Abstract
We have extensively purified a factor from conditioned medium that restores aerial mycelium formation to a mutant of Streptomyces coelicolor that is defective in morphological differentiation. Response to this factor is shown to depend on the presence of the BldK oligopeptide import system. We suggest that this substance acts at the first step in a putative cascade of developmental regulatory signals.
Multicellular organisms use intercellular signaling molecules to control many developmental processes (5). The filamentous, fungus-like bacterium Streptomyces coelicolor has a multicellular lifestyle in which extensive cell-cell signaling controls the formation of a spore-forming cell type called the aerial hyphae (11). The life cycle of S. coelicolor commences with spore germination and the formation of a branched network of vegetative hyphae called the substrate mycelium. Because cell division occurs only sporadically in the substrate mycelium, individual filaments typically contain tens or hundreds of chromosomes. Twenty-four to 36 h after germination, the colony begins to erect the aerial hyphae, in which a massive round of synchronous cell division leads to the formation of uninucleoidal spores over the next few days. A small hydrophobic molecule called SapB coats the aerial filaments and evidently contributes to their capacity to break with surface tension and stand up into the air (9a, 10). The resulting white fuzzy layer of aerial hyphae on the surface of the colony acts like a fruiting body, allowing the dispersal of the mature spores, thereby completing the life cycle (2).
The bld (from “bald”) genes (1, 6, 7, 11) are believed to be involved, at least in part, in a cascade of extracellular signals that controls events like SapB synthesis and that leads to aerial mycelium formation (11). Mutations in bld261, for example, appear to block the production of the first signal of the cascade (signal 1 [8]). Cells containing mutations in bldK, which encodes an oligopeptide importer, are believed to be blocked after the release of, but before the response to, signal 1. It has been proposed that wild-type colonies produce and export signal 1, perhaps constitutively, and that after a period during which signal 1 accumulates extracellularly, import it through the BldK oligopeptide permease, triggering the next step in the cascade. Cells containing the mutations bldA, -H, -G, -C, and -D are believed to be blocked at later steps in this cascade (8, 11).
Many of the bld mutants show a marked defect in the catabolite repression of metabolic operons by rich carbon sources that is a characteristic of wild-type S. coelicolor physiology (9). This and the fact that the developmental phenotype of some of the bld mutants can be suppressed by growth on poor carbon sources have led to the suggestion that a role of the bld gene products, and perhaps therefore the signaling cascade, is to couple morphological differentiation to the nutritional state of the substrate mycelium (1, 7, 9).
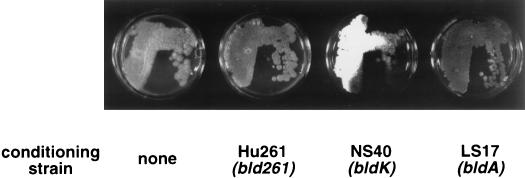
To explore this putative cell-cell signaling mechanism, we used bld mutant strains of S. coelicolor (Table 1) to condition Streptomyces growth medium and examined the effects of this conditioning on the developmental phenotype of the bld261 mutant strain HU261. These experiments were carried out by using a previously described procedure (11) with the modification that recipient cells were grown directly on conditioned medium rather than on filter discs. When equivalent amounts of medium conditioned by either a bldK mutant (NS40) or a bldA mutant (LS17) were added to fresh R2YE growth medium (4), both stimulated some degree of aerial mycelium formation in HU261, indicating that both strains produced a signal molecule(s) whose production is blocked by the bld261 mutation. Whereas medium conditioned by the bldK mutant induced HU261 to produce an abundant lawn of aerial mycelium (reminiscent of wild-type development), medium conditioned by the bldA mutant stimulated only a thin layer of aerial mycelium that was not visible unless viewed under magnification (Fig. 1). Likewise, medium conditioned by mutants believed to be blocked at later steps in the signaling cascade (bldH, bldG, bldC, or bldD) was able to stimulate only a small amount of aerial mycelium formation in HU261 (data not shown). We interpret this to mean that a signaling molecule(s) that induces aerial mycelium formation in a bld261 mutant is produced by the bldA, bldH, bldG, bldC, and bldD mutants but that most of it is then imported into the donor cells by the BldK oligopeptide importer. In contrast, because the bldK mutant is defective in oligopeptide import, the signaling molecule accumulates to a higher concentration in medium conditioned by this mutant than in medium conditioned by the mutants that are bldK+. The stimulation of aerial mycelium formation in HU261 was not due to a spurious effect of growing the cells on partially depleted medium because HU261-conditioned medium did not elicit any effect on the any of the bld mutants (Fig. 1 and data not shown).
