Abstract
Heavy metal(loid)s (HMs) have caused serious environmental pollution and health risks. Although the past few years have witnessed the achievements of studies on environmental behavior of HMs, the related toxicity mechanisms, and pollution control, their relationship remains a mystery. Researchers generally focused on one topic independently without comprehensive considerations due to the knowledge gap between environmental science and human health. Indeed, the full life cycle control of HMs is crucial and should be reconsidered with the combination of the occurrence, transport, and fate of HMs in the environment. Therefore, we started by reviewing the environmental behaviors of HMs which are affected by a variety of natural factors as well as their physicochemical properties. Furthermore, the related toxicity mechanisms were discussed according to exposure route, toxicity mechanism, and adverse consequences. In addition, the current state-of-the-art of available technologies for pollution control of HMs wastewater and solid wastes were summarized. Finally, based on the research trend, we proposed that advanced in-operando characterizations will help us better understand the fundamental reaction mechanisms, and big data analysis approaches will aid in establishing the prediction model for risk management.
Keywords: Heavy metal(loid)s, Full life cycle, Toxicity, Environment, Pollution control
Graphical abstract
Highlights
-
•
The full life cycle evaluations of HMs were reconsidered to map the links between HMs and their related health issues.
-
•
The related environmental fate, toxicity mechanisms to human health and control methods/technologies of HMs were summarized.
-
•
The perspectives to narrow the knowledge gap between HMs and their related toxic mechanism to human health were discussed.
1. Introduction
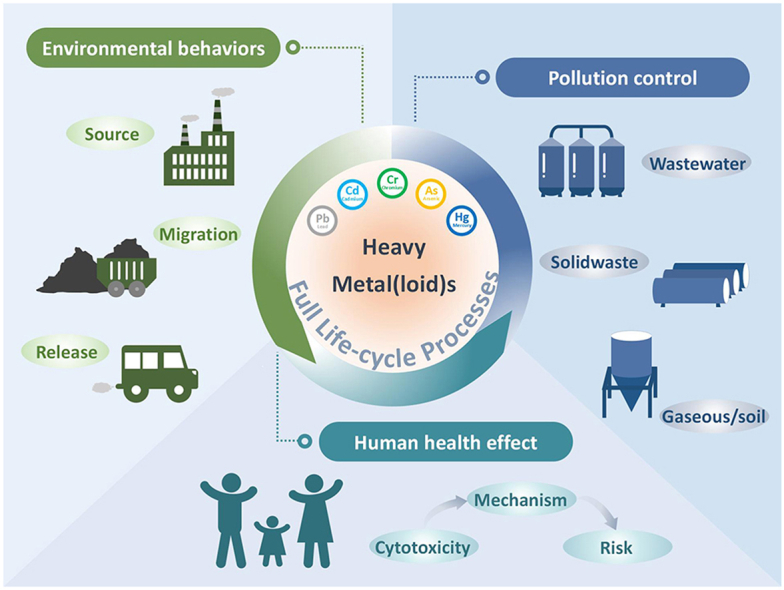
With the staggering advancements of industrialization and urbanization, our society is facing great opportunities for economic development. Nevertheless, the environment is seriously harmed by numerous pollutants due to human activities. Among all these pollutants, heavy metal(loid)s (HMs) widely produced from metallurgy, and other industries have caused great concerns due to their toxicity and bioconcentration [1]. Although numerous advanced technologies have been employed to remediate HMs in the areas of soil, water treatment, and solid waste disposal [2,3], the HMs have still caused wide-ranging health effects for humans and wildlife [4]. During the past decades, researchers have devoted on exploring the migration of HMs, the toxicity caused by HMs and their relationships. However, the considerations of estimating effective links between HMs and their derived health problems were easily ignored. To eliminate the increasing concerns of HMs pollutants, it is essential to systematically study the full life cycle control of HMs, thus enhancing the recognition of their derived problems on human health.
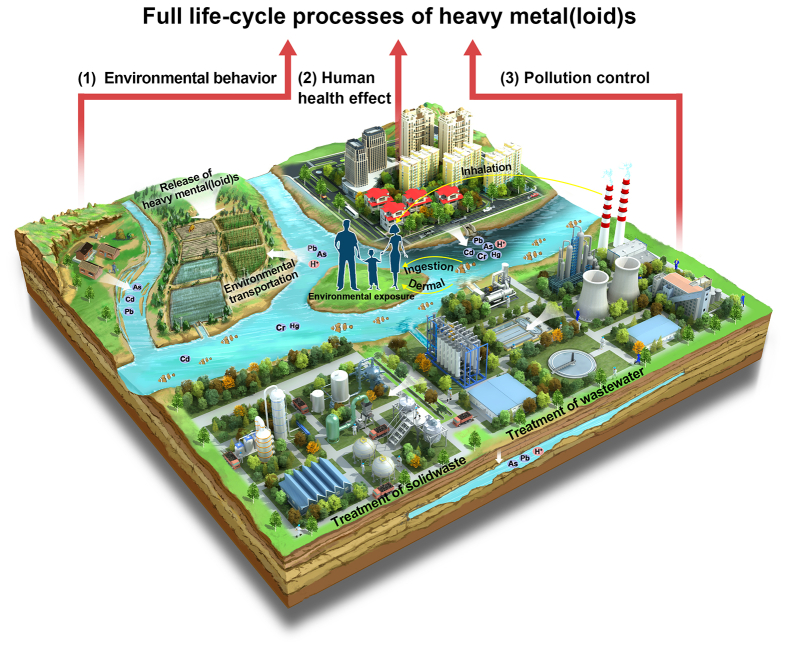
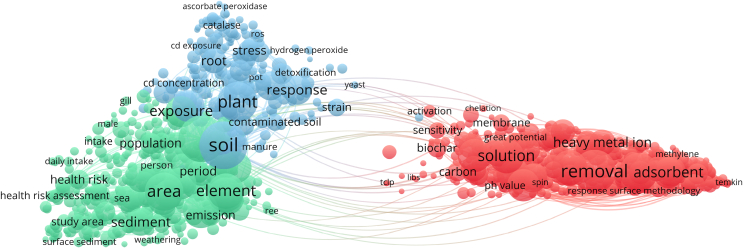
The full life cycle process of HMs control generally involves multidisciplinary subjects, including environment, geology, and medicine. It is difficult to identify the correlations and the migrations of HMs during the full life cycle process. As shown in Fig. 1, HMs, discharged into the environment, have complex behavior, such as migration, accumulation, stability, and organic reaction. The spatial and temporal transport scales of HMs depend primarily on their chemical and physical forms [5,6]. Furthermore, the intensity of interactions between HMs and environment media affects the redistribution of HMs in terrestrial, freshwater, and marine ecosystems [7]. Due to the characteristics of the universality, easy migration, and nonbiodegradability, HMs could be easily transferred and enriched through food chains and drinking water, threatening human health to some extent [[8], [9], [10]]. In addition, HMs could be easily accumulated and stabilized in the biological system via various exposure routes, including inhalation [11], ingestion [12], and dermal contact [13]. Indeed, the various chemical and physical characteristics of HMs might lead to different human exposure routes, internal accumulation, metabolism behaviors, and target/non-target organ toxicity, respectively. Interestingly, several research groups have made promising experiments on interaction with a large number of cellular components and toxicity mechanisms in the early stages [[14], [15], [16], [17]]. In response to the stress caused by the introduction of HMs into cells, several cellular pathways of pro-survival or pro-apoptotic pathways are activated to alleviate cellular damage and preserve cellular homeostasis [18]. The continued and excessive exposure will inhibit the activity of antioxidant defense enzymes [19], deplete reduced glutathione (GSH) [20], and generate excessive reactive oxygen species (ROS) [21]. The early cellular damage evolves into pathological processes [22,23]. More importantly, the exposure to HMs would result in adverse consequences of developmental abnormalities, behavior alteration, reproductive issues, and even reduction in lifespan [24]. Past years have witnessed the successful applications of advanced technologies for HMs treatment and the fundamental studies of toxicity mechanism of HMs on human health. In addition, according to a scientometric analysis of research papers about HMs over the past five years, most studies focused on environment transportation (red color), toxicity mechanism (blue color), and pollution control (green color) independently (Fig. 2). Integrated thinking is lacked in the source identifications, the control technologies, the migration path of HMs, and their effects on human exposure/health. Therefore, it is the right time to reconsider the significance of the full life cycle evaluations of HMs and further employ the comprehensive guidelines to map the links between HMs and their related health issues.
Fig. 1.
Schematic diagram of migration, human exposure pathways, and pollution control of HMs in the environment.
Fig. 2.
A scientometric analysis of the potential impact of HMs based on the literature data retrieved from the Web of Science in the past five years. Keyword analysis and visualization are conducted using VOSviewer, which is used to group keywords into different clusters.
In this review, we aim to summarize the current cutting-edge knowledge on the related environmental fate, toxicity mechanisms to human health, and control methods/technologies of HMs, respectively. Importantly, the pathways and accumulations of HMs will be introduced to track the sources and help understand the exposure route related to human health. Furthermore, the exposure route, metabolism, toxicity mechanism, and adverse consequence will be briefly summarized. The current advanced technologies for pollution control of the industrial wastewater and solid wastes will be outlined to provide the guideline for future development. At last, we will provide the perspectives to narrow the knowledge gap and build an effective link between HMs and their related toxic mechanism to human health. Additionally, the advanced in-operando characterizations and artificial intelligence will also be outlined to provide possible directions for the future development of HMs and suggest methodologies for the development of other pollutants.
2. Environmental behavior and the fate of HMs
The increasing environmental pollution caused by HMs induced numerous concerns about ecological and global public health problems [25,26]. Moreover, the complex migration routes and processes in various environmental media largely restrict the introduction of effective controlling measurements. In addition, the various physical/chemical processes, including adsorption, biotransformation, and redox reactions, could lead to toxicity transformations, thus further causing pathological changes in organs [[27], [28], [29]]. Therefore, it is essential to systematically investigate the source, migration, and fate of HMs in the environment.
HMs could enter the environment via many specific paths, such as natural processes (e.g., weathering of rocks, soil erosion, forest fire, and volcanic eruption), agriculture (e.g., fertilizers, animal manures, and pesticides), domestics use (e.g., sewage sludge and burning of fuel), and industry (e.g., metallurgy processing, automobiles, and coal burning) [30,31]. The production of HMs can be generally divided into three categories, i.e., wastewater, waste gas, and solid waste. Specifically, HMs mainly exists in the form of particles and aerosols in atmosphere, and the migration of HMs in atmosphere is the foremost long transport pathway, which always involves some complex absorption pathways [32]. For instance, PM2.5 with an aerodynamic diameter smaller than 2.5 μm is a typical atmosphere particulate [33]. HMs could be absorbed by PM2.5 in the pores and attached on the surface of silica particles [34], which are hard to capture by various conventional air pollution control devices [35,36]. The complex migration path will not only affect the atmospheric visibility but also result in easy entrance into organism and accumulation [[37], [38], [39]]. Different from the mobility of HMs in water and gas, HMs in solid waste have distinct temporal and spatial characteristics. Numerous studies have demonstrated that the release of HMs in solid waste is affected by environmental conditions, such as temperature, pH, salinity, dissolved oxygen, redox potential, and other factors [40]. Specifically, HMs in solid waste could be easily oxidized and decomposed in an open area, which leads to water and soil pollution [41].
2.1. Source identification methods for HMs
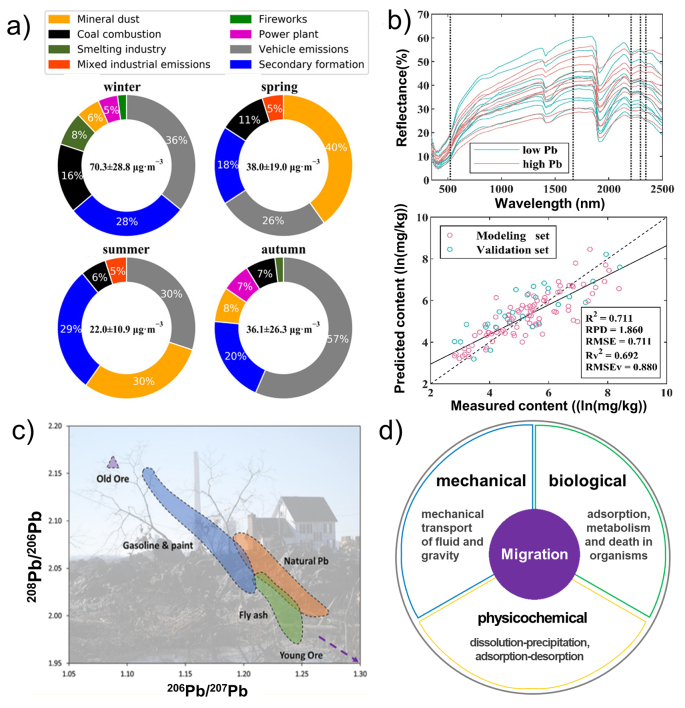
The key to environmental monitoring is the source identification [42,43]. Generally, the common analytical method combines statistical analysis means and elemental fingerprint identification to identify geographical origins. Specifically, regression analysis, hierarchical cluster analysis (HCA), and positive matrix factorization (PMF) could be employed as multivariate statistical analysis models [[44], [45], [46]]. Wang et al. demonstrated that vehicle emissions, secondary formation, mineral dust, and coal combustion are the major particulate matter (PM) sources using the PMF model (Fig. 3a) [47]. Moreover, with the rapid development of analytical technologies, remote sensing and isotopes analysis have emerged as powerful tools to monitor concentration distribution. In terms of remote sensing, multispectral aircraft and satellite systems have been successfully employed to rapidly monitor HMs in both spectral and morphological analysis at a large scale [[48], [49], [50]]. For example, Zhao et al. estimated Pb content in farmland soil using reflectance spectroscopy [51]. The researchers have also demonstrated that the estimated concentrations of HMs were consistent with the investigated results in the field (Fig. 3b). In terms of the isotope analysis, various sources and anthropogenic activities impart a distinctive isotopic composition [[52], [53], [54]]. Notably, the contaminated area could have its own “isotope signature,” and their isotope fingerprints could reflect the importance of emissions from human activities and industrial processes, as shown in Fig. 3c [55]. Gonzalez et al. used zinc and copper isotopic to trace the sources of atmospheric PM [56].
Fig. 3.
The strategies of source identification of HMs. (a) Contributions of PM sources in Lanzhou based on the PMF analysis (Reprinted from ref.[47]. Copyright (2021), with permission from Elsevier); (b) breakpoint corrected spectra of 20 samples with high and low Pb content; and scatter plot of the measured and predicted soil Pb content from optimized model evaluated by coefficient of determination (R2), residual prediction deviation (RPD), and root mean square error (RMSE) (Reproduced from ref.[51]); (c) 208Pb/206Pb versus 206Pb/207Pb ratios in fly ash samples investigated in this study compared to other Pb sources in the United States (Reprinted with permission from ref.[55]. Copyright (2019) American Chemical Society); (d) the mechanical, biological, and physicochemical migration behaviors of HMs.
In a summary, various tracing methods have been employed to identify the emission sources and their related distribution. However, the accurate and rapid trace of HMs still face great challenges in the future. Firstly, establishing uniform standards for the comparison of HMs identification is critical. In addition, developing a database based on the fingerprint of component and phase structure from the various sources is important. At last, it is necessary to construct mathematical models which could link fingerprint information including HMs and co-exist elements to trace the distribution of pollution source.
2.2. Release behaviors of HMs
The physical properties/chemical structure/phase of HMs could be varied due to the geochemical behavior (e.g., weathering and volcanic eruptions) and human activities (e.g., mining and cultivation), which might result in HMs release into the environment to some extent. Furthermore, these HMs could be migrated and transformed with various chemical forms in nature. For instance, cadmium sulfide (CdS) is commonly recognized as the main species of Cd in soil. However, S could be easily oxidized to SO42− resulting in the simultaneous release of Cd2+. Cd2+ with relatively strong migrated capability could enter the food chain via plants accumulations [57].
