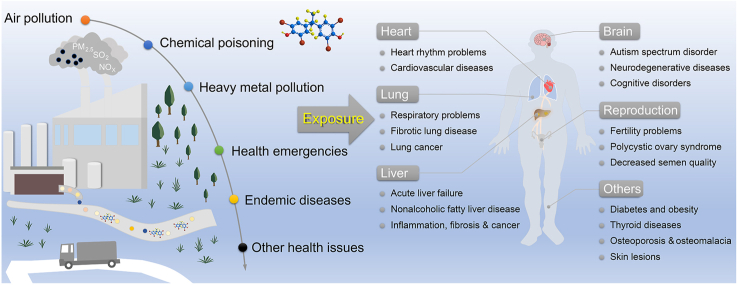
Abstract
The environmental and health impacts from the massive discharge of chemicals and subsequent pollution have been gaining increasing public concern. The unintended exposure to different pollutants, such as heavy metals, air pollutants and organic chemicals, may cause diverse deleterious effects on human bodies, resulting in the incidence and progression of different diseases. The article reviewed the outbreak of environmental pollution-related public health emergencies, the epidemiological evidence on certain pollution-correlated health effects, and the pathological studies on specific pollutant exposure. By recalling the notable historical life-threatening disasters incurred by local chemical pollution, the damning evidence was presented to criminate certain pollutants as the main culprit for the given health issues. The epidemiological data on the prevalence of some common diseases revealed a variety of environmental pollutants to blame, such as endocrine-disrupting chemicals (EDCs), fine particulate matters (PMs) and heavy metals. The retrospection of toxicological studies provided illustrative clues for evaluating ambient pollutant-induced health risks. Overall, environmental pollution, as the hidden culprit, should answer for the increasing public health burden, and more efforts are highly encouraged to strive to explore the cause-and-effect relationships through extensive epidemiological and pathological studies.
Keywords: Environmental pollution, Public health emergency, Epidemiological evidence, Pathological study, Cause-and-effect relationship
Graphical abstract
Highlights
-
•
Public health emergencies incriminate the pollutants in causing diseases.
-
•
Epidemiological data show the correlation of pollution with health problems.
-
•
Toxicological studies reveal the potential pathogenicity of typical pollutants.
1. Introduction
Since the publication of Silent Spring in 1962, the global attention has been increasingly drawn to the threat of environmental pollution to the ecosystem and human health. The pollution-driven hazards to human health, to date,remain an unsolved dilemma due to the complexity of the ever-changing environmental challenge. The global population still suffers from unprecedented health problems from the worsening situation of air, water, and soil pollution. For example, airborne fine particulate matters (PMs) increase the incidence risks of acute and chronic respiratory diseases, heart disease, stroke, and lung cancer [1]. Drinking water pollution was reported to cause nearly 900,000 diarrhea-associated deaths worldwide in 2016, including more than 470,000 child deaths [1]. Exposure to soil pollution by some heavy metals and persistent organic pollutants (POPs) poses health threats from metal poisoning or endocrine-disrupting effects, particularly affecting developmental processes during early life stages [2,3]. Environmental pollution, a hidden culprit, deserves more concern in unraveling the etiology of obstinate human diseases.
It is estimated that 24% of the global burden of disease and 23% of deaths are attributable to environmental factors, including hazardous biological, physical, and chemical factors in the environment [4,5]. Biohazard factors in the environment are classified as traditional environmental hazards [5], including pathogenic bacteria, viruses, and parasites. The notorious pandemic caused by the COVID-19 virus that emerged at the end of 2019 has swept across the world, causing a disastrous effect on global public health. According to the data released by the World Health Organization (WHO) on May 1, 2021, the COVID-19 pandemic had infected more than 153 million people and claimed over 3.2 million lives [6]. By November 5, 2021, the confirmed cases of COVID-19 infection were over 248 million, including more than 5 million deaths, despite the fact that over 7 billion vaccine doses had been administered globally. In addition to the high infectivity of the virus itself, environmental transmission is another important factor in the COVID-19 pandemic, regarding SARS-CoV-2 contamination in diverse environmental media, such as water, PMs, dust, and sewage [7]. As a matter of fact, the infectious diseases caused by other environmental and biological hazards have long been a threat to public health, including acquired immune deficiency syndrome (AIDS) caused by human immunodeficiency virus (HIV), tuberculosis (TB) caused by mycobacterium tuberculosis, malaria caused by plasmodium, chronic hepatitis B caused by hepatitis B virus (HBV), and neglected tropical diseases (NTDs), all of which were responsible for an estimated 4.3 million deaths in 2016 [1]. One tricky situation is the infectivity of asymptomatic patients. Nearly 300 million people were estimated to carry HBV globally in 2019, of which only 1/10 were diagnosed, and 1/50 received medical treatment. The majority of the infected were unaware of their infections, which could be responsible for an estimated 1.5 million new cases [6].
Radiation is another important source of environmental hazards affecting public health. Since human civilization is protected from cosmic rays by the atmosphere, the majority of the harmful radiation we receive comes from nuclear radiation. There have been four catastrophic nuclear reactor accidents in history, including the Windscale accident in the United Kingdom in 1957, the Three Mile Island accident in the United States in 1979, the Chernobyl accident in the former Soviet Union in 1986, and the most recent accident at the Fukushima nuclear power plant in Japan in 2011 [8]. These accidents resulted in the release of large amounts of radioactive material into the environment and substantial radiation exposure to the surrounding population. Epidemiological studies after the Chernobyl accident focused on thyroid disease and childhood cancer [9]. Because of the sheer amount of leaked radioactive I-131, the number of thyroid cancer cases in the surrounding area soared and reached over 4000 in the following two decades, mostly among children and adolescents [10,11]. In addition, mental illness has become one of the public health issues after the accident [12]. Compared with the external exposure caused by nuclear leakage, radionuclides can also produce internal exposure to occupational workers. A cohort study suggested that internal exposure to nuclear radiation could increase the risk of blood, lymphatic, and upper respiratory cancers in workers who engaged in long-term nuclear-related occupations [13]. Another significant source of nuclear radiation is the use of nuclear weapons in war. A cohort study on 4091 atomic bomb survivors from Hiroshima and Nagasaki in Japan showed that 44.8% of participants suffered from thyroid disease, with an estimated 28% of solid nodules, 31% of benign nodules, 25% of cysts, and 37% of malignant tumors associated with radiation exposure [14].
Apart from infectious diseases and nuclear radiation, environmental chemical hazards caused by organic and inorganic pollutants are gradually recognized worldwide. Over the past few decades, thousands of chemicals have been artificially synthesized and released into the environment [15]. With the development of science and technology, new environmental insults from the pollution of synthetic chemicals, airborne particulates, heavy metals, and E-waste are emerging [16,17]. The United States Environmental Protection Agency (USEPA) reported that the nationwide discharges of production-related toxic chemicals in 2019 were 9.8 × 108 kg, 2.72 × 108 kg, and 9.07 × 107 kg in the soil, air, and water, respectively, and the total release amount was higher than that in 2015, although the chemical emissions have been declining in recent years [18,19]. The exposure to environmental pollutants has become another important causative factor for human diseases [5]. Regardless of genetic diseases, numerous investigations have confirmed that a majority of noncommunicable diseases (NCDs), such as cancer, cardiovascular diseases, respiratory diseases, musculoskeletal diseases, neuropsychiatric disorders, and obesity, are strongly associated with the exposure to chemical pollutants, like POPs [3]. The WHO has targeted three environmental aspects for monitoring in the Sustainable Development Goals (SDG), including air pollution, water, and sanitation, as they caused 7 million, 0.9 million, and 107,000 deaths globally in 2016, respectively [1].
In view of the increasing public concern about environmental pollution-associated health burden, we systematically recalled the historical public health emergencies caused by chemical pollution, sorted out the epidemiological evidence on pollutant exposure-associated human diseases, and discussed the potential pathogenic factors of some compounds, including heavy metal and organometallic compounds, organic halogen compounds, phenolic and bisphenol compounds, and airborne fine PMs based on the pathological studies.
2. Environmental pollution-related public health emergencies
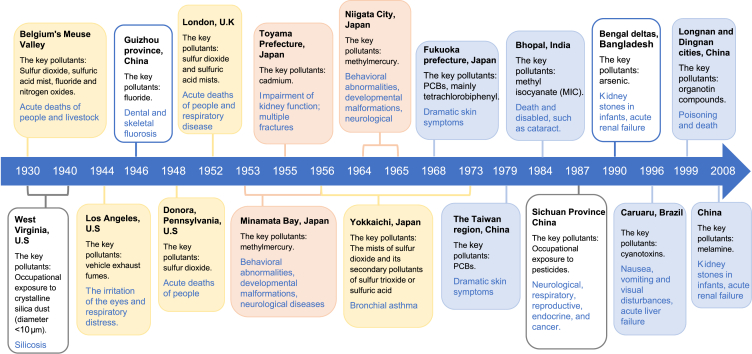
The chronicles of historical public health emergencies associated with environmental pollution (Fig. 1) provided people with profound life lessons on the intentional or unintentional chemical release into the environment. There were three categories: notorious public health events, endemic diseases, and occupational exposure-related diseases.
Fig. 1.
A diagram of public health emergencies caused by environmental pollution. The events caused by air pollution, heavy metal contamination, and chemical poisoning are shaded with yellow, orange, and blue, respectively. The incidents caused by local chemical pollution and occupational exposure are outlined with blue and grey borders, respectively.
2.1. Notorious public health events
Since the middle of the 20th century, some countries like the United States, Japan, and the United Kingdom vigorously developed industries to achieve rapid economic growth. Along with that, the massive emission of pollutants resulted in serious environmental hazards, including air pollution, heavy metal pollution, and chemical poisoning, triggering a series of emergencies that threatened public health.
2.1.1. Air pollution episodes
Air pollution has a long history as a threat to human health, resulting in several major episodes during the 1930s and 1950s. The very first documented air pollution disaster was the Meuse Valley fog of 1930 in Belgium. The windless cold weather was unfavorable for the diffusion of the atmospheric emissions from steel mills, coke plants, and foundries in the valley, thus causing a high accumulation of air pollutants, including sulfur dioxide, sulfuric acid mist, fluoride, and nitrogen oxide [20,21]. The inhalation of these pollutants was extremely hazardous and even fatal. Thousands of people suffered respiratory diseases, and more than 60 deaths occurred within one week [20]. Another similar air pollution episode happened in the southwestern Pennsylvania town of Donora in October 1948. The combination of toxic air exhaust released from the industrial plants and coal-burning homes under unfavorable meteorological conditions increased the local mortality to more than six times the normal rate [20,21].
The fog episode in London in December 1952, also known as the Great Smog, was probably the worst air pollution event recorded in human history [20]. The atmospheric pollutant emissions were from industrial production, power plants, and coal-burning homes, which contained high levels of sulfur dioxide and sulfuric acid mists [22,23]. Together with the foggy weather in the city, the air pollution caused the incidence of respiratory diseases within 12-h exposure to the smog, and an estimated death toll of 4703 people occurred during the week of this event [20,22]. An even greater challenge was that the smog caused latent health effects by decreasing the human ability to cope with diseases, thus leading to high mortality and morbidity in the following several months of the event [21]. The Great Smog episode is widely regarded as the very start of epidemiological studies on air pollution [21].
