Abstract

The economic and practical strategies of direct nucleophilic attack/addition cyclization and C–H bond activation reactions to synthesize 3-benzyl-/3-benzyl-2-phenyl-benzo[4,5]imidazo[2,1-b]thiazoles via (Z)-(2,3-dibromoprop-1-en-1-yl)benzene/(3-bromoprop-1-yn-1-yl)benzene, 1H-benzo[d]imidazole-2-thiols and halobenzenes have been developed. With the optimized reaction conditions, the nucleophilic attack/addition cyclization reaction (Cs2CO3, MeCN, 90 °C, 24 h) and C–H bond activation reaction [Pd(OAc)2/PPh3, p-xylene, 135 °C, 24 h] could tolerate various electron-donating and electron-withdrawing groups and afford new benzo[4,5]imidazo[2,1-b]thiazoles in good to excellent yields (up to 93% yield).
Introduction
Imidazo[1,2-b]thiazoles have proven to be valuable heterocyclic scaffolds due to their biological and pharmaceutical activities.1 For example, compounds (A) and (B) were identified as small molecule activators of SIRT1 and 1000-fold more potent than resveratrol.2 The derivatives (C) showed good selectivity toward melanoma cell lines than that for other cancer types.3 The 4-(imidazo[2,1-b]benzothiazol-2-yl)phenyl moiety (D) impaired survival, anchorage-independent growth, and in vivo tumorigenesis, without showing side effects (Figure 1).4
Figure 1.
Some useful benzoimidazothiazoles.
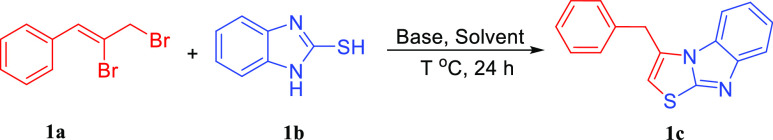
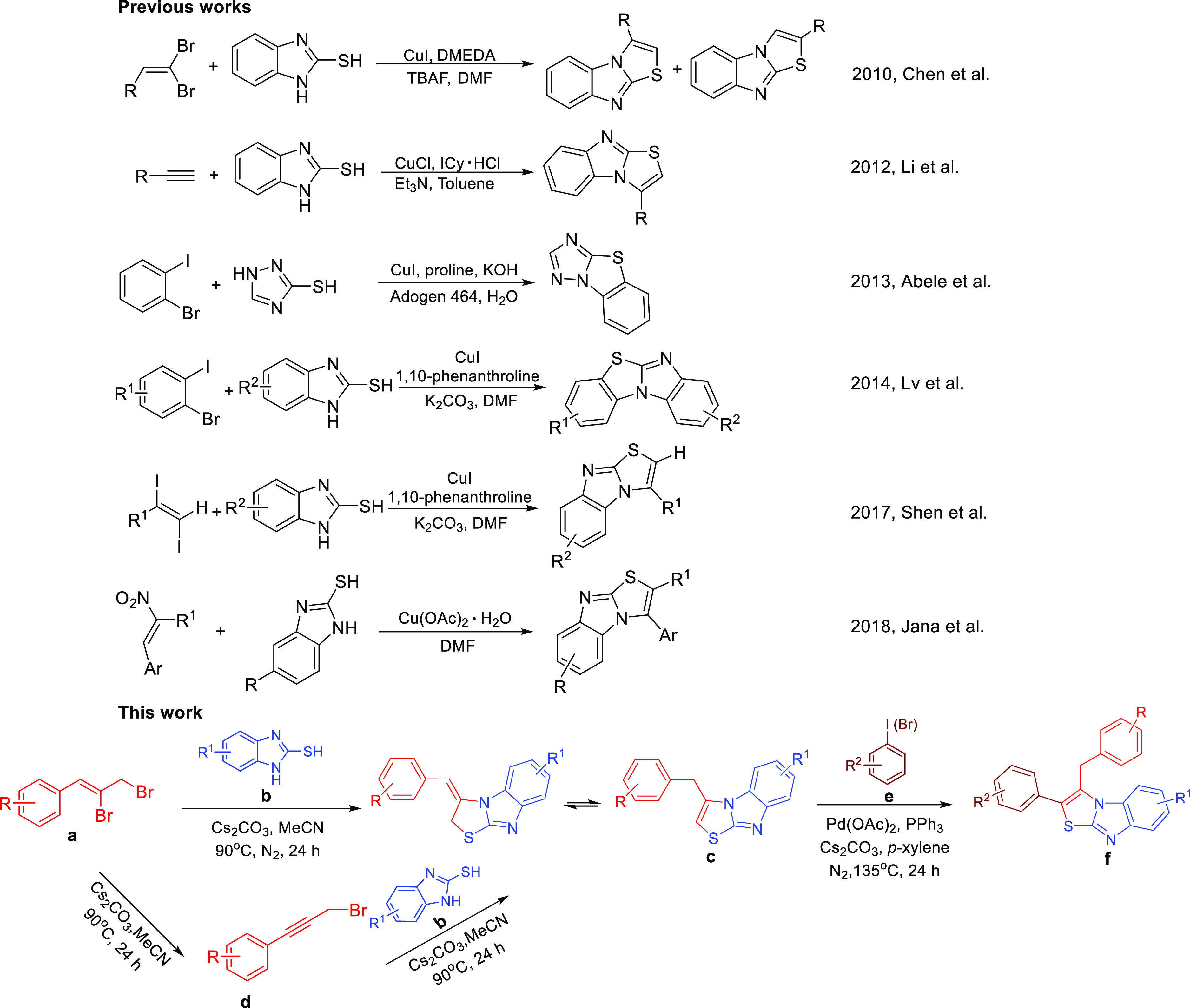
Numerous literature have reported the green synthesis of benzothiazole,5 but for imidazo[1,2-b]thiazoles, there are few economic, convenient, and practical synthetic pathways have been documented,6 and a majority of studies were reported to construct imidazole[2,1-b]-thiazoles by the copper-catalyzed cross-coupling reaction (Scheme 1 and Figure 1).7 The one-pot cascade direct nucleophilic or electrophilic attack reactions are one of the most important strategies for its easily worked up to form C–X (X = C, N, O, S, etc.) bond and construct heterocyclic without metal catalyst, ligand, and auxiliary additives.8 It is economic, convenience, practical, environmentally friendly, and attracted the research interest of chemists.9 Moreover, the C–H activation reaction have also been made great success for its efficiency and atomic economy features over the past decades.10 Numerous catalytic systems have been reported for the C–H bond activation to form the C–X bond.11 As a follow-up to prior research, we reported the nucleophilic attack/addition cyclization and C–H bond activation reactions for the synthesis 3-benzyl-/3-benzyl-2-phenyl-benzo[4,5]imidazo[2,1-b]thiazoles (c/f) via (Z)-(2,3-dibromoprop-1-en-1-yl)benzene/(3-bromoprop-1-yn-1-yl)benzene (a/d), 1H-benzo[d]imidazole-2-thiols b and halobenzenes e. The product c could be generated by the two pathway of cascade nucleophilic attack/addition cyclization reaction of a/d and b without metal catalyst. Next, c was further derivatized by the C–H bond activation reaction to synthesize f.
Scheme 1. Related Works.
Results and Discussion
First, we began the investigation using (Z)-(2,3-dibromoprop-1-en-1-yl)benzene 1a and 1H-benzo[d]imidazole-2-thiol 1b as model substrates for the optimization of the reaction conditions (Table 1). After the examination of the solvent, temperature, and base (Table 1, entries 1–12), it was found that 1c could be isolated in 93% yield with Cs2CO3 in MeCN at 90 °C for 24 h (Table 1, entry 7). Our previous work proved that e could be obtained by the elimination reaction of a,121c could also be detected in excellent yield when (3-bromoprop-1-yn-1-yl)benzene 1e was used to carry out the reaction under the same condition (Table 1, entry 13).
Table 1. Optimization of the Reaction Conditionsa.
| entry | solvent | base | T (°C) | yield (%)b |
|---|---|---|---|---|
| 1 | DMF | Cs2CO3 | 110 | 81 |
| 2 | DMSO | Cs2CO3 | 110 | 68 |
| 3 | toluene | Cs2CO3 | 110 | 12 |
| 4 | 1,4-dioxane | Cs2CO3 | 110 | 36 |
| 5 | MeCN | Cs2CO3 | 110 | 81 |
| 6 | MeCN | Cs2CO3 | 100 | 87 |
| 7 | MeCN | Cs2CO3 | 90 | 93 |
| 8 | MeCN | Cs2CO3 | 80 | 90 |
| 9 | MeCN | Na2CO3 | 90 | 63 |
| 10 | MeCN | t-BuOK | 90 | 43 |
| 11 | MeCN | 90 | 15 | |
| 12 | MeCN | Cs2CO3 | 90 | 78c |
| 13 | MeCN | Cs2CO3 | 90 | 90d |
Reaction conditions: 1a (0.3 mmol, 1.0 equiv), 1b (0.3 mmol, 1.0 equiv), and base (0.6 mmol, 2.0 equiv) in solvent (2.0 mL) for 24 h.
The yields were determined by 1H NMR analysis using 1,3,5-trimethoxybenzene as the internal standard.
Gram-scale (10 mmol scale) reaction.
(3-Bromoprop-1-yn-1-yl)benzene 1e was used.
