Abstract
There are increasing concerns over the threat of nanoplastics to environmental and human health. However, multidisciplinary barriers persist between the communities assessing the risks to environmental and human health. As a result, the hazards and risks of nanoplastics remain uncertain. Here, we identify key knowledge gaps by evaluating the exposure of nanoplastics in the environment, assessing their bio-nano interactions, and examining their potential risks to humans and the environment. We suggest considering nanoplastics a complex and dynamic mixture of polymers, additives, and contaminants, with interconnected risks to environmental and human health. We call for comprehensive integration of One Health approach to produce robust multidisciplinary evidence to nanoplastics threats at the planetary level. Although there are many challenges, this holistic approach incorporates the relevance of environmental exposure and multi-sectoral responses, which provide the opportunity to identify the risk mitigation strategies of nanoplastics to build resilient health systems.
Keywords: Nanoplastics, Environmental exposure, Human health, One health, Risk mitigation
Graphical abstract
Highlights
-
•
Nanoplastic is a complex and dynamic mixture of polymer, additive, and contaminant.
-
•
Knowledge gaps persist in exposure, bio–nano interactions, and potential risks.
-
•
A one health approach is critical to achieve environmental and human health.
-
•
Environmental exposure, multi-responses, and mitigation strategies are integrated.
1. Introduction
Plastic pollution is a global health crisis. Although the impact of the ubiquitous plastic contamination remains to be fully understood, particular attention is shifting toward particles in micro size (1 μm−5 mm) and smaller particles, in particular, nanoplastics. Nanoplastics are defined as plastic particles with a size ranging from 1 to 100 nm [1] or 1–1, 000 nm [2]. Microplastic and nanoplastic particles are released into the environment either directly from products such as paints, cosmetics, biomedical products, laundry detergents, and fabric softeners during their life cycle [3,4], or from the degradation and fragmentation of weathered plastic-containing products [5]. The latter include 3D printing, tire wear, plastic bags and bottles, and plastic mulch in agriculture [6]. The presence of plastic particles has been documented all around the world, from mountains to remote areas that are far away from direct impacts of anthropogenic activities, such as the deep sea, and from the atmosphere to human feces [[7], [8], [9], [10], [11], [12]]. This global accumulation of plastic particles will aggravate both ecosystem and human exposure.
Nanoplastics differ distinctly from microplastics, both in physicochemical behaviors and biological reactivity [13]. For instance, the abundance of nanoplastics in the environment is likely to be several orders of magnitude higher than that of microplastics [14]. Besides, a growing body of evidence shows that nanoplastics cross the gut barrier and translocate to secondary tissues after ingestion [15]. By contrast, microplastics are physically trapped in the gut after ingestion by a wide variety of species, ranging from zooplankton to mammals, although their translocation in wild-caught fish has recently been reported [[16], [17], [18]]. Thus, nanoplastics are proposed to pose a greater risk to the ecosystem and human health than microplastics [19].
The risk assessment of nanoplastics has reached crossroads: the environmental risk community is interested in their environmental behaviors and eco-toxicity to algae, plants, invertebrates, and fish, whereas the human health risk community focuses on the in vitro human cells, human tissues, and mammals in the laboratory. To date, no published study has directly examined the effects of nanoplastics on humans. Due to the differences in research focus, there exists a gap between the fields of human and environmental nanotoxicology. This is unfortunate given the exposure continuum and interconnectedness of all living species, including humans, animals, plants, and their shared environment on the planet.
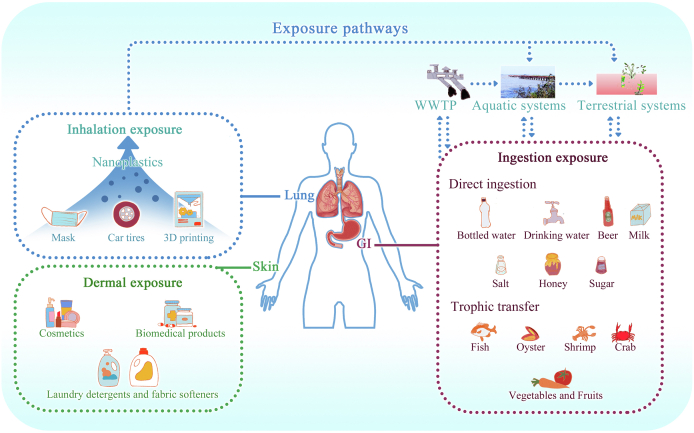
There is a strong connection between human and environmental health research, partially due to the relevance of environmental exposure pathways to humans. Three major exposure pathways to nanoplastics and microplastics, i.e., via the gastrointestinal tract (ingestion), the skin (dermal exposure), and the lung (inhalation exposure) are demonstrated in Fig. 1 [20,21]. As far as we know, the ingestion pathway is, if not the primary, at least an important route of human exposure to plastics via the consumption of contaminated seafood (such as fish, crustacean, and shellfish) and plants [17,[22], [23], [24], [25], [26]]. Likewise, other food products such as table salt, honey, and sugar are well known for microplastic contamination [[27], [28], [29], [30]]. In addition, the leaching out of microplastics and nanoplastics from packaging materials (e.g., feeding bottles, silicone-rubber baby teats, and teabag) into human food and drinks, such as rice, baby formula, drinking water and beer, contributes to this pathway [25,31,32]. The concentrations documented for nanoplastics and microplastics in various foodstuffs (Fig. 2 and Table S1) can be used to perform a dietary exposure assessment. A recent study estimated the lifetime accumulation of microplastics (1–10 μm) in children and adults via seafood, tap water, bottled water, salt, beer, milk, and inhalation using a physiologically-based pharmacokinetic sub-model. The median intake rates per capita are 553 and 883 particles/d for children and adults, respectively, leading to up to 5.01 ✕ 104 particles per capita for adults till age 70 [33]. In another study, the daily intake of microplastics is estimated to be 106–142 particles using foodstuffs such as seafood, sugar, salts, honey, alcohol, as well as tap and bottled water [24]. In particular, air, bottled water, and seafood consumption account for the majority of microplastic intake [24]. Unfortunately, these estimates do not account for commonly consumed food items, including grains, beef, and poultry, due to the lack of data. Inhalation is relevant in occupational exposure to nanoplastic-containing aerosols [34]. It could also occur through atmospheric deposition and face mask wearing [35,36]. Dermal exposure occurs via the use of personal care products, laundry detergents, and fabric softeners [37]. However, the extent to which inhalation and dermal exposure pathway contribute to the overall exposure is not fully understood. Further information on the inhalation and dermal exposure to nanoplastics has been summarized in and can be referred to the recent reviews [20,38].
Fig. 1.
Human exposure to nanoplastics. Schematic illustration showing major exposure pathways of human to nanoplastics, i.e., inhalation, dermal and ingestion pathways. Nanoplastics are released from consumer products or from the degradation of plastic-containing products due to weathering, build up in the aerosols, the wastewater treatment, aquatic environment and terrestrial environment, and accumulate in a wide range of foodstuff and drinks. WWTP, wastewater treatment plant; GI, gastrointestinal tract.
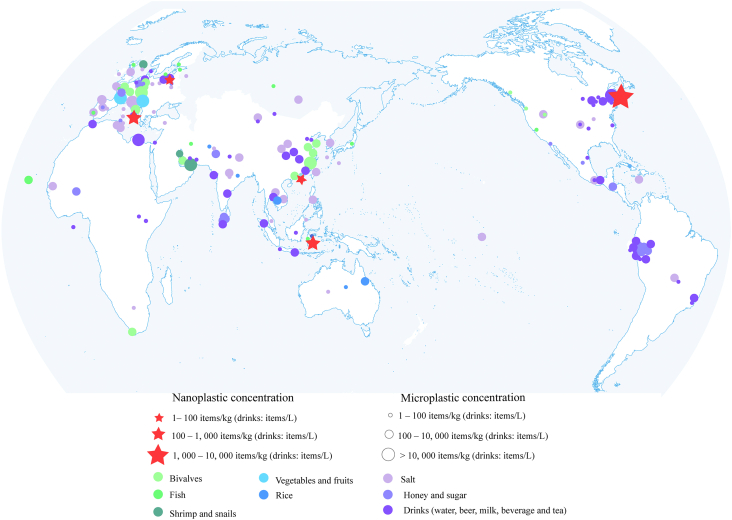
Fig. 2.
Worldwide concentrations of microplastics and nanoplastics reported in food items including salt, seafood (fish, bivalves, shrimp and snails), vegetables and fruits, honey and sugar, rice, and drinks (bottled and tap water, beer, tea and milk). Polymer types, sizes, and concentrations are also presented in Table S1.
Very limited information on the concentrations of nanoplastics in humans is available. As for microplastics, a total of 12 microplastic pigments (5–10 μm) are found in four human placentas [39]. Micro polyethylene terephthalate (PET) and polycarbonate concentrations are 5.7–82 μg/g and 0.049–2.1 μg/g for six infant stool specimens, but an order of magnitude lower in 10 adult stool samples (below the limit of quantification to 16 μg/g) [11]. Moreover, infants suffer from higher levels of daily exposure than adults (e.g., 83 vs. 5.8 μg/kg for PET, 0.86 vs. 0.2 μg/kg for polycarbonate).
In this article, we reviewed the environmental and human health research of nanoplastics and identified their gaps in terms of environmental exposure, nano–bio interactions, and effect mechanisms. We proposed a One Health approach to multidisciplinary collaboration and bridging human and environmental health. Moreover, a roadmap for One Health approach by overcoming these disconnections was also laid out. This work will provide the opportunity to identify the risk mitigation strategies to build resilient health systems nationally and internationally.
2. Gaps at exposure
Environmental assessment is critical to understand the concentrations and fate of nanoplastics to which human may be exposed. Nanoplastics are everywhere, and different exposure routes may result in much more complex exposure scenarios.
2.1. A mismatch between laboratory and environment-occurring nanoplastics
Due to separation and analytical challenges and lack of extensive surveys, we are far from understanding nanoplastic exposure in natural environmental settings (Fig. 3a). The identification of nanoplastics in complex environmental and biological matrix is highly challenging because of their dilute concentrations, small size, and the fact that their overall chemical composition is not all too different from that of organic matter. It is estimated that more than 300 million tons of nanoplastics are in the global environment [40]. However, exposure concentrations of nanoplastics are likely to be underestimated considering that the fragmentation of weathered plastics continues and that reliable methods sensitive to nanoscale size and concentrations down to nanogram per liter remain sparse. Indeed, when weathering plastic fragments into nanoplastics, the mass of a particle decreases but the particle number increases.
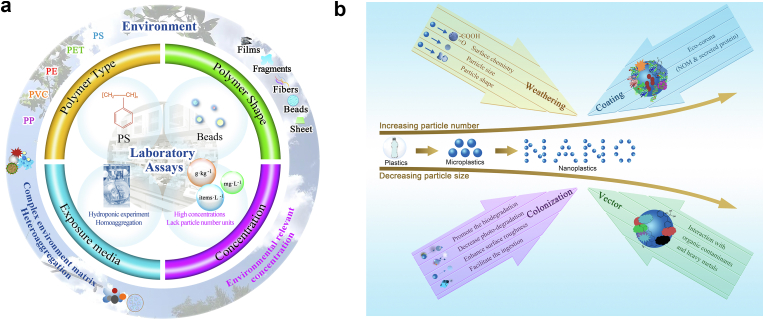
Fig. 3.
(a) Mismatch between laboratory and environment-occurring nanoplastics in the risk assessment to environmental and human health. Polyethylene (PE), polyethylene terephthalate (PET), polypropylene (PP), polyvinyl chloride (PVC), polystyrene (PS) are the most commonly detected plastics in the environment. (b) Transformation of nanoplastics in the environment. Nanoplastics are continuously produced, and undergo weathering, coating, colonization and co-exposure with other contaminants. Eco-corona forms outside an organism with high concentrations of natural organic matter and excreted proteins.
Very limited information regarding exposure levels of nanoplastics in human food is available. Nanoplastics have been detected in Zebra snails, vegetables, and fruits (particles <2 μm), likely presenting a direct route of human exposure (Table S1) [[41], [42], [43], [44], [45]]. There are several reviews on the exposure levels of microplastics in beverages and food sources [[46], [47], [48]]. Since it is common that microplastics are degraded into nanoplastics after ingestion in the gut of vertebrates [49,50], it is not surprising that the number concentrations of nanoplastics will be several orders of magnitude higher than that of microplastics in seafood. Such high exposure levels of nanoplastics and their toxic effects may be much more severe than those current estimates of microplastics. Therefore, there is an urgent need to identify the possible magnitude of nanoplastic exposure to humans.