Different from the acute health effects of previous episodes, Yokkaichi asthma was gradually recognized, due to increased morbidity of respiratory diseases, including bronchial asthma, in Yokkaichi, Japan, in the 1960s [24]. Since 1956, Yokkaichi has become a petroleum-based industrial city, with its local naval fuel plants contributing to a quarter of the total output of Japan’s petroleum industry at the time [25]. More than 100,000 tons of sulfur dioxide are emitted annually from oil-fired power plants and refineries [26]. The mists of sulfur dioxide and its secondary pollutants, such as sulfur trioxide or sulfuric acid, were responsible for more than 600 patients with respiratory diseases in the 1960s, including asthma, chronic bronchitis, and pulmonary emphysema [24,26,27]. The follow-up data during several decades after 1973 showed that 486 of 1232 patients suffered from Yokkaichi asthma and died by the year 2000 [26].
Besides the above air pollution episodes commonly caused by sulfur dioxide and its acid mists from industrial plants, another type of serious air pollution is the photochemical smog, which is caused by high concentrations of the air pollutants from the photochemical reaction of vehicle exhaust fumes in sunlight [20]. The first identified case was the Los Angeles smog in 1944 when the light brownish smog caused irritation of the eyes and respiratory distress. During the recent two decades, public health issues caused by airborne PMs have been gradually gaining high attention [21,28]. All these episodes provide clear proof that air pollution can cause both public health emergencies and long-term chronic diseases [29,30], and it is consequently one of the most important aspects of environmental health studies.
2.1.2. Heavy metal pollution
Heavy metal pollution occasionally causes unusual diseases which draw global concern. A typical example was the “Itai–itai disease” characterized by severe pain all over the body, which appeared among the local people in the Jinzu River basin of Toyama Prefecture, Japan, in 1955, and this painful epidemic lasted for decades [31]. The etiological survey showed that cadmium (Cd) pollution in the wastewater emission from the Mitsui Mining and Smelting Company was the main culprit [27]. In the 1960s, local residents were estimated to consume 600 μg Cd daily from Cd-contaminated rice [32]. Because of long-term exposure, people suffered from severe impairment of kidney function (e.g., the tubules and glomerulus), widespread osteoporosis, and multiple fractures [32,33]. A tracking survey on 757 residents from 1967 to 1984 showed that Cd exposure caused 207 deaths. Of 573 persons who had a total Cd intake of greater than 2 g, 196 died [34].
Another famous case, Minamata disease, occurred in Minamata Bay, Kumamoto prefecture, Japan, in 1953–1956 [35], due to mercury contamination in wastewater discharge from a local chemical plant [36]. There were 81.5 tons of mercury in total estimated to be released into the aquatic environment during 1932–1971 [37], which was gradually transformed into methyl mercury through biological methylation in the environment. Before the emergence of the strange sickness in human beings, large numbers of fish and other sea creatures died in Minamata Bay, and the animals in the surrounding areas began to behave abnormally, such as loss of the ability to fly in seabirds, and twitching in cats [36,38]. After consuming the contaminated fish and shellfish [37], 54 human deaths in total were recorded, and many children developed congenital diseases [35]. This event affected thousands of people. The survivors still suffered developmental disabilities, physical deformities, cognitive impairments, and neurological disorders, even decades later [36]. During 1964–1965, another minor incident of methylmercury poisoning occurred in Japan’s Niigata City [35]. By 1994, all the patients identified with Minamata disease in both incidents had died, including 1769 deaths in Kumamoto prefecture and 690 deaths in Niigata prefecture [35].
2.1.3. Chemical poisoning
With the continuous development of chemical industries, a large number of chemicals have been produced, followed by the increased risk for the emergence of poisoning events, due to improper chemical management and disposal. A well-known skin epidemic initially broke out in Fukuoka prefecture and later spread to more than 20 prefectures in Japan in 1968 [39]. The investigation indicated that a brand of Kanemi rice oil (rice-bran oil, edible oil) had been contaminated by accidental leakage of up to 2000 mg/L of polychlorinated biphenyls (PCBs), the main constituent of which was tetrachlorobiphenyl [40,41]. In total, 1057 patients were confirmed to suffer from this contaminated oil-derived disease (i.e., Yusho disease) in 1971, and the case number rose to 1788 in 1982 [39,41]. The major symptoms of these patients were dramatic skin problems, such as the swollen upper eyelid, increased eye secretions, acne-like eruptions, and enlarged follicles. Another disease, known as Yu-Cheng disease, occurred in the Taiwan region, China, in 1979. The origin of this incident was the consumption of rice-bran oil containing high contamination of PCBs [42,43], and about 1700 people consequently suffered from skin symptoms [44].
Another tragedy caused by a chemical spill occurred in Bhopal, India, in December 1984. At a local pesticide factory, water flowed into a storage tank full of methyl isocyanate (MIC) and triggered a violent reaction, resulting in a massive release of the volatile MIC [45]. The lethal gas with MIC floated at a low altitude, and quickly spread from a radius of 3 miles to 6 miles [46]. As one of the worst chemical accidents in history, the Bhopal disaster caused 2000 deaths and 100,000 injured [47]. Thousands of the survivors are still suffering from the accident with all sorts of physical disabilities, such as cataracts [45,46].
Another public health emergency caused by foodborne organic compound poisoning emerged due to the outbreak of an unusual epidemic of renal calculi in infants in some areas of China in the spring of 2008 [48]. The cause of this incident was the illegal addition of melamine in dairy products, especially for infant formula [48]. By the end of November 2008, a total of 294,000 cases of urinary stones were diagnosed in infants and children, wherein a small number of patients suffered acute renal failure, and six of them died [49].
The foodborne chemical contamination also happened in the edible lard poisoning incident in Longnan and Dingnan counties, Jiangxi province, China, at the beginning of 1999. The containers with organotin residues severely contaminated the edible lard. The highest concentration of organotin compounds in the lard was 1.94 mg/g, wherein the neurotoxic trimethyltin reached the level of 13.80 μg/g [50]. More than 1000 people were poisoned in this incident, and three died. The analysis of samples from the victims showed high levels of organotins ranging from 0.1 to 1.9 μg/g in their bodies [51].
The public health issues associated with the indirect effects of chemical pollution are also widely concerned. For example, the algal toxin poisoning occurs from time to time due to the explosive growth of microalgae in aquatic systems. The cause is the extensive use of nitrogen fertilizer, phosphate fertilizer, and urea in agriculture, animal husbandry, and aquaculture, resulting in the intensification of offshore eutrophication, the consequent outbreak of algal blooms, and the large production of algal toxins [52,53]. An outbreak of Caruaru syndrome occurred at a dialysis clinic in Caruaru, Brazil, in 1996, and the etiological study confirmed the contamination of two cyanotoxins in the water supply. The patients on routine hemodialysis suffered nausea, vomiting, and visual disturbances after treatment. According to statistics, 100 of 131 patients developed acute liver failure, and 76 of them died later [54].
2.2. Endemic diseases
Endemic diseases in some specific areas are closely related to the surrounding environment, mainly due to the contamination of certain chemical elements therein. The causative elements can be either natural or anthropogenic. The endemic diseases caused by excessive exposure to arsenic and fluorine are representative examples.
As a metalloid element, arsenic occurs in nature as inorganic and organic compounds, and the former has high toxicity to human health [55]. The natural origins of arsenic may slowly leak and pollute the surrounding areas. Some human activities, such as primarily metal mining and smelting, may also provide anthropogenic sources of arsenic [56]. Human exposure is mainly through arsenic-containing drinking water, seafood, soil, and air. Long-term arsenic ingestion can cause skin lesions, characterized by varying degrees of keratosis and melanoderma, usually on the palms and soles [55]. The most famous outbreak of endemic diseases caused by arsenic exposure occurred in the Bengal deltas, Bangladesh, in the 1990s [55,57], due to the switch of drinking water supplies to groundwater containing high levels of arsenic in the 1980s [58]. By 2000, around 40 million residents had been exposed to drinking water contaminated with excessive arsenic. Approximately 2 million of them developed the symptoms of arsenic poisoning, and nearly 0.3 million people eventually died of cancer [59]. The following investigations revealed that 1 of 18 adult deaths was caused by arsenic-contaminated drinking water [60]. Similar arsenic poisonings were also reported in some other areas like Thailand, Hungary, and the Taiwan region, China [58,61].
Another typical example is endemic fluorosis, which spreads in many countries and regions due to excessive fluoride. As fluoride is abundant in Earth’s crust, it exists in various waters, especially in groundwater, where the concentrations can be very high [62]. Environmental fluoride directly pollutes foods and drinks, thus causing unintended fluoride exposure to human beings [63]. Although small amounts of fluoride can be used to prevent caries in dental preparations, e.g., the addition of fluoride below 1.5 mg/L in salt or drinking water [62], the excessive fluoride intake can cause health problems characterized by dental fluorosis and skeletal fluorosis [64]. Nowadays, dental fluorosis is used as an indicator of early-stage skeletal fluorosis [65]. Besides the natural and artificial routes, the inhalation of PMs from high fluoride-containing coal is also a vitally important way of human exposure [62,64]. Coal burning has caused many cases of fluorosis, and one of the most well-known endemics, the fluorosis epidemic, lasted for decades in Guizhou province, Southwest China, from 1946 to the 1980s [66]. The cause was the ingestion of foods roasted using the local coal containing high levels of fluoride [66,67], and around 10 million residents consequently suffered typical symptoms of fluorosis, including dental and skeletal fluorosis [68].
2.3. Occupational exposure-related diseases
In recent years, increasing concerns have been raised about occupational safety and health risks [69]. Occupational workers, mainly engaged in industry and agriculture, may face multiple risks of acute or long-term exposure to hazardous substances.
Occupational exposure to silica dust in the industry is one representative case. Both crystalline and amorphous forms of silica can be produced during the industrial processes, including drilling, blasting, cutting, etc. [70] The inhalation of a large amount of crystalline silica dust (diameter <10 μm) can cause silicosis, a well-known fibrotic lung disease that is irreversible and potentially fatal [71,72]. Pulmonary silica exposure has also been linked to the increased risks of chronic obstructive pulmonary disease (COPD) and lung cancer [73]. Although some protective measures may decrease silica dust exposure, it still causes an estimated 200–300 silicosis deaths each year in the United States [71]. A tragedy of silicosis happened in West Virginia, the United States, in the 1930s, wherein more than 700 workers, who were involved in blasting and drilling for the construction of the Hawk’s Nest Tunnel, died of silicosis later due to the inhalation of a large amount of silica dust [74]. Ever since this disaster, occupational health issues have been gaining increasing attention.
Pesticides are the most widely-used chemicals in agriculture, which turn out to be widespread pollutants in the environment, including air, water, soil, and other environmental organisms. There are many types of pesticides, such as insecticides, herbicides, and fungicides, in single forms or combinations [75]. The pesticide exposure can happen through skin and eye contact, respiratory inhalation, and dietary ingestion [76]. Workers involved in the production and application of pesticides may encounter accidental occupational pesticide exposure through contact and inhalation, accounting for the majority (>60%) of the unintentional acute pesticide poisoning incidents [75]. It was estimated that 700,000 cases of acute poisoning were caused by occupational pesticide exposure worldwide, and the number of deaths exceeded 13,000 people, according to the data collected by the WHO in 1990 [75]. Long-term occupational exposure to pesticides is associated with health problems relating to neurological, respiratory, reproductive, endocrine systems, and even cancer [77]. Clinical manifestations may include dizziness, headache, weakness, vomiting, etc. [78]. Moreover, the biomarkers for the early warning of pesticide exposure can be monitored prior to the onset of symptoms. For example, cholinesterase inhibition can suggest organophosphorus pesticide exposure [76].