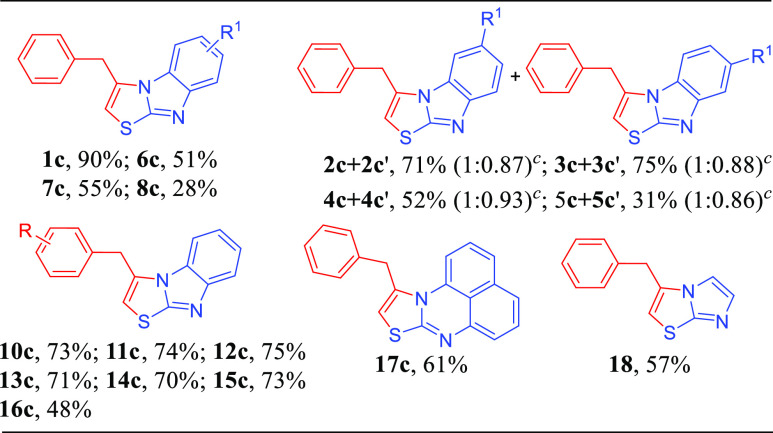
With the optimized reaction conditions, we investigated the substrate scope (Table 2). 1H-Benzo[d]imidazole-2-thiols b bearing electron-donating groups (Me, OMe) could afford the products with the mixture of two isomers (1:0.88 and 1:0.89) in good yields (Table 2, 2c + 2c′ and 3c + 3c′). When b bearing electron-withdrawing groups (F, Cl, and Br), the reaction yields decreased (Table 2, 4c + 4c′, 5c + 5c′, and 6c–8c). No corresponding product was detected for b with a strong electron-withdrawing group (NO2) (Table 2, 9c). The yields were not influenced by the electronic and steric hindrance effects of a (Table 2, 10c–15c). The lower yields of brominated substrates were due to the side reaction of the coupling reaction (Table 2, 5c + 5c′, 8c and 16c). The structure of 12c was confirmed by X-ray crystallography (CCDC no. 2254138). Fortunately, 1H-perimidine-2-thiol and 1H-imidazole-2-thiol were also suitable for the reaction, and the corresponding products were produced in moderate yields (Table 2, 17c–18c). Unfortunately, aliphatic-substituted (Z)-1,2-dibromohex-2-ene could not proceed the reaction (Table 2, 19c), and we only got the nucleophilic attack product; we speculate that the reaction could not proceed the addition cyclization step without the conjugation effect of benzene rings. From the above results, we concluded that the reaction yields were mainly influenced by the electronic effect of substrate b. Next, the substrate scope of d and b was also examined. The reactions could react well, and the yields and isomer ratios were almost the same as those of the reactions of a and b (Table 3).
Table 2. Substrate Scope of (Z)-(2,3-Dibromoprop-1-en-1-yl)benzenes and 1H-Benzo[d]imidazole-2-thiolsa,b.

Reaction conditions: a (0.3 mmol, 1.0 equiv), b (0.3 mmol, 1.0 equiv), and Cs2CO3 (2.0 mmol, 2.0 equiv) in MeCN (2.0 mL) for 24 h.
Isolated yields after flash chromatography based on a.
A mixture of two isomers was obtained (the isomeric ratio was approximately determined by 1H NMR).
Table 3. Substrate Scope of (3-Bromoprop-1-yn-1-yl)benzenes and 1H-Benzo[d]imidazole-2-thiolsa,b.
Reaction conditions: d (0.3 mmol, 1.0 equiv), b (0.3 mmol, 1.0 equiv), and Cs2CO3 (2.0 mmol, 2.0 equiv) in MeCN (2.0 mL) for 24 h.
Isolated yields after flash chromatography based on a.
A mixture of two isomers was obtained (the isomeric ratio was approximately determined by 1H NMR).
We also attempted to synthesize f via the palladium-catalyzed C–H bond activation reaction by c and e (Table 4). First, 1c and 1e were used to optimize the reaction conditions. When Pd(OAc)2 (0.015 mmol, 0.075 equiv)/PPh3 (0.03 mmol, 0.15 equiv), 1c (0.2 mmol, 1.0 equiv), iodobenzene 1e (0.30 mmol, 1.5 equiv), Cs2CO3 (0. 4 mmol, 2.0 equiv), and DMF (2 mL) were used to do the reaction at 135 °C under N2 for 24 h, 1f was isolated in 40% yield (Table 4, entry 1). The structure of 1f was confirmed by X-ray crystallography (CCDC no. 2254139). To further improve the yield, we screened different solvents (DMSO, DMA, and p-xylene), reaction temperature, and catalyst systems, and the result entry 4 showed the best reaction conditions (Table 4, entries 2–9).
Table 4. Optimization of the Reaction Conditionsa.
| entry | catalyst | solvent | T (°C) | yield(%)b |
|---|---|---|---|---|
| 1 | Pd(OAc)2/PPh3 | DMF | 135 | 40 |
| 2 | Pd(OAc)2/PPh3 | DMSO | 135 | 48 |
| 3 | Pd(OAc)2/PPh3 | DMA | 135 | 36 |
| 4 | Pd(OAc)2/PPh3 | p-xylene | 135 | 82 |
| 5 | Pd(OAc)2/PPh3 | p-xylene | 145 | 78 |
| 6 | Pd(OAc)2/PPh3 | p-xylene | 125 | 38 |
| 7 | Pd(OAc)2 | p-xylene | 135 | 21 |
| 8 | PdCl2/PPh3 | p-xylene | 135 | 57 |
| 9 | Pd(dppf)Cl2/PPh3 | p-xylene | 135 | 72 |
Reaction conditions: Pd salt (0.015 mmol, 0.075 equiv), ligand (0.03 mmol, 0.15 equiv), 1c (0.2 mmol, 1.0 equiv), 1e (0.30 mmol, 1.5 equiv), and Cs2CO3 (0.4 mmol, 2.0 equiv) in solvent (2.0 mL) under the N2 atmosphere for 24 h.
Isolated yield after flash chromatography based on 1c.
Finally, the range of suitability of the substrates was investigated, the substrate c reacted with e to produce f in moderate to good yields (Table 5). Bromobenzene could also be used for this reaction, but only 26% of 1f was isolated (Table 5, 1f). When iodine benzenes with o-, m-, p-, and di-CH3 groups were used, the desired products could be obtained in moderate yields (Table 5, 2f–5f). The reaction yields increased when substrate e reacted with electron-withdrawing groups (Table 5, 6f–11f). We also repeated the reaction with 1-iodonaphthalene, and the product 12f was obtained in moderate yield (Table 5, 12f). When the reaction was carried out with 2-iodo-1,1′-biphenyl, the yield of the product 13f decreased obviously due to the steric hindrance effect (Table 5, 13f). The reaction yields were not influenced by the functional groups (R and R1) of the benzene ring, and good yields of products were obtained (Table 5, 14f–16f, 17f + 17f′, and 18f + 18f′).
Table 5. Derivatization of 3-Benzyl-benzo[4,5]imidazo[2,1-b]thiazolesa,b.

Reaction conditions: Pd(OAc)2 (0.015 mmol, 0.075 equiv), PPh3 (0.03 mmol, 0.15 equiv), c (0.2 mmol, 1.0 equiv), e (0.3 mmol, 1.5 equiv), and Cs2CO3 (0.4 mmol, 2.0 equiv) in p-xylene (2.0 mL) at 135 °C under the N2 atmosphere for 24 h.
Isolated yield after flash chromatography based on c.
Bromobenzene was used.
A mixture of 17f and 18f was obtained (the isomeric ratio was approximately determined by 1H NMR).
Conclusions
In summary, we have presented practical and efficient strategies for the synthesis of 3-benzyl-/3-benzyl-2-phenyl-benzo[4,5]imidazo[2,1-b]thiazoles via direct nucleophilic attack/addition cyclization and C–H bond activation reactions. A wide range of new derivatives could be easily synthesized with different functional groups in moderate to excellent yields. The significance of the 3-benzyl-/3-benzyl-2-phenyl-benzo[4,5]imidazo[2,1-b]thiazoles will make the protocols appealing to synthetic and medicinal chemistry.
Experimental Section
General Remarks
Reagents and solvents were purchased from commercial sources. Reagents were used without further purification unless otherwise noted. Column chromatography was hand packed with silica gel (200–300 mesh). Reactions were monitored using Merck Kieselgel 60 F254 aluminum plates. TLC was visualized by UV fluorescence (254 nm) and then one of the following: KMnO4, phosphomolybdic acid, ninhydrin, p-anisaldehyde, and vanillin. NMR spectra were recorded on a Bruker AVANCE Neo-500 spectrometer at room temperature. 1H frequency is obtained at 500 MHz, and 13C frequency is obtained at 126 MHz. Chemical shifts (δ) were reported in parts per million relative to the residual solvent peaks rounded to the nearest 0.01 for proton and 0.1 for carbon (ref: CHCl3 [1H: 7.26, 13C: 77.16], Coupling constants (J) were reported in Hz to the nearest 0.1 Hz. Peak multiplicity was indicated as follows: s (singlet), d (doublet), t (triplet), q (quartet), quint (quintet), dd (doublet of doublets), dt (doublet of triplets), and m (multiplet). Attribution of peaks was performed using the multiplicities and integrals of the peaks. The melting points were uncorrected. High-resolution mass spectra (HRMS) were recorded on a Q-TOF Premier (ESI).
General Procedure for the Synthesis of 3-Benzylbenzo[4,5]imidazo[2,1-b]thiazoles by a with b (1c, 2c + 2c′, 3c + 3c′, 4c + 4c′, 5c + 5c′, 6c–8c, 9c + 9c′, and 10c–19c)
An Schlenk tube equipped with a Teflon valve was charged with a magnetic stir bar, a (0.3 mmol, 1.0 equiv), b (0.3 mmol, 1.0 equiv), Cs2CO3 (0.6 mmol, 2.0 equiv), and MeCN (2.0 mL). The reaction mixture was stirred at 90 °C for 24 h. The reaction was monitored by TLC. When a was consumed, the reaction was stopped and cooled to room temperature, and the crude reaction mixture was filtered through the filter paper and washed with EtOAc (20 mL) three times. The organic phase was concentrated, and the residue was purified by column chromatography on silica gel using petrol/EtOAc as the eluent to give the product c.
