Abstract
Aims
The most appropriate timing of exercise therapy to improve cardiorespiratory fitness (CRF) among patients initiating chemotherapy is not known. The effects of exercise therapy administered during, following, or during and following chemotherapy were examined in patients with breast cancer.
Methods and results
Using a parallel-group randomized trial design, 158 inactive women with breast cancer initiating (neo)adjuvant chemotherapy were allocated to receive (1:1 ratio): usual care or one of three exercise regimens—concurrent (during chemotherapy only), sequential (after chemotherapy only), or concurrent and sequential (continuous) (n = 39/40 per group). Exercise consisted of treadmill walking three sessions/week, 20–50 min at 55%–100% of peak oxygen consumption (VO2peak) for ≈16 (concurrent, sequential) or ≈32 (continuous) consecutive weeks. VO2peak was evaluated at baseline (pre-treatment), immediately post-chemotherapy, and ≈16 weeks after chemotherapy. In intention-to-treat analysis, there was no difference in the primary endpoint of VO2peak change between concurrent exercise and usual care during chemotherapy vs. VO2peak change between sequential exercise and usual care after chemotherapy [overall difference, −0.88 mL O2·kg−1·min−1; 95% confidence interval (CI): −3.36, 1.59, P = 0.48]. In secondary analysis, continuous exercise, approximately equal to twice the length of the other regimens, was well-tolerated and the only strategy associated with significant improvements in VO2peak from baseline to post-intervention (1.74 mL O2·kg−1·min−1, P < 0.001).
Conclusion
There was no statistical difference in CRF improvement between concurrent vs. sequential exercise therapy relative to usual care in women with primary breast cancer. The promising tolerability and CRF benefit of ≈32 weeks of continuous exercise therapy warrant further evaluation in larger trials.
Keywords: Aerobic training, Exercise capacity, Cardiorespiratory fitness, Treatment sequencing
Structured Graphical Abstract
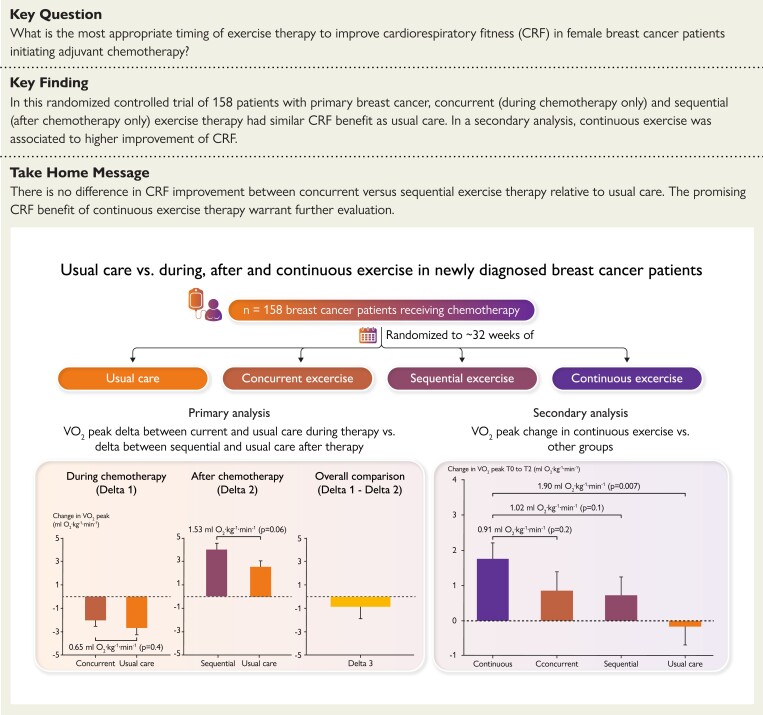
Structured Graphical Abstract.
Summary of the key findings of this study. The most appropriate timing of exercise therapy to improve cardiorespiratory fitness (VO2peak) in cancer patients initiating adjuvant chemotherapy is not known. In this randomized controlled trial of 158 patients with primary breast cancer, concurrent (during chemotherapy only) and sequential (after chemotherapy only) had similar VO2peak benefit. Continuous (concurrent plus sequential) exercise was the only schedule associated with significant VO2peak improvements compared to baseline.
See the editorial comment for this article ‘Best timing for exercise in breast cancer patients initiating chemotherapy: what is the answer?’, by A. Abreu, https://doi.org/10.1093/eurheartj/ehad136.
Introduction
Adjuvant breast cancer chemotherapy improves clinical outcomes but causes physiological toxicity.1,2 Cardiorespiratory fitness (CRF), an integrative measure of whole-body cardiovascular function, declines between ≈ 5 to ≈ 15% during four to six months of standard adjuvant chemotherapy, 3–7 the equivalent to 5–15 years of normal aging.8 The marked decline in CRF predisposes to excess non-cancer competing morbidity and mortality, 9,10 and its attendant symptom burden.11 The efficacy of prophylactic exercise therapy initiated concurrent with adjuvant breast cancer chemotherapy to attenuate the observed CRF decline is inconsistent. Exercise therapy tolerability, as defined by rates of attrition and adherence rates, is also suboptimal.3,6,12 In contrast, exercise therapy is well-tolerated and associated with consistent CRF improvements in the post-treatment setting.13,14 Pan-cancer meta-analyses indicate exercise therapy administered in the post-treatment setting is associated with superior improvements in CRF relative to exercise therapy concurrent with active therapy.15,16
The apparent superior tolerability and CRF benefit of exercise therapy in the post-treatment setting has raised questions regarding its relative merits during active treatment.17,18 Real-world studies reflect this notion. Exercise therapy and general physical activity are infrequently discussed or recommended during oncology treatment consultations.19,20 Avoidance or minimizing exercise therapy during this period may, however, heighten susceptibility and severity of physiological and symptom-related toxicity. Lack of oncologist recommendation may also discourage participation in exercise therapy—a strategy that is of great interest to patients seeking to gain some control of their disease management.21,22 Studies investigating the most appropriate timing of exercise therapy for breast cancer patients initiating adjuvant chemotherapy are required.
We conducted a Phase 2, four-arm randomized controlled trial (RCT) to compare the tolerability and efficacy of exercise therapy administered concurrent or sequential to chemotherapy, relative to general physical activity advice (usual care), in patients with primary breast cancer. We hypothesized concurrent exercise therapy would be associated with superior improvements in CRF compared with sequential exercise therapy relative to usual care. A protocol-specified secondary objective evaluated the tolerability and efficacy of exercise therapy administered concurrent and sequential (i.e. continuous) to chemotherapy.
Methods
Trial design and patients
The full methods and protocol are provided in the Supplement. Using a parallel-group, four-arm design (see Supplementary material online, Figure S1A), women with invasive, node-negative, or node-positive breast adenocarcinoma (stage I, II, or III) initiating (neo)adjuvant chemotherapy at Duke University Medical Center (DUMC) or Memorial Sloan Kettering Cancer Center (MSK) were eligible. Additional eligibility were self-reported inactivity (i.e. < 150 min of moderate or vigorous exercise per week23), and be able to complete an acceptable cardiopulmonary exercise test (CPET).24 The study was approved by the DUMC and MSK institutional review boards. All patients provided written informed consent.
Procedures
Study participation comprised two phases: (1) ‘during chemotherapy’—period between randomization (T0) and completion of the final chemotherapy cycle (≈14–20 weeks) (T1), and (2) ‘after chemotherapy’—period between the completion of the final chemotherapy cycle (T1) to post-intervention (≈28–40 weeks post-randomization) (T2). Chemotherapy regimen and additional adjuvant therapy was provided per oncologist discretion. The T1–T2 period was matched in length to the T0-T1 period for each patient; thus, the total study length (T0–T2) for all patients was ≈ 28–40 weeks (see Supplementary material online, Figure S1A). Patients were randomly allocated (1:1:1:1) to receive usual care or one of three exercise therapy regimens: (i) concurrent [administered for the length of chemotherapy (≈14–20 weeks)], (ii) sequential [initiated within 14 days of final chemotherapy cycle for ≈ 14–20 weeks], or (iii) continuous [administered during chemotherapy (≈14–20 weeks) and continued after the final chemotherapy cycle for an additional ≈ 14–20 weeks (i.e. ≈ 28–40 weeks in total)].
