Abstract

Cd(II) and Pb(II) adsorption from brackish water by activated carbon (AC) and biochars derived from bamboo (BB), palm shell (PSB), and mangrove wood (MB) in single- and bisolute systems was investigated. Physicochemical characterization including SEM, FTIR, pH, pHPZC, elemental analysis, proximate analysis, XRF, iodine number, BET surface area analysis, and TGA was carried out. The adsorption of Cd(II) and Pb(II) was in the following order: AC > BB > MB > PSB and was higher in single-solute than bisolute systems with greater Pb(II) adsorption efficiency than Cd(II). Salinity negatively affected metal sorption, particularly for Cd(II), but higher pH enhanced removal. Upon increasing the salinity from 0 to 25 ppt, the removal efficiency of BB was reduced from 75.9 to 52.2% (Cd) and 91.1 to 80.5% (Pb) in the single-solute system. In addition, the removal efficiency was decreased from 71.6 to 41.3% for Cd(II) and 90.9 to 76.3% for Pb)(II) in the bisolute system. The removal trend of the adsorption system of BB with 0 ppt salinity responded positively upon increasing pH from 5 to 8, and the removal of Cd(II) was increased from 54.4 to 75.8% and that of Pb(II) was increased from 66.3 to 91.0% in the single-solute system. The adsorption kinetic data are well explained by the pseudo-second-order model suggesting that chemisorption is the rate-limiting step. The key results of the present work suggest the applicability of BB as an alternative adsorbent to AC due to its comparable physicochemical properties, such as surface area (191.95 m2/g), pore volume (0.1038 cm3/g), pHPZC (9.27), iodine number (104.2 mg/g), and the presence of hydroxyl (−OH), amine (−NH), and COO– groups necessary for metal bonding. The adsorption performance of BB is promising, and hence, it can be considered to remove the Cd(II) and Pb(II) ions from brackish water as statistically it is the least impacted by change in salinity at a confidence level of P ≤ 0.05 compared to MB and PSB.
1. Introduction
Water quality can be affected by human activities, which are recognized as the main contributors to water pollution. Large quantities of untreated and improperly treated water loaded with heavy metals (HMs) are being discharged into both freshwater and coastal aquatic environments. These HMs usually bioaccumulate and can be biomagnified in water and sediment, eventually entering the aquatic food chains and thus causing serious health effects and even the death of fish and other aquatic animals. Among all the toxic HMs, cadmium (Cd) and lead (Pb) are commonly found in coastal areas such as in the mouth of the Chao Phraya River, Thailand, where the reported concentrations are 0.029–0.193 and 0.190–4.428 μg/L, respectively.1 The concentrations of Cd and Pb in coastal surface waters of northern Vietnam range from 0.00 to 30 μg/L and from 30 to 390 μg/L,2 and in surface seawater of the northeastern coastal region of China, the concentrations are from 0.19 to 0.43 μg/L and from 2.65 to 7.68 μg/L, respectively.3 The continuous exposure of white shrimp to exceeded levels of Cd and Pb causes oxidative stress, mucosal damage of shrimp intestine, and increased microbial variations with serious intestinal health problems of shrimp.4 Additionally, the consumption of contaminated aquatic animals can cause several health problems in humans, such as cell necrosis, renal failure, lung cancer, bone fractures, anemia, and cardiovascular diseases.5−7 Therefore, it is important to remove the toxic HMs from water feeding the aquaculture farms for increased productivity of cultured animals and for better human health.
Several techniques can be used to treat polluted water, including membrane filtration, ultrafiltration, solvent extraction, coagulation, chemical filtration, electrochemical separation, and adsorption.8 The selection of a specific technique is generally based on factors such as operational cost, reliability, feasibility, efficiency, environmental friendliness, and operational challenges.9 Adsorption is widely accepted and well-established as a technique for treating metal pollutants as it offers flexibility of operation and design with significant toxicity reduction, biological availability, and the transition of HMs from the liquid–solid phase in polluted waters. Activated carbon (AC), which is porous in nature and is generally suitable for treating various kinds of pollutants, is commonly used for adsorption. When AC is applied to remove certain types of pollutants, it is usually dependent on the nature of the starting material and its treatment (thermal, chemical, and/or physical). The AC is costly, and its use for the removal of HMs from large volumes of water can make it inaccessible for local aquaculture farmers; therefore, recently, much of the focus is on the valorization of agro-industrial waste and conversion of biomass. Thermal conversion is a conventional method being used to convert biomass by heating, under limited oxygen supply, to produce a carbon-rich material called biochar.10
Several aspects can affect the capability of adsorbents and control the output of the adsorption system while studying the efficiency of cost-effective adsorbents to remove HMs from waste and brackish water. These include physicochemical properties of adsorbents (surface area, porosity, thermal stability, elemental composition, surface active sites, presence of specific functional groups, point of zero charge, etc.), the interaction mechanism between the adsorbents and HMs, and the chemical composition of waste and brackish water (pH, organic contaminants and dissolved organic compounds, inorganic compounds, and nutrient load).11 There is considerable concern with respect to the adsorption–desorption of HMs from adsorbents in the adsorption system due to the change in pH and salinity. A number of studies have been conducted on the impact of saline concentration on the removal capacity of an adsorbent,11,12 and most of the previous studies have worked on the efficacy of the adsorbent in a single-solute system,13,14,15 with simplified matrices and important environmental parameters16,17 (e.g., salinity, pH, single and binary solute systems of Pb(II) and Cd(II)); the effect of other ionic species and environmental aspects were not considered. Salinity plays an important role in shrimp aquaculture farming, as changes in salinity can reduce growth performance, induce stress, lead to a low rate of survival, and seriously disturb the osmoregulation system of cultured species.18 Hence, it is important to find out the behavior of coexisting metal ions without salinity and under the influence of salinity, as the aquaculture farmers mix sea salt in water (canal) to prepare the brackish water to maintain the optimum conditions for cultured animals to achieve maximum growth with high survival rates. The use of biochar is feasible to improve the brackish water quality of a shrimp nursery pond. The removal of toxic HMs from aquaculture ponds contributes to the low mortality of shrimp seedlings and ensures the food safety.19 Recently, most of the focus had been on the modification of biochar to enhance its physicochemical characteristics for better performance.20 Nonetheless, the extensive modifications increase the cost and are susceptible to cause secondary pollution.21,22 Additionally, because of the tight economic situation of the majority of aquaculture farmers, the modified biochars are not a preferable option to choose; therefore, the present study is designed for the utilization of unmodified biochar.
Depending on the feedstock, production conditions (e.g., temperature and operation time), methods of carbonization, and surface activation, biochar can contain different physicochemical properties including surface functional groups, surface area, pore size and volume, chemical composition (C, H, O), alkalinity, etc., and thus can be applied as a sorbing material for different types of contaminants.23 The moisture ratio in a feedstock determines the energy required to reach the pyrolysis temperature in a shorter period of time to achieve effective pyrolysis with lower atomization on the carbon surface.24 Consequently, the biochar prepared from a feedstock with lower moisture content is highly aromatized with π-electrons for bond formation with heavy metal cations during adsorption.25 As pH plays a key role in any adsorption system, the application of alkaline biochar can be effective to remove positively charged metal cations through adsorption. Therefore, it is required to perform a complete set of physicochemical analyses to have deep insights into the adsorbent for its application in the adsorption system.
Biochar is an alternative adsorbent, has the ability to sequester contaminants from water, and is of particular interest in developing countries like Thailand, which has an abundance of biomass and agro-industrial waste materials. The production of biochar from biomass and agro-industrial waste materials and its application in water/wastewater treatment are a win–win scenario for waste minimization and the circular economy. This approach allows for the recycling of large amounts of biomass and provides value-added products for further applications. The concept of circular bioeconomy is gaining much recognition as it helps to create a closed-loop system to minimize the waste, and resources are efficiently utilized.26 Locally produced biochar can be potentially applied to treat the contaminated waters due to its physicochemical characteristics (surface area, porosity, specific functional groups, etc.). The present research aims to investigate the physicochemical properties of biochars (produced from locally available biomass and agro-industrial waste) and their adsorption capabilities for the removal of Pb(II) and Cd(II) through batch adsorption experiments. These studies are designed to optimize the conditions for the effective removal of metal ions and to understand the underlying adsorption characteristics. The mass transfer rate from bulk solution phase to adsorption sites can be a limiting factor and can slow down the adsorption process, resulting in inefficient adsorption.27 From an operational point of view, it is important to study the kinetics of the adsorption system as it is essential to design the adsorption system. There is still a dearth of information regarding adsorption kinetics in connection to brackish water involved in single- and bisolute systems. Therefore, this work studied different adsorption kinetic models and investigated the influence of salinity and pH on Cd(II) and Pb(II) removal in single- and bisolute systems.
Typically, seawater contains a variety of cations (e.g., Na+, K+, Mg2+, and Ca2+) and anions (e.g., Cl–, SO42–, and HCO3–). The presence of various cations and anions may negatively impact the adsorption–desorption of toxic metal ions to the surface of the adsorbent. In multicomponent adsorption systems, competitive cations–anions can cause a significant effect on adsorption, as some components may have a high affinity for the adsorbent, leading to reduced removal efficiency of the targeted pollutant.28 Hence, it is pertinent to study the change in the salinity of water and its effect on the removal efficiencies of Pb(II) and Cd(II) by different biochars. Moreover, the pH of brackish water can significantly affect the adsorption of HMs in the presence of various cations–anions, and a slight change in pH can influence the biochar’s surface charge, resulting in increased/decreased adsorption. The literature review depicts a lack of essential research information on the effect of different salinity and pH in single- and bisolute systems in batch adsorption of Pb(II) and Cd(II) by raw biochars produced from bamboo, mangrove, and palm shell in comparison to AC and the kinetics involved in the adsorption processes. Moreover, detailed physicochemical characteristics of all three biochars and AC can provide deep insights into each adsorbent. The present work plans to fill up the existing data gaps, which can help to design and operate an adsorption system (Pb/Cd biochar) at optimum pH in brackish water to attain better removal efficiency of HMs.
