Abstract

Effect-directed analysis (EDA) combined with nontarget screening (NTS) has established a valuable tool for the identification of unmonitored toxic substances in environmental samples. It consists of three main steps: (1) highly potent fraction identification, (2) toxicant candidate selection, and (3) major toxicant identification. Here, we discuss the methodology, current status, limitations, and future challenges of EDA combined with NTS. This method has been applied successfully to various environmental samples, such as sediments, wastewater treatment plant effluents, and biota. We present several case studies and highlight key results. EDA has undergone significant technological advancements in the past 20 years, with the establishment of its key components: target chemical analysis, bioassays, fractionation, NTS, and data processing. However, it has not been incorporated widely into environmental monitoring programs. We provide suggestions for the application of EDA combined with NTS in environmental monitoring programs and management, with the identification of further research needs.
Keywords: In vitro bioassay, Nontarget screening, Fractionation, Environmental monitoring, Effect-based monitoring, Environmental management
Short abstract
EDA combined with NTS is useful for the identification of unmonitored toxicants in environmental samples and can be applied effectively in environmental monitoring programs.
1. Effect-Directed Analysis Combined with Nontarget Screening
Effect-directed analysis (EDA) is a valuable method for identifying major toxicants in environmental samples.1−3 It enables the detection of toxic substances in samples with complex matrixes, as “finding a needle in a haystack.”1−3 The principle of EDA is to reduce sample complexity by fractionating samples that exhibit significant toxicity.1−3 Subsequently, bioassays and chemical analyses are conducted repeatedly to identify the major toxic substances in highly potent fractions.4−6 This process is challenging, but the results have far-reaching implications for the identification of toxic substances that have not been monitored previously.4−6 Target chemicals are often called the “tip of the iceberg” because they account for only a small portion of the observed biological effects, indicating that unmonitored toxic substances may be present in environmental samples. In recent years, the identification of novel toxic substances has been enabled due to the development of high-resolution mass spectrometry (HRMS).7 Nontarget screening (NTS) aims to detect and identify all substances present in a sample and can be usefully applied in EDA to identify causative toxic substances in highly potent fractions.7−9 The combined use of EDA and NTS addresses the limitations of performing only NTS, which lacks information on the potential toxicity of compounds in environmental samples.4−6,10−12 EDA combined with NTS has been applied successfully for the identification of unmonitored toxicants in sediment, wastewater, biota, etc.6,11,13 This approach will help to expand the scope of analysis to include unknown substances, moving away from existing methods that rely solely on target chemical monitoring.14,15 Furthermore, it is expected to facilitate the efficient incorporation of effect-based monitoring (EBM) into environmental monitoring programs.16,17 The purpose of this paper is to describe the current status and limitations of EDA combined with NTS, with case studies of its successful application. In addition, we provide suggestions for the integration of EDA into future environmental monitoring programs and management policies.
2. Methodology of EDA Combined with NTS: Limitations and Challenges
2.1. Step 1. Identification of Highly Potent Fractions
The first step in EDA is the collection and preparation of environmental samples (Figure 1). For liquids, such as river water and wastewater, composite samples are typically used to ensure representativeness. Alternatively, passive samplers, such as the polar organic chemical integrative sampler or a semipermeable membrane device, could be employed.18 EDA focuses primarily on organic toxic substances; organic extracts are produced using liquid–liquid or solid-phase extraction.19,20 During this process, the adverse effects of components and factors, such as metals, inorganic ions, salt, and pH, are disregarded. This limitation can be addressed by combining EDA with toxicity identification evaluation.21,22
Figure 1.
Schematic diagram of the effect-directed analysis combined with nontarget screening procedure. In Step 1, the raw organic extract is screened for toxicity using in vivo or in vitro bioassays. Fractionation is performed one or more times for samples with great toxicity. Fractions are subjected to bioassays to identify highly potent fractions. In Step 2, target and nontarget analyses are performed, and toxicant candidates for induced toxicity are derived. In Step 3, candidate substances are chemically and toxicologically confirmed. Contributions of existing and newly identified toxic substances to induced toxicity are calculated using potency balance analysis. Finally, the major toxicants in the environmental samples are identified.
For solid samples (e.g., sediment, soil, and biota), organic extracts are typically obtained using Soxhlet extraction, accelerated solvent extraction, ultrasonic extraction, etc.3,23 These methods may not fully consider the bioaccessibility and/or bioavailability of organic chemicals in environmental samples. To compensate for this limitation, bioaccessibility-based extraction methods (partial or selective extraction), such as TENAX, have been developed and applied to EDA.3,24−26 Gel permeation chromatography column cleanup is effective in removing interfering substances, such as lipids, from samples (e.g., biota or highly polluted sediments).
Various in vitro and in vivo bioassays can be employed in EDA (Table S1). The group of compounds in samples that react may vary depending on the bioassay method selected.12 When a raw extract exhibits a significant response, fractionation testing is conducted to identify major toxic fractions. Chemical fractionation is the separation of various substances in the raw organic extract based on their physicochemical properties (e.g., polarity, molecular mass, log KOW, etc.) using column chromatography.1−3,13 This process aims to reduce the complexity of the sample, enabling the identification of potential toxicants through instrumental analysis. If the sample complexity is still high after fractionation, multistep fractionation can be considered.1−3,19,27 However, as more fractionations are performed, compounds in the sample might be lost; thus, it is crucial to determine the appropriate fractionation process.
The isolation of toxic fractions is often more evident in in vitro bioassays that rely on the specific modes of action related to the chemical structures,19 as compared to in vivo bioassays which evaluate lethal or sublethal effects on organisms.28 However, in the case of highly toxic substances, like N-(1,3-dimethylbutyl)-N′-phenyl-p-phenylenediamine (6PPD)-quinone found in tire leachate, toxic fractions could be clearly isolated even in in vivo bioassays.29 Occasionally, the toxic potency is greater following fractionation than in the parent fraction, possibly due to the complex interactions, such as mixture effects and/or removal of compounds causing masking effects.17,19,30,31 Stringent quality control is necessary during bioassays; fractions should be recombined, and their toxicity is compared to that of the parent fraction. In addition, certain chemicals may simultaneously activate multiple pathways in test organisms in a nonspecific manner. As a result, toxic effects that are more pronounced than those of previously identified mechanisms are often observed.32 As EDA is performed with organic extracts, efforts should be made to minimize the influence of solvents.19 Of note, the toxicity of hydrophobic compounds may be underestimated due to their (1) low solubility in water, (2) tendency to adsorb to plastic well plates, and (3) effects on partitioning, especially in lipid-rich sample matrixes.19,33 Thus, it is important to design bioassays, including appropriate dosing techniques and the use of laboratory tools.
