Abstract
Background:
Brain metastases (BM) and lactate dehydrogenase (LDH) levels above the upper limit of normal (ULN) are associated with poor prognosis in patients with melanoma. Although treatment with the BRAF inhibitor dabrafenib and the MEK inhibitor trametinib have demonstrated long-term clinical benefit in patients with melanoma, data on their efficacy in patients with BM are limited.
Methods:
DESCRIBE Italy is an observational, retrospective, real-world study evaluating dabrafenib plus trametinib in 499 patients with BRAFV600-mutant stage III unresectable or stage IV melanoma from various sites across Italy. Here, we analyzed the clinical outcomes for the subgroup of patients receiving first-line treatment and presenting with BM at diagnosis and assessed the impact of predictive factors such as LDH levels and the presence of other metastases on median progression-free survival (mPFS).
Results:
Overall, 325 evaluable patients were on first-line therapy and are the focus of this analysis; of these, 76 patients (23.4%) had BM at baseline. mPFS was lower for patients with BM at baseline compared with overall patients (8.7 months vs 9.3 months, respectively). Patients with BM at diagnosis and LDH >ULN had a considerably shorter mPFS compared with patients with LDH ⩽ULN (5.3 months vs 9.9 months, respectively). mPFS was noticeably longer for patients with cerebral metastases only compared with patients with cerebral and other metastases (15.0 months vs 8.7 months, respectively).
Conclusions:
Dabrafenib plus trametinib showed effectiveness in a real-world population of patients with advanced BRAFV600-mutated melanoma and BM at baseline, supporting its use in this population with poor outcomes.
Keywords: BRAF, dabrafenib, melanoma, real-world, trametinib, chart review
Introduction
Melanoma is a poorly differentiated malignant tumor arising from melanin-producing cells (melanocytes), primarily in the skin; 1 incidence of melanoma has been increasing worldwide for the past few decades.2,3 Cutaneous melanoma is the second most common cancer diagnosis in Italy among people aged 0-49 years, with an annual average incidence of new diagnoses of 20.4 per 100,000 for men and 16.6 per 100,000 for women in 2020.4,5 Melanoma incidence varies across Italy, with the number of new cases decreasing from North to South; the effect of latitude is significant even after adjusting for several demographic and social variables.6,7
Although early diagnosis has improved survival rates, advanced melanoma management remains a challenge, with poor prognosis for patients in the metastatic stage. 8 About half of patients with advanced melanoma will develop symptomatic brain metastases (BM), which are associated with very poor prognosis and a median overall survival (OS) of two to five months.9,10 Before the advent of immune checkpoint inhibitors and targeted therapies (ipilimumab was approved by the FDA in 2011, and dabrafenib and trametinib in 2013), none of the available systemic treatments showed clinically meaningful efficacy, with responses only observed in approximately 10% of patients with BM.10,11
Lactate dehydrogenase (LDH) is a metabolic enzyme which catalyzes the reversible conversion of lactate into pyruvate, providing energy to rapidly proliferating cancer cells. 12 Expression of LDH is upregulated by hypoxia and is considered a biochemical marker of tumor burden. 13 Increased LDH levels are one of the most important prognostic factors for patients with malignant melanoma and are associated with poor survival.13,14
Targeted therapies are effective against BRAF mutations, which are present in 40–66% of all melanomas.15-17 The COMBI-d and COMBI-v Phase III clinical studies demonstrated superior efficacy for the BRAF inhibitor (BRAFi) dabrafenib in combination with the MEK inhibitor trametinib compared with BRAFi monotherapy in patients with BRAFV600-mutant metastatic melanoma.18,19 Analysis of pooled survival data from these two studies showed progression-free survival (PFS) rates of 19% at five years, with a five-year OS rate of 34%. 20 These results highlight the long-term clinical benefit of the dabrafenib/trametinib combination.
Safety and efficacy results from clinical trials were confirmed in a real-world retrospective study of patients with BRAFV600-mutant unresectable or metastatic melanoma who received dabrafenib plus trametinib as part of a compassionate use program (DESCRIBE II). 21 This study also showed lower OS for patients with known BM (9.5 months vs 15 months in those without; 15.5 months in BRAFi-naïve patients with known BM vs 20.0 months in overall BRAFi-naïve patients); lower PFS and overall response rates were also observed in this patient subpopulation. Patients with BM were also included in the DESCRIBE III study, a global real-world retrospective study in patients with unresectable or metastatic melanoma treated with dabrafenib monotherapy and/or dabrafenib plus trametinib as part of a managed access program. 22 This study categorized patients according to whether they derived long, intermediate or short-term benefit from therapy; findings showed that although the proportion of patients with BM was similar across groups, it was largest among those patients that derived short-term benefit from therapy (22.1% vs 18.7% in intermediate-term and 17.3% in long-term benefit).
DESCRIBE Italy was a retrospective, real-world chart review study evaluating the use of dabrafenib plus trametinib in patients with BRAFV600-mutant stage III unresectable or stage IV melanoma from various sites across Italy.23,24 The data confirmed the safety and effectiveness of dabrafenib plus trametinib treatment in a real-world setting and provided further support for the use of this therapeutic approach in patients with metastatic melanoma.
Here, we analyzed the clinical outcomes for the subgroup of patients from the DESCRIBE Italy study receiving first-line treatment and presenting with BM at baseline and assessed the impact of predictive factors such as LDH levels and the presence of other metastases on PFS.
Methods
Study design
The DESCRIBE Italy study was an observational, retrospective chart review study. Eligible patients with BRAFV600 mutation–positive unresectable or metastatic melanoma were aged ⩾18 years, received at least one dose of dabrafenib/trametinib as part of the Managed Access Program (MAP), and had provided signed written informed consent (the latter was not required for deceased patients). Patients were excluded from the study if they had not participated in the MAP, were part of a dabrafenib/trametinib clinical trial, or if their medical chart was missing, empty, or irretrievable.
