Abstract
The ATP-binding cassette (ABC) transporters belong to a large superfamily of proteins which share a common function and a common nucleotide-binding domain. The CvaB protein from Escherichia coli is a member of the bacterial ABC exporter subfamily and is essential for the export of the peptide antibiotic colicin V. Here we report that, surprisingly, the CvaB carboxyl-terminal nucleotide-binding domain (BCTD) can be preferentially cross-linked to GTP but not to ATP at low temperatures. The cross-linking is Mg2+ and Mn2+ dependent. However, BCTD possesses similar GTPase and ATPase activities at 37°C, with the same kinetic parameters and with similar responses to inhibitors. Moreover, a point mutation (D654H) in CvaB that completely abolishes colicin V secretion severely impairs both GTPase and ATPase activities in the corresponding BCTD, indicating that the two activities are from the same enzyme. Interestingly, hydrolysis activity of ATP is much more cold sensitive than that of GTP: BCTD possesses mainly GTP hydrolysis activity at 10°C, consistent with the cross-linking results. These findings suggest a novel mechanism for an ABC protein-mediated transport with specificity for GTP hydrolysis.
The CvaB transporter protein of Escherichia coli, together with CvaA and TolC proteins, mediates export of the 88-amino-acid bacteriocin, colicin V (ColV), across the bacterial cytoplasmic and outer membranes into the surrounding medium (14, 19). ColV is taken up by the target cells and kills these sensitive cells by disrupting their membrane potential (57). The export is dependent on an N-terminal double-glycine-type signal of the toxin precursor which is cleaved concomitant with secretion (9, 16, 20). The CvaB protein has been shown to play a pivotal role in this processing event (59, 60).
Previous sequence analysis revealed that CvaB is a 698-residue cytoplasmic membrane protein and belongs to the ATP-binding cassette (ABC) superfamily of prokaryotic and eukaryotic transporters (3, 14, 15, 19, 22). Eukaryotic ABC members like cystic fibrosis transmembrane conductance regulator (CFTR) and multidrug resistance P glycoprotein contain two copies of a nucleotide-binding domain (NBD), which suggests that two binding sites may also be needed in other transporters (15, 22). For bacterial ABC importers, the NBDs are autonomous as part of transporter complexes. Like most other bacterial ABC exporters, the NBD sequence of CvaB together with an N-terminal integral membrane domain forms a single polypeptide.
The NBDs of ABC transporters retain a significant degree of homology spanning approximately 200 amino acids (3, 15, 22) and contain several common features, including a Walker A motif (a glycine-rich domain) and a Walker B motif including an aspartate for Mg2+ coordination. These two conserved sites form a nucleotide-binding pocket also known as a Rossman fold (47) or Doolittle motif (13). The binding site occurs at the end of an alpha helix; the residues GXXGXGKST form a turn, bringing the lysine residue in close proximity to the phosphates in the Mg2+-ATP. The aspartic acid residue within the B site is in close proximity in space to the A site, and its negative charge may interact with the Mg2+ molecule (55). The NBD sequence also contains a third consensus element, a linker peptide LSGGQ (or C motif) which has been suggested to act as a signal transducer between the hydrophobic domain and the NBD (3, 9, 15, 22, 31). It is widely accepted that ATPase activity contributes energy to all active ABC protein-dependent transport processes. Several ABC transporters have been shown to bind ATP analogs (21, 23), and mutations within the cassette affect ATP binding and hydrolysis of some ABC transporters (5, 7, 10, 29, 30, 40, 54). Moreover, in vitro ATP binding and hydrolysis have been demonstrated by either isolated purified component (56) or fusions with a carrier protein (31).
Although intensive research has been focused on ABC protein-mediated transports, the underlying mechanism has yet to be worked out. Large variations exist within the superfamily. In the ColV system, there is no direct evidence yet that the putative ATP-binding domain of CvaB binds or hydrolyzes ATP. Recently it has been shown that GTP is as good as or better than ATP as an energy source for in vitro processing of ColV (60). To determine whether CvaB is involved in both nucleotide hydrolysis activities, we characterized the C-terminal NBD of CvaB (BCTD). Two types of fusion constructs demonstrate that this domain can bind and be cross-linked to GTP and to ATP with different efficiencies at different temperatures. Similar differences were found for their ATPase and GTPase hydrolysis activities, indicating that BCTD is a specific GTPase at low temperatures. Moreover, the nucleotide binding and hydrolysis of a Walker B site mutation (D654H) were impaired, which corresponds to the secretion defect of ColV in cells, indicating the activity of BCTD correlates with its export function.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
The E. coli strain and plasmid used in this study, DH5αF′{F′/endA1 hsdR17 (rK− mK+) supE44 thi-1 recA1 gyrA (Nalr) relA1 Δ(lacZYA-argF)U169 deoR [φ80 Δ(lacZ)M15} and pHK11-4 (pBR322 with cvaAC and cvi, cvaB::Tn5), were laboratory stocks (18, 60). LinA (48) and TB (19) media were used as both liquid and solid (with 1.5% agar) growth media except when noted otherwise. The antibiotics ampicillin, chloramphenicol, and kanamycin were used at final concentrations of 200, 30, and 50 μg/ml, respectively.
DNA techniques and plasmid construction.
DNA manipulations were generally carried out as described by Sambrook et al. (48). Site-directed oligonucleotide mutagenesis of the cvaB gene was performed by PCR, using essentially an overlapping extension method (32). The mutagenic oligonucleotides used as primers in the PCRs were P301 (5′-ATTATTTATGCATGAGGCAACCA-3′) and P302 (5′-TGGTTGCCTCATGCATAAATAAT-3′) to convert Asp654 to His654 (Asp654 is a conserved residue of CvaB Walker B site), as well as P303 (5′-CCTCGTCGACTTAAATAGAAATAACTCTATCAAC-3′) and P304 (5′-CGCGGATCCTGTTGACCTATAAGGGAGCACCTC-3′) (italicized letters indicate restriction sites for BamHI and SalI, respectively), two outside primers, using plasmid pHK11 (18) as the template. The PCR products were cloned into pACYC184 with BamHI and SalI to yield plasmid pXZ14. The mutation was verified by DNA sequencing in an ABI model 373A DNA sequencer in the Biology Department core facility.
