Abstract
Successful mitosis depends on the timely establishment of correct chromosomal attachments to microtubules. The kinetochore, a modular multiprotein complex, mediates this connection by recognizing specialized chromatin containing a histone H3 variant called Cse4 in budding yeast and CENP‐A in vertebrates. Structural features of the kinetochore that enable discrimination between Cse4/CENP‐A and H3 have been identified in several species. How and when these contribute to centromere recognition and how they relate to the overall structure of the inner kinetochore are unsettled questions. More generally, this molecular recognition ensures that only one kinetochore is built on each chromatid and that this happens at the right place on the chromatin fiber. We have determined the crystal structure of a Cse4 peptide bound to the essential inner kinetochore Okp1‐Ame1 heterodimer from budding yeast. The structure and related experiments show in detail an essential point of Cse4 contact and provide information about the arrangement of the inner kinetochore.
Keywords: centromere, kinetochore, mitosis, X‐ray crystallography
Subject Categories: Cell Cycle, Structural Biology
A crystal structure of the essential interaction between the centromeric histone and conserved inner kinetochore proteins from budding yeast provides a mechanism for centromere recognition.
Introduction
Kinetochores assemble at centromeres by interacting with a variant histone H3 known as centromere protein A (CENP‐A) in vertebrates and as Cse4 in Saccharomyces cerevisiae and other point‐centromere yeast. CENP‐A and Cse4 deviate from canonical histone H3 at residues interspersed in the histone core and in the sequence and length of the N‐terminal segment (Fig 1A). Components of the assembled kinetochore that recognize these CENP‐A‐ or Cse4‐specific features include yeast Mif2 (CENP‐C in vertebrates) and one or more subunits of the yeast Ctf19 complex (Ctf19c), which is homologous to the human CCAN (for Constitutive Centromere Associated Network). An important open question is how these factors “read” distinguishing features of Cse4 to restrict kinetochore assembly to centromeres.
Figure 1. Crystal structure of the Okp1‐Ame1‐Cse4END complex.
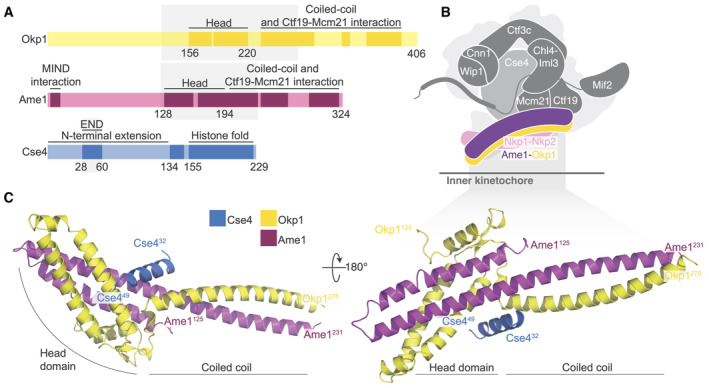
- Domain diagram showing the relevant regions of Okp1, Ame1, and Cse4. The shaded boxes demarcate the minimal Okp1‐Ame1 peptides used for crystallography. The “Head” domains correspond to the previously described four‐helix bundle (by analogy to the MIND complex; Dimitrova et al, 2016). Darker regions of the primary structure diagram indicate segments that are ordered in previously reported structures or that are known to bind partners. An N‐terminal peptide of Ame1 connects to spindle microtubules indirectly via the MIND complex (Hornung et al, 2014).
- Schematic view of the yeast inner kinetochore (Ctf19c, Mif2, and Cse4) showing the position of Okp1‐Ame1 (purple and yellow) and Nkp1‐Nkp2 (pink). N‐terminal extensions are omitted for clarity.
- Crystal structure of the Okp1‐Ame1‐Cse4END complex (this work). Protein chains are colored as in panel A.
Source data are available online for this figure.
Table 1.
Affinity measurements for Cse4END binding determined by fluorescence polarization.
| Okp1‐Ame1 version | K D | r 2 | |
|---|---|---|---|
| Okp1‐Ame1 (WT) | FL | 152 (97–240) nM | 0.9623 |
| Okp1‐Ame1 W(T) + 10 μM Nkp1‐Nkp2 | FL | 133 (80–220) nM | 0.9576 |
| Okp1(EYAA)‐Ame1 | FL | > 3 μM | n.d. |
| Okp1‐Ame1(I195Y) | FL | >> 3 μM | n.d. |
Full‐length Okp1‐Ame1 complex was used. Equilibrium constant values are given with the asymmetric 95% confidence interval shown in parentheses (K D – equilibrium dissociation constant, r 2 – goodness of fit, n.d. – not determined).
The Cse4 N‐terminal extension is much longer than its histone H3 counterpart (~140 and ~40 amino acid residues, respectively). Despite its length, a relatively short segment of the Cse4 N‐terminal extension is necessary and sufficient for cell growth (residues 28–60, Fig 1A) (Chen et al, 2000; Ichikawa et al, 2018). Accordingly, this segment was named the Cse4 Essential N‐terminal Domain (Cse4END). Early yeast two‐hybrid experiments suggested that Ctf19 binds Cse4END (Chen et al, 2000). More recent biochemical reconstitution experiments showed that the Okp1‐Ame1 heterodimer, which is a Ctf19c sub‐module that binds the Ctf19 protein, is the direct binding partner of Cse4END (Anedchenko et al, 2019; Fischbock‐Halwachs et al, 2019; Hinshaw & Harrison, 2019).
Okp1 and Ame1 are the only Ctf19c components fully required for mitosis (Ortiz et al, 1999; Pot et al, 2005). They associate as an elongated heterodimer with a globular “head” and an extended alpha‐helical coiled‐coil “shaft.” Loops in both chains interrupt the shaft and interact with Ctf19 and Mcm21 to make the COMA complex (Fig 1B) (Cheeseman et al, 2002; De Wulf et al, 2003; Hinshaw & Harrison, 2019). Nkp1 and Nkp2 bind along the length of the Okp1‐Ame1 dimer, making a six‐protein complex (Schmitzberger et al, 2017). Okp1, Ame1, Ctf19, and Mcm21 all have long, flexible N‐terminal extensions. Only that of Ame1 is essential; it indirectly recruits the microtubule‐binding Ndc80 complex to the kinetochore, thus enabling chromosomal contact with the mitotic spindle (Hornung et al, 2014).
Molecular recognition of Cse4 by the Okp1‐Ame1 heterodimer has not been resolved, despite recent cryo‐EM structures that show in detail nearly all protein contacts that contribute to inner kinetochore assembly in yeast (Yan et al, 2018, 2019; Hinshaw & Harrison, 2019, 2020; Guan et al, 2021; Dendooven et al, 2023). An experimental solution will constrain models for centromeric nucleosome binding by the Ctf19c. There are also several reported post‐translational modifications of the Cse4 N‐terminal extension that are thought to control kinetochore function (Hewawasam et al, 2010; Ranjitkar et al, 2010; Samel et al, 2012; Boeckmann et al, 2013; Hoffmann et al, 2018; Ohkuni et al, 2018; Anedchenko et al, 2019; Mishra et al, 2021). Cse4 binding to Okp1‐Ame1 can be reconstituted in vitro (Anedchenko et al, 2019; Fischbock‐Halwachs et al, 2019; Hinshaw & Harrison, 2019), but a published cryo‐EM structure that includes Cse4 and the assembled Ctf19c did not show Cse4END density (Yan et al, 2019). In our own efforts to solve this problem, we have attempted to determine the structure of the Ctf19c bound either to a reconstituted Cse4 nucleosome particle or to a minimal Cse4END peptide, but the resulting maps do not show density corresponding to Cse4END. In the current work, we have used X‐ray crystallography to identify the Cse4END binding site.
