Abstract
Background
Artificial intelligence (AI) applied to 12-lead electrocardiographs (ECGs) can detect hypertrophic cardiomyopathy (HCM).
Objectives
The purpose of this study was to determine if AI-enhanced ECG (AI-ECG) can track longitudinal therapeutic response and changes in cardiac structure, function, or hemodynamics in obstructive HCM during mavacamten treatment.
Methods
We applied 2 independently developed AI-ECG algorithms (University of California-San Francisco and Mayo Clinic) to serial ECGs (n = 216) from the phase 2 PIONEER-OLE trial of mavacamten for symptomatic obstructive HCM (n = 13 patients, mean age 57.8 years, 69.2% male). Control ECGs from 2,600 age- and sex-matched individuals without HCM were obtained. AI-ECG output was correlated longitudinally to echocardiographic and laboratory metrics of mavacamten treatment response.
Results
In the validation cohorts, both algorithms exhibited similar performance for HCM diagnosis, and exhibited mean HCM score decreases during mavacamten treatment: patient-level score reduction ranged from approximately 0.80 to 0.45 for Mayo and 0.70 to 0.35 for USCF algorithms; 11 of 13 patients demonstrated absolute score reduction from start to end of follow-up for both algorithms. HCM scores were significantly associated with other HCM-relevant parameters, including left ventricular outflow tract gradient at rest, postexercise, and with Valsalva, and NT-proBNP level, independent of age and sex (all P < 0.01). For both algorithms, the strongest longitudinal correlation was between AI-ECG HCM score and left ventricular outflow tract gradient postexercise (slope estimate: University of California-San Francisco 0.70 [95% CI: 0.45-0.96], P < 0.0001; Mayo 0.40 [95% CI: 0.11-0.68], P = 0.007).
Conclusions
AI-ECG analysis longitudinally correlated with changes in echocardiographic and laboratory markers during mavacamten treatment in obstructive HCM. These results provide early evidence for a potential paradigm for monitoring HCM therapeutic response.
Key words: artificial intelligence, electrocardiogram, hypertrophic cardiomyopathy, machine learning, mavacamten
Central Illustration
Hypertrophic cardiomyopathy (HCM) is a myocardial disorder defined by unexplained left ventricular (LV) hypertrophy and characterized by hypercontractility, impaired relaxation, and, in the obstructive variant, dynamic LV outflow tract (LVOT) obstruction. The initial evaluation and longitudinal monitoring of patients with HCM relies on clinical assessment and can include assessment with echocardiography, cardiac magnetic resonance imaging, and laboratory testing.1 The electrocardiogram (ECG) can be normal or show nonspecific abnormalities, including LV hypertrophy or diffuse Q waves.2 The nonspecific nature of these ECG changes can make it challenging to use ECGs alone for HCM diagnosis and monitoring.
Artificial intelligence (AI)-based analysis of digital data from standard 12-lead ECGs (AI-ECG) has been shown to achieve fully automated HCM diagnosis with high accuracy as AI-ECG models for the detection of HCM based on deep learning have been independently developed by research groups.3,4 However, since various disease-related physiologic and anatomic changes can be reflected in ECG changes, AI-ECG may also have the utility to track disease status over time or capture cardiac hemodynamics or structure relevant to disease-specific clinical decision making.
Mavacamten is a first-in-class cardiac myosin inhibitor targeting the underlying pathophysiology of HCM. In the phase 2 PIONEER-HCM (NCT02842242)5 and PIONEER-OLE (NCT03496168) studies and the recent phase 3 EXPLORER-HCM study (NCT03470545), mavacamten was shown to improve exercise capacity, LVOT obstruction, and health status.6 Clinical trials provide unique and highly enriched phenotyping for a subset of HCM patients through frequent, serial paired ECGs, echocardiograms, and laboratory measurements.