Once patients were deemed eligible, pseudonymized retrospective data regarding baseline characteristics, treatment patterns, disease progression, and survival status were collected from the patient’s medical charts and entered into electronic Case Report Forms (eCRFs). Data for each patient were collected from the first dose of dabrafenib/trametinib until treatment discontinuation, death, last clinical encounter or until 31 October 2017, whichever occurred first. Only the data collected in the eCRFs was available for analysis. Since the data were collected retrospectively, the start date of collection is not specified.
The overall aim of the DESCRIBE Italy study was to describe the baseline features, treatment patterns, and efficacy and safety outcomes in Italian patients with BRAFV600 mutation-positive unresectable or metastatic melanoma who received dabrafenib plus trametinib during the MAP. The present work focuses on PFS in patients treated in first line with dabrafenib and trametinib and presenting with BM at baseline.
Assessment of disease progression
All assessments were performed according to the investigator’s judgement and in accordance with local clinical practice. Local guidelines recommend that diagnosis of BM is performed by cranial magnetic resonance imaging (MRI) with and without administration of contrast agent; computed tomography (CT) imaging is only recommended in patients for whom MRI is contraindicated. 25 Disease progression was documented by the treating physician based on radiographic imaging, symptoms, and performance status.
PFS was estimated using the Kaplan-Meier product-limit method and defined as the time (in months) from the initiation of dabrafenib/trametinib treatment to the date of first documented progressive disease or death due to any cause, whichever occurred first. For patients who neither progressed nor died, PFS time was censored at treatment discontinuation, last clinical encounter or 31 October 2017, whichever occurred first. For patients who started a new anticancer treatment, PFS time was censored at the date of last adequate assessment before the start of the new treatment. For enrolled subjects with no post-baseline disease assessments who did not die, PFS time was censored at the date of dabrafenib/trametinib initiation; these patients were therefore not evaluable for PFS analyses.
PFS of patients on first-line treatment with BM at baseline was compared to that of all patients on first-line treatment (Online Supplementary Figure 1, gray boxes). Two different subgroup analyses were carried out. The first compared the PFS of patients receiving first-line therapy with BM and normal LDH value at baseline vs patients with BM and LDH value > upper limit of normal (ULN) at baseline (Online Supplementary Figure 1, blue boxes). The second analysis compared the PFS of patients receiving first-line therapy with BM and other metastatic sites at baseline vs patients with cerebral metastases only at baseline (Online Supplementary Figure 1, yellow boxes).
Statistical analysis
No statistical sample size calculations were performed. This study was observational and statistical analyses were descriptive for all endpoints. Demographic and baseline disease characteristics were summarized descriptively. PFS was estimated using the Kaplan-Meier product-limit method, and two-sided 95% confidence intervals (CIs) were calculated. All statistical analyses were performed by SAS® release 9.4 (SAS Institute, Inc, Cary, North Carolina, USA).
Ethical approvals
This study was carried out in accordance with the Guidelines for Good Pharmacoepidemiology Practices of the International Society for Pharmacoepidemiology (ISPE 2016), the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines, and ethical principles outlined in the Declaration of Helsinki. All patients provided signed written informed consent (the latter was not required for deceased patients). All required local approvals from Ethics Committees were obtained before commencing data collection at each site (see Ethical Approvals notes for the names of committees).
Results
Patients
Patient demographics and baseline characteristics have been described previously 23 and are presented in Online Supplementary Figure 2. Briefly, 499 patients were enrolled, with a median age of 59 years (range, 23‒90 years). Almost half of the patients were female (46.1%) and 54.3% of patients had a baseline Eastern Cooperative Oncology Group performance status (ECOG PS) of 0. The most frequent BRAF mutation was BRAFV600E, recorded in 81.4% of patients, followed by BRAFV600K (10.6%) and other BRAFV600 mutations (i.e., V600D, V600R, and others; 7.2%). Of the 499 patients enrolled, 390 were considered evaluable (all patients in the enrolled set who started trametinib concomitantly to dabrafenib or within 90 days after dabrafenib initiation with a comparable baseline metastatic evaluation).
The majority of patients (406/499, 81.4%) were on first-line therapy. Of these patients, 58 (14.3%) had at least one prior adjuvant therapy. Among the 87/499 (17.4%) patients on a subsequent line of therapy, the most common prior antineoplastic medications were ipilimumab (38/87 patients, 43.7%), vemurafenib (28/87, 32.2%), dacarbazine (13/87, 14.9%) and temozolomide (11/87, 12.6%). Of the 406 patients on first-line therapy, 325 were considered evaluable.
A small proportion of enrolled patients had received radiotherapy for brain metastases as prior therapy before treatment with dabrafenib/trametinib (44/499 [8.8%] had received whole brain radiotherapy and 8/499 [1.6%] had received radiotherapy on the tumor bed in brain and surrounding tissue) or concomitantly with dabrafenib/trametinib treatment (43/499 [8.6%] had received whole brain radiotherapy; 12/499 [2.4%] had received radiotherapy on the tumor bed in brain and surrounding tissue, and 10/499 [2.0%] had received radiotherapy to the head). In terms of patients receiving surgery for BM, one patient was recorded as having undergone a brain tumor operation, one patient underwent craniotomy, and one patient underwent neurosurgery concomitantly with dabrafenib/trametinib treatment; surgical incomplete excision was reported in three cases. In terms of corticosteroid use, a number of patients were recorded as having received or receiving ongoing concomitant systemic steroids (prednisone, 56; dexamethasone sodium phosphate, 52; dexamethasone, 49; methylprednisolone, 13; cortisone acetate, four; cortisone, three; beclomethasone dipropionate, two; fluocortolone, hydrocortisone sodium succinate, methylprednisolone sodium succinate, triamcinolone, one each).