Study interventions
Exercise therapy included aerobic exercise only comprised of individualized supervised treadmill walking (Jog Excite 700 or Jog Forma, Technogym, Inc.) three times weekly for 20 to 50 min/session (duration range: 60 to 125 min/week). Resistance training was not performed. The dose-intensity of each session alternated between 55% to 100% of each patient’s individually measured CRF (VO2peak) at pre-randomization (T0) or midpoint (T1) consistent with a non-linear (periodized) schedule.13 Exercise therapy dose modification was permitted and performed using standardized criteria (see Supplementary material online, Table S1).25,26 Patients allocated to usual care received home-based advice to perform unsupervised physical activity three days/week for 30 min/session for ≈ 28–40 weeks.23 General physical activity advice was provided to the usual care group, as opposed to no intervention, to facilitate accrual, and minimize lost to follow-up and exercise contamination.27
Endpoints
The primary endpoint was change in VO2peak (ml O2.kg−1.min−1) evaluated by direct measurement of expired gas analysis (ParvoMedics, TrueOne 2400, USA) using a symptom-limited CPET on an electronic motorized treadmill (GE Healthcare, T-2100, USA) with continuous 12-lead ECG analysis (GE Healthcare, Case Stress Testing System, USA).24 Secondary endpoints were other CPET variables, patient-reported outcomes (PROs), cardiac [left ventricular ejection fraction (LVEF)] function, arterial stiffness, exercise therapy tolerability, and safety. PROs were quality of life (Functional Assessment of Cancer Therapy-General), FACT-Breast, 28 fatigue, 29 pain (Brief Pain Inventory), 30 sleep quality (Pittsburgh Sleep Quality Index), 31 and physical function (Medical Outcomes Trust Short Form Health Survey).32 Resting LVEF (IE33; Philips, The Netherlands) was assessed according to standard guidelines.33 Arterial stiffness central (carotid-femoral) and peripheral (carotid-radial) pulse wave velocity were assessed using handheld tonometers (SPT-301; Millar Instruments, USA) according to standard guidelines.34 All endpoints were evaluated at T0, T1, and T2. Tolerability was evaluated by lost to follow-up, exercise therapy attendance (ratio of attended to planned sessions), and relative dose-intensity (RDI, ratio of total ‘completed’ to total ‘planned’ cumulative exercise therapy dose).25,26 Safety was evaluated by the type and prevalence of adverse events during exercise therapy.26
Statistical analysis
The sample size calculation was based on the primary analysis only: change in VO2peak between concurrent exercise therapy and usual care during chemotherapy (T0 to T1) vs. change in VO2peak between sequential exercise therapy and usual care (T1 to T2) after chemotherapy (see Supplementary material online, Figure S1B). Using a two-sample t-test with 34 patients per group provided 80% power using a two-tailed Type I error of 0.05. To account for an anticipated lost to follow-up rate of 15%, the sample size was increased to 40 patients per group. A protocol-specified secondary analysis compared continuous exercise therapy with all other groups separately from baseline to post-intervention (T0 to T2; Supplementary material online, Figure S1C) using pair-wise comparisons.
All analyses were conducted under the intention-to-treat (ITT) principle. All endpoints were modeled using linear mixed models with random intercepts for patients, an unstructured covariance matrix, and factors (fixed effects) for study group and timepoint and their interaction. All patients had one CPET measurement at T0 and all were included in the analysis with the estimation of patient-specific random intercepts. The missed time points are not included in the estimation process, but mixed effects models use maximum likelihood methods to estimate the means and differences rather than taking simple averages and differences. Differences were estimated using model-estimated marginal means, and the hypothesis tests were conducted using contrasts. Results are presented as mean ± standard error (SE) unless otherwise specified. Sensitivity analyses restricted to patients with complete CPET data (i.e. T0, and T1 and/or T2) and adjusting for chemotherapy schedule (neoadjuvant vs. adjuvant) where the model remained the same were conducted for the primary and secondary analyses of the primary endpoint. All analyses were conducted using R version 4.1.1.35–37 Statistical significance was determined by p < 0.05 except for the secondary analysis where the alpha was Bonferroni adjusted for the three pair-wise comparisons: .
Results
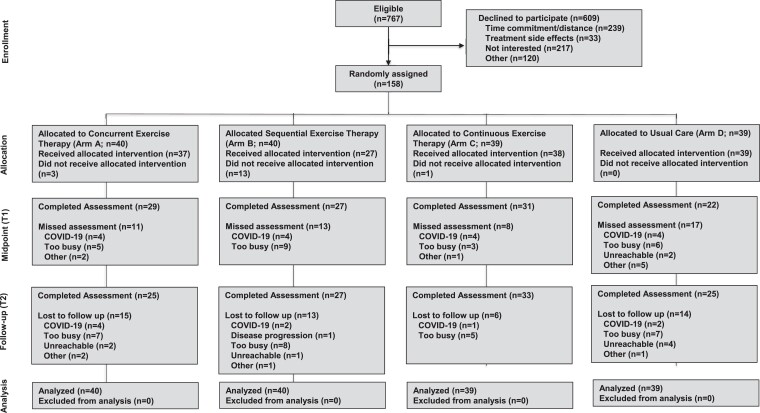
Between December 2012 and March 2020, a total of 158 patients initiating (neo)adjuvant chemotherapy were randomized to: concurrent (n = 40), sequential (n = 40), and continuous exercise therapy (n = 39) or usual care (n = 39) (Figure 1). Patient accrual was stopped early due to the COVID-19 pandemic. Participant baseline characteristics were balanced between arms (Table 1). For the overall cohort, mean pre-randomization VO2peak was 24.9 ± 5.1 mL O2·kg−1·min−1, the equivalent of 24% below normative values.38 No patient had evidence of systolic dysfunction (LVEF <50%) at baseline.39 Median [quartile (Q1–Q3]) time from start of chemotherapy to randomization for all patients was 10 (5–14) days.
Figure 1.
CONSORT flow for non-pharmacological trials. Definitions. Did not receive allocated intervention: Did not complete at least 1 exercise therapy session; Lost to follow-up: non-completion of the cardiopulmonary exercise test assessment at T2. COVID-19: coronavirus disease 2019.
Table 1.
Characteristics of the participants at baseline
| Characteristic | All | Concurrent exercise | Sequential exercise | Continuous exercise | Usual care |
|---|---|---|---|---|---|
| (n = 158) | (n = 40) | (n =40) | (n = 39) | (n = 39) | |
| Study site—no. (%) | |||||
| DUMC | 14 (9) | 4 (10) | 4 (10) | 3 (8) | 3 (8) |
| MSK | 144 (91) | 36 (90) | 36 (90) | 36 (92) | 36 (92) |
| Age (yrs)—mean (SD) | 47 (11) | 50 (11) | 46 (10) | 48 (12) | 45 (10) |
| BMI (kg/m2)—mean (SD) | 27 (6) | 27 (6) | 27 (5) | 28 (6) | 27 (7) |
| Smoking status—no. (%) | |||||
| Never | 99 (63) | 24 (60) | 25 (62) | 26 (67) | 24 (62) |
| Former | 41 (26) | 10 (25) | 11 (28) | 9 (23) | 11 (28) |
| Current | 4 (2.5) | 2 (5.0) | 0 (0) | 1 (2.6) | 1 (2.6) |
| Unknown | 14 (8.9) | 4 (10) | 4 (10) | 3 (7.7) | 3 (7.7) |
| Exercise behavior (minutes/week)a—median (range) | 0 [0, 150] | 0 [0, 140] | 0 [0, 150] | 0 [0, 135] | 0 [0, 135] |
