Abstract
We assessed phthalate-hormone associations in 382 pregnant women of the new-generation SEPAGES cohort (2014–2017, France) using improved exposure and outcome assessments. Metabolites from seven phthalate compounds and the replacement di(isononyl)cyclohexane-1,2-dicarboxylate (DINCH) were measured in within-subject pools of repeated urine samples collected at the second and third pregnancy trimesters (≈21 samples/trimester). Metabolites from five steroid hormones were measured in maternal hair samples collected at delivery, reflecting cumulative levels over the previous weeks to months. Adjusted linear regression and Bayesian weighted quantile sum (BWQS) mixture models were performed. Each doubling in third-trimester urinary mono-benzyl phthalate (MBzP) concentrations was associated with an average increase of 13.3% (95% CI: 2.65, 24.9) for ∑cortisol, 10.0% (95% CI: 0.26, 20.7) for ∑cortisone, 17.3% (95% CI: 1.67, 35.4) for 11-dehydrocorticosterone, and 16.2% (95% CI: 2.20, 32.1) for testosterone, together with a suggestive 10.5% (95% CI: −1.57, 24.1) increase in progesterone levels. Each doubling in second-trimester urinary di-isononyl phthalate (DiNP) concentrations was inversely associated with testosterone levels (−11.6%; 95% CI: −21.6, −0.31). For most hormones, a nonsignificant trend toward a positive phthalate mixture effect was observed in the third but not in the second trimester. Our study showed that exposure to some phthalate metabolites, especially MBzP, may affect adrenal and reproductive hormone levels during pregnancy.
Keywords: endocrine disruption, phthalate, DiNP, MBzP, cortisol, testosterone, steroid hormone, pregnancy
Short abstract
This study, implementing improved exposure and outcome assessment methods, showed that some phthalate metabolites can influence adrenal and reproductive hormone levels during pregnancy.
1. Introduction
Maternal steroid hormone regulation during pregnancy is critical to maintaining a healthy pregnancy and achieving optimal fetal development.1,2 For example, the equilibrium between glucocorticoids (e.g., cortisol) and their precursor progesterone is critical to maintaining pregnancy and switching the maternal immune response toward fetal tolerance.3,4 Contrastingly, a disequilibrium in progesterone and/or glucocorticoids predisposes to inflammation, which may lead to placental insufficiency and poor fetal growth.3 Regarding the hypothalamus-pituitary-adrenal (HPA) axis, corticotropin-releasing hormone (CRH) and cortisol play a major role in the timing of labor and have been associated with prematurity and low birth weight in epidemiologic studies.2,5 In relation to the hypothalamus-pituitary-gonadal (HPG) axis, women presenting higher testosterone levels, such as cases of polycystic ovarian syndrome (PCOS), also show a higher risk of preterm birth, fetal growth restriction, and pregnancy complications.6–9 Overall, disruptions in steroid hormone levels during pregnancy may result in adverse pregnancy and birth outcomes.3
Phthalates are a family of chemicals produced at high volumes used in a wide range of commercial products. However, some are considered endocrine disruptors and can interfere with hormonal regulation.10 High molecular weight phthalates (>250 Da; ester side-chain lengths of five or more carbons) are employed as plasticizers in the production of polyvinyl chloride plastics and are found in a variety of products, such as food contact materials,11 building and construction materials (e.g., vinyl flooring, floor tiles, wall coverings, and furniture upholstery), medical devices (tubing, catheters, blood/dialysis bags), and toys.12,13 Low-molecular-weight phthalates (<250 Da; ester side-chain lengths of one to four carbons) are used as solubilizing agents in the formulation of cosmetics and personal care products (e.g., fragrances) and as coatings of some pharmaceuticals.13 Despite some regulations in Europe, recent birth cohorts and biomonitoring studies show that phthalate exposure is still widespread, with more than 90% of the European population showing detectable concentrations in urine.14,15 In addition, newer phthalates such as bis(2-propylheptyl) phthalate (DPHP) and the nonphthalate substitute 1,2-cyclohexane dicarboxylic acid diisononyl ester (DINCH) have recently entered the market, showing increasing concentrations in humans over time.14,16 Since these new chemicals have a similar structure to those they replace and could potentially exert deleterious effects,17,18 there is a need for continuous biomonitoring and surveillance.19,20
Phthalates and their metabolites have been shown to interfere with steroid hormone regulation in laboratory animals through a variety of mechanisms that depend on age and sex.10,21 Through the construction of an adverse outcome pathway network, Baken et al. have shown that exposure to specific phthalates (diethyl-hexyl phthalate (DEHP), monoethyl-hexyl phthalate, and dibutyl-phthalate (DBP), among others) could be related to hormonal alterations and reproductive problems in experimental animals.21 These effects depend on sex and exposure timing and can be mediated through peroxisome-proliferator-activated receptors and other pathways implicating the estrogenic and glucocorticoid receptors.21 In addition to the ability of some phthalates to interact with the HPG axis, the adrenal gland has recently emerged as another target of phthalate toxicity, with DEHP and DBP being able to influence adrenal gland histology and steroidogenesis in both male and female rats.22,23 However, mechanistic data on the impact of phthalates on the HPA axis is still limited.24
The human evidence is very scarce, with some studies in pregnant women finding associations between specific phthalate metabolites and either increased or decreased testosterone levels in maternal serum25,26 or urine,27 as well as reduced serum progesterone levels.25,28 One study showed increased cord blood cortisol levels related to several phthalate metabolites among women carrying female fetuses. In contrast, reduced cortisol levels were reported among those who carried male fetuses in association with DEHP metabolites.29 Another study found that prenatal exposure to DEHP metabolites was associated with reduced cord blood cortisol and cortisone levels and increased adrenal androgens.30 These previous studies showed associations that varied by exposure at different pregnancy trimesters and, in some cases, were sex-specific.
Limitations of previous observational studies include the assessment of phthalate metabolites in spot urine samples, which leads to exposure misclassification and attenuation bias due to the short half-life and large within-day and within-week variability of phthalate metabolites.31,32 Another shortcoming is that most studies used a unique or few blood25,26,28–30 or urine27 sample(s) to evaluate hormone levels since many of them are influenced by circadian rhythms and time-specific confounders.33,34 Distinct collection protocols or biospecimens are needed to provide complementary information about these hormone levels over an extended period of time (i.e., weeks or months) while being less influenced by circadian rhythms and transient stressors (e.g., allergies, colds, and transient moods).
Our objective was to study the longitudinal associations between prenatal exposure to phthalates and maternal steroid hormones in a new-generation cohort. We relied on the prospective collection of multiple urine samples during pregnancy to improve exposure characterization.31,32 In addition, we collected hair samples at delivery, which allowed us to reliably measure cumulative levels of steroid hormones over the previous weeks to months.35–37
2. Methods
2.1. Study Population
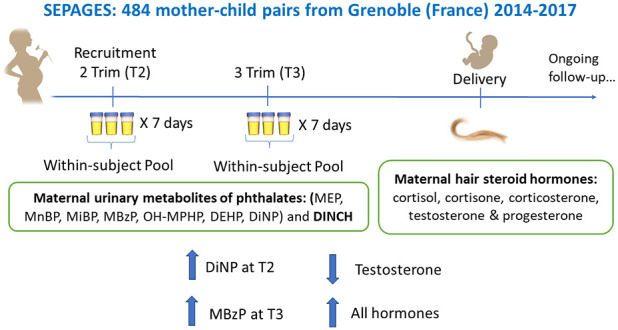
We used data from the French mother-child cohort SEPAGES that recruited 484 pregnant women at eight obstetrical ultrasonography practices in the Grenoble metropolitan area from July 2014 to July 2017. Inclusion criteria were singleton pregnancies up to gestational week 19, being older than 18 years, living in the metropolitan area of Grenoble, and planning to deliver in one of the four Grenoble maternity clinics. The research protocol was presented to 3360 women, with an eligibility and participation rate of 69 and 21%, respectively.38
The current study included mothers with adrenal and reproductive hormones assessed in hair samples collected at delivery and urinary phthalate metabolites measured at the second trimester (T2) (N = 382) and third trimester (T3) (N = 378) of pregnancy (Supporting Information Figure S1). The SEPAGES study was approved by the Ethics Committee (CPP) Sud-Est V and the National Commission on Informatics and Liberty (CNIL). After the study staff explained all details to the participants, pregnant women signed the written informed consent.
2.2. Repeated Urine Collection and Exposure Assessment
Pregnant women were asked to collect 3 urine samples per day (morning, midday, and evening) for 7 consecutive days, twice during pregnancy: at the second trimester [median (P25, P75) gestational age: 17 weeks (16–18)], and during the third trimester [33 weeks (31–34)]. Women showed excellent compliance to the urine collection protocol: the median (P25, P75) number of biospecimens collected per pregnant woman was 21 (20, 21) samples/trimester at both T2 and T3 collection weeks. Women stored urine samples in their freezers until the SEPAGES field workers picked them up at the end of each collection week for transport to a certified biobank (ISO 9001 standard, Grenoble University Hospital, bb-0033–00069). For each of the two prenatal urine collection weeks, within-subject pools were conducted by combining equal volumes of all spot urine samples obtained over a collection week (Supporting Information Figure S2). This pooling strategy, which does not consider urinary dilution of each spot sample, was previously validated as a good proxy of the urine concentrations that would have been obtained in the pool of the whole volume of all individual spot samples collected.39 We have indeed shown that when an equal volume of each individual urine sample is pooled, as was the case of the SEPAGES cohort, standardization by specific gravity or creatinine is not needed and can even be counterproductive for some compounds such as bisphenol A.39
Aliquots of weekly pools were stored at −80 °C before being sent on dry ice with a temperature sensor to the Norwegian Institute of Public Health (Oslo, Norway), a certified European laboratory that has participated in recent interlaboratory comparisons.40 The concentration of 13 phthalate and two nonphthalate plasticizer metabolites were measured (Supporting Information Table S1) using high-performance liquid chromatography coupled with mass spectrometry.41 The limits of detection (LOD) and quantification (LOQ) ranged from 0.07 to 0.4 and 0.20 to 0.50 ng/mL, respectively. Urine specific gravity was assessed in each weekly pool using a hand-held Atago PAL 10 S refractometer.
