Abstract
CADM3 has been recently reported causing a rare axonal Charcot–Marie–Tooth disease in three independent Caucasian families carrying a recurrent change. We describe the first alternative causative mutation in CADM3 in a family from black African and also observed de novo in a patient of Caucasian ancestry. The disease inheritance was consistent with autosomal dominant and sporadic patterns, respectively. Eight patients and their relatives were enroled from both families. The mean age at diagnosis was 33.9 years, and walking difficulty was commonly the first symptom. Neurological examination showed distal muscle weakness and atrophy, sensory loss and foot and hand deformities. A high clinical variability was noted, but as seen in CADM3-associated neuropathy, symptoms were more pronounced in the arms in some patients. Nerve conduction studies showed no response in most of the examined nerves, and an axonal type of neuropathy, where recorded. Whole exome sequencing revealed a novel missense variant (c.1102G>T; Gly368Cys) in CADM3, segregating with the disease. Functional analyses showed a significant decrease in CADM3-Gly368Cys protein levels in the membrane and major structural changes in its predicted secondary structure. Therefore, we extend the genotype spectrum of CADM3, underlining the need for genetic studies in underrepresented populations like in Africa.
Keywords: CMT, next-generation sequencing, CADM3, novel variant, Mali
Yalcouyé et al. report that variants in CADM3 gene cause an axonal-type neuropathy. This study provides further evidence of the implication of CADM3 in the pathogenesis of Charcot–Marie–Tooth disease and raises the importance of including diverse and underrepresented populations like African in genetic studies.
Graphical Abstract
Graphical Abstract.
Introduction
Charcot–Marie–Tooth disease (CMT) is the most common hereditary peripheral neuropathy with a high clinical and genetic heterogeneity. Characterized by progressive, distally ascending motor and sensory loss and feet deformities, its subtypes are broadly subclassified into demyelinating (CMT1 and CMT4) and axonal forms (CMT2).1-3 The genetic heterogeneity of axonal CMT makes the targeted gene testing strategy less efficient, especially in understudied populations such as African.1 Over the past few decades, the advent of next-generation sequencing has led to the identification of nearly 100 different genes.4,5 However, only 20–30% of CMT2 have been genetically diagnosed,6 and a few genetically confirmed CMT cases have been reported in the sub-Saharan African population, including Mali.7-11 More recently, whole exome sequencing has allowed the identification of a novel CMT gene, CADM3 (OMIM: 609743).12 CADM3 encodes a member of the CADM family of cell–cell adhesion molecules. The subcellular distribution showed that CADM3 is highly enriched in the cell membrane. CADM3 is expressed in axons, where it interacts primarily with CADM4 (OMIM: 609744) and plays an important role in axon guidance, myelination and maintenance of the axonal architecture.12 One recurrent CADM3 variant (Tyr172Cys) causing CMT2FF (OMIM: 619519) has been identified in three unrelated Caucasian families, with marked motor and especially upper limb involvement. In this study, we report a second pathogenic variant in the CADM3 gene in two unrelated families: one of a black African ancestry from Mali and another a Caucasian American with a CMT2 phenotype.
Material and methods
Ethical compliance
The study was conducted in full accordance with the Declaration of Helsinki and was approved by the institutional ethics committee of the Faculty of Medicine and Dentistry of Bamako, Mali (N°2020/129/CE/FMOS/FAPH). Informed consent and assent for minor participants were obtained prior to their enrolment in this project, including the permission to publish photographs or videos.
Clinical and neurophysiological assessment
All participants have undergone a careful clinical examination by a research team including a neurogeneticist and neurologists. Nerve conduction studies were performed in selected affected individuals.
Genetic analyses
DNA was extracted from peripheral blood in all available family members using the QIAGEN Puregene Blood DNA kit C (Qiagen, Germantown, MD) following manufacturer’s instructions.
For whole exome sequencing, DNA samples of five individuals (III.4, III.5, III.29, IV.20 and IV.33) of Family 1 and one (III.2) of Family 2 were exome sequenced, and Fastq data were aligned to the human reference hg19 genome using a well-established bioinformatic algorithm and software (details are provided in Supplementary material). Putative variants were confirmed through Sanger sequencing, and segregation analysis was assessed in available individuals (Fig. 1A).