| VO2peak, ml O2.kg−1.min−1—mean (SD) | 24.9 (5.1) | 25.2 (6.1) | 25.2 (4.7) | 23.9 (4.7) | 25.4 (4.8) |
| Resting left ventricular ejection fraction, %—mean (SD) | 63.3 (2.7) | 63.5 (2.1) | 63.2 (3.3) | 63.0 (3.0) | 63.3 (2.2) |
| Unknown—no. (%) | 11 (7) | 4 (10) | 1 (3) | 2 (5) | 4 (10) |
| Resting heart rate, beats.min−1—mean (SD) | 79 (11) | 77 (10) | 78 (9) | 81 (13) | 80 (10) |
| Resting systolic blood pressure, mmHg—mean (SD) | 115 (14) | 116 (13) | 115 (13) | 114 (14) | 115 (15) |
| Resting diastolic blood pressure, mmHg—mean (SD) | 70 (9) | 71 (10) | 70 (9) | 71 (8) | 69 (10) |
| Peak heart rate, beats.min−1—mean (SD) | 173 (15) | 171 (14) | 172 (14) | 173 (16) | 177 (14) |
| Peak systolic blood pressure, mmHg—mean (SD) | 172 (18) | 176 (16) | 172 (19) | 174 (19) | 167 (18) |
| Unknown—no. (%) | 22 (14) | 8 (20) | 6 (15) | 3 (8) | 5 (13) |
| Peak diastolic blood pressure, mmHg—mean (SD) | 65 (9) | 64 (8) | 64 (8) | 67 (12) | 64 (10) |
| Unknown—no. (%) | 22 (14) | 8 (20) | 6 (15) | 3 (8) | 5 (13) |
| Race and ethnicity—no. (%) b | |||||
| Asian | 16 (10) | 6 (15) | 3 (8) | 2 (5) | 5 (13) |
| Hispanic | 23 (15) | 2 (5) | 8 (20) | 4 (10) | 9 (23) |
| Native Hawaiian or other Pacific Islander | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Non-Hispanic Black | 27 (17) | 8 (20) | 6 (15) | 8 (21) | 5 (13) |
| Non-Hispanic White | 78 (49) | 21 (52) | 21 (52) | 20 (51) | 16 (41) |
| Other | 5 (3) | 0 (0) | 1 (3) | 1 (3) | 3 (8) |
| Unknown | 9 (6) | 3 (8) | 1 (3) | 4 (10) | 1 (3) |
| Disease stage—no. (%) | |||||
| IA | 51 (32) | 10 (25) | 13 (32) | 16 (41) | 12 (31) |
| IB | 5 (3) | 1 (3) | 1 (3) | 2 (5) | 1 (3) |
| IIA | 40 (25) | 13 (32) | 14 (35) | 6 (15) | 7 (18) |
| IIB | 40 (25) | 10 (25) | 6 (15) | 13 (33) | 11 (28) |
| IIIA | 16 (10) | 3 (8) | 6 (15) | 2 (5) | 5 (13) |
| IIIB | 2 (1) | 0 (0) | 0 (0) | 0 (0) | 2 (5) |
| IIIC | 4 (3) | 3 (8) | 0 (0) | 0 (0) | 1 (3) |
| Clinical subtype—no. (%) | |||||
| ER+/PR+ | 92 (58) | 19 (48) | 27 (68) | 19 (49) | 27 (69) |
| HER2+ | 18 (11) | 6 (15) | 4 (10) | 5 (13) | 3 (8) |
| ER−/PR−/HER2− | 33 (21) | 11 (28) | 6 (15) | 9 (23) | 7 (18) |
| Other | 15 (10) | 4 (10) | 3 (8) | 6 (15) | 2 (5) |
| Surgery—no. (%) | |||||
| Lumpectomy | 83 (53) | 21 (52) | 21 (52) | 19 (49) | 22 (56) |
| Mastectomy | 73 (46) | 18 (45) | 19 (48) | 20 (51) | 16 (41) |
| Other | 2 (1) | 1 (3) | 0 (0) | 0 (0) | 1 (3) |
| Chemotherapy—no. (%) | |||||
| Dose-dense | 129 (82) | 35 (88) | 31 (78) | 30 (77) | 33 (85) |
| Neoadjuvant | 49 (31) | 16 (40) | 12 (30) | 8 (21) | 13 (33) |
| Anthracycline-containing | 110 (70) | 30 (75) | 28 (70) | 24 (62) | 28 (72) |
| Anthracycline and Capecitabine | 8 (5) | 4 (10) | 1 (3) | 2 (5) | 1 (3) |
| Antibody therapy—no. (%) | 38 (24) | 9 (22) | 14 (35) | 6 (15) | 9 (23) |
| Unknown | 14 (9) | 4 (10) | 4 (10) | 3 (8) | 3 (8) |
| Radiotherapy—no. (%) | 121 (77) | 31 (78) | 29 (73) | 29 (74) | 32 (82) |
| Left-sided | 56 (35) | 10 (25) | 15 (38) | 13 (33) | 18 (46) |
| Endocrine therapy—no. (%) | 111 (70) | 29 (72) | 30 (75) | 24 (62) | 28 (72) |
| Current CVD medications—no. (%) | |||||
| Statin | 16 (10) | 8 (20) | 5 (12) | 3 (8) | 0 (0) |
| Beta-blockers | 11 (7) | 2 (5) | 3 (8) | 5 (13) | 1 (3) |
| Aspirin/anti-platelet | 8 (5) | 3 (8) | 3 (8) | 2 (5) | 0 (0) |
| Diabetes medication | 7 (4) | 5 (12) | 2 (5) | 0 (0) | 0 (0) |
| Calcium channel blocker | 7 (4) | 0 (0) | 4 (10) | 2 (5) | 1 (3) |
| Angiotensin receptor blockers | 6 (4) | 2 (5) | 2 (5) | 2 (5) | 0 (0) |
| ACE inhibitors | 5 (3) | 2 (5) | 2 (5) | 1 (3) | 0 (0) |
| Diuretic | 2 (1) | 1 (3) | 1 (3) | 0 (0) | 0 (0) |
| Pre-existing (controlled) CVD conditions—no. (%) | |||||
| Type 2 diabetes | 8 (5) | 5 (12) | 2 (5) | 1 (3) | 0 (0) |
| Hyperlipidemia | 14 (9) | 8 (20) | 4 (10) | 2 (5) | 0 (0) |
| Hypertension | 21 (13) | 5 (12) | 7 (18) | 8 (21) | 1 (3) |
| Any | 27 (17) | 9 (22) | 8 (20) | 9 (23) | 1 (3) |
aExercise defined as the total minutes of self-reported moderate/vigorous exercise per week.
bOther race category self-defined as ‘other’.
Chemotherapy, radiation, and endocrine therapy rates include only those patients receiving each treatment.
CVD, cardiovascular disease; DUMC, Duke University Medical Center; MSK, Memorial Sloan Kettering; SD, standard deviation; BMI, body mass index; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; ACE, angiotensin converting enzyme.
Primary analysis
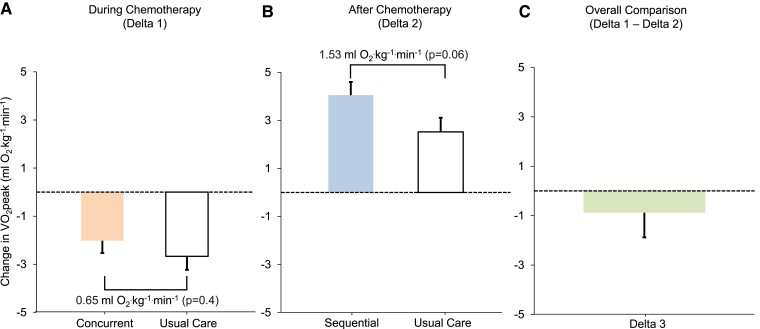
Delta VO2peak between concurrent exercise therapy and usual care during chemotherapy (i.e. T0 to T1) was 0.65 mL O2.kg−1.min−1 (± 0.75) (P = 0.4) (Figure 2A). Delta VO2peak between sequential exercise therapy and usual care after chemotherapy (i.e. T1 to T2) was 1.53 mL O2.kg−1.min−1 (± 0.80) (P = 0.06) (Figure 2B). There was no difference for delta VO2peak between concurrent exercise therapy and usual care during chemotherapy compared to delta VO2peak between sequential exercise therapy and usual care after chemotherapy (overall mean difference, −0.88 ± 1.26 mL O2.kg−1.min−1; P = 0.48 Figure 2C). Sensitivity analyses results did not differ from the primary results (see Supplementary material online, Tables S2 and S3). A similar pattern was observed for other CPET variables (Table 2), and other secondary endpoints (see Supplementary material online, Table S4). Non-protocol, self-reported exercise increased in all groups with no differences between exercise therapy groups and usual care (Table 2). Patient-level (non-model-estimated) changes in VO2peak during chemotherapy ranged from −8.10 to 2.40 mL O2·kg−1·min−1 and −9.30 to 5.70 mL O2·kg−1·min−1 in concurrent exercise therapy and usual care, respectively. Patient-level changes in VO2peak after chemotherapy ranged from −2.40 to 7.70 mL O2·kg−1·min−1 and 0.90 to 6.50 mL O2·kg−1·min−1 in sequential exercise therapy and usual care, respectively.
Figure 2.
Change in model-estimated marginal means of VO2peak. Panel A: Change in VO2peak during chemotherapy for concurrent exercise therapy and usual care (Delta 1). Panel B: Change in VO2peak after chemotherapy for sequential exercise therapy and usual care (Delta 2). Panel C: Overall comparison (Delta 3): difference between Delta 1 and Delta 2 (i.e. difference for the change in VO2peak between concurrent exercise therapy and usual care during chemotherapy vs. change in VO2peak between sequential exercise therapy and usual care after chemotherapy). Error bars indicate 1 standard error. VO2peak, peak oxygen consumption; T0; baseline; T1; immediately after last chemotherapy cycle; T2, post-intervention at ≈ 28–40 weeks post-randomization.
Table 2.
