Abstract
OBJECTIVES
The use of segmentectomy is expected to increase. However, understanding of the segmental bronchial branching is limited. Herein, we aimed to investigate bronchial branching pattern complexity and segmental volumetry of the right upper lung lobe to develop an accurate understanding of segmental anatomy and contribute to the advancement of safe and efficient lung segmentectomy.
METHODS
We evaluated chest computed tomography scans of 303 patients and categorized the branching of segmental bronchi (segment 1, apical; segment 2, posterior; and segment 3, anterior) into 4 major types (typical trifurcated, bifurcated non-defective, bifurcated defective and atypical trifurcated) and 11 subtypes. Segmental volumetry was performed to determine the predominant segment in each case (volume difference <5% was considered equal). Branching complexity was evaluated separately for volumetry-predominant and volumetry-non-predominant segments.
RESULTS
Trifurcated non-defective was the most frequent branching type (64.4%), followed by bifurcated non-defective (22.1%), bifurcated defective (8.6%) and trifurcated half-defective (4.0%). In terms of segmental volumetry, most cases had a one-segment-predominant distribution (71%) and only 5% of cases had equal distribution (segment 1 = segment 2 = segment 3). More than half of the cases had a segment 3-predominant distribution (52%). Branching complexity analysis revealed that the volumetry-non-predominant segment was associated with a higher risk of complex branching patterns compared with the volumetry-predominant segment (37% vs 19%, respectively; P < 0.005).
CONCLUSIONS
Volumetric assessment of the right upper lobe showed a heterogeneous segmental volume distribution. Care should be taken during lung segmentectomy of the volumetry-non-predominant segments because of the high risk associated with complex bronchial branching patterns.
Clinical trial registration
No. 4840.
Keywords: Bronchial branching pattern, Right upper lobe, Volumetry, Pulmonary segmentectomy
Segmentectomy is associated with significant preservation of pulmonary function.
INTRODUCTION
Segmentectomy is associated with significant preservation of pulmonary function. Therefore, it has been increasingly performed for small lung tumours, including primary lung cancer or metastatic lung tumours [1–5]. Recently, a large, multi-institutional, prospective randomized trial comparing the outcomes of lobectomy and segmentectomy for small primary lung cancers (≤2 cm) (JCOG0802/WJOG4607L) revealed that the overall survival of patients who underwent segmentectomy was significantly better than that of patients who underwent lobectomy. Despite a higher local recurrence in the segmentectomy group compared with the lobectomy group, there were no significant between-group differences in the risk of lung cancer death. More importantly, death from other cancers, respiratory disease and cerebrovascular disease occurred more frequently in the lobectomy group than in the segmentectomy group [6]. Therefore, the use of segmentectomy is expected to increase.
Preoperative prediction of resected and preserved lung volumes after lung resection is one of the most crucial functional assessments for safe surgery, and many thoracic surgeons have relied on the number of segments to be removed to estimate postoperative pulmonary function [7]. This strategy is based on the assumption that all segments have equal volumes. However, the role of segmental volumetry in these calculations has not been elucidated.
In general, lung segmentectomy is technically more challenging than lobectomy because of the complexity of the segmental bronchovascular anatomy. Although there are unlimited variations in the hilar segmental anatomy, several anatomical classifications of the lung hilum have been reported based on research using human cadaver organs [8, 9]. Our colleague demonstrated that three-dimensional computed tomography (3D-CT) imaging is useful for easy and precise measurement of anatomical branching patterns of the bronchovascular tree compared with traditional methods for studying anatomy using cadavers [10]. In addition, we developed a simplified 3D anatomical model of the right upper lobe (RUL) for appropriate intraoperative access to the intersegmental vein, which is an essential topographic landmark for lung segmentectomy [10–12]. During lung segmentectomy, surgeons should equally address the anatomical complexity and anomalies of the pulmonary artery and bronchus.
The RUL is the most lung cancer-prone lobe [13, 14], and the RUL bronchial tree is characterized by several complicated anatomical patterns (e.g. a defective branching pattern) [8, 10, 11]. In patients with a complex bronchial branching pattern, the potential risk of inappropriate segmentectomy, including excessive or insufficient resections, is high. However, understanding of the anatomical complexity of segmental bronchial branching is limited, and no studies have investigated bronchial branching patterns from the perspective of lung segmentectomy. Moreover, in previous studies, many patients showed unclassified branching patterns [8, 10, 11], which also negatively affects the approach to adequate segmentectomy.
To address the above-mentioned issues and knowledge gaps, in this study, we investigated the segmental bronchial tree of the RUL using 3D-CT. We specifically focused on segmental volumetry to determine potential variability in segmental volumes and evaluated the anatomical complexity of bronchial branching patterns to (i) develop a more thorough, comprehensive, and clinically useful anatomical classification; (ii) analyse segmental volumetry; and (iii) evaluate bronchial branching complexity from the perspective of lung segmentectomy. We believe that this investigation will contribute to the advancement of safe and effective approaches to lung segmentectomy.
MATERIALS AND METHODS
Ethical statement
This study was approved by the Institutional Review Board of Shinshu University Hospital (No. 4938). Due to the retrospective nature of the study, written consent was not necessary.