We show here that Cse4END binds at the head‐shaft junction of Okp1‐Ame1. We determined a 1.8 Å resolution crystal structure of a truncated Okp1‐Ame1 bound with a Cse4END peptide to specify in detail the amino acid residues involved, and we have shown by binding experiments and yeast genetics that the interaction is functionally relevant in cells.
Results and Discussion
The crystal structure of Cse4END with Okp1‐Ame1
We determined the crystal structure of the Cse4END peptide bound to a minimal Okp1‐Ame1 heterodimer (Fig 1C). Crystals containing all three proteins, including the full Cse4END sequence, grew in space group P 42212 (Fig EV1A and B) and yielded diffraction data to a minimum Bragg spacing of 1.8 Å. We determined the structure by molecular replacement (MR) as described in the Materials and Methods section and in Table EV1.
Figure EV1. Crystal structure of Okp1‐Ame1‐Cse4END and flexibility at the Okp1‐Ame1 head‐coiled‐coil joint and Nkp1‐Nkp2 position.
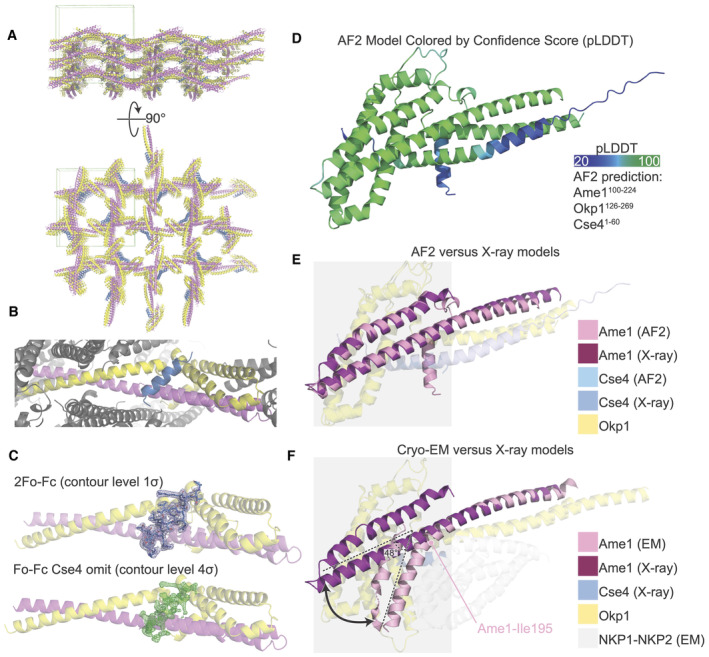
- The crystal structure of Okp1‐Ame1‐Cse4 colored as in Fig 1. The green box shows the limits of a single unit cell.
- Close‐up view of an individual biological protomer. Okp1‐Ame1‐Cse4 colored as in Fig 1. Neighboring protomers are colored gray.
- Cse4END peptide density from the final refined model (top; 2Fo‐Fc) and from the refined model lacking Cse4END (bottom; Fo‐Fc, Cse4 omitted).
- The structure of Okp1‐Ame1‐Cse4 as predicted by AlphaFold 2 (AF2) (Jumper et al, 2021). The model is colored according to confidence score (pLDDT) from low (blue) to high (green). The peptides used for prediction are given at right.
- Overlay of Okp1‐Ame1‐Cse4 from AF2 with the current Okp1‐Ame1‐Cse4 crystal structure (X‐ray). Only Ame1 (magenta and pink) is shown as an opaque chain for clarity. The gray box marks the Okp1‐Ame1 head domain.
- Overlay of the Okp1‐Ame1‐Cse4 structure from cryo‐EM (EM) with the current crystal structure. The angle between the head and coiled coil shaft is indicated for the cryo‐EM structure. Ame1‐Leu195, which is the position at which Ame1 bends in the cryo‐EM structure, is annotated. The Okp1‐Ame1 head domain is marked as in panel E. Structures were aligned on the Okp1‐Ame1 coiled coil shaft. The Nkp1‐Nkp2 structure from cryo‐EM (NKP12‐76; NKP24‐84) is shown as transparent gray chains.
In the crystal structure of the heterotrimeric complex, Cse4END binds at the junction between the globular head of Okp1‐Ame1 and the proximal part of the coiled‐coil shaft (Figs 1C and EV1C). The ordered Cse4 residues in the crystal structure are mostly α‐helical (residues 34–46). The Cse4END position is essentially as predicted by AlphaFold 2 (Jumper et al, 2021; Dendooven et al, 2023 and this work; Fig EV1D and E). The Cse4END binding site is on an external surface in structures of the assembled Ctf19c (Hinshaw & Harrison, 2019; Yan et al, 2019).
The orientation of the four‐helix bundle that makes up the Okp1‐Ame1 head domain differs from what we and others observed in cryo‐EM reconstructions of the Ctf19c (Fig EV1F) (Hinshaw & Harrison, 2019; Yan et al, 2019). In the current structure, the Okp1‐Ame1 head tilts away from the shaft. This is most evident for Ame1, where there is no discernable break in the extended helix that connects the shaft and head, whereas the same helix bends at Ame1‐I195 in the previous Ctf19c structures.
Specific contacts that enable Cse4END‐Okp1‐Ame1 binding
The three‐way interface between Cse4, Okp1, and Ame1 has a hydrophobic core surrounded by polar interactions. The Cse4END residues at the interface are conserved across point‐centromere yeast, as are most of their partners in Okp1 and Ame1 (Figs 2A and EV2). Hydrophobic contacts between Cse4 and Okp1 include Cse4‐L42 and Okp1‐I234 (Fig 2B). Likewise, Cse4‐L41 and ‐I34 contact Ame1 I195 (Fig 2C). Peripheral charged contacts include an electrostatic interaction between Cse4‐R46 and Okp1‐E235. Cse4‐R37 makes complementary charge contacts with Ame1‐D191 and ‐D194. Both contacts are conserved in yeast. This overall arrangement, with multiple charged contacts surrounding a hydrophobic core encompassing all three proteins, explains how a short Cse4END peptide binds the Okp1‐Ame1 dimer with an equilibrium dissociation constant of ~150 nM (see below).
Figure 2. Contacts between Okp1‐Ame1 and Cse4END .
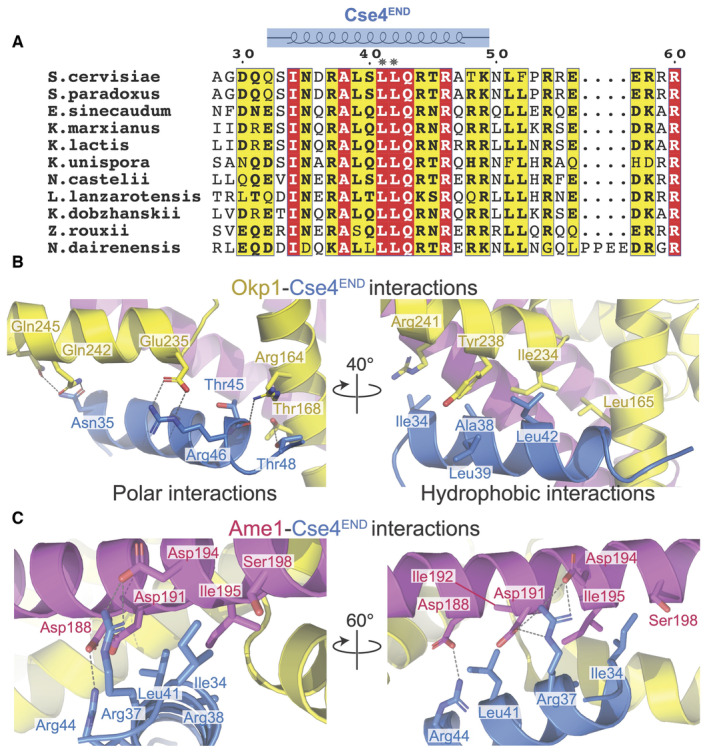
- Protein sequence alignment showing the Cse4END peptide. Numbering corresponds to the S. cerevisiae Cse4 protein. Amino acid residues 28–60 are shown. The blue diagram above shows the residues visible in the crystal structure. Asterisks mark L41 and L42.