2. Materials and Method
2.1. Biochar Preparation
Mangrove, bamboo, and palm shell were collected from local communities in Thailand. Biochars derived from mangrove (MB) and bamboo (BB) were produced in a traditional brick kiln under slow pyrolysis at 500 ± 100 °C (adopted from Be et al.29 and Pinisakul et al.30). Palm-shell-derived biochar (PSB) was produced in an iron bucket under slow pyrolysis at 400 ± 100 °C.31 The bamboo-based activated carbon (AC) was obtained from Charcoal Home Co., Ltd., Bangkok, Thailand, and was produced under slow pyrolysis at 1000 °C. The collected biochars and AC samples were crushed manually with a mortar and pestle and sieved to particle sizes of 2–3 mm. The particle size of 2–3 mm was selected as it is a suitable size for filter media to avoid clogging and agglomeration and for easy passage of water. All of the biochar and AC samples were stored in airtight containers to avoid contamination and to allow further use.
2.2. Biochar Characterization
The pH of a biochar in deionized water (pHDI) was analyzed using a pH meter (Ponpe 510PD, China) after mixing 1 g of biochar in 20 mL of deionized water and shaking at 100 rpm in an incubator shaker (Innova 42 R Inc./ref shaker, Eppendorf, USA) at 28 ± 2 °C for 12 h.32 The solid addition method33 was applied to compute the pH at the point of zero charge (pHPZC). The surface analysis of biochars and AC was performed by using a scanning electron microscope (SEM) at 500× magnification (Phenom ProX G6, Thermo Fisher Scientific, USA). The surface area and average pore diameter (Brunauer–Emmett–Teller, BET) of biochars and AC were measured by using a surface area and pore size analyzer (3Flex, Micromeritics, USA), and N2 adsorption isotherms were measured at 77 K. The iodine number of biochars and AC was determined using the ASTM Standard method D4607-94.34 Elemental analysis of each sample was performed using an elemental analyzer (Micro Corder JM10, Japan) to obtain carbon (C), hydrogen (H), and nitrogen (N) contents, and a sulfur (S) analyzer (Emia-220 V2, Horiba, Japan) was used to determine S content. Total oxygen (O) and O/C and H/C ratios were calculated from the data obtained. The chemical composition of AC and biochars was determined by an X-ray fluorescence (XRF) spectrometer (Horiba, XGT-2000W, Japan) with an X-ray tube adjusted at 50 kV and 1 mA. The dissolved organic carbon (DOC) of each biochar was analyzed by an organic carbon analyzer (TOC-Vwp, Shimadzu, Japan).35 An amount of 0.5 g of each sample was agitated with 200 mL of ultrapure water (UV- Milli-Q) at 100 rpm for 24 h and filtered with a 0.45 μm (PES, Whatman, UK) syringe filter for cation and anion analysis by ion chromatography (930 Compact IC Flex, Metrohm, Switzerland).
Thermogravimetric analysis (TGA) of BB, MB, PSB, and AC was carried out by using a thermogravimetric analyzer (TGA/DSC 3, Mettler Toledo Inc., USA) with a heating value of 10 °C/min, N2 flow rate of 20 mL/min, and temperature fractions of 30–900 °C. Differential thermogravimetric (DTG) curves were obtained by numerical derivation from the TGA curves. Fourier transform infrared (FTIR) analysis was performed at 400–4000 cm–1 wavenumbers to identify the functional groups of each sample, with 50 scans recorded at 2 cm–1 resolutions (DSC 1, Thermo Fisher Scientific Inc., USA).
2.3. Preparation of Synthetic Brackish Water
Synthetic brackish water was obtained by mixing 0.6 kg of commercial sea salt (purchased from Phetchaburi Aquaculture Demonstration Farm, Thailand) in 10 L of deionized water. The sea salt was commercially produced by evaporating the seawater in large quantity where the main constituents of sea salt are NaCl, MgCl2, CaCl2, KCl, CaSO4, etc. The brackish water was aerated with air diffusers for 3 days to precipitate CaCO3 from the solution. After removing the supernatant saline solution, the weight of the precipitate was found to be 16.5 g (2.75% of sea salt). The supernatant saline solution was further analyzed for calcium content by inductively coupled plasma-optical emission spectroscopy (ICP-OES) (Optima 8000, PerkinElmer, USA). The prepared brackish water was checked for a salinity concentration of 30 parts per thousand (ppt) before it was diluted to 0, 5, 15, and 25 ppt. Stock solutions of 1000 mg/L of Pb(II) and Cd(II) were prepared separately in single-solute and bisolute systems using Pb(NO3)2 (Kemaus, Australia) and Cd(NO3)2·4H2O (Fine-Chem, India), respectively, using the synthetic brackish water. Each metal solution was diluted to 1 mg/L, and the solution’s initial pH was maintained at 7.0 ± 0.2 using HNO3 (1–100 mM) and/or NaOH (1–100 mM).
2.4. Adsorption Kinetic Experiments
Batch experiments were performed to determine the adsorption kinetics of Cd(II) and Pb(II) on MB, PSB, BB, and AC. Each adsorbent (0.1 g) was added to 50 mL of 1 mg/L Cd(II) and Pb(II) separately in single- and bisolute solutions in screw-capped polypropylene tubes. The samples were agitated at 120 rpm in an incubator shaker (Innova 42 R Inc./ref shaker, Eppendorf, USA) at 28 ± 2 °C for 5, 10, 20, 30, 60, ..., 1440 min. The experiments were performed in duplicate with the control experiments (without adsorbent). After sorption experiments, the mixture was filtered using a 0.45 μm (PES, Whatman, UK) syringe filter, acidified with concentrated HNO3 to maintain pH values of 2–3, and measured for metal concentration by using atomic absorption spectroscopy (AAS) (AA-6300, Shimadzu Corporation, Japan) at wavelengths of 228.8 nm (for Cd) and 217.0 nm (for Pb). The adsorption equilibrium time was determined, and adsorption kinetics of the experimental data was fitted with pseudo-first-order (PFO), pseudo-second-order (PSO), Elovich, and intraparticle diffusion (IPD) models. The information on the kinetic equations is presented in Table 1. The IPD model was applied to the entire contact time and also in two phases by segregating the two linear regressions to have detailed information on adsorption characteristics. The kinetic models were fitted by using linear regression, and the model calculations were done using the kinetic model equations to plot nonlinear reverse fitting graphs to show the experimental and model calculated data, given in Table 1 by using Microsoft Excel.
Table 1. Adsorption Kinetic Model Equations.
| description | equation | linear form |
|---|---|---|
| adsorption efficiency (Qt) | 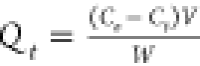 |
|
| intraparticle diffusion | Qt = kintt1/2 + C | 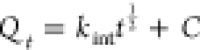 |
| Elovich | 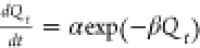 |
 |
| pseudo-first-order | 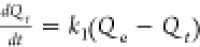 |
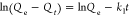 |
| pseudo-second-order | 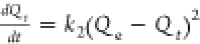 |
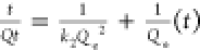 |
C0 represents the initial concentration of the adsorbate (Cd and Pb) in the solution (mg/L), Ct is the amount of the adsorbate adsorbed at time t (min), V is the volume of the solution (mL), and W is the weight of the adsorbent (g). kint is the intraparticle diffusion rate constant, and C represents the boundary layer thickness. The Elovich kinetic model equation is given in Table 1, and this model describes the adsorption processes of different contaminants in a liquid medium, where α is the initial rate constant (mg/g·min) and β is the desorption constant linked to the extent of surface coverage and activation energy needed for chemisorption.36Qe and Qt represent the amount of Cd(II) and/or Pb(II) adsorbed (mg/g) onto the adsorbate at equilibrium and at time t (min), respectively. k1 (1/min) and k2 (g/mg·min) represent the PFO and PSO adsorption rate constants.
2.5. Effect of Solution pH on Cd(II) and Pb(II) Adsorption
The effect of solution pH (5, 6, 7, 8) was tested at salinity concentrations of 0 and 15 ppt to compare the performance of BB, MB, PSB, and AC to remove Pb(II) and Cd(II) in single- and bisolute systems with 1 mg/L of initial concentration of both metal ions. Regarding high salinity concentrations in the solutions, the chelating resin, Chelex 100 resin (Bio-Rad Laboratories, USA), was used to extract Cd and Pb from brackish water after the sorption and filtration steps. The extracted solutions were further analyzed for metal concentration using AAS analysis. The solid phase extraction method using the Chelex 100 resin was adopted from the Bio-Rad Laboratories instruction manual37 and from method no. A479, Shimadzu Corporation.38
2.6. Effect of Salinity on Cd(II) and Pb(II) Adsorption
The effect of salinity concentrations (at 0, 5, 15, and 25 ppt) on Pb(II) and Cd(II) adsorption by MB, PSB, BB, and AC was studied in both single- and bisolute systems. Pb(II) and Cd(II) solutions with the previously prepared brackish water (see section 2.3) were examined under similar conditions (see section 2.5). The remaining metal ions (Pb and Cd) in solutions were analyzed by AAS after performing the solid phase extraction using the chelating resin (section 2.5). Visual MINTEQ is a software that can be used to calculate the metal speciation, sorption, and solubility equilibria in synthetic and natural aqueous systems.39 In the present study, Visual MINTEQ version 3.1 was used to check the metal speciation in brackish water at different salinity (0, 5, 15, and 25 ppt) concentrations. The input quantitative mass concentration of each component including metal ions (Cd2+, Pb2+) and cations–anions of brackish water (NaCl, MgCl2, CaCl2, KCl, and CaSO4) at different salinities were considered to predict the metal speciation. Furthermore, the cations–anions and DOC released from different biochar were also taken into consideration as the inputs of the Visual MINTEQ simulation. The calculated model output data were used to plot the predominance graphs in the form of total concentration versus pH dependencies. The concentrations of all elements and compounds are reported in the Supporting Information (SI).
