Abstract
Research on the environmental health of emerging contaminants is critical to understand their risks before causing severe harm. However, the low environmental concentrations, complex behaviors, and toxicology of emerging contaminants present enormous challenges for researchers. Here, we reviewed the research on the environmental health of engineered nanomaterials (ENMs), one of the typical emerging contaminants, to enlighten pathways for future research on emerging contaminants at their initial exploratory stage. To date, some developed pretreatment methods and detection technologies have been established for the determination of ENMs in natural environments. The mechanisms underlying the transfer and transformation of ENMs have been systematically explored in laboratory studies. The mechanisms of ENMs-induced toxicity have also been preliminarily clarified at genetic, cellular, individual, and short food chain levels, providing not only a theoretical basis for revealing the risk change and environmental health effects of ENMs in natural environments but also a methodological guidance for studying environmental health of other emerging contaminants. Nonetheless, due to the interaction of multiple environmental factors and the high diversity of organisms in natural environments, health effects observed in laboratory studies likely differ from those in natural environments. We propose a holistic approach and mesocosmic model ecosystems to systematically carry out environmental health research on emerging contaminants, obtaining data that determine the objectivity and accuracy of risk assessment.
Keywords: Emerging contaminants, Engineered nanomaterials, Holistic approach, Mesocosmic systems, Natural environments
Graphical abstract
Highlights
-
•
Lessons learned from ENMs study provide a paradigm for other emerging contaminants
-
•
Health risks depend on multiple environmental factors, media, and organisms
-
•
Environmental health research should be conducted in natural environments
-
•
A holistic approach is advocated to ensure risk assessment and management
-
•
Call for research on ecological and human health effects of emerging contaminants
1. Introduction
The concept of environmental health was proposed by the European community members of the World Health Organization (WHO) in 1993, referring to the science that identifies environmental sources and risks of hazardous agents in air, water, soil, food, or other environmental media and evaluates and controls their adverse effects on ecological and human health [1,2]. Goals of environmental health research are to (1) identify environmental exposure of key pollutants that pose threats to human health; (2) reveal the toxicity mechanism of environmental pollutants and their impacts on human health by combining environmental and genetic methodologies; (3) precisely control environmental health risks of pollutants; and (4) finally build a new “One Health” pattern of “environment–human–plants–animals”. Hazards of pollutants to environmental health, especially to human health, are often irreversible [3]. The WHO global assessment report pointed out that 24% of human diseases and 23% of deaths were caused by contaminants [4]; pregnant women were exposed to more than 43 types of generally cytotoxic and genotoxic contaminants every day, posing threats to our descendants [4].
Environmental pollutants have been produced unintentionally by human activities and raised concerns when their long-term accumulation resulted in a certain concentration threshold and pollution events. The earliest concern about environmental contaminants arose in the 1960s when dichlorodiphenyltrichloroethane (DDT) was batch produced to eradicate malaria and accumulated in the environment [5,6]. As described in Silent Spring by Rachel Carson in 1962, the non-targeted toxic effects of DDT resulted in the death of many kinds of animals, insects, fish, and even high-trophic organisms, including humans, reducing biodiversity and causing ecological imbalance [5]. The above consequences cannot be eliminated, although DDT applic2ation has been discontinued for more than half a century. Another painful lesson is learned from mercury (Hg), a metal additive commonly used in medical fillers, personal care products, thermometers, and batteries. The emergence of Hg dates back to more than a thousand years before Christ, but the environmental health research on Hg did not begin until 1956, when Hg pollution caused the well-known “Minamata disease,” leading to neuromuscular damage, renal dysfunction, and immunotoxicity of organisms [7,8]. The mortality rate caused by Hg pollution can be as high as 34% [9]. The old concept of “pollution first, mitigation later” has led to research lag and outbreak of the above pollution events. This warns us that, for the sake of environmental sustainability, research on the environmental health of new compounds/materials should be performed before their wide application.
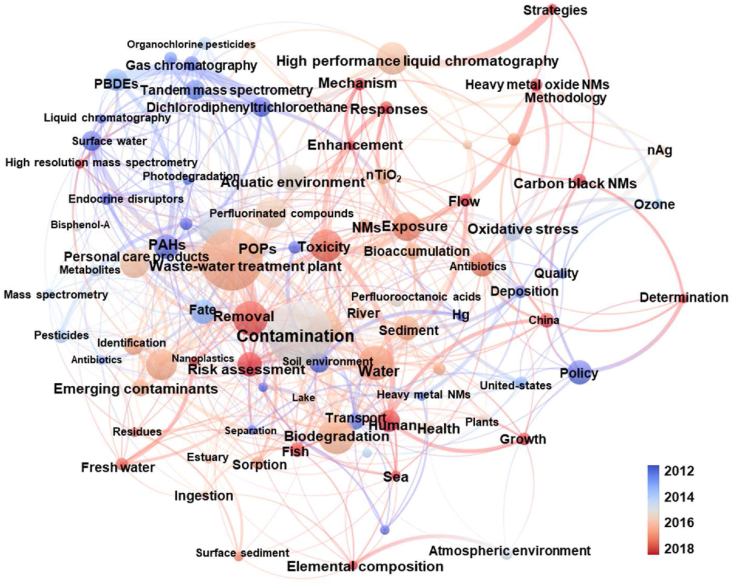
In comparison with traditional contaminants, such as DDT and Hg, emerging contaminants are a class of substances that are newly discovered or presenting new concerns, and they are toxic, accumulative, and environmentally persistent. In addition, their production and application are not yet effectively managed, their environmental behaviors are not yet fully understood, and their environmental toxicology and health risks are not comprehensively assessed [10,11]. The universally acknowledged emerging contaminants include persistent organic pollutants such as organochlorine pesticides and polybrominated diphenyl ethers, endocrine disruptors such as perfluorinated compounds, personal care products, engineered nanomaterials (ENMs), antibiotics, and resistance genes (Fig. 1).
Fig. 1.
Co-occurrence network with timeline showing overlay visualization of keywords extracted from the literature associated with emerging contaminants. The bibliographic search was conducted on the Web of Science database, and the keywords used for article searching were “New contaminant∗” or “New pollut∗” or “Emerging contaminant∗” or “Emerging pollut∗”, with only research articles published from January 1990 to December 2021 included. The circle size reflects the number of co-occurrence links of each keyword, and the line color represents the average published year of each keyword.
Environmental concentrations of emerging contaminants are generally too low to be detected or traced, let alone accurate evaluation of their environmental health risks and efficient management [10]. Once a new contaminant enters the environment, a series of biogeochemical behaviors will occur, including transfer, transformation, and transmission or accumulation along the food chain, resulting in property changes that increased difficulties in its detection and changed environmental health effects. It was reported that emerging contaminants, such as perfluorooctanoic acids, could be adsorbed by soil or dust particles, which inhibits their migration and bioavailability, and reduces their environmental risks [12,13]. Meanwhile, horizontal transfer and transmission of resistant genes among bacteria and/or trophic levels enhanced their environmental health risks [14,15]. Environmental aging of microplastics results in the co-migration of coexisting pollutants and the release of inherent components, such as plastic nanomaterials (NMs) and additives, leading to more complex environmental risks for microplastics [[16], [17], [18]]. A large number of laboratory studies have demonstrated the generally negative health effects of emerging contaminants, including nervous system disorders, cytotoxicity, and genotoxicity [5,19,20]. However, the results were generally obtained from exposure experiments with single organisms, high exposure concentration of contaminants, and controlled environmental conditions, which may not reflect the “real” environmental risk of emerging contaminants in natural settings.
In the recent 20-year trajectory of environmental health research associated with emerging contaminants, serious concerns have been raised for certain types of emerging contaminants at a specific period and have been banned from use today, while ENMs have always been the centered concern (Fig. 1). ENMs are intentionally or incidentally designed and produced by humans, which could be introduced into environments during anthropogenic processes. The highly reactive surfaces of ENMs make them easy to transform but difficult to detect [19,21]. Moreover, various ENMs exhibit different environmental behaviors, increasing the complexity of their environmental health assessment [19,22]. Since 2003, several high-impact journals have called for attention to address the environmental health effects of ENMs [21,23]. ENMs, such as nanoscale titanium dioxide (nTiO2), one of the commonly used NMs, have been proved to be a kind of carcinogen [20], which resulted in tightened control of ENMs and increased research interests in their environmental health and safety. In 2010, ENMs were included in the list of emerging contaminants by the United States Environmental Protection Agency [24]. In the past 20 years, despite many challenges, significant progress has been made in the environmental health research of ENMs. Thus, the research experiences with ENMs can be learnt and used for environmental health issues of other emerging contaminants, especially at their initial exploratory stage. Moreover, as one of the most typical emerging contaminants, ENMs exhibit the characteristics of both organic and inorganic contaminants. In the new context of unknown environmental occurrence, insufficient environmental toxicology data, and complex environmental behavior of emerging contaminants, this review aims to answer the following question: how to carry out basic research on the environmental health of emerging contaminants and obtain objective data to guarantee the accuracy and objectivity of relevant environmental health risk assessment?
Taking the example of ENMs, the environmental health literature was comprehensively reviewed to enlighten the environmental health research of other emerging contaminants and provide a cornerstone for recognizing their environmental behaviors in natural environments. A genuine understanding of the environmental geochemical process of emerging contaminants is the prerequisite for their scientific control in the future.
2. Environmental health of ENMs
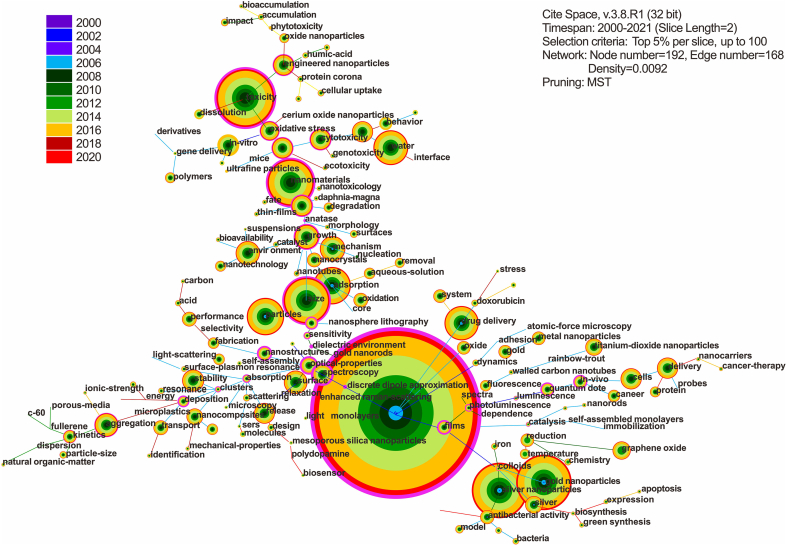
Nanotechnology has penetrated many fields, including chemical and pharmaceutical industry, medicine, agriculture, and environment [[25], [26], [27]]. Nanotechnology research is one of the most competitive scientific highlands in the world. To date, the global annual production of ENMs has exceeded 2.4 million tons, which brings environmental health risks during their production, use, and disposal [27,28]. In the past 20 years, the environmental health research of ENMs has shown great progress, especially in the following four aspects: (i) environmental source and exposure risks of ENMs, and development of corresponding detection methods to uncover the environmentally relevant concentration of ENMs; (ii) environmental behaviors, including transfer and transformation of ENMs, and the factors that may change them; (iii) toxicity of ENMs and associated mechanisms; and (iv) environmental risk assessment and management of ENMs (Fig. 2). Despite continuous research, environmental health risk assessment of ENMs still faces challenges due to evident deficiencies in previous studies. For example, the absence of ENMs trace analysis methods in environmental samples led to higher exposure concentrations set in previous laboratory studies, which failed to offer guidance for the “real” environmental risks of ENMs. Moreover, when ENMs enter environments, complex environmental behaviors inevitably occur, while the biogeochemical behaviors of ENMs, which contribute to the objective assessment of ENMs’ environmental health risks, have not yet been clarified. To date, the toxicity of ENMs has mainly been determined at the genetic, cellular, and individual levels in laboratories [23,25,28]. Their ecotoxicity and toxicity to humans have rarely been reported. Nonetheless, this chapter introduces basic research conclusions obtained in the process of studying the environmental health risks of ENMs, which can be used as guidelines for environmental health research on other emerging contaminants.
Fig. 2.
A pathfinder network of keywords associated with engineered nanomaterials (ENMs) in the field of environmental sciences. The bibliographic search was conducted on the Web of Science database, and the keywords used for article searching were (“Engineered nanoparticle∗” or “Engineered nanomaterial∗” or “Engineered nanopollut∗”) and “Environment∗”, with only research articles published from January 2000 to December 2021 included. The node threshold was set as top 25, and the co-occurrence rings and links within the same year were assigned with a unique color. The hub keywords with high centrality scores were highlighted with purple trims.
2.1. Analysis methods for preliminary determination of the potential source and environmental concentration of ENMs
Tracking the sources and determining the environmental occurrence of ENMs in natural environments are the basis for an accurate assessment of ENMs’ environmental health risks. It is well known that NMs are ubiquitous in environments, resulting from anthropogenic sources, known as ENMs, or natural sources such as volcanic eruptions, forest fires, mineral weathering, sandstorms, dust deposition, and sea surges [29]. As shown in Fig. 2, the concerned ENMs in the past 20 years include metal ENMs such as nAg0 and nFe0, metal oxide ENMs such as nTiO2, nZnO, nCeO2, and nFe2O3, and carbon-based ENMs such as carbon black NMs, graphene NMs, and plastic NMs.
ENMs enter environments directly with daily production and use of nanoscale materials, which is also a major route of environmental exposure to ENMs. For example, nTiO2 has been used as an anti-ultraviolet coating or photocatalyst in the fields of medicine, skincare, textile, food packaging, coating, and automobile industry [30,31]. As one of the most important bactericidal and disinfectants, nAg0 with concentrations as high as 15 mg/L has been added to skincare products [32]. Nanoscale additives can be released during their process of production, use, and abandonment, resulting in environmental risks. With a large specific surface area, strong reducibility, and sorption capacity, nFe0 is widely used for contaminant remediation, which results in high exposure of nFe0 to the ecosystem in contaminated sites [33]. Moreover, nano-agricultural technologies have been developed to alleviate the environmental pollution caused by traditional high-consumption agricultural activities and improve the bioavailability of fertilizer [[34], [35], [36], [37]]. However, the application of nanoscale fertilizer or pesticides will also lead to substantial exposure of various ENMs to humans, farmers in particular [36], raising many concerns about the environmental health of ENMs.
ENMs could also be produced from the breakdown of larger particles through solar photolysis, mechanical wearing, chemical corrosion, or biodegradation. One typical example is plastic pollution. Plastics are widely used in manufacturing, transportation, packaging, agriculture, and other fields [38]. The global production of plastics is about 8.3 billion tons from 1950 to 2015 [38,39], which is expected to reach 34 billion tons by 2050, and 80% of them would be accumulated in the environment [39]. About 6.7 × 107 microplastic EMs (size <5 mm) can be released after only one month of light aging of a square meter of bulk plastics [40]. The aging of microplastics can further increase the particle number by at least one order of magnitude due to the release of plastic NMs (size <100 nm) [41]. About 3.3 × 1011 (∼2.1 mg) plastic NMs can be released per gram of textiles when being washed [42], and about 1.5 × 1010 plastic NMs can be produced per gram of plastic teabags during use [43]. It was also reported that about 2.5 × 1010 plastic NMs can be generated per nursing bottle during sterilization [44]. This suggests that a baby may be exposed to up to three million plastic NMs per day when fed with milk powder from an ordinary polypropylene bottle [44]. Compared to bulk plastics, plastic NMs have higher bioavailability and thus higher environmental health risks. Another example of “broken-down” ENMs is carbon black NMs, which dominates the combustion products of carbonaceous fuels and are introduced into the environment at about 7.5 million tons per year [29]. In addition, the concentration of fullerene, another kind of nanoscale combustion product, is 2.27–10.50 pg/m3 in air, as high as 170 pg/m3 in vehicle exhaust and 108–895 pg/g (dw) in sludge [[45], [46], [47]].
The characterization and quantification of natural NMs remain difficult. This is ascribed to (i) the types and occurrence of natural NMs are unclear, and (ii) the components of natural NMs are complex and indistinguishable from natural environmental media. To date, the development of analytical methods for NMs has focused on both natural NMs and ENMs. It has been predicted that the concentration of natural NMs in surface water is likely to be orders of magnitude higher than that of ENMs [48]. The existence of natural NMs brings great challenges to the separation and detection of ENMs in natural media, resulting in difficulties in accurately assessing the environmental risks of ENMs. The main difference between ENMs and natural NMs is demonstrated by natural NMs’ generally more complex compositions, fewer modifiers, and significantly different environmental behaviors [48]. For example, unlike the surface of natural nAg0 wrapped with humic substances, the engineered nAg0 is generally coated with polyvinylpyrrolidone or sodium citrate modifiers [48]. Therefore, chemical fingerprinting analysis of the modifiers with ultra-high-resolution mass spectrometry (MS) can be used to distinguish natural and engineered nAg0 [49,50]. Another example is that a large number of rare metals generally coexist with natural metal oxide NMs, while only corresponding metals and oxygen atoms exist in metal oxide ENMs, such as nCeO2 or nTiO2 [51]. Therefore, elemental analysis can be used to identify natural and engineered metal oxide NMs. For example, the added nSiO2 ENMs can be distinguished from natural samples with an accuracy of 93.3% by combining dual isotopic fingerprint analysis and machine learning [52].