Study cohort
In this retrospective study, we investigated consecutive patients who underwent thoracic surgery at Shinshu University Hospital between January 2019 and August 2021 (n = 574). Patients with a history of pulmonary resection (n = 20), those with pulmonary inflammatory changes in the RUL (n = 8) and those without available preoperative thin-slice CT images (0.63-mm-thick images) (n = 243) were excluded. A total of 303 patients were included in this study.
Three-dimensional computed tomography reconstruction
We utilized a novel 3D-CT workstation (REVORAS, Ziosoft, Tokyo, Japan) for 3D-CT reconstruction and subsequent volumetry (details are described in the Supplementary Material).
Segmental branching pattern of the right upper lobe bronchus
Branching patterns were categorized into 4 major types: (i) typical trifurcated, (ii) bifurcated non-defective, (iii) bifurcated defective and (iv) atypical trifurcated. The non-defective type was defined as branching patterns with all subsegmental bronchi arising from their corresponding segmental bronchial branches (e.g. B1a and B1b arising from B1). The defective type was defined as branching patterns with 2 subsegmental bronchial branches arising from 2 separate non-corresponding segmental bronchial branches (e.g. B1a from B2 and B1b from B3).
We created several rules with technical terminology to develop the branching patterns (details are described in the Supplementary Material). There were 11 branching patterns classified into 4 major types: (i) ‘typical trifurcated type’ including <B1/B2/B3>; (ii) ‘bifurcated non-defective type’ including <B1 + B2/B3>, <B1 + B3/B2> and <B1/B2 + B3>; (iii) ‘bifurcated defective type’ including <BX1a + B2/BX1b + B3>, <B1 + BX2a/B3 + BX2b> and <B1 + BX3b/B2 + BX3a>; and (iv) ‘atypical trifurcated type’ including <B1a/B2/BX1b + B3>, <BX1a + B2/B1b/B3>, <B1 + BX2a/B2b/B3> and <B1/B2a/BX2b + B3>. In addition to the 4 major branching types, we defined a variant branching pattern as the displaced type in which a segmental or subsegmental branch arises from the trachea or bronchus other than the RUL bronchus (Supplementary Material, Fig. S1).
We summarized the data on branching patterns in our cohort and compared them with those from previous studies (Supplementary Material, Table S1) [10].
Segmental volumetric analysis of the right upper lobe
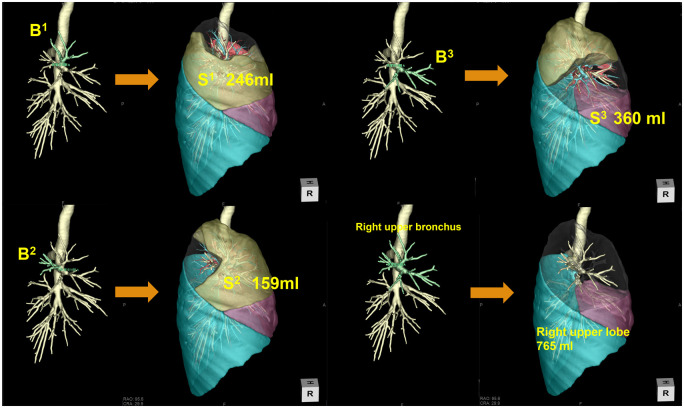
The volume of each segment was measured semi-automatically by selecting the index segmental bronchial branch on the 3D workstation (Fig. 1). If there was more than 1 subsegmental branch arising from a different bronchus, we selected all branches that entered the index segment to measure segmental volume.
Figure 1:
Actual method for segmental volumetry of the right upper lobe. First, bronchial bifurcation in the right upper lobe is identified. Subsequently, each target bronchus is selected, and the segmental volume is automatically calculated.
We measured the distribution of segmental volumes to determine the volumetric relationships between each segment, including equal (=), larger (>) and smaller (<) relationships. An equal relationship between segments (e.g. S1 = S2) was defined as a difference in volumetric distribution of ≤5%.
We classified 3 major volumetric relationships as follows: ‘equality relationship’ (S1 = S2 = S3), ‘one-segment-predominant relationship’ [S1 predominant, S2 predominant and S3 predominant; each segment-predominant relationship includes 3 patterns (e.g. S1 predominant: S1 > S2 > S3, S1 > S3 >S2, or S1 > S2 = S3)] and ‘other relationship’ [including a pattern with 2 equally larger segments and 1 smaller segment (e.g. S1 = S2 > S3)].
Segmentectomy-specific complexity based on bronchial branching patterns
Assuming there are segments α and β, and there is a patient with a small lung tumour in segment α (Sα) who is scheduled for Sα segmentectomy, a corresponding segmental bronchus (Bα) should be divided during surgery. When there are 2 subsegmental branches (Bαa and Bαb) arising from 2 separate bronchi [defective branching pattern (Bα/Bβ + BXαb)] or when Bα is a single branch but arises from a common branch with another segmental bronchus (Bβ), which should be preserved [common branching due to a bifurcated branching pattern (Bα + Bβ)], the potential risk of inappropriate surgery, including insufficient or excessive resection, is high. Therefore, the complexity of the Bα branching pattern will affect operative difficulty during Sα segmentectomy.