Out of 499 enrolled patients, 165 (33.1%) went on to receive second-line therapy, while 192 (38.5%) were recorded as not being referred for second-line therapy and 142 (28.5%) had missing information regarding second-line therapy referral.
PFS in patients receiving first-line treatment
Overall, 115/499 (23.0%) enrolled patients presented with brain metastases. This analysis focused on evaluable patients receiving first-line treatment, of which 76/325 (23.4%) presented with BM (Online Supplementary Figure 1). Median PFS was numerically lower for patients on first-line therapy with BM at baseline compared with overall patients receiving first-line treatment (8.7 months [95% CI: 6.8–10.1] vs 9.3 months [95% CI: 8.3–10.3], respectively) (Table 1). The PFS rates at one, two and three years were also consistently lower for patients with BM at baseline. Kaplan-Meier estimates of PFS for these patients are shown in Figure 1.
Table 1.
PFS in patients receiving first-line treatment.
| Subgroups | N | Number of censored patients | Number of patients with PFS events, n (%) | Median PFS (95% CI) | 1-y PFS rate, % | 2-y PFS rate, % | 3-y PFS rate, % |
|---|---|---|---|---|---|---|---|
| First-line treatment | 325 | 111 | 214 (65.9) | 9.3 | 40 | 24 | 10 |
| (8.3-10.3) | |||||||
| First-line treatment with BM at baseline | 76 | 23 | 53 (69.7) | 8.7 | 32 | 19 | 8 |
| (6.8-10.1) |
CI, confidence interval; PFS, progression-free survival; y, year.
Figure 1.
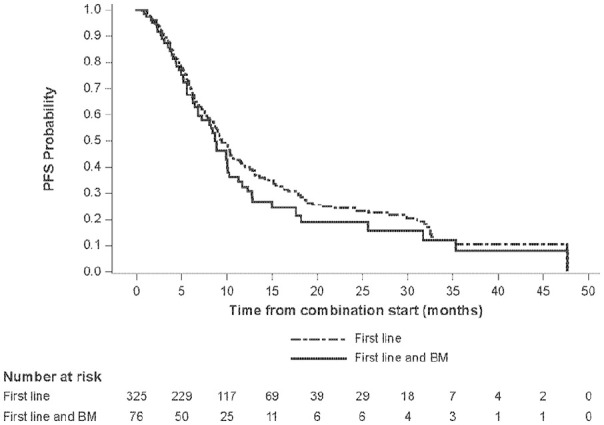
Kaplan-Meier estimate of PFS for patients receiving first-line treatment.
BM, brain metastases; PFS, progression-free survival.
PFS in patients receiving first-line treatment by LDH value at baseline
Among all enrolled patients, the median value of LDH at baseline was 318 U/L (range: 76‒4471 U/L). 23 Approximately half of all patients (45.3%) had a LDH value within or below the normal range at baseline, while 28.7% had a value greater than the ULN. LDH values were not reported for 26.1% of the patients.
Median PFS for patients on first-line therapy with BM and LDH >ULN at baseline was shorter (5.3 months [95% CI: 3.9–7.3] vs 9.9 months [95% CI: 6.9–25.6] for patients with BM and LDH values within or below the normal limit at baseline) (Table 2). This was reflected in substantially lower PFS rates at one and two years. Kaplan-Meier estimates of PFS for these patient subgroups are shown in Figure 2.
Table 2.
PFS in patients receiving first-line treatment by LDH subgroup.
| Subgroups | N | Number of censored patients | Number of patients with PFS events, n (%) | Median PFS (95% CI) | 1-y PFS rate, % | 2-y PFS rate, % | 3-y PFS rate, % |
|---|---|---|---|---|---|---|---|
| LDH within or below normal range | 32 | 13 | 19 (59.4) | 9.9 | 49 | 31 | NE |
| (6.9-25.6) | |||||||
| LDH >ULN | 26 | 4 | 22 (84.6) | 5.3 | 9 | 5 | 5 |
| (3.9-7.3) |
Survival estimates at three years for patients with LDH within or below normal range cannot be evaluated because an observation period of three years was not reached by any patient. CI, confidence interval; LDH, lactate dehydrogenase; NE, not estimable; PFS, progression-free survival; ULN, upper limit of normal; y, year.
Figure 2.
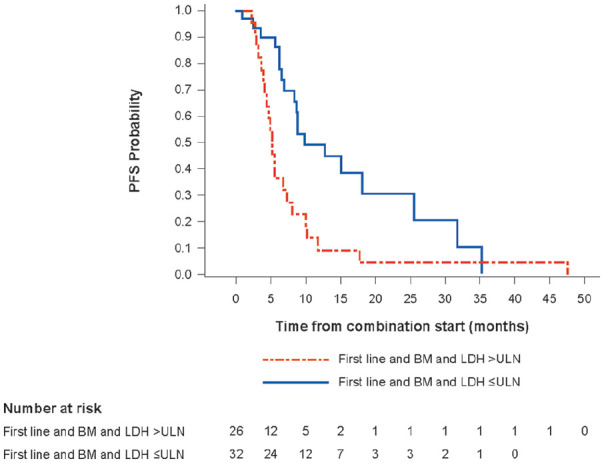
Kaplan-Meier estimate of PFS for patients receiving first-line treatment by LDH subgroup.
BM, brain metastases; LDH, lactate dehydrogenase; PFS, progression-free survival; ULN, upper limit of normal.