General Procedure for the Synthesis of 3-Benzylbenzo[4,5]imidazo[2,1-b]thiazoles by d with b (1c, 2c + 2c′, 3c + 3c′, 4c + 4c′, 5c + 5c′, 6c–8c, and 10c–18c)
An Schlenk tube equipped with a Teflon valve was charged with a magnetic stir bar, (3-Bromoprop-1-yn-1-yl)benzenes d (0.3 mmol, 1.0 equiv), b (0.3 mmol, 1.0 equiv), Cs2CO3 (0.6 mmol, 2.0 equiv), and MeCN (2.0 mL). The reaction mixture was stirred at 90 °C for 24 h. The reaction was monitored by TLC. When d was consumed, the reaction was stopped and cooled to room temperature, and the crude reaction mixture was filtered through the filter paper and washed with EtOAc (20 mL) three times. The organic phase was concentrated, and the residue was purified by column chromatography on silica gel using petrol/EtOAc as the eluent to give the product c.
General Procedure for the Synthesis of 3-Benzyl-2-phenylbenzo[4,5]imidazo[2,1-b]thiazoles by c and e (1f–16f, 17f + 17f′, and 18f + 18f′)
An Schlenk tube equipped with a Teflon valve was charged with a magnetic stir bar, c (0.20 mmol, 1.0 equiv), e (0.30 mmol, 1.5 equiv), Pd(OAc)2 (0.015 mmol), PPh3 (0.03 mmol), and Cs2CO3 (0.4 mmol, 2 equiv). The tube was placed under a vacuum for 20 min and backfilled with N2. Then, p-xylene (2.0 mL) was added via a syringe. The reaction mixture was stirred at 135 °C for 24 h. The reaction was monitored by TLC. When c was consumed, the reaction was stopped and cooled to room temperature, and the crude reaction mixture was filtered through the filter paper and washed with EtOAc (20 mL) three times. The organic phase was concentrated, and the residue was purified by column chromatography on silica gel using petrol/EtOAc as the eluent to give the product f.
3-benzylbenzo[4,5]imidazo[2,1-b]thiazole (1c)
The title compound was prepared according to the general procedure and purified by column chromatography on silica gel using petrol/EtOAc (5:1, v/v) as the eluent to afford a light yellow solid [73.6 mg; 93% yield (1a was used); 71.4 mg; 90% yield (1d was used)]. Mp: 83–85 °C [Lit: mp: 82–93 °C].7e,131H NMR (500 MHz, chloroform-d): δ 7.78 (d, J = 8.0 Hz, 1H), 7.62 (d, J = 8.5 Hz, 1H), 7.40–7.30 (m, 6H), 7.17 (t, J = 7.5 Hz, 1H), 6.16 (s, 1H), 4.41 (s, 2H). 13C NMR (126 MHz, chloroform-d): δ 157.5, 148.6, 135.1, 133.3, 130.2, 129.2, 128.9, 127.7, 123.4, 120.9, 119.3, 111.1, 106.7, 34.8.
3-Benzyl-6-methylbenzo[4,5]imidazo[2,1-b]thiazole (2c) and 3-Benzyl-7-methylbenzo[4,5]imidazo[2,1-b]thiazole (2c′)
The title compound was prepared according to the general procedure and purified by column chromatography on silica gel using petrol/EtOAc (4:1, v/v) as the eluent to afford a light yellow oil [66.8 mg; 80% yield (1a was used); 59.3 mg; 71% yield (1d was used)]. 1H NMR (500 MHz, chloroform-d): δ 7.65 (d, J = 8.0 Hz, 0.46H), 7.55 (s, 0.53H), 7.49 (d, J = 8.5 Hz, 0.59H), 7.41 (s, 0.50H), 7.40–7.33 (m, 2.44H), 7.32–7.27 (m, 2.78H), 7.15 (d, J = 8.0 Hz, 0.50H), 6.96 (d, J = 8.5 Hz, 0.53H), 6.10 (s, 0.50H), 6.05 (s, 0.44H), 4.35 (d, J = 8.0 Hz, 2H), 2.47 (s, 1.58H), 2.44 (s, 1.35H). 13C NMR (126 MHz, chloroform-d): δ 157.3, 156.9, 148.9, 146.6, 135.1, 133.4, 133.2, 133.2, 130.6, 130.3, 129.1, 129.1, 129.0, 128.8, 128.2, 127.6, 127.6, 124.8, 122.2, 119.0, 118.7, 111.1, 110.5, 106.2, 106.2, 34.8, 34.7, 21.8, 21.7. HRMS (ESI): m/z calcd for C17H15N2S, [M + H]+ 279.0951; found, 279.0963.
3-Benzyl-6-methoxybenzo[4,5]imidazo[2,1-b]thiazole (3c) and 3-Benzyl-7-methoxybenzo[4,5]imidazo[2,1-b]thiazole (3c′)
The title compound was prepared according to the general procedure and purified by column chromatography on silica gel using petrol/EtOAc (4:1, v/v) as the eluent to afford a light brown oil [73.3 mg; 83% yield (1a was used); 66.2 mg; 75% yield (1d was used)]. 1H NMR (500 MHz, chloroform-d): δ 7.61 (d, J = 9.0 Hz, 0.52H), 7.43 (d, J = 9.0z Hz, 0.51H), 7.37–7.34 (m, 2H), 7.31–7.26 (m, 3H), 7.22 (d, J = 2.0 Hz, 0.48H), 6.97 (d, J = 2.5 Hz, 0.52H), 6.93 (dd, J1 = 9.0 Hz, J2 = 2.0 Hz, 0.52H), 6.79 (dd, J1 = 9.0 Hz, J2 = 2.5 Hz, 0.50H), 6.20 (s, 0.53H), 6.20 (s, 0.47H), 4.36 (d, J = 11.5 Hz, 2H), 3.84 (s, 1.42H), 3.70 (s, 1.58H). 13C NMR (126 MHz, chloroform-d): δ 157.1, 156.8, 155.8, 155.1, 147.6, 141.6, 135.1, 134.8, 133.4, 132.7, 130.0, 129.2, 129.2, 128.8, 128.6, 127.7, 127.7, 124.2, 119.0, 112.7, 111.7, 111.1, 107.4, 107.1, 100.9, 95.7, 56.0, 55.8, 34.6, 34.6. HRMS (ESI): m/z calcd for C17H15N2OS, [M + H]+ 295.0900; found, 295.0916.
3-Benzyl-6-chlorobenzo[4,5]imidazo[2,1-b]thiazole (4c) and 3-Benzyl-7-chlorobenzo[4,5]imidazo[2,1-b]thiazole (4c′)
The title compound was prepared according to the general procedure and purified by column chromatography on silica gel using petrol/EtOAc (4:1, v/v) as the eluent to afford a light yellow solid [54.7 mg; 61% yield (1a was used); 46.6 mg; 52% yield (1d was used)]. Mp: 121.0–122.7 °C. 1H NMR (500 MHz, chloroform-d): δ 7.75 (d, J = 2.0 Hz, 0.51H), 7.68 (d, J = 8.5 Hz, 0.47H), 7.61 (d, J = 2.0 Hz, 0.47H), 7.46 (d, J = 8.5 Hz, 0.51H), 7.41–7.35 (m, 2H), 7.34–7.27 (m, 3H), 7.11 (dd, J1 = 9.0 Hz, J2 = 2.0 Hz, 0.50H), 6.22 (s, 0.49H), 6.16 (s, 0.46H), 4.37 (d, J = 5.0 Hz, 2H). 13C NMR (126 MHz, chloroform-d): δ 158.7, 158.1, 149.3, 147.1, 134.7, 134.6, 133.2, 133.1, 130.3, 129.3, 129.3, 129.1, 129.0, 128.8, 128.7, 127.9, 127.8, 126.4, 124.0, 121.1, 120.0, 119.0, 111.7, 111.3, 107.3, 34.7, 34.7.
3-Benzyl-6-bromobenzo[4,5]imidazo[2,1-b]thiazole (5c) and 3-Benzyl-7-bromobenzo[4,5]imidazo[2,1-b]thiazole (5c′)
The title compound was prepared according to the general procedure and purified by column chromatography on silica gel using petrol/EtOAc (4:1, v/v) as the eluent to afford a light yellow solid [38.1 mg; 37% yield (1a was used); 31.9 mg; 31% yield (1d was used)]. Mp: 131–132 °C. 1H NMR (500 MHz, chloroform-d): δ 7.74 (s, 0.52H), 7.67 (d, J = 8.5 Hz, 0.55H), 7.61 (s, 0.52H), 7.47 (d, J = 8.5 Hz, 0.62H), 7.41–7.35 (m, 2H), 7.34 (s, 0.47H), 7.31–7.29 (m, 2H), 7.28 (s, 0.45H), 7.11 (d, J = 9.0 Hz, 0.48H), 6.22 (s, 0.48H), 6.16 (s, 0.46H), 4.37 (d, J = 4.5 Hz, 2H). 13C NMR (126 MHz, chloroform-d): δ 158.7, 158.2, 149.3, 147.1, 134.7, 134.6, 133.3, 133.2, 130.4, 129.3, 129.3, 129.2, 129.0, 128.8, 127.9, 1f27.8, 126.5, 124.1, 121.2, 120.0, 119.0, 111.7, 111.4, 107.3, 34.8, 34.7. HRMS (ESI): m/z calcd for C16H12BrN2S, [M + H]+ 342.9900; found, 342.9886.