For glutathione S-transferase (GST) fusion constructs, primer P307 (5′-CTCGAATTCGTGGGCAGTTTTCGGAAAGAGTT-3′ (italicized letters indicate an EcoRI restriction site), primer P303, and pHK11 template generated a 0.78-kbp PCR fragment encoding the sequence of a 260-residue C-terminal domain of CvaB. This fragment was cloned into the expression vector pGEX5x-2 (Pharmacia) with EcoRI and SalI, creating plasmid pXZ7. With primer P305 (CGCGA ATTCTAAGAATAATGAGTCTGCACAATGAGCGCATT-3′; italicized letters indicate an EcoRI restriction site), primer P303, and pHK11, a PCR fragment encoding the 240-residue C-terminal domain of CvaB was generated and cloned into pGEX-5x-2 to yield plasmid pXZ8.
For the construction of His6 fusions, primer P305 primer P306 (5′-CGCGAATTCTTATTAAATAGAAATAACTCTATCAACAGT-3′), and pHK11 generated a 0.78-kbp PCR fragment which was cloned into pTrcHisB (Invitrogen) with PstI and EcoRI sites, yielding plasmid pXZ28. Using pXZ14 as a template, a PCR fragment encoding mutated BCTD (D654H) was also generated and cloned into pTrcHisB to yield plasmid pXZ29.
Overproduction and purification of GST-BCTD and His6-BCTD fusion proteins.
For the overproduction of GST-BCTD fusion protein, 1 liter of E. coli [DH5α(pXZ7), DH5α(pXZ8), and DH5α(pXZ27)] was grown at 37°C to an optical density at 600 nm of 0.5 in LinA medium supplemented with 1% glucose and ampicillin (200 μg/ml). Isopropylthiogalactopyranoside (IPTG; 1 mM) was added for 4 h to induce expression of GST-BCTD. Cells were pelleted and suspended in 50 mM Tris-HCl (pH 7.6)–500 mM NaCl buffer and then passed through a French pressure cell twice at 17,000 lb/in2. The cell lysate was centrifuged at 30,000 × g for 10 min, and the pellet containing GST-BCTD inclusion body was washed twice with 20 ml of 50 mM Tris-HCl (pH 7.6)–500 mM NaCl buffer. Then GST-BCTD inclusion body was extracted with 2 ml of 6 M urea–50 mM Tris-HCl (pH 7.6). By using the procedures described previously (27), except that glutathione was replaced with dithiothreitol (DTT), the urea-extracted GST-BCTD was renatured by dialysis in 50 mM Tris-acetate (pH 7.6)–20% glycerol–2 mM DTT. Then the GST-BCTD was affinity purified by glutathione-agarose chromatography in parallel with GST protein [in IPTG-induced DH5α(pGEX-5x-2) cells] as described previously (31).
For overproduction and purification of His6-BCTD, a 1-liter culture of E. coli DH5α(pXZ28) or DH5α(pXZ29) was grown and induced with IPTG, and the lysates were prepared as described above. The pellet after centrifugation was washed with 20 mM sodium phosphate buffer (pH 7.6) twice and extracted with 10 ml of 6 M guanidine-HCl–20 mM sodium phosphate buffer (pH 7.6)–500 mM NaCl. The extract was mixed with 2 ml of a 50% slurry of nickel metal Probond resin (Invitrogen), which had previously been equilibrated in the same buffer. After stirring for 30 min, the resin was loaded into a column. The column was washed at least 10 bed volumes with 20 mM sodium phosphate buffer (pH 7.6) containing 6 M urea and 500 mM NaCl until the A280 of the flowthrough was less than 0.03. The column was further washed with 5 bed volumes of 20 mM sodium phosphate buffer (pH 4.0) containing 6 M urea and 500 mM NaCl. The His6-BCTD was eluted with 6 ml of same buffer containing 300 mM imidazole. By using the procedure described above, the His6-BCTD was renatured in 50 mM Tris-acetate (pH 7.6)–20% glycerol–2 mM DTT.
UV cross-linking of protein to GTP or ATP.
Cross-linking was performed as described by Yue and Schimmel (58). Samples (20 μl) containing 1 to 2 μg of protein and 1 μM α- or γ-32P-labeled nucleoside triphosphate (NTP; 30 Ci mmol−1; NEN) in buffer (20 mM Tris-acetate [pH 8.0], 5% glycerol, 5 mM MgCl2, 0.5 mM DTT) were irradiated on ice for 30 min or at other temperatures for 20 min in a UV cross-linker (Stratagene Stratalinker 2400; 254-nm-wavelength bulbs). Proteins were precipitated with 10% trichloroacetic acid. The pellets were washed with acetone, and photoadducts were analyzed by sodium dodecyl sulfate (SDS)–10% polyacrylamide gel electrophoresis and autoradiography.
GTPase and ATPase activity measurements.
For GTPase and ATPase assays, the complete reaction mixture (20 μl) contained 5 mM magnesium chloride, 100 mM potassium acetate, 40 mM Tris-acetate (pH 7.5), 0.1 mg of ovalbumin per ml, 1 mM DTT, and 0.5 μg of His6-BCTD. [γ-32P]GTP or [γ-32P]ATP (1 μCi; Amersham) was added at various concentrations. The assay was carried out as described previously (37). Briefly, after a 10-min incubation at the temperatures indicated, the reaction mixtures were mixed with 800 μl of a 5% suspension of activated charcoal (Sigma) in 20 mM phosphoric acid, incubated on ice for 10 min, and then centrifuged for 10 min to pellet the charcoal and bound nucleotide. A 200-μl aliquot of supernatant fraction containing the liberated radioactive phosphate was analyzed by a scintillation counter and corrected for background counts from reactions performed with buffer only. The rate of nucleotide hydrolysis at 5 μM is linear up to 20 min at 37°C.
ColV assay.
A plate assay as previously described (16, 24) was used to quantitate the levels of active ColV secreted from the wild-type or mutant CvaB. Briefly, a 0.5-ml suspension of ColV-sensitive cells (E. coli 71-18) was mixed with 3 ml of H-top agar (38) and spread on an agar plate of TB medium (10 g of tryptone and 8 g of NaCl/liter). A single colony of the strain to be assayed was picked onto a lawn of sensitive cells. After overnight growth at 37°C, a circular halo of growth inhibition, resulting from the radial diffusion of active ColV, formed around the ColV-producing cells.
Chemicals and reagents.
All chemicals and reagents were of reagent grade and obtained from Sigma unless otherwise specified.
RESULTS
Overproduction and purification of GST-BCTD fusion proteins.