We recently reported the application of 2 independently developed AI-ECG algorithms from 2 different institutions on the baseline and on-treatment ECGs of HCM patients enrolled in the PIONEER-OLE study.7 Both algorithms demonstrated reductions in mean AI-ECG HCM scores during treatment, averaged across each time point, with reductions in patient-level scores from 0.85 to 0.37 for the Mayo algorithm (mean per-patient reduction 56%) and 0.67 to 0.38 for the USCF algorithm (mean per-patient reduction 43%). The AI-ECG score reductions mirrored the on-treatment reductions in the provoked LVOT gradient and N-terminal pro B-type natriuretic peptide (NT-proBNP) measurements. In the current study, we aimed to further investigate patient-level trends in AI-ECG scores and examine longitudinal associations with important echocardiographic and laboratory metrics in the PIONEER-OLE cohort.
Methods
Overall study design
We applied 2 AI-ECG algorithms developed and trained independently at 2 institutions (University of California-San Francisco [UCSF], [San Francisco, California, USA]4 and Mayo Clinic [Rochester, Minnesota, USA]3) to XML format ECG data from the PIONEER-OLE trial which enrolled patients between May and October 2018. Institutional review board approval was obtained in both institutions. Both algorithms were applied as they were trained, without additional refinement or tuning. The AI-ECG algorithms provide an output from 0 to 1 for each ECG, referred to as the HCM score, representing the probability that the ECG belongs to a patient with HCM (Central Illustration).
Central Illustration.
Using Artificial Intelligence-Enhanced Electrocardiography in Hypertrophic Cardiomyopathy to Assess Disease Status and Treatment Response
Two independently trained AI-ECG HCM algorithms were applied to ECGs from patients with obstructive hypertrophic cardiomyopathy in the phase 2 PIONEER-OLE trial of mavacamten. Longitudinal scores from each AI-ECG algorithm calculated from HCM ECGs obtained during the clinical trial were significantly associated with changes in most parameters including left ventricular outflow tract gradient postexercise and NT-proBNP Level, Independent of age and sex (all P < 0.01). AI-ECG = artificial intelligence-enhanced electrocardiograms; AUC = area under the receiver operating characteristic curve; ECG = electrocardiogram; HCM = hypertrophic cardiomyopathy; NT-proBNP = N-terminal pro-B-type natriuretic peptide; UCSF = University of California-San Francisco.
For each patient in the ongoing PIONEER-OLE study, both algorithms were applied to available ECGs acquired pretreatment and during on-treatment follow-up through a datacut date of January 29, 2020. If multiple ECGs were obtained during a single visit, the algorithms were applied to all ECGs for a single visit date, and the outputs were averaged. Patient data were collected for visits starting at screening through a maximal follow-up at week-72. Nine out of 13 patients had reached the week-72 study visit as of the datacut date, and a few patients had longer available follow-up. At time of the datacut, the total duration on-study per patient ranged from 25 to 90 weeks (median 79 weeks) of mavacamten treatment, depending on the date of each patient’s trial enrollment.
Clinical trial overview
Mavacamten was initially evaluated in 21 patients with symptomatic obstructive HCM in PIONEER-HCM, a phase 2, multicenter, open-label dose-ranging study.5 Following PIONEER-HCM completion, the ongoing 5-year PIONEER-OLE study enrolled 13 patients at 4 U.S. clinical sites from PIONEER-HCM to examine the longer-term safety and effectiveness of mavacamten.8
PIONEER-OLE is being conducted in accordance with the International Council for Harmonisation Good Clinical Practice guidelines, the principles of the Declaration of Helsinki, and applicable regulatory requirements. An independent ethics committee or institutional review board (UCSF and Mayo Clinic) reviewed and approved the study protocol, informed consent forms, recruitment documents, and other information provided to patients. All patients provided written informed consent.
Trial procedures
Patients were evaluated at baseline (Day 0) before starting 5 mg daily oral mavacamten and at weeks 4, 8, and 12, and every 12 weeks thereafter to monitor safety, efficacy, and mavacamten plasma concentration. Dose titration occurred at week 6 if indicated per protocol (dose strengths 5, 10, and 15 mg). The latest study visit with assessments included in this analysis was at 72 weeks. Efficacy and pharmacokinetic assessments included 12-lead ECGs, echocardiographic variables (LV ejection fraction, LVOT gradient), NYHA functional class, and blood samples for drug plasma concentration and NT-proBNP level. Resting echocardiography was performed at weeks 4, 8, 12, 24, 36, 48, and 72, and postexercise stress echocardiography was performed at weeks 4, 48, and 72. Echocardiograms were acquired at each site according to a detailed acquisition protocol, and images were assessed by a central core imaging laboratory (Brigham and Women’s Hospital).