Differences in study endpoints for the primary and protocol-specified analyses
| Primary analysis (concurrent and sequential vs. usual care) | Secondary analysis (continuous vs. other arms, T2-T0)c | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Difference between groups | Overall differencea | |||||||||||
| Variable | Concurrent vs. UC (T1-T0) | P | Sequential vs. UC (T2-T1) | P | Concurrent and UC (T1-T0) vs. sequential and UC (T2-T1) | P b | Delta continuous vs. concurrent | P | Delta continuous vs. sequential | P | Delta continuous vs. UC | P |
| Primary endpoint | ||||||||||||
| VO2peak, ml O2.kg−1.min−1 | 0.65 (−0.83, 2.13) | 0.39 | 1.53 (−0.04, 3.11) | 0.06 | −0.88 (−3.36, 1.59) | 0.48 | 0.91 (−0.79, 2.61) | 0.21 | 1.02 (−0.62, 2.66) | 0.14 | 1.90 (0.22, 3.57) | 0.007 |
| Secondary endpoints | ||||||||||||
| Resting cardiovascular function | ||||||||||||
| Heart rate, beats.min−1 | −1.80 (−7.76, 4.16) | 0.55 | −2.99 (−9.47, 3.49) | 0.37 | 1.19 (−8.91, 11.29) | 0.82 | −7.08 (−13.89, −0.27) | 0.01 | −2.91 (−9.61, 3.80) | 0.31 | −1.63 (−8.47, 5.20) | 0.57 |
| Systolic blood pressure, mmHg | −5.26 (−12.34, 1.82) | 0.15 | 6.78 (−0.91, 14.46) | 0.08 | −12.04 (−24.02, −0.05) | 0.05 | 0.64 (−7.44, 8.72) | 0.85 | 3.96 (−4.00, 11.92) | 0.24 | 1.50 (−6.62, 9.61) | 0.66 |
| Diastolic blood pressure, mmHg | −4.15 (−8.74, 0.45) | 0.08 | 1.51 (−3.48, 6.50) | 0.55 | −5.66 (−13.44, 2.13) | 0.15 | −0.87 (−6.12, 4.37) | 0.69 | −1.65 (−6.82, 3.52) | 0.45 | −1.98 (−7.25, 3.29) | 0.38 |
| Other peak CPET variables | ||||||||||||
| VO2peak, L O2.min−1 | 0.04 (−0.06, 0.14) | 0.45 | 0.10 (−0.01, 0.20) | 0.07 | −0.06 (−0.23, 0.10) | 0.47 | 0.09 (−0.02, 0.21) | 0.05 | 0.06 (−0.05, 0.17) | 0.22 | 0.12 (0.01, 0.23) | 0.01 |
| RER | −0.04 (−0.09, 0.01) | 0.16 | −0.01 (−0.07, 0.04) | 0.68 | −0.02 (−0.11, 0.06) | 0.57 | 0.01 (−0.05, 0.07) | 0.63 | 0.00 (−0.06, 0.05) | 0.90 | 0.01 (−0.05, 0.06) | 0.77 |
| Ventilation, L O2.min−1 | −2.65 (−8.11, 2.80) | 0.34 | 5.21 (−0.64, 11.05) | 0.08 | −7.86 (−17.01, 1.30) | 0.09 | 5.45 (−0.83, 11.74) | 0.04 | 3.46 (−2.61, 9.53) | 0.18 | 6.11 (−0.08, 12.30) | 0.02 |
| Heart rate, beats.min−1 | −1.79 (−6.85, 3.28) | 0.49 | −1.59 (−7.00, 3.81) | 0.56 | −0.19 (−8.68, 8.29) | 0.96 | 0.86 (−4.97, 6.69) | 0.73 | 2.05 (−3.58, 7.68) | 0.39 | 0.92 (−4.82, 6.66) | 0.71 |
| Systolic blood pressure, mmHg | −1.26 (−12.12, 9.61) | 0.82 | −3.09 (−14.59, 8.42) | 0.60 | 1.83 (−16.29, 19.95) | 0.84 | 5.99 (−6.63, 18.60) | 0.26 | 6.53 (−5.42, 18.47) | 0.20 | 0.39 (−11.58, 12.37) | 0.94 |
| Diastolic blood pressure, mmHg | −0.65 (−6.22, 4.93) | 0.82 | −3.74 (−9.65, 2.17) | 0.22 | 3.09 (−6.21, 12.40) | 0.51 | −2.64 (−9.10, 3.83) | 0.34 | −3.16 (−9.29, 2.97) | 0.22 | −4.22 (−10.37, 1.93) | 0.01 |
| Exercise behavior, minutes/week | −18.29 (−47.15, 10.56) | 0.21 | −5.88 (−37.57, 25.81) | 0.72 | −12.41 (−61.36, 36.54) | 0.62 | −16.80 (−50.33, 16.74) | 0.24 | −0.41 (−33.97, 33.15) | 0.98 | 0.76 (−33.12, 34.65) | 0.96 |
Data presented are model-estimated marginal means (95% confidence interval).
aChange in endpoint between concurrent exercise and usual care during chemotherapy (T0 to T1) vs. change in endpoint between sequential exercise and usual care (T1 to T2) after chemotherapy.
b P-value for delta between concurrent and usual care (T1-T0) vs. vs. sequential and usual care (T2-T1).
c P-values significant at Bonferroni adjusted alpha level (<0.017).
T0; baseline; T1; immediately after last chemotherapy cycle; T2, post-intervention at ≈ 28–40 weeks post-randomization; CPET, cardiopulmonary exercise test; RER, respiratory exchange ratio; UC, usual care.
Protocol-specified secondary analysis
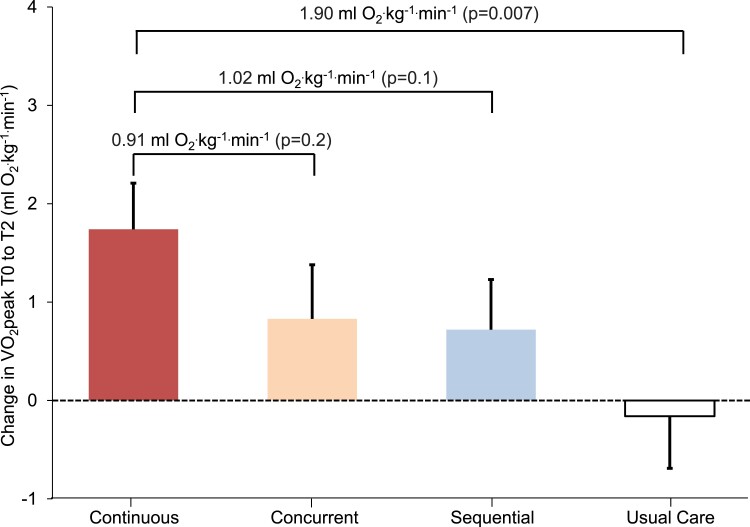
Continuous exercise therapy was associated with a 1.74 mL O2.kg−1.min−1 (± 0.47) (P < 0.001) increase in VO2peak from baseline to post-intervention (i.e. T0 to T2) compared with a 0.83 mL O2.kg−1.min−1 (± 0.55) (P = 0.13), 0.72 mL O2.kg−1.min−1 (± 0.51) (P = 0.16), and −0.16 mL O2.kg−1.min−1 (± 0.53) (P = 0.77) change in concurrent exercise therapy, sequential exercise therapy, and usual care, respectively (Table 2; Figure 3). The comparison between continuous exercise therapy and usual care was significant (mean difference: 1.90 ± 0.71 mL O2.kg−1.min−1; P = 0.007). Similar patterns were observed for secondary endpoints (see Supplementary material online, Table S5). VO2peak either returned to baseline levels or beyond at post-intervention (T2) in 70% of patients in the continuous exercise therapy group compared with 60%, 44%, and 40% in concurrent exercise therapy, sequential exercise therapy, and usual care, respectively.
Figure 3.
Change in model-estimated marginal means of VO2peak from baseline to post-intervention (T0 to T2). Error bars indicate 1 standard error. VO2peak, peak oxygen consumption; T0; baseline; T2, post-intervention at ≈ 28–40 weeks post-randomization.
Tolerability and safety
Lost to follow-up was significantly lower with continuous exercise therapy (13%) compared with all other groups (32% to 40%; P = 0.04). Median RDI was 70% (range, 0% to 100%) and 84% (range, 0% to 100%) with concurrent exercise therapy and sequential exercise therapy, respectively compared with 83% (range, 0% to 98%; 81% during chemotherapy, 82% after chemotherapy) with continuous exercise therapy (P = 0.78; Table 3). No serious adverse events were observed. The prevalence of exercise-induced tachycardia, the most common reported non-serious adverse event was 39%, 3%, and 39% with concurrent, sequential, and continuous exercise therapy, respectively (P < 0.001; Table 4).
Table 3.
Tolerability of exercise regimens
| Variable | All | Concurrent exercise | Sequential exercise | Continuous exercise | Usual care | Pa |
|---|---|---|---|---|---|---|
| (n = 158) | (n = 40) | (n = 40) | (n = 39) | (n = 39) | ||
| Intervention length, weeks—median (range) | 20 (9, 46) | 16 (9, 22) | 15 (9, 24) | 28 (14, 42) | 28 (12, 46) | N/A |
| Lost to follow-up—no. (%) | 48 (30) | 16 (40) | 13 (32) | 5 (13) | 14 (36) | 0.04 |
| Attendance, %—median (range) | 78 (0, 100) | 71 (0, 100) | 84 (0, 100) | 82 (0, 97) | N/A | 0.74 |
| Permanent discontinuation—no. (%) | 40 (34) | 13 (32) | 17 (42) | 10 (26) | N/A | 0.28 |
| Dose interruption—no. (%) | 77 (65) | 28 (70) | 16 (40) | During: 23 (59) After: 28 (72) Overall: 33 (87) | N/A | <0.001 |
| Dose modification—no. (%) | 12 (10) | 4 (10) | 4 (10) | 4 (10) | N/A | >0.99 |
| Pre-treatment dose modification—no. (%) | 2 (2) | 0 (0) | 0 (0) | 2 (5) | N/A | 0.11 |
| Early session termination—no. (%) | 44 (37) | 17 (42) | 7 (18) | 20 (51) | N/A | 0.005 |
| Relative dose-intensity, %—median (range) | 78 (0, 100) | 70 (0, 100) | 84 (0, 100) | During: 81 (0, 100) After: 82 (0, 100) Overall: 83 (0, 98) | N/A | 0.78 |
Definitions. Lost to follow-up: non-completion of the cardiopulmonary exercise test assessment at post-intervention; attendance: ratio of total number of attended to planned treatments; permanent discontinuation: permanent discontinuation of treatment prior to T1 (concurrent) or T2 (sequential and continuous); dose interruption: missing ≥3 consecutive sessions; dose modification: ≥ 10% of sessions requiring modification (reduction/escalation) of intensity or duration; pre-treatment dose modification: reduction of pre-treatment session intensity; early session termination: early termination of planned session duration; relative dose-intensity, the ratio of total ‘completed’ to total ‘planned’ cumulative dose.
aKruskal–Wallis rank sum test; Pearson's chi-squared test for differences across all applicable groups.
bAll variables are collectively counted as 1 entity in the same patient unless otherwise indicated.
no, number; N/A, not applicable.