3-Benzyl-6,7-difluorobenzo[4,5]imidazo[2,1-b]thiazole (6c)
The title compound was prepared according to the general procedure and purified by column chromatography on silica gel using petrol/EtOAc (1:1, v/v) as the eluent to afford a light yellow solid [46.8 mg; 52% yield (1a was used); 46.0 mg; 51% yield (1d was used)]. Mp: 172–174 °C. 1H NMR (500 MHz, chloroform-d): δ 7.53 (dd, J1 = 10.5 Hz, J2 = 7.5 Hz, 1H), 7.40–7.32 (m, 4H), 7.28 (d, J = 7.0 Hz, 2H), 6.25 (s, 1H), 4.35 (s, 2H). 13C NMR (126 MHz, chloroform-d): δ 158.5, 149.4, 149.3, 147.5, 147.4, 147.4, 145.6, 145.5, 144.0, 134.5, 132.7, 129.4, 128.7, 127.9, 107.5, 106.6, 106.5, 99.6, 99.4, 34.6. HRMS (ESI): m/z calcd for C16H11F2N2S, [M + H]+ 301.0606; found, 301.0623.
3-Benzyl-6,7-dichlorobenzo[4,5]imidazo[2,1-b]thiazole (7c)
The title compound was prepared according to the general procedure and purified by column chromatography on silica gel using petrol/EtOAc (1:1, v/v) as the eluent to afford a light yellow solid [62.0 mg; 62% yield (1a was used); 55.0 mg; 55% yield (1d was used)]. Mp: 202–203 °C [Lit: mp: 199–201 °C].141H NMR (500 MHz, chloroform-d): δ 7.85 (s, 1H), 7.68 (s, 1H), 7.42–7.33 (m, 3H), 7.30 (d, J = 7.5 Hz, 2H), 6.23 (s, 1H), 4.36 (s, 2H). 13C NMR (126 MHz, chloroform-d): δ 159.3, 147.5, 134.3, 133.2, 129.4, 128.9, 128.9, 128.0, 127.7, 124.8, 120.2, 112.5, 107.9, 34.7.
3-Benzyl-6,7-dibromobenzo[4,5]imidazo[2,1-b]thiazole (8c)
The title compound was prepared according to the general procedure and purified by column chromatography on silica gel using petrol/EtOAc (1:1, v/v) as the eluent to afford a light yellow solid [46.9 mg; 37% yield (1a was used); 35.5 mg; 28% yield (1d was used)]. Mp: 216–217 °C. 1H NMR (500 MHz, chloroform-d): δ 8.09 (s, 1H), 7.90 (s, 1H), 7.41 (t, J = 7.0 Hz, 2H), 7.36 (t, J = 7.5 Hz, 1H), 7.30 (d, J = 7.0 Hz, 2H), 6.30 (s, 1H), 4.39 (s, 2H). 13C NMR (126 MHz, chloroform-d): δ 157.3, 146.7, 132.4, 131.2, 127.9, 127.4, 126.9, 126.0, 121.5, 116.9, 113.7, 113.6, 105.8, 32.8. HRMS (ESI): m/z calcd for C16H11Br2N2S, [M + H]+ 422.8984; found, 422.9002.
3-(4-Methylbenzyl)benzo[4,5]imidazo[2,1-b]thiazole (10c)
The title compound was prepared according to the general procedure and purified by column chromatography on silica gel using petrol/EtOAc (2:1, v/v) as the eluent to afford a light yellow solid [66.8 mg; 80% yield (2a was used); 61.0 mg; 73% yield (2d was used)]. Mp: 149–150 °C [Lit: mp: 151–152 °C].141H NMR (500 MHz, chloroform-d): δ 7.78 (d, J = 8.0 Hz, 1H), 7.64 (d, J = 8.0 Hz, 1H), 7.34 (t, J = 7.5 Hz, 1H), 7.20–7.16 (m, 5H), 6.15 (s, 1H), 4.37 (s, 2H), 2.35 (s, 3H). 13C NMR (126 MHz, chloroform-d): δ 157.6, 148.6, 137.4, 133.7, 131.9, 130.3, 129.9, 128.8, 123.4, 120.8, 119.3, 111.2, 106.5, 34.5, 21.2. HRMS (ESI): m/z calcd for C17H15N2S, [M + H]+ 279.0951; found, 279.0937.
3-(4-Methoxybenzyl)benzo[4,5]imidazo[2,1-b]thiazole (11c)
The title compound was prepared according to the general procedure and purified by column chromatography on silica gel using petrol/EtOAc (1:1, v/v) as the eluent to afford a light yellow solid [73.3 mg; 83% yield (3a was used); 65.4 mg; 74% yield (3d was used)]. Mp: 119–121 °C. 1H NMR (500 MHz, chloroform-d): δ 7.78 (d, J = 8.0 Hz, 1H), 7.62 (d, J = 8.5 Hz, 1H), 7.33 (t, J = 7.5 Hz, 1H), 7.21 (d, J = 8.5 Hz, 2H), 7.17 (t, J = 8.0 Hz, 1H), 6.90 (d, J = 8.0 Hz, 2H), 6.12 (s, 1H), 4.33 (s, 2H), 3.80 (s, 3H). 13C NMR (126 MHz, chloroform-d): δ 159.1, 157.6, 148.6, 133.9, 130.2, 130.0, 126.9, 123.4, 120.8, 119.2, 114.6, 111.2, 106.4, 55.4, 34.0. HRMS (ESI): m/z calcd for C17H15N2OS, [M + H]+ 295.0900; found, 295.0915.
3-(4-Fluorobenzyl)benzo[4,5]imidazo[2,1-b]thiazole (12c)
The title compound was prepared according to the general procedure and purified by column chromatography on silica gel using petrol/EtOAc (1:1, v/v) as the eluent to afford a light yellow solid [68.6 mg; 81% yield (4a was used); 63.5 mg; 75% yield (4d was used)]. Mp: 113–115 °C. 1H NMR (500 MHz, chloroform-d): δ 7.79 (d, J = 8.0 Hz, 1H), 7.59 (d, J = 8.5 Hz, 1H), 7.34 (t, J = 7.5 Hz, 1H), 7.29–7.26 (m, 2H), 7.17 (t, J = 7.0 Hz, 1H), 7.07 (t, J = 8.5 Hz, 2H), 6.15 (d, J = 1.0 Hz, 1H), 4.8 (s, 2H). 13C NMR (126 MHz, chloroform-d): δ 163.3, 161.3, 157.5, 148.6, 133.1, 130.7, 130.7, 130.5, 130.5, 130.2, 123.5, 120.9, 119.4, 116.3, 116.1, 111.0, 106.7, 34.0. HRMS (ESI): m/z calcd for C16H12FN2S, [M + H]+ 283.0700; found, 283.0689.
3-(4-Chlorobenzyl)benzo[4,5]imidazo[2,1-b]thiazole (13c)
The title compound was prepared according to the general procedure and purified by column chromatography on silica gel using petrol/EtOAc (4:1, v/v) as the eluent to afford a light yellow solid [72.6 mg; 81% yield (5a was used); 63.6 mg; 71% yield (5d was used)]. Mp: 136–138 °C. 1H NMR (500 MHz, chloroform-d): δ 7.77 (d, J = 8.5 Hz, 1H), 7.55 (d, J = 8.5 Hz, 1H), 7.35–7.31 (m, 3H), 7.22 (d, J = 8.5 Hz, 2H), 7.15 (t, J = 8.0 Hz, 1H), 6.15 (s, 1H), 4.35 (s, 2H). 13C NMR (126 MHz, chloroform-d): δ 157.4, 148.5, 133.6, 133.5, 132.7, 130.2, 130.0, 129.4, 123.5, 120.9, 119.3, 110.9, 106.9, 34.1.
3-(3-Chlorobenzyl)benzo[4,5]imidazo[2,1-b]thiazole (14c)
The title compound was prepared according to the general procedure and purified by column chromatography on silica gel using petrol/EtOAc (4:1, v/v) as the eluent to afford a light yellow solid [71.7 mg; 80% yield (6a was used); 62.7 mg; 70% yield (6d was used)]. Mp: 90–92 °C. 1H NMR (500 MHz, chloroform-d): δ 7.78 (d, J = 8.0 Hz, 1H), 7.56 (d, J = 8.0 Hz, 1H), 7.35–7.29 (m, 4H), 7.18–7.15 (m, 2H), 6.18 (s, 1H), 4.36 (s, 2H). 13C NMR (126 MHz, chloroform-d): δ 157.3, 148.5, 137.0, 135.0, 132.3, 130.4, 130.0, 129.0, 127.9, 127.0, 123.5, 120.9, 119.3, 110.9, 107.0, 34.3. HRMS (ESI): m/z calcd for C16H12ClN2S, [M + H]+ 299.0405; found, 299.0418.
3-(2-Chlorobenzyl)benzo[4,5]imidazo[2,1-b]thiazole (15c)
The title compound was prepared according to the general procedure and purified by column chromatography on silica gel using petrol/EtOAc (4:1, v/v) as the eluent to afford a light yellow solid [73.5 mg; 82% yield (7a was used); 65.4 mg; 73% yield (7d was used)]. Mp: 117–119 °C. 1H NMR (500 MHz, chloroform-d): δ 7.79 (d, J = 8.5 Hz, 1H), 7.55 (d, J = 8.5 Hz, 1H), 7.49 (d, J = 8.0 Hz, 1H), 7.34 (t, J = 8.0 Hz, 1H), 7.30–7.26(m, 1H), 7.22–7.15 (m, 3H), 6.14 (s, 1H), 4.50 (s, 2H). 13C NMR (126 MHz, chloroform-d): δ 157.4, 148.6, 134.3, 133.1, 131.5, 130.4, 130.2, 130.1, 129.3, 127.6, 123.5, 121.0, 119.4, 110.9, 106.8, 32.5. HRMS (ESI): m/z calcd for C16H12ClN2S, [M + H]+ 299.0405; found, 299.0421.