- Close‐up views of the Okp1‐Cse4 interface. Polar interactions are shown on the left. Hydrophobic contacts are shown on the right.
- Two views of the Ame1‐Cse4 interface.
Source data are available online for this figure.
Figure EV2. Protein sequence alignments for Okp1 and Ame1 covering the Cse4END contacts shown in Fig 2 .
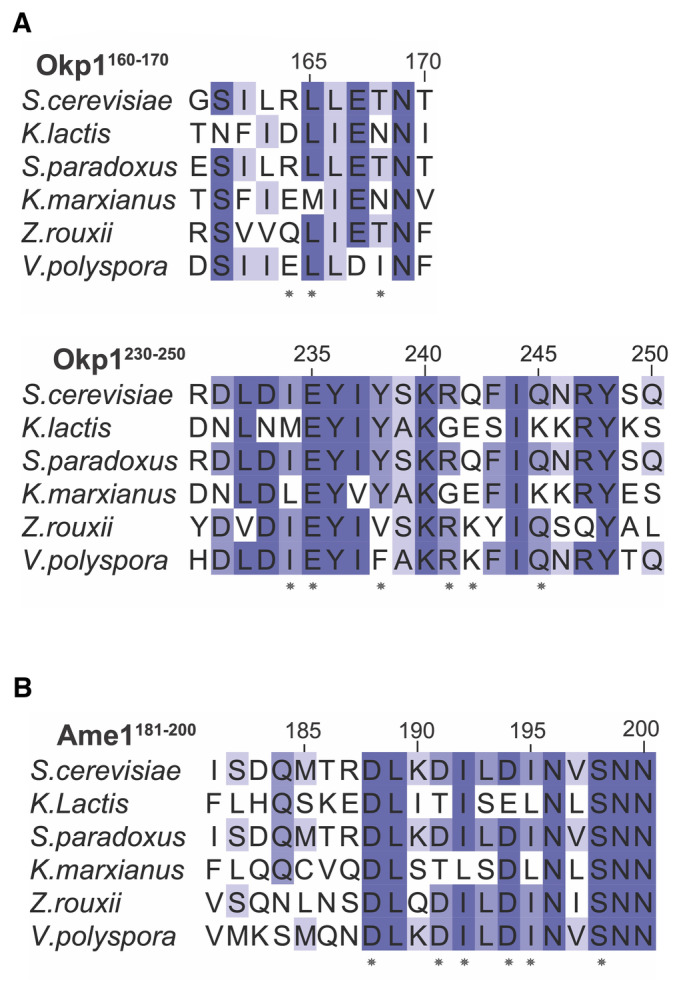
-
A, BAsterisks mark the Okp1‐Ame1 residues shown as sticks in Fig 2.
Comparison with Cryo‐EM structures of the Ctf19c (Hinshaw & Harrison, 2019; Yan et al, 2019) shows that Nkp1 and Nkp2 bind close to the Cse4END binding site on Okp1‐Ame1. Because the Okp1‐Ame1 head domain is shifted in the crystal structure as described above, it is not clear from these comparisons whether Nkp1‐Nkp2 would need to partly dissociate from Okp1‐Ame1 to accommodate Cse4END (Fig EV1F). Indeed, a recent cryo‐EM structure of COMA‐Nkp1‐Nkp2 shows partial Nkp1‐Nkp2 dissociation from Okp1‐Ame1 is possible (Dendooven et al, 2023). We used pulldown assays to test whether Nkp1‐Nkp2 and Cse4END compete for Okp1‐Ame1 binding. Consistent with published data (Anedchenko et al, 2019; Fischbock‐Halwachs et al, 2019; Hinshaw & Harrison, 2019), a glutathione‐S‐transferase (GST) fusion of Cse4END bound Okp1‐Ame1 (Fig 3A). GST‐Cse4END bound the four‐protein COMA complex and the six‐protein COMA‐Nkp1‐Nkp2 complex equally well. Truncation of Okp1‐Ame1 to the minimal heterodimeric complex used for crystallization (Okp1125‐275‐Ame1124‐231) preserved GST‐Cse4END binding (Fig EV3A). Control pulldowns confirmed that none of the tested prey proteins bind GST (Fig EV3B).
Figure 3. Biochemical investigation of Okp1‐Ame1‐Cse4END binding and disruptive point mutations.
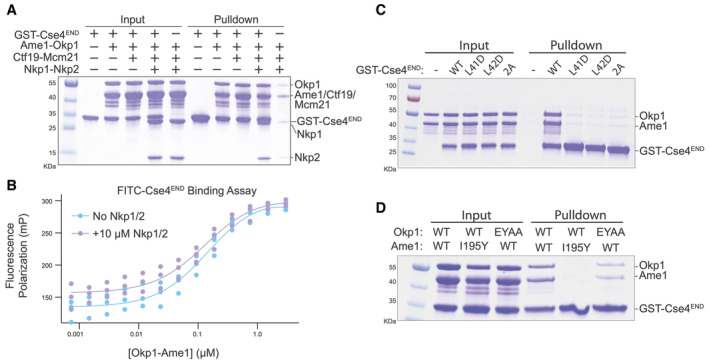
-
AGST pulldown assay for Cse4 binding. The indicated COMA complex proteins were tested for their association with immobilized GST‐Cse4END.
-
BFluorescence polarization assay for quantification of Cse4 binding affinities (n = 3 independent replicates). FITC‐labeled Cse4END peptide was incubated with increasing concentrations of the indicated protein complexes. See also Table 1.
-
C, DGST pulldown assays as in panel A. Cse4END and its mutants (L41D, L42D, or L41,42A) were tested in panel C. The indicated Okp1‐Ame1 complexes were tested for their association with GST‐Cse4END in panel D.
Source data are available online for this figure.
Figure EV3. Further biochemical characterization of the Okp1‐Ame1‐Cse4END interaction.
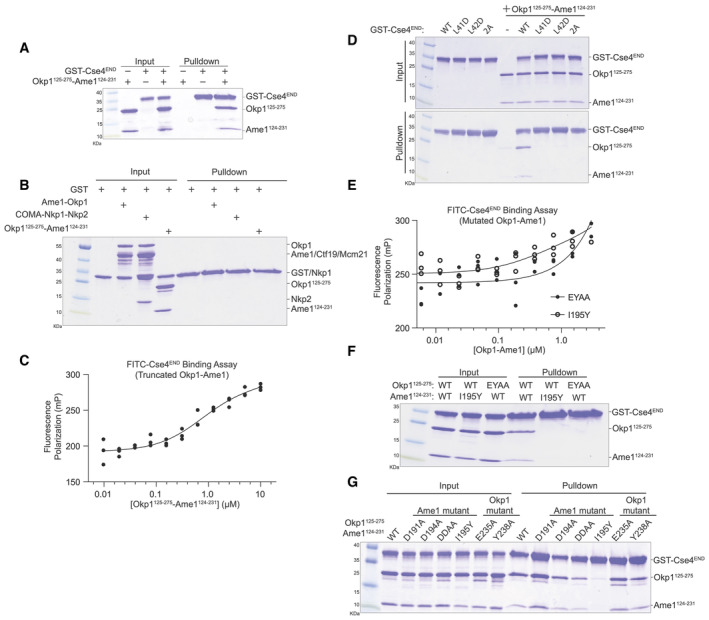
- Pulldown assay showing binding between the truncated Okp1‐Ame1 complex used for crystallography (Okp1125‐275l‐Ame1124‐231) and GST‐Cse4END.