Figure 1.
Pedigree of Family 1 showing a dominant inheritance pattern (arrow indicates the proband and asterisks the participants seen in clinic).
Functional analyses
Protein modelling
Since there is no solved structure for CADM3 protein, the CADM3 isoform 1 (NM_021189.5) protein sequence (NP_067012.1) was retrieved from the NCBI GenePept database in FASTA format, and secondary structures were predicted for the wild-type (WT) as well as mutant sequences using the PSIPRED workbench (http://bioinf.cs.ucl.ac.uk/psipred). The 3D structures of CADM3 G368 WT and C368 mutant proteins were modeled on the Swiss Model server using the Alpha Fold-predicted CADM3 3D structure (https://alphafold.ebi.ac.uk/entry/Q8N126) as a template. The 3D structures were then refined using the Seok lab’s GalaxyRefine algorithm.13 The PyMol software was used for hydrogen bond analysis and structure visualization.14
Generation of constructs
Plasmid encoding the open reading frame of CADM3 (NM_021189.4) fused to an HA tag was obtained from GeneCopoeia. Site-directed mutagenesis was performed to generate plasmids with the novel mutation corresponding to Gly368Cys. The point mutation was introduced by polymerase chain reaction using the Q5 Site-Direct Mutagenesis kit (NEB) with the following primers: 5′-CGCCATCATCTGTGGGATCGTG-3′ and 5′-TGGTAGGTGCTGGAGGAG-3′.
Cell surface protein isolation and western blot
HEK293 cells were transfected with CADM3 plasmids, WT, Gly368Cys and Tyr172Cys using Lipofectamine 3000 (Thermo Fisher). The next day, cells were harvested for cell surface protein extraction using Pierce Cell Surface Protein Isolation Kit (Thermo Scientific) according to the manufacturer’s protocol. Plasma membrane proteins were biotinylated and extracted using the Pierce Cell Surface Biotinylation and Isolation Kit (Thermo Scientific) following the manufacturer’s protocol. Briefly, cells were incubated with the biotinylation reagent, Sulfo-NHS-SS-Bio, for 10 min. Cells were subsequently washed and lysed on ice for 30 min. Biotinylated proteins were captured with neutravidin resin for 30 min. Resin was washed, and proteins were eluted with dithiothreitol. Western blot was performed with the following antibodies: HA (3724, Cell Signaling), ATP1A1 (ab7671, Abcam) and GAPDH (sc-47724, Santa Cruz). Membranes were visualized using the FluorChem E FE0436 imaging system (ProteinSimple).
Immunofluorescence
We used human oesteosarcoma cell lines (U2OS cells) that were plated in an eight-well slide chamber (1 cm2 per well) and transfected with CADM3 plasmids. Cells were fixed with 4% paraformaldehyde for 15 min and permeabilized with cold methanol for 5 min. Cells were incubated with the monoclonal anti-HA primary antibody, Alexa Fluor 555 secondary antibody (Thermo Fisher) and 4′,6-diamidino-2-phenylindole (DAPI). Cells were imaged by confocal microscopy with a ×60 objective lens (Zeiss LSM710). Intercellular contact sites formed between two cells were counted using a tally counter for a total of 15 min for each well (three WT and three mutants) in a blinded experiment.
Statistical analysis
Student’s t-test was performed to determine the statistical significance of the mean difference of the quantitative data between two groups using Prism. A P-value less than 0.05 was considered statistically significant.