PFS in patients receiving first-line treatment by metastatic site at baseline
Among all patients enrolled, 240/499 (48.1%) patients had ⩽3 metastatic sites without BM and 78/499 (15.6%) had >3 metastatic sites without BM, while 193/499 (38.7%) had >3 metastatic sites and/or BM. 23 At baseline, BM were present in 115/499 (23.0%) patients, of which 67 (13.4%) had ⩽3 metastatic sites and 48 (9.6%) had >3 metastatic sites.
For this analysis, we tried to assess the impact of additional metastatic sites to the outcomes of patients with BM at baseline. Median PFS was noticeably longer for patients with cerebral metastases only compared to those patients with cerebral and other metastases at baseline (15.0 months [95% CI: 4.4–not estimable] vs 8.7 months [95% CI: 6.2–10.0]) (Table 3); however, it should be noted that just 13 patients presented with cerebral metastases only. PFS rates at one, two and three years reflected this improvement in survival. Kaplan-Meier estimates of PFS for these patient subgroups are shown in Figure 3.
Table 3.
PFS in patients receiving first-line treatment by metastatic site.
| Subgroups | N | Number of censored patients | Number of patients with PFS events, n (%) | Median PFS (95% CI) | 1-y PFS rate, % | 2-y PFS rate, % | 3-y PFS rate, % |
|---|---|---|---|---|---|---|---|
| Cerebral metastases only | 13 | 6 | 7 (53.8) | 15.0 | 51 | 38 | 19 |
| (4.4-NE) | |||||||
| Cerebral and other metastases | 63 | 17 | 46 (73.0) | 8.7 | 28 | 15 | 6 |
| (6.2-10.0) |
CI, confidence interval; NE, not estimable; PFS, progression-free survival; y, year.
Figure 3.
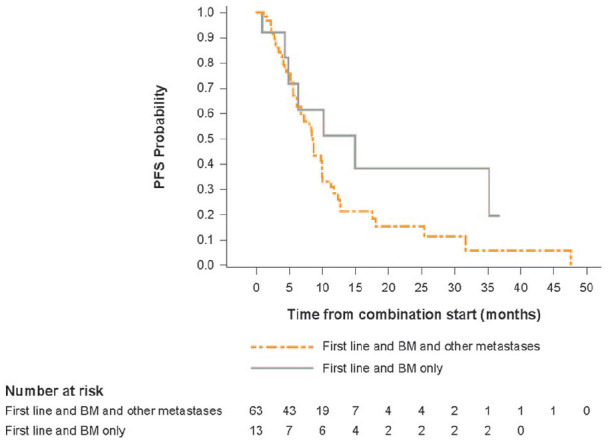
Kaplan-Meier estimate of PFS for patients receiving first-line treatment by metastatic site.
BM, brain metastases; PFS, progression-free survival.
Discussion
Our results confirm the effectiveness of dabrafenib/trametinib treatment for patients with advanced BRAFV600-mutated melanoma and BM at baseline outside of the clinical trial setting. Moreover, clinical benefit was observed in this population, which has been previously reported as having very poor prognosis and limited survival.9,10
The median PFS for patients receiving first-line treatment was very similar to that reported in the ADMIRE retrospective study including 382 Russian patients with advanced BRAFV600-mutant melanoma who received combination therapy with a BRAFi and a MEK inhibitor as first-line treatment in a real-world setting (9.3 months vs 9.2 months). 26 Although in our study the median PFS for patients in the first-line treatment setting with BM at baseline was shorter than that for all patients in first-line therapy (8.7 months vs 9.3 months), the difference was minimal. Median PFS for this population was also similar to the median PFS for all patients included in the DESCRIBE Italy study (9.3 months). 23
A pooled analysis of 563 treatment-naïve patients with BRAFV600E/K-mutant metastatic melanoma who received dabrafenib/trametinib in the COMBI-d and COMBI-v trials revealed a median PFS of 11.1 months and PFS rates of 48%, 30% and 23% at one, two and three years, respectively. 27 These results suggest slightly longer PFS in clinical trials compared with that observed in our study. However, although patients with BM were included in the clinical trials, only those patients with asymptomatic BM that had been treated and remained stable for at least 12 weeks were eligible to participate. The inclusion of patients with active BM could explain, at least in part, the shorter median PFS observed in our study. It is also worth pointing out that patients in the real-world setting are usually in poorer health than those recruited for clinical trials; for example, the DESCRIBE Italy study included patients with ECOG PS of 2 and 3. 23
The COMBI-MB study specifically assessed the effectiveness of dabrafenib/trametinib in patients with BRAFV600-mutant metastatic melanoma and active BM. 28 Median PFS ranged from 4.2 to 7.2 months across the four different study cohorts; median PFS was 5.6 months in cohort A, which included 76 patients with BRAFV600E-mutant, asymptomatic melanoma BM without previous local brain-directed therapy, and an ECOG performance status of 0 or 1. This is considerably lower than the median PFS reported here for patients with BM at baseline receiving first-line treatment (8.7 months); however, it should be noted that patients with up to two previous systemic therapies for metastatic melanoma (except for BRAF or MEK inhibitors), previous temozolomide therapy for brain metastases, adjuvant interferon and/or previous systemic treatment in the adjuvant setting were all eligible for COMBI-MB, while the present work focused on patients in the first-line treatment setting.