Generally, the release of HMs is regulated by environmental conditions, such as pH, redox potential (Eh), salinity, and temperature [58,59]. In naturally polluted rocks, sedimentary aquifers, and fractured bedrocks, it has been reported that the HMs could easily leach from the solid phase due to the variation of pH values [60]. The strong correlation between HMs leaching and pH is attributed to the synergistic effects of three pH-dependent processes. Firstly, HMs oxyanion undergoes protonation and deprotonation reactions, while HMs cation could form insoluble precipitate. For example, arsenic exists as a positive five-valent oxyanion in an oxidizing environment that undergoes a protonation and deprotonation reaction to produce four oxyanion species: H3AsO4 (pH < 2.5), H2AsO4− (pH 2.5–7.0), HAsO42− (pH 7.0–11.8), and AsO43− (pH > 11.8) [61]. Pb will form the carbonate minerals cerussite (PbCO3) and hydrocerussite (Pb3(CO3)2(OH)2) between pH 4 and 8 [62]. Furthermore, HMs undergo adsorption–desorption reactions under the change of pH. The speciation of arsenic strongly affects its adsorption on the surface of ferric hydroxide/oxide, as ferric hydride (a known precursor mineral of goethite and hematite) exhibits a net positive surface and arsenic exists in the form of negatively charged oxygen-bearing anions [63]. With the increase of pH, the surface positive charge of the ferric hydroxide/oxide decreases, which facilitates the desorption of arsenic [64]. At last, the pH of environment could destructure the crystals phase (e.g., Fe-oxyhydroxides, metal oxides, and silicate minerals). When the host crystal lattices are destroyed due to the proton-promoted dissolution reaction under acidic conditions, the naturally contaminated rocks containing minerals could release both adsorbed and incorporated HMs [62]. Due to the relatively redox sensitivity of the minerals, the release of their loaded HMs (e.g., Pb2+, Cd2+, Cu2+, and Zn2+) is also highly dependent on the Eh of the system [65]. Briefly, HMs in sulfides minerals are stable under reduction conditions but could rapidly react with oxidants and dissolve via electrochemical pathway. By contrast, HMs in oxides and carbonates minerals are stable under oxidation conditions but could rapidly react with reductants [66]. However, the prediction model and the links between the release of HMs and Eh of the systems are still quite confusing to the researchers and deserve to be estimated and explored in the future.
2.3. Migration behavior of HMs
After being released into the environment, the temporal and spatial transportation of HMs primarily depend on its chemical/physical properties, environmental medium properties, and the properties of contacting materials. The transportation processes are categorized into mechanical migration [67], physical/chemical migration, and biological migration [29], as shown in Fig. 3d. In terms of mechanical migration, the mechanical force plays an important role in migrations and re-distribution of HMs, which could be divided into fluid and gravity force. Fluid migrations are the main mechanical transportation, which includes free diffusion and transportation. The meteorological/conservancy conditions, emission concentrations, and total amounts might regulate the migration distance [68,69]. Additionally, the concentration of HMs is proportional to the average fluid speed and inversely proportional to the diffusion height/distance from pollution sources [70,71]. At last, the particles of HMs appear to be settled by the gravity migration [72]. Another migration process for environmental pollutants is the physical and chemical changes of HMs. In this process, HMs undergo dissolution–precipitation and adsorption–desorption, and they are further adsorbed and migrated along with colloids or particles [73,74]. Moreover, the migration of HMs will be controlled by acid–base interaction, which might be attributed to the variations of the pH values in environment [58]. The acidic condition could stimulate the pollutants to soluble substances, thus promoting their migration in environments. When the environmental pH value increase, many pollutants may precipitate and relatively be enriched in the sediment. Redox is another key factor affecting the migration of HMs [75]. Under oxidizing conditions, some elements (e.g., chromium, vanadium, and selenium) form soluble compounds (e.g., chromate, vanadate, and selenate) and show strong migration abilities. In the reduced environment, these elements become insoluble compounds. Since HMs are persistent in the environment, they could be transported through living organisms. On one hand, microorganisms could change HMs into chemical states that are easier to transport. On the other hand, HMs could enter organism directly from the abiotic environment (i.e., soil-to-plant transfer and water-to-fish transfer). At last, HMs could be transported into the biosphere through ingestion, metabolism, and food chains [76].
Overall, HMs will be enriched from the environment into the organisms under a certain level. The individual organisms will accumulate HMs at different stages of their growth and development [77]. Notably, bioaccumulation occurs when uptake is greater than elimination. The concentration of HMs will gradually increase along the food chain of the ecosystem with the increase in nutrient levels [78]. Since most of these HMs will be accumulated into human body, the accumulation level leads to an increase in toxicity and significantly threaten human health [79,80].
3. Potential toxicity of humans
Human beings are exposed to HMs through various routine, which lead to adverse consequences. Understanding the correlations between exposure routine and HMs is probably the most effective way to confront and alleviate the harm for human health. The main exposure routes of HMs to human are ingestion, inhalation, and dermal contact. The HMs migrate/assimilate into the drinking water [81], seeds/fruits of crops [82], and organisms [8], eventually enter the human body through ingestion. Specifically, the HMs are adsorbed on the airborne fine PM and microplastics, which increase the exposure risk of inhalation [83,84]. Dermal contact is another route of exposure and is considered a less significant pathway due to the stratum corneum. Although the exposure dose of human could be acquired by related statistics, some actual exposure parameters on the regional dependence well deserve to be investigated by questionnaire or fieldwork.
3.1. Impacts of bioavailability/speciation on cytotoxicity
The toxicity of HMs is highly correlated with bioavailability. Luo et al. demonstrated that various sources influence the human bioaccessibility of PM2.5 simulated by lung fluid extraction and DGT method [85]. The solubility of trace metals in the matrix, such as aluminosilicate mineral, is typically inferior to 2% due to the substitution in the crystalline network [86]. Xie et al. found that the bioavailability of As in PM2.5 varied with the seasons, as the concentration of As in PM2.5 is significantly higher in winter than that in summer, but the bioavailability is significantly lower [87]. The bioavailability strongly depends on their redox state and bound form [88]. Taking chromium as an example, Cr(III) is an essential element for the proper function of living organisms, while Cr(VI) shows strong toxicity on biological system. Cr(VI) usually possesses higher solubility compared to stable Cr(III) [89]. Indeed, the solubility of Cr(VI) combined with different anions show significant diversity. Lead chromate, calcium chromate, and barium chromate are extremely insoluble while dichromate are highly soluble [90]. In terms of Hg, several chemical forms of Hg consist of elemental Hg (metallic), inorganic and organic Hg. Organic Hg (e.g., methylmercury and ethylmercury) with high bioaccumulation could cause toxic action in the neurons [91]. In addition to the neurotoxicity, elemental mercury could lead to the adverse consequences on the kidneys and thyroid [92]. HgS was recognized as the low bioavailable Hg species due to the low solubility (pKsp = 55.9–50.9) [93].
3.2. Toxicity mechanism of HMs to humans
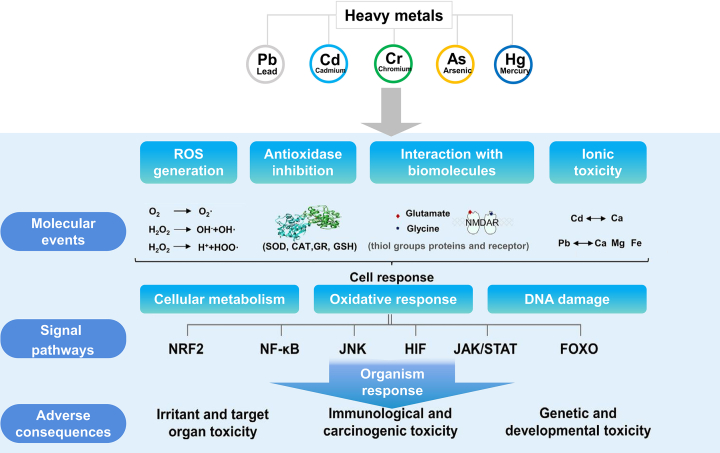
HMs will cause certain toxicity due to their interaction with cellular components. Furthermore, the continued or high-level exposure will induce adverse health consequences [94]. ROS generation, reduced GSH decrease, and activity inhibition of antioxidant defense enzymes are commonly recognized as the reaction mechanisms for metal-induced cell toxicities. The toxic effect is mostly induced by oxidative stress due to the excessive generation of ROS. On the one hand, HMs or intermediate reactive species lead to the formation of free radicals. On the other hand, HMs disrupt the antioxidant enzyme system, reduce ROS scavengers, and endorse the oxidation condition [95]. Generally, the dynamic equilibrium for the redox station is controlled by enzymatic reactants and non-enzymatic antioxidants. The enzymatic reactants, such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx), are the first defense against oxidation stress. SOD could catalyze the disintegration of superoxide radicals (·O2)22− into hydrogen peroxide (H2O2) and oxygen (O2). Meanwhile, CAT and GPx could decompose H2O2 into H2O and O2. The non-enzymatic antioxidant GSH is a thiol tripeptide to reduce superoxide radicals, hydroxyl radicals, and peroxynitrites [96]. The imbalance between ROS and antioxidant defense leads to overgeneration of the free radicals, which would further cause oxidative stress produced from HMs to some extent. The exposure of Pb could inhibit the activity of antioxidant enzymes (e.g., SOD, CAT, GPx, and GR) and thus increase the levels of ROS [97]. The elevated oxidative response is related to the destabilization of cellular events, such as cell division, cell cycle, immune response, and cell death.
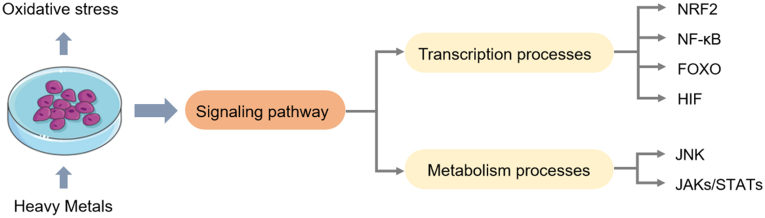
The imbalance in redox homeostasis is well known as a center of HMs-associated toxicity [18]. With the imbalance of redox conditions, the cells could remediate various damages via the activation of signaling pathways. The involvement of various signaling pathways has been recognized in HMs-induced pathological processes. At the early stage, the attempt is to repair damages via the activation of pro-survival pathways. However, cells activate the death pathways to clear up the damaged cells when the degree of damage is high [98]. In recent years, researchers have focused on the relationship between oxidative stress and associated cellular regulators/pathways. Several regulatory molecules have been uncovered in response to HMs-induced oxidative stress, as shown in Fig. 4.
Fig. 4.
HMs induce cells damage through disturbing transcription or metabolism processes. NRF2, Keap1-NRF2 signaling; FOXO, forkhead box O; JNK, Jun N-terminal kinase; NF-κB, nuclear factor kappa B; HIF, hypoxia-inducible factors; JAKs/STATs, Janus kinase/signal transducer and activator of transcription proteins.
The exposure of HMs could activate or inhibit the transcription route, which leads to different disorder conditions. The transcription initiation processes are complex and often require the assistance of transcription factors. Kelch-like ECH-associated protein 1 (Keap1)-NRF2 signaling (NRF2), nuclear factor kappa B (NF-κB), forkhead box O (FOXO), and hypoxia-inducible factors (HIF) are families of transcription factors. Generally, the activities of transcription factors are controlled by functional proteins, which are oxidated or functionalized in the stress condition. The activity of NRF2 is maintained by Keap1. However, this free NRF2 combines with specific DNA sequence and regulates the transcription of more than 150 cytoprotective/detoxification genes, where Keap1 is oxidized during free radical stress [99]. Recent studies revealed that NRF2 is strongly activated to trigger the pro-survival gene after exposure of human liver cancer cells to Cr6+ [100]. In addition, NF-κB regulates numerous genes associated with stress response, cell metabolism, inflammation, apoptosis, and tumorigenesis. The interaction between stress-associated ligands and related cell receptors leads to the activation of IκB kinase (IKK), which modulates the phosphorylation of NF-κB inhibitors [101]. Cr6+ can induce hypersensitivity in human keratinocyte cells, leading to the activation of NF-κB signaling, apoptosis, and autophagy via ROS production. In another study, the exposure of Cd has been reported to damage renal function via oxidative stress and enhance NF-κB expression for rats [102]. FOXO is another transcription factor, which could be expressed in most of the tissues. Importantly, FOXO could govern the downstream molecules that are involved in different biological processes. During the oxidative stress, FOXOs easily suffer from phosphorylate, which leads to its entrance into the nucleus and controls the homeostasis of antioxidant enzymes (CAT and SOD) and cell process. It has been proved that As-acid leads to the nuclear translocation of FOXO1/3a in mouse skin fibroblast cellular, which triggers cellular aging in these cells [103]. Moreover, it has been proved that the upregulation of FOXO1 is related to mitochondrial biogenesis where human hepatocellular carcinomas (HepG2) cells are in a chromium-containing environment. HIF is an active protein of the cellular adaptive response to a hypoxic statement. HIF, with HIF-1α (an oxygen induction) and HIF-1β (a constitutively expressed subunit) as two subunits, maintains and regulates ATP levels and transcription processes under the anoxic condition [104]. Li et al. demonstrated that the induction of ROS upon arsenic exposure leads to the decrease of Fe2+ and ascorbate in cellular, which might stabilize HIF-1α [105]. Lead could induce oxidative stress and subsequently activate the HIF-1α signaling, which results in the induction of hepatic dysfunction [106].
Besides the above considerations, some regulators could also be affected by heavy metals (e.g., Jun N-terminal kinase [JNK] and Janus kinase [JAKs]/signal transducer and activator of transcription proteins [STATs]). The defect in regulators signaling will contribute to different cell events. JNK, mitogen-activated protein kinase (MAPK), could be activated by various stress factors, which affects embryogenesis, neuronal functions, and diet-induced obesity [[107], [108], [109]]. Datta et al. demonstrated that the exposure of arsenic leads to the death of macrophages via the activation of NADPH oxidase in Clarias batrachus, which further activates the JNK signal to mediate apoptosis [110]. JAK-STATs, an evolutionarily conserved pathway, execute several cellular responses against growth factors. The interaction between JAK/STATs signaling and stress response is unclear due to the complex biological systems. However, it was found that the inhibition of JAK/STAT signaling occurs through oxidative stress induced by cadmium [111]. Another evidence demonstrated that arsenic interacts with JAK tyrosine kinase, which suppresses the activity of JAK kinase [112]. In general, various kinds of HMs modulate JAK/STAT signaling at a certain level. Under regular physiological condition, ROS, signaling molecules, normally runs cellular machinery. However, with the exposure of HMs, the redox imbalance activates/inactivates a series of signaling pathways. The interaction between ROS and signal transmission is a central toxic mechanism behind HMs’ associated health consequences. An investigation between cell signaling and HMs would help understand how the exposure of HMs switches themselves from molecular level into pathological expression.
Besides the oxidative response, the toxicity is closely related to the structure characteristics of HMs. For example, because of its structural resemblance with Ca2+, Cd2+ might destroy the metabolism of vitamin D and the estrogenic signaling. In additions, it could substitute Ca2+ in the bones, which lead to abnormal metabolism in bone. Moreover, lead also has similar toxic mechanism to substitutes bivalent cations (e.g., Ca2+ and Mg2+) and monovalent cation (e.g., Na+ and K+). Pb2+ could disturb neuronal voltage-gated Ca2+ channels, which leads to the obstacle of learning and memory.
The oxidative stress and interaction between the biological molecular/analogs lead to a range of pathological conditions. The epidemiological data have revealed the adverse health consequence. A typical example was the “itai-itai disease” in Japan, which was caused by the long-term exposure of cadmium [113]. Local resident suffered from severe impairment of kidney function, osteoporosis, and multiple fractures [114]. Minamata disease, induced by mercury, had affected thousands of people who still suffer from developmental disabilities, physical deformities, cognitive impairments, and neurological disorders [115]. Ou et al. demonstrated that significantly negative associations between Hg levels in some matrixes and anthropometry of neonates (weight and height) and infants (height) (P < 0.05) [116]. Wang et al. found that lead exposure play an important role in DNA methylation (DNAm) with children’s intelligence based on 333 children aged 9–11 [117]. Previous researches has revealed that long-term arsenic ingestion can cause skin lesions (e.g., keratosis and melanoderma) through the alteration of miRNA, protein, and metabolite profiles [118]. The toxicity mechanism and adverse consequences of HMs (e.g., chromium, mercury, arsenic, lead, and cadmium) are summarized as shown in Fig. 5.
Fig. 5.
The molecule event, cell effects, signal pathway, and organism damages of HMs. SOD, superoxide dismutase; CAT, catalase; GPx, glutathione peroxidase; GSH, glutathione.
