Abstract
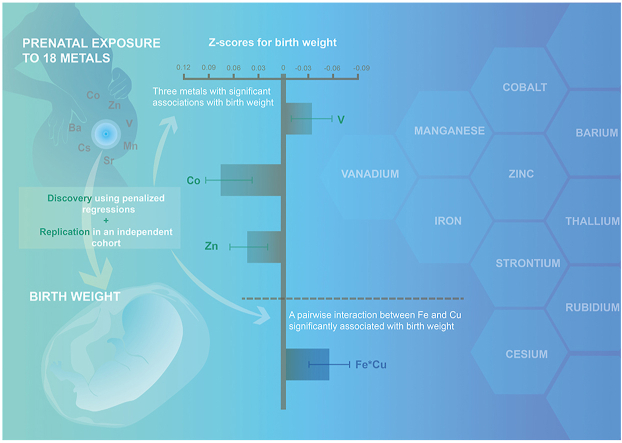
The understanding of the impact of prenatal exposure to metal mixtures on birth weight is limited. We aimed to identify metal mixture components associated with birth weight and to determine additional pairwise interactions between metals showing such associations. Concentrations of 18 metals were measured using inductively coupled plasma mass spectrometry in urine samples collected in the 3rd trimester from a prenatal cohort (discovery; n = 1849) and the Healthy Baby Cohort (replication; n = 7255) in Wuhan, China. In the discovery set, we used two penalized regression models, i.e., elastic net regression for main effects and a lasso for hierarchical interactions, to identify important mixture components associated with birth weight, which were then replicated. We observed that 8 of the 18 measured metals were retained by elastic net regression, with five metals (vanadium, manganese, iron, cesium, and barium) showing negative associations with Z-scores for birth weight and three metals (cobalt, zinc, and strontium) showing positive associations. In replication set, associations remained significant for vanadium (β = −0.035; 95% confidence interval [CI], −0.059 to −0.010), cobalt (β = 0.073; 95% CI, 0.049 to 0.097), and zinc (β = 0.040; 95% CI, 0.016 to 0.065) after Bonferroni correction. We additionally identified and replicated a single pairwise interaction between iron and copper exposure on birth weight (P < 0.001). Using a two-stage analysis, we identified and replicated individual metals and additional pairwise interactions-associated birth weight. The approach could be used in other studies estimating the effect of complex mixtures on human health.
Keywords: Prenatal exposure, Metal mixture, Birth cohort, Two-stage analysis
Graphical abstract
Highlights
-
•
Prenatal exposure to metal mixtures impacts newborns' birth weight.
-
•
As mixture components, metals (V, Co, and Zn) and their pairwise interactions (Fe × Cu) are associated with birth weight.
-
•
Our approach could be used to examine the health effects of complex mixtures of metals, chemicals, and nutrients.
1. Introduction
Metals are a group of essential and toxic elements that generally occur in the environment as mixtures [1]. Metals are released into the environment through both natural and anthropogenic sources, and are detected in the atmosphere, waterways, soils, and food [2,3]. In addition, dozens of metals are detected in urine and blood samples of humans [4,5], indicating that humans are simultaneously exposed to different metals.
It is well acknowledged that the developing fetus is more susceptible to environmental pollutants than adults [6,7]. Adverse birth outcomes, such as low birth weight, have been considered an important indicator of intrauterine growth restriction for fetuses, which has been associated with an increased risk of childhood diseases and long-term adverse health outcomes [8,9]. Prenatal exposure to higher levels of metals, especially toxic metals such as lead, cadmium, arsenic, vanadium, chromium, and thallium, has been associated with an increased risk of adverse birth outcomes and restricted fetal growth [[10], [11], [12], [13], [14], [15], [16], [17], [18]]. A few studies have also investigated whether there are periods of heightened vulnerability to the effect of some toxic metals on fetal growth [[19], [20], [21], [22]]. In recent epidemiological studies, several statistical methods have been used to examine associations between exposure to chemical mixtures during pregnancy and birth outcomes [[23], [24], [25]], such as principal component analysis [26], sparse partial least squares [27], weighted quantile sum regression [28], Bayesian kernel machine regression (BKMR) [29,30], and elastic net regression [31]. These methods differ in their assumptions and inferential goals. Principal component analysis and sparse partial least squares assume linearity of exposure effects and aim to reduce the dimensionality of exposures [24]. Weighted quantile sum regression constructs a weighted sum of the sample quantiles for each exposure of interest and estimates its overall association with the outcome [32]. BKMR seeks to estimate pairwise and higher-order interactive effects of all mixture components while incorporating exposure-response nonlinearities [33], and has recently been extended to identifying periods of heightened vulnerability to mixtures [34]. Finally, elastic net regression is a penalized regression approach that performs variable selection and shrinkage in the presence of groups of correlated exposures [35]. The Hierarchical Interaction Lasso, as implemented in the R package hierNet [36], is a penalized regression model that can fit sparse interactions under a hierarchy restriction. This method has never before been utilized to examine the impact of metal mixture exposures during pregnancy on birth weight, and may be particularly useful in identifying novel pairwise between-metal interactions associated with birth weight.
In this study, based on data from an ongoing prospective cohort in China, we applied two penalized regression models to identify metal mixture components associated with birth weight, including the linear associations of individual metals and their pairwise interactions. Then, we replicated these findings in an independent population using data from the Healthy Baby Cohort (HBC) study, a birth cohort from China.
2. Materials and methods
2.1. Study participants
Participants in the discovery set were pregnant women from an ongoing, prospective prenatal cohort study established at Wuhan Children’s Hospital (Wuhan Maternal and Child Healthcare Hospital), Wuhan, Hubei Province, China. Between October 2013 and October 2016, we included 1849 women when they had their first prenatal care visits at the study hospital. All these participants did not smoke tobacco or comsume alcohol during pregnancy, took their first prenatal care visits with a gestational age less than 16 weeks at the study hospital, provided one urine sample at prenatal care visits in the 3rd trimester, and delivered live singletons without congenital disorders at the study hospital [37].