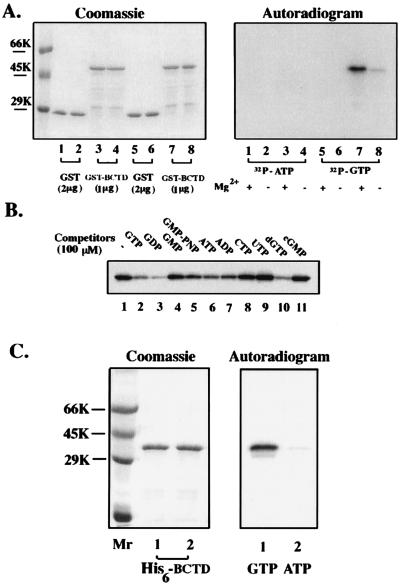
Like other ABC transporters, the CvaB protein has been very difficult to characterize biochemically because of its low abundance and extreme hydrophobicity (14, 19). To test whether BCTD is indeed an ATPase, we fused the coding sequence of NBD in frame to the Schistosoma japonicum GST gene in the expression vector pGEX-5x-2, creating plasmid pXZ7. The resulting fusion protein (GST-BCTD) has a deduced molecular mass of 57.6 kDa comprising the 218-amino-acid GST, a 12-residue linker (SDLIEGRGIPGI), and the 260 C-terminal amino acids of CvaB. E. coli DH5α(pXZ7) cells with IPTG induction generated abundant inclusion body of GST-BCTD. (Even when growth conditions were varied or a new fusion construct with 240 residues [pXZ8] was used, little soluble GST-BCTD could be obtained.) The GST-BCTD was extracted from the inclusion body with 6 M urea, and the urea-extracted GST-BCTD was renatured in solution such that it could be purified by glutathione-agarose affinity chromatography (Fig. 1A, left, lanes 3, 4, 7, and 8), in parallel with GST (lanes 1, 2, 5, and 6). The low-molecular-weight protein bands below GST-BCTD were probably degradation products, since they reacted with CvaB antibodies (data not shown).
FIG. 1.
BCTD can be cross-linked to [α-32P]GTP in much higher affinity than to [α-32P]ATP. (A) Left, Coomassie blue-stained SDS-gel showing protein profiles used in the cross-linking. The molecular size markers are bovine serum albumin (66 kDa [66K]), ovalbumin (45 kDa), and carbonic anhydrase (29 kDa). Right, autoradiogram of UV cross-linking with [α-32P]ATP and [α-32P]GTP at 0°C. (B) Competitive binding of GTP to GST-BCTD. GST-BCTD was irradiated with UV (254 nm) in the presence of [α-32P]GTP and Mg2+. The unlabeled nucleotides as indicated (100 μM) were added to the complete reaction mixtures before cross-linking. (C) Left, Coomassie blue-stained gel of His6-BCTD; right, autoradiogram of UV cross-linking with [α-32P]GTP (lane 1) and [α-32P]ATP (lane 2).
GST-BCTD fusion protein can be cross-linked to GTP in much higher affinity than to ATP.
To determine whether GST-BCTD can specifically bind nucleotides, cross-linking with [α-32P]ATP or [α-32P]GTP by UV irradiation was carried out. Surprisingly, SDS-gel electrophoresis revealed a band labeled with [α-32P]GTP whose position corresponded to that of GST-BCTD (Fig. 1A, right, lanes 7 and 8), but little photoadduct was detected for the [α-32P]ATP reaction (lanes 3 and 4). Similar findings were observed with [γ-32P]ATP or -GTP, and no phosphorylation was observed (data not shown). Cross-linking with both nucleotides was equally efficient since a 70-kDa band (probably DnaK) in the crude protein sample could also be labeled with both nucleotides (data not shown). GST protein itself did not cross-link with either nucleotide (lanes 1, 2, 5, and 6), indicating that the GTP cross-linking of GST-BCTD is specifically due to NBD of CvaB. Moreover, the GTP cross-linking of GST-BCTD was greatly enhanced with Mg2+ (compare lane 7 to lane 8). The Mg2+-dependent GTP binding is distinct from that for other GTP-binding proteins (11, 44).
To determine the specificity of GTP-cross-linking, 100-fold molar excesses of various nucleotides were added as competitors during UV irradiation. As shown in Fig. 1B, GDP, GTP, and dGTP had the strongest competition effect, while GMP-PCP competed less well. Interestingly, ATP and ADP could also compete though to lesser extents, whereas GMP, CTP, UTP, and cGMP competed poorly. The dGTP competition is consistent with results for other GTP-binding proteins, such as Ha-Ras p21 (36) or YPT1 (51), but differs from results for FtsZ (11, 44). On the other hand, the ATP competition is quite unique for the GTP cross-linking of GST-BCTD in comparison to other G proteins.
His6-BCTD also preferentially cross-links to GTP.
Since it is generally believed that ABC transporters are ATP-binding proteins (12, 28, 31, 34, 46, 53, 56), we considered the possibility that the GST fusion had altered the conformation of the NBD such that it changed its binding specificity from ATP to GTP. Therefore, we made another, much smaller gene fusion without an additional defined functional domain, i.e., 260-residue BCTD with an N-terminal six-histidine tag. His6-BCTD could be affinity purified through an Ni2+-agarose column (Fig. 1C, left, lane 1). The purified His6-BCTD fusion protein also cross-linked to GTP to a much greater extent than to ATP (Fig. 1C, right). The lower band, which can also be cross-linked with nucleotides, is probably a minor degradation product of His6-BCTD since it also reacted with CvaB antibody (data not shown).
GTPase and ATPase activities of His6-BCTD.