Development of neural network ECG algorithms
To develop the UCSF HCM model, 3,945 ECGs from 323 patients who met guideline-based criteria for HCM1,9 were obtained, along with 5,931 ECGs from 3,762 age- and sex-matched subjects without HCM, as has been previously described.4 Data were split into 70% training, 10% validation, and 20% testing datasets, with no overlapping patients. Standard 10-second, 12-lead ECG voltage data were converted to a 2,500 × 12 matrix and input into a 1-dimensional convolutional neural network (CNN) whose first layer convolves 64 8 × 12 filters across the 2,500 × 12 input followed by 8 × 64 filters, and onward in a ResNet architecture.10,11 The neural network was implemented using TensorFlow 1.12 (Google) and Python 3.6 (Python Software Foundation). Model hyperparameters were tuned in the validation dataset.
To develop the Mayo Clinic HCM model, a total of 3,060 patients with a validated HCM diagnosis and 63,941 controls (without HCM) were age- and sex-matched and split into 70% training, 10% validation, and 20% testing dataset groups. Digitally stored, standard, 10-second, 12-lead ECGs acquired in the supine position were converted to a 12 × 5,000 matrix, and CNN using the Keras Framework with a TensorFlow backend and Python was applied. One ECG per patient was included. Convolutions occurred within each lead and across different leads of the 12-lead recording. After initial training, the model was fine-tuned in the internal validation dataset.
ECG algorithm PIONEER-OLE cohorts
To preliminarily validate the 2 CNN algorithms on HCM ECG data from PIONEER-OLE, cohorts were constructed by combining HCM ECGs from PIONEER-OLE (1 pretreatment ECG from each of the 13 patients) and non-HCM ECGs (the first available ECG) from patients randomly drawn from each respective institution that were age- and sex-matched to the PIONEER-OLE cohort using a 1:200 case:control ratio, simulating HCM population prevalence. For the Mayo algorithm,3 an ECG was identified as belonging to a patient with HCM if the AI-ECG HCM score output was ≥0.11, the optimal threshold specified in the initial model derivation study; for the UCSF algorithm, the optimal threshold was ≥0.54.
Statistical analysis
Descriptive summary statistics were reported for continuous variables. Nominal categorical variables were summarized using counts and percentages. Scatter plots were created to show the association of pretreatment and on-treatment scores with LVOT gradients and NT-proBNP levels prior to treatment and longitudinally during treatment. Regression lines and corresponding confidence bands for scatter plots were generated by fitting a mixed effects model described below.
A mixed effects model was run to predict LVOT gradient with Valsalva, at rest and postexercise, and log NT-proBNP. In each model, the HCM score was treated as a fixed effect and the patient as a random effect, thus accounting for the potential correlation between ECG or echocardiographic measurements from the same patient. To explore the role of age and sex in the observed association, these variables were added to this mixed model as fixed effect covariates. The presented longitudinal correlation value is a slope estimate of normalized scores and normalized echocardiography and laboratory values from a mixed model (accounting for longitudinal measurements from patients over time). Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc). P values <0.05 were considered statistically significant.
Results
During treatment with mavacamten (median 79 weeks [range 25.0-90.1 weeks]), each patient underwent a mean of 7.8 ECGs depending on enrollment date. A total of 216 ECGs were obtained during the study. At baseline, the mean age of the PIONEER-OLE cohort was 57.8 years; 69.2% were male, and 92.3% had NYHA class II symptoms (Table 1).
Table 1.
Baseline Characteristics of PIONEER-OLE Cohort (N = 13)
| Age, mean (SD), y | 57.8 ± 13.3 |
| Male | 9 (69) |
| Body mass index, kg/m2 | 30.9 ± 5.4 |
| Systolic BP, mm Hg | 123.2 ± 10.9 |
| Diastolic BP, mm Hg | 72.6 ± 7.1 |
| Heart rate, beats/min | 61.8 ± 10.6 |
| NYHA functional class | |
| II | 12 (92) |
| III | 1 (7) |
| Background HCM therapy while on study drug | |
| Metoprolol | 11 (84) |
| Bisoprolol | 1 (7) |
| Echocardiography parameters | |
| Resting LVEF, % | 72.0 ± 4 |
| LV mass index, g/m2 | 103.0 ± 25.8 |
| LA volume index, mL/m2 | 40.9 ± 16.4 |
| Maximum wall thickness, mm | 20.9 ± 2.1 |
| LVOT gradient, mm Hg | |
| Resting | 67.3 ± 42.8 |
| Valsalva | 89.9 ± 30.7 |
| Postexercise | 127.5 ± 33.4 |
| NT-proBNP, pg/mL | 594 (110-10,132) |
Values are mean ± SD, n (%), or median (range).