The HBC, the replication set, was established between September 2012 and October 2014 at the Wuhan Children’s Hospital. Pregnant women were recruited at admission to the study hospital for deliveries [15]. This study included 7255 pregnant women who did not smoke tobacco or consume alcohol during pregnancy, provided urine samples during their hospital stay prior to delivery, and gave birth to live singletons without congenital disorders [12].
Women in both studies agreed to participate after invitation and provided signed informed consent at the time of enrollment. The study protocols of both cohorts have been approved by the ethics committee of Tongji Medical College, Huazhong University of Science and Technology, and the study hospital.
2.2. Urine collection and assessment
Urine samples of women from the discovery set were collected at 37.9 ± 1.8 weeks [37], and those of women from the HBC were obtained before deliveries with an average of 39.0 ± 1.2 weeks [12]. All urine samples were stored at −20 °C until quantification of metal concentrations. Urinary concentrations of creatinine were measured using Mindray BS-200 CREA Kit to control for variations in urine dilution (Shenzhen Mindray Bio-medical Electronics, Shenzhen, China).
Urinary concentrations of 18 metals were assessed using inductively coupled plasma mass spectrometry (ICP-MS; Agilent 7700, Agilent Technologies, Santa Clara, CA, USA), i.e., aluminum (Al), vanadium (V), chromium (Cr), manganese (Mn), iron (Fe), cobalt (Co), nickel (Ni), copper (Co), zinc (Zn), arsenic (As), selenium (Se), rubidium (Rb), strontium (Sr), cadmium (Cd), cesium (Cs), barium (Ba), thallium (Tl), and lead (Pb). Details on the methods of sample preparation, analysis, ICP-MS operating condition, and quality controls were as described previously [12,20]. Briefly, urine samples were thawed, nitrated (3% HNO3, overnight), sonicated (40 °C for 1 h, by ultrasound), and centrifuged. Supernatants of the resulting samples were obtained and analyzed by the ICP-MS in helium mode, with 27Al, 51V, 52Cr, 55Mn, 56Fe, 59Co, 60Ni, 63Cu, 66Zn, 75As, 78Se, 85Rb, 88Sr, 111Cd, 133Cs, 137Ba, 205Tl, and 208Pb monitored simultaneously. The detection limit of quantification (LOQ) of each metal was calculated using a previously published formula [38]. For both discovery and replication sets, the detection rates of all 18 metals were generally higher than 90% or 95%; the spike recoveries and intra-day and inter-day precisions of all 18 metals were satisfactory (Table S1).
2.3. Neonatal outcomes
Information on newborn sex, birth weight (in grams), and gestational age at delivery (in days) of each newborn were retrieved from medical records. For each participant, gestational age at delivery was calculated as days from the first day of the last menstrual period (LMP) to the date of delivery. The dates of the LMP were self-reported by pregnant women and were also corrected by obstetricians based on the first-trimester ultrasound measures using clinical criteria. The ultrasound-corrected gestational age was used when the difference between self-reported and ultrasound-corrected gestational ages was larger than seven days. We constructed the sex- and gestational age-adjusted standard deviation scores (Z-score) for birth weight using an international standard (http://intergrowth21.ndog.ox.ac.uk/) developed by the INTERGROWTH-21st Project [39].
2.4. Covariates
In-person interviews were carried out by trained nurses at enrollment, including information on maternal age, education, annual household income, active smoking, secondhand tobacco smoke (SHS) exposure, alcohol consumption, and folic acid supplement use during pregnancy. SHS during pregnancy was defined as being exposed to secondhand smoke from either family members (at home) or colleagues (at work). Maternal height (self-reported; in meters) and pre-pregnancy weight (in kilograms) were also collected during the interviews, and pre-pregnancy body mass index (BMI, kg/m2) was subsequently calculated as weight divided by the square of height. Additionally, information on parity and pregnancy complications (anemia during pregnancy, hypertensive disorders of pregnancy, and gestational diabetes mellitus) was retrieved from medical records.
2.5. Statistical analysis
Before analyzing the data, concentrations of each metal below the LOQ were imputed as the LOQ divided by the square root of 2 [40]. Urinary concentrations of each metal were standardized by dividing them by urinary creatinine concentrations and were then natural log-transformed to increase the normality and reduce the influence of outliers for each metal. The resulting concentrations for each metal were then centered by their first quartile and scaled by their inter-quartile range to make regression coefficients directly comparable across different metals in each cohort. Pearson correlation coefficients for urinary metal concentrations were calculated in both discovery and replication sets. Statistical analyses were performed using R (version 3.5.1) or SAS (version 9.4).
Identify individual metals associated with birth weight (discovery). In ordinary least squares regression, P-values for individual regression coefficients are conditional on the set of predictors included in the model and, therefore, can be highly unstable when computed from strongly correlated metal exposures. To minimize multicollinearity, we applied elastic net regression, a penalized regression approach capable of simultaneously performing coefficient shrinkage and variable selection, to estimate associations between metal mixture components and Z-scores for birth weight [31,35]. We used the R package glmnet to fit elastic net regression models, and the optimal model was selected based on 10-fold cross-validation, a procedure that estimates the optimal values of the regularization parameter (λ) and mixing parameter (γ) based on minimizing deviance [41]. We performed 100 iterations in order to reduce the impact of random seeds in the cross-validation procedure of elastic net regression.
Identify pairwise interactions of metals associated with birth weight (discovery). We used a Hierarchical Interaction Lasso, as implemented in the R package hierNet [36]. This method adds a set of convex constraints to the lasso and fits sparse interaction models under a hierarchy restriction. We used a strong hierarchy restriction that includes a pairwise interaction in the optimal model only if one or both exposure variables have significant linear (main) associations [36]. As the package does not allow for covariate adjustment, we calculated the residual of Z-scores for the birth weight from a linear regression model including all covariates and used it as the outcome in the hierarchical lasso model.
