. (B) Without initial sucrose. Lanes: 1 and 2, 300 mM 1-kestose; 3 and 4, 50 mM 1-kestose. Lanes 2 and 4 are controls of the reaction mixture that were not incubated with A. foetidus 1-SST.
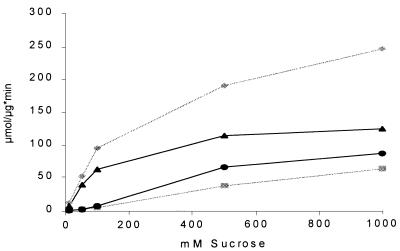
To quantify the substrate dependence of the A. foetidus 1-SST reaction, we incubated 1 μg of the protein with different sucrose concentrations and measured glucose and fructose release in a coupled photometric assay. The synthesis of kestose was calculated from the excess of free glucose compared to the amount of fructose. As shown in Fig. 7, kestose synthesis by A. foetidus 1-SST started at substrate concentrations above 100 mM and reached 70% of fructose release at a substrate concentration of 1 M, whereas kestose production by yeast invertase reached only 25% of the hydrolytic activity.
FIG. 7.
Influence of substrate concentration on fructose and kestose production by A. foetidus 1-SST (1 μg/ml) and commercial yeast invertase (1 μg/ml). x axis, sucrose concentration; y axis, micromoles of sugar released per microgram of protein in 1 min. Sugar release by A. foetidus 1-SST is given in black; invertase is indicated in gray. Circles, kestose production by A. foetidus 1-SST; squares, kestose production by invertase; diamonds, fructose release by invertase; triangles, fructose release by A. foetidus 1-SST.
DISCUSSION
In this report, we describe the purification of 1-SST from A. foetidus NRRL 337 and the isolation of a cDNA and a genomic DNA of the corresponding gene. Twenty-two amino acids of the N terminus of the purified protein were determined. This sequence could also be found in the deduced amino acid sequence of the cloned cDNA, and furthermore, the cDNA encoded an enzyme with the same catalytic properties as the purified 1-SST when it was expressed in an invertase-deficient yeast strain under the control of the ADH promoter. This is convincing evidence that the cloned cDNA and genomic clones represent the 1-SST gene of A. foetidus.
The A. foetidus 1-SST gene is a low-copy-number gene as revealed by a DNA blot experiment with digested genomic DNA that was hybridized to the CK14 sequence (data not shown). A single band hybridized to CK14 even under low-stringency conditions, suggesting that no additional copies or homologous genes are present in the genome of A. foetidus. This finding is in agreement with our finding that no sucrolytic activity other than that of 1-SST was detectable in culture filtrate or protein extracts of fungal mycelia. The ability of A. foetidus to grow on sucrose as a sole carbon source therefore seems to be entirely dependent on the 1-SST activity.
Growth experiments with the fungus demonstrated that secretion of 1-SST seems to be regulated through the physiological status of the culture. When the medium was not buffered or barely buffered and the sucrose concentration was low, only small amounts of 1-SST were secreted and the pH of the medium quickly dropped to pH 2. Higher concentrations of sucrose stimulated production of 1-SST.
An in-frame stop codon is present in the A. foetidus 1-SST gene 75 bp upstream of the 1,611-bp ORF of the cDNA clone CK14. We therefore believe that the cDNA encodes the entire 1-SST protein. The encoded protein consists of 537 amino acids and thus has a calculated mass of 59,150 Da, including a 1,900-Da signal peptide.
Due to glycosylation, the apparent molecular mass of the protein by SDS-PAGE was much higher than that calculated for the ORF of CK14. Enzymatic deglycosylation revealed two protein species with molecular masses of around 60 kDa. Currently, we have no explanation for the double band that was obtained for the deglycosylated protein. Compared to the sizes of other secreted proteins of fungi from the genus Aspergillus, an increase in mass of about 40 kDa seems extraordinarily high given that only eight possible glycosylation sites could be identified within the sequence of the mature peptide. Glucose oxidase of Aspergillus niger also contains eight consensus motifs for glycosylation, but the increase in molecular mass caused by posttranslational modification of the protein is only around 10 kDa (7).
