Abstract

Flame retardant (FR) exposure has been linked to several environmental and human health effects. Because of this, the production and use of several FRs are regulated globally. We reviewed the available records of polybrominated diphenyl ethers (PBDEs) and hexabromocyclododecanes (HBCDDs) in human breast milk from literature to evaluate the efficacy of regulation to reduce the exposure of FRs to humans. Two-hundred and seven studies were used for analyses to determine the spatial and temporal trends of FR exposure. North America consistently had the highest concentrations of PBDEs, while Asia and Oceania dominated HBCDD exposure. BDE-49 and -99 indicated decreasing temporal trends in most regions. BDE-153, with a longer half-life than the aforementioned isomers, typically exhibited a plateau in breast milk levels. No conclusive trend could be established for HBCDD, and insufficient information was available to determine a temporal trend for BDE-209. Breakpoint analyses indicated a significant decrease in BDE-47 and -99 in Europe around the time that regulation has been implemented, suggesting a positive effect of regulation on FR exposure. However, very few studies have been conducted globally (specifically in North America) after 2013, during the time when the most recent regulations have been implemented. This meta-analysis provides insight into global trends in human exposure to PBDEs and HBCDD, but the remaining uncertainty highlights the need for ongoing evaluation and monitoring, even after a compound group is regulated.
Keywords: flame retardant, polybrominated diphenyl ether, hexabromocyclododecane, breast milk, biomonitoring, temporal trends, effectiveness evaluation
Short abstract
Flame retardant levels in breast milk differ globally due to past flammability standards, and time trends suggest recent regulatory action has been effective at reducing some exposures.
Introduction
Flame retardants are added to a wide range of consumer products and materials in order to reduce ignition or flammability of a material or fulfill fire safety requirements.1 However, past efforts to reduce flammability through the addition of synthetic organic flame retardants have led to negative impacts on human and environmental health due to exposure to harmful chemicals.2
Polybrominated diphenyl ethers (PBDEs) and hexabromocyclododecanes (HBCDDs) were among the dominant FRs used for decades.3,4 The three technical mixtures of PBDEs (penta-, octa-, and deca-BDE) had multiple uses, including polyurethane foam, electrical and electronic equipment, building materials, and vehicle parts.5 Technical HBCDD (a mixture of the stereoisomers, α-, β-, and γ-HBCDD, with γ-HBCDD being the most abundant) was primarily used in electronics, textiles, and especially in expanded (EPS) and extruded polystyrene (XPS) applied as construction and packing materials.6,7
PBDEs and HBCDDs are known to be persistent, bioaccumulative, and subject to long-range transport in the environment and are ubiquitous across environmental systems.8,9 PBDEs have been reported in human blood,10−12 adipose tissues,13−15 and milk16−19 since the early 1990s, and evidence of human exposure to HBCDDs arose shortly thereafter.20−23
PBDE exposure has been associated with numerous adverse health outcomes, including alterations to thyroid function, reproductive systems, and breast cancer,24−26 with strong evidence for neurodevelopmental impacts, including lower IQ and ADHD.27,28 Elevated levels of PBDEs in breast milk have specifically been associated with neurodevelopmental effects and alterations to the gut microbiome in young children.29,30 Similar adverse effects are associated with elevated HBCDD concentrations, including endocrine disruption, specifically thyroid, neurobehavioral, and developmental disorders.26,31
In response to concerns regarding the environmental and human health impacts of certain FRs, actions were taken to reduce production.32 In 2004, the European Union stated that “in order to protect health and the environment the placing on the market and the use of pentaBDE and octaBDE and the placing on the market of articles containing one or both of these substances should be prohibited”,33 and in the same year these mixtures were voluntarily withdrawn from the U.S. marketplace by their manufacturers.34 The lower brominated PBDE congeners, tetra- and penta-BDE (main components of commercial penta-BDE435) and hexa- and hepta-BDEs (main components of commercial octa-BDE4)36 were listed in the Stockholm Convention on Persistent Organic Pollutants (POPs) in 2009, requiring parties to eliminate the production and use of the compounds. Deca-BDE, the fully brominated PBDE molecule and main component of the decaBDE commercial product,37 was similarly listed in 2017.38 In 2008, HBCDDs were recognized as substances of very high concern (SVHC) in the EU due to environmental and human health risks39,40 and were added to the Stockholm Convention in 2013.41 In specific cases, individual countries were permitted continued production of HBCDDs until 2024.7
The evaluation of temporal patterns of a chemical’s concentration in a predetermined medium is an effective tool to determine the efficiency of policy in mitigating chemical exposure.42 Recent studies have identified declines in components of the penta- and octa-BDE technical mixtures,9 in air (1993–2018),43 soil (1998–2008),44 sediment (2002–2012),45 sewage sludge (2004–2010),46 and fish (1980–2009),47 while BDE-209 has been stable or increasing in many matrices.46,48,49 In humans, declines in levels of less brominated PBDEs and a more recent plateau have been identified in several countries, however, this is not uniform across regions or matrices:9,22,50,51 there is a lack of understanding of how generalizable these regional trends are. For HBCDDs, there is even less evidence of a global trend. Although time trends of HBCDD concentrations in multiple environmental matrices52−54 have been determined, analysis of temporal patterns of HBCDDs has been limited to regional scales. No consensus or clear global time trend of HBCDD concentrations in humans has been identified,9 with some studies reporting an increase of HBCDDs,54−56 others a decrease57 or no trend.58,59
Studying the effectiveness of policies concerning FRs through time trend analysis of biomonitoring data presents inherent complexities. Different FRs, each with distinct chemical structures, are incorporated into diverse applications such as furniture, electronics, and building insulation, which are associated with different emission and exposure routes.4 After restriction, continued presence of FR-containing products further complicates this, as different product types have very different replacement rates (e.g., smartphones, 2–6 years60 vs building insulation, 30–50 yrs61). The environmental persistence of compounds is generally longer indoors62,63 and indoor levels are sustained until active removal of sources.64,65 Exposure to FRs is further influenced by regional factors like building and cleaning practices and dietary patterns.66 Moreover, the differing persistence of FRs within the body complicates our understanding of exposure,67 with some FRs possessing longer half-lives in human tissues68 and partitioning within body tissues varying by compound/congener.69,70 Concentrations of POPs in human tissues typically reflect long-term exposures, for example, variations of PCB concentrations in breast milk levels can be explained by differences in early life exposures rather than current dietary exposures.71 Despite the above-mentioned complexities, our insight into the effectiveness of restrictions and the remaining risks to human populations from legacy FRs can be improved by multistudy analyses to understand and interpret the global time patterns of PBDEs and HBCDDs in humans.
In this analysis, we first review available records of PBDE and HBCDD in human milk to determine time patterns of global human exposure to FRs. Second, we evaluate the impact of regulations that were introduced over the past 20 years on exposure to these legacy FRs. Finally, we investigate the regional differences in exposure to FRs in relation to use. We supplement this with a review of past studies evaluating temporal patterns of PBDEs and HBCDDs in human matrices, to provide a comprehensive review of trends in global exposure.
Methods
Rationalization of Study Matrix
It is impossible to select a single biological matrix that encompasses the global population, as well as all target compounds. Due to the lipophilic nature of PBDEs and HBCDDs, they are best evaluated through matrices such as blood serum or breast/maternal milk.72 Breast milk has a high lipid content and can be collected noninvasively, making it a reliable and accessible matrix for assessing body burdens of PBDEs and HBCDDs, as well as many other POPs. The Stockholm Convention, in cooperation with the World Health Organization, has identified human milk as a core matrix of its Global Monitoring Plan and supported with routine quantification in pooled milk samples.70,73
In addition to its importance in routine monitoring programs, maternal milk is an ideal matrix for meta-analyses of biomonitoring data because of the relative homogeneity of the study population: all female, with an age range generally spanning 18–45 years. Moreover, breast milk is not only an indicator of human exposure but also represents a direct exposure route to infants, and breast milk is typically the most important exposure pathway of young children to POPs, including PBDEs.74,75
Search Strategy and Selection Criteria–Human Milk Meta-Analysis
A literature search of peer-reviewed studies and reports produced by regulatory bodies (e.g., UNEP, German Federal Environment Agency) was conducted using ISI Web of Science and Google Scholar. The search was not limited by years or language of publication. The search was initially conducted in March 2020 and updated in September 2022. The following search terms were used.
For HBCDDs
All fields: [hexabromocyclododecane* OR HBCD*] AND [(human milk) or (breast milk)]. This search produced 168 results, which were evaluated for their appropriateness. The criteria for inclusion were that the studies provided lipid-standardized HBCDD levels in human milk (either isomer-specific HBCDD or ∑HBCDD) and included basic information on the study population (country of residence, sampling year). Of the initial 168 studies identified, 49 met the criteria and were used for further analysis (Figure S1, data sources listed in Table 1).
Table 1. Countries Used in This Study Grouped According to the United Nations Geoscheme.
| region | countries included | PBDEs | HBCDDs | ||
|---|---|---|---|---|---|
| Africa | Congo | South Africa | South Africa | 7 studies79−85 | 5 studies79−83 |
| Cote d’Ivoire | Mauritius | Tanzania | |||
| Djibouti | Morocco | Togo | |||
| Egypt | Niger | Tunisia | |||
| Ethiopia | Nigeria | Uganda | |||
| Ghana | Senegal | Zambia | |||
| Kenya | |||||
| Asia | China | Japan | Syria | 53 studies18,81,86−136 | 15 studies81,89,95,132−135,137−144 |
| Georgia | Macao | Taiwan | |||
| India | Philippines | Tajikistan | |||
| Indonesia | Russiaa | Vietnam | |||
| Israel | South Korea | ||||
| Central and South America and Caribbean | Antigua and Barbuda | Chile | Peru | 3 studies73,81,145 | 2 studies73,81 |
| Barbados | Haiti | Suriname | |||
| Brazil | Jamaica | Uruguay | |||
| Europe | Belgium | Greece | Romania | 61 studies1,19,29,30,59,81,146−200 | 25 studies23,59,76,81,146−151,153−159,167,185,189,193,201−204 |
| Bulgaria | Hungary | Russiaa | |||
| Croatia | Ireland | Slovakia | |||
| Cyprus | Italy | Spain | |||
| Czechia | Lithuania | Sweden | |||
| Denmark | Luxembourg | Switzerland | |||
| Faroe Islands | Moldova | Turkey | |||
| Finland | Netherlands | UK | |||
| France | Norway | Ukraine | |||
| Germany | Poland | ||||
| North America | Canada | Mexico | USA | 25 studies17,51,70,77,81,205−222 | 5 studies51,77,78,81,83 |
| Oceania | Australia | Kiribati | Tonga | 7 studies223−228 | 2 studies81,226 |
| Fiji | New Zealand | Tuvalu | |||
General samples from Russia were included within the European category. When a study specified a geographic region that was within the Asian part of Russia, it was included in Asia.
For PBDEs
All fields: [polybrominated diphenyl ether* OR PBDE*] AND [(human milk) or (breast milk)]. This search identified 1204 results, which were then evaluated for their appropriateness. Only studies reporting individual PBDE congeners were included, and four congeners were selected as indicators due to their prevalence in literature: BDE-47, BDE-99, BDE-153, and BDE-209. Studies also had to include lipid-standardized concentrations for at least one of these congeners and include information on the study population (country of residence, sampling year). Of the initial 1204 studies, 158 met the criteria and were used for further analysis (Figure S1, data sources listed in Table 1).
All available data (either primary data reporting individual concentrations or all summary statistics) were extracted from the articles to a spreadsheet database. Data reported from pooled samples were treated as mean values.
Data Set Standardization
Statistical evaluation was carried out by R software (version R 4.1.2). The data sources were separated by compound, sampling location, and/or date of sample collection. These records were aggregated by country and date of sampling, characterized either by mean or median value, minimum, 5th, 10th, 25th, 75th, 90th, and 95th quantile and maximum or any combination of these descriptive statistics. If all primary data/summary statistics within an aggregate record were taken during one year, the aggregated record was assigned to that year. In the other cases, the aggregated record was assigned to the middle point between the years of the oldest and the newest primary data/summary statistic.
For HBCDDs, 41 aggregated records included both α-HBCDD and the sum of α, β, and γ-isomers. These 41 records were used for estimating the contribution of α-HBCDD to the sums. This contribution was 94.0%, showing clearly that α-HBCDD dominates over the β and γ isomers. This 94.0% was then used to extrapolate α-HBCDD from records where only ∑HBCDDs were reported for the original data set, resulting in 260 aggregated records for α-HBCDD.
Primary FR data from five locations76−78 were used to establish that α-HBCDD and PBDE concentrations in breast milk have a general log-normal distribution of primary data within an aggregate data record. On the basis of the log-normal distribution assumption, the maximum likelihood estimation was used to apply a log-normal distribution for each aggregated sample and thus estimate its median value in cases where only other summary statistics were reported. In a few cases, the maximum likelihood estimate was not directly applicable since the only descriptive statistic characterizing the aggregated sample was an arithmetic mean. In such cases, a median standard deviation based on the rest of the samples was used (0.62 ng/g lipid weight; lw for α-HBCDD, 0.32 ng/g lw for BDE-47, 0.23 ng/g lw for BDE-99, 0.35 ng/g lw for BDE 153, and 50.15 ng/g lw for BDE-209) to derive quantiles describing the expected log-normal distribution. Finally, since there are no theoretical minimal and maximal values for the log-normal statistical distribution, the min and max values were considered as (1/n)th and (1–1/n)th quantiles for aggregated samples with known n; for aggregated samples with an unknown number of primary samples, n = 40 was used as it was the median value of studies where n was specified (Figure S2).
Temporal Pattern Meta-Analysis
Data were grouped regionally following the United Nations geoscheme (Table 1). With the use of the aggregate data by region and sampling year, a weighted Theil-Sen trend analysis was conducted, assigning each aggregated sample a weight in the range of 0.1 to 1.0 for 10 to 100 primary samples and 1.0 for more than 100 primary samples. A weighted Mann-Kendall test was then used for assessing the trends’ significance.
Breakpoint Analysis
An additional type of temporal pattern analysis was applied to European and Asian data sets, as these continents had the most complete records for both PBDEs and HBCDDs and are of particular interest given the early introduction of FR regulations. To evaluate whether this early introduction of restrictions led to a change in the FR time patterns over time, breakpoint analysis was applied to find a time point when the slope of the trend breaks,229 i.e., suggesting a change in the rate of change of a given FR concentration in human milk. The breakpoint analysis identifies the breakpoint by aiming for normally distributed residuals of both linear trends before and after the breakpoint, which indicates an optimal fit. The method searches for all possible breakpoints (in this case in increments of whole years only) and selects the breakpoint with the smallest sum of squares of residuals. Only significant results according to the difference of halving times before and after the breakpoint are considered.229
Analysis of Geographic Patterns
Additional comparative statistical analyses were conducted using Graphpad Prism 8.0.2 using all studies after the year 2000. Data were grouped according to geographic region (Table 1). The concentrations from the different regions were compared using Kruskall-Wallis nonparametric ANOVA tests, and individual regions were compared with all others using Dunn’s multiple comparison tests. For geographic patterns, significance was set at p < 0.05.
Limitations
The quality of the analytical work performed in individual studies was not evaluated, in favor of allowing for a greater breadth of data to be incorporated in the meta-analysis. All studies were published in peer-reviewed journals or as reports available from reputable national/international organizations, leading to the assumption of an acceptable level of data quality. Some reports (notably the UNEP/WHO data included in the Stockholm Convention GMP reports) do not include analytical information, although data are produced by recognized, accredited laboratories, and exclusion of this data would lead to a substantial loss in geographic coverage. Additionally, older studies reflecting very early analyses of PBDEs and HBCDDs may have more generous allowances in terms of QA/QC; however, it was important for the temporal analyses that these could be included. However, a consequence of this is that not all studies will meet the most stringent QA/QC standards.
While breastfeeding mothers present a relatively homogeneous population with respect to age and sex, some additional factors can impact breast milk concentrations of FRs, and these could not be incorporated into our meta-analysis, primarily because of inconsistent data across studies.
Representativeness
Data from breastmilk only reflects the chemical burden in the breast-feeding female population. The prevalence of breastfeeding mothers also varies by socioeconomic status and cultural background.230
Parity
Some studies have shown that parity is related to differences in levels of persistent compounds in breast milk. Primiparous mothers have been shown to have higher HBCDD concentrations than multiparous mothers,132 while studies focused on PBDEs have not found a relationship.84,187,207,231 As information on parity was not consistently recorded for all data sources, we did not include this confounder in our analyses and used all available data, regardless of parity.
Duration of Lactation and Sample Collection
The timing of breast milk collection within the lactation period varied widely, from 1 week to 10 months after birth, although the most typical was 3–8 weeks after birth, following the guidance of the WHO/UNEP breast milk surveys.232 Whether this impacts levels of PBDEs and HBCDDs in milk is unclear. Some studies have reported variability166,207 or significant decreases233 in PBDE levels in breast milk up to a year postpartum; however, Harrad and Abdallah151 reported no change in HBCDDs in milk over 12 months of lactation.
Maternal Age
This is often identified as a determinant of breast milk levels; however, this is directly related to the understanding of the persistence of these FRs in the body and temporal changes in exposures within a country.234 Older maternal age has been associated with higher PBDE levels in some studies in breast milk129,204 and sera,235 while others have found no association187 or an inverse association of lower levels in breast milk from older mothers.84,207 Similarly for HBCDDs, Fujii et al. identified age-dependency of γ-HBCDD in milk,132 but not other HBCDD isomers, while Drage et al.235 found no age-dependency in HBCDDs in sera.