3. Epidemiological evidence on the relevance of environmental pollutants in human diseases
The epidemiological studies may help provide important clues for elucidating the etiology of environment-relevant diseases. With the increasing concern on environmental health issues, numerous epidemiological studies have been performed to establish the link between environmental pollutant exposure and human health problems, including pulmonary and cardiovascular, endocrine, reproductive, nerve system diseases, and some other clinic symptoms (Table 1).
Table 1.
Epidemiological evidence on the relevance of environmental pollutant exposure with disease incidence.
| Classification | Diseases | Main chemicals | Related Population | Morbidity | References |
|---|---|---|---|---|---|
| Pulmonary and cardiovascular diseases | Pulmonary diseases | Diesel exhaust, a mixture of burning gas, and ultrafine particulate matters coated with organic compounds | Railroad workers in the United States | The global deaths from chronic respiratory diseases (CRDs) and cardiovascular diseases (CVDs) were 4.1 million and 17.9 million in 2019, increased by 10% and 25% since 2000, respectively. | [82] |
| PM2.5 and nitrogen dioxide | Patients with COPD | [83] | |||
| PM10 | Men | [88] | |||
| Sulfur dioxide, nitrogen dioxide and PM2.5 | Children aged 9 to 14 with asthma | [84] | |||
| Cardiovascular diseases | PM2.5, PM10, sulfur dioxide and ozone | Adults | [[85], [86], [87], [88]] | ||
| Endocrine diseases | Diabetes (T2D) | PCBs, DDT, DDE, DDD, Dioxins and dioxin-like chemicals, Trans-Nonachlor, PM2.5, Cd | Adults | An estimated 46.3% of people over the age of 20 already have been suffering from diabetes in the United States. | [93,96,97] |
| Thyroid disease | PCB congeners (99, 139, 153, 180, 183, and 187) | Pregnant woman | Spontaneous hypothyroidism: 0.35% in women and 0.06% in men; hyperthyroidism: 0.5%–2% in women and 0.05%–0.2% in men; nodular goiter: 0.1%; thyroid cancer: 1 to 10 per 100,000. | [99] | |
| PCB congeners (99, 139, 153, 180, 183, 187, 194, and 199) | Adults | [104] | |||
| PCBs and dioxins | Adults over 60 years of age | [105] | |||
| Obesity | DDT, DDE | Pregnant mothers and 7-year-old children | As of 2008, among the population over the age of 20 worldwide, an estimated 1.5 billion people were overweight, of which about 33% were obese. | [110,114] | |
| Atmospheric fine particulate matter | Veterans in the United States | [111] | |||
| Phthalates | Children aged 4 to 7 exposed during pregnancy | [115] | |||
| Reproductive diseases | Decreased semen quality | BPA | Young men | 20%–40% in young men. | [117,118] |
| PM2.5, PM10 | [119] | ||||
| Success rates of IVF | Methylparaben | Fathers involved in IVF | – | [123] | |
| Menstruation disorders | PFASs (PFOS, PFOA, PFHxS, PFNA) | Prepregnant women | – | [122] | |
| Endometriosis | PCBs (118, 138, 153 and 170), p,p′-DDE, PFBS | Women | 10%–15% in women of childbearing age | [120,121] | |
| Infertility | Pesticides (organophosphate and pyrethroids) | Women | Infertility in developed and developing countries is 3.5%–16.7% and 6.9%–9.3%, respectively. | [124] | |
| Nervous system diseases | Autism spectrum disorder (ASD) | Heavy metals (mercury, cadmium, nickel, etc.), chlorinated solvents (vinyl chloride, trichloroethylene), air pollutants, phthalates | Perinatal women and children | ASD in 8-year-old children in the United States has increased from 1 in 2500 to 1 in 59 children in recent 40 years. | [[128], [129], [130], [131], [132]] |
| Attention-deficit/hyperactivity disorder (ADHD) | BPA, phthalates and PFOA | Children | Childhood exposure was associated with ADHD, and boys seemed to be more vulnerable than girls. | [[137], [138], [139], [140]] | |
| Alzheimer’s disease (AD) | Aluminum, PM2.5, Air pollution | The aged | – | [143,145,146] | |
| Pesticides, fumigants or defoliant, organophosphate | Occupational populations | – | [[147], [148], [149]] | ||
| Others | Nonalcoholic fatty liver disease (NAFLD) | BPA, POPs (PCDD, PCDF, PCBs) | Adults | 30% of the total population in the United States were suffering from NAFLD. | [[152], [153], [154]] |
3.1. Pulmonary and cardiovascular diseases
Pulmonary and cardiovascular diseases are currently the most worrying NCDs. According to the WHO Health Statistics report (2021), the global deaths from chronic respiratory diseases (CRDs) and cardiovascular diseases (CVDs) have increased by 10% and 25% since 2000, reaching 4.1 million and 17.9 million, respectively, in 2019 [6]. Furthermore, according to the latest Global Cancer Statistics released in 2020, new cases (2.2 million) and deaths (1.8 million) from lung cancer ranked second and first among 36 cancers, respectively [79]. Pulmonary and cardiovascular diseases are often closely related, as the lungs are responsible for the gas exchange of the blood circulatory system [80]. In addition to some undesirable personal habits, such as smoking [6,81], environmental pollutant exposure is an important risk factor for the development and progression of pulmonary and cardiovascular diseases.
Air pollution is commonly blamed for causing cardiopulmonary diseases. A study of COPD cases among railroad workers in the United States showed that exposure to diesel exhaust, a mixture of burning gas and ultrafine PMs containing organic compounds, led to an increase in COPD mortality of the train conductors and engineers, which was correlated with the length of job service [82]. Exposure to ambient air containing PM2.5 (11.65 μg/m3) and nitrogen dioxide (11.97 mg/m3) increased heart rate variability (HRV) in patients with COPD by 8.3% and 7.7%, respectively [83]. Another study on children aged 9 to 14 with asthma revealed that exposure to ambient air containing sulfur dioxide (5.4 mg/m3), nitrogen dioxide (6.8 mg/m3), and PM2.5 (5.4 μg/m3) was associated with the reduced pulmonary function, with 25%–75% drop in lung capacity [84]. The air pollution may also contribute to a significant impact on CVD development. A recent study indicated that every 10 μg/m3 increase in the average PM2.5 concentration in surrounding air was associated with a 0.39% increase in hypertension-related mortality [85]. In addition to PM2.5, PM10 (per 19 μg/m3 increase), sulfur dioxide (per 20 μg/m3 increase), and ozone (per 22 μg/m3 increase) were suggested to be significantly associated with an increased risk of hypertension in humans [86,87]. A cohort study of more than 70,000 men in China showed that long-term exposure to PM10 was also significantly associated with cardiopulmonary disease mortality [88]. By contrast, the increase in the residential area greening has been recently found to be related to a decrease in the incidence of acute myocardial infarction and heart failure among local residents, and the patients with CVD could benefit from the good air quality evidenced by a reduced risk of death [89].
3.2. Endocrine diseases
The endocrine glands release hormones, which consist of a highly complex and tightly controlled network throughout the body, and play important roles in maintaining homeostasis in vivo. Any disturbances or disorders in the endocrine system may have negative impacts on human health. With the widespread existence of environmental endocrine-disrupting chemicals (EDCs) [90,91], the correlation between EDC exposure and the incidence of human diseases has been gaining increasing attention. Three major endocrine diseases, including diabetes mellitus, thyroid disease, and obesity, have been revealed to be highly associated with exposure to some environmental pollutants.
Diabetes, including type 1 and type 2 diabetes (T1D and T2D), has become a major threat to public health worldwide [92]. An estimated 11.3% of people over the age of 20 have developed diabetes in the United States, while another 35% have developed prediabetes due to above-standard blood glucose levels [93]. T2D is more susceptible to epigenetic factors than T1D, which is an autoimmune disease. In addition to the traditional risk factors, such as genetics, obesity, poor diet, and lifestyle, environmental pollutants are also considered to be associated with the development of diabetes [94,95]. A review of the epidemiological studies showed that T2D incidence was positively correlated with multiple POP exposure, including polychlorinated biphenyls (PCBs), dichlorodiphenyltrichloroethane (DDT), dichlorodiphenyldichloroethylene (DDE), dichlorodiphenyldichloroethane (DDD), dioxins and dioxin-like chemicals, and trans-Nonachlor [93]. Another study evaluated the contribution of air pollution to the incidence of diabetes by investigating the evidence from 13 related epidemiological studies in Europe or North America, and the results showed an 8%–10% increase in T2D risk for the additional exposure to 10 μg/m3 PM2.5, suggesting a positive correlation between air pollution and T2D [96]. In addition, a cohort study of 3140 adults revealed a significantly positive dose–response relationship between urinary Cd concentration and the risk of developing T2D [97], suggesting that diverse pollutants could be the suspicious predisposing causes for this kind of metabolic disease.
Thyroid disease, often associated with improper iodine intake [98], is one of the most common diseases worldwide, including hypothyroidism, hyperthyroidism, goiter, thyroid nodules, thyroiditis, Graves’ disease, and thyroid cancer. Thyroid diseases are mostly determined by the abnormal blood concentrations of tetraiodothyronine (T4) and thyroid-stimulating hormone (TSH) [99]. The estimated annual incidence of spontaneous hypothyroidism is 0.35% in women, which is about six times the percentage in men [100]. In an iodine-replete community, hyperthyroidism in women is 0.5%–2%, 10 times the percentage in men [101]. The prevalence of single thyroid nodules is 3% and multinodular goiter is 1% [101]. The annual incidence of thyroid cancer falls in the range of 1–10 per 100,000 [102,103]. In addition to iodine, chemical exposure has also been suggested to be correlated with thyroid diseases. Prenatal exposure to PCB congeners, including 99, 139, 153, 180, 183, and 187, was found to be associated with TSH levels in the offspring, according to an epidemiological study performed in the USA [99]. A study on a Mexican-American population showed that PCB congeners 99, 139, 153, 180, 183, 187, 194, and 199 were positively correlated with TSH levels in offspring [104]. The exposure to PCBs and dioxins was also considered to be associated with a higher risk of the incidence of thyroid disease in adults over 60 years old, and total T4 levels were negatively correlated with chemical levels in these old people. Moreover, TSH levels were positively correlated with the chemicals in females but negatively correlated with PCBs in males [105]. The negative association between PCB exposure and thyroid hormone levels was also reported in some other references [99,106]. The controversial findings could probably be due to the difference in factors like age, gender, the race of the studied population, etc. More studies are still in urgent need to clarify the role of chemicals in the development of thyroid diseases.