2.2. Step 2. Selection of Toxicant Candidates
The selection of target and suspect analytes in EDA focuses on compound groups that align with the toxicity endpoint. Their concentrations in fractions are quantified, and potency balance analysis is performed by comparing the observed toxicity using the concentrations and relative potency values (RePs) of the target compounds (Figure 2).34 When the known compounds do not sufficiently explain the observed toxicity, NTS can be applied to expand the range of substances of interest.35−37 During NTS, optimization of the instrumental conditions of HRMS is crucial, as the proficiency of the user can significantly impact the outcomes.38 The data processing of NTS is also a critical step in identifying toxicant candidates.4,5 Despite the complexity reduction achieved through fractionation, hundreds of compounds are often detected in the fractions.4,5 If additional suitable fractionations are available, they can be employed to further reduce complexity. Otherwise, the detected compounds should be filtered using specific criteria based on the bioassay endpoints to derive potential toxicants.12,18 For example, in a study to identify unknown aryl hydrocarbon receptor (AhR) active substances in environmental samples, the criteria for selecting candidate compounds were the presence and number of aromatic rings.39
Figure 2.
Principle of potency balance analysis to determine the toxicity contributions of individual compounds. The toxicity contribution is calculated by comparing the total induced toxicity in the sample [bioassay-derived bioanalytical equivalent (BEQbio)] and the toxicity of known toxic substances [instrument-derived bioanalytical equivalent (BEQchem)]. When the BEQchem exceeds the BEQbio (Case 1), a mixture toxic effect is suspected. When the BEQbio and BEQchem are similar (Case 2), the known substances account for most of the toxicity. When the BEQchem is much lower than the BEQbio (Case 3), the sample contains unknown toxic substances (RePs, relative potency values; EC, effective concentration).
Next, candidate compounds can be selected by matching with mass spectral libraries. When the components of a compound cannot form a combination structure or the fragment ions do not match the spectrum of the library, these compounds could be excluded from the list of candidates.40,41 Mass libraries for product-derived substances, including pharmaceuticals and pesticides, are relatively well developed; those for byproducts and transformation products (TPs) are less comprehensive.42 If there is no match in the mass spectral library, the unknown substances could be identified through further identification techniques, such as in silico fragmentation tools (e.g., MetFrag and MetFusion).43,44 Toxicant candidates are selected based on the results of target analysis and NTS, typically on the scale of a few dozen compounds. Recent advancements have led to the proposal of more systematic prioritization through machine learning, artificial neural network analysis, or in silico modeling.45
2.3. Step 3. Identification of Major Toxicants
In Step 3, chemical and toxicological confirmation is carried out on the toxicant candidates (Figure 2). However, this step can be performed only on compounds for which standard materials are available. Such materials are frequently unavailable11,13 or cannot be purchased due to their high cost or export/import restrictions.46 In such instances, in silico modeling and toxicity prediction databases can be utilized to predict the toxicological potencies of chemicals, providing insights into their toxicity mechanism, effective concentration (EC), and inhibitory concentration.6 Chemical confirmation involves the comparison of retention times of compounds on gas chromatography (GC) or liquid chromatography (LC) using standard materials and the examination of the masses of fragment ions utilizing Full MS/ddMS2.4,10 When these analyses confirm that the substance matches the compound detected in the sample, quantitative analysis is performed. Toxicological confirmation is conducted with bioassays using diluted standards. The ReP value is calculated by comparing the EC (e.g., EC20 or EC50) of the newly identified toxicant with the reference compound.34 It enables the conversion of the bioassay response to an equivalent concentration.34
Potency balance analysis, also referred to as “iceberg modeling,” involves the quantitative comparison of the bioanalytical equivalent concentration in the highly potent fraction derived from bioassays (BEQbio) with the instrument-derived bioanalytical equivalent (BEQchem).16,47 This comparison assumes that the effects of toxic substances are additive.48 Mixture toxic effects are suspected when the BEQchem is significantly greater than the BEQbio (Figure 2, Case 1).6,10 When the BEQchem and BEQbio are similar, the analyzed compounds account for most of the responses (Figure 2, Case 2).11,30 For most environmental samples, the BEQchem is much smaller than the BEQbio (Figure 2, Case 3), indicating the presence of unknown toxic substances.4,49 When the newly identified toxic substances explain a significant portion of the induced toxicity, EDA combined with NTS can be considered successful; when these compounds do not substantially increase the explanatory power, the major toxic substance has not been identified. This limitation can be attributed to several factors, including the lack of mass spectral libraries, unavailability of standard materials, and selection criteria constraints.46,50 Through potency balance analysis, the toxicity contributions of individual compounds can be calculated, and the substance with the greatest contribution can be considered the major toxic substance.
3. Applications of EDA Combined with NTS: Case Studies
3.1. Sediments
Sediments are commonly used in environmental pollution monitoring because they accumulate organic pollutants over a long period (Table S2).4,5,51 Using the H4IIE-luc bioassay, Kim et al.5 identified major AhR-active compounds in coastal sediments near industrial complexes in Ulsan Bay, Korea. AhR-mediated potencies were found mainly in fractions of aromatics with log KOW values of 5–7. Through GC–quadrupole time-of-flight mass spectrometry (QTOFMS), the researchers identified seven novel AhR-active substances that accounted for up to 16% of the induced AhR-mediated potency. In another study, Cha et al.49 identified polar AhR agonists in sediments from Lake Sihwa, Korea. Eight polar AhR agonists that explained an average of 5.9% of the total AhR-mediated potency were identified. These polar AhR agonists were associated primarily with pharmaceuticals and pesticides.