Nanoplastics in the environment are heterogeneous in polymer type, shape, and size, which might not be the case in the laboratory. In the absence of reliable exposure levels, most studies in the laboratory use standardized nanoplastics such as PS beads, which are characterized by homogeneity of size, polymer type, and physical/chemical structure, at unrealistically high concentrations. Unfortunately, a single type of plastics does not represent the diversity of plastics in nature. In fact, the most commonly detected plastics in the environment are polyethylene (PE), polyvinyl chloride (PVC), polypropylene (PP), PET, and polystyrene (PS) [20,51]. In addition, the shapes used in the laboratory are not always consistent with those observed in the environmental samples (see the section of Intrinsic Properties of Nanoplastics). Although reference nanoplastics such as PS beads are of importance to provide comparable data across studies, these benchmark data help to identify the key parameters responsible for the biological outcomes of interest. The question is how representative these models and pristine nanoplastics are.
The lack of consensus on the characteristics of nanoplastics, e.g., different units and exposure media in the literature, results in ambiguous communication. Nanoplastics have been reported on the basis of total mass (e.g., milligram per liter) or particle number concentrations (e.g., items per liter). However, a more detailed analysis to provide information, such as particle size, particle number, surface charge, and surface chemistry, is required for particulate contaminants, whose behaviors are based on the theories in colloid or particle science. Although the unit conversion can be possible for some forms of particles (e.g., beads and fibers), it is still a significant challenge for the conversion between units due to heterogeneous composition and size distribution of nanoplastics, the dynamics of weathering (e.g., associated with biofilms), and the complex environmental matrices in the real world [52]. This hampers reproducible and statistically significant results. Another example is the health risk assessment of human cell lines. Particle number concentrations should be carefully chosen not only to ensure their reliable detection by the available analytical techniques but also to avoid the exaggerated homo/heteroaggregation in aquatic media which may not represent the real exposure conditions in vivo. The latter is of particular importance given that representative nanoplastics such as PE, PP, PET, PVC, and PS have a density close to water (0.88–1.50 g/cm3), leading to downstream transport in suspension rather than deposition [53]. Standardizing these assays and the methods could offer a pathway toward reproductive and representative experiments.
2.2. Highly dynamic characteristics of nanoplastics in the environment
The generally prevailing assumption that plastics per se behave as an “inert” material may not be valid [54]. Plastics are dynamic systems whose size varies with organic and inorganic compounds, as well as microorganisms in the environment. As a result, plastics may play an important role in their interactions with biota including humans during weathering, coating, colonization, and co-exposure with other contaminants in the environment (Fig. 3b).
Environmental weathering, under abiotic or biotic conditions, leads to differences in the surface chemistry, structure, particle size and shape, as well as the release of chemicals present in the plastics. For example, an expanded PS box declines 5% in weight during outdoor weathering within 1 month and produces 6.7 × 107 microparticles and nanoparticles/cm2 [55]. Photo-oxidation leads to the formation of carbonyl groups on plastics [56], which decreases their surface hydrophobicity but increases the reductive capability [57]. Hence, weathered nanoplastics will have different adverse effects compared to pristine particles [58,59]. More importantly, weathering processes result in complex chemical mixtures (e.g., secondary nanoparticles and additives). For example, carbonyl groups newly formed at PS plastic surface under natural sunlight exposure reduce ionic silver to metallic silver nanoparticles in the surface water and sand matrix [21], causing synergistic toxicity of PS nanoplastics and silver nanoparticles toward two freshwater algae Chlamydomonas reinhardtii and Ochromonas Danica [60]. The additives, introduced as stabilizers, pigments, flame retardants, and antibiotics to improve the properties of plastics, are susceptible to leaching during weathering because they are not chemically bound to the polymer [61,62]. However, whether these chemicals are hazardous depends on their release rate from plastics and their bioavailability to biota. A recent study has shown that PE and PVC plastic pellets release additives at higher rates in surface waters, while those under the deep sea represent a slow but continuous source of additives over much longer periods [63]. Zimmermann et al. [64] have detected 260 chemicals tentatively in 34 plastic extracts, which triggered toxicity in vitro, but most chemicals remained largely unidentified. As a result, the complexity of the mixtures impedes the identification of the main effect drivers. Overall, the potential adverse effects due to plastic weathering are missing, and the components that drive the adverse effects remain unknown.
Similar to other nanomaterials, nanoplastics have physical and chemical interactions with the surrounding environment, e.g., interactions with microorganisms, coatings with exo-organic materials and macromolecules (i.e., eco-corona; see the section of Transformation), and heteroaggregation with natural colloids (e.g., clays and aerosols in the atmosphere) [65]. Thus, the physical and chemical parameters of particles may change, triggering different adverse effects than pristine particles [66]. To date, our understanding of microbial interactions with plastics has been achieved in samples that are a few hundred micrometers or millimeters in size. Microorganisms change the characteristics of plastics in diverse ways. Microorganisms can excrete extracellular enzymes that break down the particle surface, thus promoting the biodegradation of plastics [67,68]. For instance, Archaea, Bacteria, and Eukarya (fungi) contribute to the degradation of poly(butylene succinate-co-adipate) under field soil conditions [69]. Meanwhile, microorganisms can decrease the photo-oxidation of plastics by shielding the particles’ surface from ultraviolet radiation [70,71]. Moreover, the extracellular polymer substances released by microorganisms can facilitate plastic aggregation, alter their size and density, and enhance surface roughness [72]. As a result, biofilm-associated particles sorb more contaminants such as perfluorooctane sulfonate than virgin ones, thus introducing more contaminants to organisms [73]. In parallel, microbial colonization on low-density polyethylene (50 μm film thickness) enhances the dissipation rates of DDTs and PAHs such as phenanthrene, fluoranthene, and pyrene in the sediments [74]. Moreover, microorganisms can facilitate the ingestion of nanoplastics for primary consumers such as nematodes [75]. It remains largely unknown how microorganisms interact with nanoplastics that are smaller than microorganisms themselves.
In addition, nanoplastics can serve as vectors for a wide variety of contaminants such as heavy metals and organic pollutants, as well as antibiotic resistance genes [[76], [77], [78], [79]]. Nanoplastics can concentrate environmental contaminants and modify their distribution, transport, transformation, and effects upon concurrent exposure [21,[80], [81], [82]]. These nanoplastics that humans are exposed to are substantially different from pristine nanoplastics used directly in consumer products, increasing the difficulty of human health assessment. Currently, there have been several reviews focusing on the interactions of plastics and contaminants [[83], [84], [85]]; however, few studies concerning the effects of nanoplastics of environmental origin are available [86].
3. Gaps at the nano-bio interactions
Nano-bio interactions depend not only on the intrinsic properties of nanoplastics (e.g., size, surface charge, chemistry, shape, and type), but also on the complex neighboring conditions where nanoplastics transform, and on the internalization by biota (Fig. 4).
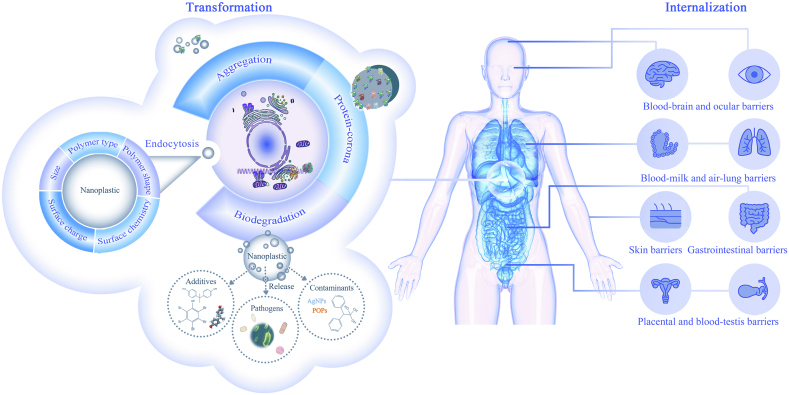
Fig. 4.
Transformation of nanoplastics in human barriers. Nanoplastics undergo aggregation, formation of protein-corona and degradation at the nano-bio interface. Protein-coronas are formed upon nanoplastic internalization by a biota with endogenous proteins. With the biodegradation, the additives, pathogens and other contaminants in nanoplastics are released. Nanoplastics come across the cell membrane by endocytosis and potentially other pathways, which are highly dependent on the polymer type, size and shape, and surface charge and chemistry. AgNPs, metallic silver nanoparticles; POPs, persistent organic pollutants.
3.1. Intrinsic properties of nanoplastics
Size is a key factor in the nano–bio interactions. Intestinal translocation in Caco-2 cells reaches up to 7.8% for 50 nm PS nanoparticles and to 0.8% for 100 nm PS [87,88]. Compared to 500 nm PS, smaller PS nanoparticles (<200 nm) accumulate more in the brain tissues of rats 3 h after intravenous injection [89]. Compared to larger nanoplastics (202 and 535 nm), 64 nm PS induces significantly greater pro-inflammatory effects to the lung upon instillation in rats [90]. Pacific oyster Crassostrea gigas larvae of 3 days post fertilization have an average load of 10.5 PS nanoparticles (160 nm) per larva, in contrast to 0.26 microplastics (1.8 μm) per larva [91]. It is thus proposed that the effects of nanoplastics with a certain size in the laboratory will differ distinctly from those with a wide range of sizes found in nature since laboratory work may underestimate the complex particle–particle interactions and biota–particle interactions [92].
In addition to size, surface charge and surface chemistry are important considerations. Upon oral exposure, rats accumulate a greater amount of negatively charged PS nanoparticles (50 nm) than positively charged nanoparticles [87]. Although positively charged PS nanoparticles (71 nm) have a more significant impact on gene expression and physiological alterations in Arabidopsis thaliana, their uptake is lower than those of negatively charged PS (55 nm) [93]. This can be explained by the fact that positively charged PS stimulates high levels of root exudates, reducing the stability of PS and limit its uptake [93]. Similarly, positively charged PS nanoparticles cause cellular apoptosis and embryonic malformations, resulting in a higher toxicity to developing embryos of sea urchin Paracentrorus lividus than negatively charged counterparts [94]. It has also been reported that two types of negatively charged 50 nm PS have a greater than 30-fold difference in their translocation rates using in vitro intestinal cell models, as a result of differences in the surface chemistry [88].
Plastics have a wide range of shapes in nature, such as fibers, fragments, beads, and film. Fiber is the most prominent plastic shape identified in field studies, but less than 10% of effect studies use plastic fibers [92]. PE fibers (100–400 μm) show greater adverse effects than PE beads (1–4 μm), via carapace and antenna deformation in Ceriodaphnia dubia without ingestion [95]. Similarly, PP fibers (20–75 μm in length and 20 μm in diameter) are more toxic to amphipod Hyalella azteca than PE beads (10–27 μm): The 10-d LC50 for beads and fibers are 4.64 ✕ 104 items/mL and 71 items/mL, respectively; fibers also induce slower egestion and less growth. This is attributed to a longer residence time of fibers in the gut [96]. In another study, the ingestion of 34 μm PP fibers induces increased mortality of grass shrimp Palaemonetes pugio than that of fragments, while fibers with a size of 93 μm are less toxic [97]. However, very limited information is available in the literature on nanoplastic shape-driven toxicity.
The polymer type is also an important factor affecting nano-bio interactions. PVC nanoparticles reduce the lifetime of acorn barnacle Amphibalanus Amphitrite with a mortality rate of 99% at 25 mg/L, compared to minimal toxicity of poly(methyl methacrylate) and PS nanoparticles, at equivalent diameters (ca. 100 nm) [98]. Insights into the relationships of nanoplastic effects with size, surface charge, and chemistry, polymer shape, and type are important for advancing the risk assessments and facilitating a safe-by-design strategy (see the section of Multi-sectoral Responses).
3.2. Transformation
The toxicity of nanoplastics within biota highly depends on their transformation at nano-bio interfaces, as a result of physical, chemical, and biological interactions with various biomolecules (e.g., proteins, lipids, and carbohydrates) in membranes, organelles, cells, tissues, or biological fluids. The nanoplastic transformation includes aggregation, eco-corona and protein-corona formation, degradation, and possibly redox reaction (for weathered polymers with oxidative/reductive groups). It will greatly change the physicochemical properties of nanoplastics and influence their biological activity.
Aggregation is an important physical transformation of nanoplastics in body fluids. The extracellular aggregation of few-layer graphene decreases the cellular uptake and cytokine release in the gastrointestinal tract [99], while the intracellular aggregation of nanoplastics appears to prolong the clearance of nanomaterials [100]. The aggregation of 50 nm PS nanoplastics by human immunoglobulin G, however, does not reduce PS toxicity to Daphnia magna [101]. The biological reactivity is most probably caused by the mixture of single nanoplastics and their aggregations [102].
Eco-corona and protein-corona formation gives nanoplastics new identities and properties and allows the organisms to respond to a complex rather than nanoplastic itself. Eco-corona is formed with exo-organic materials and macromolecules (e.g., natural organic matter and secreted proteins). Studies on the implications of eco-corona formation on the fate, bioavailability, and toxicity of nanoplastics are relatively scarce. Eco-corona formed with secreted proteins enhances the uptake and toxicity of PS nanoparticles to Daphnia magna [103], but that formed with humic substances protects Daphnia magna against PS toxicity [104,105].