Replication in the HBC. To replicate the results from elastic net regression, we applied multivariable linear regression models to estimate the associations between each selected metal and Z-scores for birth weight. When replicating the findings from the lasso for hierarchical interactions, we calculated the residual of Z-scores for birth weight on all the prespecified covariates to make the regression coefficients comparable across the discovery and replication sets. Then, we fit one linear regression model that included all selected metals and their interactions. Additionally, we used contour plots to show the interaction effects of metal mixtures on birth weight in the prenatal cohort and the HBC. In both replication analyses, we used Bonferroni corrections to control the family-wise error rate at the 5% level of significance. In addition, 16 women were participants in both the prenatal cohort and the HBC. Thus, we did a sensitivity analysis, excluding these 16 women from the HBC when performing the replication analyses.
Adjustment for covariates. All the statistical models in the discovery and replication sets were adjusted for the same set of covariates, including maternal age at recruitment (continuous), parity (nulliparous/multiparous), pre-pregnancy BMI (categorized using the Chinese standard: <18.5, 18.5–23.9, ≥24.0 kg/m2), active smoking before pregnancy (no/yes), SHS during pregnancy (no/yes), alcohol consumption before pregnancy (no/yes), folic acid supplementation during pregnancy (no/only in the first trimester/only in the second and third trimester/during the entire pregnancy), and education (≤9, 10–12, >12 years).
3. Results
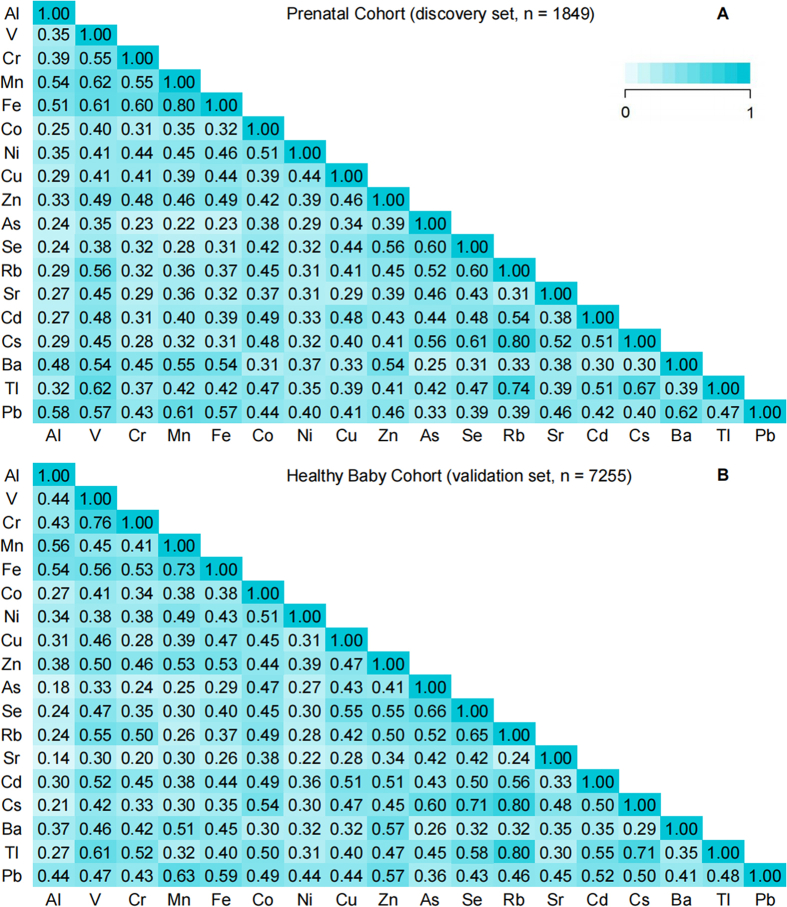
Maternal and neonatal characteristics in the prenatal cohort are similar to those in the HBC, but women from the prenatal cohort had a lower prevalence of alcohol consumption before pregnancy (0.9%; vs. 2.5% in the HBC), a slightly higher prevalence of SHS during pregnancy (29.7%; vs. 22.9% in the HBC), and higher education levels (78.9% women with over 12 years of education; vs. 67.3% in the HBC). The prevalence of pregnancy complications in the prenatal cohort was slightly lower than that of the HBC (Table 1). Urinary concentrations of the 18 measured metals in the prenatal cohort were similar to those in the HBC (Table S2). Pearson correlation coefficients for urinary metal concentrations in the discovery set ranged from 0.22 to 0.80, and similar results were observed for urinary metal concentrations in the HBC (Fig. 1).
Table 1.
Characteristics of the prenatal cohort and the Healthy Baby Cohort.
| Prenatal Cohort Mean ± SD or No. (%) |
Healthy Baby Cohort Mean ± SD or No. (%) |
|
|---|---|---|
| All individuals | 1849 | 7255 |
| Maternal characteristics | ||
| Age at recruitment, years a | ||
| <25 | 222 (12.0) | 799 (11.0) |
| 25–29 | 1123 (60.7) | 3972 (54.7) |
| 30–34 | 415 (22.4) | 2001 (27.6) |
| ≥35 | 89 (4.8) | 483 (6.7) |
| Pre-pregnancy BMI, kg/m2 | ||
| <18.5 | 346 (18.7) | 1524 (21.0) |
| 18.5–23.9 | 1253 (67.8) | 4824 (66.5) |
| ≥24.0 | 250 (13.5) | 907 (12.5) |
| Nulliparous | 1557 (84.2) | 6140 (84.6) |
| Alcohol consumption before pregnancy | 16 (0.9) | 183 (2.5) |
| Active smoking before pregnancy | 10 (0.5) | 52 (0.7) |
| Secondhand tobacco smoke during pregnancy | 550 (29.7) | 1665 (22.9) |
| Anemia during pregnancy | 70 (3.8) | 335 (4.6) |
| Gestational diabetes mellitus | 172 (9.3) | 864 (11.9) |
| Hypertensive disorders of pregnancy | ||
| Hypertension | 42 (2.3) | 175 (2.4) |
| Pre-eclampsia | 18 (1.0) | 108 (1.5) |
| Education | ||
| ≤9 years | 117 (6.3) | 987 (13.6) |
| 10–12 years | 274 (14.8) | 1382 (19.0) |
| >12 years |
1458 (78.9) |
4886 (67.3) |
| Neonatal characteristics | ||
| Girls | 871 (47.1) | 3388 (46.7) |
| Gestational age, week | 39.4 ± 1.0 | 39.2 ± 1.2 |
| Birth weight, g | 3348 ± 415 | 3352 ± 434 |
SD, standard deviation; No., Number; BMI, body mass index.