Table 4.
Adverse events during exercise therapy sessions
| Event | Overall | Concurrent exercise | Sequential exercise | Continuous exercise | P b |
|---|---|---|---|---|---|
| (n = 108)a | (n = 36) | (n = 36) | (n = 36) | ||
| Cardiopulmonary | |||||
| Exercise-induced tachycardia | 29 (27%) | 14 (39%) | 1 (2.8%) | 14 (39%) | <0.001 |
| Resting tachycardia | 18 (17%) | 8 (22%) | 3 (8.3%) | 7 (19%) | 0.25 |
| Fatigue | 9 (8.3%) | 3 (8.3%) | 0 (0%) | 6 (17%) | 0.04 |
| Dizziness | 7 (6.5%) | 2 (5.6%) | 1 (2.8%) | 4 (11%) | 0.50 |
| Hypertension | 5 (4.6%) | 1 (2.8%) | 1 (2.8%) | 3 (8.3%) | 0.62 |
| Dyspnea | 3 (2.8%) | 0 (0%) | 0 (0%) | 3 (8.3%) | 0.10 |
| Post-exercise tachycardia | 2 (1.9%) | 0 (0%) | 1 (2.8%) | 1 (2.8%) | >0.99 |
| Musculoskeletal/other | |||||
| Arthralgia | 6 (5.6%) | 2 (5.6%) | 1 (2.8%) | 3 (8.3%) | 0.87 |
| Back pain | 4 (3.7%) | 1 (2.8%) | 1 (2.8%) | 2 (5.6%) | >0.99 |
| Abdominal pain | 3 (2.8%) | 1 (2.8%) | 1 (2.8%) | 1 (2.8%) | >0.99 |
| Nausea | 3 (2.8%) | 1 (2.8%) | 0 (0%) | 2 (5.6%) | 0.77 |
| Foot pain | 2 (1.9%) | 1 (2.8%) | 0 (0%) | 1 (2.8%) | >0.99 |
| Generalized muscle weakness | 1 (0.9%) | 0 (0%) | 0 (0%) | 1 (2.8%) | >0.99 |
| Hot flashes | 1 (0.9%) | 0 (0%) | 0 (0%) | 1 (2.8%) | >0.99 |
| Leg fatigue | 1 (0.9%) | 1 (2.8%) | 0 (0%) | 0 (0%) | >0.99 |
| Myalgia | 1 (0.9%) | 0 (0%) | 0 (0%) | 1 (2.8%) | >0.99 |
| Peripheral sensory neuropathy | 1 (0.9%) | 0 (0%) | 1 (2.8%) | 0 (0%) | >0.99 |
Data presented as number of patients (%). Events counted once per patient as one entity.
aAdverse events are summarized for Memorial Sloan Kettering Cancer Center patients allocated to exercise therapy.
bFisher's exact test; Pearson's chi-squared test.
Definitions. Adverse categorized according to Common Terminology Criteria for Adverse Events and Exercise Oncology Exercise Physiology standard guidelines which included: Exercise-induced tachycardia: Exercise session heart rate ≥ 10 beats per min outside of prescribed range; post-exercise tachycardia: heart rate not recovered to below 100 beats per minute within 20 min post-exercise.
Discussion
International organizations recommend exercise therapy both during and following adjuvant chemotherapy, 23,40–42 yet the most appropriate timing of exercise therapy relative to treatment initiation is not known. We employed a novel design evaluating exercise therapy in the context of changes observed within usual care in the same setting and patient cohort. This permitted direct comparison of concurrent vs. sequential use of exercise therapy relative to chemotherapy administration. This trial failed to support the primary hypothesis: there was no statistical difference in CRF improvement between exercise therapy administered concurrent vs. sequential to chemotherapy, relative to usual care (Structured Graphical Abstract). Several findings warrant discussion in this paradigm.
First, findings of our study are consistent with several prior contemporary trials showing concurrent use of exercise therapy is associated with attenuation as opposed to complete abrogation of declines in CRF during adjuvant breast cancer chemotherapy.6,12,43,44 In the Combined Aerobic and Resistance Exercise (CARE) trial, all exercise regimens investigated (standard dose aerobic training; combined aerobic and resistance training, and high dose aerobic training) failed to attenuate significant declines in CRF during taxane-based chemotherapy.6 Similarly, in the Physical exercise during Adjuvant Chemotherapy Effectiveness Study (PACES), a three-arm RCT of either a clinic-based or home-based exercise regimen vs. usual care, CRF declined in all groups but to lesser extent in the exercise groups.12 Finally, the Optimal Timing of Physical Activity in Cancer Treatment (ACT) Trial evaluated 24 weeks of exercise training initiated during or after chemotherapy in patients with breast, testicular, or colon cancer. From baseline to immediately post-chemotherapy CRF declined significantly in both groups; however, this decrease was significantly attenuated by exercise during chemotherapy.43 These findings are contrary to the earlier Supervised Trial of Aerobic vs. Resistance Training (START) trial, reporting standard dose aerobic training but not resistance training completely abrogated the CRF decline observed in the usual care group.4 The reasons for the discrepant findings may relate to differences in timing of exercise therapy relative to chemotherapy administration, patient characteristics, and regional differences in standard use of chemotherapeutics. Any benefit of exercise therapy requires interpretation in the context of tolerability.25 Prior trials have assessed exercise therapy tolerability via a single metric: attendance (ratio of completed to planned sessions). In this context, findings of the present study corroborate the findings of the CARE, START, ACT, and PACES trials showing an attendance rate of ≈ 75%, 4,6,12,43 a rate typically considered to be acceptable tolerability. However, application of metrics adapted from oncology drug trials26 in the present trial revealed approximately one-third of patients permanently discontinued exercise therapy; the rate of dose interruption (missing ≥3 consecutive sessions) was 70%. This underscores the significant time, financial, or physiological toxicities faced by patients during adjuvant chemotherapy present major barriers to participation and/or tolerability of exercise therapy in this setting.17,18 Rigorous monitoring and reporting of feasibility/tolerability is essential to adequately evaluate the overall benefit of exercise therapy during definitive cancer therapy.
Second, our findings corroborate prior work demonstrating exercise therapy significantly improves CRF and other physiological outcomes in the post-treatment setting.13,14 Direct comparisons are limited since all prior post-treatment exercise therapy trials enrolled patients ≥ 1 year following definitive therapy; the present study is the first to investigate sequential use of exercise therapy in any cancer setting. Our findings support prior work in adjuvant breast cancer3,12,43 reporting CRF recovers to ‘near’ pre-chemotherapy (baseline) levels ≈ 3 to 4 months after therapy cessation, at least in a proportion of patients. While recovery to near pre-chemotherapy levels may be clinically satisfying it is suboptimal since baseline CRF in the present study was 24% below age–sex-matched normative values plus only 40% of usual care patients fully returned to their baseline VO2peak. Hence, most primary breast cancer patients likely exhibit persistent marked CRF impairments predisposing to a plethora of treatment late-effects.45 Conversely, sequential use of exercise therapy increased CRF beyond pre-treatment values highlighting that without intervention treatment-induced physiological impairments are unlikely to fully recover.5,46
Third, although no statistical difference between concurrent vs. sequential exercise therapy, there was a CRF benefit of 0.9 mL O2.kg−1.min−1 favoring sequential exercise therapy, a clinically important change in asymptomatic women.47 The inferior benefit of concurrent exercise therapy likely reflects poorer tolerability (reflecting major barriers to exercise therapy participation during chemotherapy) and chemotherapy-induced multisystem toxicity impairing normal physiological response to exercise therapy.2 Taxane-anthracycline-containing regimens are well known to cause varying degrees of direct injury to the cardiac-pulmonary-blood-skeletal muscle axis, the major determinants of CRF.2 Mechanistic studies reveal that both central (e.g. cardiac output, myocardial fibrosis)46,48 and peripheral (e.g. arterio-venous O2 extraction, skeletal muscle composition)49,50 limitations are important contributors to impaired CRF in patients previously exposed to anthracyclines. Overall, the tolerability-to-benefit ratio favors recommendation of sequential use of exercise therapy.
A secondary objective of the present study evaluated the effects and tolerability of continuous exercise therapy. Although our study was not powered to detect superiority, this regimen was associated with significant, clinically meaningful improvements in CRF compared with usual care, and numerical improvements in comparison with the other exercise therapy sequencing regimens. Tolerability of continuous exercise therapy was also excellent despite being twice the length of the other exercise therapy regimens. These findings are, however, hypothesis-generating. The promising tolerability and CRF benefit of continuous exercise require validation in larger, adequately powered trials.