TABLE 1.
Strains and plasmids used in this work
| Strain or plasmid | Genotype or features | Background | Source |
|---|---|---|---|
| Streptomyces coelicolor | |||
| HU261 | bld261 hisA1 uraA1 strA1 NF SCP2* | J. Willey | |
| LS17 | bldA39 hisA1 uraA1 strA1 Pgl− | K. Chater | |
| NS17 | bldK1::aadA | Lab collection | |
| NS40 | bldK1::aad hisA1 uraA1 strA1 | This study | |
| NS47 | bld261 hisA1 uraA1 strA1 NF SCP2* pJRM10 | This study | |
| NS48 | bld261 hisA1 uraA1 strA1 NF SCP2* pbldK22 | This study | |
| NS49 | bld261 bldK1::aadA hisA1 uraA1 strA1 NF SCP2* pJRM10 | This study | |
| NS50 | bld261 bldK1::aadA hisA1 uraA1 strA1 NF SCP2* pbldK22 | This study | |
| Escherichia coli | |||
| DH5α | supE44 ΔlacU169 (φ80lacZΔM15)hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | Lab collection | |
| ER-2 | F′ lacIq leuB6 thi-1 fhuA31 lacY1 tsx-78 galK2 galT22 supE44 hisG4 rpsL136 (Strr) xyl-5 mtl-1 dam13::Tn9(Cmr) dcm-6 mcrB1 mcrA hsdR2(rK− mK+) | J. McCormick | |
| Plasmids | |||
| pJRM10 | bla tsr | pIISK+ and pIJ911 | Lab collection |
| pbldK22 | bldKABCDE tsr | pJRM-10 | Lab collection |
FIG. 1.
Stimulation of aerial mycelium formation in a bld261 mutant by conditioned media. bld261 recipient cells were grown on control medium or a 1:4 mixture of bld261-, bldK-, or bldA-conditioned medium and fresh R2YE agar (4) as indicated.
To determine whether the capacity of a bld261 mutant to respond to the factor present in conditioned medium requires the BldK oligopeptide importer, we created strains doubly mutant for bld261 and bldK. NS47 and NS48 contain the bld261 mutation and, respectively, the control plasmid pJRM10 and the BldK-expressing plasmid pbldK22. NS49 and NS50 contain the bld261 mutation, the bldK1 mutation, and plasmids pJRM10 and pbldK22, respectively. When these strains were grown on medium conditioned by a bldK mutant, only those bld261 mutant cells with an active BldK oligopeptide importer were able to produce the aerial mycelium (Table 2). This suggests that the signaling molecule present in this medium might be an oligopeptide and that it must be imported into cells through the BldK oligopeptide importer to bring about aerial mycelium formation. The dependence of the activity of this factor on uptake by BldK further suggests that it is the first signal of the cascade that has previously been designated signal 1 (8).
TABLE 2.
Requirement of bldK for signal response
| Assay strain | Genotype | Aerial mycelium in response to bldK-conditioned mediuma |
|---|---|---|
| NS47 | bld261(pJRM10) | + |
| NS48 | bld261(pbldK22) | + |
| NS49 | bld261 bldK(pJRM10) | − |
| NS50 | bld261 bldK(pbldK22) | + |
Aerial mycelium was (+) or was not (−) produced.
