Abstract
Exposure of cells of Schizosaccharomyces pombe to heat shock or osmotic upshift results in an increased level of neutral trehalase activity, which is responsible for hydrolysis of intracellular trehalose. We constructed S. pombe mutants lacking neutral trehalase activity by gene replacement at the newly defined ntp1+ locus. Analysis of these mutants revealed that a twofold increase in trehalose accumulation, enhanced acquired thermoresistance, and marked salt tolerance characterized their ability to grow in liquid and solid media. Analysis of the expression of the trehalase gene under heat shock and osmotic upshift revealed the transcriptional activation of ntp1+ in response to both stresses.
The function of neutral trehalase in yeasts is to control the intracellular concentration of trehalose, which plays an important role in the life of yeast. Considerable evidence over the last few years indicates that trehalose may serve both as a potential carbon and energy source and as a protectant metabolite able to counteract deleterious effects of environmental stresses (11, 22–24). Rapid mobilization of this reserve carbohydrate is associated with growth resumption, suggesting that its energy supply function may be a critical factor in overcoming nutritional imbalance during stress. Heat shock enhances trehalase in Schizosaccharomyces pombe (7, 20). Also, trehalase increases markedly upon the exposure of cells of the fission yeast to media containing high salinity levels, suggesting that this effect might be a component of the osmotic-stress response (9). At present, the biochemical mechanisms responsible for these increases in S. pombe are poorly understood, although available evidence indicates that the heat shock-induced increase in trehalase might be due to a mechanism different from that underlying the response of trehalase under osmotic-stress conditions (9).
In the budding yeast Saccharomyces cerevisiae the gene encoding the neutral cytosolic trehalase, NTH1, has been cloned and the gene product has been identified (2, 13). Furthermore, S. cerevisiae mutants deficient in neutral trehalase activity have been characterized (16, 17). In contrast, no similar tasks have so far been accomplished for the fairly unrelated fission yeast S. pombe. In this work we identify the gene encoding neutral trehalase in S. pombe and present some features of mutants lacking trehalase obtained by one-step gene disruption. Finally, the expression of the trehalase gene under heat and osmotic stress was also determined.
Disruption of the neutral trehalase gene from S. pombe.
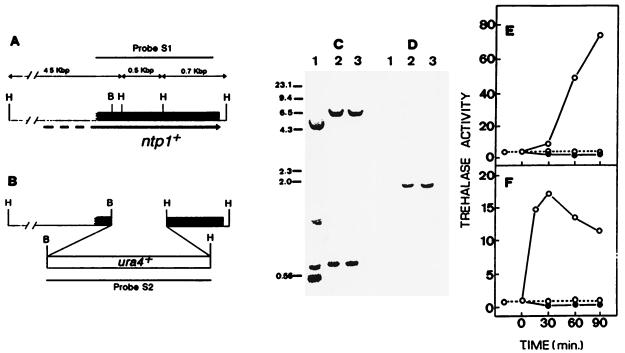
A search in nucleic acid databases disclosed a clone from an S. pombe cDNA library (GenBank accession no., D89273) with an insert of 1,537 bp which was highly similar to the 3′ end of the coding region of S. cerevisiae neutral trehalase gene NTH1 (56% identity in the overall sequence) (13, 24a). We considered that this sequence might be part of the coding region for the neutral trehalase gene of fission yeast. To check for this possibility, we first amplified by PCR the 1.5-kb fragment with 5′ oligonucleotide GAA TCA CTG GGT TTG CTT and 3′ oligonucleotide CGT AAG GGA ATA TTC GCC as the primers and genomic DNA from wild-type strain 972h− as the template. A 1.5-kb band was selectively amplified, gel purified, and later cloned into pGEM-T vector (Promega) to form pMTM-8. The insert was then sequenced to confirm sequence identity with the cDNA clone. To determine whether the cloned S. pombe fragment included part of the coding region for the neutral trehalase gene, an internal 0.6-kb region was replaced by the ura4+ gene (Fig. 1A and B) and integrated via homologous recombination into haploid h+ and h− strains, since neutral trehalases do not appear to be essential for survival of yeasts (1, 13). pMTM-8 was digested with BamHI and HindIII, thereby releasing an internal fragment of 673 bp from the cloned S. pombe sequence, which was replaced with the S. pombe BamHI-HindIII fragment containing the ura4+ gene (10), thus creating plasmid pMTM-9. pMTM-9 contains the S. pombe ura4+ gene flanked by 370 and 630 bp of the coding region of the trehalase gene at the 5′ and 3′ ends, respectively. This plasmid was linearized with BstXI and NcoI, releasing a 2.7-kb fragment (ntp1::ura4+) that was gel purified and transformed into haploid strains JY742 (h+ ade6-M216 leu1-32 