Obesity is another endocrine-related disease that has received much attention. As of 2008, there was an estimated 1.5 billion overweight population over the age of 20 worldwide, of which about 500 million were obese [99]. The prevalence of obesity in developed countries has significantly increased over the past 20 years, led by the United States, with 30% of adults being obese and 65% being overweight [107,108]. Not only is obesity aesthetically unattractive, but it is also a significant threat to public health. Billions of cases, such as high blood pressure, insulin resistance, and glucose intolerance, are linked to obesity around the world [99]. However, as a complex endocrine disease, the development of obesity is also caused by a variety of factors, including congenital genetics, personal lifestyle, and some environmental pollutants with endocrine-disrupting activities [109]. Obesogens, termed by Blumberg and Grun in 2006, now refer to the chemicals that disrupt the body’s normal homeostatic controls to promote adipogenesis and lipid accumulation. A meta-analysis of seven epidemiological studies showed a positive association between the metabolite p,p′-DDE and obesity assessed by body mass index (BMI), and the analysis of in vitro and in vivo data also provided the biological evidence on the association of p,p′-DDT, and p,p′-DDE exposure with the development of obesity [110]. In addition, the atmospheric fine PMs are also a potential obesogenic factor. A recent cohort study involving more than 3.9 million veterans in the United States showed that an annual average of PM2.5 concentration higher than 10 μg/m3 was associated with the increased obesity risk and high BMI change, suggesting that the health hazards of the airborne PM2.5 include obesogenic effects [111]. Furthermore, the sharply rising rate of obesity in childhood is of deep concern, which implies that the obesity risks may emerge in the early stage of life [112,113]. A cohort study on the association between DDT and DDE exposure in pregnant mothers and the obesity occurrence in 7-year-old children showed that 96 out of 270 children (35.6%) were obese, although the maternal exposure to DDT and DDE was not significantly correlated with the incidence of the corresponding childhood obesity [114]. Another larger-scale cohort study aimed to investigate the association between urinary phthalate metabolite levels of the pregnant mothers and the weights of their children aged 4 to 7, and the result showed a positive correlation between mono-3-carboxypropyl phthalate concentrations and the childhood overweight or obesity [115]. The epidemiological evidence on environmental chemicals-induced obesity is ambiguous, and more proof is still required.
3.3. Reproductive diseases
With the worldwide decline in human fertility, increasing attention has been paid to reproductive health. The statistics show that the proportions of couples suffering from infertility in developed and developing countries are 3.5%–16.7% and 6.9%–9.3%, respectively [116]. Reproductive health problems in females mainly originate from diseases, including polycystic ovary syndrome (PCOS, 3%–15% in women of childbearing age), endometriosis (10%–15% in women of childbearing age), and uterine fibroids (25%–50% in pre-menopausal women), and those in males are mainly caused by testicular germ cell carcinoma (TGC, increasing up to 400% in young men in industrialized countries during the past 40–50 years), congenital cryptorchidism (1%–9% in newborns) and decreased semen quality (20%–40% in young men) [99]. Given that endogenous estrogens and androgens are strongly associated with reproductive development, it is reasonable to speculate that some estrogen- or androgen-like pollutants may affect the reproductive health of both men and women. A cohort study of 308 young men showed that the urinary bisphenol A (BPA) levels were positively correlated with the reproduction-related hormone levels, including testosterone, estradiol, and luteinizing hormone, although BPA was not associated with semen quality [117]. In a similar study, environmental BPA concentrations were found to be negatively correlated with free androgen index but positively related with sex hormone-binding globulin [118]. Furthermore, a retrospective cohort study summarized the relationship between PM and semen quality, and the results showed that PM2.5 and PM10 were negatively correlated with semen quality and sperm concentration during spermatogenesis [119]. A case–control study on organochlorine POPs and endometriosis showed that the elevated serum concentrations of PCBs (118, 138, 153, and 170) and p,p′-DDE exposure were associated with the increased endometriosis risk [120]. In addition, the exposure concentrations of perfluorobutane sulfonic acid (PFBS) were also found to be positively associated with the risk of endometriosis [121]. In addition to the causative effect on endometriosis, per- and polyfluoroalkyl substances (PFASs) might interfere with menstruation. A study on the correlation of PFAS exposure levels with menstrual regularity, cycle length, and amount of menstrual bleeding in prepregnant women showed that the levels of four PFASs, including perfluorooctane sulfonate (PFOS), perfluorooctanoate (PFOA), perfluorohexanesulfonate (PFHxS) and perfluorononanoic acid (PFNA), were negatively correlated with menorrhagia [122]. Another study indicated that the concentrations of methylparaben exposure in fathers were associated with the success rates of in vitro fertilization (IVF) and safe birth [123]. Examining the metabolites in the urine of the prepregnant women showed that exposure to pesticides, including organophosphate and pyrethroids, was associated with increased infertility in women [124]. Diverse evidence has been continuously gathered on environmental pollutant-incurred reproductive problems, due to the paramount biological significance of this system in human beings.
3.4. Nervous system disease
Over the past few decades, neurodevelopmental disorders in childhood and neurodegenerative diseases at older ages have attracted increasing attention. Autism spectrum disorder (ASD) is a complex neurodevelopmental disorder characterized by deficits in social interaction, and restrictive and repetitive behavior patterns, mainly occurring in infancy and lasting throughout life [125]. According to the statistics, the prevalence of ASD in 8-year-old children in the United States increased from 1/2500 in the 1970s to 1/59 in 2014, making it one of the fastest growing diseases in recent years [126]. However, the etiology of ASD remains poorly understood [127]. In addition to the genetic factors, more and more epidemiological evidence has indicated that environmental pollutants are associated with the occurrence of ASD [127]. Given that fetal brain development is extremely sensitive to environmental insults, chemical exposure during pregnancy might seriously affect neurodevelopment [128]. The study on the association between residential air pollution and childhood ASD showed that the elevated levels of some heavy metals (mercury, Cd, nickel, etc.) and chlorinated compounds (vinyl chloride, trichloroethylene, etc.) in the air during the maternal, perinatal period contributed to the increased ASD risk in the children [129]. A large case–control study in the United States also suggested a significant correlation between air pollutants and ASD in children, which also exhibited gender differences [130]. In addition, the epidemiological data has also showed exposure to EDCs, e.g., phthalates, is associated with ASD traits [131,132]. More investigations on the correlation of some other EDCs with the incidence of ASD, considering the importance of endocrine homeostasis in neurodevelopment, are needed in the future.
The association of another neurodevelopmental disorder, attention-deficit/hyperactivity disorder (ADHD), with environmental pollutants is also concerning [133]. Similar to ASD, ADHD begins to appear in childhood and persists into adulthood, which is characterized by inattention and impulsivity [134]. The rate of ADHD in children and adolescents worldwide is 5.29%, while that in adults decreases to 2.5% [135,136]. Besides the influence of genetic factors, chemical exposure is considered the causative factor for the incidence of this disease [137]. A nationwide study on the children in the United States showed that childhood exposure to BPA was associated with ADHD, and boys seemed to be more vulnerable than girls [138]. A similar result was found in a case–control study of school-age children in China [139]. Some other chemicals, like phthalates and PFOA, were also reported to be correlated with ADHD [140].
Neurodegenerative disease, mostly occurring in the elderly, is another heavy public health burden. This type of disease, such as Alzheimer’s disease (AD), results in nervous system dysfunction through the gradual and progressive loss of nerve cells [141]. An estimated 5.8 million elderly Americans are living with AD, and 13.8 million cases are expected by 2050 as the population ages [142]. Besides the known biological factors, such as age and genetics, the role of environmental factors in causing the neurodegenerative disease risk cannot be ignored [141]. A recent comprehensive retrospective epidemiological study suggested a negative correlation between PM2.5 and cognition levels [143]. The increasing evidence showed that air pollution could be blamed for the incidence of Alzheimer’s disease and related impaired cognitive functions. However, the causal relationship was not very clear, and some available findings were still controversial [144]. A recent study on Parkinson’s disease (PD) and amyotrophic lateral sclerosis (ALS) cases suggested that the ambient air pollution could worsen the diseases of the patients [145]. Furthermore, some chemical exposure could also contribute to the development of neurodegenerative diseases. An epidemiological analysis suggested metallic aluminum exposure from antiperspirants could be correlated with AD [146]. A cohort study on elderly people with occupational pesticide exposure showed an increased risk of cognitive decline [147]. Occupational exposure to fumigants or defoliants was also found to significantly increase the risk of AD [148]. Another study showed that occupational exposure to organophosphate (OP) pesticides was significantly associated with peripheral nervous system damage [149]. All the available findings suggest the potential causative role of environmental pollution in the development of neurodegenerative disease, which needs further investigation.
3.5. Other diseases
Nonalcoholic fatty liver disease (NAFLD) is a reversible fatty liver disease first characterized in the 1980s [150]. The prevalence of NAFLD is quite common in countries with high-fat diets. For example, the incidence of NAFLD has reached 30% of the total population in the United States [151]. Although poor personal lifestyles, such as overnutrition and lack of exercise, are commonly believed to contribute to NAFLD [152], some environmental pollutants may also be responsible for the rapid prevalence of this disease. Some epidemiological studies suggested that the serum concentrations of BPA, polychlorinated dibenzo-p-dioxins (PCDD), and polychlorinated dibenzofurans (PCDF) could be associated with the incidence of NAFLD [153,154]. Some other POPs like PCBs were also found to be associated with the abnormal levels of serum alanine transaminase (ALT) and aspartate transaminase (AST), suggesting the occurrence of liver injury [152]. Regarding the complexity of environmental pollution-involved etiology, more latent deleterious effects induced by chemical exposure on human health are to be explored.
4. Potential pathogenicity of environmental pollutants
The studies on the toxicological effects and the related mechanisms of some environmental pollutants are commonly performed using human-derived tissue, cell samples, as well as mammalian animals, especially rodents, which may partially provide experimental evidence on the potential etiology of the related diseases mentioned above. From the toxicological research aspect, four types of compounds, including heavy metal and organometallic compounds, organic halogen compounds, phenolic and bisphenolcompounds, and airborne fine PMs, were discussed, considering their massive production, universal environmental contamination, and high potential in causing the deleterious health outcomes (Table 2).
Table 2.
Toxicological evidence on the potential pathogenicity of environmental pollutants.