3-(4-Bromobenzyl)benzo[4,5]imidazo[2,1-b]thiazole (16c)
The title compound was prepared according to the general procedure and purified by column chromatography on silica gel using petrol/EtOAc (6:1, v/v) as the eluent to afford a light yellow solid [65.9 mg; 64% yield (8a was used); 49.4 mg; 48% yield (8d was used)]. Mp: 142–144 °C. 1H NMR (500 MHz, chloroform-d): δ 7.79 (d, J = 8.0 Hz, 1H), 7.58 (d, J = 8.0 Hz, 1H), 7.51 (d, J = 8.0 Hz, 2H), 7.35 (t, J = 7.5 Hz, 1H), 7.19 (d, J = 8.0 Hz, 3H), 6.19 (s, 1H), 4.38 (s, 2H). 13C NMR (126 MHz, chloroform-d): δ 156.4, 147.6, 133.1, 131.6, 131.4, 129.6, 129.1, 122.5, 120.7, 119.9, 118.4, 109.9, 105.9, 33.2. HRMS (ESI): m/z calcd for C16H12BrN2S, [M + H]+ 342.9900; found, 342.9887.
10-Benzylthiazolo[3,2-a]perimidine (17c)
The title compound was prepared according to the general procedure and purified by column chromatography on silica gel using petrol/EtOAc (6:1, v/v) as the eluent to afford a light yellow solid [64.1 mg; 68% yield (9a was used); 57.5 mg; 61% yield (9d was used)]. Mp: 155–157 °C. 1H NMR (500 MHz, chloroform-d): δ 7.37 (t, J = 7.0 Hz, 2H), 7.30 (t, J = 7.0 Hz, 1H), 7.26–7.22 (m, 3H), 7.18 (d, J = 8.5 Hz, 1H), 7.07 (d, J = 8.0 Hz, 1H), 7.01 (t, J = 8.0 Hz, 1H), 6.91 (d, J = 7.5 Hz, 1H), 6.75 (d, J = 7.5 Hz, 1H), 5.66 (s, 1H), 4.28 (s, 2H). 13C NMR (126 MHz, chloroform-d): δ 162.5, 143.2, 137.8, 136.8, 136.3, 136.2, 129.2, 129.1, 129.0, 127.5, 126.7, 122.0, 121.3, 119.2, 113.8, 105.4, 103.1, 38.3.
3-Benzylimidazo[2,1-b]thiazole (18c)
The title compound was prepared according to the general procedure and purified by column chromatography on silica gel using petrol/EtOAc (1:1, v/v) as the eluent to afford a light brown oil [32.8 mg; 51% yield (9a was used); 36.6 mg; 57% yield (9d was used)]. 1H NMR (500 MHz, chloroform-d): δ 7.37–7.34 (m, 2H), 7.31–7.28 (m, 2H), 7.26–7.22 (m, 3H), 6.38 (d, J = 1.0 Hz, 1H), 4.05 (s, 2H). 13C NMR (126 MHz, chloroform-d): δ 149.8, 134.9, 134.7, 130.9, 129.1, 128.9, 127.6, 111.1, 108.7, 34.5.
3-Benzyl-2-phenylbenzo[4,5]imidazo[2,1-b]thiazole (1f)
The title compound was prepared according to the general procedure and purified by column chromatography on silica gel using petrol/EtOAc (6:1, v/v) as the eluent to afford a light yellow solid [55.8 mg; 82% yield (1e was used); 17.7 mg; 26% yield (bromobenzene was used)]. Mp: 215–217 °C. 1H NMR (500 MHz, chloroform-d): δ 7.77 (d, J = 8.5 Hz, 1H), 7.48 (d, J = 7.0 Hz, 2H), 7.44–7.40 (m, 3H), 7.35 (t, J = 7.5 Hz, 2H), 7.29–7.25 (m, 5H), 7.02 (t, J = 7.5 Hz, 1H), 4.53 (s, 2H). 13C NMR (126 MHz, chloroform-d): δ 155.3, 148.1, 136.3, 131.5, 130.1, 129.5, 129.3, 129.1, 129.0, 127.6, 127.4, 126.5, 124.6, 123.4, 121.0, 119.2, 111.4, 32.3. HRMS (ESI): m/z calcd for C22H17N2S, [M + H]+: 341.1107; found, 341.1118.
3-Benzyl-2-(o-tolyl)benzo[4,5]imidazo[2,1-b]thiazole (2f)
The title compound was prepared according to the general procedure and purified by column chromatography on silica gel using petrol/EtOAc (4:1, v/v) as the eluent to afford a light yellow solid (39.7 mg; 56% yield). Mp: 151–153 °C. 1H NMR (500 MHz, chloroform-d): δ 7.78 (d, J = 8.0 Hz, 1H), 7.38–7.34 (m, 2H), 7.31–7.19 (m, 7H), 7.13 (d, J = 7.5 Hz, 2H), 7.04 (t, J = 8.0 Hz, 1H), 4.29 (s, 2H), 2.33 (s, 3H). 13C NMR (126 MHz, chloroform-d): δ 156.1, 147.8, 138.9, 136.0, 131.8, 130.8, 130.1, 129.9, 129.9, 129.2, 127.7, 127.6, 127.2, 126.3, 123.3, 120.9, 119.1, 111.4, 31.9, 20.4. HRMS (ESI): m/z calcd for C23H19N2S, [M + H]+ 355.1264; found, 355.1262.
3-Benzyl-2-(m-tolyl)benzo[4,5]imidazo[2,1-b]thiazole (3f)
The title compound was prepared according to the general procedure and purified by column chromatography on silica gel using petrol/EtOAc (4:1, v/v) as the eluent to afford a light yellow solid (35.2 mg; 51% yield). Mp: 178–179 °C. 1H NMR (500 MHz, chloroform-d): δ 7.77 (d, J = 8.0z Hz, 1H), 7.36–7.20 (m, 11H), 7.03 (t, J = 8.0 Hz, 1H), 4.53 (s, 2H), 2.37 (s, 3H). 13C NMR (126 MHz, chloroform-d): δ 155.4, 147.9, 139.1, 136.4, 131.4, 130.0, 129.9, 129.9, 129.5, 129.2, 127.7, 127.4, 126.5, 126.1, 124.9, 123.4, 121.0, 119.1, 111.4, 32.4, 21.6. HRMS (ESI): m/z calcd for C23H19N2S, [M + H]+ 355.1264; found, 355.1275.
3-Benzyl-2-(p-tolyl)benzo[4,5]imidazo[2,1-b]thiazole (4f)
The title compound was prepared according to the general procedure and purified by column chromatography on silica gel using petrol/EtOAc (4:1, v/v) as the eluent to afford a light yellow solid (43.2 mg; 61% yield.) mp: 208–209 °C. 1H NMR (500 MHz, chloroform-d): δ 7.76 (d, J = 8.5 Hz, 1H), 7.38–7.33 (m, 4H), 7.29–7.21 (m, 7H), 7.02 (t, J = 7.5 Hz, 1H), 4.51 (s, 2H), 2.39 (s, 3H). 13C NMR (126 MHz, chloroform-d): δ 155.3, 147.5, 139.3, 136.3, 130.0, 129.5, 129.0, 128.5, 127.6, 127.4, 126.2, 125.0, 123.4, 121.0, 119.0, 111.4, 32.3, 21.4. HRMS (ESI): m/z calcd for C23H19N2S, [M + H]+ 355.1264; found, 355.1272.
3-Benzyl-2-(3,4-dimethylphenyl)benzo[4,5]imidazo[2,1-b]thiazole (5f)
The title compound was prepared according to the general procedure and purified by column chromatography on silica gel using petrol/EtOAc (4:1, v/v) as the eluent to afford a light yellow solid (36.8 mg; 50% yield). Mp: 143–144 °C. 1H NMR (500 MHz, chloroform-d): δ 7.77 (d, J = 8.0 Hz, 1H), 7.36–7.23 (m, 8H), 7.20–7.16 (m, 2H), 7.03 (t, J = 7.5 Hz, 1H), 4.52 (s, 2H), 2.28 (d, J = 13.0 Hz, 6H). 13C NMR (126 MHz, chloroform-d): δ 155.3, 147.7, 137.9, 137.7, 136.5, 130.5, 130.3, 130.0, 129.4, 128.9, 127.7, 127.3, 126.4, 126.1, 125.1, 123.4, 121.0, 119.0, 111.4, 32.3, 19.9, 19.7. HRMS (ESI): m/z calcd for C24H21N2S, [M + H]+ 369.1420; found, 369.1433.
3-Benzyl-2-(2-chlorophenyl)benzo[4,5]imidazo[2,1-b]thiazole (6f)
The title compound was prepared according to the general procedure and purified by column chromatography on silica gel using petrol/EtOAc (3:1, v/v) as the eluent to afford a light yellow solid (60.7 mg; 81% yield). Mp: 115–117 °C. 1H NMR (500 MHz, chloroform-d): δ 7.78 (d, J = 8.5 Hz, 1H), 7.52–7.48 (m, 2H), 7.8 (td, J1 = 8.0, J2 = 2.0 Hz, 1H), 7.33–7.25 (m, 5H), 7.22–7.17 (m, 3H), 7.05–7.01 (m, 1H), 4.32 (s, 2H). 13C NMR (126 MHz, chloroform-d): δ 156.0, 148.0, 135.6, 135.6, 133.2, 131.1, 130.4, 130.0, 129.6, 129.2, 128.9, 127.6, 127.2, 123.3, 120.9, 120.8, 119.1, 111.4, 32.2. HRMS (ESI): m/z calcd for C22H16ClN2S, [M + H]+ 375.0718; found, 375.0699.
