Graphical abstract

Highlights
• The reaction rate of PFAS can be high up to 2.400 h−1 in hydrothermal treatment.
• Additives can greatly enhance the performance of hydrothermal treatment for PFAS.
• Hydrothermal treatment reduced 71% of energy consumption than waste incineration.
Per- and polyfluoroalkyl substances (PFAS), consisting of at least one perfluoroalkyl group (CnF2n+1), are a class of synthesized organic compounds with hydrogen atoms partially or entirely replaced by fluorine atoms [1]. Short-chain PFAS refers to the perfluoroalkane and -alkyl sulfonic acids (PFSAs) with less than six carbon atoms and the perfluoroalkyl carboxylic acids (PFCAs) with less than eight carbon atoms, while long-chain PFAS include the PFSAs with more than six carbon atoms and the PFCAs with more than eight carbon atoms [2]. With high resistance to stains, oil, and water, PFAS exhibit unique physical and chemical properties and thus have been widely utilized in industry and consumer products, for example, waterproof clothing, cosmetics, and fire-fighting material [3]. PFAS contamination has been found in air [4], drinking water [5], and soil [6]. A growing body of studies demonstrates that exposure to PFAS can cause permanent damage and severe diseases in the human body, such as thyroid disease, kidney cancer, and reproductive and immune system damage [7].
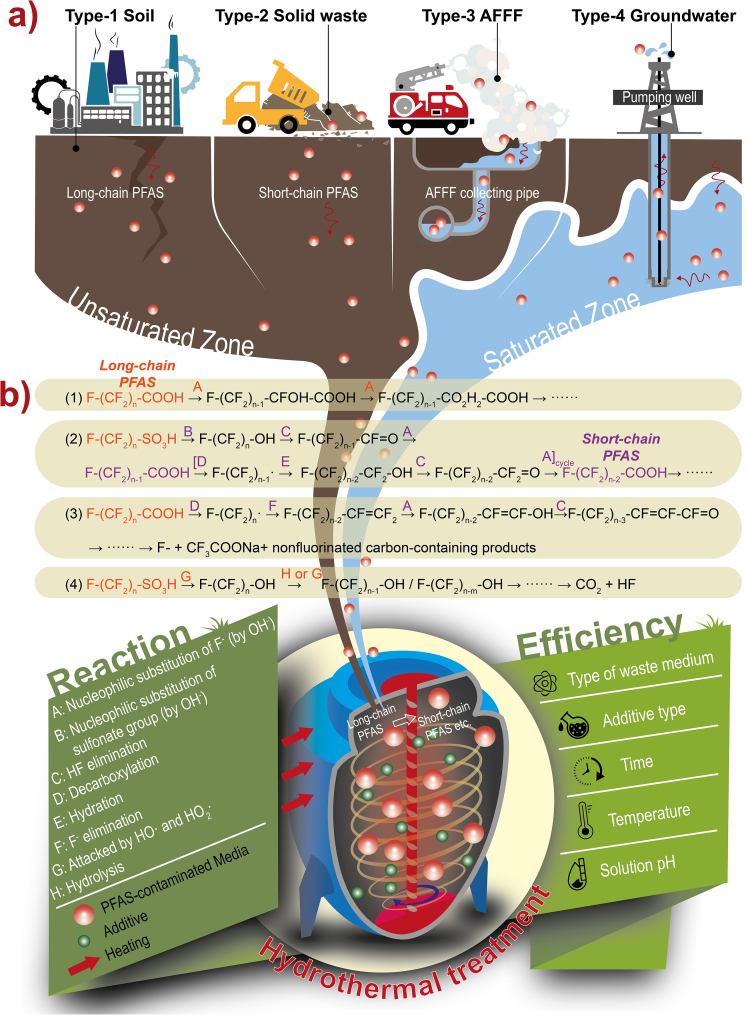
The high bonding energy of the C–F bond (485 kJ/mol) makes PFAS stable and recalcitrant. Photolysis [8], plasma-based technology [9], and electrochemical oxidation [10] were adopted for destructing PFAS. However, these methods have the disadvantages of incomplete degradation and high cost. Hydrothermal treatment is a promising technology for PFAS removal because of its outstanding performance. Using subcritical (100–374 °C) or supercritical (>374 °C) water, hydrothermal treatment can be environmentally benign and highly efficient for degrading recalcitrant PFAS due to the unique properties different from ambient conditions. It has been employed to achieve the highly efficient remediation of various PFAS-containing waste media, for instance, aqueous film-forming foam (AFFF) [11], sewage sludge [12], and soil and groundwater (Fig. 1) [6]. Widely used for rapidly extinguishing fire, AFFF is a complicated water-based mixture of PFAS, hydrocarbon surfactants, and other