Abstract
Pseudoloma neurophilia (Pn), the causative agent of the most commonly reported disease of zebrafish, is a microsporidian parasite that confounds research by inducing behavioral and physiologic changes in zebrafish. Although a treatment for P. neurophilia has not been documented in zebrafish, albendazole (ALB) and fumagillin (FUM) have been used to treat microsporidian infections of other fish species. To investigate the efficacy of oral ALB and FUM in the treatment of Pn, we performed a pilot study that demonstrated the safety and palatability of novel gel-based diets containing FUM or ALB in adult AB zebrafish. In a subsequent study, approximately 250 adult AB zebrafish (previously infected with Pn) were treated with these medicated diets for 4 wk. At 4 different time points (weeks 0, 5, 10, and 16 of the study), fish were euthanized and whole-body qPCR was performed to assess Pn prevalence across treatment and control groups. There was no statistically significant association between treatment group and Pn prevalence at any time point, although potential biologically relevant reductions in Pn prevalence occurred in the combination therapy group at weeks 5 and 16 and in the ALB group at week 5. Based on high-performance liquid chromatography analyses, the medicated diets contained less ALB and more FUM than expected, highlighting the importance of validating medicated feed concentrations to ensure safety, efficacy, and consistency. While Pn remains challenging to eradicate and control, results of this study warrant further investigation into the utility of ALB and FUM as potential treatments for this pathogen.
Abbreviations and Acronyms: ALB, albendazole; FUM, fumagillin; Pn, Pseudoloma neurophilia; WB-qPCR, whole-body qPCR; high-performance liquid chromatography (HPLC)
Introduction
Pseudoloma neurophilia (Pn) is the most commonly reported pathogen of laboratory-maintained zebrafish and has been identified in 45% of research laboratories that report to the Zebrafish International Resource Center (ZIRC).15 In zebrafish, Pn may be transmitted both vertically and horizontally.20 Horizontal transmission of Pn has been reported most frequently, and transmission can occur when fish ingest free Pn spores in the environment or cannibalize infected tissues.25 After ingestion, the parasite disseminates to multiple tissues, and spores may be found in the skeletal muscle, ovaries, kidney, and liver, among other tissues.19,22 The spores commonly develop in immune-privileged sites, including the eye and neural tissue.17 On histology, spores are typically found in aggregated complexes, called parasite clusters, in the hindbrain, spinal cord, meninges, and nerve roots.17 Previous work has demonstrated that exposure of naïve zebrafish to a low concentration of infective Pn spores results in a detectable infection within 60 d after exposure, with 40% prevalence in the population at 92 to 334 d after initial exposure.22 These data reflect observations of natural exposure events. Wild-type zebrafish at ZIRC have shown no evidence of Pn infection via histology or on clinical presentation at 4 mo after experimental exposure to infective spores. However, one study reported that Pn was detected in 59% of zebrafish 8 mo after a natural exposure event.15 This finding suggests that Pn can be maintained at a low prevalence in a population for months, with a gradual increase in prevalence as the pathogen spreads within the population over time.
Also referred to as “skinny disease,” Pn infection may induce nonspecific clinical signs, including emaciation, scoliosis, and occasional deaths.15 Pn infections are often subclinical, although histologic lesions in fish include myositis, meningitis, myelitis, encephalitis, and granulomatous inflammation.25 Importantly, structural changes secondary to Pn infection may affect anatomic, physiologic, and behavioral data collected from zebrafish models of biomedical research, ultimately contributing to non-protocol induced variation.15 For example, Pn-infected zebrafish exposed to cortisol or stressed by crowding, handling, and simulated transport demonstrated significantly reduced fecundity and body condition as compared with their uninfected counterparts.17 Previous work also found that Pn infection affects the immune system and suggests that immune activation, even in subclinical infection, may affect study outcomes.12 Finally, Pn-infected fish demonstrate altered behavior including reduced activity, increased freezing behavior, and altered shoaling behavior, suggesting that Pn infection induces a high-stress or anxious phenotype in zebrafish that may interfere with behavior interpretation.11,24
Pn infection threatens animal welfare by contributing to increased zebrafish mortality and reduced fecundity while also acting as a source of non-protocol-induced variation in research. The environmentally resistant spore, multiple modes of transmission, and subclinical nature of Pn infection make this pathogen challenging to detect and control. PCR of zebrafish tissue is the most sensitive method for detection and screening for Pn. Recent work demonstrated that quantitative PCR of the whole zebrafish carcass can diagnose this pathogen with sensitivity comparable to use of brain or spinal cord alone, thereby eliminating the time-intensive process of nervous tissue dissection.22 Histology is a less sensitive diagnostic method that can be used for monitoring the general health of a zebrafish colony.15
Excluding and controlling Pn infection is challenging and requires a multifactorial approach involving the quarantine of imported fish, surface-sanitization of eggs with sodium hypochlorite, maintaining UV sterilization of the filtration system to prevent Pn spread, removing sick and aged fish that may serve as Pn reservoirs, routine health surveillance including screening for and eliminating Pn-positive populations, and strict disinfection of tanks when spawning or moving fish.9,13,15 Despite strict biosecurity practices, Pn is an insidious pathogen that may evade surveillance for months until the infection has spread throughout a population.22 An effective treatment for Pn has not been documented; therefore, when a Pn-positive tank or population is identified, it should be replaced as soon as possible.15
Although Pn has no treatment, other microsporidian infections have been successfully treated with specific antimicrobials. Fumagillin (FUM), an antibiotic derived from Aspergillus fumigatus, inhibits methionine aminopeptidase 2, a eukaryotic enzyme that is important for methionine function, tissue repair, and angiogenesis.6 FUM has been administered orally to treat intestinal microsporidiosis in people and is used topically to treat keratoconjunctivitis associated with human ocular encephalitozoonosis.14 Benzimidazoles have also been used to treat human microsporidiosis and encephalitozoonosis of humans and rabbits.4 Benzimidazoles, such as albendazole (ALB), inhibit microsporidia by binding to tubulin, thus inhibiting its microtubule polymerization and assembly.6 Previous work used a stereomicroscope to count visible Loma salmonae clusters in the gills, demonstrating that oral FUM and ALB each reduced the number of L. salmonae microsporidian parasite clusters in rainbow trout.26 A separate study demonstrated that Chinook salmon infected with the microsporidian pathogen Nucleospora salmonis had fewer clinical signs and a reduced parasite burden when treated with a FUM analogue.7
Given the success of previous work that used ALB and FUM to treat microsporidiosis of other species, the goal of this study was to evaluate these drugs as potential treatments for Pn in zebrafish. To achieve this goal, we had 3 study aims: (1) establish a Pn-infected study population because zebrafish at our institution have historically tested negative for this pathogen on routine health surveillance; (2) generate palatable feeds containing ALB and/or FUM at appropriate doses, and verify that oral administration of these medicated diets was not associated with adverse clinical events; and (3) assess the effect of oral administration of the ALB- and/or FUM-containing diets on Pn prevalence in a treated population of infected zebrafish.