3.2. Wastewater Treatment Plant Effluents
Wastewater treatment plant (WWTP) effluent is a major point source of toxic substances in aquatic environments, and the identification of unmonitored toxicants is crucial.7,11,52 Mijangos et al.7 identified toxic substances in WWTP effluents that caused growth inhibition and skeletal malformation in sea urchin embryos. For fractions showing high toxicity, NTS was performed with the use of LC–HRMS. Six toxic substances affecting sea urchin embryogenesis, including pesticides, antidepressants, and anthelmintic agents, were newly identified. In an artificial mixture test, these substances were found to account for 79% of the total observed toxicity. Gwak et al.11 identified two unmonitored estrogen receptor (ER) agonists in sewage treatment plant effluents using T47D-kbluc bioassays and LC–QTOFMS analysis. These novel ER agonists explained only 4% of the total ER-mediated potency, indicating that unknown ER agonists may still be present in the samples.
3.3. Biota
Toxic substances can accumulate in wild animals and humans through bioaccumulation and biomagnification.53−55 In some cases, top predators can accumulate exceptionally high levels of toxic substances.53 The application of EDA to biological samples has been employed in polar bears,15 seabirds,13 cetaceans,56 and humans.57 Simon et al.15 identified thyroid hormone-disrupting compounds in polar bear blood plasma samples (Svalbard, Norway). They conducted NTS with LC–TOFMS and identified branched nonylphenols and mono- and dihydroxylated-octachlorinated biphenyls as major toxicants, explaining 32% of the total measured transthyretin-binding potencies in the extracts. To date, relatively fewer EDA studies have been conducted on biota samples than on other environmental samples.23 This is due to various interfering substances, including endogenous compounds and metabolites, present in biological samples,15,23 making it difficult for fractionations, bioassays, and instrumental analyses.13
3.4. Others and Future EDA Application
In addition to the cases mentioned above, EDA has been applied successfully to oil and oil-contaminated sediments,58 microplastics,59 indoor household dust,25 river water,60 and atmospheric fine particles (Table S2).61 Furthermore, its potential applicability to road dust and household chemical products is being recognized. In addition to the applicability of EDA to these marine–terrestrial–atmospheric environments, EDA needs to be performed more to identify unknown toxic substances accumulated in human and human-related samples. EDA can be employed for the identification of not only artificial chemicals but also natural biotoxins. As for all sample types, proper sample collection and preparation with the consideration of exposure scenarios, use of appropriate bioassays and endpoints, and target compound selection are important in the application of EDA combined with NTS to such samples.
4. Future Perspectives on EDA Combined with NTS
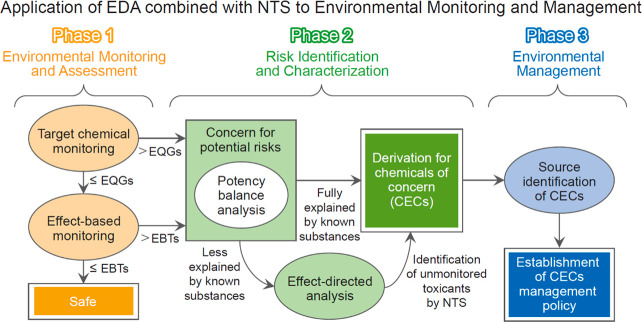
EDA combined with NTS can be applied in environmental monitoring and management for ecosystem protection along with existing target chemical monitoring and EBM. We propose three phases (Phases 1–3) for the application (Figure 3).
Figure 3.
Applicability of EDA to environmental monitoring and management. In Phase 1, environmental contamination by toxic substances is diagnosed and evaluated through target chemical and effect-based monitoring. When the concentrations of target substances exceed environmental quality guidelines (EQGs) or the bioassay results exceed effect-based triggers (EBTs), the potential risk exists. When the target concentrations are below EQGs, and the bioassay results are below EBTs, the situation is considered safe. In Phase 2, a potency balance analysis is performed to determine the explanatory power of known toxic substances and identify chemicals of emerging concern (CECs). When known toxicants do not adequately account for the overall toxicity, EDA should be performed to identify unmonitored toxicants. In Phase 3, the sources of CECs are identified, and environmental policies are established for CECs reduction and management.
4.1. Phase 1. Environmental Monitoring and Assessment
Target chemical monitoring for organic pollutants in aquatic environments is used widely in many countries as a primary tool.19,38 It is a straightforward and intuitive approach to the designation and management of pollutants with concentrations exceeding environmental quality guidelines (EQGs) as chemicals of emerging concern (CECs). However, there are thousands of toxic substances in the environment, including metabolites and TPs, while target chemical monitoring evaluates only a small portion of them (about hundreds).38,62 Furthermore, chemical analysis cannot account for the mixture effects of chemicals in the environment. Even if individual compounds are present below the EQGs and detection limits, they can cause significant toxicity due to mixture effects.17 To compensate for this limitation, EBM has recently been proposed, and related elements are actively being developed.16,17
The establishment of effect-based trigger (EBT) values is crucial for EBM application. Concentrations obtained with different bioassays, which assess various toxicological endpoints, cannot be compared directly due to the use of different reference compounds.12,16 Thus, endpoint-specific EBT values should be determined, and the amounts to which they are exceeded in various bioassays can be compared. When chemical analysis yields results that do not exceed EQGs, but EBM reveals potential toxicity through the exceeding of EBTs, further evaluation of potential risks to the ecosystem is necessary (Figure 3). A situation can be deemed “safe” when the results of target chemical monitoring do not surpass EQGs, and there are no potential toxicity endpoints exceeding EBTs, as indicated by EBM. More ecologically relevant and practical EBT values are necessary for an accurate evaluation of potential risks to the ecosystem. Furthermore, considering the bioavailability of chemicals when establishing EBT values can provide a more refined perspective in assessing the potential risk of environmental contamination and identifying CECs.
4.2. Phase 2. Risk Identification and Characterization
Risk identification and characterization are conducted for samples containing toxic substances whose concentrations have been found to exceed EQGs by chemical analysis and whose endpoints have been found to exceed EBTs by EBM. When the target compounds account for a significant proportion of the overall toxic response, the compounds with significant toxicity contributions are recognized as CECs (Figure 3). When the target compounds explain only a small proportion of the overall induced toxicity, EDA combined with NTS is performed to identify unmonitored toxic substances. Thus, toxic substances identified through EBM and EDA are designated CECs. In some cases, however, major toxic substances remain unidentified after EDA is performed. In such instances, mixture toxicity testing or further confirmation of the toxicity of compounds not confirmed by NTS is recommended.7,14 Furthermore, when parent compounds are introduced into the aquatic environment, exposure to environmental factors and chemical reactions can lead to the formation of TPs.29 These TPs may still possess toxicity or even exhibit increased toxicity due to photooxidation and microbial activity.63,64 Identifying these TPs is challenging due to the lack of established methods for isolating and purifying these products from the organic extracts. In addition, many such products do not match mass library entries, and standard materials are often unavailable.42 To address this issue, the development of more extensive compound libraries and standard materials, and methods of isolating and purifying TPs from parent compounds, is necessary.