3.3. Health risk assessment for exposure humans
According to the aforementioned discussions, human health has been seriously threatened by HMs. The hazard level of HMs exposure could be quantitively evaluated by human health risk evaluation models (e.g., Rabinowitz, Bert, Stern, and Leggett model). Specially, health risk assessment (HRA) has been widely applied in the health evaluation of HMs pollution [119,120]. HRA, proposed by National Academy of Sciences, utilize the knowledge of toxicity and epidemiology to quantitively predicts health effects of pollutants via numerical model [121]. Li et al. systematically assessed the soil pollution levels and quantified the health risks of HMs pollutants to humans from mines in China [122]. Hence, the evaluation models could be employed as an effective tools to help identify the degree of hazard levels. Firstly, sensitive population could be identified by the daily exposure dose calculated via exposure parameters. Zhang et al. evaluated the probabilistic risk assessment of HMs for receptor populations under different land uses in urban and suburban soils of Changsha [123]. Secondly, the reference value of external exposure to the environment corresponding to the control limit standards could be calculated on the basis of HRA models. Zhang et al. used the Integration Exposure Uptake Biokinetic Model (IEUBK) and the Adult Lead Model (ALM) to calculate the lead criteria values for residual land and commercial/industrial land [124]. Finally, health risk evaluation aims at risk management. The identified or potential risk could give rise to some effective suggestion for HMs emission and pollutions control methods.
4. Pollution control technologies in HMs remediation
Implementing efficient pollution blocking technology at the source of HMs is the most effective way to reduce the health risk of human. Originating from the industrial process, the HMs with high toxicity generally flow into wastewater (e.g., mineral acid wastewater and beneficiation wastewater) and waste solid (e.g., nano sludge and mineral tailings). In this section, the blocking technologies for HMs pollution were systematically summarized to better understand waste treatment for sustainable economic and environment development.
4.1. Multi-stages water treatment processes
The wastewater treatment could be realized in various technologies from a simple chemical precipitation to complex electrochemical or membrane technology. Generally, various pre-treatments, biological treatments, purification methods, and different combinations have been proposed to provide efficient wastewater treatment.
4.1.1. Pre-treatments
The pre-treatment is a critical process before further refined wastewater treatments, including coagulation, flocculation, chemical precipitation, and physical filtration. Coagulation and flocculation would alter the physical-chemical state of dissolved and suspended solids, which involve the addition of inorganic and organic chemicals to facilitate their removal. Coagulants are water-soluble polymers with low molecular weight and high positive charge density, which are employed to neutralize the negative charge on the surface of the particles. The particles could react with each other through collision, surface adsorption, and van der Waals, thus facilitating separation from water. On the other side, the flocculation utilizes the chains of polymers to bridge suspended particles, and the flocculants are organic polymers, which generally possess higher molecular weights, specific electrical properties, and charge density. Chemical precipitation is also widely used to treat wastewater with high HMs concentration due to its easy operation and low cost. During this process, the HMs react with chemical reagents (such as hydroxides, sulfides, and ferrite) and form insoluble particles. However, this method is relatively adaptable for the treatment of wastewater with high concentrations and might not be employed to selectively remove HMs. Electrodeposition could be used to recover metals from metal-containing industrial wastes with high recovery efficiencies for metals and a small amount of leaching medium [125,126]. The conventional electrodeposition has lower energy efficiency and selectivity due to electrochemical and concentration polarization. Micucci et al. demonstrated that copper and lead could be recovered with high efficiency by using rotating cylinder electrode reactors, which alleviate the concentration gradient between the electrode and solution [127]. Fogarasi et al. developed a direct and mediated electrochemical oxidation method, which was carried out by utilizing two different types of reactors coupled in series, for the recovery of copper and the enrichment of silver and gold [128].
4.1.2. Biological treatment
According to the oxygen requirement of microorganisms, biological treatments can be classified into anaerobic and aerobic treatments. The related microorganisms are divided into non-methanogenic bacteria and methanogenic bacteria. The microorganisms transform complex organic matter (e.g., carbohydrates, proteins, and lipids) into biogas (methane and carbon dioxide) in anaerobic or anoxic conditions. Among them, methane-rich biogas could be employed as a renewable energy source. However, relatively large reactor is required due to the sluggish reaction kinetics. In the aerobic treatment, microorganisms degrade organic matter into humus-like substances and carbon dioxide. Aerobic microorganisms require suitable conditions, such as proper carbon-nitrogen ratio, water content, and dissolved oxygen concentration. The distinct difference between anaerobic and aerobic treatments is in the dissolved oxygen concentration. The air must be continuously aerated and circulated in the aerobic tanks, where forced air from an air blower or compressor is mixed with the wastewater. The biological treatments are most generally used in removing organic pollutants. In addition, microorganisms are utilized as biomaterials of HMs and may be explored as a removal or detoxification technology of HMs in industrial wastewater. However, industrial-scale application of microbial treatment has been limited for HMs wastewaters due to the high sensitivity of microorganisms to operational conditions and low removal efficiency of HMs.
4.1.3. Purification treatment
In terms of the low-concentration wastewater, further deep purification and technologies are needed, such as adsorption, electrochemical deionization, ion exchange, crystallization, distillation, and membrane technologies. Adsorption is a widely accepted economical method due to its simple operation, high removal efficiency, and regeneration. The interaction between HMs and adsorbents mainly depends on the surface characteristics of the adsorbent. Designing adsorption materials with high specific surface area, hierarchical porous structure, and high-density functional group is the most important hotspot in the adsorption method for wastewater treatment. As a low-energy-consumption water treatment technology, ion exchange resin immobilizes HMs by releasing the pre-saturated ions. After the adsorption is saturated, the regenerant is used to elute the pollutant ions and restore resin exchange capacity. The unit structure of ion exchange resin is composed of three parts: insoluble three-dimensional space network skeleton, functional groups connected to the skeleton, and exchangeable ions. To achieve the industry emission standards, the mixed bed approach, including cation exchange resins and anion exchange resin, is typically used to remove both the cations and anions. However, ion exchange technology is highly sensitive to the process parameters (e.g., pH, temperature, ionic strength, and contact time). To achieve high adsorption capacity and high cycling stability, the efficient and economical regeneration methods and ion exchange resins are the keys to improve the application of ion exchange method in the future.
Membrane separation technology refers to the processes of separation, purification, and concentration through different pressure or electric potentials. Based on the type of membrane and separation mechanism, membrane technologies can be categorized into microfiltration (MF), ultrafiltration (UF), nanofiltration (NF), liquid filtration (LF), reverse osmosis (RO), nano-hybrid membranes, and electrodialysis deionization (EDI). EDI is well known for its cyclic efficiency and reversibility. Porous materials are used as the electrode in EDI process, and the HMs migrate to the electrode with a reverse charge under the electric field. To overcome the limitations of desalination efficiency through physical electrosorption, researchers have started exploring different EDI processes. Processes such as surface redox binding, ion insertion, conversion reactions, or charge compensation with redox-active electrolytes are added to EDI [[129], [130]]. High desalination efficiency electrodes need to be developed to guarantee high energy efficiency. However, the economic cost of electricity and device hinder the future development of EDI. Generally, the separation mechanisms of the membrane technologies include size sieving, solution diffusion, and Donnan exclusion. Nevertheless, membrane technologies are subjected to permeability trade-offs, fouling of membranes, and high energy consumption. The most typical technologies of purification treatment are derived from distillation and crystallization, which are used to treat the high-concentration brine after membrane concentration and thermal evaporation. The technologies mainly include natural evaporation, multi-stage flash (MSF), multiple-effect evaporation (MED), mechanical vapor recompression evaporation (MVR), and membrane distillation (MD) technologies. In these technologies, sufficient energy is provided to increase the temperature or decrease the vapor pressure of polluted water to achieve its boiling temperature. However, the distillation technology only converses dissolved ions into miscellaneous salts, which may result in hazardous solids hard to utilize in the future. The hazardous waste could only be accumulated, landfilled, and treated, leading to the huge loss of valuable metals.
The pre-treatment and biological treatment could remove the major portion of HMs, which is suitable for the high-concentration wastewater. The purification technologies could be employed to dispose the low and trace concentration wastewater. These technologies are restricted by the sensitive operation condition and the high energy consumption. More importantly, it is critical to understand that, in most cases, one technique could not meet the high standard of wastewater control under complex conditions. The combination of various technologies may significantly address the current concerns of HMs wastewater.
4.2. Analysis methods and treatment strategies of heavy metals solid waste
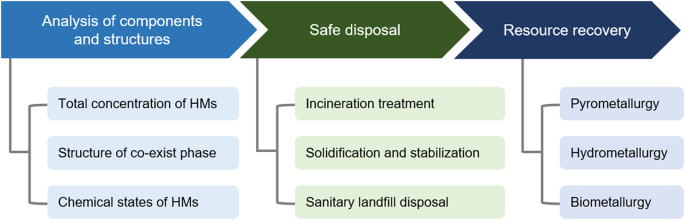
After the treatment of wastewater, HMs eventually flow into heavy metals solid waste (HMSWs), which are mostly classified as hazardous waste according to the policy and legal laws. The HMSWs will be generated by millions of tons every year approximately [131]. The HMSWs have the characteristics of complex composition, strong mobility, and difficult treatment. Due to soil erosion, infiltration of river groundwater, and natural weathering, it is an urgent need to develop high-efficiency technology for the HMSWs disposal. This section mainly reviews three parts: (i) the analysis of components and structures of HMSWs; (ii) the recycling methods; (iii) the harmless disposal technologies, as shown in Fig. 6. It is expected to provide a reference for the improvement and design of the treatment process of HMSWs in the future.
Fig. 6.
The treatment strategies of HMSWs.
4.2.1. Characteristic of components and structures
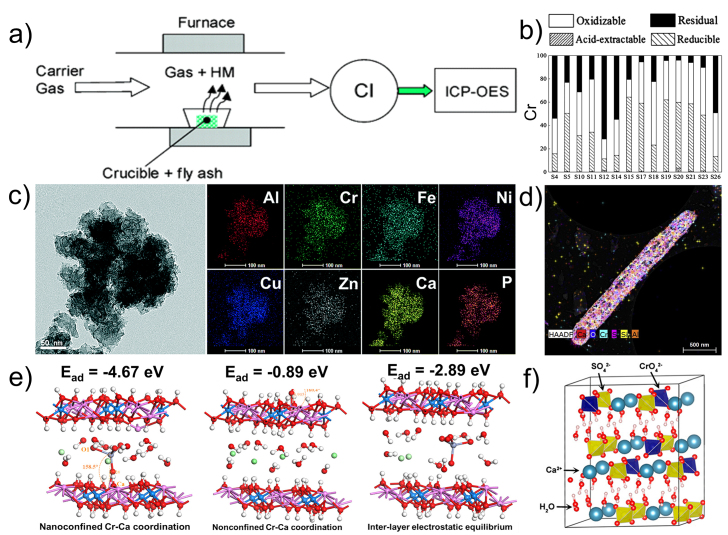
The total concentration of HMs is an important evaluation factor that shows their characteristic. Struits et al. evaluated the resource characteristics of fly ash via the in situ inductively coupled plasma optical emission spectrometer (ICP-OES) detections for the evaporation of HMs (Fig. 7a) [132]. However, using total concentration to assess the potential value of HMSWs indicates that HMs have an equal chemical state. Such a result is unclear to analyze the chemical structure of HMs. To classify different chemical forms, the sequential selective extractions (e.g., BCR four-step continuous extraction method and Tessier five-step continuous extraction method) could furnish the structure information. Tou et al. assessed the risk of metals in sewage sludge based on analysis via modified BCR sequential extraction (Fig. 7b) [133]. In addition, mineralogical ingredients are other crucial characteristics of HMSWs. From the resource’s perspective, HMSWs are a potential complex ore, which consists of natural minerals, artificial minerals, and mixtures [134]. Modern analytical testing methods, such as chemical analysis, polarized light microscopic analysis, and mineral dissociation analysis, are used to analyze the main elemental composition, mineral species, inlay, and encapsulation properties. Mineralogical analysis of HMSWs could provide a mineralogical theoretical basis for the separation of HMs in the HMSWs.
Fig. 7.
The analysis methods of HMSWs. (a) A setup of the online detection for the evaporation of HMs from fly ash by ICP-OES (Reprinted with permission from ref. [132]. Copyright (2004) American Chemical Society); (b) distribution of the different metal fractions in sludge samples (Reprinted with permission from ref. [133]. Copyright (2017) American Chemical Society); (c) the TEM image and elemental maps of the sludge (Used with permission of Royal Society of Chemistry, from ref. [140], permission conveyed through Copyright Clearance Center, Inc.); (d) Cs-STEM-XEDS image of the reduced chromite ore processing residue (Reprinted from ref. [141], Copyright (2021), with permission from Elsevier); (e) the binding modes and adsorption energies (Ead) of CrO42− on hydrocalumite (Ca4Al2Cl2(OH)12·6H2O) (Reprinted with permission from ref. [138]. Copyright (2021) American Chemical Society); (f) the crystal structure model of CrO42--encapsulated gypsum sludge (Reprinted with permission from ref. [139]. Copyright (2018) American Chemical Society).
The dominant problems of comprehensive recovery are the microscopic features, which results in complicated but strong interactions between HMs and coexisting component. The different chemical states reflect the coordination, which directly impacts the interaction with other elements as well as the stability. For mercury as an example, the mobility of dissolved organic matter complex is highest among general states while HgS is less mobile [135]. In addition, understanding the structure and interaction features of the coexisting phases are prerequisites for selecting the remediate methods. HMs stubbornly combine with coexisting phases. The structure parameters (e.g., crystal structure, surface morphology, particle size, and pore structure) influence the adsorption type and strength. Zhang et al. demonstrated that Cr(VI) was immobilized in the Portland cement because of the similar tetrahedral structure and the comparable thermochemical radii of CrO42− and SO42− [136]. Another study has shown that the adsorption energy of nano-Mg(OH)2 is much higher than that of Mg(OH)2 [137]. According to the structural properties of HMs and coexisting phase, the interactions encompass encapsulation/aggregation, strong adsorption, and substituted doping [131]. The encapsulation is generally understood as HMs being wrapped by coexisting phases, while aggregation refers to amorphous HMs gathered with nano/microparticles coexisting phases through interface interactions. Zheng et al. demonstrated that the abundant amorphous particles of HMs coexist in the electroplating sludge (Fig. 7c) [140]. Another study revealed the chromate could incorporates into the ettringite via the elemental mapping of Cs-STEM-XEDS as shown in Fig. 7d. Strong adsorption is another interaction in that HMs are adsorbed on coexisting phases by the chemical binding (e.g., ion exchange and coordination), which generally appears on the surface of porous coexisting phases. Tian et al. found that the hydrocalumite has a high affinity to CrO42− via the spatial restriction and shielding effects, which enhanced the Cr−Ca coordination (Fig. 7e) [138]. Substituted doping is a common interaction between HMs and coexisting phases. In the substituted doping, HMs substitute the similar ions with ionic radii and balanced charges Goldschmidt’s rules. For chromium as an example, the CrO42− could replace SO42− in the lattice of gypsum crystal due to the identical charge, similar tetrahedral structure and comparable thermochemical radii (Fig. 7f) [139]. The substituted doping HMs are strongly retained in the lattice of coexisting phases, making them tricky to extract.
4.2.2. Harmless treatment
Through the above-mentioned analysis of the structure and component features, the remediation methods are divided into safe treatments and resource recovery. The former focuses on pollution characteristics while the latter focuses on resource properties. The commonly used methods are discussed as follows.