We hypothesized that we would successfully establish a population of Pn-infected zebrafish and that the medicated diets would be safe and palatable to adult zebrafish. We also hypothesized that high-performance liquid chromatography (HPLC) results would reveal that the diets contained appropriate ALB and/or FUM concentrations based on dosing calculations, and that these drugs would remain stable in the diets over 28 d of frozen storage. Finally, we hypothesized that combination therapy with both ALB and FUM would be most effective at reducing Pn prevalence over time.
Materials and Methods
Ethics statement.
This study was designed with consideration of the 3Rs (replacement, reduction, and refinement) to test potential treatments for Pn. All procedures were approved by the IACUC at Cornell University, an AAALAC-accredited institution.
Animals and husbandry.
A population of 330 wild-type AB zebrafish was reared at Cornell University for the purpose of infection with Pn. This population was reared from embryos that were generated and surface-disinfected in November 2021. At our institution, a group of apparently healthy prefiltration zebrafish are submitted annually for health monitoring, including PCR for infectious agents (Aeromonas hydrophila, Edwardsiella ictaluri, Flavobacterium columnare, Mycobacterium spp., Ichthyophthirius multifiliis, Pleistophora hyphessobryconis, Pseudocapillaria tomentosa, Piscinoodinium pillulare, Pseudoloma neurophilia, and Saprolegnia brachydanis). In the past 5 y, fish from this facility have tested positive for Mycobacterium chelonae and have historically tested negative for Pn. All fish obtained from this facility were apparently healthy.
To ensure that the uninfected control fish were definitively free of Pn, 100 wild-type AB zebrafish embryos specific pathogen free (SPF) of Pn were obtained from the Sinnhuber Aquatic Research Laboratory (Corvallis, OR). These SPF zebrafish were spawned in January 2022 at a zebrafish facility that maintains strict quarantine and testing requirements to exclude Pn, as previously described.9 As with the embryos generated in house, SPF embryos were surface disinfected with sodium hypochlorite.
At the time of Pn exposure, all 330 fish were housed in a 40-gallon static aquarium. SPF embryos were reared and maintained in a 20-gallon static aquarium. Air stones were present in static tanks, both of which were maintained in Living Stream Systems (LS-700, Frigid Units, Toledo, OH); each system acted as a flow-through basin, receiving water of a species-appropriate temperature that maintained water temperature in each static tank.
Adult zebrafish enrolled in the pilot study or experiment were housed in 1.4 L zebrafish tanks maintained on a flow-through rack system (Aquaneering, San Diego, CA). All fish were housed in the same room and received water from the same source. SPF fish were maintained in a system that was separate from the rack system. Husbandry was performed first for SPF fish, such that personnel did not return to the SPF fish after working with other fish in the room.
Water temperature and chemistry were maintained within standard ranges established as appropriate for this species and were used for all fish in both the pilot study and the experiment.9 These conditions were as follows: 27 to 30 °C, pH 7 to 8, dissolved oxygen > 6 mg/L, conductivity 500 to 2,000 μS, ammonia < 0.01 ppm, nitrite < 0.5 ppm, and nitrate ≤ 50 ppm). All fish were maintained on a 12:12-h light cycle (lights on at 08:00 and off at 20:00). Daily water changes of 25 to 30% were performed in the static aquaria. Except when exposed to Pn inoculum or medicated feeds, juvenile and adult zebrafish were fed a combination of artemia (premium-grade brine shrimp eggs, Brine Shrimp Direct, Ogden, UT) hatched in house and commercially available flake feed (TetraMin Tropical Flakes, Spectrum Brands Pet, Blacksburg, VA) twice daily. Pn-free larvae were fed powdered feed (Gemma Micro 75, Skretting USA, Tooele, UT) 3 times a day until the standard diet was introduced at 21 d after fertilization.
Pn exposure.
The institutional zebrafish facility that supplied fish for this study has historically tested negative for Pn. Therefore, zebrafish used in treatment and infected control groups had to first be infected with Pn. To achieve this, approximately 50 wild-type AB zebrafish carcasses were sourced from a colony of Pn-infected zebrafish maintained at Oregon State University. A parasite inoculum was prepared by finely mincing dissected spinal cords and hindbrains using sterile razor blades, as previously described.22 The spore concentration of this inoculum was not determined. Approximately 48 h later, the inoculum was added to the static aquarium of 330 zebrafish (approximately 2-mo-old), which were fasted for 24 h prior to inoculum exposure. No additional feed was added to this aquarium for 24 h and no water changes were performed until 5 d after the addition of the inoculum.
Medicated diet generation.
Diets containing no drug (control), FUM, ALB, or both drugs were prepared for the pilot study and the subsequent experiment. A gel-based fish diet (Gelly Belly, Florida Aqua Farms, Dade City, FL) was prepared, as previously described, but with the addition of clam juice (Snow’s, San Diego, CA) to enhance palatability.8
For the pilot study, an ALB dose of 1.5 mg/kg body weight and FUM dose of 15 mg/kg body weight were selected based on a previous study that used these doses in rainbow trout to control another microsporidian pathogen, L. salmonae.26 For this zebrafish study, an assumed approximate weight of 0.6 g/fish and a feeding rate of 2% body weight once daily were used to generate the medicated feeds. Based on preliminary trials, the authors determined that a ratio of 2 parts dry gel-based fish diet:3 parts water or stock solution:1 part clam juice created a feed consistency amenable to mincing, freezing, and thawing. An editable spreadsheet was developed to calculate the volumes of dry gel-based fish diet, water, clam juice, and ALB or FUM required based on the number of fish in each study group. This editable tool is available to others who wish to create medicated fish feeds and can be accessed at https://github.com/IvanekLab/Diet-Formulation-Calculator.
To create the medicated diets, ALB (Valbazen 113.6 mg/mL; Zoetis, Parsipanny, NJ) was mixed in 9 mL of deionized water to create a 1:10 diluted stock solution. The ALB stock solution was then diluted a second time to produce the appropriate concentration. For the FUM-containing diet, 7.5-g FUM powder (Fumidil-B 20 g FUM/kg powder; KBNP, Chungcheongnam-do, South Korea) was added to 100 mL deionized water to create a stock solution. A stock solution was not created for the control diet. The corresponding solutions (or deionized water alone for the control diet) and clam juice were each heated to 60 °C and then aliquoted into a portion of dry gel-based fish diet for each group. After mixing, this solution was allowed to set for 5 to 10 min at 40 °C, followed by freezing for 20 min at -20 °C to facilitate mincing of the diet using a razor blade to generate approximately 1-mm3 pieces. The daily aliquots of feed required for each tank were added to 1.5 mL microcentrifuge tubes stored at -20 °C until feeding time, as previously described.1
Based on HPLC results from the pilot study, the medicated diets created for the subsequent experiment used an ALB dose of 2 mg/kg body weight and FUM dose of 15 mg/kg body weight. The medicated feeds for the experiment were otherwise prepared as described for the pilot study, except for the combination diet. For the combination diet provided during the experiment, an ALB dilution was created as described for the pilot study, and a FUM stock solution was created by adding 15-g FUM powder to 100 mL deionized water. Both solutions were added in equal parts to the mixture of dry gel-based fish diet and clam juice.