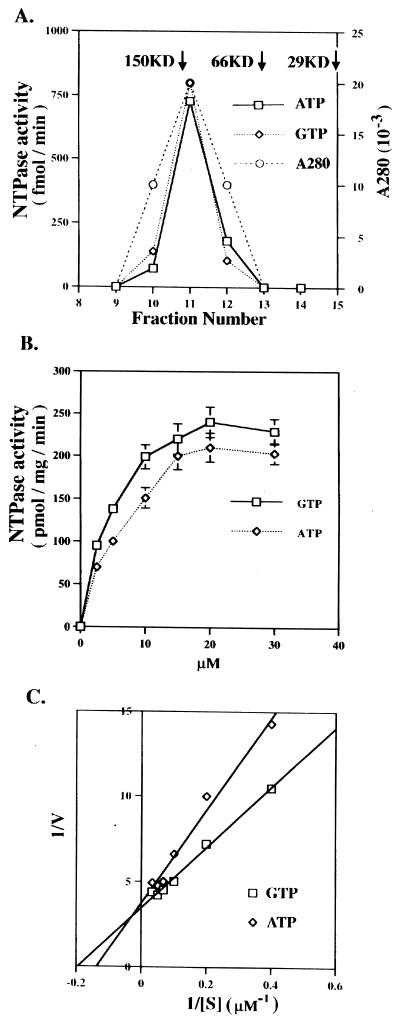
The ability of BCTD to cross-link GTP specifically but not ATP is quite surprising, given that ATP has always been assumed to be the reacting nucleotide. To determine whether BCTD can hydrolyze GTP or ATP, we measured GTPase and ATPase activities of His6-BCTD. The purified His6-BCTD preparation was further fractionated over a calibrated FPLC Superdex-200 column. His6-BCTD corresponded to one protein peak which was consistent with the position of a tetramer of His6-BCTD (Fig. 2A), similar to those reported for the NBD of P glycoprotein (53). The GTPase activity was indeed copurified with His6-BCTD. However, in contrast to its cross-linking activity, the ATP hydrolysis activity of His6-BCTD was almost as high as that for GTP at 37°C (Fig. 2A and B).
FIG. 2.
GTPase and ATPase activities of His6-BCTD at 37°C. (A) Copurification of His6-BCTD with nucleotide hydrolysis activities. The His6-BCTD preparation was fractionated on a calibrated FPLC Superdex-200 gel filtration column (Pharmacia; 0.5 ml min−1) in 50 mM Tris-HCl (pH 7.6)–20 mM KCl–20 mM NH4Cl–1 mM DTT, and fractions of 40 μl were assayed for activity. Size standards included carbonic anhydrase (29 kDa [29KD]), bovine serum albumin (66 kDa), and alcohol dehydrogenase (150 kDa). (B) Substrate concentration titration of GTP and ATP hydrolysis of His6-BCTD at 37°C. (C) Lineweaver-Burk plot of the GTPase and ATPase activities.
Kinetic parameters of the GTPase and ATPase activities of His6-BCTD.
The kinetic parameters of the reactions were estimated from Lineweaver-Burk plots (Fig. 2B and C). The Vmax values for GTPase and ATPase are approximately 0.29 and 0.27 nmol of nucleotide hydrolyzed/min/mg of protein, respectively, and the estimated Kms are 5.0 and 7.0 μM, respectively.
The His6-BCTD-associated GTPase and ATPase activities were strongly dependent on the presence of Mg2+ ions in the assay buffer; little activity was detected in its absence (Table 1). When Mg2+ was replaced by Mn2+, the activities were even higher. Co2+ could partially substitute for Mg2+. In contrast, both Ca2+ and Zn2+ failed to support GTP or ATP hydrolysis.
TABLE 1.
Effects of divalent cations on His6-BCTD GTPase and ATPase activitiesa
| Cation | % Activity (mean ± SE; n = 6)
|
|
|---|---|---|
| GTPase | ATPase | |
| None | 0 | 0 |
| Mg2+ | 100 | 100 |
| Mn2+ | 165 ± 14.5 | 125 ± 9.8 |
| Ca2+ | 0 | 0 |
| Zn2+ | 0 | 0 |
| Co2+ | 15 ± 1.8 | 20 ± 2.8 |
GTPase and ATPase activities were measured with a nucleotide concentration of 10 μM as described in Materials and Methods; cations (5 mM) in their chloride form were used. Activities are standardized to that of the reaction mixture containing Mg2+.
The His6-BCTD-associated GTPase and ATPase activities were further characterized with respect to sensitivity toward typical inhibitors of F-, P-, and V-type ATPases (41). The results are summarized in Table 2. None of these inhibitors has significant effect on either GTP or ATP hydrolysis. However, the sulfhydryl reagent N-ethylmaleimide (NEM) strongly inhibited both activities (Table 2).
TABLE 2.
Effects of ATPase inhibitors on His6-BCTD GTPase and ATPase activitiesa
| Compound | % Activity (mean ± SE; n = 6)
|
||
|---|---|---|---|
| GTPase | ATPase | Inhibitor forb: | |
| None | 100 | 100 | |
| Vanadate (100 μM) | 74 ± 8 | 90 ± 11 | P |
| NaN3 (50 mM) | 80 ± 7.6 | 90 ± 5.8 | F |
| KNO3 (2 mM) | 76 ± 12.3 | 84 ± 10.9 | V |
| NEM (1 mM) | 10 ± 2 | 18 ± 3.5 | |
Reaction mixtures contained the same protein and nucleotides as those for Table 1. His6-BCTD was preincubated with the compounds for 10 min on ice before addition of nucleotides to initiate the reaction at 37°C.
P, phosphorylation-type ATPase; F, F0F1-type ATPase; V, vacuolar-type ATPase.
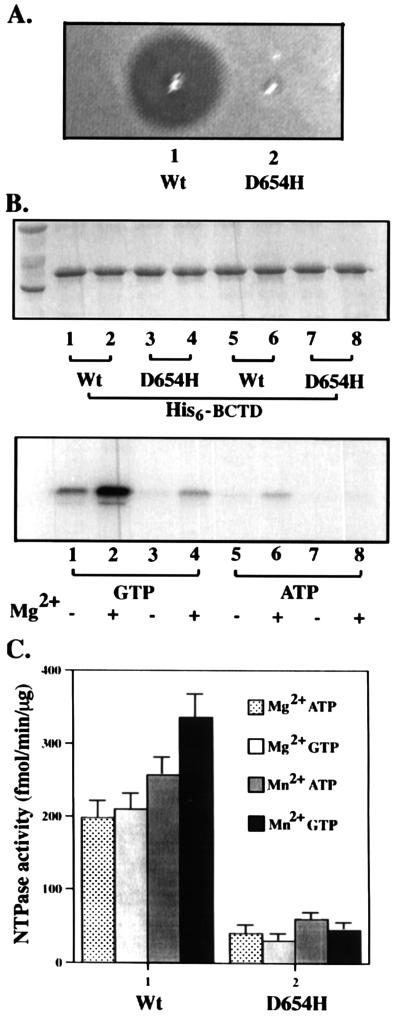
Walker B mutation (D654H) impairs both nucleotide binding and hydrolysis of BCTD.