2.7. Statistical Approach
The biochar’s characteristics and adsorption efficiencies of both metal ions (Cd and Pb) were measured in duplicate, and both averages and standard deviations were reported. Experiments were repeated when variations exceeded 10%. The constants of the adsorption parameters were calculated using Microsoft Excel. Statistical analysis of the experimental data was performed using a two-way ANOVA (IBM SPSS Statistics 26) at a statistical significance confidence of P ≤ 0.05. Furthermore, the root-mean-square error (RMSE) was determined to validate the experimental data and calculated data obtained from different models in this study. The details of the RMSE equation are given in the SI.
3. Results and Discussion
3.1. Biochar Characterization
The pHDI, pHPZC, XRF, BET surface area, iodine number, and ultimate analysis of AC, BB, MB, and PSB are presented in Table 2. The high pHDI values of AC (10.06 ± 0.04) and BB (9.89 ± 0.06) are reported (Table 2). The pHPZC values of AC and BB are 10.01 ± 0.03 and 9.27 ± 0.01, respectively, whereas comparatively low pHPZC values were obtained for MB (7.58 ± 0.07) and PSB (7.69 ± 0.07). This is likely due to higher pyrolysis temperatures for AC (1000 °C) and BB (500 ± 100 °C), providing more oxides and ash than PSB, for which pyrolysis temperature was 400 ± 100 °C. However, even if the MB was produced at similar conditions like BB, it had lower pHPZC (7.58 ± 0.07) compared to BB (9.27 ± 0.01), implying the more alkaline nature of BB than MB. Hassan et al.40 and Ghodake et al.41 also reported that the nature of feedstock and pyrolysis temperature determine the properties of biochars, which can be related to the findings of the present research.
Table 2. Characterization Data of Activated Carbon and Biocharsa.
| parameter | AC | BB | MB | PSB |
|---|---|---|---|---|
| pHDI | 10.06 ± 0.04 | 9.89 ± 0.06 | 7.94 ± 0.06 | 7.97 ± 0.03 |
| pHPZC | 10.01 ± 0.03 | 9.27 ± 0.01 | 7.58 ± 0.07 | 7.69 ± 0.07 |
| iodine number (mg/g) | 106.5 | 104.2 | 52.3 | 21.5 |
| BET surface area (m2/g) | 136.02 | 191.95 | 11.74 | 2.46 |
| pore volume (cm3/g) | 0.1098 | 0.1038 | 0.0065 | 0.0006 |
| pore size (Å) | 19.52 | 21.64 | 55.00 | 15.56 |
| DOC (mg/L) | 0.9 | 1.6 | 0.6 | 1.3 |
| ultimate analysis (% wt) | ||||
| C | 86.0 | 77.2 | 68.0 | 72.0 |
| H | 4.2 | 3.5 | 4.3 | 4.8 |
| N | 0.3 | 0.5 | 0.8 | 0.7 |
| S | 0.1 | 0.3 | 0.2 | 0.1 |
| O | 9.4 | 18.5 | 26.7 | 22.4 |
| O/C | 0.11 | 0.24 | 0.39 | 0.30 |
| H/C | 0.05 | 0.05 | 0.06 | 0.07 |
| XRF analysis (oxide % wt) | ||||
| Ca | 18.30 | 7.39 | 84.83 | 84.44 |
| K | 60.95 | 76.82 | 10.05 | 6.02 |
| Cl | 3.40 | 2.83 | 4.26 | 0.73 |
| S | 0.21 | 3.49 | 0.86 | 0.74 |
| Si | 11.90 | 4.92 | ND | 7.61 |
| P | 0.62 | 4.55 | ND | 0.46 |
| Al | 4.62 | ND | ND | ND |
| Pb | ND | ND | ND | ND |
| Cd | ND | ND | ND | ND |
ND: not detected.
The XRF results also show the high content (%) of potassium (K) in AC (60.9%) and BB (76.82%), whereas PSB has the lowest K (6.0%). The AC and BB were produced at a higher pyrolysis temperature, which released the alkali minerals out of its structure, and oxygen bound functional groups disintegrated, resulting in the total reduction of acidic functional groups and vice versa.42 In addition, potassium is alkaline in nature and can possibly form potassium hydroxide, which is a strong base. Therefore, the pHDI's of AC and BB are higher compared to MB and PSB. Furthermore, BB has the highest amount of phosphorus (P, 4.5%) and sulfur (S, 3.49%). However, no Cd and Pb were detected by XRF for all samples. Experimental data of Prakongkep et al. (2020) support the results of the present research that some biochar had higher Ca and lower K and vice versa.43 The DOC released from AC and biochars was in the range of 0.6–1.6 mg/L (see Table 2).
The surface area (SA) of BB was 191.95 m2/g, which was higher than those of MB (11.74 m2/g) and PSB (2.46 m2/g) (Table 2). This is similar to the reported results in the literature,44 where it was found that biochar’s SA increased with higher production temperatures. However, different feedstocks also induced variations in SA between BB and MB. In addition, compared to BB, AC (136.02 m2/g) produced at 1000 °C had lower SA, likely due to the increase of the production temperature to a certain limit that causes structural changes and leads to a decline in SA.45 AC had the highest iodine number (106.5 mg/g) followed by the BB (104.2 mg/g), MB (52.3 mg/g), and PSB (21.5 mg/g), in alignment with the order of metal adsorption (i.e., AC > BB > MB > PSB; see section 3.2). These results affirm that the iodine number provides a more realistic measurement of the adsorptive power of the adsorbent than BET surface area.46
The O/C ratios were in the following order: AC < BB < PSB < MB. Generally, biochars prepared at higher temperatures have lower O/C ratios, which directly correlate with greater stability and higher heating value.47,48 The H/C ratios were given in the following order: PSB > MB > BB > AC. In biochar, a low H/C ratio indicates high aromaticity, and vice versa.49 This indicates that AC, which had the lowest O/C and H/C ratios, had the highest stability and aromaticity. The BB has lower stability and aromaticity of 0.24 and 0.05, respectively, compared to MB and PSB having higher O/C and H/C ratios (Table 2). The O/C ratios of biochars measured in the present study are similar to a previous study where corncob and coconut husk biochars recorded an O/C ratio of 0.3; however, in the case of rice straw biochar, the O/C ratio of 0.6 was higher compared to the results obtained in this study.50
The SEM images of AC showed irregular, highly porous, and rough-looking surfaces. Similarly, BB had a highly porous structure but with more uniformity and regularity than the other biochars (Figure 1), which may help to induce the sorption of metals. MB and PSB had irregular shapes with flaky surfaces and less porous structures. According to the recommendations of the International Union of Pure and Applied Chemistry (IUPAC), AC and PSB were microporous, as their pore sizes were less than 20 Å. The average pore sizes of BB and MB were 21.64 and 55.00 Å, and they were thus classified as mesoporous and macroporous, respectively.
Figure 1.
SEM analysis of (a) AC, (b) BB, (c) MB, and (d) PSB.
TGA results indicating the thermal stability of biochars and AC over the temperatures range of 25–900 °C are shown in Figure 2a. A minor weight loss was observed from 150 to 500 °C, whereas a major weight loss occurred between 600 and 900 °C, particularly for PSB and MB. Most of the weight loss was due to the decomposition of carbon hydrates and lignin and the burning of residual carbon (organic matter) in the sample. AC lost less weight than all biochars, likely due to its higher pyrolysis temperature. Overall, it can be concluded that AC and BB were more thermally stable than PSB and MB. Moreover, MB lost more weight (final weight 57%) than BB (final weight 84%), where the production temperatures of MB and BB were similar (500 ± 100 °C). This indicates that different biochar materials play equally important roles in variations of thermal stability than pyrolysis temperature.41
Figure 2.
Thermogravimetric (a) and FTIR (b) graphs before and after adsorption by activated carbon (AC), bamboo biochar (BB), palm shell biochar (PSB), and mangrove biochar (MB).
The FTIR spectra of AC, BB, MB, and PSB shows strong peaks in the range of 3419–3454 cm–1, indicating the existence of carboxylic acids, phenols or alcohols, and adsorbed water in the form of hydroxyl (−OH) and amine (−NH) groups51 (Figure 2b). Another prominent peak was found in all four adsorbent samples at around 1631 to 1636 cm–1, which was due to COO asymmetric stretching and C=C aromatic ring stretching vibrations and C=O stretching vibrations of carbonyl and carboxylate ion groups.52 Furthermore, the comparatively small peaks found in all biochar samples at the range of 475 to 602 cm–1 can be designated to surface aromatic −CH and δ−C–H groups.51 The relatively small peaks observed in all the samples at ranges of 2068 to 2080 cm–1 and 1399 to 1400 cm–1 were due to the C=N stretching vibrations and C–H bonds, respectively.53 The characteristic peaks observed in the BB sample at 1119 cm–1 and in the MB sample at around 1241 cm–1 were due to the presence of a C–O–C group. The presence of hydroxyl (−OH), amine (−NH), and carboxylic (COO−) groups in biochar offers active sites that can bind metal ions in aqueous solutions.54 The slight shifting of wavenumbers of all the prominent peaks found in the present study is indicative of Cd and Pb adsorption.
3.2. Adsorption Kinetic Studies
The Cd(II) and Pb(II) adsorptions by biochars and AC at various contact times in both systems (single- and bisolute) are shown in the Supporting Information (Figure S1). AC and BB exhibited faster adsorption kinetics than MB and PSB: the equilibrium time was attained after 8 h for AC, 12 h for BB and MB, and 16 h for PSB, and all of the kinetics plateaued within 24 h (Figure S1).
The linearized pseudo-first-order (PFO) kinetic model was applied to fit the results of the adsorption kinetic experiment by plotting log(Qe – Qt) against time and is given in Figure S2a,b, and the kinetic parameters are shown in Table 3. There were slight differences in parameter K1 among all the biochars and AC for both Cd(II) and Pb(II) adsorption in both single- and bisolute systems (Table 3). This model is based on the assumption that one adsorption active site occupies one adsorbate molecule.55 The nonlinear reverse fitting graphs are given in Figure 3, where the experimental data points largely differ from model calculations. The comparatively inferior results of kinetic data obtained by the PFO kinetic model suggest that physical adsorption may not be the rate controlling step, as the correlation coefficient (R2) values were lower and RMSEs were higher than the pseudo-second-order (PSO) values for all adsorbents.