BP = blood pressure; HCM = hypertrophic cardiomyopathy; LA = left atrial; LV = left ventricular; LVEF = left ventricular ejection fraction; LVOT = left ventricular outflow tract; NT-proBNP = N-terminal pro B-type natriuretic peptide.
Both algorithms exhibited similar overall performance in the preliminary validation data of baseline ECGs of PIONEER-OLE HCM patients and the ECGs of age- and sex-matched controls without HCM.7 The UCSF and Mayo algorithms correctly classified 11/13 and 12/13 patients as having HCM, respectively. Notably, 1 patient (Patient 6) exhibited a less typical phenotype without substantially elevated LVOT gradients (LVOT gradients at rest and with Valsalva were 8.6 and 32.0 mmHg, respectively) or NT-proBNP (172 ng/mL) at baseline. For this patient, both algorithms predicted HCM scores lower than their respective thresholds for HCM detection (Supplemental Figure 1).
As evidenced by the individual HCM score trajectories (Figure 1), individual variations were observed in the longitudinal patterns of HCM score changes for individual patients. Upon inspection, patient-level score reduction ranged from approximately 0.80 to 0.45 for the Mayo algorithm and 0.70 to 0.35 for the USCF algorithm. Eleven of 13 patients demonstrated absolute score reduction from start to end of follow-up per the Mayo algorithm and 11 of 13 patients per the UCSF algorithm.
Figure 1.
Longitudinal Changes to AI-ECG Algorithm Scores and Clinical Measures in a Clinical Trial
The dashed lines represent the optimal diagnostic thresholds for each algorithm as determined during algorithm development at the respective institutions. AI-ECG = artificial intelligence–based electrocardiogram analysis; HCM = hypertrophic cardiomyopathy; LOESS = locally estimated scatter plot smoothing; LVOT = left ventricular outflow tract; NT-proBNP = N-terminal pro B-type natriuretic peptide; PK = pharmacokinetic levels; UCSF = University of California-San Francisco.
The individual patient-level longitudinal AI-ECG HCM score trends generally mirrored the decreasing trends over time in LVOT gradient with Valsalva and NT-proBNP (Figure 1), though the AI-ECG score changes lagged the more rapid change of these measurements. The HCM scores decreased over time then plateaued at about 200 days (∼28.6 weeks) since treatment initiation, mirroring the LVOT gradient with Valsalva and NT-proBNP plateau at ∼100 days. Mavacamten plasma concentration remained relatively stable in most patients following treatment initiation and titration.
AI-ECG HCM scores were associated with clinical metrics reflective of HCM disease status prior to treatment (Figure 2) and longitudinally during treatment. When ECG data were examined across the full study duration (216 total ECGs), linear associations between AI-ECG HCM scores and clinical metrics were stronger than pretreatment correlations (Figure 3 and Central Illustration). For both algorithms, the strongest longitudinal correlation was for LVOT gradient postexercise (UCSF 0.70 [95% CI: 0.45-0.96], P < 0.0001; Mayo 0.40 [95% CI: 0.11-0.68], P = 0.007) (Figure 3). AI-ECG HCM scores were also correlated with LVOT gradient with Valsalva and log NT-proBNP. AI-ECG HCM scores (normalized) also showed weaker correlations with other clinical measures: Mayo, LV mass index (0.17; 95% CI: 0.06-0.29, P = 0.004), left atrial (LA) volume index (0.22; 95% CI: 0.11-0.33, P < 0.001), maximum wall thickness (0.16; 95% CI: 0.02-0.29, P = 0.02); UCSF: LV mass index (0.32; 95% CI: 0.19-0.46, P < 0.001), LA volume index (0.38; 95% CI: 0.24-0.51, P < 0.001), maximum wall thickness (0.15; 95% CI: −0.03 to 0.34, P = 0.10). In mixed effects models adjusted for age and sex, both algorithms showed significant associations with clinical metrics including LVOT gradients at rest, with Valsalva, and post exercise, log-transformed NT-proBNP, LV mass index, LA volume index, and maximum wall thickness (P < 0.05 for all, except the UCSF score for maximum wall thickness, P = 0.07).