Selection of Breast Milk as Biomonitoring Matrix
While breast milk has the advantage of being noninvasive and widely monitored, BDE-209 preferentially partitions to serum lipids rather than milk lipids,69 leading to proportionally lower levels in breast milk compared with exposures. However, while milk:serum partitioning can vary by congener/compound,69 the relative geographic patterns and temporal trends (1227 samples from 1973 to 2019) should be appropriately captured by either matrix, as milk and serum concentrations are typically well-correlated.215 The limited number of spatial and temporal studies conducted on certain compounds, such as BDE-209 and HBCDD in human breast milk is also a limitation for this study.
Uncertainties Regarding Partitioning and Half-Lives of PBDEs in Human Breast Milk
Very few studies have been conducted on the human biological distributions and half-lives of PBDE and HBCDDs. The existing evidence suggests higher persistence of BDE-153 in the human body68,236,237 and decreased partitioning to milk for higher molecular weight FRs.69 As a result, comparisons of concentrations across congeners would not necessarily reflect exposure trends; however, the bulk of our analysis relies on trends built individually for each congener and thus should not be impacted by the uncertainty in partitioning and half-lives.
Temporal Patterns: Search Strategy and Selection Criteria
We supplemented our meta-analysis of temporal patterns of PBDE and HBCDD exposure with a comprehensive review of published time trends. A literature search was conducted using ISI Web of Science and Google Scholar, initially in March 2020, updated in September 2022. Search terms for the temporal trend studies were a combination of chemical-related terms (polybrominated diphenyl ether*, PBDE*, hexabromocyclododecane*, HBCD*), matrix-related (human, blood, serum, plasma, milk), and trend-related (time-trend* or temporal*).
For the temporal trend analysis of PBDEs, 268 data sources were identified, and the data were further examined to identify only studies that reported any of the four indicator PBDEs (−47, −99, −153, or −209), reflected the general population (not occupational exposure), reported basic biomonitoring parameters (e.g., geographic region, matrix, year of sample collection, number of samples), and had at least two time points with harmonized analyses (e.g., by the same laboratory). This resulted in 24 studies which were included in the overview of temporal trends (Table S2). For the temporal trend analysis of HBCDDs, the literature search identified 88 data sources, and the inclusion criteria (reported either α- or ∑HBCDD, not occupational exposure, reported basic biomonitoring parameters) led to the inclusion of 20 studies (Table S3). The trends from these studies were extracted, using the interpretation of the study authors to determine whether a trend is classified as increasing or decreasing over time, or whether no time trend is apparent.
Results
Spatial Patterns
Available data were not equally distributed across all geographic regions. Most studies were conducted in Europe and Asia, while Central and South America, North America, and Oceania had only limited data (Table 1). Very few studies on human biomonitoring of HBCDDs have been conducted in North America, which is surprising, considering that the region is known to have had stringent flame retardant regulations and historically high use of BFRs.3
Oceania had the highest median α-HBCDD concentration in breast milk (2.7 ng/g lipid), followed by Asia (1.5 ng/g lipid) and Europe (0.9 ng/g lipid) (Figure 1). The elevated concentrations of α-HBCDD in breast milk from Oceania were unexpected but may reflect a common market with many products from Asian manufacturers and Oceania implementing HBCDD regulations years later than Europe.238 Breast milk from Asia had significantly higher concentrations of α-HBCDD than milk from Africa, and Europe had significantly higher concentrations than Central and South America (Figure 1).
Figure 1.
Box-and-whiskers (horizontal lines are medians, 95% confidence intervals, minima, and maxima) of (A) BDE-47, (B) BDE-99, (C) BDE-153, (D) BDE-209, and (E) α-HBCDD concentrations in different regions (CSA = Central and South America and the Caribbean), in breast milk data collected after year 2000. Nonparametric ANOVA tests (Kruskal–Wallis with Dunn’s post-tests) were conducted to determine significant differences.
The concentrations of BDE-47, -99, and -153 in breast milk from North America were significantly higher than those from Europe, Africa, Asia, and Central- and South America: BDE-47, and -99 concentrations in North America were 39 and 65 times higher, respectively, than concentrations in Asia (Figure 1, Table S1). For the higher brominated compounds, North America had 50 and 138 times higher concentrations than Africa for BDE-153 and -209, respectively (Figure 1, Table S1). Europe had significantly higher concentrations of BDE-99 than Africa, and Africa had significantly lower concentrations of BDE-153 than all the other regions. For BDE-209, there were fewer data records which limited the comparison. The fact that BDE-209 is notoriously difficult to quantify due to high molecular mass and chemical instability likely contributes to the lack of data on levels in breast milk.239,240 For BDE-209, concentrations in milk from Asia were substantially lower than in North America, and no other regions had significant differences (Figure 1).
Temporal Patterns
Africa, Oceania, and Central and South America had limited or no data on BDE-209 concentrations in breast milk, and temporal patterns could not be fully evaluated (Table 1; Figures S5, S7, and S8). The most prominent differences in trends are seen between Europe and North America for BDE-47, -153, and α-HBCDD. The temporal trends of BDE-99 and -209 are depicted elsewhere (Figures S3 and S4).
In Europe, BDE-47 and -99 decreased significantly (p = 0.0001; annual change of −9.3% and −10.1% respectively), while BDE-153 and -209 had no change over time (Figures 2 and S3). α-HBCDD concentrations increased significantly in the European population (p < 0.0001; 13.9% per annum) (Figure 2).
Figure 2.
Weighted temporal trends of BDE-47, BDE-153, and α-HBCDD concentrations (ng/g lipid weight, lw) in breast milk from Europe and North America from literature. Shaded area indicates 95% confidence interval.
No significant changes (p < 0.05) were observed for any compound in North America (Figures 2 and S4), although we note that North American data was generally very sparse, limiting the ability to distinguish temporal trends. Notably, North America was the region with the least available α-HBCDD data points in breastmilk. The western hemisphere regions were the only regions where BDE-47 and -99 did not show a decreasing trend (Figures 2 and S4). The temporal patterns of all other geographical regions are presented in the Figures S5–S8.
Africa had a decrease in BDE-47 (p = 0.022; −7.8% per annum). Africa was the only continent where a significant decrease of α-HBCDD was observed (p < 0.0001; −33.6% per annum) (Figure S5). A similar pattern to Europe was seen in Asia. BDE-47 decreased (p = 0.002; −9.7 per annum), while BDE-153 and BDE-209 both increased, with BDE-209 increasing by 12.8% per annum (p = 0.05). α-HBCDD concentrations increased at a rate of 7.9% per annum (p = 0.006) (Figure S6).
In Central and South America and the Caribbean, BDE-153 increased significantly (p = 0.08) with an annual change of 14.8% (Figure S7). Oceania was the only region with a consistent decrease in all PBDE congeners. BDE-47 and -99 decreased significantly with an annual change of −13.1% and −15.5%, respectively. BDE-153 decreased at a rate of −6% per annum (p = 0.054) (Figure S8).
Breakpoint Analysis
Only Asia and Europe had sufficient data for breakpoint analyses (Figure 3). Breakpoints were calculated for other regions to identify the timing of concentration peaks (Figures S9–S12), but the trends are of limited value due to scarce data and are not discussed in detail.
Figure 3.
Results of breakpoint analysis for PBDEs in human milk for Asia and Europe for BDE-47, BDE-99, BDE-153, BDE-209, and α-HBCDD. The shaded area indicates the 95th percent confidence interval. The dotted blue line indicates when regulation was implemented by the Stockholm Convention (2009: BDE-47, -99, and -153; 2013: HBCDD; 2019: BDE-209). Please note that the Stockholm Convention date does not directly indicate the introduction of restrictions in each country; these may be earlier due to national/regional initiatives, or later, as Stockholm Convention parties enact regulations to implement the Convention. Thus, they are only indicative of the general timing of global restrictions.
In Europe, an increase in α-HBCDD of 13.9% per annum was observed from 1980 to 2010. In 2010, there was a change (breakpoint) in the temporal trends of α-HBCDD (Figure 3). The post-2010 decrease is not statistically significant due to limited data collected since 2010; thus, we cannot determine if recent concentrations are stable or declining. Although the breakpoint is not statistically significant, it represents the best fit for the available data and suggests a shift in exposure post-2010. In Asia, the α-HBCDD increase was even sharper (+31.6% per year; p = 0.007 Figure 3) and the breakpoint was identified earlier (1998), suggesting Asian concentrations reached a plateau at this point. Like Europe, the modeled decrease since the breakpoint in Asia is not statistically significant.
In both Asian and European breast milk data, the breakpoint in concentrations for BDE-47 and 99 was substantially earlier than for HBCDDs, close to 1990 for BDE-47 and between 1995 and 2000 for BDE-99. In all cases, concentrations decreased after the breakpoint for both congeners, but this postbreakpoint decrease is only significant for BDE-47 and BDE-99 in Europe (p > 0.0001).
However, there is a clear contrast between the breakpoints and before/after trends for BDE-153. The breakpoint for BDE-153 reflects only a change in the rate of increase of BDE-153 (Asia) or plateau (Europe) with no evidence of declining breast milk levels. Temporal patterns for BDE-209 in Asia were not significant, indicating no clear time trends. The European breakpoint for BDE-209 indicated a shift from the significant increase before 2004 to a current plateau or declining phase.
Comparison with Other Reported Time Trends
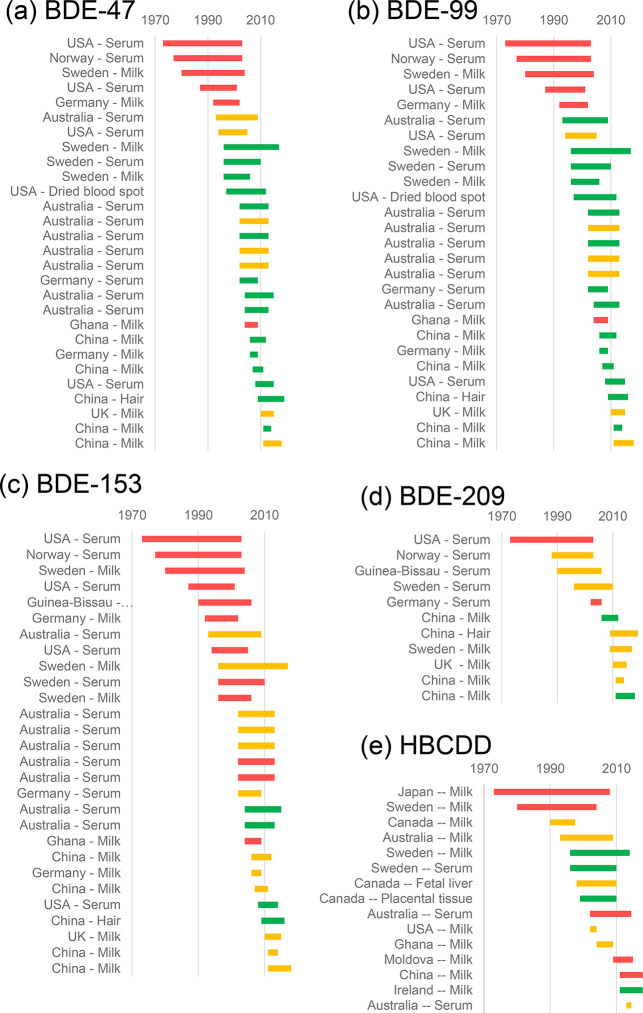
For the PBDEs, clear differences in the time trends by congener and by study timing are seen. For BDE-47 and -99 (Figure 4), there is a clear shift from early increasing time trends to more recent plateaus or decreasing time trends. The same pattern of early increasing trends and more recent reports of decreasing time trends is visible for BDE-153 (Figure 4), but the first decreases are not reported until much more recently. Most time trends for BDE-153 indicate a plateau. Relatively few studies report time trend analysis for HBCDD and BDE-209. For BDE-209 and HBCDD, no discernible time trend can be derived from literature for human matrices; trends were variable and not generalizable by region or duration of the time trend (Figure 4).
Figure 4.
Time trends reported in human matrices in literature for (a) BDE-47, (b) BDE-99, (c) BDE-153, (d) BDE-209, and (e) HBCDD. Red bars indicate an increasing trend, yellow bars indicate no trend or a plateau, and green bars indicate a decreasing trend. Trends are classified based on the interpretations of the authors of each study. References for all studies can be found in Tables S2 and S3.
Discussion and Implications
Temporal Patterns
The analysis of published studies suggests that BDE-47 and -99 have a global decreasing trend. Decreasing temporal patterns were found in Europe, Asia, and Oceania. BDE-47 and -99 have human elimination half-lives of approximately 0.37–3 and 0.77–8 years, respectively.68,236,237 In the time since the penta- and octa-PBDEs were included in the Stockholm Convention in 2004, the population would have been exposed to lower concentrations of the compounds, and the existing compounds would have been eliminated from their bodies. The United States is a signatory to the Stockholm Convention but has yet to ratify or implement the convention in national legislation.241 The North American region was the only region with a continuous increase in BDE-47 and -99 (Figure 2).
While BDE-153 was also added to the Stockholm Convention in 2004, it does not share the same decreasing trends as the lower brominated congeners. All regions exhibited either a plateau or an increase in BDE-153 concentrations. Beyond the reduction in exposure due to the introduction of chemical restrictions, congener-specific differences in metabolism and storage in the body also affect breast milk trends differently. The human elimination half-life of BDE-153 is between 3.5 and 11.7 years.68,236,237 When considering worst case scenario, a half-life of up to 11.7 years would result in a 4-fold reduction (in the absence of continued exposure) of concentrations measured around 23 years ago, at the time of the implementation of the Stockholm Convention. Therefore, a full elimination would not be expected by the time of writing. This, coupled with continued exposure from existing products, could explain the lack of a decrease of BDE-153 in breast milk (Figures 2 and 4).
Global restrictions on penta- and octa-BDE technical mixtures, which are dominated by lower brominated congeners were generally between 2004 and 2013,33,242,243 whereas deca-BDE/BDE-209 was restricted only in 2017.244 Conclusions on BDE-209 are limited because of the lack of data and likely because the temporal changes are not yet significant enough to be identified in the generally short-time trend analyses that have been performed (Figure 4). The short half-life of BDE-209 in the body (e.g., 15 days in blood245), combined with the lack of a visible decline in BDE-209 levels in milk, suggests ongoing consistent BDE-209 exposures, despite recent restrictions in production, particularly in Asia, where the temporal trend (Figure S6) and the inverse breakpoint (Figure 3) indicated increasing concentrations in BDE-209 in breast milk. This agrees with our understanding of the recent high use of BDE-209 in consumer products and building materials on a global scale and the lag time between chemical restrictions and product replacements: emission of BDE-209 from in-use and waste stocks is estimated to continue until 2050.3
It is concerning how few studies investigated HBCDDs in residents from the Americas (n = 7), Africa (n = 5), and Oceania (n = 2) (Table 1). A similar problem was observed with PBDEs, where Africa (n = 7), Oceania (n = 7), and Central- and South America (n = 3) had limited studies, while Asia and Europe had more studies (n = 53 and 61, respectively) (Table 1). Without the proper information on the state of contamination in the Americas, particularly the highly developed and industrialized North America (n = 25 studies on PBDE), it is impossible to determine a global perspective on the state of human exposure.
Breakpoint analyses are useful tools to determine whether the accumulation trend of a compound has increased, plateaued, or decreased over time, highlighting the approximate time when the change occurred. In Asia and Europe, the broad time trends of FR concentrations in breast milk are closely tied to the timing of chemical restrictions (Figure 3). The European trend indicated a breakpoint in ∼2010 for α-HBCDD (Figure 3), which coincides with the increase in restrictions in Europe (identified as SVHC in 2009 and listed in Annex XIV of REACH in 2011)40 and provides evidence that the restrictions impacted HBCDD use and thus exposures in Europe. After 2010, it is unclear whether α-HBCDDs are in a plateau phase or whether we begin seeing evidence of a decrease in Europe and Asia (Figure 3), but it suggests ongoing human exposure at levels close to the European peak.
It is important to highlight that in Figure 3, none of the breakpoints observed for Europe or Asia align with the timing of the Stockholm Convention’s implementation (represented by the blue dotted line) but rather occurred earlier. This observation suggests that regional restrictions likely had a more pronounced impact than global restrictions. However, it should be acknowledged that the number of studies conducted after the implementation of the Stockholm Convention is limited compared to those conducted before its implementation, making it challenging to precisely assess the Convention’s effectiveness, especially concerning HBCDD and deca-BDE.