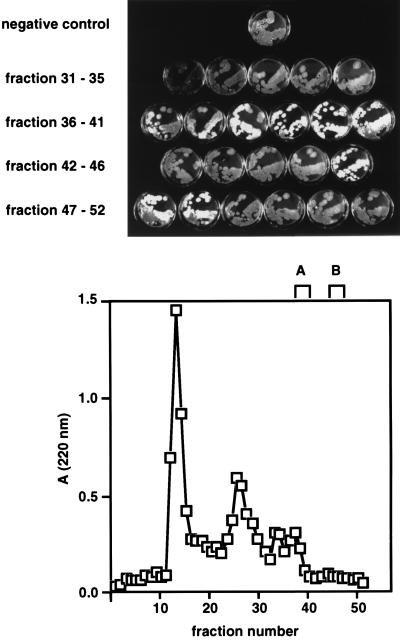
To isolate the signal 1 molecule, NS17-conditioned medium was converted to a form in which it could be applied to a C18 column. This involved melting the medium at 80°C for 1 h and spinning it at 35,000 rpm and 15°C, allowing the agar to resolidify and form a pellet. In preparative-scale experiments, 4 liters of signal-containing supernatant was applied to a series of C18 columns and eluted with increasingly shallow gradients of acetonitrile (Table 3). Fractions collected from the eluates were lyophilized, resuspended in 0.01 Tris (pH 7.0)–0.01 M NH4Cl, added to fresh R2YE growth medium, and assayed for aerial mycelium-stimulating activity by using strain HU261. Interestingly, during the second chromatographic step of the purification, factors having the capacity to induce aerial mycelium formation in the bld261 mutant were detected in two discrete peaks (peaks A and B) (Fig. 2), both of which eluted later than the majority of the UV-absorbing material that had been loaded on the column. Fractions containing the factors corresponding to peak A (fractions 38 to 41) and peak B (fractions 46 to 48) were pooled separately and subjected to high-performance liquid chromatography with a C18 column, where they eluted at 28 and 42%, respectively. It proved impossible to isolate sufficient amounts of the factor corresponding to peak A for further chromatographic or biophysical analysis. The factor present in peak B was purified to apparent homogeneity in one further chromatography step (Fig. 3). Each of the UV-absorbing peaks in this final elution profile was assayed for the stimulation of aerial mycelium formation in HU261. Only the peak indicated in Fig. 3, eluting at 40% acetonitrile, showed any such activity. Fractions flanking either side of this peak showed no activity. It was concluded that this fraction contained an essentially pure signaling molecule.
TABLE 3.
Purification of peak A and peak B signal molecules
| Step | Column | Flow rate (ml/min) | Elution method | Comment |
|---|---|---|---|---|
| 1 | 150-ml C18 Waters 125 A bulk packing materiala | 8 | 200 ml of 80% acetonitrile | |
| 2 | 50-ml C18 Waters 125 A bulk packing materiala | 1 | 0 to 80% acetonitrile gradient; 0.8%/ml over 100 ml | Two activity peaks |
| 3A | 4.6- by 250-mm Vydac 218TP C18 (peak A)a | 0.5 | 20 to 34% acetonitrile gradient; 0.18%/min over 75 min | Eluted at 28% acetonitrile |
| 3B | 4.6- by 250-mm Vydac 218TP C18 (peak B)a | 0.5 | 35 to 48% acetonitrile gradient; 0.18%/min over 75 min | Eluted at 42% acetonitrile |
| 4 | 1- by 150-mm Zorbax C18 (peak B only)b | 0.05 | 0 to 100% acetonitrile gradient; 0.25%/min over 400 min | Eluted at 40% acetonitrile |
Run and eluted in the presence of 0.02 M phosphate buffer at pH 7.
Run and eluted in the presence of 0.1% trifluoroacetic acid.
FIG. 2.
C18 chromatography resolves two discrete factors in conditioned medium. Signal activity from the first step in the purification procedure was passed over a 50-ml C18 column and eluted with a gradient of acetonitrile. Fractions were assayed for developmental activity against bld261 mutant recipient cells.
FIG. 3.
Purification of the signaling molecule contained in peak B to homogeneity. The column eluate from the third chromatography step in the purification that was highly enriched for the peak B factor was passed over a high-performance liquid chromatography C18 column. Each UV-absorbing peak was assayed for the presence of a signal molecule that could induce aerial mycelium formation in bld261 mutant recipient cells. mAU, milliabsorbance units.
We anticipated that this signaling molecule would be an oligopeptide since it rescues the phenotype of bld261 mutants in a manner that depends on active oligopeptide uptake. Mass spectrometry and amino acid composition analyses suggested that the purified factor from peak B contains a molecule with a mass of 655 Da that contains serine and glycine, properties that would be consistent with import by an oligopeptide importer such as BldK (3). Unfortunately, however, repeated attempts at obtaining an amino acid sequence by sequential Edman degradation were unsuccessful, suggesting that if this factor is a peptide, its amino terminus is blocked by some sort of covalent modification.