In this study, we investigated the incidence of complex branching patterns in each segmental bronchus during an assumed, corresponding segmentectomy. Assuming Sα segmentectomy is planned (Sβ and Sγ are segments to be preserved), a complex branching pattern of Bα was defined as follows: (i) ‘defective branching pattern’, 2 separate subsegmental branches (Bαa and Bαb) arising from 2 separate bronchi (defective pattern [Bβ + BXαa/Bγ + BXαb] or [Bαa/Bβ + BXαb/Bγ]); (ii) ‘common branching pattern’, a segmental bronchial branch (Bα) arising from a common bronchus (Bα + Bβ), in which Bβ should be preserved. In contrast, a simple branching pattern of Bα was defined as cases with Bα that arises directly from the lobar bronchus.
We hypothesized that an uneven volumetric distribution could be related to the bronchial branching pattern, based on our surgical experience with lung segmentectomy. Therefore, we focused on the potential relationship between volumetric predominance and branching complexity and compared the incidence of complex branching patterns between segmentectomies of volumetry-predominant and volumetry-non-predominant segments.
Statistical analysis
All statistical analyses were performed using SPSS version 27 (Chicago, IL, USA). Details are described in the Supplementary Material.
RESULTS
Patient characteristics and bronchial branching patterns
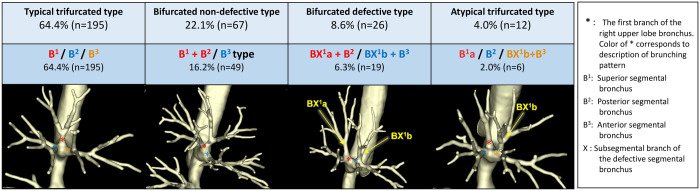
Table 1 shows the characteristics and bronchial branching patterns of the study participants. We investigated 574 consecutive patients who underwent thoracic surgery. Patients with a history of pulmonary resection (n = 20), those with pulmonary inflammatory changes in the RUL (n = 8) and those without available preoperative thin-slice CT images (n = 243) were excluded. Eventually, a total of 303 patients were included in this study. Figure 2 shows a representative 3D image of the 4 major classifications of RUL bronchus. The detailed classifications are shown in Supplementary Material, Figs S1 and S2. The proportions for each branching pattern were as follows:
Table 1:
Patient characteristics and branching patterns of the right upper lobar bronchus
| N = 303 | |
|---|---|
| Age (years) | 72 (25–94) |
| Sex | |
| Male | 138 (46%) |
| Female | 165 (54%) |
| Smoking status | |
| Never | 161 (53%) |
| Former/current | 142 (47%) |
| Pulmonary function test | |
| FVC (l) | 3.1 (1.2–5.4) |
| %FVC (%) | 107 (53–171) |
| FEV1.0 (l) | 2.7 (1.1–4.3) |
| FEV1.0/FVC (%) | 77 (35–97) |
| Diagnosis | |
| Primary lung cancer | 230 (76%) |
| Metastatic lung tumour | 49 (16%) |
| Mediastinal tumour | 12 (4%) |
| Others | 12 (4%) |
| Bronchial branching type | |
| Typical trifurcated type | |
| B1 + B2 + B3 | 195 (64%) |
| Bifurcated non-defective type | |
| B1 + B2/B3 | 49 (16%) |
| B1 + B3/B2 | 10 (3%) |
| B2 + B3/B1 | 8 (3%) |
| Bifurcated defective type | |
| BX1a + B2/BX1b + B3 | 19 (6%) |
| B1 + BX2a/B3 + BX2b | 5 (2%) |
| B1 + BX3b/B2 + BX3a | 2 (0.7%) |
| Atypical Trifurcated type | |
| B1a/B2/BX1b + B3 | 6 (2%) |
| BX1a + B2/B1b/B3 | 1 (0.3%) |
| B1 + BX2a/B2b/B3 | 3 (1%) |
| B1/B2a/BX2b + B3 | 2 (0.7%) |
| Displaced type | |
| B1 displaced | 1 (0.3%) |
| B2 displaced | 0 |
| B3 displaced | 2 (0.7%) |
Data are presented as median (interquartile range) or number (%).
B1: apical branch; B2: posterior branch; B3: anterior branch; BX: defective bronchial branch; FEV1.0: forced expiratory volume in 1 second; FVC: forced vital capacity.
Figure 2:
Major classification and representative 3D image of right upper lobe bronchus. *The first branch of the right upper lobe bronchus.
Typical trifurcated type (B1/B2/B3): 64.4% (n = 195).
Bifurcated non-defective type: 22.1% (n = 67) [B1 + B2/B3 type: 16.2% (n = 49), B1 + B3/B2 type: 3.3% (n = 10), B1/B2 + B3 type: 2.6% (n = 8)].
Bifurcated defective type: 8.6% (n = 26) [BX1a + B2/BX1b + B3 type: 6.3% (n = 19), B1 + BX2a/B3 + BX2b type: 1.7% (n = 5), B1 + BX3b/B2 + BX3a type: 0.7% (n = 2)].