3-Benzyl-2-(3-chlorophenyl)benzo[4,5]imidazo[2,1-b]thiazole (7f)
The title compound was prepared according to the general procedure and purified by column chromatography on silica gel using petrol/EtOAc (3:1, v/v) as the eluent to afford a light yellow solid (60.0 mg; 80% yield). Mp: 149–151 °C. 1H NMR (500 MHz, chloroform-d): δ 7.77 (d, J = 8.0 Hz, 1H), 7.7 (s, 1H), 7.40–7.33 (m, 5H), 7.31–7.26 (m, 3H), 7.23 (d, J = 7.0 Hz, 2H), 7.05 (t, J = 7.5 Hz, 1H), 4.52 (s, 2H). 13C NMR (126 MHz, chloroform-d): δ 155.1, 148.0, 135.9, 135.2, 133.3, 130.5, 130.0, 129.6, 129.2, 129.2, 127.6, 127.6, 127.5, 127.2, 123.6, 123.0, 121.2, 119.2, 111.4, 32.3. HRMS (ESI): m/z calcd for C22H16ClN2S, [M + H]+ 375.0718; found, 375.0711.
3-Benzyl-2-(4-chlorophenyl)benzo[4,5]imidazo[2,1-b]thiazole (8f)
The title compound was prepared according to the general procedure and purified by column chromatography on silica gel using petrol/EtOAc (3:1, v/v) as the eluent to afford a light yellow solid (66.7 mg; 89% yield). Mp: 226–227 °C. 1H NMR (500 MHz, chloroform-d): δ 7.77 (d, J = 8.0 Hz, 1H), 7.39 (s, 4H), 7.35 (t, J = 7.0 Hz, 2H), 7.30–7.22 (m, 5H), 7.03 (t, J = 8.0 Hz, 1H), 4.49 (s, 2H). 13C NMR (126 MHz, chloroform-d): δ 155.0, 148.1, 136.0, 135.2, 130.3, 130.0, 129.6, 129.5, 127.5, 127.5, 127.0, 123.5, 123.2, 121.1, 119.2, 111.4, 32.3. HRMS (ESI): m/z calcd for C22H16ClN2S, [M + H]+ 375.0718; found, 375.0729.
3-Benzyl-2-(2-bromophenyl)benzo[4,5]imidazo[2,1-b]thiazole (9f)
The title compound was prepared according to the general procedure and purified by column chromatography on silica gel using petrol/EtOAc (4:1, v/v) as the eluent to afford a light yellow solid (45.3 mg; 54% yield). Mp: 134–136 °C. 1H NMR (500 MHz, chloroform-d): δ 7.78 (d, J = 8.0 Hz, 1H), 7.70 (dd, J1 = 8.0 Hz, J2 = 1.5 Hz, 1H), 7.49 (dd, J1 = 7.5 Hz, J2 = 2.0 Hz, 1H), 7.36 (td, J1 = 7.5 Hz, J2 = 1.0 Hz, 1H), 7.32–7.25 (m, 5H), 7.22–7.17 (m, 3H), 7.04 (t, J = 7.5 Hz, 1H), 4.32 (s, 2H). 13C NMR (126 MHz, chloroform-d): δ 155.9, 147.7, 135.6, 133.6, 133.3, 131.6, 131.3, 129.2, 128.8, 127.8, 127.7, 127.3, 125.9, 123.5, 122.9, 121.0, 119.0, 111.5, 77.4, 77.2, 76.9, 32.2. HRMS (ESI): m/z calcd for C22H16BrN2S, [M + H]+ 419.0213; found, 419.0199.
2-(3-Benzylbenzo[4,5]imidazo[2,1-b]thiazol-2-yl)benzonitrile (10f)
The title compound was prepared according to the general procedure and purified by column chromatography on silica gel using petrol/EtOAc (1:1, v/v) as the eluent to afford a light yellow solid (65.8 mg; 90% yield). Mp: 161–162 °C. 1H NMR (500 MHz, chloroform-d): δ 7.79 (t, J = 8.0 Hz, 2H), 7.68–7.53 (m, 3H), 7.32 (t, J = 8.5 Hz 2H), 7.29–7.24 (m, 2H), 7.20 (t, J = 7.5 Hz, 1H), 7.14 (d, J = 7.5 Hz, 2H), 7.07 (t, J = 7.5 Hz, 1H), 4.44 (s, 2H). 13C NMR (126 MHz, chloroform-d): δ 155.4, 147.9, 135.2, 134.5, 133.9, 133.3, 132.4, 130.0, 129.8, 129.4, 127.7, 127.5, 123.8, 121.3, 119.4, 119.3, 117.4, 114.7, 111.6, 32.4. HRMS (ESI): m/z calcd for C23H16N3S, [M + H]+ 366.1060; found, 366.1074.
3-Benzyl-2-(2-nitrophenyl)benzo[4,5]imidazo[2,1-b]thiazole (11f)
The title compound was prepared according to the general procedure and purified by column chromatography on silica gel using petrol/EtOAc (1:1, v/v) as the eluent to afford a light yellow oil (62.4 mg; 81% yield). 1H NMR (500 MHz, chloroform-d): δ 8.04 (dd, J1 = 7.5 Hz, J2 = 1.0 Hz, 1H), 7.77 (d, J = 8.5 Hz, 1H), 7.68–7.57 (m, 3H), 7.31–7.25 (m, 4H), 7.21 (t, J = 7.5 Hz, 1H), 7.13 (d, J = 7.5 Hz, 2H), 7.04 (m, 1H), 4.31 (s, 2H). 13C NMR (126 MHz, chloroform-d): δ 155.7, 150.1, 148.1, 135.2, 134.2, 133.2, 130.9, 130.0, 129.4, 128.8, 127.6, 127.4, 125.4, 125.2, 123.6, 121.2, 119.3, 118.5, 111.5, 32.3. HRMS (ESI): m/z calcd for C22H16N3O2S, [M + H]+ 386.0958; found, 386.0966.
3-Benzyl-2-(naphthalen-1-yl)benzo[4,5]imidazo[2,1-b]thiazole (12f)
The title compound was prepared according to the general procedure and purified by column chromatography on silica gel using petrol/EtOAc (4:1, v/v) as the eluent to afford a colorless oil (53.1 mg; 68% yield). 1H NMR (500 MHz, chloroform-d): δ 8.03 (d, J = 8.0 Hz, 1H), 7.96–7.92 (m, 2H), 7.81 (d, J = 8.0 Hz, 1H), 7.64 (d, J = 7.0 Hz, 1H), 7.56–7.48 (m, 3H), 7.35–7.29 (m, 2H), 7.26–7.23 (m, 3H), 7.20–7.13 (m, 3H), 7.06 (t, J = 7.5 Hz, 1H), 4.31 (s, 2H). 13C NMR (126 MHz, chloroform-d): δ 156.3, 148.0, 136.0, 133.9, 132.9, 130.4, 130.1, 129.2, 128.8, 128.7, 127.8, 127.6, 127.3, 127.2, 126.7, 125.4, 125.4, 123.4, 121.9, 121.0, 119.2, 111.5, 32.2. HRMS (ESI): m/z calcd for C26H19N2S, [M + H]+ 391.1264; found, 391.1276.
2-([1,1′-Biphenyl]-2-yl)-3-benzylbenzo[4,5]imidazo[2,1-b]thiazole (13f)
The title compound was prepared according to the general procedure and purified by column chromatography on silica gel using petrol/EtOAc (3:1, v/v) as the eluent to afford a colorless oil (14.2 mg; 17% yield). 1H NMR (500 MHz, chloroform-d): δ 7.72 (d, J = 8.0 Hz, 1H), 7.54 (d, J = 8.0 Hz, 1H), 7.52–7.44 (m, 2H), 7.41 (td, J1 = 7.5, J2 = 1.5 Hz, 1H), 7.29–7.15 (m, 9H), 7.0 (d, J = 8.0 Hz, 1H), 6.95 (t, J = 6.5 Hz, 3H), 4.11 (s, 2H). 13C NMR (126 MHz, chloroform-d): δ 156.2, 147.8, 143.4, 140.3, 135.8, 132.4, 131.0, 129.9, 129.2, 129.2, 129.1, 128.6, 127.8, 127.6, 127.5, 127.5, 127.0, 125.6, 123.9, 123.2, 120.7, 119.0, 111.5, 53.6, 31.8. HRMS (ESI): m/z calcd for C28H21N2S, [M + H]+ 417.1420; found, 417.1392.
3-(4-Methylbenzyl)-2-phenylbenzo[4,5]imidazo[2,1-b]thiazole (14f)
The title compound was prepared according to the general procedure and purified by column chromatography on silica gel using petrol/EtOAc (4:1, v/v) as the eluent to afford a light yellow solid (56.7 mg; 80% yield). Mp: 183–184 °C. 1H NMR (500 MHz, chloroform-d): δ 7.77 (d, J = 8.0 Hz, 1H), 7.49–7.47 (m, 2H), 7.44–7.39 (m, 3H), 7.30 (t, J = 8.0 Hz, 2H), 7.14 (s, 4H), 7.04 (t, J = 7.5 Hz, 1H), 4.49 (s, 2H), 2.33 (s, 3H). 13C NMR (126 MHz, chloroform-d): δ 155.4, 148.0, 137.0, 133.1, 131.6, 130.2, 130.1, 129.3, 129.1, 129.0, 127.5, 126.8, 124.5, 123.4, 121.0, 119.1, 111.5, 32.0, 21.2.
2-(4-Chlorophenyl)-3-(4-methylbenzyl)benzo[4,5]imidazo[2,1-b]thiazole (15f)
The title compound was prepared according to the general procedure and purified by column chromatography on silica gel using petrol/EtOAc (3:1, v/v) as the eluent to afford a light yellow solid (63.0 mg; 81% yield). Mp: 190–192 °C. 1H NMR (500 MHz, chloroform-d): δ 7.77 (d, J = 8.0 Hz, 1H), 7.39 (s, 4H), 7.33–7.26 (m, 2H), 7.16–7.10 (m, 4H), 7.05 (t, J = 7.5 Hz, 1H), 4.45 (s, 2H), 2.33 (s, 3H). 13C NMR (126 MHz, chloroform-d): δ 155.0, 147.8, 137.2, 135.2, 132.8, 130.3, 130.3, 130.0, 129.5, 127.4, 127.3, 123.6, 123.2, 121.2, 119.1, 111.5, 31.9, 21.2.