- Recombinant proteins used for pulldowns in Fig 3 were tested for their association with GST to determine the level of non‐specific binding. Results of a GST pulldown assay are shown.
- The minimal Okp1‐Ame1 complex used for crystallography was tested for its association with FITC‐Cse4END in a fluorescence polarization experiment. The measured dissociation constant is ~750 nM (see Materials and Methods; n = 3 independent experiments).
- GST‐Cse4END and its mutants were tested for Okp1‐Ame1 binding.
- Full‐length mutant Okp1‐Ame1 complex (EYAA or I195Y as indicated) was tested for its association with FITC‐Cse4END.
- Various Okp1‐Ame1 mutants (indicated above) were tested for binding to GST‐Cse4END.
- Various Okp1‐Ame1 mutants were tested for Cse4END binding as in panel F.
Source data are available online for this figure.
To quantify Cse4END binding to Okp1‐Ame1, we created a fluorescence polarization assay using a fluorescent Cse4END peptide (Fig 3B). An equilibrium dissociation constant of ~150 nM describes the binding event, and this value matches one previously reported (Anedchenko et al, 2019). Adding excess Nkp1‐Nkp2 protein to the binding reaction did not change the dissociation constant, consistent with the pulldown results. Therefore, Cse4 can bind the assembled COMA‐Nkp1‐Nkp2 complex. Because Nkp1‐Nkp2 do not displace Cse4END from Okp1‐Ame1, their inclusion cannot explain the inability of Cse4END to bind the reconstituted Ctf19c in biochemical experiments (Dendooven et al, 2023).
We also measured Cse4 affinity for the truncated Okp1‐Ame1 complex used for crystallography. The measured dissociation constant was ~750 nM (Fig EV3C). The ~5‐fold higher value versus full‐length Okp1‐Ame1 could partly be due to the higher salt concentration required for the truncated Okp1‐Ame1 sample.
Effects of mutations on affinity of Cse4END for Okp1‐Ame1
We tested the importance of residues at the interface between Cse4END and Okp1‐Ame1 using the biochemical assays described above. The cse4‐L41D, cse4‐L42D, and cse4‐L41,42A (cse4‐2A) mutations disrupt central hydrophobic interactions with Okp1‐Ame1. The corresponding Cse4END peptides failed to bind recombinant Okp1‐Ame1 in the pulldown assay (Fig 3C). The same was true in a pulldown assay carried out with the minimal Okp1‐Ame1 complex used for crystallography (Fig EV3D). These experiments confirm the importance of hydrophobic contacts at the core of the three‐protein interface.
We used the crystal structure to create Okp1‐Ame1 mutations that specifically interfere with Cse4END binding. Among those tested, ame1‐I195Y and okp1‐E235,Y238A (okp1‐EYAA) were the most potent disruptors of Cse4 binding. Both mutations prevented Cse4END binding in the pulldown and fluorescence polarization assays (Figs 3D and EV3E). They also prevented Cse4END binding when introduced into the minimal Okp1‐Ame1 complex (Fig EV3F). In addition to these mutations, we tested the ame1‐D191A and ‐D194A mutations, which prevent coordination of the conserved Cse4‐R37 via hydrogen bonding interactions. Neither mutation perturbed Cse4END binding, and the combination (ame1‐DDAA) disrupted Cse4 binding in the pulldown assay, but only slightly (Fig EV3G).
Effects of Cse4END binding mutants on cell growth
To test whether Cse4END mutations that disrupt Okp1‐Ame1 binding interfere with cell division, we used a plasmid shuffling assay in which a plasmid‐borne complementing CSE4 allele restores the viability of cse4∆ cells. Selection against the complementing allele reveals the phenotype associated with a CSE4 test allele carried on a second plasmid. The cse4‐2A test allele did not support cell growth, and the cse4‐L41A (cse4‐1A) test allele produced slower‐growing cells than did the CSE4 allele (Fig 4A). Therefore, Cse4END hydrophobic residues required for Ame1‐Okp1 binding are also required for cell viability.
Figure 4. Cellular fitness of yeast with disrupted Cse4END binding.
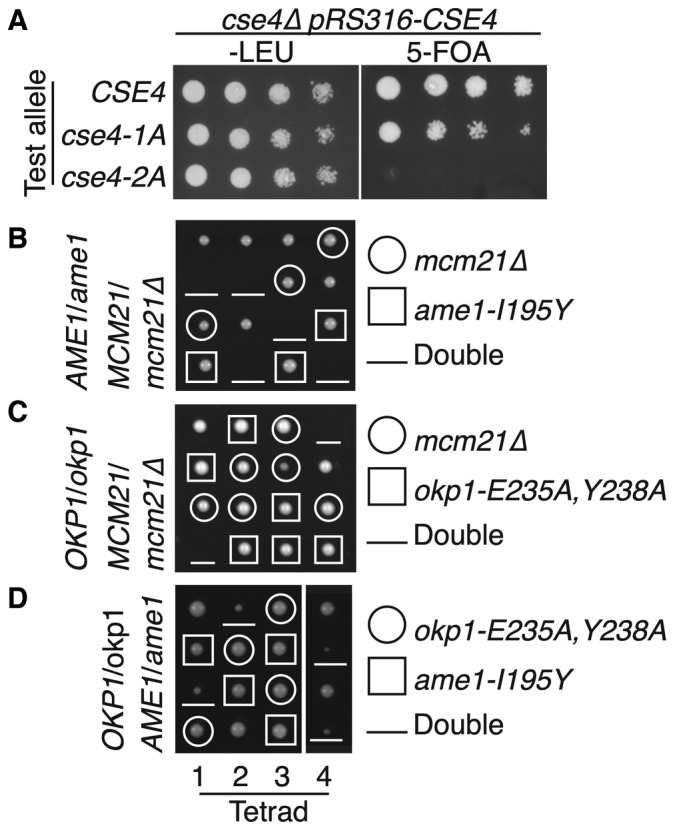
-
AGrowth of cse4 mutants. Loss of a complementing CSE4 plasmid (pRS316‐CSE4) was induced by growth on 5‐FOA. Test cse4 alleles are indicated at left (cse4‐1A – L41A; cse4‐2A – L41,42A).
-
B–DHaploid progeny from sporulation of the indicated heterozygous diploid strain (left). Spore genotypes and mutant alleles are given at right. Meiotic products from each tetrad are arranged vertically.
Source data are available online for this figure.
We next tested whether Okp1‐Ame1 residues that interact with Cse4END are essential. To do so, we sporulated diploid cells heterozygous for the ame1‐I195Y and okp1‐E235A,Y238A mutations and examined their haploid progeny (Fig 4B–D). Neither mutation produced obvious dominant defects in the parental heterozygous cells, and the expression levels of these mutant proteins matched their wild type counterparts (Fig EV4A). Both mutations permitted cell growth in haploid spores but were lethal in combination with mcm21Δ (Fig 4C). This result matches the previous observation that non‐lethal Cse4END mutations are synthetic‐lethal with Ctf19c mutations (e.g., cse4‐R37A chl4Δ double mutant cells are inviable) (Samel et al, 2012; Anedchenko et al, 2019).