Different from eco-corona, protein-corona is formed with endogenous proteins. Different protein-binding on negatively charged 50 nm PS led to an over 30-fold difference in their intestinal translocation [88]. This could pave the way for unanticipated effects. In another study, a total of 166 different plasma corona proteins were found upon 110 nm PS incubation with human plasma for 0.5 min, but corona formation only increased the uptake of positively charged PS in human endothelial cells at an early exposure time, with less impact on negatively charged PS [102]. Coronated PS nanoparticles (13–135 nm) in human blood cells are more genotoxic and cytotoxic than virgin nanoplastics due to protein-induced coalescence in nanoplastics [106]. In contrast, fetal bovine serum coating on the surface of 300 nm PS nanoparticles acts as a membrane protector and mitigates the cytotoxicity of PS [107]. After cellular internalization, PS nanoplastic corona may eventually degrade in subcellular compartments such as lysosomes, and cytotoxicity of nanoplastics occurs [107]. These results illustrate the significant effect of protein-corona on nanoplastic toxicity.
Degradation within organisms is important for nanoplastic transformation. PE microplastics (32 μm) are degraded into nanoplastics (<1 μm) in the digestive system of Antarctic krill, and particle size in the krill is averagely 78% smaller than the original size [49]. The degradation of microplastics most likely occurs in humans. However, it remains unclear to what extent the nanoplastics can be degraded in the acidic stomach and neutral intestine. In addition, 44 nm PS nanoplastics were reported to be degraded into smaller particles in the rhizosphere of A. thaliana upon co-exposure to Cd and nanoplastics. This is attributed to the enrichment of soil bacteria with a metabolic preference for aromatic compounds and the increase in low-molecular-weight organic acids in root exudates [108]. The degradation of nanoplastics, presumably, depends on their locations in tissues and cells, and the presence of suitable enzymes, which is a research frontier. These degradation processes can reduce the molecular weight of nanoplastics and change their size, shape, and chemical composition. Accompanied with degradation, the possible leaching of chemicals (including additives and adsorbed contaminants) from the plastics may cause harm to humans. Many chemical additives and monomers are reproductively toxic (e.g., bis(2-ethylhexyl) phthalate and bisphenol A), carcinogenic (vinyl chloride and butadiene), and even mutagenic (benzene and phenol) [109,110]. This may add considerable value in assessing environmental and human health risks.
3.3. Internalization
Nanoplastics could overcome environmental and physiological barriers and be internalized in a variety of species such as plants, invertebrates, vertebrates including fish and mammals [111]. Environmental barriers include but are not limited to the barriers along the food chain; that is, the interface between the environment and primary producers (i.e., algae and plants), and the interface between primary producers and consumers at higher trophic levels. Physiological barriers within biota include gastrointestinal, blood–brain, placental, air–lung, skin, blood–ocular, blood–milk, and blood–testis barriers (Fig. 4) [112,113]. For example, PS nanoparticles (20–330 nm) overcome the blood–brain barrier in rats and fish [114]. The passage of nanoplastics (40–100 nm) across blood–brain barriers in various species has been recently reviewed by Prust et al [115]. PS particles sized 50–300 nm can cross the placental barrier and transport from the fetal to the maternal blood circulation by in vitro placental perfusion [116]. For example, 42 nm PS is transferred from mother zebrafish Danio rerio to offspring [117]. In terms of skin barriers, fluorescent PS nanoparticles concentrate in the top layers of the skin at a depth of 2–3 μm and cannot permeate into deeper skin tissue, unless the skin is damaged [118].
The mechanisms of nanoplastics that cross various barriers seem contradictory. PET nanoparticles (27 nm) enter the Caco-2 intestinal epithelial cells mainly through the endocytosis pathway [15]. PS with a size of 44 nm enters the mammalian cells, i.e., bovine oviductal epithelial cells and human colon fibroblasts, through ATP-independent processes [119]. Such differences in uptake and translocation mechanisms depend on the intrinsic properties of nanoplastics as shown previously, cell types, and protein-corona [120].
4. Gaps at the effect mechanisms
Following external and internal exposure to nanoplastics, adverse effects have been demonstrated in the laboratory at various levels of biological organizations (cells, tissues, and individuals) for a wide range of species. Most studies on human health focus on in vitro studies using human cell lines, and mammalians such as mice and rodents. Upon uptake, the immune cells confuse nanoplastics with viruses due to their similarity in size, and thus capture these particles, which induce inflammatory responses [121]. In addition, nanoplastics induce oxidative stress, immunological responses, genotoxicity and DNA damage, neurotoxicity, and reproductive abnormities [122]. However, most cell cultures are of cancer origin, and the effects need to be carefully and cautiously interpreted because cancer cells show distinct differences in metabolism and reactive oxidation stress. Moreover, it remains a challenge to extrapolate the in vitro effects of nanoplastics on the whole organism. Indeed, the translocation rates of negatively charged 50 nm PS are ca. 211-fold difference in a Caco-2 mono-culture, 78-fold in a co-culture with mucus-secreting HT29-MTX cells, and 30-fold in a tri-culture with M-cells. As a result, up to 8% of 50 nm PS nanoparticles are internalized by in vitro intestinal cell models, while 0.2%–1.7% is assimilated by rats [87,88]. In addition, there is little evidence of nanoplastic toxicity at realistic doses or under chronic exposure.
The effects of nanoplastics on the toxicity of adsorbents under multi-stressor exposures remain a debate. Co-exposure to 44 nm PS and a complex PAH mixture decreases the developmental deformities and impairs vascular development induced by PAH in zebrafish larvae. This is linked to the low PAH accumulation in the co-exposed zebrafish [123]. Ingestion of 50 nm PS induces oxidative stress on lipid membranes and inhibits membrane defense by decreasing the activity in P-glycoproteins and multidrug resistance proteins. As a result, it enhances the toxicity of 2,2′,4,4′-tetrabromodiphenyl ether and triclosan in monogonont rotifer B. koreanus [124]. Koelmans et al. [85] suggest that plastics such as PS with low affinity for persistent organic pollutants have a marginal decreasing effect on the bioaccumulation of persistent organic pollutants, while stronger sorbents such as PE have a more substantial effect.
The colonization of human pathogens (e.g., Vibrio spp.) [125] and microorganisms on nanoplastics may also contribute to the pernicious effects. For instance, ingestion of these nanoplastics may enhance the release of antibiotics and the development of antibiotic-resistant bacteria in the human gut, and favor human infection related to resistant bacteria [126]. Currently, increasing evidence has shown the microbiome shift in the gut of soil fauna. PS nanoparticles (50–100 nm) modify the microbiome in soil oligochaete Enchytraeus crypticus at a high concentration (10% of dry soil weight) [127]. The decline in Rhizobiaceae, Xanthobacteraceae, and Isosphaeraceae in E. crypticus may have significant implications because they play a fundamental role in nitrogen cycling and organic matter decomposition. Similarly, PS plastics (2–2.9 μm) not only change the gut microbial community and diversity but also increase antibiotic-resistant genes accumulation in collembolan Folsomia candida gut, especially when PS nanoparticles hold adsorbed sulfamethoxazole [128]. Poly(lactic acid) (PLA) is a substitute for non-degradable plastics such as PE and PP. However, the bacterial abundance and antibiotic-resistant genes on PLA are significantly greater than those on PP and PE in the indoor environment, posing health risks to humans especially in hospital wards [129]. Trophic transfer of 44 nm PS nanoplastics from mussel Mytilus edulis to whiteleg shrimp Litopenaeus vannamei also change the microbial activities in L. vannamei [130].
Given that nanoplastics represent a complex mixture of particles, chemicals, and microorganisms, knowledge of particle toxicity alone is not sufficient to get a full understanding of nanoplastic toxicity. Therefore, it is urgent to identify the possible magnitude of potential exposure and assess quantitatively where the thresholds of the hazard are, what the toxic mechanisms are, and the key factors triggering them. These systems-level evaluations can play important roles in risk assessments and a safe-by-design approach.
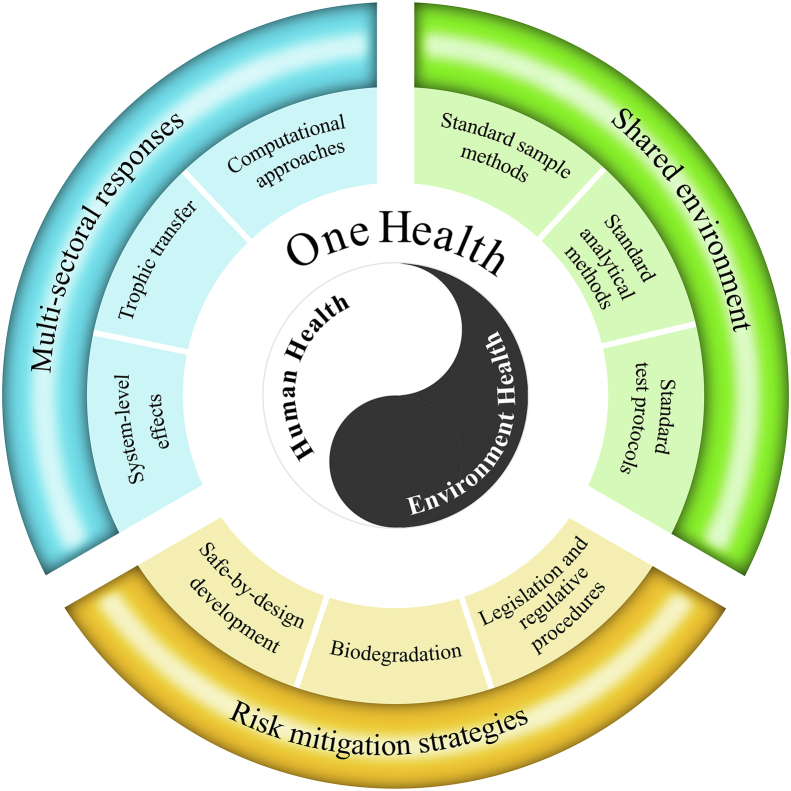
5. One Health approach
Even though cross-disciplinary interaction will promote multi-sectoral responses to nanoplastic threats at complex systems, and provide guidance on mitigating these risks, such information is still discipline-specific with few interactions. Realizing the exposure continuum and interlinkages between humans, animals, plants, and the shared environment on the planet, a closer transdisciplinary and interdisciplinary collaboration approach—the concept of One Health—at the human-animal-ecosystem interface is necessary. One Health has been recognized and promoted by the World Health Organization (WHO), the Food and Agriculture Organization of the United Nations (FAO), the World Organization for Animal Health (OIE), among several others. The One Health approach illustrates a sustainably healthy future for all species facing the nanoplastic threat.
Currently, removing the interdisciplinary barriers that still divide environmental and human health is a major challenge to the One Health framework. A major barrier is the lack of communication between the fields of human and environmental nanotoxicity. The communities could benefit from the harmonization of methodologies and approaches, given that there is no consensus even on laboratory protocols. Thus, there will be a shift from research generating isolated and basic knowledge to those leading to systematic and implicational knowledge. Based on the disconnections stated above, we will offer our recommendations for future research in those fields, to achieve the One Health framework (Fig. 5).
Fig. 5.
A possible One Health framework for the risk assessments of nanoplastics and risk mitigation strategies.
5.1. The shared environment among all species
Plants and animals share a common environment with humans. Within this space, accurate exposure levels of all species are of critical importance. This requires establishing standard sampling and analytical methods to reduce the variability across different geographies [131]. The standardization for each environmental matrix (e.g., soil, aquatic environments, sewage sludge, and air; Fig. 1) can be used for all nanoplastic types, allowing for more relevant comparisons between studies in different laboratories. Although considerable advances have been made over the past few years, only a few studies have successfully measured nanoplastics in real field samples, including seawater, snow, air, sand, and soils [19,132]. The current spectroscopic methods such as Fourier-transform infrared spectroscopy and Raman spectroscopy are limited to particles ranging from 1 μm to 5 mm [8,133,134]. Coupled with electron microscopy, nanoplastics in the size of 100 nm can be successfully identified [135]. Asymmetric flow field-flow fractionation coupled to multi-angle light scattering was used to determine the size of PS spiked in fish after the enzymatic digestion by proteinase K [136]. Mass spectrometry methods, such as pyrolysis gas chromatography mass spectrometry, have been increasingly used to identify and quantify nanoplastics in water samples [8,137], as well as in soils and animals when coupled to matrix digestion such as alkaline digestion (sodium hydroxide/tetramethylammonium hydroxide) or enzymatic extraction [137,138]. Matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF MS) was employed to detect nanoplastics spiked in water and fish, and nanoplastics in aviation cups and river sediment [139,140]. However, the information on the particle number and/or morphology is lacking [141].