Participants of the prenatal cohort were recruited at their first prenatal care visits (less than 16 weeks of gestation) in the study hospital; participants of the Healthy Baby Cohort were recruited at their admission to the study hospital for deliveries.
Fig. 1.
Pearson correlation coefficients between each metal in A) the prenatal cohort (discovery set) and B) the Healthy Baby Cohort (replication set). Urine samples for women from the prenatal cohort were collected during 3rd-trimester prenatal care visits. Urine samples from the Healthy Baby Cohort were obtained before delivery. Al, aluminum; Se, selenium; Rb, rubidium; Cd, cadmium; V, vanadium; Mn, manganese; Fe, iron; Co, cobalt; Zn, zinc; Sr, strontium; Cs, cesium; Ba, barium; Ni, nickel; Pb, lead; Cr, chromium; Co, copper; Tl, thallium; As, arsenic.
In the prenatal cohort, we identified eight metals associated with Z-scores for birth weight using elastic net regression, and the findings were replicated in the HBC (Table 2). Five of these metals (V, Mn, Fe, Cs, and Ba) showed negative associations with birth weight, whereas the other three metals (Co, Zn, and Sr) showed positive associations. In the HBC, the adjusted associations between these eight identified metals with Z-scores for birth weight were in the same directions with those observed in the prenatal cohorts, and the associations for V (β = −0.035; 95% CI, −0.059 to −0.010), Co (β = 0.073; 95% CI, 0.049 to 0.097), Zn (β = 0.040; 95% CI, 0.016 to 0.065), and Sr (β = 0.028; 95% CI, 0.005 to 0.052) were statistically significant (P ≤ 0.02). Of these metals, the associations between V, Co, and Zn with Z-scores for birth weight remained significant after Bonferroni correction for multiple testing (P < 0.00625 for eight comparisons). In the sensitivity analysis excluding 16 women who were participants of both cohorts from the HBC, the results for the replication analyses did not change (data not shown).
Table 2.
Associations between urinary metal concentrations and Z-scores for birth weight in the prenatal cohort (discovery set) and the Healthy Baby Cohort (replication set).
| β in the optimal elastic net model in the prenatal cohort a | Association between metals and birth weight in the Healthy Baby Cohort b |
||
|---|---|---|---|
| β (95% CI) | P | ||
| V | −0.011 | −0.035 (−0.059, −0.010) | 0.006∗ |
| Mn | −0.007 | −0.020 (−0.045, 0.005) | 0.12 |
| Fe | −0.082 | −0.013 (−0.040, 0.014) | 0.35 |
| Co | 0.034 | 0.073 (0.049, 0.097) | <0.001∗ |
| Zn | 0.072 | 0.040 (0.016, 0.065) | 0.001∗ |
| Sr | 0.089 | 0.028 (0.005, 0.052) | 0.02 |
| Cs | −0.046 | −0.015 (−0.034, 0.004) | 0.12 |
| Ba | −0.031 | −0.007 (−0.030, 0.016) | 0.55 |
β, regression coefficient; CI, confidence interval; FDR, false discovery rate.
∗ P-values remained significant after Bonferroni correction (P < 0.00625; 0.05/8 metals).
Only metals with non-zero regression coefficients in the optimal elastic net model were shown.
As a validation, associations between each metal and Z-scores for birth weight were estimated using linear regression models. All pairwise interactions were estimated jointly in the validation set using a single linear regression model.
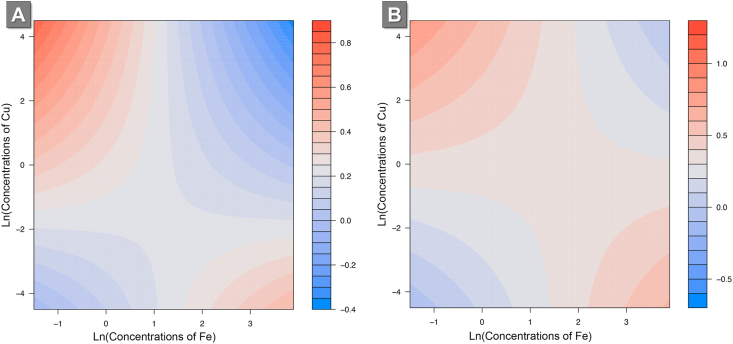
To identify possible pairwise interactions of metals and associations with birth weight, we performed the lasso for hierarchical interaction analysis using urinary metal concentrations in the prenatal cohort. These findings were also validated in the HBC (Table 3). In the prenatal cohort, we identified 15 pairwise interactions between metals and Z-scores for birth weight. In the HBC, most of the interaction effects were in the same direction as those in the prenatal cohort. Only one interaction (Fe × Cu) was significantly associated with Z-scores for birth weight (P < 0.001) after Bonferroni correction for multiple testing (P < 0.003 for 15 comparisons) in the HBC. Finally, we used contour plots to interpret the identified two-way Fe × Cu interaction effect on birth weight. Findings suggest potential antagonism at high levels of Fe and Cu, consistent across cohorts (Fig. 2).
Table 3.