The incineration treatment, an exothermic oxidation process, is still the common disposal approach, which typically reduces the weight of the original HMSWs by 80%–85% and volume by 95%–96%. Upon incineration, HMSWs are converted into fly ash, bottom ash, flue gas, and slag. The fly ash and bottom ash considerably show various compositions which largely depend on the source of HMSWs and operating conditions of incineration. The ash consists of inorganic components, including amounts of metals (e.g., Pb, Mg, Se, Co, and Cr), minerals (e.g., Al2O3, CaO, SiO2, and asbestos), and organic compounds (dioxins, furans, and polycyclic aromatic hydrocarbons) [142]. In addition, the ash generated from solid wastes exhibits severe health effects to humans and animals [[143], [144], [145]]. The solidification/stabilization technology exploits suitable solidification substrates and chemical reagents to immobilize HMs, which includes cement solidification and thermal treatment. Traditional cement solidification has been widely used due to relatively low cost, easy operation, high mechanical strength, and hard biodegradation [146]. The mechanism of solidification mainly relies on cohesion to fix HMs into the matrix of the cement, including ordinary Portland, magnesium-based, calcium aluminate, and alkali-activated cement. The immobilization mechanisms of HMs solidification treatment encompass adsorption, isomorphous substitution, (co-)precipitation, surface complexation, and encapsulation. Among these processes, the hydration products (e.g., calcium silicate hydrate and ettringite) play a critical role [147]. However, the considerable increase in volume and the uncertain durability under natural environments are unavoidable. The thermal treatment for HMSWs could be classified into (i) vitrification and crystallization, (ii) sintering where glass-ceramics and lightweight ash are the main products. The thermal treatment is an endothermic reaction, which mainly include three processes of dehydration (100–200 °C) polymorphic transition (480–670 °C) and fusion (1100–1250 °C) [148]. HMs could be chemically bounded into the amorphous network, and the efficiency of immobilization highly relies on the composition/structure of HMSW [149]. Additionally, HMs could be stabilized by phase re-forming and transition in the fly ash. Compared with cement solidification, the thermal treatment has good comprehensive physical/chemical properties and excellent solidification effect. However, the complex operation and high energy consumption constrain its market application. Sanitary landfill remains a common solid waste disposal method because of low cost, large treatment capacity, and simple disposal procedure [150,151]. During the disposal in the landfill, HMs will interact with chemical matters under complex conditions [152,153]. The harsh environment will induce the transformation of chemical states of HMs, such as aggregation, dissolution, complexation, sulfidation, and reduction/oxidation. In the process, leachates are considered a potentially polluted source in which the dissolved, nanoparticulate, and colloidal HMs are transported to the environment [154,155]. The environmental problems still exist in the final treatment [150].
Several limitations for the safe treatment methods are as shown below. Firstly, the valuable metals are not recycled, resulting in a waste of resources. Secondly, the safe treatment process requires multi-step linkage and energy consumption. Finally, landfill disposal causes a long-term release of HMs with complex chemical states into the environment. “Waste” can also be regarded as a “resource” [156]. The recovery of HMs could not only prevent the occurrence of second pollution but also rationally utilize natural resources [157]. Therefore, it is a tendency to develop new technology to recovers high-value metals with easy operation steps. Three main methods have been developed for the recovery of valuable metals from HMSWs, including pyrometallurgy [158,159], hydrometallurgy [160,161], and biometallurgy [162,163].
4.2.3. Resource recovery strategies
As the conventional recovery method, pyrometallurgy recycles metals via high-temperature chemical reactions [164]. Pyrometallurgy can be classified into three different categories: high-temperature incineration, vacuum carbon-thermal reduction, and chlorination volatilization methods. The high-temperature incineration contains four steps of calcination, roasting, smelting, and refining. In the vacuum reduction metal recovery method, metal oxides are reduced into metals using reducing agents via thermal routes. It should be noted that the process does not include a toxic chemical agent, signifying a green approach [165]. Chlorination volatilization methods generate volatile chlorides in the presence of chlorination agents via heating HMSWs. The metals are recovered by hydrometallurgy. Experimental conditions, such as heating time, smelting temperature, vacuum level, and concentration of chlorinating agent, affects the yield and purity of recovery products [166]. Pyrometallurgy is relatively easy and has a broad range of applications. However, the process has low products-energy rate and cannot selectively recover HMs. There are several key issues hindering the future development of pyrometallurgy. Hydrometallurgy leaching is one kind of chemical leaching. Initially, the metal compounds are selectively dissolved using an appropriate leaching agent (e.g., acid [166], alkaline [167], and ionic liquid [168]). The leaching efficiency of HMs is closely associated with pH, time, leaching agents, and contact temperature [169]. Subsequently, the dissolved metal ions are separated and purified using several different methods (e.g., precipitation [170], solvent extraction [171], ion adsorption [172], ion exchange [173], and electrochemical reduction [174]). The leaching of Ni, Co, Mn, and other metals could be performed using acidic and alkaline solutions [175,176]. Das et al. used organo-phosphoric acid reagents to extract scandium(III) from acidic solutions [177]. However, it suffers from an environmental issue due to the generations of NOx, Cl2, and acid fumes. Biometallurgy is one of the most novel and ecofriendly biotechnologies for the recovery of HMs via microbiological processes [[178], [179], [180]]. The bioleaching process contains several reactions of acidolysis, redoxolysis, and complexolysis [181]. In acidolysis, the metals of solid phase are substituted by proton and transferred into the liquid phase. Autotrophic microorganisms utilize elemental sulfur as an energy source to produce sulfuric acid through the metabolic reaction [178]. In contrast, heterotrophic microorganisms need an organic matter as a carbon resource to generate various organic acid molecules [182]. The metabolites create local strong acid conditions on the attachment point of microbes, rendering the solid more prone to dissolution [183]. Likewise, the complexing molecules coordinate with the HMs through ion exchange, which accelerates the release of HMs [184]. The HMs in the leachate are further purified by suitable processes [177,185]. Although bioleaching avoids the use of concentrated acid/base and does not emit harmful gases [183], several drawbacks still exist: (1) slow processing and low bioleaching yield in some cases due to refractory properties of ores/wastes; (2) slow diffusion of oxidants to ore surface and inert formation of jarosite and iron hydroxides on ore surface.
Processing technology has always been updated iteratively to develop superior and feasible treatment methods. To effectively recover or separate HMs, numerous new recovery methods have been developed to extract valuable metals (e.g., supercritical fluid extraction [186,187] and plasma technology [188,189]). Wang et al. conducted supercritical fluid experiments on the separation and recovery of salts, in which up to 96% of alkali salt can be recovered with the addition of potassium [190]. Szałatkiewicz et al. used high-temperature plasma technology to treat printed circuit boards without the need for preprocessing [191]. It is worth noting that the HMs in HMSWs exist in various states, which directly affect the efficiency of HMSWs treatment. The regulation of nanocrystal growth has been an efficient method under surface/interfacial control [192]. Liu et al. used Na2CO3 and NaHCO3 as mineralizers to render Mg(OH)2 into the Na2Mg(CO3)2, releasing adsorbed-Cr(VI) into the solution [193]. Zhuang et al. adopted a selective fast growth strategy for SnO2 by employing NaOH as surface/interfacial regulating agents to achieve selective separation of HMs [194]. Liu et al. firstly provides a feasible strategy to fully extract encapsulated Cr(VI) from the solid waste by regulating the phase transformation and chromium species under hydrothermal conditions [139]. The nanocrystal growth manipulation with surface/interface regulating agents provides an exciting reference for research and the development of new technology.
4.3. Control technologies for gaseous HMs
HMs of raw materials are volatilized into air pollutants during high-temperature processes, which can mostly be converted into particles with the cooling of gas. Fine particles generally contain heavy metals and oxidized condensates. To date, a series of technologies have been developed for fine particles of HMs. Physical blocking (e.g., cyclone dust collector, bag filler, and electrostatic precipitator) is one of the mainstream conventional dust removal strategies [195]. Xie et al. proposed a new dust collector combining a cyclone separator and cartridge filter, which is 70%–80% higher than that of a common cyclone [196]. The temperature, flow rate, particle size, and other process conditions influence the removal efficiency of HMs [197,198]. However, the removal efficiency of the physical blocking technologies still fails to satisfy the practical requirements. In particular, elemental mercury (Hg0) is difficult to remove by the above treatment due to its high volatility and insolubility. Wet scrubbing technology, as a pipe-end treatment, is widely employed for gas purification. Liu et al. designed a series of transition metal-thiourea solutions to selectively remove Hg0, which displayed a significantly high Hg0 removal efficiency of 88.7% [199]. Liu et al. proposed a novel technique for the oxidation of Hg0 through thermally activated ammonium persulfate [200]. Recently, mercury adsorption over various adsorbents (e.g., activated carbon, metal oxides, and metal sulfides) has received great attention for its portability and reversibility [201]. Hong et al. exploited magnetic pyrrhotite to reclaim Hg0 from Zn-SFG because of the surface unsaturation coordination sites [202].
4.4. Soil remediation technologies
Human activities, such as heavy industry, sewage irrigation, and fertilizer application, have led to severe soil pollution of HMs [203]. The remediation methods for soil polluted with HMs can be mainly divided into physical, chemical, and biological remediation [45]. Physical remediation, including oil replacement, desorption methods, soil barrier landfill technology, and electrodynamic remediation technologies, separates HM pollutants from soil through various physical processes. Physical remediation is generally combined with other technologies to enhance the remediation effect of HMs [204]. Compared with physical remediation, chemical remediation was developed earlier [205]. It involves solidification/stabilization technology, leaching methods, and the addition of chelating agents [206,207]. However, these technologies, costing tons of chemicals, may cause irreversible changes in soil chemical and biological composition. Furthermore, the application of soil amendments may also bring second environmental and health risks. Biological remedial techniques mainly include microorganisms and phytoremediation. The process of microbial remediation technology reduces or eliminates HMs through biotic activity [208]. Most microbial remediation is still not carried out in the practical field due to multiple impact factors. Currently, phytoremediation has received great attention for the remediation of contaminated soil, which does not damage the soil structure and causes minimal disturbance to the soil system [209,210]. However, its development and application are severely limited because of its low remediation efficiency and ineffective disposal technology for HM-contaminated biomass. Therefore, it is difficult for a single remediation to satisfy the actual requirement due to the complexity of contaminants and conditions. Based on a remediation feasibility analysis, it may be a future trend to combine remediation technologies and take advantage of maximizing remediation efficiency.
5. Conclusions and perspectives: what is next?
The HMs have attracted considerable attention in environmental behavior, health toxicity, and environmental remediation. Although pioneer studies have been conducted to reveal the fundamental mechanisms of their migration and toxicity and to develop a series of treatment technologies, the current understandings and technologies are still far from the requirements for remediating HMs in the future.
In terms of tracing the sources of HMs, the combination of isotopes and multivariate statistical analysis is the most common method. For instance, Liu et al. have revealed the different Ag isotope fractionation effects between natural Ag nanoparticles and engineered Ag nanoparticles in an aquatic environment [211]. Vaněk et al. have demonstrated that Tl contamination could be traced in soils through isotope analysis [212]. However, the natural and anthropogenic ZnO and CeO2 showed no obvious difference in stable isotopic compositions [213]. The analysis of monoisotopic may narrow down or ignore other environmental varieties. It is noted that the pre-treatment is a vital process to improve the analytical efficiency. The HMs in natural systems normally presents at very low concentrations. The complex matrices might cause serious interference to the instrument detection. Hence, it is necessary to uniform the characterization analysis in complex systems. In addition, the single characterization might not offer enough data to support the complex information to some extent due to the high diversity of HMs in nature. The combination of multiple characterization techniques to form a multi-dimensional characterization platform will be an important trend in the future.
While HMs is toxic to a variety of biosystems such as nervous, immune, endocrine, respiratory, integumentary, cardiovascular, and reproductive systems [214], the stress responses can alleviate the harm to body functions and induce resistance to drugs. It has been demonstrated that both arsenic detoxification enzyme and microbiome members play an important role in protection against arsenic toxicity in mouse models [215]. Rubin found that sulfate-reducing bacteria in human gastrointestinal tract was sufficient and necessary to induce arsenic thiolation [216]. Perry et al. found that chronic exposure to arsenic in drinking water can result in resistance to antimonial drugs in a mouse model of visceral leishmaniasis [217]. The multifaceted interplays of host factors and metabolism functioned with HMs are complex. Since the effect of HMs on the human body is bidirectional, unilateral feedback toward the harm of HMs could not comprehensively explain the toxic mechanism. Uncovering the antagonistic mechanism on the selective response of physical functions to HMs is necessary. Moreover, revealing the antagonistic mechanism could help evaluate the harm and understand the special pathway of response. More importantly, epidemiology of region influenced by HMs pollutions deserve to be explored. In the process of epidemiology study, including occurrence, epidemic process, transmission route, and epidemic factors, studies should be systematically conducted on toxic characteristics to guide the implementation of relevant control strategies.
Indeed, the natural characteristics and the relevant environmental factors to HMs are complex, which results in difficulty in constructing prediction model for tracing HMs. With a strong capacity to draw numerical information from multidimensional data, machine learning has a wide range of applications, such as predicting pollutant distribution and nanotoxicity [218,219]. Nevertheless, the collection and noise reduction of data is a time-consuming process. Hence, the establishment of normalized database is necessary. Machine learning often involves integrating diverse data from several sources, which are varied in terms of type, format, and semantics. In addition, gathering data would introduce uncertainty which depends on different means and methods (e.g., instrument operation or data analysis). Therefore, a normalization dataset will be critical to enormously improve the efficiency of machine learning. The interpretation about machine learning is also critical. Although high-precision predictions are achieved by configuring the appropriate parameters and big data, the internal operations of the model remain obscure [220]. Current interpretable studies are mainly devoted to judging the rationality of decision-making, which lacks the interpretation of the interaction among multiple features.
Current studies of HMs and their derived source tracking, toxicity mechanisms, and controlling technologies are quickly approaching their limits. Luckily, the developing advanced in-operando characterizations provides us great opportunities to understand the fundamental reaction mechanisms at nano/microscale, which would be of benefit to guide the future research directions either for toxic mechanisms or materials design for pollution control [[221], [222], [223]]. In addition, in terms of the macroscale understanding, machine learning and big data analysis offer us a new insight to quickly obtain predictable data, which benefits the establishment of intuitive module and guides the experimental design.
Declaration of competing interests
The authors declare no competing financial interests.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (Grant No. 2019YFA0210400), the National Natural Science Foundation of China (Grant Nos. 21836002 and 52104315), the Foundation for Innovative Research Groups of the National Natural Science Foundation of China (Grant No. 52121004) and the Major program Natural Science Foundation of Hunan Province of China (No. 2021JC0001).
Contributor Information
Wenchao Zhang, Email: wenchao.zhang@csu.edu.cn.
Zhang Lin, Email: zhang_lin@csu.edu.cn.