Table 3. Adsorption Kinetic Rate Constants of Pseudo-first-order, Pseudo-second-order and Elovich Models in Single- and Bisolute Systemsa.
| metal | kinetic model | parameter | sample |
|||
|---|---|---|---|---|---|---|
| AC | BB | MB | PSB | |||
| Cd | PFO | K1 (1/min) | 0.006 (0.005) | 0.005 (0.006) | 0.005 (0.004) | 0.004 (0.003) |
| Qe (mg/g) | 0.462 (0.458) | 0.390 (0.376) | 0.330 (0.312) | 0.265 (0.251) | ||
| R2 | 0.898 (0.877) | 0.909 (0.876) | 0.959 (0.879) | 0.884 (0.994) | ||
| RMSE | 0.027 (0.027) | 0.023 (0.020) | 0.012 (0.012) | 0.005 (0.003) | ||
| PSO | K2 (g/mg min) | 0.075 (0.065) | 0.077 (0.086) | 0.051 (0.050) | 0.018 (0.017) | |
| Qe (mg/g) | 0.472 (0.490) | 0.399 (0.385) | 0.345 (0.326) | 0.295 (0.285) | ||
| h | 0.017 (0.014) | 0.012 (0.013) | 0.006 (0.005) | 0.002 (0.001) | ||
| R2 | 0.999 (0.999) | 0.999 (0.998) | 0.999 (0.999) | 0.985 (0.985) | ||
| RMSE | 0.003 (0.002) | 0.001 (0.002) | 0.001 (0.003) | 0.004 (0.004) | ||
| Elovich | α (mg/g·min) | 0.055 (0.046) | 0.040 (0.037) | 0.017 (0.015) | 0.006 (0.005) | |
| β (g/mg) | 13.7 (13.5) | 15.8 (16.1) | 16.9 (17.5) | 20.7 (21.3) | ||
| R2 | 0.9453 (0.9516) | 0.9428 (0.9373) | 0.9760 (0.9661) | 0.9446 (0.9391) | ||
| RMSE | 0.009 (0.009) | 0.008 (0.008) | 0.005 (0.005) | 0.006 (0.006) | ||
| Pb | PFO | K1 (1/min) | 0.007 (0.006) | 0.005 (0.005) | 0.005 (0.005) | 0.003 (0.004) |
| Qe (mg/g) | 0.484 (0.481) | 0.459 (0.446) | 0.364 (0.359) | 0.294 (0.279) | ||
| R2 | 0.889 (0.886) | 0.873 (0.927) | 0.897 (0.985) | 0.977 (0.980) | ||
| RMSE | 0.034 (0.033) | 0.022 (0.015) | 0.013 (0.011) | 0.009 (0.007) | ||
| PSO | K2 (g/mg min) | 0.126 (0.106) | 0.053 (0.036) | 0.043 (0.038) | 0.031 (0.031) | |
| Qe (mg/g) | 0.490 (0.488) | 0.473 (0.468) | 0.381 (0.379) | 0.311 (0.297) | ||
| h | 0.030 (0.025) | 0.012 (0.008) | 0.006 (0.005) | 0.003 (0.003) | ||
| R2 | 0.999 (0.999) | 0.999 (0.999) | 0.998 (0.999) | 0.996 (0.996) | ||
| RMSE | 0.005 (0.004) | 0.004 (0.004) | 0.005 (0.004) | 0.004 (0.004) | ||
| Elovich | α (mg/g·min) | 0.140 (0.104) | 0.032 (0.022) | 0.020 (0.017) | 0.010 (0.009) | |
| β (g/mg) | 14.9 (14.3) | 12.5 (12.2) | 15.6 (15.5) | 19.1 (19.8) | ||
| R2 | 0.8913 (0.9000) | 0.9490 (0.9730) | 0.9814 (0.9865) | 0.9847 (0.9792) | ||
| RMSE | 0.012 (0.012) | 0.010 (0.007) | 0.005 (0.004) | 0.003 (0.004) | ||
Note: Data are shown in the single-solute system followed by the bisolute system in parentheses. RMSE: root-mean-square error.
Figure 3.
Adsorption kinetic studies of Cd(II) and Pb(II) adsorption in single-solute (a, b) and bisolute (c, d) systems (C0 = 1 mg/L, dosage 2 g/L, pH 7.0 ± 0.2).
The linearized PSO model graphs are presented in Figure S2c,d, and the equations and kinetic constants are shown in Tables 1 and 3, respectively. The results denote that AC and BB exhibited the fastest kinetic rates for both Cd and Pb(II), respectively, as the highest K2 values were recorded for these samples, whereas the slowest kinetic rates observed were for PSB, in both systems (single- and bisolute) (Table 3). The K2 values were slightly low in bisolute systems for each adsorbent and for both metal ions, which can be because of competition between both metal ions. The higher K2 values of AC and BB than MB and PSB for both metal ions suggest a faster initial adsorption that can be attributed to the availability of more active sites due to the high porosity and surface area of AC and BB (Table 2). The PSO kinetic model was suitable to explain Cd(II) and Pb(II) adsorption for AC, BB, and MB as the system achieved high R2 (>0.99) and the lowest RMSE, suggesting the involvement of chemisorption. Moreover, the nonlinear reverse fitting graphs of different kinetic models are given in Figure 3, where the calculated and experimental data points are in agreement compared to other models (with the lowest RMSE), suggesting a strong indication of chemisorption. This indicates that chemical adsorption or chemisorption was playing an effective role to influence the rate of reaction, where the Cd(II) and Pb(II) may have interacted to form bonds by π-electrons, ion exchange, surface complexation, and electrostatic attraction with the negatively charged adsorbent.56 The initial adsorption rate constant (h) was calculated from the rate constants derived from the PSO graph. The h values were in the order AC > BB > MB > PSB for both metal ions in both systems (single- and bisolute). However, the h values were higher for each biochar sample for Pb(II) in both solute system (single-solute and bisolute) than for Cd(II) in either system (Table 3). High h values express a greater tendency to adsorb a contaminant.57 These results thus show that all adsorbents adsorbed more Pb(II) than Cd(II). The greater overall adsorption of Pb(II) than Cd(II) can be due to the sequence of atomic radii, which is larger for Pb(II) (1.20 Å) than for Cd(II) (0.97 Å). It can be due to the higher atomic weight, paramagnetic force, and electronegativity of Pb(II) (1.80 eV) compared with Cd(II) (1.59 eV).58,59 The metal cations are generally solvated in aqueous media, and to adsorb onto the substrate, they should be denuded of the hydration sheath covering it. Therefore, because of the higher electronegativity and paramagnetic forces of Pb(II), comparatively lower energy is needed for the dehydration process, which makes the adsorption of Pb(II) easier than that of Cd(II). The comparatively lower affinity of AC and biochars toward Cd(II) might be linked to the lower electronegativity and polarizing power of Cd(II) resulting in lower adsorption. This means that the biochar–Cd(II) interplay forces are weaker than the biochar–Pb(II) forces, resulting in comparatively lower adsorption of Cd(II).
The intraparticle diffusion plot of Qt against t1/2 was graphed for the Cd(II) and Pb(II) adsorption in Figure S2a–f. The intraparticle diffusion plots were expressed in two phases. The first phase (Figure S3c,d) was related to fast adsorption onto the easily accessible macropores of biochar, indicating film diffusion followed by macroporous diffusion, whereas the second phase represented micropore diffusion (Figure S3e,f). The intraparticle diffusion constants are presented in Table 4. Intraparticle diffusion constants (Kint), the adsorption at the adsorbent’s surface in the rate-determining step for both metal ions (Cd and Pb), were in the order AC > BB > MB > PSB for phase 1 and in the reverse order of PSB > MB > BB > AC for phase 2 (Figure S3e,f). This indicates that intraparticle diffusion partially controls the first phase of reaction, with a higher Kint than in phase 2 (Table 4). The boundary layer thickness constant C calculated from this model was comparatively lower in the first phase than the second phase of contact time, and the large C value is directly proportional to the large film diffusion resistance.60 In this study, as the adsorption system proceeds from the first phase to the second phase, the intraparticle diffusion constants (Kint's) decrease, indicating the disappearing role of the intraparticle diffusion model and signaling the increasing role of either physisorption or chemisorption.36,61 However, the negative C values were also observed during the first phase of reaction, which can be explained by the combined effects of film diffusion and surface reaction control.61,62 The pore sizes of AC and BB are nearly similar, and MB has the highest; however, PSB has the lowest pore size, and the adsorption of cations could have been affected by its pore size. The nonlinear reverse fitting graphs of this model are given in Figure 3, confirming that the intraparticle diffusion was dominant in the first half of reaction time and the deviation of model fitting line in the second half is due to either chemisorption or physisorption. Additionally, the results show that the linear plot lines did not meet at the origin (Figure S3a,b), stipulating that the diffusion through pores was not solely controlling the adsorption at that particular time. This deviation of straight lines can be due to differences between mass transfer rates at the start and end stages of adsorption. Adsorption of Pb (II) in single and bisolute systems by PSB may have followed PSO, whereas Cd(II) sorption in single-solute systems can be explained by intraparticle diffusion (R2 > 0.98) with comparatively the lowest RMSE of 0.004 that could be due to the smaller ionic size of Cd ions that may have adsorbed into micropores of PSB (PSB is categorized as microporous, see section 3.1).