ura4-D18) (14) and MM-2 (h− ade6-M210 leu1-32 ura4-D18) (our stock) by the lithium acetate method (15). Transformants were recovered at a high frequency on the basis of uracil prototrophy, and effective disruption of the putative neutral trehalase gene was verified by PCR and Southern blot hybridization (Fig. 1C and D). Two of these transformants were selected for further studies and designated MMT-3 (h+ ade6-M216 leu1-32 ura4-D18 Δntp1::ura4+) and MMT-8 (h− ade6-M210 leu1-32 ura4-D18 Δntp1::ura4+). Final proof that the selected sequence corresponded to the structural gene for neutral trehalase came from direct determinations of neutral trehalase activity for both control and disrupted strains (Fig. 1E and F). Neutral trehalase activity increases in S. pombe following a glucose pulse (4, 5) or a heat shock (7, 20). Accordingly, control strain JY742 showed a typical activation of neutral trehalase after each one of these treatments, whereas no activity was detectable at any condition in the disrupted strains. Thus, the sequence isolated from S. pombe is a part of a gene coding for the neutral trehalase. We have termed this gene ntp1+ (for neutral trehalase of S. pombe) and will refer to it in this way hereafter. The possibility that ntp1+ encodes a regulatory factor instead of being the structural gene for trehalase is highly unlikely in view of the sequence identity to part of the coding region of NTH1 by which the initial clone was selected.
FIG. 1.
Disruption of the ntp1+ gene from S. pombe. (A) Restriction map of the genomic region of S. pombe containing the ntp1+ gene. The solid bar indicates the DNA fragment amplified and sequenced. The arrow below indicates the direction of transcription of the ntp1+ ORF. (B) Construct used in the disruption of ntp1+. The 3′ end of the gene was replaced by the ura4+ cassette. Restriction sites B and H correspond to BamHI and HindIII, respectively. (C) Southern hybridization analysis of HindIII-digested genomic DNA (10 μg) from strain JY742 (ntp1+; lane 1) and transformants MMT-3 (Δntp1::ura4+; lane 2) and MMT-8 (Δntp1::ura4+; lane 3), with S1 as a digoxigenin-labelled probe for the ntp1+ gene (panel A), yielded the pattern expected for gene replacement at the ntp1+ locus. (D) The same analysis as that shown in panel C was performed, except that in this case the genomic DNAs were digested with HindIII and BamHI and hybridized with digoxigenin-labelled probe S2 (panel B), which corresponds to the ura4+ gene. (E) Specific activity of neutral trehalase (in units per milligram of protein) for control strain JY742 (ntp1+) (○) and for disruptant MMT-3 (Δntp1::ura4+) (•) following treatment at 40°C for different times. (F) Specific activity of neutral trehalase after addition, at zero time, of 100 mM glucose to cultures of control strain JY742 (ntp1+) (○) and disruptant MMT-3 (Δntp1::ura4+) (•). The discontinuous line in panels E and F shows trehalase activity in control cells not subjected to any of the treatments referred to above.
Properties of ntp1+-disrupted mutants.
The ntp1+ gene is not essential for the viability of S. pombe. Growth rates in a range of 25 to 37°C were found to be similar for control and ntp1+-disrupted strains. Also, under standard growth conditions (27°C), the doubling time for control JY742 and trehalase-less MMT-3 and MMT-8 strains was close to 181 ± 2.3 min. when the strains were cultured in rich medium (yeast extract plus supplements [YES]) and was 203 ± 3.6 min when the strains were grown in minimal medium (15). Although trehalase mutants of S. cerevisiae grow poorly on glycerol (16), we did not find a similar behavior for Δntp1+ strains of S. pombe. Similarly, no significant differences in mating and sporulation frequencies between wild-type cells and trehalase-less mutants were observed. To examine if the ntp1+ gene product was essential for spore germination, mutant strain MMT-3 was crossed with wild-type strain MM-2 and diploids were isolated in minimal medium without adenine. Sporulation was performed in malt extract (ME) medium, and spores were purified by glusulase treatment (15). Random spore analysis using uracil prototrophy as a marker yielded a 2:2 segregation for this characteristic. Therefore, unlike the tps1+ gene (3), the ntp1+ gene is not essential for spore germination in S. pombe. In addition, disruption of the ntp1+ gene was without noticeable effect on cell morphology.