Figure EV4. In vivo consequences of Okp1‐Ame1 mutations.
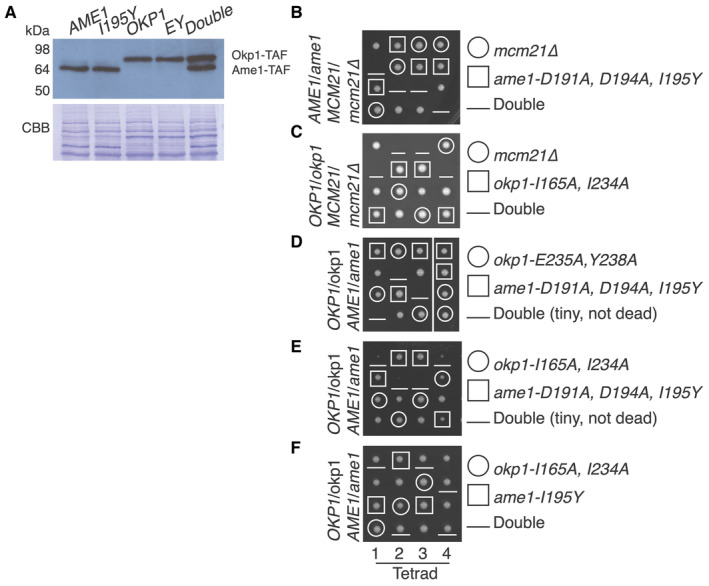
-
AWestern blot showing expression of Ame1, Okp1, and their mutants in whole cell extracts (TAF – protein A‐FLAG tag; anti‐Protein A used for detection).
-
B–FTetrad dissection results as in Fig 4B–D. The mutants tested and the resulting spore genotypes are shown at right.
Source data are available online for this figure.
If ame1‐I195Y and okp1‐E235A, Y238A each support weak Cse4 binding below the detection limit of our biochemical assay, then introduction of both mutations might completely ablate the interaction, yielding inviable spores. To test this idea, we examined okp1‐E235A, Y238A ame1‐I195Y double mutants and found that these spores produced tiny colonies (Fig 4D). We carried out the same experiments for the ame1‐D191A, D194A, I195Y and okp1‐I165A, I234A mutations and observed the same set of double mutant phenotypes in progeny spores (Fig EV4B–F). Therefore, the Cse4 binding site on Okp1‐Ame1 is required for cell viability. As demonstrated by the synthetic lethality when combined with mcm21Δ, an intact Ctf19c can compensate for partial disruption of the Cse4 binding site in vivo.
We have determined the crystal structure of an essential segment of Cse4 bound to the inner kinetochore proteins Okp1 and Ame1. This segment, Cse4END, binds the junction between the Okp1‐Ame1 coiled‐coil shaft and head domains. Minimal Okp1‐Ame1 mutations that disrupt Cse4 binding (this work) or MIND binding (Hornung et al, 2014), are lethal, indicating that the main essential function of Okp1‐Ame1 is to connect centromeric DNA (via Cse4) to spindle microtubules (via MIND‐Ndc80). Force transmission through these connections has been reconstituted in a minimal biochemical system (Hamilton et al, 2020). Whether this axis is the essential conduit for the force that moves chromosomes in vivo or if its essential function is regulatory remains to be seen.
Contacts between Cse4END and Okp1‐Ame1 seen in the crystal structure clarify observations from genetics experiments. The cse4‐R37A mutation weakens Cse4‐Okp1 binding and is lethal in genetic backgrounds with compromised kinetochores (e.g., mcm21Δ) (Anedchenko et al, 2019). Charge complementarity between Cse4‐R37 and Ame1‐D191/194 provides a structural basis for this finding. The okp1‐R164C mutation suppresses lethality in cse4‐R37A mcm21Δ cells (Anedchenko et al, 2019). The mutant Okp1 cysteine would have a set of non‐polar contacts with T45 and T48 of Cse4, potentially compensating for the loss of a salt bridge between Cse4 R37 and Ame1 D191/194. Cse4 lysine acetylation also regulates Okp1‐Ame1 binding, but the acetylated residue (Cse4‐K49) is not visible in the crystal structure, preventing detailed analysis of this phenotype. Identification of Ame1 point mutations that prevent Cse4‐R37 recognition (ame1‐D191A,D194A) provides a tool useful for studying the function of Cse4 arginine methylation.
The Cse4‐Okp1‐Ame1 structure prompted us to revisit previous models for centromeric nucleosome recognition (Fig EV5). An initial Ctf19c structure in the absence of the Cse4 nucleosome suggested a straightforward docking model for Ctf19c‐Cse4 binding, with which the observed position of Cse4END in the current structure is compatible (Hinshaw & Harrison, 2019). This model requires repositioning of the Ctf3c‐Cnn1‐Wip1 complex and the Okp1‐Ame1 head domains, along with partial DNA unwrapping from the nucleosome itself. Flexibility of the Ctf3 module and partial DNA unwrapping have been observed (Hinshaw & Harrison, 2020; Migl et al, 2020; Dendooven et al, 2023), and we report that the Okp1‐Ame1 head domain is indeed mobile with respect to the adjacent coiled‐coil shaft.
Figure EV5. Model for Cse4 nucleosome contact by the Ctf19c and relation to cse4 alleles.
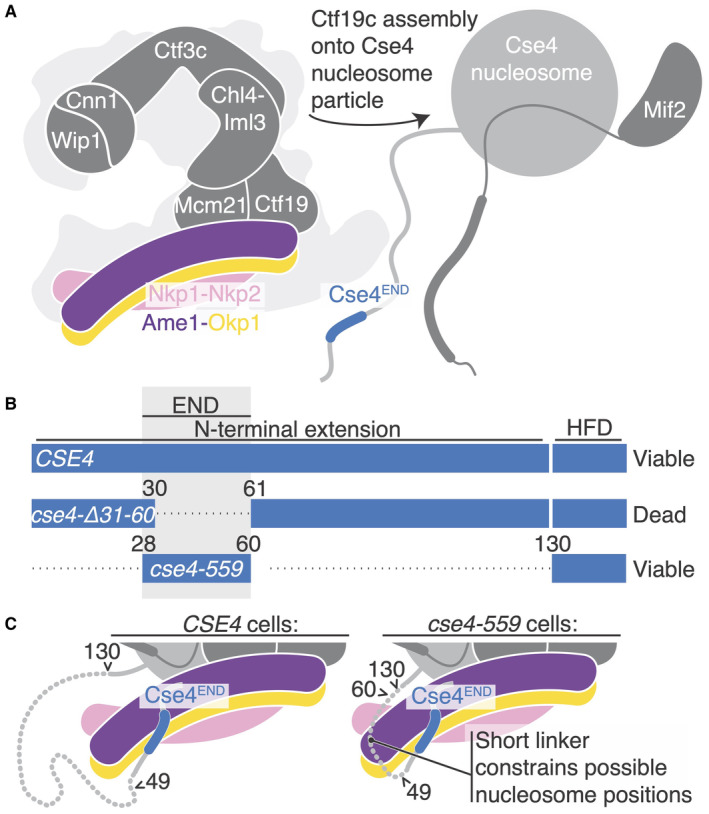
- Schematic showing Ctf19c assembly onto the Cse4‐Mif2 complex. The assembly occurs via a largely unknown biochemical mechanism.
