Abstract

This work highlighted the counterion association of diphenhydramine hydrochloride (DPC) and chlorpheniramine maleate (CPM) with anionic sodium tetradecyl sulfate (STS) by conductivity, fluorescence, and UV spectrophotometer measurements. The presence of drugs and the formation of premicellar aggregates of STS were highlighted. The modified Corrin–Harkins CH approaches assessed the STS counterion binding values B = 0.300 for DPC and 0.379 for CPM in the aqueous media at 25 °C. The counterion binding constant (βc) and Gibb’s free energy of micellization (ΔGmic°) were increased and became more negative, suggesting that the drug–surfactant interaction was controlled by electrostatic interaction. Furthermore, the spectral study evaluated that the three isosbestic points for CPM and one isosbestic point for DPC in the STS micelles were observed, which confirmed that CPM was more binding than DPC with the STS micelles. The differential absorbance spectra study was applied to UV spectra to determine the binding constants (Kb) of 2.232 and 2.837 and partition coefficients (Kx) of 286.64 and 3209.21 for DPC and CPM in the presence of STS micelles. The findings demonstrated that the CPM molecules have been associated with the Palisade layer of the STS micelles, and the DPC molecules were bound to the Stern layer of the STS micelles. Finally, we came to the conclusion that ionic drugs could improve the micellization capabilities of surfactants, which might be useful for choosing the best excipients for pharmaceutical applications.
1. Introduction
Numerous drug–surfactant assemblies have been researched recently because of the vital significance of the pharmacological and therapeutic interactions between the drugs and the surfactant.1−6 Combining a drug and a surfactant shows interaction and provides greater features than the drug or surfactant functioning independently.7−12 Ionic medications have grown in importance recently as organic counterions or additions to enhance the interfacial and micellization properties of a charged surfactant and also to boost the concentration of pharmaceuticals that are only partially dissolved in the aqueous medium.13,14 Whenever there are extra ingredients such as electrolytes, the critical micelle concentration (CMC) of the surfactants decreases.15,16 Since they decrease their efficacy surface area per headgroup by limiting undesirable contact between each headgroup, additives, remarkably, make it simpler to induce an appearance change in surfactant micelles. The total amount of counterions that are physically associated with a micelle is compared to the amount of surfactant molecules (aggregate number) that reside in an ionic micelle to determine the counterion binding constant.13−20 The most often used acceptable methods for estimating the counterion binding constant are the Corrin–Harkins (CH),21 modified CH approach,21 and the slope–ratio of conductance method.22
Many more investigators have shown in recent years which organic counterions can improve the micellization functions of surfactants in the aqueous medium, for example, tetraethyl and tetra-n-propylammonium,23n-alkyl carboxylate ions24 and tetraalkylammonium counterions.25−27 Our research groups are currently investigating the micellization properties of surfactants in the presence of organic additives such as ionic drugs, dyes, and polyelectrolytes in the aqueous medium.13,14,19,28,29
Antihistaminic medications were widely used for their ability to make weakly water-soluble substances soluble in an aqueous environment. Shah and Flanagan30 evaluated the impact of self-associations of antihistamine drug salts on the dissolution of salicylamide and acetaminophen in water-based solutions. We have reported that DPC and cetirizine hydrochloride (CTZ) drugs were used as organic counterions to reduce the CMC ionic surfactants.13,14 We observed that counterions reduced the CMC of sodium dilauramidoglutamide lysine (SDGL), sodium dodecyl sulfate (SDS), and sodium dodecylbenzenesulfonate (SDBS). Another use of ionic drugs (CTZ, CPM, and DPC) as counterions in surfactant interactions was to increase the attraction and occupancy of poorly water-soluble drugs, such as anti-inflammatory (ethenzamide, ibuprofen) and antifungal drugs (clotrimazole and itraconazole).13,14
Based on these considerations, current research work intended to explore the micellization abilities of sodium tetradecyl sulfate in the presence of organic counterions chlorpheniramine maleate (CPM) and diphenhydramine hydrochloride (DPC). Diphenhydramine, an ethanolamine and first-generation histamine antagonist with antiallergic properties, is available as DPC in the salt form. By competitively inhibiting H1-receptors, DPC decreases histamine action on bronchial smooth muscle, capillaries, and gastrointestinal smooth muscle. This lessens the effects of histamine on bronchoconstriction, vasodilation, increased capillary permeability, and gastrointestinal smooth muscle spasms. An alkylamine derivative called CPM is used to treat allergic responses, such as hay fever, rhinitis, urticaria, and asthma. Chlorpheniramine Maleate is an antihistamine with anticholinergic, moderate sedative, and competitive histamine H1 receptor antagonist effects. In this study, the DPC and CPM molecule used as organic counterions on STS in the aqueous media was investigated using conductivity, fluorescence, and UV absorbance experiments. The differential absorption spectral study was used to determine the binding constant (Kb) and partition coefficients (Kx) parameters of DPC and CPM in the existing STS micelle.
2. Experimental Section
2.1. Materials
STS (CAS no: 1191-50-0, Purity: 95%) was purchased from Sigma-Aldrich, India. DPC (CAS no. 147-24-0, Purity: 98%) and CPM (CAS no: 113-92-8, Purity: 99%) were purchased from Research-Lab Fine Chem. industries, India. Water used was triple-deionized and had a specific conductivity of between 0.5 and 1.0 μS/cm (±5% μS/cm) at 25 °C. In Figure 1, the compound structures are exposed.
Figure 1.
Chemical structure of (A) STS, (B) DPC and (C) CPM.