Table 4. Intraparticle Diffusion Model Constants in Single- and Bisolute Systemsa.
| adsorbent | Cd(II) |
Pb(II) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Kint | C | R2 | RMSE | Kint | C | R2 | RMSE | ||
| (mg/g min1/2) | (mg/g) | (mg/g min1/2) | (mg/g) | ||||||
| phase 1 | AC | 0.0347 (0.0343) | 0.0165 (0.0074) | 0.9755 (0.9707) | na | 0.0396 (0.0403) | 0.0472 (0.0297) | 0.9493 (0.9482) | na |
| BB | 0.0319 (0.0343) | –0.0054 (−0.0073) | 0.9661 (0.9631) | na | 0.0359 (0.0292) | –0.0240 (−0.0185) | 0.9956 (0.9866) | na | |
| MB | 0.0230 (0.0200) | –0.0164 (−0.0147) | 0.9947 (0.9861) | na | 0.0203 (0.0206) | 0.0088 (−0.0022) | 0.9311 (0.9503) | na | |
| PSB | 0.0094 (0.0087) | –0.0060 (−0.0064) | 0.9928 (0.9950) | na | 0.0144 (0.0128) | –0.0044 (−0.0018) | 0.9840 (0.9603) | na | |
| phase 2 | AC | 0.0010 (0.0013) | 0.4257 (0.4096) | 0.9246 (0.9813) | na | 0.0006 (0.0008) | 0.4640 (0.4511) | 0.9052 (0.9166) | na |
| BB | 0.0014 (0.0009) | 0.3404 (0.3444) | 0.8581 (0.5721) | na | 0.0013 (0.0027) | 0.4127 (0.3563) | 0.7676 (0.5757) | na | |
| MB | 0.0022 (0.0016) | 0.2563 (0.2552) | 0.7996 (0.7242) | na | 0.0032 (0.0035) | 0.2581 (0.2429) | 0.6433 (0.7378) | na | |
| PSB | 0.0058 (0.0057) | 0.0581 (0.0541) | 0.9487 (0.9162) | na | 0.0043 (0.0041) | 0.1402 (0.1357) | 0.8930 (0.8674) | na | |
| overall | AC | 0.0098 (0.0099) | 0.1766 (0.1641) | 0.7220 (0.7351) | 0.020 (0.020) | 0.0086 (0.0090) | 0.2370 (0.2216) | 0.6282 (0.6424) | 0.022 (0.022) |
| BB | 0.0084 (0.0082) | 0.1416 (0.1371) | 0.7155 (0.7039) | 0.018 (0.018) | 0.0109 (0.0115) | 0.1394 (0.1038) | 0.7458 (0.8152) | 0.021 (0.018) | |
| MB | 0.0083 (0.0080) | 0.0815 (0.0698) | 0.8137 (0.8112) | 0.013 (0.013) | 0.0091 (0.0092) | 0.0874 (0.0770) | 0.8506 (0.8617) | 0.013 (0.012) | |
| PSB | 0.0075 (0.0073) | 0.0093 (0.0065) | 0.9781 (0.9738) | 0.004 (0.004) | 0.0078 (0.0075) | 0.0418 (0.0372) | 0.9264 (0.9252) | 0.007 (0.007) | |
Note: Data are shown in the single-solute system followed by the bisolute system in parentheses. RMSE: root-mean-square error; na: not available.
The linearized Elovich model graphs are presented in Figure S4a,b, and nonlinear reverse fitting graphs are given in Figure 3. The adsorption parameters α and β are given in Table 3, where α is related to the initial sorption rate and β is related to the size of binding surface and the activation energy for chemisorption. The Elovich model kinetic fittings showed comparably lower RMSE and higher correlation coefficient (R2) than PFO. The comparatively better fitting of nonlinear reverse fitting of the Elovich model to experimental data points indicates that the sorption of both Cd(II) and Pb(II) tends to be largely influenced by chemisorption during the interaction. Additionally, it suggests that the interaction between the adsorbent and adsorbate ions occurred because of the formation of a chemical bond. Similar results have been previously reported in the literature to support the outcome of the present findings.63,64
3.3. Effect of Solution pH on Cd(II) and Pb(II) Adsorption
Generally, all the adsorbents exhibited increased adsorption efficiency for both metal ions following increasing pH values from 5 to 8 in both systems (single- and bisolute) (Figure 4). It can be due to the low concentration of H+ at high pH levels, which decreases competition with adsorbate ions for negatively charged binding points on the adsorbent’s surface, resulting in higher adsorption of both metal ions. AC and BB were found to be the least affected by low pH (pH 5) in terms of the adsorption of both metal ions in both systems (single- and bisolute) compared to MB and PSB. This can be linked to the high pHPZC and iodine numbers of both AC and BB, which result in more available active sites (see Table 2) than MB and PSB. The change of pH directly impacts the protonation of surface functional groups and net charge of adsorbents. The hindrance and repulsion of positively charged cations by H+ ions intensified as the pH of the adsorption system was decreased, and vice versa. On decreasing pH, the oxygen functional groups were protonated, and the carboxylic functional groups are deprotonated at pKa 4.7–10.1.65 On increasing pH, there was a reduction in positive charges (H+) in the solution, which resulted in low electrostatic repulsive power among the protons themselves and with metal ions bearing a positive charge on the active areas of the adsorbent’s surface. At the experimental pH range of 5–7, despite having a net positive charge on biochars due to solution pH being less than pHPZC, the biochar surface was negatively charged at specific points because of the deprotonation of carboxylic functional groups (pKa 4.7–10.1). Because of more favorable electrostatic drawing forces between the adsorbent’s surface (MB and PSB) with negative charge and adsorbate ions with positive charge, the adsorption of cations is enhanced as the pH of the system is increased.66 The low removal in bisolute systems (Figure 4a,b,d) is due to the repulsion caused by metal ions carrying positive charge, competing to bind with the negatively charged adsorbent. Additionally, in the bisolute systems, cation uptake by all four adsorbents was reduced significantly (P ≤ 0.05) at pH 5, which may be due to the presence of a more positive charge on adsorbent’s surface at low pH and also due to the increased repulsion from H+ ions and repulsion/competition between both metal ions. It is also important to note that Pb(II) removal by all biochar samples was higher than Cd(II) removal in bisolute systems. This is likely due to the higher electronegativity (2.33 for Pb and 1.69 for Cd) and higher effective ionic radius (0.143 nm for Pb and 0.124 nm for Cd) of Pb(II) compared to Cd(II). Additionally, it is important to focus on the relationship among the surface properties (functional groups) of adsorbents, pH levels, and Cd(II) and Pb(II) removal tendency. The interactions between surface functional groups and metal ions are complicated and highly dependent on the heterogeneity and chemical nature of the adsorbent surface and the nature and ionic strength of the solution, where the metal ions may be adsorbed by forming a surface complex or by ion exchange (chemisorption).67 Generally, the adsorption of metal ions on the oxide surfaces is related to the hydrolysis reaction and adsorption affinity, which surge with the rise in hydrolysis constant of the metal ions.
Figure 4.
Effect of initial pH on the sorption of Cd(II) and Pb(II) in single- (si) and bisolute (bi) systems at 0 ppt salinity (a, b) and 15 ppt salinity (c, d).
At a pH range of 5–8, Pb2+ and Pb(OH)+ are the dominant species for Pb and Cd2+ is the dominant species for Cd.68 The Pb2+ and Cd2+ speciation predicted by Visual MINTEQ software is given in Figure 5. In the absence of salinity, Cd2+ was in dominant form until pH 8 and gradually started to decline beyond pH 8 (Figure 5a). However, at 5 ppt salinity (Figure 5b), CdCl+ and CdCl2 were the dominant species until pH 10. These predictions from MINTEQ are consistent with the Cd forming ligands with Cd-chloride, which resulted in the reduced adsorption of Cd2+ in the present study. Moreover, the Pb2+ was the dominant form (Figure 5c) at 0 ppt salinity, and PbOH+ started to increase in concentration when the pH was more than 7. In the presence of salinity, the PbCl+ and PbCl2 were the dominant species (Figure 5d); however, the concentration of Pb2+ was comparatively higher than that of Cd2+, and this is the reason why the adsorption of Pb(II) is higher compared to Cd(II) in the present study. In the presence of salinity, Cd and Pb dominantly exist in CdCl+ and PbCl+ forms, respectively (Figure 5b–d). However, the chemical bonding with negatively charged surface functional groups still happened, and adsorption was impacted less for AC and BB than for MB and PSB. Furthermore, the results show that the final pH of the adsorption system (solution with adsorbent) was changed little from the initial pH of less than 0.4 for all adsorbents (Table S3). Thus, at pH < 7, the surfaces of MB and PSB exhibited a negative charge and attracted heavy metal ions. AC and BB exhibited a slight positive charge (pHDI < pHpzc) at all pH ranges tested in this study; however, the adsorption of metal ions was higher because of the alkaline nature bearing a higher number of negatively charged functional groups.
Figure 5.
Cd2+ and Pb2+ speciation at 0 ppt salinity (a, c) and at 5 ppt salinity (b, d) predicted by Visual MINTEQ (version 3.1).
The electrostatic interactions are relatively weak, and their contribution to adsorb metal cations onto carbon-rich adsorbents is secondary,69,70 as the surface of the adsorbent is differently charged and, most importantly, the surface charge is directly linked to solution pH and pHPZC of the adsorbent.71 Furthermore, the soft Lewis base functional groups (e.g., carbonyl and aromatic structures) favor the dipole–dipole interactions including cation-π bonding for metal ions; however, the hard Lewis base functional groups (e.g., deprotonated carboxylic acids and phenols) favor the adsorption of metal ions by cation exchange.72 The possible interaction between metal ions (M2+) and the different surface functional groups can be expressed in eqs 1–4.57,73
| 1 |
| 2 |
| 3 |
| 4 |
In addition, once the Pb2+ and Cd2+ diffused to the inner pores of AC and BB, a small amount of precipitation forms of Pb(OH)2 and Cd(OH)2 may have occurred on the surfaces of AC and BB, as the pHDI was higher than 9.0 (Table 2).