Associations of pairwise interactions between metals with Z-scores for birth weight in the prenatal cohort (discovery set) and the Healthy Baby Cohort (replication set).
| Association/Metal | Selection in the prenatal cohort a |
Associations between metal mixtures and birth weight in the Healthy Baby Cohort b |
|
|---|---|---|---|
| β | β (95% CI) | P | |
| V × Ni | −0.009 | −0.010 (−0.041, 0.021) | 0.53 |
| V × Pb | −0.003 | −0.009 (−0.045, 0.027) | 0.62 |
| Cr × Co | 0.026 | 0.049 (0.009, 0.088) | 0.02 |
| Mn × Zn | −0.005 | −0.005 (−0.048, 0.037) | 0.81 |
| Fe × Co | −0.006 | −0.052 (−0.078, −0.025) | <0.001∗ |
| Fe × Ba | 0.013 | 0.037 (−0.006, 0.080) | 0.09 |
| Co × Sr | −0.006 | −0.028 (−0.066, 0.010) | 0.14 |
| Ni × Zn | −0.009 | 0.022 (−0.007, 0.051) | 0.14 |
| Ni × Sr | −0.003 | 0.001 (−0.027, 0.028) | 0.96 |
| Co × Sr | 0.019 | 0.013 (−0.013, 0.039) | 0.32 |
| Co × Tl | −0.0003 | −0.013 (−0.042, 0.015) | 0.35 |
| Zn × Sr | 0.001 | 0.008 (−0.029, 0.044) | 0.68 |
| Zn × Pb | −0.018 | −0.020 (−0.064, 0.023) | 0.35 |
| As × Sr | 0.006 | 0.003 (−0.024, 0.031) | 0.81 |
| Cs × Ba | −0.032 | 0.029 (0.003, 0.056) | 0.03 |
∗ P-values remained significant after Bonferroni correction (P < 0.003; 0.05/15 pair-wise interactions).
The metal mixture selection was performed using a lasso for hierarchical testing of interactions. The hierarchical lasso does not allow for covariate adjustment. Therefore, we calculated the residual of birth weight (used as the outcome) from a linear regression model including all covariates described at Statistical Analysis section.
The selected associations between each pairwise interaction and Z-scores for birth weight were estimated in the validation set using a single linear regression model that estimates all the selected interactions.
Fig. 2.
Associations between Fe × Cu interactions and Z-scores for birth weight in A) the prenatal cohort (discovery set) and B) the Healthy Baby Cohort (replication set). In these two contour plots, the X-axis is the natural log-transformed creatinine-standardized urinary concentration of Fe, and the Y-axis is the natural log-transformed creatinine-standardized urinary concentration of Cu. The color and color depth in the contour plots are the Z-scores for birth weight
4. Discussion
In this study, we used penalized regression approaches to estimate the associations between prenatal exposure to metal mixtures and birth weight based on a two-stage analysis design. In the discovery stage, we applied two penalized regression models, elastic net regression and a lasso for hierarchical interactions, in an ongoing prospective prenatal cohort study to identify associations between mixtures of metal exposures in the 3rd trimester and birth weight. The identified associations were then replicated in the HBC, an independent birth cohort. Our findings suggested that three metals (V, Co, and Zn) were associated with birth weight, among which V was inversely associated with birth weight, but Co and Zn were positively associated with birth weight. We also observed an additional two-way interaction (Fe × Cu) associated with birth weight as a part of the metal mixture.
4.1. Identification of individual metals associated with birth weight
In our previous studies, higher concentrations of urinary V, Cr, Mn, As, Cd, Pb, and Tl in the 3rd trimester have been associated with lower birth weight or increased risk of low birth weight (< 2500 g) [12,[14], [15], [16], [17], [18],21,37,[42], [43], [44]]. All these studies used a single-metal strategy for their statistical analysis. In these studies, we observed that associations between a certain metal and birth weight did not change after mutually adjusting for other metals. However, using such a single-metal strategy, we were unable to conclude which metal had stronger associations with birth weight when compared to other metals. Additionally, some of these metals were highly correlated with each other, which could result in multicollinearity when estimating associations using linear or logistic regression models. Therefore, in this study, we used elastic net regression, a penalized regression model that overcomes the multicollinearity problem when performing variable selections [31], which could identify metals associated with birth weight, independent of other metals in a mixture.
In a recent nested case-control study, the authors used elastic net regression and observed that 15 of the 22 metals measured in maternal serum samples were associated with low birth weight risk. They also applied conditional logistic regression models for the same study participants, and the results suggested that lower serum Co and higher serum titanium levels were associated with an increased risk of low birth weight [45]. Consistently, we observed positive associations between urinary Co concentrations and birth weight, but data on urinary titanium concentrations were not available in this study.
It is worth noting that coefficients estimated from elastic net regression are biased in terms of magnitude, unlike ordinary least squares regression coefficients, and their standard errors do not account for the variable selection aspect of the model-fitting process [35,46]. As a result, the associations between variables selected by elastic net regression with the outcome of interest need to be replicated using ordinary linear regression methods in an independent replication sample. Therefore, we applied linear regression models in the HBC, an independent population-based birth cohort, and successfully replicated the directions of all the identified metal-birth weight associations, and three of these associations (V, Co, and Zn) remained significant in the replication set after Bonferroni correction. Moreover, in both the prenatal cohort and the HBC, urinary V concentrations showed negative associations with birth weight, while urinary Co and Zn concentrations showed positive associations. The magnitudes of these regression coefficients were not directly comparable across cohorts, not only because of different estimation methods, but also because the inter-quartile ranges for each metal differed across cohorts and affected the exposure standardization scheme (Table S2). Nevertheless, our findings not only demonstrate the agreement between elastic net and linear regression models, but also suggest that associations between urinary concentrations of V, Co, and Zn in the 3rd trimester and birth weight are robust to replication across independent cohorts.
V and Co in the environment are both released through natural and anthropogenic sources, to which humans can be exposed through the air, drinking water, and food [47,48]. Zn is enriched in several foods, and dietary intake is a main source of Zn for humans [49]. Additionally, V has been associated with impaired fetal growth in our previous studies [12,21]. Zn is the essential component of Zn-binding proteins in the human body, and has been recognized as essential for prenatal and postnatal growth [50]. Co is an essential trace element for humans, as it is a key constituent of vitamin B12 [51], but the role of Co in fetal development needs further investigation.