Preparations of active A. foetidus 1-SST show a single protein band of 180 kDa by seminative PAGE. This band disappears when gels are run under denaturing conditions (2% SDS, 100 mM dithiothreitol). As only one peptide species with a molecular mass of 90 kDa results from reductive denaturation, we believe that the active A. foetidus 1-SST is a dimer with identical subunits.
Dimerization has also been shown for secreted inulinase (Inu1) of Kluyveromyces marxianus CBS 6556 (27), which shows 36% amino acid identity to A. foetidus 1-SST. Both proteins belong to a large family of sucrose-cleaving enzymes that share extensive homology in certain protein domains. For example, amino acids 38 to 45 are 100% identical to amino acids 50 to 57 of Inu1 of K. marxianus (19) or amino acids 39 to 46 of invertase (Suc2) from S. cerevisiae (35). The highest degree of homology to sequences in the database was found between A. foetidus 1-SST and the fructanase gene levJ of Actinomyces naeslundii T14V (23). Despite an identity of 41% at the amino acid level, these proteins do not share enzymatic specificity. The levJ gene product hydrolyzes sucrose, raffinose, inulin, and levan (23) but does not synthesize fructan, whereas A. foetidus 1-SST does not hydrolyze levan or inulin.
In the deduced peptide sequence of A. foetidus 1-SST, we could recognize the six domains which are conserved among bacterial and fungal β-fructofuranosidases (12). As these boxes can be identified in fructosyltransferases of plant and bacterial origin also, it was interesting to investigate whether it would be possible to discriminate sucrolytic enzymes from transfructosylating enzymes. The fructosyltransferase of S. mutans as an example of a bacterial fructosyltransferase and the plant 1-SST from artichoke (14) and Jerusalem artichoke (PCT/NL961/00012) and sucrose:fructan 6-fructosyltransferase from barley (31) were compared to sucrose-cleaving enzymes, e.g., cell wall invertase of N. tabacum (11), suc2 invertase of S. cerevisiae (35), and levanase of B. subtilis (22). The sequence comparison does not reveal fructosyltransferase-specific motifs, as the similarity is higher for sequences of similar origins (e.g., plant versus bacterium) than for sequences of similar functions. Interestingly, the invertase of A. niger contains only one of these domains (2) and shares a sequence homology of only 38% with A. foetidus 1-SST, clearly demonstrating that these two enzymes are barely related. Enzymatic specificity of A. foetidus 1-SST is more similar to that of plant 1-SST, which is a key enzyme in fructan synthesis (6, 21, 36). The irreversible fructosyl transfer between two sucrose units to form 1-kestose and glucose provides the substrate for synthesis of higher fructans of the inulin series but probably also provides a substrate for levan synthesis (16, 30). A plant 1-SST has been partially purified from barley and analyzed in vitro (30). The main reaction catalyzed by the enzyme is fructosyl transfer with sucrose as donor and acceptor, but cleavage of sucrose was also detectable. As the enzyme was purified from plant extracts which also contain different types of invertases, it cannot be ruled out that contaminating invertases gave rise to the sucrose cleavage. In contrast, Koops and Jonker (17) purified 1-SST from Helianthus tuberosus and found no hydrolysis of sucrose, at least for short incubation periods. The question of whether 1-SST can catalyze fructosyl transfer from sucrose to water could probably be answered by expression of a 1-SST gene in an invertase-free background.
To allow discrimination of the A. foetidus 1-SST-encoded fructosyltransferase activity from invertase, we decided to produce the 1-SST in the invertase-deficient yeast strain YSH 2.64-1A (10). The cDNA insert of pCK14 could be functionally expressed in yeast when the 5′ untranslated region was extended by 70 bp taken from the sucrose transporter gene of spinach (24). This insertion readjusts the structure of yeast genes, which usually contain long 5′ leader sequences separating the upstream activating sequence and the ATG start codon (8).