The effect of pH at a salinity of 15 ppt on the adsorption efficiency of all of the adsorbents played a considerable role. In regard to Pb(II) at a salinity of 15 ppt, the single- and bisolute systems followed similar patterns except that the removal of Pb(II) was more in single-solute than bisolute systems, which may be due to the repulsion of salt ions by the change in pH. However, Cd(II) removal at 15 ppt was higher than that at 0 ppt in bisolute systems at pH 7 and 8. This enhanced removal of Cd(II) at 15 ppt in bisolute systems may be due to the Pb(II) forming ligands with dissolved organic compounds that were released (see section 3.1) at different pH values (Figure 4), leaving the active sites vacant for Cd(II) to occupy. The formation of Pb(II) ligands by organic compounds can inhibit adsorption and affect the overall removal efficiency of an adsorption system.74
3.4. Effect of Salinity on Cd(II) and Pb(II) Adsorption
The results show that AC was the most efficient adsorbent for both metal ions at various salinity concentrations (Figure 6). The adsorption efficiency of Pb(II) by AC was 0.50 mg/g in a single-solute system and was unaffected (P ≤ 0.05) by changes in the salinity concentration from 0 to 25 ppt.
Figure 6.

Effect of salinity on the adsorption of Pb(II) (a) and Cd(II) (b) in single- (si) and bisolute (bi) systems (C0 1 mg/L, dosage 2 g/L, pH 7.0 ± 0.2). Different letters are assigned to denote the significant differences produced by Duncan’s test (P < 0.05).
The overall adsorption of Pb(II) by BB, MB, and PSB biochars showed downward trends when the salinity was increased from 0 to 25 ppt (Figure 6a). The Pb(II) removal by BB was reduced from 0.45 to 0.40 mg/g in the single-solute system upon increasing salinity from 5 to 25 ppt. However, the Pb(II) removal by BB further came down in the bisolute system: it was measured as 0.44 mg/g at 5 ppt salinity and dropped to 0.38 mg/g at 25 ppt salinity. Differences in removal efficiency between 5 and 25 ppt salinity and between single and bisolute systems were found to be significant at a P ≤ 0.05 confidence level. MB and PSB followed similar patterns, with decreased adsorption in bisolute systems and also upon increasing the salinity of the adsorption system. PSB was the worst performing biochar in both systems (single- and bisolute), resulting in 0.29 and 0.28 mg/g removal rates (Pb (II)) in single- and bisolute systems, respectively, at 5 ppt salinity (Figure 6a). It was further reduced to 0.26 mg/g in single-solute and to 0.25 mg/g in bisolute systems with 25 ppt salinity.
Figure 6b shows the Cd(II) removal onto AC and different biochars in both systems (single- and bisolute), with AC exhibiting the highest adsorption in both solute systems at different salinity concentrations. The adsorption of Cd(II) by AC dropped from 0.47 mg/g (single-solute) to 0.44 mg/g (bisolute) at 5 ppt salinity, and the amount of Cd(II) adsorbed onto AC fell further in both systems (single- and bisolute) at 15 and 25 ppt salinities, which can be linked to the increased hindrance and competition of cations–anions from salt ions and the presence of Cd(II) in the solution.75 It is interesting to note that all adsorbents followed a similar pattern of adsorption in both solute systems, in the order AC > BB > MB > PSB. The least performing biochar for the Cd(II) removal in a bisolute system at 25 ppt salinity was PSB (adsorption efficiency 0.17 mg/g) followed by MB (0.19 mg/g). The adsorption of Pb(II) was always greater than Cd(II) adsorption, which corresponds to the reverse order of the hydrated ionic radii, Pb(II) > Cd(II). The adsorption of Pb(II) onto different adsorbents is comparatively higher than the adsorption of Cd(II) because of the larger ionic radius and higher Pauling electronegativity value of Pb(II).59 This leads to greater adsorption of Pb(II) in comparison to Cd(II) in bisolute systems. Similar trends of competitive adsorption have been reported in the literature: natural zeolites76 and black carbon from wheat waste77 have been observed to remove more Pb(II) than Cu(II) and more Cu(II) than Cd(II) and can be compared to the trend of more Pb(II) removal than Cd(II) that was observed in the present study. Furthermore, the results show that the competitive adsorption of Pb(II) and Cd(II) from brackish water can be linked to the physicochemical properties of both the adsorbent and the adsorbate. The results obtained from this study reveal that, at any salinity concentration, AC and BB are more effective adsorbents than MB or PSB.
4. Conclusions
In the present work, the biomass and agro-industrial wastes were converted to biochars in slow pyrolysis, and the physicochemical properties of biochars in comparison with AC were determined to check their suitability for adsorption of Cd(II) and Pb(II) from brackish water in single- and bisolute systems. The following conclusions can be drawn:
-
1.
The physicochemical properties of biochars are dependent on feedstock, pyrolysis temperature, and time. The BB produced at 500 ± 100 °C has higher thermal stability, surface area (191.95 m2/g), pore volume (0.1038 cm3/g), pHPZC (9.27), and iodine number (104.2 mg/g) compared to MB and PSB.
-
2.
The BB effectively adsorbed Cd(II) and Pb(II) in both single- and bisolute systems and is comparable with commercially produced AC. The adsorption of Pb(II) by any adsorbent was found to be higher compared to Cd(II) in both solute systems at zero salinity compared to the salinity range of 5–25 ppt. The adsorption of both metal ions followed the order AC > BB > MB > PSB in both solute systems at any salinity.
-
3.
The PSO model explains the kinetic data well and suggests chemical adsorption between metals and adsorbents. The adsorption efficiency was found to be dependent on changes in pH (5–8) and salinity (0–25 ppt).
Acknowledgments
The authors highly appreciate and acknowledge the Petchra Pra Jom Klao Doctoral Scholarship (grant no. 34/2563) and King Mongkut’s University of Technology Thonburi (KMUTT) for providing the scholarship to the first author to undertake the present study.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c03335.
Plots of the pHPZC and time profile data of Pb(II) and Cd(II) in single- and bisolute systems; linearized fittings of data to pseudo-first-order, pseudo-second-order, intraparticle diffusion, and Elovich models; tables of the effect of salinity (0–25 ppt) and pH (5–8) on Pb(II) and Cd(II) removal and final pH values at the end of the experiment in connection to salinity and adsorbent; Visual MINTEQ input data; oxygen calculation in adsorbents; and RMSE equations (PDF)
Author Contributions
Soydoa Vinitnantharat and Suchanya Wongrod: conceptualization, supervision, review, editing, and validation. Sohail Rafiq: experimental work, data analysis, and manuscript writing (revision, formatting, and final editing).
The authors declare no competing financial interest.
Supplementary Material
References
- Asokbunyarat V.; Sirivithayapakorn S. Heavy metals in sediments and water at the Chao Phraya River mouth, Thailand. Thai. Environ. Eng. J. 2020, 34, 33–44. [Google Scholar]
- Ngoc N.-T.-M.; Chuyen N.-V.; Thao N.-T.-T.; Duc N.-Q.; Trang N.-T.-T.; Binh T.-T.-B.; Sa G.-C.; Tran N.-B.; Ba N.-V.; Khai N.-V.; Son H.-A.; Han P.-V.; Wattenberg E.-V.; Nakamura H.; Thuc P.-V. Chromium, cadmium, lead and arsenic concentrations in water, vegetables and seafood consumed in a coastal area in northern Vietnam. Environ. Health Insights 2020, 14, 1–9. 10.1177/1178630220921410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X.; Wang J.; Cai W.; Xu X.; Sun M. The Pollution Status of Heavy Metals in the Surface Seawater and Sediments of the Tianjin Coastal Area, North China. Int. J. Environ. Res. Public Health. 2021, 18, 11243. 10.3390/ijerph182111243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Y.; Wang Y.; Huang J.; Li H.; Dong H.; Zhang J. Toxic effects of cadmium and lead exposure on intestinal histology, oxidative stress response, microbial community of Pacific white shrimp Litopenaeus vannamei. Mar. Pollut. Bull. 2021, 167, 112220 10.1016/j.marpolbul.2021.112220. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO). Exposure to cadmium: a major public health concern. 2019a. https://apps.who.int/iris/bitstream/handle/10665/329480/WHO-CED-PHE-EPE-19.4.3-eng.pdf.
- World Health Organization (WHO). Exposure to lead: a major public health concern. 2019b. https://www.who.int/publications/i/item/9789240037632.