New strategies for nanoplastic quantification include doping nanoplastics with metals before single-particle inductively coupled plasma mass spectrometry (ICP-MS) analysis [142,143], stable/radioactive isotope techniques, and time-of-flight/nanoscale secondary ion mass spectrometry (TOF/nano SIMS) [144]. The use of “labeled” nanoplastics (e.g., the stable isotope 13C) allows selective tracking of the plastic carbon through the transformation, transport, and internalization processes. The biodegradation of poly(butylene adipate-co-terephthalate), important polyester used in commercial polymeric mulch films in agriculture, by soil microorganisms is demonstrated using intrinsic 13C-labeling. Nanoscale secondary ion mass spectrometry (nanoSIMS) further shows the incorporation of polymer-derived 13C into the biomass of film-colonizing microorganisms [67]. More information on the analysis of environmental nanoplastics can be referred to a recent review of Cai et al. [19]. Based on these analytical frameworks, the environmental risk assessment will demonstrate the biological and environmental relevance of nanoplastics. However, these methods have only been tested in the laboratory but not in field environmental samples.
Standardized test protocols can ensure the risk assessments are of sufficient quality and avoid biased assessments [92]. Recently, Koelmans et al. [145] demonstrated the lack of reliability of ecological effects of microplastics, partly due to technical shortcomings in the experimental design such as verification of background contamination in the laboratory, and limitations in environmentally relevant testing conditions. Guidance on standardizing testing protocols is provided, which may also be validated for nanoplastics, either for environmental or human health risk assessment.
Nanoplastic weathering, coating, and carriers in the neighboring environment are also the pressing research priorities in the protocols. A less pronounced reduction in the growth of plants by nanoplastics is reported in soil than in aquatic culture medium, indicating the mediating effect of soil matrix on nanoplastic biological reactivity [93]. Aged nanoplastics induce more malformed offspring than pristine counterparts to Daphnia magna, illustrating the importance of environmental behaviors of nanoplastics [122]. Thus, the development of a conceptual risk assessment framework for nanoplastics is very challenging but can shape the research field and affect the mitigation measures [146].
5.2. Multi-sectoral responses
Humans feed on plants and animals. As such, integrated multiscale approaches are urgently needed to ensure food safety and food security, especially the accumulation of nanoplastics in edible parts of crops and seafood. A growing body of evidence has shown that nanoplastics not only have physiological effects on individuals but also reduce population growth and have severe alterations in reproduction [122]. The effects of nanoplastics on environmental and human health could vary up to several orders of magnitude among endpoints [147], illustrating the complexity of exposures and the inter-connection between environmental and human health. For example, 50% effective concentration (EC50) of fertilization and embryogenesis inhibition of Pacific oyster C. gigas ranged from 0.15 to 4.9 mg/L (50 nm positively charged beads) [146,148]. These concentrations were much greater than the community level effect thresholds, i.e., 5.4 μg/L or 5.97 × 1010 particles/L [149]. However, it is highly challenging to measure the effects of nanoplastics at community level and ecosystem level in the field, partially due to the difficulties in identifying and quantifying nanoplastics in different environmental matrix and biota, as well as multiple stressors in nature [150]. The possible magnitude of potential exposure and the hazards at various levels, i.e., from the molecules to cells, organs, individuals, population, community, and ecosystem, is vital to enhance a sustainable reconnection between plants, animals, humans, and our shared environment with increased exposure to nanoplastics.
Of particular concern is the transfer of nanoplastics along the food web [151]. Such studies would need a high priority because the ingestion pathway is an important route of human exposure to nanoplastics. Hitherto, there are a few reports on the transfer of PS nanoparticles at extremely high concentrations [152]. Such increasing evidence demonstrates the trophic availability of PS nanoparticles along the food chain and the associated toxicity (e.g., behavioral disorders and morphological changes in the brain, and reduced growth) to consumers at higher trophic levels [114,153], providing implications for food safety and human health.
In addition to in vivo or in vitro testing, alternative approaches include artificial intelligence (AI) and machine learning. These computational approaches are being used to predict the environmental and human risks of engineered nanomaterials and facilitate safe-by-design nanomaterials in consumer products and medicines. The features of engineered nanomaterials (e.g., size, shape, and surface chemistry) are linked quantitatively to biological responses via different models (such as quantitative structure–activity relationships, QSAR). Compared to expensive and time-consuming experiments, AI and machine learning can integrate existing large data and predict future outcomes. In line with engineered nanomaterials, the diversity of nanoplastics and their dynamic interactions with surrounding complex environments demand computational approaches for risk assessments [154]. For example, machine learning was used to predict the degradation of ocean plastics, using a hierarchy of surface erosion predictors and plastic features (e.g., molecular weight and hydrophobicity) [155]. QSAR has been used in predicting cellular responses to polymers and bacterial biofilm formation, providing guidance on biopolymer design [156,157]. Similarly, micropore volume and O/C ratio of 18 microplastics were identified as key parameters controlling bio-accessibility of pyrene and 4-nonylphenol using a deep learning neural network approach [158]. A key challenge is the scarcity of empirical data and the reliance on databases of nanoplastics, which impedes the application of AI and machine learning approaches. Attempts to overcome this scarcity are the first step for the computational approach. Training the models under environmental conditions at realistic nanoplastic exposure concentrations will identify key specific properties that determine the effects of nanoplastics at multiscale levels. This will ultimately provide important new insights and suggestions to mitigate the risks of nanoplastics efficiently.
5.3. Risk mitigation strategies
Given the harm of nanoplastics to environmental and human health, the priority now is how to develop novel techniques to remediate the risks of nanoscale plastics. It is estimated that about 9% of plastics produced globally from 1950 to 2015 have been recycled, 12% incinerated, and 79% accumulated in landfills or the natural environment [159]. To date, solutions mainly target the plastic management at their end-of-life, rather than the safe-by-design strategies of intelligent plastics for easy chemical or biological recycling [160]. In fact, chemical and mechanical recycling could extend the lifetime of plastics over their entire life cycle before final disposal but could not prevent waste production [160]. Biodegradable plastics are often considered as the desired solution but only account for 2% of all materials produced per year [161]. The biodegradable rate of plastics is lower in the field than that in the laboratory, due to different environmental conditions (e.g., temperature, pH, and oxygen levels) and microbial structure; more importantly, the resulting products are not well known [162,163]. Further, plastic carbon is not always completely incorporated into microbiota mass or converted into carbon dioxide/methane. More likely, plastics returning to the environment are degraded into smaller particles, which are more readily ingestible and pose higher risks. The development of bioengineered microorganisms (especially Ideonella sakaiensis) and engineered enzymes that can hydrolyze PET is an area of active research [[164], [165], [166]].
Another feasible solution is minimizing the release of plastics into the environment. For example, economic stimuli like tax benefits in the reuse of plastics have been applied in the UK and China [167,168]. International organizations have made recommendations to limit international trade of plastic waste (UN Basel Convention Annex II), ban particular single-use plastic items (European Parliament Resolution P9 TA (2019)0305), and reduce the amount of plastic in the environment (EU 2018/0172). On March 2, 2022, the resumed fifth session of the United Nations Environmental Assembly (UNEA-5) adopted a resolution setting up the path to a global treaty to end plastic pollution and forge an international legally binding agreement by 2024 [169]. Overall, the remediation of plastic particles in the environment is a great challenge and requires the One Health approach, in which polymer scientists, industry, environmental engineers, and policy-makers work together to design, produce and remediate environmental plastics to ensure that plastics do not have unintended negative consequences.
One Health approach provides a systematic path forward that will be critical for mitigating the risks of nanoplastics and achieving the UN Sustainable Development Goals. We, therefore, ask for the comprehensive integration of One Health across scientific communities. This is the only way toward mitigating the impacts of these emerging contaminants, which in fact have been accumulated in the environment for decades.
Conflicts of Interest
The authors have declared no conflicts of interest.
Acknowledgments
The authors sincerely thank the anonymous reviewers for their critical and constructive comments on this manuscript. This work was supported by the Youth Innovation Promotion Association, Chinese Academy of Sciences (2020314), the National Natural Science Foundation of China (41977355), China Postdoctoral Science Foundation (2021M703303) and the USDA Hatch program (MAS 00549).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.eehl.2022.02.001.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.European Commission . 2018. Definition of a Nanomaterial.https://ec.europa.eu/environment/chemicals/nanotech/faq/definition_en.htm [Google Scholar]
- 2.Gigault J., Halle A.t., Baudrimont M., Pascal P.-Y., Gauffre F., Phi T.-L., El Hadri H., Grassl B., Reynaud S. Current opinion: what is a nanoplastic? Environ. Pollut. 2018;235:1030–1034. doi: 10.1016/j.envpol.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 3.Hernandez L.M., Yousefi N., Tufenkji N. Are there nanoplastics in your personal care products? Environ. Sci. Technol. Lett. 2017;4:280–285. doi: 10.1021/acs.estlett.7b00187. [DOI] [Google Scholar]
- 4.Hosseinkhani B., Callewaert C., Vanbeveren N., Boon N. Novel biocompatible nanocapsules for slow release of fragrances on the human skin. N. Biotechnol. 2015;32:40–46. doi: 10.1016/j.nbt.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Duan J., Bolan N., Li Y., Ding S., Atugoda T., Vithanage M., Sarkar B., Tsang D.C.W., Kirkham M.B. Weathering of microplastics and interaction with other coexisting constituents in terrestrial and aquatic environments. Water Res. 2021;196:117011–117027. doi: 10.1016/j.watres.2021.117011. [DOI] [PubMed] [Google Scholar]