2.2. Experimental Procedure
2.2.1. Conductivity Technique
An STI-475 (Sky Technology India) conductivity meter was used for determining the conductance values with every addition of diluted STS in the DPC and CPM solutions. We utilized a known volume of water (20 mL) in the sample vessels and slowly introduced STS solution at 25 ± 0.2 °C to evaluate conductance. Then, we gradually added STS solution containing DPC/CPM (0.1–20 mmol L–1) to sample vessels at 25 °C while using DPC/CPM (0.1–20 mmol L–1) solution as a solvent. After every sample insertion, the conductance values were recorded. A stock solution of STS, DPC, and CPM in distilled water was first formed at a concentration of 50 mmol L–1. In the CMC experiment, 10 mL (25.0 mmol L–1) of STS was combined with the solution in a 50 mL volumetric flask. In the preceding solution, DPC/CPM solutions in various quantities of 0.1 mL (0.1 mmol L–1), 0.3 mL (0.3 mmol L–1), 0.6 mL (0.6 mmol L–1), 1.0 mL (1.0 mmol L–1), 3.0 mL (3.0 mmol L–1), 6 mL (6.0 mmol L–1), 10 mL (10.0 mmol L–1), 15 mL (15.0 mmol L–1), and 20 mL (20.0 mmol L–1) were added. After careful mixing of the aforementioned solutions, the sample was marked up in 50 mL of water.
2.2.2. Steady-State Fluorescence Measurements
Using a pyrene fluorescence probe, we determined the CMCs of STS in the presence and absence of various DPC and CPM concentrations that varied from 0.1 to 15 mmol L–1. In brief, fixed pyrene concentrations (0.0375 mmol L–1) were used for making several sets of STS containing DPC/CPM solutions. A fluorescence spectrometer (Hitachi F-7000) was then used to measure the fluorescence at an excitation wavelength of 336 nm and record spectra between 350 and 400 nm. Fluorescence intensity ratios of I373/I384 (I1/I3) to micelle concentrations were used to represent the CMC values. According to Aguair et al.’s approach,31 CMCs were calculated using I1/I3 data, and I1/I3 data were fitted to the Boltzmann equation (a sigmoid type equation) as follows:
| 1 |
where I1/I3 ratios A1 and A2 represent the ratios at low and high concentrations, respectively.
2.2.3. Spectral Study
The Kb and Kx values for DPC and CPM in the presence of STS micelles were determined by using a Shimadzu UV-1800 UV spectrophotometer. In this investigation, a baseline for the UV experiment was established using water. The STS solution was selected higher than CMC in the aqueous medium for UV examinations. In the prepared STS solutions, the DPC and CPM concentrations were held constant at 0.1 mmol L–1. DPC and CPM measurements were conducted at 260 nm for all samples.13,14,32
3. Result and Discussion
3.1. Effect of DPC/CPM on the Micellization of STS
The conductivity measurement (specific conductance (κ) μS cm–1 vs CSTS mmol L–1) and fluorescence measurements (I1/I3 vs DPC/CPM concentrations (mmol L–1)) were assessed to find out the CMC of the STS in the aqueous and the DPC and CPM medium. When the STS solution contains DPC and CPM, it exhibits a distinct form of aggregation behavior with two conductance breakdowns, as seen in Figures 2 and 3. The formation of pre-micellar deviation points known as critical aggregation concentration (CAC) indicated that drug–surfactant monomeric interaction was detected at extremely low STS concentrations.33 The surfactant CMC was determined by the post-micellar deviation points (second break) at greater concentrations of STS, as illustrated in Figures 2 and 3 and Table 1. The CMC of STS was quite close to the values reported in the literature.34−36
Figure 2.
Plots of the specific conductance (κ) of STS (mmol L–1) in varying DPC concentrations (mmol L–1) in an aqueous medium at 25 °C. Standard deviation, ur = ±5%.
Figure 3.
Plots of the specific conductance (κ) of STS (mmol L–1) in varying CPM concentrations (mmol L–1) in an aqueous medium at 25 °C. Standard deviation, ur = ± 5%.
Table 1. Values of CAC (mmol L–1), CMC (mmol L–1), βc, and ΔG°mic (kJ mol–1) for STS in the DPC and CPM Medium at 25 °Ca.
| drugs(mmol L–1) | CAC(mmol L–1) | CMC(mmol L–1) | CAC(mmol L–1) | CMC(mmol L–1) | βc | ΔG°mic |
|---|---|---|---|---|---|---|
| conductance | fluorescence | |||||
| DPC | ||||||
| 0.000 | 2.200 | 2.390 | 0.631 | –24.73 | ||
| 0.100 | 0.884 | 2.130 | 1.985 | 0.640 | –24.99 | |
| 0.300 | 0.861 | 1.820 | 1.400 | 0.657 | –25.91 | |
| 0.600 | 0.708 | 1.610 | 1.251 | 0.670 | –26.62 | |
| 1.000 | 0.600 | 1.350 | 1.017 | 0.698 | –27.80 | |
| 3.000 | 0.536 | 0.900 | 0.731 | 0.716 | –29.82 | |
| 6.000 | 0.342 | 0.780 | 0.514 | 0.704 | –30.22 | |
| 10.00 | 0.221 | 0.590 | 0.439 | 0.665 | –30.68 | |
| 15.00 | 0.217 | 0.530 | 0.303 | 0.670 | –31.21 | |
| 20.00 | 0.220 | 0.500 | 0.677 | –31.58 | ||
| CPM | ||||||
| 0.000 | 2.200 | 2.390 | 0.631 | –24.73 | ||
| 0.100 | 0.718 | 1.800 | 1.556 | 0.723 | –26.98 | |
| 0.300 | 0.686 | 1.640 | 1.259 | 0.745 | –27.73 | |
| 0.600 | 0.600 | 1.410 | 1.019 | 0.755 | –28.54 | |
| 1.000 | 0.501 | 0.920 | 0.769 | 0.763 | –30.54 | |
| 3.000 | 0.750 | 0.603 | 0.778 | –31.70 | ||
| 6.000 | 0.450 | 0.328 | 0.790 | –34.18 | ||
| 10.00 | 0.390 | 0.294 | 0.774 | –34.50 | ||
| 15.00 | 0.340 | 0.275 | 0.779 | –35.22 | ||
| 20.00 | 0.310 | 0.771 | –35.45 | |||
Standard deviations (u) were u(T) = ± 0.2 °C. Standard deviations, ur for CAC/CMC = ±5%, ur for βc = ±5%, and ur for (ΔGmic°) = ±5%.