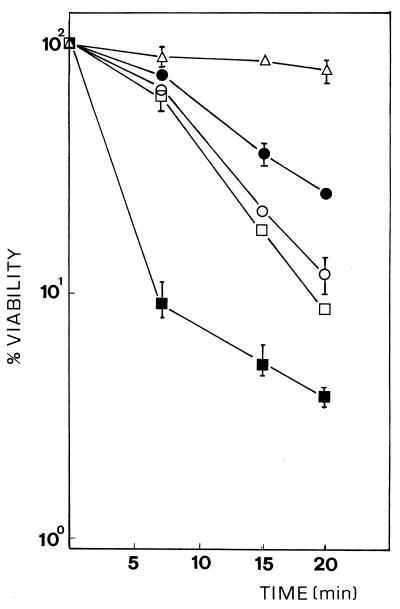
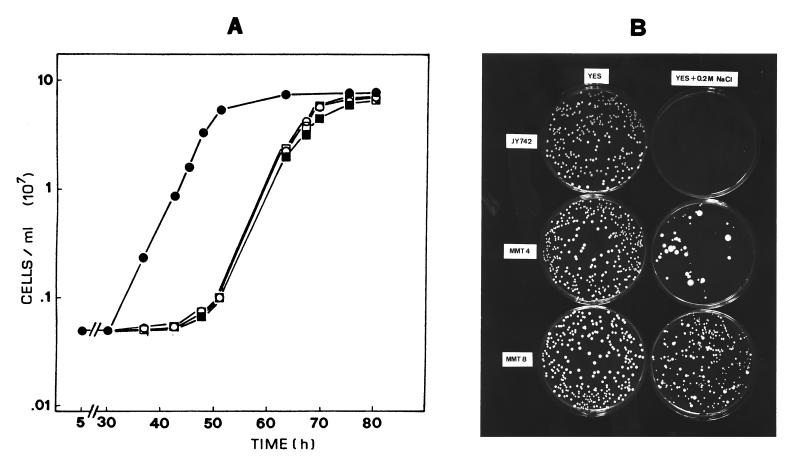
Measurement of intracellular trehalose (8) indicated that ntp1+-disrupted cells from mid-log-phase cultures contained about twice the amount of trehalose present in control cells (Table 1). Furthermore, trehalose accumulated both in wild-type control and mutant strains following a temperature increase (7). The disaccharide was also overproduced by increasing the salt concentration of the medium (Table 1), a result which is in agreement with increased tps1+ transcription during the osmotic-stress response (6). Trehalose hyperaccumulation in Δntp1 cells was paralleled by a relative increase in induced thermotolerance compared with control cells (Fig. 2) but not in intrinsic thermotolerance to heat (i.e., direct resistance at 48°C), which slightly decreased in both fermenting and nonfermenting conditions (data not shown). Figure 2 includes comparative determinations of viability for heat-hypersensitive strain PBU13 (Δtps1::ura4+) and heat-resistant strain JZ636 (Δpka1::ura4+) (8, 18). Loss of trehalase function was also accompanied by enhanced recovery from osmotic stress and better adaptation for growth in both liquid and solid minimal and rich media supplemented with NaCl (Fig. 3). These results suggest that trehalose may contribute to cell survival not only as a thermoprotective agent during a temperature upshift (7, 18) but also as an osmolyte during osmotic adjustment.
TABLE 1.
Trehalose content in control and ntp1-disrupted strains during heat shock and under osmotic stress
| Straina (relevant genotype) | Trehalose contentb (nmol/107 cells) after:
|
||
|---|---|---|---|
| No treatment | Heat shock | Osmotic stress | |
| JY742 (control) | 3.5 ± 0.3 | 73.3 ± 4.0 | 21.2 ± 1.2 |
| MMT-3 (Δntp1::ura4+) | 7.2 ± 0.9 | 138.6 ± 9.2 | 57.7 ± 6.4 |
| MMT-8 (Δntp1::ura4+) | 7.7 ± 0.5 | 129.1 ± 6.7 | 53.1 ± 5.5 |
Mid-exponential-phase cultures growing on YES medium were extracted for trehalose content measurement before and after either a 60-min treatment at 40°C (heat shock) or the addition of 0.75 M NaCl (osmotic stress).