- Gidlow D.-A. Lead toxicity. Occup. Med. 2015, 65, 348–356. 10.1093/occmed/kqv018. [DOI] [PubMed] [Google Scholar]
- Alkhadra M.-A.; Su X.; Suss M.-E.; Tian H.; Guyes E.-N.; Shocron A.-N.; Conforti K.-M.; De Souza J.-P.; Kim N.; Tedesco M.; Khoiruddin K.; Wenten G.; Santiago J.-G.; Hatton T.-A.; Bazant M.-Z. Electrochemical methods for water purification, ion separations, and energy conversion. Chem. Rev. 2022, 122 (16), 13547–13635. 10.1021/acs.chemrev.1c00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crini G.; Lichtfouse E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. 10.1007/s10311-018-0785-9. [DOI] [Google Scholar]
- Wan S.; Wang S.; Li Y.; Gao B. Functionalizing biochar with Mg-Al and Mg-Fe layered double hydroxides for removal of phosphate from aqueous solutions. J. Ind. Eng. Chem. 2017, 47, 246–253. 10.1016/j.jiec.2016.11.039. [DOI] [Google Scholar]
- Esfandiar N.; Suri R.; McKenzie E.-R. Competitive sorption of Cd, Cr, Cu, Ni, Pb and Zn from stormwater runoff by five low-cost sorbents; effects of co-contaminants, humic acid, salinity and pH. J. Hazard. Mater. 2022, 423, 126938 10.1016/j.jhazmat.2021.126938. [DOI] [PubMed] [Google Scholar]
- Behbani A.; Ryan R.-J.; McKenzie E.-R. Impacts of salinity on the dynamics of fine particles and their associated metals during stormwater management. Sci. Total Environ. 2021, 777, 146135 10.1016/j.scitotenv.2021.146135. [DOI] [Google Scholar]
- Valencia A.; Kilner J.; Chang N. B.; Wanielista M. P. Chemophysical evaluation of green sorption media for copper removal in stormwater runoff for improving ecosystem and human health. Water Air Soil Pollut. 2019, 230, 1–28. 10.1007/s11270-018-4047-z. [DOI] [Google Scholar]
- Hasan M.-S.; Geza M.; Vasquez R.; Chilkoor G.; Gadhamshetty V. Enhanced heavy metal removal from synthetic stormwater using nanoscale zerovalent iron–modified biochar. Water Air Soil Pollut. 2020, 231, 1–15. 10.1007/s11270-020-04588-w. [DOI] [Google Scholar]
- Hong J.; Ko D.; Hwang Y. Disulfide polymer grafted polypropylene/polyethylene filter media for selective cadmium removal. J. Hazard. Mater. 2020, 399, 123060 10.1016/j.jhazmat.2020.123060. [DOI] [PubMed] [Google Scholar]
- Ramdani A.; Taleb S.; Benghalem A.; Ghaffour N. Removal of excessive fluoride ions from Saharan brackish water by adsorption on natural materials. Desalination. 2010, 250, 408–413. 10.1016/j.desal.2009.09.066. [DOI] [Google Scholar]
- Chebbi R.; Fadel A.; Aidi A. The elimination by natural Algerian clay of chromium ions from salt water. Ann. Chim. – Sci. Mater. 2021, 45, 105–112. 10.18280/acsm.450202. [DOI] [Google Scholar]
- Gao W.; Tian L.; Huang T.; Yao M.; Hu W.; Xu Q. Effect of salinity on the growth performance, osmolarity and metabolism-related gene expression in white shrimp Litopenaeus vannamei. Aquacult. Rep. 2016, 2016 (4), 125–129. 10.1016/j.aqrep.2016.09.001. [DOI] [Google Scholar]
- Frías-Espericueta M.-G.; Abad-Rosales S.; Nevárez-Velázquez A.-C.; Osuna-López I.; Páez-Osuna F.; Lozano-Olvera R.; Voltolina D. Histological effects of a combination of heavy metals on Pacific white shrimp Litopenaeus vannamei juveniles. Aquat. Toxicol. 2008, 89, 152–157. 10.1016/j.aquatox.2008.06.010. [DOI] [PubMed] [Google Scholar]
- Sun T.; Xu Y.; Sun Y.; Wang L.; Liang X.; Zheng S. Cd immobilization and soil quality under Fe-modified biochar in weakly alkaline soil. Chemosphere. 2021, 280, 130606 10.1016/j.chemosphere.2021.130606. [DOI] [PubMed] [Google Scholar]
- Mafiana M.-O.; Dodkins I.-R.; Dirisu C.-G.; Li S.-W. Use of biochar for limiting the pathway of exposure and reducing the risk of heavy metal contamination from mines. Water Air Soil Pollut. 2021, 232, 94. 10.1007/s11270-021-05004-7. [DOI] [Google Scholar]
- Xu X.; Wu Y.; Wu X.; Sun Y.; Huang Z.; Li H.; Wu Z.; Zhang X.; Qin X.; Zhang Y.; Deng J.; Huang J. Effect of physicochemical properties of biochar from different feedstock on remediation of heavy metal contaminated soil in mining area. Surf. Interfaces. 2022, 32, 102058 10.1016/j.surfin.2022.102058. [DOI] [Google Scholar]
- Rajapaksha A. U.; Chen S. S.; Tsang D. C. W.; Zhang M.; Vithanage M.; Mandal S.; Gao B.; Bolan N. S.; Ok Y. S. Engineered/designer biochar for contaminants removal/immobilization from soil and water: potential and implication of biochar modification. Chemosphere 2016, 148, 276–291. 10.1016/j.chemosphere.2016.01.043. [DOI] [PubMed] [Google Scholar]
- Tomczyk A.; Sokołowska Z.; Boguta P. Biochar physicochemical properties: pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Biotechnol 2020, 19, 191–215. 10.1007/s11157-020-09523-3. [DOI] [Google Scholar]
- Yang Y.; Cui M.; Ren Y.; Guo J.; Zheng Z.; Liu H. Towards understanding the mechanism of heavy metals immobilization in biochar derived from co-pyrolysis of sawdust and sewage sludge. Bull. Environ. Contam. Toxicol. 2020, 104, 489–496. 10.1007/s00128-020-02801-4. [DOI] [PubMed] [Google Scholar]
- Yang M.; Chen L.; Wang J.; Msigwa G.; Osman A.-I.; Fawzy S.; Rooney D.-W.; Yap P.-S. Circular economy strategies for combating climate change and other environmental issues. Environ. Chem. Lett. 2023, 21, 55–80. 10.1007/s10311-022-01499-6. [DOI] [Google Scholar]
- Inglezakis V.-J.; Balsamo M.; Montagnaro F. Liquid-solid mass transfer in adsorption system-An overlooked resistance?. Ind. Eng. Chem. Res. 2020, 59, 22007–22016. 10.1021/acs.iecr.0c05032. [DOI] [Google Scholar]
- Girish C. R. Various isotherm models for multicomponent adsorption: A review. Int. J. Civil Eng. Technol. 2017, 8, 80–86. [Google Scholar]
- Be S.; Vinitnantharat S.; Pinisakul A. Effect of mangrove biochar residue amended shrimp pond sediment on nitrogen adsorption and leaching. Sustainability. 2021, 13, 7230. 10.3390/su13137230. [DOI] [Google Scholar]
- Pinisakul A.; Kruatong N.; Vinitnantharat S.; Wilamas P.; Neamchan R.; Sukkhee N.; Werner D.; Sanghaisuk S. Arsenic, iron, and manganese adsorption in single and trinary heavy metal solution systems by bamboo-derived biochars. C 2023, 9, 40. 10.3390/c9020040. [DOI] [Google Scholar]
- Prakongkep N.; Gilkes R.-J.; Wiriyakitnateekul W. Agronomic benefits of durian shell biochar. J. Met. Mater. Miner. 2014, 24, 7–11. [Google Scholar]
- Tang P.-L.Removal of chromium (VI), copper (II) and arsenic (V) from aqueous solution and wastewater by ethylenediamine modified rice hull. 2001, Ph.D. Thesis, Universiti Putra Malaysia, Selangor, Malaysia. [Google Scholar]
- Balistrieri L.-S.; Murray J.-W. The surface chemistry of goethite (αFeOOH) in major ion seawater. Am. J. Sci. 1981, 281, 788–806. 10.2475/ajs.281.6.788. [DOI] [Google Scholar]
- The American Society for Testing and Materials (ASTM). Standard test method for determination of iodine number of activated carbon. ASTM committee on standards, ASTM D4607–94; 2006, Philadelphia, PA, USA.
- Afnor. Qualite de I’eau. Tome 2. Lignes directrices pour le dosage du carbone organique total (TOC) et carbone organique dissous (COD). 1997, Norme EN 1484, 6th ed. France.
- Tan K.-L.; Hameed B.-H. Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J. Taiwan. Inst. Chem. Eng. 2017, 74, 25–48. 10.1016/j.jtice.2017.01.024. [DOI] [Google Scholar]
- Bio-Rad Laboratories. Alfred Nobel Dr., Hercules, CA 94547 LIT200 Rev B. Chelex 100 and Chelex 20 Chelating Ion Exchange Resin Instruction Manual, 2000. https://www.bio-rad.com/webroot/web/pdf/lsr/literature/LIT200.pdf.
- Shimadzu. Method number A479. Flame AA analysis of cadmium (Cd) and lead (Pb) in simulated seawater and polished rice using chelating polymer solid phase extraction, 2014. https://www.shimadzu.com/an/sites/shimadzu.com.an/files/pim/pim_document_file/applications/application_note/12380/jpa314006.pdf.
- Gustafsson J. P.; Visual MINTEQ Version 3.0. KTH Royal Institute of Technology, 2010.