- cse4 alleles (white text), their corresponding Cse4 proteins (blue bars), and their reported abilities to support cell viability (right). The alleles were reported by Chen et al (2000) and Fischbock‐Halwachs et al (2019). The dotted lines indicate omitted fragments. The numbers correspond to full length Cse4. A gray box marks the boundaries of Cse4END. HFD – histone fold domain.
- Structural view of the Cse4 N‐terminal extension in CSE4 (left) or cse4‐559 (right) cells. Arrows and nearby numbers point to Cse4 amino acid positions according to their numbering in the full‐length protein.
More recent cryo‐EM structures of the yeast inner kinetochore (Yan et al, 2019; Dendooven et al, 2023) have raised the possibility of alternative Ctf19c‐nucleosome poses. These rely principally on contact between Ctf19c proteins and the DNA phosphate backbone. In one case, the Cbf1 and Cep3 proteins contribute DNA sequence specificity (Dendooven et al, 2023). In these published structures, a ~60 Å peptide linker would be required to connect the Cse4 histone core to the Cse4END peptide. This contradicts genetic evidence, which shows that fusion of the Cse4END segment directly to the histone core (at residue 130) supports cell viability at a level indistinguishable from that of a wild type CSE4 allele (Fig EV5B and C) (Chen et al, 2000). Dendooven et al have proposed an alternative assembly, in which an extra and presumably essential COMA complex orbits the Ctf19c and attaches through Cse4END to satisfy this discrepancy (Dendooven et al, 2023), but there is at present no evidence for such an arrangement. Indeed, it is unclear what physiological context might support such an assembly, since the imaged samples were generated by mixing recombinant protein complexes without regard for cell cycle state, a known regulator of kinetochore assembly. Considering the existing structural data, we suggest two possibilities: (i) the heterotrimeric contacts we have described serve a cell cycle‐specific function and do not occur in the fully assembled Ctf19c or (ii) the published Ctf19c‐Cse4 structures do not recapitulate the physiologically relevant positions of the centromeric histone proteins and of the centromere itself.
This work serves to re‐emphasize the question, what distinguishes the structure of the assembled Ctf19c, which is known, from its structure when bound to the centromeric nucleosome? There are likely several answers to this question, depending on cell cycle context. Neither the published Ctf19c‐Cse4 structures nor our own unpublished structures, in which we have attempted to visualize just the Cse4END peptide bound to the assembled Ctf19c, show any discernable Cse4END density. That Cse4END binds recombinant COMA but not the assembled Ctf19c suggests that a structural switch accompanies nucleosome engagement in vivo. The altered orientation of the Okp1‐Ame1 head domain in the current structure is one candidate for such a switch. Relevant post‐translational modifications of Ctf19c proteins, Cse4, and Mif2 have all been reported (Boeckmann et al, 2013; de Albuquerque et al, 2016; Anedchenko et al, 2019; Hinshaw et al, 2023). Mif2 is of particular interest; it is heavily phosphorylated, and any models of kinetochore assembly must account for this and its essentiality in vivo. Overall, it will be important to determine which modifications are required for inner kinetochore assembly and under what cell cycle conditions they contribute.
Materials and Methods
Protein expression and purification
Heterodimers of full‐length Okp1‐Ame1, Ctf19‐Mcm21, and Nkp1‐Nkp2 were expressed and purified in E.coli as previously described (Hinshaw & Harrison, 2019). Briefly, transformed cells were grown to an OD600 of 0.5 at 37°C in 2XYT media with antibiotic selection, induced with 0.5 mM of Isopropyl β‐D‐1‐thiogalactopyranoside (IPTG) at 18°C for ∼16 h, and harvested by centrifugation for 20 min at 4000 rpm. Pelleted cells were lysed by sonication in the lysis buffer containing 25 mM HEPES, pH 7.5, 800 mM NaCl, 10 mM imidazole,1 mM tris(2‐carboxyethyl)phosphine (TCEP), 0.1 mg/ml PMSF, 5 mM β‐mercaptoethanol, 1 mM PMSF, 1 μg/ml pepstatin, 1 μg/ml aprotinin, 1 μg/ml leupeptin, 30 μg/ml DNase I, and an EDTA‐free protease inhibitor tablet (Roche, cOmplete™). After sonication, lysate clarified by centrifugation was passed over TALON Metal Affinity Resin (Takara), washed with 10 column volumes (CV) of lysis buffer, and eluted with 400 mM imidazole. The eluate was further purified on a HiTrap SP or Q ion‐exchange column (Cytiva). The peak fractions were pooled for gel filtration in 20 mM Tris, pH 8.5, 200 mM NaCl, 1 mM TCEP. Peak fractions were collected, concentrated, snap‐frozen, and stored at −80°C until use.
For pulldown assays, a DNA fragment coding for Cse4 residues 28–60 (Cse4END) was cloned into a pLIC vector coding for a TEV‐cleavable N‐terminal Glutathione S‐transferase tag and transformed into Rosetta™ 2 (pLysS) competent cells (Sigma‐Aldrich). GST‐Cse4END was purified as described above, using Pierce Glutathione Agarose (Thermo Scientific) and eluting with L‐Glutathione.
To find a minimal dimeric construct of Okp1‐Ame1 that could bind Cse4, we cloned various truncated constructs of Okp1 and Ame1 genes into the pet‐Duet bacterial expression vector, with a poly‐histidine tag and tobacco etch virus (TEV) protease site at the N terminus of Okp1 and no tag on Ame1. We found that Okp1125‐275 – Ame1124‐231 can be expressed in Rosetta™ 2 (pLysS) competent cells (Sigma‐Aldrich) and purified as described above, except that the N‐His6 tag on Okp1 was cleaved with TEV protease at 30°C for 1 h before the ion‐exchange step. Final proteins after gel filtration were concentrated to ~15 mg/ml, snap frozen, and stored in 100 mM HEPES, pH 7.5, 300 mM ammonium sulfate, and 1 mM TCEP, at −80°C until use.
Pulldown assays
GST‐Cse428‐60 was mixed with Okp1‐Ame1, COMA, COMA‐Nkp1‐Nkp2, or Okp1125‐275 – Ame1124‐231 and the mixed samples were incubated with glutathione agarose resin at 4°C for 1 h in binding buffer containing 20 mM Tris, pH 8.5, 200 mM NaCl and 1 mM TCEP. Resin was washed at least three times with the same buffer to remove unbound protein, and bound protein then eluted with glutathione. Samples of input and eluate were collected for analysis by SDS–PAGE.
Fluorescence polarization assays
FITC‐Cse428‐60 peptide was synthesized by the Tufts University Core Facility. 20 nM of FITC‐Cse428‐60 peptide was used in all reactions, and Okp1‐Ame1 concentrations were varied to determine the dissociation constant (K D ), in reaction buffer containing 100 mM HEPES, pH 7.5, 200 mM NaCl, 1 mM TCEP (full‐length Okp1‐Ame1 complex) or reaction buffer with 600 mM NaCl (truncated Okp1‐Ame1 complex). The reactions were analyzed after incubating for 30 min at room temperature. Readings were recorded with a Perkin Elmer EnVision (ICCB‐Longwood Screening Facility, Harvard University), and each curve was repeated in triplicate. EnVision data collection protocols were optimized separately for each titrated complex (Okp1‐Ame1 full‐length or its mutants, Okp1‐Ame1 with Nkp1/2, and truncated Okp1‐Ame1). GraphPad Prism was used for all data fitting (one site – total binding model). The 20 μM Okp1‐Ame1 data points were eliminated for the truncated Okp1‐Ame1 dataset due to high apparent non‐specific binding.