As previously mentioned, the environmental concentration of emerging contaminants is commonly low. For accurate detection, ENMs should be extracted and isolated from environmental samples and then enriched to a detectable concentration. However, to date, efficient pretreatment methods including separation and enrichment of ENMs from natural environmental samples are rare [56]. In natural environments, the low concentration and active environmental behaviors of ENMs, along with complex environmental matrix, also bring great challenges to the determination of ENMs [53,54]. For example, natural organic matters (NOMs) have similar composition, structure, and properties as carbon-based ENMs, increasing the difficulty of ENMs separation from environmental samples [54]. Moreover, the physicochemical properties, including particle size, morphology, and composition of ENMs, would be altered during the heterogeneous agglomeration or redox reactions between ENMs and the coexisting environmental substrates, such as NOMs, minerals, and microorganisms [55]. It was reported that ENMs could be separated from complex sediment matrices by strong acid/base digestion. However, this method changes the morphology and properties of ENMs, which jeopardizes the evaluation of ENMs occurrence in environments. Another method, cloud point extraction, does not change the physicochemical properties of ENMs [57] but fails to meet the enrichment requirement to make low-concentration ENMs in environmental samples detectable. Combined with microporous membrane extraction, the enrichment factors of this method can be enhanced by thousands of times [57,58]. Nonetheless, due to strong membrane adsorption, the elution and separation factors of this combined method are still too low for the accurate detection of ENMs. Advanced pretreatment methods are still needed to address issues on the separation of ENMs from environmental samples and the increase of enrichment factors of ENMs. Insights into the structural characteristics of ENMs in natural environments are likely feasible (Fig. 3).
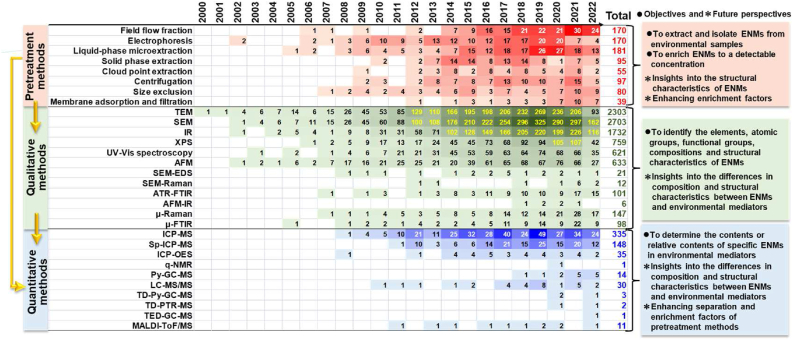
Fig. 3.
Emergence and development timeline of analytical methods that are commonly used for the detection of ENMs. The color of each square represents the number of articles related to the corresponding method, which is the number in the box. The darker the color, the larger the number of related articles. TEM, transmission electron microscopy; SEM, scanning electron microscopy; SEM-EDS, SEM-energy dispersive spectrometer; IR, infrared spectroscopy; AFM, atomic force microscopy; FTIR, Fourier transform infrared spectroscopy; ICP-MS, inductively coupled plasma mass spectrometry; Sp-ICP-MS, single-particle ICP-MS; ICP-OES, ICP optical emission spectrometry; q-NMR, quantitative proton-nuclear magnetic resonance; Py-GC-MS, pyrolysis gas chromatography-mass spectrometry; LC-MS/MS, liquid chromatography MS/MS; TD-Py-GC-MS, thermal desorption-Py-GC-MS; TD-PTR-MS, TD-proton transfer reaction-MS; TED-GC-MS, thermal extraction-desorption-GC-MS; MALDI-ToF/MS, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry.
Apart from pretreatment methods, many analytical methods have also been developed for the qualitative and quantitative detection of ENMs in environmental samples after pretreatment (Fig. 3). Specifically, transmission electron microscopy (TEM) and scanning electron microscopy (SEM) are widely used to observe the morphological characters of ENMs. With plastic NMs developed as an emerging topic in environmental science, atomic force microscopyinfrared spectroscopy (AFM-IR) is emerging and being used for plastic NMs characterization. Single-particle inductively coupled plasma mass spectrometry (Sp-ICP-MS) and ICP-MS technologies are currently one of the most widely used methods for particle size measurement and quantitative determination of low-concentration metal and metal oxides ENMs (Fig. 3) [[59], [60], [61]]. As for carbon-based ENMs, such as plastic NMs, their quantification mainly relies on MS technologies, such as pyrolysis gas chromatography mass spectrometry (Py-GC-MS) and liquid chromatography (LC)-MS/MS, by comparing the characteristic fingerprint of the samples with the standard database [54,62]. The quantitative proton-nuclear magnetic resonance (q-NMR), thermal desorption (TD)-Py-GC-MS, and thermal extraction-desorption (TED)-GC-MS are emerging and being used for plastic NMs quantification. However, taking plastic NMs as an example, the Py fragments of different plastic particles could overlap, making it still difficult to distinguish the variation of plastic types [57]. Insights into the differences in composition and structural characteristics between ENMs and environmental mediators are critical for the development of advanced analysis methods.
Overall, the detection of ENMs in natural environments is still one of the main bottlenecks in revealing the sources, exposure risks, and environmental health effects of ENMs. This requires the development of efficient enrichment and elution methods to make the low concentrations of ENMs in samples detectable. In addition, to distinguish the source of ENMs from natural samples, an in-depth understanding of the production process and structural characteristics of ENMs is crucial. Moreover, improving the detection accuracy of instruments is the key to the determination of low-concentration ENMs. To overcome the limitation of analytical methods, some exposure assessment and mass balance models could be used to determine the source and distribution of ENMs [63]. For example, the substance flow analysis model can be used to calculate the exposure flux of ENMs based on the whole life cycle of ENMs. The environmental fate model can also predict the environmental occurrence of ENMs based on the aggregation and migration kinetics of ENMs under multiple environmental factors in the environment [64,65]. Compared to single case studies, using big data analysis to establish suitable models is one of the most effective ways to predict the source, occurrence, exposure risks, and environmental health effects of emerging contaminants, such as ENMs. In this case, researchers need to thoroughly consider the coexistence of multiple exposure pathways of ENMs and complex environmental factors that change the flow and dynamic transformation of ENMs. This contributes to the establishment, optimization, and development of a feasible assessment model. Moreover, deep machine learning algorithms are needed for source prediction and risk assessments of ENMs in a large area. However, due to the lack of detection methods for ENMs in natural environments, the model accuracy cannot be verified.
2.2. Environmental behaviors of ENMs mediated by certain environmental factors
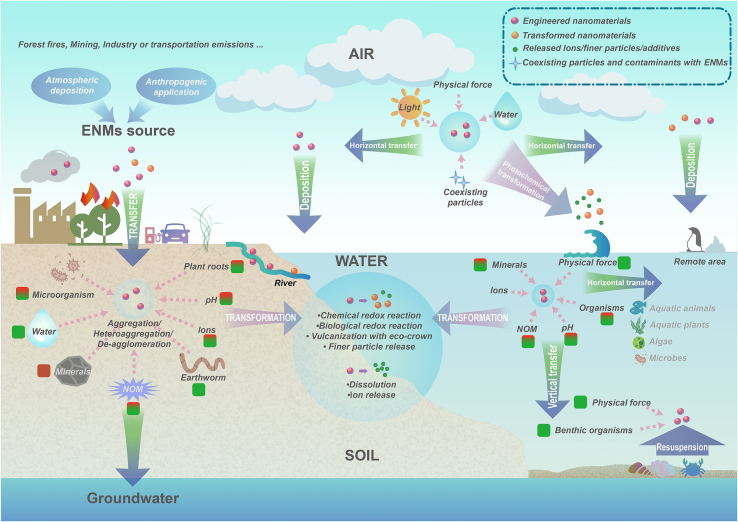
ENMs undergo complex environmental processes among air-soil-water environmental matrix, which result in the accumulation of ENMs and change their physicochemical properties (Fig. 4) [28,39,66]. Environmental behaviors of ENMs, including transfer and transformation, can be affected by complex biotic and abiotic environmental factors [67]. This changes the environmental occurrence of ENMs, such as morphology, surface charge, and aggregate structure, and determines their “real” environmental health risks. Moreover, this also adds difficulties to the detection of ENMs and the objective assessment of their environmental health risks. The biogeochemical process of ENMs has always been a focus of attention and one of the most significant difficulties. Establishing a standardized system to carry out environmental behavior research on ENMs and obtain data close to that in the natural environment is key to the objective evaluation of environmental risks of such emerging contaminants as ENMs.
Fig. 4.
Environmental transfer and transformation of ENMs. The green and red color in boxes next to each environmental factor represents the promotion and inhibition effects of the corresponding environmental factor on ENMs transfer, respectively. The higher proportion of green in a box, the higher number of reported studies on an environmental factor to promote ENMs transfer according to the collected papers in the past 20 years. NOM, natural organic matter.
2.2.1. Transfer of ENMs
2.2.1.1. Transfer of ENMs in atmospheric environments
ENMs with particle diameters less than 100 nm dominate particulate matters that transport in the atmosphere. There are 1 × 1012 – 10 × 1012 g of NMs introduced into the atmosphere through forest fires, mining, industry, or transportation emissions each year [28]. ENMs reside in the atmosphere for a very short time and are then quickly condensed and deposited into the terrestrial or aquatic environment by gravity forces. Through skin contact or respiration, human exposure to ENMs in the atmosphere poses threats to human health [68]. As for certain ENMs, such as plastic NMs, whose density is lower than natural dust, a long-distance horizontal migration with airflow can occur. Therefore, plastic NMs are detectable in remote Antarctica and Mount Everest [69].
ENMs migrate not as a single particle in the atmosphere, and environmental factors, such as humidity, light, coexisting particles, and other contaminants, could interact with them during the process (Fig. 4). Therefore, modeling the migration pattern of ENMs is complex, and the fate of ENMs in the atmosphere is hard to predict. Moreover, both vertical and horizontal migration occurs at the same time, and sample collection and detection of ENMs in the atmosphere are not easy. All the above challenges limit the development of models for evaluating the transfer of ENMs in atmospheres. To date, there is still no suitable model to determine the temporal and spatial concentration distribution of ENMs in the atmosphere.
2.2.1.2. Transfer of ENMs in terrestrial environments
ENMs enter the terrestrial environment through atmospheric deposition, surface runoff, and sludge application, and then participate in complex biogeochemical cycles [28]. Environmental behaviors of ENMs in soil are mediated by factors, such as soil organic matter (OM), minerals, dissolved ions, pH, redox, and microorganisms (Fig. 4) [[70], [71], [72], [73], [74]]. It has been shown that humic acids in soil pore water determined the dispersion and stability of ENMs [70]. ENMs are wrapped with humic acids to increase the steric hindrance between particles, which improves the stability and bioavailability of ENMs. Such enhancement increases with soil osmotic potential due to the swelling of humic acids [33,75]. Some studies suggest that the increased stability of nTiO2 by soil OMs is only applicable in soil with low ion concentrations [67,70]. This might be attributed to the fact that ions build bridges between ENMs or compress the double layers of ENMs to promote their agglomeration. Moreover, the adsorption of nAg0 to hydrophilic NOMs also promotes the settlement of ENMs in soil, with the increased gravitational effects being the main cause of such destabilization [76,77]. Generally, the high concentration and cation exchange capacity of OMs, along with high soil pH, promote the migration of nAg0 in soil, which could be inhibited by the coexisting clay minerals, such as iron oxide minerals [[77], [78], [79]]. This is due to the adsorption and fixation of ENMs by soil particles, which reduce ENMs mobility. Compared to the aggregated ENMs, the dispersed ENMs have higher mobility, causing pollution risks to groundwater. Brownian motion drives the collision between ENMs and soil particles, and the probability of collision is proportional to the aggregation rate of particles [72,80]. Generally, the transfer of ENMs in terrestrial environments is determined by both soil properties and ENMs characteristics, including particle size, shape, and surface charge [67,72,[81], [82], [83]].
Apart from abiotic factors, the activities of abundant organisms, including feeding, excretion, movement, and growth in the soil can cause bioturbation and thus promote the migration of ENMs. Microorganisms dominate the soil organisms and are likely the principal driver of ENMs migration [72,[84], [85], [86]]. For example, the formation of soil microbial film could carry ENMs and lead to co-migration [24,71,87]. In addition, the ENMs entering bacteria can be fused with organic components of bacterial DNA, and thus become a residue in bacteria and migrate with bacterial communities [88]. Moreover, microorganisms exposed to ENMs secrete diverse organic macromolecules, which can affect the migration of ENMs as NOMs.
Earthworms are another active organism in the soil. They can drive the migration of ENMs through activities, such as burrowing, swallowing, and excretion. For example, the burrowing activity of Lumbricus terrestris earthworm resulted in a 30 cm downward migration of plastic NMs within one week, which could reach up to 100 cm with the extension of time [89]. Another study suggested that earthworms can carry plastic NMs to soil areas with low humidity, stressing soil organisms with both drought and plastic NMs pollutants [89,90]. In addition, earthworms can also secrete surface mucus that primarily consists of inorganic salts, sugars, amino acids, and proteins, in which the active groups such as carboxyl and amino could modify the properties of ENMs, such as hydrophobicity and electronegativity [91]. This likely changes the migration of ENMs in soil. Moreover, after entering the earthworm gut and being excreted, the surface properties of ENMs could be changed, which also alters migration patterns and bioavailability [90]. The contribution of earthworms to the migration of ENMs has not been thoroughly evaluated, and the effects of earthworms on the environmental health of ENMs are still unknown.
In agricultural soil, the impact of plants on the migration of ENMs is highly concerning. On one hand, ENMs adsorbed on the surface of plant roots have low mobility and can only transport downward with the growth and lengthening of plant roots [92]. On the other hand, the properties of ENMs could be changed by root exudates, which affect their dissolution and agglomeration processes [83]. To date, the uptake of ENMs by plant roots has been widely proved, but the rhizosphere behavior of ENMs is still unclear [82,92,93]. For example, polystyrene (PS) plastic NMs (100 nm, 50 mg/L) were detected in cucumber tissues after 14 days of exposure, which was ascribed to the root uptake and aboveground migration of plastic NMs along vascular [93]. The uptake of ENMs by plants threatens human health along the food chain.
Despite the above research progress, most of the current studies on the transfer of ENMs in soil were conducted in laboratory soil columns, ignoring the complex interaction among ENMs, soil, and the surrounding ecosystem under field hydrogeology. Related laboratory findings are thus likely far from reflecting the environmental transfer of ENMs in natural environments. Therefore, ENM transfer in in-situ soil environments, where complex biological and physicochemical factors coexist, should be further studied.
2.2.1.3. Transfer of ENMs in aquatic environments
Transfer of ENMs in aquatic environments includes vertical, horizontal, and biological migrations (Fig. 4) [71,94,95]. Aquatic environments are among the most important sinks of ENMs, as they bring together the ENMs in terrestrial or atmospheric environments through surface runoff and deposition processes. In addition, ENMs have longer migration distances and stronger bioavailability, thus processing higher environmental health risks in aquatic environments than in soil environments. Compared to that in atmospheric environments, the migration of ENMs in aquatic environments also lasts longer and is mediated by more complex environmental factors.
Vertical migration of ENMs refers to an up-bottom gravitational sedimentation process of ENMs caused by self-agglomeration or heterogeneous agglomeration or a bottom-up resuspension process of ENMs caused by the hydrodynamics or bioturbation, which leads to different environmental exposure risks of ENMs to organisms at different water depths. Many environmental factors, including NOMs, minerals, pH, ionic strength, physical force, and organisms, determine the migration of ENMs in aquatic environments [70,78,[96], [97], [98], [99]]. A laboratory study found that the shear force of water promoted the resuspension of graphene NMs and nCeO2, which increased the residence time and bioavailability of both ENMs and consequently their toxicity in water [95]. In general, NOMs significantly promote the suspension of ENMs through electrostatic repulsion while minerals promote the sedimentation of ENMs through heterogeneous agglomeration, which also depends on the composition and types of the NOMs or minerals [70]. For example, among the high-molecular-weight humic acids, including Suwannee River fulvic acid (SRFA), Pony Lake fulvic acid (PLFA), and Suwannee River humic acid (SRHA), only the latter two that contain reducing sulfur and nitrogen groups can specifically bind to Ag ions and thus significantly improve the stability of nAg0 [78,100]. In addition, the negatively charged kaolin showed electrostatic repulsion with graphene NMs, inhibiting their heterogeneous agglomeration, while the positively charged kaolin interacted with graphene NMs under the action of Al–O bridging, promoting their vertical migration [97,98]. The interaction mechanisms between ENMs and NOMs or minerals need to be further studied. For example, how would electron transfer occur between various ENMs and minerals or NOMs? What is the resultant steady-state conformation of ENMs and minerals or NOMs during co-migration?