HPLC analysis.
Medicated feed samples were analyzed using HPLC at the Iowa State University Veterinary Diagnostic Laboratory. Briefly, the control and medicated gel-based diet samples were prepared by adding pesticide-grade acetonitrile to a tube of each diet. Extraction was performed by vortexing, followed by centrifugation at 3,000 × g. A 100 µL portion of the supernatant was transferred into an amber autosampler vial with an insert. HPLC analysis was then performed using a Vanquish UHPLC System (ThermoFisher Scientific) with a detection wavelength of 294 nm for ALB and 336 nm for FUM. Retention times were 12.5 min for ALB and 13.6 min for FUM. Both ALB and FUM were detected with a photodiode array.
Pilot study design.
To achieve the first 2 study aims, a pilot study was performed to evaluate the safety, palatability, and efficacy of oral ALB and FUM and to assess Pn prevalence in the Pn-exposed population. This 4-wk study was performed 12 wk following initial Pn exposure. Males and females were included in the pilot study, which occurred when fish were approximately 5-mo-old. Three days before treatment began, 30 fish previously exposed to Pn were randomly assigned to one of 3 groups, each represented by a single tank of 10 fish: 1) ALB at 1.5 mg/kg, 2) FUM at 15 mg/kg, or 3) control (no medication). At day 0, the 2 medicated diets (ALB or FUM) and control diets were prepared, and approximately 4 g of each diet was immediately submitted to the Iowa State University Veterinary Diagnostic Laboratory for HPLC analysis. An additional 4 g of each diet was stored in 50-mL conical tubes for 28 d, at which time these frozen diets were also submitted to the Iowa State University Veterinary Diagnostic Laboratory for HPLC analysis. From days 0 to 28 of the experiment, each group was fed its corresponding medicated or control diet once daily, and no other feed was given during this period. At feeding time, water was shut off to each tank and the appropriate aliquot of feed was added to each tank. Although the diet was finely minced prior to storage, it typically fell to the bottom of the tank as a discrete mass. Fish were observed readily feeding from this mass of feed by tearing off pieces. Water flow was turned back on 60 min after feeding.
On days 0 to 28, an observer checked each tank daily for any mortalities. In addition, after feeding each group its corresponding diet, the observer would return at 5, 10, 15, and 60 min after feeding and record whether any feed material remained in each tank. On day 29 of the experiment, all 30 fish were euthanized for WB-qPCR to determine Pn prevalence across groups. Therefore, Pn prevalence results from the pilot study were determined 16 wk after exposure to Pn.
Experimental design.
To assess the third study aim, a larger experiment was performed 16 wk after the pilot study (therefore 32 wk after the initial exposure to Pn). For this experiment, fish were assigned to one of 5 groups: 1) ALB at 2 mg/kg, 2) FUM at 15 mg/kg, 3) combined treatment with both ALB 2 mg/kg + FUM 15 mg/kg, 4) infected control, or 5) uninfected control. Both males and females were used in the experiment; Pn-infected fish were approximately 10-mo-old, and SPF fish were approximately 8-mo-old. Three days before treatment began, fish that had previously been exposed to Pn were randomly allocated to the ALB, FUM, combination, or infected control groups (groups 1 to 4). Each of these 4 groups was represented by 4 tanks (n = 14 fish/tank, n = 56 fish/group). In addition, 3 d before treatment began, SPF fish were randomly allocated to the uninfected control group, which was represented by 2 tanks (n = 14 fish/tank, n = 28 fish/uninfected control group). At day 0, the medicated diets (ALB, FUM, and combination) and control (no medication) were prepared, and approximately 4 g of each diet was immediately submitted to the Iowa State University Veterinary Diagnostic Laboratory for HPLC analysis. An additional 4 grams of each diet was stored in 50-mL conical tubes for 28 d at −20 °C. On day 28 of the experiment, these frozen diets were also submitted to the Iowa State University Veterinary Diagnostic Laboratory for HPLC analysis.
In addition, on day 0 of the experiment, 2 fish from each tank (n = 36) were euthanized and WB-qPCR was performed to obtain a baseline Pn prevalence prior to beginning treatment. From days 0 to 28 of the experiment, each group was fed its corresponding diet once daily. Feeding procedures for the experiment were identical to that of the pilot study.
On day 29 of the experiment, the gel-based diets were discontinued, and the standard diet was resumed. On day 29 (week 5) and at weeks 10 and 16 of the experiment, fish from each tank were euthanized for WB-qPCR to determine Pn prevalence across groups. At weeks 5 and 10, 4 fish from each tank were euthanized. At week 16, the remaining fish were euthanized for WB-qPCR. At this final week 16 time point, only 2 to 4 fish were available in each tank because 8 fish had died during the experiment.
qPCR to determine Pn infection status.
Whole-body qPCR (WB-qPCR) was used to perform the analysis, as previously described,22 except that stainless steel grinding beads (SPW Industrial, Laguna Hills, CA) were used instead of mortar and pestle to macerate the tissues prior to sonication of each sample. Each carcass was processed individually, and 500 µl of RNAlater (ThermoFisher Scientific, Waltham, MA) rather than phosphate-buffered saline was used for tissue storage in 1.5-mL microcentrifuge tubes. A sample of extracted Pn DNA was used as a positive control for qPCR assays performed in the pilot study and the subsequent experiment. qPCR analysis was blinded when processing experimental samples but was not blinded for the pilot study.
Histology.
Histology was performed on fish that were either euthanized for welfare concerns or found dead without severe autolysis. An incision was made in the ventral body wall to allow fixative to penetrate the body cavity. The whole fish was then fixed in Davidson’s fixative for 24 h, followed by sagittal bisection of the fish using a razor blade. The bisected fish was then placed in a cassette and returned to Davidson’s fixative for an additional 12 h, followed by 5 to 7 d of immersion in Cal-Ex II (ThermoFisher Scientific) decalcifying solution. The cassette was then transferred to 10% neutral buffered formalin and submitted to the Cornell University Animal Health Diagnostic Center, Ithaca, NY, for standard histologic processing. Sagittal sections were obtained to visualize the central nervous system. The tissue sections were stained with hematoxylin and eosin (H and E), Luna, and Fite-Faraco stains.
Euthanasia.
For either research endpoints or welfare concerns, fish were euthanized in accordance with institutional and AVMA guidelines by using tricaine methanesulfonate immersion at a concentration of 250 mg/L buffered to a pH of 7 to 7.5 with sodium bicarbonate. Fish were declared dead after 30 min of immersion and loss of rhythmic opercular movement.
Statistical analysis.
HPLC data from the pilot study and experiment are reported using descriptive statistics. Prevalence and observational data from the pilot study were analyzed by Fisher exact test. P values < 0.05 were considered statistically significant.