| Type | Chemical | Biological effects/potential mechanism | References |
|---|---|---|---|
| Heavy metals and organometallic compounds | Cadmium (Cd) | Cause liver diseases such as oxidative stress, fibrosis, and lipid deposition. | [[155], [156], [157], [158], [159], [160], [161], [162]] |
| Induce osteoporosis and osteomalacia. | [163,164] | ||
| Reproductive toxicities such as premature delivery and fetal growth inhibition. | [[165], [166], [167]] | ||
| Lead (Pb) | Neurotoxic effects, including the influence of hippocampal neurogenesis and functional plasticity, defects of learning and memory in offspring mice, and cognitive deficits in later life among rodents and primates. | [[170], [171], [172], [173]] | |
| Methylmercury (MeHg) | High neurotoxic effects, including mitochondrial dysfunction, behavioral changes, and blood–brain barrier damage. | [[175], [176], [177], [178], [179]] | |
| Organic halogen compounds | Tetrabromobisphenol A (TBBPA) | Thyroid morphological abnormalities and thyroid dysfunction | [182] |
| Neurotoxic effects, including impairment of spatial learning ability and activity habits in adult rats, the impact of Ca2+ imbalance, amino acid receptors and oxidative stress in SH-SY5Y cells, and damage of the blood–brain barrier. | [181,183,184] | ||
| Hepatorenal toxicity in rodents. | [185,186] | ||
| Polychlorinated biphenyls (PCBs) | Endocrine-disrupting effects such as (ant)agonistic activities of ER. | [187,188] | |
| Neurobehavioral toxic effects, including longer reflexes and anxious behaviors in offspring mice. | [189,190] | ||
| Reproductive toxicities such as affect semen quality in male rats. | [191] | ||
| Per- and polyfluoroalkyl substances (PFASs) | Neurotoxic effects such as increased motor activity and decreased habituation in offspring, impaired spatial memory and learning, and neurological dysfunction and neurobehavioral changes in rodents. | [[193], [194], [195], [196]] | |
| Reproductive toxicities, including the influence of oocyte quality and fertilization failure in pig. | [197] | ||
| Endocrine-disrupting effects such as interference with the human thyroid hormone system. | [198,199] | ||
| Hematotoxicological effects by perturbing the plasma kallikrein-kinin system (KKS) and increasing the permeability of vascular endothelial cells. | [200,201] | ||
| Phenolic and bisphenolic compounds | Bisphenol A (BPA) | Endocrine-disrupting effects, including the agonistic activity of ER and antagonistic activities of AR, TR, and PPARγ. | [[205], [206], [207], [208], [209]] |
| Reproductive toxicities such as reducing adult sexual activity levels, promoting sexual maturation, affecting the menstrual cycle, and inhibiting embryonic development in female rodents, and also affecting the meiosis process of spermatocytes in male rodents. | [[210], [211], [212], [213]] | ||
| Neurotoxic effects such as behavioral abnormalities, neuronal apoptosis, and cognitive decline. | [[214], [215], [216]] | ||
| Synthetic phenolic antioxidants (SPAs) | Lipid metabolism disorders such as promoting the adipogenic differentiation of 3T3-L1 preadipocytes and accelerating the accumulation of intracellular triglycerides in mice. | [219,220] | |
| Endocrine-disrupting effects such as perturb steroidogenesis. | [221] | ||
| Reproductive toxicity such as weight reduction of uterine and uterine adnexa in immature female rats. | [222] | ||
| Airborne fine PMs | PM2.5 | Cause cardiovascular and respiratory problems. | [226] |
| Neurotoxic effects such as induced cellular damage in SH-SY5Y cells and compromised learning and cognitive performance. | [227,228] | ||
| Reproductive toxicity such as reducing the serum testosterone levels, the sperm count and motility, and promoting the sperm malformation and germ cell apoptosis in male SD rats. | [[229], [230], [231]] | ||
| Metabolism disorders of glucose and lipid such as impaired glucose tolerance, hindered glycogen synthesis, increased plasma glucose levels, and induced inflammatory response of visceral adipose tissue and liver lipid metabolism in male mice. | [232,233] |
4.1. Heavy metal and organometallic compounds
As a toxic heavy metal pollutant, Cd exists widely in industrial production and human routine activities, which makes its inevitable exposure to human bodies. Cd can enter the organisms through various routes, including skin contact, ingestion, and inhalation, and may accumulate in the liver, kidney, and skeleton, resulting in the consequent hazardous effects, including hepatotoxicity, nephrotoxicity, and even carcinogenesis. Since the liver is the main target organ of Cd-induced injury, a large number of studies involving Cd-induced liver diseases have been reported so far. The excessive reactive oxygen species (ROS) production, the reduction of the antioxidant enzyme activities, and the consequent irreversible oxidative stress were considered the main toxic mechanism for Cd-induced hepatic damage in mammals [155,156]. Along with the worsening hepatotoxicity from the continuous cellular oxidative stress, the liver could develop the symptom of fibrosis due to Cd exposure [157]. In addition, the toxicological study also indicated that Cd treatment could induce hepatocellular lipid deposition and the alignment disorder of the hepatic cord and cause immune deficiency and dysfunction in the liver by disturbing the production of proinflammatory cytokines [[158], [159], [160]]. Cd was also reported to potentially induce liver cancer by inhibiting endoplasmic reticulum stress [161,162]. The accumulation of Cd in bones could disturb the metabolic balance of osteoclasts and decrease bone density, thus increasing the risk of osteoporosis and osteomalacia [163,164]. As for the reproductive toxicity, Cd accumulated in the ovary and testis could affect the development and function of the reproductive organs and reduce gametogenesis [165]. In addition, Cd could also enter the placental tissue through umbilical cord blood and cause adverse effects on the pregnancy process, leading to risks of premature delivery and fetal growth inhibition [166,167].
Lead (Pb) is widely used in industry because of its good ductility, low melting point, and strong corrosion resistance. As a well-known toxic metal element, Pb released into the environment can be stable for long periods of time, thus causing a serious threat to public health [168]. The main target organs of lead exposure include the liver, kidneys, nervous system, and cardiovascular system [169]. The neurotoxicological study showed that Pb exposure could induce the proliferation of the microglia and astrocytes in the hippocampus of young mice by triggering the TLR4-MyD88-NFκB signaling pathway, thus interfering with hippocampal neurogenesis and functional plasticity [170]. Pb exposure during maternal pregnancy could affect the offspring’s emotional behaviors and impair their learning and memory abilities. The expressions of cadherin 2 and cadherin 3, the members of the cadherin superfamily that regulated postsynaptic activity, were significantly altered, and the learning and memory abilities were compromised in the offspring due to the maternal Pb exposure during the period from gestation to weaning [171]. Exposure to Pb in the early life stage might affect the activities of the proteins associated with the development of Alzheimer’s disease, leading to cognitive deficits in the elder life stage among rodents and primates [172,173].
Mercury is a ubiquitous, persistent heavy metal with organic and inorganic species in the environment [174]. As a representative toxic organometallic compound, methylmercury (MeHg) exhibited high neurotoxicity, especially when the exposure occurred during fetal development [175]. MeHg exposure could induce oxidative damage and cause genomic instability, DNA damage, glutathione metabolism disturbance, and mitochondrial dysfunction in the cerebral cortex of rodents, thus leading to neurobehavioral abnormality [176,177]. Both in vitro and in vivo studies showed that MeHg exposure in rodents could upregulate vascular endothelial growth factor (VEGF) and induce damage to the blood–brain barrier, consequently causing the brain edema, bleeding, and microcirculatory failure [178]. The neurotoxicity induced by MeHg exposure might involve multiple molecular mechanisms, including the disturbances in the ubiquitin-proteasome system, pyruvate transport to mitochondria, and chemokines [179].
4.2. Organic halogen compounds
As a new brominated flame retardant, tetrabromobisphenol A (TBBPA) and its derivatives are widely used in industrial products, such as circuit boards, furniture, plastic tableware, and other materials. Organisms can be exposed to TBBPA released in the environment through ingestion, respiration, and direct contact and may affect the endocrine system, neurodevelopment, thyroid function, and tumorigenesis [180,181]. As a potential EDC, TBBPA has a similar structure to the thyroid hormone and could affect the expressions of the related genes regulated by the thyroid receptors, leading to thyroid morphological abnormalities and thyroid dysfunction [182]. In addition, TBBPA was also reported to cause the impairment of spatial learning ability in adult rats, exerting potential neurotoxicity [183]. A human neuroblastoma cell (SH-SY5Y)-based study showed that TBBPA stimulation caused intracellular Ca2+ imbalance, the formation of β-amyloid peptide, and oxidative stress [184]. Another study showed that TBBPA could change the function of ATP binding cassette (ABC) transporters in the blood–brain barrier, interfering with the brain homeostasis and the central nervous system in rats [181]. Some other target organ toxicity, like hepatorenal toxicity, was also reported for TBBPA in rodents [185,186].
Polychlorinated biphenyls (PCBs) are a class of synthetic chlorinated compounds composed of more than 200 congeners used in lubricants, insulation, and coolants. Although PCBs have been banned or restricted in many countries, they are still ubiquitous and persistent in the environment. Long-term contamination of PCBs in the environment may pose potential risks to human health by causing endocrine-disrupting effects, neurotoxicity, and reproductive toxicity. A variety of PCB congeners have been recognized to display (anti)estrogenic activities, which could be responsible for the reproductive disease observed in epidemiological studies [120]. For example, using an in vitro luciferase reporter screening assay, nine PCB congeners exhibited estrogen receptor (ER) agonistic activities, and another nine congeners showed antagonistic activities [187]. The study on the chemical structure-related effect showed that PCB congeners with less chlorination exhibited stronger estrogenic activities based on an ER-CALUX (estrogen receptor-chemical activated luciferase expression) bioassay [188]. A study on neurobehavioral toxicity showed that the offspring with maternal exposure to six nondioxin-like-PCBs (NDL-PCBs) mixture during lactation displayed significantly longer turning reflexes than the control and exhibited anxious behaviors, which might be associated with ryanodine receptor 3 (RyR3) overexpression in cerebellar neurons and the resultant disturbance in the calcium signaling pathway [189,190]. Regarding reproductive toxicity, the subcutaneous injection of PCB-77 was found to affect semen quality in male rats, including a decreased sperm count and an increased proportion of abnormal sperm, such as abnormal sperm tails [191]. The underlying mechanism of the initial molecular event of PCBs was presumed to be the downregulation of intercellular connexins, which disrupted the integrity of the cell membrane, and compromised the cell barrier function [192].
PFASs are fluorinated compounds used in a wide range of industrial manufacture and daily life, including hydraulic fluids, leather, food packaging, shampoo, etc. Due to their unique chemical properties and high toxicity, some PFAS compounds, such as PFOS, PFOA, and their salts, precursors, and related products, have drawn global attention and been listed in the Stockholm Convention. The currently available toxicological studies showed that PFASs might exert neurotoxicity, reproductive toxicity, endocrine disturbance, and hematotoxicity. In concern of the neurotoxicity of PFASs, a neurobehavioral study showed PFOS exposure during pregnancy could result in increased motor activity and decreased habituation in the offspring [193]. PFOS exposure also caused a decline in spatial memory and learning ability in adult mice [194]. The neurological dysfunction and neurobehavioral changes could be induced by PFOS through increasing intracellular Ca2+ concentration, activating the ROS-dependent protein kinase C (PKC) signaling pathway, and inducing neuronal apoptosis [195,196]. The reproductive toxicity of PFASs has been rarely reported so far. A recent study showed that PFOS could affect the meiotic process, mitochondrial function, and the distribution of cortical particles (CGs) in porcine oocytes, leading to poor oocyte quality and fertilization failure [197]. This could be relevant to the adverse reproductive outcome revealed by the epidemiological study [122]. In addition, in vitro studies showed that PFASs could affect the thyroid hormone system, resulting in endocrine-disrupting effects [198,199]. Recent studies also indicated that PFASs could cause hematotoxicological effects by perturbing the plasma kallikrein-kinin system (KKS) and increasing the permeability of vascular endothelial cells [200,201].
4.3. Phenolic and bisphenol compounds
BPA, as the main representative of bisphenol compounds, is an important raw material that can be widely used to produce polycarbonates, epoxy resins, polymer materials, plasticizers, antioxidants, and other chemical products [202]. Human exposure to BPA is mainly through the routes of contaminated water and food-packaging materials [203] and might cause a variety of toxic effects, including endocrine-disrupting effects, reproductive toxicity, and neurotoxicity [204]. As an environmental EDC, the estrogenic activity of BPA has been fully demonstrated by both in vitro and in vivo studies [205]. Besides the agonistic binding with ERs [206], BPA could bind with some other nuclear receptors, including androgen receptor (AR), thyroid hormone receptor (TR), and peroxisome proliferator-activated receptor γ (PPARγ), to display the antagonistic effects [[207], [208], [209]]. As for reproductive toxicity, neonatal exposure to BPA significantly reduced adult sexual activities through an estrogen-mediated pathway in female rats [210]. Other reports suggested that BPA exposure in female rodents could promote sexual maturation, induce changes in the menstrual cycle [211], and inhibit embryonic development by producing aneuploid chromosomal mutations [212]. Furthermore, BPA could affect spermatogenesis by slowing down the meiosis process of spermatocytes, resulting in decreased semen quality and sperm count and abnormal offspring [213]. These pathological results might support the epidemiological findings on the positive correlation of reproductive problems with BPA exposure [117,118]. The neurotoxicity of BPA in rodents was proven by the findings on its toxic effects on neurons, including the increased neuronal apoptosis, behavioral abnormalities [214,215], and the cognitive decline in offspring due to the perinatal chemical exposure [216].