Results
Clinical phenotype
Family 1
Seven patients (six males and one female) and their relatives from a large family of Fulani ethnicity were enroled. The disease distribution was consistent with an autosomal dominant pattern of inheritance (Fig. 1). The mean age at diagnosis was 35.7 years, ranging from 12 to 65 years, and walking difficulty was the common starting symptom. The neurological examination showed distal muscle weakness and wasting and mild sensory impairment, steppage gait, decreased to absent tendon reflexes throughout, foot deformities (pes cavus and planus) and hammer toes in all affected individuals (Fig. 2A–C). An important clinical variability was noted within the family with some younger patients appearing to be more affected than the older ones (Fig. 2A and B). However, some patients had more pronounced upper limb involvement than lower limb symptoms (Fig. 2B and Video 1). One patient (IV.36) was wheelchair bound at age of 18 years, while his 50-year-old father (III.23) and the oldest enroled individual (III.4, 65 years) had mild symptoms. Moreover, besides having distal muscle weakness, one patient displayed a severe lower limb motor deficit likely due to the sequelae of poliomyelitis (Fig. 2C). In fact, parents reported that at age of 2 years, he presented an acute diarrhoeal syndrome with fever after which they noticed muscle weakness that worsened gradually. Nerve conduction studies were performed in five patients, and no response was recorded in most of the examined nerves, in severely affected individuals. However, where recorded, responses were consistent with an axonal type of neuropathy with reduced amplitude of the compound motor action potential and sensory nerve action potential while conduction velocities were in the normal range. All phenotypic and genotypic characteristics are summarized in Table 1.
Figure 2.
Clinical findings. (A–C) Images of the Patients IV.20, IV.36 and III.29 showing phenotypic variability between patients from different generations with foot and hand deformities and muscle atrophy and weakness.
Table 1.
Phenotypic and genetic findings in patients with CMT2FF
| Clinical examination findings | Laboratory findings | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscle weakness UL | Muscle weakness LL | Sensory loss | Tendon reflexes | Skeletal deformities | Nerve conduction studies | CADM3 variant | ||||||||||||
| Patients | Age (years) | Sex | Age of onset (years) | CMTNSv2 | First symptom | Proximal R/L | Distal R/L | Proximal R/L | Distal R/L | Median | Sural | Peroneal | c.1102G>T; Gly368Cys | |||||
| SNAP Amp | CMAP Amp | CV m/s | SNAP Amp | CMAP Amp | ||||||||||||||
| F1/III.5 | 65 | M | Fifth decade | 6 | Hand weakness | 5/5 | 3/4 | 5/5 | 3+/4 | Minimal | Decreased | Pes cavus, hammer toes | 6 | 9.1 | 48 | 3 | 3.2 | c.1102G>T; Gly368Cys |
| F1/III.23 | 50 | M | Fourth decade | 2 | Muscle atrophy | 5/5 | 5/5 | 5/5 | 5/5 | Mild | Decreased | Absent | 14 | 14.9 | 51 | 11 | 9 | c.1102G>T; Gly368Cys |
| F1/III.29 | 45 | M | First decade | 16 | Walking difficulty | 5/5 | 0/0 | 5/5 | 0/0 | Mild | Absent | Pes planus | 5 | NR | NR | NR | NR | c.1102G>T; Gly368Cys |
| F1/IV.20 | 35 | M | First decade | 22 | Hand weakness | 3/3 | 0/0 | 1/1 | 0/0 | Mild | Absent | Pes cavus | NR | NR | NR | 5 | NR | c.1102G>T; Gly368Cys |
| F1/IV.33 | 25 | M | First decade | 10 | Walking difficulty and hand weakness | 5/5 | 3/3 | 5/5 | 3+/5 | Minimal | Decreased | Absent | 15 | 17 | 51 | 10 | 2.6 | c.1102G>T; Gly368Cys |
| F1/IV.36 | 18 | M | First decade | 18 | Walking difficulty | 5/5 | 0/0 | 4/4 | 0/0 | Mild | Absent | Pes cavus, hammer toes, claw hands | ND | ND | ND | ND | ND | c.1102G>T; Gly368Cys |
| F1/IV.37 | 12 | F | Second decade | 5 | Walking difficulty | 5/5 | 4−/4− | 5/5 | 3+/3+ | Mild | Absent | Pes cavus | ND | ND | ND | ND | ND | c.1102G>T; Gly368Cys |
| F2/III.2 | 9 | M | First decade | 13 | Foot deformity | 4−/4− | 3/3 | 3/3 | 2/2 | Absent | Absent | Pes valgus | c.1102G>T; Gly368Cys | |||||
Muscle weakness based on Medical Research Council Scale (0–5). Note: CMTNSv2 was estimated only based on clinical signs and symptoms and did not include electrophysiological items. Amp, amplitude; SNAP, sensory nerve action potential; CMAP, compound motor action potential; CMTNSv2, Charcot–Marie–Tooth Neuropathy Score version 2; F, family; UL, upper limb; LL, lower limb, R, right; L, left; CV, conduction velocity; NR, no response; ND, not done; m/s, metre per second.