4.3. Phase 3. Environmental Management
In the selection of CECs, the identification of the source and contamination pathway is crucial. Effective CECs management can be achieved only with the identification of the sources of major toxic substances, which can be challenging when multiple sources exist. Initially, the pollution source is estimated based on the compound group concentration and composition and statistical results.6,10 Product-derived CECs are easier to manage compared to byproducts and TPs;65,66 their introduction into the environment can be reduced by banning their use when alternatives exist. For CECs that are byproducts and TPs, the process by which they were produced from the parent compounds needs to be clarified. Studies of the multimedia distribution of CECs in aquatic environments, accompanied by the examination of their persistence, bioaccumulation, and biomagnification, are needed.
5. Implications
The ultimate goal of EDA combined with NTS is to contribute to the effective management of healthy ecosystems through the identification of toxic substances that pose potential risks but are not currently monitored. Over the past 20 years, many research efforts have contributed to the advancement of EDA methods, resulting in the accumulation of knowledge and expertise. To further enhance the application of EDA and its integration into environmental monitoring programs and policies, additional research is needed to address the following current limitations.
-
1.
Environmental relevance should be further considered during the EDA process, including sampling, extraction, and bioassays.
-
2.
Active substances should be identified and listed based on their toxicity, enabling the simultaneous application of chemical monitoring and EBM.
-
3.
The range of target substances should be expanded, and EQGs should be developed for a broader array of compounds.
-
4.
Assay-specific EBTs should be developed to enable comparison across bioassays.
-
5.
The processing of NTS data should be systematized to ensure comprehensive analysis.
As EDA involves the use of techniques from various research fields, such as environmental analytical chemistry, environmental toxicology, and environmental policy, multidisciplinary collaboration among relevant researchers is essential. The future application of EDA can be enhanced by addressing limitations and fostering collaboration, leading to improved ecosystem management and protection.
Acknowledgments
The authors thank the four anonymous reviewers and the handling editor for their valuable comments and suggestions for improving the quality of this paper. This study was supported by grants from the National Research Foundation of Korea (2021R1C1C1005977 and 2021R1I1A1A01049680) and the Korea Institute of Marine Science & Technology Promotion (KIMST) funded by the Ministry of Oceans and Fisheries (2021-0427, 2022-0534, and RS-2023-00256330).
Biographies

Seongjin Hong is an associate professor of the Department of Marine Environmental Science at Chungnam National University, South Korea. His main research interests are (1) the distribution, fate, and bioaccumulation of persistent toxic substances in the environment; (2) the occurrence and fate of biotoxins and their causative microalgae; (3) the source identification of pollutants using compound-specific stable isotope ratios; and (4) the identification of key toxicants in environmental samples using effect-directed analysis. For the past five years, he has identified several novel endocrine disruptors in environmental samples, such as sediment, wastewater, and biological samples, using effect-directed analysis. He was awarded honorable mention in the 2023 James Morgan Early Career Award from Environmental Science & Technology. The ultimate goal of his research is to identify unknown toxic substances in the environment through the application of more EDA techniques and to reflect them in environmental management policies.

Jong Seong Khim is a professor in the School of Earth and Environmental Sciences at Seoul National University (SNU). He obtained a B.S. degree in oceanography and master’s and doctoral degrees in marine biology from SNU. His research interests encompass a broad spectrum of ecological and environmental topics with keywords of (1) biodiversity, (2) biological assay, (3) ecological quality, (4) marine pollution, (5) sediment assessment, and (6) marine ecosystem services. He has edited several books and published >300 peer-reviewed journal articles, with total citations of >10 000 and an h-index of 53 at present. He is a recipient of several research awards including the 2014 International Cooperation Award for Young Scientist from the Chinese Academy of Sciences, China. He was selected as World Expert in Environmental Monitoring, Top 0.01% in 2021, and elected as a fellow of the Korean Academy of Marine Science in 2021. He is currently co-editor-in-chief of Regional Studies in Marine Science.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.3c05035.