- 6.Qi Y., Ossowicki A., Yang X., Huerta Lwanga E., Dini-Andreote F., Geissen V., Garbeva P. Effects of plastic mulch film residues on wheat rhizosphere and soil properties. J. Hazard Mater. 2020;387:121711–121718. doi: 10.1016/j.jhazmat.2019.121711. [DOI] [PubMed] [Google Scholar]
- 7.Allen S., Allen D., Phoenix V.R., Le Roux G., Durántez Jiménez P., Simonneau A., Binet S., Galop D. Atmospheric transport and deposition of microplastics in a remote mountain catchment. Nat. Geosci. 2019;12:339–344. doi: 10.1038/s41561-019-0335-5. [DOI] [Google Scholar]
- 8.Materic D., Kasper-Giebl A., Kau D., Anten M., Greilinger M., Ludewig E., van Sebille E., Rockmann T., Holzinger R. Micro- and nanoplastics in alpine snow: a new method for chemical identification and (semi)quantification in the nanogram range. Environ. Sci. Technol. 2020;54:2353–2359. doi: 10.1021/acs.est.9b07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ter Halle A., Jeanneau L., Martignac M., Jardé E., Pedrono B., Brach L., Gigault J. Nanoplastic in the north atlantic subtropical gyre. Environ. Sci. Technol. 2017;51:13689–13697. doi: 10.1021/acs.est.7b03667. [DOI] [PubMed] [Google Scholar]
- 10.Dris R., Gasperi J., Saad M., Mirande C., Tassin B. Synthetic fibers in atmospheric fallout: a source of microplastics in the environment? Mar. Pollut. Bull. 2016;104:290–293. doi: 10.1016/j.marpolbul.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J., Wang L., Trasande L., Kannan K. Occurrence of polyethylene terephthalate and polycarbonate microplastics in infant and adult feces. Environ. Sci. Technol. Lett. 2021;8:989–994. doi: 10.1021/acs.estlett.1c00559. [DOI] [Google Scholar]
- 12.Liu C.G., Li J., Zhang Y.L., Wang L., Deng J., Gao Y., Yu L., Zhang J.J., Sun H.W. Widespread distribution of PET and PC microplastics in dust in urban China and their estimated human exposure. Environ. Int. 2019;128:116–124. doi: 10.1016/j.envint.2019.04.024. [DOI] [PubMed] [Google Scholar]
- 13.Facciolà A., Visalli G., Pruiti Ciarello M., Di Pietro A. Newly emerging airborne pollutants: current knowledge of health impact of micro and nanoplastics. Int. J. Environ. Res. Publ. Health. 2021;18:2997–3014. doi: 10.3390/ijerph18062997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gigault J., El Hadri H., Nguyen B., Grassl B., Rowenczyk L., Tufenkji N., Feng S., Wiesner M. Nanoplastics are neither microplastics nor engineered nanoparticles. Nat. Nanotechnol. 2021;16:501–507. doi: 10.1038/s41565-021-00886-4. [DOI] [PubMed] [Google Scholar]
- 15.Magrì D., Sánchez-Moreno P., Caputo G., Gatto F., Veronesi M., Bardi G., Catelani T., Guarnieri D., Athanassiou A., Pompa P.P., Fragouli D. Laser ablation as a versatile tool to mimic polyethylene terephthalate nanoplastic pollutants: characterization and toxicology assessment. ACS Nano. 2018;12:7690–7700. doi: 10.1021/acsnano.8b01331. [DOI] [PubMed] [Google Scholar]
- 16.Caron A.G.M., Thomas C.R., Berry K.L.E., Motti C.A., Ariel E., Brodie J.E. Ingestion of microplastic debris by green sea turtles (Chelonia mydas) in the Great Barrier Reef: validation of a sequential extraction protocol. Mar. Pollut. Bull. 2018;127:743–751. doi: 10.1016/j.marpolbul.2017.12.062. [DOI] [PubMed] [Google Scholar]
- 17.McIlwraith H.K., Kim J., Helm P., Bhavsar S.P., Metzger J.S., Rochman C.M. Evidence of microplastic translocation in wild-caught fish and implications for microplastic accumulation dynamics in food webs. Environ. Sci. Technol. 2021;55:12372–12382. doi: 10.1021/acs.est.1c02922. [DOI] [PubMed] [Google Scholar]
- 18.Cole M., Lindeque P., Fileman E., Halsband C., Goodhead R., Moger J., Galloway T.S. Microplastic ingestion by zooplankton. Environ. Sci. Technol. 2013;47:6646–6655. doi: 10.1021/es400663f. [DOI] [PubMed] [Google Scholar]
- 19.Cai H., Xu E.G., Du F., Li R., Liu J., Shi H. Analysis of environmental nanoplastics: progress and challenges. Chem. Eng. Sci. 2021;410:128208–128220. doi: 10.1016/j.cej.2020.128208. [DOI] [Google Scholar]
- 20.Lehner R., Weder C., Petri-Fink A., Rothen-Rutishauser B. Emergence of nanoplastic in the environment and possible impact on human health. Environ. Sci. Technol. 2019;53:1748–1765. doi: 10.1021/acs.est.8b05512. [DOI] [PubMed] [Google Scholar]
- 21.Huang Y., Dang F., Yin Y., Fang G., Wang Y., Yu G., Zhou D., Xing B. Weathered microplastics induce silver nanoparticle formation. Environ. Sci. Technol. Lett. 2021;9:179–185. doi: 10.1021/acs.estlett.1c00766. [DOI] [Google Scholar]
- 22.Wegner A., Besseling E., Foekema E.M., Kamermans P., Koelmans A.A. Effects of nanopolystyrene on the feeding behavior of the blue mussel (Mytilus edulis L.) Environ. Toxicol. Chem. 2012;31:2490–2497. doi: 10.1002/etc.1984. [DOI] [PubMed] [Google Scholar]
- 23.Ribeiro F., Okoffo E.D., O'Brien J.W., Fraissinet-Tachet S., O'Brien S., Gallen M., Samanipour S., Kaserzon S., Mueller J.F., Galloway T., Thomas K.V. Quantitative analysis of selected plastics in high-commercial-value australian seafood by pyrolysis gas chromatography mass spectrometry. Environ. Sci. Technol. 2020;54:9408–9417. doi: 10.1021/acs.est.0c02337. [DOI] [PubMed] [Google Scholar]
- 24.Cox K.D., Covernton G.A., Davies H.L., Dower J.F., Juanes F., Dudas S.E. Human consumption of microplastics. Environ. Sci. Technol. 2019;53:7068–7074. doi: 10.1021/acs.est.9b01517. [DOI] [PubMed] [Google Scholar]
- 25.Li L.Z., Luo Y.M., Li R.J., Zhou Q., Peijnenburg W., Yin N., Yang J., Tu C., Zhang Y.C. Effective uptake of submicrometre plastics by crop plants via a crack-entry mode. Nat. Sustain. 2020;3:929–937. doi: 10.1038/s41893-020-0567-9. [DOI] [Google Scholar]
- 26.Alexander J., Barregard L., Bignami M., Ceccatelli S., Cottrill B., Dinovi M., Edler L., Grasl-Kraupp B., Hogstrand C., Hoogenboom L., Knutsen H.K., Nebbia C.S., Oswald I., Petersen A., Rogiers V.M., Rose M., Roudot A.-C., Schwerdtle T., Vleminckx C., Vollmer G., Wallace H., Chain E.P.C.F. Presence of microplastics and nanoplastics in food, with particular focus on seafood. EFSA J. 2016;14:4501–4531. doi: 10.2903/j.efsa.2016.4501. [DOI] [Google Scholar]
- 27.Liebezeit G., Liebezeit E. Non-pollen particulates in honey and sugar. Food Addit. Contam. Part A. 2013;30:2136–2140. doi: 10.1080/19440049.2013.843025. [DOI] [PubMed] [Google Scholar]
- 28.Lee H., Kunz A., Shim W.J., Walther B.A. Microplastic contamination of table salts from Taiwan, including a global review. Sci. Rep. 2019;9:10145–101154. doi: 10.1038/s41598-019-46417-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mintenig S.M., Löder M.G.J., Primpke S., Gerdts G. Low numbers of microplastics detected in drinking water from ground water sources. Sci. Total Environ. 2019;648:631–635. doi: 10.1016/j.scitotenv.2018.08.178. [DOI] [PubMed] [Google Scholar]
- 30.Diaz-Basantes M.F., Conesa J.A., Fullana A. Microplastics in honey, beer, milk and refreshments in Ecuador as emerging contaminants. Sustainability. 2020;12:5514–5531. doi: 10.3390/su12145514. [DOI] [Google Scholar]
- 31.Li D., Shi Y., Yang L., Xiao L., Kehoe D.K., Gun’ko Y.K., Boland J.J., Wang J.J. Microplastic release from the degradation of polypropylene feeding bottles during infant formula preparation. Nat. Food. 2020;1:746–754. doi: 10.1038/s43016-020-00171-y. [DOI] [PubMed] [Google Scholar]
- 32.Su Y., Hu X., Tang H., Lu K., Li H., Liu S., Xing B., Ji R. Steam disinfection releases micro(nano)plastics from silicone-rubber baby teats as examined by optical photothermal infrared microspectroscopy. Nat. Nanotechnol. 2021;17:76–85. doi: 10.1038/s41565-021-00998-x. [DOI] [PubMed] [Google Scholar]
- 33.Mohamed Nor N.H., Kooi M., Diepens N.J., Koelmans A.A. Lifetime accumulation of microplastic in children and adults. Environ. Sci. Technol. 2021;55:5084–5096. doi: 10.1021/acs.est.0c07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prata J.C. Airborne microplastics: consequences to human health? Environ. Pollut. 2018;234:115–126. doi: 10.1016/j.envpol.2017.11.043. [DOI] [PubMed] [Google Scholar]
- 35.Gasperi J., Wright S.L., Dris R., Collard F., Mandin C., Guerrouache M., Langlois V., Kelly F.J., Tassin B. Microplastics in air: are we breathing it in? Curr. Opin. Environ. Sci. Health. 2018;1:1–5. doi: 10.1016/j.coesh.2017.10.002. [DOI] [Google Scholar]
- 36.Ma J., Chen F., Xu H., Jiang H., Liu J., Li P., Chen C.C., Pan K. Face masks as a source of nanoplastics and microplastics in the environment: quantification, characterization, and potential for bioaccumulation. Environ. Pollut. 2021;288:117748–117754. doi: 10.1016/j.envpol.2021.117748. [DOI] [PubMed] [Google Scholar]
- 37.Kelly M.R., Lant N.J., Kurr M., Burgess J.G. Importance of water-volume on the release of microplastic fibers from laundry. Environ. Sci. Technol. 2019;53:11735–11744. doi: 10.1021/acs.est.9b03022. [DOI] [PubMed] [Google Scholar]
- 38.Lett Z., Hall A., Skidmore S., Alves N.J. Environmental microplastic and nanoplastic: exposure routes and effects on coagulation and the cardiovascular system. Environ. Pollut. (Barking, Essex : 1987) 2021;291:118190–118204. doi: 10.1016/j.envpol.2021.118190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ragusa A., Svelato A., Santacroce C., Catalano P., Notarstefano V., Carnevali O., Papa F., Rongioletti M.C.A., Baiocco F., Draghi S., D'Amore E., Rinaldo D., Matta M., Giorgini E. Plasticenta: first evidence of microplastics in human placenta. Environ. Int. 2021;146:106274–106282. doi: 10.1016/j.envint.2020.106274. [DOI] [PubMed] [Google Scholar]
- 40.Toumey C. Notes on environmental nanoscience. Nat. Nanotechnol. 2020;15:250–251. doi: 10.1038/s41565-020-0677-6. [DOI] [PubMed] [Google Scholar]
- 41.Shupe H.J., Boenisch K.M., Harper B.J., Brander S.M., Harper S.L. Effect of nanoplastic type and surface chemistry on particle agglomeration over a salinity gradient. Environ. Toxicol. Chem. 2021;40:1820–1826. doi: 10.1002/etc.5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Al-Sid-Cheikh M., Rowland S.J., Stevenson K., Rouleau C., Henry T.B., Thompson R.C. Uptake, whole-body distribution, and depuration of nanoplastics by the scallop Pecten maximus at environmentally realistic concentrations. Environ. Sci. Technol. 2018;52:14480–14486. doi: 10.1021/acs.est.8b05266. [DOI] [PubMed] [Google Scholar]
- 43.Gamarro E., Ryder J., Elvevoll E., Olsen R. Microplastics in fish and shellfish–A threat to seafood safety? J. Aquat. Food Prod. Technol. 2020;29:1–9. doi: 10.1080/10498850.2020.1739793. [DOI] [Google Scholar]
- 44.Oliveri Conti G., Ferrante M., Banni M., Favara C., Nicolosi I., Cristaldi A., Fiore M., Zuccarello P. Micro- and nano-plastics in edible fruit and vegetables. The first diet risks assessment for the general population. Environ. Res. 2020;187:109677–109684. doi: 10.1016/j.envres.2020.109677. [DOI] [PubMed] [Google Scholar]
- 45.Zhou C.-Q., Lu C.-H., Mai L., Bao L.-J., Liu L.-Y., Zeng E.Y. Response of rice (Oryza sativa L.) roots to nanoplastic treatment at seedling stage. J. Hazard Mater. 2021;401:123412–123422. doi: 10.1016/j.jhazmat.2020.123412. [DOI] [PubMed] [Google Scholar]
- 46.Mortensen N.P., Fennell T.R., Johnson L.M. NanoImpact; 2021. Unintended Human Ingestion of Nanoplastics and Small Microplastics through Drinking Water, Beverages, and Food Sources; pp. 100302–100323. [DOI] [PubMed] [Google Scholar]
- 47.Jin M., Wang X., Ren T., Wang J., Shan J. Microplastics contamination in food and beverages: direct exposure to humans. J. Food Sci. 2021;86:2816–2837. doi: 10.1111/1750-3841.15802. [DOI] [PubMed] [Google Scholar]