Values are means ± standard deviations of at least three independent determinations for each strain.
FIG. 2.
Sensitivity of ntp1+-disrupted strain MMT-3 (•) to a thermal shock at 48°C for the times indicated after a conditioning pretreatment (37°C, 60 min). Acquired thermotolerance is indicated by the survival fraction (viability), which is calculated as a percentage relative to the survival of control samples that received no heat treatment. Results represent the mean values ± standard deviations from three independent experiments. In some cases error bars are omitted because deviations were so small that they fell within the corresponding symbols. For comparison, strains PBU13 (h+ ade6-M216 leu1-32 ura4-D18 Δtps1::ura4+) (▪) and JZ636 (h+ ade6-M210 leu1-32 ura4-D18 Δpka1::ura4+) (▵) and control strains JY742 (□) and MMT-4 (h+ ade6-M216 leu1-32) (○) were examined in a similar manner.
FIG. 3.
Loss of neutral trehalase activity causes an osmotic-stress-resistant phenotype in S. pombe. (A) Growth of strains JY742 (control) (▪), MMT-3 (Δntp1::ura4+) (•), PBU13 (Δtps1::ura4+) (○), and MMT-4 (ura4+) (□) in YES medium supplemented with 0.3 M NaCl. (B) Strains JY742 (control), MMT-8 (Δntp1::ura4+), and MMT-4 (ura4+) were grown in YES medium to mid-log phase, and approximately 300 viable cells were seeded on YES or YES plus 0.2 M NaCl agar plates that were incubated at 28°C for 10 days.
Stress conditions induce transcription of the trehalase gene.
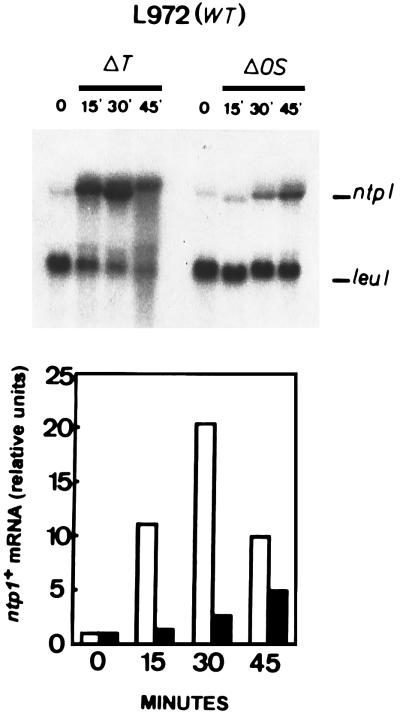
Previous work has established that exponentially growing cells of S. pombe in rich medium increase notably their basal values of neutral trehalase activity when subjected to either heat shock treatment (7, 20, 21) or osmotic stress (9). We addressed this issue by investigating whether the increment in trehalase activity includes transcriptional control. The internal 0.673-kb BamHI-HindIII fragment from the ntp1+ gene (Fig. 1A) was labelled with [α-32P]dCTP and used as a probe to analyze the expression of trehalase under normal and stressed conditions. First, we determined the size of the ntp1+ mRNA in wild-type strain 972h−. A single transcript of approximately 2.3 kb was detected by Northern blot hybridization (19) in cells exponentially growing in YES medium (Fig. 4, zero time). This mRNA species was absent when total RNA from Δntp1::ura4+ strains was analyzed (data not shown). The basal level of expression of the ntp1+ gene was relatively low compared with that of the leu1+ gene, which was used as an internal control (12). Contrary to what happens in S. cerevisiae (25), trehalase activity in S. pombe increases under osmotic-stress conditions (9). Northern blot analysis of the expression of trehalase in S. pombe during thermal or saline stress revealed that the levels of ntp1+ mRNA were increased by each of these treatments (Fig. 4). Moreover, taking into account earlier observations (8, 9), we found that during heat shock or osmotic stress the levels of trehalase mRNA and enzyme activity are modulated together, indicating a primary regulation at the transcriptional level. These results are clearly at variance with a model of trehalase activation by heat shock regulated mainly at the posttranslational level (18). Another interesting point of this study concerns the kinetics of ntp1+ transcription, which were much slower during osmotic stress than during heat shock treatment under conditions of maximal induction. The former triggered a steady increase in the level of ntp1+ mRNA that lasted for about 2 h, while the latter resulted in maximum expression 30 min after the temperature upshift (Fig. 4). This might be taken as further indication that signalling pathways regulating the expression of ntp1+ operate through different mechanisms in each case.