Effect-based assessment tools available for effect-directed analysis and case studies for effect-directed analysis combined with nontarget screening (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Brack W. Effect-directed analysis: a promising tool for the identification of organic toxicants in complex mixtures?. Anal. Bioanal. Chem. 2003, 377, 397–407. 10.1007/s00216-003-2139-z. [DOI] [PubMed] [Google Scholar]
- Brack W.; Schirmer K. Effect-directed identification of oxygen and sulfur heterocycles as major polycyclic aromatic cytochrome P4501A-inducers in a contaminated sediment. Environ. Sci. Technol. 2003, 37, 3062–3070. 10.1021/es020248j. [DOI] [PubMed] [Google Scholar]
- Hong S.; Giesy J. P.; Lee J.-S.; Lee J-H; Khim J. S. Effect-Directed Analysis: Current status and future challenges. Ocean Sci. J. 2016, 51, 413–433. 10.1007/s12601-016-0038-4. [DOI] [Google Scholar]
- Cha J.; Hong S.; Kim J.; Lee J.; Yoon S. J.; Lee S.; Moon H. B.; Shin K. H.; Hur J.; Giesy J. P.; Khim J. S. Major AhR-active chemicals in sediments of Lake Sihwa, South Korea: Application of effect-directed analysis combined with full-scan screening analysis. Environ. Int. 2019, 133, 105199. 10.1016/j.envint.2019.105199. [DOI] [PubMed] [Google Scholar]
- Kim J.; Hong S.; Cha J.; Lee J.; Kim T.; Lee S.; Moon H. B.; Shin K. H.; Hur J.; Lee J. S.; Giesy J. P.; Khim J. S. Newly identified AhR-active compounds in the sediments of an industrial area using effect-directed analysis. Environ. Sci. Technol. 2019, 53, 10043–10052. 10.1021/acs.est.9b02166. [DOI] [PubMed] [Google Scholar]
- Lee J.; Hong S.; Kim T.; Park S. Y.; Cha J.; Kim Y.; Gwak J.; Lee S.; Moon H. B.; Hu W.; Wang T.; Giesy J. P.; Khim J. S. Identification of AhR agonists in sediments of the Bohai and Yellow Seas using advanced effect-directed analysis and in silico prediction. J. Hazard. Mater. 2022, 435, 128908. 10.1016/j.jhazmat.2022.128908. [DOI] [PubMed] [Google Scholar]
- Mijangos L.; Krauss M.; de Miguel L.; Ziarrusta H.; Olivares M.; Zuloaga O.; Izagirre U.; Schulze T.; Brack W.; Prieto A.; Etxebarria N. Application of the sea urchin embryo test in toxicity evaluation and effect-directed analysis of wastewater treatment plant effluents. Environ. Sci. Technol. 2020, 54, 8890–8899. 10.1021/acs.est.0c01504. [DOI] [PubMed] [Google Scholar]
- Gallampois C. M.; Schymanski E. L.; Krauss M.; Ulrich N.; Bataineh M.; Brack W. Multicriteria approach to select polyaromatic river mutagen candidates. Environ. Sci. Technol. 2015, 49, 2959–2968. 10.1021/es503640k. [DOI] [PubMed] [Google Scholar]
- Xiao H.; Krauss M.; Floehr T.; Yan Y.; Bahlmann A.; Eichbaum K.; Brinkmann M.; Zhang X.; Yuan X.; Brack W.; Hollert H. Effect-directed analysis of aryl hydrocarbon receptor agonists in sediments from the three Gorges Reservoir. China. Environ. Sci. Technol. 2016, 50, 11319–11328. 10.1021/acs.est.6b03231. [DOI] [PubMed] [Google Scholar]
- Gwak J.; Cha J.; Lee J.; Kim Y.; An S. A.; Lee S.; Moon H. B.; Hur J.; Giesy J. P.; Hong S.; Khim J. S. Effect-directed identification of novel aryl hydrocarbon receptor-active aromatic compounds in coastal sediments collected from a highly industrialized area. Sci. Total Environ. 2022, 803, 149969. 10.1016/j.scitotenv.2021.149969. [DOI] [PubMed] [Google Scholar]
- Gwak J.; Lee J.; Cha J.; Kim M.; Hur J.; Cho J.; Kim M. S.; Jang K. S.; Giesy J. P.; Hong S.; Khim J. S. Molecular characterization of estrogen receptor agonists during sewage treatment processes using effect-directed analysis combined with high-resolution full-scan screening. Environ. Sci. Technol. 2022, 56, 13085–13095. 10.1021/acs.est.2c03428. [DOI] [PubMed] [Google Scholar]
- Lee J.; Hong S.; Kim T.; Lee C.; An S. A.; Kwon B. O.; Lee S.; Moon H. B.; Giesy J. P.; Khim J. S. Multiple bioassays and targeted and nontargeted analyses to characterize potential toxicological effects associated with sediments of Masan Bay: Focusing on AhR-mediated potency. Environ. Sci. Technol. 2020, 54, 4443–4454. 10.1021/acs.est.9b07390. [DOI] [PubMed] [Google Scholar]
- Cha J.; Hong S.; Gwak J.; Kim M.; Lee J.; Kim T.; Han G. M.; Hong S. H.; Hur J.; Giesy J. P.; Khim J. S. Identification of novel polar aryl hydrocarbon receptor agonists accumulated in liver of black-tailed gulls in Korea using advanced effect-directed analysis. J. Hazard. Mater. 2022, 429, 128305. 10.1016/j.jhazmat.2022.128305. [DOI] [PubMed] [Google Scholar]
- Muz M.; Krauss M.; Kutsarova S.; Schulze T.; Brack W. Mutagenicity in surface waters: Synergistic effects of carboline alkaloids and aromatic amines. Environ. Sci. Technol. 2017, 51, 1830–1839. 10.1021/acs.est.6b05468. [DOI] [PubMed] [Google Scholar]
- Simon E.; van Velzen M.; Brandsma S. H.; Lie E.; Loken K.; de Boer J.; Bytingsvik J.; Jenssen B. M.; Aars J.; Hamers T.; Lamoree M. H. Effect-directed analysis to explore the polar bear exposome: identification of thyroid hormone disrupting compounds in plasma. Environ. Sci. Technol. 2013, 47, 8902–8912. 10.1021/es401696u. [DOI] [PubMed] [Google Scholar]
- Escher B. I.; Aït-Aïssa S.; Behnisch P. A.; Brack W.; Brion F.; Brouwer A.; Buchinger S.; Crawford S. E.; Du Pasquier D.; Hamers T.; van der Oost R.; Vermeirssen E.; Neale P. A.; et al. Effect-based trigger values for in vitro and in vivo bioassays performed on surface water extracts supporting the environmental quality standards (EQS) of the European Water Framework Directive. Sci. Total Environ. 2018, 628–629, 748–765. 10.1016/j.scitotenv.2018.01.340. [DOI] [PubMed] [Google Scholar]