2.3. Hair Steroid Hormones
Around delivery (1–3 days postpartum), at the maternity ward, mothers collected their own maternal hair samples using material and protocol provided by SEPAGES field workers. Once collected, hair samples were stored at ambient temperature by the maternity staff until it was transported to the biobank, where they were stored at −80 °C. Hair samples from 408 SEPAGES mothers were sent to the Applied Metabolomics Research Laboratory of the IMIM-Hospital del Mar Medical Research Institute (Barcelona, Spain), where the first 3 cm (scalp side, corresponding to the hair growth of the third trimester42,43) were used to assess 10 steroid hormones (five hormones and five metabolites, Table 2) using a previously validated liquid chromatography-tandem mass spectrometry (LC–MS/MS) methodology.44 The hormones and metabolites assessed were cortisol and its metabolites 20α-dihydrocortisol and 20β-dihydrocortisol; cortisone and its metabolites 20α-dihydrocortisone, 20β-dihydrocortisone, and β-cortolone; 11-dehydrocorticosterone; testosterone; and progesterone. The selection of these hormones was based on their reliable measurement in hair samples,44 their relevance to pregnancy, and their representativeness of both HPG and HPA hormonal axes. During the analytical procedure, two samples broke during centrifugation, and 10 had a sample weight below the minimum required. Additionally, 12 samples did not pass analytical quality controls for most hormones and were excluded from our analysis. Thus, data on steroid hormones were available for 384 women. Figure S3 graphically depicts the relationships among these hormones (Supporting Information).
Table 2. Hormone Levels in the Study Population (N = 382).
| Hormone levels in maternal hair at delivery | LOD | % > LOD | P25 | Median | P75 |
|---|---|---|---|---|---|
| ∑Cortisol (nmol/g)a | 0.009 | 0.017 | 0.035 | ||
| Cortisol (ng/g) | 0.06 | 98 | 0.73 | 1.71 | 4.06 |
| 20α-dihydrocortisol (ng/g) | 0.07 | 85 | 0.23 | 0.63 | 1.48 |
| 20β-dihydrocortisol (ng/g) | 0.01 | 98 | 1.85 | 3.69 | 6.71 |
| ∑Cortisone (nmol/g)a | 0.050 | 0.091 | 0.156 | ||
| Cortisone (ng/g) | 0.10 | 95 | 4.77 | 12.0 | 22.5 |
| 20α-dihydrocortisone (ng/g) | 0.02 | 99 | 3.80 | 8.27 | 15.7 |
| 20β-dihydrocortisone (ng/g) | 0.02 | 97 | 2.60 | 5.74 | 9.82 |
| β-cortolone (ng/g) | 0.50 | 74 | <LOD | 4.54 | 7.66 |
| 11-dehydrocorticosterone (ng/g) | 0.10 | 88 | 0.91 | 1.82 | 2.98 |
| Testosterone (ng/g) | 0.10 | 89 | 0.23 | 0.42 | 0.65 |
| Progesterone (ng/g) | 0.10 | 100 | 30.8 | 76.5 | 166.6 |
Molar sum of cortisol and cortisone metabolites. Median ∑cortisol and ∑cortisone levels expressed as ng/g were 6.1 and 32.9 ng/g, respectively.
2.4. Statistical Analysis
2.4.1. Phthalate Biomarkers
Concentrations below the LOD and between the LOD and LOQ were imputed by values randomly selected between 0 and LOD and between LOD and LOQ, respectively, considering the underlying distribution of concentrations.45,46
If needed (i.e., sample processing and analytical conditions affecting the measured concentrations), phthalate metabolite concentrations were standardized using a two-step approach to correct for between-sample variations in the processing and chemical analysis of urine samples.47,48 The following sampling conditions were considered: time of urine sample transportation between the home of participants to the biobank, the time that samples were maintained at 4 °C in the course of the pooling process, and analytical batches. First, the associations between each natural-log-transformed phthalate biomarker and the three above-mentioned conditions were estimated running separate linear regressions adjusted for maternal age, education, prepregnancy body mass index (BMI), parity, date, season, pregnancy trimester of sample collection, and urinary specific gravity. Second, we used the measured phthalate concentrations and the estimated effects of processing/assay conditions associated with phthalate concentrations (p-value < 0.20) to predict standardized concentrations, that is, concentrations that would have been obtained if all samples had been processed under the same conditions and assayed in the same batch. Details have been described in Guilbert et al. (2021) and Mortamais et al. (2012).47,48 Since high correlations (Spearman rho > 0.80) between these standardized and nonstandardized phthalate concentrations have been previously reported,49 the current work provides descriptive statistics only for standardized concentrations, which were used in all phthalate-hormone analyses. In the case of phthalate metabolites belonging to the same parent compound, molar sums (μmol/L) were computed [i.e., ∑DEHP, the sum of diisononyl phthalate metabolites (∑DiNP) and ∑DINCH]. Urinary phthalate metabolite concentrations were natural log-transformed to reduce the skewness of distributions and the influence of extreme values.
2.4.2. Hormone Biomarkers
Some steroids presented a moderate percentage of nondetected values (range between 0 and 26%). As for exposure biomarkers, nondetects were imputed by values randomly selected between 0 and the LOD, considering the estimated distribution of the quantified hormone concentrations.45,46 Such a method results in a balanced distribution of imputed values, providing unbiased or minimally biased parameter estimates when 30% or less of data are below detection limits.46
To reduce the number of statistical comparisons, we computed the molar sums of cortisol and cortisone and their metabolites by summing their concentrations divided by their molecular weight. Five outcomes were thus included in the main analysis: ∑cortisol, ∑cortisone, 11-dehydrocorticosterone, testosterone, and progesterone. In addition, two hormone ratios were computed: the ∑cortisol to ∑cortisone ratio as an indicator of corticoid activity that may be predictive of hypertensive disorders50 and the testosterone to ∑cortisol ratio as an indicator of the general (im)balance between the mutually inhibiting HPG and HPA axes that has been related to antisocial and risk-taking behaviors.51 The two ratios were considered secondary outcomes to be interpreted alongside the primary outcomes. All hormones and ratios were natural-log-transformed to reduce the skewness of distributions.51
2.4.3. Covariates and Confounding Factors
Data on potential covariates were self-reported using questionnaires administered by a field worker during study visits and clinical examinations. Confounding factors and outcome predictors were selected a priori based on causal knowledge using a Directed Acyclic Graph (DAG)52 (Supporting Information Figure S4). All models were adjusted for maternal age at conception (continuous), prepregnancy BMI (underweight/normal weight vs overweight/obese mothers), education level (below vs Master’s degree or above), active smoking during pregnancy (smoking at any trimester vs nonsmoker), passive tobacco exposure (exposed to indoor tobacco smoke ≥1 h/week at any trimester vs nonexposed), parity (nulliparous vs uniparous/multiparous), maternal anxiety/depression score at the third trimester using the French version of the Hospital Anxiety and Depression scale (continuous),53 infant sex, and season of hair collection (winter vs remaining seasons since hair samples collected in winter showed significantly lower corticosteroid levels compared to other seasons).
We additionally controlled for technical variables, such as the hair analysis batch (dichotomous) and time elapsed (continuous) between hair sampling and hormone measurement. The slight number of missing values for some covariates (see Table 1 footnote) was singly imputed with the variable median or mode value.
Table 1. Study Population Characteristics (N = 382)a,b.
| Characteristics | Included (N = 382) | Excluded (N = 102) | P-valuec |
|---|---|---|---|
| N (%) or mean (SD) | N (%) or mean (SD) | ||
| Mother’s age at conception of index pregnancy (years) | 32.6 (3.9) | 32.2 (3.8) | 0.41 |
| Mother’s anxiety/depression symptoms in 3rd trimesterd | 10.4 (4.8) | 11.0 (4.6) | 0.29 |
| Mother’s prepregnancy BMI (kg/m2) | 22.3 (3.6) | 22.8 (4.4) | 0.09 |
| <25 | 320 (84%) | 78 (76%) | |
| ≥25 | 62 (16%) | 24 (24%) | |
| Mother’s education | 0.40 | ||
| Less than Master’s degree | 162 (42%) | 48 (47%) | |
| Master’s degree or higher | 220 (58%) | 54 (53%) | |
| Mother’s tobacco consumption during pregnancy (=consumers ≥1 cigarette/day) | 23 (6%) | 6 (6%) | 0.96 |
| Mother’s passive tobacco exposure (=exposed ≥ 1h/week) | 64 (17%) | 24 (24%) | 0.12 |
| Parity | 0.07 | ||
| Nulliparous | 167 (44%) | 55 (54%) | |
| Uniparous/multiparous | 215 (56%) | 47 (46%) | |
| Season of hair collection | NA | ||
| Winter | 75 (20%) | NA | |
| Remaining seasons | 307 (80%) | NA | |
| Child sex (=boys) | 206 (54%) | 52 (53%) | 0.88 |
| Time elapsed from hair collection to assessment (years) | 4.3 (0.78) | ||
| Analytical batch of hair analysis | |||
| Batch 1 | 288 (75%) | ||
| Batch 2 | 94 (25%) |
Abbreviations: BMI (body mass index); N (number); NA (Not applicable); SD (standard deviation).
Note: percentage of missing covariates for included participants: maternal anxiety/depression score (5.0%), education (0.5%), prepregnancy BMI (0.5%), active (8.1%), and passive (5.5%) smoking. The remaining covariates had no missing values.
Categorical variables compared using the two-sample proportion test and continuous variables compared using the two-sample t-test.
Hospital anxiety and depression score rated on a scale of 21. Anxiety and depression symptoms were summed, so the scores should be interpreted on a scale of 42.
2.4.4. Associations between Phthalates and Hormones
The linearity of the relationship between phthalate metabolites and hormones was tested by using generalized additive models (GAMs). Most associations showed effective degrees of freedom below 2,54,55 supporting linear relationships and the modeling of phthalates as continuous variables (Supporting Information Figure S5). Additionally, the few possible nonlinear associations identified were not statistically significant (GAM p-value >0.05).