References
- 1.Podgorski J., Berg M. Global threat of arsenic in groundwater. Science. 2020;368:845–850. doi: 10.1126/science.aba1510. [DOI] [PubMed] [Google Scholar]
- 2.Bolisetty S., Peydayesh M., Mezzenga R. Sustainable technologies for water purification from heavy metals: review and analysis. Chem. Soc. Rev. 2019;48:463–487. doi: 10.1039/C8CS00493E. [DOI] [PubMed] [Google Scholar]
- 3.Liu W.Z., Weng C.Z., Zheng J.Y., Peng X.Q., Zhang J., Lin Z. Emerging investigator series: treatment and recycling of heavy metals from nanosludge. Environ. Sci.: Nano. 2019;6:1657–1673. doi: 10.1039/c9en00120d. [DOI] [Google Scholar]
- 4.Liu J., Luo X., Wang J., Xiao T.F., Chen D.Y., Sheng G.D., et al. Thallium contamination in arable soils and vegetables around a steel plant—a newly-found significant source of Tl pollution in South China[J] Environ. Pollut. 2017;224:445–453. doi: 10.1016/j.envpol.2017.02.025. [DOI] [PubMed] [Google Scholar]
- 5.Driscoll C.T., Mason R.P., Chan H.M., Jacob D.J., Pirrone N. Mercury as a global pollutant: sources, pathways, and effects. Environ. Sci. Technol. 2013;47:4967–4983. doi: 10.1021/es305071v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhitkovich A. Chromium in drinking water: sources, metabolism, and cancer risks. Chem. Res. Toxicol. 2011;24:1617–1629. doi: 10.1021/tx200251t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang H., Huang K., Zhang K., Weng Q., Zhang H., Wang F. Predicting heavy metal adsorption on soil with machine learning and mapping global distribution of soil adsorption capacities. Environ. Sci. Technol. 2021;55:14316–14328. doi: 10.1021/acs.est.1c02479. [DOI] [PubMed] [Google Scholar]
- 8.Blum J.D. Marine mercury breakdown. Nat. Geosci. 2011;4:139–140. doi: 10.1038/ngeo1093. [DOI] [Google Scholar]
- 9.Qin J., Lehr C.R., Yuan C., Le X.C., McDermott T.R., Rosen B.P. Biotransformation of arsenic by a Yellowstone thermoacidophilic eukaryotic alga. Proc. Natl. Acad. Sci. USA. 2009;106:5213–5217. doi: 10.1073/pnas.0900238106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McConville M.J., Ralph S.A. Chronic arsenic exposure and microbial drug resistance. Proc. Natl. Acad. Sci. USA. 2013;110:19666–19667. doi: 10.1073/pnas.1319659110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh N., Kumar A., Gupta V.K., Sharma B. Biochemical and molecular bases of lead-induced toxicity in mammalian systems and possible mitigations. Chem. Res. Toxicol. 2018;31:1009–1021. doi: 10.1021/acs.chemrestox.8b00193. [DOI] [PubMed] [Google Scholar]
- 12.González Rendón E.S., Cano G.G., Alcaraz-Zubeldia M., Garibay-Huarte T., Fortoul T.I. Lead inhalation and hepatic damage: morphological and functional evaluation in mice. Toxicol. Ind. Health. 2018;34:128–138. doi: 10.1177/0748233717750981. [DOI] [PubMed] [Google Scholar]
- 13.Hegazy A.M.S., Fouad U.A. Evaluation of lead hepatotoxicity; histological, histochemical and ultrastructural study. Forensic Med. Anat. Res. 2014;2:70–79. doi: 10.4236/fmar.2014.23013. [DOI] [Google Scholar]
- 14.Zhang Y., Liu Q., Yin H., Li S. Cadmium exposure induces pyroptosis of lymphocytes in carp pronephros and spleens by activating NLRP3. Ecotoxicol. Environ. Saf. 2020;202 doi: 10.1016/j.ecoenv.2020.110903. [DOI] [PubMed] [Google Scholar]
- 15.Bishak Y.K., Payahoo L., Osatdrahimi A., Nourazarian A. Mechanisms of cadmium carcinogenicity in the gastrointestinal tract. Asian Pac. J. Cancer Prev. APJCP. 2015;16:9–21. doi: 10.7314/apjcp.2015.16.1.9. [DOI] [PubMed] [Google Scholar]
- 16.Wani A.L., Ara A., Usmani J.A. Lead toxicity: a review. Interdiscipl. Toxicol. 2015;8:55–64. doi: 10.1515/intox-2015-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flora G., Gupta D., Tiwari A. Toxicity of lead: a review with recent updates. Interdiscipl. Toxicol. 2012;5:47–58. doi: 10.2478/v10102-012-0009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paithankar J.G., Saini S., Dwivedi S., Sharma A., Chowdhuri D.K. Heavy metal associated health hazards: an interplay of oxidative stress and signal transduction. Chemosphere. 2021;262 doi: 10.1016/j.chemosphere.2020.128350. [DOI] [PubMed] [Google Scholar]
- 19.Cao X., Wang S., Bi R., Tian S., Huo Y., Liu J. Toxic effects of Cr(VI) on the bovine hemoglobin and human vascular endothelial cells: molecular interaction and cell damage. Chemosphere. 2019;222:355–363. doi: 10.1016/j.chemosphere.2019.01.137. [DOI] [PubMed] [Google Scholar]
- 20.Jindal R., Handa K. Hexavalent chromium-induced toxic effects on the antioxidant levels, histopathological alterations and expression of Nrf2 and MT2 genes in the branchial tissue of Ctenopharyngodon idellus. Chemosphere. 2019;230:144–156. doi: 10.1016/j.chemosphere.2019.05.027. [DOI] [PubMed] [Google Scholar]
- 21.Ren X., Wang S., Zhang C., Hu X., Zhou L., Li, et al. Y. Selenium ameliorates cadmium-induced mouse leydig TM3 cell apoptosis via inhibiting the ROS/JNK/c-Jun signaling pathway. Ecotoxicol. Environ. Saf. 2020;192 doi: 10.1016/j.ecoenv.2020.110266. [DOI] [PubMed] [Google Scholar]
- 22.Deng P., Ma Q., Xi Y., Yang L., Lin M., Yu Z., et al. Transcriptomic insight into cadmium-induced neurotoxicity in embryonic neural stem/progenitor cells. Toxicol. Vitro. 2020;62:104686. doi: 10.1016/j.tiv.2019.104686. [DOI] [PubMed] [Google Scholar]
- 23.Zhao H., Wang Y., Liu J., Guo M., Fei D., Yu H. The cardiotoxicity of the common carp (Cyprinus carpio) exposed to environmentally relevant concentrations of arsenic and subsequently relieved by zinc supplementation. Environ. Pollut. 2019;253:741–748. doi: 10.1016/j.envpol.2019.07.065. [DOI] [PubMed] [Google Scholar]
- 24.Wang L., Hitron J.A., Wise J.T., Son Y.O., Roy R.V., Kim D., et al. Ethanol enhances arsenic-induced cyclooxygenase-2 expression via both NFAT and NF-κB signalings in colorectal cancer cells. Toxicol. Appl. Pharmacol. 2015;288:232–239. doi: 10.1016/j.taap.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carvalho F.P. Mining industry and sustainable development: time for change. Food Energy Secur. 2017;6:61–77. doi: 10.1002/fes3.109. [DOI] [Google Scholar]
- 26.Tchounwou P.B., Yedjou C.G., Patlolla A.K., Sutton D.J. Heavy metal toxicity and the environment. Experientia Suppl. 2012;101:133–164. doi: 10.1007/978-3-7643-8340-4_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qin W., Han D., Song X., Liu S. Sources and migration of heavy metals in a Karst water system under the threats of an abandoned Pb-Zn mine, Southwest China. Environ. Pollut. 2021;277 doi: 10.1016/j.envpol.2021.116774. [DOI] [PubMed] [Google Scholar]
- 28.Soriano A., Pallarés S., Pardo F., Vicente A.B., Sanfeliu T., Bech J. Deposition of heavy metals from particulate settleable matter in soils of an industrialised area. J. Geochem. Explor. 2012;113:36–44. doi: 10.1016/j.gexplo.2011.03.006. [DOI] [Google Scholar]
- 29.Tian L., Guan W., Ji Y., He X., Chen W., Alvarez P.J.J., et al. Microbial methylation potential of mercury sulfide particles dictated by surface structure. Nat. Geosci. 2021;14:409–416. doi: 10.1038/s41561-021-00735-y. [DOI] [Google Scholar]
- 30.Hsu-Kim H., Kucharzyk K.H., Zhang T., Deshusses M.A. Mechanisms regulating mercury bioavailability for methylating microorganisms in the aquatic environment: a critical review. Environ. Sci. Technol. 2013;47:2441–2456. doi: 10.1021/es304370g. [DOI] [PubMed] [Google Scholar]
- 31.Gilmour C., Bell J.T., Soren A.B., Riedel G., Riedel G., Kopec A.D., et al. Distribution and biogeochemical controls on net methylmercury production in Penobscot River marshes and sediment. Sci. Total Environ. 2018;640–641:555–569. doi: 10.1016/j.scitotenv.2018.05.276. [DOI] [PubMed] [Google Scholar]
- 32.Yang X., Lu D., Tan J., Sun X., Zhang Q., Zhang L. et al.,Two-dimensional silicon fingerprints reveal dramatic variations in the sources of particulate matter in Beijing during 2013-2017. Environ. Sci. Technol. 2020;54:7126–7135. doi: 10.1021/acs.est.0c00984. [DOI] [PubMed] [Google Scholar]
- 33.Lu D.W., Liu Q., Yu M., Yang X.Z., Fu Q., Zhang X.S. et al.,Natural silicon isotopic signatures reveal the sources of airborne fine particulate matter. Environ. Sci. Technol. 2018;52:1088–1095. doi: 10.1021/acs.est.7b06317. [DOI] [PubMed] [Google Scholar]
- 34.Lu D., Luo Q., Chen R., Zhuansun Y., Jiang J., Wang W. et al.,Chemical multi-fingerprinting of exogenous ultrafine particles in human serum and pleural effusion. Nat. Commun. 2020;11:2567. doi: 10.1038/s41467-020-16427-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nzihou A., Stanmore B. The fate of heavy metals during combustion and gasification of contaminated biomass—a brief review. J. Hazard Mater. 2013;256–257:56–66. doi: 10.1016/j.jhazmat.2013.02.050. [DOI] [PubMed] [Google Scholar]
- 36.Yoshiie R., Yamamoto Y., Uemiya S., Kambara S., Moritomi H. Simple and rapid analysis of heavy metals in sub-micron particulates in flue gas. Powder Technol. 2008;180:135–139. doi: 10.1016/j.powtec.2007.03.020. [DOI] [Google Scholar]
- 37.Hu X., Zhang Y., Ding Z., et al. Bioaccessibility and health risk of arsenic and heavy metals (Cd, Co, Cr, Cu, Ni, Pb, Zn and Mn) in TSP and PM2.5 in Nanjing, China, Atmos. Environ. Times. 2012;57:146–152. doi: 10.1016/j.atmosenv.2012.04.056. [DOI] [Google Scholar]
- 38.Jia Y., Huang H., Zhong M., Wang F.H., Zhang L.M., Zhu Y.G. Microbial arsenic methylation in soil and rice rhizosphere. Environ. Sci. Technol. 2013;47:3141–3148. doi: 10.1021/es303649v. [DOI] [PubMed] [Google Scholar]
- 39.Jaishankar M., Tseten T., Anbalagan N., Mathew B.B., Beeregowda K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscipl. Toxicol. 2014;7:60–72. doi: 10.2478/intox-2014-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Durães N., Novo L.A.B., Candeias C., da Silva E.F. Soil Pollution. Elsevier; Amsterdam: 2018. Distribution, transport and fate of pollutants; pp. 29–57. [DOI] [Google Scholar]
- 41.García-Giménez R., Jiménez-Ballesta R. Mine tailings influencing soil contamination by potentially toxic elements. Environ. Earth Sci. 2017;76:1–12. doi: 10.1007/s12665-016-6376-9. [DOI] [Google Scholar]
- 42.Wu J., Li J., Teng Y., Chen H., Wang Y. A partition computing-based positive matrix factorization (PC-PMF) approach for the source apportionment of agricultural soil heavy metal contents and associated health risks. J. Hazard Mater. 2020;388 doi: 10.1016/j.jhazmat.2019.121766. [DOI] [PubMed] [Google Scholar]
- 43.Jiang H.H., Cai L.M., Wen H.H., Hu G.C., Chen L.G., Luo J. An integrated approach to quantifying ecological and human health risks from different sources of soil heavy metals. Sci. Total Environ. 2020;701 doi: 10.1016/j.scitotenv.2019.134466. [DOI] [PubMed] [Google Scholar]
- 44.Sun L., Ma X., Jin H.Y., Fan C.J., Li X.D., Zuo T.T., et al. Geographical origin differentiation of Chinese Angelica by specific metal element fingerprinting and risk assessment. Environ. Sci. Pollut. Res. Int. 2020;27:45018–45030. doi: 10.1007/s11356-020-10309-x. [DOI] [PubMed] [Google Scholar]
- 45.Wu Y., Li X., Yu L., Wang T.Q., Wang J.N., Liu T.T. Review of soil heavy metal pollution in China: spatial distribution, primary sources, and remediation alternatives. Resour. Conserv. Recycl. 2022;181 doi: 10.1016/j.resconrec.2022.106261. [DOI] [Google Scholar]
- 46.Wang L., Ma L., Yang Z. Spatial variation and risk assessment of heavy metals in paddy rice from Hunan Province, Southern China. Int. J. Environ. Sci. Technol. 2018;15:1561–1572. doi: 10.1007/s13762-017-1504-y. [DOI] [Google Scholar]
- 47.Wang M., Tian P., Wang L., Yu Z., Du T., Chen Q., et al. High contribution of vehicle emissions to fine particulate pollutions in Lanzhou, Northwest China based on high-resolution online data source appointment. Sci. Total Environ. 2021;798 doi: 10.1016/j.scitotenv.2021.149310. [DOI] [PubMed] [Google Scholar]
- 48.Filippelli G., Anenberg S., Taylor M., van Geen A., Khreis H. New approaches to identifying and reducing the global burden of disease from pollution. GeoHealth. 2020;4 doi: 10.1029/2018GH000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun W., Liu S., Zhang X., Zhu H.T. Performance of hyperspectral data in predicting and mapping zinc concentration in soil. Sci. Total Environ. 2022;824 doi: 10.1016/j.scitotenv.2022.153766. [DOI] [PubMed] [Google Scholar]
- 50.Tan K., Ma W., Chen L., Wang H., Du Q., Du P., et al. Estimating the distribution trend of soil heavy metals in mining area from HyMap airborne hyperspectral imagery based on ensemble learning. J. Hazard Mater. 2021;401:123288. doi: 10.1016/j.jhazmat.2020.123288. [DOI] [PubMed] [Google Scholar]
- 51.Zhao D.Y., Xie D.N., Yin F., Liu L., Feng J.L., Ashraf T. Estimation of Pb content using reflectance spectroscopy in farmland soil near metal mines, central China. Remote. Sensors. 2022;14:2420. doi: 10.3390/rs14102420. [DOI] [Google Scholar]
- 52.Long Z., Zhu H., Bing H., Tian X., Wang Z., Wang X., et al. Contamination, sources and health risk of heavy metals in soil and dust from different functional areas in an industrial city of Panzhihua City, Southwest China. J. Hazard Mater. 2021;420:126638. doi: 10.1016/j.jhazmat.2021.126638. [DOI] [PubMed] [Google Scholar]
- 53.Huang Y., Li T., Wu C., He Z., Japenga J., Deng M. An integrated approach to assess heavy metal source apportionment in peri-urban agricultural soils. J. Hazard Mater. 2015;299:540–549. doi: 10.1016/j.jhazmat.2015.07.041. [DOI] [PubMed] [Google Scholar]
- 54.Wang P., Li Z., Liu J., Bi X., Ning Y., Yang S., et al. Apportionment of sources of heavy metals to agricultural soils using isotope fingerprints and multivariate statistical analyses. Environ. Pollut. 2019;249:208–216. doi: 10.1016/j.envpol.2019.03.034. [DOI] [PubMed] [Google Scholar]
- 55.Wang Z., Dwyer G.S., Coleman D.S., Vengosh A. Lead isotopes as a new tracer for detecting coal fly ash in the environment. Environ. Sci. Technol. Lett. 2019;6:714–719. doi: 10.1021/acs.estlett.9b00512. [DOI] [Google Scholar]
- 56.Gonzalez R.O., Strekopytov S., Amato F., Querol X., Reche C., Weiss D. New insights from zinc and copper isotopic compositions into the sources of atmospheric particulate matter from two major European Cities. Environ. Sci. Technol. 2016;50:9816–9824. doi: 10.1021/acs.est.6b00863. [DOI] [PubMed] [Google Scholar]