solvents. Most PFAS in AFFF was degraded to nondetectable levels at near-critical conditions (350 °C, 16.5 MPa) within 15 min, and the reaction rate k ranged from 1.000 to 2.400 h−1 [11]. Similarly, hydrothermal treatment can be applied to treat sewage sludge with 100% PFCAs degradation at 300 °C within 30 min [12]. However, high temperatures also resulted in a rise in the concentration of perfluoroalkyl acids (PFAAs) precursors, which can be removed by the addition of Ca(OH)2. Furthermore, hydrothermal treatment can efficiently treat the real and complex PFAS-contaminated groundwater and soil matrix [6]. Near-complete degradation of 148 PFAS identified in those samples was achieved within 90 min at 350 °C. Notably, the removal efficiency of PFAS was not affected by the groundwater or soil matrix. The first-order reaction rate k (0.7200–2.1600 h−1) in groundwater GW2 samples for the most recalcitrant PFAS was significantly increased compared with the k (0.2880–0.5580 h−1) of treatment in soil A samples. The greatest increment of k (0.2880–1.440 h−1) reached 400% [6], indicating that complex soil components may affect k of PFAS [6]. The matrix effect was also observed in the hydrothermal treatment of sewage sludge and the k of treatment in sewage sludge (e.g., perfluorooctanesulfonic acid [PFOS], 0.4200 h−1) [12] decreased by 22% compared with the k (0.5580 h−1) in soil samples [6]. Collectively, soil and sewage sludge have highly heterogeneous components, which can significantly affect the PFAS reaction rate. However, the k (0.2880–2.4000 h−1) of PFAS in AFFF [11], soil, and groundwater [6] by hydrothermal treatment were still significantly greater than that of ion exchange (0.0030–0.0240 h−1), constructed wetlands (0.0024–0.0060 h−1), biodegradation (0.0002–0.0040 h−1), phytoremediation (0.0200–0.0250 h−1), mechanochemical technology (0.0700–1.3000 h−1), multifunctional material (0.3000–2.0000 h−1) (Figure S1). To enhance the reaction rate, future studies should prioritize considering the heterogeneity and complexity of waste media.
Fig. 1.
Field application designs of hydrothermal treatment for treating the contaminated soil, solid waste (e.g., sewage sludge), AFFF, and groundwater (a); and proposed key reaction mechanisms of PFAS (PFSAs and PFCAs), transformation processes, and controlling factors in the hydrothermal treatment system (b).