Notably, very small amounts of the factors in peaks A and B (we presume nanomolar concentrations on the basis of UV absorbance) were able to reproducibly induce a large amount of aerial mycelium in bld261 mutants, supporting the idea that these are bona fide components of signal 1. Finally, there is at present no explanation for the existence of two factors, peaks A and B, having the activity of signal 1, and it remains possible that one of these molecules is a biologically active fragment of the other. We anticipate that future molecular genetic studies, including especially the cloning and sequencing of the bld261 locus, will shed further light on the nature of signal 1.
Acknowledgments
We thank Jan Westpheling, Joanne Willey, and Julie Schwedock for their critical reading of the manuscript. We are also grateful to Jonathan Solomon and Beth Lazazzera for assistance at several stages of this work and to Julie Schwedock and Joe McCormick for advice concerning the handling of S. coelicolor. We also thank the Harvard University microchemistry group, including Bill Lane, John Neveu, Renee Robinson, and Eric Spooner, for their expert technical assistance.
This work was supported by a grant from the Eli Lilly Corporation and by NIH grant GM18568.
REFERENCES
- 1.Champness W. New loci required for Streptomyces coelicolor morphological and physiological differentiation. J Bacteriol. 1988;170:1168–1174. doi: 10.1128/jb.170.3.1168-1174.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chater K F. Genetics differentiation in Streptomyces. Annu Rev Microbiol. 1993;47:685–713. doi: 10.1146/annurev.mi.47.100193.003345. [DOI] [PubMed] [Google Scholar]
- 3.Higgins C. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 4.Hopwood D A, Bibb M J, Chater K F, Keiser T, Bruton C J, Keiser H M, Lydiate D J, Smith C P, Ward J M, Schrempf H. Genetic manipulation of streptomyces: a laboratory manual. Norwich, England: The John Innes Foundation; 1985. [Google Scholar]
- 5.Kenyon C. A perfect vulva every time: gradients and signaling cascades in C. elegans. Cell. 1995;82:171–174. doi: 10.1016/0092-8674(95)90302-x. [DOI] [PubMed] [Google Scholar]
- 6.Lawlor E J, Baylis H A, Chater K F. Pleiotropic morphological and antibiotic deficiencies result from mutations in a gene encoding a tRNA-like product in Streptomyces coelicolor A(3)2. Genes Dev. 1987;1:1305–1310. doi: 10.1101/gad.1.10.1305. [DOI] [PubMed] [Google Scholar]
- 7.Merrick M J. A morphological and genetic mapping study of bald colony mutants of Streptomyces coelicolor. J Gen Microbiol. 1976;96:299–315. doi: 10.1099/00221287-96-2-299. [DOI] [PubMed] [Google Scholar]
- 8.Nodwell J R, McGovern K, Losick R. An oligopeptide permease responsible for the import of an extracellular signal governing aerial mycelium formation in Streptomyces coelicolor. Mol Microbiol. 1996;22:881–893. doi: 10.1046/j.1365-2958.1996.01540.x. [DOI] [PubMed] [Google Scholar]
- 9.Pope M K, Green B D, Westpheling J. The bld mutants of Streptomyces coelicolor are defective in the regulation of carbon utilization, morphogenesis and cell-cell signaling. Mol Microbiol. 1996;19:747–756. doi: 10.1046/j.1365-2958.1996.414933.x. [DOI] [PubMed] [Google Scholar]
- 9a.Richter, M., and J. Willey. Personal communication.
- 10.Willey J W, Santamaria R, Guijarro J, Geislich M, Losick R. Extracellular complementation of a developmental mutation implicates a small sporulation protein in aerial mycelium formation by S. coelicolor. Cell. 1991;65:641–650. doi: 10.1016/0092-8674(91)90096-h. [DOI] [PubMed] [Google Scholar]
- 11.Willey J W, Schwedock J, Losick R. Multiple extracellular signals govern the production of a morphogenetic protein involved in aerial mycelium formation by Streptomyces coelicolor. Genes Dev. 1993;7:895–903. doi: 10.1101/gad.7.5.895. [DOI] [PubMed] [Google Scholar]