Spatial Patterns
The strong contrast between PBDE concentrations in North America, particularly the USA, and most other regions is directly related to differences in flammability standards and PBDE use. BFRs have been quantified at higher concentrations in North America than in Europe in multiple matrices, including human tissue,26 house dust,246 and bird eggs.247 The USA has historically had higher concentrations of flame retardants in its consumer products compared with other regions due to stricter flammability standards.248−250 The fact that PBDE congeners still show an increasing time trend in North America (Figure 2) is likely linked to the large past use of PBDEs, combined with the later introduction of regulations. The United States has not ratified the Stockholm Convention and does not have any federal regulations on FR in existing uses, although 13 states have internal, state-wide concentration limits on FRs in selected products.251 Significant New Use Rules (SNURs), implemented by the United States Environmental Protection Agency in 2012, aim to ensure that any new uses of specific flame retardant chemicals undergo a thorough review and approval process prior to manufacturing or processing. The US EPA implemented SNURs for Penta and OctaBDE in 2006, ensuring no new use or manufacturing of these compounds.252 All manufacture, import, processing and distribution of decaBDE was under the US TSCA in 2021; however, significant exemptions remain, e.g., in motor vehicle parts until 2036.253 Large numbers of products containing PBDEs are likely still in use or circulation, which leads to continuous exposure and a higher body burden.
Surprisingly, breast milk from the Oceania region was also significantly higher than most other global regions, save North America (Figure 1). While most of the world reduced PBDE use in 2004, whether through regulation voluntary action, Australia only began implementing regulation on PBDE use, manufacturing, and import in 2007.238 Even though the import of PBDEs is banned, no regulation exists for the import of products that potentially could contain PBDEs, such as automobile parts, textiles, or electronic products.254
BDE-47 and -99, both primary components of penta-BDE, displayed similar spatial patterns (Figure 1), attributed to patterns in the use of technical penta-BDE formula worldwide.
General Observations
A substantial lag-time exists between cessation of production and cessation of use of FRs because of the long half-lives of the compounds and the lifespan of products that they are used in. Significant reductions in production have a slower effect on use and emissions because of the large stock of PBDE-containing materials in use. Abbasi et al.3 estimated that the peak in PBDE use occurred in 2003; however, thousands of tons of PBDEs will remain in use in consumer products for decades. Plastic, textile, and electronic products containing FRs are still in use, to say nothing of buildings’ thermal insulation containing EPS or XPS, which accounts for more than 97% of the global HBCDD volume used.255 The ongoing human exposure to HBCDDs will be further mediated by HBCDD exposure through the renovation and demolition of buildings. Demolition activity can release significant amounts of building material-associated chemicals and demolition waste, including EPS or XPS panels, which are estimated to stay in place for ∼50 years before renovation takes place.61 Thus, direct exposures to HBCDDs in indoor spaces and environmental release will continue for many decades,256 effectively slowing the decreasing concentration levels through constant primary exposures. These can either contribute directly to either occupational or local population exposures, as well as increase the burden of secondary environmental exposures through landfill disposal and subsequent leaching of HBCDDs from the products.31
Furthermore, as we move toward a circular economy, there is significant potential for FRs to be incorporated into new consumer products made from recycled materials.257,258 Abbasi et al.3 estimated that 45000 t of PBDEs may reappear in new products made from recycled materials, such as plastics, food contact materials,259,260 and children’s toys.261,262 Due to the persistence of FRs, all environmental releases can contribute to secondary FR contamination in the surrounding air, soil, and water sources and lead to human exposure via dietary sources.263
The lack of recent biomonitoring studies on PBDEs and HBCDDs limits the evaluation of current population exposures and the impact of regulation on time trends. Of the human biomonitoring studies (Table 1 and Figure 4), less than 10% of the records cover the period after 2013, limiting our ability to evaluate the effectiveness of legislation on the global exposure trend of FRs. This may be due to the perception that once chemicals have been regulated, the problem has been dealt with, and it can be difficult to maintain interest and/or financial support for chemicals that are perceived to have been already addressed.110
Regulation has a quantifiable effect on the concentrations of FRs in human breast milk. Regions such as Asia and Europe where earlier regional regulations, prior to the Stockholm Convention, have been implemented had the clearest decline of most of the compounds that were considered in this study. Australia, where regulations were implemented later still shows elevated concentrations in human matrices, as does North America, which had the historically highest FR use globally. On top of use and regulation, the chemical characteristics of specific PBDE congeners also affect the response to regulation and can impact the time needed to evaluate the efficacy of policy actions. Although PBDEs and HBCDD are regulated through a ban on production, regulation will have different outcomes for different individual compounds based on their dominant use, the volume of historical use, and biological half-lives. However, it is encouraging to see that existing regulation and policy, when implemented early and comprehensively, had a positive impact on the decreasing human body burden of lower brominated flame retardants.
Acknowledgments
This study has received funding from the European Union’s Horizon 2020 research and innovation programme under Grant no. 733032 (HBM4EU). The authors also thank the RECETOX Research Infrastructure (LM2023069) financed by the Ministry of Education, Youth and Sports, and Operational Programme Research, Development and Innovation - project CETOCOEN EXCELLENCE (CZ.02.1.01/0.0/0.0/17_043/0009632) for supportive background. The work was also supported by the European Union’s Horizon 2020 research and innovation programme under Grant no. 857560. This publication reflects only the authors’ views, and the European Commission is not responsible for any use that may be made of the information it contains.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.3c02896.
Figures showing temporal trend and breakpoint analysis for all flame retardants from all regions and tables detailing references used in literature review and meta-analysis (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Wemken N.; Drage D. S.; Cellarius C.; Cleere K.; Morrison J. J.; Daly S.; Abdallah M. A. E.; Tlustos C.; Harrad S.; Coggins M. A. Emerging and Legacy Brominated Flame Retardants in the Breast Milk of First Time Irish Mothers Suggest Positive Response to Restrictions on Use of HBCDD and Penta- and Octa-BDE Formulations. Environ. Res. 2020, 180, 108805 10.1016/j.envres.2019.108805. [DOI] [PubMed] [Google Scholar]
- Montalbano A. M.; Albano G. D.; Anzalone G.; Moscato M.; Gagliardo R.; Di Sano C.; Bonanno A.; Ruggieri S.; Cibella F.; Profita M. Cytotoxic and Genotoxic Effects of the Flame Retardants (PBDE-47, PBDE-99 and PBDE-209) in Human Bronchial Epithelial Cells. Chemosphere 2020, 245, 125600 10.1016/j.chemosphere.2019.125600. [DOI] [PubMed] [Google Scholar]
- Abbasi G.; Li L.; Breivik K. Global Historical Stocks and Emissions of PBDEs. Environ. Sci. Technol. 2019, 53 (11), 6330–6340. 10.1021/acs.est.8b07032. [DOI] [PubMed] [Google Scholar]
- De Wit C. A. An Overview of Brominated Flame Retardants in the Environment. Chemosphere 2002, 46 (5), 583–624. 10.1016/S0045-6535(01)00225-9. [DOI] [PubMed] [Google Scholar]
- Abbasi G.; Buser A. M.; Soehl A.; Murray M. W.; Diamond M. L. Stocks and Flows of PBDEs in Products from Use to Waste in the U.S. and Canada from 1970 to 2020. Environ. Sci. Technol. 2015, 49 (3), 1521–1528. 10.1021/es504007v. [DOI] [PubMed] [Google Scholar]
- Covaci A.; Gerecke A. C.; Law R. J.; Voorspoels S.; Kohler M.; Heeb N. V.; Leslie H.; Allchin C. R.; de Boer J. Hexabromocyclododecanes (HBCDs) in the Environment and Humans: A Review. Environ. Sci. Technol. 2006, 40 (12), 3679–3688. 10.1021/es0602492. [DOI] [PubMed] [Google Scholar]
- Koch C.; Schmidt-Kötters T.; Rupp R.; Sures B. Review of Hexabromocyclododecane (HBCD) with a Focus on Legislation and Recent Publications Concerning Toxicokinetics and -Dynamics. Environ. Pollut. 2015, 199, 26–34. 10.1016/j.envpol.2015.01.011. [DOI] [PubMed] [Google Scholar]
- de Wit C. A.; Herzke D.; Vorkamp K. Brominated Flame Retardants in the Arctic Environment - Trends and New Candidates. Sci. Total Environ. 2010, 408 (15), 2885–2918. 10.1016/j.scitotenv.2009.08.037. [DOI] [PubMed] [Google Scholar]
- Law R. J.; Covaci A.; Harrad S. J.; Herzke D.; Abdallah M. A.-E.; Fernie K.; Toms L.-M. L.; Takigami H. Levels and Trends of PBDEs and HBCDs in the Global Environment: Status at the End of 2012. Environ. Int. 2014, 65, 147–158. 10.1016/j.envint.2014.01.006. [DOI] [PubMed] [Google Scholar]
- Sjodin A; Hagmar L; Klasson-Wehler E; Kronholm-Diab K; Jakobsson E; Bergman A Flame Retardant Exposure: Polybrominated Diphenyl Ethers in Blood from Swedish Workers. Environ. Health Perspect 1999, 107 (8), 643–648. 10.1289/ehp.99107643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasson-Wehler E.; Hovander L.; Bergman A. New Organohalogens in Human Plasma - Identification and Quantification. Organohalogen Compd. 1997, 33, 420–425. [Google Scholar]
- Schroter-Kermani C.; Helm D.; Herrmann T.; Papke O. The German Environmental Specimen Bank-Application in Trend Monitoring of Polybrominated Diphenyl Ethers in Human Blood. Organohalogen Compd. 2000, 47, 49–52. [Google Scholar]
- Stanley J. S.; Cramer P. H.; Thornburg K. R.; Remmers J. C.; Breen J. J.; Schwemberger J. Mass Spectral Confirmation of Chlorinated and Brominated Diphenylethers in Human Adipose Tissues. Chemosphere 1991, 23 (8–10), 1185–1195. 10.1016/0045-6535(91)90143-2. [DOI] [Google Scholar]
- Haglund P. S.; Zook D. R.; Buser H. R.; Hu J. Identification and Quantification of Polybrominated Diphenyl Ethers and Methoxy-Polybrominated Diphenyl Ethers in Baltic Biota. Environ. Sci. Technol. 1997, 31 (11), 3281–3287. 10.1021/es9702834. [DOI] [Google Scholar]
- Meneses M.; Wingfors H.; Schuhmacher M.; Domingo J.L.; Lindstrom G.; Bavel B.v. Polybrominated Diphenyl Ethers Detected in Human Adipose Tissue from Spain. Chemosphere 1999, 39 (13), 2271–2278. 10.1016/S0045-6535(99)00150-2. [DOI] [PubMed] [Google Scholar]
- Norén K.; Meironyté D. Certain Organochlorine and Organobromine Contaminants in Swedish Human Milk in Perspective of Past 20–30 Years. Chemosphere 2000, 40 (9–11), 1111–1123. 10.1016/S0045-6535(99)00360-4. [DOI] [PubMed] [Google Scholar]
- Siddiqi M. A.; Laessig R. H.; Reed K. D. Polybrominated Diphenyl Ethers (PBDEs): New Pollutants-Old Diseases. Clin Med. Res. 2003, 1, 281–290. 10.3121/cmr.1.4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K.; Harada K.; Takenaka K.; Uehara S.; Kono M.; Shimizu T.; Takasuga T.; Senthilkumar K.; Yamashita F.; Koizumi A. Levels and Concentration Ratios of Polychlorinated Biphenyls and Polybrominated Diphenyl Ethers in Serum and Breast Milk in Japanese Mothers. Environ. Health Perspect 2006, 114 (8), 1179–1185. 10.1289/ehp.9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind Y.; Darnerud P. O.; Atuma S.; Aune M.; Becker W.; Bjerselius R.; Cnattingius S.; Glynn A. Polybrominated Diphenyl Ethers in Breast Milk from Uppsala County, Sweden. Environ. Res. 2003, 93 (2), 186–194. 10.1016/S0013-9351(03)00049-5. [DOI] [PubMed] [Google Scholar]
- Bjermo H.; Aune M.; Cantillana T.; Glynn A.; Lind P. M.; Ridefelt P.; Darnerud P. O. Serum Levels of Brominated Flame Retardants (BFRs: PBDE, HBCD) and Influence of Dietary Factors in a Population-Based Study on Swedish Adults. Chemosphere 2017, 167, 485–491. 10.1016/j.chemosphere.2016.10.008. [DOI] [PubMed] [Google Scholar]
- Barghi M.; Shin E. su; Choi S. D.; Dahmardeh Behrooz R.; Chang Y. S. HBCD and TBBPA in Human Scalp Hair: Evidence of Internal Exposure. Chemosphere 2018, 207, 70–77. 10.1016/j.chemosphere.2018.05.032. [DOI] [PubMed] [Google Scholar]
- Drage D. S.; Mueller J. F.; Hobson P.; Harden F. A.; Toms L. M. L. Demographic and Temporal Trends of Hexabromocyclododecanes (HBCDD) in an Australian Population. Environ. Res. 2017, 152, 192–198. 10.1016/j.envres.2016.10.015. [DOI] [PubMed] [Google Scholar]
- Inthavong C.; Hommet F.; Bordet F.; Rigourd V.; Guérin T.; Dragacci S. Simultaneous Liquid Chromatography–Tandem Mass Spectrometry Analysis of Brominated Flame Retardants (Tetrabromobisphenol A and Hexabromocyclododecane Diastereoisomers) in French Breast Milk. Chemosphere 2017, 186, 762–769. 10.1016/j.chemosphere.2017.08.020. [DOI] [PubMed] [Google Scholar]
- Dorman D. C.; Chiu W.; Hales B. F.; Hauser R.; Johnson K. J.; Mantus E.; Martel S.; Robinson K. A.; Rooney A. A.; Rudel R.; Sathyanarayana S.; Schantz S. L.; Waters K. M. Polybrominated Diphenyl Ether (PBDE) Neurotoxicity: A Systematic Review and Meta-Analysis of Animal Evidence. Journal of Toxicology and Environmental Health, Part B 2018, 21 (4), 269–289. 10.1080/10937404.2018.1514829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan M. L. Y.; Co V. A.; El-Nezami H. Endocrine Disrupting Chemicals and Breast Cancer: A Systematic Review of Epidemiological Studies. Crit Rev. Food Sci. Nutr 2022, 62 (24), 6549–6576. 10.1080/10408398.2021.1903382. [DOI] [PubMed] [Google Scholar]
- Lyche J. L.; Rosseland C.; Berge G.; Polder A.. Human Health Risk Associated with Brominated Flame-Retardants (BFRs). Environment International. Elsevier Ltd, 2015; pp 170–180, 10.1016/j.envint.2014.09.006. [DOI] [PubMed] [Google Scholar]
- Lam J.; Lanphear B. P.; Bellinger D.; Axelrad D. A.; McPartland J.; Sutton P.; Davidson L.; Daniels N.; Sen S.; Woodruff T. J.. Developmental PBDE Exposure and IQ/ADHD in Childhood: A Systematic Review and Meta-Analysis. Environ. Health Perspect 2017, 125 ( (8), ), 10.1289/EHP1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S.; Paalanen L.; Melymuk L.; Katsonouri A.; Kolossa-Gehring M.; Tolonen H. The Association between ADHD and Environmental Chemicals—A Scoping Review. Int. J. Environ. Res. Public Health 2022, 19 (5), 2849. 10.3390/ijerph19052849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iszatt N.; Janssen S.; Lenters V.; Dahl C.; Stigum H.; Knight R.; Mandal S.; Peddada S.; González A.; Midtvedt T.; Eggesbø M. Environmental Toxicants in Breast Milk of Norwegian Mothers and Gut Bacteria Composition and Metabolites in Their Infants at 1 Month. Microbiome 2019, 7, 34. 10.1186/s40168-019-0645-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascon M.; Fort M.; Martinez D.; Carsin A.-E.; Forns J.; Grimalt J. O.; Santa Marina L.; Lertxundi N.; Sunyer J.; Vrijheid M. Polybrominated Diphenyl Ethers (PBDEs) in Breast Milk and Neuropsychological Development in Infants. Environ. Health Perspect 2012, 120 (12), 1760–1765. 10.1289/ehp.1205266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feiteiro J.; Mariana M.; Cairrão E. Health Toxicity Effects of Brominated Flame Retardants: From Environmental to Human Exposure. Environ. Pollut. 2021, 285, 117475 10.1016/j.envpol.2021.117475. [DOI] [PubMed] [Google Scholar]
- Brown P.; Cordner A. Lessons Learned from Flame Retardant Use and Regulation Could Enhance Future Control of Potentially Hazardous Chemicals. Health Aff 2011, 30 (5), 906–914. 10.1377/hlthaff.2010.1228. [DOI] [PubMed] [Google Scholar]
- Directive 2003/11/EC of the European Parliament and of the Council of February 6 2003 Amending for the 24th Time Council Directive 76/669/EEC Relating to Restrictions on the Marketing and Use of Certain Dangerous Substances and Preparations (Pentabromodip. Off J. Eur. Union OJ. L 2003, 16 (1), 45–46. [Google Scholar]
- ATSDR. Toxic Substances Portal. ToxFAQs. https://wwwn.cdc.gov/TSP/ToxFAQs/ToxFAQsDetails.aspx?faqid=1462&toxid=183 (accessed 2023-01-28).
- Stockholm Convention; TetraBDE and pentaBDE. https://chm.pops.int/Implementation/Alternatives/AlternativestoPOPs/ChemicalslistedinAnnexA/TetraBDEandpentaBDE/tabid/5868/Default.aspx (accessed 2023-07-24).
- Stockholm Convention: HexaBDE and heptaBDE. https://chm.pops.int/Implementation/Alternatives/AlternativestoPOPs/ChemicalslistedinAnnexA/HexaBDEandheptaBDE/tabid/5862/Default.aspx (accessed 2023-07-24).