Surfactant micellization in the presence of the organic additive has been demonstrated to exhibit similar behavior below the CMC37−39 Shahir et al.37 reported two breakdowns for tetradecyltrimethylammonium bromide (TTAB) with the addition of 2 × 10–5 mol L–1 tartrazine dye, which was identical to the breaks seen in both systems STS-CPM and STS-DPC. The two TTAB breaks in the tartrazine solution were thought to correlate to two CMC values, the initial owing to the creation of dye-rich micelles and the second due to the development of surfactant-rich micelles. The distinction in the two breaks was interpreted as the result of TTAB replacing the TTAB-tartrazine ion pairs at the self-assembled monolayer.37 A comparable explanation was suggested for the breaks observed in CTAB (cetyltrimethylammonium bromide), TTAB, CPB (cetylpyridinium bromide), and CPC in a fixed concentration of Congo red aqueous solution.38 Our research group earlier showed that in the presence of 3.6 × 10–5 mol L–1 PR, CPC exhibits two breakdowns in the surface tension experiment.19 Bhattarai39 investigated the micellization characteristics of cetyltrimethylammonium bromide in the presence of sodium polystyrenesulfonate in water at 298.15 K and discovered two breakdowns in the conductance studies.
In the current systems, STS-DPC and STS-CPM, the appearance of first break (CAC) in STS conductance plots was observed with increasing of DPC from 0.1 to 20 mmol L–1 and CPM from 0.1 to 1.0 mmol L–1 in the micellar solutions. As indicated in Table 1, the concentrations of CAC dropped as the concentration of DPC increased in the micellar medium. We previously reported that the CMC of DPC was 92.2 mmol L–1 and CPM was 66.8 mmol L–1 in water,40,41 and that at lower concentrations of STS molecules, monomeric interactions between STS-DPC and STS-CPM were discovered, with the first break occurring. The CMC of STS was thought to be the second or most prominent break in the conductance graphs. Due to decreased hydrophobicity, the DPC molecules may be forced to occupy the in-between the Stern layer and outer Palisade layer of the STS micelle. As the concentration of DPC increased from 0.1 to 20 mmol L–1, two breaks were seen, as shown in Figure 2. The excess free DPC molecules remained in the micellar medium, which was responsible for the pre- and postmicellar interaction. CPM had higher hydrophobicity than DPC, and as STS concentration increased, CPM was more solubilized inside the STS micelle. This could be the possible reason that at >1.0 mmol L–1 of CPM, only one break was found in the conductance plots, as shown in Figure 3.
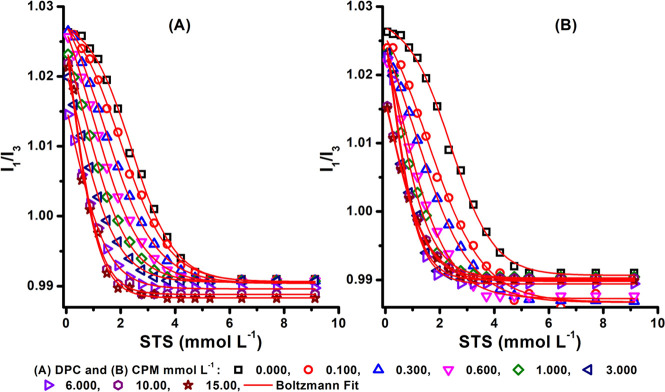
From Figure 4A, B it was observed that the I1/I3 polarity of STS-DPC and STS-CPM was decreased with increased concentration of STS in the mixtures, and CMC values were also decreased in all mixtures of DPC/CPM from 0.1 to 15 mmol L–1. This indicates the formation of STS micelles to be due to the penetration of DPC and CPM molecules into the micelles, which favored the formation of drug-based micelle in the solution. There was no CAC break observed in the fluorescence measurements. A similar behavior has also been reported for drug–surfactant interaction in our previous study.13,14
Figure 4.
Representative plots of pyrene intensity (I1/I3) ratios vs STS concentrations (mmol L–1) in varying (A) DPC and (B) CPM concentrations (mmol L–1) in an aqueous medium at 25 °C. Standard deviation, ur = ± 5%.
As DPC and CPM concentrations increased, the CMC of the STS began to decrease, as seen in Table 1. Due to variances in the methods used, the CMC values from conductivity studies and fluorescence measurements were not exactly the same.42 The STS CMC was lower in CPM than in DPC, as shown in Table 1 and Figure 5A. A comparable noteworthy decline in CMCs of ionic surfactants in organic drugs has been reported in our group. We reported that the CMC of sodium dodecylbenzenesulfonate (SDBS) was significantly reduced in the existence of DPC and cetirizine hydrochloride (CTZ) as counterion ions.13
Figure 5.
(A) Difference of the CMC of STS, plots of the (B) Corrin–Harkins (CH) and (C) modified CH equations for the STS-DPC and STS-CPM systems. Standard deviations, ur = ±5%.
3.2. Counterion Binding of STS in the Presence of DPC and CPM
The Corrin–Harkins (CH) equation15 is frequently used to calculate the counterion binding constant (β) of a surfactant.
| 2 |
The term ce represents the concentration of added organic ionic additives (DPC/CPM) and A is a constant associated with the standard free energy of micellization. Eq 2 is applicable for an ionic surfactant + organic ionic additive system only if the added ionic additives contain the same counterion. When the ionic surfactant (STS) and additional ionic additives (DPC/CPM) contain distinct counterions, the modified eq 3 for mixed counterions system was utilized.16,21
| 3 |
where A′ = ΔG°m/[(1 + β1)RT] and B = β2/(1 + β1). ΔG°m is the standard energy of micellization per mole of an ionic surfactant. β1 and β2 are the counterion binding constants of the counterion from STS and the counterion from the added ionic additives (DPC/CPM), respectively. The total counterion binding constant was β = β1 + β2. When ce = 0, B likewise went to zero, and eq 3 became lncmc = A′, giving the commonly used equation for the standard free energy of ionic surfactant micellization. Interestingly, unlike the CH equation, the modified form of the CH equation cannot obtain the value β directly from the slope.16Figure 5B,C depicts the plots of the CH and modified CH equations. According to Figure 5 B, the CH equation was not appropriate in the cases of STS-DPC and STS-CPM. We observed uniformity in the characteristics of the plots for STS in the presence of diphenhydramine (DP+) and chlorpheniramine (CP+) counterions by using a modified CH equation, as shown in Figure 5C. The modified CH equation plot for STS-DPC and STS-CPM occurs on linear regions, comparable to the modified CH plot for SDBS-DPC and SDBS-CTZ systems.13
By the least-squares fitting data shown in Figure 5C, we got B = 0.300 in the case of DPC and B = 0.379 in the case of CPM, respectively. Earlier, we reported13 that B = 0.414 and 0.52, respectively, in the case of SDBS-DPC and SDBS-CTZ systems, where SDBS was the surfactant and DPC and CTZ were counterions. Similarly, we reported B = 0.594 and 0.148 in the case of DPC as a counterion in the presence of surfactants sodium dilauramidoglutamide lysine and sodium dodecyl sulfate,14 respectively.