- Neale P. A.; Escher B. I.; de Baat M. L.; Dechesne M.; Dingemans M. M. L.; Enault J.; Pronk G. J.; Smeets P.; Leusch F. D. L. Application of effect-based methods to water quality monitoring: Answering frequently asked questions by water quality managers, regulators, and policy makers. Environ. Sci. Technol. 2023, 57, 6023–6032. 10.1021/acs.est.2c06365. [DOI] [PubMed] [Google Scholar]
- Zwart N.; Nio S. L.; Houtman C. J.; de Boer J.; Kool J.; Hamers T.; Lamoree M. H. High-throughput effect-directed analysis using downscaled in vitro reporter gene assays to identify endocrine disruptors in surface water. Environ. Sci. Technol. 2018, 52, 4367–4377. 10.1021/acs.est.7b06604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack W.; Ait-Aissa S.; Burgess R. M.; Busch W.; Creusot N.; Di Paolo C.; Escher B. I.; Mark Hewitt L.; Hilscherova K.; Hollender J.; Neumann S.; Rostkowski P.; Ruttkies C.; Schollee J.; Schymanski E. L.; Schulze T.; Seiler T. B.; Tindall A. J.; De Aragão Umbuzeiro G.; Vrana B.; Krauss M.; et al. Effect-directed analysis supporting monitoring of aquatic environments–An in-depth overview. Sci. Total Environ. 2016, 544, 1073–1118. 10.1016/j.scitotenv.2015.11.102. [DOI] [PubMed] [Google Scholar]
- Burgess R. M.; Ho K. T.; Brack W.; Lamoree M. Effects-directed analysis (EDA) and toxicity identification evaluation (TIE): Complementary but different approaches for diagnosing causes of environmental toxicity. Environ. Toxicol. Chem. 2013, 32, 1935–1945. 10.1002/etc.2299. [DOI] [PubMed] [Google Scholar]
- Li H.; Yi X.; Cheng F.; Tong Y.; Mehler W. T.; You J. Identifying organic toxicants in sediment using effect-directed analysis: A combination of bioaccessibility-based extraction and high-throughput midge toxicity testing. Environ. Sci. Technol. 2019, 53, 996–1003. 10.1021/acs.est.8b05633. [DOI] [PubMed] [Google Scholar]
- Qi H.; Li H.; Wei Y.; Mehler W. T.; Zeng E. Y.; You J. Effect-directed analysis of toxicants in sediment with combined passive dosing and in vivo toxicity testing. Environ. Sci. Technol. 2017, 51, 6414–6421. 10.1021/acs.est.7b00540. [DOI] [PubMed] [Google Scholar]
- Simon E.; Lamoree M. H.; Hamers T.; de Boer J. Challenges in effect-directed analysis with a focus on biological samples. Trac-Trend Anal. Chem. 2015, 67, 179–191. 10.1016/j.trac.2015.01.006. [DOI] [Google Scholar]
- Cui X.; Mayer P.; Gan J. Methods to assess bioavailability of hydrophobic organic contaminants: Principles, operations, and limitations. Environ. Pollut. 2013, 172, 223–234. 10.1016/j.envpol.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q.; Shen Y.; Chou L.; Guo J.; Zhang X.; Shi W. Identification of glucocorticoid receptor antagonistic activities and responsible compounds in house dust: Bioaccessibility should not be ignored. Environ. Sci. Technol. 2022, 56, 16768–16779. 10.1021/acs.est.2c04183. [DOI] [PubMed] [Google Scholar]
- Hong S.; Yim U. H.; Ha S. Y.; Shim W. J.; Jeon S.; Lee S.; Kim C.; Choi K.; Jung J.; Giesy J. P.; Khim J. S. Bioaccessibility of AhR-active PAHs in sediments contaminated by the Hebei Spirit oil spill: Application of Tenax extraction in effect-directed analysis. Chemosphere 2016, 144, 706–712. 10.1016/j.chemosphere.2015.09.043. [DOI] [PubMed] [Google Scholar]
- Brack W.; Kind T.; Hollert H.; Schrader S.; Möder M. Sequential fractionation procedure for the identification of potentially cytochrome P4501A-inducing compounds. J. Chromatogr. A 2003, 986, 55–66. 10.1016/S0021-9673(02)01909-X. [DOI] [PubMed] [Google Scholar]
- An S. A.; Lee J.; Cha J.; Gwak J.; Kim M.; Hur J.; Hong S.; Khim J. S. Characterization of microalgal toxicants in the sediments from an industrial area: Application of advanced effect-directed analysis with multiple endpoint bioassays. Environ. Int. 2023, 173, 107833. 10.1016/j.envint.2023.107833. [DOI] [PubMed] [Google Scholar]
- Tian Z.; Zhao H.; Peter K. T.; Gonzalez M.; Wetzel J.; Wu C.; Hu X.; Prat J.; Mudrock E.; Hettinger R.; Cortina A. E.; Biswas R. G.; Kock F. V. C.; Soong R.; Jenne A.; Du B.; Hou F.; He H.; Lundeen R.; Gilbreath A.; Sutton R.; Scholz N. L.; Davis J. W.; Dodd M. C.; Simpson A.; McIntyre J. K.; Kolodziej E. P. A ubiquitous tire rubber–derived chemical induces acute mortality in coho salmon. Science 2021, 371, 185–189. 10.1126/science.abd6951. [DOI] [PubMed] [Google Scholar]
- Hashmi M. A. K.; Krauss M.; Escher B. I.; Teodorovic I.; Brack W. Effect-directed analysis of progestogens and glucocorticoids at trace concentrations in river water. Environ. Toxicol. Chem. 2020, 39, 189–199. 10.1002/etc.4609. [DOI] [PubMed] [Google Scholar]
- Hashmi M. A. K.; Escher B. I.; Krauss M.; Teodorovic I.; Brack W. Effect-directed analysis (EDA) of Danube River water sample receiving untreated municipal wastewater from Novi Sad, Serbia. Sci. Total Environ. 2018, 624, 1072–1081. 10.1016/j.scitotenv.2017.12.187. [DOI] [PubMed] [Google Scholar]