Atypical trifurcated type: 4.0% (n = 12) [B1a/B2/BX1b + B3 type: 2.0% (n = 6), BX1a + B2/B1b/B3: 0.3% (n = 1), B1 + BX2a/B2b/B3 type: 1.0% (n = 3), B1/B2a/BX2b + B3 type: 0.7% (n = 2)].
Displaced type: 1.0% (n = 3).
Segmental volumetric analysis of the right upper lobe—predominant and non-predominant segments
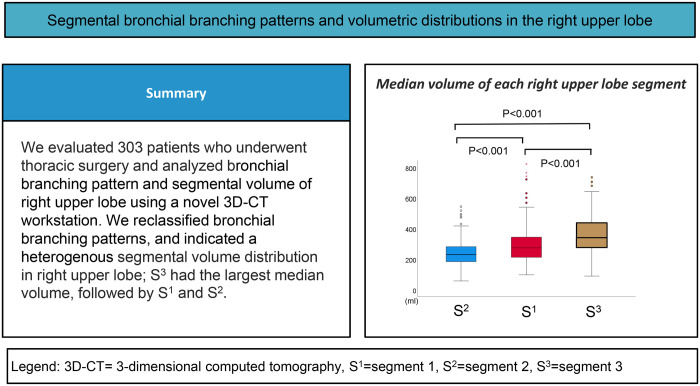
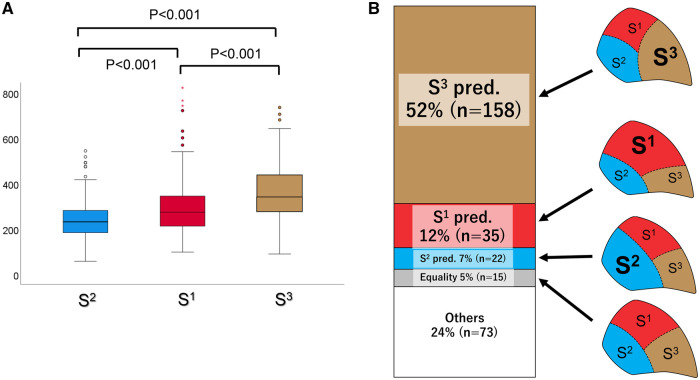
Figure 3A shows the mean volumes of the S1, S2 and S3 segments in the RUL of the 303 patients. S3 had the largest median volume [342.6 ml; interquartile range (IQR), 280.2–438.7 ml], followed by S1 (median volume, 273.6 ml; IQR, 211.5–344.9 ml) and S2 (median volume, 231.2 ml; IQR, 182.2–281.8 ml) (P < 0.001). Figure 3B shows the volume distribution pattern in the RUL, with the following frequencies:
Figure 3:
(A) Median volume of each right upper lobe segment. (B) Volume distribution of the 3 segments and their frequencies in the right upper lobe.
Equality relationship (S1=S2=S3): 5% (n = 15).
Segment-predominant relationship: 71% (n = 215) [S1 predominant: 12% (n = 35), S2 predominant: 7% (n = 22), S3 predominant: 52% (n = 158)].
Other relationship: 24% (n = 73).
Of the cases, 10.8% (n = 32) had an S3 volume exceeding 50% of the RUL (S3>S1 + S2 pattern; termed ‘mega S3’). Supplementary Material, Figure S3 shows the cases of mega S3, which occupied 61.6% of the RUL. A comparison of the predicted segmental volume in the RUL between the conventional method based on the number of segments or subsegments, and the volumetric analysis in the current study by a novel 3D-CT workstation has also been presented. In this case, when calculated using the conventional method, the volume of S1 and S2 was overestimated and the volume of S3 was underestimated when compared to those calculated based on 3D-CT volumetry. Table 2 shows the detailed distribution of all patterns.
Table 2:
Distribution of each volumetric segment pattern in the right upper lobe
| Predominant type | n (%) |
|---|---|
| Equally relationship (S1 = S2 = S3) | 15 (5%) |
| One-segment-predominant relationship | 215 (71%) |
| S3 (anterior segment) predominant | 158 (52%) |
| S3 > S1 > S2 | 64 |
| S3 > S2 > S1 | 30 |
| S3 > S1 = S2a | 64 |
| S1 (apical segment) predominant | 35 (12%) |
| S1 > S2 > S3 | 1 |
| S1 > S3 > S2 | 17 |
| S1 > S2 = S3a | 17 |
| S2 (posterior segment) predominant | 22 (7%) |
| S2 > S1 > S3 | 3 |
| S2 > S3 > S1 | 13 |
| S2 > S1 = S3a | 6 |
| Other relationship | 73 (24%) |
| S1 = S2 > S3a | 21 |
| S1 = S3 > S2a | 46 |
| S2 = S3 > S1a | 6 |
‘=’ represents an equal volumetric relationship, which is defined as a volumetric difference of <5% between 2 segments.