- 57.Zhou J.W., Li Z., Liu M.S., Yu H.M., Wu L.H., Huang F., et al. Cadmium isotopic fractionation in the soil-plant system during repeated phytoextraction with a cadmium hyperaccumulating plant species. Environ. Sci. Technol. 2020;54:13598–13609. doi: 10.1021/acs.est.0c03142. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Y., Zhang H., Zhang Z., Liu C., Sun C., Zhang W., et al. pH effect on heavy metal release from a polluted sediment. J. Chem. 2018;2018:7597640. doi: 10.1155/2018/7597640. [DOI] [Google Scholar]
- 59.Acosta J.A., Jansen B., Kalbitz K., Faz A., Martinez-Martinez S. Salinity increases mobility of heavy metals in soils. Chemosphere. 2011;85:1318–1324. doi: 10.1016/j.chemosphere.2011.07.046. [DOI] [PubMed] [Google Scholar]
- 60.Ho G.D., Tabelin C.B., Tangviroon P., Tamamura S., Igarashi T. Effects of cement addition on arsenic leaching from soils excavated from projects employing shield-tunneling method. Geoderma. 2021;385 doi: 10.1016/j.geoderma.2020.114896. [DOI] [Google Scholar]
- 61.Tanaka M., Takahashi Y., Yamaguchi N., Kim K.W., Zheng G., Sakamitsu M. The difference of diffusion coefficients in water for arsenic compounds at various pH and its dominant factors implied by molecular simulations. Geochem. Cosmochim. Acta. 2013;105:360–371. doi: 10.1016/j.gca.2012.12.004. [DOI] [Google Scholar]
- 62.Tabelin C.B., Igarashi T., Villacorte-Tabelin M., Park I., Opiso E.M., Ito M., et al. Arsenic, selenium, boron, lead, cadmium, copper, and zinc in naturally contaminated rocks: a review of their sources, modes of enrichment, mechanisms of release, and mitigation strategies. Sci. Total Environ. 2018;645:1522–1553. doi: 10.1016/j.scitotenv.2018.07.103. [DOI] [PubMed] [Google Scholar]
- 63.Hao L., Liu M., Wang N., Li G. A critical review on arsenic removal from water using iron-based adsorbents. RSC Adv. 2018;8:39545–39560. doi: 10.1039/C8RA08512A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cheng W., Zhang W., Hu L., Ding W., Wu F., Li J. Etching synthesis of iron oxide nanoparticles for adsorption of arsenic from water. RSC Adv. 2016;6:15900–15910. doi: 10.1039/C5RA26143K. [DOI] [Google Scholar]
- 65.Markelova E. UWSpace; 2017. Interpretation of Redox Potential and Assessment of Oxyanion (As, Sb, Cr) Mobility during Oxic-Anoxic Oscillations [D] [Google Scholar]
- 66.Lottermoser B.G. Mine Wastes. Springer Berlin Heidelberg; Berlin, Heidelberg: 2010. Sulfidic mine wastes; pp. 43–117. [DOI] [Google Scholar]
- 67.Mao S., Gu W., Bai J., Dong B., Huang Q., Zhao J., et al. Migration characteristics of heavy metals during simulated use of secondary products made from recycled e-waste plastic. J. Environ. Manag. 2020;266:110577. doi: 10.1016/j.jenvman.2020.110577. [DOI] [PubMed] [Google Scholar]
- 68.Fu X., Wang S., Chang X., Cai S., Xing J., Hao J. Modeling analysis of secondary inorganic aerosols over China: pollution characteristics, and meteorological and dust impacts. Sci. Rep. 2016;6 doi: 10.1038/srep35992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nguyen M.V., Park G.H., Lee B.K. Correlation analysis of size-resolved airborne particulate matter with classified meteorological conditions. Meteorol. Atmos. Phys. 2017;129:35–46. doi: 10.1007/s00703-016-0456-y. [DOI] [Google Scholar]
- 70.Kim J.J., Hann T., Lee S.J. Effect of flow and humidity on indoor deposition of particulate matter. Environ. Pollut. 2019;255 doi: 10.1016/j.envpol.2019.113263. [DOI] [PubMed] [Google Scholar]
- 71.Naveen B.P., Sumalatha J., Malik R.K. A study on contamination of ground and surface water bodies by leachate leakage from a landfill in Bangalore, India. Int. J. Geotech. Eng. 2018;9:1–20. doi: 10.1186/s40703-018-0095-x. [DOI] [Google Scholar]
- 72.Goonetilleke A., Lampard J.L. Elsevier; Amsterdam: 2019. Stormwater Quality, Pollutant Sources, Processes, and Treatment Options. Approaches to Water Sensitive Urban Design; pp. 49–74. [DOI] [Google Scholar]
- 73.Huang B., Yuan Z., Li D., Zheng M., Nie X., Liao Y. Effects of soil particle size on the adsorption, distribution, and migration behaviors of heavy metal(loid)s in soil: a review. Environ. Sci. Process. Impacts. 2020;22:1596–1615. doi: 10.1039/d0em00189a. [DOI] [PubMed] [Google Scholar]
- 74.Pakulski D., Czepa W., Witomska S., Aliprandi A., Pawluć P., Patroniak V., et al. Graphene oxide-branched polyethylenimine foams for efficient removal of toxic cations from water. J. Mater. Chem. 2018;6:9384–9390. doi: 10.1039/C8TA01622D. [DOI] [Google Scholar]
- 75.Yuan Y., Bolan N., Prévoteau A., Vithanage M., Biswas J.K., Ok Y.S., et al. Applications of biochar in redox-mediated reactions. Bioresour. Technol. 2017;246:271–281. doi: 10.1016/j.biortech.2017.06.154. [DOI] [PubMed] [Google Scholar]
- 76.Ali H., Khan E., Ilahi I. Environmental chemistry and ecotoxicology of hazardous heavy metals: environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019 doi: 10.1155/2019/6730305. 2019. [DOI] [Google Scholar]
- 77.Ali H., Khan E. Trophic transfer, bioaccumulation, and biomagnification of non-essential hazardous heavy metals and metalloids in food chains/webs—concepts and implications for wildlife and human health. Hum. Ecol. Risk Assess. 2019;25:1353–1376. doi: 10.1080/10807039.2018.1469398. [DOI] [Google Scholar]
- 78.Beesley L., Hough R., Deacon C.M., Norton G.J. Health11; 2019. The Impacts of Applying Metal(loid) Enriched Wood Ash to Soils on the Growth and Elemental Accumulation of Rice, Expo; pp. 311–324. [DOI] [Google Scholar]
- 79.Bi B., Liu X., Guo X., Lu S. Occurrence and risk assessment of heavy metals in water, sediment, and fish from Dongting Lake, China. Environ. Sci. Pollut. Res. 2018;25:34076–34090. doi: 10.1007/s11356-018-3329-8. [DOI] [PubMed] [Google Scholar]
- 80.Rai P.K., Lee S.S., Zhang M., Tsang Y.F., Kim K.H. Heavy metals in food crops: health risks, fate, mechanisms, and management. Environ. Int. 2019;125:365–385. doi: 10.1016/j.envint.2019.01.067. [DOI] [PubMed] [Google Scholar]
- 81.Cheema A.I., Liu G., Yousaf B., Abbas Q., Zhou H. A comprehensive review of biogeochemical distribution and fractionation of lead isotopes for source tracing in distinct interactive environmental compartments. Sci. Total Environ. 2020;719 doi: 10.1016/j.scitotenv.2019.135658. [DOI] [PubMed] [Google Scholar]
- 82.Rothenberg S.E., Yin R.S., Hurley J.P., Krabbenhoft D.P., Ismawati Y., Hong C., Donohue A. Stable mercury isotopes in polished rice (Oryza sativa L.) and hair from rice consumers. Environ. Sci. Technol. 2017;51:6480–6488. doi: 10.1021/acs.est.7b01039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xu H.M., Sonke J.E., Guinot B., Fu X.W., Sun R.Y., Lanzanova A., et al. Seasonal and annual variations in atmospheric Hg and Pb isotopes in xi’an, China. Environ. Sci. Technol. 2017;51:3759–3766. doi: 10.1021/acs.est.6b06145. [DOI] [PubMed] [Google Scholar]
- 84.Cheng Z., Chen L.J., Li H.H., Lin J.Q., Yang Z.B., Yang Y.X., et al. Characteristics and health risk assessment of heavy metals exposure via household dust from urban area in Chengdu, China. Sci. Total Environ. 2018;619–620:621–629. doi: 10.1016/j.scitotenv.2017.11.144. [DOI] [PubMed] [Google Scholar]
- 85.Luo X., Zhao Z., Xie J., Luo J., Chen Y., Li H., et al. Pulmonary bioaccessibility of trace metals in PM2.5 from different megacities simulated by lung fluid extraction and DGT method. Chemosphere. 2019;218:915–921. doi: 10.1016/j.chemosphere.2018.11.079. [DOI] [PubMed] [Google Scholar]
- 86.Desboeufs K.V., Sofikitis A., Losno R., Colin J., Ausset P. Dissolution and solubility of trace metals from natural and anthropogenic aerosol particulate matter. Chemosphere. 2005;58:195–203. doi: 10.1016/j.chemosphere.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 87.Xie J.J., Yuan C.G., Shen Y.W., Xie J., He K.Q., Zhu H.T., et al. Bioavailability/speciation of arsenic in atmospheric PM2.5 and their seasonal variation: a case study in Baoding City, China. Ecotoxicol, Environ. Saf. 2019;169:487–495. doi: 10.1016/j.ecoenv.2018.11.026. [DOI] [PubMed] [Google Scholar]
- 88.Liu X., Ouyang W., Zhang T. Chemical speciation and health effect of heavy metals in atmospheric particulate matter. Huanjing Huaxue-Environmental Chemistry. 2021;40(4):974–989. [Google Scholar]
- 89.Kotaś J., Stasicka Z. Chromium occurrence in the environment and methods of its speciation. Environ. Pollut. 2000;107:263–283. doi: 10.1016/S0269-7491(99)00168-2. [DOI] [PubMed] [Google Scholar]
- 90.Liang J.L., Huang X.M., Yan J.W., Li Y.Y., Zhao Z.W., Liu Y.Y., et al. A review of the formation of Cr(VI) via Cr(III) oxidation in soils and groundwater. Sci. Total Environ. 2021;774:145762. doi: 10.1016/j.scitotenv.2021.145762. [DOI] [Google Scholar]
- 91.Bjørklund G., Dadar M., Mutter J., Aaseth J. The toxicology of mercury: current research and emerging trends. Environ. Res. 2017;159:545–554. doi: 10.1016/j.envres.2017.08.051. [DOI] [PubMed] [Google Scholar]
- 92.Pamphlett R. The prevalence of inorganic mercury in human cells increases during aging but decreases in the very old. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-96359-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lei P., Zou N., Liu Y., Cai W., Wu M., Tang W., et al. Understanding the risks of mercury sulfide nanoparticles in the environment: formation, presence, and environmental behaviors. J. Environ. Sci. (China) 2022;119:78–92. doi: 10.1016/j.jes.2022.02.017. [DOI] [PubMed] [Google Scholar]
- 94.Chen C.H.S., Kuo T.C., Kuo H.C., Tseng Y.J., Kuo C.H., Yuan T.H., et al. Metabolomics of children and adolescents exposed to industrial carcinogenic pollutants. Environ. Sci. Technol. 2019;53:5454–5465. doi: 10.1021/acs.est.9b00392. [DOI] [PubMed] [Google Scholar]
- 95.Chen Q.Y., Murphy A., Sun H., Costa M. Molecular and epigenetic mechanisms of Cr(VI)-induced carcinogenesis. Toxicol. Appl. Pharmacol. 2019;377 doi: 10.1016/j.taap.2019.114636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Smeyne M., Smeyne R.J. Glutathione metabolism and Parkinson’s disease. Free Radic. Biol. Med. 2013;62:13–25. doi: 10.1016/j.freeradbiomed.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Patra R.C., Rautray A.K., Swarup D. Oxidative stress in lead and cadmium toxicity and its amelioration. Vet. Med. Int. 2011;2011 doi: 10.4061/2011/457327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lin Y.C., Wang F.F. Mechanisms underlying the pro-survival pathway of p53 in suppressing mitotic death induced by adriamycin. Cell. Signals. 2008;20:258–267. doi: 10.1016/j.cellsig.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 99.Li J.Y., Zheng X.Y., Ma X.Y., Xu X.Y., Du Y., Lv Q.J., et al. Melatonin protects against chromium(VI)-induced cardiac injury via activating the AMPK/Nrf2 pathway. J. Inorg. Biochem. 2019;197:110698. doi: 10.1016/j.jinorgbio.2019.110698. [DOI] [PubMed] [Google Scholar]
- 100.Han B., Li S.Y., Lv Y.Y., Yang D.Q., Li J.Y., Yang Q.Y., et al. Dietary melatonin attenuates chromium-induced lung injuryviaactivating the Sirt1/Pgc-1α/Nrf2 pathway. Food Funct. 2019;10:5555–5565. doi: 10.1039/c9fo01152h. [DOI] [PubMed] [Google Scholar]
- 101.Lee Y.H., Su S.B., Huang C.C., Sheu H.M., Tsai J.C., Lin C.H., et al. N-acetylcysteine attenuates hexavalent chromium-induced hypersensitivity through inhibition of cell death, ROS-related signaling and cytokine expression. PLoS One. 2014;9 doi: 10.1371/journal.pone.0108317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ansari M.N., Aloliet R.I., Ganaie M.A., Khan T.H., Najeeb-Ur-Rehman, Imam F., et al. Roflumilast, a phosphodiesterase 4 inhibitor, attenuates cadmium-induced renal toxicity via modulation of NF-κB activation and induction of NQO1 in rats. Hum. Exp. Toxicol. 2019;38:588–597. doi: 10.1177/0960327119829521. [DOI] [PubMed] [Google Scholar]
- 103.Yamaguchi Y., Madhyastha H., Madhyastha R., Choijookhuu N., Hishikawa Y., Pengjam Y., et al. Arsenic acid inhibits proliferation of skin fibroblasts, and increases cellular senescence through ROS mediated MST1-FOXO signaling pathway. J. Toxicol. Sci. 2016;41:105–113. doi: 10.2131/jts.41.105. [DOI] [PubMed] [Google Scholar]
- 104.Morten K.J., Badder L., Knowles H.J. Differential regulation of HIF-mediated pathways increases mitochondrial metabolism and ATP production in hypoxic osteoclasts. J. Pathol. 2013;229:755–764. doi: 10.1002/path.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li Y.N., Xi M.M., Guo Y., Hai C.X., Yang W.L., Qin X.J. NADPH oxidase-mitochondria axis-derived ROS mediate arsenite-induced HIF-1α stabilization by inhibiting prolyl hydroxylases activity. Toxicol. Lett. 2014;224:165–174. doi: 10.1016/j.toxlet.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 106.Das K.K., Jargar J.G., Saha S., Yendigeri S.M., Singh S.B. Α-tocopherol supplementation prevents lead acetate and hypoxia-induced hepatic dysfunction. Indian J. Pharmacol. 2015;47:285–291. doi: 10.4103/0253-7613.157126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dou Y., Jiang X., Xie H., He J., Xiao S. The Jun N-terminal kinases signaling pathway plays a seesaw role in ovarian carcinoma: a molecular aspect. J. Ovarian Res. 2019;12:99. doi: 10.1186/s13048-019-0573-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schellino R., Boido M., Vercelli A. JNK signaling pathway involvement in spinal cord neuron development and death. Cells. 2019;8:1576. doi: 10.3390/cells8121576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Solinas G., Becattini B. JNK at the crossroad of obesity, insulin resistance, and cell stress response. Mol. Metabol. 2017;6:174–184. doi: 10.1016/j.molmet.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Datta S., Mazumder S., Ghosh D., Dey S., Bhattacharya S. Low concentration of arsenic could induce caspase-3 mediated head kidney macrophage apoptosis with JNK-p38 activation in Clarias batrachus. Toxicol. Appl. Pharmacol. 2009;241:329–338. doi: 10.1016/j.taap.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 111.Monroe R.K., Halvorsen S.W. Cadmium blocks receptor-mediated Jak/STAT signaling in neurons by oxidative stress. Free Radic. Biol. Med. 2006;41:493–502. doi: 10.1016/j.freeradbiomed.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 112.Cheng H.Y., Li P., David M., Smithgall T.E., Feng L., Lieberman M.W. Arsenic inhibition of the JAK-STAT pathway. Oncogene. 2004;23:3603–3612. doi: 10.1038/sj.onc.1207466. [DOI] [PubMed] [Google Scholar]