The destruction rate of PFAS under hydrothermal treatment is not only controlled by the medium types, solution pH, residence time, and temperature [13] but also by the additives (e.g., dimethyl sulfoxide [DMSO], Fe, NaOH, H2O2), which are key factors for affecting PFAS destruction and defluorination [11]. The role of additives in promoting the hydrothermal treatment of PFAS is that of catalysts and amendments, which can enhance the degradation and defluorination of PFAS. For the most recalcitrant PFOS, additives can significantly lower the reaction temperature, as PFOS degradation requires 600 °C without additives [14]. Furthermore, some exogenous impurities probably exist in additives, but they are more likely not to affect the removal efficiency of hydrothermal treatment for their little amount at present research. The polar aprotic solvents (DMSO) can achieve milder reaction conditions by inducing the low-temperature mineralization of PFCAs by producing reactive perfluoroalkyl ion intermediates at 80–120 °C within 24 h, with most ion intermediates (78%–100%) degraded into F− [1]. It is suggested that decarboxylation is the rate-limiting step, and the following processes of defluorination and chain-shortening can also take place at near-ambient temperature (40 °C). Adding Fe also can enhance the PFAS (e.g., PFOS) destruction and achieve 51.4% of F− yield, whereas treated without Fe, the yield of F− was only 0.16% [15]. Compared with other metals (Al, Zn, Cu) (remaining 93.6%, 84.7%, and 23.1% PFOS, respectively), Fe exhibited the highest activity Fe (only remaining 0.54% PFOS). In addition, alkalis were also studied as catalysts under subcritical conditions, a new process known as hydrothermal alkaline treatment (HALT). After testing various additives (acids, alkalis, oxidants, and reductants), the solution pH was increased to alkaline conditions (pH ≥ 9) after reaction with amendments, indicating that HALT is a base-promoted mechanism [13]. High defluorination and PFOS removal (>70%) were achieved with the addition of NaOH, which showed significant enhancement compared to other ineffective additives (<20%). Compared to subcritical water, supercritical water can shorten reaction time by sharply increasing reaction temperature. On the flip side, temperature increase also results in reactor erosion, high energy consumption, and toxic byproducts and intermediates (e.g., CHF3) [16].
Generally, the reaction mechanism of PFAS is the nucleophilic substitution of F− by OH−, resulting in an unstable hydroxylated structure that leads to the breakage of the C–C bond (350 °C, NaOH) (Fig. 1) [11]. Moreover, the degradation process (long-chain to short-chain) of PFAS (e.g., PFOS) was a nucleophilic substitution of the sulfonate group with a series of decarboxylation reactions, hydration, HF elimination, and nucleophilic substitution of F− (by OH−) [13]. The OH− initiates and facilitates the reaction by substituting the sulfonate group, producing unstable perfluorinated alcohol. The HF elimination of perfluorinated alcohol produced perfluorinated ketone, and the hydration of perfluorinated ketone releases F− to form PFCAs (e.g., PFOA), which is further decarboxylated, hydrated, HF-eliminated, F− nucleophilically substituted, until complete degradation of the C–F chain (350 °C, NaOH) [13]. However, Trang et al. found that the decarboxylation reaction of PFAS (e.g., PFCAs) produced perfluoroalkyl ion intermediates, suggesting a new reaction mechanism of PFCAs does not conform to the commonly assumed one carbon chain shortening mechanisms (80–120 °C, NaOH, DMSO) (Fig. 1) [1]. When this reaction was performed in the presence of NaOH, the PFCAs mineralized to F−, sodium trifluoroacetate (CF3COONa), and nonfluorinated carbon-containing products (formate, carbonate, oxalate, glycolate, and tartronate) [1]. The reaction pathway in higher temperatures differs from reactions under the above subcritical conditions (100–374 °C). Under supercritical conditions (500 °C, H2O2), the cleavage of the C–S bond is the initial and limiting reaction step, and then the C–C bond is hydrolyzed with water or attacked by hydroxyl (HO·) and hydroperoxyl (HO2·) radicals (generated by H2O2) to break, and the carbon chain is gradually shortened to produce final products HF and CO2 [17]. The complex composition of the contaminated medium may not have a decisive influence on the reaction mechanisms since the order of bond cleavage mainly depends on energy barriers and the reactivity of the bond [18]. However, it is worth noting that the medium’s heterogeneity may impact the rate of adsorption–desorption of PFAS [6]. The above speculations on the degradation mechanism are based on detecting intermediates during the reaction. However, the actual pathway by which degradation occurs during the reaction process needs to be further clarified, and more precise detection and tracking of the behavior of intermediates in the liquid and gas phases are needed.