Complex bronchial branching pattern for each right upper lobe segmentectomy according to volumetric predominance
Table 3 shows the incidence of bronchial branching patterns based on volumetry-predominant segmental patterns. In the S3-predominant type (n = 158), 22 (14%) cases had a B3 complex bronchial pattern (BX1a + B3/BX1b/B2 type: 3, B1/BX2a/BX2b + B3 type: 1, B1 + B3/B2 type: 1, B1/B2 + B3 type: 4, BX1a + B2/BX1b + B3 type: 11, B1 + BX2a/B3 + BX2b type: 1, displaced B3: 1). In the S1-predominant type (n = 35), 10 (29%) cases had a B1 complex bronchial pattern (BX1a + B3/B1b/B2 type: 2, B1 + BX2a/B2b/B3 type: 1, B1/B2a/BX2b + B3 type: 1, B1 + B3/B2 type: 1, BX1a + B2/BX1b + B3 type: 2, B1 + BX2a/B3 + BX2b type: 2, B1 + BX3b/B2 + BX3a type: 1). In the S2-predominant type (n = 22), 6 (27%) cases had a B2 complex bronchial pattern (B1 + BX2a/B2b/B3 type: 2, B1/B2a/BX2b + B3 type: 1, BX1a + B2/BX1b + B3 type: 1, B1 + BX2a/B3 + BX2b type: 2).
Table 3:
Incidence of bronchial branching patterns based on volumetry-predominant segmental patterns
| Equality | S1 pred. | S2 pred. | S3 pred. | Others | |
|---|---|---|---|---|---|
| N = 15 | N = 35 | N = 22 | N = 158 | N = 73 | |
| Bronchial branching pattern | |||||
| Typical trifurcated | |||||
| B1/B2/B3 | 12 (80%) | 20 (57%) | 11 (50%) | 96 (61%) | 56 (77%) |
| Bifurcated non-defective | |||||
| B1+B2/B3 | 1 (7%) | 0 | 0 | 39 (25%) | 9 (12%) |
| B1+B3/B2 | 2 (13%) | 1 (3%) | 5 (23%) | 1 (0.6%) | 1 (1%) |
| B2+B3/B1 | 0 | 4 (11%) | 0 | 4 (3%) | 0 |
| Bifurcated defective | |||||
| BX1a + B2/BX1b+B3 | 0 | 2 (6%) | 1 (5%) | 11 (7%) | 5 (7%) |
| B1 + BX2a/B3 + BX2b | 0 | 2 (6%) | 2 (9%) | 1 (0.6%) | 0 |
| B1 + BX3b / B2 + BX3a | 0 | 1 (3%) | 0 | 0 | 1 (0%) |
| Atypical trifurcated | |||||
| B1a/B2/BX1b + B3 | 0 | 2 (6%) | 0 | 3 (2%) | 1 (1%) |
| BX1a + B2/BX1b/B3 | 1 (3%) | 0 | 0 | 0 | |
| B1 + BX2a/BX2b/B3 | 0 | 1 (3%) | 2 (9%) | 0 | 0 |
| B1/BX2a/BX2b + B3 | 0 | 0 | 1 (5%) | 1 (0.6%) | 0 |
| Displaced | |||||
| B1 displaced | 0 | 0 | 0 | 1 (0.6%) | 0 |
| B2 displaced | 0 | 0 | 0 | 0 | 0 |
| B3 displaced | 0 | 1 (3%) | 0 | 1 (0.6%) | 0 |
| Segmentectomy-specific bronchial branching complexitya | |||||
| B3 complexity during S3 segmentectomy | Non-pred. | Non-pred. | Pred. | ||
| Complexa | NA | 12 (34%) | 9 (41%) | 22 (14%) | NA |
| Simplea | NA | 23 (66%) | 13 (59%) | 136 (86%) | NA |
| B1 complexity during S1 segmentectomy | Pred. | Non-pred. | Non-pred. | ||
| Complexa | NA | 10 (29%) | 10 (45%) | 56 (35%) | NA |
| Simplea | NA | 25 (71%) | 12 (55%) | 102 (65%) | NA |
| B2 complexity during S2 segmentectomy | Non-pred. | Pred. | Non-pred. | ||
| Complexa | NA | 8 (23%) | 6 (27%) | 56 (35%) | NA |
| Simplea | NA | 27 (77%) | 16 (73%) | 102 (65%) | NA |
Data are presented as number (%).
Bronchial branching patterns in the predominant segment are defined as follows: complex, any bronchial branching pattern that requires surgical division of >1 bronchial branch or only 1 branch but requires preservation of the proximal branch(es) after the first segmental branch that belongs to another segment during segmentectomy of the index segment; simple, any bronchial branching pattern that requires surgical division of only 1 index segmental bronchus without preservation of any branches after the first segmental branch. Underlines represent cases of complex bronchial patterns in the predominant segment.
B1: apical branch; B2: posterior branch; B3: anterior branch; BX: defective bronchial branch; NA: not applicable; Pred.: predominant; SX pred.: one-segment-predominant relationship (S1 predominant, S2 predominant, and S3 predominant).