Family 2
A 9-year-old male patient from an American Caucasian family and his unaffected parents were enroled. Patient was born at term following an unremarkable pregnancy and without perinatal complications. The disease distribution in the family was suggestive of a sporadic case (Supplementary Fig. 1). He had a normal early development until around 2 years of age. He was noted to have progressive valgus deformity of the feet initially improved with bracing but followed by a progressive decline in gait stability and strength at around 3 years of age accompanied by weight loss, a proximal muscle weakness and atrophy, without significant sensory or pain complaint. He had reduced reflexes in upper limbs but did not present clinically apparent sensory abnormalities. Nerve conduction studies showed a motor-predominant, primarily axonal neuropathy. Right sural nerve biopsy was unrevealing, and quadriceps muscle biopsy showed Type 2 fibre predominance and Type 2 atrophy.
Genetic data
Whole exome sequencing revealed a novel missense variant at position c.1102G>T, p.Gly368Cys, in the CADM3 gene in both families. This variant was confirmed by Sanger sequencing in all 15 available members of the respective families (Fig. 3A) and was found to co-segregate with the disease. However, parents of the patient in the Family 2 did not have the variant, making it a de novo in that family. This variant is located in a highly conserved domain of the protein (Fig. 3B) with a phyloP100way score = 9.23 and is predicted to be deleterious using several in silico prediction tools including CADD score = 31 and SIFT = 0 (Supplementary Table 1). The variant is absent from gnomAD.
Figure 3.
Genetic data. (A) Chromatogram showing the nucleotide G to T change (asterisk). (B) Amino acid conservation across a wide range of species.
Protein modelling
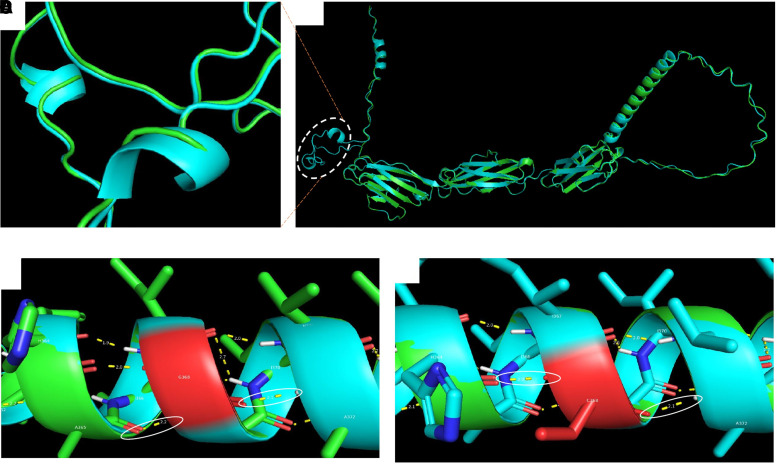
Several major structural changes were observed in the mutant. The helix structure at 6ASLL9 was completely changed to a beta strand, which was extended by two amino acids. In the predicted structures gained, there were two additional helices in the mutant structure that were not found in the WT structure (Fig. 4A and B). In addition, hydrogen bond analysis of the refined structures revealed that the WT Gly368 and mutant Cys368 formed two hydrogen bonds with histidine-364 and alanine-372 in a helical structure (Fig. 4C and D). However, this helical structure consists of residues forming a transmembrane helix (UniProt), meaning that it is supposed to be inserted in the membrane. Other minor changes were observed as depicted in the black boxes (Supplementary Fig. 2).
Figure 4.