Figure 2.
Pretreatment Spearman Correlations Between AI-ECG and Clinical Scores
The regression lines and confidence bands shown in the lower panels correspond to the mixed effects model run to predict each of the following: LVOT gradient with Valsalva, at rest, and post-exercise, and log NT-proBNP. P values for significance of slope estimate <0.01 for all measurements shown. AI-ECG = artificial intelligence–based electrocardiogram analysis; CI = confidence interval; CNN = convolutional neural network; DNN = deep neural network; HCM = hypertrophic cardiomyopathy; LOESS = locally estimated scatter plot smoothing; LVOT = left ventricular outflow tract; NT-proBNP = N-terminal pro B-type natriuretic peptide; UCSF = University of California-San Francisco.
Figure 3.
Post-Treatment Initiation Correlations of AI-ECG Algorithm HCM Scores and HCM-Relevant Clinical Metrics
Strength of association was determined by the slope estimate of normalized scores from a mixed effects model accounting for longitudinal measurements from patients over time. The regression lines and confidence bands correspond to the mixed effects model run to predict each of the following: LVOT gradient with Valsalva, at rest, and post-exercise, and log NT-proBNP. P values for significance of slope estimate <0.01 for all measurements shown. AI-ECG = artificial intelligence–enhanced electrocardiography; CI = confidence interval; HCM = hypertrophic cardiomyopathy; LVOT = left ventricular outflow tract; NT-proBNP = N-terminal pro-B-type natriuretic peptide; UCSF = University of California-San Francisco.
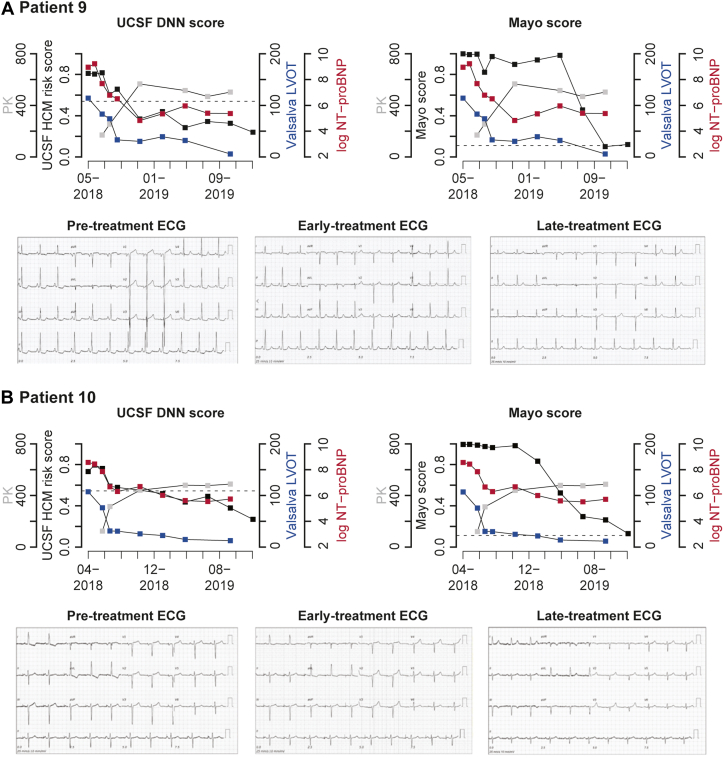
Figure 4 highlights the longitudinal patterns of the AI-ECG HCM scores and primary HCM clinical metrics alongside pretreatment and on-treatment ECGs for 2 example patients (data for all patients are shown in Supplemental Figure 1). Pretreatment ECGs for both patients exhibited stereotypical changes associated with HCM, such as LV hypertrophy with strain patterns, T-wave inversion, ST-segment depression and repolarization abnormalities, and p-wave abnormalities (Figure 4). Over time, participants’ HCM scores from both algorithms decreased below their respective thresholds used to detect HCM by the end of treatment (indicated by dotted lines). The decrease in LVOT gradient with Valsalva appeared to precede substantial drops in HCM score from either algorithm. The UCSF HCM score trend temporally correlated with log NT-proBNP and LVOT gradient trends with Valsalva and continued to decrease for both patients after NT-proBNP plateaued. The Mayo algorithm’s HCM score decreased initially alongside early decreases in clinical metrics, then plateaued before decreasing dramatically for both patients later in the study period. After 8 to 12 weeks of treatment, ECGs for both patients continued to show HCM-related abnormalities, although they improved compared to baseline, and HCM scores from both algorithms were lower than baseline. At the 84-week visit for these 2 patients who had longer follow-up, there was substantial normalization of HCM-related ECG abnormalities for both, which was similarly captured quantitatively by both algorithms as further reduction in AI-ECG HCM scores.