Solution pH and ionic strength also affect the migration of ENMs by altering the surface charge of ENMs [101]. Metal ions tend to be dissolved in acidic water from metal or metal oxide ENMs and the released ions compressed the double layers of the ENMs to reduce their stability [71,72]. As for carbon-based ENMs, taking plastic NMs as an example, the stability of plastic NMs increases with rising pH due to the enhanced electronegativity of plastic NMs surfaces at higher pH [101]. Cations, such as Al3+, Ca2+, and Na+ can, however, compress the double layers on the surface of plastic NMs, thus promoting the agglomeration of plastic NMs [96,99].
It is noted that different environmental factors could interact in natural water environments, and which factor dominates the migration of ENMs remains unclear. Preliminary laboratory results have suggested that the interaction of multiple environmental factors could cause completely reversed effects on the migration of ENMs. For example, NOMs alone promote the suspension of plastic NMs, and the coexisting Ca2+ can bridge between NOMs and plastic NMs, facilitating their co-aggregation and reducing the stability of the latter [99]. The promotion of resuspension of graphene NMs by shear force was decreased by Ca2+ and Mg2+ while increased by NOMs [67,102]. Moreover, even if environmental factors affect the migration of ENMs in the same way, the mechanisms might be different. For example, cations, such as Ca2+ and Al3+, promoted the agglomeration of plastic NMs in aquatic environments, but the mechanisms depend on pH [99]. At pH < 5, the metal ions render the distribution of negative charges on plastic NMs surface to reduce their stability, while at pH > 7, the metal ions undergo crystal agglomeration that captures the plastic NMs and, therefore, reduces their stability.
In natural water environments, especially in oceans and rivers, the horizontal migration of ENMs is the main process of long-distance transport of ENMs. However, studies on the horizontal migration of ENMs have been less reported compared to vertical migration. This is because, on one hand, most current research on the migration of ENMs in aquatic environments is conducted in microcosmic systems, where horizontal migration is almost negligible. On the other hand, vertical migration with the action of gravity inevitably occurs during horizontal migration. To date, there is still no scientific or accurate theoretical model to describe and predict horizontal migration [103].
Biological activities can affect both the vertical and horizontal migration of ENMs. The suspended ENMs with high bioaccessibility could co-migrate with water organisms, such as algae, phytoplankton, and microorganisms via adsorption–desorption or uptake–excretion processes. In addition, organisms can release various biological macromolecules, such as proteins, lipids, polysaccharides, and other extracellular polymers, to form ecological crowns on the surface of ENMs, which indirectly changes their migration. For example, studies have shown that the ecological coronas around plastic NMs and nTiO2 surface increased the steric hindrance between particles, which increased their stability in water and promoted their migration in different porous media [104,105]. However, there are also some contradictory findings showing that biological macromolecules could first form large heterogeneous aggregates with plankton and minerals, which drive the sink of plastic NMs during the sedimentation process of aggregates and thus reduce the suspension stability of plastic NMs [106]. Moreover, it has been well known that ENMs are generally indigestible by organisms, resulting in the transfer and accumulation of ENMs along with different trophic levels [107]. ENMs could accumulate in many biological organs, such as gills, stomach, and intestines [107]. Biomagnification effects of ENMs in two-level food chains, such as Scenedesmus obliquus to Megaflea, Nerei to Turbot, and Microalgae to Scallops, have been proved [67,68,107]. However, the biomagnification of nTiO2 did not occur between Daphnia magna and Danio rerio [108]. Using 14C-isotope labeling technology, Dong et al. only observed significant biomagnification of graphene NMs from Escherichia coli to Tetrahymena thermophila, but not from T. thermophila to D. magna and further to D. rerio [109]. The lipophilicity of ENMs, their ability to cross cell membranes, and their ability to retain in a digest tract or other tissues determine their propensity for biomagnification along food chains [110,111].
Transfer of ENMs determines the environmental distribution and exposure risks of ENMs. Although the effects of many environmental factors, including light, oxygen, soil pH, NOMs, minerals, and organisms, on the transfer of ENMs in different environmental media have been systematically explored, few of the current studies have considered how the interaction between multiple coexisting environmental factors affects the migration of ENMs. ENMs undergo active biogeochemical processes in aquatic environments, and the sampling and detection methods of ENMs in aquatic environments are the most mature. Thus, an in-depth investigation of how multiple environmental factors mediate the transfer of ENMs and the corresponding changes in environmental risks is suitable to be carried out in a natural water environment.
2.2.2. Transformation of ENMs
Transfer mainly changes the bioavailability of ENMs in the environment, while transformation changes the physical and chemical properties of ENMs, such as their structures. With high reactivity, the environmental transformation of ENMs commonly occurs in the form of physicochemical processes such as fragmentation, dissolution, sulfidation and photochemical [25], and biological alteration such as degradation (Fig. 4). The transformation of ENMs determines the persistence and toxicity of ENMs in the environment. For example, plastic NMs and toxic plastic additives could be released during the physical fragmentation of large plastic particles, resulting in increased toxicity [39,68]. Physical force, light, dissolved oxygen, coexisting ions, and temperature are the main environmental factors driving the physicochemical transformation of ENMs [40,71,112,113]. Since the photocatalytic transformation of ENMs is inevitable in the atmosphere, on the surface of the soil, and in the water environment, studies have been conducted on how it affects the surface properties, redox states, and stability of the ENMs. It was reported that visible light oxidized nAg0 to more bioavailable Ag+, causing increased heavy toxicity, while ultraviolet light reduced Ag+ to less toxic Ag0 [112,114]. Photocatalysis increases surface functional groups and reactive sites on plastic NMs, thus increasing their bioavailability and toxicity [92].
Dissolved oxygen significantly affects redox reactions on metal ENMs. It was reported that the release of metal ions from nCuO, nAg0, and nTiO2 under aerobic conditions is 10.4–34.5 times higher than that under anaerobic conditions [113]. It was also reported that nCuO mainly existed in the forms of toxic CuO and humic acid-CuO complexes in aerobic soil while in the forms of less toxic Cu2S and goethite-Cu2+ complexes in the flooded rice planting season [115]. In addition, the interaction of ENMs with coexisting ions also affects their transformation and consequently their toxic risks. For instance, phosphate inhibited the release of toxic Zn2+ from nZnO by forming amorphous Zn3(PO4)2 on nZnO surface; carbonate also inhibited the release of toxic Ag+ from nAg0 by covering surface reactive sites; coexisting sulfhydryl groups on mineral particles converted nAg0 to less toxic nAg2S [114,[116], [117], [118]].
Temperature is another critical factor affecting the transformation of ENMs. It was reported that in an alternate freezing–thawing process, toxic metal ions were released from ENMs during the soil thawing process, indicating that high temperature promoted the release of metal ions from ENMs [118]. The interaction of environmental factors in natural environments is complex, and exactly how it drives the transformation of ENMs is still unknown. For example, the forms of NOMs and contents of dissolved oxygen in environments change with temperature. NOMs inhibit the transformation of ENMs by masking the reactive sites on the surface of ENMs. At the same time, NOMs can catalyze the formation of superoxide from oxygen, which facilitates the transformation of ENMs [119].
Biotransformation of ENMs occurs everywhere. To date, however, research on the biotransformation of ENMs usually focuses on the degradation of carbon-based ENMs and redox change of metal ENMs by a single microorganism. For instance, the white-rot fungi (Phlebia tremella and Trametes versicolor) can degrade fullerol by incorporating it into lipid metabolism [120]. Fungi exhibit greater resistance to plastic NMs than prokaryotes, likely because the secretion of β-glucosidase, glycine-aminopeptidase, and phenoloxidase by fungi induces enzymatic reactions for the degradation of plastic NMs [121]. The biotransformation of metal or metal oxide ENMs is mainly achieved through redox changes. For example, halophilic and sulfur-associated bacteria can initiate sulfurization reactions, which convert nAg0 to less toxic nAg2S [122]. Moreover, the biotransformation of ENMs also occurs in or on the surface of some animals and plants, which changes the toxicity of ENMs. It was reported that nAg0 can be ingested by large fleas and oxidized into Ag+ in its intestine, causing increased toxicity [123]. There are few reports on the transformation of ENMs in plant tissues. The rhizosphere and phyllosphere are the key gateways for plants to respond to ENMs. For example, nAu was oxidized to Au(CN)2− by biofilm on Egeria densa leaves [124], while nCuO was reduced to Cu(I) compounds by biofilm in the rhizosphere of Eichhornia crassipes [125]. Generally, microbial extracellular secretions can form protein coronas around ENMs, which changes the bioavailability and toxicity of ENMs. Protein coronas have an affinity for cell membranes, which facilitates the absorption of ENMs by plant roots through cell membranes via phagocytosis, pinocytosis, or passive transport [126]. However, the increased bioavailability of ENMs did not correspondingly increase their toxicity. This is because protein corona masked the reactive sites on ENMs and protected the cell from toxicity [105]. With protein coronas protection, the fatality rate decreased by 13% and 5% for bacteria exposed to 5 μg/mL nAg0 and nematodes exposed to 10 μg/mL nAg0, respectively [105].
In natural environments, the biotransformation and physicochemical transformation of ENMs occur simultaneously; yet, the contribution of each transformation process to particle toxicity change and their interactive mechanisms remain unknown. The complex environmental transformation processes of ENMs also make it extremely difficult to assess the bioavailability, toxicity, and environmental health of ENMs in natural environments.
2.3. Environmental and human health effects of ENMs in toxicological research
The most essential basic data for ENMs’ environmental health risk assessment can be obtained from their toxicological studies. The toxicological studies of ENMs began in the 2000s, and a large number of laboratory studies have confirmed that different ENMs have unequal toxic effects and varied toxic mechanisms toward different organisms (Fig. 5) [23]. For a decade, a risk assessment model has been applied to evaluate and predict the environmental health risks of ENMs, while a stable and comprehensive model is still lacking due to limitations in appropriate and quantitative risk assessment experimental designs and in available and comparable data. Since 2016, positive environmental effects of ENMs, such as nano-fertilizers, are increasingly identified, accompanied by the development of nano-agricultural technologies. To date, ENMs demonstrate plentiful advantages in terms of both industrial approaches and consumer confidence, and evidence of consumer opposition to the application of ENMs remains few, which supports the continued development of nanotechnology [34,66,127]. Simultaneously, this will also lead to the continuous introduction of a large amount of ENMs into environments and pose resultant potential risks to environmental health. With continuous and in-depth research, people have come to realize that the environmental health risks of ENMs are not only the toxicity risks to a single organism but also the threats to the ecological environment and human health. However, the biological toxicity of ENMs at environment-related concentrations remains unclear, and it is still difficult to carry out ecological and human health risk assessments of emerging contaminants, such as ENMs. Herein, we reviewed nearly 20 years of research on the environmental and human health effects of ENMs and outlined the knowledge gaps that need to be filled to propose precise management strategies. Nonetheless, we also noted the possible bias in the review due to the fact that current experiments were designed in vitro or conducted from model animal organs and publication bias was likely to exist.
Fig. 5.
Emergence and development timeline of (A) the toxicological studies of ENMs in the past 20 years and (B) exposure risks and toxicity of ENMs to humans. The specific data in (B) are cited from references [29,30,68,[190], [191], [192], [193],196,199,200,202]. nTiO2, nanoscale titanium dioxide.
2.3.1. Toxicity of ENMs
The toxicity of ENMs originates from the nanoscale size of ENMs and the accompanying high bioavailability, strong biokinetics, and toxicological properties of the particular surface [128]. Numerous researchers have suggested that the immunotoxicity, cytotoxicity, neurotoxicity, and genotoxicity of ENMs can result in physical damage, inflammation, and metabolic disorder of organisms [19,[129], [130], [131], [132], [133], [134], [135]]. The phytotoxicity of ENMs was observed in 2011, showing that the photosynthetic processes of plants were inhibited by ENMs. Oxidative stress is one of the most common toxicity mechanisms [132,136]. Briefly, the interaction between O2 and ENMs leads to the formation of reactive oxygen species (ROS) in organisms through disproportionation or Fenton reaction, resulting in oxidative stress. It was reported that nTiO2 passed through the cell membrane of algal and destroyed cells involved in antioxidant processes, inducing the production of intracellular ROS. The generated ROS damaged the cells by oxidizing lipids, proteins, and nucleic acids [134,137]. Moreover, ENMs could target the mitochondria system and initiate apoptosis and programmed death of cells [19]. The cytotoxicity of ENMs is manifested in the inhibition of nitrogen metabolism and decrease in cell growth [114,116,131,138].
The toxicity of ENMs at the individual level is mainly reflected by the disorder of the metabolic process and inhibition of metabolic activity. For example, graphene NMs inhibited the growth of freshwater fish, such as Geophagus iporangensis, Anabas testudineus, and D. rerio by inhibiting enzymatic activities participating in lipid and amino acid metabolisms [133]. Fatty acids and proteins are typical markers for evaluating the metabolic changes of individuals in response to stress [137]. It was also reported that graphene NMs can increase the contents of lipid peroxides but decrease that of protein in D. rerio and Anabas testudineus, resulting in oxidative stress [132,139]. Intestinal tissues are at the most risk since ingestion is the primary exposure pathway of organisms to ENMs [140]. When the ingested ENMs penetrate the mucosal membrane of intestinal tissues and transport across the cells, many other systems of organisms, including circulatory and lymphatic systems, would be attacked, causing immunotoxicity. For example, PS plastic NMs caused immunotoxicity of organisms, such as D. rerio, Tegillarca granosa, and rats, which was ascribed to the downregulated expression of the functional genes participating in signaling and apoptosis [141]. Immunotoxicity caused by ENMs is also accompanied by liver infection and other chronic toxicity in organisms [131,[142], [143], [144]].
The toxicity of ENMs depends on the exposure concentration of ENMs. According to meta-analysis studies, ENMs cause toxicity only at high concentrations, and low concentrations of ENMs may even promote the growth of organisms [138,145]. For example, Ghosh et al. found that DNA damage in tobacco (Nicotiana tabacum) occurred when the exposure concentrations of nTiO2 were higher than 319 mg/L [146]. It was reported that 10 μg/L of nAg0 promoted the growth of algae and zooplankton, while nAg0 with concentrations higher than 500 μg/L inhibited the photosynthesis of phytoplankton [147,148]. At low concentrations, other ENMs, such as carbon nanotubes, nMgO, and nFe2O3, also promoted the growth of algae [149,150]. After oral or skin exposure, fullerene ENMs with low concentrations did not show toxicity to animals such as Oysters in a short term, while the increase of fullerene ENMs concentration along with long-term pulsed exposure-induced production of superoxide free radicals and lipid peroxidation, leading to cytotoxicity [151]. Environmental concentrations of emerging contaminants, such as ENMs, are often low. It was reported that the concentrations of ENMs in the aqueous and terrestrial environments are in the order of μg/L and mg/kg, respectively [152]. However, most of the current toxicology studies are carried out under conditions of high exposure concentration in the order of mg/L or mg/kg levels [131,137,146]. This suggests that the toxicity results from existing laboratory studies likely overestimate the environmental risks of ENMs in natural environments.
When entering environments, ENMs interact with other pollutants and cause joint toxicity to organisms [153]. The real partition of organic pollutants in an aqueous phase can be comparably increased by ENMs adsorption. ENMs can act as a vector to increase the bioaccessibility of pollutants [154]. Simultaneously, the interaction between ENMs and pollutants can also influence the physicochemical properties or bioavailability of ENMs, resulting in indirect and varied toxicity to organisms [155,156]. With model organisms, including human hepatoma cells, aquatic organisms, such as alga and D. rerio, and plants, such as wheat, many studies have observed synergistic toxic effects of ENMs and pollutants, showing that ENMs enhance the risks of metabolic disorder, structural damage, or lethality of organisms by pollutants [153]. Moreover, ENMs can also change metabolism pathways of pollutants in organisms and thus modify the toxicity of pollutants. For example, graphene NMs induced a higher activity of arsenate reductase in wheat roots and contributed to the reduction of As(V) to As(III), and the latter causes inhibition of carbohydrates metabolism in the wheat [157]. ENMs also damaged the function of efflux transporter of cells, resulting in increased accumulation and toxicity of pollutants in cells [158,159]. Opposite findings show that ENMs alleviated toxicity of pollutants to organisms, which can be ascribed to, on one hand, aggregation of ENMs down-regulating the bioavailability of pollutants, on the other hand, ENMs enhancing the defense ability of organisms against the coexisting pollutants [160,161]. It was reported that nTiO2 induced a state of slow metabolism and H+ transport in vacuolar cells by inhibiting the expression of ATP synthase, alleviating the attack of phoxim on nerve systems and reducing neurotoxicity and genotoxicity of phoxim [162].