DESCRIBE II was a retrospective chart review of 271 patients with BRAFV600-mutated unresectable stage III/IV melanoma receiving dabrafenib/trametinib in a compassionate-use setting, in multiple sites around the world. 21 Among patients receiving first-line dabrafenib/trametinib, median PFS was 8.1 months, which is slightly lower than that observed in the DESCRIBE Italy study (9.3 months). Median PFS was 7.5 months among all BRAFi-naïve patients participating in DESCRIBE II (n=162), while median PFS was 6.2 months for BRAFi-naïve patients with known BM (n=62) and 8.0 months for those without BM (n=100). It should be noted that the median PFS for all BRAFi-naïve patients was quite similar to that of BRAFi-naïve patients without BM. Interestingly, a German observational retrospective study of 672 patients with BM from malignant melanoma reported an OS of 5.0 months, 29 which is considerably shorter than that observed in our study. Since the German study included patients diagnosed between 1986 and 2007, the improvement in survival could reflect the advent of targeted therapies, which had not been approved at the time. Of note, a retrospective analysis of 531 Italian and Polish patients with melanoma brain metastases showed that prognosis for these patients has improved since 2017, the year when systemic therapies (BRAF-targeting and immunotherapy) became widely available for patients with melanoma. 30 Furthermore, the longest median OS in this study was achieved by patients harboring BRAF mutations; this result suggests that BRAF-targeting therapies can dramatically improve outcomes for this patient population.
Recent studies have shown improvement in OS for patients with melanoma and BM following treatment with immunotherapy, particularly in combination with other approaches such as surgery or chemotherapy.30-33 Ipilimumab and nivolumab may be an attractive therapeutic option for patients with melanoma and BM, and further studies are warranted to assess their efficacy and safety in combination with BRAF-targeted therapies.
Our results showed that patients with BM and elevated LDH levels or metastases other than cerebral at baseline showed decreased PFS rates. These factors had already been reported as predictors of poor prognosis in pooled analyses of patients from the COMBI-d and COMBI-v clinical trials.20,27 Furthermore, another pooled analysis including patients from the BRF113220, COMBI-d and COMBI-v studies found that patients with LDH levels within normal limits and metastases located in fewer than three organ sites at baseline had the longest PFS. 34 In terms of real-world patients, results from the Danish Metastatic Melanoma Database showed that both LDH <ULN and no extracranial disease were associated with significantly improved overall survival in patients with melanoma BM; 35 improved overall survival was also longer for patients with LDH <ULN in the ADMIRE retrospective study. 26 These results are in agreement with our observations, which showed that patients with LDH levels below the ULN or cerebral metastases only at baseline had better outcomes in terms of median PFS. However, LDH values were missing for 26.1% of patients in this study and the number of patients with cerebral metastases only was very low, so our results should be interpreted cautiously.
Both of these predictive factors reflect widespread disease, since high LDH levels are associated with hypoxia (given that glycolytic activity is enhanced under hypoxic conditions) and tumor necrosis, which in turn is associated with tumor burden.13,36 Our results therefore highlight the importance of early diagnosis of late-stage melanoma, which may result in improved clinical benefit for patients. Furthermore, our results may indicate that elevated LDH levels at baseline are associated with decreased response to systemic therapy in melanoma patients with BM; this suggests that stratification of patients with BM according to LDH levels may provide a more accurate assessment of response.
Limitations of this study are typical of observational, retrospective studies. The data collected in the eCRFs were limited and did not include information on intracranial responses or BM response to local/locoregional treatment; it is therefore not possible to assess the effects of dabrafenib/trametinib treatment on BM progression. Furthermore, information on health services received outside of the MAP setting was not collected. Assessment of progression was carried out by attending physicians according to local guidelines and was not uniform across patients as it would have been in a clinical trial. Moreover, given that brain imaging at baseline was not mandatory, it is possible that some of the enrolled patients had asymptomatic brain metastases at diagnosis that went undetected. Information on known prognostic factors for patients with melanoma and brain metastases such as size, number of lesions, presence of leptomeningeal disease and presence of symptoms was not available, which hinders interpretation of our results. Patient numbers in subgroups were low because of censoring, particularly at the three-year mark; for this reason, survival curves and estimates should be interpreted cautiously. However, it should be noted that many patients were still receiving treatment at data cut-off.
In conclusion, our results confirm the activity of dabrafenib/trametinib in patients with advanced BRAFV600-mutated melanoma and BM at baseline, given the effectiveness observed in a real-world setting for this population with poor outcomes.
Supplemental Material
Supplemental material, sj-pdf-1-tmj-10.1177_03008916231179251 for Outcomes in patients with BRAFV600–mutated melanoma and brain metastases at baseline treated with dabrafenib plus trametinib by Massimo Aglietta, Vanna Chiarion-Sileni, Paolo Fava, Massimo Guidoboni, Roberta Depenni, Alessandro Minisini, Francesca Consoli, Paolo Antonio Ascierto, Gaetana Rinaldi, Maria Banzi, Riccardo Marconcini, Rossana Gueli, Virginia Ferraresi, Marco Tucci, Giuseppe Tonini, Giovanni Lo Re, Michele Guida, Michele Del Vecchio, Ilaria Gioia Marcon and Paola Queirolo in Tumori Journal
Supplemental material, sj-pdf-2-tmj-10.1177_03008916231179251 for Outcomes in patients with BRAFV600–mutated melanoma and brain metastases at baseline treated with dabrafenib plus trametinib by Massimo Aglietta, Vanna Chiarion-Sileni, Paolo Fava, Massimo Guidoboni, Roberta Depenni, Alessandro Minisini, Francesca Consoli, Paolo Antonio Ascierto, Gaetana Rinaldi, Maria Banzi, Riccardo Marconcini, Rossana Gueli, Virginia Ferraresi, Marco Tucci, Giuseppe Tonini, Giovanni Lo Re, Michele Guida, Michele Del Vecchio, Ilaria Gioia Marcon and Paola Queirolo in Tumori Journal
Acknowledgments
The authors would like to thank Paola Amore, of Novartis Farma SpA, for support and assistance in the review process. They would also like to thank Vanesa Martinez Lopez, of Novartis Ireland Ltd, for medical writing support, which was funded by Novartis Farma SpA, Italy in accordance with Good Publication Practice (GPP 2022) guidelines (https://www.ismpp.org/gpp-2022).