Figure 4.
Longitudinal Trends in AI-ECG Algorithm Scores, Clinical Metrics, and ECGs
(A) Patient 9. (B) Patient 10. Trends for 2 example patients in AI-ECG algorithm scores and clinical metrics over time. Black squares show AI-ECG score trends. Grey squares show PK Levels. Blue squares show left ventricular outflow tract gradients with valsalva maneuver. Red squares show serum NT-proBNP levels. ECG examples for each patient are shown at varying treatment stages below. AI-ECG = artificial intelligence–based electrocardiogram analysis; CNN = convolutional neural network; DNN = deep neural network; ECG = electrocardiogram; HCM = hypertrophic cardiomyopathy; LVOT = left ventricular outflow tract; NT-proBNP = N-terminal pro-B-type natriuretic peptide; PK = pharmacokinetic levels; UCSF = University of California-San Francisco.
Discussion
Our results demonstrate that the AI-ECG HCM scores correlate with disease status, hemodynamic changes in LVOT gradients, and serum NT-proBNP levels in patients with obstructive HCM on mavacamten treatment, even when ECG changes are less apparent. The longitudinal correlation of AI-ECG HCM scores with these markers shows for the first time that AI captures previously unappreciated disease-related physiologic changes using standard 12-lead ECGs. AI-ECG may thus provide a novel paradigm to monitor disease status, cardiac hemodynamics, and drug therapeutic response in a widely accessible, noninvasive manner.
As we reported previously, both UCSF and Mayo algorithms performed similarly well without fine-tuning on PIONEER-OLE data to detect HCM.7 These data provide preliminary evidence of external validity, though formal external validation is pending in larger independent HCM cohorts and non-HCM controls. The significant and strong association of the HCM score with echocardiographically-measured LVOT gradients and NT-proBNP likely reflects the importance of these clinical metrics to aspects of disease pathophysiology and severity reflected in the raw ECG waveform. In particular, both algorithms demonstrated the strongest correlation with changes in postexercise LVOT gradients. The LVOT gradient is a critical measure in the HCM phenotype that correlates with treatment response and symptoms. Further, postexercise LVOT gradient may better discriminate subtle differences in HCM physiology, hence potentially explaining the observed stronger correlation with the AI-ECG HCM scores.
As hypothesized, changes in AI-ECG occurred gradually over time, following earlier changes in hemodynamic and biomarker measures. These associations suggest that ECG data could potentially capture more information related to obstructive HCM pathophysiology (or correlates thereof) than is currently appreciated (eg, LV or LA mass/volume, degree of septal hypertrophy, or even cellular changes). ECG changes associated with HCM previously described in the literature have largely been nonspecific.12 The degree of association we observed with AI-ECG varied by metric for obstructive HCM. For instance, there was a relatively uniform decline in LVOT gradient and NT-proBNP on mavacamten over time, whereas the reduction in HCM scores varied between patients. Some of these differences are likely due to the unique characteristics of the populations used to train the algorithms at each institution.