As a critical consideration in large-scale applications, the energy consumption required for hydrothermal treatment exhibits its potential for commercial and industrial applications. Heating liquid water (∼1100 kJ/kg) requires less than 58% of the heat input for vaporizing water (∼2600 kJ/kg) to hydrothermal reaction temperature (e.g., 350 °C) [13]. For the same liquid waste streams, energy input is 71% lower by hydrothermal treatment (127 kW·h/m3) than waste incineration (440 kW·h/m3), where the required temperatures are higher than 750 °C generally, based on the assumption that raising the temperature to the setting reaction conditions is the major energy consumption process [11]. To reduce the energy demand for PFAS degradation, the continuous hydrothermal treatment processing system is suggested, as it can achieve heat recovery and is critical for large-scale implementation [14]. In addition, lower temperatures and milder conditions are also essential methods of decreasing energy requirements. Further studies for large-scale pilot experiments are required for detailed energy calculation and comparison versus other promising technologies.
High temperature and pressure result in the deposition of inorganic salts. Moreover, the final product HF causes corrosion of the reactor and requires neutralization of the effluent flow [17]. Reactors that can withstand supercritical water conditions are usually costly and energy-intensive, leading to concerns about the economic feasibility of scale-up and application. The current research is limited to the experimental stage without practical application cases. The technology’s overall environmental impact and operating costs should be life-cycle analyzed to better promote hydrothermal treatment. Hydrothermal treatment, as a low-carbon, efficient, and economical technology, uses relatively simple experimental conditions and reagents to achieve near-complete degradation of PFAS. In future applications, attempts can be made to optimize hydrothermal treatment with other additives (e.g., biochar, iron sulfides, and iron oxides) to further lower reaction temperature and shorten the residual time, as it must strike a balance between temperature, time, and energy consumption while ensuring PFAS degradation effects. In addition, reactor corrosion and excessive water input should be taken into consideration when conducting large-scale applications. Taken together, hydrothermal treatment of PFAS shows excellent potential to be a more sustainable and effective option for PFAS removal from various environmental media.
Declaration of competing interests
The authors declare no conflicts of interest.
Acknowledgments
The research was supported by the National Natural Science Foundation of China (Nos. 42177386, 41907165) and the National Key Research and Development Program of China (No. 2020YFC1808200).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.eehl.2023.02.002.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Trang B., Li Y., Xue X.S., Ateia M., Houk K.N., Dichtel W.R. Low-temperature mineralization of perfluorocarboxylic acids. Science. 2022;377(6608):839–845. doi: 10.1126/science.abm8868. [DOI] [PubMed] [Google Scholar]
- 2.Zheng G., Schreder E., Dempsey J.C., Uding N., Chu V., Andres G., et al. Per- and polyfluoroalkyl substances (PFAS) in breast milk: concerning trends for current-use PFAS. Environ. Sci. Technol. 2021;55(11):7510–7520. doi: 10.1021/acs.est.0c06978. [DOI] [PubMed] [Google Scholar]
- 3.Whitehead H.D., Venier M., Wu Y., Eastman E., Urbanik S., Diamond M.L., et al. Fluorinated compounds in north American cosmetics. Environ. Sci. Technol. Lett. 2021;8(7):538–544. [Google Scholar]