Footnotes
Author contributions: IGM had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. MA, VC-S, PF, MGuidoboni, RD, AM, FC, PAA, GR, MB, RM, RG, VF, MT, GT, GLR, MGuida, MDV, IGM and PQ acquired, analyzed or interpreted data; critically reviewed and drafted the manuscript; provided final approval, and agreed to be accountable for all aspects of the work. Concept and design: Novartis Farma SpA.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MA has received research funding from AstraZeneca and Pharmamar; has received consulting fees from Bayer, BMS, Merck and Novartis; and has received travel support from BMS, Merck and Tesaro. VC-S has received honoraria from MDS, Merck Serono and Novartis; has received travel support from BMS, Novartis and Pierre Fabre; and has participated in advisory boards for Pierre Fabre. MGuidoboni has received consulting fees from BMS and Novartis; has received honoraria from BMS and Pierre Fabre; has received travel support from Pierre Fabre; and has participated in advisory boards for BMS. RD has received honoraria from BMS, MSD, Novartis, Pierre Fabre and Sanofi. AM has received honoraria from Merck, Pierre Fabre and Sun Pharma, and has participated in advisory boards for BMS, MSD, Novartis and Pierre Fabre. FC has received honoraria from BMS, Merck, Novartis and Pierre Fabre, and has participated in advisory boards for BMS, Merck and Novartis. PAA has received research funding from BMS, Pfizer, Roche-Genentech and Sanofi; has received consulting fees from 4SC, AstraZeneca, BMS, Boehringer-Ingelheim, Daiichi Sankyo, Eisai, Idera, Immunocore, Italfarmaco, iTeos, Lunaphore, Merck Serono, Merck Sharp & Dohme, Nektar, Nouscom, Novartis, Oncosec, Pfizer, Pierre Fabre, Regeneron, Roche-Genentech, Sandoz, Sanofi, Seagen and Sun Pharma; and has received travel support from MSD. RM has received consulting fees from BMS, Ipsen, MSD, Novartis and Pierre Fabre; has received honoraria from BMS, Ipsen, MSD, Novartis, Pierre Fabre and Sanofi; has received travel support from BMS, Ipsen, MSD and Novartis; has participated in advisory boards for BMS, Ipsen, MSD, Novartis and Pierre Fabre. VF has received honoraria from BMS, MSD, Novartis and Pierre Fabre; has received travel support from BMS and Pierre Fabre; and has participated in advisory boards for BMS, MSD and Novartis. MT has received institutional research funding from Novartis; has received honoraria from BMS, Novartis and Pierre Fabre; has received travel support from MSD; and has participated in advisory boards for Novartis. GT has received honoraria from Molteni, Novartis and Pharmamar. MGuida has participated in advisory boards for BMS, Merck, Novartis and Pierre Fabre. MDV has received consulting fees from BMS, Merck, Novartis, Pierre Fabre and Sanofi; has received honoraria from BMS, Merck, Novartis, Pierre Fabre and Sanofi; has received travel support from BMS, Merck, Novartis, Pierre Fabre and Sanofi; and has participated in advisory boards for BMS, Merck, Novartis, Pierre Fabre and Sanofi. IGM is a Novartis employee. PQ has received consulting fees from BMS, Merck, Novartis, Pierre Fabre, Roche and Sanofi; has received honoraria from BMS, Merck, Novartis, Pierre Fabre, Roche and Sanofi; has received travel support from BMS, Merck, Novartis, Pierre Fabre, Roche and Sanofi; and has participated in advisory boards for BMS, Merck, Novartis, Pierre Fabre, Roche and Sanofi. MB, PF, RG, GLR, and GR have no conflict of interest to declare.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Novartis Farma SpA.
Ethical Approvals: All required local approvals from Ethics Committees were obtained before commencing data collection at each site. The Ethics Committees were Comitato Etico Regionale della Liguria c/o IRCCS AOI San Martino - IST Istituto Nazionale per la Ricerca sul Cancro (Genova), Comitato Etico Indipendente dell’IRCCS Istituto Clinico Humanitas di Rozzano, Comitato Etico Unico Regionale (CEUR) c/o Direzione Scientifica del Centro di riferimento Oncologico di Aviano, Comitato Etico Interaziendale della AO SS. Antonio e Biagio e Cesare Arrigo di Alessandria, Comitato Etico dell’AOU Consorziale Policlinico di Bari, Comitato Etico dell’Area Vasta Emilia Nord, Comitato Etico dell‘IRCCS Istituto Dermopatico dell`Immacolata di Roma, Comitato Etico della Provincia di Brescia c/o AO Spedali Civili, Comitato Etico della Fondazione IRCCS Istituto Nazionale dei Tumori di Milano, Comitato Etico dell’Insubria c/o Ospedale di Circolo e Fondazione Macchi (Varese), Comitato Etico della Provincia di Treviso e Belluno, Comitato Etico della Romagna (CEROM), Comitato Etico per la Sperimentazione Clinica delle Province di Verona e Rovigo Presso AOUI Verona, Comitato Etico per la Sperimentazione Clinica dell’IRCCS Istituto Oncologico Veneto di Padova, Comitato Regione Toscana - Area Vasta Nord Ovest c/o Azienda Ospedaliero Universitaria Pisana di Pisa, Comitato Etico dell`IRCCS Ospedale Oncologico di Bari, Comitato Etico degli IRCCS Istituto Europeo di Oncologia e Centro Cardiologico Monzino, Comitato Etico IRCCS Pascale - A.O.R. N. Santobono-Pausilipon, Comitato Etico Valpadana c/o AO di Cremona, Comitato Etico di Area Vasta Emilia Centro della Regione Emilia-Romagna CE-AVEC, Comitato Etico Indipendente dell’Azienda Ospedaliera Universitaria di Cagliari, Comitato Etico Centrale IRCCS Lazio - Sezione IFO-Bietti c/o IRCCS Istituti Fisioterapici Ospitalieri di Roma, Comitato Etico Palermo 1 c/o AOU Policlinico P. Giaccone di Palermo, Comitato Etico dell’IRCCS di Candiolo, Comitato Etico dell’ Universita’ ’Campus Bio-Medico’ di Roma, Comitato Etico Interaziendale A.O. Citta’ della Salute e della Scienza di Torino, Comitato Etico Interaziendale dell’Azienda Ospedaliera Universitaria Maggiore della Carita’ di Novara, Comitato Etico di Bergamo c/o A.S.S.T. Papa Giovanni XXIII, Comitato Etico per la Sperimentazione Clinica della Provincia di Venezia e IRCCS San Camillo Presso ULSS 12 Veneziana, Comitato Etico della Brianza c/o Ospedale S. Gerardo di Monza - Ospedale San Gerardo, Comitato Etico Interaziendale AO S. Croce e Carle di Cuneo - AA.SS.LL. CN1, CN2 e AT.