In natural environments, ENMs undergo a complex environmental transformation, which changes ENMs properties and the resultant toxicity. Under the same concentration, the toxicity of ENMs in soils is much less than that in an aquatic environment [163]. This can be attributed to the interaction of ENMs with soil particles and the heterogeneous agglomeration of soil minerals and ENMs, which reduce the bioavailability of ENMs and thus alleviate their toxicity in soils. In addition, the ENMs could be wrapped by soil OMs, which work as natural barriers to reduce the bioaccessibility of ENMs through steric hindrance. NOMs can, however, also decrease the toxicity of ENMs by improving the dispersion of ENMs in aquatic environments through electrostatic or steric hindrance [164], or increase the toxicity of ENMs by promoting the release of toxic metal ions or additives from ENMs, and acting as electron shuttles between ENMs and organisms to generate oxygen free radicals [165]. Therefore, results from laboratory studies involving direct exposure of original ENMs to organisms either overestimate or underestimate the toxicity and risks of ENMs in natural environments. It is also worth noting that the air-soil-water ecosystems are interconnected, and the transfer of ENMs among different environmental media increases the exposure pathways of ENMs to organisms. For example, the toxicity of ENMs to plants likely results from combined exposure toxicity of ENMs in soils (through roots) and atmosphere (through leaves or fruits) in natural environments. Moreover, although residence times of ENMs in atmosphere are short, natural environmental factors including moisture or aerosol particles change the real-time concentration and properties of ENMs and thus affect their toxic risks in atmosphere. The inevitable atmospheric exposure pathways in natural environments are often ignored in many toxicology case studies; therefore, the current findings likely underestimate the environmental risks of ENMs.
2.3.2. Ecotoxicity of ENMs
ENMs pose unequal exposure risks to different organisms, and the responses and adaption strategies of different organisms to ENMs are also different [166]. Ecotoxicity of ENMs could be evaluated by changes in the composition and evolution of species, diversity and function of communities, elemental transformation, and energy flow of an ecosystem. Although the importance of ecotoxicological research of ENMs was recognized at the beginning of toxicological study of ENMs, it was not until 2014 that the researchers began to investigate the ecotoxicity of ENMs in terms of energy flows and material cycles in ecosystems (Fig. 5A). To date, there are a lot of knowledge gaps in research on the ecotoxicity of ENMs due to the lack of data on environmental concentrations, occurrence forms, and environmental behaviors of ENMs in natural environments.
Microorganisms are one of the most sensitive biological components in response to ENMs. At environment-relevant concentrations, nAg0 and nCuO particles were found to significantly reduce microbial biomass and diversity in soil [167,168]. Microorganisms are also one of the most important drivers of biogeochemical processes. The high biodiversity is conducive to efficient resource capture and energy utilization, which facilitates biogeochemical cycles in an ecosystem [169,170]. Therefore, the impacts of ENMs on microbial communities can change the ecological functions of environmental media, and the decline in biodiversity by ENMs is indicative of the deterioration of ecosystem sustainability [[171], [172], [173]]. It was also reported that the abundance of functional genes involved in the ammonia transformation process was significantly reduced by long-term exposure of nAg0 in a loamy soil, suggesting an inhibition of ENMs to the soil nitrogen cycle [167].
The toxicity of ENMs to organisms at different trophic levels disturbs ecosystem stability and weakens ecological service functions. Primary producers in marine systems provide more than 80% of the global oxygen [174,175]. It was reported that nTiO2 can inhibit the photosynthesis and reproduction of primary producers such as microalgae (Chaetoceros muelleri) in ocean by shading the light and damaging cells [137], which likely reduces the oxygen supply capacity of the ocean. The introduction of plastic NMs can reduce the carbon sequestration and productivity of the ocean [174]. This is because plastic NMs reduced the biodiversity of primary producers and the sinking rate of marine organic carbon such as biological excrements, which further altered the ecological functions of the ocean, including the carbon cycle and energy flow [[175], [176], [177], [178]]. Most of the studies on the ecological effects of ENMs in terrestrial environments still focus on changes in primary producers (e.g., plants) and decomposers (e.g., microorganisms). However, ENMs can also decrease the biodiversity of consumers by increasing the abundance of predators or downgrading the trophics [179,180], which threatens ecological stability and functions.
Transfer, bioaccumulation, and biomagnification of ENMs along food chains are a critical issue in ENMs ecotoxicity. It was reported that 45% of nAg0 can be transferred from microalgae to D. magna, resulting in nAg0 accumulation in and toxicity to D. magna [114]. The assimilation efficiency of ENMs such as nTiO2 from D. magna to D. rerio is even higher [116]. The feeding to consumers (e.g., bivalves and copepods) with ENMs-absorbed microalgae caused oxidative stress and genotoxicity, decreasing their reproduction and survival. To date, the effects of ENMs on biological communities are still mostly studied with single model organisms or short food chains, while the ecological effects of ENMs based on an experimental system, including multi-species food networks, are rarely reported. In addition, whether and how the effects of ENMs on low-trophic-level biomes indirectly affect higher-trophic-level biomes are still unclear.
In order to elucidate the shifts in ecosystem functions in response to ENMs, mesocosmic experiments can be conducted and some advanced technologies used in ecological fields can be borrowed. For example, environmental DNA metabarcoding and multi-omics technologies have been widely used in ecological fields to reveal changes in biodiversity and monitor the distribution of new species among different trophic levels in the food chains. The establishment of ecologically relevant systems such as mesocosmic systems can provide critical data to support the design of laboratory experiments and model building [181,182]. With the aid of these technologies, the responses of biological communities and functional diversity to ENMs are likely to be clarified in real ecosystems.
Another critical concern is biological evolution. It was well known that organisms undergo beneficial gene mutation or recombination to adapt to changes in environments. ENMs are refractory in ecosystems [183,184] as they can damage DNA and cause ectopic and aberration of chromosomes, which induces gene mutation and recombination [185,186]. The genotoxicity of ENMs might facilitate the evolution of organisms, which has been ignored in exploring the ecotoxic effects of ENMs. To test gene mutations in the process of biological evolution, certain single-celled organisms such as Tetrahymena can be used as model organisms. Furthermore, a structure–activity model can be built to illustrate the relationships between various ENMs and biological evolution. This requires the disclosure of more genetic information databases of biomes. To date, the impacts of ENMs on ecological function and evolution from a global ecosystem perspective are still lacking.
2.3.3. Human health effects of ENMs
Negative effects of ENMs on the environment eventually threaten human health (Fig. 5B). With the rapid development of nanotechnology, beneficial uses of ENMs in costume, skincare, and medical therapy have made great progress. For example, carbon-based ENMs including fullerene are promising in capacity to induce antimicrobial ability and regeneration of biological tissues [187]. But the application of ENMs in the above fields also causes direct ENMs exposure to humans. Without an appropriate risk assessment of the ENMs to human health, nanotechnology cannot be developed with public confidence. Therefore, research on the effects of ENMs on human health has attracted increasing attention.
Inhalation is one of the main exposure pathways of ENMs to humans [29,188]. The annual amount of inhalation of nTiO2 by humans is about 55,000 particles per capita [30]. Therefore, the respiratory system is usually the main target of atmosphere ENMs, which results in diseases, including asthma, pulmonary fibrosis, pneumothorax, and chronic bronchitis [189]. A large number of ENMs have been detected in human lungs, attracting much attention to evaluate their health risks [190]. It was reported that PS plastic NMs induced the release of reactive oxygen species in BEAS-2B cells, which caused cytotoxicity and genotoxicity in the epithelium of the respiratory lining and macrophages, and consequently cellular inflammatory [191]. In addition, nanofibers can deposit in terminal bronchioles, alveolar ducts, and alveoli of the lungs of humans, leading to chronic inflammation, granuloma, and pulmonary fibrosis [192]. Exposure to carbon black NMs also induced serious accumulation of superoxide anions in endothelial cells of the pulmonary artery, causing cell apoptosis and cardiovascular disease [193].
Skin adsorption is another main exposure pathway of ENMs in humans [29]. The concentration of ENMs is about 0.3–1.5 grains/m3 and as high as 59.4 grains/m3 in outdoor and indoor atmospheric environments, respectively, causing skin absorption of ENMs [29]. In addition, applications of personal care products and wearing of functional clothing also led to skin contact with ENMs [68]. It was reported that the skin exposure of nCuO released from paint and coating leads to abnormal development of children’s weight [194]. An in vitro Calu-3 epithelial cell incubation experiment showed that 31 μg/mL of plastic NMs could enter the cells through endocytosis to produce semi-lethal toxicity [195]. The DNA double-strand structure of the epithelial cells was broken, and the cell metabolism under the stress of nSiO2 consumed more than two times of glutathione compared to the control. Moreover, 150 μg/mL of plastic NMs resulted in the death of macrophages differentiated from THP-1, leading to impaired immunity processes in the cells [195].
The potential ingested amount of ENMs by humans is about 49,000 particles per capita every year, which mainly originates from the consumption of drinking water, vegetables, fruits, crops, aquatic products, and meats [29,30]. In this case, the gastrointestinal tract becomes the main target of ENMs. Therefore, exploring the bioavailability and transformation mechanism of ENMs in the human digestive system is important in evaluating the human health risks of ENMs. It was reported that nAg0 particles are prone to be transformed into more bioavailable and toxic Ag+ in the acidic gastric juice [196]. Moreover, metal ions are prone to be released from metal oxide ENMs such as nFe2O3 and nZnO in gastric juice, causing toxic effects on the digestive system of organisms [197,198].
The interaction between ENMs and epithelial cells generally determines the transport efficiency and toxicity of ENMs to the intestine and other organs. ENMs can be absorbed by the intestinal cell barrier, disordering the functions of the intestine. For example, plastic NMs compromised the permeability of intestinal epithelium and resulted in peroxidation and inflammation of the intestine [199]. Moreover, the microbial community structure in the intestine was changed by plastic NMs, causing cytotoxicity of the immune system [199]. ENMs with positive charges tend to enter cells through endocytosis and those with negative charges tend to cross the intestinal cell barrier through the cell gap [198,200]. When the ingested ENMs passed through the epithelial cells, they would pose severe threats to organs, including liver, kidney, heart, and even nerve systems. For example, nCuO exposure damaged the gastrointestinal tract and respiratory system and the functions of the liver and kidney of humans [194]. The exposure of nAl2O3 inhibited the activity of human lymphocytes, which is one of the main manifestations of ENMs immunotoxicity [201]. It was also reported that nAg0 with concentrations higher than 15 μg/mL caused oxidative damage and accelerated the death of colon cells [197]. Moreover, ENMs may damage the reproductive organs of humans and cause growth delay or cancer [196,201,202]. Neurological disorders and oxidative DNA damage by ENMs have been proved, which likely cause various genetic diseases in humans and even influence the health of future generations [30,195,201].
Views on human health effects of ENMs predominately come from laboratory studies with certain organs or tissue of model animals exposed to ENMs in vivo or machine learning studies based on in vitro model predictions. Few studies have considered the influences of environmental factors on the properties of ENMs, such as particle sizes, shape, charge properties, exposure dose, and bioavailability, which likely leads to biased toxic predictions of ENMs. In addition, differences in cell types, cultural systems, gender, age, or species of model animals in different studies could also lead to inconsistent understandings of potential ENMs toxicity. Moreover, there are diverse exposure pathways of ENMs to humans; yet, their contributions to the toxicological consequences are unknown. To fill the above knowledge gaps, the latest dynamic human environmental exposome can be applied to better understand the life-cycle toxicity of ENMs and the related mechanisms to different genes, cells, and tissues. This technology can systematically track different organisms exposed to various ENMs, describing associations between biological responses and ENMs and proposing an exposome network of ENMs based on interactions between environmental and individual factors. Identifying the indicator organisms and toxicity targets of different ENMs is also critical for the health risk assessment of ENMs.
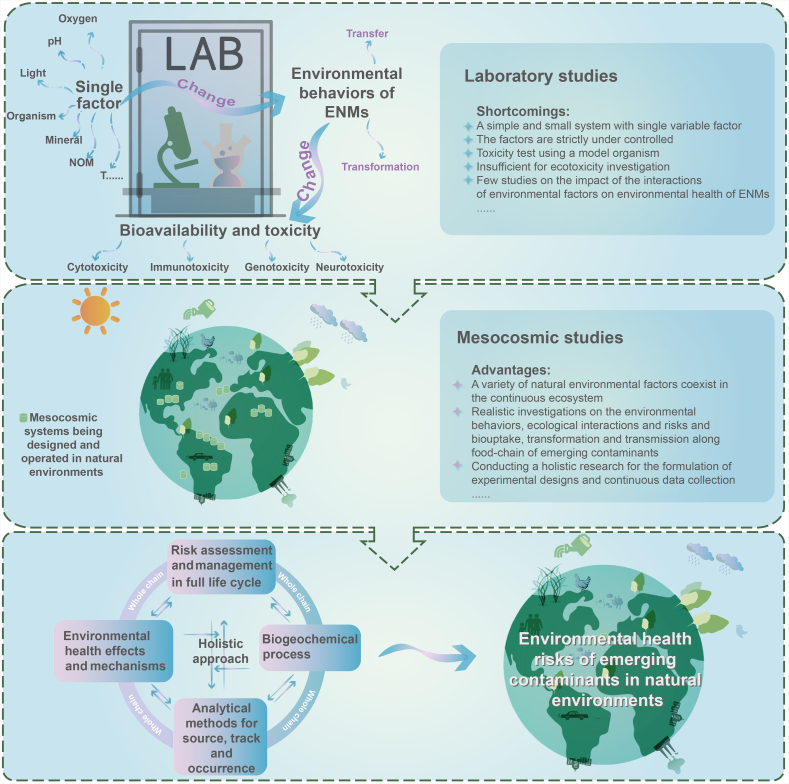
3. Implications for basic environmental health research on emerging contaminants to guide their objective assessment and scientific control
In the past 20 years, great progress has been made in the environmental health research of ENMs. Some developed pretreatment methods and detection technologies of ENMs, particularly inorganic EMMs, have been established for the qualitative and quantitative determination of ENMs in natural environments. In addition, the mechanisms underlying the transformation and transfer of ENMs in different environmental media under different conditions have been systematically explored in laboratory studies, which provides theoretical guidance for revealing the environmental behaviors and fates of ENMs in natural environments. Moreover, the mechanisms of ENMs-induced toxicity to a single organism and the transmitted toxicity along relatively simple food chains have been preliminarily clarified, which is critical for systematically understanding the environmental health effects of ENMs. Nonetheless, there are still many unresolved scientific issues in the environmental health research of ENMs. First and foremost, the determination of exposure flux and occurrence of ENMs in natural environments is still under exploration, which is one of the bottlenecks for environmental health risk identification of ENMs. Second, how would numerous data from laboratory studies guide the health risk assessment of ENMs in natural environments? Third, the environmental health effects of ENMs under the interaction of environmental factors (e.g., oxygen, light, and temperature) and genetic factors (e.g., genetic characteristics and evolution of organisms) have not yet been revealed. Therefore, research on the transmission and accumulation of ENMs along food chains and generations should be strengthened in the future. Fourth, it is well known that ENMs generally act as carriers of other coexisting pollutants or pathogens, resulting in joint toxicity to organisms. However, the corresponding mechanisms have not been elucidated. Fifth, the hazard thresholds of ENMs to organisms in natural environments are lacking, which also leads to the lack of standards for environmental health risk assessment and management of ENMs.
Environmental health research of other emerging contaminants faces the same scientific challenges as those of ENMs. For example, the majority of emerging contaminants have relatively low environmental concentrations and undergo complex transfer and transformation processes in natural environments, which leads to significant challenges in determining their occurrence and toxicity in natural environments. In addition, emerging contaminants are also accumulative and environmentally persistent, which causes their biotransmission along food chains and poses ongoing threats to environmental health and even influences biological evolution. Therefore, a lot of lessons learned from the environmental health research on ENMs can be borrowed to guide the environmental health research of other emerging contaminants. To this end, a roadmap for environmental health research on emerging contaminants is now proposed:
3.1. Methodological guarantee: developing advanced analytical methods to determine the occurrence of emerging contaminants in natural environments
One of the biggest characteristics of emerging contaminants is the low environmental relevant concentration and unclear environmental occurrence. Accurate detection of the source and occurrence of emerging contaminants in different environments provides a guarantee for the assessment of their environmental health risks and the scientific design of follow-up basic research on environmental health. It is well known that the toxicity of contaminants depends on their structural characteristics and exposure concentration and is affected by complex environmental properties. Similar to ENMs, the chemical composition of an emerging contaminant can be detected based on its specific structural characteristics. Generally, high-resolution MS or NMR technologies can be used to recognize and determine the occurrence of organic emerging contaminants. One challenge is the trace concentrations of emerging contaminants and their co-existence with other pollutants or environmental substances such as NOMs in natural environments. Therefore, it is critical to develop efficient pretreatment methods for the extraction and isolation of emerging contaminants from complex environmental media and for their enrichment to detectable concentrations. A feasible research strategy is coupling various pretreatment methods, such as structural modification, selective extraction, and functional membrane filtration of the target contaminants. This requires a comprehensive consideration of the structural and composition characteristics of the target contaminants and environmental matrix. Improving the detection accuracy of instruments is another key direction of future research on the determination of emerging contaminants. Another challenge is the absence of standards for the concerned emerging contaminants. In this case, the structure of emerging contaminants can be obtained by deconvolution and mining of mass spectral fingerprints, matching with compounds with similar structures in databases and conducting deep machine learning based on extensive experiences. If it is still difficult to use instruments to determine the concentration of emerging contaminants, effect-directed screening models incorporating environmental economics are recommended to deduce the occurrence of emerging contaminants in natural environments.