- Stockholm Convention: Decabromodiphenyl ether (commercial mixture, c-decaBDE). https://chm.pops.int/Implementation/Alternatives/AlternativestoPOPs/ChemicalslistedinAnnexA/cdecaBDE/tabid/5985/Default.aspx (accessed 2023-07-24).
- UNEP. Stockholm Convention: United Nations Environment Programme (UNEP). www.pops.int (accessed 2023-02-13). [Google Scholar]
- Risk Assessment: Hexabromocyclododecane, Final report, May 2008. https://echa.europa.eu/documents/10162/661bff17-dc0a-4475-9758-40bdd6198f82. (accessed 2023-01-28).
- Kemmlein S.; Herzke D.; Law R. J. Brominated Flame Retardants in the European Chemicals Policy of REACH-Regulation and Determination in Materials. Journal of Chromatography A 2009, 1216 (3), 320–333. 10.1016/j.chroma.2008.05.085. [DOI] [PubMed] [Google Scholar]
- UNEP. Stockholm Convention Global Monitoring Plan for Persistent Organic Pollutants: First Global Monitoring Report; Geneva, 2009. https://chm.pops.int/implementation/globalmonitoringplan/monitoringreports/tabid/525/default.aspx. (accessed 2023-03-30).
- Sharma B. M.; Kalina J.; Whaley P.; Scheringer M. Towards Guidelines for Time-Trend Reviews Examining Temporal Variability in Human Biomonitoring Data of Pollutants. Environ. Int. 2021, 151, 106437 10.1016/j.envint.2021.106437. [DOI] [PubMed] [Google Scholar]
- Wong F.; Hung H.; Dryfhout-Clark H.; Aas W.; Bohlin-Nizzetto P.; Breivik K.; Mastromonaco M. N.; Lundén E. B.; Ólafsdóttir K.; Sigurosson Á.; Vorkamp K.; Bossi R.; Skov H.; Hakola H.; Barresi E.; Sverko E.; Fellin P.; Li H.; Vlasenko A.; Zapevalov M.; Samsonov D.; Wilson S. Time Trends of Persistent Organic Pollutants (POPs) and Chemicals of Emerging Arctic Concern (CEAC) in Arctic Air from 25 Years of Monitoring. Sci. Total Environ. 2021, 775, 145109 10.1016/j.scitotenv.2021.145109. [DOI] [PubMed] [Google Scholar]
- Schuster J. K.; Gioia R.; Moeckel C.; Agarwal T.; Bucheli T. D.; Breivik K.; Steinnes E.; Jones K. C. Has the Burden and Distribution of PCBs and PBDEs Changed in European Background Soils between 1998 and 2008? Implications for Sources and Processes. Environ. Sci. Technol. 2011, 45 (17), 7291–7297. 10.1021/es200961p. [DOI] [PubMed] [Google Scholar]
- Sutton R.; Sedlak M. D.; Yee D.; Davis J. A.; Crane D.; Grace R.; Arsem N. Declines in Polybrominated Diphenyl Ether Contamination of San Francisco Bay Following Production Phase-Outs and Bans. Environ. Sci. Technol. 2015, 49 (2), 777–784. 10.1021/es503727b. [DOI] [PubMed] [Google Scholar]
- Olofsson U.; Bignert A.; Haglund P. Time-Trends of Metals and Organic Contaminants in Sewage Sludge. Water Res. 2012, 46 (15), 4841–4851. 10.1016/j.watres.2012.05.048. [DOI] [PubMed] [Google Scholar]
- Crimmins B. S.; Pagano J. J.; Xia X.; Hopke P. K.; Milligan M. S.; Holsen T. M. Polybrominated Diphenyl Ethers (PBDEs): Turning the Corner in Great Lakes Trout 1980–2009. Environ. Sci. Technol. 2012, 46 (18), 9890–9897. 10.1021/es302415z. [DOI] [PubMed] [Google Scholar]
- Hale R. C.; La Guardia M. J.; Harvey E.; Chen D.; Mainor T. M.; Luellen D. R.; Hundal L. S. Polybrominated Diphenyl Ethers in U.S. Sewage Sludges and Biosolids: Temporal and Geographical Trends and Uptake by Corn Following Land Application. Environ. Sci. Technol. 2012, 46 (4), 2055–2063. 10.1021/es203149g. [DOI] [PubMed] [Google Scholar]
- Leslie H. A.; Brandsma S. H.; Barber J. L.; Gabrielsen G. W.; Bersuder P.; Barry J.; Shore R. F.; Walker L. A.; de Boer J. Decabromodiphenylether Trends in the European Environment: Bird Eggs, Sewage Sludge and Surficial Sediments. Sci. Total Environ. 2021, 774, 145174 10.1016/j.scitotenv.2021.145174. [DOI] [PubMed] [Google Scholar]
- Turyk M. E.; Anderson H. A.; Steenport D.; Buelow C.; Imm P.; Knobeloch L. Longitudinal Biomonitoring for Polybrominated Diphenyl Ethers (PBDEs) in Residents of the Great Lakes Basin. Chemosphere 2010, 81 (4), 517–522. 10.1016/j.chemosphere.2010.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecter A.; Papke O.; Tung K. C.; Joseph J.; Harris T R.; Dahlgren J. Polybrominated Diphenyl Ether Flame Retardants in the U.S. Population: Current Levels, Temporal Trends, and Comparison with Dioxins, Dibenzofurans, and Polychlorinated Biphenyls. J. Occup Environ. Med. 2005, 47 (3), 199–211. 10.1097/01.jom.0000158704.27536.d2. [DOI] [PubMed] [Google Scholar]
- Law R. J.; Barry J.; Barber J. L.; Bersuder P.; Deaville R.; Reid R. J.; Brownlow A.; Penrose R.; Barnett J.; Loveridge J.; Smith B.; Jepson P. D. Contaminants in Cetaceans from UK Waters: Status as Assessed within the Cetacean Strandings Investigation Programme from 1990 to 2008. Mar. Pollut. Bull. 2012, 64 (7), 1485–1494. 10.1016/j.marpolbul.2012.05.024. [DOI] [PubMed] [Google Scholar]
- Munschy C.; Marchand P.; Venisseau A.; Veyrand B.; Zendong Z. Levels and Trends of the Emerging Contaminants HBCDs (Hexabromocyclododecanes) and PFCs (Perfluorinated Compounds) in Marine Shellfish along French Coasts. Chemosphere 2013, 91 (2), 233–240. 10.1016/j.chemosphere.2012.12.063. [DOI] [PubMed] [Google Scholar]
- Smythe T. A.; Loseto L. L.; Bignert A.; Rosenberg B.; Budakowski W.; Halldorson T.; Pleskach K.; Tomy G. T. Temporal Trends of Brominated and Fluorinated Contaminants in Canadian Arctic Beluga Delphinapterus Leucas. Arct Sci. 2018, 4 (3), 388–404. 10.1139/AS-2017-0044. [DOI] [Google Scholar]
- Johansson A.-K.; Sellström U.; Lindberg P.; Bignert A.; de Wit C. A. Temporal Trends of Polybrominated Diphenyl Ethers and Hexabromocyclododecane in Swedish Peregrine Falcon (Falco Peregrinus Peregrinus) Eggs. Environ. Int. 2011, 37 (4), 678–686. 10.1016/j.envint.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Vorkamp K.; Falk K.; Møller S.; Rigét F. F.; Sørensen P. B. Regulated and Unregulated Halogenated Flame Retardants in Peregrine Falcon Eggs from Greenland. Environ. Sci. Technol. 2018, 52 (2), 474–483. 10.1021/acs.est.7b04866. [DOI] [PubMed] [Google Scholar]
- Rüdel H.; Müller J.; Nowak J.; Ricking M.; Klein R.; Kotthoff M. Hexabromocyclododecane Diastereomers in Fish and Suspended Particulate Matter from Selected European Waters—Trend Monitoring and Environmental Quality Standard Compliance. Environmental Science and Pollution Research 2017, 24 (22), 18048–18062. 10.1007/s11356-017-9469-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aznar-Alemany Ò.; Sala B.; Jobst K. J.; Reiner E. J.; Borrell A.; Aguilar À.; Eljarrat E. Temporal Trends of Halogenated and Organophosphate Contaminants in Striped Dolphins from the Mediterranean Sea. Sci. Total Environ. 2021, 753, 142205 10.1016/j.scitotenv.2020.142205. [DOI] [PubMed] [Google Scholar]
- Tao F.; Abou-Elwafa Abdallah M.; Ashworth D. C.; Douglas P.; Toledano M. B.; Harrad S. Emerging and Legacy Flame Retardants in UK Human Milk and Food Suggest Slow Response to Restrictions on Use of PBDEs and HBCDD. Environ. Int. 2017, 105, 95–104. 10.1016/j.envint.2017.05.010. [DOI] [PubMed] [Google Scholar]
- Magnier L.; Mugge R. Replaced Too Soon? An Exploration of Western European Consumers’ Replacement of Electronic Products. Resour Conserv Recycl 2022, 185, 106448 10.1016/j.resconrec.2022.106448. [DOI] [Google Scholar]
- Charbonnet J. A.; Weber R.; Blum A. Flammability Standards for Furniture, Building Insulation and Electronics: Benefit and Risk. Emerg. Contam. 2020, 6, 432–441. 10.1016/j.emcon.2020.05.002. [DOI] [Google Scholar]
- Abbatt J. P. D.; Wang C. The Atmospheric Chemistry of Indoor Environments. Environ. Sci.: Processes Impacts 2020, 22, 25–48. 10.1039/C9EM00386J. [DOI] [PubMed] [Google Scholar]
- Melymuk L.; Demirtepe H.; Jílková S. R. Indoor Dust and Associated Chemical Exposures. Current Opinion in Environmental Science and Health 2020, 15, 1–6. 10.1016/j.coesh.2020.01.005. [DOI] [Google Scholar]
- Rodgers K. M.; Bennett D.; Moran R.; Knox K.; Stoiber T.; Gill R.; Young T. M.; Blum A.; Dodson R. E. Do Flame Retardant Concentrations Change in Dust after Older Upholstered Furniture Is Replaced?. Environ. Int. 2021, 153, 106513 10.1016/j.envint.2021.106513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett D. H.; Moran R. E.; Wu X. M.; Tulve N. S.; Clifton M. S.; Colon M.; Weathers W.; Sjodin A.; Jones R.; Hertz-Picciotto I. Polybrominated Diphenyl Ether (PBDE) Concentrations and Resulting Exposure in Homes in California: Relationships among Passive Air, Surface Wipe and Dust Concentrations, and Temporal Variability. Indoor Air 2015, 25 (2), 220–229. 10.1111/ina.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Schyff V.; Kalina J.; Govarts E.; Gilles L.; Schoeters G.; Castaño A.; Esteban-López M.; Kohoutek J.; Kukučka P.; Covaci A.; Koppen G.; Andrýsková L.; Piler P.; Klánová J.; Jensen T. K.; Rambaud L.; Riou M.; Lamoree M.; Kolossa-Gehring M.; Vogel N.; Weber T.; Göen T.; Gabriel C.; Sarigiannis D. A.; Sakhi A. K.; Haug L. S.; Murinova L. P.; Fabelova L.; Tratnik J. S.; Mazej D.; Melymuk L. Exposure to Flame Retardants in European Children — Results from the HBM4 EU Aligned Studies. International Journal of Hygiene and Environmental Health 2023, 247, 114070 10.1016/j.ijheh.2022.114070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H. M.; McKone T. E.; Tulve N. S.; Clifton M. S.; Bennett D. H. Indoor Residence Times of Semivolatile Organic Compounds: Model Estimation and Field Evaluation. Environ. Sci. Technol. 2013, 47 (2), 859–867. 10.1021/es303316d. [DOI] [PubMed] [Google Scholar]
- Geyer H. J.; Schramm K.-W.; Darnerud O.; Aune M.; Feicht E. A.; Fried K. W.; Henkelmann B.; Lenoir D.; Schmid P.; Mcdonald T. A. Terminal Elimination Half-Lives of the Brominated Flame Retardants TBBPA, HBCD, and Lower Brominated PBDEs in Humans. Organohalogen Compd. 2004, 66, 3820–3825. [Google Scholar]
- t’Mannetje A.; Coakley J.; Mueller J. F.; Harden F.; Toms L.-M.; Douwes J. Partitioning of Persistent Organic Pollutants (POPs) between Human Serum and Breast Milk: A Literature Review. Chemosphere 2012, 89 (8), 911–918. 10.1016/j.chemosphere.2012.06.049. [DOI] [PubMed] [Google Scholar]
- Schecter A.; Colacino J.; Sjödin A.; Needham L.; Birnbaum L. Partitioning of Polybrominated Diphenyl Ethers (PBDEs) in Serum and Milk from the Same Mothers. Chemosphere 2010, 78 (10), 1279–1284. 10.1016/j.chemosphere.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Komprda J.; Komprdová K.; Domínguez-Romero E.; Mikeš O.; Řiháčková K.; Čupr P.; Černá M.; Scheringer M. Dynamics of PCB Exposure in the Past 50 Years and Recent High Concentrations in Human Breast Milk: Analysis of Influencing Factors Using a Physiologically Based Pharmacokinetic Model. Sci. Total Environ. 2019, 690, 388–399. 10.1016/j.scitotenv.2019.06.504. [DOI] [PubMed] [Google Scholar]
- Hajeb P.; Castaño A.; Cequier E.; Covaci A.; López M. E.; Antuña A. G.; Haug L. S.; Henríquez-Hernández L. A.; Melymuk L.; Pérez Luzardo O.; Thomsen C.; Vorkamp K. Critical Review of Analytical Methods for the Determination of Flame Retardants in Human Matrices. Anal. Chim. Acta 2022, 1193, 338828 10.1016/j.aca.2021.338828. [DOI] [PubMed] [Google Scholar]
- UNEP . Global Monitoring Plan for Persistent Organic Pollutants under the Stockholm Convention Article 16 on Effectiveness Evaluation - Third Regional Monitoring Report - Latin America and the Caribbean; 2021. https://chm.pops.int/implementation/globalmonitoringplan/monitoringreports/tabid/525/default.aspx. (accessed 2023-03-30).
- Johnson-Restrepo B.; Kannan K. An Assessment of Sources and Pathways of Human Exposure to Polybrominated Diphenyl Ethers in the United States. Chemosphere 2009, 76 (4), 542–548. 10.1016/j.chemosphere.2009.02.068. [DOI] [PubMed] [Google Scholar]
- Sahlström L. M. O.; Sellström U.; de Wit C. A.; Lignell S.; Darnerud P. O. Brominated Flame Retardants in Matched Serum Samples from Swedish First-Time Mothers and Their Toddlers. Environ. Sci. Technol. 2014, 48 (13), 7584–7592. 10.1021/es501139d. [DOI] [PubMed] [Google Scholar]
- Eljarrat E.; Guerra P.; Martínez E.; Farré M.; Alvarez J. G.; López-Teijón M.; Barceló D. Hexabromocyclododecane in Human Breast Milk: Levels and Enantiomeric Patterns. Environ. Sci. Technol. 2009, 43 (6), 1940–1946. 10.1021/es802919e. [DOI] [PubMed] [Google Scholar]
- Ryan J. J.; Rawn D. F. K. The Brominated Flame Retardants, PBDEs and HBCD, in Canadian Human Milk Samples Collected from 1992 to 2005; Concentrations and Trends. Environ. Int. 2014, 70, 1–8. 10.1016/j.envint.2014.04.020. [DOI] [PubMed] [Google Scholar]
- Carignan C. C.; Abdallah M. A. E.; Wu N.; Heiger-Bernays W.; McClean M. D.; Harrad S.; Webster T. F. Predictors of Tetrabromobisphenol-A (TBBP-A) and Hexabromocyclododecanes (HBCD) in Milk from Boston Mothers. Environ. Sci. Technol. 2012, 46 (21), 12146–12153. 10.1021/es302638d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asante K. A.; Adu-Kumi S.; Nakahiro K.; Takahashi S.; Isobe T.; Sudaryanto A.; Devanathan G.; Clarke E.; Ansa-Asare O. D.; Dapaah-Siakwan S.; Tanabe S. Human Exposure to PCBs, PBDEs and HBCDs in Ghana: Temporal Variation, Sources of Exposure and Estimation of Daily Intakes by Infants. Environ. Int. 2011, 37 (5), 921–928. 10.1016/j.envint.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Darnerud P. O.; Aune M.; Larsson L.; Lignell S.; Mutshatshi T.; Okonkwo J.; Botha B.; Agyei N. Levels of Brominated Flame Retardants and Other Pesistent Organic Pollutants in Breast Milk Samples from Limpopo Province, South Africa. Sci. Total Environ. 2011, 409 (19), 4048–4053. 10.1016/j.scitotenv.2011.05.054. [DOI] [PubMed] [Google Scholar]
- Hůlek R.; Borůvková J.; Gregor J.; Kalina J.; Bednářová Z.; Šebková K.; Melkes O.; Šalko M.; Novák R.; Jarkovský J.; Dušek L.; Klánová J.. Global Monitoring Plan of the Stockholm Convention on Persistent Organic Pollutants: visualisation and on-line analysis of global levels of chemicals in air, water, breast milk and blood [online]. Masaryk University, 2014, http://visualization.pops-gmp.org/2014/ (accessed 2022-07-14).