Adjusted linear regression models were then performed for each phthalate-hormone pair at both the second and third trimester of pregnancy since previous studies showed associations may differ by trimester.27–29 Regression estimates were expressed as percent change (PC) for each twofold increase in standardized urinary phthalate biomarkers using the following formula: ([e(ln2×β) – 1] × 100).
A phthalate–sex interaction term was added to the model to identify potential sex-specific associations since previous studies also showed effect modification by this variable.27,29 A p-value below 0.10 for the interaction term was considered evidence of effect modification,56 in which case a sex-stratification of the specific association was conducted.
2.4.5. Mixture Model
Given the possibility of cumulative phthalate effects,57 the overall mixture effect of prenatal exposure to phthalates and DINCH on maternal hair hormone levels was estimated using adjusted Bayesian weighted quantile sum (BWQS) regression. BWQS is a quantile-based approach that combines multiple independent variables additively into a weighted index, with weights estimating the contribution of each component to the mixture.58 This model represents a novel Bayesian extension of the classic WQS regression, counteracting its main limitations, including higher stability of estimates, higher statistical power (no data splitting into training and validation sets), and ability to estimate mixture associations considering multiple chemicals with individual positive, negative, and/or null effects simultaneously.59 Phthalate and DINCH metabolite concentrations were modeled in quartiles. Similar to linear regressions, BWQS effect estimates (Beta1 and 95% Credible Intervals) were expressed as the PC in hormone levels for each quartile increase in the mixture: [(exp(β1) – 1) × 100].
2.4.6. Sensitivity Analyses
The robustness of the main models was tested after (1) additional adjustment for urine-specific gravity (continuous) to rule out any potential influence of urine dilution;60 (2) exclusion of extreme exposure and hormone values corresponding to percentiles 1 and 99 of their respective distributions.
2.4.7. Interpretation and Software
Statistical significance was set as p-value < 0.05. A p-value between 0.05 and 0.10 was considered possibly suggestive of an association. However, results were interpreted not solely depending on statistical significance but considering patterns of associations and the previous evidence available to contextualize the findings.61 Thus, associations with p-value <0.10 will only be considered meaningful if there is enough previous toxicological or epidemiological support or if it follows a pattern of associations (i.e., a nonisolated association). Regression analyses were carried out with Stata version 14.2 (Stata Corp), while GAM (“mgcv” package) and BWQS models using RStudio version 4.0.3 (RStudio Team, 2020).
3. Results
3.1. Characteristics of the Study Population
Mothers showed a high education level (58% reached a Master’s degree or higher), 16% were overweight/obese (≥25 kg/m2), and conceived at a mean (SD) age of 32.6 (3.9) years (Table 1). About half of the mothers (44%) were nulliparous at the time of conception, and a slightly higher number of boys (54%) were born in the cohort. The prevalence of maternal active smoking was low (6%) compared to the data in France,38 while 17% of the women were exposed to environmental tobacco smoke.
Phthalates and DINCH metabolites were quantified in more than 95% of the urine samples in both pregnancy trimesters. The highest median urinary concentrations were observed for the sum of DEHP metabolites and the low molecular weight metabolite MEP (Supporting Information Table S2). Phthalate metabolites showed low-to-moderate estimates of Spearman correlation (rho range: 0.27–0.59) between the two pregnancy trimesters (Supporting Information Figure S6). In general, phthalate exposure levels were lower or similar to other contemporary European cohorts.14,62 An extended description of phthalate exposure levels in the SEPAGES cohort has been previously reported.14
Regarding hormones measured in hair samples, progesterone showed the highest levels (median: 76.5 ng/g), followed by ∑cortisone (median: 32.9 ng/g) and ∑cortisol (median: 6.1 ng/g) (Table 2). ∑Cortisol and ∑cortisone were highly correlated with their respective metabolites (rho >0.84), with the exception of ∑cortisone and β-cortolone, which were moderately correlated (rho: 0.43). ∑Cortisol and ∑cortisone levels were also highly correlated (rho: 0.86). While ∑cortisol and ∑cortisone moderately correlated with 11-dehydrocorticosterone (rho: 0.56 and 0.67, respectively), their correlations with testosterone and progesterone levels tended to be lower (Supporting Information Figure S7).
3.2. Associations in the Whole Population
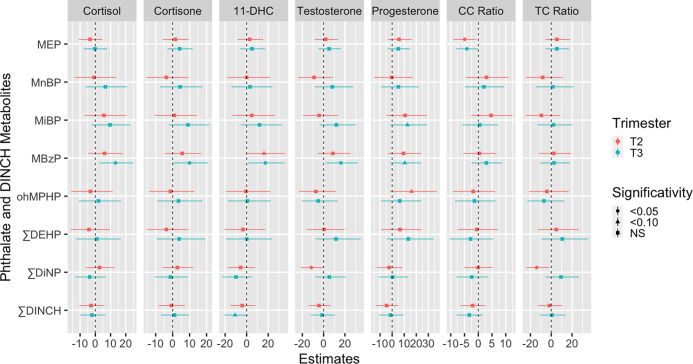
MBzP showed the most consistent pattern of associations with hair hormones (Figure 1 and Supporting Information Table S3). Second-trimester exposure to MBzP tended to be associated with higher levels of all hormones but only reached borderline-statistical significance for 11-dehydrocorticosterone levels (PC = 16.1%; 95% CI: −1.60, 35.6). Third-trimester MBzP exposure was robustly and positively associated with most of the hormones investigated. Each doubling in third-trimester urinary MBzP concentrations was associated with average increases of 13.3% (95% CI: 2.65, 24.9) for ∑cortisol, 10.0% (95% CI: 0.26, 20.7) for ∑cortisone, 17.3% (95% CI: 1.67, 35.4) for 11-dehydrocorticosterone and 16.2% (95% CI: 2.20, 32.1) for testosterone, together with a suggestive 10.5% (95% CI: −1.57, 24.1) increase in progesterone levels (Figure 1 and Table S3). No associations with hormone ratios were found for MBzP in the second or third trimester.
Figure 1.
Adjusted associations between maternal prenatal urinary phthalate metabolite concentrations and hair hormones at delivery. Geometric figures (circles, triangles, and squares) and error bars represent linear regression effect estimates with their corresponding 95% confidence intervals expressed as percent change (PC) in hormone levels for each doubling in exposure biomarkers. Models adjusted for maternal age at conception (continuous), prepregnancy BMI (normal weight vs overweight/obese), education level (below vs Master’s degree or above), active smoking during pregnancy (smoker vs nonsmoker), passive tobacco exposure (exposed vs nonexposed), parity (nulliparous vs uniparous/multiparous), maternal anxiety/depression score at third trimester (continuous), infant sex and season of hair collection (winter vs remaining seasons), hair analysis batch (dichotomous), and time elapsed (continuous) between hair sampling and hormone measurement. Abbreviations: 11-DHC (11-dehydrocorticosterone); CC (cortisol to cortisone); TC (testosterone to cortisol); mono-ethyl phthalate (MEP); mono-n-butyl phthalate (MnBP); mono-isobutyl phthalate (MiBP); mono-benzyl phthalate (MBzP); 6-hydroxy-mono-propyl-heptyl phthalate (ohMPHP); di(2-ethylhexyl) phthalate (DEHP); di-isononylphthalate (DiNP); di(isononyl)cyclohexane-1,2-dicarboxylate (DINCH).
Although without reaching statistical significance, third-trimester urinary MiBP concentrations tended to be positively associated with most of the hormones assessed in hair samples (Figure 1 and Table S3), with a suggestive association (i.e., p-value <0.10) being observed for progesterone (PC = 12.7%; 95% CI: −1.51, 28.9).
Second-trimester urinary ∑DiNP concentrations were inversely associated with testosterone levels (PC = −11.6%; 95% CI: −21.6, −0.31) and the testosterone to cortisol ratio (TC) ratio (PC = −14.0%; 95% CI%: −24.4, −2.15). Third-trimester ∑DiNP exposure did not follow the same pattern, nor was it associated with any hormone outcome.
Although maternal MEP exposure was associated with a lower cortisol to cortisone ratio (CC) ratio at both second (PC = −4.98%; 95% CI: −9.05, −0.72) and third (PC = −4.18%; 95% CI: −8.12, −0.07) trimesters, this association appeared isolated since MEP was not associated with any of the primary hormone outcomes examined. Additionally, no meaningful associations were observed for MnBP and ∑DEHP during any pregnancy trimester (Figure 1 and Supporting Information Table S3).
3.3. Sex-Specific Associations
Few sex-specific associations were observed (Supporting Information Table S4). Sex-stratified models showed that the previously observed second-trimester inverse ∑DiNP-testosterone association was driven by boys (PC = −21.9%; 95% CI: −34.3, −7.15), while a null association was observed in girls (PC = 0.28%; 95% CI: −15.7, 19.3).
A sex-specific pattern was identified for MnBP, a compound not associated with any hormone when boys and girls were studied together. In women carrying male fetuses, second-trimester MnBP exposure was positively associated with 11-dehydrocorticosterone (PC = 40.4%; 95% CI: 3.53, 91.9), together with nonsignificant increases in ∑cortisol (PC = 14.9%; 95% CI: −7.30, 41.4) and ∑cortisone (PC = 8.67%; 95% CI: −9.88, 32.0) levels, while women carrying female fetuses showed opposite trends including significantly lower 11-dehydrocorticosterone levels (PC = −22.6%; 95% CI: −40.5, 0.00) (Table S4).
3.4. Mixture Associations
BWQS models showed that the phthalate mixtures measured at the second and third trimesters of pregnancy were not significantly associated with any of the hormones investigated (Table 3). Notwithstanding, several suggestive positive associations were observed for the third-trimester mixture and testosterone (PC = 17.0%; 95% CI: −3.63, 41.6), ∑cortisone (PC = 11.0%; 95% CI: −4.28, 27.3), and progesterone (PC = 10.3%; 95% CI: −7.36, 30.2) (Table 3). Although, in general, phthalates showed similar mixture weights, MBzP and MiBP tended to show slightly higher weights, in line with single-pollutant models (Supporting Information Figure S8). The phthalate mixture in the third trimester also tended to be associated with a lower CC ratio, which was mostly driven by MEP in accordance with single-pollutant models (Supporting Information, Figure S8).