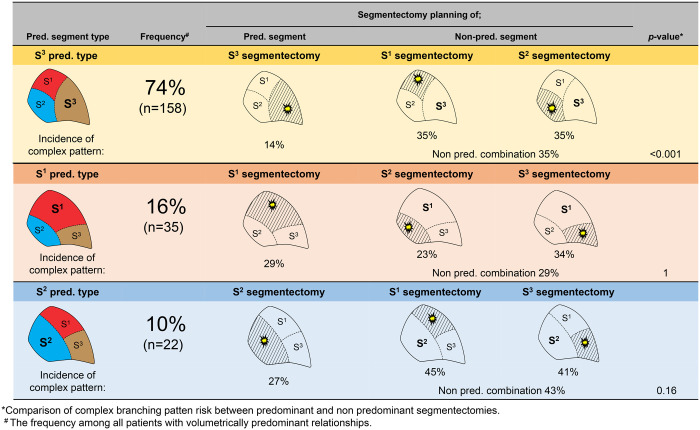
Figure 4 shows the frequencies of bronchial patterns in segmentectomy planning for each predominant pattern. In all patients with the one-segment-predominant relationship (n = 215), the incidence of complex bronchial patterns in the volumetry-predominant segment was significantly lower than that in the volumetry-non-predominant segments (18% and 35%, respectively, P < 0.001; Table 4). Particularly, in the S3-predominant type, the incidence of the B3 complex bronchial pattern was only 14%, whereas those of B1 and B2 were 35% and 35%, respectively (P < 0.001). Similarly, in the S2-predominant type, the incidence of the B2 complex bronchial pattern was higher than that of B1 or B3 (45% or 41%, respectively); however, this was not statistically significant (P = 0.16).
Figure 4:
Frequencies of bronchial patterns in segmentectomy planning for each predominant pattern. In the S3-predominant type, the frequency of the B3 simple bronchial pattern is 86%, while that of the B3 complex pattern is 16%; in S1 or S2 segmentectomy, the frequency of B1 and B2 complex patterns is 35% (P < 0.001). In the S1-predominant type, the B1 complex bronchial pattern frequency is 29%; in S2 or S3 segmentectomy, the frequency of B2 and B3 complex patterns is 29% (P = 1). In the S2-predominant type, the B2 complex pattern frequency is 27%; in S1 or S3 segmentectomy, the frequency of B1 and B2 complex patterns is 43% (P = 0.16).
Table 4:
Incidence of complex bronchial patterns in predominant and non-predominant segments according to volumetry-predominant segment status
| Bronchial branching complexity during segmentectomy | Segmentectomy planning for |
P-Value | |
|---|---|---|---|
| Predominant segment (n = 215) | Non-predominant segmenta (n = 430) | ||
| Complex | 18% (n = 38) | 35% (n = 150) | <0.001 |
| Simple | 82% (n = 177) | 65% (n = 280) | |
There are 2 non-predominant segments per case.
DISCUSSION
Our topographical anatomical investigation and volumetric analysis of the RUL revealed previously unknown findings. The significance and novelty of this study are as follows: (i) our simple branching pattern classification comprising 4 major categories (i.e. typical trifurcated, bifurcated non-defective, bifurcated defective and atypical trifurcated) covers 99% of patients; (ii) volumetric analysis revealed an uneven segmental distribution, demonstrating a frequent S3-predominant relationship; and (iii) segmentectomy-specific bronchial branching complexity demonstrated a significant association between volumetry-non-predominant segments and a higher risk of complex branching patterns, suggesting the potential utility of volumetry in predicting surgical challenges associated with segmentectomy.
Our literature review of bronchial branching patterns revealed 3 studies describing segmental branching classifications of the RUL and their frequencies [8–10]. Among these studies, the earliest (published in 1948) investigated cadavers, whereas the most recent study investigated 3D-CT images to differentiate anatomical patterns similar to our study (published in 2015) [10]. Supplementary Material, Table S1 shows a comparison between our study and that of Nagashima et al. [10]. Although the distribution of the typical trifurcated category was slightly higher in our study than that in the study by Nagashima et al. (63% vs 44%, respectively), a significant difference between the 2 studies is that in the latter, 19% of patients were unclassified, compared with only 1% in our study. We also suggest that the significant difference in diagnostic performance between the 2 studies could be partially attributed to the difference in 3D-CT workstations. In our study, we utilized a newly developed 3D-CT workstation (REVORAS, Ziosoft, Tokyo, Japan), which enables a more vivid and clear analysis of bronchial bifurcations by measuring extrabronchial contours, compared with conventional 3D-CT workstations that measure intraluminal contours. Measurement of intraluminal contours to evaluate the bronchial tree may cause misidentification of small segmental bronchial branches, which might have affected the incidence of unclassified cases (Supplementary Material, Fig. S4).
In our study cohort, we observed 3 cases of displaced branching, which is defined as a branching pattern in which a segmental bronchus arises from a different lobar bronchus. There were 2 cases of B3 downwards-shifting malformation, in which B3 arises from the middle lobar bronchus. Yaginuma investigated the incidence of displaced bronchi using CT scans. He analysed 6072 patients and reported that displaced bronchi were more frequent in the RUL (frequency: 0.64%; n = 39) [15]. Another study reported an incidence of displaced bronchi in the RUL of 0.15% [16]. The incidence of the displaced pattern in our study was 1%, which is consistent with these studies.