4.2. Identification of interaction associations of metal mixtures with birth weight
When estimating the associations between exposure to metal mixtures and birth weight, it is important to take into account the interactions between metals. However, the impact of two-way interactions between metal pairs on birth weight is not fully understood. Recent studies on interaction associations for metals have focused predominantly on only three or four metals. For example, one of our recent studies indicated a potential antagonistic effect between Ni and Se on the risk of preterm low birth weight [52]. Another recent study using BKMR suggested a potential joint effect between As and Mn on birth weight [29].
In the present study, we used a lasso for hierarchical interactions to estimate the combined effect of exposure to metal mixtures during pregnancy on birth weight [36]. This approach allowed us to identify mixture components with stronger associations (compared to other components) with birth weight by simultaneously examining the linear associations and pairwise interactions. In order to avoid overfitting and obtain unbiased regression coefficients, we first performed model selection in the prenatal cohort and then replicated the associations between selected mixture components and Z-scores for birth weight in the HBC. Using this approach, we identified and replicated a two-way interaction (Fe × Cu) significantly associated with Z-scores for birth weight. Fe and Cu are essential trace elements for humans. Although several studies have estimated their associations with fetal growth, the specific roles of these elements remain unclear [53]. Our findings suggest potential antagonism between Fe and Cu. However, further studies are needed to replicate these results, as well as investigate the common underlying biological mechanisms for these heavy metals and trace elements. Our findings also indicate the necessity of estimating the interactive effects of environmental pollutants and essential elements on fetal growth, especially in studies evaluating the impact of mixture exposures on human health.
4.3. Strengths and limitations
In this study, we included two large, well-characterized, and independent groups of participants that had exposure, outcome, and covariate information collected in comparable ways. The large sample size in both studies facilitated our ability to estimate small effect sizes. The similarity in the model fitting approach used across the two studies reduces the likelihood that differences in our findings might be due to study methods. Further, we used a stringent approach (i.e., Bonferroni correction) to account for multiple comparisons in the replication set. Additionally, the penalized regression methods used in this study have been well established and are robust in the presence of collinearity between variables.
Pregnant women from the discovery and the replication sets were all from the same hospital but were recruited independently using different strategies. Although some sociodemographic and perinatal factors differed between discovery and replication sets, such differences were not strong enough to confound the direction of the associations between metal mixture components and birth weight, which was similar in the discovery and replication sets. Future studies on other populations would be helpful, such as populations from other cities or rural areas in China, or from other countries. Additionally, as in all observational cohort studies, residual confounding from unmeasured factors is possible. Another limitation of this study is the lack of diet information and nutritional status during pregnancy, as certain metals included in this study are considered important nutrients. Future studies with nutritional and environmental data are needed to address interactions between nutritional factors and environmental exposures on fetal growth.
5. Conclusions
In this study, we used a two-stage analysis design with penalized regression models to identify metal mixture components associated with birth weight. Using this approach, we identified and replicated several components of a metal mixture associated with birth weight, including individual metals (V, Co, Zn) and a two-way interaction between Fe and Cu. Our findings need to be validated in other populations. Furthermore, the approaches used in this study could be applied in studies examining the health effects of complex mixtures of heavy metals and trace elements, endocrine-disrupting chemicals, and nutritional factors.
Author contributions
JH, GP, TZ, JMB, and YL conceived the study. JH, GP, and JMB drafted and revised the manuscript. JH and GP analyzed the data. JH, TZ, and JMB interpreted the results. WL led the measurements of urinary concentrations of metals and creatinine. CW and AZ led the collection of urine samples and data used in this study. SL, SLB, KS, and WX participated in the preparation, revision, and finalization of the manuscript. SX, BZ, and YL are coprincipal investigators of the prenatal cohort study, who were responsible for data collection, quality control, and study design. All authors have read and approved the manuscript.
Declaration of competing interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests. The authors have no conflict of interest to declare. JMB’s institution was financially compensated for his services as an expert witness for plaintiffs in litigation related to PFAS-contaminated drinking water; these funds were not paid to JMB directly.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (42077398), Program for HUST Academic Frontier Youth Team (2018QYTD12) and the National Institute of Environmental Health Sciences (R01 ES024381 and R01 ES025214). We thank all the staff and students who made contributions to the cohort study and the study participants for their support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.eehl.2022.09.001.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Nys C., Versieren L., Cordery K.I., Blust R., Smolders E., De Schamphelaere K. Systematic evaluation of chronic metal-mixture toxicity to three species and implications for risk assessment. Environ. Sci. Technol. 2017;51(8):4615–4623. doi: 10.1021/acs.est.6b05688. [DOI] [PubMed] [Google Scholar]
- 2.Nriagu J.O., Pacyna J.M. Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature. 1988;333(6169):134–139. doi: 10.1038/333134a0. [DOI] [PubMed] [Google Scholar]