Table 3. Mixture Associations Using BWQS Regression Modelsa.
| Hormones | Second trimester | Third trimester |
|---|---|---|
| PC (95% CrI) | PC (95% CrI) | |
| ∑Cortisol | –2.33 (−18.3, 16.2) | 5.74 (−9.89, 23.5) |
| ∑Cortisone | 0.06 (−14.0, 16.7) | 11.0 (−4.28, 27.3) |
| 11-dehydrocorticosterone | –0.39 (−22.2, 27.5) | 5.55 (−17.2, 32.9) |
| Testosterone | –4.50 (−22.3, 17.5) | 17.0 (−3.63, 41.6) |
| Progesterone | 4.64 (−12.9, 26.9) | 10.3 (−7.36, 30.2) |
| CC Ratio | –3.22 (−13.4, 7.68) | –5.50 (−13.6, 3.44) |
| TC Ratio | –3.15 (−23.5, 23.3) | 10.3 (−10.3, 35.9) |
Note: Percent change (PC) in hormone levels per quartile increase of the phthalate mixture, with its related 95% Credible Interval (95% CrI). The weight contributed by each chemical compound to the most relevant mixture associations can be consulted in Figure S8. Mixture models were adjusted for the same set of covariates included in single-pollutant models. Abbreviations: CC (cortisol to cortisone); TC (testosterone to cortisol).
3.5. Sensitivity Analyses
The additional inclusion of urine-specific gravity as a covariate in the models did not materially change associations (Supporting Information Table S5).
The exclusion of extreme values attenuated the isolated associations observed between MEP and the CC ratio that were no longer significant (Supporting Information Figure S9). Associations observed at the third trimester for MBzP did not materially change, while associations for third trimester MiBP were notably strengthened, showing significant associations with ∑cortisol (PC = 13.6%; 95% CI: 0.92, 28.0), ∑cortisone (PC = 15%; 95% CI: 2.71, 28.7), 11-dehydrocorticosterone (PC = 26.1%; 95% CI: 6.34, 49.4), testosterone (PC = 16.9%; 95% CI: 0.45, 36.1), and progesterone (PC = 15.9%; 95% CI: 0.81, 33.2) levels (Supporting Information Table S6 and Figure S9).
4. Discussion
This cohort is the first to rely on within-subject pools of multiple urine samples to assess phthalate exposure during gestation and to examine its association with maternal hair samples collected at delivery to assess cumulative hormone levels. In the second trimester, ∑DiNP concentrations were associated with lower testosterone levels in the whole poopulation, and especially among women carrying male fetuses. In the third trimester, urinary MBzP concentrations were robustly and consistently associated with higher levels of all the adrenal and reproductive hormones investigated. Third-trimester MiBP exposure followed a similar pattern of positive associations with all hormones that reached statistical significance after the exclusion of extreme values.
In line with single-pollutant results, a nonsignificant trend toward a positive association with most hormones was observed for the phthalate mixture in the third but not in the second trimester. Nevertheless, the lack of a significant mixture association seems to imply that, in this study population, specific phthalate metabolites (∑DiNP, MBzP, and to some extent MiBP) rather than a “cocktail effect” are the drivers of changes in specific hormones.
4.1. Biological Plausibility and Literature Comparison
MBzP and other phthalate monoesters can exert a dose-dependent increase in the gene expression of peroxisome proliferator-activated receptor gamma in the mouse Granulosa cells at environmentally relevant concentrations,63 which may reduce aromatase expression, leading to increased testosterone levels by reducing its conversion to estradiol.21 Although this hypothesis could explain the increased testosterone levels reported in our study, it does not explain the MBzP-related systematic increase observed in all adrenal and reproductive hormones. Our MBzP findings could result from one of the following modes of action: (1) a central simultaneous increase in both hypothalamic gonadotropin- and corticotropin-releasing hormones or their pituitary downstream hormones (for which we did not find supporting evidence); (2) a peripheral increase in the rate-limiting step of steroidogenesis, that is, an increased cholesterol delivery to the inner mitochondrial membrane by Steroidogenic Acute Regulatory Proteins (StAR), or upregulation of CYP450scc (cholesterol side-chain cleavage enzyme) that converts cholesterol into pregnenolone, the common precursor of all steroid hormones (Supporting Information Figure S3).64 We found in vitro and epidemiological evidence supporting that MBzP can upregulate P450scc in placental cells and tissue at human-relevant concentrations.65,66 In contrast, no relevant data was found in relation to StAR. Although, to our best knowledge, this MBzP-upregulation of P450scc has not been studied in other steroid organs with high P450scc expression, such as the adrenal glands and ovary,67 a molecular docking study supported the ability of MBzP to interact with P450scc.68 Therefore, an MBzP-driven increase in steroidogenic hormones through a multiorgan (placenta, adrenal glands, and/or ovary) upregulation of P450scc appears as a biologically plausible explanation that could be further investigated in future experimental studies.
Although the current work is difficult to compare with previous studies due to differences in matrices for hormone assessment (use of blood25,26,29 or urine27 instead of hair), our MBzP results are coherent with Pacyga et al., who reported a trend toward positive associations between maternal prenatal urinary MBzP concentrations and the sum of urinary testosterone metabolites measured three times during pregnancy among 434 U.S. women.27 Other studies reported nonsignificant associations with serum testosterone and progesterone. However, the trend in estimates was again toward higher hormone levels.25,26 Regarding corticosteroids, a positive association was reported between first-trimester urinary MBzP concentrations and cord blood CC ratio measured at birth among 553 Chinese mother–newborn pairs.29 Despite the small number of studies, the literature appears supportive of possible associations between prenatal MBzP exposure and increased adrenal and reproductive hormone levels during pregnancy.
MiBP showed a pattern of increased steroidogenesis similar to that of MBzP, which was notably strengthened after exclusion of extreme exposure and hormone values. Compared to MBzP, both the epidemiological25–27,29 and toxicological evidence for hormonal effects during pregnancy is more limited and equivocal.69 Although MiBP has also been shown to interact with the P450scc enzyme, the specific study tested very high doses in adult male mice,70 being not comparable to our work. Additional data will help to confirm or rule out our MiBP results.
DiNP is a phthalate replacement for DEHP. Although it is a less potent antiandrogenic chemical than DEHP,71–74 DiNP still shows endocrine-disrupting abilities. In adult female mice, DiNP exposure altered steroid hormone levels and ovarian folliculogenesis at moderate doses, with DiNP decreasing serum testosterone levels at a low-to-moderate dose of 100 μg/kg/day.75,76 In the current work, we also observed reduced testosterone concentrations in response to higher DiNP exposure, especially among women carrying male fetuses. Since the human male fetus is able to synthesize its own testicular testosterone between the first and second trimester,77 we hypothesize this association could reflect an antiandrogenic effect that may affect not only the mother but also the developing male fetus. Although biologically plausible, this association needs to be confirmed in future studies, given the limited toxicologic and epidemiologic data available for this phthalate replacement.
MnBP is known to exert antiandrogenic actions in male21,78,79 and female reproductive organs.78,80–82 However, interspecies differences in glucocorticoids83 precluded us from comparing our MnBP results to the rodent studies available.23,84 Since comparison with other epidemiological studies is neither possible, the MnBP-related increase in 11-dehydrocorticosterone levels among women carrying male fetuses is difficult to interpret and needs to be confirmed in future studies.
Although MEP was negatively associated with the CC ratio at both the second and third trimesters, which could indicate a higher conversion of cortisol to its less active metabolite cortisone, no associations were observed between MEP and cortisol or cortisone individually. The lack of toxicological support, together with the fact that when outliers were excluded, the MEP-CC ratio associations disappeared, decreased our confidence in this potential association.
4.2. Hair vs Other Matrices for Hormone Assessment
Compared to saliva, urine, and blood, cortisol in hair is thought to reflect mid to long-term cumulative levels, which are less affected by circadian rhythms and time-varying stressors.43 Although there are still some doubts about whether hair cortisol represents long or midterm cumulative concentrations,85,86 hair seems to provide information at least on the previous weeks, constituting an advantage over saliva, urine, and blood spot samples, which only represents the past few hours.87 In a recent study with a longitudinal design comparing hair and multiple saliva samples, Singh Solorzano et al. (2023) confirmed that hair cortisol is a reliable retrospective biomarker of at least the previous 6 weeks.87 Recent evidence suggests that not only corticosteroids but also other steroids, such as reproductive hormones, can be assessed in hair samples, possibly leading to an improved hormone assessment in clinical and population studies.42
In the current study, hair corticosteroids and reproductive hormones were assessed in the last 3 cm of maternal hair obtained around delivery. Assuming an average hair growth of 1 cm per month, the samples would reflect cumulative hormone levels over the previous 3 months (the third trimester) or at least the previous 6 weeks.43,87 Indeed, we observed more associations when phthalate metabolites were assessed in the third trimester compared to those in the second trimester. Since phthalates have short half-lives, a possible explanation is that phthalate exposure in the third trimester may be more biologically relevant to the cumulative period reflected by hair hormone analysis.
4.3. Clinical Relevance
Regarding the extrapolation of population-based results to the clinic, it should be taken into account that even moderate disturbances in the hormonal milieu can have relevant effects, particularly during fetal development.2,3,5,88 Additionally, exposure to phthalates and their replacements is pervasive, with virtually all human populations showing quantifiable levels.14–16 In this setting, small shifts in the mean of a distribution can produce large effects in their tails.89–92
The ovarian synthesis of maternal testosterone increases about 70% throughout pregnancy.93 Notwithstanding, testosterone levels are especially elevated in cases of pre-eclampsia and PCOS. Since these hyperandrogenic pregnancy states have been related to preterm birth, fetal growth restriction, and pregnancy complications including diabetes,6–9 as well as a higher risk of metabolic and behavioral problems in the offspring,94,95 the positive associations observed with MBzP (and suggested for MiBP) could be of clinical relevance, especially at high-end exposure levels.