To further ascertain that both GTPase and ATPase activities are from BCTD and to test whether such nucleotide binding and hydrolysis of BCTD are relevant to CvaB transport function, we introduced one mutation into the nucleotide-binding motif (D654H, a Walker B mutation). As a result, this mutation completely abolished ColV secretion (Fig. 3A) and the CvaB protein with this mutation was stable within the cells (data not shown), indicating that this conserved residue in the nucleotide-binding motif is important for CvaB function. We also constructed the corresponding D654H mutation in BCTD, purified the His6-BCTD, and tested its nucleotide-binding and hydrolysis activities. In comparison to the wild type, nucleotide cross-linking and both GTPase and ATPase activities of His6-BCTD (D654H) were severely impaired (Fig. 3B and C), indicating that the same mutation affects both enzymatic activities. These results also rule out the possibility that either of the nucleotide hydrolysis activities is due to the contamination with some other E. coli NTPase.
FIG. 3.
Effects of point mutation D654H. (A) Effect on ColV secretion. Wild-type (Wt) cvaB strain DH5α(pHK11-4, pXZ11) and D654H mutant DH5α(pHK11-4, pXZ15) were spotted for the ColV assay. (B) Effect on the GTP binding of His6-BCTD. His6-BCTD (wild type and D654H) was subjected to UV cross-linking with [α-32P]ATP and [α-32P]GTP. (C) Effects on GTPase and ATPase activities of His6-BCTD. His6-BCTD (wild type and D654H) was assayed for nucleotide hydrolysis activities in the presence of Mg2+ or Mn2+.
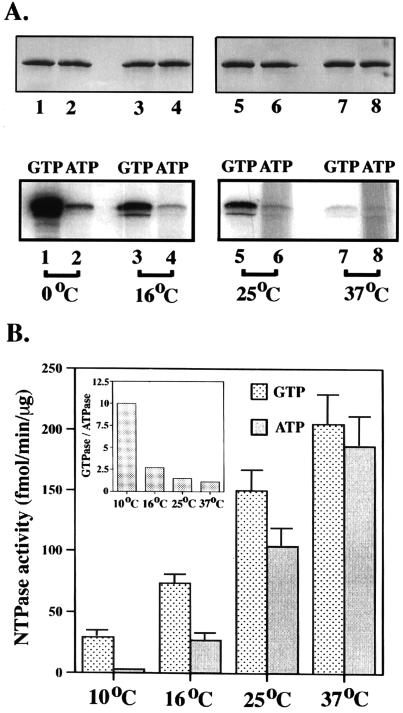
GTP is the best substrate for His6-BCTD at low temperatures.
Ironically, cross-linking data showed that BCTD cross-linked to GTP with much higher affinity than to ATP, whereas enzymatic data indicated that GTPase and ATPase activities were similar. We reasoned that the difference may be due to the temperature effect, since cross-linking was done on ice while enzymatic activities were assayed at 37°C. Therefore, to determine whether the substrate specificity of His6-BCTD is temperature dependent, we carried out the hydrolysis and UV cross-linking at different temperatures. As shown in Fig. 4A, the efficiency of cross-linking to both nucleotides decreased with increasing temperatures under the conditions used. Indeed, the high cross-linking affinity of His6-BCTD for GTP at 0°C decreased much more drastically than that for ATP with increasing temperatures to such an extent that levels of cross-linking of GTP and ATP were similar at 37°C. On the other hand, as shown in Fig. 4B, at 37°C ATP and GTP are similar with respect to nucleotide hydrolysis efficiency. As the assayed temperatures were lowered, the activities, especially that of ATPase, decreased. Remarkably, it appeared that the ATPase activity of BCTD was much more cold sensitive; GTPase activity was about 10 times higher than ATPase activity at 10°C (Fig. 4B, insert), consistent with the previous cross-linking results (Fig. 1C). The Kms of GTPase and ATPase at 10°C are similar, but the Vmax values of GTPase and ATPase at 10°C are around 0.12 and 0.01 nmol of nucleotide hydrolyzed/min/mg, respectively, corresponding approximately to the difference in activities.
FIG. 4.
Temperature effects of nucleotide cross-linking and hydrolysis. (A) The upper panel is the Coomassie blue-stained gel of protein samples used for the lower panel, which is the autoradiogram of [α-32P]ATP and [α-32P]GTP cross-linkings at different temperatures. (B) Nucleotide hydrolysis activities at different temperatures. Data are means ± standard errors (bars) (n = 4). The GTPase/ATPase ratio at each temperature is shown in the insert.
DISCUSSION
In this study, the C-terminal NBD of CvaB is characterized in a GST-BCTD fusion and a His6-BCTD version, from which large amounts of purified proteins can be readily obtained. We have shown that, surprisingly, BCTD preferably binds (as manifested by cross-linking) and hydrolyzes GTP at low temperatures, whereas it hydrolyzes ATP almost as efficiently as GTP at 37°C. Moreover, a Walker B mutant (D654H) of CvaB completely abolishes ColV secretion. The nucleotide-binding and hydrolysis activities of the corresponding His6-BCTD (D654H) fusion are impaired. These results indicate that the GTP-binding and hydrolysis activities of BCTD are correlated with secretion activity and that CvaB may be a specific GTPase at low temperatures. These findings are unexpected, since it is generally believed that ATP is the preferred nucleotide substrate for the ABC transporter to function.
The GTPase and ATPase activities of CvaBCTD are in the range of those for the NBDs of P glycoprotein (53) and purified CFTR protein (34) and close to the intrinsic rate reported for the low-molecular-weight GTPases such as Ras (17). On the other hand, the activities are lower than those for other NTPases (6, 31, 33, 49). It is possible that the N-terminal transmembrane domains of CvaB are required for full activity of nucleotide hydrolysis when CvaB in the membrane is associated with other accessory proteins or in the presence of ColV precursor. SecA ATPase is known to be enhanced greatly in the presence of membrane lipid and precursor (35). Moreover, the enzymatic activity may be enhanced by other protein factors; some nucleotide-binding protein can bind nucleotides but fail to hydrolyze them in the absence of an activating factor (42, 52).