- 3.Tchounwou P.B., Yedjou C.G., Patlolla A.K., Sutton D.J. Heavy metal toxicity and the environment. Experientia Suppl. 2012;101:133–164. doi: 10.1007/978-3-7643-8340-4_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fourth Report on Human Exposure to Environmental Chemicals. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, GA: 2009. https://www.cdc.gov/exposurereport/ Available online: [Google Scholar]
- 5.Morton J., Tan E., Leese E., Cocker J. Determination of 61 elements in urine samples collected from a non-occupationally exposed UK adult population. Toxicol. Lett. 2014;231(2):179–193. doi: 10.1016/j.toxlet.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 6.Braun J.M. Early-life exposure to EDCs: role in childhood obesity and neurodevelopment. Nat. Rev. Endocrinol. 2017;13(3):161–173. doi: 10.1038/nrendo.2016.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gluckman P.D., Hanson M.A., Cooper C., Thornburg K.L. Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 2008;359(1):61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu L., Johnson H.L., Cousens S., Perin J., Scott S., Lawn J.E., et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379(9832):2151–2161. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 9.Romero R., Dey S.K., Fisher S.J. Preterm labor: one syndrome, many causes. Science. 2014;345(6198):760–765. doi: 10.1126/science.1251816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis M.A., Higgins J., Li Z., Gilbert-Diamond D., Baker E.R., Das A., et al. Preliminary analysis of in utero low-level arsenic exposure and fetal growth using biometric measurements extracted from fetal ultrasound reports. Environ. Health : a global access science source. 2015;14:12. doi: 10.1186/1476-069X-14-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cabrera-Rodríguez R., Luzardo O.P., González-Antuña A., Boada L.D., Almeida-González M., Camacho M., et al. Occurrence of 44 elements in human cord blood and their association with growth indicators in newborns. Environ. Int. 2018;116:43–51. doi: 10.1016/j.envint.2018.03.048. [DOI] [PubMed] [Google Scholar]
- 12.Hu J., Xia W., Pan X., Zheng T., Zhang B., Zhou A., et al. Association of adverse birth outcomes with prenatal exposure to vanadium: a population-based cohort study. Lancet Planet. Health. 2017;1(6):e230–e241. doi: 10.1016/S2542-5196(17)30094-3. [DOI] [PubMed] [Google Scholar]
- 13.Kippler M., Tofail F., Gardner R., Rahman A., Hamadani J.D., Bottai M., et al. Maternal cadmium exposure during pregnancy and size at birth: a prospective cohort study. Environ. Health Perspect. 2012;120(2):284–289. doi: 10.1289/ehp.1103711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan X., Hu J., Xia W., Zhang B., Liu W., Zhang C., et al. Prenatal chromium exposure and risk of preterm birth: a cohort study in Hubei, China. Sci. Rep. 2017;7(1):3048. doi: 10.1038/s41598-017-03106-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xia W., Du X., Zheng T., Zhang B., Li Y., Bassig B.A., et al. A case-control study of prenatal thallium exposure and low birth weight in China. Environ. Health Perspect. 2016;124(1):164–169. doi: 10.1289/ehp.1409202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang B., Xia W., Li Y., Bassig B.A., Zhou A., Wang Y., et al. Prenatal exposure to lead in relation to risk of preterm low birth weight: a matched case-control study in China. Reprod. Toxicol. 2015;57:190–195. doi: 10.1016/j.reprotox.2015.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xia W., Hu J., Zhang B., Li Y., WiseSr J.P., Bassig B.A., et al. A case-control study of maternal exposure to chromium and infant low birth weight in China. Chemosphere. 2016;144:1484–1489. doi: 10.1016/j.chemosphere.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang J., Huo W., Zhang B., Zheng T., Li Y., Pan X., et al. Maternal urinary cadmium concentrations in relation to preterm birth in the Healthy Baby Cohort Study in China. Environ. Int. 2016;94:300–306. doi: 10.1016/j.envint.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Cantonwine D., Hu H., Sánchez B.N., Lamadrid-Figueroa H., Smith D., Ettinger A.S., et al. Critical windows of fetal lead exposure: adverse impacts on length of gestation and risk of premature delivery. J. Occup. Environ. Med. 2010;52(11):1106–1111. doi: 10.1097/JOM.0b013e3181f86fee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng L., Zhang B., Zheng T., Hu J., Zhou A., Bassig B.A., et al. Critical windows of prenatal exposure to cadmium and size at birth. Int. J. Environ. Res. Publ. Health. 2017;14(1) doi: 10.3390/ijerph14010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu J., Peng Y., Zheng T., Zhang B., Liu W., Wu C., et al. Effects of trimester-specific exposure to vanadium on ultrasound measures of fetal growth and birth size: a longitudinal prospective prenatal cohort study. Lancet Planet. Health. 2018;2(10):e427–e437. doi: 10.1016/S2542-5196(18)30210-9. [DOI] [PubMed] [Google Scholar]
- 22.Peng Y., Hu J., Li Y., Zhang B., Liu W., Li H., et al. Exposure to chromium during pregnancy and longitudinally assessed fetal growth: findings from a prospective cohort. Environ. Int. 2018;121(Pt 1):375–382. doi: 10.1016/j.envint.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Chiu Y.H., Bellavia A., James-Todd T., Correia K.F., Valeri L., Messerlian C., et al. Evaluating effects of prenatal exposure to phthalate mixtures on birth weight: a comparison of three statistical approaches. Environ. Int. 2018;113:231–239. doi: 10.1016/j.envint.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stafoggia M., Breitner S., Hampel R., Basagaña X. Statistical approaches to address multi-pollutant mixtures and multiple exposures: the state of the science. Curr Environ Health Rep. 2017;4(4):481–490. doi: 10.1007/s40572-017-0162-z. [DOI] [PubMed] [Google Scholar]
- 25.Agier L., Portengen L., Chadeau-Hyam M., Basagaña X., Giorgis-Allemand L., Siroux V., et al. A systematic comparison of linear regression-based statistical methods to assess exposome-health associations. Environ. Health Perspect. 2016;124(12):1848–1856. doi: 10.1289/EHP172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maresca M.M., Hoepner L.A., Hassoun A., Oberfield S.E., Mooney S.J., Calafat A.M., et al. Prenatal exposure to phthalates and childhood body size in an urban cohort. Environ. Health Perspect. 2016;124(4):514–520. doi: 10.1289/ehp.1408750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lenters V., Portengen L., Smit L.A.M., Jönsson B.A.G., Giwercman A., Rylander L., et al. Phthalates, perfluoroalkyl acids, metals and organochlorines and reproductive function: a multipollutant assessment in Greenlandic, Polish and Ukrainian men. Occup. Environ. Med. 2015;72(6):385–393. doi: 10.1136/oemed-2014-102264. [DOI] [PubMed] [Google Scholar]