- 4.Morales-McDevitt M.E., Becanova J., Blum A., Bruton T.A., Vojta S., Woodward M., et al. The air that we breathe: neutral and volatile PFAS in indoor air. Environ. Sci. Technol. Lett. 2021;8(10):897–902. doi: 10.1021/acs.estlett.1c00481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y.-L., Sun M. Ion exchange removal and resin regeneration to treat per- and polyfluoroalkyl ether acids and other emerging PFAS in drinking water. Water Res. 2021;207:117781. doi: 10.1016/j.watres.2021.117781. [DOI] [PubMed] [Google Scholar]
- 6.Hao S., Choi Y.J., Deeb R.A., Strathmann T.J., Higgins C.P. Application of hydrothermal alkaline treatment for destruction of per- and polyfluoroalkyl substances in contaminated groundwater and soil. Environ. Sci. Technol. 2022;56(10):6647–6657. doi: 10.1021/acs.est.2c00654. [DOI] [PubMed] [Google Scholar]
- 7.Lohmann R., Cousins I.T., DeWitt J.C., Glüge J., Goldenman G., Herzke D., et al. Are fluoropolymers really of low concern for human and environmental health and separate from other PFAS? Environ. Sci. Technol. 2020;54(20):12820–12828. doi: 10.1021/acs.est.0c03244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Z., Chen Z., Gao J., Yu Y., Men Y., Gu C., et al. Accelerated degradation of perfluorosulfonates and perfluorocarboxylates by UV/sulfite + iodide: reaction mechanisms and system efficiencies. Environ. Sci. Technol. 2022;56(6):3699–3709. doi: 10.1021/acs.est.1c07608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh R.K., Fernando S., Baygi S.F., Multari N., Thagard S.M., Holsen T.M. Breakdown products from perfluorinated alkyl substances (PFAS) degradation in a plasma-based water treatment process. Environ. Sci. Technol. 2019;53(5):2731–2738. doi: 10.1021/acs.est.8b07031. [DOI] [PubMed] [Google Scholar]
- 10.Radjenovic J., Duinslaeger N., Avval S.S., Chaplin B.P. Facing the challenge of poly- and perfluoroalkyl substances in water: is electrochemical oxidation the answer? Environ. Sci. Technol. 2020;54(23):14815–14829. doi: 10.1021/acs.est.0c06212. [DOI] [PubMed] [Google Scholar]
- 11.Hao S., Choi Y.J., Wu B., Higgins C.P., Deeb R., Strathmann T.J. Hydrothermal alkaline treatment for destruction of per- and polyfluoroalkyl substances in aqueous film-forming foam. Environ. Sci. Technol. 2021;55(5):3283–3295. doi: 10.1021/acs.est.0c06906. [DOI] [PubMed] [Google Scholar]
- 12.Zhang W., Liang Y. Effects of hydrothermal treatments on destruction of per- and polyfluoroalkyl substances in sewage sludge. Environ. Pollut. 2021;285:117276. doi: 10.1016/j.envpol.2021.117276. [DOI] [PubMed] [Google Scholar]
- 13.Wu B., Hao S., Choi Y., Higgins C.P., Deeb R., Strathmann T.J. Rapid destruction and defluorination of perfluorooctanesulfonate by alkaline hydrothermal reaction. Environ. Sci. Technol. Lett. 2019;6(10):630–636. [Google Scholar]
- 14.Li J., Pinkard B.R., Wang S., Novosselov I.V. Review: hydrothermal treatment of per- and polyfluoroalkyl substances (PFAS) Chemosphere. 2022;307(Pt 2):135888. doi: 10.1016/j.chemosphere.2022.135888. [DOI] [PubMed] [Google Scholar]
- 15.Hori H., Nagaoka Y., Yamamoto A., Sano T., Yamashita N., Taniyasu S., et al. Efficient decomposition of environmentally persistent perfluorooctanesulfonate and related fluorochemicals using zerovalent iron in subcritical water. Environ. Sci. Technol. 2006;40(3):1049–1054. doi: 10.1021/es0517419. [DOI] [PubMed] [Google Scholar]
- 16.Li J., Austin C., Moore S., Pinkard B.R., Novosselov I.V. PFOS destruction in a continuous supercritical water oxidation reactor. Chem. Eng. J. 2023;451:139063. doi: 10.1016/j.chemosphere.2023.138358. [DOI] [PubMed] [Google Scholar]
- 17.Pinkard B.R., Shetty S., Stritzinger D., Bellona C., Novosselov I.V. Destruction of perfluorooctanesulfonate (PFOS) in a batch supercritical water oxidation reactor. Chemosphere. 2021;279:130834. doi: 10.1016/j.chemosphere.2021.130834. [DOI] [PubMed] [Google Scholar]
- 18.Carter K.E., Farrell J. Oxidative destruction of perfluorooctane sulfonate using boron-doped diamond film electrodes. Environ. Sci. Technol. 2008;42(16):6111–6115. doi: 10.1021/es703273s. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.