Future directions
Result of the present trial corroborate prior work4–51) highlighting the need for investigation of alternative approaches that optimize the efficacy and tolerability of exercise therapy in breast, and other oncology, settings. For instance, whether the superior effects of continuous exercise on CRF relative to other regimes was due to longer program length or timing (i.e. during and after chemotherapy) is not known. Meta-analyses evaluating the effects of exercise therapy generally report longer programs (greater than ∼15 weeks) are not associated with superior improvements relative to shorter programs; 15,52,53 however, increasing exercise program length alone may allow more time for physiological adaptation and augment CRF response.54–56 Trials directly investigating the most appropriate length of exercise programs are needed.42,57 As in other clinical settings, 58 exercise-induced improvements in the present trial may be due to a combination of early exercise initiation and longer program length. Specifically, the modest exercise therapy attenuation of CRF decline during chemotherapy resulted in patients having a higher VO2peak at chemotherapy cessation. This, in turn, may have potentiated physiological adaptation in the post-chemotherapy setting, permitting prescription and tolerance of greater exercise therapy doses. In contrast, chemotherapy-induced decline in CRF was unabated among patients allocated to sequential exercise therapy which subsequently attenuated exercise therapy benefit after treatment cessation. Thus, the exercise-associated mitigation of fitness declines during chemotherapy observed in the present trial likely provides a critical foundation/cardiovascular base for superior adaptation to exercise after therapy. A trial evaluating whether CRF improvement with 32 weeks of exercise therapy initiated only after chemotherapy would be as efficacious as 32 weeks continuous exercise therapy (i.e. during and after chemotherapy) could address this important question. An ongoing clinical trial will evaluate whether increasing aerobic exercise therapy length and/or dose improves CRF response in post-treatment breast cancer (ClinicalTrials.gov Identifier: NCT04458532); however, increasing exercise therapy dose and/or volume alone may be inadequate to ameliorate dysfunction across specific systems (i.e. cardiopulmonary–vascular–muscular axis) manifest during chemotherapy.59 Identifying central and/or peripheral components contributing to poor CRF via imaging and/or invasive hemodynamic monitoring during CPET may facilitate the design of more personalized exercise therapy prescriptions to augment CRF benefit.59 Finally, we selected supervised exercise sessions to maximize exercise therapy fidelity and safety/tolerability; however, the primary reason for non-participation and loss to follow-up was related to inconvenience (i.e. time commitment) of attending in-person exercise sessions or study assessments. A fully digitally enabled, decentralized clinical trial solution could lower patient burden by reducing or eliminating site-based visits and allowing for implementation of high-fidelity exercise therapy delivery. Ongoing clinical trials testing remotely delivered site-less exercise therapy models may address major challenges to trial participation and enhance exercise therapy adherence.
Study limitations
Our study has important limitations. First, our findings are limited to less active patients with primary breast cancer initiating chemotherapy and do not generalize either to those engaging in regular exercise therapy or initiating other types of systemic or localized cancer therapies. Additional work is needed to evaluate the efficacy of exercise therapy to offset cardiovascular toxicities during contemporary therapies.60,61 Furthermore, unlike prior adjuvant exercise therapy trials where patients received standard dosing, 3,6,12 over 80% of patients in our trial received dose-dense regimens. The tolerability and response to exercise therapy in these settings is likely distinct. Second, generalizability of our findings may be limited by recruitment of a cohort of patients highly motivated to voluntarily participate in a lifestyle intervention. This well-established ‘healthy volunteer effect’ may be associated with more favorable changes in clinical trial settings than those observed when the intervention is implemented in the community.62 Third, our lost to follow-up rate was like prior comparable trials (e.g. ACT); 43 however, this may have impacted study power. Nevertheless, it is important to note that the estimated effect size is much smaller than the pre-specified effect size used to design this trial.63 Furthermore, the study over-enrolled to account for potential dropout. Consequently, the negative finding is likely not a result of low power due to attrition. Finally, we evaluated the acute effects of a relatively short exercise intervention. Longer-term evaluation of exercise therapy tolerability and efficacy is required.
Conclusions
Prevention, mitigation, and recovery of treatment-induced multisystem physiological toxicity is recognized as an important clinical need in the management of patients with cancer.64,65 Within this paradigm, exercise therapy is a potential non-pharmacological strategy that may complement other supportive care therapies to offset toxicity.59 We found no statistical difference in CRF improvement between exercise therapy administered concurrent vs. sequential to chemotherapy, relative to usual care. As such, this is a negative trial based on the primary analysis. The promising tolerability and benefit of exercise therapy following a continuous schedule warrants further evaluation. Our findings have important implications for the clinical management of breast cancer patients initiating adjuvant chemotherapy.
Authors contribution
Elisabeth Comen (Investigation: Supporting; Writing—review & editing: Supporting), Gabriella D'Andrea (Investigation: Supporting; Writing—review & editing: Supporting), Shanu Modi (Investigation: Supporting; Writing—review & editing: Supporting), Rachel Sanford (Investigation: Supporting; Writing—review & editing: Supporting), Devika Gajria (Investigation: Supporting; Writing—review & editing: Supporting), Victoria Blinder (Investigation: Supporting; Writing—review & editing: Supporting), Chau Dang (Investigation: Supporting; Writing—review & editing: Supporting), Lee Jones (Data curation: Lead; Funding acquisition: Lead; Investigation: Lead; Methodology: Lead; Project administration: Lead; Supervision: Lead; Writing—original draft: Lead), Chaya Moskowitz (Formal analysis: Lead; Methodology: Supporting; Writing—review & editing: Supporting), Neil Eves (Conceptualization: Supporting; Investigation: Supporting; Writing—review & editing: Supporting), Jeffrey Peppercorn (Investigation: Supporting; Writing—review & editing: Supporting), Ayca Gucalp (Investigation: Supporting; Writing—review & editing: Supporting), Meghan Michalski (Investigation: Supporting; Writing—review & editing: Supporting), Catherine Lee (Investigation: Supporting; Writing—review & editing: Supporting), James Herndon (Conceptualization: Supporting; Formal analysis: Supporting; Writing—review & editing: Supporting), Jessica Scott, PhD (Investigation: Supporting; Methodology: Supporting; Writing—original draft: Supporting), Jasme Lee (Formal analysis: Supporting; Writing—review & editing: Supporting), Kelly A. O’Brien, MA (Investigation: Supporting; Writing—review & editing: Supporting), Jacqueline Bromberg (Investigation: Supporting; Writing—review & editing: Supporting), Tiffany Traina (Investigation: Supporting; Writing—review & editing: Supporting), Kylie Rowed (Investigation: Supporting; Writing—review & editing: Supporting), John Sasso (Investigation: Supporting; Writing—review & editing: Supporting), and Anthony Yu (Investigation: Supporting; Writing—review & editing: Supporting)
Supplementary data
Supplementary data is available at European Heart Journal online.
Trial Registration
Clinicaltrials.gov Identifier: NCT01943695
Supplementary Material
Contributor Information
Jessica M Scott, Department of Medicine, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA; Department of Medicine, Weill Cornell Medical College, 418 E 71st St, New York, NY 10021, USA.
Jasme Lee, Department of Medicine, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA.
James E Herndon, Department of Biostatistics and Bioinformatics, Duke University Medical Center, 2424 Erwin Road, 8020 Hock Plaza, Durham, NC 27705, USA.
Meghan G Michalski, Department of Medicine, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA.
Catherine P Lee, Department of Medicine, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA.
Kelly A O’Brien, Department of Medicine, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA.
John P Sasso, School of Health and Exercise Sciences, University of British Columbia, 1147 Research Road, Kelowna, BC V1V 1V7, Canada.
Anthony F Yu, Department of Medicine, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA; Department of Medicine, Weill Cornell Medical College, 418 E 71st St, New York, NY 10021, USA.
Kylie A Rowed, Department of Medicine, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA.
Jacqueline F Bromberg, Department of Medicine, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA; Department of Medicine, Weill Cornell Medical College, 418 E 71st St, New York, NY 10021, USA.
Tiffany A Traina, Department of Medicine, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA; Department of Medicine, Weill Cornell Medical College, 418 E 71st St, New York, NY 10021, USA.
Ayca Gucalp, Department of Medicine, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA; Department of Medicine, Weill Cornell Medical College, 418 E 71st St, New York, NY 10021, USA.
Rachel A Sanford, Department of Medicine, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA.
Devika Gajria, Department of Medicine, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA; Department of Medicine, Weill Cornell Medical College, 418 E 71st St, New York, NY 10021, USA.
Shanu Modi, Department of Medicine, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA; Department of Medicine, Weill Cornell Medical College, 418 E 71st St, New York, NY 10021, USA.
Elisabeth A Comen, Department of Medicine, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA; Department of Medicine, Weill Cornell Medical College, 418 E 71st St, New York, NY 10021, USA.
Gabriella D'Andrea, Department of Medicine, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA; Department of Medicine, Weill Cornell Medical College, 418 E 71st St, New York, NY 10021, USA.
Victoria S Blinder, Department of Medicine, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA; Department of Medicine, Weill Cornell Medical College, 418 E 71st St, New York, NY 10021, USA.
Neil D Eves, School of Health and Exercise Sciences, University of British Columbia, 1147 Research Road, Kelowna, BC V1V 1V7, Canada.
Jeffrey M Peppercorn, Division of Hematology/Oncology, Massachusetts General Hospital, 55 Fruit St., Boston, MA 02114, USA.
Chaya S Moskowitz, Department of Medicine, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA.
Chau T Dang, Department of Medicine, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA; Department of Medicine, Weill Cornell Medical College, 418 E 71st St, New York, NY 10021, USA.
Lee W Jones, Department of Medicine, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA; Department of Medicine, Weill Cornell Medical College, 418 E 71st St, New York, NY 10021, USA.
Data availability
The corresponding author will consider requests for collaborative study and analysis of de-identified individual participant data to investigators who sign a data access agreement and provide a methodologically sound proposal. Interested researchers should submit proposals and analytic plans to the corresponding author. A data use agreement will be issued and will be compliant with relevant patient confidentiality and privacy regulations.