As food additives, synthetic phenolic antioxidants (SPAs) have been widely consumed around the world in recent years due to their excellent antioxidant activity without affecting the taste and color of food [217]. The lipophilic SPAs can be directly consumed in large amounts by the human body through diet, causing potential health issues, although most of these compounds exhibit low acute toxicity [217,218]. The toxicological studies indicated SPAs might cause lipid metabolism disorder and reproductive toxicity. For example, a recent study showed that butyl hydroxyanisole (BHA), one of the SPAs, could promote the adipogenic differentiation of 3T3-L1 preadipocytes [219] and accelerate the accumulation of intracellular triglycerides in white fat tissues of high fat-diet mice, suggesting that BHA could be a new environmental obesogen [220]. In regard to endocrine-disrupting effects and reproductive toxicity, BHA was reported to perturb steroidogenesis by inducing E2 secretion [221]. Another study indicated that this chemical could exert antiestrogenic activity, resulting in the significant weight reduction of uterine and uterine adnexa among immature female rats, suggesting that BHA might cause the risk of infertility [222].
4.4. Airborne fine PMs
Airborne fine PMs have become one of the globally leading health threats [223], and more than 90% of the population worldwide is suffering from polluted air, with PM2.5 levels exceeding the WHO standard of 10 μg/m3 [224]. A recent study has confirmed that these airborne particles can indeed migrate into the human circulatory system, increasing the risk of internal exposure [225]. Long-term exposure to airborne PM2.5 is commonly believed to cause cardiovascular and respiratory problems [226]. However, as the blood circulates, PM2.5 can be carried to other organs, causing neurotoxicity, reproductive toxicity, and systemic metabolism disorders of glucose and lipid. In concern of neurotoxicity, a study based on human SH-SY5Y nerve cells showed PM2.5 caused cellular damage by inducing mitochondrial dysfunction, oxidative stress response, and inflammatory response [227]. Furthermore, researchers found that the male rats exposed to PM2.5 had significantly compromised learning and cognitive performance due to the increased levels of heavy metals and glutamate in the hippocampus and abnormal expressions of glutamate receptors [228]. There has been increasing concern about the reproductive toxicity of PM2.5 in recent years. PM2.5 exposure in male Sprague–Dawley (SD) rats reduced the serum testosterone levels and semen quality by causing apoptosis and autophagy in testicular tissue [229]. Another study indicated that the decreases in the sperm count and motility and the increase in the sperm malformation rate were caused in the male SD rats with PM2.5 exposure, and the in vitro and in vivo results suggested that PM2.5 could induce DNA damage by increasing ROS, elevating the expressions of apoptosis-related proteins, and disturbing mitochondrial function [230]. The induction of endoplasmic reticulum stress was also reported to contribute to PM2.5-caused germ cell apoptosis [231], thus potentially incurring the male infertility problem. Combined with the related epidemiological finding [119], the cause-and-effect relationship could be possibly recognized between airborne fine PMs and reproductive disease. Additionally, the exposure to PM2.5 was found to be related to the dysregulation of glucose and lipid metabolism in vivo, as evidenced by the findings on the impaired glucose tolerance, hindered glycogen synthesis, and increased plasma glucose level in male mice [232]. The underlying mechanism study showed that PM2.5 exposure caused the increase of Nrf2 expression, induced oxidative stress, then activated JNK signaling and inhibited the IRS-1/ATK pathway, leading to insulin resistance and incidence risk of type 2 diabetes [232]. Similar findings were observed in the male mice with PM2.5 treatment with a different mechanism explanation that PM2.5 regulated the inflammatory response of visceral adipose tissue and liver lipid metabolism in vivo [233].
Altogether, the toxicological studies may provide hints of the latent threats from environmental pollution to human health. The pollutants which may have deleterious effects on human bodies and cause health issues include but are not limited to the chemicals mentioned above. More toxicological data are still urgently needed for those chemicals with widespread occurrence in the environment and high exposure risks to human beings. The relevance between the pathological data and the epidemiological evidence needs to be further investigated to clarify the cause-and-effect relationship between the pollutants and related diseases.
5. Perspectives
Environmental pollution may cause diverse health problems in humans. The former or ongoing public health emergencies signal the dreadful threat from the pollution incidents; more suspicious disease-causative pollutants may be discovered from epidemiological studies, and toxicological research may provide important information on the potentials of the chemicals causing adverse health effects. Although a massive body of data has been gathered on environmental health hazards, a concerted effort is still required to resolve the yet unsettled issues. The future focus may be placed on the integrated analysis of adverse health effects from the combined pollutant exposure, individual-specific environmental insult-induced morbidity, and new health threats from emerging chemicals of concern. Unveiling the hidden culprit for human health problems from the environment has been, and will continue to be, the arduous mission for the sake of a better life.
Declaration of competing interests
The authors declare no competing financial interests.
Acknowledgments
This work was financially supported by the National Key R&D Program of China (2018YFA0901101) and the National Natural Science Foundation of China (22193050, 92143301, 22176203).
References
- 1.World health statistics 2019 . World Health Organization; Geneva: 2019. Monitoring health for the sdgs, sustainable development goals. [Google Scholar]
- 2.Mielke H.W., Reagan P.L. Soil is an important pathway of human lead exposure. Environ Health Perspect. 1998;106:217–229. doi: 10.1289/ehp.98106s1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goralczyk K., Majcher A. Are the civilization diseases the result of organohalogen environmental pollution? Acta Biochim Pol. 2019;66:123–127. doi: 10.18388/abp.2018_2776. [DOI] [PubMed] [Google Scholar]
- 4.Prüss-Üstün A., Corvalán C.F. World Health Organization; Geneva: 2006. Preventing disease through healthy environments: towards an estimate of the environmental burden of disease. [Google Scholar]
- 5.Laborde A., Tomasina F., Bianchi F., Brune M.N., Buka I., Comba P., et al. Children's health in Latin America: the influence of environmental exposures. Environ Health Perspect. 2015;123:201–209. doi: 10.1289/ehp.1408292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World health statistics . World Health Organization; Geneva: 2021. Monitoring health for the sdgs, sustainable development goals. [Google Scholar]
- 7.Qu G.B., Li X.D., Hu L.G., Jiang G.B. An imperative need for research on the role of environmental factors in transmission of novel coronavirus (COVID-19) Environ Sci Technol. 2020;54:3730–3732. doi: 10.1021/acs.est.0c01102. [DOI] [PubMed] [Google Scholar]
- 8.Bouville A., Linet M.S., Hatch M., Mabuchi K., Simon S.L. Guidelines for exposure assessment in health risk studies following a nuclear reactor accident. Environ Health Perspect. 2014;122:1–5. doi: 10.1289/ehp.1307120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cardis E., Hatch M. The Chernobyl accident - an epidemiological perspective. Clin Oncol. 2011;23:251–260. doi: 10.1016/j.clon.2011.01.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams D. Radiation carcinogenesis: lessons from Chernobyl. Oncogene. 2008;27:S9–S18. doi: 10.1038/onc.2009.349. [DOI] [PubMed] [Google Scholar]
- 11.Jacob P., Bogdanova T.I., Buglova E., Chepurniy M., Demidchik Y., Gavrilin Y., et al. Thyroid cancer among Ukrainians and Belarusians who were children or adolescents at the time of the Chernobyl accident. J Radiol Prot. 2006;26:51–67. doi: 10.1088/0952-4746/26/1/003. [DOI] [PubMed] [Google Scholar]
- 12.Bromet E.J. Mental health consequences of the Chernobyl disaster. J Radiol Prot. 2012;32:N71–N75. doi: 10.1088/0952-4746/32/1/N71. [DOI] [PubMed] [Google Scholar]
- 13.Ritz B., Morgenstern H., Crawford-Brown D., Young B. The effects of internal radiation exposure on cancer mortality in nuclear workers at rocketdyne/atomics international. Environ Health Perspect. 2000;108:743–751. doi: 10.1289/ehp.00108743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imaizumi M., Usa T., Tominaga T., Neriishi K., Akahoshi M., Nakashima E., et al. Radiation dose-response relationships for thyroid nodules and autoimmune thyroid diseases in Hiroshima and Nagasaki atomic bomb survivors 55-58 years after radiation exposure. JAMA. 2006;295:1011–1022. doi: 10.1001/jama.295.9.1011. [DOI] [PubMed] [Google Scholar]
- 15.Landrigan P.J., Goldman L.R. Children's vulnerability to toxic chemicals: a challenge and opportunity to strengthen health and environmental policy. Health Aff. 2011;30:842–850. doi: 10.1377/hlthaff.2011.0151. [DOI] [PubMed] [Google Scholar]
- 16.Barreto M.L. The globalization of epidemiology: critical thoughts from Latin America. Int J Epidemiol. 2004;33:1132–1137. doi: 10.1093/ije/dyh113. [DOI] [PubMed] [Google Scholar]
- 17.Barreto S.M., Miranda J.J., Figueroa J.P., Schmidt M.I., Munoz S., Kuri-Morales P.P., et al. Epidemiology in Latin America and the caribbean: current situation and challenges. Int J Epidemiol. 2012;41:557–571. doi: 10.1093/ije/dys017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.2019 TRI national analysis materials; United States Environmental Protection Agency. 2019. Washington, DC. [Google Scholar]
- 19.2015 TRI national analysis materials; United States Environmental Protection Agency. 2015. Washington, DC. [Google Scholar]
- 20.Brimblecombe P. In: Encyclopedia of environmental health. Nriagu J., editor. Elsevier; Norwich: 2019. Air pollution episodes; pp. 41–48. [Google Scholar]
- 21.Bell M.L., Davis D.L. Reassessment of the lethal London fog of 1952: novel indicators of acute and chronic consequences of acute exposure to air pollution. Environ Health Perspect. 2001;109:389–394. doi: 10.2307/3434786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin A.E. Mortality and morbidity statistics and air pollution. Proc Roy Soc Med. 1964;57:969–975. doi: 10.1001/jama.1934.02750480063026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lioy P.J., Waldman J.M. Acidic sulfate aerosols: characterization and exposure. Environ Health Perspect. 1989;79:15–34. doi: 10.2307/3430526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kitagawa T. Cause analysis of the Yokkaichi asthma episode in Japan. J Air Pollut Control Assoc. 1984;34:743–746. doi: 10.1080/00022470.1984.10465807. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida K., Oshima H., Imai M. Air pollution and asthma in Yokkaichi, air pollution and asthma in Yokkaichi. Arch Environ Health. 1966;13:763–768. doi: 10.1080/00039896.1966.10664661. [DOI] [PubMed] [Google Scholar]