- 48.Shruti V.C., Perez-Guevara F., Elizalde-Martinez I., Kutralam-Muniasamy G. Toward a unified framework for investigating micro(nano)plastics in packaged beverages intended for human consumption. Environ. Pollut. 2021;268:115811–115824. doi: 10.1016/j.envpol.2020.115811. [DOI] [PubMed] [Google Scholar]
- 49.Dawson A.L., Kawaguchi S., King C.K., Townsend K.A., King R., Huston W.M., Bengtson Nash S.M. Turning microplastics into nanoplastics through digestive fragmentation by Antarctic krill. Nat. Commun. 2018;9:1001–1009. doi: 10.1038/s41467-018-03465-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yong C.Q.Y., Valiyaveetill S., Tang B.L. Toxicity of microplastics and nanoplastics in mammalian systems. Int. J. Environ. Res. Publ. Health. 2020;17:1509–1533. doi: 10.3390/ijerph17051509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rochman C.M., Hoh E., Hentschel B.T., Kaye S. Long-term field measurement of sorption of organic contaminants to five types of plastic pellets: implications for plastic marine debris. Environ. Sci. Technol. 2013;47:1646–1654. doi: 10.1021/es303700s. [DOI] [PubMed] [Google Scholar]
- 52.Leusch F.D.L., Ziajahromi S. Converting mg/L to particles/L: reconciling the occurrence and toxicity literature on microplastics. Environ. Sci. Technol. 2021;55:11470–11472. doi: 10.1021/acs.est.1c04093. [DOI] [PubMed] [Google Scholar]
- 53.Stubbins A., Law K.L., Munoz S.E., Bianchi T.S., Zhu L. Plastics in the earth system. Science. 2021;373:51–55. doi: 10.1126/science.abb0354. [DOI] [PubMed] [Google Scholar]
- 54.Ramsperger A., Narayana V.K.B., Gross W., Mohanraj J., Thelakkat M., Greiner A., Schmalz H., Kress H., Laforsch C. Environmental exposure enhances the internalization of microplastic particles into cells. Sci. Adv. 2020;6 doi: 10.1126/sciadv.abd1211. eabd1211-eabd1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Song Y.K., Hong S.H., Eo S., Han G.M., Shim W.J. Rapid production of micro- and nanoplastics by fragmentation of expanded polystyrene exposed to sunlight. Environ. Sci. Technol. 2020;54:11191–11200. doi: 10.1021/acs.est.0c02288. [DOI] [PubMed] [Google Scholar]
- 56.Yousif E., Haddad R. Photodegradation and photostabilization of polymers, especially polystyrene: Review. SpringerPlus. 2013;2:398–430. doi: 10.1186/2193-1801-2-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilkes R.A., Aristilde L. Degradation and metabolism of synthetic plastics and associated products by Pseudomonas sp.: capabilities and challenges. J. Appl. Microbiol. 2017;123:582–593. doi: 10.1111/jam.13472. [DOI] [PubMed] [Google Scholar]
- 58.Zhang X., Xia M., Zhao J., Cao Z., Zou W., Zhou Q. Photoaging enhanced the adverse effects of polyamide microplastics on the growth, intestinal health, and lipid absorption in developing zebrafish. Environ. Int. 2022;158:106922–106933. doi: 10.1016/j.envint.2021.106922. [DOI] [PubMed] [Google Scholar]
- 59.Wang X., Zheng H., Zhao J., Luo X., Wang Z., Xing B. Photodegradation elevated the toxicity of polystyrene microplastics to grouper (Epinephelus moara) through disrupting hepatic lipid homeostasis. Environ. Sci. Technol. 2020;54:6202–6212. doi: 10.1021/acs.est.9b07016. [DOI] [PubMed] [Google Scholar]
- 60.Huang B., Wei Z.-B., Yang L.-Y., Pan K., Miao A.-J. Combined toxicity of silver nanoparticles with hematite or plastic nanoparticles toward two freshwater algae. Environ. Sci. Technol. 2019;53:3871–3879. doi: 10.1021/acs.est.8b07001. [DOI] [PubMed] [Google Scholar]
- 61.Gunaalan K., Fabbri E., Capolupo M. The hidden threat of plastic leachates: a critical review on their impacts on aquatic organisms. Water Res. 2020;184:116170–116185. doi: 10.1016/j.watres.2020.116170. [DOI] [PubMed] [Google Scholar]
- 62.Lambert S., Sinclair C., Boxall A. In: Whitacre D.M., editor. Vol. 227. Springer International Publishing; Cham: 2014. Occurrence, degradation, and effect of polymer-based materials in the environment; pp. 1–53. (Rev. Environ. Contam. Toxicol.). [DOI] [PubMed] [Google Scholar]
- 63.Fauvelle V., Garel M., Tamburini C., Nerini D., Castro-Jiménez J., Schmidt N., Paluselli A., Fahs A., Papillon L., Booth A.M., Sempéré R. Organic additive release from plastic to seawater is lower under deep-sea conditions. Nat. Commun. 2021;12:4426–4434. doi: 10.1038/s41467-021-24738-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zimmermann L., Dierkes G., Ternes T.A., Voelker C., Wagner M. Benchmarking the in vitro toxicity and chemical composition of plastic consumer products. Environ. Sci. Technol. 2019;53:11467–11477. doi: 10.1021/acs.est.9b02293. [DOI] [PubMed] [Google Scholar]
- 65.Alimi O.S., Budarz J.F., Hernandez L.M., Tufenkji N. Microplastics and nanoplastics in aquatic environments: aggregation, deposition, and enhanced contaminant transport. Environ. Sci. Technol. 2018;52:1704–1724. doi: 10.1021/acs.est.7b05559. [DOI] [PubMed] [Google Scholar]
- 66.Junaid M., Wang J. Interaction of nanoplastics with extracellular polymeric substances (eps) in the aquatic environment: a special reference to eco-corona formation and associated impacts. Water Res. 2021;201:117319–117337. doi: 10.1016/j.watres.2021.117319. [DOI] [PubMed] [Google Scholar]
- 67.Zumstein M.T., Schintlmeister A., Nelson T.F., Baumgartner R., Woebken D., Wagner M., Kohler H.-P.E., McNeill K., Sander M. Biodegradation of synthetic polymers in soils: tracking carbon into CO2 and microbial biomass. Sci. Adv. 2018;4:9024–9032. doi: 10.1126/sciadv.aas9024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Amaral-Zettler L.A., Zettler E.R., Mincer T.J. Ecology of the plastisphere. Nat. Rev. Microbiol. 2020;18:139–151. doi: 10.1038/s41579-019-0308-0. [DOI] [PubMed] [Google Scholar]
- 69.Purahong W., Wahdan S.F.M., Heinz D., Jariyavidyanont K., Sungkapreecha C., Tanunchai B., Sansupa C., Sadubsarn D., Alaneed R., Heintz-Buschart A., Schaedler M., Geissler A., Kressler J., Buscot F. Back to the future: decomposability of a biobased and biodegradable plastic in field soil environments and its microbiome under ambient and future climates. Environ. Sci. Technol. 2021;55:12337–12351. doi: 10.1021/acs.est.1c02695. [DOI] [PubMed] [Google Scholar]
- 70.Nelson T.F., Reddy C.M., Ward C.P. Product formulation controls the impact of biofouling on consumer plastic photochemical fate in the ocean. Environ. Sci. Technol. 2021;55:8898–8907. doi: 10.1021/acs.est.1c02079. [DOI] [PubMed] [Google Scholar]
- 71.Ghosh S.K., Pal S., Ray S. Study of microbes having potentiality for biodegradation of plastics. Environ. Sci. Pollut. Control Ser. 2013;20:4339–4355. doi: 10.1007/s11356-013-1706-x. [DOI] [PubMed] [Google Scholar]
- 72.Rummel C.D., Jahnke A., Gorokhova E., Kühnel D., Schmitt-Jansen M. Impacts of biofilm formation on the fate and potential effects of microplastic in the aquatic environment. Environ. Sci. Technol. Lett. 2017;4:258–267. doi: 10.1021/acs.estlett.7b00164. [DOI] [Google Scholar]
- 73.Bhagwat G., Carbery M., Tran T.K.A., Grainge I., O'Connor W., Palanisami T. Fingerprinting plastic-associated inorganic and organic matter on plastic aged in the marine environment for a decade. Environ. Sci. Technol. 2021;55:7407–7417. doi: 10.1021/acs.est.1c00262. [DOI] [PubMed] [Google Scholar]
- 74.Wu C.C., Bao L.J., Liu L.Y., Shi L., Tao S., Zeng E.Y. Impact of polymer colonization on the fate of organic contaminants in sediment. Environ. Sci. Technol. 2017;51:10555–10561. doi: 10.1021/acs.est.7b03310. [DOI] [PubMed] [Google Scholar]
- 75.Li D., Deng Y., Wang S., Du H., Xiao G., Wang D. Assessment of nanopolystyrene toxicity under fungal infection condition in Caenorhabditis elegans. Ecotoxicol. Environ. Saf. 2020;197:110625–110634. doi: 10.1016/j.ecoenv.2020.110625. [DOI] [PubMed] [Google Scholar]
- 76.Koelmans A.A., Bakir A., Burton G.A., Janssen C.R. Microplastic as a vector for chemicals in the aquatic environment: critical review and model-supported reinterpretation of empirical studies. Environ. Sci. Technol. 2016;50:3315–3326. doi: 10.1021/acs.est.5b06069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sarkar D.J., Das Sarkar S., Das B.K., Sahoo B.K., Das A., Nag S.K., Manna R.K., Behera B.K., Samanta S. Occurrence, fate and removal of microplastics as heavy metal vector in natural wastewater treatment wetland system. Water Res. 2021;192:116853–116867. doi: 10.1016/j.watres.2021.116853. [DOI] [PubMed] [Google Scholar]
- 78.Ossmann B.E., Sarau G., Holtmannspotter H., Pischetsrieder M., Christiansen S.H., Dicke W. Small-sized microplastics and pigmented particles in bottled mineral water. Water Res. 2018;141:307–316. doi: 10.1016/j.watres.2018.05.027. [DOI] [PubMed] [Google Scholar]
- 79.Zhang H.B., Zhou Q., Xie Z.Y., Zhou Y., Tu C., Fu C.C., Mi W.Y., Ebinghaus R., Christie P., Luo Y.M. Occurrences of organophosphorus esters and phthalates in the microplastics from the coastal beaches in north China. Sci. Total Environ. 2018;616:1505–1512. doi: 10.1016/j.scitotenv.2017.10.163. [DOI] [PubMed] [Google Scholar]
- 80.Liu J., Ma Y., Zhu D.Q., Xia T.J., Qi Y., Yao Y., Guo X.R., Ji R., Chen W. Polystyrene Nanoplastics-enhanced contaminant transport: role of irreversible adsorption in glassy polymeric domain. Environ. Sci. Technol. 2018;52:2677–2685. doi: 10.1021/acs.est.7b05211. [DOI] [PubMed] [Google Scholar]
- 81.Liu J., Zhang T., Tian L.L., Liu X.L., Qi Z.C., Ma Y.N., Ji R., Chen W. Aging Significantly Affects mobility and contaminant-mobilizing ability of nanoplastics in saturated loamy sand. Environ. Sci. Technol. 2019;53:5805–5815. doi: 10.1021/acs.est.9b00787. [DOI] [PubMed] [Google Scholar]
- 82.Ma Y.N., Huang A.N., Cao S.Q., Sun F.F., Wang L.H., Guo H.Y., Ji R. Effects of nanoplastics and microplastics on toxicity, bioaccumulation, and environmental fate of phenanthrene in fresh water. Environ. Pollut. 2016;219:166–173. doi: 10.1016/j.envpol.2016.10.061. [DOI] [PubMed] [Google Scholar]
- 83.Wang F., Wong C.S., Chen D., Lu X.W., Wang F., Zeng E.Y. Interaction of toxic chemicals with microplastics: a critical review. Water Res. 2018;139:208–219. doi: 10.1016/j.watres.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 84.Eder M.L., Oliva-Teles L., Pinto R., Carvalho A.P., Almeida C.M.R., Hornek-Gausterer R., Guimaraes L. Microplastics as a vehicle of exposure to chemical contamination in freshwater systems: current research status and way forward. J. Hazard Mater. 2021;417 doi: 10.1016/j.jhazmat.2021.125980. 125980-112593. [DOI] [PubMed] [Google Scholar]
- 85.Koelmans A.A., Besseling E., Wegner A., Foekema E.M. Plastic as a carrier of POPs to aquatic organisms: a model analysis. Environ. Sci. Technol. 2013;47:7812–7820. doi: 10.1021/es401169n. [DOI] [PubMed] [Google Scholar]
- 86.Oliveira M., Almeida M., Miguel I. A micro(nano)plastic boomerang tale: a never ending story? TrAC Trends Anal. Chem. (Reference Ed.) 2019;112:196–200. doi: 10.1016/j.trac.2019.01.005. [DOI] [Google Scholar]
- 87.Walczak A.P., Hendriksen P.J.M., Woutersen R.A., van der Zande M., Undas A.K., Helsdingen R., van den Berg H.H.J., Rietjens I.M.C.M., Bouwmeester H. Bioavailability and biodistribution of differently charged polystyrene nanoparticles upon oral exposure in rats. J. Nanopart. Res. 2015;17:231–244. doi: 10.1007/s11051-015-3029-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Walczak A.P., Kramer E., Hendriksen P.J.M., Tromp P., Helsper J.P.F.G., van der Zande M., Rietjens I.M.C.M., Bouwmeester H. Translocation of differently sized and charged polystyrene nanoparticles in in vitro intestinal cell models of increasing complexity. Nanotoxicology. 2015;9:453–461. doi: 10.3109/17435390.2014.944599. [DOI] [PubMed] [Google Scholar]