Protein modelling. (A, B) Superimposed 3D structures of CADM3 WT (green) and mutant (light blue) showing some regions with secondary structural changes (gain of helices in the mutant protein). (C, D) Hydrogen bond analysis of CADM3 WT and mutant protein structures. Hydrogen bonds involving the mutant site are indicated by the white circles. The protein cysteine residue has a free thiol group, which likely interferes with the insertion of the helix in the membrane. The WT G368 and mutant C368 formed two hydrogen bonds with histidine-364 and alanine-372 in a helical structure. The mutant C368 is a polar uncharged amino acid with a thiol (SH) side chain, which may affect the insertion of the helical structure into the membrane.
Membrane isolation, western blot and immunofluorescence
The newly identified CADM3 variant, Gly368Cys, is located in the protein transmembrane domain. Therefore, we decided to investigate whether the mutation affects membrane targeting. Cell surface proteins were isolated from HEK293 cells transfected with plasmids encoding the WT and mutants (Gly368Cys and Tyr172Cys). Western blot shows that both WT and mutant proteins are predominantly enriched in the cell surface (plasma membrane); however, reduced levels of membrane CADM3 were detected in the mutants compared to the WT (Fig. 5A; Supplementary Fig. 3A–C). We performed immunofluorescence to analyze protein co-localization and intercellular contact sites. Quantification of CADM3 staining at intercellular contact sites revealed a significantly decreased amount of the mutant protein at contact sites compared to WT (Fig. 5B). Our results demonstrate that the mutant protein reaches the cell surface to a considerably lesser extent compared to the WT. Interestingly, the variant Gly368Cys also creates a new cysteine, similar to the previously reported Tyr172Cys variant, which might interfere with the native disulphide bond important for the formation of the Ig-like loops.
Figure 5.
Functional studies. (A) Western blot analysis of membrane and cytoplasmic proteins extracted from HEK293-transfected cells showing that both WT and mutant proteins are predominantly enriched in the membrane compared to the cytoplasm, with a decrease level of the mutant. (B) Immunofluorescence of CADM3 (red) and 4′,6-diamidino-2-phenylindole (blue) in U2OS cells transfected with WT-CADM3-HA and G368C-CADM3-HA. White arrows indicate intercellular contact sites. Scale bars = 10 μm. Quantification of CADM3 staining cell–cell contact sites in WT and mutant (P < 0.001, two-tailed t-test, n = 3). The quantification of CADM3 staining at intercellular contact sites revealed an important decrease number of the mutant protein at contact sites compared to WT. This demonstrates that the mutant protein reaches the cell surface to a considerably lesser extent compared to the WT.
Discussion
CMT2 is a clinical and genetic umbrella of inherited peripheral neuropathies caused by primarily axonal dysfunction of the peripheral nerves. In contrast to CMT1, only one-third of CMT2 patients have a genetic diagnosis.3,4 CADM3 is a recently identified CMT gene with the characteristic feature of upper limb involvement.12 The CADM3 gene encodes a member of the CADM family of cell–cell adhesion molecules and plays an important role in axon guidance, myelination and maintenance of the axonal architecture.12 Pathological studies have shown that the removal of axonal Cadm3 promotes Schwann cell myelination in the in vitro dorsal root ganglion neuron/Schwann cell myelinating system in addition to its roles in axon.15 Furthermore, using an animal model, Rebelo et al.12 demonstrated that knockin mice carrying a heterozygous Tyr170Cys mutation in the CADM3 gene (corresponding to the human Tyr172Cys mutation) showed a decreased expression of mutant Cadm3 in the sciatic nerve compared to controls and were consistent with abnormal axon–glia interactions. In this study, we investigated two families from different ethnic backgrounds, one of Fulani ethnicity from Mali and the other from American Caucasian descent with CMT2 phenotype, segregating the same pathogenic variant in CADM3. The clinical symptoms included distal muscle weakness and atrophy, foot and hand deformities, decreased or absent tendon reflexes and walking difficulties. Notably, a high phenotypic variability was seen within and between the families similar to the previous