3-(4-Chlorobenzyl)-2-phenylbenzo[4,5]imidazo[2,1-b]thiazole (16f)
The title compound was prepared according to the general procedure and purified by column chromatography on silica gel using petrol/EtOAc (3:1, v/v) as the eluent to afford a light yellow solid (61.5 mg; 82% yield). Mp: 250–252 °C. 1H NMR (500 MHz, chloroform-d): δ 7.80 (d, J = 8.0 Hz, 1H), 7.47–7.40 (m, 5H), 7.35–7.30 (m, 3H), 7.28 (s, 2H), 7.20 (d, J = 8.0 Hz, 2H), 7.07 (t, J = 7.5 Hz, 1H), 4.50 (s, 2H). 13C NMR (126 MHz, chloroform-d): δ 155.2, 148.0, 134.8, 133.3, 131.3, 129.9, 129.7, 129.4, 129.2, 129.1, 129.0, 125.9, 125.1, 123.5, 121.1, 119.3, 111.1, 31.7. HRMS (ESI): m/z calcd for C22H16ClN2S, [M + H]+ 375.0718; found, 375.0736.
3-Benzyl-6-methyl-2-phenylbenzo[4,5]imidazo[2,1-b]thiazole and 3-Benzyl-7-methyl-2-phenylbenzo[4,5]imidazo[2,1-b]thiazole (17f)
The title compound was prepared according to the general procedure and purified by column chromatography on silica gel using petrol/EtOAc (4:1, v/v) as the eluent to afford a light yellow solid (56.7 mg; 80% yield). Mp: 177–179 °C. 1H NMR (500 MHz, chloroform-d): δ 7.63 (d, J = 8.5 Hz, 0.48H), 7.55 (s, 0.55H), 7.49–7.46 (m, 2H), 7.43–7.39 (m, 3H), 7.37–7.33 (m, 2H), 7.30–7.24 (m, 3H), 7.14 (d, J = 8.5 Hz, 0.55H), 7.10–7.07 (m, 0.96H), 6.84 (d, J = 8.0 Hz, 0.54H), 4.51 (d, J = 2.0 Hz, 2H), 2.44 (s, 1.69H), 2.31 (s, 1.42H). 13C NMR (126 MHz, chloroform-d): δ 155.2, 154.7, 148.4, 146.2, 136.4, 136.3, 133.2, 131.6, 131.6, 130.7, 130.2, 129.5, 129.4, 129.3, 129.1, 129.1, 129.0, 128.9, 128.1, 127.7, 127.6, 127.4, 126.5, 126.5, 124.8, 124.3, 124.2, 122.4, 119.0, 118.6, 111.5, 110.8, 32.3, 32.3, 21.8, 21.7. HRMS (ESI): m/z calcd for C23H19N2S, [M + H]+ 355.1264; found, 355.1256.
3-Benzyl-6-methoxy-2-phenylbenzo[4,5]imidazo[2,1-b]thiazole and 3-Benzyl-7-methoxy-2-phenylbenzo[4,5]imidazo[2,1-b]thiazole (18f)
The title compound was prepared according to the general procedure and purified by column chromatography on silica gel using petrol/EtOAc (3:1, v/v) as the eluent to afford a light yellow solid (60.8 mg; 82% yield). Mp: 147–148 °C. 1H NMR (500 MHz, chloroform-d): δ 7.61 (d, J = 9.0 Hz, 0.58H), 7.51–7.46 (m, 2H), 7.44–7.33 (m, 5H), 7.30–7.26 (m, 2H), 7.23 (d, J = 7.0 Hz, 1.48H), 7.11 (d, J = 9.0 Hz, 0.49H), 6.89 (dd, J1 = 8.5 Hz, J2 = 2.0 Hz, 0.45H), 6.69 (d, J = 2.0 Hz, 0.48H), 6.65 (dd, J1 = 9.0 Hz, J2 = 2.5 Hz, 0.49H), 4.49 (d, J = 6.0 Hz, 2H), 3.82 (s, 1.56H), 3.54 (s, 1.47H). 13C NMR (126 MHz, chloroform-d): δ 156.9, 155.1, 155.0, 153.7, 148.4, 142.0, 136.4, 136.1, 131.5, 131.4, 130.0, 129.5, 129.5, 129.3, 129.1, 129.0, 129.0, 127.6, 127.6, 127.5, 127.4, 126.5, 126.0, 124.8, 124.5, 119.2, 112.8, 111.7, 110.7, 101.3, 95.5, 55.8, 55.7, 32.3, 32.2. HRMS (ESI): m/z calcd for C23H19N2OS, [M + H]+ 371.1213; found, 371.1224.
Acknowledgments
This work was financially supported by the Natural Science Foundation of Shandong Province (ZR2019QB022) and the College Students’ Innovation and Entrepreneurship Training Program of Liaocheng University (CXCY2022190).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c06594.
1H NMR and 13C NMR spectra of all products (PDF)
Accession Codes CCDC 2254138−2254139 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033. (TXT, TXT)
The authors declare no competing financial interest.
Supplementary Material
References
- Balkan A.; Uma S.; Ertan M.; Wiegrebe W. Thiazolo[3,2-a]Pyrimidine Derivatives as Calcium-Antagonists. Pharmazie 1992, 47, 687–688. [PubMed] [Google Scholar]
- Milne J. C.; Lambert P. D.; Schenk S.; Carney D. P.; Smith J. J.; Gagne D. J.; Jin L.; Boss O.; Perni R. B.; Vu C. B.; Bemis J. E.; Xie R.; Disch J. S.; Ng P. Y.; Nunes J. J.; Lynch A. V.; Yang H.; Galonek H.; Israelian K.; Choy W.; Iffland A.; Lavu S.; Medvedik O.; Sinclair D. A.; Olefsky J. M.; Jirousek M. R.; Elliott P. J.; Westphal C. H. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 2007, 450, 712–716. 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. H.; El-Gamal M. I.; Lee Y. S.; Oh C. H. New imidazo[2,1-b]thiazole derivatives: synthesis, in vitro anticancer evaluation, and in silico studies. Eur. J. Med. Chem. 2011, 46, 5769–5777. 10.1016/j.ejmech.2011.08.024. [DOI] [PubMed] [Google Scholar]
- Furlan A.; Colombo F.; Kover A.; Issaly N.; Tintori C.; Angeli L.; Leroux V.; Letard S.; Amat M.; Asses Y.; Maigret B.; Dubreuil P.; Botta M.; Dono R.; Bosch J.; Piccolo O.; Passarella D.; Maina F. Identification of new aminoacid amides containing the imidazo[2,1-b]benzothiazol-2-ylphenyl moiety as inhibitors of tumorigenesis by oncogenic Met signaling. Eur. J. Med. Chem. 2012, 47, 239–254. 10.1016/j.ejmech.2011.10.051. [DOI] [PubMed] [Google Scholar]
- a Kashiyama E.; Hutchinson I.; Chua M.-S.; Stinson S. F.; Phillips L. R.; Kaur G.; Sausville E. A.; Bradshaw T. D.; Westwell A. D.; Stevens M. F. G. Antitumor Benzothiazoles. 8. Synthesis, Metabolic Formation, and Biological Properties of the C- and N-Oxidation Products of Antitumor 2-(4-Aminophenyl)benzothiazoles. J. Med. Chem. 1999, 42, 4172–4184. 10.1021/jm990104o. [DOI] [PubMed] [Google Scholar]; b Azizi N.; Amiri A. K.; Baghi R.; Bolourtchian M.; Hashemi M. M. PTSA catalyzed simple and green synthesis of benzothiazole derivatives in water. Monatsh. Chem 2009, 140, 1471–1473. 10.1007/s00706-009-0209-4. [DOI] [Google Scholar]; c Blacker A. J.; Farah M. M.; Hall M. I.; Marsden S. P.; Saidi O.; Williams J. M. J. Synthesis of Benzazoles by Hydrogen-Transfer Catalysis. Org. Lett. 2009, 11, 2039–2042. 10.1021/ol900557u. [DOI] [PubMed] [Google Scholar]; d Bozorov K.; Zhao J.; Aisa H. A. 1,2,3-Triazole-containing hybrids as leads in medicinal chemistry: A recent overview. Bioorg. Med. Chem. 2019, 27, 3511–3531. 10.1016/j.bmc.2019.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Chitti S.; Van Calster K.; Cappoen D.; Nandikolla A.; Khetmalis Y. M.; Cos P.; Kumar B. K.; Murugesan S.; Gowri Chandra Sekhar K. V. Design, synthesis and biological evaluation of benzo-[d]-imidazo-[2,1-b]-thiazole and imidazo-[2,1-b]-thiazole carboxamide triazole derivatives as antimycobacterial agents. RSC Adv. 2022, 12, 22385–22401. 10.1039/D2RA03318F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Afsina Abdulla C. M.; Neetha M.; Aneeja T.; Anilkumar G. Synthesis and Applications of Imidazothiazoles: An Overview. ChemistrySelect 2020, 5, 10374–10386. 10.1002/slct.202002842. [DOI] [Google Scholar]; b Claudio-Catalán M. Á.; Pharande S. G.; Quezada-Soto A.; Kishore K. G.; Rentería-Gómez A.; Padilla-Vaca F.; Gámez-Montaño R. Solvent- and Catalyst-Free One-Pot Green Bound-Type Fused Bis-Heterocycles Synthesis via Groebke–Blackburn–Bienaymé Reaction/SNAr/Ring-Chain Azido-Tautomerization Strategy. ACS Omega 2018, 3, 5177–5186. 10.1021/acsomega.8b00170. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Wang J.; Li J.; Zhu Q. Copper-Promoted Cycloaddition of α-Methylenyl Isocyanides with Benzothiazoles: Tunable Access to Benzo[d]imidazothiazoles. Org. Lett. 2015, 17, 5336–5339. 10.1021/acs.orglett.5b02694. [DOI] [PubMed] [Google Scholar]; d Ma Y. C.; Na M.; Gu Y. F.; Huang G. L.; Li X.; Chen Y. G. Direct arylation of imidazo[2,1-b]thiazoles and thiazoles with aryl iodides via CuCl2/PPh3-catalyzed C-H bond functionalization. Appl. Organomet. Chem. 2015, 29, 165–169. 10.1002/aoc.3263. [DOI] [Google Scholar]