ORCID iD: Massimo Aglietta  https://orcid.org/0000-0002-3616-7377
https://orcid.org/0000-0002-3616-7377
Supplemental material: Supplemental material for this article is available online.
References
- 1. Garbe C, Amaral T, Peris K, et al. European consensus-based interdisciplinary guideline for melanoma. Part 1: Diagnostics - Update 2019. Eur J Cancer 2020; 126: 141-158. [DOI] [PubMed] [Google Scholar]
- 2. Forsea AM, Del Marmol V, de Vries E, et al. Melanoma incidence and mortality in Europe: new estimates, persistent disparities. Br J Dermatol 2012; 167: 1124-1130. [DOI] [PubMed] [Google Scholar]
- 3. Erdmann F, Lortet-Tieulent J, Schüz J, et al. International trends in the incidence of malignant melanoma 1953-2008–are recent generations at higher or lower risk? Int J Cancer 2013; 132: 385-400. [DOI] [PubMed] [Google Scholar]
- 4. Morgese F, Sampaolesi C, Torniai M, et al. Gender differences and outcomes in melanoma patients. Oncol Ther 2020; 8: 103-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. AIOM-CCM-AIRTUM. I numeri del cancro in Italia 2020, http://www.registri-tumori.it/cms/
- 6. Crocetti E, Buzzoni C, Chiarugi A, et al. Relationship between latitude and melanoma in Italy. ISRN Oncol 2012; 2012: 864680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Piscitelli P, Neglia C, Falco A, et al. Melanoma in the Italian population and regional environmental influences: a national retrospective survey on 2001-2008 hospitalization records. Int J Environ Res Public Health 2015; 12: 9102-9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Di Lorenzo S, Fanale D, Corradino B, et al. Absence of germline CDKN2A mutation in Sicilian patients with familial malignant melanoma: Could it be a population-specific genetic signature? Cancer Biol Ther 2016; 17: 83-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davies MA, Liu P, McIntyre S, et al. Prognostic factors for survival in melanoma patients with brain metastases. Cancer 2011; 117: 1687-1696. [DOI] [PubMed] [Google Scholar]
- 10. Spagnolo F, Picasso V, Lambertini M, et al. Survival of patients with metastatic melanoma and brain metastases in the era of MAP-kinase inhibitors and immunologic checkpoint blockade antibodies: A systematic review. Cancer Treat Rev 2016; 45: 38-45. [DOI] [PubMed] [Google Scholar]
- 11. Vosoughi E, Lee JM, Miller JR, et al. Survival and clinical outcomes of patients with melanoma brain metastasis in the era of checkpoint inhibitors and targeted therapies. BMC Cancer 2018; 18: 490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xu J, Zhao J, Wang J, et al. Prognostic value of lactate dehydrogenase for melanoma patients receiving anti-PD-1/PD-L1 therapy: A meta-analysis. Medicine (Baltimore) 2021; 100: e25318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Petrelli F, Ardito R, Merelli B, et al. Prognostic and predictive role of elevated lactate dehydrogenase in patients with melanoma treated with immunotherapy and BRAF inhibitors: a systematic review and meta-analysis. Melanoma Res 2019; 29: 1-12. [DOI] [PubMed] [Google Scholar]
- 14. Knispel S, Gassenmaier M, Menzies AM, et al. Outcome of melanoma patients with elevated LDH treated with first-line targeted therapy or PD-1-based immune checkpoint inhibition. Eur J Cancer 2021; 148: 61-75. [DOI] [PubMed] [Google Scholar]
- 15. Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature 2002; 417: 949-954. [DOI] [PubMed] [Google Scholar]
- 16. Jakob JA, Bassett RL, Jr., Ng CS, et al. NRAS mutation status is an independent prognostic factor in metastatic melanoma. Cancer 2012; 118: 4014-4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Network CGA. Genomic classification of cutaneous melanoma. Cell 2015; 161: 1681-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med 2014; 371: 1877-1888. [DOI] [PubMed] [Google Scholar]
- 19. Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 2015; 372: 30-39. [DOI] [PubMed] [Google Scholar]
- 20. Robert C, Grob JJ, Stroyakovskiy D, et al. Five-Year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med 2019; 381: 626-636. [DOI] [PubMed] [Google Scholar]
- 21. Atkinson V, Sandhu S, Hospers G, et al. Dabrafenib plus trametinib is effective in the treatment of BRAF V600-mutated metastatic melanoma patients: analysis of patients from the dabrafenib plus trametinib named patient program (DESCRIBE II). Melanoma Res 2020; 30: 261-267. [DOI] [PubMed] [Google Scholar]