For the experimental prevalence results, the proportion of infected fish was compared between the 5 treatments at 0, 5, 10, and 16 wk using a linear mixed model. The model contained fixed effects of treatment, week, and the interaction between treatment and week, and a random effect of tank due to the repeated sampling from the tanks over the study weeks. Fixed effects were tested using F tests with the Kenward-Roger approximation for the degrees of freedom. Although in theory, the infection data could have been analyzed at the fish level using a generalized linear mixed model with a binomial distribution, this model would suffer from separation because some treatment groups had no infections (i.e., no variability) at some weeks.
All analyses were performed using the R statistical software package, version 4.2.3 (R Core Team, Vienna, Austria). All graphs and plots were created using the GraphPad Prism software package (GraphPad Software, Boston, MA).
Results
Pilot study.
Table 1 displays results of HPLC analysis of feeds used in the pilot study. The concentration of ALB in the feed was 66% of the expected concentration after 1 d of frozen storage and 58% of the expected concentration after 28 d of frozen storage. After 1 d of frozen storage, the FUM diet contained 298% of the expected concentration based on a dose of 15 mg/kg body weight. After 28 d of frozen storage, the FUM concentration was 234% of the expected concentration. As discussed below, zebrafish in the pilot study tolerated this higher concentration of FUM in the diet; therefore, a FUM dose of 15 mg/kg was used in the subsequent experiment. Given that the dietary ALB concentration was lower than expected, the dose of ALB was increased to 2 mg/kg for the subsequent experiment.
Table 1.
High-performance liquid chromatography results for medicated feeds generated for the (A) pilot study and (B) experiment
| A. Pilot study | |||
|---|---|---|---|
| Expected concentration (ppm) | Actual concentration (ppm) | % of expected | |
| 1-d frozen | |||
| ALB | 72 | 47 | 66% |
| FUM | 750 | 2,233 | 298% |
| 28-d frozen | |||
| ALB | 72 | 41 | 58% |
| FUM | 750 | 1,754 | 234% |
| % change in concentration after 4-wk frozen | |||
| ALB | −13% | ||
| FUM | −21% | ||
| B. Experiment | ||||
|---|---|---|---|---|
| Expected concentration (ppm) | Actual concentration (ppm) | % of expected | ||
| 1-d frozen | ||||
| ALB | 100 | 46 | 46% | |
| FUM | 750 | 1762 | 235% | |
| combination | ALB FUM |
100 750 |
51 1,258 |
51% 168% |
| 28-d frozen | ||||
| ALB | 100 | 47 | 47% | |
| FUM | 750 | 2,098 | 280% | |
| combination | ALB FUM |
100 750 |
47 1,639 |
47% 219% |
| % change in concentration after 4-wk frozen | ||||
| ALB | 3% | |||
| FUM | 19% | |||
| combination | ALB FUM |
−7% 30% |
||
The expected concentration (ppm) is the concentration of ALB or FUM in the feeds based on dosing calculations. The control diet contained no measurable FUM or ALB, and those results are omitted from this table.
Each group was observed daily at 5, 10, 15, and 60 min after feeding to determine the time required for each group to finish the medicated or control diet. All groups finished their corresponding diets within 60 min on all 28 d of the pilot study (Figure 1). On day 26 of the 28-d study, the ALB group finished their diet in less than 5 min. In contrast, on day 22 of the 28-d study, the FUM group finished their diet in 15 to 60 min. The control group finished their diet in less than 10 min on 27 of the 28 d. A Fisher exact test in which ALB and FUM were each compared with the control demonstrated a significant association between the administered diet and duration of time to finish the diet; the ALB group required significantly less time (P = 0.0043) and the FUM group required significantly more time (P < 0.0001) to finish their respective diets as compared with the control group (Figure 1).
Figure 1.

For the 28-d pilot study, each study group was observed daily and the time required to finish the diet recorded. On most days, the albendazole group took less than 5 min to finish the diet (no feed observed in the tank after 5-min post-feeding), whereas on most days the fumagillin group took over 15 min to finish the diet. All groups finished the diet by 60-min post-feeding. Fisher exact test was used for statistical analysis where albendazole and fumagillin were each compared to the control and post hoc analysis using a Benjamini-Hochberg correction. A P value < 0.05 was considered significant.
Table 2 displays the results of WB-qPCR performed on fish enrolled in the pilot study. Of the 30 fish, one fish from the ALB group was found dead on day 26 of the study. Histopathology of this fish demonstrated large aggregates of acid-fast bacilli in the coelom, consistent with Mycobacterium infection. No Pn spores or clusters were observed on histopathology of this fish. The 29 fish remaining at the end of the experiment had an overall Pn prevalence of 7% (Table 2). A Fisher exact test comparing prevalence results between the ALB or FUM groups and the control group found no statistically significant difference between the Pn prevalence between treated and untreated (control) groups. However, the overall Pn prevalence of 7% demonstrates that the initial Pn exposure, which occurred 16 wk prior to the conclusion of the pilot study, was successful in establishing Pn infection in this naïve zebrafish population.
Table 2.
Pn prevalence of zebrafish after the pilot study
| Number of fish exposed | Number of fish that died during the study | Number of fish that tested positive for Pn after 28-d pilot study | Prevalence | P a | |
|---|---|---|---|---|---|
| ALB | 10 | 1 | 1 | 11% | > 0.99 |
| FUM | 10 | 0 | 0 | 0% | > 0.99 |
| Control | 10 | 0 | 1 | 10% | |
| Total | 30 | 1 | 2 | 7% |
Prevalence was calculated as the number of Pn-infected fish identified on whole-body qPCR divided by the total number of live fish at the end of the 28-d pilot study. One fish that died from the ALB group in the pilot study was excluded from the calculations of ALB group prevalence and total prevalence.
Fisher exact tests were used to analyze results from either the ALB or FUM groups compared with those of the infected control group. A P < 0.05 was considered significant.
Experiment.
Similar to HPLC results from the pilot study, HPLC results from the experiment (Table 1B) showed that the ALB diet contained less ALB than expected based on a dose of 2 mg/kg body weight. As with the pilot study, the FUM diet prepared for the experiment contained more FUM than expected. Over 4 wk of frozen storage, the measured concentration of FUM in the experimental diet samples increased by 19%, whereas the measured ALB concentration increased by 3%. For the combination diet, the ALB concentration decreased by 7% and the FUM concentration increased by 30% after storage (Table 1B). These results differ from the pilot study results, which demonstrated reductions in ALB and FUM concentrations in the diets after frozen storage.
The experiment began 32 wk after initial Pn exposure and 16 wk after the pilot study. At the beginning of the experiment, an overall baseline prevalence of 44% was calculated as the average pretreatment (week 0) prevalence among all groups (Table 3). Figure 2 shows that Pn prevalence was 0% at the time of initial exposure, increased to 7% based on results from the pilot study, and increased to 44% among all groups in the experiment at week 0. Notable changes in prevalence occurred at week 10 of the experiment when the prevalence in the infected control group and ALB treatment group were lower than prevalence values at other time points (Figures 2 and 3). The Pn prevalence for the uninfected control group was 0% at all time points.