3.2. System guarantee: conducting research jointly mediated by multiple natural environmental factors and an environmentally relevant concentration of emerging contaminants
Current studies on emerging contaminants usually use high-dosage exposure while the environmental concentrations of emerging contaminants are generally low. In addition, although laboratory studies can mimic environmental conditions to a certain extent, they are limited in controlled environmental conditions, small reaction space, short-term exposure period, and simplification of the biological system. Moreover, the majority of emerging contaminants are persistent and bioaccumulative, causing ongoing ecotoxicity and toxic risks to human health. Considering the genetic evolution and ecological succession, the prediction of emerging contaminants’ toxicity is challenging. All the above also makes the related laboratory findings far from reflecting the environmental health effects of emerging contaminants in natural environments. A comprehensive and reliable research system should be established to obtain data to support the objective environmental health risk assessment of emerging contaminants.
To fill the knowledge gaps, mesocosmic model ecosystems are recommended to reveal the environmental effects of ENMs in natural environments (Fig. 6). In a mesocosmic ecosystem, a variety of natural environment factors, including natural light, environmental media, and biological components (e.g., representative biological responders), are introduced at the same time, and the complex biological processes are characterized in a long-term period to support the objective evaluation of life-scale biogeochemical behaviors, biological response, and ecological effects of emerging contaminants. In addition to overcoming the shortcomings of traditional laboratory assays, research conducted in these ecosystems also avoids the unrepeatable and uncontrollable problems of in situ field experiments. How to build a reasonable ecosystem with a self-sufficient and complete food network ensure ecosystem continuity and monitor all input and output parameters in the operating system are the priorities and the biggest challenges for constructing a mesocosmic ecosystem. Biological factors (e.g., interacting organisms among different trophic levels, including primary producer, consumer, and decomposer) and environmental parameters (e.g., dissolved oxygen, organic carbon, temperature, pH, and conductivity) are generally included in the integrated ecosystem. All parameters, including environmental behaviors of the target contaminants (e.g., exposure flux and fates, aggregation, sorption–desorption, and oxidation–reduction), activities of organisms (e.g., metabolism, predation, vitality, mortality, and evolution), and ecosystem development and stability, are monitored and recorded dynamically to comprehensively evaluate the mass flow and ecological risks of emerging contaminants.
Fig. 6.
Mesocosmic model ecosystems are recommended to be used to conduct a holistic environmental health study of emerging contaminants in natural environments. Oxygen, light, organisms, natural organic matter (NOM), minerals, temperature (T), pH, and physical forces are typical environmental factors that have been proved to change the environmental behaviors of ENMs in laboratory studies.
3.3. Theoretical guarantee: establishing a standard holistic approach for objective risk assessment and precise management of emerging contaminants in a global view
Although risk assessment research of ENMs has been carried out for more than ten years, to date, the theoretical and methodological system for ENMs environmental risk assessment has been unestablished and lacking suitable risk management, indicating that the control strategy for ENMs still needs further research.
There is no systematic environmental health risk assessment and management for emerging contaminants such as ENMs due to the lack of basic data about the source, emission flux, occurrence, biological processes, health effects, and hazard thresholds. Significant differences in exposure concentration and periods of emerging contaminants and model organisms as well as experimental factors among different studies lead to inconsistency and incomparability of results between these studies. In this case, a holistic approach (named whole-chain research methodology) is proposed (Fig. 6). Specifically, the source, track, environmental concentration, and emission flux of emerging contaminants are obtained based on the analysis of environmental samples and follow-up modeling. The results are used to guide the setting of exposure concentration, periods, and pathways during laboratory research of environmental health effects and biogeochemical processes of emerging contaminants. Dynamic changes in environmental occurrences such as concentration, speciation, and bioavailability during biogeochemical processes are captured from continuous mesocosmic studies. The results are utilized for the development of analytical technologies and for understanding varied health effects of emerging contaminants in different natural environments. Thresholds of toxicity concern determined by health effects and mechanism studies are used to assess the risk of emerging contaminants in natural environments. In return, based on risk assessment results, researchers can choose suitable indicator organisms or toxicity targets in toxicology studies. Therefore, the findings obtained from all components in the whole-chain research are verifiable and consistent, which jointly guarantee the proposal of scientific management policies and strategies. When researchers in this field use a standardized system to carry out environmental health research on emerging contaminants, the data obtained can be summarized into a big data system for comparison, which is conducive to guiding subsequent research and putting forward scientific management and control strategies for emerging contaminants from a global view. It is also worth mentioning that close-loop management of the whole life cycle of the compounds from their raw materials to products and to the disposal process is warranted to propose a practical management strategy for risk control of emerging contaminants. In this case, environmental risk assessment of emerging contaminants in different producing taches is needed, and renewing the list of emerging contaminants on time and forming a clear management protocol for each type of emerging contaminant are urgently needed.
Environmental health effects of emerging contaminants are complex as reviewed above. One of the most feasible and efficient ways to address the challenges is establishing interdisciplinary collaboration among researchers from the fields of environmental science, materials science, geochemistry, biology, and medical science. A series of comprehensive projects should be launched to deepen scientific understanding in terms of biogeochemical processes and health risks of emerging contaminants and to ensure environmental sustainability. We call for the establishment of research paradigms and benchmarking methods for emerging contaminants to provide scientific and theoretical support for the risk management and control of emerging contaminants.
The roadmap proposed in this review facilitates researchers to properly conduct environmental health research on emerging contaminants and obtain systematic and objective basic data as well as a scientifically corrected, accurate risk assessment model, thus laying a scientific and instructive foundation for the government to formulate emerging contaminants control policies.
Declaration of competing interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Zhenyu Wang reports that financial support was provided by the National Natural Science Foundation of China. Xiaona Li reports that financial support was provided by the National Natural Science Foundation of China and the Natural Science Foundation of Jiangsu Province. Baoshan Xing reports that financial support was provided by the USDA Hatch Program.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (42192572, 41820104009, and 42107244), the Natural Science Foundation of Jiangsu Province (BK20210486), and the USDA Hatch Program (MAS 00549).
References
- 1.Yassi A., Kjellstrom T., deKok T., Guidotti T. WHO/United Nations Environment Programme. UNEP/UNESCO/Council of Rectors of European Universities, Vol. 7. CRE; Geneva: 1998. Basic environmental health. Prelimimary version. [Google Scholar]
- 2.Wagener S., World Health Organization . 1993. A Report Pf a World Health Organisation Consultation on Health and Environmnet in Preparation for 2nd European Conference on Environment and Health. Helsinki 20-22 June,1994. Sofia, Bulgaria. [Google Scholar]
- 3.Joubert B.R., Mantooth S.N., McAllister K.A. Environmental health research in Africa: important progress and promising opportunities. Front. Genet. 2020;10:29. doi: 10.3389/fgene.2019.01166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pruss-Ustun A., Wolf J., Corvalan C., Neville T., Bos R., Neira M. Diseases due to unhealthy environments: an updated estimate of the global burden of disease attributable to environmental determinants of health. J. Public Health. 2017;39(3):464–475. doi: 10.1093/pubmed/fdw085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pereira L.C., de Souza A.O., Bernardes M.F.F., Pazin M., Tasso M.J., Pereira P.H., Dorta D.J. A perspective on the potential risks of emerging contaminants to human and environmental health. Environ. Sci. Pollut. Res. 2015;22(18):13800–13823. doi: 10.1007/s11356-015-4896-6. [DOI] [PubMed] [Google Scholar]
- 6.Fitzhugh O.G., Nelson A.A. The chronic oral toxicity of DDT (2,2-bis(p-chlorophenyl-1,1,1-trichloroethane) J. Pharmacol. Exp. Therapeut. 1947;89(1):18. [PubMed] [Google Scholar]
- 7.Al-Batanony M.A., Abdel-Rasul G.M., Abu-Salem M.A., Al-Dalatony M.M., Allam H.K. Occupational exposure to mercury among workers in a fluorescent lamp factory, quisna industrial zone, Egypt. Int. J. Occup. Environ. Med. 2013;4(3):149–156. [PubMed] [Google Scholar]
- 8.Bose-O’Reilly S., Bernaudat L., Siebert U., Roider G., Nowak D., Drasch G. Signs and symptoms of mercury-exposed gold miners. Int. J. Occup. Med. Environ. Health. 2017;30(2):249–269. doi: 10.13075/ijomeh.1896.00715. [DOI] [PubMed] [Google Scholar]
- 9.Zeitz P., Orr M.F., Kaye W.E. Public health consequences of mercury spills: hazardous substances emergency events surveillance system, 1993-1998. Environ. Health Perspect. 2002;110(2):129–132. doi: 10.1289/ehp.02110129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gavrilescu M., Demnerova K., Aamand J., Agathoss S., Fava F. Emerging pollutants in the environment: present and future challenges in biomonitoring, ecological risks and bioremediation. N. Biotech. 2015;32(1):147–156. doi: 10.1016/j.nbt.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Federal U.S.E.P.A., Restoration F., Ffrro R.O. PBB; 2008. Emerging Contaminants–Polybrominated Diphenyl Ethers (PBDE) and Polybrominated Biphenyls. [Google Scholar]
- 12.Zareitalabad P., Siemens J., Hamer M., Amelung W. Perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) in surface waters, sediments, soils and wastewater - a review on concentrations and distribution coefficients. Chemosphere. 2013;91(6):725–732. doi: 10.1016/j.chemosphere.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 13.Lyu Y., Brusseau M.L., Chen W., Yan N., Fu X.R., Lin X.Y. Adsorption of PFOA at the air-water Interface during transport in unsaturated porous media. Environ. Sci. Technol. 2018;52(14):7745–7753. doi: 10.1021/acs.est.8b02348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de la Cruz F., Davies J. Horizontal gene transfer and the origin of species: lessons from bacteria. Trends Microbiol. 2000;8(3):128–133. doi: 10.1016/s0966-842x(00)01703-0. [DOI] [PubMed] [Google Scholar]
- 15.McAdam P.R., Templeton K.E., Edwards G.F., Holden M.T.G., Feil E.J., Aanensen D.M., et al. Molecular tracing of the emergence, adaptation, and transmission of hospital-associated methicillin-resistant Staphylococcus aureus. Proc. Natl. Acad. Sci. U.S.A. 2012;109(23):9107–9112. doi: 10.1073/pnas.1202869109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song Y.K., Hong S.H., Eo S., Han G.M., Shim W.J. Rapid production of micro- and nanoplastics by fragmentation of expanded polystyrene exposed to sunlight. Environ. Sci. Technol. 2020;54(18):11191–11200. doi: 10.1021/acs.est.0c02288. [DOI] [PubMed] [Google Scholar]
- 17.Romera-Castillo C., Pinto M., Langer T.M., Alvarez-Salgado X.A., Herndl G.J. Dissolved organic carbon leaching from plastics stimulates microbial activity in the ocean. Nat. Commun. 2018;9:7. doi: 10.1038/s41467-018-03798-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Town R.M., van Leeuwen H.P. Uptake and release kinetics of organic contaminants associated with micro- and nanoplastic particles. Environ. Sci. Technol. 2020;54(16):10057–10067. doi: 10.1021/acs.est.0c02297. [DOI] [PubMed] [Google Scholar]
- 19.Nel A., Xia T., Madler L., Li N. Toxic potential of materials at the nanolevel. Science. 2006;311(5761):622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 20.Rocco L., Santonastaso M., Mottola F., Costagliola D., Suero T., Pacifico S., Stingo V. Genotoxicity assessment of TiO2 nanoparticles in the teleost Danio rerio. Ecotoxicol. Environ. Saf. 2015;113:223–230. doi: 10.1016/j.ecoenv.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 21.Brumfiel G. Nanotechnology: a little knowledge. Nature. 2003;424(6946):246–248. doi: 10.1038/424246a. [DOI] [PubMed] [Google Scholar]
- 22.Colvin V.L. The potential environmental impact of engineered nanomaterials. Nat. Biotechnol. 2003;21(10):1166–1170. doi: 10.1038/nbt875. [DOI] [PubMed] [Google Scholar]
- 23.Service R.F. American Chemical Society meeting: nanomaterials show signs of toxicity. Science. 2003;300(5617) doi: 10.1126/science.300.5617.243a. 243-243. [DOI] [PubMed] [Google Scholar]
- 24.Aamand J. EPA; 2010. United States Environmental Protection Agency, Emerging Contaminants-Nanomaterials. Solid Waste and Emergency Response (5106P) p. 505. F-10-008. [Google Scholar]
- 25.Solano R., Patino-Ruiz D., Tejeda-Benitez L., Herrera A. Metal- and metal/oxide-based engineered nanoparticles and nanostructures: a review on the applications, nanotoxicological effects, and risk control strategies. Environ. Sci. Pollut. Res. 2021;28(14):16962–16981. doi: 10.1007/s11356-021-12996-6. [DOI] [PubMed] [Google Scholar]
- 26.Husen A. Chapter 1–Introduction and techniques in nanomaterials formulation. Nanomater. Agric. Forest. Appl. 2020:1–14. [Google Scholar]
- 27.Valsami-Jones E., Lynch I. How safe are nanomaterials? Science. 2015;350(6259):388–389. doi: 10.1126/science.aad0768. [DOI] [PubMed] [Google Scholar]
- 28.Hochella M.F., Mogk D.W., Ranville J., Allen I.C., Luther G.W., Marr L.C., et al. Natural, incidental, and engineered nanomaterials and their impacts on the Earth system. Science. 2019;363(6434):eaau8299. doi: 10.1126/science.aau8299. [DOI] [PubMed] [Google Scholar]