- Müller M. H. B.; Polder A.; Brynildsrud O. B.; Lie E.; Løken K. B.; Manyilizu W. B.; Mdegela R. H.; Mokiti F.; Murtadha M.; Nonga H. E.; Skaare J. U.; Lyche J. L. Brominated Flame Retardants (BFRs) in Breast Milk and Associated Health Risks to Nursing Infants in Northern Tanzania. Environ. Int. 2016, 89–90, 38–47. 10.1016/j.envint.2015.12.032. [DOI] [PubMed] [Google Scholar]
- UNEP. Global Monitoring Plan for Persistent Organic Pollutants under the Stockholm Convention Article 16 on Effectiveness Evaluation - Third Regional Monitoring Report - Africa Region; 2021. https://chm.pops.int/implementation/globalmonitoringplan/monitoringreports/tabid/525/default.aspx. (accessed 2023-03-30).
- Hassine S. B.; Ameur W. B.; Gandoura N.; Driss M. R. Determination of Chlorinated Pesticides, Polychlorinated Biphenyls, and Polybrominated Diphenyl Ethers in Human Milk from Bizerte (Tunisia) in 2010. Chemosphere 2012, 89 (4), 369–377. 10.1016/j.chemosphere.2012.05.035. [DOI] [PubMed] [Google Scholar]
- Matovu H.; Sillanpää M.; Ssebugere P. Polybrominated Diphenyl Ethers in Mothers’ Breast Milk and Associated Health Risk to Nursing Infants in Uganda. Science of The Total Environment 2019, 692, 1106–1115. 10.1016/j.scitotenv.2019.07.335. [DOI] [PubMed] [Google Scholar]
- Horng C.; Chao H.; Chang C.; Govindasamy A.; Tzeng T.; Shieh P. Polybrominated Diphenyl Ethers in Human Milk in Taiwan. Asian J. Chem. 2010, 22 (4), 2869–2878. [Google Scholar]
- Zhang J. G.; Sun X. W.; Ai H. Levels and Congener Profiles of Polybrominated Diphenyl Ethers (PBDEs) in Primipara Breast Milk from Shenzhen and Exposure Risk for Breast-Fed Infants. Journal of Environmental Monitoring 2012, 14, 893–900. 10.1039/c2em10739b. [DOI] [PubMed] [Google Scholar]
- Sudaryanto A.; Kajiwara N.; Takahashi S.; Muawanah; Tanabe S. Geographical Distribution and Accumulation Features of PBDEs in Human Breast Milk from Indonesia. Environ. Pollut. 2008, 151 (1), 130–138. 10.1016/j.envpol.2007.02.016. [DOI] [PubMed] [Google Scholar]
- UNEP. Global monitoring plan for Persistent Organic Pollutants. Third regional monitoring report Asia-Pacific region. http://chm.pops.int/implementation/globalmonitoringplan/monitoringreports/tabid/525/default.aspx (accessed 2023-03-30).
- Zhu L.; Ma B.; Li J.; Wu Y.; Gong J. Distribution of Polybrominated Diphenyl Ethers in Breast Milk from North China: Implication of Exposure Pathways. Chemosphere 2009, 74, 1429–1434. 10.1016/j.chemosphere.2008.12.030. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Zhang K.; Yang D.; Ma L.; Lei B.; Zhang X.; Zhou J.; Fang X.; Yu Y. Polybrominated Biphenyl Ethers in Breast Milk and Infant Formula from Shanghai, China: Temporal Trends, Daily Intake, and Risk Assessment. Sci. Total Environ. 2014, 497, 508–515. 10.1016/j.scitotenv.2014.08.034. [DOI] [PubMed] [Google Scholar]
- Yin S.; Guo F.; Aamir M.; Liu Y.; Tang M.; Liu W. Multicenter Biomonitoring of Polybrominated Diphenyl Ethers (PBDEs) in Colostrum from China: Body Burden Profile and Risk Assessment. Environ. Res. 2019, 179, 108828 10.1016/j.envres.2019.108828. [DOI] [PubMed] [Google Scholar]
- Yang L.; Lu Y.; Wang L.; Chang F.; Zhang J.; Liu Y. Levels and Profiles of Polybrominated Diphenyl Ethers in Breast Milk During Different Nursing Durations. Bull. Environ. Contam. Toxicol. 2016, 97, 510–516. 10.1007/s00128-016-1908-2. [DOI] [PubMed] [Google Scholar]
- Wang Y.-F.; Wang S.-L.; Chen F.-A.; Chao H. A.; Tsou T.-C.; Shy C.-G.; Päpke O.; Kuo Y.-M.; Chao H.-R. Associations of Polybrominated Diphenyl Ethers (PBDEs) in Breast Milk and Dietary Habits and Demographic Factors in Taiwan. Food Chem. Toxicol. 2008, 46, 1925–1932. 10.1016/j.fct.2008.01.032. [DOI] [PubMed] [Google Scholar]
- Tue N. M.; Sudaryanto A.; Minh T. B.; Isobe T.; Takahashi S.; Viet P. H.; Tanabe S. Accumulation of Polychlorinated Biphenyls and Brominated Flame Retardants in Breast Milk from Women Living in Vietnamese E-Waste Recycling Sites. Science of the Total Environment, The 2010, 408, 2155–2162. 10.1016/j.scitotenv.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Tsydenova O. V.; Sudaryanto A.; Kajiwara N.; Kunisue T.; Batoev V. B.; Tanabe S. Organohalogen Compounds in Human Breast Milk from Republic of Buryatia, Russia. Environ. Pollut. 2007, 146, 225–232. 10.1016/j.envpol.2006.04.036. [DOI] [PubMed] [Google Scholar]
- Sun S.; Zhao J.; Leng J.; Wang P.; Wang Y.; Fukatsu H.; Liu D.; Liu X.; Kayama F. Levels of Dioxins and Polybrominated Diphenyl Ethers in Human Milk from Three Regions of Northern China and Potential Dietary Risk Factors. Chemosphere 2010, 80, 1151–1159. 10.1016/j.chemosphere.2010.06.021. [DOI] [PubMed] [Google Scholar]
- Sudaryanto A.; Kajiwara N.; Tsydenova O. V.; Isobe T.; Yu H.; Takahashi S.; Tanabe S. Levels and Congener Specific Profiles of PBDEs in Human Breast Milk from China: Implication on Exposure Sources and Pathways. Chemosphere 2008, 73 (10), 1661–1668. 10.1016/j.chemosphere.2008.07.088. [DOI] [PubMed] [Google Scholar]
- Shy C.-G.; Huang H.-L.; Chao H.-R.; Chang-Chien G.-P. Cord Blood Levels of Thyroid Hormones and IGF-1 Weakly Correlate with Breast Milk Levels of PBDEs in Taiwan. Int. J. Hyg Environ. Health 2012, 215, 345–351. 10.1016/j.ijheh.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Shi Z.; Wang Y.; Niu P.; Wang J.; Sun Z.; Zhang S.; Wu Y. Concurrent Extraction, Clean-up, and Analysis of Polybrominated Diphenyl Ethers, Hexabromocyclododecane Isomers, and Tetrabromobisphenol A in Human Milk and Serum. J. Sep Sci. 2013, 36 (20), 3402–3410. 10.1002/jssc.201300579. [DOI] [PubMed] [Google Scholar]
- Shi Z.; Jiao Y.; Hu Y.; Sun Z.; Zhou X.; Feng J.; Li J.; Wu Y. Levels of Tetrabromobisphenol A, Hexabromocyclododecanes and Polybrominated Diphenyl Ethers in Human Milk from the General Population in Beijing, China. Sci. Total Environ. 2013, 452–453, 10–18. 10.1016/j.scitotenv.2013.02.038. [DOI] [PubMed] [Google Scholar]
- Shi Z.; Zhang L.; Zhao Y.; Sun Z.; Zhou X.; Li J.; Wu Y. A National Survey of Tetrabromobisphenol-A, Hexabromocyclododecane and Decabrominated Diphenyl Ether in Human Milk from China: Occurrence and Exposure Assessment. Sci. Total Environ. 2017, 599–600, 237–245. 10.1016/j.scitotenv.2017.04.237. [DOI] [PubMed] [Google Scholar]
- Shen H.; Ding G.; Wu Y.; Pan G.; Zhou X.; Han J.; Li J.; Wen S. Polychlorinated Dibenzo-p-Dioxins/Furans (PCDD/Fs), Polychlorinated Biphenyls (PCBs), and Polybrominated Diphenyl Ethers (PBDEs) in Breast Milk from Zhejiang, China. Environ. Int. 2012, 42, 84–90. 10.1016/j.envint.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Ohta S.; Ishizuka D.; Nishimura H.; Nakao T.; Aozasa O.; Shimidzu Y.; Ochiai F.; Kida T.; Nishi M.; Miyata H. Comparison of Polybrominated Diphenyl Ethers in Fish, Vegetables, and Meats and Levels in Human Milk of Nursing Women in Japan. Chemosphere 2002, 46, 689–696. 10.1016/S0045-6535(01)00233-8. [DOI] [PubMed] [Google Scholar]
- Ma S.; Yu Z.; Zhang X.; Ren G.; Peng P.; Sheng G.; Fu J. Levels and Congener Profiles of Polybrominated Diphenyl Ethers (PBDEs) in Breast Milk from Shanghai: Implication for Exposure Route of Higher Brominated BDEs. Environ. Int. 2012, 42, 72–77. 10.1016/j.envint.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Lin Y.; Feng C.; Xu Q.; Lu D.; Qiu X.; Jin Y.; Wang G.; Wang D.; She J.; Zhou Z. A Validated Method for Rapid Determination of Dibenzo-p-Dioxins/Furans (PCDD/Fs), Polybrominated Diphenyl Ethers (PBDEs) and Polychlorinated Biphenyls (PCBs) in Human Milk: Focus on Utility of Tandem Solid Phase Extraction (SPE) Cleanup. Anal Bioanal Chem. 2016, 408, 4897–4906. 10.1007/s00216-016-9576-y. [DOI] [PubMed] [Google Scholar]
- Li X.; Tian Y.; Zhang Y.; Ben Y.; Lv Q. Accumulation of Polybrominated Diphenyl Ethers in Breast Milk of Women from an E-Waste Recycling Center in China. Journal of Environmental Sciences 2017, 52, 305–313. 10.1016/j.jes.2016.10.008. [DOI] [PubMed] [Google Scholar]
- Li J.; Yu H.; Zhao Y.; Zhang G.; Wu Y. Levels of Polybrominated Diphenyl Ethers (PBDEs) in Breast Milk from Beijing, China. Chemosphere 2008, 73, 182–186. 10.1016/j.chemosphere.2008.05.019. [DOI] [PubMed] [Google Scholar]
- Li J.; Ma W.; Zhao Y.; Jin Y.; Xiao X.; Ge W.; Shi H.; Zhang Y. Lactational Exposure of Polybrominated Diphenyl Ethers and Its Association with Infant Developmental Measurements. J. Hazard Mater. 2020, 388, 122031 10.1016/j.jhazmat.2020.122031. [DOI] [PubMed] [Google Scholar]
- Leung A. O. W.; Chan J. K. Y.; Xing G. H.; Xu Y.; Wu S. C.; Wong C. K. C.; Leung C. K. M.; Wong M. H. Body Burdens of Polybrominated Diphenyl Ethers in Childbearing-Aged Women at an Intensive Electronic-Waste Recycling Site in China. Environmental Science and Pollution Research 2010, 17, 1300–1313. 10.1007/s11356-010-0310-6. [DOI] [PubMed] [Google Scholar]
- Lee S.; Kim S.; Kim E.; Lee I.-S.; Choi G.; Kim H.-J.; Park J.; Jae Lee J.; Choi S.; Young Kim S.; Kim S.; Kim S.; Choi K.; Moon H.-B. Polybrominated Diphenyl Ethers (PBDEs) in Breast Milk of Korea in 2011: Current Contamination, Time Course Variation, Influencing Factors and Health Risks. Environ. Res. 2013, 126, 76–83. 10.1016/j.envres.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Koh T.-W.; Chih-Cheng Chen S.; Chang-Chien G.-P.; Lin D.-Y.; Chen F.-A.; Chao H.-R. Breast-Milk Levels of Polybrominated Diphenyl Ether Flame Retardants in Relation to Women’s Age and Pre-Pregnant Body Mass Index. Int. J. Hyg Environ. Health 2010, 213, 59–65. 10.1016/j.ijheh.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Kim U.-J.; Lee I.-S.; Kim H. S.; Oh J.-E. Monitoring of PBDEs Concentration in Umbilical Cord Blood and Breast Milk from Korean Population and Estimating the Effects of Various Parameters on Accumulation in Humans. Chemosphere 2011, 85, 487–493. 10.1016/j.chemosphere.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Kim T. H.; Bang D. Y.; Lim H. J.; Jin Won A.; Ahn M. Y.; Patra N.; Chung K. K.; Kwack S. J.; Park K. L.; Han S. Y.; Choi W. S.; Han J. Y.; Lee B. M.; Oh J. E.; Yoon J. H.; Lee J.; Kim H. S. Comparisons of Polybrominated Diphenyl Ethers Levels in Paired South Korean Cord Blood, Maternal Blood, and Breast Milk Samples. Chemosphere 2012, 87 (1), 97–104. 10.1016/j.chemosphere.2011.11.074. [DOI] [PubMed] [Google Scholar]
- KAWASHIRO Y.; FUKATA H.; OMORI-INOUE M.; KUBONOYA K.; JOTAKI T.; TAKIGAM H.; SAKAI S.-i.; MORI C. Perinatal Exposure to Brominated Flame Retardants and Polychlorinated Biphenyls in Japan. Endocr J. 2008, 55 (6), 1071–1084. 10.1507/endocrj.K08E-155. [DOI] [PubMed] [Google Scholar]
- Kao C.-C.; Chen C.-C.; Avelino J. L.; Cortez M. P.; Tayo L. L.; Lin Y.-H.; Tsai M.-H.; Lin C.-W.; Hsu Y.-C.; Hsieh L.-T.; Lin C.; Wang L.-C.; Yu K.-L. J.; Chao H.-R. Infants’ Neurodevelopmental Effects of PM2.5 and Persistent Organohalogen Pollutants Exposure in Southern Taiwan. Aerosol Air Qual Res. 2019, 19, 2793–2803. 10.4209/aaqr.2019.10.0550. [DOI] [Google Scholar]
- Kang C. S.; Lee J.-H.; Kim S.-K.; Lee K.-T.; Lee J. S.; Park S.; Yun H.; Kannan K.; Yoo Y. W.; Ha Y.; Lee S. W. Polybrominated Diphenyl Ethers and Synthetic Musks in Umbilical Cord Serum, Maternal Serum, and Breast Milk from Seoul, South Korea. Chemosphere 2010, 80, 116–122. 10.1016/j.chemosphere.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Jin J.; Wang Y.; Yang C.; Hu J.; Liu W.; Cui J.; Tang X. Polybrominated Diphenyl Ethers in the Serum and Breast Milk of the Resident Population from Production Area, China. Environ. Int. 2009, 35, 1048–1052. 10.1016/j.envint.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Hedley A. J.; Hui L. L.; Kypke K.; Malisch R.; van Leeuwen F. X. R.; Moy G.; Wong T. W.; Nelson E. A. S. Residues of Persistent Organic Pollutants (POPs) in Human Milk in Hong Kong. Chemosphere 2010, 79 (3), 259–265. 10.1016/j.chemosphere.2010.01.047. [DOI] [PubMed] [Google Scholar]
- Haraguchi K.; Koizumi A.; Inoue K.; Harada K. H.; Hitomi T.; Minata M.; Tanabe M.; Kato Y.; Nishimura E.; Yamamoto Y.; Watanabe T.; Takenaka K.; Uehara S.; Yang H. R.; Kim M. Y.; Moon C. S.; Kim H. S.; Wang P.; Liu A.; Hung N. N. Levels and Regional Trends of Persistent Organochlorines and Polybrominated Diphenyl Ethers in Asian Breast Milk Demonstrate POPs Signatures Unique to Individual Countries. Environ. Int. 2009, 35 (7), 1072–1079. 10.1016/j.envint.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Fujii Y.; Ito Y.; Harada K. H.; Hitomi T.; Koizumi A.; Haraguchi K. Regional Variation and Possible Sources of Brominated Contaminants in Breast Milk from Japan. Environ. Pollut. 2012, 162, 269–274. 10.1016/j.envpol.2011.11.022. [DOI] [PubMed] [Google Scholar]
- Eslami B.; Koizumi A.; Ohta S.; Inoue K.; Aozasa O.; Harada K.; Yoshinaga T.; Date C.; Fujii S.; Fujimine Y.; Hachiya N.; Hirosawa I.; Koda S.; Kusaka Y.; Murata K.; Nakatsuka H.; Omae K.; Saito N.; Shimbo S.; Takenaka K.; Takeshita T.; Todoriki H.; Wada Y.; Watanabe T.; Ikeda M. Large-Scale Evaluation of the Current Level of Polybrominated Diphenyl Ethers (PBDEs) in Breast Milk from 13 Regions of Japan. Chemosphere 2006, 63 (4), 554–561. 10.1016/j.chemosphere.2005.09.067. [DOI] [PubMed] [Google Scholar]