- 113.H.Q. Xu, Y. Jia, Z.D. Sun, J.H. Su, Q.S. Liu, Q.F. Zhou, G.B. Jiang, Environmental pollution, a hidden culprit for health issues. Eco Environ. Health. 2022;1:31–45. doi: 10.1016/j.eehl.2022.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Inaba T., Kobayashi E., Suwazono Y., Uetani M., Oishi M., Nakagawa H., Nogawa K. Estimation of cumulative cadmium intake causing Itai-itai disease. Toxicol. Lett. 2005;159:192–201. doi: 10.1016/j.toxlet.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 115.Kessler R. The Minamata Convention on Mercury: a first step toward protecting future generations. Environ. Health Perspect. 2013;121 doi: 10.1289/ehp.121-A304. A304–A309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ou L., Chen C., Chen L., Wang H., Yang T., Xie H., et al. Low-level prenatal mercury exposure in North China: an exploratory study of anthropometric effects. Environ. Sci. Technol. 2015;49:6899–6908. doi: 10.1021/es5055868. [DOI] [PubMed] [Google Scholar]
- 117.Wan C., Pan S., Lin L., Li J., Dong G., Jones K.C., et al. DNA methylation biomarkers of IQ reduction are associated with long-term lead exposure in school aged children in Southern China. Environ. Sci. Technol. 2021;55:412–422. doi: 10.1021/acs.est.0c01696. [DOI] [PubMed] [Google Scholar]
- 118.Zhou Y., Wang Y., Su J., Wu Z., Wang C., Zhong W., et al. Integration of microRNAome, proteomics and metabolomics to analyze arsenic-induced malignant cell transformation. Oncotarget. 2017;8:90879–90896. doi: 10.18632/oncotarget.18741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.National Research Council . Risk Assessment in the Federal Government: Managing the Process. The National Academies Press; Washington, DC: 1983. [DOI] [PubMed] [Google Scholar]
- 120.Kan X., Dong Y., Feng L., Zhou M., Hou H. Contamination and health risk assessment of heavy metals in China’s lead-zinc mine tailings: a meta-analysis. Chemosphere. 2021;267 doi: 10.1016/j.chemosphere.2020.128909. [DOI] [PubMed] [Google Scholar]
- 121.Liu L., Liu Q., Ma J., Wu H., Qu Y., Gong Y., et al. Heavy metal(loid)s in the topsoil of urban parks in Beijing, China: concentrations, potential sources, and risk assessment. Environ. Pollut. 2020;260:114083. doi: 10.1016/j.envpol.2020.114083. [DOI] [PubMed] [Google Scholar]
- 122.Li Z., Ma Z., van der Kuijp T.J., et al. A review of soil heavy metal pollution from mines in China: pollution and health risk assessment. Sci. Total Environ. 2014;468–469:843–853. doi: 10.1016/j.scitotenv.2013.08.090. [DOI] [PubMed] [Google Scholar]
- 123.Zhang Y., Guo Z., Peng C., Deng H., Xiao X. A questionnaire based probabilistic risk assessment (PRA) of heavy metals in urban and suburban soils under different land uses and receptor populations. Sci. Total Environ. 2021;793 doi: 10.1016/j.scitotenv.2021.148525. [DOI] [PubMed] [Google Scholar]
- 124.Zhang H.Z., Luo Y.M., Zhang H.B., Song J., Xia J.Q., Zhao Q.G. Development of lead benchmarks for soil based on human blood lead level in China. Huanjing Kexue. 2009;30:3036–3042. [PubMed] [Google Scholar]
- 125.Gunarathne V., Rajapaksha A.U., Vithanage M., Alessi D.S., Selvasembian R., Naushad M., et al. Hydrometallurgical processes for heavy metals recovery from industrial sludges. Crit. Rev. Environ. Sci. Technol. 2022;52:1022–1062. doi: 10.1080/10643389.2020.1847949. [DOI] [Google Scholar]
- 126.Fogarasi S., Imre-Lucaci F., Egedy A., Imre-Lucaci Á., Ilea P. Eco-friendly copper recovery process from waste printed circuit boards using Fe3+/Fe2+ redox system. Waste Manag. 2015;40:136–143. doi: 10.1016/j.wasman.2015.02.030. [DOI] [PubMed] [Google Scholar]
- 127.Mecucci A., Scott K. Leaching and electrochemical recovery of copper, lead and tin from scrap printed circuit boards. J. Chem. Technol. Biotechnol. 2002;77:449–457. doi: 10.1002/jctb.575. [DOI] [Google Scholar]
- 128.Fogarasi S., Imre-Lucaci F., Imre-Lucaci Á., Ilea P. Copper recovery and gold enrichment from waste printed circuit boards by mediated electrochemical oxidation. J. Hazard Mater. 2014;273:215–221. doi: 10.1016/j.jhazmat.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 129.Srimuk P., Su X., Yoon J., Aurbach D., Presser V. Charge-transfer materials for electrochemical water desalination, ion separation and the recovery of elements. Nat. Rev. Mater. 2020;5:517–538. doi: 10.1038/s41578-020-0193-1. [DOI] [Google Scholar]
- 130.Zhang W., Lu J., Guo Z. Challenges and future perspectives on sodium and potassium ion batteries for grid-scale energy storage. Mater. Today. 2021;50:400–417. doi: 10.1016/j.mattod.2021.03.015. [DOI] [Google Scholar]
- 131.Deng H., Tian C., Li L., Liang Y.J., Yan S.J., Hu M., et al. Microinteraction analysis between heavy metals and coexisting phases in heavy metal containing solid wastes. ACS EST Eng. 2022;2:547–563. doi: 10.1021/acsestengg.1c00343. [DOI] [Google Scholar]
- 132.Struis R.P.W.J., Ludwig C., Lutz H., Scheidegger A.M. Speciation of zinc in municipal solid waste incineration fly ash after heat treatment: an X-ray absorption spectroscopy study. Environ. Sci. Technol. 2004;38:3760–3767. doi: 10.1021/es0346126. [DOI] [PubMed] [Google Scholar]
- 133.Tou F., Yang Y., Feng J., Niu Z., Pan H., Qin Y., et al. Environmental risk implications of metals in sludges from waste water treatment plants: the discovery of vast stores of metal-containing nanoparticles. Environ. Sci. Technol. 2017;51:4831–4840. doi: 10.1021/acs.est.6b05931. [DOI] [PubMed] [Google Scholar]
- 134.Lin L., Liu X.M., Liang Y.J., Xu W.B., Li Y., Lin Z. Analysis of mineral phases in heavy-metal hazardous waste under the interdisciplinary scope of data science and chemistry [J] Prog. Chem. 2021;33(12):2163–2172. [Google Scholar]
- 135.Gai K., Hoelen T.P., Hsu-Kim H., Lowry G.V. Mobility of four common mercury species in model and natural unsaturated soils. Environ. Sci. Technol. 2016;50:3342–3351. doi: 10.1021/acs.est.5b04247. [DOI] [PubMed] [Google Scholar]
- 136.Zhang M., Yang C., Zhao M., Yang K., Shen R., Zheng Y. Immobilization of Cr(VI) by hydrated Portland cement pastes with and without calcium sulfate. J. Hazard Mater. 2018;342:242–251. doi: 10.1016/j.jhazmat.2017.07.039. [DOI] [PubMed] [Google Scholar]
- 137.Liu W., Huang F., Wang Y., Zou T., Zheng J., Lin Z. Recycling MgOH2 nanoadsorbent during treating the low concentration of CrVI. Environ. Sci. Technol. 2011;45:1955–1961. doi: 10.1021/es1035199. [DOI] [PubMed] [Google Scholar]
- 138.Tian C., Tu J., Qiu P., Wang S., Song H., Xu Y., et al. Ultrastrong anion affinity of anionic clay induced by its inherent nanoconfinement. Environ. Sci. Technol. 2021;55:930–940. doi: 10.1021/acs.est.0c03775. [DOI] [PubMed] [Google Scholar]
- 139.Liu W.Z., Zheng J.Y., Ou X.W., Liu X.M., Song Y., Tian C., et al. Effective extraction of Cr(VI) from hazardous gypsum sludge via controlling the phase transformation and chromium species. Environ. Sci. Technol. 2018;52:13336–13342. doi: 10.1021/acs.est.8b02213. [DOI] [PubMed] [Google Scholar]
- 140.Zheng J., Lv J., Liu W., Dai Z., Liao H., Deng H. et al.,Selective recovery of Cr from electroplating nanosludge via crystal modification and dilute acid leaching. Environ. Sci.: Nano. 2020;7:1593–1601. doi: 10.1039/D0EN00196A. [DOI] [Google Scholar]
- 141.Song Y., Li J., Peng M., Deng Z., Yang J., Liu W., et al. Identification of Cr(VI) speciation in ferrous sulfate-reduced chromite ore processing residue (rCOPR) and impacts of environmental factors erosion on Cr(VI) leaching. J. Hazard Mater. 2019;373:389–396. doi: 10.1016/j.jhazmat.2019.03.097. [DOI] [PubMed] [Google Scholar]
- 142.Nanda S., Berruti F. A technical review of bioenergy and resource recovery from municipal solid waste. J. Hazard Mater. 2021;403 doi: 10.1016/j.jhazmat.2020.123970. [DOI] [PubMed] [Google Scholar]
- 143.Chen J.C., Huang J.S. Partitioning characteristics and particle size distributions of heavy metals in the O2/RFG waste incineration system. J. Hazard Mater. 2009;172:826–832. doi: 10.1016/j.jhazmat.2009.07.074. [DOI] [PubMed] [Google Scholar]
- 144.Liu H., Li S., Guo G., Gong L., Zhang L., Qie Y., et al. Ash formation and the inherent heavy metal partitioning behavior in a 100 t/d hazardous waste incineration plant. Sci. Total Environ. 2022;814:151938. doi: 10.1016/j.scitotenv.2021.151938. [DOI] [PubMed] [Google Scholar]
- 145.Qian G., Cao Y., Chui P., Tay J. Utilization of MSWI fly ash for stabilization/solidification of industrial waste sludge. J. Hazard Mater. 2006;129:274–281. doi: 10.1016/j.jhazmat.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 146.Wang X.P., Yu R., Shui Z.H., Zhao Z.M., Song Q.L., Yang B., Fan D.Q. Development of a novel cleaner construction product: ultra-high performance concrete incorporating lead-zinc tailings. J. Clean. Prod. 2018;196:172–182. doi: 10.1016/j.jclepro.2018.06.058. [DOI] [Google Scholar]
- 147.Isteri V., Ohenoja K., Hanein T., Kinoshita H., Tanskanen P., Illikainen M., Fabritius T. Production and properties of ferrite-rich CSAB cement from metallurgical industry residues. Sci. Total Environ. 2020;712 doi: 10.1016/j.scitotenv.2019.136208. [DOI] [PubMed] [Google Scholar]
- 148.Li R., Wang L., Yang T., Raninger B. Investigation of MSWI fly ash melting characteristic by DSC-DTA. Waste Manag. 2007;27:1383–1392. doi: 10.1016/j.wasman.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 149.Zhang Z.K., Li A.M., Wang X.X., Zhang L. Stabilization/solidification of municipal solid waste incineration fly ash via co-sintering with waste-derived vitrified amorphous slag. Waste Manag. 2016;56:238–245. doi: 10.1016/j.wasman.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 150.Yang K., Zhu Y., Shan R.R., Shao Y.Q., Tian C. Heavy metals in sludge during anaerobic sanitary landfill: speciation transformation and phytotoxicity. J. Environ. Manag. 2017;189:58–66. doi: 10.1016/j.jenvman.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 151.Benn T.M., Westerhoff P. Nanoparticle silver released into water from commercially available sock fabrics. Environ. Sci. Technol. 2008;42:4133–4139. doi: 10.1021/es7032718. [DOI] [PubMed] [Google Scholar]
- 152.Omar H., Rohani S. Treatment of landfill waste, leachate and landfill gas: a review. Front. Chem. Sci. Eng. 2015;9:15–32. doi: 10.1007/s11705-015-1501-y. [DOI] [Google Scholar]
- 153.Caballero-Guzman A., Sun T., Nowack B. Flows of engineered nanomaterials through the recycling process in Switzerland. Waste Manag. 2015;36:33–43. doi: 10.1016/j.wasman.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 154.Baun D.L., Christensen T.H. Speciation of heavy metals in landfill leachate: a review. Waste Manag. Res. 2004;22:3–23. doi: 10.1177/0734242X04042146. [DOI] [PubMed] [Google Scholar]
- 155.Mitrano D.M., Mehrabi K., Dasilva Y.A.R., Nowack B. Mobility of metallic (nano)particles in leachates from landfills containing waste incineration residues. Environ. Sci.: Nano. 2017;4:480–492. doi: 10.1039/C6EN00565A. [DOI] [Google Scholar]
- 156.Zhang W., Hu C., Guo Z., Dai L. High-performance K-CO2 batteries based on metal-free carbon electrocatalysts. Angew. Chem., Int. Ed. 2020;59:3470–3474. doi: 10.1002/anie.201913687. [DOI] [PubMed] [Google Scholar]
- 157.Song H., Ou X., Han B., Deng H., Zhang W., Tian C., et al. An overlooked natural hydrogen evolution pathway: Ni2+ boosting H2 O reduction by Fe(OH)2 oxidation during low-temperature serpentinization. Angew. Chem., Int. Ed. Engl. 2021;60:24054–24058. doi: 10.1002/anie.202110653. [DOI] [PubMed] [Google Scholar]
- 158.Lane D.J., Sippula O., Koponen H., Heimonen M., Peräniemi S., Lähde A., et al. Volatilisation of major, minor, and trace elements during thermal processing of fly ashes from waste- and wood-fired power plants in oxidising and reducing gas atmospheres. Waste Manag. 2020;102:698–709. doi: 10.1016/j.wasman.2019.11.025. [DOI] [PubMed] [Google Scholar]
- 159.Kurashima K., Matsuda K., Kumagai S., Kameda T., Saito Y., Yoshioka T. A combined kinetic and thermodynamic approach for interpreting the complex interactions during chloride volatilization of heavy metals in municipal solid waste fly ash. Waste Manag. 2019;87:204–217. doi: 10.1016/j.wasman.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 160.Zhu S., Wang Z., Lin X., Sun T., Qu Z., Chen Y., et al. Effective recycling of Cu from electroplating wastewater effluent via the combined Fenton oxidation and hydrometallurgy route. J. Environ. Manag. 2020;271:110963. doi: 10.1016/j.jenvman.2020.110963. [DOI] [PubMed] [Google Scholar]
- 161.Luo J., Duan N., Xu F.Y., Jiang L.H., Zhang C.M., Ye W.Q. System-level analysis of the generation and distribution for Pb, Cu, and Ag in the process network of zinc hydrometallurgy: implications for sustainability. J. Clean. Prod. 2019;234:755–766. doi: 10.1016/j.jclepro.2019.06.250. [DOI] [Google Scholar]
- 162.Li J., Xu T., Liu J., Wen J., Gong S. Bioleaching metals from waste electrical and electronic equipment (WEEE) by Aspergillus Niger: a review. Environ. Sci. Pollut. Res. Int. 2021;28:44622–44637. doi: 10.1007/s11356-021-15074-z. [DOI] [PubMed] [Google Scholar]
- 163.Priya A., Hait S. Comparative assessment of metallurgical recovery of metals from electronic waste with special emphasis on bioleaching. Environ. Sci. Pollut. Res. 2017;24:6989–7008. doi: 10.1007/s11356-016-8313-6. [DOI] [PubMed] [Google Scholar]
- 164.Garole D.J., Hossain R., Garole V.J., Sahajwalla V., Nerkar J., Dubal D.P. Recycle, recover and repurpose strategy of spent Li-ion batteries and catalysts: current status and future opportunities. ChemSusChem. 2020;13:3079–3100. doi: 10.1002/cssc.201903213. [DOI] [PubMed] [Google Scholar]
- 165.Li J., Wang G., Xu Z. Environmentally-friendly oxygen-free roasting/wet magnetic separation technology for in situ recycling cobalt, lithium carbonate and graphite from spent LiCoO2/graphite lithium batteries. J. Hazard Mater. 2016;302:97–104. doi: 10.1016/j.jhazmat.2015.09.050. [DOI] [PubMed] [Google Scholar]
- 166.Ma E., Lu R., Xu Z. An efficient rough vacuum-chlorinated separation method for the recovery of indium from waste liquid crystal display panels. Green Chem. 2012;14:3395–3401. doi: 10.1039/C2GC36241D. [DOI] [Google Scholar]
- 167.Antuñano N., Cambra J.F., Arias P.L. Development of a combined solid and liquid wastes treatment integrated into a high purity ZnO hydrometallurgical production process from Waelz oxide. Hydrometallurgy. 2017;173:250–257. doi: 10.1016/j.hydromet.2017.09.002. [DOI] [Google Scholar]