Table 3.
Pn prevalence at each time point for each experimental group
| Prevalence before treatment | Fumagillin | Albendazole | Combination | Infected Control | |
|---|---|---|---|---|---|
| Week 0 | 44% | 38% | 50% | 38% | 50% |
| Week 5 | 44% | 25% | 13% | 31% | |
| Week 10 | 31% | 0% | 56% | 6% | |
| Week 16 | 14% | 44% | 15% | 40% |
The prevalence in the uninfected control group was 0 at all 4 time points and is not included in this table or the overall prevalence calculation. The average Pn prevalence before treatment (week 0) across all groups was 44%. There was no significant interaction between treatment and time point (P = 0.22), or significant main effect of treatment or time point (P = 0.43, P = 0.15, respectively) where a P value < 0.05 was considered significant.
Figure 2.
The left side of the Y axis shows Pn prevalence following Pn exposure. At the beginning of the experiment (week 0), overall Pn prevalence across groups was 44%. The right side of the Y axis demonstrates Pn prevalence for each experimental group over time following treatment. The Pn prevalence obtained for the uninfected control group was 0 at all time points, and this group is omitted from this figure.
Figure 3.
Plots demonstrating Pn prevalence over time as determined by whole-body qPCR at 5, 10, and 16-wk time points. The figure shows predicted means and associated standard error bars from raw data. Prevalence of the infected control group was compared to each of the treatment groups. There was no significant association between treatment group and Pn prevalence. The Pn prevalence obtained for the uninfected control group was 0 at all time points, and this group is omitted from this figure.
The proportion of Pn-infected fish was compared among the 5 groups at the 4 experimental time points using a linear mixed model that included fixed effects of treatment, week, and interaction between treatment and week, and a random effect of tank due to the repeated samplings from the tanks over the study weeks. Fixed effects were tested using F tests with the Kenward-Roger approximation for the degrees of freedom. A statistically significant association was not detected between any experimental group and Pn prevalence at any time point. However, several notable reductions in Pn prevalence occurred in different treatment groups at several time points. For example, the prevalence for the combination group was 38% at week 0, fell to 13% at week 5 and rose to 15% at week 16 (Table 3). Similarly, the prevalence for the FUM group was 38% at week 0, and fell to 14% at week 16. In addition, the Pn prevalence in the ALB group decreased by 25% between weeks 0 and 5.
During the experiment, 7 fish were found dead and one fish was euthanized for clinical reasons. These deaths and euthanasia occurred outside of the 4 experimental time points. WB-qPCR of these 8 fish was uniformly negative for Pn. Despite the loss of these 8 fish, the proportion of fish remaining in each group at each time point was relatively uniform. Furthermore, there was not one group lost notably more fish than others.
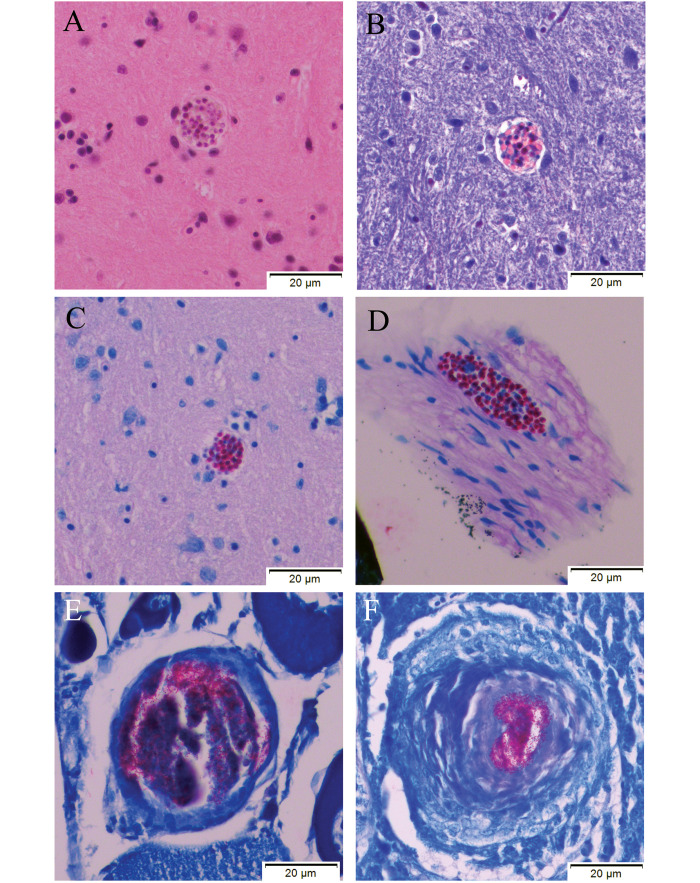
At the conclusion of the experiment, a small number of zebrafish that had been exposed to the initial Pn inoculum remained. This population of fish had not been enrolled in the pilot study or experiment. Approximately 12 mo after exposure to Pn, one fish from this population was found unmoving at the bottom of its tank with multifocal erosions over the coelom. This fish was euthanized, and histopathology demonstrated Pn parasite clusters in the hindbrain and meninges (Figure 4 A-D). In addition, incidental granulomas containing acid-fast bacilli (suspected Mycobacterium spp.) were found in the ovary and liver (Figure 4 E and F). Ten clinically-normal fish from this population were euthanized approximately 13 mo after exposure to Pn and were evaluated histologically for teaching purposes. Of these 10 fish, 4 demonstrated concurrent infection with Pn and acid-fast bacilli. Figure 5 shows a large Pn parasite cluster (circled) and aggregates of acid-fast bacilli (arrows: suspected Mycobacterium spp.) in the ovary of one of these 4 fish.
Figure 4.
Histology results for a zebrafish that was exposed to the Pn inoculum and was found at the bottom of a tank with multifocal superficial erosions over the coelom approximately 12-mo post-exposure. This fish was not enrolled in either the pilot study or the experiment, although it had been exposed to the same inoculum as infected fish enrolled in these studies. This animal was euthanized and processed for histology. All images were obtained at 40× magnification. (A-C) Images show a Pn xenoma within the hindbrain of the fish (A) H and E, (B) Luna, (C) Fite Faraco. Image D shows another xenoma at the level of the meninges (D) Fite Faraco. Incidental granulomas containing acid-fast bacteria (suspected Mycobacterium sp.) were seen in the ovaries (E) Fite Faraco and liver (F) Fite Faraco. Zebrafish exposed to Pn in this study were reared in a facility with endemic M. chelonae identified on previous surveillance monitoring reports.
Figure 5.

Histology results for a zebrafish that was exposed to the Pn inoculum but was not enrolled in either the pilot study or the experiment, although it had been exposed to the same inoculum as infected fish enrolled in these studies. This animal did not demonstrate any clinical signs and was euthanized 13-mo post-exposure. This histology section, stained with Fite Faraco, demonstrates numerous Pn spores within a parasite cluster (circled) in the ovary, as well as aggregates of acid-fast bacilli (arrows: suspected Mycobacterium sp).