- Cui C.; Tian Y.; Zhang L.; Gao Y.; Jin J.; Wang P.; Ding W.; Wang X.; Shi R.; Wang Y. Polybrominated Diphenyl Ethers Exposure in Breast Milk in Shanghai, China: Levels, Influencing Factors and Potential Health Risk for Infants. Science of The Total Environment 2012, 433, 331–335. 10.1016/j.scitotenv.2012.06.075. [DOI] [PubMed] [Google Scholar]
- Chen Z. J.; Liu H. Y.; Cheng Z.; Man Y. B.; Zhang K. S.; Wei W.; Du J.; Wong M. H.; Wang H. S. Polybrominated Diphenyl Ethers (PBDEs) in Human Samples of Mother-Newborn Pairs in South China and Their Placental Transfer Characteristics. Environ. Int. 2014, 73, 77–84. 10.1016/j.envint.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Chen T.; Huang M.; Li J.; Li J.; Shi Z. Polybrominated Diphenyl Ethers and Novel Brominated Flame Retardants in Human Milk from the General Population in Beijing, China: Occurrence, Temporal Trends, Nursing Infants’ Exposure and Risk Assessment. Science of The Total Environment 2019, 689, 278–286. 10.1016/j.scitotenv.2019.06.442. [DOI] [PubMed] [Google Scholar]
- Chen M.-W.; Castillo B. A. A.; Lin D.-Y.; Chao H.-R.; Tayo L. L.; Gou Y.-Y.; Chen F.-A.; Huang K.-L. Levels of PCDD/Fs, PBDEs, and PBDD/Fs in Breast Milk from Southern Taiwan. Bull. Environ. Contam. Toxicol. 2018, 100, 369–375. 10.1007/s00128-018-2278-8. [DOI] [PubMed] [Google Scholar]
- Chao H. R.; Wang S. L.; Lee W. J.; Wang Y. F.; Päpke O. Levels of Polybrominated Diphenyl Ethers (PBDEs) in Breast Milk from Central Taiwan and Their Relation to Infant Birth Outcome and Maternal Menstruation Effects. Environ. Int. 2007, 33 (2), 239–245. 10.1016/j.envint.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Chao H.-R.; Tsou T.-C.; Huang H.-L.; Chang-Chien G.-P. Levels of Breast Milk PBDEs From Southern Taiwan and Their Potential Impact on Neurodevelopment. Pediatr. Res. 2011, 70 (6), 596–600. 10.1203/PDR.0b013e3182320b9b. [DOI] [PubMed] [Google Scholar]
- Chao H. A.; Chen S. C. C.; Chang C. M.; Koh T. W.; Chang-Chien G. P.; Ouyang E.; Lin S. L.; Shy C. G.; Chen F. A.; Chao H. R. Concentrations of Polybrominated Diphenyl Ethers in Breast Milk Correlated to Maternal Age, Education Level, and Occupational Exposure. J. Hazard Mater. 2010, 175 (1–3), 492–500. 10.1016/j.jhazmat.2009.10.032. [DOI] [PubMed] [Google Scholar]
- Bi X.; Qu W.; Sheng G.; Zhang W.; Mai B.; Chen D.; Yu L.; Fu J. Polybrominated Diphenyl Ethers in South China Maternal and Fetal Blood and Breast Milk. Environ. Pollut. 2006, 144 (3), 1024–1030. 10.1016/j.envpol.2005.12.056. [DOI] [PubMed] [Google Scholar]
- Akutsu K.; Kitagawa M.; Nakazawa H.; Makino T.; Iwazaki K.; Oda H.; Hori S. Time-Trend (1973–2000) of Polybrominated Diphenyl Ethers in Japanese Mother’s Milk. Chemosphere 2003, 53 (6), 645–654. 10.1016/S0045-6535(03)00764-1. [DOI] [PubMed] [Google Scholar]
- Fujii Y.; Kato Y.; Masuda N.; Harada K. H.; Koizumi A.; Haraguchi K. Contamination Trends and Factors Affecting the Transfer of Hexabromocyclododecane Diastereomers, Tetrabromobisphenol A, and 2,4,6-Tribromophenol to Breast Milk in Japan. Environ. Pollut. 2018, 237, 936–943. 10.1016/j.envpol.2018.03.015. [DOI] [PubMed] [Google Scholar]
- Devanathan G.; Subramanian A.; Sudaryanto A.; Takahashi S.; Isobe T.; Tanabe S. Brominated Flame Retardants and Polychlorinated Biphenyls in Human Breast Milk from Several Locations in India: Potential Contaminant Sources in a Municipal Dumping Site. Environ. Int. 2012, 39 (1), 87–95. 10.1016/j.envint.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Malarvannan G.; Isobe T.; Covaci A.; Prudente M.; Tanabe S. Accumulation of Brominated Flame Retardants and Polychlorinated Biphenyls in Human Breast Milk and Scalp Hair from the Philippines: Levels, Distribution and Profiles. Sci. Total Environ. 2013, 442, 366–379. 10.1016/j.scitotenv.2012.10.005. [DOI] [PubMed] [Google Scholar]
- Malarvannan G.; Kunisue T.; Isobe T.; Sudaryanto A.; Takahashi S.; Prudente M.; Subramanian A.; Tanabe S. Organohalogen Compounds in Human Breast Milk from Mothers Living in Payatas and Malate, the Philippines: Levels, Accumulation Kinetics and Infant Health Risk. Environ. Pollut. 2009, 157 (6), 1924–1932. 10.1016/j.envpol.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Tsai M. H.; Chao H. R.; Hsu W. L.; Tsai C. C.; Lin C. W.; Chen C. H. Analysis of Polybrominated Diphenyl Ethers and Lipid Composition in Human Breast Milk and Their Correlation with Infant Neurodevelopment. Int. J. Environ. Res. Public Health 2021, 18 (21), 11501. 10.3390/ijerph182111501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M.; Li J.; Xiao Z.; Shi Z. Tetrabromobisphenol A and Hexabromocyclododecane Isomers in Breast Milk from the General Population in Beijing, China: Contamination Levels, Temporal Trends, Nursing Infant’s Daily Intake, and Risk Assessment. Chemosphere 2020, 244, 125524 10.1016/j.chemosphere.2019.125524. [DOI] [PubMed] [Google Scholar]
- Kakimoto K.; Akutsu K.; Konishi Y.; Tanaka Y. Time Trend of Hexabromocyclododecane in the Breast Milk of Japanese Women. Chemosphere 2008, 71 (6), 1110–1114. 10.1016/j.chemosphere.2007.10.035. [DOI] [PubMed] [Google Scholar]
- Kakimoto K.; Akutsu K.; Konishi Y.; Toriba A.; Hayakawa K. Hexabromocyclododecane Levels in Foodstuff and Human Breast Milk in Osaka, Japan. Interdisciplinary Studies on Environmental Chemistry 2012, 6, 253–259. 10.1021/bk-2016-1244.ch006. [DOI] [Google Scholar]
- Lu S.; Tan Z.; Jiang Y.; Wu D.; Zhang J.; Zhou J.; Lin X. Hexabromocyclododecanes in Breast Milk from Residents in Shenzhen, China: Implications for Infant Exposure. Sci. Total Environ. 2018, 622–623, 1090–1097. 10.1016/j.scitotenv.2017.11.277. [DOI] [PubMed] [Google Scholar]
- Shi Z.; Wang Y.; Niu P.; Wang J.; Sun Z.; Zhang S.; Wu Y. Concurrent Extraction, Clean-up, and Analysis of Polybrominated Diphenyl Ethers, Hexabromocyclododecane Isomers, and Tetrabromobisphenol A in Human Milk and Serum. J. Sep. Sci. 2013, 36, 3402–3410. 10.1002/jssc.201300579. [DOI] [PubMed] [Google Scholar]
- Shi Z.; Zhang L.; Zhao Y.; Sun Z.; Zhou X.; Li J.; Wu Y. A National Survey of Tetrabromobisphenol-A, Hexabromocyclododecane and Decabrominated Diphenyl Ether in Human Milk from China: Occurrence and Exposure Assessment. Sci. Total Environ. 2017, 599–600, 237–245. 10.1016/j.scitotenv.2017.04.237. [DOI] [PubMed] [Google Scholar]
- Shi Z. X.; Wu Y. N.; Li J. G.; Zhao Y. F.; Feng J. F. Dietary Exposure Assessment of Chinese Adults and Nursing Infants to Tetrabromobisphenol-A and Hexabromocyclododecanes: Occurrence Measurements in Foods and Human Milk. Environ. Sci. Technol. 2009, 43 (12), 4314–4319. 10.1021/es8035626. [DOI] [PubMed] [Google Scholar]
- Shi Z.; Jiao Y.; Hu Y.; Sun Z.; Zhou X.; Feng J.; Li J.; Wu Y. Levels of Tetrabromobisphenol A, Hexabromocyclododecanes and Polybrominated Diphenyl Ethers in Human Milk from the General Population in Beijing, China. Sci. Total Environ. 2013, 452–453, 10–18. 10.1016/j.scitotenv.2013.02.038. [DOI] [PubMed] [Google Scholar]
- Souza M. C. O.; Devóz P. P.; Ximenez J. P. B.; Bocato M. Z.; Rocha B. A.; Barbosa F. Potential Health Risk to Brazilian Infants by Polybrominated Diphenyl Ethers Exposure via Breast Milk Intake. Int. J. Environ. Res. Public Health 2022, 19 (17), 11138. 10.3390/ijerph191711138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antignac J. P.; Main K. M.; Virtanen H. E.; Boquien C. Y.; Marchand P.; Venisseau A.; Guiffard I.; Bichon E.; Wohlfahrt-Veje C.; Legrand A.; Boscher C.; Skakkebæk N. E.; Toppari J.; Le Bizec B. Country-Specific Chemical Signatures of Persistent Organic Pollutants (POPs) in Breast Milk of French, Danish and Finnish Women. Environ. Pollut. 2016, 218, 728–738. 10.1016/j.envpol.2016.07.069. [DOI] [PubMed] [Google Scholar]
- Aune M.; Barregård L.; Claesson A.; Darnerud P. O.. Resultatrapport till Miljöövervakningen: Organiska Miljögifter i Bröstmjölk Från Göteborg 2001; Livsmedelsverket: 2002.
- Darnerud P. O.; Lignell S.; Aune M.; Isaksson M.; Cantillana T.; Redeby J.; Glynn A. Time Trends of Polybrominated Diphenylether (PBDE) Congeners in Serum of Swedish Mothers and Comparisons to Breast Milk Data. Environ. Res. 2015, 138, 352–360. 10.1016/j.envres.2015.02.031. [DOI] [PubMed] [Google Scholar]
- Eggesbø M.; Thomsen C.; Jørgensen J. V.; Becher G.; Øyvind Odland J.; Longnecker M. P. Associations between Brominated Flame Retardants in Human Milk and Thyroid-Stimulating Hormone (TSH) in Neonates. Environ. Res. 2011, 111 (6), 737–743. 10.1016/j.envres.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn A.; Lignell S.; Darnerud P. O.; Aune M.; Halldin Ankarberg E.; Bergdahl I. A.; Barregård L.; Bensryd I. Regional Differences in Levels of Chlorinated and Brominated Pollutants in Mother’s Milk from Primiparous Women in Sweden. Environ. Int. 2011, 37 (1), 71–79. 10.1016/j.envint.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Harrad S.; Abdallah M. A. E. Concentrations of Polybrominated Diphenyl Ethers, Hexabromocyclododecanes and Tetrabromobisphenol-A in Breast Milk from United Kingdom Women Do Not Decrease over Twelve Months of Lactation. Environ. Sci. Technol. 2015, 49 (23), 13899–13903. 10.1021/acs.est.5b00539. [DOI] [PubMed] [Google Scholar]
- Hoopmann M.; Albrecht U. V.; Gierden E.; Huppmann R.; Suchenwirth R. Time Trends and Individual Characteristics Associated with Polybrominated Diphenyl Ethers in Breast Milk Samples 2006–2009 in Lower Saxony, Germany. Int. J. Hyg Environ. Health 2012, 215 (3), 352–359. 10.1016/j.ijheh.2011.08.019. [DOI] [PubMed] [Google Scholar]
- Lignell S.; Aune M.; Darnerud P. O.; Cnattingius S.; Glynn A. Persistent Organochlorine and Organobromine Compounds in Mother’s Milk from Sweden 1996–2006: Compound-Specific Temporal Trends. Environ. Res. 2009, 109 (6), 760–767. 10.1016/j.envres.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Polder A.; Thomsen C.; Lindström G.; Løken K. B.; Skaare J. U. Levels and Temporal Trends of Chlorinated Pesticides, Polychlorinated Biphenyls and Brominated Flame Retardants in Individual Human Breast Milk Samples from Northern and Southern Norway. Chemosphere 2008, 73 (1), 14–23. 10.1016/j.chemosphere.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Polder A.; Gabrielsen G. W.; Odland J.; Savinova T. N.; Tkachev A.; Løken K. B.; Skaare J. U. Spatial and Temporal Changes of Chlorinated Pesticides, PCBs, Dioxins (PCDDs/PCDFs) and Brominated Flame Retardants in Human Breast Milk from Northern Russia. Sci. Total Environ. 2008, 391 (1), 41–54. 10.1016/j.scitotenv.2007.10.045. [DOI] [PubMed] [Google Scholar]
- Pratt I.; Anderson W.; Crowley D.; Daly S.; Evans R.; Fernandes A.; Fitzgerald M.; Geary M.; Keane D.; Morrison J. J.; Reilly A.; Tlustos C. Brominated and Fluorinated Organic Pollutants in the Breast Milk of First-Time Irish Mothers: Is There a Relationship to Levels in Food?. Food Addit Contam Part A Chem. Anal Control Expo Risk Assess 2013, 30 (10), 1788–1798. 10.1080/19440049.2013.822569. [DOI] [PubMed] [Google Scholar]
- Thomsen C.; Liane V. H.; Becher G. Automated Solid-Phase Extraction for the Determination of Polybrominated Diphenyl Ethers and Polychlorinated Biphenyls in Serum-Application on Archived Norwegian Samples from 1977 to 2003. J. Chromatogr B Analyt Technol. Biomed Life Sci. 2007, 846 (1–2), 252–263. 10.1016/j.jchromb.2006.09.011. [DOI] [PubMed] [Google Scholar]
- UNEP. Global monitoring report for Persistent Organic Pollutants. Third regional monitoring report Western Europe and Others Group (WEOG) region. https://chm.pops.int/implementation/globalmonitoringplan/monitoringreports/tabid/525/default.aspx (accessed 2023-03-30).
- UNEP. Global monitoring report for Persistent Organic Pollutants. Third regional monitoring report Central and Eastern European region. 2021. http://chm.pops.int/implementation/globalmonitoringplan/monitoringreports/tabid/525/default.aspx (accessed 2023-03-30).
- Čechová E.; Vojta Š.; Kukučka P.; Kočan A.; Trnovec T.; Murínová L. P.; de Cock M.; van de Bor M.; Askevold J.; Eggesbø M.; Scheringer M. Legacy and Alternative Halogenated Flame Retardants in Human Milk in Europe: Implications for Children’s Health. Environ. Int. 2017, 108, 137–145. 10.1016/j.envint.2017.08.008. [DOI] [PubMed] [Google Scholar]
- Fürst P. Dioxins, Polychlorinated Biphenyls and Other Organohalogen Compounds in Human Milk. Mol. Nutr Food Res. 2006, 50 (10), 922–933. 10.1002/mnfr.200600008. [DOI] [PubMed] [Google Scholar]
- Strandman T.; Koistinen J.; Vartiainen T. Polybrominated Flame Retardants (PBDEs) in Placenta and Human Milk. Organohalogen Compd. 2000, 47, 61–64. [Google Scholar]
- AMAP Assessment 2002 : The Influence of Global Change on Contaminant Pathways to within, and from the Arctic; Arctic Monitoring and Assessment Programme, 2003.