- Judson R.; Houck K.; Martin M.; Richard A. M.; Knudsen T. B.; Shah I.; Little S.; Wambaugh J.; Woodrow Setzer R.; Kothya P.; Phuong J.; Filer D.; Smith D.; Reif D.; Rotroff D.; Kleinstreuer N.; Sipes N.; Xia M.; Huang R.; Crofton K.; Thomas R. S. Editor’s Highlight: Analysis of the Effects of Cell Stress and Cytotoxicity on In Vitro Assay Activity Across a Diverse Chemical and Assay Space. Toxicol. Sci. 2016, 152, 323–339. 10.1093/toxsci/kfw092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. E. C.; Oostingh G. J.; Mayer P. Passive Dosing for Producing Defined and Constant Exposure of Hydrophobic Organic Compounds during in Vitro Toxicity Tests. Chem. Res. Toxicol. 2010, 23, 55–65. 10.1021/tx900274j. [DOI] [PubMed] [Google Scholar]
- Villeneuve D. L.; Kannan K.; Khim J. S.; Falandysz J.; Nikiforov V. A.; Blankenship A. L.; Giesy J. P. Relative potencies of individual polychlorinated naphthalenes to induce dioxin-like responses in fish and mammalian in vitro bioassays. Arch. Environ. Contam. Toxicol. 2000, 39, 273–281. 10.1007/s002440010105. [DOI] [PubMed] [Google Scholar]
- Hernández F.; Bakker J.; Bijlsma L.; de Boer J.; Botero-Coy A. M.; Bruinen de Bruin Y.; Fischer S.; Hollender J.; Kasprzyk-Hordern B.; Lamoree M.; López F. J.; Laak T. L. t.; van Leerdam J. A.; Sancho J. V.; Schymanski E. L.; de Voogt P.; Hogendoorn E. A. The role of analytical chemistry in exposure science: Focus on the aquatic environment. Chemosphere 2019, 222, 564–583. 10.1016/j.chemosphere.2019.01.118. [DOI] [PubMed] [Google Scholar]
- Narváez A.; Izzo L.; Rodríguez-Carrasco Y.; Ritieni A. Citrinin Dietary Exposure Assessment Approach through Human Biomonitoring High-Resolution Mass Spectrometry-Based Data. J. Agr. Food Chem. 2021, 69, 6330–6338. 10.1021/acs.jafc.1c01776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postigo C.; Andersson A.; Harir M.; Bastviken D.; Gonsior M.; Schmitt-Kopplin P.; Gago-Ferrero P.; Ahrens L.; Ahrens L.; Wiberg K. Unraveling the chemodiversity of halogenated disinfection by-products formed during drinking water treatment using target and non-target screening tools. J. Hazard. Mater. 2021, 401, 123681. 10.1016/j.jhazmat.2020.123681. [DOI] [PubMed] [Google Scholar]
- Escher B. I.; Stapleton H. M.; Schymanski E. L. Tracking complex mixtures of chemicals in our environment. Science 2020, 367, 388–392. 10.1126/science.aay6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekenyan O. G.; Veith G. D.; Call D. J.; Ankley G. T. A QSAR evaluation of Ah receptor binding of halogenated aromatic xenobiotics. Environ. Health Persp. 1996, 104, 1302–1310. 10.1289/ehp.961041302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booij P.; Vethaak A. D.; Leonards P. E.; Sjollema S. B.; Kool J.; de Voogt P.; Lamoree M. H. Identification of photosynthesis inhibitors of pelagic marine algae using 96-well plate microfractionation for enhanced throughput in effect-directed analysis. Environ. Sci. Technol. 2014, 48, 8003–8011. 10.1021/es405428t. [DOI] [PubMed] [Google Scholar]
- Moschet C.; Lew B. M.; Hasenbein S.; Anumol T.; Young T. M. LC- and GC-QTOF-MS as Complementary Tools for a Comprehensive Micropollutant Analysis in Aquatic Systems. Environ. Sci. Technol. 2017, 51, 1553–1561. 10.1021/acs.est.6b05352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández F.; Ibáñez M.; Gracia-Lor E.; Sancho J. V. Retrospective LC-QTOF-MS analysis searching for pharmaceutical metabolites in urban wastewater. J. Sep. Sci. 2011, 34, 3517–3526. 10.1002/jssc.201100540. [DOI] [PubMed] [Google Scholar]
- Wolf S.; Schmidt S.; Müller-Hannemann M.; Neumann S. In silico fragmentation for computer assisted identification of metabolite mass spectra. BMC Bioinformatics 2010, 11, 148. 10.1186/1471-2105-11-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlich M.; Neumann S. MetFusion: integration of compound identification strategies. J. Mass Spectrom. 2013, 48, 291–298. 10.1002/jms.3123. [DOI] [PubMed] [Google Scholar]
- Dávila-Santiago E.; Shi C.; Mahadwar G.; Medeghini B.; Insinga L.; Hutchinson R.; Good S.; Jones G. D. Machine learning applications for chemical fingerprinting and environmental source tracking using non-target chemical data. Environ. Sci. Technol. 2022, 56, 4080–4090. 10.1021/acs.est.1c06655. [DOI] [PubMed] [Google Scholar]
- Zwart N.; Jonker W.; Broek R. T.; de Boer J.; Somsen G.; Kool J.; Hamers T.; Houtman C. J.; Lamoree M. H. Identification of mutagenic and endocrine disrupting compounds in surface water and wastewater treatment plant effluents using high-resolution effect-directed analysis. Water Res. 2020, 168, 115204. 10.1016/j.watres.2019.115204. [DOI] [PubMed] [Google Scholar]
- Neale P. A.; Braun G.; Brack W.; Carmona E.; Gunold R.; Konig M.; Krauss M.; Liebmann L.; Liess M.; Link M.; Schafer R. B.; Schlichting R.; Schreiner V. C.; Schulze T.; Vormeier P.; Weisner O.; Escher B. I. Assessing the mixture effects in in vitro bioassays of chemicals occurring in small agricultural streams during rain events. Environ. Sci. Technol. 2020, 54, 8280–8290. 10.1021/acs.est.0c02235. [DOI] [PubMed] [Google Scholar]
- Larsson M.; Orbe D.; Engwall M. Exposure time-dependent effects on the relative potencies and additivity of PAHs in the Ah receptor-based H4IIE-luc bioassay. Environ. Toxicol. Chem. 2012, 31, 1149–1157. 10.1002/etc.1776. [DOI] [PubMed] [Google Scholar]
- Cha J.; Hong S.; Lee J.; Gwak J.; Kim M.; Kim T.; Hur J.; Giesy J. P.; Khim J. S. Novel polar AhR-active chemicals detected in sediments of an industrial area using effect-directed analysis based on in vitro bioassays with full-scan high resolution mass spectrometric screening. Sci. Total Environ. 2021, 779, 146566. 10.1016/j.scitotenv.2021.146566. [DOI] [PubMed] [Google Scholar]