Considering β1 (=m1/n) and β2 (=m2/n) reflected the surfactant’s counterion binding constants for sodium ion (Na+) and DP+ or CP+ counterions,16 respectively. The STS aggregate number (n) was ≈66.34 The amount of drugs coupled for each micelle (β = m/n) for STS-CPM was 34.49 and that for STS-DPC was 27.3, correspondingly. The number of CP+ ions in STS micelles was greater than the number of DP+ ions. In the occurrence of DPC, the B value for STS micelles was revealed to contain a greater electrostatic interaction through CP+ than DP+. We hypothesized that the counterion of the cationic drug would prefer to bind to the STS micelle and be responsible for decreasing counterion (Na+) binding to the STS micelle. Collins’ law of equivalent aqueous affinities indicated the preferential binding of DP+ to TS– and CP+ to TS– micelles.43 The Hofmeister series44 was frequently parallel to the ionic activity. Kosmotropes stayed hydrated above the exterior of the water, whereas chaotropes lost their hydration cover. According to Collins’ law, kosmotropic ion-kosmotropic ion and chaotropic ion-chaotropic ion association were strong because both produced ion-pair connection without intermediary water molecules, whereas kosmotropic ion-chaotropic ion synergy developed solvent extraction ion-pairs. DP+ and CP+ ions were also chaotropic, but Na+ ions were Kosmotropic. In light of Collins’ rule, STS head groups were projected to include extra relevance for DP+ and CP+ than Na+, explaining the inhibition of Na+ binding in the existence of DPC and CPM.
We needed the value of the βc, to calculate the ΔG°mic of the STS surfactant. For ionic surfactants, βc was calculated using a straightforward approach known as the slope-ratio method, in which βc = 1 – S2/S1, in which S2 and S1 correspond to the post-micellar and pre-micellar slopes of the κ vs CSTS (Figures 2 and 3) plots.22 As illustrated in Figure 6A, the slope–ratio process provided βc values for the STS-DPC and STS-CPM systems. The plot of βc vs drug demonstrated that when STS micelle was formed with DPC and CPM, βc of STS micelle increased confirming that the overall charge of TS– + DP+ and TS– + CP+ micelle was greatly raised caused by electrostatic interaction.
Figure 6.
Plots of (A) βc, and (B) ΔG°mic of STS in the presence of varying DPC and CPM concentrations (mmol L–1) at 25 ± 0.2 °C.
Using eq 4,22 ΔG°mic was determined. Results are shown in Table 1.
| 4 |
where Xcmc, R, and T are, respectively, the mole fraction units of surfactants, the gas constant, and the temperature in Kelvin. Figure 6 depicts the plots of βc and ΔG°mic for STS in the presence and absence of DPC and CPM in the aqueous medium, and Table 1 lists the results. The values of βc and ΔG°mic for STS have been found with values of 0.63 and −24.74 kJ mol–1,34,35 While it was shown in Table 1, the STS βc and ΔG°mic values improved when DPC and CPM concentrations improved from 0.1 to 20.0 mmol L–1.
The ΔG°mic values were entirely negative, indicating that STS and drugs (DPC/CPM) had a complete synergistic effect. The STS system βc values were greater in the CPM than in the DPC medium. The optimal occupancy of CP+ ions in STS micelles relative to DP+ ions could influence it.13
3.3. Spectroscopy Approach for STS Interaction with DPC/CPM
3.3.1. Simple Absorption Spectra of DPC and CPM with STS
We obtained visible spectra of DPC and CPM in an aqueous solution to identify the spectral characteristics of the system under investigation. The visible spectra shift from 200 to 300 nm was used to analyze the interaction of DPC/CPM (0.1 mmol L–1) with the STS micelle, as shown in Figure 7A, B. The two noticeable peaks in the STS micelles were detected at ≈222 and 260 nm for DPC and 226 and 260 nm for CPM. The strong host–guest interaction between DPC/CPM and STS has led to the observation of the hyperchromic shift at 260 nm in spectra. When STS concentrations increased along with the presence of DPC, the hypsochromic shift (blue shift) was seen from 222 to 212 nm. Similar to this, STS-CPM showed a hypsochromic effect from 226 to 223 nm. When DPC and CPM were combined with the STS micelle, isosbestic spots were seen, indicating a strong interaction between the two substances. As seen in Figure 7A, the isosbestic point for the STS-DPC system was discovered at 231.9 nm in the aqueous medium. As illustrated in Figure 7B, three isosbestic points for CPM were detected in the presence of STS micelles at 206, 222, and 237 nm. The appearance of three ionic species—a chloro-bound complex in the first instance, a pyridine-bound complex in the second, and an amino-bound complex in the third—led to the discovery of three isosbestic points. The development of a single STS-DPC complex, such as an amino-bound complex, was used to detect the existence of isosbestic points.
Figure 7.
UV spectra of (A) DPC and (B) CPM at (0.1 mmol L–1) in a different concentration of STS (mmol L–1) at 25 °C ± 0.2 °C in the aqueous medium.
3.3.2. Differential Absorption Spectra of DPC/CPM with STS
Drug partitioning between the aqueous and micellar phases and the binding of drugs to surfactants in terms of the binding constant and partition coefficient, respectively, may be conveniently assessed using the differential spectroscopic technique.