report.12 Some affected individuals showed more severe upper limb involvement when compared to the lower limbs. However, one patient in the Family 1 displayed a distinguishable phenotype with a pronounced segmental lower limb motor deficit, likely due to an overlap of CMT with poliomyelitis sequelae. In the first report of CMT2FF, an early onset of the disease was reported in three of the four patients with marked upper limb involvement. However, in one family, brisk reflexes suggested upper motor neuron involvement.12 In the families we report here, no symptoms of upper motoneuron involvement were noted, but the patient in Family 2 presented with foot deformities and a proximal weakness consistent with muscle involvement, pointing towards a clinical variability of CMT2FF. CADM3 is a cell adhesion molecule involved in maintaining the structure and function of cell junctions. Therefore, being membrane bound is an essential property of the protein. The variant described herein (Gly368Cys) is predicted to occur in a membrane-bound helix that is apparently crucial for the function of CADM3. The variant is a cysteine residue, and typically cysteine residues do not occur free in membrane proteins but rather form cysteine bridges.16 Therefore, the substitution to cysteine as a free residue might interfere with the function of CADM3 as a cell–cell adhesion molecule. More interestingly, western blot and immunofluorescence analyses showed that the mutant protein (Gly368Cys) reaches the cell surface to a considerably lesser extent compared to the WT protein. In addition, the variant Gly368Cys also creates a new cysteine, like the previously reported Tyr172C variant, which might interfere with the native disulphide bond important for the formation of the Ig-like loops.12
This is the second report of CADM3 neuropathy worldwide and the first that included a population of black ancestry. We present a comprehensive whole exome sequencing approach to describe the only second pathogenic variant in the CADM3 gene causing CMT2. Our study provides further evidence of the implication of CADM3 in the pathogenesis of CMT and raises the importance of collaborative studies including underrepresented populations like in Africa that can lead to the discovery of disease genes for more inclusivity in future therapeutic endeavour.
Supplementary Material
Acknowledgements
The authors thank the patients and families for their participation in this study and the Centre Hospitalier Universitaire du Point ‘G’, Bamako, Mali.
Contributor Information
Abdoulaye Yalcouyé, Faculté de Médecine et d’Odontostomatologie, USTTB, Bamako, Mali; Division of Human Genetics, Department of Pathology, University of Cape Town, Cape Town, South Africa.
Adriana P Rebelo, Dr. John T. Macdonald Foundation Department of Human Genetics, John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, Miami, USA.
Lassana Cissé, Faculté de Médecine et d’Odontostomatologie, USTTB, Bamako, Mali.
Lynette Rives, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, USA.
Salia Bamba, Faculté de Médecine et d’Odontostomatologie, USTTB, Bamako, Mali.
Joy Cogan, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, USA.
Kevin Esoh, Division of Human Genetics, Department of Pathology, University of Cape Town, Cape Town, South Africa.
Salimata Diarra, Faculté de Médecine et d’Odontostomatologie, USTTB, Bamako, Mali; Neurogenetics Branch, National Institutes of Neurological Disorders and Stroke, Bethesda, USA.
Kimberly M Ezell, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, USA.
Abdoulaye Taméga, Faculté de Médecine et d’Odontostomatologie, USTTB, Bamako, Mali.
Cheick O Guinto, Faculté de Médecine et d’Odontostomatologie, USTTB, Bamako, Mali; Service de Neurologie, Centre Hospitalier Universitaire Point ‘G’, Bamako, Mali.
Maike F Dohrn, Dr. John T. Macdonald Foundation Department of Human Genetics, John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, Miami, USA; Department of Neurology, Medical Faculty RWTH Aachen University, Aachen, Germany.
Rizwan Hamid, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, USA.
Kenneth H Fischbeck, Neurogenetics Branch, National Institutes of Neurological Disorders and Stroke, Bethesda, USA.
Stephan Zuchner, Dr. John T. Macdonald Foundation Department of Human Genetics, John P. Hussman Institute for Human Genomics, University of Miami Miller School of Medicine, Miami, USA.