At the end of gestation, glucocorticoid levels increase up to 20-fold compared to those of midpregnancy, and pregnancy is thus conceptualized as a period of transient hypercortisolism.3 Indeed, the placenta acts as a stress-sensitive organ that increasingly secretes CRH levels that, in turn, increase adrenocorticotrophic hormone (ACTH) secretion at the maternal pituitary gland, with consequently increased cortisol levels at the adrenal glands, which become gradually hypertrophic throughout pregnancy. The activation of the HPA axis, especially the release of CRH by the placenta, has been proposed to function as a biological clock that can determine a preterm, term, or post-term delivery.2 Thus, our findings of increased hair cortisol and cortisone in response to MBzP exposure could have an impact on the timing of labor, possibly leading to an increased risk of preterm birth as shown by a recent meta-analysis [OR (95% CI) = 1.18 (1.01, 1.37)].96
4.4. Strengths and Limitations
The main strengths of this work are the improved methodologies that limited bias arising due to daily variations occurring in both the phthalate metabolites and the hormones examined. This new-generation cohort is the first to perform a within-subject pooling of multiple repeated urine samples, designed to reduce phthalate exposure misclassification and attenuation bias.31,32 At the same time, our study is the first in the field of endocrine disruption to use maternal hair samples obtained at delivery to measure cumulative adrenal and reproductive hormone levels over the third trimester of pregnancy.42,43 Among the limitations is the medium size of the cohort, especially when examining sex-stratified associations. However, we anticipate a substantially increased statistical power compared to similar-sized cohorts collecting few urine samples.31 Another limitation was the lack of data for related steroid hormones such as estradiol or the adrenal androgen dehydroepiandrosterone. Finally, although we controlled for the most relevant covariates reported in the literature, we cannot fully exclude the possibility of residual or unmeasured confounding (e.g., diet quality or medications containing phthalates). Additionally, the low number of women reporting hair dyes during pregnancy (n = 7) prevented us from adjusting models with this variable. However, we relied on a 7-day recall questionnaire completed during the second and third pregnancy trimesters to estimate hair dye treatments, which may have probably underestimated its use over the whole pregnancy.
Overall, this prospective study with improved exposure and outcome assessment showed that maternal third-trimester urinary MBzP concentrations were robustly and consistently associated with increased levels of all of the adrenal and reproductive hormones evaluated in maternal hair samples at delivery, deserving further confirmation and investigation of its possible clinical implications.
Acknowledgments
Vicente Mustieles was under a postdoctoral contract with the ANR EDeN project (19-CE36-0003-01). This work was supported by ANR (EDeN project ANR-19-CE36-0003-01) and ANSES (HyPAxE project EST-2019/1/039). The SEPAGES cohort was supported by the European Research Council (N°311765-E-DOHaD), the European Community’s Seventh Framework Programme (FP7/2007-206—N°308333-892 HELIX), the European Union’s Horizon 2020 research and innovation programme (N° 874583 ATHLETE Project, N°825712 OBERON Project), the French Research Agency—ANR (PAPER project ANR-12-PDOC-0029-01, SHALCOH project ANR-14-CE21-0007, ANR-15-IDEX-02 and ANR-15-IDEX5, GUMME project ANR-18-CE36-005, ETAPE project ANR-18-CE36-0005—EDeN project ANR-19-CE36-0003-01—MEMORI project ANR 21-CE34-0022), the French Agency for Food, Environmental and Occupational Health & Safety—ANSES (CNAP project EST-2016-121, PENDORE project EST-2016-121, HyPAxE project EST-2019/1/039), the Plan Cancer (Canc’Air project), the French Cancer Research Foundation Association de Recherche sur le Cancer—ARC, the French Endowment Fund AGIR for chronic diseases–APMC (projects PRENAPAR and LCI-FOT), the French Endowment Fund for Respiratory Health, the French Fund—Fondation de France (CLIMATHES—00081169, SEPAGES 5–00099903, ELEMENTUM—00124527). We thank the SEPAGES study group: E. Eyriey, A. Licinia, A. Vellement (Groupe Hospitalier Mutualiste, Grenoble), I. Pin, S. Bayat, P. Hoffmann, E. Hullo, C. Llerena (Grenoble Alpes University Hospital, La Tronche), X. Morin (Clinique des Cèdres, Echirolles), A. Morlot (Clinique Belledonne, Saint-Martin d’Hères), J. Lepeule, S. Lyon-Caen, C. Philippat, I. Pin, J. Quentin, V. Siroux and R. Slama (Grenoble Alpes University, Inserm, CNRS, IAB). We thank Matthieu Rolland, Karine Supernant, Anne Boudier for data management, and Nicolas Jovanovic for code sharing to build Figure 1. SEPAGES biospecimens are stored at Grenoble University Hospital (CHU-GA) biobank (bb-0033-00069); we would like to thank the entire CRB team and, in particular, the technicians for the huge work of biospecimens processing and pooling. SEPAGES data are stored thanks to the Inserm RE-CO-NAI platform funded by Commissariat Général à l’Investissement. Finally, and importantly, we would like to express our sincere thanks to the participants of the SEPAGES study.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.3c03401.
Phthalate biomarkers assessed in SEPAGES, urinary phthalate metabolite concentrations, adjusted main associations for single-pollutant models, sex-stratified phthalate-hormone associations, sensitivity analysis of main model with additional adjustment for specific gravity, sensitivity analysis of the main model with exclusion of outliers, study population flow-chart, Cohort design figure, DAG for covariate selection, GAMs, Spearman correlation heat plot of phthalate metabolites, Spearman correlation heat plot of hair hormones, relative weights of BWQS mixture models, and figure of sensitivity analysis excluding outliers (ZIP)
The authors declare no competing financial interest.
Supplementary Material
References
- Monticone S.; Auchus R. J.; Rainey W. E. Adrenal Disorders in Pregnancy. Nat. Rev. Endocrinol. 2012, 8 (11), 668–678. 10.1038/nrendo.2012.155. [DOI] [PubMed] [Google Scholar]
- Mastorakos G.; Ilias I. Maternal Hypothalamic-Pituitary-Adrenal Axis in Pregnancy and the Postpartum Period: Postpartum-Related Disorders. Ann. N.Y. Acad. Sci. 2006, 900 (1), 95–106. 10.1111/j.1749-6632.2000.tb06220.x. [DOI] [PubMed] [Google Scholar]
- Solano M. E.; Arck P. C. Steroids, Pregnancy and Fetal Development. Front. Immunol. 2020, 10, 3017. 10.3389/fimmu.2019.03017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah N. M.; Lai P. F.; Imami N.; Johnson M. R. Progesterone-Related Immune Modulation of Pregnancy and Labor. Front. Endocrinol. 2019, 10 (MAR), 198. 10.3389/fendo.2019.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R.; Nicholson R. C. Corticotrophin Releasing Hormone and the Timing of Birth. Front. Biosci. 2007, 12 (1), 912–918. 10.2741/2113. [DOI] [PubMed] [Google Scholar]
- Yamamoto M.; Feigenbaum S. L.; Crites Y.; Escobar G. J.; Yang J.; Ferrara A.; Lo J. C. Risk of Preterm Delivery in Non-Diabetic Women with Polycystic Ovarian Syndrome. J. Perinatol. 2012, 32 (10), 770–776. 10.1038/jp.2011.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen S. M.; Jacobsen G.; Romundstad P. Maternal Testosterone Levels during Pregnancy Are Associated with Offspring Size at Birth. Eur. J. Endocrinol. 2006, 155 (2), 365–370. 10.1530/eje.1.02200. [DOI] [PubMed] [Google Scholar]
- Kumar S.; Gordon G. H.; Abbott D. H.; Mishra J. S. Androgens in Maternal Vascular and Placental Function: Implications for Preeclampsia Pathogenesis. Reproduction 2018, 156 (5), R155–R167. 10.1530/REP-18-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vejrazkova D.; Vcelak J.; Vankova M.; Lukasova P.; Bradnova O.; Halkova T.; Kancheva R.; Bendlova B. Steroids and Insulin Resistance in Pregnancy. J. Steroid Biochem. Mol. Biol. 2014, 139, 122–129. 10.1016/j.jsbmb.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Benjamin S.; Masai E.; Kamimura N.; Takahashi K.; Anderson R. C.; Faisal P. A.. Phthalates Impact Human Health: Epidemiological Evidences and Plausible Mechanism of Action. Journal of Hazardous Materials; Elsevier B.V., 2017; pp 360–383. 10.1016/j.jhazmat.2017.06.036. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Ruan Z.; Jing J.; Yang Y.; Li Z.; Zhang S.; Yang J.; Ai S.; Luo N.; Peng Y.; Fang P.; Lin H.; Zou Y. High-Temperature Soup Foods in Plastic Packaging Are Associated with Phthalate Body Burden and Expression of Inflammatory MRNAs: A Dietary Intervention Study. Environ. Sci. Technol. 2022, 56 (12), 8416–8427. 10.1021/acs.est.1c08522. [DOI] [PubMed] [Google Scholar]