The ATPase activities of several ABC transporters, e.g., P glycoprotein, HlyB, PrtD, and MalK, are resistant to many specific inhibitors of F-, P-, and V-type ATPases but sensitive to vanadate (1, 12, 31). The enzymatic activity of mammalian CFTR transporter is resistant to vanadate but inhibited by sodium azide (4, 34). BCTD is resistant to all of these inhibitors. The difference in sensitivity to the inhibitors reflects the variations within the superfamily. BCTD contains four cysteine residues (Cys-540, Cys-571, Cys-588, and Cys-602) as putative targets for modification by NEM, which strongly inhibits the enzymatic activity. P glycoprotein and MalK both have a cysteine residue in Walker A motif, and their ATPase activities are also sensitive to NEM (1, 39). However, the ATPase activities of HlyB and PrtD (although they all contain cysteine residues) are not inhibited by 1 mM NEM (12, 31). The GTPase of CvaBCTD has exactly the same pattern for the inhibitors as its ATPase, which is consistent with the notion that the two activities come from the same enzyme. It is worth noting that processing of ColV-1 in vitro utilizes GTP and can be completely inhibited by NEM (60). Blocking the GTPase and ATPase activities of CvaB may be one explanation for the inhibition of processing of the precursors. The divalent cation specificity of CvaBCTD shows significant differences from those of most F-type ATPases, which hydrolyze ATP in the presence of any one of the divalent cations tested in this study (26). On the other hand, the divalent results are quite similar to the results for HlyB ATPase (31). In all, the CvaB ATPase and GTPase activities appear to be quite unique among the ABC transporters but to share various characteristics with a number of other ATPases.
The finding that BCTD is a specific GTPase is quite unexpected. However, it has been shown that ATP- and GTP-binding proteins share some common nucleotide-binding motifs (50). The phosphate-binding loop (G1 motif) of many GTP-binding proteins is similar to the Walker A motif (50). Certain GTP-binding proteins such as tubulin, FtsZ, and FstY, which deviate significantly from certain consensus motifs of canonical GTPases (8), have additional Mg2+-binding motifs similar to the nucleotide-binding motifs of ATP-binding protein (45). Substrate specificity of these nucleotide protein is probably quite subtle. Even though it has a potential GTP-binding domain, the Hrs-2 protein is in fact an ATPase (6). A point mutation in the P loop converts E. coli FtsZ GTPase to an ATPase (45). Moreover, ATP is the best substrate for several ABC transporters, but GTP as well as some other nucleotides can also be utilized effectively (2, 4, 31, 39); some ABC transporters can bind GTP as well as ATP (28), indicating that this type of ATPase displays rather broad substrate specificity. Indeed, it has been reported that the NBD2 of CFTR is a G protein that binds GTP as well as ATP (43). Thus, it is possible that as the nucleotide-binding loops of GTPase and ATPase differ slightly, the NBD of an ABC transporter may become more restricted to ATP at low temperatures, as is the case of CvaB here.
Under physiological conditions, the intracellular concentration of ATP is higher than that of GTP, which suggests that at 37°C, BCTD functions as an ATPase; at low temperatures, however, it likely acts as a GTPase. The feature of this soluble domain presumably is representative of the whole CvaB transporter, in conjunction with accessory protein CvaA and TolC, in the membrane. If this is true, the result also suggests that the physiological effect of nucleotide hydrolysis for CvaB-mediated transport at low temperatures may be different from that at 37°C. It is worth noting that GTP may directly provide energy to the ABC transporter. It is not clear whether this reflects a common feature for other ABC transporters, including multidrug-resistant P glycoprotein and CFTR. On the other hand, it is also possible that CvaB represents a new subset of ABC transporters, as significant variations in the NBD are observed among the ABC transporters (3, 15, 22). In this context, CvaB may be a unique GTPase that can also function as an ATPase at 37°C but not at low temperatures. Although the physiological consequences are not yet clear, it is tempting to speculate that the GTPase may be related to the regulatory functions in the ColV secretory complex, in a manner similar to the roles of GTP hydrolysis on G proteins.
ACKNOWLEDGMENTS
We thank R. Kolter for strains and plasmids, J. Houghton for critical review of the manuscript, and P. Kaur for helpful discussion. We also acknowledge the numerous discussion of the laboratory members.
This work was supported in part by a grant from National Institutes of Health and by equipment grants from Georgia Research Alliance.
REFERENCES
- 1.Al-Shawi M K, Senior A E. Characterization of the adenosine triphosphatase activity of Chinese hamster P-glycoprotein. J Biol Chem. 1993;268:4197–4206. [PubMed] [Google Scholar]
- 2.Ames G F-L, Nikaido K, Groarke J, Petithory J. Reconstitution of periplasmic transport in inside-out membrane vesicles. J Biol Chem. 1989;264:3998–4002. [PubMed] [Google Scholar]
- 3.Ames G F-L, Mimura C S, Holbrook S R, Shyamala V. Traffic ATPases: a superfamily of transport proteins operating from Escherichia coli to humans. Adv Enzymol. 1992;65:1–47. doi: 10.1002/9780470123119.ch1. [DOI] [PubMed] [Google Scholar]
- 4.Anderson M P, Berger H A, Rich D P, Gregory R J, Smith A E, Welsh M J. Nucleoside triphosphates are required to open the CFTR chloride channel. Cell. 1991;67:775–784. doi: 10.1016/0092-8674(91)90072-7. [DOI] [PubMed] [Google Scholar]
- 5.Anderson M P, Welsh M J. Regulation by ATP and ADP of CFTR chloride channels that contain mutant nucleotide-binding domains. Science. 1992;257:1701–1704. doi: 10.1126/science.1382316. [DOI] [PubMed] [Google Scholar]
- 6.Bean A J, Selfert R, Chen Y A, Sack R, Scheller R H. Hrs-2 is an ATPase implicated in calcium-regulated secretion. Nature. 1997;385:826–829. doi: 10.1038/385826a0. [DOI] [PubMed] [Google Scholar]