- 22. Atkinson VG, Quaglino P, Aglietta M, et al. A retrospective analysis of dabrafenib and/or dabrafenib plus trametinib combination in patients with metastatic melanoma to characterize patients with long-term benefit in the individual patient program (DESCRIBE III). Cancers (Basel) 2021; 13: 2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aglietta M, Chiarion-Sileni V, Fava P, et al. Retrospective chart review of dabrafenib plus trametinib in patients with metastatic BRAF v600-mutant melanoma treated in the individual patient program (DESCRIBE Italy). Target Oncol 2021; 16: 789-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aglietta M C-SV, Fava P, Guidoboni M, et al. Retrospective chart review of dabrafenib plus trametinib in patients with metastatic BRAF V600–mutant melanoma treated in the Individual Patient Program (DESCRIBE Italy). Targeted Oncol. 2021; 16(6): 789-799. doi: 10.1007/s11523-021-00850-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Le Rhun E, Guckenberger M, Smits M, et al. EANO-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with brain metastasis from solid tumours. Ann Oncol 2021; 32: 1332-1347. [DOI] [PubMed] [Google Scholar]
- 26. Orlova KV, Ledin EV, Zhukova NV, et al. Real-world experience with targeted therapy in BRAF mutant advanced melanoma patients: results from a multicenter retrospective observational study advanced melanoma in Russia (Experience) (ADMIRE). Cancers (Basel) 2021; 13(11): 2529. doi: 10.3390/cancers13112529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schadendorf D, Long GV, Stroiakovski D, et al. Three-year pooled analysis of factors associated with clinical outcomes across dabrafenib and trametinib combination therapy phase 3 randomised trials. Eur J Cancer 2017; 82: 45-55. [DOI] [PubMed] [Google Scholar]
- 28. Davies MA, Saiag P, Robert C, et al. Dabrafenib plus trametinib in patients with BRAF(V600)-mutant melanoma brain metastases (COMBI-MB): a multicentre, multicohort, open-label, phase 2 trial. Lancet Oncol 2017; 18: 863-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eigentler TK, Figl A, Krex D, et al. Number of metastases, serum lactate dehydrogenase level, and type of treatment are prognostic factors in patients with brain metastases of malignant melanoma. Cancer 2011; 117: 1697-1703. [DOI] [PubMed] [Google Scholar]
- 30. Placzke J, Teterycz P, Quaglino P, et al. The analysis of trends in survival for patients with melanoma brain metastases with introduction of novel therapeutic options before the era of combined immunotherapy-multicenter Italian-Polish report. Cancers (Basel) 2022; 14(23): 5763. doi: 10.3390/cancers14235763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maio M, Grob JJ, Aamdal S, et al. Five-year survival rates for treatment-naive patients with advanced melanoma who received ipilimumab plus dacarbazine in a phase III trial. J Clin Oncol 2015; 33: 1191-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tawbi HA, Forsyth PA, Algazi A, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med 2018; 379: 722-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Amaral T, Kiecker F, Schaefer S, et al. Combined immunotherapy with nivolumab and ipilimumab with and without local therapy in patients with melanoma brain metastasis: a DeCOG* study in 380 patients. J Immunother Cancer 2020; 8(1): e000333. doi: 10.1136/jitc-2019-000333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Long GV, Grob JJ, Nathan P, et al. Factors predictive of response, disease progression, and overall survival after dabrafenib and trametinib combination treatment: a pooled analysis of individual patient data from randomised trials. Lancet Oncol 2016; 17: 1743-1754. [DOI] [PubMed] [Google Scholar]
- 35. Pedersen S, Møller S, Donia M, et al. Real-world data on melanoma brain metastases and survival outcome. Melanoma Res 2022; 32: 173-182. [DOI] [PubMed] [Google Scholar]
- 36. Van Wilpe S, Koornstra R, Den Brok M, et al. Lactate dehydrogenase: a marker of diminished antitumor immunity. Oncoimmunology 2020; 9: 1731942. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tmj-10.1177_03008916231179251 for Outcomes in patients with BRAFV600–mutated melanoma and brain metastases at baseline treated with dabrafenib plus trametinib by Massimo Aglietta, Vanna Chiarion-Sileni, Paolo Fava, Massimo Guidoboni, Roberta Depenni, Alessandro Minisini, Francesca Consoli, Paolo Antonio Ascierto, Gaetana Rinaldi, Maria Banzi, Riccardo Marconcini, Rossana Gueli, Virginia Ferraresi, Marco Tucci, Giuseppe Tonini, Giovanni Lo Re, Michele Guida, Michele Del Vecchio, Ilaria Gioia Marcon and Paola Queirolo in Tumori Journal
Supplemental material, sj-pdf-2-tmj-10.1177_03008916231179251 for Outcomes in patients with BRAFV600–mutated melanoma and brain metastases at baseline treated with dabrafenib plus trametinib by Massimo Aglietta, Vanna Chiarion-Sileni, Paolo Fava, Massimo Guidoboni, Roberta Depenni, Alessandro Minisini, Francesca Consoli, Paolo Antonio Ascierto, Gaetana Rinaldi, Maria Banzi, Riccardo Marconcini, Rossana Gueli, Virginia Ferraresi, Marco Tucci, Giuseppe Tonini, Giovanni Lo Re, Michele Guida, Michele Del Vecchio, Ilaria Gioia Marcon and Paola Queirolo in Tumori Journal