- Darbre P. D.What Are Endocrine Disrupters and Where Are They Found?. Endocrine Disruption and Human Health; Elsevier, 2015; pp 3–26. 10.1016/B978-0-12-801139-3.00001-6. [DOI] [Google Scholar]
- Hauser R.; Calafat A. M. Phthalates and Human Health. Occup. Environ. Med. 2005, 62 (11), 806–818. 10.1136/oem.2004.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippat C.; Rolland M.; Lyon-Caen S.; Pin I.; Sakhi A. K.; Sabaredzovic A.; Thomsen C.; Slama R. Pre- and Early Post-Natal Exposure to Phthalates and DINCH in a New Type of Mother-Child Cohort Relying on within-Subject Pools of Repeated Urine Samples. Environ. Pollut. 2021, 287, 117650. 10.1016/j.envpol.2021.117650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weise P.; Apel P.; Kolossa-Gehring M. [Human Biomonitoring for Europe (HBM4 EU)-First Insights into the Results of the Initiative]. Bundesgesundheitsblatt. Gesundheitsforschung. Gesundheitsschutz 2022, 65, 936–939. 10.1007/s00103-022-03578-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu H.; Jönsson B. A.; Gennings C.; Svensson Å.; Nånberg E.; Lindh C. H.; Knutz M.; Takaro T. K.; Bornehag C. G. Temporal Trends of Phthalate Exposures during 2007–2010 in Swedish Pregnant Women. J. Expo. Sci. Environ. Epidemiol. 2018, 28 (5), 437–447. 10.1038/S41370-018-0020-6. [DOI] [PubMed] [Google Scholar]
- Engel A.; Buhrke T.; Kasper S.; Behr A.-C.; Braeuning A.; Jessel S.; Seidel A.; Völkel W.; Lampen A. The urinary metabolites of DINCH ® have an impact on the activities of the human nuclear receptors ERα, ERβ, AR, PPARα and PPARγ. Toxicol. Lett. 2018, 287, 83–91. 10.1016/j.toxlet.2018.02.006. [DOI] [PubMed] [Google Scholar]
- Schaffert A.; Karkossa I.; Ueberham E.; Schlichting R.; Walter K.; Arnold J.; Blüher M.; Heiker J. T.; Lehmann J.; Wabitsch M.; Escher B. I.; von Bergen M.; Schubert K. Di-(2-Ethylhexyl) Phthalate Substitutes Accelerate Human Adipogenesis through PPARγ Activation and Cause Oxidative Stress and Impaired Metabolic Homeostasis in Mature Adipocytes. Environ. Int. 2022, 164, 107279. 10.1016/j.envint.2022.107279. [DOI] [PubMed] [Google Scholar]
- Qadeer A.; Kirsten K. L.; Ajmal Z.; Jiang X.; Zhao X. Alternative Plasticizers As Emerging Global Environmental and Health Threat: Another Regrettable Substitution?. Environ. Sci. Technol. 2022, 56 (3), 1482–1488. 10.1021/acs.est.1c08365. [DOI] [PubMed] [Google Scholar]
- Mustieles V.; Arrebola J. P.; Porta M. From Old Pollutants to the Regulation of Bisphenol A: Lessons Learned for Health Promotion and Disease Prevention. Prev. Med. 2023, 169, 107460. 10.1016/j.ypmed.2023.107460. [DOI] [PubMed] [Google Scholar]
- Baken K. A.; Lambrechts N.; Remy S.; Mustieles V.; Rodríguez-Carrillo A.; Neophytou C. M.; Olea N.; Schoeters G. A Strategy to Validate a Selection of Human Effect Biomarkers Using Adverse Outcome Pathways: Proof of Concept for Phthalates and Reproductive Effects. Environ. Res. 2019, 175, 235–256. 10.1016/j.envres.2019.05.013. [DOI] [PubMed] [Google Scholar]
- Lee B. Y.; Jo J. B.; Choi D.; Lee S.-H.; Cheon Y.-P. A Chronic-Low-Dose Exposing of DEHP with OECD TG 443 Altered the Histological Characteristics and Steroidogeic Gene Expression of Adrenal Gland in Female Mice. Dev. Reprod. 2021, 25 (4), 257–268. 10.12717/DR.2021.25.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad S.; Sharma S.; Afjal M. A.; Habib H.; Akhter J.; Goswami P.; Parvez S.; Akhtar M.; Raisuddin S. MRNA Expression and Protein-Protein Interaction (PPI) Network Analysis of Adrenal Steroidogenesis in Response to Exposure to Phthalates in Rats. Environ. Toxicol. Pharmacol. 2022, 89, 103780. 10.1016/j.etap.2021.103780. [DOI] [PubMed] [Google Scholar]
- Graceli J. B.; Dettogni R. S.; Merlo E.; Niño O.; da Costa C. S.; Zanol J. F.; Ríos Morris E. A.; Miranda-Alves L.; Denicol A. C. The Impact of Endocrine-Disrupting Chemical Exposure in the Mammalian Hypothalamic-Pituitary Axis. Mol. Cell. Endocrinol. 2020, 518, 110997. 10.1016/j.mce.2020.110997. [DOI] [PubMed] [Google Scholar]
- Cathey A. L.; Watkins D.; Rosario Z. Y.; Velez C.; Alshawabkeh A. N.; Cordero J. F.; Meeker J. D. Associations of Phthalates and Phthalate Replacements With CRH and Other Hormones Among Pregnant Women in Puerto Rico. J. Endocr. Soc. 2019, 3 (6), 1127–1149. 10.1210/js.2019-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyanarayana S.; Butts S.; Wang C.; Barrett E.; Nguyen R.; Schwartz S. M.; Haaland W.; Swan S. H. Early Prenatal Phthalate Exposure, Sex Steroid Hormones, and Birth Outcomes. J. Clin. Endocrinol. Metab. 2017, 102 (6), 1870–1878. 10.1210/jc.2016-3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacyga D. C.; Gardiner J. C.; Flaws J. A.; Li Z.; Calafat A. M.; Korrick S. A.; Schantz S. L.; Strakovsky R. S. Maternal Phthalate and Phthalate Alternative Metabolites and Urinary Biomarkers of Estrogens and Testosterones across Pregnancy. Environ. Int. 2021, 155, 106676. 10.1016/j.envint.2021.106676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns L. E.; Ferguson K. K.; Soldin O. P.; Cantonwine D. E.; Rivera-González L. O.; Del Toro L. V. A.; Calafat A. M.; Ye X.; Alshawabkeh A. N.; Cordero J. F.; Meeker J. D. Urinary Phthalate Metabolites in Relation to Maternal Serum Thyroid and Sex Hormone Levels during Pregnancy: A Longitudinal Analysis. Reprod. Biol. Endocrinol. 2015, 13 (1), 4. 10.1186/1477-7827-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X.; Li J.; Jin S.; Li Y.; Liu W.; Zhao H.; Zhou Y.; Jiang Y.; Liu H.; Xia W.; Cai Z.; Xu S.; Shen X. Associations between Repeated Measures of Maternal Urinary Phthalate Metabolites during Pregnancy and Cord Blood Glucocorticoids. Environ. Int. 2018, 121 (Pt 1), 471–479. 10.1016/J.ENVINT.2018.09.037. [DOI] [PubMed] [Google Scholar]
- Araki A.; Mitsui T.; Goudarzi H.; Nakajima T.; Miyashita C.; Itoh S.; Sasaki S.; Cho K.; Moriya K.; Shinohara N.; Nonomura K.; Kishi R. Prenatal Di(2-Ethylhexyl) Phthalate Exposure and Disruption of Adrenal Androgens and Glucocorticoids Levels in Cord Blood: The Hokkaido Study. Sci. Total Environ. 2017, 581-582, 297–304. 10.1016/j.scitotenv.2016.12.124. [DOI] [PubMed] [Google Scholar]
- Perrier F.; Giorgis-Allemand L.; Slama R.; Philippat C. Within-Subject Pooling of Biological Samples to Reduce Exposure Misclassification in Biomarker-Based Studies. Epidemiology 2016, 27 (3), 378–388. 10.1097/EDE.0000000000000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernet C.; Philippat C.; Agier L.; Calafat A. M.; Ye X.; Lyon-Caen S.; Hainaut P.; Siroux V.; Schisterman E. F.; Slama R. An Empirical Validation of the Within-Subject Biospecimens Pooling Approach to Minimize Exposure Misclassification in Biomarker-Based Studies. Epidemiology 2019, 30 (5), 756–767. 10.1097/EDE.0000000000001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanakis G. A.; Tsametis C. P.; Goulis D. G. Measuring Testosterone in Women and Men. Maturitas 2019, 125, 41–44. 10.1016/j.maturitas.2019.04.203. [DOI] [PubMed] [Google Scholar]
- Vanaelst B.; De Vriendt T.; Huybrechts I.; Rinaldi S.; De Henauw S. Epidemiological Approaches to Measure Childhood Stress. Paediatr. Perinat. Epidemiol. 2012, 26 (3), 280–297. 10.1111/j.1365-3016.2012.01258.x. [DOI] [PubMed] [Google Scholar]
- Peng F. J.; Palazzi P.; Mezzache S.; Bourokba N.; Soeur J.; Appenzeller B. M. R. Profiling Steroid and Thyroid Hormones with Hair Analysis in a Cohort of Women Aged 25 to 45 Years Old. Eur. J. Endocrinol. 2022, 186 (5), K9–K15. 10.1530/EJE-22-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalder T.; Kirschbaum C. Analysis of Cortisol in Hair–State of the Art and Future Directions. Brain. Behav. Immun. 2012, 26 (7), 1019–1029. 10.1016/j.bbi.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Zhang Q.; Chen Z.; Chen S.; Xu Y.; Deng H. Intraindividual Stability of Cortisol and Cortisone and the Ratio of Cortisol to Cortisone in Saliva, Urine and Hair. Steroids 2017, 118, 61–67. 10.1016/j.steroids.2016.12.008. [DOI] [PubMed] [Google Scholar]