Protein crystallization and diffraction data collection
Purified Okp1125‐275 – Ame1124‐231 was mixed with peptide Cse428‐60 (synthesized at the Tufts University Core facility) in a ratio of 1: 1.3 and diluted to 9 mg/ml. The best crystals were grown within ∼3 days by hanging drop vapor diffusion at 18°C against reservoir solution containing 19% PEG 8000, 0.55 M lithium sulfate and a 1:1 sample:well drop ratio. Crystals were transferred into mother liquor supplemented with 25% glycerol and flash‐frozen in liquid nitrogen. Diffraction data to 1.8 Å minimum Bragg spacing were collected on beamline 24‐ID‐E at the Advanced Photon Source (Argonne National Laboratory). The complex crystallized in space group P 42 21 2 (a = 154.52, b = 154.52, c = 37.18). Data collection statistics are in Table EV1.
Structure determination
X‐ray diffraction data processing was carried out with xia2 (https://xia2.github.io/index.html) using DIALS (Winter et al, 2022) and Aimless (Evans & Murshudov, 2013). The Okp1162‐275 – Ame1124‐231 structure from the yeast the Ctf19c/CCAN structure (PDB: 6NUW) failed to yield a molecular replacement solution in Phenix (Adams et al, 2010), but the AlphaFold2 (Jumper et al, 2021) prediction for Okp1125‐275 – Ame1124‐231 yielded a robust solution with clear density for the Cse4 peptide. Model building was carried out in Coot (Emsley et al, 2010) and refined in Phenix (Adams et al, 2010). Coordinates and diffraction data have been deposited in the protein data bank (PDB ID: 8T0P). Refinement statistics are in Table EV1.
Construction of plasmids and yeast strains
Centromeric plasmids (pRS315 and pRS316) were linearized by NotI and repaired by PCR products of Cse4 (ChrXI: 345945‐347290), Ame1 (ChrII: 646026‐647638) and Okp1 (ChrVII: 853524‐855435) flanked by 34 bp homology via homologous recombination in yeast using standard yeast transformation method. Plasmids were rescued from successful transformants via electroporation, sequenced, and then used to make other plasmid derivatives. These plasmid derivatives, including insertion of 3xFlag, TAF tag (3xFlag‐TEV‐ProteinA), KanMX6 marker and later point mutants, were generated by combining PCR fragments with overlapping homology regions (> 33 bp), via homologous recombination in yeast. Specifically, a 3xFlag tag was inserted at an internal XbaI site in Cse4 (Wisniewski et al, 2014) and then a KanMX6 marker was inserted in the promoter region of Cse4 (ChrXI: 347222) to generate HZE2719. A TAF tag followed by KanMX marker was appended to the C‐terminus of Ame1 (HZE2663) or Okp1 (HZE3198). These plasmids were then used to generate various point mutants as indicated.
A diploid of W303 strain (HZY1079) was used to generate a heterozygous deletion of Cse4, Ame1 or Okp1 using a natMX6 marker. After introducing pRS316‐Cse4, pRS316‐Ame1, or pRS316‐Okp1, the transformed cells were sporulated to obtain the haploid cse4, ame1 and okp1 null mutants that were kept alive by their respective complementing plasmids. PCR products of ame1 and okp1 point mutants (with C‐terminal TAF tag and G418 marker) were amplified from the mutant plasmids using a high‐fidelity DNA polymerase (SuperFi, Invitrogen) and transformed into the respective haploid ame1 and okp1 null mutants. Correct integrations were identified by resistance to G418 and 5‐Fluoroorotic acid (5‐FOA), and nourseothricin‐sensitivity, and confirmed by sequencing the genomic DNA of Ame1 and Okp1. Standard yeast genetic methods, including mating, sporulation, and dissection with a Singer dissection microscope, were used to construct various double mutants, as described by Lichten (2014). Plates were incubated at 30°C for 2 or 3 days before taking images using a BioRad ChemiDoc MP imaging system. The spores were identified based on the selection markers used to create each mutant. Yeast strains used are summarized in Table EV2. Plasmids used are summarized in Table EV3.
Plasmid shuffling using URA3/5‐FOA
Haploid strains cse4Δ::natMX6 pRS316‐Cse4 (HZY2970), were transformed with the indicated plasmids bearing cse4 mutants. Successful transformants were grown up in Sc‐Leu media to an OD600 ∼ 1, normalized based on cell density, and then spotted on the indicated plates following 5‐fold serial dilutions. 5‐fluoroorotic acid (Sc supplemented with 0.1% 5‐FOA treatment removes the complementing URA plasmid), exposing the phenotypes of cse4 mutants. Plates were incubated at 30°C for 2 or 3 days before taking images using a BioRad ChemiDoc MP imaging system.
Author contributions
Sunbin Deng: Data curation; formal analysis; validation; investigation; visualization; methodology; writing – review and editing. Jiaxi Cai: Data curation; formal analysis; investigation; methodology; writing – review and editing. Stephen C Harrison: Formal analysis; writing – original draft; writing – review and editing. Huilin Zhou: Data curation; formal analysis; investigation; methodology; writing – review and editing. Stephen M Hinshaw: Visualization; methodology; writing – original draft; writing – review and editing.
Disclosure and competing interests statement
The authors declare that they have no conflict of interest.
Supporting information
Expanded View Figures PDF
PDF+
Table EV1
Table EV2
Table EV3
Source Data for Expanded View
Source Data for Figure 1
Source Data for Figure 2
Source Data for Figure 3
Source Data for Figure 4
Acknowledgements
We thank Cindy Zhang for technical assistance. We thank the ICCB‐Longwood Screening Facility (Harvard Medical School) for help collecting fluorescence polarization data. X‐ray diffraction data were recorded at beamline ID‐24‐E (operated by the Northeast Collaborative Access team: NE‐CAT) at the Advanced Photon Source (APS, Argonne National Laboratory). We thank the staff of the NE‐CAT beamlines for help in data collection. NE‐CAT is supported by NIH grant P30 GM124165, using resources of the APS, operated by Argonne National Laboratory under Contract DE‐AC02‐06CH11357. JC and HZ are supported by RO1 GM116897. SCH is an Investigator in the Howard Hughes Medical Institute.
EMBO reports (2023) 24: e57702
Contributor Information
Huilin Zhou, Email: huzhou@health.ucsd.edu.
Stephen M Hinshaw, Email: hinshaw@stanford.edu.
Data availability
Coordinates of the structure described in this article have been deposited in the PDB with accession number (PDB ID: 8T0P; https://www.rcsb.org/structure/8T0P).