- 29.Malakar A., Kanel S.R., Ray C., Snow D.D., Nadagouda M.N. Nanomaterials in the environment, human exposure pathway, and health effects: a review. Sci. Total Environ. 2021;759:19. doi: 10.1016/j.scitotenv.2020.143470. [DOI] [PubMed] [Google Scholar]
- 30.Baranowska-Wojcik E., Szwajgier D., Oleszczuk P., Winiarska-Mieczan A. Effects of titanium dioxide nanoparticles exposure on human health-a review. Biol. Trace Elem. Res. 2020;193(1):118–129. doi: 10.1007/s12011-019-01706-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Kattan A., Wichser A., Vonbank R., Brunner S., Ulrich A., Zuind S., Nowack B. Release of TiO2 from paints containing pigment-TiO2 or nano-TiO2 by weathering. Environ. Sci.-Process Impacts. 2013;15(12):2186–2193. doi: 10.1039/c3em00331k. [DOI] [PubMed] [Google Scholar]
- 32.Gajbhiye S., Sakharwade S. Silver nanoparticles in cosmetics. J. Chem. Dermatol. Sci. Appl. 2016;(1):48–53. [Google Scholar]
- 33.Saleh N., Kim H.J., Phenrat T., Wski K.M.Y., Tilton R.D., Lowry G.V. Ionic strength and composition affect the mobility of surface-modified Fe0 nanoparticles in water-saturated sand columns. Environ. Sci. Technol. 2008;42(9):3349. doi: 10.1021/es071936b. [DOI] [PubMed] [Google Scholar]
- 34.DeRosa M.C., Monreal C., Schnitzer M., Walsh R., Sultan Y. Nanotechnology in fertilizers. Nat. Nanotechnol. 2010;5(2) doi: 10.1038/nnano.2010.2. 91-91. [DOI] [PubMed] [Google Scholar]
- 35.Ghafariyan M.H., Malakouti M.J., Dadpour M.R., Stroeve P., Mahmoudi M. Effects of magnetite nanoparticles on soybean chlorophyll. Environ. Sci. Technol. 2013;47(18):10645–10652. doi: 10.1021/es402249b. [DOI] [PubMed] [Google Scholar]
- 36.Kah M., Kookana R.S., Gogos A., Bucheli T.D. A critical evaluation of nanopesticides and nanofertilizers against their conventional analogues. Nat. Nanotechnol. 2018;13(8):677–684. doi: 10.1038/s41565-018-0131-1. [DOI] [PubMed] [Google Scholar]
- 37.Taha R.A., Hassan M.M., Ibrahim E.A., Abou Baker N.H., Shaaban E.A. Carbon nanotubes impact on date palm in vitro cultures. Plant Cell Tissue Organ Cult. 2016;127(2):525–534. [Google Scholar]
- 38.Wright S.L., Kelly F.J. Plastic and human health: a micro issue? Environ. Sci. Technol. 2000;51(12):6634–6647. doi: 10.1021/acs.est.7b00423. [DOI] [PubMed] [Google Scholar]
- 39.Stubbins A., Law K.L., Munoz S.E., Bianchi T.S., Zhu L.X. Plastics in the earth system. Science. 2021;373(6550):51–55. doi: 10.1126/science.abb0354. [DOI] [PubMed] [Google Scholar]
- 40.Wagner S., Reemtsma T. Things we know and don't know about nanoplastic in the environment. Nat. Nanotechnol. 2019;14(4):300–301. doi: 10.1038/s41565-019-0424-z. [DOI] [PubMed] [Google Scholar]
- 41.Enfrin M., Lee J., Gibert Y., Basheer F., Kong L.X., Dumee L.F. Release of hazardous nanoplastic contaminants due to microplastics fragmentation under shear stress forces. J. Hazard Mater. 2020:384. doi: 10.1016/j.jhazmat.2019.121393. [DOI] [PubMed] [Google Scholar]
- 42.Yang T., Luo J.L., Nowack B. Characterization of nanoplastics, fibrils, and microplastics released during washing and abrasion of polyester textiles. Environ. Sci. Technol. 2021;55(23):15873–15881. doi: 10.1021/acs.est.1c04826. [DOI] [PubMed] [Google Scholar]
- 43.Hernandez L.M., Xu E.G., Larsson H.C.E., Tahara R., Maisuria V.B., Tufenkji N. Plastic teabags release billions of microparticles and nanoparticles into tea. Environ. Sci. Technol. 2019;53(21):12300–12310. doi: 10.1021/acs.est.9b02540. [DOI] [PubMed] [Google Scholar]
- 44.Brachner A., Fragouli D., Duarte I.F., Farias P.M.A., Dembski S., Ghosh M., et al. Assessment of human health risks posed by nano-and microplastics is currently not feasible. Int. J. Environ. Res. Publ. Health. 2020;17(23):8832. doi: 10.3390/ijerph17238832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tiwari A.J., Ashraf-Khorassani M., Marr L.C. C60 fullerenes from combustion of common fuels. Sci. Total Environ. 2016;547:254–260. doi: 10.1016/j.scitotenv.2015.12.142. [DOI] [PubMed] [Google Scholar]
- 46.Encinas D., Gómez-De-Balugera Z. Archives of Environmental Contamination & Toxicology; 2018. Fullerene C60 in Atmospheric Aerosol and its Relationship to Combustion Processes. [DOI] [PubMed] [Google Scholar]
- 47.Sanchis J., Milacic R., Zuliani T., Vidmar J., Abad E., Farre M., Barcelo D. Occurrence of C-60 and related fullerenes in the Sava River under different hydrologic conditions. Sci. Total Environ. 2018;643:1108–1116. doi: 10.1016/j.scitotenv.2018.06.285. [DOI] [PubMed] [Google Scholar]
- 48.Wagner S., Gondikas A., Neubauer E., Hofmann T., von der Kammer F. Spot the difference: engineered and natural nanoparticles in the environment-release, behavior, and fate. Angew. Chem.-Int. Edit. 2014;53(46):12398–12419. doi: 10.1002/anie.201405050. [DOI] [PubMed] [Google Scholar]
- 49.Zhou H.L., Jin W.Q. Membranes with Intrinsic micro-porosity: structure, solubility, and applications. Membranes. 2019;9(1):28. doi: 10.3390/membranes9010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou X.X., Liu R., Liu J.F. Rapid chromatographic separation of dissoluble Ag(I) and silver-containing nanoparticles of 1-100 nanometer in antibacterial products and environmental waters. Environ. Sci. Technol. 2014;48(24):14516–14524. doi: 10.1021/es504088e. [DOI] [PubMed] [Google Scholar]
- 51.Yi Z.B., Loosli F., Wang J.J., Berti D., Baalousha M. How to distinguish natural versus engineered nanomaterials: insights from the analysis of TiO2 and CeO2 in soils. Environ. Chem. Lett. 2020;18(1):215–227. [Google Scholar]
- 52.Yang X.Z., Liu X., Zhang A.Q., Lu D.W., Li G., Zhang Q.H., et al. Distinguishing the sources of silica nanoparticles by dual isotopic fingerprinting and machine learning. Nat. Commun. 2019;10:9. doi: 10.1038/s41467-019-09629-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou X., Liu J. Research advances in separation and determination of metallic nanomaterials in the environment. Chin. Sci. Bull. 2017;62(24):2758–2769. [Google Scholar]
- 54.Ivleva N.P. Chemical analysis of microplastics and nanoplastics: challenges, advanced methods, and perspectives. Chem. Rev. 2021;121(19):11886–11936. doi: 10.1021/acs.chemrev.1c00178. [DOI] [PubMed] [Google Scholar]
- 55.Liu L.H., He B., Liu Q., Yun Z.J., Yan X.T., Long Y.M., Jiang G.B. Identification and accurate size characterization of nanoparticles in complex media. Angew. Chem.-Int. Edit. 2014;53(52):14476–14479. doi: 10.1002/anie.201408927. [DOI] [PubMed] [Google Scholar]
- 56.Gray E.P., Coleman J.G., Bednar A.J., Kennedy A.J., Ranville J.F., Higgins C.P. Extraction and analysis of silver and gold nanoparticles from biological tissues using single particle inductively coupled plasma mass spectrometry. Environ. Sci. Technol. 2013;47(24):14315–14323. doi: 10.1021/es403558c. [DOI] [PubMed] [Google Scholar]
- 57.Zhou X.X., Hao L.T., Wang H.Y.Z., Li Y.J., Liu J.F. Cloud-point extraction combined with thermal degradation for nanoplastic analysis using pyrolysis gas chromatography-mass spectrometry. Anal. Chem. 2019;91(3):1785–1790. doi: 10.1021/acs.analchem.8b04729. [DOI] [PubMed] [Google Scholar]
- 58.Zhou X.X., Lai Y.J., Liu R., Li S.S., Xu J.W., Liu J.F. Polyvinylidene fluoride micropore membranes as solid-phase extraction disk for preconcentration of nanoparticulate silver in environmental waters. Environ. Sci. Technol. 2017;51(23):13816–13824. doi: 10.1021/acs.est.7b04055. [DOI] [PubMed] [Google Scholar]
- 59.Zhou X.X., Liu J.F., Jiang G.B. Elemental mass size distribution for characterization, quantification and identification of trace nanoparticles in serum and environmental waters. Environ. Sci. Technol. 2017;51(7):3892–3901. doi: 10.1021/acs.est.6b05539. [DOI] [PubMed] [Google Scholar]
- 60.Soto-Alvaredo J., Montes-Bayon M., Bettmer J. Speciation of silver nanoparticles and silver(I) by reversed-phase liquid chromatography coupled to ICPMS. Anal. Chem. 2013;85(3):1316–1321. doi: 10.1021/ac302851d. [DOI] [PubMed] [Google Scholar]
- 61.Franze B., Engelhard C. Fast separation, characterization, and speciation of gold and silver nanoparticles and their ionic counterparts with micellar electrokinetic chromatography coupled to ICP-MS. Anal. Chem. 2014;86(12):5713–5720. doi: 10.1021/ac403998e. [DOI] [PubMed] [Google Scholar]
- 62.Li Q.C., Lai Y.J., Yu S.J., Li P., Zhou X.X., Dong L.J., et al. Sequential isolation of microplastics and nanoplastics in environmental waters by membrane filtration, followed by cloud-point extraction. Anal. Chem. 2021;93(10):4559–4566. doi: 10.1021/acs.analchem.0c04996. [DOI] [PubMed] [Google Scholar]
- 63.Wigger H., Kagi R., Wiesner M., Nowack B. Exposure and possible risks of engineered nanomaterials in the environment-current knowledge and directions for the future. Rev. Geophys. 2020;58(4):25. [Google Scholar]
- 64.Baalousha M., Cornelis G., Kuhlbusch T.A.J., Lynch I., Nickel C., Peijnenburg W., et al. Modeling nanomaterial fate and uptake in the environment: current knowledge and future trends. Environ. Sci.-Nano. 2016;3(2):323–345. [Google Scholar]
- 65.Kooi M.B.,E., Kroeze C., van Wezel A.P., Koelmans A.A. In: Freshwater Microplastics : Emerging Environmental Contaminants? Wagner M., Lambert S., editors. Springer International Publishing; Cham: 2018. Modeling the fate and transport of plastic debris in freshwaters: review and guidance; pp. 125–152. [Google Scholar]
- 66.Svendsen C., Walker L.A., Matzke M., Lahive E., Harrison S., Crossley A., et al. Key principles and operational practices for improved nanotechnology environmental exposure assessment. Nat. Nanotechnol. 2020;15(9):731–742. doi: 10.1038/s41565-020-0742-1. [DOI] [PubMed] [Google Scholar]
- 67.Abbas Q., Yousaf B., Amina, Ali M.U., Munir M.A.M., El-Naggar A., et al. Transformation pathways and fate of engineered nanoparticles (ENPs) in distinct interactive environmental compartments: a review. Environ. Int. 2020;138:18. doi: 10.1016/j.envint.2020.105646. [DOI] [PubMed] [Google Scholar]
- 68.Shi Q.Y., Tang J.C., Liu R.T., Wang L. Toxicity in vitro reveals potential impacts of microplastics and nanoplastics on human health: a review. Crit. Rev. Environ. Sci. Technol. 2021;33 [Google Scholar]
- 69.Materic D., Ludewig E., Brunner D., Rockmann T., Holzinger R. Nanoplastics transport to the remote, high-altitude Alps. Environ. Pollut. 2021;288:11. doi: 10.1016/j.envpol.2021.117697. [DOI] [PubMed] [Google Scholar]
- 70.Philippe A., Schaumann G.E. Interactions of dissolved organic matter with natural and engineered inorganic colloids: a review. Environ. Sci. Technol. 2014;48(16):8946–8962. doi: 10.1021/es502342r. [DOI] [PubMed] [Google Scholar]
- 71.Lin D.H., Tian X.L., Wu F.C., Xing B.S. Fate and transport of engineered nanomaterials in the environment. J. Environ. Qual. 2010;39(6):1896–1908. doi: 10.2134/jeq2009.0423. [DOI] [PubMed] [Google Scholar]
- 72.Tourinho P.S., van Gestel C.A.M., Lofts S., Svendsen C., Soares A., Loureiro S. Metal-based nanoparticles in soil: fate, behavior, and effects on soil invertebrates. Environ. Toxicol. Chem. 2012;31(8):1679–1692. doi: 10.1002/etc.1880. [DOI] [PubMed] [Google Scholar]
- 73.Dinesh R., Anandaraj M., Srinivasan V., Hamza S. Engineered nanoparticles in the soil and their potential implications to microbial activity. Geoderma. 2012;173:19–27. [Google Scholar]
- 74.Pachapur V.L., Larios A.D., Cledon M., Brar S.K., Verma M., Surampalli R.Y. Behavior and characterization of titanium dioxide and silver nanoparticles in soils. Sci. Total Environ. 2016;563:933–943. doi: 10.1016/j.scitotenv.2015.11.090. [DOI] [PubMed] [Google Scholar]
- 75.Birk S., Chapman D., Carvalho L., Spears B.M., Andersen H.E., Argillier C., et al. Impacts of multiple stressors on freshwater biota across spatial scales and ecosystems. Nat. Ecol. Evol. 2020;4(8):1060–1068. doi: 10.1038/s41559-020-1216-4. [DOI] [PubMed] [Google Scholar]
- 76.Schlich K., Hund-Rinke K. Influence of soil properties on the effect of silver nanomaterials on microbial activity in five soils. Environ. Pollut. 2015;196:321–330. doi: 10.1016/j.envpol.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 77.Degenkolb L., Kaupenjohann M., Klitzke S. The variable fate of Ag and TiO2 nanoparticles in natural soil SolutionsSorption of organic matter and nanoparticle stability. Water Air Soil Pollut. 2019;230(3):14. [Google Scholar]
- 78.Gutierrez L., Schmid A., Zaouri N., Garces D., Croue J.P. Colloidal stability of capped silver nanoparticles in natural organic matter-containing electrolyte solutions. NanoImpact. 2020;19:9. [Google Scholar]
- 79.Furtado L.M., Norman B.C., Xenopoulos M.A., Frost P.C., Metcalfe C.D., Hintelmann H. Environmental fate of silver nanoparticles in boreal lake ecosystems. Environ. Sci. Technol. 2015;49(14):8441–8450. doi: 10.1021/acs.est.5b01116. [DOI] [PubMed] [Google Scholar]
- 80.Peralta-Videa J.R., Zhao L.J., Lopez-Moreno M.L., de la Rosa G., Hong J., Gardea-Torresdey J.L. Nanomaterials and the environment: a review for the biennium 2008-2010. J. Hazard Mater. 2011;186(1):1–15. doi: 10.1016/j.jhazmat.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 81.Wu X.L., Lyu X.Y., Li Z.Y., Gao B., Zeng X.K., Wu J.C., et al. Transport of polystyrene nanoplastics in natural soils: effect of soil properties, ionic strength and cation type. Sci. Total Environ. 2020;707:7. doi: 10.1016/j.scitotenv.2019.136065. [DOI] [PubMed] [Google Scholar]
- 82.Morimoto Y., Kobayashi N., Shinohara N., Myojo T., Tanaka I., Nakanshi J. Hazard assessments of manufactured nanomaterials. J. Occup. Health. 2010;52(6):325–334. doi: 10.1539/joh.r10003. [DOI] [PubMed] [Google Scholar]
- 83.Sun X.D., Yuan X.Z., Jia Y.B., Feng L.J., Zhu F.P., Dong S.S., et al. Differentially charged nanoplastics demonstrate distinct accumulation inArabidopsis thaliana. Nat. Nanotechnol. 2020;15(9):755–+. doi: 10.1038/s41565-020-0707-4. [DOI] [PubMed] [Google Scholar]
- 84.Gwinn M.R., Vallyathan V. Nanoparticles: health effects - pros and cons. Environ. Health Perspect. 2006;114(12):1818–1825. doi: 10.1289/ehp.8871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ge Y.G., Schimel J.P., Holden P.A. Evidence for negative effects of TiO2 and ZnO nanoparticles on soil bacterial communities. Environ. Sci. Technol. 2011;45(4):1659–1664. doi: 10.1021/es103040t. [DOI] [PubMed] [Google Scholar]
- 86.Calder A.J., Dimkpa C.O., McLean J.E., Britt D.W., Johnson W., Anderson A.J. Soil components mitigate the antimicrobial effects of silver nanoparticles towards a beneficial soil bacterium, Pseudomonas chlororaphis O6. Sci. Total Environ. 2012;429:215–222. doi: 10.1016/j.scitotenv.2012.04.049. [DOI] [PubMed] [Google Scholar]
- 87.Diao J.J., Hua D., Lin J.Q., Teng H.H., Chen D.C. Nanoparticle delivery by controlled bacteria. J. Nanosci. Nanotechnol. 2005;5(10):1749–1751. doi: 10.1166/jnn.2005.168. [DOI] [PubMed] [Google Scholar]