There is a significant opportunity to further refine these algorithms for specific clinical metrics or tasks of interest in the target HCM population. It is likely that if the algorithms were specifically refined to detect HCM in the PIONEER-OLE cohort or retrained for each clinically important HCM metric, they would exhibit higher performance for those tasks than achieved herein. While clinicians commonly compare ECGs between 2 time points (eg, current vs immediately prior ECG), there is no established quantitative approach to characterize changes between serial ECGs beyond global visual comparison. Our AI-ECG analysis for HCM uniquely provides a quantitative metric of HCM disease risk (and potentially severity) that can be easily tracked using serial ECGs over various time points. Further, we demonstrate that these algorithms can begin to quantify improvements of HCM physiology with treatment even when disease-specific ECG abnormalities are still clearly present.
The AI-ECG approach has further potential to provide insights beyond HCM score reductions to improve outcomes. Finally, since ECGs may also be performed by patients at home via smartphone-enabled electrodes, AI-ECG analysis of remotely obtained ECGs may potentially permit assessment of disease status or treatment response in the future. However, further studies would be required to determine if such an approach could potentially guide drug titration to enhance safety. Future directions of this research include formal external validation in larger independent diverse HCM and non-HCM control cohorts, demonstration of algorithm performance using single-lead ECG inputs (such as those derived from wearables), and prediction of adverse events in HCM.
Study Limitations
This study has several limitations. We applied our algorithms to a small sample of PIONEER-OLE patients to leverage the longer-term follow-up serial ECG and echocardiogram measurements in this subset of the larger PIONEER-HCM trial. While the total number of patients with HCM was small, the uniquely rich phenotyping across a longer follow-up was valuable to demonstrate associations between ECG-based scores and clinical metrics. In our validations of both algorithms, we were limited in drawing ECGs for patients without HCM from the respective institutions at which the algorithms were trained. However, patients without HCM were age- and sex-matched and randomly selected from databases from the 2 large institutions, so these were generally representative of patients without HCM.
Conclusions
In this study, we demonstrated that 2 independently derived AI-ECG algorithms performed well to identify HCM. The resulting AI-ECG HCM scores significantly declined over time during treatment with mavacamten. The longitudinal, on-treatment trends of the AI-ECG HCM scores correlated well with trends in laboratory and echocardiographically measured metrics indicating treatment response. AI-based analysis of ECGs may represent an effective and scalable approach for tracking HCM disease state during mavacamten treatment and possibly for other therapeutics. This work shows that AI-ECG may provide a new paradigm for monitoring disease status, cardiac hemodynamics, and therapeutic response.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE: Deep neural network algorithm analysis of ECGs captures information regarding HCM disease severity and therapeutic response, including correlations with objective hemodynamic and laboratory abnormalities.
TRANSLATIONAL OUTLOOK: This may offer a potentially new paradigm of ECG-based disease and monitoring of therapeutic response in HCM and possibly other diseases.
Funding support and author disclosures
Dr Tison has received support for this work from the National Institutes of Health NHLBI K23HL135274. The Tison lab has received funding from MyoKardia Inc, a wholly owned subsidiary of Bristol Myers Squibb to support this analysis. The work to derive the Mayo Clinic AI-ECG algorithm was funded by the Louis V. Gerstner, Jr Fund at Vanguard Charitable. The PIONEER-OLE study was funded by MyoKardia Inc, a wholly owned subsidiary of Bristol Myers Squibb. Co-authors employed by MyoKardia Inc, a wholly owned subsidiary of Bristol Myers Squibb were involved in study design, data collection, data analysis, data interpretation, and review of the manuscript in collaboration with academic coauthors. Drs Noseworthy, Siontis, Attia, and Friedman are coinventors of the Mayo Clinic AI-ECG algorithm utilized in this work. Dr Noseworthy has received research funding from the National Institutes of Health (NIH, including the National Heart, Lung, and Blood Institute [NHLBI] and the National Institute on Aging [NIA]), Agency for Healthcare Research and Quality (AHRQ), Food and Drug Administration (FDA), and the American Heart Association (AHA; and is a study investigator in an ablation trial sponsored by Medtronic. Dr Noseworthy and the Mayo Clinic are involved in a potential equity/royalty relationship with AliveCor. Dr Tison has previously received research grants from General Electric, Janssen Pharmaceuticals, and MyoKardia Inc, a wholly owned subsidiary of Bristol Myers Squibb; and has received consulting fees from MyoKardia. Dr Abraham has received consulting fees from Adagio Medical and Farapulse. Drs Balasubramanyam and Sehnert are employees of and report stock and stock options from BMS. Drs Agarwal, Li, and Edelberg were employees of BMS at the time of analysis. Dr Masri has received grants from Pfizer, Akcea, and Ultromics; and consulting fees from Eidos, Ionis, Alnylam, Cytokinetics, and Pfizer. Dr Wang has received grants from Cytokinetics and MyoKardia Inc, a wholly owned subsidiary of Bristol Myers Squibb, personal fees from MyoKardia; and consulting fees from Cytokinetics. Dr Olgin has received research funding from the NIH (U2CEB021881) and from Samsung and iBeat. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgment
The authors thank the patients and their families, the investigators, and the clinical study teams for making the study possible. Editorial support was provided by Kim Fuller, PhD of Cello Health Communications/SciFluent, funded by MyoKardia Inc, a wholly owned subsidiary of Bristol Myers Squibb.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental appendix, please see the online version of this paper.