The main product of A. foetidus 1-SST reactions is 1-kestose, the smallest of the inulin-type fructans. Other kestose isomers are also synthesized by A. foetidus 1-SST but occur only in trace amounts at the pH optimum of the enzyme. Hydrolysis of sucrose by A. foetidus 1-SST shows saturation at a substrate concentration of about 500 mM. In contrast, yeast invertase does not seem to saturate even at 1 M sucrose. Furthermore, kestose production is a side reaction of invertase, never exceeding 20% of the sucrose turnover.
In addition to the extracellular 1-SST protein, intracellular localization could be obtained by fusing an N-terminal signal sequence of the patatin gene B33 from Solanum tuberosum (26) to the A. foetidus 1-SST coding region. Products formed by intracellular and extracellular A. foetidus 1-SST did not differ. This allows the production of fructo-oligosaccharides in the baker’s yeast S. cerevisiae. As oligofructans are well-known for their health benefits in human nutrition (15), this finding is also of considerable biotechnological interest.
REFERENCES
- 1.Bennetzen J L, Hall B D. The primary structure of the Saccharomyces cerevisiae gene for alcohol dehydrogenase. J Biol Chem. 1982;257:3018–3025. [PubMed] [Google Scholar]
- 2.Boddy L M, Bergès T, Barreau C, Vainstein M H, Dobson M J, Ballance D J, Peberd J F. Purification and characterization of an Aspergillus niger invertase and its DNA sequence. Curr Genet. 1993;24:60–66. doi: 10.1007/BF00324666. [DOI] [PubMed] [Google Scholar]
- 3.Cairns A J, Howarth C J, Pollock C J. Characterization of acid invertase from the snow mould Monographella nivalis: a mesophilic enzyme from a psychophilic fungus. New Phytol. 1995;129:299–308. doi: 10.1111/j.1469-8137.1995.tb04300.x. [DOI] [PubMed] [Google Scholar]
- 4.Cairns A J, Ashton J E. The interpretation of in vitro measurements of fructosyl transferase activity: an analysis of patterns of fructosyl transfer by fungal invertase. New Phytol. 1991;118:23–34. [Google Scholar]
- 5.Dohmen R J, Strasser A W M, Hoener C B, Hollenberg C P. An efficient transformation procedure enabling long term storage of competent cells of various yeast genera. Yeast. 1992;7:691–692. doi: 10.1002/yea.320070704. [DOI] [PubMed] [Google Scholar]
- 6.Edelman J, Jefford T G. The mechanism of fructosan metabolism in higher plants as exemplified in Helianthus tuberosus. New Phytol. 1968;67:517–531. [Google Scholar]
- 7.Frederick K R, Tung J, Emerick R S, Masiarz F R, Chamberlain S H, Vasavada A, Rosenberg S, Chakraborty S, Schopter L M, Massey V. Glucose oxidase from Aspergillus niger cloning gene sequence secretion from Saccharomyces cerevisiae and kinetic analysis of a yeast-derived enzyme. J Biol Chem. 1990;265:3793–3802. [PubMed] [Google Scholar]
- 8.Furter-Graves E M, Hall B D. DNA sequence elements required for transcription initiation of the Schizosaccharomyces pombe ADH gene in Saccharomyces cerevisiae. Mol Gen Genet. 1990;223:407–416. doi: 10.1007/BF00264447. [DOI] [PubMed] [Google Scholar]
- 9.Geier G, Geider K K. Characterization and influence on virulence of the levansucrase gene from the fireblight pathogen Erwinia amylovora. Physiol Mol Plant Pathol. 1993;42:387–404. [Google Scholar]
- 10.Gozalbo D, Hohmann S. Nonsense suppressors partially revert the decrease of the messenger RNA level of a nonsense mutant allele in yeast. Curr Genet. 1990;17:77–80. doi: 10.1007/BF00313252. [DOI] [PubMed] [Google Scholar]