Funding
This study was supported by a research grant from the National Cancer Institute (R01CA164751) awarded to LWJ and grants from AKTIV Against Cancer and the Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748).
References
- 1. Scott JM, Dolan LB, Norton L, Charles JB, Jones LW. Multisystem toxicity in cancer: lessons from NASA's countermeasures program. Cell 2019;179:1003–1009. 10.1016/j.cell.2019.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jones LW, Eves ND, Haykowsky M, Freedland SJ, Mackey JR. Exercise intolerance in cancer and the role of exercise therapy to reverse dysfunction. Lancet Oncol 2009;10:598–605. 10.1016/S1470-2045(09)70031-2 [DOI] [PubMed] [Google Scholar]
- 3. Moller T, Andersen C, Lillelund C, Bloomquist K, Christensen KB, Ejlertsen B, et al. Physical deterioration and adaptive recovery in physically inactive breast cancer patients during adjuvant chemotherapy: a randomised controlled trial. Sci Rep 2020;10:9710. 10.1038/s41598-020-66513-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Courneya KS, Segal RJ, Mackey JR, Gelmon K, Reid RD, Friedenreich CM, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol 2007;25:4396–4404. 10.1200/JCO.2006.08.2024 [DOI] [PubMed] [Google Scholar]
- 5. Jones LW, Courneya KS, Mackey JR, Muss HB, Pituskin EN, Scott JM, et al. Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J Clin Oncol 2012;30:2530–2537. 10.1200/JCO.2011.39.9014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Courneya KS, McKenzie DC, Mackey JR, Gelmon K, Friedenreich CM, Yasui Y, et al. Effects of exercise dose and type during breast cancer chemotherapy: multicenter randomized trial. J Natl Cancer Inst 2013;105:1821–1832. 10.1093/jnci/djt297 [DOI] [PubMed] [Google Scholar]
- 7. Travier N, Velthuis MJ, Steins Bisschop CN, van den Buijs B, Monninkhof EM, Backx F, et al. Effects of an 18-week exercise programme started early during breast cancer treatment: a randomised controlled trial. BMC Med 2015;13:121. 10.1186/s12916-015-0362-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fleg JL, Morrell CH, Bos AG, Brant LJ, Talbot LA, Wright JG, et al. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation 2005;112:674––682.. 10.1161/CIRCULATIONAHA.105.545459 [DOI] [PubMed] [Google Scholar]
- 9. Afifi AM, Saad AM, Al-Husseini MJ, Elmehrath AO, Northfelt DW, Sonbol MB. Causes of death after breast cancer diagnosis: a US population-based analysis. Cancer 2020;126:1559–1567. 10.1002/cncr.32648 [DOI] [PubMed] [Google Scholar]
- 10. Kwan ML, Cheng RK, Iribarren C, Neugebauer R, Rana JS, Nguyen-Huynh M, et al. Risk of cardiometabolic risk factors in women with and without a history of breast cancer: the pathways heart study. J Clin Oncol 2022;40:1635–1646. 10.1200/JCO.21.01738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abrahams HJG, Gielissen MFM, Schmits IC, Verhagen C, Rovers MM, Knoop H. Risk factors, prevalence, and course of severe fatigue after breast cancer treatment: a meta-analysis involving 12 327 breast cancer survivors. Ann Oncol 2016;27:965–974. 10.1093/annonc/mdw099 [DOI] [PubMed] [Google Scholar]
- 12. van Waart H, Stuiver MM, van Harten WH, Geleijn E, Kieffer JM, Buffart LM, et al. Effect of low-intensity physical activity and moderate- to high-intensity physical exercise during adjuvant chemotherapy on physical fitness, fatigue, and chemotherapy completion rates: results of the PACES randomized clinical trial. J Clin Oncol 2015;33:1918–1927. 10.1200/JCO.2014.59.1081 [DOI] [PubMed] [Google Scholar]
- 13. Scott JM, Thomas SM, Peppercorn JM, Herndon JE II, Douglas PS, Khouri MG, et al. Effects of exercise therapy dosing schedule on impaired cardiorespiratory fitness in patients with primary breast cancer: a randomized controlled trial. Circulation 2020;141:560–570. 10.1161/CIRCULATIONAHA.119.043483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life outcomes. J Clin Oncol 2003;21:1660–1668. 10.1200/JCO.2003.04.093 [DOI] [PubMed] [Google Scholar]
- 15. Scott JM, Zabor EC, Schwitzer E, Koelwyn GJ, Adams SC, Nilsen TS, et al. Efficacy of exercise therapy on cardiorespiratory fitness in patients with cancer: a systematic review and meta-analysis. J Clin Oncol 2018;36:2297–2305. 10.1200/JCO.2017.77.5809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv 2010;4:87–100. 10.1007/s11764-009-0110-5 [DOI] [PubMed] [Google Scholar]
- 17. The Lancet Oncology . Exercise and cancer treatment: balancing patient needs. Lancet Oncol 2018;19:715. 10.1016/S1470-2045(18)30376-0 [DOI] [PubMed] [Google Scholar]
- 18. Demark-Wahnefried W. Move onward, press forward, and take a deep breath: can lifestyle interventions improve the quality of life of women with breast cancer, and how can we be sure? J Clin Oncol 2007;25:4344–4345. 10.1200/JCO.2007.13.1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jones LW, Courneya KS. Exercise discussions during cancer treatment consultations. Cancer Pract 2002;10:66–74. 10.1046/j.1523-5394.2002.102004.x [DOI] [PubMed] [Google Scholar]
- 20. Ligibel JA, Jones LW, Brewster AM, Clinton SK, Korde LA, Oeffinger KC, et al. Oncologists’ attitudes and practice of addressing diet, physical activity, and weight management with patients with cancer: findings of an ASCO survey of the oncology workforce. J Oncol Pract 2019;15:e520–e528. 10.1200/JOP.19.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jones LW, Courneya KS. Exercise counseling and programming preferences of cancer survivors. Cancer Pract 2002;10:208–215. 10.1046/j.1523-5394.2002.104003.x [DOI] [PubMed] [Google Scholar]
- 22. Wong EC, Kaplan CP, Barulich M, Melisko M. Assessing preferences for receiving supportive care resources among patients seen at a breast care center. Breast Cancer Res Treat 2020;183:381–389. 10.1007/s10549-020-05786-0 [DOI] [PubMed] [Google Scholar]
- 23. Campbell KL, Winters-Stone KM, Wiskemann J, May AM, Schwartz AL, Courneya KS, et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc 2019;51:2375–2390. 10.1249/MSS.0000000000002116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. American Thoracic Society; American College of Chest Physicians . ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 2003;167:211–277. 10.1164/rccm.167.2.211 [DOI] [PubMed] [Google Scholar]
- 25. Scott JM, Iyengar NM, Nilsen TS, Michalski M, Thomas SM, Herndon J II, et al. Feasibility, safety, and efficacy of aerobic training in pretreated patients with metastatic breast cancer: a randomized controlled trial. Cancer 2018;124:2552–2560. 10.1002/cncr.31368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nilsen TS, Scott JM, Michalski M, Capaci C, Thomas S, Herndon JE II, et al. Novel methods for reporting of exercise dose and adherence: an exploratory analysis. Med Sci Sports Exerc 2018;50:1134–1141. 10.1249/MSS.0000000000001545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bisschop CN S, Courneya KS, Velthuis MJ, Monninkhof EM, Jones LW, Friedenreich C, et al. Control group design, contamination and drop-out in exercise oncology trials: a systematic review. PLoS One 2015;10:e0120996. 10.1371/journal.pone.0120996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, Lloyd SR, et al. Reliability and validity of the functional assessment of cancer therapy-breast quality-of-life instrument. J Clin Oncol 1997;15:974–986. 10.1200/JCO.1997.15.3.974 [DOI] [PubMed] [Google Scholar]
- 29. Lai JS, Cella D, Chang CH, Bode RK, Heinemann AW. Item banking to improve, shorten and computerize self-reported fatigue: an illustration of steps to create a core item bank from the FACIT-fatigue scale. Qual Life Res 2003;12:485–501. 10.1023/A:1025014509626 [DOI] [PubMed] [Google Scholar]
- 30. Cleeland CS. Measurement and prevalence of pain in cancer. Semin Oncol Nurs 1985;1:87–92. 10.1016/S0749-2081(85)80041-3 [DOI] [PubMed] [Google Scholar]
- 31. Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 32. Ware JE J, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, Raczek A. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the medical outcomes study. Med Care 1995;33:AS264–AS279. [PubMed] [Google Scholar]
- 33. Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr 2014;27:911–939. 10.1016/j.echo.2014.07.012 [DOI] [PubMed] [Google Scholar]
- 34. Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, et al. Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American heart association. Hypertension 2015;66:698–722. 10.1161/HYP.0000000000000033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Team RC . R: A Language and Environment for Statistical Computing.: R Foundation for Statistical Computing; 2021. [Google Scholar]
- 36. Bates D, Machler M, Bolker BM, Walker SC. Fitting linear mixed-effects models using lme4. J Stat Soft 2015;67:1–48. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- 37. Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biom J 2008;50:346–363. 10.1002/bimj.200810425 [DOI] [PubMed] [Google Scholar]