- Lyon-Caen S.; Siroux V.; Lepeule J.; Lorimier P.; Hainaut P.; Mossuz P.; Quentin J.; Supernant K.; Meary D.; Chaperot L.; Bayat S.; Cassee F.; Valentino S.; Couturier-Tarrade A.; Rousseau-Ralliard D.; Chavatte-Palmer P.; Philippat C.; Pin I.; Slama R.; The SEPAGES Study Group Deciphering the Impact of Early-Life Exposures to Highly Variable Environmental Factors on Foetal and Child Health: Design of SEPAGES Couple-Child Cohort. Int. J. Environ. Res. Public Health 2019, 16 (20), 3888. 10.3390/ijerph16203888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippat C.; Calafat A. M. Comparison of Strategies to Efficiently Combine Repeated Urine Samples in Biomarker-Based Studies. Environ. Res. 2021, 192, 110275. 10.1016/j.envres.2020.110275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban López M.; Göen T.; Mol H.; Nübler S.; Haji-Abbas-Zarrabi K.; Koch H. M.; Kasper-Sonnenberg M.; Dvorakova D.; Hajslova J.; Antignac J. P.; Vaccher V.; Elbers I.; Thomsen C.; Vorkamp K.; Pedraza – Díaz S.; Kolossa-Gehring M.; Castaño A. The European Human Biomonitoring Platform - Design and Implementation of a Laboratory Quality Assurance/Quality Control (QA/QC) Programme for Selected Priority Chemicals. Int. J. Hyg. Environ. Health 2021, 234, 113740. 10.1016/J.IJHEH.2021.113740. [DOI] [PubMed] [Google Scholar]
- Sabaredzovic A.; Sakhi A. K.; Brantsæter A. L.; Thomsen C. Determination of 12 Urinary Phthalate Metabolites in Norwegian Pregnant Women by Core-Shell High Performance Liquid Chromatography with on-Line Solid-Phase Extraction, Column Switching and Tandem Mass Spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 1002, 343–352. 10.1016/j.jchromb.2015.08.040. [DOI] [PubMed] [Google Scholar]
- Wang W.; Moody S. N.; Kiesner J.; Tonon Appiani A.; Robertson O. C.; Shirtcliff E. A. Assay Validation of Hair Androgens across the Menstrual Cycle. Psychoneuroendocrinology 2019, 101, 175–181. 10.1016/j.psyneuen.2018.10.029. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C.; Tietze A.; Skoluda N.; Dettenborn L. Hair as a Retrospective Calendar of Cortisol Production-Increased Cortisol Incorporation into Hair in the Third Trimester of Pregnancy. Psychoneuroendocrinology 2009, 34 (1), 32–37. 10.1016/j.psyneuen.2008.08.024. [DOI] [PubMed] [Google Scholar]
- Gomez-Gomez A.; Pozo O. J. Determination of Steroid Profile in Hair by Liquid Chromatography Tandem Mass Spectrometry. J. Chromatogr. A 2020, 1624, 461179. 10.1016/j.chroma.2020.461179. [DOI] [PubMed] [Google Scholar]
- Helsel D. R. Less than Obvious - Statistical Treatment of Data below the Detection Limit. Environ. Sci. Technol. 1990, 24 (12), 1766–1774. 10.1021/es00082a001. [DOI] [Google Scholar]
- Lubin J. H.; Colt J. S.; Camann D.; Davis S.; Cerhan J. R.; Severson R. K.; Bernstein L.; Hartge P. Epidemiologic Evaluation of Measurement Data in the Presence of Detection Limits. Environ. Health Perspect. 2004, 112 (17), 1691–1696. 10.1289/ehp.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortamais M.; Chevrier C.; Philippat C.; Petit C.; Calafat A. M.; Ye X.; Silva M. J.; Brambilla C.; Eijkemans M. J.; Charles M. A.; Cordier S.; Slama R. Correcting for the Influence of Sampling Conditions on Biomarkers of Exposure to Phenols and Phthalates: A 2-Step Standardization Method Based on Regression Residuals. Environ. Health: Global Access Sci. Source 2012, 11 (1), 29. 10.1186/1476-069X-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilbert A.; Rolland M.; Pin I.; Thomsen C.; Sakhi A. K.; Sabaredzovic A.; Slama R.; Guichardet K.; Philippat C. Associations between a Mixture of Phenols and Phthalates and Child Behaviour in a French Mother–Child Cohort with Repeated Assessment of Exposure. Environ. Int. 2021, 156, 106697. 10.1016/j.envint.2021.106697. [DOI] [PubMed] [Google Scholar]
- Mustieles V.; Rolland M.; Pin I.; Thomsen C.; Sakhi A.; Sabaredzovic A.; Muckle G.; Guichardet K.; Slama R.; Philippat C. Early-Life Exposure to a Mixture of Phenols and Phthalates in Relation to Child Social Behavior: Applying an Evidence-Based Prioritization to a Cohort with Improved Exposure Assessment. Environ. Health Perspect. 2023, 131 (8), 87006. 10.1289/EHP11798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dötsch J.; Dörr H. G.; Stalla G. K.; Sippell W. G. Effect of Glucocorticoid Excess on the Cortisol/Cortisone Ratio. Steroids 2001, 66 (11), 817–820. 10.1016/S0039-128X(01)00117-9. [DOI] [PubMed] [Google Scholar]
- Sollberger S.; Ehlert U. How to Use and Interpret Hormone Ratios. Psychoneuroendocrinology 2016, 63, 385–397. 10.1016/j.psyneuen.2015.09.031. [DOI] [PubMed] [Google Scholar]
- Textor J.; van der Zander B.; Gilthorpe M. S.; Liskiewicz M.; Ellison G. T. Robust Causal Inference Using Directed Acyclic Graphs: The R Package “Dagitty. Int. J. Epidemiol. 2017, 45 (6), 1887–1894. 10.1093/ije/dyw341. [DOI] [PubMed] [Google Scholar]
- Zigmond A. S.; Snaith R. P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67 (6), 361–370. 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- Zuur A. F.Mixed Effects Models and Extensions in Ecology with R; Springer: New York, USA, 2009. https://books.google.com/books/about/Mixed_Effects_Models_and_Extensions_in_E.html?hl=es&id=vQUNprFZKHsC. [Google Scholar]
- Hunsicker M. E.; Kappel C. V.; Selkoe K. A.; Halpern B. S.; Scarborough C.; Mease L.; Amrhein A. Characterizing Driver-Response Relationships in Marine Pelagic Ecosystems for Improved Ocean Management. Ecol. Appl. 2016, 26 (3), 651–663. 10.1890/14-2200. [DOI] [PubMed] [Google Scholar]
- Kaufman J. S.; MacLehose R. F. Which of These Things Is Not like the Others?. Cancer 2013, 119, 4216–4222. 10.1002/cncr.28359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne-Sturges D.; De Saram S.; Cory-Slechta D. A. Cumulative Risk Evaluation of Phthalates Under TSCA. Environ. Sci. Technol. 2023, 57 (16), 6403–6414. 10.1021/acs.est.2c08364. [DOI] [PubMed] [Google Scholar]
- Colicino E.; Pedretti N. F.; Busgang S. A.; Gennings C. Per- and Poly-Fluoroalkyl Substances and Bone Mineral Density: Results from the Bayesian Weighted Quantile Sum Regression. Environ. Epidemiol. 2020, 4 (3), e092 10.1097/EE9.0000000000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennings C.; Carrico C.; Factor-Litvak P.; Krigbaum N.; Cirillo P. M.; Cohn B. A. A Cohort Study Evaluation of Maternal PCB Exposure Related to Time to Pregnancy in Daughters. Environ. Health 2013, 12 (1), 66. 10.1186/1476-069X-12-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper J. R.; O’Brien K. M.; Ferguson K. K.; Buckley J. P. Urinary Specific Gravity Measures in the U.S. Population: Implications for the Adjustment of Non-Persistent Chemical Urinary Biomarker Data. Environ. Int. 2021, 156, 106656. 10.1016/j.envint.2021.106656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrhein V.; Greenland S.; McShane B.. Scientists Rise up against Statistical Significance. Nature. Nature Publishing Group; March 21, 2019; pp 305–307. 10.1038/d41586-019-00857-9. [DOI] [PubMed] [Google Scholar]
- Warembourg C.; Basagaña X.; Seminati C.; de Bont J.; Granum B.; Lyon-Caen S.; Manzano-Salgado C. B.; Pin I.; Sakhi A. K.; Siroux V.; Slama R.; Urquiza J.; Vrijheid M.; Thomsen C.; Casas M. Exposure to Phthalate Metabolites, Phenols and Organophosphate Pesticide Metabolites and Blood Pressure during Pregnancy. Int. J. Hyg. Environ. Health 2019, 222 (3), 446–454. 10.1016/j.ijheh.2018.12.011. [DOI] [PubMed] [Google Scholar]
- Meling D. D.; De La Torre K. M.; Arango A. S.; Gonsioroski A.; Deviney A. R. K.; Neff A. M.; Laws M. J.; Warner G. R.; Tajkhorshid E.; Flaws J. A. Phthalate Monoesters Act through Peroxisome Proliferator-Activated Receptors in the Mouse Ovary. Reprod. Toxicol. 2022, 110, 113–123. 10.1016/j.reprotox.2022.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugaeva K. V.; Sluchanko N. N. Steroidogenic Acute Regulatory Protein: Structure, Functioning, and Regulation. Biochemistry 2019, 84 (S1), 233–253. 10.1134/S0006297919140141. [DOI] [PubMed] [Google Scholar]
- Adibi J. J.; Buckley J. P.; Lee M. K.; Williams P. L.; Just A. C.; Zhao Y.; Bhat H. K.; Whyatt R. M. Maternal Urinary Phthalates and Sex-Specific Placental MRNA Levels in an Urban Birth Cohort. Environ. Health 2017, 16 (1), 35. 10.1186/s12940-017-0241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adibi J. J.; Zhao Y.; Zhan L. V.; Kapidzic M.; Larocque N.; Koistinen H.; Huhtaniemi I. T.; Stenman U. H. An Investigation of the Single and Combined Phthalate Metabolite Effects on Human Chorionic Gonadotropin Expression in Placental Cells. Environ. Health Perspect. 2017, 125 (10), 107010. 10.1289/EHP1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickery L. E.Cholesterol Side Chain Cleavage Cytochrome P450 (P450scc); Springer: Berlin, Heidelberg, 1993; pp 651–665. 10.1007/978-3-642-77763-9_42. [DOI] [Google Scholar]
- Ahmad S.; Khan M. F.; Parvez S.; Akhtar M.; Raisuddin S. Molecular Docking Reveals the Potential of Phthalate Esters to Inhibit the Enzymes of the Glucocorticoid Biosynthesis Pathway. J. Appl. Toxicol. 2017, 37 (3), 265–277. 10.1002/jat.3355. [DOI] [PubMed] [Google Scholar]