3.3.2.1. Binding Constant of DPC and CPM
The UV absorbance method was used to estimate the binding constants (Kb) of DPC and CPM in the STS solution. DPC and CPM in aqueous media were chosen to operate at wavelengths of 260 ± 2 nm. Figure 7A, B shows the UV spectra of DPC and CPM in STS micellar solution. As implied above from the values of the B and βc, the amount of drug ions required to an ionic micelle may be greater than one in the event of drug–micelle synergism, mainly when the drug and micelle were charged and the drug functions as a counterion to the ionic micelle.19 If drug cation binding to micelles were considered a distinct equilibrium, it might be represented as eq 5:
| 5 |
where Dfree+ stands for drug cation in the free state (free CP+/DP+), Mmic for the TS– micelle, Dmic+ for drugs cation bound to the micelle (binding CP+/DP+), and Nm for the ratio of micelle moles to bound drug moles, respectively. The activity terms for the aforementioned equilibrium (eq 6) may be replaced with the appropriate concentration terms because the concentrations of the various species were relatively low.
| 6 |
| 7 |
Considering that each micelle could be bound to multiple Dfree+ ions (CP+/DP+), all of the micelles were accessible for utilization during the drug binding process, and the micelle concentration was thus taken to be equal to the total STS micelle concentration. According to Srivastava and Ismail,19 the measured absorbance, A, corresponding to Dfree+ (CP+/DP+) might be represented as follows:
| 8 |
A is the measured absorbance in STS solutions, and Aw is the absorbance of the drugs in the aqueous medium. Amic is the drug’s absorption when combined with micelles. The STS concentration is c (above the CMC), where c0 is the STS CMC. Kb functions as a binding constant. The results in Figure 8 were plotted as 1/(A – Aw) versus 1/(c – c0); the linearity of the graph showed that eq 8 was suitable when the Nm amount was 1.0. The absorbance experiments revealed a 1:1-type coupling between the drugs and the micelle, even though the stoichiometry term (Nm) of the drug–micelle association is incorporated in eq 8. In this study, a value of 0.9 for Nm was found to be the best match for the data. Therefore, it was chosen that the fitted value of Nm was approximately equivalent to 1, and the absorbance data showed that one drug was bound to each micelle. Other studies have already reported on the variance of Nm values.13,14,19,28,29,32,40,41 For instance, Caetano and Tabak45 used a relation similar to eq 8 to analyze the absorbance data of the drugs trifluoperazine and chlorpromazine in aqueous cetyltrimethylammonium chloride solution, resulting in found values of Nm in the variety of 0.90–1.14 for chlorpromazine and in the range of 1.00–1.12 for trifluoperazine. Pellosi et al.46 reported values of Nm around 1 for the four xanthenes dyes fluorescein, eosin Y, erythrosin B, and rose bengal B in an aqueous pluronic P-123 solution, however, they observed values of Nm close to 2 in an aqueous cetyltrimethylammonium bromide solution.
Figure 8.

Plots STS-DPC (black square) and STS-CPM (red circle) comparable to eq 8 exhibit the distinction of 1/(A – Aw) with 1/(c – c0)Nm. The red line represents the linear fit to determine the Kb from the intercept and slope ratio.
Furthermore, the Kb value was determined using the slope and intercept data from Figure 8. For DPC and CPM, the values of log Kb were 2.232 and 2.837, respectively. Kb values revealed that CPM had higher Kb values than DPC in the STS micelle derived from eq 8. Because the CPM contained three binding sites and the DPC contained just one, it could be possible that the electrostatic interaction between TS– and CP+ is greater than that between TS– and DP+.
Additionally, it seemed noteworthy that the Kmb values had been determined at CMC through the following equation.19,47
| 9 |
where n represents the surfactant aggregation number and Kmb represents the binding constant at CMC. For DPC and CPM, the value of Kmb (log Kmb) was determined to be 4.053 and 4.663, respectively, which was greater than the value derived from eq 8. The two binding constant values (2.232 and 4.053 for DPC and 2.837 and 4.663 for CPM) differed because eq 8 offers the Kb value at STS concentrations above the CMC whereas eq 9 gives the Kmb value at the CMC. As the limiting value of the equilibrium constant for a certain drug-micelle association, the value of Kmb at the CMC may be taken into consideration.
3.3.2.2. Partition Coefficient of DPC and CPM
UV absorbance from Figure 7A, B in terms of the partition coefficient (Kx) was used to assess the partitioning function of DPC and CPM between the aqueous and micellar phases. As seen in Figure 7, the enhanced solubility of the drug molecules in the STS micelle essentially prompted the absorbance of DPC and CPM to increase with the increase in the STS concentration. Using the Kawamura model,48 the coefficient for the partition (Kx) was calculated:
| 10 |
When ΔA is used to indicate differential absorbance, ΔA∞ is used to represent differential absorbance at infinity, and cms is used to represent the total concentration of (c – c0). The partition coefficient, Kx, is obtained by multiplying Kc by nw (Kx = Kc× nw),49 where Kc is the partition constant with unit dm3 mol–1. The water molarity in this equation, nw, is 55.5 mol dm–3. The partition coefficient data were analyzed based on how much the micellar medium had been dispersed. The value of Kc was used to calculate the intercept and slope values of the plot between 1/ΔA and 1/(ce + (c – c0)), as shown in Figure 9. For DPC and CPM in the presence of STS micelle, the values of Kc were 5.164 and 57.82 dm3 mol–1 and Kx was observed to be 286.64 and 3209.21, respectively. Because of the substantial electrostatic association among TS– and CP+, the value of CPM was significantly greater than that of DPC, indicating that the CP+ ions were occupied inside the palisade layer of the micelle with the greatest number possible. The DP+ molecules’ lower Kx values suggested that they were kept in the Stern layer of the STS micelle even though they had an electrostatic interaction.
Figure 9.

Kawamura plot between 1/ΔA and 1/(ce + (c – c0)) for the calculation of Kx for STS-DPC and STS-CPM.