- 28.Yang X., Li Y., Li J., Bao S., Zhou A., Xu S., et al. Associations between exposure to metal mixtures and birth weight. Environ. Pollut. 2020;263 [Google Scholar]
- 29.Valeri L., Mazumdar M.M., Bobb J.F., Henn B.C., Wright R.O. The joint effect of prenatal exposure to metal mixtures on neurodevelopmental outcomes at 20-40 Months of age: evidence from rural Bangladesh. Environ. Health Perspect. 2017;125(6) doi: 10.1289/EHP614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rahman M.L., Oken E., Hivert M.F., Rifas-Shiman S., Lin P.D., Colicino E., et al. Early pregnancy exposure to metal mixture and birth outcomes - a prospective study in Project Viva. Environ. Int. 2021;156 doi: 10.1016/j.envint.2021.106714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lenters V., Portengen L., Rignell-Hydbom A., Jönsson B.A., Lindh C.H., Piersma A.H., et al. Prenatal phthalate, perfluoroalkyl acid, and organochlorine exposures and term birth weight in three birth cohorts: multi-pollutant models based on elastic net regression. Environ. Health Perspect. 2016;124(3):365–372. doi: 10.1289/ehp.1408933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carrico C., Gennings C., Wheeler D.C., Factor-Litvak P. Characterization of weighted quantile sum regression for highly correlated data in a risk analysis setting. J. Agric. Biol. Environ. Stat. 2015;20(1):100–120. doi: 10.1007/s13253-014-0180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bobb J.F., Valeri L., Claus Henn B., Christiani D.C., Wright R.O., Mazumdar M., et al. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics. 2015;16(3):493–508. doi: 10.1093/biostatistics/kxu058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu S.H., Bobb J.F., Lee K.H., Gennings C., Claus Henn B., Bellinger D., et al. Lagged kernel machine regression for identifying time windows of susceptibility to exposures of complex mixtures. Biostatistics. 2018;19(3):325–341. doi: 10.1093/biostatistics/kxx036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zou H., Hastie T. Regularization and variable selection via the elastic net. J. Roy. Stat. Soc. B. 2005;67(2):301–320. [Google Scholar]
- 36.Bien J., Taylor J., Tibshirani R. A lasso for hierarchical interactions. Ann. Stat. 2013;41(3):1111–1141. doi: 10.1214/13-AOS1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu J., Wu C., Zheng T., Zhang B., Xia W., Peng Y., et al. Critical windows for associations between manganese exposure during pregnancy and size at birth: a longitudinal cohort study in wuhan, China. Environ. Health Perspect. 2018;126(12) doi: 10.1289/EHP3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boumans P.W.J.M., Ivaldi J.C., Slavin W. Measuring detection limits in inductively coupled plasma emission spectrometry-II. Experimental data and their interpretation. Spectrochim. Acta B Atom Spectrosc. 1991;46(5):641–665. [Google Scholar]
- 39.Villar J., Ismail L.C., Victora C.G., Ohuma E.O., Bertino E., Altman D.G., et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014;384(9946):857–868. doi: 10.1016/S0140-6736(14)60932-6. [DOI] [PubMed] [Google Scholar]
- 40.Hornung R.W., Reed L.D. Estimation of average concentration in the presence of nondetectable values. Appl. Occup. Environ. Hyg. 1990;5(1):46–51. [Google Scholar]
- 41.Friedman J., Hastie T., Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J. Stat. Software. 2010;33(1):1–22. [PMC free article] [PubMed] [Google Scholar]
- 42.Liu H., Lu S., Zhang B., Xia W., Liu W., Peng Y., et al. Maternal arsenic exposure and birth outcomes: a birth cohort study in Wuhan, China. Environ. Pollut. 2018;236:817–823. doi: 10.1016/j.envpol.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 43.Jiang M.M., Li Y.Y., Zhang B., Zhou A.F., Zheng T.Z., Qian Z.M., et al. A nested case-control study of prenatal vanadium exposure and low birthweight. Hum. Reprod. 2016;31(9):2135–2141. doi: 10.1093/humrep/dew176. [DOI] [PubMed] [Google Scholar]
- 44.Xia W., Zhou Y., Zheng T., Zhang B., Bassig B.A., Li Y., et al. Maternal urinary manganese and risk of low birth weight: a case-control study. BMC Publ. Health. 2016;16:142. doi: 10.1186/s12889-016-2816-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hou Q.Z., Huang L.L., Ge X.T., Yang A.M., Luo X.Y., Huang S.F., et al. Associations between multiple serum metal exposures and low birth weight infants in Chinese pregnant women: a nested case-control study. Chemosphere. 2019;231:225–232. doi: 10.1016/j.chemosphere.2019.05.103. [DOI] [PubMed] [Google Scholar]
- 46.Lazarevic N., Barnett A.G., Sly P.D., Knibbs L.D. Statistical methodology in studies of prenatal exposure to mixtures of endocrine-disrupting chemicals: a review of existing approaches and new alternatives. Environ. Health Perspect. 2019;127(2) doi: 10.1289/EHP2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.ATSDR. Toxicological . 2012. Profile for Vanadium. [PubMed] [Google Scholar]
- 48.ATSDR. Toxicological . 2004. Profile for Cobalt. [Google Scholar]
- 49.ATSDR. Toxicological . 2005. Profile for Zinc. [PubMed] [Google Scholar]
- 50.Hambidge K.M., Krebs N.F. Zinc deficiency: a special challenge. J. Nutr. 2007;137(4):1101–1105. doi: 10.1093/jn/137.4.1101. [DOI] [PubMed] [Google Scholar]
- 51.Yamada K. Cobalt: its role in health and disease. Met Ions Life Sci. 2013;13:295–320. doi: 10.1007/978-94-007-7500-8_9. [DOI] [PubMed] [Google Scholar]
- 52.Sun X., Jiang Y., Xia W., Jin S., Liu W., Lin X., et al. Association between prenatal nickel exposure and preterm low birth weight: possible effect of selenium. Environ. Sci. Pollut. Res. Int. 2018;25(26):25888–25895. doi: 10.1007/s11356-018-2622-x. [DOI] [PubMed] [Google Scholar]
- 53.Bermúdez L., García-Vicent C., López J., Torró M.I., Lurbe E. Assessment of ten trace elements in umbilical cord blood and maternal blood: association with birth weight. J. Transl. Med. 2015;13:291. doi: 10.1186/s12967-015-0654-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.