Discussion
In line with our hypotheses, we established a population of Pn-infected zebrafish and found that the medicated diets appear palatable and safe for use in adult zebrafish. In both the pilot study and experiment, the HPLC results demonstrated that diets contained more FUM and less ALB than was expected based on dosing calculations. Finally, as discussed below, no treatment was significantly associated with reduced Pn prevalence at any time, although biologically relevant reductions in Pn may have occurred in groups treated with FUM.
According to the product label, the FUM product used to create the FUM diet is water soluble. This FUM product is manufactured for the treatment of the microsporidian parasite Nosema apis of honeybees, and product instructions state that the powder should be dissolved in water before feeding to honeybees. The water solubility of the ALB solution used to formulate the ALB diet is 0.54 mg/L at 25 °C at a pH of 7.28 We did not measure the pH of the diets that contained ALB or FUM. The medicated diets were formulated at room temperature (approximately 20 °C). At the time of this study, pharmaceutical-grade FUM was not available. The FUM product used in this study contains 20 g/kg FUM powder. This product may have contained more than the labeled 20 g/kg of FUM, resulting in the increased FUM concentration measured by HPLC.
The FUM and ALB products were shaken as recommended prior to use, and the medicated diets were thoroughly mixed by hand after the addition of ALB and/or FUM. However, if the products were not thoroughly mixed before or after their addition to the dietary vehicle, the concentrations of ALB and/or FUM in the diets may not have been homogeneous. Therefore, HPLC analyses of the diets may have detected concentrations of FUM or ALB that were artifactually high or low due to mixing errors.
In the pilot study, the concentrations of both FUM and ALB in the diets decreased over time (-21 and -13% change in concentration of the FUM and ALB diets, respectively, over 4 wk of frozen storage). In the experiment, FUM concentrations increased over time, and ALB concentration increased in the ALB-only diet but decreased in the combination diet (Table 1B). These findings may reflect mixing errors. A previous study measured the drug concentration of pelleted zebrafish feed that used vegetable oil to coat the feed with emamectin and ivermectin.2 HPLC analysis of the feed revealed that ivermectin and emamectin concentrations were 4.5 to 12.5 times greater than the expected concentrations based on dose calculations.2 The results from this study and ours emphasize the importance of verifying drug concentrations in medicated feeds to obtain accurate drug dosing.
For unknown reasons, the concentration of FUM and ALB in the diets depreciated over time in the pilot study but increased over time in the experiment. A previous study found that the concentration of ALB in an oral suspension formulation decreased below the recommended level (per the United States Pharmacopeia) following 9 mo of storage according to manufacturer instructions.27 The same bottle of ALB was used in both the pilot study and experiment, and this product was stored in manufacturer-recommended conditions between our 2 studies. This storage period may, in part, account for the reduced ALB concentration in the pilot study samples but does not account for the higher concentration found in the experimental HPLC results for the ALB diet. We are unaware of studies that evaluate the stability of FUM during prolonged storage. Validating drug concentrations in medicated diets, such as those used in this study, is essential to generate reproducible research. We encourage the use of HPLC or other methods to verify drug concentrations in investigational diets administered to zebrafish and other species.
In the pilot study, zebrafish took significantly longer to finish the FUM diet than the ALB or control diets. Therefore, the increased concentration of FUM in that diet (based on HPLC results) may have been unpalatable or aversive to fish, such that they took longer to consume the FUM diet. This finding may also be related to low study numbers, as we included only 10 fish/group in the pilot study, with no replicate tanks for each group. Fish in the FUM group may have been slower to consume the feed due to individual variation or environmental variables (i.e., fish in this group preferred to consume algae over the FUM diet). In the pilot study, groups finished the diet (no leftover feed was observed) within 60 min. Therefore, we conclude that the medicated diets are palatable and will be consumed by adult zebrafish.
During the pilot study, one fish died. Histopathology of this fish, which was in the ALB group, revealed multifocal coelomic granulomas containing acid-fast bacilli, consistent with Mycobacterium infection. No other deaths or evidence of morbidity was observed during the pilot study for any group. During the experiment, one fish was euthanized for clinical reasons, and 7 fish were found dead. WB-qPCR of these 8 experimental fish was negative for Pn. The 8 losses were associated with different experimental groups; therefore, no one group (control or treatment) had more deaths than did the others.
The limited morbidity and mortality observed in the pilot study and experiment across treatment and infected control groups supports the safety of the medicated diets and the safety of oral administration of FUM and ALB. Our study is limited in that we did not characterize FUM and ALB tissue distribution in zebrafish or investigate the effects of these drugs on reproduction and other physiologic parameters. Future toxicologic work should investigate the effects of FUM and ALB in zebrafish.
The pilot study revealed no statistically significant difference in Pn prevalence across groups. This may be attributed to low study numbers, low initial (pretreatment) prevalence or reflect a true lack of statistical significance across groups. Similarly, no statistically significant associations between treatment group and Pn prevalence were observed at any time point in the experiment. However, despite lacking statistical significance, biologically relevant reductions in Pn prevalence may have occurred at some time points for the combination and FUM groups as compared with the baseline, pretreatment prevalence. A biologically relevant effect has been defined as “… as an effect considered by expert judgement as important and meaningful for human, animal, plant, or environmental health. It therefore implies a change that may alter how decisions for a specific problem are taken.”3 In the context of our study, reductions in Pn prevalence, while not statistically significant, are noteworthy as they may impact zebrafish health and reduce Pn prevalence in a population, thereby impacting the significance of Pn as a confounding factor in zebrafish research.
In the experiment, the week 5 time point immediately followed the 28-d treatment period. Therefore, any immediate effect of the treatments should have been observed at this time point. At week 5, the prevalence of Pn in the combination group was 25% lower than the baseline prevalence of this group. Compared with the overall pretreatment prevalence across groups, the combination group prevalence at week 5 decreased by 31% (Table 3). Although these reductions were not statistically significant, the reduction in prevalence at week 5 in the combination therapy group may represent a biologically relevant finding. This finding may indicate that combination therapy may be useful for quarantine treatment for Pn in zebrafish.
After week 5, any residual effects of treatment would have been observed at weeks 10 and 16. In week 10 of the experiment, the Pn prevalence in ALB and infected control groups fell to 0 and 6%, respectively (Table 3). Though not statistically significant, the low prevalence in the ALB group may reflect possible therapeutic effects of the treatment; however, potential therapeutic effects were apparently lost at later time points when Pn prevalence increased for this group (Figure 3). In addition, Pn prevalence was markedly higher in the combination group at week 10 as compared with previous and later time points.