- 89.Kulkarni S.A., Feng S.-S. Effects of particle size and surface modification on cellular uptake and biodistribution of polymeric nanoparticles for drug delivery. Pharm. Res. 2013;30:2512–2522. doi: 10.1007/s11095-012-0958-3. [DOI] [PubMed] [Google Scholar]
- 90.Xu M., Halimu G., Zhang Q., Song Y., Fu X., Li Y., Li Y., Zhang H. Internalization and toxicity: a preliminary study of effects of nanoplastic particles on human lung epithelial cell. Sci. Total Environ. 2019;694:133794–133804. doi: 10.1016/j.scitotenv.2019.133794. [DOI] [PubMed] [Google Scholar]
- 91.Cole M., Galloway T.S. Ingestion of nanoplastics and microplastics by Pacific oyster larvae. Environ. Sci. Technol. 2015;49:14625–14632. doi: 10.1021/acs.est.5b04099. [DOI] [PubMed] [Google Scholar]
- 92.de Ruijter V.N., Redondo-Hasselerharm P.E., Gouin T., Koelmans A.A. Quality criteria for microplastic effect studies in the context of risk assessment: a critical review. Environ. Sci. Technol. 2020;54:11692–11705. doi: 10.1021/acs.est.0c03057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sun X.D., Yuan X.Z., Jia Y., Feng L.J., Zhu F.P., Dong S.S., Liu J., Kong X., Tian H., Duan J.L., Ding Z., Wang S.G., Xing B. Differentially charged nanoplastics demonstrate distinct accumulation in Arabidopsis thaliana. Nat. Nanotechnol. 2020;15:755–760. doi: 10.1038/s41565-020-0707-4. [DOI] [PubMed] [Google Scholar]
- 94.Della Torre C., Bergami E., Salvati A., Faleri C., Cirino P., Dawson K.A., Corsi I. Accumulation and embryotoxicity of polystyrene nanoparticles at early stage of development of sea urchin embryos Paracentrotus lividus. Environ. Sci. Technol. 2014;48:12302–12311. doi: 10.1021/es502569w. [DOI] [PubMed] [Google Scholar]
- 95.Ziajahromi S., Kumar A., Neale P.A., Leusch F.D.L. Impact of microplastic beads and fibers on waterflea (Ceriodaphnia dubia) survival, growth, and reproduction: implications of single and mixture exposures. Environ. Sci. Technol. 2017;51:13397–13406. doi: 10.1021/acs.est.7b03574. [DOI] [PubMed] [Google Scholar]
- 96.Au S.Y., Bruce T.F., Bridges W.C., Klaine S.J. Responses of Hyalella azteca to acute and chronic microplastic exposures. Environ. Toxicol. Chem. 2015;34:2564–2572. doi: 10.1002/etc.3093. [DOI] [PubMed] [Google Scholar]
- 97.Gray A.D., Weinstein J.E. Size- and shape-dependent effects of microplastic particles on adult daggerblade grass shrimp (Palaemonetes pugio) Environ. Toxicol. Chem. 2017;36:3074–3080. doi: 10.1002/etc.3881. [DOI] [PubMed] [Google Scholar]
- 98.Yip Y.J., Lee S.S.C., Neo M.L., Teo S.L.-M., Valiyaveettil S. A comparative investigation of toxicity of three polymer nanoparticles on acorn barnacle (Amphibalanus amphitrite) Sci. Total Environ. 2021:150965. doi: 10.1016/j.scitotenv.2021.150965. [DOI] [PubMed] [Google Scholar]
- 99.Guarnieri D., Sánchez-Moreno P., Del Rio Castillo A.E., Bonaccorso F., Gatto F., Bardi G., Martín C., Vázquez E., Catelani T., Sabella S., Pompa P.P. Biotransformation and biological interaction of graphene and graphene oxide during simulated oral ingestion. Small. 2018;14:1800227–1800238. doi: 10.1002/smll.201800227. [DOI] [PubMed] [Google Scholar]
- 100.Gustafson H.H., Holt-Casper D., Grainger D.W., Ghandehari H. Nanoparticle uptake: the phagocyte problem. Nano Today. 2015;10:487–510. doi: 10.1016/j.nantod.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Frankel R., Ekvall M.T., Kelpsiene E., Hansson L.-A., Cedervall T. Controlled protein mediated aggregation of polystyrene nanoplastics does not reduce toxicity towards Daphnia magna. Environ. Sci. Nano. 2020;7:1518–1524. doi: 10.1039/C9EN01236B. [DOI] [Google Scholar]
- 102.Tenzer S., Docter D., Kuharev J., Musyanovych A., Fetz V., Hecht R., Schlenk F., Fischer D., Kiouptsi K., Reinhardt C., Landfester K., Schild H., Maskos M., Knauer S.K., Stauber R.H. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat. Nanotechnol. 2013;8:772–781. doi: 10.1038/nnano.2013.181. [DOI] [PubMed] [Google Scholar]
- 103.Nasser F., Lynch I. Secreted protein eco-corona mediates uptake and impacts of polystyrene nanoparticles on Daphnia magna. J. Proteonomics. 2016;137:45–51. doi: 10.1016/j.jprot.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 104.Saavedra J., Stoll S., Slaveykova V.I. Influence of nanoplastic surface charge on eco-corona formation, aggregation and toxicity to freshwater zooplankton. Environ. Pollut. 2019;252:715–722. doi: 10.1016/j.envpol.2019.05.135. [DOI] [PubMed] [Google Scholar]
- 105.Fadare O.O., Wan B., Liu K., Yang Y., Zhao L., Guo L.-H. Eco-corona vs protein corona: effects of humic substances on corona formation and nanoplastic particle toxicity in Daphnia magna. Environ. Sci. Technol. 2020;54:8001–8009. doi: 10.1021/acs.est.0c00615. [DOI] [PubMed] [Google Scholar]
- 106.Gopinath P.M., Saranya V., Vijayakumar S., Mythili Meera M., Ruprekha S., Kunal R., Pranay A., Thomas J., Mukherjee A., Chandrasekaran N. Assessment on interactive prospectives of nanoplastics with plasma proteins and the toxicological impacts of virgin, coronated and environmentally released-nanoplastics. Sci. Rep. 2019;9:8860–8875. doi: 10.1038/s41598-019-45139-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tan Y., Zhu X., Wu D., Song E., Song Y. Compromised autophagic effect of polystyrene nanoplastics mediated by protein corona was recovered after lysosomal degradation of corona. Environ. Sci. Technol. 2020;54:11485–11493. doi: 10.1021/acs.est.0c04097. [DOI] [PubMed] [Google Scholar]
- 108.Yoon H., Kim J.-T., Chang Y.-S., Kim E.-J. Fragmentation of nanoplastics driven by plant–microbe rhizosphere interaction during abiotic stress combination. Environ. Sci. Nano. 2021;8:2802–2810. doi: 10.1039/D1EN00230A. [DOI] [Google Scholar]
- 109.Lithner D., Larsson A., Dave G. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci. Total Environ. 2011;409:3309–3324. doi: 10.1016/j.scitotenv.2011.04.038. [DOI] [PubMed] [Google Scholar]
- 110.Wright S.L., Kelly F.J. Plastic and human health: a micro issue? Environ. Sci. Technol. 2017;51:6634–6647. doi: 10.1021/acs.est.7b00423. [DOI] [PubMed] [Google Scholar]
- 111.Lu Y.-Y., Li H., Ren H., Zhang X., Huang F., Zhang D., Huang Q., Zhang X. Size-dependent effects of polystyrene nanoplastics on autophagy response in human umbilical vein endothelial cells. J. Hazard Mater. 2022;421:126770–126780. doi: 10.1016/j.jhazmat.2021.126770. [DOI] [PubMed] [Google Scholar]
- 112.André P., Saubaméa B., Cochois-Guégan V., Marie-Claire C., Cattelotte J., Smirnova M., Schinkel A.H., Scherrmann J.-M., Cisternino S. Transport of biogenic amine neurotransmitters at the mouse blood-retina and blood-brain barriers by uptake1 and uptake2. J. Cerebr. Blood Flow Metabol. 2012;32:1989–2001. doi: 10.1038/jcbfm.2012.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mruk D.D., Cheng C.Y. The mammalian blood-testis barrier: its biology and regulation. Endocr. Rev. 2015;36:564–591. doi: 10.1210/er.2014-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mattsson K., Johnson E.V., Malmendal A., Linse S., Hansson L.-A., Cedervall T. Brain damage and behavioural disorders in fish induced by plastic nanoparticles delivered through the food chain. Sci. Rep. 2017;7:11452–11459. doi: 10.1038/s41598-017-10813-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Prust M., Meijer J., Westerink R.H.S. The plastic brain: neurotoxicity of micro- and nanoplastics. Part. Fibre Toxicol. 2020;17:24–40. doi: 10.1186/s12989-020-00358-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Grafmueller S., Manser P., Diener L., Diener P.-A., Maeder-Althaus X., Maurizi L., Jochum W., Krug H.F., Buerki-Thurnherr T., von Mandach U., Wick P. Bidirectional transfer study of polystyrene nanoparticles across the placental barrier in an ex vivo human placental perfusion model. Environ. Health Perspect. 2015;123:1280–1286. doi: 10.1289/ehp.1409271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pitt J.A., Trevisan R., Massarsky A., Kozal J.S., Levin E.D., Di Giulio R.T. Maternal transfer of nanoplastics to offspring in zebrafish (Danio rerio): a case study with nanopolystyrene. Sci. Total Environ. 2018;643:324–334. doi: 10.1016/j.scitotenv.2018.06.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mortensen L.J., Oberdorster G., Pentland A.P., DeLouise L.A. In vivo skin penetration of quantum dot nanoparticles in the murine model: the effect of UVR. Nano Lett. 2008;8:2779–2787. doi: 10.1021/nl801323y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fiorentino I., Gualtieri R., Barbato V., Mollo V., Braun S., Angrisani A., Turano M., Furia M., Netti P.A., Guarnieri D., Fusco S., Talevi R. Energy independent uptake and release of polystyrene nanoparticles in primary mammalian cell cultures. Exp. Cell Res. 2015;330:240–247. doi: 10.1016/j.yexcr.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 120.Shi Q., Tang J., Liu R., Wang L. Toxicity in vitro reveals potential impacts of microplastics and nanoplastics on human health: a review. Crit. Rev. Environ. Sci. Technol. 2021:1–33. doi: 10.1080/10643389.2021.1951528. [DOI] [Google Scholar]
- 121.Yu X., Feizpour A., Ramirez N.-G.P., Wu L., Akiyama H., Xu F., Gummuluru S., Reinhard B.M. Glycosphingolipid-functionalized nanoparticles recapitulate Cd169-dependent Hiv-1 uptake and trafficking in dendritic cells. Nat. Commun. 2014;5:4136–4148. doi: 10.1038/ncomms5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Besseling E., Wang B., Lurling M., Koelmans A.A. Nanoplastic affects growth of S. obliquus and reproduction of D. magna. Environ. Sci. Technol. 2014;48:12336–12343. doi: 10.1021/es503001d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Trevisan R., Voy C., Chen S., Di Giulio R.T. Nanoplastics decrease the toxicity of a complex pah mixture but impair mitochondrial energy production in developing zebrafish. Environ. Sci. Technol. 2019;53:8405–8415. doi: 10.1021/acs.est.9b02003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jeong C.-B., Kang H.-M., Lee Y.H., Kim M.-S., Lee J.-S., Seo J.S., Wang M., Lee J.-S. Nanoplastic ingestion enhances toxicity of persistent organic pollutants (POPs) in the monogonont rotifer brachionus koreanus via multixenobiotic resistance (MXR) disruption. Environ. Sci. Technol. 2018;52:11411–11418. doi: 10.1021/acs.est.8b03211. [DOI] [PubMed] [Google Scholar]
- 125.Zettler E.R., Mincer T.J., Amaral-Zettler L.A. Life in the "Plastisphere": microbial communities on plastic marine debris. Environ. Sci. Technol. 2013;47:7137–7146. doi: 10.1021/es401288x. [DOI] [PubMed] [Google Scholar]
- 126.Fournier E., Etienne-Mesmin L., Grootaert C., Jelsbak L., Syberg K., Blanquet-Diot S., Mercier-Bonin M. Microplastics in the human digestive environment: a focus on the potential and challenges facing in vitro gut model development. J. Hazard Mater. 2021;415:125632–125647. doi: 10.1016/j.jhazmat.2021.125632. [DOI] [PubMed] [Google Scholar]
- 127.Zhu B.-K., Fang Y.-M., Zhu D., Christie P., Ke X., Zhu Y.-G. Exposure to nanoplastics disturbs the gut microbiome in the soil oligochaete Enchytraeus crypticus. Environ. Pollut. 2018;239:408–415. doi: 10.1016/j.envpol.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 128.Xiang Q., Zhu D., Chen Q.L., O'Connor P., Yang X.R., Qiao M., Zhu Y.G. Adsorbed sulfamethoxazole exacerbates the effects of polystyrene (similar to 2 μm) on gut microbiota and the antibiotic resistome of a soil collembolan. Environ. Sci. Technol. 2019;53:12823–12834. doi: 10.1021/acs.est.9b04795. [DOI] [PubMed] [Google Scholar]