With the global increase in high-risk elderly patients with early-stage lung cancer, the importance of predicting postoperative pulmonary function has increased. Conventional prediction of resected lung volume is based on the number of resected segments (or subsegments) [7]. Our volumetric findings suggest that the conventional prediction of postoperative pulmonary function may provide an invalid predictive value. Equally distributed RUL segments were seen in only 5% of patients, and more than two-thirds (71%) of patients had volumetric-predominant segments. In addition, 11% of patients had ‘mega S3’, defined as predominant S3 that occupies more than half of the RUL. These findings indicate that the conventional prediction method, which is based on the number of resected segments or subsegments, could lead to the underestimation or overestimation of the resected lung volume. We present a representative case of a patient with an S3 predominant distribution in Supplementary Material, Fig. 4. In this case, the predominant S3 segmentectomy removed 11% of the whole lung guided by 3D-CT volumetry. In contrast, only 4.8% was resected based on conventional methods (using subsegments). Conversely, the actual resected volume by non-predominant S1 or S2 segmentectomies was overestimated compared to the conventional method. We believe that thoracic surgeons should be aware of these findings when making decisions on surgical indications, particularly in patients who are elderly or have poor pulmonary function.
This study also provides surgeons with clinically useful information regarding the potential surgical difficulties associated with RUL segmentectomy. If a tumour develops in the non-predominant RUL segment in patients with a one-segment-predominant relationship (including 71% of patients in our cohort), the risk of having a complex bronchial branching pattern would be higher than in cases where a tumour develops in the predominant segment, particularly in the S3-predominant type (Fig. 4). In cases with complex bronchial branching patterns, sufficient preoperative simulation and intraoperative navigation using 3D-CT planning should be considered to avoid inappropriate segmentectomy based on misidentification of the segmental bronchial anatomy.
Limitations
This study had several limitations. First, segmental volumetry might have been affected by patient posture (generally supine) at the time of CT scanning. Yamada et al. [17] analysed the differences in lung volume between the supine and standing positions using a CT scan. They showed that the volume of each lobe (except for the middle lobe) was significantly greater in the standing than in the supine position. S2, which is located on the dorsal side when the patient is in the supine position, could have been influenced by the effects of gravity. Second, our segmental volumetry results were based only on 3D-CT analysis. We conducted an independent study to investigate the accuracy of predicting postoperative pulmonary function based on 3D-CT volumetry, the results of which will be analysed and published later. Third, the number of patients, particularly those with minor branching patterns, was relatively small, which negatively affected the generalizability of our findings. Lastly, regarding the concept of surgical complexity, our study did not directly correlate anatomical variations with objective parameters, such as surgical time and margin distance. This limits our ability to comprehensively evaluate the relationship between anatomical variations and surgical complexity. Future studies should incorporate these objective parameters to provide more detailed insights into the impact of anatomical variations on pulmonary segmentectomy outcomes.
CONCLUSIONS
We successfully classified all patients based on RUL segmental bronchial branching patterns. Thoracic surgeons should exercise extreme care when interpreting the relatively frequent presentation of heterogeneous volumetric distribution of RUL segments and the risk of complex bronchial branching patterns when planning RUL segmentectomy.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Editage (www.editage.com) for English language editing.
Conflict of interest: none declared.
Glossary
ABBREVIATIONS
- 3D-CT
Three-dimensional computed tomography
- IQR
Interquartile range
- RUL
Right upper lobe
Contributor Information
Kentaro Miura, Division of General Thoracic Surgery, Department of Surgery, Shinshu University School of Medicine, Matsumoto, Japan.
Takashi Eguchi, Division of General Thoracic Surgery, Department of Surgery, Shinshu University School of Medicine, Matsumoto, Japan.
Shogo Ide, Division of General Thoracic Surgery, Department of Surgery, Shinshu University School of Medicine, Matsumoto, Japan.
Shuji Mishima, Division of General Thoracic Surgery, Department of Surgery, Shinshu University School of Medicine, Matsumoto, Japan.
Shunichiro Matsuoka, Division of General Thoracic Surgery, Department of Surgery, Shinshu University School of Medicine, Matsumoto, Japan.
Tetsu Takeda, Division of General Thoracic Surgery, Department of Surgery, Shinshu University School of Medicine, Matsumoto, Japan.
Kazutoshi Hamanaka, Division of General Thoracic Surgery, Department of Surgery, Shinshu University School of Medicine, Matsumoto, Japan.
Kimihiro Shimizu, Division of General Thoracic Surgery, Department of Surgery, Shinshu University School of Medicine, Matsumoto, Japan.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
DATA AVAILABILITY
The data underlying this article cannot be shared publicly due to the privacy of individuals that participated in the study. The data will be shared on reasonable request to the corresponding author.
Author contributions
Kentaro Miura: Data curation; Investigation; Methodology; Writing—original draft. Takashi Eguchi: Data curation; Investigation; Methodology. Shogo Ide: Data curation; Investigation. Shuji Mishima: Investigation. Shunichiro Matsuoka: Data curation; Investigation; Software. Tetsu Takeda: Investigation. Kazutoshi Hamanaka: Investigation. Kimihiro Shimizu: Methodology; Supervision; Writing—original draft; Writing—review & editing.