- a Xu H.; Zhang Y.; Huang J. Q.; Chen W. Z. Copper-Catalyzed Synthesis of N-Fused Heterocycles through Regioselective 1,2-Aminothiolation of 1,1-Dibromoalkenes. Org. Lett. 2010, 12, 3704–3707. 10.1021/ol101563f. [DOI] [PubMed] [Google Scholar]; b Xiao D. M.; Han L. Q.; Sun Q.; Chen Q. X.; Gong N. B.; Lv Y.; Suzenet F.; Guillaumet G.; Cheng T. M.; Li R. T. Copper-mediated synthesis of N-fused heterocycles via C-sp-S coupling reaction and 5-endo-dig cyclization sequence. RSC Adv. 2012, 2, 5054–5057. 10.1039/c2ra20254a. [DOI] [Google Scholar]; c Beresneva T.; Popelis J.; Abele E. Novel Cu-catalyzed methods for the synthesis of fused thiazoles using S,N-diarylation reaction. Chem. Heterocycl. Compd. 2013, 49, 345–347. 10.1007/s10593-013-1253-x. [DOI] [Google Scholar]; d Gao J. L.; Zhu J. Y.; Chen L. B.; Shao Y. Y.; Zhu J. Q.; Huang Y. J.; Wang X. X.; Lv X. Synthesis of benzimidazo[2,1-b]benzothiazole derivatives through sequential Cu-catalyzed domino coupling and Pd-catalyzed Suzuki reaction. Tetrahedron Lett. 2014, 55, 3367–3373. 10.1016/j.tetlet.2014.04.070. [DOI] [Google Scholar]; e Shen G. D.; Yang B. C.; Huang X. Q.; Hou Y. X.; Gao H.; Cui J. C.; Cui C. S.; Zhang T. X. Copper- and Palladium-Catalyzed Cross-Coupling Reactions for the Synthesis of N-Fused Benzo[4,5]imidazo[2,1-b]thiazole Derivatives via Substituted trans-1,2-Diiodoalkenes, 1H-Benzo[d]imidazole-2-thiols, and Halobenzenes. J. Org. Chem. 2017, 82, 3798–3805. 10.1021/acs.joc.7b00162. [DOI] [PubMed] [Google Scholar]; f Jana S.; Chakraborty A.; Shirinian V. Z.; Hajra A. Synthesis of Benzo[4,5]imidazo[2,1-b]thiazole by Copper(II)-Catalyzed Thioamination of Nitroalkene with 1H-Benzo[d]imidazole-2-thiol. Adv. Synth. Catal. 2018, 360, 2402–2408. 10.1002/adsc.201800393. [DOI] [Google Scholar]
- a Shrinidhi A.; Perrin C. L. Nucleophilic Addition of Enolates to 1,4-Dehydrobenzene Diradicals Derived from Enediynes: Synthesis of Functionalized Aromatics. ACS Omega 2022, 7, 22930–22937. 10.1021/acsomega.2c02916. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Worch J. C.; Stubbs C. J.; Price M. J.; Dove A. P. Click Nucleophilic Conjugate Additions to Activated Alkynes: Exploring Thiol-yne, Amino-yne, and Hydroxyl-yne Reactions from (Bio)Organic to Polymer Chemistry. Chem. Rev. 2021, 121, 6744–6776. 10.1021/acs.chemrev.0c01076. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Zhan Y.; Zhao Y.; Du Q.; Rui J. C.; Chen R. Z.; Zheng X. T.; Wu X. J. Chemo- and regioselective nucleophilic hydrofunctionalization of unactivated aliphatic alkenes under transition-metal-free catalysts. Green Chem. 2021, 23, 3250–3255. 10.1039/D1GC00474C. [DOI] [Google Scholar]; d Alcaide B.; Almendros P.; Martínez del Campo T.; Martin L.; Palop G.; Toledano-Pinedo M. Oxidative selenofunctionalization of allenes: convenient access to 2-(phenylselanyl)-but-2-enals and 4-oxo-3-(phenylselanyl)pent-2-enoates. Org. Chem. Front. 2019, 6, 2447–2451. 10.1039/c9qo00561g. [DOI] [Google Scholar]; e Smyshliaeva L. A.; Varaksin M. V.; Slepukhin P. A.; Chupakhin O. N.; Charushin V. N. Transition metal-free oxidative and deoxygenative C-H/C-Li cross-couplings of 2H-imidazole 1-oxides with carboranyl lithium as an efficient synthetic approach to azaheterocyclic carboranes. Beilstein J. Org. Chem. 2018, 14, 2618–2626. 10.3762/bjoc.14.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Borlinghaus N.; Ansari T. N.; von Garrel L. H.; Ogulu D.; Handa S.; Wittmann V.; Braje W. M. Nucleophilic aromatic substitution reactions under aqueous, mild conditions using polymeric additive HPMC. Green Chem. 2021, 23, 3955–3962. 10.1039/d1gc00128k. [DOI] [Google Scholar]; b Moseev T. D.; Varaksin M. V.; Gorlov D. A.; Charushin V. N.; Chupakhin O. N. Transition-Metal-Free C-H/C-Li Coupling of Nonaromatic 2H-Imidazole 1-Oxides with Pentafluorophenyl Lithium in the Design of Novel Fluorophores with Intramolecular Charge Transfer Effect. J. Org. Chem. 2020, 85, 11124–11133. 10.1021/acs.joc.0c01042. [DOI] [PubMed] [Google Scholar]
- a Peng X. P.; Rahim A.; Peng W. J.; Jiang F.; Gu Z. H.; Wen S. J. Recent Progress in Cyclic Aryliodonium Chemistry: Syntheses and Applications. Chem. Rev. 2023, 123, 1364–1416. 10.1021/acs.chemrev.2c00591. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Lu M. Z.; Goh J.; Maraswami M.; Jia Z. H.; Tian J. S.; Loh T. P. Recent Advances in Alkenyl sp2 C-H and C-F Bond Functionalizations: Scope, Mechanism, and Applications. Chem. Rev. 2022, 122, 17479–17646. 10.1021/acs.chemrev.2c00032. [DOI] [PubMed] [Google Scholar]; c Maegawa T.; Mizui R.; Urasaki M.; Fujimura K.; Nakamura A.; Miki Y. Direct Synthesis of Chalcones from Anilides with Phenyl Vinyl Ketones by Oxidative Coupling Through C–H Bond Activation. ACS Omega 2018, 3, 5375–5381. 10.1021/acsomega.8b00594. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Liu T.; Myers M. C.; Yu J. Q. Copper-Catalyzed Bromination of C(sp3)-H Bonds Distal to Functional Groups. Angew. Chem., Int. Ed. 2017, 56, 306–309. 10.1002/anie.201608210. [DOI] [PubMed] [Google Scholar]; e Wang F.; Yu S. J.; Li X. W. Transition metal-catalysed couplings between arenes and strained or reactive rings: combination of C-H activation and ring scission. Chem. Soc. Rev. 2016, 45, 6462–6477. 10.1039/C6CS00371K. [DOI] [PubMed] [Google Scholar]
- a Johansson Seechurn C. C. C.; Kitching M. O.; Colacot T. J.; Snieckus V. Palladium-Catalyzed Cross-Coupling: A Historical Contextual Perspective to the 2010 Nobel Prize. Angew. Chem., Int. Ed. 2012, 51, 5062–5085. 10.1002/anie.201107017. [DOI] [PubMed] [Google Scholar]; b Lv X. L.; Wang K. C.; Wang B.; Su J.; Zou X. D.; Xie Y. B.; Li J. R.; Zhou H. C. A Base-Resistant Metalloporphyrin Metal-Organic Framework for C-H Bond Halogenation. J. Am. Chem. Soc. 2017, 139, 211–217. 10.1021/jacs.6b09463. [DOI] [PubMed] [Google Scholar]
- Li Z. J.; Zhao L. Y.; Zhang Y. L.; Yan H.; Huang X. Q.; Shen G. D. Cascade Nucleophilic Attack/Addition Cyclization Reactions to Synthesize Oxazolidin-2-imines via (Z)-2-Bromo-3-phenylprop-2-en-1-ols/3-phenylprop-2-yn-1-ols and Diphenyl Carbodiimides. J. Org. Chem. 2022, 87, 12721–12732. 10.1021/acs.joc.2c01268. [DOI] [PubMed] [Google Scholar]
- Heravi M. M.; Keivanloo A.; Rahimizadeh M.; Bakavoli M.; Ghassemzadeh M. Pd-Cu catalyzed heterocyclization during Sonogashira coupling: synthesis of 3-benzylthiazolo[3,2-a]benzimidazole. Tetrahedron Lett. 2004, 45, 5747–5749. 10.1016/j.tetlet.2004.05.094. [DOI] [Google Scholar]
- Omar M. A.; Frey W.; Conrad J.; Beifuss U. Transition-Metal-Free Synthesis of Imidazo[2,1-b]thiazoles and Thiazolo[3,2-a]benzimidazoles via an S-Propargylation/5-exo-dig Cyclization/lsomerization Sequence Using Propargyl Tosylates as Substrates. J. Org. Chem. 2014, 79, 10367–10377. 10.1021/jo501980w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.