- 7.Berkower C, Michaelis S. Mutational analysis of the yeast a-factor transporter STE6, a member of the ATP binding cassette (ABC) protein superfamily. EMBO J. 1991;10:3777–3785. doi: 10.1002/j.1460-2075.1991.tb04947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bourne H R, Sander A A, McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 1991;349:117–126. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- 9.Browne B L, McClendon V, Bedwell D M. Mutations within the first LSGGQ motif of Ste6p cause defects in a-factor transport and mating in Saccharomyces cerevisiae. J Bacteriol. 1996;178:1712–1719. doi: 10.1128/jb.178.6.1712-1719.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng S H, Gregory R J, Marshall J, Paul S, Souza D W, White G A, O’Riordan C R, Smith A E. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell. 1990;63:827–834. doi: 10.1016/0092-8674(90)90148-8. [DOI] [PubMed] [Google Scholar]
- 11.de Boer P, Crossley R, Rothfield L. The essential bacteria cell-division protein FtsZ is a GTPase. Nature. 1992;359:254–256. doi: 10.1038/359254a0. [DOI] [PubMed] [Google Scholar]
- 12.Delepelaire P. PrtD, the integral membrane ATP-binding cassette component of the Erwinia chrysanthemi metalloprotease secretion system, exhibits a secreton signal-regulated ATPase activity. J Biol Chem. 1994;269:27952–27957. [PubMed] [Google Scholar]
- 13.Doolittle R F, Johnson M S, Husain I, Houten B V, Thomas D C, Sancar A. Domainal evolution of a prokaryotic DNA repair protein and its relationship to active-transport proteins. Nature. 1986;323:451–453. doi: 10.1038/323451a0. [DOI] [PubMed] [Google Scholar]
- 14.Fath M J, Skvirsky R, Gilson L, Mahanty H K, Kolter R. The secretion of colicin V. In: James R, Lazdunski C, Pattus F, editors. Bacteriocins, microcins, and lantibiotics. NATO ASI series. Heidelberg, Germany: Springer-Verlag; 1991. pp. 331–348. [Google Scholar]
- 15.Fath M J, Kolter R. ABC transporters: bacterial exporters. Microbiol Rev. 1993;57:995–1017. doi: 10.1128/mr.57.4.995-1017.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fath M J, Zhang L H, Rush J, Kolter R. Purification and characterization of colicin V from Escherichia coli culture supernatants. Biochemistry. 1994;33:6911–6917. doi: 10.1021/bi00188a021. [DOI] [PubMed] [Google Scholar]
- 17.Gideon P, John J, Frech M, Lautwein A, Clark R, Scheffler J E, Wittinghofer A. Mutational and kinetic analyses of the GTPase-activating protein (GAP)-p21 interaction: the C-terminal domain of GAP is not sufficient for full activity. Mol Cell Biol. 1992;12:2050–2056. doi: 10.1128/mcb.12.5.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilson L, Mahanty H K, Kolter R. Four plasmid genes are required for colicin V synthesis, export, and immunity. J Bacteriol. 1987;169:2466–2470. doi: 10.1128/jb.169.6.2466-2470.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilson L, Mahanty H K, Kolter R. Genetics analysis of an MDR-like export system: the secretion of colicin V. EMBO J. 1990;7:3997–4004. doi: 10.1002/j.1460-2075.1990.tb07606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Havarstein L S, Holo H, Nef I F. The leader peptide of colicin V shares consensus sequences with leader peptides that are common among peptide bacteriocins produced by Gram-positive bacteria. Microbiology. 1994;140:2383–2389. doi: 10.1099/13500872-140-9-2383. [DOI] [PubMed] [Google Scholar]
- 21.Higgins C F, Hiles I D, Salmond G P C, Gill D R, Evans J A D I J, Holland I B, Garay L, Buckel S D, Bell A W, Hermodson M A. A family of related ATP-binding subunits coupled to many distinct biological processes in bacteria. Nature. 1986;323:448–450. doi: 10.1038/323448a0. [DOI] [PubMed] [Google Scholar]
- 22.Higgins C F. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 23.Hobson A C, Weatherwax R, Ames G F L. ATP-binding sites in the membrane components of histidine permease, a periplasmic transport system. Proc Natl Acad Sci USA. 1984;81:7333–7337. doi: 10.1073/pnas.81.23.7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hwang J, Manuvakhova M, Tai P C. Characterization of in-frame proteins encoded by cvaA, an essential gene in the colicin V secretion system: CvaA* stabilizes CvaA to enhance secretion. J Bacteriol. 1997;179:689–696. doi: 10.1128/jb.179.3.689-696.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hwang J, Zhong X, Tai P C. Interactions of dedicated export membrane proteins: CvaA, a member of the membrane fusion protein family, interacts with CvaB and TolC to form colicin V secretion complex. J Bacteriol. 1997;179:6264–6270. doi: 10.1128/jb.179.20.6264-6270.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kagawa Y, Sone N, Hirata H, Yoshida M. Structure and function of H+-ATPase. J Bioenerg Biomembr. 1979;11:39–78. doi: 10.1007/BF00743196. [DOI] [PubMed] [Google Scholar]
- 27.Kaur P, Rosen B P. In vitro assembly of an anion-stimulated ATPase from peptide fragments. J Biol Chem. 1994;269:9698–9704. [PubMed] [Google Scholar]
- 28.Kaur P. Expression and characterization of DrrA and DrrB proteins of Streptomyces peucetius in Escherichia coli: DrrA is an ATP binding protein. J Bacteriol. 1997;179:569–575. doi: 10.1128/jb.179.3.569-575.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koronakis E, Huges C, Milisav I, Koronakis V. Protein exporter function and in vitro ATPase activity are correlated in ABC-domain mutants of HlyB. Mol Microbiol. 1995;16:87–96. doi: 10.1111/j.1365-2958.1995.tb02394.x. [DOI] [PubMed] [Google Scholar]
- 30.Koronakis V, Koronakis E, Hughes C. Comparison of the haemolysin secretion protein HlyB from Proteus vulgaris and Escherichia coli: site-directed mutagenesis causing impairment of export function. Mol Gen Genet. 1988;213:551–555. doi: 10.1007/BF00339631. [DOI] [PubMed] [Google Scholar]
- 31.Koronakis V, Hughes C, Koronakis E. ATPase activity and ATP/ADP-induced conformational change in the soluble domain of the bacterial protein translocator HlyB. Mol Microbiol. 1993;8:1163–1175. doi: 10.1111/j.1365-2958.1993.tb01661.x. [DOI] [PubMed] [Google Scholar]