- 88.Chae Y., An Y.J. Current research trends on plastic pollution and ecological impacts on the soil ecosystem: a review. Environ. Pollut. 2018;240:387–395. doi: 10.1016/j.envpol.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 89.Heinze W.M., Mitrano D.M., Lahive E., Koestel J., Cornelis G. Nanoplastic transport in soil via bioturbation by Lumbricus terrestris. Environ. Sci. Technol. 2021;55(24):16423–16433. doi: 10.1021/acs.est.1c05614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kwak J.I., An Y.J. Microplastic digestion generates fragmented nanoplastics in soils and damages earthworm spermatogenesis and coelomocyte viability. J. Hazard Mater. 2021;402:8. doi: 10.1016/j.jhazmat.2020.124034. [DOI] [PubMed] [Google Scholar]
- 91.Zhang D.G., Chen Y.X., Ma Y.H., Guo L., Sun J.Y., Tong J. Earthworm epidermal mucus: rheological behavior reveals drag-reducing characteristics in soil. Soil Tillage Res. 2016;158:57–66. [Google Scholar]
- 92.Li J., Song Y., Cai Y.B. Focus topics on microplastics in soil: analytical methods, occurrence, transport, and ecological risks. Environ. Pollut. 2020;257:12. doi: 10.1016/j.envpol.2019.113570. [DOI] [PubMed] [Google Scholar]
- 93.Li C.J., Gao Y., He S., Chi H.Y., Li Z.C., Zhou X.X., Yan B. Quantification of nanoplastic uptake in cucumber plants by pyrolysis gas chromatography/mass spectrometry. Environ. Sci. Technol. Lett. 2021;8(8):633–638. [Google Scholar]
- 94.Hua Z.L., Tang Z.Q., Bai X., Zhang J.A., Yu L., Cheng H.M. Aggregation and resuspension of graphene oxide in simulated natural surface aquatic environments. Environ. Pollut. 2015;205:161–169. doi: 10.1016/j.envpol.2015.05.039. [DOI] [PubMed] [Google Scholar]
- 95.Lv B.W., Wang C., Hou J., Wang P.F., Miao L.Z., Xing B.S. Development of a comprehensive understanding of aggregation settling movement of CeO2 nanoparticles in natural waters. Environ. Pollut. 2020;257:11. doi: 10.1016/j.envpol.2019.113584. [DOI] [PubMed] [Google Scholar]
- 96.Cai L., Hu L.L., Shi H.H., Ye J.W., Zhang Y.F., Kim H. Effects of inorganic ions and natural organic matter on the aggregation of nanoplastics. Chemosphere. 2018;197:142–151. doi: 10.1016/j.chemosphere.2018.01.052. [DOI] [PubMed] [Google Scholar]
- 97.Zhao J., Liu F.F., Wang Z.Y., Cao X.S., Xing B.S. Heteroaggregation of graphene oxide with minerals in aqueous phase. Environ. Sci. Technol. 2015;49(5):2849–2857. doi: 10.1021/es505605w. [DOI] [PubMed] [Google Scholar]
- 98.Sotirelis N.P., Chrysikopoulos C.V. Heteroaggregation of graphene oxide nanoparticles and kaolinite colloids. Sci. Total Environ. 2017;579:736–744. doi: 10.1016/j.scitotenv.2016.11.034. [DOI] [PubMed] [Google Scholar]
- 99.Singh N., Tiwari E., Khandelwal N., Darbha G.K. Understanding the stability of nanoplastics in aqueous environments: effect of ionic strength, temperature, dissolved organic matter, clay, and heavy metals. Environ. Sci.-Nano. 2019;6(10):2968–2976. [Google Scholar]
- 100.Rong H., Garg S., Waite T.D. Impact of light and Suwanee River fulvic acid on O2 and H2O2 mediated oxidation of silver nanoparticles in simulated natural waters. Environ. Sci. Technol. 2019;53(12):6688–6698. doi: 10.1021/acs.est.8b07079. [DOI] [PubMed] [Google Scholar]
- 101.Dong S.N., Cai W.W., Xia J.H., Sheng L.T., Wang W.M., Liu H. Aggregation kinetics of fragmental PET nanoplastics in aqueous environment: complex roles of electrolytes, pH and humic acid. Environ. Pollut. 2021;268:9. doi: 10.1016/j.envpol.2020.115828. [DOI] [PubMed] [Google Scholar]
- 102.Li J., Ghoshal S. Comparison of the transport of the aggregates of nanoscale zerovalent iron under vertical and horizontal flow. Chemosphere. 2016;144:1398–1407. doi: 10.1016/j.chemosphere.2015.09.103. [DOI] [PubMed] [Google Scholar]
- 103.Williams R.J., Harrison S., Keller V., Kuenen J., Lofts S., Praetorius A., et al. Models for assessing engineered nanomaterial fate and behaviour in the aquatic environment. Curr. Opin. Environ. Sustain. 2019;36:105–115. [Google Scholar]
- 104.Wheeler K.E., Chetwynd A.J., Fahy K.M., Hong B.S., Tochihuitl J.A., Foster L.A., et al. Environmental dimensions of the protein corona. Nat. Nanotechnol. 2021;16(6):617–629. doi: 10.1038/s41565-021-00924-1. [DOI] [PubMed] [Google Scholar]
- 105.Spagnoletti N.F., Kronberg F., Spedalieri C., Munarriz E., Giacometti R. Protein corona on biogenic silver nanoparticles provides higher stability and protects cells from toxicity in comparison to chemical nanoparticles. J. Environ. Manag. 2021;297:12. doi: 10.1016/j.jenvman.2021.113434. [DOI] [PubMed] [Google Scholar]
- 106.Dong Z.Q., Hou Y.Z., Han W.H., Liu M.P., Wang J.L., Qiu Y.P. Protein corona-mediated transport of nanoplastics in seawater-saturated porous media. Water Res. 2020;182:9. doi: 10.1016/j.watres.2020.115978. [DOI] [PubMed] [Google Scholar]
- 107.Wang Z.Y., Yin L.Y., Zhao J., Xing B.S. Trophic transfer and accumulation of TiO2 nanoparticles from clamworm (Perinereis aibuhitensis) to juvenile turbot (Scophthalmus maximus) along a marine benthic food chain. Water Res. 2016;95:250–259. doi: 10.1016/j.watres.2016.03.027. [DOI] [PubMed] [Google Scholar]
- 108.Zhu X.S., Wang J.X., Zhang X.Z., Chang Y., Chen Y.S. Trophic transfer of TiO2 nanoparticles from daphnia to zebrafish in a simplified freshwater food chain. Chemosphere. 2010;79(9):928–933. doi: 10.1016/j.chemosphere.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 109.Dong S.P., Xia T., Yang Y., Lin S.J., Mao L. Bioaccumulation of C-14-Labeled graphene in an aquatic food chain through direct uptake or trophic transfer. Environ. Sci. Technol. 2018;52(2):541–549. doi: 10.1021/acs.est.7b04339. [DOI] [PubMed] [Google Scholar]
- 110.Mortimer M., Petersen E.J., Buchholz B.A., Orias E., Holden P.A. Bioaccumulation of multiwall carbon nanotubes in Tetrahymena thermophila by direct feeding or trophic transfer. Environ. Sci. Technol. 2016;50(16):8876–8885. doi: 10.1021/acs.est.6b01916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mader S.S. fifth ed. Wm. C. Brown Publishers; Dubuque, IA, USA: 1996. Biology. [Google Scholar]
- 112.Odzak N., Kistler D., Sigg L. Influence of daylight on the fate of silver and zinc oxide nanoparticles in natural aquatic environments. Environ. Pollut. 2017;226:1–11. doi: 10.1016/j.envpol.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 113.Mulenos M.R., Liu J.Q., Lujan H., Guo B.L., Lichtfouse E., Sharma V.K., et al. Copper, silver, and titania nanoparticles do not release ions under anoxic conditions and release only minute ion levels under oxic conditions in water: evidence for the low toxicity of nanoparticles. Environ. Chem. Lett. 2020;18(4):1319–1328. [Google Scholar]
- 114.Zhao J., Wang X.J., Hoang S.A., Bolan N.S., Kirkham M.B., Liu J.N., et al. Silver nanoparticles in aquatic sediments: occurrence, chemical transformations, toxicity, and analytical methods. J. Hazard Mater. 2021;418:15. doi: 10.1016/j.jhazmat.2021.126368. [DOI] [PubMed] [Google Scholar]
- 115.Peng C., Xu C., Liu Q.L., Sun L.J., Luo Y.M., Shi J.Y. Fate and transformation of CuO nanoparticles in the soil-rice system during the life cycle of rice plants. Environ. Sci. Technol. 2017;51(9):4907–4917. doi: 10.1021/acs.est.6b05882. [DOI] [PubMed] [Google Scholar]
- 116.Zhao J., Lin M.Q., Wang Z.Y., Cao X.S., Xing B.S. Engineered nanomaterials in the environment: are they safe? Crit. Rev. Environ. Sci. Technol. 2021;51(14):1443–1478. [Google Scholar]
- 117.Dong B., Liu G.F., Zhou J.T., Wang J., Jin R.F. Transformation of silver ions to silver nanoparticles mediated by humic acid under dark conditions at ambient temperature. J. Hazard Mater. 2020;383:9. doi: 10.1016/j.jhazmat.2019.121190. [DOI] [PubMed] [Google Scholar]
- 118.Zhang W.C., Ke S., Sun C.Y., Xu X., Chen J.B., Yao L.G. Fate and toxicity of silver nanoparticles in freshwater from laboratory to realistic environments: a review. Environ. Sci. Pollut. Res. 2019;26(8):7390–7404. doi: 10.1007/s11356-019-04150-0. [DOI] [PubMed] [Google Scholar]
- 119.Sangani M.F., Owens G., Fotovat A. Transport of engineered nanoparticles in soils and aquifers. Environ. Rev. 2019;27(1):43–70. [Google Scholar]
- 120.Schreiner K.M., Filley T.R., Blanchette R.A., Bowen B.B., Bolskar R.D., Hockaday W.C., et al. White-rot basidiomycete-mediated decomposition of C60 fullerol. Environ. Sci. Technol. 2009;43(9):3162–3168. doi: 10.1021/es801873q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Du J.J., Qv W.R., Niu Y.L., Qv M.X., Jin K., Xie J.Y., et al. Nanoplastic pollution inhibits stream leaf decomposition through modulating microbial metabolic activity and fungal community structure. J. Hazard Mater. 2022;424:9. doi: 10.1016/j.jhazmat.2021.127392. [DOI] [PubMed] [Google Scholar]
- 122.Miclaus T., Beer C., Chevallier J., Scavenius C., Bochenkov V.E., Enghild J.J., et al. Dynamic protein coronas revealed as a modulator of silver nanoparticle sulphidation in vitro. Nat. Commun. 2016;7:10. doi: 10.1038/ncomms11770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yan N., Tang B.Z., Wang W.X. Vivo bioimaging of silver nanoparticle dissolution in the gut environment of zooplankton. ACS Nano. 2018;12(12):12212–12223. doi: 10.1021/acsnano.8b06003. [DOI] [PubMed] [Google Scholar]
- 124.Avellan A., Simonin M., McGivney E., Bossa N., Spielman-Sun E., Rocca J.D., et al. Gold nanoparticle biodissolution by a freshwater macrophyte and its associated microbiome. Nat. Nanotechnol. 2018;13(11):1072–1077. doi: 10.1038/s41565-018-0231-y. [DOI] [PubMed] [Google Scholar]
- 125.Desmau M., Carboni A., Le Bars M., Doelsch E., Benedetti M.F., Auffan M., et al. How microbial biofilms control the environmental fate of engineered nanoparticles? Front. Environ. Sci. 2020;8:20. [Google Scholar]
- 126.Zhao J., Ren W.T., Dai Y.H., Liu L.J., Wang Z.Y., Yu X.Y., et al. Uptake, distribution, and transformation of CuO NPs in a floating plant Eichhornia crassipes and related stomatal responses. Environ. Sci. Technol. 2017;51(13):7686–7695. doi: 10.1021/acs.est.7b01602. [DOI] [PubMed] [Google Scholar]
- 127.Giles E.L., Kuznesof S., Clark B., Hubbard C., Frewer L.J. Consumer acceptance of and willingness to pay for food nanotechnology: a systematic review. J. Nanoparticle Res. 2015;17(12) doi: 10.1007/s11051-015-3270-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sharifi S., Behzadi S., Laurent S., Forrest M.L., Stroeve P., Mahmoudi M. Toxicity of nanomaterials. Chem. Soc. Rev. 2012;41(6):2323–2343. doi: 10.1039/c1cs15188f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yan N., Wang W.X. Novel Imaging of silver nanoparticle uptake by a unicellular alga and trophic transfer to Daphnia magna. Environ. Sci. Technol. 2021;55(8):5143–5151. doi: 10.1021/acs.est.0c08588. [DOI] [PubMed] [Google Scholar]
- 130.Rai P.K., Lee J., Brown R.J.C., Kim K.H. Micro- and nano-plastic pollution: behavior, microbial ecology, and remediation technologies. J. Clean. Prod. 2021;291:22. [Google Scholar]
- 131.Souza J.P., Venturini F.P., Santos F., Zucolotto V. Chronic toxicity in Ceriodaphnia dubia induced by graphene oxide. Chemosphere. 2018;190:218–224. doi: 10.1016/j.chemosphere.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 132.Souza J.P., Mansano A.S., Venturini F.P., Santos F., Zucolotto V. Antioxidant metabolism of zebrafish after sub-lethal exposure to graphene oxide and recovery. Fish Physiol. Biochem. 2019;45(4):1289–1297. doi: 10.1007/s10695-019-00678-7. [DOI] [PubMed] [Google Scholar]
- 133.de Medeiros A.M.Z., Coa F., Alves O.L., Martinez D.S.T., Barbieri E. Metabolic effects in the freshwater fish Geophagus iporangensis in response to single and combined exposure to graphene oxide and trace elements. Chemosphere. 2020;243:8. doi: 10.1016/j.chemosphere.2019.125316. [DOI] [PubMed] [Google Scholar]
- 134.Li Z.Q., Hu M.H., Song H.T., Lin D.H., Wang Y.J. Toxic effects of nano-TiO2 in bivalves-A synthesis of meta-analysis and bibliometric analysis. J. Environ. Sci. 2021;104:188–203. doi: 10.1016/j.jes.2020.11.013. [DOI] [PubMed] [Google Scholar]
- 135.Valdiglesias V., Kilic G., Costa C., Fernandez-Bertolez N., Pasaro E., Teixeira J.P., et al. Effects of iron oxide nanoparticles: cytotoxicity, genotoxicity, developmental toxicity, and neurotoxicity. Environ. Mol. Mutagen. 2015;56(2):125–148. doi: 10.1002/em.21909. [DOI] [PubMed] [Google Scholar]
- 136.Gallo A., Manfra L., Boni R., Rotini A., Migliore L., Tosti E. Cytotoxicity and genotoxicity of CuO nanoparticles in sea urchin spermatozoa through oxidative stress. Environ. Int. 2018;118:325–333. doi: 10.1016/j.envint.2018.05.034. [DOI] [PubMed] [Google Scholar]
- 137.Baharlooeian M., Kerdgari M., Shimada Y. Ecotoxicological effects of TiO2 nanoparticulates and bulk Ti on microalgae Chaetoceros muelleri. Environ. Technol. Innovat. 2021;23:12. [Google Scholar]
- 138.Chen G.C., Vijver M.G., Peijnenburg W. Summary and analysis of the currently existing literature data on metal-based nanoparticles published for selected aquatic organisms: applicability for toxicity prediction by (Q)SARs. ATLA, Altern. Lab. Anim. 2015;43(4):221–240. doi: 10.1177/026119291504300404. [DOI] [PubMed] [Google Scholar]
- 139.Paital B., Guru D., Mohapatra P., Panda B., Panda N., Rath S., et al. Ecotoxic impact assessment of graphene oxide on lipid peroxidation at mitochondrial level and redox modulation in fresh water fish Anabas testudineus. Chemosphere. 2019;224:796–804. doi: 10.1016/j.chemosphere.2019.02.156. [DOI] [PubMed] [Google Scholar]
- 140.Yausheva E., Sizova E., Lebedev S., Skalny A., Miroshnikov S., Plotnikov A., et al. Influence of zinc nanoparticles on survival of worms Eisenia fetida and taxonomic diversity of the gut microflora. Environ. Sci. Pollut. Res. 2016;23(13):13245–13254. doi: 10.1007/s11356-016-6474-y. [DOI] [PubMed] [Google Scholar]
- 141.Tang Y., Zhou W.S., Sun S.G., Du X.Y., Han Y., Shi W., Liu G.X. Immunotoxicity and neurotoxicity of bisphenol A and microplastics alone or in combination to a bivalve species, Tegillarca granosa. Environ. Pollut. 2020;265:9. doi: 10.1016/j.envpol.2020.115115. [DOI] [PubMed] [Google Scholar]
- 142.Lu L., Wan Z.Q., Luo T., Fu Z.W., Jin Y.X. Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice. Sci. Total Environ. 2018;631–632:449–458. doi: 10.1016/j.scitotenv.2018.03.051. [DOI] [PubMed] [Google Scholar]
- 143.Mattsson K., Hansson L.A., Cedervall T. Nano-plastics in the aquatic environment. Environ. Sci.-Process Impacts. 2015;17(10):1712–1721. doi: 10.1039/c5em00227c. [DOI] [PubMed] [Google Scholar]
- 144.Prata J.C., da Costa J.P., Lopes I., Duarte A.C., Rocha-Santos T. Environmental exposure to microplastics: an overview on possible human health effects. Sci. Total Environ. 2020;702:9. doi: 10.1016/j.scitotenv.2019.134455. [DOI] [PubMed] [Google Scholar]
- 145.Liu Y.L., Xiao Z.G., Chen F.R., Yue L., Zou H., Lyu J.Z., et al. Metallic oxide nanomaterials act as antioxidant nanozymes in higher plants: trends, meta-analysis, and prospect. Sci. Total Environ. 2021;780:14. doi: 10.1016/j.scitotenv.2021.146578. [DOI] [PubMed] [Google Scholar]
- 146.Ghosh M., Bandyopadhyay M., Mukherjee A. Genotoxicity of titanium dioxide (TiO2) nanoparticles at two trophic levels Plant and human lymphocytes. Chemosphere. 2010;81(10):1253–1262. doi: 10.1016/j.chemosphere.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 147.Lu T., Zhang Q., Zhang Z.Y., Hu B.L., Chen J.M., Chen J., Qian H.F. Pollutant toxicology with respect to microalgae and cyanobacteria. J. Environ. Sci. 2021;99:175–186. doi: 10.1016/j.jes.2020.06.033. [DOI] [PubMed] [Google Scholar]