- Zeilmaker M.; Moermond C.; Brandon E.; Hoogerhuis P.; Razenberg L.; Janssen M.. Persistent Organic Pollutants in Human Milk RIVM Letter Report 2020–0025; Rijksinstituut voor Volksgezondheid en Milieu RIVM, 2020, 10.21945/RIVM-2020-0025. [DOI]
- Weber H.; Heseker H. Analysis of Polybrominated Diphenyl Ethers in Breast Milk of German Mothers - Results of a Pilot Study. Fresenius Environmental Bulletin and Advances in Food Sciences 2004, 13 (4), 356–360. [Google Scholar]
- Thomsen C.; Haug L. S.; Stigum H.; Frøshaug M.; Broadwell S. L.; Becher G. Changes in Concentrations of Perfluorinated Compounds, Polybrominated Diphenyl Ethers, and Polychlorinated Biphenyls in Norwegian Breast-Milk during Twelve Months of Lactation. Environ. Sci. Technol. 2010, 44, 9550–9556. 10.1021/es1021922. [DOI] [PubMed] [Google Scholar]
- Sahlström L. M. O.; Sellström U.; de Wit C. A.; Lignell S.; Darnerud P. O. Estimated Intakes of Brominated Flame Retardants via Diet and Dust Compared to Internal Concentrations in a Swedish Mother-Toddler Cohort. Int. J. Hyg Environ. Health 2015, 218 (4), 422–432. 10.1016/j.ijheh.2015.03.011. [DOI] [PubMed] [Google Scholar]
- Raab U.; Preiss U.; Albrecht M.; Shahin N.; Parlar H.; Fromme H. Concentrations of Polybrominated Diphenyl Ethers, Organochlorine Compounds and Nitro Musks in Mother’s Milk from Germany (Bavaria). Chemosphere 2008, 72 (1), 87–94. 10.1016/j.chemosphere.2008.01.053. [DOI] [PubMed] [Google Scholar]
- Ozcan S.; Tor A.; Aydin M. E. Levels of Organohalogenated Pollutants in Human Milk Samples from Konya City, Turkey. Clean (Weinheim) 2011, 39 (10), 978–983. 10.1002/clen.201100068. [DOI] [Google Scholar]
- Meironyté Guvenius D.; Aronsson A.; Ekman-Ordeberg G.; Bergman Å.; Norén K. Human Prenatal and Postnatal Exposure to Polybrominated Diphenyl Ethers, Polychlorinated Biphenyls, Polychlorobiphenylols, and Pentachlorophenol. Environ. Health Perspect 2003, 111 (9), 1235–1241. 10.1289/ehp.5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Main K. M.; Kiviranta H.; Virtanen H. E.; Sundqvist E.; Tuomisto J. T.; Tuomisto J.; Vartiainen T.; Skakkebæk N. E.; Toppari J. Flame Retardants in Placenta and Breast Milk and Cryptorchildism in Newborn Boys. Environ. Health Perspect 2007, 115 (10), 1519–1526. 10.1289/ehp.9924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lignell S.; Aune M.; Darnerud P. O.; Stridsberg M.; Hanberg A.; Larsson S. C; Glynn A. Maternal Body Burdens of PCDD/Fs and PBDEs Are Associated with Maternal Serum Levels of Thyroid Hormones in Early Pregnancy: A Cross-Sectional Study. Environmental Health 2016, 15, 1–11. 10.1186/s12940-016-0139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z.-M.; Albrecht M.; Fromme H.; Schramm K.-W.; De Angelis M. Persistent Organic Pollutants in Human Breast Milk and Associations with Maternal Thyroid Hormone Homeostasis. Environ. Sci. Technol. 2020, 54, 1111–1119. 10.1021/acs.est.9b06054. [DOI] [PubMed] [Google Scholar]
- Lacorte S.; Ikonomou M. G. Occurrence and Congener Specific Profiles of Polybrominated Diphenyl Ethers and Their Hydroxylated and Methoxylated Derivatives in Breast Milk from Catalonia. Chemosphere 2009, 74 (3), 412–420. 10.1016/j.chemosphere.2008.09.050. [DOI] [PubMed] [Google Scholar]
- Kazda R.; Hajšlová J.; Poustka J.; Čajka T. Determination of Polybrominated Diphenyl Ethers in Human Milk Samples in the Czech Republic: Comparative Study of Negative Chemical Ionisation Mass Spectrometry and Time-of-Flight High-Resolution Mass Spectrometry. Anal. Chim. Acta 2004, 520 (1–2), 237–243. 10.1016/j.aca.2004.04.069. [DOI] [Google Scholar]
- Kalantzi O. I.; Martin F. L.; Thomas G. O.; Alcock R. E.; Tang H. R.; Drury S. C.; Carmichael P. L.; Nicholson J. K.; Jones K. C. Different Levels of Polybrominated Diphenyl Ethers (PBDEs) and Chlorinated Compounds in Breast Milk from Two U.K. Regions. Environ. Health Perspect 2004, 112 (10), 1085–1091. 10.1289/ehp.6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaraczewska K.; Lulek J.; Covaci A.; Voorspoels S.; Kaluba-Skotarczak A.; Drews K.; Schepens P. Distribution of Polychlorinated Biphenyls, Organochlorine Pesticides and Polybrominated Diphenyl Ethers in Human Umbilical Cord Serum, Maternal Serum and Milk from Wielkopolska Region, Poland. Sci. Total Environ. 2006, 372 (1), 20–31. 10.1016/j.scitotenv.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Jakobsson K., Athanasiadou M., Christiansson A., Athanassiadis I., Bergman Å, Hagmar L.. Polybrominated diphenyl ethers (PBDEs) in serum from Swedish men 1988–2002 : A longitudinal study. Lunds universitet, 2005, https://www.diva-portal.org/smash/record.jsf?pid=diva2%3A657721&dswid=-8348.
- Ingelido A. M.; Ballard T.; Dellatte E.; di Domenico A.; Ferri F.; Fulgenzi A. R.; Herrmann T.; Iacovella N.; Miniero R.; Päpke O.; Porpora M. G.; Felip E. De. Polychlorinated Biphenyls (PCBs) and Polybrominated Diphenyl Ethers (PBDEs) in Milk from Italian Women Living in Rome and Venice. Chemosphere 2007, 67 (9), S301–S306. 10.1016/j.chemosphere.2006.05.111. [DOI] [PubMed] [Google Scholar]
- Hernik A.; Struciński P.; Buckley B. T.; Góralczyk K.; Czaja K.; Korcz W.; Matuszak M.; Łyczewska M.; Minorczyk M.; Liszewska M.; Ludwicki J. K. Relationship between Paired Cord Blood and Milk POPs Levels as a Tool for Assessing Perinatal Exposure, a Pilot Study. Human and Ecological Risk Assessment: An International Journal 2016, 22 (7), 1456–1468. 10.1080/10807039.2016.1185688. [DOI] [Google Scholar]
- Hernik A.; Goralczyk K.; Strucinski P.; Czaja K.; Kucharska A.; Korcz W.; Snopczynski T.; Ludwicki J. K. Polybrominated Diphenyl Ethers, Polychlorinated Biphenyls and Organochlorine Pesticides in Human Milok as Markers of Environmental Exposure to These Compounds. Ann. Agric. Environ. Med. 2011, 18 (1), 113–118. [PubMed] [Google Scholar]
- Gómara B.; Herrero L.; Pacepavicius G.; Ohta S.; Alaee M.; González M. J. Occurrence of Co-Planar Polybrominated/Chlorinated Biphenyls (PXBs), Polybrominated Diphenyl Ethers (PBDEs) and Polychlorinated Biphenyls (PCBs) in Breast Milk of Women from Spain. Chemosphere 2011, 83 (6), 799–805. 10.1016/j.chemosphere.2011.02.080. [DOI] [PubMed] [Google Scholar]
- Gómara B.; Herrero L.; Ramos J. J.; Mateo J. R.; Fernandez M. A.; Garcia J. F.; Gonzalez M. J. Distribution of Polybrominated Diphenyl Ethers in Human Umbilical Cord Serum, Paternal Serum, Maternal Serum, Placentas, and Breast Milk from Madrid Population, Spain. Environ. Sci. Technol. 2007, 41 (20), 6961–6968. 10.1021/es0714484. [DOI] [PubMed] [Google Scholar]
- Fängström B.; Strid A.; Grandjean P.; Weihe P.; Bergman Å. Environmental Health: A Global Access Science Source A Retrospective Study of PBDEs and PCBs in Human Milk from the Faroe Islands. Environmental health 2005, 4, 1–9. 10.1186/1476-069X-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fängström B.; Athanassiadis I.; Odsjö T.; Norøn K.; Bergman Š. Temporal Trends of Polybrominated Diphenyl Ethers and Hexabromocyclododecane in Milk from Stockholm Mothers, 1980–2004. Mol. Nutr. Food Res. 2008, 52, 187–193. 10.1002/mnfr.200700182. [DOI] [PubMed] [Google Scholar]
- Erdoǧrul Ö.; Covaci A.; Kurtul N.; Schepens P. Levels of Organohalogenated Persistent Pollutants in Human Milk from Kahramanmaraş Region, Turkey. Environ. Int. 2004, 30 (5), 659–666. 10.1016/j.envint.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Dimitriadou L.; Malarvannan G.; Covaci A.; Iossifidou E.; Tzafettas J.; Zournatzi-Koiou V.; Kalantzi O. I. Levels and Profiles of Brominated and Chlorinated Contaminants in Human Breast Milk from Thessaloniki, Greece. Sci. Total Environ. 2016, 539, 350–358. 10.1016/j.scitotenv.2015.08.137. [DOI] [PubMed] [Google Scholar]
- Croes K.; Colles A.; Koppen G.; Govarts E.; Bruckers L.; Van de Mieroop E.; Nelen V.; Covaci A.; Dirtu A. C.; Thomsen C.; Haug L. S.; Becher G.; Mampaey M.; Schoeters G.; Van Larebeke N.; Baeyens W. Persistent Organic Pollutants (POPs) in Human Milk: A Biomonitoring Study in Rural Areas of Flanders (Belgium). Chemosphere 2012, 89 (8), 988–994. 10.1016/j.chemosphere.2012.06.058. [DOI] [PubMed] [Google Scholar]
- Colles A.; Koppen G.; Hanot V.; Nelen V.; Dewolf M. C.; Noël E.; Malisch R.; Kotz A.; Kypke K.; Biot P.; Vinkx C.; Schoeters G. Fourth WHO-Coordinated Survey of Human Milk for Persistent Organic Pollutants (POPs): Belgian Results. Chemosphere 2008, 73 (6), 907–914. 10.1016/j.chemosphere.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Chovancová J.; Čonka K.; Kočan A.; Sejáková Z. S. PCDD, PCDF, PCB and PBDE Concentrations in Breast Milk of Mothers Residing in Selected Areas of Slovakia. Chemosphere 2011, 83 (10), 1383–1390. 10.1016/j.chemosphere.2011.02.070. [DOI] [PubMed] [Google Scholar]
- Bramwell L.; Fernandes A.; Rose M.; Harrad S.; Pless-Mulloli T. PBDEs and PBBs in Human Serum and Breast Milk from Cohabiting UK Couples. Chemosphere 2014, 116, 67–74. 10.1016/j.chemosphere.2014.03.060. [DOI] [PubMed] [Google Scholar]
- Bordajandi L. R.; Abad E.; González M. J. Occurrence of PCBs, PCDD/Fs, PBDEs and DDTs in Spanish Breast Milk: Enantiomeric Fraction of Chiral PCBs. Chemosphere 2008, 70 (4), 567–575. 10.1016/j.chemosphere.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Björklund J. A.; Sellström U.; de Wit C. A.; Aune M.; Lignell S.; Darnerud P. O. Comparisons of Polybrominated Diphenyl Ether and Hexabromocyclododecane Concentrations in Dust Collected with Two Sampling Methods and Matched Breast Milk Samples. Indoor Air 2012, 22 (4), 279–288. 10.1111/j.1600-0668.2011.00765.x. [DOI] [PubMed] [Google Scholar]
- Bergman Å.; Hovander L.; Sundström M.; Athanassiadis I.; Athanasiadou M.; Sällsten G.; Bignert A.; Nyberg E. Insamling Och Kemisk Analys Av Miljöföroreningar i Svensk Modersmjölk; Resultat från 2008–2010. Rapport till Naturvårdsverket 2010–11–24 2008, 20. [Google Scholar]
- Athanasiadou M.; Bergman Å.. Insamling Av Bröstmjölksprover Från Stockholm, Göteborg, Lund Och Umeå Samt Analyser Av Insamlade Bröstmjölksprover Resultat Från 2007 Års Arbete; Department of Environmental Chemistry, Stockholm University: 2008.
- Alivernini S.; Battistelli C. L.; Turrio-Baldassarri L. Human Milk as a Vector and an Indicator of Exposure to PCBs and PBDEs: Temporal Trend of Samples Collected in Rome. Bull. Environ. Contam. Toxicol. 2011, 87, 21–25. 10.1007/s00128-011-0262-7. [DOI] [PubMed] [Google Scholar]
- Abdallah M. A. E.; Harrad S. Polybrominated Diphenyl Ethers in UK Human Milk: Implications for Infant Exposure and Relationship to External Exposure. Environ. Int. 2014, 63, 130–136. 10.1016/j.envint.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Desalegn A. A.; Collet B.; Iszatt N.; Stigum H.; Jensen T. K.; Jonker L.; Besselink H.; van der Burg B.; Eggesbø M. Aryl Hydrocarbon Receptor Activity in Human Breast Milk and Cryptorchidism: A Case-Control Study within the Prospective Norwegian HUMIS Cohort. Environ. Res. 2022, 214, 113861 10.1016/j.envres.2022.113861. [DOI] [PubMed] [Google Scholar]
- Fromme H.; Fuchs V.; Albrecht M.; Aschenbrenner B.; Röhl C.; Janitzki N.; Herber-Jonat S.; Wöckner M.; Völkel W.; Flemmer A. W.; Schober W. Polychlorinated Dioxins and Dibenzofurans (PCDD/F), Polybrominated Dioxins and Dibenzofurans (PBDD/F), Polychlorinated Biphenyls (PCB), Polybrominated Diphenyl Ethers (PBDE), and per- and Polyfluoroalkyl Substances (PFAS) in German Breast Milk Samples (LUPE 8). Sci. Total Environ. 2022, 825, 154066 10.1016/j.scitotenv.2022.154066. [DOI] [PubMed] [Google Scholar]
- Rovira J.; Martínez M. Á.; Mari M.; Cunha S. C.; Fernandes J. O.; Marmelo I.; Marques A.; Haug L. S.; Thomsen C.; Nadal M.; Domingo J. L.; Schuhmacher M. Mixture of Environmental Pollutants in Breast Milk from a Spanish Cohort of Nursing Mothers. Environ. Int. 2022, 166, 107375 10.1016/j.envint.2022.107375. [DOI] [PubMed] [Google Scholar]
- Roosens L.; D’Hollander W.; Bervoets L.; Reynders H.; Van Campenhout K.; Cornelis C.; Van Den Heuvel R.; Koppen G.; Covaci A. Brominated Flame Retardants and Perfluorinated Chemicals, Two Groups of Persistent Contaminants in Belgian Human Blood and Milk. Environ. Pollut. 2010, 158 (8), 2546–2552. 10.1016/j.envpol.2010.05.022. [DOI] [PubMed] [Google Scholar]
- Stoffmonographie Für 1,2,5,6,9,10-Hexabromcyclododecan (HBCDD) - HBM-Werte Für HBCDD Im Fettanteil Der Muttermilch Oder Des Blutplasmas. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2015, 58, 889–907, 10.1007/s00103-015-2193-7. [DOI] [PubMed] [Google Scholar]
- Lankova D.; Lacina O.; Pulkrabova J.; Hajslova J. The Determination of Perfluoroalkyl Substances, Brominated Flame Retardants and Their Metabolites in Human Breast Milk and Infant Formula. Talanta 2013, 117, 318–325. 10.1016/j.talanta.2013.08.040. [DOI] [PubMed] [Google Scholar]
- Thomsen C.; Stigum H.; Frøshaug M.; Broadwell S. L.; Becher G.; Eggesbø M. Determinants of Brominated Flame Retardants in Breast Milk from a Large Scale Norwegian Study. Environ. Int. 2010, 36 (1), 68–74. 10.1016/j.envint.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Butryn D. M.; Gross M. S.; Chi L. H.; Schecter A.; Olson J. R.; Aga D. S. One-Shot” Analysis of Polybrominated Diphenyl Ethers and Their Hydroxylated and Methoxylated Analogs in Human Breast Milk and Serum Using Gas Chromatography-Tandem Mass Spectrometry. Anal. Chim. Acta 2015, 892, 140–147. 10.1016/j.aca.2015.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butryn D. M.; Chi L. H.; Gross M. S.; McGarrigle B.; Schecter A.; Olson J. R.; Aga D. S. Retention of Polybrominated Diphenyl Ethers and Hydroxylated Metabolites in Paired Human Serum and Milk in Relation to CYP2B6 Genotype. J. Hazard Mater. 2020, 386, 121904 10.1016/j.jhazmat.2019.121904. [DOI] [PubMed] [Google Scholar]
- Daniels J. L.; Pan I. J.; Jones R.; Anderson S.; Patterson D. G.; Needham L. L.; Sjödin A. Individual Characteristics Associated with PBDE Levels in U.S. Human Milk Samples. Environ. Health Perspect 2010, 118 (1), 155–160. 10.1289/ehp.0900759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn R. L.; Huwe J. K.; Carey G. B. Biomonitoring Polybrominated Diphenyl Ethers in Human Milk as a Function of Environment, Dietary Intake, and Demographics in New Hampshire. Chemosphere 2010, 80 (10), 1175–1182. 10.1016/j.chemosphere.2010.06.017. [DOI] [PubMed] [Google Scholar]
- Guo W.; Holden A.; Smith S. C.; Gephart R.; Petreas M.; Park J. S. PBDE Levels in Breast Milk Are Decreasing in California. Chemosphere 2016, 150, 505–513. 10.1016/j.chemosphere.2015.11.032. [DOI] [PubMed] [Google Scholar]