- 11.Greiner S, Weil M, Krausgrill S, Rausch T. A tobacco cDna coding for cell-wall invertase. Plant Physiol. 1995;108:825–826. doi: 10.1104/pp.108.2.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gunasekaran P, Gunasekaran T, Caimi B, Mukundan A G, Preziosi L, Baratti J. Cloning and sequencing of the sacA gene: characterization of a sucrase from Zymomonas mobilis. J Bacteriol. 1990;172:6727–6735. doi: 10.1128/jb.172.12.6727-6735.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harada T, Suzuki S, Taniguchi H, Sasaki T. Characteristics and applications of a polyfructan synthesized from sucrose by Aspergillus sydowi conidia. In: Nishinara K, Doi E, editors. Food hydrocolloids: structures, properties, and functions. New York, N.Y: Plenum Press; 1994. pp. 77–82. [Google Scholar]
- 14.Hellwege E M, Gritscher D, Willmitzer L, Heyer A. Transgenic potato tubers accumulate high levels of 1-kestose and nystose: functional identification of a sucrose:sucrose 1-fructosyltransferase of artichoke (Cynara scolymus) blossom disk. Plant J. 1997;12:1057–1065. doi: 10.1046/j.1365-313x.1997.12051057.x. [DOI] [PubMed] [Google Scholar]
- 15.Hiramaya M, Nishizawa K, Hidaka H. Production and characteristics of fructo-oligosaccharides. In: Fuchs A, editor. Inulin and inulin-containing crops. Amsterdam, The Netherlands: Elsevier Publishing; 1993. pp. 347–354. [Google Scholar]
- 16.Housley T L, Pollock C J. The metabolism of fructan in higher plants. In: Suzuki M, Chatterton J, editors. Science and technology of fructans. Boca Raton, Fla: CRC Press; 1993. pp. 192–223. [Google Scholar]
- 17.Koops A J, Jonker H H. Purification and characterization of the enzymes of fructan biosynthesis in tubers of Helianthus tuberosus Colombia. II. Purification of sucrose:sucrose 1-fructosyltransferase and reconstitution of fructan synthesis in vitro with purified sucrose:sucrose 1-fructosyltransferase and fructan:fructan 1-fructosyl-transferase. Plant Physiol. 1996;110:1167–1175. doi: 10.1104/pp.110.4.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 19.Laloux O, Cassart Delcour J-P, Beeumen J, Vandenhaute J. Cloning and sequencing of the inulinase gene of kluyveromyces-marxianus-var-marxianus ATCC 12424. FEBS Lett. 1991;289:64–68. doi: 10.1016/0014-5793(91)80909-m. [DOI] [PubMed] [Google Scholar]
- 20.Logemann J, Schell J, Willmitzer L. Improved method for the isolation of RNA from plant tissue. Anal Biochem. 1987;163:16–20. doi: 10.1016/0003-2697(87)90086-8. [DOI] [PubMed] [Google Scholar]
- 21.Lüscher M, Erdin C, Sprenger N, Hochstrasser U, Boller T, Wiemken A. Inulin synthesis by a combination of purified fructosyltransferases from tubers of Helianthus tuberosus. FEBS Lett. 1996;385:39–42. doi: 10.1016/0014-5793(96)00343-2. [DOI] [PubMed] [Google Scholar]
- 22.Martin I, Debarbouille M, Ferrari E, Klier A, Rapoport G. Characterization of the levanase gene of Bacillus subtilis which shows homology to yeast invertase. Mol Gen Genet. 1987;208:177–184. doi: 10.1007/BF00330439. [DOI] [PubMed] [Google Scholar]
- 23.Norman J M, Bunny K L, Giffard P M. Characterization of levJ, a sucrase-fructanase-encoding gene from Actinomyces naeslundii T14V, and comparison of its product with other sucrose-cleaving enzymes. Gene. 1995;152:93–98. doi: 10.1016/0378-1119(94)00695-o. [DOI] [PubMed] [Google Scholar]
- 24.Riesmeier J W, Willmitzer L, Frommer W B. Isolation and characterization of a sucrose carrier cDNA from spinach by functional expression in yeast. EMBO J. 1992;11:4705–4713. doi: 10.1002/j.1460-2075.1992.tb05575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Röber M, Geider K, Mueller-Röber B, Willmitzer L. Synthesis of fructans in tubers of transgenic starch-deficient potato plants does not result in an increased allocation of carbohydrates. Planta (Heidelberg) 1996;199:528–536. doi: 10.1007/BF00195183. [DOI] [PubMed] [Google Scholar]