Reviewer information
Interdisciplinary CardioVascular and Thoracic Surgery thanks Gonzalo Varela, Tevfik Kaplan, Dominique Gossot and the other anonymous reviewer(s) for their contribution to the peer review process of this article.
REFERENCES
- 1.Okada M, Koike T, Higashiyama M, Yamato Y, Kodama K, Tsubota N.. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg 2006;132:769–75. [DOI] [PubMed] [Google Scholar]
- 2.Harada H, Okada M, Sakamoto T, Matsuoka H, Tsubota N.. Functional advantage after radical segmentectomy versus lobectomy for lung cancer. Ann Thorac Surg 2005;80:2041–5. [DOI] [PubMed] [Google Scholar]
- 3.Keenan RJ, Landreneau RJ, Maley RH Jr, Singh D, Macherey R, Bartley S. et al. Segmental resection spares pulmonary function in patients with stage I lung cancer. Ann Thorac Surg 2004;78:228–33; discussion 228–33. [DOI] [PubMed] [Google Scholar]
- 4.Ohtaki Y, Shimizu K.. Anatomical thoracoscopic segmentectomy for lung cancer. Gen Thorac Cardiovasc Surg 2014;62:586–93. [DOI] [PubMed] [Google Scholar]
- 5.Nakazawa S, Shimizu K, Mogi A, Kuwano H.. VATS segmentectomy: past, present, and future. Gen Thorac Cardiovasc Surg 2018;66:81–90. [DOI] [PubMed] [Google Scholar]
- 6.Saji H, Okada M, Tsuboi M, Nakajima R, Suzuki K, Aokage K. et al. ; West Japan Oncology Group and Japan Clinical Oncology Group. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607–17. [DOI] [PubMed] [Google Scholar]
- 7.Bolliger CT, Gückel C, Engel H, Stöhr S, Wyser CP, Schoetzau A. et al. Prediction of functional reserves after lung resection: comparison between quantitative computed tomography, scintigraphy, and anatomy. Respiration 2002;69:482–9. [DOI] [PubMed] [Google Scholar]
- 8.Boyden EA, Scannell JG.. An analysis of variations in the bronchovascular pattern of the right upper lobe of 50 lungs. Am J Anat 1948;82:27–73. [DOI] [PubMed] [Google Scholar]
- 9.Yamashita H. Variations in the Pulmonary Segments and the Bronchovascular Trees. Roentgenologic Anatomy of the Lung. Tokyo: Igaku-Syoin, 1978. [Google Scholar]
- 10.Nagashima T, Shimizu K, Ohtaki Y, Obayashi K, Kakegawa S, Nakazawa S. et al. An analysis of variations in the bronchovascular pattern of the right upper lobe using three-dimensional CT angiography and bronchography. Gen Thorac Cardiovasc Surg 2015;63:354–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimizu K, Nagashima T, Ohtaki Y, Obayashi K, Nakazawa S, Kamiyoshihara M. et al. Analysis of the variation pattern in right upper pulmonary veins and establishment of simplified vein models for anatomical segmentectomy. Gen Thorac Cardiovasc Surg 2016;64:604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakazawa S, Shimizu K, Kawatani N, Obayashi K, Ohtaki Y, Nagashima T. et al. Right upper lobe segmentectomy guided by simplified anatomic models. JTCVS Tech 2020;4:288–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okami J, Shintani Y, Okumura M, Ito H, Ohtsuka T, Toyooka S. et al. ; Japanese Joint Committee of Lung Cancer Registry. Demographics, safety and quality, and prognostic information in both the seventh and eighth editions of the TNM classification in 18,973 surgical cases of the Japanese joint committee of lung cancer registry database in 2010. J Thorac Oncol 2019;14:212–22. [DOI] [PubMed] [Google Scholar]
- 14.Okada M, Mimura T, Ikegaki J, Katoh H, Itoh H, Tsubota N.. A novel video-assisted anatomic segmentectomy technique: selective segmental inflation via bronchofiberoptic jet followed by cautery cutting. J Thorac Cardiovasc Surg 2007;133:753–8. [DOI] [PubMed] [Google Scholar]
- 15.Yaginuma H. Investigation of displaced bronchi using multidetector computed tomography: associated abnormalities of lung lobulations, pulmonary arteries and veins. Gen Thorac Cardiovasc Surg 2020;68:342–9. [DOI] [PubMed] [Google Scholar]
- 16.Ghaye B, Szapiro D, Fanchamps JM, Dondelinger RF.. Congenital bronchial abnormalities revisited. Radiographics 2001;21:105–19. [DOI] [PubMed] [Google Scholar]
- 17.Yamada Y, Yamada M, Yokoyama Y, Tanabe A, Matsuoka S, Niijima Y et al Differences in lung and lobe volumes between supine and standing positions scanned with conventional and newly developed 320-detector-row upright CT: intra-individual comparison. Respiration 2020;99:598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly due to the privacy of individuals that participated in the study. The data will be shared on reasonable request to the corresponding author.