FIG. 4.
Expression of the ntp1+ gene in S. pombe 972h− (wild type) increases during heat (ΔT) and osmotic (ΔOS) stress. The cells were grown in YES medium to mid-log phase (zero time) and were heat shocked (40°C) or osmotically shocked (0.75 M NaCl) for the times indicated (in minutes). Total RNA was extracted from each sample, and 20 μg was applied to each lane in a 1.5% agarose-formaldehyde gel. The denatured RNAs were transferred to a nylon membrane and hybridized with ntp1+ and leu1+ (32P)-labelled probes. The upper panel shows the autoradiogram following Northern blot hybridization. The lower panel indicates in arbitrary units (histogram) a quantitative estimate of the amount of ntp1+ mRNA based on the expression of leu1+ as an internal standard; a PhosphorImager (Molecular Dynamics) was used for the estimate. The solid bars represent the heat shock data, while the open bars represent the osmotic-shock data.
The apparent contradiction that heat and salt stresses increase expression of ntp1+ while disruption of the gene increases tolerance can be explained by taking into account the fact that under such conditions tps1+ transcription is greatly enhanced and a net increase in trehalose occurs (6, 18) (this study). Lack of a functional ntp1+ gene would thus favor the accumulation of trehalose, which is known to function as an stress metabolite (23, 24).
Acknowledgments
This work was financied in part by a grant from DGICYT (PB94-1151), Spain. T.S. and J.F. are postdoctoral fellows supported by CajaMurcia.
We thank C. Gancedo and M. Yamamoto for supplying yeast strains.
REFERENCES
- 1.Amaral F C, Van Dijck P, Nicoli J R, Thevelein J M. Molecular cloning of the neutral trehalase from Kluyveromyces lactis and the distinction between neutral and acid trehalases. Arch Microbiol. 1997;167:202–208. doi: 10.1007/s002030050436. [DOI] [PubMed] [Google Scholar]
- 2.App H, Holzer H. Purification and characterization of neutral trehalase from yeast ABS1 mutants. J Biol Chem. 1989;264:17583–17588. [PubMed] [Google Scholar]
- 3.Blázquez M A, Stucka R, Feldmann H, Gancedo C. Trehalose-6-P synthase is dispensable for growth on glucose but not for spore germination in Schizosaccharomyces pombe. J Bacteriol. 1994;176:3895–3902. doi: 10.1128/jb.176.13.3895-3902.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carrillo D, Vicente-Soler J, Gacto M. Activation of neutral trehalase by fermentable sugars and cAMP in the fission yeast Schizosaccharomyces pombe. FEMS Microbiol Lett. 1992;98:61–66. [Google Scholar]
- 5.Carrillo D, Vicente-Soler J, Gacto M. Cyclic AMP signalling pathway and trehalase activation in the fission yeast Schizosaccharomyces pombe. Microbiology. 1994;140:1467–1472. doi: 10.1099/00221287-140-6-1467. [DOI] [PubMed] [Google Scholar]
- 6.Degols G, Shiozaki K, Russell P. Activation and regulation of the Spc-1 stress-activated protein kinase in Schizosaccharomyces pombe. Mol Cell Biol. 1996;16:2870–2877. doi: 10.1128/mcb.16.6.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Virgilio C, Simmen U, Hottiger T, Boller T, Wiemken A. Heat shock induces enzymes of trehalose metabolism, trehalose accumulation, and thermo-tolerance in Schizosaccharomyces pombe, even in the presence of cycloheximide. FEBS Lett. 1990;273:107–110. doi: 10.1016/0014-5793(90)81062-s. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez J, S.t. T, Vicente-Soler J, Cansado J, Gacto M. Heat shock response in Schizosaccharomyces pombe cells lacking cyclic-AMP dependent phosphorylation. Curr Genet. 1997;31:112–118. doi: 10.1007/s002940050183. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez J, Soto T, Vicente-Soler J, Cansado J, Gacto M. Osmo-stress-induced changes in neutral trehalase activity of the fission yeast Schizosaccharomyces pombe. Biochim Biophys Acta. 1997;1357:41–48. doi: 10.1016/s0167-4889(97)00010-4. [DOI] [PubMed] [Google Scholar]