A plausible explanation for the week 10 results in the ALB, combination, and infected control groups is natural variation in Pn prevalence over time. Despite the dogma that Pn prevalence generally increases in populations over time given the horizontal and vertical transmission routes of this pathogen,22 previous work has demonstrated considerable variability in Pn prevalence in zebrafish populations over time.22 For example, another study found that Pn prevalence at 60-d post-exposure was 100% among tested males but only 60% at 304-d post-exposure in males tested from the same tank.22 Furthermore, a review of Pn transmission in zebrafish indicates that Pn prevalence varies widely in association with a number of factors, including the type of exposure (natural or experimental), tissues tested, days postexposure, diagnostic test used (histology compared with PCR), and zebrafish line or strain.22
The results of our experiment, particularly in the infected control group, support previous work demonstrating variation in reported Pn prevalence at different time points in a population.22 Future work should study why this variation in Pn prevalence occurs (diagnostic method, cyclical nature of the parasite, environmental factors influencing Pn prevalence, etc.) and how this information could be used to control this pathogen in zebrafish facilities. Newer, nonlethal diagnostic methods, such as environmental water sampling, may also provide information that contributes to our understanding of Pn prevalence in populations over time.21,23
At the final experimental time point, week 16, Pn prevalence decreased by more than 20% for the combination and FUM treatment groups as compared with week 0 prevalence values for those groups (Table 3). Compared with the overall pretreatment prevalence of 44%, the reductions in Pn prevalence at weeks 5 and 16 for the combination group and week 16 for the FUM group are even more striking. Although not statistically significant, these differences may represent biologically relevant residual effects of FUM or combination therapy on Pn prevalence. However, the reductions in prevalence at these time points could also reflect natural variation of Pn prevalence over time. Regardless, the results of our experiment merit replication. Despite our discussion of potential biologic relevance of FUM and combination treatments at multiple time points, we reiterate that no statistically significant associations were detected in this study between any treatment and Pn prevalence at any time point.
Microsporidian parasites of salmonids have been successfully treated with FUM with or without ALB.7,26 These microsporidian parasites include L. salmonae and N. salmonis, which infect salmonid endothelial and hematopoietic cells, respectively.10 In contrast, Pn spores are characteristically found in the central nervous system, though spores may be detected in other tissues.19,20,22 Therefore, unlike therapies required to treat L. salmonae or N. salmonis, therapies targeting Pn must cross the blood-brain barrier to achieve therapeutic concentrations in the central nervous system. As in other species, zebrafish have a blood-brain barrier that excludes toxins and promotes neuronal homeostasis.16 A metabolite of ALB, albendazole sulfoxide, is reported to cross the blood-brain barrier in humans.5 It is unclear whether therapeutic concentrations of ALB and FUM were achieved in the plasma and neural tissues of zebrafish used in our study. Future studies of Pn in zebrafish should investigate the pharmacokinetics and distribution of these drugs in zebrafish blood and the central nervous system.
Like many zebrafish used in biomedical research, those used in this study were sourced from a colony that consistently tests positive for Mycobacterium spp. on routine pathogen surveillance. Multiple fish exposed to the initial Pn inoculum were later found with evidence of coinfection with a Mycobacterium sp. and Pn on histopathology (Figures 4 and 5). Previous work describes the role of Pn as a stressor that may induce immunosuppression in fish; Pn infection and associated clinical signs may also be exacerbated by environmental stress.17,18 Mycobacterium spp., opportunistic pathogens ubiquitous in zebrafish facilities, may have served as a confounding variable in this study by inducing clinical signs and morbidity distinct from those induced by Pn infection. Given the opportunistic nature of both Mycobacterium spp. and Pn, complex and multifactorial variables are likely to influence coinfection of fish with these pathogens. The influence and effects of opportunistic pathogens in Pn infection and disease progression warrant further investigation, including how coinfection may alter or confound zebrafish research.
In our experiment, the reduction in Pn prevalence at select time points in the FUM and combination treatment groups may have biological relevance. These findings warrant further investigation into these treatments, particularly FUM, as potential therapies for Pn. Other methods of oral dosing, such as top-coating pelleted feeds with FUM or ALB, may be alternatives to the gel-based diet used in this study. Top-coating pelleted feeds, previously used to administer oral emamectin and ivermectin to zebrafish, involves spraying the feed with vegetable oil.2 Given the low water solubility of ALB, this top-coating method may be superior to the gel-based formulation that we used with regard to achieving therapeutic doses of ALB in the feed. However, given the water solubility of FUM, the gel-based dietary vehicle may be superior to top-coating for oral delivery of FUM to zebrafish.
As previously discussed, HPLC results from our study and a previous publication suggest that using medicated diets likely results in inaccurate drug dosing of zebrafish.2 Creating medicated feeds for exploratory research on treatments for fish diseases presents several challenges, including the logistical challenges of incorporating small drug volumes into small batches of hand-mixed fish feed. Although microsporidia are treated orally in other species, for zebrafish, using immersion therapy for Pn may be less time intensive than oral dosing with potentially more accurate drug dose delivery, but has not been investigated to our knowledge.
Pn remains a threat to animal welfare and a confounder in zebrafish research, thereby impacting the quality and reproducibility of research that uses zebrafish. The best method to control this pathogen continues to be strict quarantine and biosecurity practices in zebrafish facilities, as well as routine pathogen surveillance using a variety of diagnostic modalities.13 The findings of this study suggest that FUM or combination FUM and ALB therapy may be associated with biologically relevant reductions in Pn prevalence at certain time points after treatment; further investigations of these therapies are warranted.
Acknowledgments
We thank Dwayne Schrunk and Thomas Olsen of the Iowa State Veterinary Diagnostic Lab for assistance in dose calculations and HPLC analysis of medicated diet samples. We also thank Dr. Lynn Johnson of the Cornell Statistical Consulting Unit for her assistance in performing statistical analyses. This research was supported by the Grants for Laboratory Animal Science (GLAS) from the American Association for Laboratory Animal Science.
References
- 1.Chang CT, Benedict S, Whipps CM. 2019. Transmission of Mycobacterium chelonae and Mycobacterium marinum in laboratory zebrafish through live feeds. J Fish Dis 42: 1425–1431. 10.1111/jfd.13071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collymore C, Watral V, White JR, Colvin ME, Rasmussen S, Tolwani RJ, Kent ML. 2014. Tolerance and efficacy of emamectin benzoate and ivermectin for the treatment of Pseudocapillaria tomentosa in laboratory zebrafish (Danio rerio). Zebrafish 11: 490–497. 10.1089/zeb.2014.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.EFSA Scientific Committee. 2011. Statistical significance and biological relevance. EFSA J 9: 17. [Google Scholar]
- 4.Didier ES, Maddry JA, Brindley PJ, Stovall ME, Didier PJ. 2005. Therapeutic strategies for human microsporidia infections. Expert Rev Anti Infect Ther 3: 419–434. 10.1586/14787210.3.3.419. [DOI] [PubMed] [Google Scholar]
- 5.González-Hernández I, Ruiz-Olmedo MI, Cardenas G, Jung-Cook H. 2012. A simple LC-MS/MS method to determine plasma and cerebrospinal fluid levels of albendazole metabolites (albendazole sulfoxide and albendazole sulfone) in patients with neurocysticercosis. Biomed Chromatogr 26: 267–272. 10.1002/bmc.1659. [DOI] [PubMed] [Google Scholar]
- 6.Han B, Weiss LM. 2018. Therapeutic targets for the treatment of microsporidiosis in humans. Expert Opin Ther Targets 22: 903–915. 10.1080/14728222.2018.1538360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Higgins MJ, Kent ML, Moran JD, Weiss LM, Dawe SC. 1998. Efficacy of the fumagillin analog TNP-470 for Nucleospora salmonis and Loma salmonae infections in chinook salmon Oncorhynchus tshawytscha. Dis Aquat Organ 34: 45–49. 10.3354/dao034045. [DOI] [PubMed] [Google Scholar]
- 8.Sciarra JB, Tyler A, Kolb A. 2014. A gelatin-based diet for oral dosing juvenile to adult zebrafish (Danio rerio). [Cited 29 September 2023]. https://www.aalas.org/articles/2014/06/01/a-gelatinbased-diet-for-oral-dosing-juvenile-to-adult-zebrafish-danio-rerio.