References
- Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse‐Kunstleve RW et al (2010) PHENIX: a comprehensive Python‐based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr 66: 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Albuquerque CP, Liang J, Gaut NJ, Zhou H (2016) Molecular circuitry of the SUMO (small ubiquitin‐like modifier) pathway in controlling sumoylation homeostasis and suppressing genome rearrangements. J Biol Chem 291: 8825–8835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anedchenko EA, Samel‐Pommerencke A, Tran Nguyen TM, Shahnejat‐Bushehri S, Popsel J, Lauster D, Herrmann A, Rappsilber J, Cuomo A, Bonaldi T et al (2019) The kinetochore module Okp1(CENP‐Q)/Ame1(CENP‐U) is a reader for N‐terminal modifications on the centromeric histone Cse4(CENP‐A). EMBO J 38: e98991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeckmann L, Takahashi Y, Au WC, Mishra PK, Choy JS, Dawson AR, Szeto MY, Waybright TJ, Heger C, McAndrew C et al (2013) Phosphorylation of centromeric histone H3 variant regulates chromosome segregation in Saccharomyces cerevisiae . Mol Biol Cell 24: 2034–2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman IM, Anderson S, Jwa M, Green EM, Kang J, Yates JR 3rd, Chan CS, Drubin DG, Barnes G (2002) Phospho‐regulation of kinetochore‐microtubule attachments by the Aurora kinase Ipl1p. Cell 111: 163–172 [DOI] [PubMed] [Google Scholar]
- Chen Y, Baker RE, Keith KC, Harris K, Stoler S, Fitzgerald‐Hayes M (2000) The N terminus of the centromere H3‐like protein Cse4p performs an essential function distinct from that of the histone fold domain. Mol Cell Biol 20: 7037–7048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wulf P, McAinsh AD, Sorger PK (2003) Hierarchical assembly of the budding yeast kinetochore from multiple subcomplexes. Genes Dev 17: 2902–2921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dendooven T, Zhang Z, Yang J, McLaughlin SH, Schwab J, Scheres SHW, Yatskevich S, Barford D (2023) Cryo‐EM structure of the complete inner kinetochore of the budding yeast point centromere. Sci Adv 9: eadg7480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrova YN, Jenni S, Valverde R, Khin Y, Harrison SC (2016) Structure of the MIND complex defines a regulatory focus for yeast kinetochore assembly. Cell 167: 1014–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P, Lohkamp B, Scott WG, Cowtan K (2010) Features and development of Coot. Acta Crystallogr D Biol Crystallogr 66: 486–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans PR, Murshudov GN (2013) How good are my data and what is the resolution? Acta Crystallogr D Biol Crystallogr 69: 1204–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbock‐Halwachs J, Singh S, Potocnjak M, Hagemann G, Solis‐Mezarino V, Woike S, Ghodgaonkar‐Steger M, Weissmann F, Gallego LD, Rojas J et al (2019) The COMA complex interacts with Cse4 and positions Sli15/Ipl1 at the budding yeast inner kinetochore. Elife 8: e42879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan R, Lian T, Zhou BR, He E, Wu C, Singleton M, Bai Y (2021) Structural and dynamic mechanisms of CBF3‐guided centromeric nucleosome formation. Nat Commun 12: 1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton GE, Helgeson LA, Noland CL, Asbury CL, Dimitrova YN, Davis TN (2020) Reconstitution reveals two paths of force transmission through the kinetochore. Elife 9: e56582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewawasam G, Shivaraju M, Mattingly M, Venkatesh S, Martin‐Brown S, Florens L, Workman JL, Gerton JL (2010) Psh1 is an E3 ubiquitin ligase that targets the centromeric histone variant Cse4. Mol Cell 40: 444–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinshaw SM, Harrison SC (2019) The structure of the Ctf19c/CCAN from budding yeast. Elife 8: e44239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinshaw SM, Harrison SC (2020) The structural basis for kinetochore stabilization by Cnn1/CENP‐T. Curr Biol 30: e3423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinshaw SM, Quan Y, Cai J, Zhou AL, Zhou H (2023) Multi‐site phosphorylation of yeast Mif2/CENP‐C promotes inner kinetochore assembly. Curr Biol 33: 688–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann G, Samel‐Pommerencke A, Weber J, Cuomo A, Bonaldi T, Ehrenhofer‐Murray AE (2018) A role for CENP‐A/Cse4 phosphorylation on serine 33 in deposition at the centromere. FEMS Yeast Res 18 10.1093/femsyr/fox094 [DOI] [PubMed] [Google Scholar]
- Hornung P, Troc P, Malvezzi F, Maier M, Demianova Z, Zimniak T, Litos G, Lampert F, Schleiffer A, Brunner M et al (2014) A cooperative mechanism drives budding yeast kinetochore assembly downstream of CENP‐A. J Cell Biol 206: 509–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa Y, Saitoh N, Kaufman PD (2018) An asymmetric centromeric nucleosome. Elife 7: e37911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Zidek A, Potapenko A et al (2021) Highly accurate protein structure prediction with AlphaFold. Nature 596: 583–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichten M (2014) Tetrad, random spore, and molecular analysis of meiotic segregation and recombination. Methods Mol Biol 1205: 13–28 [DOI] [PubMed] [Google Scholar]
- Migl D, Kschonsak M, Arthur CP, Khin Y, Harrison SC, Ciferri C, Dimitrova YN (2020) Cryoelectron microscopy structure of a yeast centromeric nucleosome at 2.7 a resolution. Structure 28: 363–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra PK, Wood H, Stanton J, Au WC, Eisenstatt JR, Boeckmann L, Sclafani RA, Weinreich M, Bloom KS, Thorpe PH et al (2021) Cdc7‐mediated phosphorylation of Cse4 regulates high‐fidelity chromosome segregation in budding yeast. Mol Biol Cell 32: ar15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkuni K, Levy‐Myers R, Warren J, Au WC, Takahashi Y, Baker RE, Basrai MA (2018) N‐terminal sumoylation of centromeric histone H3 variant Cse4 regulates its proteolysis to prevent mislocalization to non‐centromeric chromatin. G3 (Bethesda) 8: 1215–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz J, Stemmann O, Rank S, Lechner J (1999) A putative protein complex consisting of Ctf19, Mcm21, and Okp1 represents a missing link in the budding yeast kinetochore. Genes Dev 13: 1140–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pot I, Knockleby J, Aneliunas V, Nguyen T, Ah‐Kye S, Liszt G, Snyder M, Hieter P, Vogel J (2005) Spindle checkpoint maintenance requires Ame1 and Okp1. Cell Cycle 4: 1448–1456 [DOI] [PubMed] [Google Scholar]
- Ranjitkar P, Press MO, Yi X, Baker R, MacCoss MJ, Biggins S (2010) An E3 ubiquitin ligase prevents ectopic localization of the centromeric histone H3 variant via the centromere targeting domain. Mol Cell 40: 455–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samel A, Cuomo A, Bonaldi T, Ehrenhofer‐Murray AE (2012) Methylation of CenH3 arginine 37 regulates kinetochore integrity and chromosome segregation. Proc Natl Acad Sci U S A 109: 9029–9034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitzberger F, Richter MM, Gordiyenko Y, Robinson CV, Dadlez M, Westermann S (2017) Molecular basis for inner kinetochore configuration through RWD domain‐peptide interactions. EMBO J 36: 3458–3482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter G, Beilsten‐Edmands J, Devenish N, Gerstel M, Gildea RJ, McDonagh D, Pascal E, Waterman DG, Williams BH, Evans G (2022) DIALS as a toolkit. Protein Sci 31: 232–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski J, Hajj B, Chen J, Mizuguchi G, Xiao H, Wei D, Dahan M, Wu C (2014) Imaging the fate of histone Cse4 reveals de novo replacement in S phase and subsequent stable residence at centromeres. Elife 3: e02203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan K, Zhang Z, Yang J, McLaughlin SH, Barford D (2018) Architecture of the CBF3‐centromere complex of the budding yeast kinetochore. Nat Struct Mol Biol 25: 1103–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan K, Yang J, Zhang Z, McLaughlin SH, Chang L, Fasci D, Ehrenhofer‐Murray AE, Heck AJR, Barford D (2019) Structure of the inner kinetochore CCAN complex assembled onto a centromeric nucleosome. Nature 574: 278–282 [DOI] [PMC free article] [PubMed] [Google Scholar]