- 10.Grimm C, Kohli J. Observations on integrative transformation in Schizosaccharomyces pombe. Mol Gen Genet. 1988;215:87–93. doi: 10.1007/BF00331308. [DOI] [PubMed] [Google Scholar]
- 11.Hottiger T, De Virgilio C, Hall M N, Boller T, Wienkem A. The role of trehalose synthesis for the acquisition of thermotolerance in yeast. II. Physiological concentrations of trehalose increase the thermal stability of protein in vitro. Eur J Biochem. 1994;219:187–193. doi: 10.1111/j.1432-1033.1994.tb19929.x. [DOI] [PubMed] [Google Scholar]
- 12.Kikuchi Y, Kitazawa Y, Shimatake H, Yamamoto M. The primary structure of the leu1+ gene of Schizosaccharomyces pombe. Curr Genet. 1988;14:375–379. doi: 10.1007/BF00419995. [DOI] [PubMed] [Google Scholar]
- 13.Kopp M, Muller H, Holzer H. Molecular analysis of the neutral trehalase gene from Saccharomyces cerevisiae. J Biol Chem. 1993;268:4766–4774. [PubMed] [Google Scholar]
- 14.Maeda T, Watanabe Y, Kunitomo H, Yamamoto M. Cloning of the pka1 gene encoding the catalytic subunit of the cAMP-dependent protein kinase in Schizosaccharomyces pombe. J Biol Chem. 1994;269:9632–9637. [PubMed] [Google Scholar]
- 15.Moreno S, Klar A, Nurse P. Molecular genetic analysis of the fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- 16.Nwaka S, Mechler B, Destruelle M, Holzer H. Phenotypic features of trehalase mutants in Saccharomyces cerevisiae. FEBS Lett. 1995;360:286–290. doi: 10.1016/0014-5793(95)00105-i. [DOI] [PubMed] [Google Scholar]
- 17.Nwaka S, Kopp M, Holzer H. Expression and function of the trehalase genes NTH1 and YBR0106 in Saccharomyces cerevisiae. J Biol Chem. 1995;270:10193–10198. doi: 10.1074/jbc.270.17.10193. [DOI] [PubMed] [Google Scholar]
- 18.Ribeiro M J S, Reinders A, Boller T, Wiemken A, De Virgilio C. Trehalose synthesis is important for the acquisition of thermotolerance in Schizosaccharomyces pombe. Mol Microbiol. 1997;25:571–581. doi: 10.1046/j.1365-2958.1997.4961856.x. [DOI] [PubMed] [Google Scholar]
- 19.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 20.Soto T, Fernandez J, Vicente-Soler J, Cansado J, Gacto M. Protein kinase SCK1 is involved in trehalase activation by glucose and nitrogen sources in the fission yeast Schizosaccharomyces pombe. Microbiology. 1997;143:2457–2463. doi: 10.1099/00221287-143-7-2457. [DOI] [PubMed] [Google Scholar]
- 21.Soto T, Fernandez J, Vicente-Soler J, Cansado J, Gacto M. Glucose-induced, cyclic AMP-independent signalling pathway for activation of neutral trehalase in the fission yeast Schizosaccharomyces pombe. Microbiology. 1995;141:2665–2671. [Google Scholar]
- 22.Thevelein J M. Regulation of trehalose mobilization in fungi. Microbiol Rev. 1984;48:42–59. doi: 10.1128/mr.48.1.42-59.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Laere A. Trehalose, reserve and/or stress metabolite? FEMS Microbiol Rev. 1989;63:201–210. [Google Scholar]
- 24.Wienkem A. Trehalose in yeast, stress protectant rather than reserve carbohydrate. Antonie Leeuwenhoek. 1990;58:209–217. doi: 10.1007/BF00548935. [DOI] [PubMed] [Google Scholar]
- 24a.Yohioka, S., K. Kato, and H. Okayama. Unpublished data.
- 25.Zähringer H, Burgert M, Holzer H, Nwaka S. Neutral trehalase Nth1p of Saccharomyces cerevisiae encoded by the NTH1 gene is a multiple stress responsive protein. FEBS Lett. 1997;412:615–620. doi: 10.1016/s0014-5793(97)00868-5. [DOI] [PubMed] [Google Scholar]