- Hartle J. C.; Cohen R. S.; Sakamoto P.; Barr D. B.; Carmichael S. L. Chemical Contaminants in Raw and Pasteurized Human Milk. Journal of Human Lactation 2018, 34 (2), 340–349. 10.1177/0890334418759308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K.; Adgent M.; Goldman B. D.; Sjödin A.; Daniels J. L. Lactational Exposure to Polybrominated Diphenyl Ethers and Its Relation to Social and Emotional Development among Toddlers. Environ. Health Perspect 2012, 120 (10), 1438–1442. 10.1289/ehp.1205100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K.; Mendez M.; Siega-Riz A. M.; Herring A. H.; Sjödin A.; Daniels J. L. Lactational Exposure to Polybrominated Diphenyl Ethers and Its Relation to Early Childhood Anthropometric Measurements. Environ. Health Perspect 2016, 124 (10), 1656–1661. 10.1289/EHP201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Restrepo B.; Addink R.; Wong C.; Arcaro K.; Kannan K. Polybrominated Diphenyl Ethers and Organochlorine Pesticides in Human Breast Milk from Massachusetts, USA. Journal of Environmental Monitoring 2007, 9, 1205–1212. 10.1039/b711409p. [DOI] [PubMed] [Google Scholar]
- LaKind J. S.; Berlin C. M.; Sjödin A.; Turner W.; Wang R. Y.; Needham L. L.; Paul I. M.; Stokes J. L.; Naiman D. Q.; Patterson D. G. Do Human Milk Concentrations of Persistent Organic Chemicals Really Decline during Lactation? Chemical Concentrations during Lactation and Milk/Serum Partitioning. Environ. Health Perspect 2009, 117 (10), 1625–1631. 10.1289/ehp.0900876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchitti S. A.; Fenton S. E.; Mendola P.; Kenneke J. F.; Hines E. P. Polybrominated Diphenyl Ethers in Human Milk and Serum from the U.S. EPA MAMA Study: Modeled Predictions of Infant Exposure and Considerations for Risk Assessment. Environ. Health Perspect 2017, 125 (4), 706–713. 10.1289/EHP332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecter A.; Pavuk M.; Päpke O.; Ryan J. J.; Birnbaum L.; Rosen R. Polybrominated Diphenyl Ethers (PBDEs) in U.S. Mothers’ Milk. Environ. Health Perspect 2003, 111 (14), 1723–1729. 10.1289/ehp.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She J.; Holden A.; Sharp M.; Tanner M.; Williams-Derry C.; Hooper K. Polybrominated Diphenyl Ethers (PBDEs) and Polychlorinated Biphenyls (PCBs) in Breast Milk from the Pacific Northwest. Chemosphere 2007, 67 (9), S307. 10.1016/j.chemosphere.2006.05.154. [DOI] [PubMed] [Google Scholar]
- Sjödin A.; Jones R. S.; Focant J. F.; Lapeza C.; Wang R. Y.; McGahee E. E.; Zhang Y.; Turner W. E.; Slazyk B.; Needham L. L.; Patterson D. G. Retrospective Time-Trend Study of Polybrominated Diphenyl Ether and Polybrominated and Polychlorinated Biphenyl Levels in Human Serum from the United States. Environ. Health Perspect 2004, 112 (6), 654–658. 10.1289/ehp.6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S. N.; Buchar A.; Siddique S.; Takser L.; Abdelouahab N.; Zhu J. Measurements of Selected Brominated Flame Retardants in Nursing Women: Implications for Human Exposure. Environ. Sci. Technol. 2014, 48, 8873–8880. 10.1021/es5016839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu N.; Herrmann T.; Paepke O.; Tickner J.; Hale R.; Harvey E.; La Guardia M.; McClean M. D.; Webster T. F. Human Exposure to PBDEs: Associations of PBDE Body Burdens with Food Consumption and House Dust Concentrations. Environ. Sci. Technol. 2007, 41 (5), 1584–1589. 10.1021/es0620282. [DOI] [PubMed] [Google Scholar]
- Park J.-S.; She J.; Holden A.; Sharp M.; Gephart R.; Souders-Mason G.; Zhang V.; Chow J.; Leslie B.; Hooper K. High Postnatal Exposures to Polybrominated Diphenyl Ethers (PBDEs) and Polychlorinated Biphenyls (PCBs) via Breast Milk in California: Does BDE-209 Transfer to Breast Milk?. Environ. Sci. Technol. 2011, 45, 4579–4585. 10.1021/es103881n. [DOI] [PubMed] [Google Scholar]
- Martínez C.; Martínez Arroyo A.; Barrientos Alemán D.; Gavilán García A.; Caba M.; Calderón Garcidueñas A. L.; Mora A.; Zenteno E. Persistent Organic Compounds in Human Milk and Evaluation of the Effectiveness of the Stockholm Convention in Mexico. Environmental Advances 2022, 8, 100190 10.1016/j.envadv.2022.100190. [DOI] [Google Scholar]
- Mannetje A.; Coakley J.; Bridgen P.; Brooks C.; Harrad S.; Smith A. H.; Pearce N.; Douwes J. Current Concentrations, Temporal Trends and Determinants of Persistent Organic Pollutants in Breast Milk of New Zealand Women. Sci. Total Environ. 2013, 458–460, 399–407. 10.1016/j.scitotenv.2013.04.055. [DOI] [PubMed] [Google Scholar]
- Coakley J. D.; Harrad S. J.; Goosey E.; Ali N.; Dirtu A. C.; Van den Eede N.; Covaci A.; Douwes J.; Mannetje A. Concentrations of Polybrominated Diphenyl Ethers in Matched Samples of Indoor Dust and Breast Milk in New Zealand. Environ. Int. 2013, 59, 255–261. 10.1016/j.envint.2013.06.020. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Wang X.; Li Y.; Toms L. M. L.; Gallen M.; Hearn L.; Aylward L. L.; McLachlan M. S.; Sly P. D.; Mueller J. F. Persistent Organic Pollutants in Matched Breast Milk and Infant Faeces Samples. Chemosphere 2015, 118 (1), 309–314. 10.1016/j.chemosphere.2014.09.076. [DOI] [PubMed] [Google Scholar]
- Toms L. M. L.; Guerra P.; Eljarrat E.; Barceló D.; Harden F. A.; Hobson P.; Sjodin A.; Ryan E.; Mueller J. F. Brominated Flame Retardants in the Australian Population: 1993–2009. Chemosphere 2012, 89 (4), 398–403. 10.1016/j.chemosphere.2012.05.053. [DOI] [PubMed] [Google Scholar]
- Toms L. M. L.; Hearn L.; Kennedy K.; Harden F.; Bartkow M.; Temme C.; Mueller J. F. Concentrations of Polybrominated Diphenyl Ethers (PBDEs) in Matched Samples of Human Milk, Dust and Indoor Air. Environ. Int. 2009, 35 (6), 864–869. 10.1016/j.envint.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Toms L. M. L.; Harden F. A.; Symons R. K.; Burniston D.; Fürst P.; Müller J. F. Polybrominated Diphenyl Ethers (PBDEs) in Human Milk from Australia. Chemosphere 2007, 68 (5), 797–803. 10.1016/j.chemosphere.2007.02.059. [DOI] [PubMed] [Google Scholar]
- Hites R. A. Break Point Analyses of Human or Environmental Temporal Trends of POPs. Science of The Total Environment 2019, 664, 518–521. 10.1016/j.scitotenv.2019.01.353. [DOI] [PubMed] [Google Scholar]
- Heck K. E.; Braveman P.; Cubbin C.; Chávez G. F.; Kiely J. L. Socioeconomic Status and Breastfeeding Initiation among California Mothers. Public Health Reports 2006, 121 (1), 51–59. 10.1177/003335490612100111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao H. A.; Chen S. C. C.; Chang C. M.; Koh T. W.; Chang-Chien G. P.; Ouyang E.; Lin S. L.; Shy C. G.; Chen F. A.; Chao H. R. Concentrations of Polybrominated Diphenyl Ethers in Breast Milk Correlated to Maternal Age, Education Level, and Occupational Exposure. J. Hazard Mater. 2010, 175 (1–3), 492–500. 10.1016/j.jhazmat.2009.10.032. [DOI] [PubMed] [Google Scholar]
- Malisch K.; Malisch R.; Fiedler H.. Guidelines for Organization, Sampling and Analysis of Human Milk on Persistent Organic Pollutants. Chemicals Branch Division of Technology, Industry and Economics (DTIE) United Nations Environment Programme (UNEP) 2017, 39. [Google Scholar]
- Hooper K.; She J.; Sharp M.; Chow J.; Jewell N.; Gephart R.; Holden A. Depuration of Polybrominated Diphenyl Ethers (PBDEs) and Polychlorinated Biphenyls (PCBs) in Breast Milk from California First-Time Mothers (Primiparae). Environ. Health Perspect 2007, 115 (9), 1271–1275. 10.1289/ehp.10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L.; Hoang C.; Arnot J. A.; Wania F. Clarifying Temporal Trend Variability in Human Biomonitoring of Polybrominated Diphenyl Ethers through Mechanistic Modeling. Environ. Sci. Technol. 2020, 54, 166–175. 10.1021/acs.est.9b04130. [DOI] [PubMed] [Google Scholar]
- Drage D. S.; Harden F. A.; Jeffery T.; Mueller J. F.; Hobson P.; Toms L. M. L. Human Biomonitoring in Australian Children: Brominated Flame Retardants Decrease from 2006 to 2015. Environ. Int. 2019, 122, 363–368. 10.1016/j.envint.2018.11.044. [DOI] [PubMed] [Google Scholar]
- Wong F.; Cousins I. T.; MacLeod M. Bounding Uncertainties in Intrinsic Human Elimination Half-Lives and Intake of Polybrominated Diphenyl Ethers in the North American Population. Environ. Int. 2013, 59, 168–174. 10.1016/j.envint.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Trudel D.; Scheringer M.; von Goetz N.; Hungerbuhler K. Total Consumer Exposure to Polybrominated Diphenyl Ethers in North America and Europe. Environ. Sci. Technol. 2011, 45, 2391–2397. 10.1021/es1035046. [DOI] [PubMed] [Google Scholar]
- McGrath T. J.; Morrison P. D.; Ball A. S.; Clarke B. O. Concentrations of Legacy and Novel Brominated Flame Retardants in Indoor Dust in Melbourne, Australia: An Assessment of Human Exposure. Environ. Int. 2018, 113, 191–201. 10.1016/j.envint.2018.01.026. [DOI] [PubMed] [Google Scholar]
- Pietroń W. J.; Małagocki P. Quantification of Polybrominated Diphenyl Ethers (PBDEs) in Food. A Review. Talanta 2017, 167, 411–427. 10.1016/j.talanta.2017.02.043. [DOI] [PubMed] [Google Scholar]
- Stapleton H. M. Instrumental Methods and Challenges in Quantifying Polybrominated Diphenyl Ethers in Environmental Extracts: A Review. Anal. Bioanal. Chem. 2006, 386, 807–817. 10.1007/s00216-006-0400-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Department of State. Stockholm Convention on Persistent Organic Pollutants. https://www.state.gov/key-topics-office-of-environmental-quality-and-transboundary-issues/stockholm-convention-on-persistent-organic-pollutants/ (accessed 2022-12-05).
- Undeman E.; Johansson J. Polybrominated diphenyl ethers (PBDEs) in the Baltic Sea – Sources, transport routes and trends. Helcom Baltic Sea Environment Proceedings 2020, 172, 1–22. [Google Scholar]
- The 16 New POPs. In Stockholm Convention on Persistent Organic Pollutants (POPs), UN Environment; Stockholm Convention Secretariat United Nations Environment, 2017; pp 1–25.
- UNEP. Eighth Meeting of the Conference of the Parties to the Stockholm Convention. http://chm.pops.int/theconvention/conferenceoftheparties/meetings/cop8/tabid/5309/default.aspx (accessed 2023-01-06).
- Thuresson K.; Höglund P.; Hagmar L.; Sjödin A.; Bergman Å.; Jakobsson K. Apparent Half-Lives of Hepta- to Decabrominated Diphenyl Ethers in Human Serum as Determined in Occupationally Exposed Workers. Environ. Health Perspect 2006, 114 (2), 176–181. 10.1289/ehp.8350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalachova K.; Hradkova P.; Lankova D.; Hajslova J.; Pulkrabova J. Occurrence of Brominated Flame Retardants in Household and Car Dust from the Czech Republic. Sci. Total Environ. 2012, 441, 182–193. 10.1016/j.scitotenv.2012.09.061. [DOI] [PubMed] [Google Scholar]
- Guerra P.; Alaee M.; Jiménez B.; Pacepavicius G.; Marvin C.; MacInnis G.; Eljarrat E.; Barceló D.; Champoux L.; Fernie K. Emerging and Historical Brominated Flame Retardants in Peregrine Falcon (Falco Peregrinus) Eggs from Canada and Spain. Environ. Int. 2012, 40 (1), 179–186. 10.1016/j.envint.2011.07.014. [DOI] [PubMed] [Google Scholar]
- Dodson R. E.; Van Den Eede N.; Covaci A.; Perovich L. J.; Brody J. G.; Rudel R. A. Urinary Biomonitoring of Phosphate Flame Retardants: Levels in California Adults and Recommendations for Future Studies. Environ. Sci. Technol. 2014, 48 (23), 13625–13633. 10.1021/es503445c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X.; Bennett D. H.; Moran R. E.; Sjödin A.; Jones R. S.; Tancredi D. J.; Tulve N. S.; Clifton M. S.; Colón M.; Weathers W.; Hertz-Picciotto I.. Polybrominated Diphenyl Ether Serum Concentrations in a Californian Population of Children, Their Parents, and Older Adults: An Exposure Assessment Study. Environ. Health 2015, 14 ( (1), ), 10.1186/s12940-015-0002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry E.; Zota A. R.; Park J. S.; Woodruff T. J. Polybrominated Diphenyl Ethers (PBDEs) and Hydroxylated PBDE Metabolites (OH-PBDEs): A Six-Year Temporal Trend in Northern California Pregnant Women. Chemosphere 2018, 195, 777–783. 10.1016/j.chemosphere.2017.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey M.; Harrad S.; Abou-Elwafa Abdallah M.; Drage D. S.; Berresheim H. Phasing-out of Legacy Brominated Flame Retardants: The UNEP Stockholm Convention and Other Legislative Action Worldwide. Environ. Int. 2020, 144, 106041 10.1016/j.envint.2020.106041. [DOI] [PubMed] [Google Scholar]
- Significant New Use and Test Rules: Certain Polybrominated Diphenylethers. Federal Register 2012, 77 (63), 19862–19899. [Google Scholar]
- Decabromodiphenyl Ether (DecaBDE); Regulation of Persistent, Bioaccumulative, and Toxic Chemicals Under TSCA Section 6(h). Federal Register 2021, 86 (3), 880–894. [Google Scholar]
- Interim Public Health Risk Assessment of Certain PBDE Congeners 2007, 62. [Google Scholar]
- Li L.; Wania F. Elucidating the Variability in the Hexabromocyclododecane Diastereomer Profile in the Global Environment. Environ. Sci. Technol. 2018, 52 (18), 10532–10542. 10.1021/acs.est.8b03443. [DOI] [PubMed] [Google Scholar]
- Ali N.; Dirtu A. C.; Eede N. V. d.; Goosey E.; Harrad S.; Neels H.; Mannetje A.; Coakley J.; Douwes J.; Covaci A. Occurrence of Alternative Flame Retardants in Indoor Dust from New Zealand: Indoor Sources and Human Exposure Assessment. Chemosphere 2012, 88 (11), 1276–1282. 10.1016/j.chemosphere.2012.03.100. [DOI] [PubMed] [Google Scholar]
- Hennebert P. Concentrations of Brominated Flame Retardants in Plastics of Electrical and Electronic Equipment, Vehicles, Construction, Textiles and Non-Food Packaging: A Review of Occurrence and Management. Detritus 2020, 12, 34–50. 10.31025/2611-4135/2020.13997. [DOI] [Google Scholar]
- Drage D. S.; Sharkey M.; Abdallah M. A.-E.; Berresheim H.; Harrad S. Brominated Flame Retardants in Irish Waste Polymers: Concentrations, Legislative Compliance, and Treatment Options. Sci. Total Environ. 2018, 625, 1535–1543. 10.1016/j.scitotenv.2018.01.076. [DOI] [PubMed] [Google Scholar]
- Samsonek J.; Puype F. Occurrence of Brominated Flame Retardants in Black Thermo Cups and Selected Kitchen Utensils Purchased on the European Market. Food Additives & Contaminants: Part A 2013, 30 (11), 1976–1986. 10.1080/19440049.2013.829246. [DOI] [PubMed] [Google Scholar]
- Kuang J.; Abdallah M. A.-E.; Harrad S. Brominated Flame Retardants in Black Plastic Kitchen Utensils: Concentrations and Human Exposure Implications. Sci. Total Environ. 2018, 610–611, 1138–1146. 10.1016/j.scitotenv.2017.08.173. [DOI] [PubMed] [Google Scholar]
- Ionas A. C.; Dirtu A. C.; Anthonissen T.; Neels H.; Covaci A. Downsides of the Recycling Process: Harmful Organic Chemicals in Children’s Toys. Environ. Int. 2014, 65, 54–62. 10.1016/j.envint.2013.12.019. [DOI] [PubMed] [Google Scholar]
- Fatunsin O. T.; Oluseyi T. O.; Drage D.; Abdallah M. A.-E.; Turner A.; Harrad S. Children’s Exposure to Hazardous Brominated Flame Retardants in Plastic Toys. Sci. Total Environ. 2020, 720, 137623 10.1016/j.scitotenv.2020.137623. [DOI] [PubMed] [Google Scholar]
- Zhang B.; Lu S.; Huang M.; Zhou M.; Zhou Z.; Zheng H.; Jiang Y.; Bai X.; Zhang T. Urinary Metabolites of Organophosphate Flame Retardants in 0–5-Year-Old Children: Potential Exposure Risk for Inpatients and Home-Stay Infants. Environ. Pollut. 2018, 243, 318–325. 10.1016/j.envpol.2018.08.051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.