4. Conclusions
This investigation showed that the CMC of STS in aqueous media at 25 °C was decreased by the synergistic action of drugs (DPC/CPM). Since a drug and an STS were opposing charges, the CH and modified CH approaches were employed to analyze the binding of drugs (DP+/CP+) to ionic TS– micelles. The CH approach revealed that the difference appeared at lower drug concentrations. The change of STS’s CMC with drugs (DPC/CPM) concentration was satisfactorily described by the modified CH equation. Na+ counterions were shown to adhere to Collins’ ion-specificity rule, but their binding to TS–micelles was suppressed by the binding of DP+ and CP+ counterions, which were more prevalent. The values of Kb were obtained through the UV absorbance technique, and it was shown that electrostatic associations govern the binding of DP+ and CP+ to TS– micelles. Analyzing the absorbance values revealed almost 1:1 type binding between drugs and STS micelle while taking into consideration the stoichiometry of the drug-micelle binding. The partition coefficient (Kx) figures showed that the amount of drugs encapsulated increased in the presence of the STS micelle. They were computed using differential absorbance data. The Kb and Kx values indicated that CPM had a greater level of binding and encapsulation than DPC, demonstrating that CPM molecules were incorporated into the palisade layers of micelles, whereas DPC molecules were supported on the Stern layer. Finally, this study suggested that the CMC values of surfactants in the existence of ionic drugs could potentially be used to lower the amount of surfactants (disintegrating agents) in pharmaceutical formulations.
Acknowledgments
The work was supported by the Researchers Supporting Project number (RSP2023R393), King Saud University, Riyadh, Saudi Arabia.
Author Contributions
A.S.: conceptualization, data curation, writing-original draft, review and editing, M.K.: experimental, data curation, review and editing, A.B.: experimental, D.E.: experimental, O.G.S.: data curation, S.K.S.: data analysis, M.K.G.: data curation and review and editing, M.I.: data curation and review and editing, N.S.: review and editing.
The authors declare no competing financial interest.
References
- Pokhrel D. R.; Sah M. K.; Gautam B.; Basak H. K.; Bhattarai A.; Chatterjee A. A recent overview of surfactant–drug interactions and their importance. RSC Adv. 2023, 13, 17685–17704. 10.1039/D3RA02883F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra N.; Ray D.; Aswal V. K.; Ghosh S. Exploring physicochemical interactions of different salts with sodium n-dodecanoyl sarcosinate in aqueous solution. ACS Omega 2018, 3 (8), 9256–9266. 10.1021/acsomega.8b00718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed I.; Das N.; Maidul Islam A. K. M.; Plaisier J. R.; Parisse P.; Bal J. K. Interfacial interactions of a Myoglobin/DOPC hybrid system at the air–water interface and its physicochemical properties. ACS Omega 2023, 8, 30199–30212. 10.1021/acsomega.3c02909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharova L. Ya.; Voloshina A. D.; Ibatullina M. R.; Zhiltsova E. P.; Lukashenko S. S.; Kuznetsova D. A.; Kutyreva M. P.; Sapunova A. S.; Kufelkina A. A.; Kulik N. V.; Kataeva O.; Ivshin K. A.; Gubaidullin A. T.; Salnikov V. V.; Nizameev I. R.; Kadirov M. K.; Sinyashin O. G. Self-Assembling metallocomplexes of the amphiphilic 1,4- diazabicyclo[2.2.2]octane derivative as a platform for the development of nonplatinum anticancer drugs. ACS. Omega 2022, 7, 3073–3082. 10.1021/acsomega.1c06465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teoman B.; Muneeswaran Z. P.; Verma G.; Chen D.; Brinzari T. V.; Almeda-Ahmadi A.; Norambuena J.; Xu S.; Ma S.; Boyd J. M.; Armenante P. M.; Potanin A.; Pan L.; Asefa T.; Dubovoy V. Cetylpyridinium Trichlorostannate: Synthesis, antimicrobial properties, and controlled-release properties via electrical resistance tomography. ACS Omega 2021, 6, 35433–35441. 10.1021/acsomega.1c04034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afzal S.; Lone M. S.; Nazir N.; Dar A. A. pH changes in the micelle–water interface of surface-active ionic liquids dictate the stability of encapsulated Curcumin: an insight through a unique interfacial reaction between arenediazonium ions and t-butyl hydroquinone. ACS Omega 2021, 6, 14985–15000. 10.1021/acsomega.1c01119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rub M. A.; Sheikh M. S.; Khan F.; Azum N.; Alghamdi Y. G.; Asiri A. M. Impact on micellization between promethazine hydrochloride and ester bonded gemini surfactant in distinct solvents: A multi-faceted procedure. J. Mol. Liq. 2021, 342, 117477 10.1016/j.molliq.2021.117477. [DOI] [Google Scholar]
- Rub M. A.; Khan F.; Asiri A. M. The influence of various solvents on the interaction between gemini surfactant (ester-bonded) and imipramine hydrochloride: an aggregational, interfacial, and thermodynamic study. J. Mol. Liq. 2021, 334, 116524 10.1016/j.molliq.2021.116524. [DOI] [Google Scholar]
- Rub M. A. Effect of additives on the aggregation phenomena of amphiphilic drug and hydrotrope mixtures. J. Mol. Liq. 2020, 298, 112049 10.1016/j.molliq.2019.112049. [DOI] [Google Scholar]