- 9.Kent ML, Buchner C, Watral VG, Sanders JL, Ladu J, Peterson TS, Tanguay RL. 2011. Development and maintenance of a specific pathogen-free (SPF) zebrafish research facility for Pseudoloma neurophilia. Dis Aquat Organ 95: 73–79. 10.3354/dao02333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kent ML, Shaw RW, Sanders JL. Fish Microsporidia. In: Weiss LM, Becnel JJ. editors. Microsporidia: Pathogens of Opportunity. Hoboken (NJ): John Wiley & Sons, Inc. 10.1002/9781118395264.ch20. [DOI] [Google Scholar]
- 11.Midttun HLE, Vindas MA, Nadler LE, Øverli Ø, Johansen IB. 2020. Behavioural effects of the common brain-infecting parasite Pseudoloma neurophilia in laboratory zebrafish (Danio rerio). Sci Rep 10: 8083. 10.1038/s41598-020-64948-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Midttun HLE, Vindas MA, Whatmore PJ, Øverli Ø, Johansen IB. 2020. Effects of Pseudoloma neurophilia infection on the brain transcriptome in zebrafish (Danio rerio). J Fish Dis 43: 863–875. 10.1111/jfd.13198. [DOI] [PubMed] [Google Scholar]
- 13.Mocho JP, Collymore C, Farmer SC, Leguay E, Murray KN, Pereira N. 2022. FELASA-AALAS recommendations for monitoring and reporting of laboratory fish diseases and health status, with an emphasis on zebrafish (Danio rerio). Comp Med 72: 127–148. 10.30802/AALAS-CM-22-000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Molina JM, Tourneur M, Sarfati C, Chevret S, de Gouvello A, Gobert JG, Balkan S, Derouin F, Balkan S, Derouin F. Agence Nationale de Recherches sur le SIDA 090 Study Group 2002. Fumagillin treatment of intestinal microsporidiosis. N Engl J Med 346: 1963–1969. 10.1056/NEJMoa012924. [DOI] [PubMed] [Google Scholar]
- 15.Murray KN, Dreska M, Nasiadka A, Rinne M, Matthews JL, Carmichael C, Bauer J, Varga ZM, Westerfield M. 2011. Transmission, diagnosis, and recommendations for control of Pseudoloma neurophilia infections in laboratory zebrafish (Danio rerio) facilities. Comp Med 61: 322–329. [PMC free article] [PubMed] [Google Scholar]
- 16.Quiñonez-Silvero C, Hubner K, Herzog W. 2020. Development of the brain vasculature and the blood-brain barrier in zebrafish. Dev Biol 457: 181–190. 10.1016/j.ydbio.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Ramsay JM, Watral V, Schreck CB, Kent ML. 2009. Pseudoloma neurophilia infections in zebrafish Danio rerio: Effects of stress on survival, growth, and reproduction. Dis Aquat Organ 88: 69–84. 10.3354/dao02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanders JL, Monteiro JF, Martins S, Certal AC, Kent ML. 2020. The Impact of Pseudoloma neurophilia infection on body condition of zebrafish. Zebrafish 17: 139–146. 10.1089/zeb.2019.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanders JL, Peterson TS, Kent ML. 2014. Early development and tissue distribution of Pseudoloma neurophilia in the zebrafish, Danio rerio. J Eukaryot Microbiol 61: 238–246. 10.1111/jeu.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanders JL, Watral V, Clarkson K, Kent ML. 2013. Verification of intraovum transmission of a microsporidium of vertebrates: Pseudoloma neurophilia infecting the Zebrafish, Danio rerio. PLoS One 8: e76064. 10.1371/journal.pone.0076064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schuster CJ, Kent ML, Peterson JT, Sanders JL. 2022. Multi-state occupancy model estimates probability of detection of an aquatic parasite using environmental DNA: Pseudoloma neurophilia in zebrafish aquaria. J Parasitol 108: 527–538. 10.1645/22-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schuster CJ, Kreul TG, Al-Samarrie CE, Peterson JT, Sanders JL, Kent ML. 2022. Progression of infection and detection of Pseudoloma neurophilia in zebrafish Danio rerio Hamilton by PCR and histology. J Fish Dis 45: 1463–1475. 10.1111/jfd.13675. [DOI] [PubMed] [Google Scholar]
- 23.Schuster CJ, Murray KN, Sanders JL, Kent ML. 2023. Application of an eDNA assay for the detection of Pseudoloma neurophilia (Microsporidia) in zebrafish (Danio rerio) facilities. Aquaculture 564: 739044. 10.1016/j.aquaculture.2022.739044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spagnoli S, Sanders J, Kent ML. 2017. The common neural parasite Pseudoloma neurophilia causes altered shoaling behaviour in adult laboratory zebrafish (Danio rerio) and its implications for neurobehavioural research. J Fish Dis 40: 443–446. 10.1111/jfd.12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spagnoli ST, Xue L, Murray KN, Chow F, Kent ML. 2015. Pseudoloma neurophilia: A retrospective and descriptive study of nervous system and muscle infections, with new implications for pathogenesis and behavioral phenotypes. Zebrafish 12: 189–201. 10.1089/zeb.2014.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Speare DJ, Athanassopoulou F, Daley J, Sanchez JG. 1999. A preliminary investigation of alternatives to fumagillin for the treatment of Loma salmonae infection in rainbow trout. J Comp Pathol 121: 241–248. 10.1053/jcpa.1999.0325. [DOI] [PubMed] [Google Scholar]
- 27.Yagoub M, Abdoun S, Seri H. 2013. Effect of storage conditions on the stability of albendazole and oxytetracycline veterinary products marketed in Sudan. Bull Pharm Sc Assiut Universityi 36: 49–57. 10.21608/bfsa.2013.63198. [DOI] [Google Scholar]
- 28.Zoetisus.com. Safety Data Sheet. [Cited 14 May 2023]. https://www.zoetisus.com/content/_assets/docs/vmips/safety-data-sheets/valbazen.pdf.