Contributor Information
Peter A. Noseworthy, Email: noseworthy.peter@mayo.edu.
Geoffrey H. Tison, Email: geoff.tison@ucsf.edu.
Supplementary data
References
- 1.Ommen S.R., Mital S., Burke M.A., et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2020;76:e159–e240. doi: 10.1016/j.jacc.2020.08.045. [DOI] [PubMed] [Google Scholar]
- 2.McLeod C.J., Ackerman M.J., Nishimura R.A., Tajik A.J., Gersh B.J., Ommen S.R. Outcome of patients with hypertrophic cardiomyopathy and a normal electrocardiogram. J Am Coll Cardiol. 2009;54:229–233. doi: 10.1016/j.jacc.2009.02.071. [DOI] [PubMed] [Google Scholar]
- 3.Ko W.Y., Siontis K.C., Attia Z.I., et al. Detection of hypertrophic cardiomyopathy using a convolutional neural network-enabled electrocardiogram. J Am Coll Cardiol. 2020;75:722–733. doi: 10.1016/j.jacc.2019.12.030. [DOI] [PubMed] [Google Scholar]
- 4.Tison G.H., Zhang J., Delling F.N., Deo R.C. Automated and interpretable patient ECG profiles for disease detection, tracking, and discovery. Circ Cardiovasc Qual Outcomes. 2019;12 doi: 10.1161/CIRCOUTCOMES.118.005289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heitner S.B., Jacoby D., Lester S.J., et al. Mavacamten treatment for obstructive hypertrophic cardiomyopathy: a clinical trial. Ann Intern Med. 2019;170:741–748. doi: 10.7326/M18-3016. [DOI] [PubMed] [Google Scholar]
- 6.Olivotto I., Oreziak A., Barriales-Villa R., et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2020;396:759–769. doi: 10.1016/S0140-6736(20)31792-X. [DOI] [PubMed] [Google Scholar]
- 7.Tison G.H., Siontis K.C., Abreau S., et al. Assessment of disease status and treatment response with artificial intelligence-enhanced electrocardiography in obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2022;79:1032–1034. doi: 10.1016/j.jacc.2022.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heitner S.B., Lester S., Wang A., et al. Abstract 13962: precision pharmacological treatment for obstructive hypertrophic cardiomyopathy with mavacamten: one-year results from PIONEER-OLE. Circulation. 2019;140 [Google Scholar]
- 9.Gersh B.J., Maron B.J., Bonow R.O., et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;58:e212–e260. doi: 10.1016/j.jacc.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 10.He K. Paper presented at: 2016 IEEE Conference on Computer vision and pattern Recognition (CVPR) 2016. June 27-30, 2016. Deep residual learning for image recognition. Las Vegas, NV. [Google Scholar]
- 11.Hannun A.Y., Rajpurkar P., Haghpanahi M., et al. Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nat Med. 2019;25:65–69. doi: 10.1038/s41591-018-0268-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finocchiaro G., Sheikh N., Biagini E., et al. The electrocardiogram in the diagnosis and management of patients with hypertrophic cardiomyopathy. Heart Rhythm. 2020;17:142–151. doi: 10.1016/j.hrthm.2019.07.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.