- 148.Baptista M.S., Miller R.J., Halewood E.R., Hanna S.K., Almeida C.M.R., Vasconcelos V.M., et al. Impacts of silver nanoparticles on a natural estuarine plankton community. Environ. Sci. Technol. 2015;49(21):12968–12974. doi: 10.1021/acs.est.5b03285. [DOI] [PubMed] [Google Scholar]
- 149.Agathokleous E., Feng Z.Z., Iavicoli I., Calabrese E.J. The two faces of nanomaterials: a quantification of hormesis in algae and plants. Environ. Int. 2019;131:10. doi: 10.1016/j.envint.2019.105044. [DOI] [PubMed] [Google Scholar]
- 150.Latef A., Srivastava A.K., Abd El-Sadek M.S., Kordrostami M., Tran L.S.P. Titanium dioxide nanoparticles improve growth and enhance tolerance of broad bean plants under saline soil conditions. Land Degrad. Dev. 2018;29(4):1065–1073. [Google Scholar]
- 151.Ringwood A.H., Levi-Polyachenko N., Carroll D.L. Fullerene exposures with Oysters: embryonic, adult, and cellular responses. Environ. Sci. Technol. 2009;43(18):7136–7141. doi: 10.1021/es900621j. [DOI] [PubMed] [Google Scholar]
- 152.Gottschalk F., Sun T.Y., Nowack B. Environmental concentrations of engineered nanomaterials: review of modeling and analytical studies. Environ. Pollut. 2013;181:287–300. doi: 10.1016/j.envpol.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 153.Liu Y., Nie Y.G., Wang J.J., Wang J., Wang X., Chen S.P., et al. Mechanisms involved in the impact of engineered nanomaterials on the joint toxicity with environmental pollutants. Ecotoxicol. Environ. Saf. 2018;162:92–102. doi: 10.1016/j.ecoenv.2018.06.079. [DOI] [PubMed] [Google Scholar]
- 154.Limbach L.K., Wick P., Manser P., Grass R.N., Bruinink A., Stark W.J. Exposure of engineered nanoparticles to human lung epithelial cells: influence of chemical composition and catalytic activity on oxidative stress. Environ. Sci. Technol. 2007;41(11):4158–4163. doi: 10.1021/es062629t. [DOI] [PubMed] [Google Scholar]
- 155.Cui X.J., Wan B., Guo L.H., Yang Y., Ren X.M. Insight into the mechanisms of combined toxicity of single-walled carbon nanotubes and nickel ions in macrophages: role of P2X(7) receptor. Environ. Sci. Technol. 2016;50(22):12473–12483. doi: 10.1021/acs.est.6b03842. [DOI] [PubMed] [Google Scholar]
- 156.Moussa H., Merlin C., Dezanet C., Balan L., Medjandi G., Ben-Attia M., et al. Trace amounts of Cu2+ ions influence ROS production and cytotoxicity of ZnO quantum dots. J. Hazard Mater. 2016;304:532–542. doi: 10.1016/j.jhazmat.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 157.Duan G.L., Zhu Y.G., Tong Y.P., Cai C., Kneer R. Characterization of arsenate reductase in the extract of roots and fronds of Chinese brake fern, an arsenic hyperaccumulator. Plant Physiol. 2005;138(1):461–469. doi: 10.1104/pp.104.057422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Liu S., Jiang W., Wu B., Yu J., Yu H.Y., Zhang X.X., et al. Low levels of graphene and graphene oxide inhibit cellular xenobiotic defense system mediated by efflux transporters. Nanotoxicology. 2016;10(5):597–606. doi: 10.3109/17435390.2015.1104739. [DOI] [PubMed] [Google Scholar]
- 159.Ferreira J.L.R., Lonne M.N., Franca T.A., Maximilla N.R., Lugokenski T.H., Costa P.G., et al. Co-exposure of the organic nanomaterial fullerene C-60 with benzo a pyrene in Danio rerio (zebrafish) hepatocytes: evidence of toxicological interactions. Aquat. Toxicol. 2014;147:76–83. doi: 10.1016/j.aquatox.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 160.Park J.W., Henry T.B., Menn F.M., Compton R.N., Sayler G. No bioavailability of 17 alpha-ethinylestradiol when associated with nC(60) aggregates during dietary exposure in adult male zebrafish (Danio rerio) Chemosphere. 2010;81(10):1227–1232. doi: 10.1016/j.chemosphere.2010.09.036. [DOI] [PubMed] [Google Scholar]
- 161.Liu Y., Wang X.N., Wang J., Nie Y.G., Du H., Dai H., et al. Graphene oxide attenuates the cytotoxicity and mutagenicity of PCB 52 via activation of genuine autophagy. Environ. Sci. Technol. 2016;50(6):3154–3164. doi: 10.1021/acs.est.5b03895. [DOI] [PubMed] [Google Scholar]
- 162.Xie Y., Wang B.B., Li F.C., Ma L., Ni M., Shen W.D., et al. Molecular mechanisms of reduced nerve toxicity by titanium dioxide nanoparticles in the phoxim-exposed brain of Bombyx mori. PLoS One. 2014;9(6) doi: 10.1371/journal.pone.0101062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Amde M., Liu J.F., Tan Z.Q., Bekana D. Transformation and bioavailability of metal oxide nanoparticles in aquatic and terrestrial environments. A review. Environ. Pollut. 2017;230:250–267. doi: 10.1016/j.envpol.2017.06.064. [DOI] [PubMed] [Google Scholar]
- 164.Lee B.C., Hong G., Lee H., Kim P., Seo D.Y., Hwang G., et al. Influence of NOM on the stability of zinc oxide nanoparticles in ecotoxicity tests. Applied Sciences-Basel. 2020;10(18) [Google Scholar]
- 165.Kansara K., Bolan S., Radhakrishnan D., Palanisami T., Al-Muhtaseb A.H., Bolan N., et al. A critical review on the role of abiotic factors on the transformation, environmental identity and toxicity of engineered nanomaterials in aquatic environment. Environ. Pollut. 2022:296. doi: 10.1016/j.envpol.2021.118726. [DOI] [PubMed] [Google Scholar]
- 166.Jahan S., Alias Y.B., Bin Abu Bakar A.F., Bin Yusoff I. Toxicity evaluation of ZnO and TiO2 nanomaterials in hydroponic red bean (Vigna angularis) plant: physiology, biochemistry and kinetic transport. J. Environ. Sci. 2018;72:140–152. doi: 10.1016/j.jes.2017.12.022. [DOI] [PubMed] [Google Scholar]
- 167.Grun A.L., Straskraba S., Schulz S., Schloter M., Emmerling C. Long-term effects of environmentally relevant concentrations of silver nanoparticles on microbial biomass, enzyme activity, and functional genes involved in the nitrogen cycle of loamy soil. J. Environ. Sci. 2018;69:12–22. doi: 10.1016/j.jes.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 168.Simonin M., Cantarel A.A.M., Crouzet A., Gervaix J., Martins J.M.F., Richaume A. Negative effects of copper oxide nanoparticles on carbon and nitrogen cycle microbial activities in contrasting agricultural soils and in presence of plants. Front. Microbiol. 2018;9:11. doi: 10.3389/fmicb.2018.03102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cardinale B.J., Duffy J.E., Gonzalez A., Hooper D.U., Perrings C., Venail P., et al. Biodiversity loss and its impact on humanity. Nature. 2012;486(7401):59–67. doi: 10.1038/nature11148. [DOI] [PubMed] [Google Scholar]
- 170.Hooper D.U., Adair E.C., Cardinale B.J., Byrnes J.E.K., Hungate B.A., Matulich K.L., et al. A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature. 2012;486(7401):105–U129. doi: 10.1038/nature11118. [DOI] [PubMed] [Google Scholar]
- 171.Rogers K.L., Carreres-Calabuig J.A., Gorokh E., Posth N.R. Micro-by-micro interactions: how microorganisms influence the fate of marine microplastics. Limnol. Oceanogr. Lett. 2020;5(1):18–36. [Google Scholar]
- 172.Seeley M.E., Song B., Passie R., Hale R.C. Microplastics affect sedimentary microbial communities and nitrogen cycling. Nat. Commun. 2020;11(1):10. doi: 10.1038/s41467-020-16235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Oberdorster E., Zhu S.Q., Blickley T.M., McClellan-Green P., Haasch M.L. Ecotoxicology of carbon-based engineered nanoparticles: effects of fullerene (C-60) on aquatic organisms. Carbon. 2006;44(6):1112–1120. [Google Scholar]
- 174.Shen M.C., Ye S.J., Zeng G.M., Zhang Y.X., Xing L., Tang W.W., et al. Can microplastics pose a threat to ocean carbon sequestration? Mar. Pollut. Bull. 2020;150:3. doi: 10.1016/j.marpolbul.2019.110712. [DOI] [PubMed] [Google Scholar]
- 175.Sjollema S.B., Redondo-Hasselerharm P., Leslie H.A., Kraak M.H.S., Vethaak A.D. Do plastic particles affect microalgal photosynthesis and growth? Aquat. Toxicol. 2016;170:259–261. doi: 10.1016/j.aquatox.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 176.Sun X.M., Chen B.J., Li Q.F., Liu N., Xia B., Zhu L., et al. Toxicities of polystyrene nano- and microplastics toward marine bacterium Halomonas alkaliphila. Sci. Total Environ. 2018;642:1378–1385. doi: 10.1016/j.scitotenv.2018.06.141. [DOI] [PubMed] [Google Scholar]
- 177.Wieczorek A.M., Croot P.L., Lombard F., Sheahan J.N., Doyle T.K. Microplastic ingestion by gelatinous zooplankton may lower efficiency of the biological pump. Environ. Sci. Technol. 2019;53(9):5387–5395. doi: 10.1021/acs.est.8b07174. [DOI] [PubMed] [Google Scholar]
- 178.Rahman M.M., Zimmer M., Ahmed I., Donato D., Kanzaki M., Xu M. Co-benefits of protecting mangroves for biodiversity conservation and carbon storage. Nat. Commun. 2021;12(1):9. doi: 10.1038/s41467-021-24207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Estes J.A., Terborgh J., Brashares J.S., Power M.E., Berger J., Bond W.J., et al. Trophic downgrading of planet earth. Science. 2011;333(6040):301–306. doi: 10.1126/science.1205106. [DOI] [PubMed] [Google Scholar]
- 180.Nietch C.T., Quinlan E.L., Lazorchak J.M., Impellitteri C.A., Raikow D., Walters D. Effects of a chronic lower range of triclosan exposure on a stream mesocosm community. Environ. Toxicol. Chem. 2013;32(12):2874–2887. doi: 10.1002/etc.2385. [DOI] [PubMed] [Google Scholar]
- 181.Saakian D.B., Cheong K.H. A modified Crow-Kimura evolution model with reduced fitness for the smooth distribution of population. Physica A. 2020;544:10. [Google Scholar]
- 182.Smerlak M. Quasi-species evolution maximizes genotypic reproductive value (not fitness or flatness) J. Theor. Biol. 2021;522:4. doi: 10.1016/j.jtbi.2021.110699. [DOI] [PubMed] [Google Scholar]
- 183.Bernhardt E.S., Colman B.P., Hochella M.F., Cardinale B.J., Nisbet R.M., Richardson C.J., et al. An ecological perspective on nanomaterial impacts in the environment. J. Environ. Qual. 2010;39(6):1954–1965. doi: 10.2134/jeq2009.0479. [DOI] [PubMed] [Google Scholar]
- 184.Baun A., Hartmann N.B., Grieger K., Kusk K.O. Ecotoxicity of engineered nanoparticles to aquatic invertebrates: a brief review and recommendations for future toxicity testing. Ecotoxicology. 2008;17(5):387–395. doi: 10.1007/s10646-008-0208-y. [DOI] [PubMed] [Google Scholar]
- 185.da Costa J.P., Santos P.S.M., Duarte A.C., Rocha-Santos T. (Nano)plastics in the environment - sources, fates and effects. Sci. Total Environ. 2016;566:15–26. doi: 10.1016/j.scitotenv.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 186.Holden P.A., Nisbet R.M., Lenihan H.S., Miller R.J., Cherr G.N., Schimel J.P., et al. Ecological nanotoxicology: Integrating nanomaterial hazard considerations across the subcellular, population, community, and ecosystems levels. Accounts Chem. Res. 2013;46(3):813–822. doi: 10.1021/ar300069t. [DOI] [PubMed] [Google Scholar]
- 187.Serrano-Aroca A., Takayama K., Tunon-Molina A., Seyran M., Hassan S.S., Choudhury P.P., et al. Carbon-based nanomaterials: promising antiviral agents to combat COVID-19 in the microbial-resistant era. ACS Nano. 2021;15(5):8069–8086. doi: 10.1021/acsnano.1c00629. [DOI] [PubMed] [Google Scholar]
- 188.Landsiedel R., Ma-Hock L., Haussmann H.J., van Ravenzwaay B., Kayser M., Wiench K. Inhalation studies for the safety assessment of nanomaterials: status quo and the way forward. Wiley Interdiscip. Rev.-Nanomed. Nanobiotechnol. 2012;4(4):399–413. doi: 10.1002/wnan.1173. [DOI] [PubMed] [Google Scholar]
- 189.Nho R. Pathological effects of nano-sized particles on the respiratory system. Nanomed. Nanotechnol. Biol. Med. 2020;29:16. doi: 10.1016/j.nano.2020.102242. [DOI] [PubMed] [Google Scholar]
- 190.Liu K., Wang X.H., Fang T., Xu P., Zhu L.X., Li D.J. Source and potential risk assessment of suspended atmospheric microplastics in Shanghai. Sci. Total Environ. 2019;675:462–471. doi: 10.1016/j.scitotenv.2019.04.110. [DOI] [PubMed] [Google Scholar]
- 191.Dong C.D., Chen C.W., Chen Y.C., Chen H.H., Lee J.S., Lin C.H. Polystyrene microplastic particles: in vitro pulmonary toxicity assessment. J. Hazard Mater. 2020;385:8. doi: 10.1016/j.jhazmat.2019.121575. [DOI] [PubMed] [Google Scholar]
- 192.Xu M.K., Halimu G., Zhang Q.R., Song Y.B., Fu X.H., Li Y.Q., et al. Internalization and toxicity: a preliminary study of effects of nanoplastic particles on human lung epithelial cell. Sci. Total Environ. 2019;694:10. doi: 10.1016/j.scitotenv.2019.133794. [DOI] [PubMed] [Google Scholar]
- 193.Deweirdt J., Quignard J.F., Lacomme S., Gontier E., Mornet S., Savineau J.P., et al. In vitro study of carbon black nanoparticles on human pulmonary artery endothelial cells: effects on calcium signaling and mitochondrial alterations. Arch. Toxicol. 2020;94(7):2331–2348. doi: 10.1007/s00204-020-02764-9. [DOI] [PubMed] [Google Scholar]
- 194.Ameh T., Sayes C.M. The potential exposure and hazards of copper nanoparticles: a review. Environ. Toxicol. Pharmacol. 2019;71:8. doi: 10.1016/j.etap.2019.103220. [DOI] [PubMed] [Google Scholar]
- 195.Elizalde-Velazquez G.A., Gomez-Olivan L.M. Microplastics in aquatic environments: a review on occurrence, distribution, toxic effects, and implications for human health. Sci. Total Environ. 2021;780:18. doi: 10.1016/j.scitotenv.2021.146551. [DOI] [PubMed] [Google Scholar]
- 196.Wu W.H., Zhang R.J., McClements D.J., Chefetz B., Polubesova T., Xing B.S. Transformation and speciation analysis of silver nanoparticles of dietary supplement in simulated human gastrointestinal tract. Environ. Sci. Technol. 2018;52(15):8792–8800. doi: 10.1021/acs.est.8b01393. [DOI] [PubMed] [Google Scholar]
- 197.Zhang J., Guo W.L., Li Q.Q., Wang Z., Liu S.J. The effects and the potential mechanism of environmental transformation of metal nanoparticles on their toxicity in organisms. Environ. Sci.-Nano. 2018;5(11):19. [Google Scholar]
- 198.Bannunah A.M., Vllasaliu D., Lord J., Stolnik S. Mechanisms of nanoparticle Internalization and transport across an intestinal epithelial cell model: effect of size and surface charge. Mol. Pharm. 2014;11(12):4363–4373. doi: 10.1021/mp500439c. [DOI] [PubMed] [Google Scholar]
- 199.Hirt N., Body-Malapel M. Immunotoxicity and intestinal effects of nano- and microplastics: a review of the literature. Part. Fibre Toxicol. 2020;17(1):22. doi: 10.1186/s12989-020-00387-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Yao M.F., He L.L., McClements D.J., Xiao H. Uptake of gold nanoparticles by intestinal epithelial cells: impact of particle size on their absorption, accumulation, and toxicity. J. Agric. Food Chem. 2015;63(36):8044–8049. doi: 10.1021/acs.jafc.5b03242. [DOI] [PubMed] [Google Scholar]
- 201.Sohal I.S., O'Fallon K.S., Gaines P., Demokritou P., Bello D. Ingested engineered nanomaterials: state of science in nanotoxicity testing and future research needs. Part. Fibre Toxicol. 2018;15:31. doi: 10.1186/s12989-018-0265-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ortega M.T., Riviere J.E., Choi K., Monteiro-Riviere N.A. Biocorona formation on gold nanoparticles modulates human proximal tubule kidney cell uptake, cytotoxicity and gene expression. Toxicol. Vitro. 2017;42:150–160. doi: 10.1016/j.tiv.2017.04.020. [DOI] [PubMed] [Google Scholar]