- Hassan M.; Liu Y.; Naidu R.; Parikh S.-J.; Du J.; Qi F.; Willett I. Influences of feedstock sources and pyrolysis temperature on the properties of biochar and functionality as adsorbents: a meta-analysis. Sci. Total Environ. 2020, 744, 140714 10.1016/j.scitotenv.2020.140714. [DOI] [PubMed] [Google Scholar]
- Ghodake G.-S.; Shinde S.-K.; Kadam A.-A.; Saratale R.-G.; Saratale G.-D.; Kumar M.; Palem R.-R.; AL-Shwaiman H.; Elgorban A.; Syed A.; Kim D. Review on biomass feedstocks, pyrolysis mechanism and physicochemical properties of biochar: state-of-the-art framework to speed up vision of circular bioeconomy. J. Clean. Prod. 2021, 297, 126645 10.1016/j.jclepro.2021.126645. [DOI] [Google Scholar]
- Zhang X.; Zhao B.; Liu H.; Zhao Y.; Li L. Effects of pyrolysis temperature on biochar’s characteristics and speciation and environmental risks of heavy metals in sewage sludge biochars. Environ. Technol. Innov. 2022, 26, 102288 10.1016/j.eti.2022.102288. [DOI] [Google Scholar]
- Prakongkep N.; Gilkes R.; Wisawapipat W.; Leksungnoen P.; Kerdchana C.; Inboonchuay T.; Delbos E.; Strachan L.-J.; Ariyasakul P.; Ketdan C.; Hammecker C. Effects of Biochar on Properties of Tropical Sandy Soils Under Organic Agriculture. J. Agric. Sci. 2020, 13, 1–17. 10.5539/jas.v13n1p1. [DOI] [Google Scholar]
- Chatterjee R.; Sajjadi B.; Chen W.-Y.; Mattern D.-L.; Hammer N.; Raman V.; Dorris A. Effect of pyrolysis temperature on physicochemical properties and acoustic-based amination of biochar for efficient CO2 adsorption. Front. Energy Res. 2020, 8, 85. 10.3389/fenrg.2020.00085. [DOI] [Google Scholar]
- Gale M.; Nguyen T.; Moreno M.; Gilliard-AbdulAziz K.-L. Physicochemical properties of biochar and activated carbon from biomass residue: Influence of process conditions to adsorbent properties. ACS Omega. 2021, 6, 10224–10233. 10.1021/acsomega.1c00530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Maglinao J.; Capareda S. C. Improving the surface areas and pore volumes of bio-char produced from pyrolysis of cotton gin trash via steam activation process. Int. J. Sci. Eng. Sci. 2019, 3, 15–18. [Google Scholar]
- González-Arias J.; Sánchez M.-E.; Martínez E.-J.; Covalski C.; Alonso-Simón A.; González R.; Cara-Jiménez J. Hydrothermal carbonization of olive tree pruning as a sustainable way for improving biomass energy potential: Effect of reaction parameters on fuel properties. Processes. 2020, 8, 1201. 10.3390/pr8101201. [DOI] [Google Scholar]
- Praspaliauskas M.; Pedisius N.; Cepauskiene D.; Valantinavicius M. Study of chemical composition of agricultural residues from various agro-mass types. Biomass Convers. Biorefin. 2020, 10, 937–948. 10.1007/s13399-019-00457-7. [DOI] [Google Scholar]
- Steimer S.-S.; Patton D.-J.; Vu T.-V.; Panagi M.; Monks P.-S.; Harrison R.-M.; Fleming Z.-L.; Shi Z.; Kalberer M. Seasonal differences in the composition of organic aerosols in Beijing: A study by direct infusion ultrahigh resolution mass spectrometry. Atmos. Chem. Phys. 2020, 20, 13303–13318. 10.5194/acp-20-13303-2020. [DOI] [Google Scholar]
- Khawkomol S.; Neamchan R.; Thongsamer T.; Vinitnantharat S.; Panpradit B.; Sohsalam P.; Werner D.; Mrozik W. Potential of biochar derived from agricultural residues for sustainable management. Sustainability. 2021, 13, 8147. 10.3390/su13158147. [DOI] [Google Scholar]
- Mopoung S.; Dejang N. Activated carbon preparation from eucalyptus wood chips using continuous carbonization–steam activation process in a batch intermittent rotary kiln. Sci. Rep. 2021, 11, 13948. 10.1038/s41598-021-93249-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo W.; Lei H.; Villota E.; Qian M.; Zhao Y.; Huo E.; Zhang Q.; Lin X.; Wang C.; Huang Z. Synthesis and characterization of sulfonated activated carbon as a catalyst for bio-jet fuel production from biomass and waste plastics. Bioresour. Technol. 2020, 297, 122411 10.1016/j.biortech.2019.122411. [DOI] [PubMed] [Google Scholar]
- Li Z.; Zhang J.; Mu T.; Du J.; Liu Z.; Han B.; Chen J. Preparation of polyvinylpyrrolidone-protected prussian blue nanocomposites in microemulsion. Colloids Surf. A: Physicochem. Eng. Asp. 2004, 243, 63–66. 10.1016/j.colsurfa.2004.05.010. [DOI] [Google Scholar]
- Xie R.; Jin Y.; Chen Y.; Jiang W. The importance of surface functional groups in the adsorption of copper onto walnut shell derived activated carbon. Water Sci. Technol. 2017, 11, 3022–3034. 10.2166/wst.2017.471. [DOI] [PubMed] [Google Scholar]
- Khamwichit A.; Dechapanya W.; Dechapanya W. Adsorption kinetics and isotherms of binary metal ion aqueous solution using untreated venus shell. Heliyon 2022, 8, e09610 10.1016/j.heliyon.2022.e09610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambaye T.-G.; Vaccari M.; van Hullebusch E.D.-V.; Amrane A.; Rtimi S. Mechanisms and adsorption capacities of biochar for the removal of organic and inorganic pollutants from industrial wastewater. Int. J. Environ. Sci. Technol. 2021, 18, 3273–3294. 10.1007/s13762-020-03060-w. [DOI] [Google Scholar]
- Vázquez G.; Sonia Freire M.; González-Alvarez J.; Antorrena G. Equilibrium and kinetic modelling of the adsorption of Cd2+ ions onto chestnut shell. Desalination 2009, 249, 855–860. 10.1016/j.desal.2009.09.007. [DOI] [Google Scholar]
- Kavand M.; Eslami P.; Razeh L. The adsorption of cadmium and lead ions from the synthesis wastewater with the activated carbon: Optimization of the single and binary systems. J. Water Process. Eng. 2020, 34, 101151 10.1016/j.jwpe.2020.101151. [DOI] [Google Scholar]
- Jalali M.; Moharrami S. Competitive adsorption of trace elements in calcareous soils of western Iran. Geoderma. 2007, 140, 156–163. 10.1016/j.geoderma.2007.03.016. [DOI] [Google Scholar]
- McKay G.; Otterburn M.-S.; Sweeney A.-G. The removal of colour from effluent using various adsorbents-III. Silica Rate processes. Water Res. 1980, 14, 15–20. 10.1016/0043-1354(80)90037-8. [DOI] [Google Scholar]
- Yang G.; Chen H.; Qin H.; Feng Y. Amination of activated carbon for enhancing phenol adsorption: effect of nitrogen-containing functional groups. Appl. Surf. Sci. 2014, 293, 299–305. 10.1016/j.apsusc.2013.12.155. [DOI] [Google Scholar]
- Rudzinzki W.; Plazinski W. Kinetics of solute adsorption at solid/solution interfaces: on the special features of the initial adsorption kinetics. Langmuir 2008, 24, 6738–6744. 10.1021/la800743a. [DOI] [PubMed] [Google Scholar]
- Aguiar A.B.-S.; Costa J.-M.; Santos G.-E.; Sancinetti G.-P.; Rodriguez R.-P. Removal of metals by biomass derived adsorbent in its granular and powdered forms: Adsorption capacity and kinetics analysis. Sustain. Chem. 2022, 3, 535–550. 10.3390/suschem3040033. [DOI] [Google Scholar]
- Elkady M.-F.; Ibrahim A.-M.; El-Latif M.M.-A. Assessment of the adsorption kinetics, equilibrium and thermodynamic for the potential removal of reactive red dye using eggshell biocomposite beads. Desalination. 2011, 278, 412–423. 10.1016/j.desal.2011.05.063. [DOI] [Google Scholar]
- Mandal A.; Singh N.; Purakayastha T.-J. Characterization of pesticide sorption behaviour of slow pyrolysis biochars as low cost adsorbent for atrazine and imidacloprid removal. Sci. Total Environ. 2017, 577, 376–385. 10.1016/j.scitotenv.2016.10.204. [DOI] [PubMed] [Google Scholar]
- Qasem N. A.-A.; Mohammed R.-H.; Lawal D.-U. Removal of heavy metal ions from wastewater: a comprehensive and critical review. NPJ Clean Water 2021, 4, 36. 10.1038/s41545-021-00127-0. [DOI] [Google Scholar]
- Yang X.; Wan Y.; Zheng Y.; He F.; Yu Z.; Huang J.; Wang H.; Ok Y.-K.; Jiang Y.; Gao B. Surface functional groups of carbon-based adsorbents and their roles in the removal of heavy metals from aqueous solutions: A critical review. Chem. Eng. J. 2019, 366, 608–621. 10.1016/j.cej.2019.02.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y.; Jun M.; Liu W. Enhanced removal of lead(II) and cadmium(II) from water in alum coagulation by ferrate(VI) pretreatment. Water Res. 2007, 79, 2420–2426. 10.2175/106143007X212148. [DOI] [PubMed] [Google Scholar]
- Li K.; Cao J.; Li H.; Liu J.; Lu M.; Tang D. Nitrogen functionalized hierarchical microporous/mesoporous carbon with a high surface area and controllable nitrogen content for enhanced lead (II) adsorption. RSC Advances. 2016, 6, 92186–92196. 10.1039/C6RA19166E. [DOI] [Google Scholar]
- Cui L.; Wang Y.; Gao L.; Hu L.; Yan L.; Wei Q.; Du B. EDTA functionalized magnetic graphene oxide for removal of Pb (II), Hg (II) and Cu (II) in water treatment: Adsorption mechanism and separation property. Chemical engineering journal. 2015, 281, 1–10. 10.1016/j.cej.2015.06.043. [DOI] [Google Scholar]
- Mukherjee A.; Zimmerman A.; Harris W. Surface chemistry variations among a series of laboratory-produced biochars. Geoderma. 2011, 163, 247–255. 10.1016/j.geoderma.2011.04.021. [DOI] [Google Scholar]
- Harvey O.-R.; Herbert B.-E.; Rhue R.-D.; Li-Jung K. Metal interactions at the biochar-water interface: energetics and structure-sorption relationships elucidated by flow adsorption microcalorimetry. Environ. Sci. Technol. 2011, 45, 5550. 10.1021/es104401h. [DOI] [PubMed] [Google Scholar]
- Goel J.; Kadirvelu K.; Rajagopal C.; Garg V.-K. Removal of lead (II) from aqueous solution by adsorption on carbon aerogel using a response surface methodological approach. Ind. Eng. Chem. Res. 2005, 44, 1987–1994. 10.1021/ie0490684. [DOI] [Google Scholar]
- Wongrod S.; Simon S.; Guibaud G.; Lens P. N. L.; Pechaud Y.; Huguenot D.; van Hullebusch E. D. Lead sorption by biochar produced from digestates: Consequences of chemical modification and washing. J. Environ. Manage. 2018, 219, 277–284. 10.1016/j.jenvman.2018.04.108. [DOI] [PubMed] [Google Scholar]
- Choi J.; Septian A.; Shin W.-S. influence of salinity on the removal of Ni and Zn by phosphate-intercalated nano montmorillonite (PINM). Minerals. 2020, 10, 980. 10.3390/min10110980. [DOI] [Google Scholar]
- Lee D.-H.; Moon H. Adsorption equilibrium of heavy metals on natural zeolites. Korean J. Chem. Eng. 2001, 18, 247–256. 10.1007/BF02698467. [DOI] [Google Scholar]
- Wang X.-S.; Miao H.-H.; He W.; Shen H.-L. Competitive adsorption of Pb(II), Cu(II), and Cd(II) ions on wheat residue derived black carbon. J. Chem. Eng. Data 2011, 56, 444–449. 10.1021/je101079w. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.