- Yost E. E.; Euling S. Y.; Weaver J. A.; Beverly B. E. J.; Keshava N.; Mudipalli A.; Arzuaga X.; Blessinger T.; Dishaw L.; Hotchkiss A.; Makris S. L. Hazards of Diisobutyl Phthalate (DIBP) Exposure: A Systematic Review of Animal Toxicology Studies. Environ. Int. 2019, 125, 579–594. 10.1016/j.envint.2018.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y.; Wang X.; Yeung L. W. Y.; Sheng N.; Cui Q.; Cui R.; Zhang H.; Dai J. Dietary Exposure to Di-Isobutyl Phthalate Increases Urinary 5-Methyl-2’-Deoxycytidine Level and Affects Reproductive Function in Adult Male Mice. J. Environ. Sci. 2017, 61, 14–23. 10.1016/j.jes.2017.04.036. [DOI] [PubMed] [Google Scholar]
- van den Driesche S.; Shoker S.; Inglis F.; Palermo C.; Langsch A.; Otter R. Systematic Comparison of the Male Reproductive Tract in Fetal and Adult Wistar Rats Exposed to DBP and DINP in Utero during the Masculinisation Programming Window. Toxicol. Lett. 2020, 335, 37–50. 10.1016/j.toxlet.2020.10.006. [DOI] [PubMed] [Google Scholar]
- EFSA Panel on Food Contact Materials Enzymes and Processing Aids CEP; Silano V.; Barat Baviera J. M.; Bolognesi C.; Chesson A.; Cocconcelli P. S.; Crebelli R.; Gott D. M.; Grob K.; Lampi E.; Mortensen A.; Rivière G.; Steffensen I. L.; Tlustos C.; Van Loveren H.; Vernis L.; Zorn H.; Cravedi J. P.; Fortes C.; Tavares Poças M. d. F.; Waalkens-Berendsen I.; Wölfle D.; Arcella D.; Cascio C.; Castoldi A. F.; Volk K.; Castle L. Update of the Risk Assessment of Di-Butylphthalate (DBP), Butyl-Benzyl-Phthalate (BBP), Bis(2-Ethylhexyl)Phthalate (DEHP), Di-Isononylphthalate (DINP) and Di-Isodecylphthalate (DIDP) for Use in Food Contact Materials. EFSA J. 2019, 17 (12), e05838 10.2903/J.EFSA.2019.5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell R. A.; Sochaski M.; Edwards K.; Creasy D. M.; Willson G.; Andersen M. E. Disposition of Diiosononyl Phthalate and Its Effects on Sexual Development of the Male Fetus Following Repeated Dosing in Pregnant Rats. Reprod. Toxicol. 2013, 35 (1), 56–69. 10.1016/j.reprotox.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Albert O.; Nardelli T. C.; Lalancette C.; Hales B. F.; Robaire B. Effects of In Utero and Lactational Exposure to New Generation Green Plasticizers on Adult Male Rats: A Comparative Study With Di(2-Ethylhexyl) Phthalate. Toxicol. Sci. 2018, 164 (1), 129–141. 10.1093/toxsci/kfy072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C.; Lewis L. R.; Borkowski G.; Flaws J. A. Exposure to Di(2-Ethylhexyl) Phthalate and Diisononyl Phthalate during Adulthood Disrupts Hormones and Ovarian Folliculogenesis throughout the Prime Reproductive Life of the Mouse. Toxicol. Appl. Pharmacol. 2020, 393, 114952. 10.1016/j.taap.2020.114952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C.; Lewis L. R.; Borkowski G.; Flaws J. A. Late-Life Consequences of Short-Term Exposure to Di(2-Ethylhexyl) Phthalate and Diisononyl Phthalate during Adulthood in Female Mice. Reprod. Toxicol. 2020, 93, 28–42. 10.1016/j.reprotox.2019.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines M.; Spencer D.; Kung K. T.; Browne W. V.; Constantinescu M.; Noorderhaven R. M. The Early Postnatal Period, Mini-Puberty, Provides a Window on the Role of Testosterone in Human Neurobehavioural Development. Curr. Opin. Neurobiol. 2016, 38, 69–73. 10.1016/j.conb.2016.02.008. [DOI] [PubMed] [Google Scholar]
- Wang J.; Zhang X.; Li Y.; Liu Y.; Tao L. Exposure to Dibutyl Phthalate and Reproductive-Related Outcomes in Animal Models: Evidence From Rodents Study. Front. Physiol. 2021, 12, 684532. 10.3389/fphys.2021.684532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czubacka E.; Czerczak S.; Kupczewska-Dobecka M. M. The Overview of Current Evidence on the Reproductive Toxicity of Dibutyl Phthalate. Int. J. Occup. Med. Environ. Health 2021, 34 (1), 15–37. 10.13075/ijomeh.1896.01658. [DOI] [PubMed] [Google Scholar]
- Repouskou A.; Panagiotidou E.; Panagopoulou L.; Bisting P. L.; Tuck A. R.; Sjödin M. O. D.; Lindberg J.; Bozas E.; Rüegg J.; Gennings C.; Bornehag C. G.; Damdimopoulou P.; Stamatakis A.; Kitraki E. Gestational Exposure to an Epidemiologically Defined Mixture of Phthalates Leads to Gonadal Dysfunction in Mouse Offspring of Both Sexes. Sci. Rep. 2019, 9 (1), 6424. 10.1038/s41598-019-42377-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adir M.; Combelles C. M. H.; Mansur A.; Ophir L.; Hourvitz A.; Orvieto R.; Dor J.; Machtinger R. Dibutyl Phthalate Impairs Steroidogenesis and a Subset of LH-Dependent Genes in Cultured Human Mural Granulosa Cell in Vitro. Reprod. Toxicol. 2017, 69, 13–18. 10.1016/j.reprotox.2016.12.007. [DOI] [PubMed] [Google Scholar]
- Sen N.; Liu X.; Craig Z. R. Short Term Exposure to Di-n-Butyl Phthalate (DBP) Disrupts Ovarian Function in Young CD-1 Mice. Reprod. Toxicol. 2015, 53, 15–22. 10.1016/j.reprotox.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joëls M.; Karst H.; Sarabdjitsingh R. A. The Stressed Brain of Humans and Rodents. Acta Physiol. 2018, 223 (2), e13066 10.1111/APHA.13066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. J.; Ross S. M.; Hensley J.; Liu K.; Heinze S. C.; Young S. S.; Gaido K. W. Differential Steroidogenic Gene Expression in the Fetal Adrenal Gland versus the Testis and Rapid and Dynamic Response of the Fetal Testis to Di(n-Butyl) Phthalate. Biol. Reprod. 2005, 73 (5), 908–917. 10.1095/biolreprod.105.042382. [DOI] [PubMed] [Google Scholar]
- Colding-Jørgensen P.; Hestehave S.; Abelson K. S. P.; Kalliokoski O. Hair Glucocorticoids Are Not a Historical Marker of Stress – Exploring the Time-Scale of Corticosterone Incorporation into Hairs in a Rat Model. Gen. Comp. Endrocrinol. 2023, 341, 114335. 10.1016/j.ygcen.2023.114335. [DOI] [PubMed] [Google Scholar]
- Kalliokoski O.; Jellestad F. K.; Murison R. A Systematic Review of Studies Utilizing Hair Glucocorticoids as a Measure of Stress Suggests the Marker Is More Appropriate for Quantifying Short-Term Stressors. Sci. Rep. 2019, 9 (1), 11997. 10.1038/s41598-019-48517-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh Solorzano C.; Serwinski B.; Grano C.; Steptoe A. Longitudinal Association between Saliva and Hair Cortisol Concentration : A Systematic Comparison. Psychoneuroendocrinology 2023, 156 (June), 106340. 10.1016/j.psyneuen.2023.106340. [DOI] [PubMed] [Google Scholar]
- Gore A. C.; Chappell V. A.; Fenton S. E.; Flaws J. A.; Nadal A.; Prins G. S.; Toppari J.; Zoeller R. T. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36 (6), E1–E150. 10.1210/er.2015-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger D. C. A Strategy for Comparing the Contributions of Environmental Chemicals and Other Risk Factors to Neurodevelopment of Children. Environ. Health Perspect. 2012, 120 (4), 501–507. 10.1289/ehp.1104170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger D. C. Interpretation of Small Effect Sizes in Occupational and Environmental Neurotoxicology: Individual versus Population Risk. Neurotoxicology 2007, 28 (2), 245–251. 10.1016/j.neuro.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Bellinger D. C. D. C. What Is an Adverse Effect? A Possible Resolution of Clinical and Epidemiological Perspectives on Neurobehavioral Toxicity. Environ. Res. 2004, 95 (3), 394–405. 10.1016/j.envres.2003.07.013. [DOI] [PubMed] [Google Scholar]
- Rose G. Sick Individuals and Sick Populations. Int. J. Epidemiol. 2001, 30 (3), 427–432. 10.1093/ije/30.3.427. [DOI] [PubMed] [Google Scholar]
- Kallak T. K.; Hellgren C.; Skalkidou A.; Sandelin-Francke L.; Ubhayasekhera K.; Bergquist J.; Axelsson O.; Comasco E.; Campbell R. E.; Sundström Poromaa I. Maternal and Female Fetal Testosterone Levels Are Associated with Maternal Age and Gestational Weight Gain. Eur. J. Endocrinol. 2017, 177 (4), 379–388. 10.1530/EJE-17-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsham W.; Dalton S.; Bilder D. A. The Prenatal Hormone Milieu in Autism Spectrum Disorder. Front. Mol. Psychiatry 2021, 12, 655438. 10.3389/fpsyt.2021.655438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abruzzese G. A.; Silva A. F.; Velazquez M. E.; Ferrer M. J.; Motta A. B. Hyperandrogenism and Polycystic Ovary Syndrome: Effects in Pregnancy and Offspring Development. WIREs Mech. Dis. 2022, 14 (5), e1558 10.1002/wsbm.1558. [DOI] [PubMed] [Google Scholar]
- Wu Y.; Wang J.; Wei Y.; Chen J.; Kang L.; Long C.; Wu S.; Shen L.; Wei G. Maternal Exposure to Endocrine Disrupting Chemicals (EDCs) and Preterm Birth: A Systematic Review, Meta-Analysis, and Meta-Regression Analysis. Environ. Pollut. 2022, 292 (PA), 118264. 10.1016/j.envpol.2021.118264. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.