Guida Landouré, Faculté de Médecine et d’Odontostomatologie, USTTB, Bamako, Mali; Neurogenetics Branch, National Institutes of Neurological Disorders and Stroke, Bethesda, USA; Service de Neurologie, Centre Hospitalier Universitaire Point ‘G’, Bamako, Mali.
Supplementary material
Supplementary material is available at Brain Communications online.
Funding
This work was supported by grants U01HG007044, funded by the National Institute of Neurological Disorders and Stroke (NINDS) and administered by the National Human Genome Research Institute under the H3Afria initiative, 5U54NS065712 and 5R01NS105755, also funded by the National Institute of Neurological Disorders and Stroke intramural research funds. M.F.D. received a scholarship by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG, DO 2386/1-1).
Competing interests
The authors report no competing interests.
Data availability
All data included in this study are available from the corresponding author upon reasonable request.
References
- 1.Bird TD.. Charcot-Marie-Tooth (CMT) hereditary neuropathy overview. In: Adam MPArdinger HH and Pagon RA, et al. eds. GeneReviews(®) [Internet]. University of Washington, Seattle; 1993. [Google Scholar]
- 2.Nagappa M, Sharma S, Taly AB. Charcot Marie Tooth. StatPearls. StatPearls Publishing. Copyright © 2021, StatPearls Publishing LLC.; 2021. [Google Scholar]
- 3.Pipis M, Rossor AM, Laura M, Reilly MM. Next-generation sequencing in Charcot-Marie-Tooth disease: Opportunities and challenges. Nat Rev Neurol. 2019;15(11):644–656. [DOI] [PubMed] [Google Scholar]
- 4.Lin S, Xu LQ, Xu GR, et al. Whole exome sequencing reveals a broader variant spectrum of Charcot-Marie-Tooth disease type 2. Neurogenetics. 2020;21(2):79–86. [DOI] [PubMed] [Google Scholar]
- 5.McCray BA, Scherer SS. Axonal Charcot-Marie-Tooth disease: From common pathogenic mechanisms to emerging treatment opportunities. Neurotherapeutics. 2021;18(4):2269–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fridman V, Bundy B, Reilly MM, et al. CMT subtypes and disease burden in patients enrolled in the Inherited Neuropathies Consortium natural history study: A cross-sectional analysis. J Neurol Neurosurg Psychiatry. 2015;86(8):873–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guinto CO, Diarra S, Diallo S, et al. A novel mutation in KIF5A in a Malian family with spastic paraplegia and sensory loss. Ann Clin Transl Neurol. 2017;4(4):272–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landouré G, Dembélé K, Diarra S, et al. A novel variant in the spatacsin gene causing SPG11 in a Malian family. J Neurol Sci. 2020;411:116675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landouré G, Mochel F, Meilleur K, et al. Novel mutation in the ATM gene in a Malian family with ataxia telangiectasia. J Neurol. 2013;260(1):324–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Traoré M, Landouré G, Motley W, et al. Novel mutation in the NHLRC1 gene in a Malian family with a severe phenotype of Lafora disease. Neurogenetics. 2009;10(4):319–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yalcouye A, Diallo SH, Coulibaly T, et al. A novel mutation in the GARS gene in a Malian family with Charcot-Marie-Tooth disease. Mol Genet Genom Med. 2019;7(7):e00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rebelo AP, Cortese A, Abraham A, et al. A CADM3 variant causes Charcot-Marie-Tooth disease with marked upper limb involvement. Brain. 2021;144(4):1197–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heo L, Park H, Seok C. GalaxyRefine: Protein structure refinement driven by side-chain repacking. Nucleic Acids Res. 2013;41(Web Server issue):W384–W388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schrödinger . PyMOL | pymol.org. 2019.
- 15.Stavrou M, Sargiannidou I, Christofi T, Kleopa KA. Genetic mechanisms of peripheral nerve disease. Neurosci Lett. 2021;742:135357. [DOI] [PubMed] [Google Scholar]
- 16.Grazhdankin E, Stepniewski M, Xhaard H. Modeling membrane proteins: The importance of cysteine amino-acids. J Struct Biol. 2020;209(1):107400. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data included in this study are available from the corresponding author upon reasonable request.