- 38. Fitzgerald MD, Tanaka H, Tran ZV, Seals DR. Age-related declines in maximal aerobic capacity in regularly exercising vs. Sedentary women: a meta-analysis. J Appl Physiol (1985) 1997;83:160–165. 10.1152/jappl.1997.83.1.160 [DOI] [PubMed] [Google Scholar]
- 39. Zamorano JL, Lancellotti P, Rodriguez Munoz D, Aboyans V, Asteggiano R, Galderisi M, et al. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC committee for practice guidelines: the task force for cancer treatments and cardiovascular toxicity of the European society of cardiology (ESC). Eur Heart J 2016;37:2768–2801. 10.1093/eurheartj/ehw211 [DOI] [PubMed] [Google Scholar]
- 40. Cormie P, Atkinson M, Bucci L, Cust A, Eakin E, Hayes S, et al. Clinical oncology society of Australia position statement on exercise in cancer care. Med J Aust 2018;209:184–187. 10.5694/mja18.00199 [DOI] [PubMed] [Google Scholar]
- 41. Ligibel JA, Bohlke K, May AM, Clinton SK, Demark-Wahnefried W, Gilchrist SC, et al. Exercise, diet, and weight management during cancer treatment: aSCO guideline. J Clin Oncol 2022;40:2491–2507. 10.1200/JCO.22.00687 [DOI] [PubMed] [Google Scholar]
- 42. D'Ascenzi F, Anselmi F, Fiorentini C, Mannucci R, Bonifazi M, Mondillo S. The benefits of exercise in cancer patients and the criteria for exercise prescription in cardio-oncology. Eur J Prev Cardiol 2019;28:725–735. 10.1177/2047487319874900 [DOI] [PubMed] [Google Scholar]
- 43. van der Schoot GGF, Ormel HL, Westerink NL, May AM, Elias SG, Hummel YM, et al. Optimal timing of a physical exercise intervention to improve cardiorespiratory fitness: during or after chemotherapy. JACC CardioOncol 2022;4:491–503. 10.1016/j.jaccao.2022.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Foulkes SJ, Howden EJ, Haykowsky MJ, Antill Y, Salim A, Nightingale SS, et al. Exercise for the prevention of anthracycline-induced functional disability and cardiac dysfunction: the BReast cancer randomized EXercise InTervention (BREXIT) study. Circulation 2022;147:532–545. 10.1161/CIRCULATIONAHA.122.062814 [DOI] [PubMed] [Google Scholar]
- 45. Abdel-Qadir H, Austin PC, Lee DS, Amir E, Tu JV, Thavendiranathan P, et al. A population-based study of cardiovascular mortality following early-stage breast cancer. JAMA Cardiol 2017;2:88–93. 10.1001/jamacardio.2016.3841 [DOI] [PubMed] [Google Scholar]
- 46. Foulkes SJ, Howden EJ, Bigaran A, Janssens K, Antill Y, Loi S, et al. Persistent impairment in cardiopulmonary fitness after breast cancer chemotherapy. Med Sci Sports Exerc 2019;51:1573–1581. 10.1249/MSS.0000000000001970 [DOI] [PubMed] [Google Scholar]
- 47. Imboden MT, Harber MP, Whaley MH, Finch WH, Bishop DA, Fleenor BS, et al. The influence of change in cardiorespiratory fitness with short-term exercise training on mortality risk from the ball state adult fitness longitudinal lifestyle study. Mayo Clin Proc 2019;94:1406–1414. 10.1016/j.mayocp.2019.01.049 [DOI] [PubMed] [Google Scholar]
- 48. Kirkham AA, Paterson DI, Haykowsky MJ, Beaudry RI, Mackey JR, Pituskin E, et al. Aerobic fitness is related to myocardial fibrosis post-anthracycline therapy. Med Sci Sports Exerc 2021;53:267–274. 10.1249/MSS.0000000000002469 [DOI] [PubMed] [Google Scholar]
- 49. Beaudry RI, Kirkham AA, Thompson RB, Grenier JG, Mackey JR, Haykowsky MJ. Exercise intolerance in anthracycline-treated breast cancer survivors: the role of skeletal muscle bioenergetics, oxygenation, and composition. Oncologist 2020;25:e852–e860. 10.1634/theoncologist.2019-0777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Brubaker P, Jensen A, Jordan J, Lamar Z, Mihalko S, Haykowsky M, et al. Exercise capacity is reduced in cancer survivors previously treated with anthracycline-based chemotherapy despite a preserved cardiac output response. JACC Cardiovasc Imaging 2019;12:2267–2269. 10.1016/j.jcmg.2019.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Scott JM, Thomas SM, Herndon JE II, Douglas PS, Yu AF, Rusch V, et al. Effects and tolerability of exercise therapy modality on cardiorespiratory fitness in lung cancer: a randomized controlled trial. J Cachexia Sarcopenia Muscle 2021;12:1456–1465. 10.1002/jcsm.12828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lin X, Zhang X, Guo J, Roberts CK, McKenzie S, Wu WC, et al. Effects of exercise training on cardiorespiratory fitness and biomarkers of cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc 2015;4:e002014. 10.1161/JAHA.115.002014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Anderson L, Oldridge N, Thompson DR, Zwisler AD, Rees K, Martin N, et al. Exercise-Based cardiac rehabilitation for coronary heart disease: cochrane systematic review and meta-analysis. J Am Coll Cardiol 2016;67:1–12. 10.1016/j.jacc.2015.10.044 [DOI] [PubMed] [Google Scholar]
- 54. Howden EJ, Sarma S, Lawley JS, Opondo M, Cornwell W, Stoller D, et al. Reversing the cardiac effects of sedentary aging in middle age-A randomized controlled trial: implications for heart failure prevention. Circulation 2018;137:1549–1560. 10.1161/CIRCULATIONAHA.117.030617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sisson SB, Katzmarzyk PT, Earnest CP, Bouchard C, Blair SN, Church TS. Volume of exercise and fitness nonresponse in sedentary, postmenopausal women. Med Sci Sports Exerc 2009;41:539–545. 10.1249/MSS.0b013e3181896c4e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ross R, de Lannoy L, Stotz PJ. Separate effects of intensity and amount of exercise on interindividual cardiorespiratory fitness response. Mayo Clin Proc 2015;90:1506–1514. 10.1016/j.mayocp.2015.07.024 [DOI] [PubMed] [Google Scholar]
- 57. Pelliccia A, Sharma S, Gati S, Back M, Borjesson M, Caselli S, et al. 2020 ESC guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J 2021;42:17–96. 10.1093/eurheartj/ehaa605 [DOI] [PubMed] [Google Scholar]
- 58. Haykowsky M, Scott J, Esch B, Schopflocher D, Myers J, Paterson I, et al. A meta-analysis of the effects of exercise training on left ventricular remodeling following myocardial infarction: start early and go longer for greatest exercise benefits on remodeling. Trials 2011;12:92. 10.1186/1745-6215-12-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Scott JM, Nilsen TS, Gupta D, Jones LW. Exercise therapy and cardiovascular toxicity in cancer. Circulation 2018;137:1176–1191. 10.1161/CIRCULATIONAHA.117.024671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Herrmann J, Lenihan D, Armenian S, Barac A, Blaes A, Cardinale D, et al. Defining cardiovascular toxicities of cancer therapies: an international cardio-oncology society (IC-OS) consensus statement. Eur Heart J 2022;43:280–299. 10.1093/eurheartj/ehab674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lyon AR, Lopez-Fernandez T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, et al. 2022 ESC guidelines on cardio-oncology developed in collaboration with the European hematology association (EHA), the European society for therapeutic radiology and oncology (ESTRO) and the international cardio-oncology society (IC-OS). Eur Heart J 2022;43:4229–4361. 10.1093/eurheartj/ehac244 [DOI] [PubMed] [Google Scholar]
- 62. Anglemyer A, Horvath HT, Bero L. Healthcare outcomes assessed with observational study designs compared with those assessed in randomized trials. Cochrane Database Syst Rev 2014;2014:MR000034. 10.1002/14651858.MR000034.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hornsby WE, Douglas PS, West MJ, Kenjale AA, Lane AR, Schwitzer ER, et al. Safety and efficacy of aerobic training in operable breast cancer patients receiving neoadjuvant chemotherapy: a phase II randomized trial. Acta Oncol (Madr) 2014;53:65–74. 10.3109/0284186X.2013.781673 [DOI] [PubMed] [Google Scholar]
- 64. Guida JL, Agurs-Collins T, Ahles TA, Campisi J, Dale W, Demark-Wahnefried W, et al. Strategies to prevent or remediate cancer and treatment-related aging. J Natl Cancer Inst 2021;113:112–122. 10.1093/jnci/djaa060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gilchrist SC, Barac A, Ades PA, Alfano CM, Franklin BA, Jones LW, et al. Cardio-Oncology rehabilitation to manage cardiovascular outcomes in cancer patients and survivors: a scientific statement from the American heart association. Circulation 2019;139:e997–e1012. 10.1161/CIR.0000000000000679 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The corresponding author will consider requests for collaborative study and analysis of de-identified individual participant data to investigators who sign a data access agreement and provide a methodologically sound proposal. Interested researchers should submit proposals and analytic plans to the corresponding author. A data use agreement will be issued and will be compliant with relevant patient confidentiality and privacy regulations.