- 26.Rosahl S, Schmidt R, Schell J, Willmitzer L. Isolation and characterisation of a gene from Solanum tuberosum encoding patatin, the major storage protein of potato tubers. Mol Gen Genet. 1986;203:214–220. [Google Scholar]
- 27.Rouwenhorst R J, Hensing M, Verbakel J, Scheffers W A, Van Dijken J P. Structure and properties of the extracellular inulinase of Kluyveromyces marxianus CBS 6556. Appl Environ Microbiol. 1990;56:3337–3345. doi: 10.1128/aem.56.11.3337-3345.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 29.Shirozawa T, Kuramitsu H K. Sequence analysis of the Streptococcus mutans fructosyltransferase gene and flanking regions. J Bacteriol. 1988;170:810–816. doi: 10.1128/jb.170.2.810-816.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simmen U, Obenland D, Boller T, Wiemken A. Fructan synthesis in excised barley leaves. Identification of two sucrose-sucrose fructosyltransferases induced by light and their separation from constitutive invertases. Plant Physiol. 1993;101:459–468. doi: 10.1104/pp.101.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sprenger N, Bortlik K, Brandt A, Boller T, Wiemken A. Purification, cloning, and functional expression of sucrose:fructan 6-fructosyltransferase, a key enzyme of fructan synthesis in barley. Proc Natl Acad Sci USA. 1995;92:11652–11656. doi: 10.1073/pnas.92.25.11652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steinmetz M, Le Coq D, Aymerich S, Gonzy Treboul G, Gay P. The DNA sequence of the gene for the secreted Bacillus subtilis enzyme levansucrase and its genetic control sites. Mol Gen Genet. 1985;200:220–228. doi: 10.1007/BF00425427. [DOI] [PubMed] [Google Scholar]
- 33.Stitt M, McLilley R, Gerhartdt R, Heldt H W. Biomembranes. Methods Enzymol. 1989;174:518–552. [Google Scholar]
- 34.Tang L, Lenstra R, Borechert T, Nagarajan V. Isolation and characterization of levansucrase-encoding gene from Bacillus amyloliquefaciens. Gene (Amsterdam) 1990;96:89–94. doi: 10.1016/0378-1119(90)90345-r. [DOI] [PubMed] [Google Scholar]
- 35.Taussig R, Carlson M. Nucleotide sequence of the yeast suc2 gene for invertase. Nucleic Acids Res. 1983;11:1943–1954. doi: 10.1093/nar/11.6.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van den Ende W, Van Laere A. De novo synthesis of fructans from sucrose in vitro by a combination of two purified enzymes (sucrose sucrose 1-fructosyl transferase and fructan fructan 1-fructosyl transferase) from chicory roots (Cichorium intybus L.) Planta. 1996;200:335–342. [Google Scholar]
- 37.Vernet T, Dignard D, Thomas D V. A family of yeast expression vectors containing the phage f1 intergenic region. Gene. 1987;52:225–233. doi: 10.1016/0378-1119(87)90049-7. [DOI] [PubMed] [Google Scholar]
- 38.Vijn I, van Dijken A, Sprenger N, van Dun K, Weisbeek P, Wiemken A, Smeekens S. Fructan of the inulin neoseries is synthesized in transgenic chicory plants (Cichorium intybus L.) harbouring onion (Allium cepa L.) fructan:fructan 6G-fructosyltransferase. Plant J. 1997;11:387–398. doi: 10.1046/j.1365-313x.1997.11030387.x. [DOI] [PubMed] [Google Scholar]
- 39.Wagner W, Wiemken A. Properties and subcellular localisation of fructan hydrolase in the leaves of barley Hordeum vulgare cultivar Gerbel. J Plant Physiol. 1986;123:429–440. [Google Scholar]