- Rub M. A.; Pulikkal A. K.; Azum N.; Asiri A. M. The assembly of amitriptyline hydrochloride + triton X-45 (non-ionic surfactant) mixtures: Effects of simple salt and urea. J. Mol. Liq. 2022, 356, 118997 10.1016/j.molliq.2022.118997. [DOI] [Google Scholar]
- Rub M. A.; Azum N.; Alfaifi S. Y. M.; Sakib M. M. H.; Ali M. A.; Mahbub S.; Halim M. A.; Hoque M. A.; Asiri A. M. Assessment of mixed micellization behavior of amitriptyline hydrochloride and TX-165 surfactant mixture in various media (aqueous/NaCl/urea). J. Mol. Liq. 2023, 383, 122073 10.1016/j.molliq.2023.122073. [DOI] [Google Scholar]
- Kumar D.; Khan F.; Rub M. A.; Azum N.; Asiri A. M. Interactions between promethazine hydrochloride drug and sodium benzoate hydrotrope mixtures in various solvent media at different temperatures. J. Mol. Liq. 2021, 325, 115188 10.1016/j.molliq.2020.115188. [DOI] [Google Scholar]
- Srivastava A.; Kumar M.; Deb D. K.; Muzaffar F.; Singh S. Utilization of amphiphilic antihistamines drugs to enhance micellization of anionic surfactant and improve the binding and solubility of Itraconazole drug. J. Mol. Liq. 2022, 348, 118018 10.1016/j.molliq.2021.118018. [DOI] [Google Scholar]
- Srivastava A.; Uchiyama H.; Imano H.; Satone H.; Iimura K.; Kadota K.; Tozuka Y. Enhanced micellization of gemini surfactants using diphenhydramine hydrochloride as an organic counterion. J. Mol. Liq. 2020, 300, 112288 10.1016/j.molliq.2019.112288. [DOI] [Google Scholar]
- Corrin M. L.; Harkins W. D. The effect of salts on the critical concentration for the formation of micelles in colloidal electrolytes. J. Am. Chem. Soc. 1947, 69, 684–688. 10.1021/ja01195a065. [DOI] [PubMed] [Google Scholar]
- Ismail K. Influence of counterions on the aggregation of ionic surfactants and the corrin–harkins equation. J. Indian Chem. Soc. 2018, 95, 1471–1480. 10.5281/zenodo.5644547. [DOI] [Google Scholar]
- Lunkenheimer K.; Prescher D.; Geggel K. Role of counterions in the adsorption and micellization behavior of 1:1 ionic surfactants at fluid interfaces—demonstrated by the standard amphiphile system of alkali perfluoro–n–octanoates. Langmuir 2022, 38, 891–902. 10.1021/acs.langmuir.1c00527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana N.; Dey J.; Ismail K. Effect of added counterions on the micellization of cetylpyridinium chloride in the presence of acetonitrile–water mixture. J. Chem. Eng. Data 2019, 64, 4193–4205. 10.1021/acs.jced.9b00260. [DOI] [Google Scholar]
- Srivastava A.; Ismail K. Binding of phenol red to cetylpyridinium chloride at air solution and micelle–solution interfaces in aqueous ethylene glycol media. Colloids Surf., A 2014, 462, 115–123. 10.1016/j.colsurfa.2014.08.021. [DOI] [Google Scholar]
- Thapa U.; Kumar M.; Chaudhary R.; Singh V.; Singh S.; Srivastava A. Binding behaviour of hydrophobic drug tetracaine hydrochloride used as organic counterion on ionic surfactants. J. Mol. Liq. 2021, 335, 116564 10.1016/j.molliq.2021.116564. [DOI] [Google Scholar]
- Mukhim T.; Dey J.; Das S.; Ismail K. Aggregation and adsorption behavior of cetylpyridinium chloride in aqueous sodium salicylate and sodium benzoate solutions. J. Colloid Interface Sci. 2010, 350, 511–515. 10.1016/j.jcis.2010.06.070. [DOI] [PubMed] [Google Scholar]
- Singh O. G.; Ismail K. Micellization behavior of mixtures of sodium dioctylsulfosuccinate with sodium dodecylsulfate in water. J. Surfact. Deterg. 2008, 11, 89–96. 10.1007/s11743-007-1058-y. [DOI] [Google Scholar]
- Mukerjee P.; Mysels K. J.; Kapauan P. Counterion specificity in the formation of ionic micelles–size, hydration, and hydrophobic bonding effects. J. Phys. Chem. 1967, 71, 4166–4175. 10.1021/j100872a702. [DOI] [Google Scholar]
- Anacker E. W.; Underwood A. L. Organic counterions and micellar parameters. n–alkyl carboxylates. J. Phys. Chem. 1981, 85, 2463–2466. 10.1021/j150617a011. [DOI] [Google Scholar]
- Liu J.; Li X.; Hou J.; Liu F. Electric–field–induced interface behavior of dodecyl sulfate with large organic counterions: a molecular dynamics study. J. Phys. Chem. B 2020, 124, 5498–5506. 10.1021/acs.jpcb.0c00129. [DOI] [PubMed] [Google Scholar]
- Thapa U.; Dey J.; Kumar S.; Hassan P. A.; Aswal V. K.; Ismail K. Tetraalkylammonium ion induced micelle–to–vesicle transition in aqueous sodium dioctylsulfosuccinate solutions. Soft Matter 2013, 9, 11225–11232. 10.1039/c3sm52215f. [DOI] [Google Scholar]
- Ullah S.; Yao K.; Zhang P.; Wang Y.; Chen Z.; Liu C.; Wang C.; Xu B. Effect of added tetraalkylammonium counterions on the dilational rheological behaviors of N–Cocoyl Glycinate. J. Oleo Sci. 2020, 69, 883–891. 10.5650/jos.ess20031. [DOI] [PubMed] [Google Scholar]
- Kumar M.; Raj S.; Thapa U.; Singh S.; Srivastava A. Investigation the effect of sodium carboxymethylcellulose as polycounterion on cetirizine hydrochloride–sodium dodecyl sulphate mixed micelle. J. Mol. Liq. 2021, 322, 114973 10.1016/j.molliq.2020.114973. [DOI] [Google Scholar]
- Srivastava A.; Elahi D.; Kumar M.; Raghav S.; Singh O. G.; Singh N. Binding influence of sunset yellow dye on the sodium tetradecyl sulphate micelles in the presence of sodium carboxymethyl cellulose medium. J. Mol. Liq. 2023, 385, 122375 10.1016/j.molliq.2023.122375. [DOI] [Google Scholar]
- Shah S. P.; Flanagan D. R. Solubilization of salicylamide and acetaminophen by antihistamines in aqueous solution. J. Pharm. Sci. 1990, 79, 889–892. 10.1002/jps.2600791009. [DOI] [PubMed] [Google Scholar]