- 32.Kunkel T A, Roberts J D, Zkour R A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- 33.Kusters R, Lentzen G, Eppens E, van Geel A, van der Weijden C C, Wintermeyer W, Luirink J. The functioning of the SRP receptor FtsY in protein-targeting in E. coli is correlated with its ability to bind and hydrolyse GTP. FEBS Lett. 1995;372:253–258. doi: 10.1016/0014-5793(95)00997-n. [DOI] [PubMed] [Google Scholar]
- 34.Li C, Ramjeesingh M, Wang W, Garami E, Hewryk M, Lee D, Rommens J M, Galley K, Bear C E. ATPase activity of the cystic fibrosis transmembrane conductance regulator. J Biol Chem. 1996;271:28463–28468. doi: 10.1074/jbc.271.45.28463. [DOI] [PubMed] [Google Scholar]
- 35.Lill R, Dowhan W, Wickner W. The ATPase activity of SecA is regulated by acidic phospholipids, SecY, and the leader and mature domains of precursor proteins. Cell. 1990;60:271–280. doi: 10.1016/0092-8674(90)90742-w. [DOI] [PubMed] [Google Scholar]
- 36.McGrath J P, Capon D J, Goeddel D V, Levinson A D. Comparative biochemical properties of normal and activated human ras p21 protein. Nature. 1984;310:644–649. doi: 10.1038/310644a0. [DOI] [PubMed] [Google Scholar]
- 37.Miller J D, Gernstein H D, Walter P. Interaction of E. coli Ffh/4.5S ribonucleoprotein and FtsY mimics that of mammalian signal recognition particle and its receptor. Nature. 1994;367:657–659. doi: 10.1038/367657a0. [DOI] [PubMed] [Google Scholar]
- 38.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 39.Morbach S, Tebbe S, Schneider E. The ATP-binding cassette (ABC) transporter for maltose/maltodextrins of Salmonella typhimurium. J Biol Chem. 1993;268:18617–18621. [PubMed] [Google Scholar]
- 40.Panagiotidis C H, Reyes M, Sievertsen A, Boos W, Shuman H A. Characterization of the structural requirements for assembly and nucleotide-binding of an ATP-binding cassette transporter. J Biol Chem. 1993;268:23685–23696. [PubMed] [Google Scholar]
- 41.Pederson P L, Carafoli E. Ion motive ATPase. I. Ubiquity, properties, and significance to cell function. Trends Biochem Sci. 1987;12:146–150. [Google Scholar]
- 42.Powers T, Walter P. Reciprocal stimulation of GTP hydrolysis by two directly interacting GTPase. Science. 1995;269:1422–1424. doi: 10.1126/science.7660124. [DOI] [PubMed] [Google Scholar]
- 43.Randak C, Neth P, Auerswald E A, Assfalg-Machleidt I, Roscher A A, Hadorn H B, Machleidt W. A recombinant polypeptide model of the second predicted nucleotide binding fold of the cystic fibrosis transmembrane conductance regulator is a GTP-bind protein. FEBS Lett. 1996;398:97–100. doi: 10.1016/s0014-5793(96)01217-3. [DOI] [PubMed] [Google Scholar]
- 44.RayChaudhuri D, Park J T. Escherichia coli cell-division gene ftsZ encodes a novel GTP-binding protein. Nature. 1992;359:251–254. doi: 10.1038/359251a0. [DOI] [PubMed] [Google Scholar]
- 45.RayChaudhuri D, Park J T. A point mutation converts Escherichia coli FtsZ septation GTPase to an ATPase. J Biol Chem. 1994;269:22941–22944. [PubMed] [Google Scholar]
- 46.Rosen B P, Weigel U, Karkaria C, Gangola P. Molecular characterization of an anion pump. J Biol Chem. 1988;263:3067–3070. [PubMed] [Google Scholar]
- 47.Rossman M G, Liljas A, Branden C I, Babaszak L J. Evolutionary and structural relationships among dehydrogenases. In: Boyer P D, editor. The proteins. New York, N.Y: Academic Press, Inc.; 1975. pp. 62–103. [Google Scholar]
- 48.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 49.Samuelsson T, Olsson M, Wikstom P M, Johansson B R. The GTPase activity of the Escherichia coli Ffh protein is important for normal growth. Biochim Biophys Acta. 1995;1267:83–91. doi: 10.1016/0167-4889(95)00034-p. [DOI] [PubMed] [Google Scholar]
- 50.Saraste M, Sibbald P R, Wittinghofer A. The P-loop—a common motif in ATP- and GTP-binding proteins. Trends Biochem Sci. 1990;15:430–434. doi: 10.1016/0968-0004(90)90281-f. [DOI] [PubMed] [Google Scholar]
- 51.Schmitt H D, Wagner P, Pfaff E, Gallwitz D. The ras-related YPT1 gene product in yeast: a GTP-binding protein that might be involved in microtubule organization. Cell. 1986;47:401–412. doi: 10.1016/0092-8674(86)90597-0. [DOI] [PubMed] [Google Scholar]
- 52.Schurmann A, Brauers A, Mabmann S, Becker W, Joost H-G. Cloning of a novel family of mammalian GTP-binding protein (RagA, RagBs, RagB1) with remote similarity to the Ras-related GTPases. J Biol Chem. 1996;270:28982–28988. doi: 10.1074/jbc.270.48.28982. [DOI] [PubMed] [Google Scholar]
- 53.Sharma S, Rose D R. Cloning, overexpression, purification, and characterization of the carboxyl-terminal nucleotide binding domain of P-glycoprotein. J Biol Chem. 1995;270:14085–14093. doi: 10.1074/jbc.270.23.14085. [DOI] [PubMed] [Google Scholar]
- 54.Shyamala V, Baichwal V, Beall E, Ames G F-L. Structure-function analysis of the histidine permease and comparison with cystic fibrosis mutations. J Biol Chem. 1991;266:18714–18719. [PubMed] [Google Scholar]
- 55.Walker J E, Sarste M, Runswick M J, Gay N J. Distantly related sequences in the α and β-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide-binding fold. EMBO J. 1982;1:945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walter C, Honer zu Bentrup K, Schneider E. Large scale purification, nucleotide binding properties, and ATPase activity of the MalK subunit of Salmonella typhimurium maltose transport complex. J Biol Chem. 1992;267:8863–8869. [PubMed] [Google Scholar]
- 57.Yang C C, Konisky J. Colicin V-treated Escherichia coli does not generate membrane potential. J Bacteriol. 1984;158:757–759. doi: 10.1128/jb.158.2.757-759.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yue V T, Schimmel P R. Direct and specific photochemical cross-linking of adenosine 5′-triphosphate to an aminoacyl-tRNA synthetase. Biochemistry. 1977;16:4678–4684. doi: 10.1021/bi00640a023. [DOI] [PubMed] [Google Scholar]
- 59.Zhang L H, Fath M J, Mahanty H K, Tai P C, Kolter R. Genetics analysis of the colicin V secretion pathway. Genetics. 1995;141:25–32. doi: 10.1093/genetics/141.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhong X, Kolter R, Tai P C. Processing of colicin V-1, a secretable marker protein of a bacterial ATP binding cassette export system, requires membrane integrity, energy, and cytosolic factors. J Biol Chem. 1996;271:28057–28063. doi: 10.1074/jbc.271.45.28057. [DOI] [PubMed] [Google Scholar]