- Gago-Ferrero P.; Schymanski E. L.; Bletsou A. A.; Aalizadeh R.; Hollender J.; Thomaidis N. S. Extended suspect and non-target strategies to characterize emerging polar organic contaminants in raw wastewater with LC-HRMS/MS. Environ. Sci. Technol. 2015, 49, 12333–12341. 10.1021/acs.est.5b03454. [DOI] [PubMed] [Google Scholar]
- Niu L.; Carmona E.; Konig M.; Krauss M.; Muz M.; Xu C.; Zou D.; Escher B. I. Mixture risk drivers in freshwater sediments and their bioavailability determined using passive equilibrium sampling. Environ. Sci. Technol. 2020, 54, 13197–13206. 10.1021/acs.est.0c05124. [DOI] [PubMed] [Google Scholar]
- Tousova Z.; Froment J.; Oswald P.; Slobodnik J.; Hilscherova K.; Thomas K. V.; Tollefsen K. E.; Reid M.; Langford K.; Blaha L. Identification of algal growth inhibitors in treated waste water using effect-directed analysis based on non-target screening techniques. J. Hazard. Mater. 2018, 358, 494–502. 10.1016/j.jhazmat.2018.05.031. [DOI] [PubMed] [Google Scholar]
- Bytingsvik J.; Simon E.; Leonards P. E.; Lamoree M.; Lie E.; Aars J.; Derocher A. E.; Wiig O.; Jenssen B. M.; Hamers T. Transthyretin-binding activity of contaminants in blood from polar bear (Ursus maritimus) cubs. Environ. Sci. Technol. 2013, 47, 4778–4786. 10.1021/es305160v. [DOI] [PubMed] [Google Scholar]
- Misaki K.; Suzuki G.; Tue N. M.; Takahashi S.; Someya M.; Takigami H.; Tajima Y.; Yamada T. K.; Amano M.; Isobe T.; Tanabe S. Toxic identification and evaluation of androgen receptor antagonistic activities in acid-treated liver extracts of high-trophic level wild animals from Japan. Environ. Sci. Technol. 2015, 49, 11840–11848. 10.1021/acs.est.5b02288. [DOI] [PubMed] [Google Scholar]
- Wang H.; Xia X.; Liu R.; Wang Z.; Zhai Y.; Lin H.; Wen W.; Li Y.; Wang D.; Yang Z.; Muir D. C. G.; Crittenden J. C. Dietary uptake patterns affect bioaccumulation and biomagnification of hydrophobic organic compounds in fish. Environ. Sci. Technol. 2019, 53, 4274–4284. 10.1021/acs.est.9b00106. [DOI] [PubMed] [Google Scholar]
- Suzuki G.; Tue N. M.; van der Linden S.; Brouwer A.; van der Burg B.; van Velzen M.; Lamoree M.; Someya M.; Takahashi S.; Isobe T.; Tajima Y.; Yamada T. K.; Takigami H.; Tanabe S. Identification of major dioxin-like compounds and androgen receptor antagonist in acid-treated tissue extracts of high trophic-level animals. Environ. Sci. Technol. 2011, 45, 10203–10211. 10.1021/es2024274. [DOI] [PubMed] [Google Scholar]
- Dusza H. M.; Manz K. E.; Pennell K. D.; Kanda R.; Legler J. Identification of known and novel nonpolar endocrine disruptors in human amniotic fluid. Environ. Int. 2022, 158, 106904. 10.1016/j.envint.2021.106904. [DOI] [PubMed] [Google Scholar]
- Hong S.; Lee S.; Choi K.; Kim G. B.; Ha S. Y.; Kwon B. O.; Ryu J.; Yim U. H.; Shim W. J.; Jung J.; Giesy J. P.; Khim J. S. Effect-directed analysis and mixture effects of AhR-active PAHs in crude oil and coastal sediments contaminated by the Hebei Spirit oil spill. Environ. Pollut. 2015, 199, 110–118. 10.1016/j.envpol.2015.01.009. [DOI] [PubMed] [Google Scholar]
- Rummel C. D.; Escher B. I.; Sandblom O.; Plassmann M. M.; Arp H. P. H.; MacLeod M.; Jahnke A. Effects of leachates from UV-weathered microplastic in cell-based bioassays. Environ. Sci. Technol. 2019, 53, 9214–9223. 10.1021/acs.est.9b02400. [DOI] [PubMed] [Google Scholar]
- Muschket M.; Di Paolo C.; Tindall A. J.; Touak G.; Phan A.; Krauss M.; Kirchner K.; Seiler T. B.; Hollert H.; Brack W. Identification of unknown antiandrogenic compounds in surface waters by effect-directed analysis (EDA) using a parallel fractionation approach. Environ. Sci. Technol. 2018, 52, 288–297. 10.1021/acs.est.7b04994. [DOI] [PubMed] [Google Scholar]
- Durant J. L.; Lafleur A. L.; Plummer E. F.; Taghizadeh K.; Busby W. F.; Thilly W. G. Human Lymphoblast Mutagens in Urban Airborne Particles. Environ. Sci. Technol. 1998, 32, 1894–1906. 10.1021/es9706965. [DOI] [Google Scholar]
- Escher B. I.; Neale P. A.; Leusch F. D. Effect-based trigger values for in vitro bioassays: Reading across from existing water quality guideline values. Water Res. 2015, 81, 137–148. 10.1016/j.watres.2015.05.049. [DOI] [PubMed] [Google Scholar]
- Feng X.; Li D.; Liang W.; Ruan T.; Jiang G. Recognition and prioritization of chemical mixtures and transformation products in Chinese estuarine waters by suspect screening analysis. Environ. Sci. Technol. 2021, 55, 9508–9517. 10.1021/acs.est.0c06773. [DOI] [PubMed] [Google Scholar]
- Wang X.; Yu N.; Yang J.; Jin L.; Guo H.; Shi W.; Zhang X.; Yang L.; Yu H.; Wei S. Suspect and non-target screening of pesticides and pharmaceuticals transformation products in wastewater using QTOF-MS. Environ. Int. 2020, 137, 105599. 10.1016/j.envint.2020.105599. [DOI] [PubMed] [Google Scholar]
- Escher B. I.; Fenner K. Recent advances in environmental risk assessment of transformation products. Environ. Sci. Technol. 2011, 45, 3835–3847. 10.1021/es1030799. [DOI] [PubMed] [Google Scholar]
- Guo J.; Deng D.; Qiu J.; Shen J.; Wang L.; Wei S.; Zhang X.; Zhou Q.; Yu H.; Shi W. Biodirected identification of untargeted toxicants in industrial wastewater guides the upgrading of water treatments. Environ. Sci. Technol. 2021, 8, 474–481. 10.1021/acs.estlett.1c00277. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.