- Aguiar J.; Carpena P.; Molina-Bolívar J. A.; Ruiz C. C. On the determination of the critical micelle concentration by the pyrene 1:3 ratio method. J. Colloid Interface Sci. 2003, 258, 116–122. 10.1016/S0021-9797(02)00082-6. [DOI] [Google Scholar]
- Srivastava A.; Liu C.; Fang H.; Lv J.; Qiao W. Interaction and binding efficiency of cationic drug chlorpheniramine maleate – anionic amino acid gemini surfactants mixture as media for the synthesis of silver nanoparticles. Colloids Surf., A 2017, 529, 686–695. 10.1016/j.colsurfa.2017.06.053. [DOI] [Google Scholar]
- Jain N.; Trabelsi S.; Guillot S.; McLoughlin D.; Langevin D.; Letellier P.; Turmine M. Critical aggregation concentration in mixed solutions of anionic polyelectrolytes and cationic surfactants. Langmuir 2004, 20, 8496–8503. 10.1021/la0489918. [DOI] [PubMed] [Google Scholar]
- Patra N.; Mal A.; Dey A.; Ghosh S. Influence of solvent, electrolytes, β–CD, OTAB on the krafft temperature and aggregation of sodium tetradecyl sulfate. J. Mol. Liq. 2019, 280, 307–313. 10.1016/j.molliq.2019.02.002. [DOI] [Google Scholar]
- Kumar H.; Kaur G. Induced alterations in the aggregation behavior and thermodynamic properties of anionic surfactant sodium tetradecyl sulfate (STS) in presence of imidazolium based ionic liquid in aqueous media: A conductometric study. J. Physics: Conference Series. 2020, 1531, 012100 10.1088/1742-6596/1531/1/012100. [DOI] [Google Scholar]
- Rafati A. A.; Gharibi H.; Iloukhani H.; Safdari L. Aggregation number of ionic surfactants and its application for alkyltrimethyl ammonium bromides and sodium tetradecyl sulfate by potentiometric technique. Phys. Chem. Liquids 2003, 41, 227–238. 10.1080/0031910021000044438. [DOI] [Google Scholar]
- Shahir A.; Javadian S.; Razavizadeh B. B. M.; Gharibi H. Comprehensive study of tartrazine/cationic surfactant interaction. J. Phys. Chem. B 2011, 115, 14435–14444. 10.1021/jp2051323. [DOI] [PubMed] [Google Scholar]
- Rashidi Alavijeh M.; Javadian S.; Gharibi H.; Moradi M.; Tehrani Bagha A. R.; Shahir A. A. Intermolecular interactions between a dye and cationic surfactants: Effects of alkyl chain, head group, and counterion. Colloid Surf. A 2011, 380, 119–127. 10.1016/j.colsurfa.2011.02.011. [DOI] [Google Scholar]
- Bhattarai A. Micellization behavior of cetyltrimethylammonium bromide in the absence and presence of sodium polystyrene sulfonate in water and methanol–water mixture: A conductivity approach. J. Mol. Liq. 2019, 292, 111352 10.1016/j.molliq.2019.111352. [DOI] [Google Scholar]
- Kumar M.; Khushi K.; Bhardwaj A.; Deb D. K.; Singh N.; Elahi D.; Sharma S.; Bajpai G.; Srivastava A. In–vitro study for ibuprofen encapsulation, controlled release and cytotoxicity improvement using excipient–drugs mixed micelle. Colloids Surf., A 2022, 654, 130057 10.1016/j.colsurfa.2022.130057. [DOI] [Google Scholar]
- Kumar M.; Singh V.; Choudhary R.; Deb D. K.; Singh S.; Srivastava A. Mixed micellization of drug–excipients and its application to enhance the binding and encapsulation efficacy of ibuprofen in aqueous media. Colloids Surf., A 2021, 628, 127268 10.1016/j.colsurfa.2021.127268. [DOI] [Google Scholar]
- Sharma R.; Mahajan R. K. An investigation of binding ability of ionic surfactants with trifluoperazine dihydrochloride: insights from surface tension, electronic absorption and fluorescence measurements. RSC Adv. 2012, 2, 9571–9583. 10.1039/c2ra21020g. [DOI] [Google Scholar]
- Collins K. D. Ions from the Hofmeister series and osmolytes: effects on proteins in solution and in the crystallization process. Methods 2004, 34, 300–311. 10.1016/j.ymeth.2004.03.021. [DOI] [PubMed] [Google Scholar]
- Collins K. D.; Neilson G. W.; Enderby J. E. Ions in water: characterizing the forces that control chemical processes and biological structure. Biophys. Chem. 2007, 128, 95–104. 10.1016/j.bpc.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Caetano W.; Tabak M. Interaction of chlorpromazine and trifluoperazine with anionic sodium dodecyl sulfate (SDS) micelles: electronic absorption and fluorescence studies. J. Colloid Interface Sci. 2000, 225, 69–81. 10.1006/jcis.2000.6720. [DOI] [PubMed] [Google Scholar]
- Pellosi D. S.; Estevão B. M.; Semensato J.; Severino D.; Baptista M. S.; Politi M. J.; Hioka N.; Caetano W. Photophysical properties and interactions of xanthene dyes in aqueous micelles. J. Photochem. Photobiol., A 2012, 247, 8–15. 10.1016/j.jphotochem.2012.07.009. [DOI] [Google Scholar]
- Piñeiro L.; Freire S.; Bordello J.; Novo M.; Al-Soufi W. Dye exchange in micellar solutions. Quantitative analysis of bulk and single molecule fluorescence titrations. Soft Matter 2013, 9, 10779–10790. 10.1039/c3sm52092g. [DOI] [Google Scholar]
- Kawamura H.; Manabe M.; Miyamoto Y.; Fujita Y.; Tokunaga S. Partition coefficients of homologous omega–phenylalkanols between water and sodium dodecyl sulfate micelles. J. Phys. Chem. 1989, 93, 5536–5540. 10.1021/j100351a042. [DOI] [Google Scholar]
- Sharapova A.; Ol'khovich M.; Blokhina S.; Zhirova E.; Perlovich G. Solubility and partition behavior of moxifloxacin: Experimental results and thermodynamics properties. J. Mol. Liq. 2021, 339, 116814 10.1016/j.molliq.2021.116814. [DOI] [Google Scholar]