- 26.Guo P., Yokoyama K., Suenaga M., Kida H. Mortality and life expectancy of Yokkaichi asthma patients, Japan: late effects of air pollution in 1960–70s. Environ Health. 2008;7:8. doi: 10.1186/1476-069X-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kagawa J. Atmospheric pollution due to mobile sources and effects on human health in Japan. Environ Health Perspect. 1994;102:93–99. doi: 10.2307/3431936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwartz J. Air pollution and daily mortality: a review and meta analysis. Environ Res. 1994;64:36–52. doi: 10.1006/enrs.1994.1005. [DOI] [PubMed] [Google Scholar]
- 29.Rall David P. Review of the health effects of sulfur oxides. Environ Health Perspect. 1974;8:97–121. doi: 10.1289/ehp.74897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pope C.A. Epidemiology of fine particulate air pollution and human health: biologic mechanisms and who's at risk? Environ Health Perspect. 2000;108:713–723. doi: 10.1289/ehp.108-1637679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nogawa K., Kido T. Biological monitoring of cadmium exposure in Itai-itai disease epidemiology. Int Arch Occup Environ Health. 1993;65:S43–S46. doi: 10.1007/BF00381306. [DOI] [PubMed] [Google Scholar]
- 32.Satarug S., Garrett Scott H., Sens Mary A., Sens Donald A. Cadmium, environmental exposure, and health outcomes. Environ Health Perspect. 2010;118:182–190. doi: 10.1289/ehp.0901234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inaba T., Kobayashi E., Suwazono Y., Uetani M., Oishi M., Nakagawa H., et al. Estimation of cumulative cadmium intake causing Itai–itai disease. Toxicol Lett. 2005;159:192–201. doi: 10.1016/j.toxlet.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi E., Okubo Y., Suwazono Y., Kido T., Nishijo M., Nakagawa H., et al. Association between total cadmium intake calculated from the cadmium concentration in household rice and mortality among inhabitants of the cadmium-polluted Jinzu river basin of Japan. Toxicol Lett. 2002;129:85–91. doi: 10.1016/s0378-4274(01)00520-3. [DOI] [PubMed] [Google Scholar]
- 35.Eto K. Pathology of minamata disease. Toxicol Pathol. 1997;25:614–623. doi: 10.1289/ehp.121-A304. [DOI] [PubMed] [Google Scholar]
- 36.Kessler R. The minamata convention on mercury: a first step toward protecting future generations. Environ Health Perspect. 2013;121:A304–A309. doi: 10.1289/ehp.121-A304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor D. Minamata disease. Environ Sci Technol. 1982;16 doi: 10.1021/es00096a701. 81A-81A. [DOI] [PubMed] [Google Scholar]
- 38.Grandjean P., Satoh H., Murata K., Eto K. Adverse effects of methylmercury: environmental health research implications. Environ Health Perspect. 2010;118:1137–1145. doi: 10.1289/ehp.0901757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuratsune M., Yoshimura T., Matsuzaka J., Yamaguchi A. Epidemiologic study on Yusho, a poisoning caused by ingestion of rice oil contaminated with a commercial brand of polychlorinated biphenyls. Environ Health Perspect. 1972;1:119–128. doi: 10.1289/ehp.7201119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakanishi Y., Shigematsu N., Kurita Y., Matsuba K., Kanegae H., Ishimaru S., et al. Respiratory involvement and immune status in Yusho patients. Environ Health Perspect. 1985;59:31–36. doi: 10.1289/ehp.59-1568074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Urabe H., Asahi M. Past and current dermatological status of Yusho patients. Environ Health Perspect. 1985;59:11–15. doi: 10.1289/ehp.59-1568099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen P.H., Wong C.K., Rappe C., Nygren M. Polychlorinated biphenyls, dibenzofurans and quaterphenyls in toxic rice-bran oil and in the blood and tissues of patients with PCB poisoning (Yu-Cheng) in Taiwan. Environ Health Perspect. 1985;59:59–65. doi: 10.1289/ehp.59-1568079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lü Y.C., Wu Y.C. Clinical findings and immunological abnormalities in Yu-Cheng patients. Environ Health Perspect. 1985;59:17–29. doi: 10.1289/ehp.59-1568085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miyata H., Fukushima S., Kashimoto T., Kunita N. PCBs, PCQs and PCDFs in tissues of Yusho and Yu-Cheng patients. Environ Health Perspect. 1985;59:67–72. doi: 10.1289/ehp.59-1568097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crabb C. Revisiting the bhopal tragedy. Science. 2004;306:1670–1671. doi: 10.1126/science.306.5702.1670. [DOI] [PubMed] [Google Scholar]
- 46.Tachakra S. The bhopal disaster. J Roy Soc Health. 1987;107:1–2. doi: 10.1177/146642408710700101. [DOI] [PubMed] [Google Scholar]
- 47.Murphy J.F., Hendershot D., Berger S., Summers A.E., Willey R.J. Bhopal revisited. Process Saf Prog. 2014;33:310–313. doi: 10.1002/prs.11716. [DOI] [Google Scholar]
- 48.Toxicological and health aspects of melamine and cyanuric acid: report of a who expert meeting in collaboration with. FAO; World Health Organization; Geneva: 2009. [Google Scholar]
- 49.Wu Y.N., Zhao Y.F., Li J.G. A survey on occurrence of melamine and its analogues in tainted infant formula in China, Biomed. Environ Sci. 2009;22:95–99. doi: 10.1016/S0895-3988(09)60028-3. [DOI] [PubMed] [Google Scholar]
- 50.Jiang G., Zhou Q. Direct Grignard pentylation of organotin-contaminated lard samples followed by capillary gas chromatography with flame photometric detection. J. Chromator. A. 2000;886:197–205. doi: 10.1016/S0021-9673(00)00494-5. [DOI] [PubMed] [Google Scholar]
- 51.Jiang G., Zhou Q., He B. Speciation of organotin compounds, total tin, and major trace metal elements in poisoned human organs by gas chromatography-flame photometric detector and inductively coupled plasma-mass spectrometry. Environ Sci Technol. 2000;34:2697–2702. doi: 10.1021/es0008822. [DOI] [Google Scholar]
- 52.Gobler C.J., Sunda W.G. Ecosystem disruptive algal blooms of the brown tide species, aureococcus anophagefferens and aureoumbra lagunensis. Harmful Algae. 2012;14:36–45. doi: 10.1016/j.hal.2011.10.013. [DOI] [Google Scholar]
- 53.Our nutrient world: the challenge to produce more food and energy with less pollution. Centre for Ecology and Hydrology; Edinburgh: 2013. [Google Scholar]
- 54.Carmichael W.W., Azevedo S.M., An J.S., Molica R.J., Jochimsen E.M., Lau S., et al. Human fatalities from cyanobacteria: chemical and biological evidence for cyanotoxins. Environ Health Perspect. 2001;109:663–668. doi: 10.1289/ehp.01109663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Caussy D., Priest N.D. Introduction to arsenic contamination and health risk assessment with special reference to Bangladesh. Rev Environ Contam Toxicol. 2008;197:1–15. doi: 10.1007/978-0-387-79284-2_1. [DOI] [PubMed] [Google Scholar]
- 56.Garelick H., Jones H., Dybowska A., Valsami-Jones E. Arsenic pollution sources. Rev Environ Contam Toxicol. 2008;197:17–60. doi: 10.1007/978-0-387-79284-2_2. [DOI] [PubMed] [Google Scholar]
- 57.Yu G., Sun D., Zheng Y. Health effects of exposure to natural arsenic in groundwater and coal in China: an overview of occurrence. Environ Health Perspect. 2007;115:636–642. doi: 10.1289/ehp.9268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jones H., Visoottiviseth P., Bux K., Foldenyi R., Kovats N., Borbely G., et al. Case reports: arsenic pollution in Thailand, Bangladesh, and Hungary. Rev Environ Contam Toxicol. 2008;197:163–187. doi: 10.1007/978-0-387-79284-2_6. [DOI] [PubMed] [Google Scholar]
- 59.Liu R., Tushar M.S.K., Saifur M.R., Qu J. Strategy and development direction for arsenic pollution control in drinking water in Bangladesh. J Environ Chem Eng. 2020;14:2075–2080. doi: 10.12030/j.cjee.202006063. [DOI] [Google Scholar]
- 60.Zheng Y. Global solutions to a silent poison. Science. 2020;368:818–819. doi: 10.1126/science.abb9746. [DOI] [PubMed] [Google Scholar]
- 61.Steven H.L., Engel A., Cecilia A.P., Chen R., Feinleib M. Arsenic cancer risk confounder in southwest Taiwan data set. Environ Health Perspect. 2006;114:1077–1082. doi: 10.1289/ehp.8704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guidelines for drinking-water quality. World Health Organization; Geneva: 2011. [Google Scholar]
- 63.Marian K.M., Scheidegger R., Julshamn K., Bader H.P. Substance flow analysis: a case study of fluoride exposure through food and beverages in young children living in Ethiopia. Environ Health Perspect. 2011;119:579–584. doi: 10.1289/ehp.1002365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Finkelman R.B., Orem W., Castranova V., Tatu C.A., Belkin H.E., Zheng B., et al. Health impacts of coal and coal use: possible solutions. Int J Coal Geol. 2002;50:425–443. doi: 10.1016/S0166-5162(02)00125-8. [DOI] [Google Scholar]
- 65.Cao J., Liu J.W., Tang L.L., Sangbu D.Z., Yu S., Zhou S., et al. Dental and early-stage skeletal fluorosis in children induced by fluoride in brick-tea. Fluoride. 2005;38:44–47. [Google Scholar]
- 66.Guo J., Wu H., Zhao Z., Wang J., Liao H. Review on health impacts from domestic coal burning: emphasis on endemic fluorosis in Guizhou province, southwest China. Rev Environ Contam Toxicol. 2021;258:1–25. doi: 10.1007/398_2021_71. [DOI] [PubMed] [Google Scholar]
- 67.Endemic fluorosis in Guizhou province, China. World Health Forum; 1996. [PubMed] [Google Scholar]
- 68.Dai S., Ren D., Ma S. The cause of endemic fluorosis in western Guizhou province, southwest China. Fuel. 2004;83:2095–2098. doi: 10.1016/j.fuel.2004.03.016. [DOI] [Google Scholar]
- 69.Occupational safety and health in public health emergencies: a manual for protecting health workers and responders. World Health Organization; Geneva: 2018. [Google Scholar]
- 70.Merget R., Bauer T., Küpper H., Philippou S., Bauer H., Breitstadt R., et al. Health hazards due to the inhalation of amorphous silica. Arch Toxicol. 2002;75:625–634. doi: 10.1007/s002040100266. [DOI] [PubMed] [Google Scholar]
- 71.Greenberg M.I., Waksman J., Curtis J. Silicosis: a review. Dis. Mon. 2007;53:394–416. doi: 10.1016/j.disamonth.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 72.Leung C.C., Yu I.T.S., Chen W. Silicosis, Lancet. 2012;379:2008–2018. doi: 10.1016/S0140-6736(12)60235-9. [DOI] [PubMed] [Google Scholar]
- 73.Salahuddin M., Cawasji Z., Kaur S., Estrada-Y-Martin R.M., Cherian S.V. Current concepts in pathogenesis, diagnosis, and management of silicosis and its subtypes. Curr. Pulmonol. Rep. 2021;10:135–142. doi: 10.1007/s13665-021-00279-x. [DOI] [Google Scholar]
- 74.Kerr L. Review of the hawk's nest incident: America's worst industrial disaster, by M. Cherniack. J Publ Health Pol. 1987;8:581–582. https://doi-org-443.webvpn.las.ac.cn/10.2307/3342283 [Google Scholar]
- 75.Public health impact of pesticides used in agriculture. World Health Organization; Geneva: 1990. [Google Scholar]