- 129.Peng C., Zhang X., Zhang X., Liu C., Chen Z., Sun H., Wang L. Bacterial community under the influence of microplastics in indoor environment and the health hazards associated with antibiotic resistance genes. Environ. Sci. Technol. 2021;56:422–432. doi: 10.1021/acs.est.1c04520. [DOI] [PubMed] [Google Scholar]
- 130.Chae Y., Kim D., Choi M.-J., Cho Y., An Y.-J. Impact of nano-sized plastic on the nutritional value and gut microbiota of whiteleg shrimp Litopenaeus vannamei via dietary exposure. Environ. Int. 2019;130:104848–104856. doi: 10.1016/j.envint.2019.05.042. [DOI] [PubMed] [Google Scholar]
- 131.Watkins L., Sullivan P.J., Walter M.T. What you net depends on if you grab: a meta-analysis of sampling method's impact on measured aquatic microplastic concentration. Environ. Sci. Technol. 2021;55:12930–12942. doi: 10.1021/acs.est.1c03019. [DOI] [PubMed] [Google Scholar]
- 132.Zhou X.X., He S., Gao Y., Li Z.C., Chi H.Y., Li C.J., Wang D.J., Yan B. Protein corona-mediated extraction for quantitative analysis of nanoplastics in environmental waters by pyrolysis gas chromatography/mass spectrometry. Anal. Chem. 2021;93:6698–6705. doi: 10.1021/acs.analchem.1c00156. [DOI] [PubMed] [Google Scholar]
- 133.Gangadoo S., Owen S., Rajapaksha P., Plaisted K., Cheeseman S., Haddara H., Truong V.K., Ngo S.T., Vu V.V., Cozzolino D., Elbourne A., Crawford R., Latham K., Chapman J. Nano-plastics and their analytical characterisation and fate in the marine environment: from source to sea. Sci. Total Environ. 2020;732:138792–138813. doi: 10.1016/j.scitotenv.2020.138792. [DOI] [PubMed] [Google Scholar]
- 134.Fang C., Sobhani Z., Zhang X., Gibson C.T., Tang Y., Naidu R. Identification and visualisation of microplastics/nanoplastics by Raman imaging (ii): smaller than the diffraction limit of laser? Water Res. 2020;183:116046–116057. doi: 10.1016/j.watres.2020.116046. [DOI] [PubMed] [Google Scholar]
- 135.Sarau G., Kling L., Ossmann B.E., Unger A.-K., Vogler F., Christiansen S.H. Correlative microscopy and spectroscopy workflow for microplastics. Appl. Spectrosc. 2020;74:1155–1160. doi: 10.1177/0003702820916250. [DOI] [PubMed] [Google Scholar]
- 136.Correia M., Loeschner K. Detection of nanoplastics in food by asymmetric flow field-flow fractionation coupled to multi-angle light scattering: possibilities, challenges and analytical limitations. Anal. Bioanal. Chem. 2018;410:5603–5615. doi: 10.1007/s00216-018-0919-8. [DOI] [PubMed] [Google Scholar]
- 137.Blancho F., Davranche M., Hadri H.E., Grassl B., Gigault J. Nanoplastics identification in complex environmental matrices: strategies for polystyrene and polypropylene. Environ. Sci. Technol. 2021;55:8753–8759. doi: 10.1021/acs.est.1c01351. [DOI] [PubMed] [Google Scholar]
- 138.Zhou X.-X., He S., Gao Y., Chi H.-Y., Wang D.-J., Li Z.-C., Yan B. Quantitative analysis of polystyrene and poly (methyl methacrylate) nanoplastics in tissues of aquatic animals. Environ. Sci. Technol. 2021;55:3032–3040. doi: 10.1021/acs.est.0c08374. [DOI] [PubMed] [Google Scholar]
- 139.Lin Y., Huang X., Liu Q., Lin Z., Jiang G. Thermal fragmentation enhanced identification and quantification of polystyrene micro/nanoplastics in complex media. Talanta. 2020;208:120478–120486. doi: 10.1016/j.talanta.2019.120478. [DOI] [PubMed] [Google Scholar]
- 140.Wu P., Tang Y., Cao G., Li J., Wang S., Chang X., Dang M., Jin H., Zheng C., Cai Z. Determination of environmental micro(nano)plastics by matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry. Anal. Chem. 2020;92:14346–14356. doi: 10.1021/acs.analchem.0c01928. [DOI] [PubMed] [Google Scholar]
- 141.Li P., Li Q., Hao Z., Yu S., Liu J. Analytical methods and environmental processes of nanoplastics. J. Environ. Sci. 2020;94:88–99. doi: 10.1016/j.jes.2020.03.057. [DOI] [PubMed] [Google Scholar]
- 142.Jimenez-Lamana J., Marigliano L., Allouche J., Grassl B., Szpunar J., Reynaud S. A novel strategy for the detection and quantification of nanoplastics by single particle inductively coupled plasma mass spectrometry (ICP-MS) Anal. Chem. 2020;92:11664–11672. doi: 10.1021/acs.analchem.0c01536. [DOI] [PubMed] [Google Scholar]
- 143.Lai Y., Dong L., Li Q., Li P., Hao Z., Yu S., Liu J. Counting nanoplastics in environmental waters by single particle inductively coupled plasma mass spectroscopy after cloud-point extraction and in situ labeling of gold nanoparticles. Environ. Sci. Technol. 2021;55:4783–4791. doi: 10.1021/acs.est.0c06839. [DOI] [PubMed] [Google Scholar]
- 144.Sander M., Kohler H.-P.E., McNeill K. Assessing the environmental transformation of nanoplastic through C-13-labelled polymers. Nat. Nanotechnol. 2019;14:301–303. doi: 10.1038/s41565-019-0420-3. [DOI] [PubMed] [Google Scholar]
- 145.Koelmans A.A., Redondo-Hasselerharm P.E., Nor N.H.M., Kooi M. Solving the nonalignment of methods and approaches used in microplastic research to consistently characterize risk. Environ. Sci. Technol. 2020;54:12307–12315. doi: 10.1021/acs.est.0c02982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rist S., Baun A., Almeda R., Hartmann N.B. Ingestion and effects of micro- and nanoplastics in blue mussel (Mytilus edulis) larvae. Mar. Pollut. Bull. 2019;140:423–430. doi: 10.1016/j.marpolbul.2019.01.069. [DOI] [PubMed] [Google Scholar]
- 147.Miao L., Hou J., You G., Liu Z., Liu S., Li T., Mo Y., Guo S., Qu H. Acute effects of nanoplastics and microplastics on periphytic biofilms depending on particle size, concentration and surface modification. Environ. Pollut. 2019;255:113300–113309. doi: 10.1016/j.envpol.2019.113300. [DOI] [PubMed] [Google Scholar]
- 148.Tallec K., Huvet A., Di Poi C., González-Fernández C., Lambert C., Petton B., Le Goïc N., Berchel M., Soudant P., Paul-Pont I. Nanoplastics impaired oyster free living stages, gametes and embryos. Environ. Pollut. 2018;242:1226–1235. doi: 10.1016/j.envpol.2018.08.020. [DOI] [PubMed] [Google Scholar]
- 149.Besseling E., Redondo-Hasselerharm P., Foekema E.M., Koelmans A.A. Quantifying ecological risks of aquatic micro- and nanoplastic. Crit. Rev. Environ. Sci. Technol. 2019;49:32–80. doi: 10.1080/10643389.2018.1531688. [DOI] [Google Scholar]
- 150.Lusher A. In: Marine Anthropogenic Litter. Bergmann M., Gutow L., Klages M., editors. Springer International Publishing; Cham: 2015. Microplastics in the marine environment: distribution, interactions and effects; pp. 245–307. [Google Scholar]
- 151.Lehel J., Murphy S. Microplastics in the food chain: food safety and environmental aspects. Rev. Environ. Contam. Toxicol. 2021;259:1–49. doi: 10.1007/398_2021_77. [DOI] [PubMed] [Google Scholar]
- 152.Chae Y., Kim D., Kim S.W., An Y.J. Trophic transfer and individual impact of nano-sized polystyrene in a four-species freshwater food chain. Sci. Rep. 2018;8:284–295. doi: 10.1038/s41598-017-18849-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chae Y., An Y.J. Nanoplastic ingestion induces behavioral disorders in terrestrial snails: trophic transfer effects via vascular plants. Environ. Sci. Nano. 2020;7:975–983. doi: 10.1039/c9en01335k. [DOI] [Google Scholar]
- 154.Winkler D.A. Role of artificial intelligence and machine learning in nanosafety. Small. 2020;16:2001883–2001896. doi: 10.1002/smll.202001883. [DOI] [PubMed] [Google Scholar]
- 155.Min K., Cuiffi J.D., Mathers R.T. Ranking environmental degradation trends of plastic marine debris based on physical properties and molecular structure. Nat. Commun. 2020;11:727–738. doi: 10.1038/s41467-020-14538-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kholodovych V., Smith J.R., Knight D., Abramson S., Kohn J., Welsh W.J. Accurate predictions of cellular response using QSPR: a feasibility test of rational design of polymeric biomaterials. Polymer. 2004;45:7367–7379. doi: 10.1016/j.polymer.2004.09.002. [DOI] [Google Scholar]
- 157.Dundas A.A., Sanni O., Dubern J.-F., Dimitrakis G., Hook A.L., Irvine D.J., Williams P., Alexander M.R. Validating a predictive structure–property relationship by discovery of novel polymers which reduce bacterial biofilm formation. Adv. Mater. 2019;31:1903513–1903519. doi: 10.1002/adma.201903513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Liu X., Gharasoo M., Shi Y., Sigmund G., Hüffer T., Duan L., Wang Y., Ji R., Hofmann T., Chen W. Key physicochemical properties dictating gastrointestinal bioaccessibility of microplastics-associated organic xenobiotics: insights from a deep learning approach. Environ. Sci. Technol. 2020;54:12051–12062. doi: 10.1021/acs.est.0c02838. [DOI] [PubMed] [Google Scholar]
- 159.Geyer R., Jambeck J.R., Law K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017;3:e1700782–e1700787. doi: 10.1126/sciadv.1700782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Law K.L., Narayan R. Reducing environmental plastic pollution by designing polymer materials for managed end-of-life. Nat. Rev. Mater. 2021;7:104–116. doi: 10.1038/s41578-021-00382-0. [DOI] [Google Scholar]
- 161.Dilkes-Hoffman L.S., Pratt S., Lant P.A., Laycock B. In: Plastics to Energy. Al-Salem S.M., editor. William Andrew Publishing; 2019. 19 - the role of biodegradable plastic in solving plastic solid waste accumulation; pp. 469–505. [Google Scholar]
- 162.Cheng J., Jacquin J., Conan P., Pujo-Pay M., Barbe V., George M., Fabre P., Bruzaud S., Ter Halle A., Meistertzheim A.-L., Ghiglione J.-F. Relative influence of plastic debris size and shape, chemical composition and phytoplankton-bacteria interactions in driving seawater plastisphere abundance, diversity and activity. Front. Microbiol. 2021;11:610231–610248. doi: 10.3389/fmicb.2020.610231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhou Y., Kumar M., Sarsaiya S., Sirohi R., Awasthi S.K., Sindhu R., Binod P., Pandey A., Bolan N.S., Zhang Z., Singh L., Kumar S., Awasthi M.K. Challenges and opportunities in bioremediation of micro-nano plastics: a review. Sci. Total Environ. 2022;802:149823–149838. doi: 10.1016/j.scitotenv.2021.149823. [DOI] [PubMed] [Google Scholar]
- 164.Yoshida S., Hiraga K., Takehana T., Taniguchi I., Yamaji H., Maeda Y., Toyohara K., Miyamoto K., Kimura Y., Oda K. A bacterium that degrades and assimilates poly(ethylene terephthalate) Science. 2016;351:1196–1199. doi: 10.1126/science.aad6359. [DOI] [PubMed] [Google Scholar]
- 165.Knott B.C., Erickson E., Allen M.D., Gado J.E., Graham R., Kearns F.L., Pardo I., Topuzlu E., Anderson J.J., Austin H.P., Dominick G., Johnson C.W., Rorrer N.A., Szostkiewicz C.J., Copie V., Payne C.M., Woodcock H.L., Donohoe B.S., Beckham G.T., McGeehan J.E. Characterization and engineering of a two-enzyme system for plastics depolymerization. P. Natl. Acad. Sci. 2020;117:25476–25485. doi: 10.1073/pnas.2006753117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tournier V., Topham C.M., Gilles A., David B., Folgoas C., Moya-Leclair E., Kamionka E., Desrousseaux M.L., Texier H., Gavalda S., Cot M., Guemard E., Dalibey M., Nomme J., Cioci G., Barbe S., Chateau M., Andre I., Duquesne S., Marty A. An engineered pet depolymerase to break down and recycle plastic bottles. Nature. 2020;580:216–219. doi: 10.1038/s41586-020-2149-4. [DOI] [PubMed] [Google Scholar]
- 167.Uk H.M. 2020. Revenue & Customs, Introduction of a New Plastic Packing Tax.https://www.gov.uk/government/publications/introduction-of-plastic-packaging-tax-from-april-2022/introduction-of-plastic-packaging-tax-2021 [Google Scholar]
- 168.The State Council . 2021. People's Republic of China, Regulations of Mail and Delivery Packaging.https://www.gov.cn/zhengce/zhengceku/2021-02/25/content_5588767.htm [in Chinese] [Google Scholar]
- 169.United Nations . 2022. https://news.un.org/en/story/2022/02/1113142 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.