- 168.Zhang D.J., Dong L., Li Y.T., Wu Y.F., Ma Y.X., Yang B. Copper leaching from waste printed circuit boards using typical acidic ionic liquids recovery of e-wastes’ surplus value. Waste Manag. 2018;78:191–197. doi: 10.1016/j.wasman.2018.05.036. [DOI] [PubMed] [Google Scholar]
- 169.Xu X., Yang Y., Wang G., Zhang S., Cheng Z., Li T., et al. Removal of heavy metals from industrial sludge with new plant-based washing agents. Chemosphere. 2020;246:125816. doi: 10.1016/j.chemosphere.2020.125816. [DOI] [PubMed] [Google Scholar]
- 170.Chen X., Zhang J., Yan B. A clean method of precipitation vanadium from the vanadium bearing oxalic acid leaching solution. Miner. Eng. 2021;165 doi: 10.1016/j.mineng.2021.106864. [DOI] [Google Scholar]
- 171.Rao M.J., Zhang T., Li G.H., Zhou Q., Luo J., Zhang X., et al. Solvent extraction of Ni and co from the phosphoric acid leaching solution of laterite ore by P204 and P507. Metals. 2020;10:545. doi: 10.3390/met10040545. [DOI] [Google Scholar]
- 172.Das N., Jana R.K. Adsorption of some bivalent heavy metal ions from aqueous solutions by manganese nodule leached residues. J. Colloid Interface Sci. 2006;293:253–262. doi: 10.1016/j.jcis.2005.06.064. [DOI] [PubMed] [Google Scholar]
- 173.Zhang B., Liu H.Z., Wang W., Gao Z.G., Cao Y.H. Recovery of rhenium from copper leach solutions using ion exchange with weak base resins. Hydrometallurgy. 2017;173:50–56. doi: 10.1016/j.hydromet.2017.08.002. [DOI] [Google Scholar]
- 174.Meng Q., Zhang Y.J., Dong P., Liang F. A novel process for leaching of metals from LiNi1/3Co1/3Mn1/3O2 material of spent lithium ion batteries: process optimization and kinetics aspects. J. Ind. Eng. Chem. 2018;61:133–141. doi: 10.1016/j.jiec.2017.12.010. [DOI] [Google Scholar]
- 175.Han H.S., Sun W., Hu Y.H., Tang H.H., Yue T. Magnetic separation of iron precipitate from nickel sulfate solution by magnetic seeding. Hydrometallurgy. 2015;156:182–187. doi: 10.1016/j.hydromet.2015.07.001. [DOI] [Google Scholar]
- 176.Keber S., Brückner L., Elwert T., Kuhn T. Concept for a hydrometallurgical processing of a copper-cobalt-nickel alloy made from manganese nodules. Chem. Ing. Tech. 2020;92:379–386. doi: 10.1002/cite.201900125. [DOI] [Google Scholar]
- 177.Das S., Behera S.S., Murmu B.M., Mohapatra R.K., Mandal D., Samantray R., et al. Extraction of scandium(III) from acidic solutions using organo-phosphoric acid reagents: a comparative study. Separ. Purif. Technol. 2018;202:248–258. doi: 10.1016/j.seppur.2018.03.023. [DOI] [Google Scholar]
- 178.Potysz A., Van Hullebusch E.D., Kierczak J. Perspectives regarding the use of metallurgical slags as secondary metal resources—a review of bioleaching approaches. J. Environ. Manag. 2018;219:138–152. doi: 10.1016/j.jenvman.2018.04.083. [DOI] [PubMed] [Google Scholar]
- 179.Watling H.R. The bioleaching of sulphide minerals with emphasis on copper sulphides—a review. Hydrometallurgy. 2006;84:81–108. doi: 10.1016/j.hydromet.2006.05.001. [DOI] [Google Scholar]
- 180.Ye X., Lin Z., Liang S., Huang X., Qiu X., Qiu Y., et al. Upcycling of electroplating sludge into ultrafine Sn@C nanorods with highly stable lithium storage performance. Nano Lett. 2019;19:1860–1866. doi: 10.1021/acs.nanolett.8b04944. [DOI] [PubMed] [Google Scholar]
- 181.Sethurajan M., Van Hullebusch E.D., Nancharaiah Y.V. Biotechnology in the management and resource recovery from metal bearing solid wastes: recent advances. J. Environ. Manag. 2018;211:138–153. doi: 10.1016/j.jenvman.2018.01.035. [DOI] [PubMed] [Google Scholar]
- 182.Guo Z.H., Zhang L., Cheng Y., Xiao X.Y., Pan F.K., Jiang K.Q. Effects of pH, pulp density and particle size on solubilization of metals from a Pb/Zn smelting slag using indigenous moderate thermophilic bacteria. Hydrometallurgy. 2010;104:25–31. doi: 10.1016/j.hydromet.2010.04.006. [DOI] [Google Scholar]
- 183.Srichandan H., Mohapatra R.K., Parhi P.K., Mishra S. Bioleaching approach for extraction of metal values from secondary solid wastes: a critical review. Hydrometallurgy. 2019;189 doi: 10.1016/j.hydromet.2019.105122. [DOI] [Google Scholar]
- 184.Hao X.D., Zhu P., Zhang H.Z., Liang Y.L., Yin H.Q., Liu X.D., et al. Mixotrophic acidophiles increase cadmium soluble fraction and phytoextraction efficiency from cadmium contaminated soils. Sci. Total Environ. 2019;655:347–355. doi: 10.1016/j.scitotenv.2018.11.221. [DOI] [PubMed] [Google Scholar]
- 185.Le W.H., Kuang S.T., Zhang Z.F., Wu G.L., Li Y.L., Liao C.F., et al. Selective extraction and recovery of scandium from sulfate medium by Cextrant 230. Hydrometallurgy. 2018;178:54–59. doi: 10.1016/j.hydromet.2018.04.005. [DOI] [Google Scholar]
- 186.Ma H.R., Li X.J., Zhu C., Chen F.Y., Yang Y.L., Chen X.P. Liberation and recovery of Cr from real tannery sludge by ultrasound-assisted supercritical water oxidation treatment. J. Clean. Prod. 2020;267:122064. doi: 10.1016/j.jclepro.2020.122064. [DOI] [Google Scholar]
- 187.Chen J., Meng T., Leng E., Jiaqiang E. Review on metal dissolution characteristics and harmful metals recovery from electronic wastes by supercritical water. J. Hazard Mater. 2022;424 doi: 10.1016/j.jhazmat.2021.127693. [DOI] [PubMed] [Google Scholar]
- 188.Du C.M., Shang C., Gong X.J., Wang T., Wei X.G. Plasma methods for metals recovery from metal-containing waste. Waste Manag. 2018;77:373–387. doi: 10.1016/j.wasman.2018.04.026. [DOI] [PubMed] [Google Scholar]
- 189.Sanito R.C., You S.J., Wang Y.F. Application of plasma technology for treating e-waste: a review. J. Environ. Manag. 2021;288 doi: 10.1016/j.jenvman.2021.112380. [DOI] [PubMed] [Google Scholar]
- 190.Wang R.Y., Deplazes R., Vogel F., Baudouin D. Continuous extraction of black liquor salts under hydrothermal conditions. Ind. Eng. Chem. Res. 2021;60:4072–4085. doi: 10.1021/acs.iecr.0c05203. [DOI] [Google Scholar]
- 191.Szałatkiewicz J. Metals recovery from artificial ore in case of printed circuit boards, using plasmatron plasma reactor. Materials. 2016;9:683. doi: 10.3390/ma9080683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wang Y., Liu W., Huang F., Zou T., Lin Z. The “jump of size” phenomenon in aqueous-nanoparticle reaction system: phase transformation from nano-Mg(OH)2 to bulk MgCO3·3H2O. CrystEngComm. 2012;14:7165–7169. doi: 10.1039/C2CE26054A. [DOI] [Google Scholar]
- 193.Liu W., Huang F., Liao Y., Zhang J., Ren G., Zhuang Z., et al. Treatment of Cr(VI)-containing Mg(OH)2 nanowaste. Angew. Chem., Int. Ed. Engl. 2008;47:5619–5622. doi: 10.1002/anie.200800172. [DOI] [PubMed] [Google Scholar]
- 194.Zhuang Z.Y., Xu X.J., Wang Y.J., Wang Y.D., Huang F., Lin Z. Treatment of nanowaste via fast crystal growth: with recycling of nano-SnO2 from electroplating sludge as a study case. J. Hazard Mater. 2012;211–212:414–419. doi: 10.1016/j.jhazmat.2011.09.036. [DOI] [PubMed] [Google Scholar]
- 195.Liu H., Shen F.H., Li Q.Z., Wen M.N., Zhang H.L., Jiang L.H., et al. Systematic control technologies for gaseous pollutants from non-ferrous metallurgy. J. Environ. Sci. 2022 doi: 10.1016/j.jes.2022.01.035. [DOI] [PubMed] [Google Scholar]
- 196.Xie B., Li S.H., Jin H., Hu S.D., Wang F., Zhou F.B. Analysis of the performance of a novel dust collector combining cyclone separator and cartridge filter. Powder Technol. 2018;339:695–701. doi: 10.1016/j.powtec.2018.07.103. [DOI] [Google Scholar]
- 197.Wang P., Liu J.J., Wang C.H., Zhang Z.W., Li J.Y. A holistic performance assessment of duct-type electrostatic precipitators. J. Clean. Prod. 2022;357:131997. doi: 10.1016/j.jclepro.2022.131997. [DOI] [Google Scholar]
- 198.Abrahamson J., Jones R., Lau A., Reveley S. Influence of entry duct bends on the performance of return-flow cyclone dust collectors. Powder Technol. 2002;123:126–137. doi: 10.1016/S0032-5910(01)00435-1. [DOI] [Google Scholar]
- 199.Liu Z.L., Peng B., Chai L.Y., Liu H., Yang S., Yang B.T. Selective removal of elemental mercury from high-concentration SO2 flue gas by thiourea solution and investigation of mechanism. Ind. Eng. Chem. Res. 2017;56:4281–4287. doi: 10.1021/acs.iecr.7b00044. [DOI] [Google Scholar]
- 200.Liu Y., Wang Q. Removal of elemental mercury from flue gas by thermally activated ammonium persulfate in a bubble column reactor. Environ. Sci. Technol. 2014;48:12181–12189. doi: 10.1021/es501966h. [DOI] [PubMed] [Google Scholar]
- 201.Xie X.F., Zhang Z.H., Chen Z.K., Wu J.Y., Li Z.L., Zhong S.P., et al. In-situ preparation of zinc sulfide adsorbent using local materials for elemental mercury immobilization and recovery from zinc smelting flue gas. Chem. Eng. J. 2022;429:132115. doi: 10.1016/j.cej.2021.132115. [DOI] [Google Scholar]
- 202.Hong Q.Q., Zhang X.F., Zhu R.L., Wang C., Mei J., Yang S.J. Resource utilization of natural pyrite (FeS2) as the tailings after flotation of natural sphalerite (ZnS) for reclaiming high concentrations of gaseous Hg0 from Zn smelting flue gas. Chem. Eng. J. 2022;427:131644. doi: 10.1016/j.cej.2021.131644. [DOI] [Google Scholar]
- 203.Wang Z., Luo P.P., Zha X.B., Xu C.Y., Kang S.X., Zhou M.M., et al. Overview assessment of risk evaluation and treatment technologies for heavy metal pollution of water and soil. J. Clean. Prod. 2022;379:134043. doi: 10.1016/j.jclepro.2022.134043. [DOI] [Google Scholar]
- 204.Wei Y.L., Yang Y.W., Cheng N. Study of thermally immobilized Cu in analogue minerals of contaminated soils. Environ. Sci. Technol. 2001;35:416–421. doi: 10.1021/es0008721. [DOI] [PubMed] [Google Scholar]
- 205.Dhaliwal S.S., Singh J., Taneja P.K., Mandal A. Remediation techniques for removal of heavy metals from the soil contaminated through different sources: a review. Environ. Sci. Pollut. Res. Int. 2020;27:1319–1333. doi: 10.1007/s11356-019-06967-1. [DOI] [PubMed] [Google Scholar]
- 206.Hamid Y., Tang L., Sohail M.I., Cao X.R., Hussain B., Aziz M.Z., et al. An explanation of soil amendments to reduce cadmium phytoavailability and transfer to food chain. Sci. Total Environ. 2019;660:80–96. doi: 10.1016/j.scitotenv.2018.12.419. [DOI] [PubMed] [Google Scholar]
- 207.Yang Z., Rui-Lin M., Wang-Dong N., Hui W. Selective leaching of base metals from copper smelter slag. Hydrometallurgy. 2010;103:25–29. doi: 10.1016/j.hydromet.2010.02.009. [DOI] [Google Scholar]
- 208.Yin K., Wang Q.N., Lv M., Chen L.X. Microorganism remediation strategies towards heavy metals. Chem. Eng. J. 2019;360:1553–1563. doi: 10.1016/j.cej.2018.10.226. [DOI] [Google Scholar]
- 209.Shen X., Dai M., Yang J., Sun L., Tan X., Peng C., et al. A critical review on the phytoremediation of heavy metals from environment: performance and challenges. Chemosphere. 2022;291:132979. doi: 10.1016/j.chemosphere.2021.132979. [DOI] [PubMed] [Google Scholar]
- 210.Gavrilescu M. Enhancing phytoremediation of soils polluted with heavy metals. Curr. Opin. Biotechnol. 2022;74:21–31. doi: 10.1016/j.copbio.2021.10.024. [DOI] [PubMed] [Google Scholar]
- 211.Zhang W.C., Liu Y.J., Guo Z.P. Approaching high-performance potassium-ion batteries via advanced design strategies and engineering. Sci. Adv. 2019;5 doi: 10.1126/sciadv.aav7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Vaněk A., Grösslová Z., Mihaljevič M., Trubač J., Ettler V., Teper L., et al. Isotopic tracing of thallium contamination in soils affected by emissions from coal-fired power plants. Environ. Sci. Technol. 2016;50:9864–9871. doi: 10.1021/acs.est.6b01751. [DOI] [PubMed] [Google Scholar]
- 213.Laycock A., Coles B., Kreissig K., Rehkämper M. High precision 142Ce/140Ce stable isotope measurements of purified materials with a focus on CeO2 nanoparticles. J. Anal. At. Spectrom. 2016;31:297–302. doi: 10.1039/C5JA00098J. [DOI] [Google Scholar]
- 214.Du X., Zhang J., Zhang X., Schramm K.W., Nan B., Huang Q., et al. Persistence and reversibility of arsenic-induced gut microbiome and metabolome shifts in male rats after 30-days recovery duration. Sci. Total Environ. 2021;776:145972. doi: 10.1016/j.scitotenv.2021.145972. [DOI] [PubMed] [Google Scholar]
- 215.Coryell M., McAlpine M., Pinkham N.V., McDermott T.R., Walk S.T. The gut microbiome is required for full protection against acute arsenic toxicity in mouse models. Nat. Commun. 2018;9:5424. doi: 10.1038/s41467-018-07803-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Rubin S.S.D.C., Alava P., Zekker I., Du Laing G., Van de Wiele T. Arsenic thiolation and the role of sulfate-reducing bacteria from the human intestinal tract. Environ. Health Perspect. 2014;122:817–822. doi: 10.1289/ehp.1307759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Perry M.R., Wyllie S., Raab A., Feldmann J., Fairlamb A.H. Chronic exposure to arsenic in drinking water can lead to resistance to antimonial drugs in a mouse model of visceral leishmaniasis. Proc. Natl. Acad. Sci. USA. 2013;110:19932–19937. doi: 10.1073/pnas.1311535110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ban Z., Zhou Q.X., Sun A.Q., Mu L., Hu X.G. Screening priority factors determining and predicting the reproductive toxicity of various nanoparticles. Environ. Sci. Technol. 2018;52:9666–9676. doi: 10.1021/acs.est.8b02757. [DOI] [PubMed] [Google Scholar]
- 219.Yu F., Wei C., Deng P., Peng T., Hu X. Deep exploration of random forest model boosts the interpretability of machine learning studies of complicated immune responses and lung burden of nanoparticles. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abf4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Zhong S., Zhang K., Bagheri M., Burken J.G., Gu A., Li B., et al. Machine learning: new ideas and tools in environmental science and engineering. Environ. Sci. Technol. 2021;55:12741–12754. doi: 10.1021/acs.est.1c01339. [DOI] [PubMed] [Google Scholar]
- 221.Zhang F., Zhang W., Wexler D., Guo Z. Recent progress and future advances on aqueous monovalent-ion batteries towards safe and high-power energy storage. Adv. Mater. 2022;34 doi: 10.1002/adma.202107965. [DOI] [PubMed] [Google Scholar]
- 222.Yang B., Liu K., Li H., Liu C., Fu J., Li H., et al. Accelerating CO2 electroreduction to multicarbon products via synergistic electric-thermal field on copper nanoneedles. J. Am. Chem. Soc. 2022;144:3039–3049. doi: 10.1021/jacs.1c11253. [DOI] [PubMed] [Google Scholar]
- 223.Zhang W.C., Mao J.F., Li S., Chen Z.X., Guo Z.P. Phosphorus-based alloy materials for advanced potassium-ion battery anode. J. Am. Chem. Soc. 2017;139:3316–3319. doi: 10.1021/jacs.6b12185. [DOI] [PubMed] [Google Scholar]