- 76.Anwar W.A. Biomarkers of human exposure to pesticides. Environ Health Perspect. 1997;104:801–806. doi: 10.1289/ehp.97105s4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McCauley L.A., Anger W.K., Keifer M., Langley R., Robson M.G., Rohlman D. Studying health outcomes in farmworker populations exposed to pesticides. Environ Health Perspect. 2006;114:953–960. doi: 10.1289/ehp.8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Beshwari M.M.M., Bener A., Ameen A., Al-Mehdi A.M., Ouda H.Z., Pasha M.A.H. Pesticide-related health problems and diseases among farmers in the United Arab Emirates. Int J Environ Health Res. 1999;9:213–221. doi: 10.1080/09603129973182. [DOI] [Google Scholar]
- 79.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 80.Kilner P.J. Pulmonary resistance in cardiovascular context. Int J Cardiol. 2004;97:3–6. doi: 10.1016/j.ijcard.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 81.Cosselman K.E., Navas-Acien A., Kaufman J.D. Environmental factors in cardiovascular disease. Nat Rev Cardiol. 2015;12:627–642. doi: 10.1038/nrcardio.2015.152. [DOI] [PubMed] [Google Scholar]
- 82.Hart J.E., Laden F., Schenker M.B., Garshick E. Chronic obstructive pulmonary disease mortality in diesel-exposed railroad workers. Environ Health Perspect. 2006;114:1013–1017. doi: 10.1097/00001648-200611001-00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wheeler A., Zanobetti A., Gold Diane R., Schwartz J., Stone P., Suh H.H. The relationship between ambient air pollution and heart rate variability differs for individuals with heart and pulmonary disease. Environ Health Perspect. 2006;114:560–566. doi: 10.1289/ehp.8337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu L., Poon R., Chen L., Frescura A.M., Montuschi P., Ciabattoni G., et al. Acute effects of air pollution on pulmonary function, airway inflammation, and oxidative stress in asthmatic children. Environ Health Perspect. 2009;117:668–674. doi: 10.1289/ehp11813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen R., Yin P., Meng X., Liu C., Wang L., Xu X., et al. Fine particulate air pollution and daily mortality. A nationwide analysis in 272 Chinese cities. Am J Respir Crit Care Med. 2017;196:73–81. doi: 10.1164/rccm.201609-1862OC. [DOI] [PubMed] [Google Scholar]
- 86.Dong G.H., Qian Z., Xaverius P.K., Trevathan E., Maalouf S., Parker J., et al. Association between long-term air pollution and increased blood pressure and hypertension in China. Hypertension. 2013;61:578–584. doi: 10.1161/HYPERTENSIONAHA.111.00003. [DOI] [PubMed] [Google Scholar]
- 87.Yang B.Y., Qian Z., Vaughn M.G., Nelson E.J., Dharmage S.C., Heinrich J., et al. Is prehypertension more strongly associated with long-term ambient air pollution exposure than hypertension? Findings from the 33 communities Chinese health study. Environ Pollut. 2017;229:696–704. doi: 10.1016/j.envpol.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhou M., Liu Y., Wang L., Kuang X., Xu X., Kan H. Particulate air pollution and mortality in a cohort of Chinese men. Environ Pollut. 2014;186:1–6. doi: 10.1016/j.envpol.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 89.Chen H., Burnett R.T., Bai L., Kwong J.C., Crouse D.L., Lavigne E., et al. Residential greenness and cardiovascular disease incidence, readmission, and mortality. Environ Health Perspect. 2020;128:87005. doi: 10.1289/EHP6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Matuszczak E., Komarowska M.D., Debek W., Hermanowicz A. The impact of bisphenol a on fertility, reproductive system, and development: a review of the literature. Internet J Endocrinol. 2019;10:4068717. doi: 10.1155/2019/4068717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Calafat A.M., Kuklenyik Z., Reidy J.A., Caudill S.P., Ekong J., Needham L.L. Urinary concentrations of bisphenol a and 4-nonylphenol in a human reference population. Environ Health Perspect. 2005;113:391–395. doi: 10.1289/ehp.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Danaei G., Finucane M.M., Lu Y., Singh G.M., Cowan M.J., Paciorek C.J., et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 93.Taylor K.W., Novak R.F., Anderson H.A., Birnbaum L.S., Blystone C., DeVito M., et al. Evaluation of the association between persistent organic pollutants (POPs) and diabetes in epidemiological studies: a national toxicology program workshop review. Environ Health Perspect. 2013;121:774–783. doi: 10.1289/ehp.1205502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thayer K.A., Heindel J.J., Bucher J.R., Gallo M.A. Role of environmental chemicals in diabetes and obesity: a national toxicology program workshop review. Environ Health Perspect. 2012;120:779–789. doi: 10.1289/ehp.1104597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kuo C.C., Moon K., Thayer K.A., Navas-Acien A. Environmental chemicals and type 2 diabetes: an updated systematic review of the epidemiologic evidence. Curr Diabetes Rep. 2013;13:831–849. doi: 10.1007/s11892-013-0432-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Eze I.C., Hemkens L.G., Bucher H.C., Hoffmann B., Schindler C., Künzli N., et al. Association between ambient air pollution and diabetes mellitus in Europe and North America: systematic review and meta-analysis. Environ Health Perspect. 2015;123:381–389. doi: 10.1289/ehp.1307823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xiao L., Zhou Y., Ma J., Cao L., Zhu C., Li W., et al. Roles of c-reactive protein on the association between urinary cadmium and type 2 diabetes. Environ Pollut. 2019;255:113341. doi: 10.1016/j.envpol.2019.113341. [DOI] [PubMed] [Google Scholar]
- 98.Laurberg P., Pedersen I.B., Knudsen N., Ovesen L., Andersen S. Environmental iodine intake affects the type of nonmalignant thyroid disease. Thyroid. 2001;11:457–469. doi: 10.1089/105072501300176417. [DOI] [PubMed] [Google Scholar]
- 99.State of the science of endocrine disrupting chemicals 2012. World Health Organization; Geneva: 2013. [Google Scholar]
- 100.Vanderpump M.P.J., Tunbrldge W.M.G., French J.M., Appleton D., Bates D., Clark F., et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham survey. Clin Endocrinol. 1995;43:55–68. doi: 10.1111/j.1365-2265.1995.tb01894.x. [DOI] [PubMed] [Google Scholar]
- 101.Vanderpump M.P.J. The epidemiology of thyroid disease. Br Med Bull. 2011;99:39–51. doi: 10.1093/bmb/ldr030. [DOI] [PubMed] [Google Scholar]
- 102.Perros P., Boelaert K., Colley S., Evans C., Evans R.M., Gerrard G., et al. Special issue: British thyroid association guidelines for the management of thyroid cancer introduction. Clin Endocrinol. 2014;81:1–122. doi: 10.1111/cen.12515. [DOI] [PubMed] [Google Scholar]
- 103.Haugen B.R., Alexander E.K., Bible K.C., Doherty G.M., Mandel S.J., Nikiforov Y.E., et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chevrier J., Eskenazi B., Bradman A., Fenster L., Barr D.B. Associations between prenatal exposure to polychlorinated biphenyls and neonatal thyroid-stimulating hormone levels in a Mexican-American population, Salinas Valley, California. Environ Health Perspect. 2007;115:1490–1496. doi: 10.1289/ehp.9843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Turyk M.E., Anderson H.A., Persky V.W. Relationships of thyroid hormones with polychlorinated biphenyls, dioxins, furans, and DDE in adults. Environ Health Perspect. 2007;115:1197–1203. doi: 10.1289/ehp.10179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nahar M.S., Dolinoy D.C. In: Comprehensive toxicology. 3rd ed. McQueen C.A., editor. Elsevier; Oxford: 2018. Endocrine disruptors and critical windows: development and disruption of the thyroid hormone pathway in early life; pp. 257–276. [Google Scholar]
- 107.The obesity epidemic: analysis of past and projected future trends in selected oecd countries. OECD; Paris: 2009. [Google Scholar]
- 108.Cunningham E. Where can I find obesity statistics? J Am Diet Assoc. 2010;110:656. doi: 10.1016/j.jada.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 109.Stanley S., Wynne K., McGowan B., Bloom S. Hormonal regulation of food intake. Physiol Rev. 2005;85:1131–1158. doi: 10.1152/physrev.00015.2004. [DOI] [PubMed] [Google Scholar]
- 110.Cano-Sancho G., Salmon A.G., La M.M. Association between exposure to p,p′-DDT and its metabolite p,p′-DDE with obesity: integrated systematic review and meta-analysis. Environ Health Perspect. 2017;125 doi: 10.1289/EHP527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bowe B., Gibson A.K., Xie Y., Yan Y., Van D.A., Martin R.V., et al. Ambient fine particulate matter air pollution and risk of weight gain and obesity in United States veterans: an observational cohort study. Environ Health Perspect. 2021;129:47003. doi: 10.1289/EHP7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Oken E., Gillman M.W. Fetal origins of obesity. Obes Res. 2003;11:496–506. doi: 10.1038/oby.2003.69. [DOI] [PubMed] [Google Scholar]
- 113.Trasande L., Cronk C., Durkin M., Weiss M., Schoeller D.A., Gall E.A., et al. Environment and obesity in the national children's study. Environ Health Perspect. 2009;117:159–166. doi: 10.1289/ehp.11839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Warner M., Schall R.A., Harley K.G., Bradman A., Barr D., Eskenazi B. In utero DDT and DDE exposure and obesity status of 7-year-old Mexican-American children in the chamacos cohort. Environ Health Perspect. 2013;121:631–636. doi: 10.1289/ehp.1205656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Buckley J.P., Engel S.M., Braun J.M., Whyatt R.M., Daniels J.L., Mendez M.A., et al. Prenatal phthalate exposures and body mass index among 4- to 7-year-old children: a pooled analysis. Epidemiology. 2016;27:449–458. doi: 10.1097/EDE.0000000000000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Boivin J., Bunting L., Collins J.A., Nygren K.G. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod. 2007;22:1506–1512. doi: 10.1093/humrep/dem046. [DOI] [PubMed] [Google Scholar]
- 117.Lassen T.H., Frederiksen H., Jensen T.K., Petersen J.H., Joensen U.N., Main K.M., et al. Urinary bisphenol A levels in young men: association with reproductive hormones and semen quality. Environ Health Perspect. 2014;122:478–484. doi: 10.1289/ehp.1307309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mendiola J., Jørgensen N., Andersson A.M., Calafat A.M., Ye X., Redmon J.B., et al. Are environmental levels of bisphenol A associated with reproductive function in fertile men? Environ Health Perspect. 2010;118:1286–1291. doi: 10.1289/ehp.1002037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Guan Q., Chen S., Wang B., Dou X., Lu Y., Liang J., et al. Effects of particulate matter exposure on semen quality: a retrospective cohort study. Ecotoxicol Environ Saf. 2020;193:10319. doi: 10.1016/j.ecoenv.2020.110319. [DOI] [PubMed] [Google Scholar]
- 120.Porpora M.G., Medda E., Abballe A., Bolli S., De A.I., di D.A., et al. Endometriosis and organochlorinated environmental pollutants: a case–control study on Italian women of reproductive age. Environ Health Perspect. 2009;117:1070–1075. doi: 10.1289/ehp.0800273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang B., Zhang R., Jin F., Lou H., Mao Y., Zhu W., et al. Perfluoroalkyl substances and endometriosis-related infertility in Chinese women. Environ Int. 2017;102:207–212. doi: 10.1016/j.envint.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 122.Zhou W., Zhang L., Tong C., Fang F., Zhao S., Tian Y., et al. Plasma perfluoroalkyl and polyfluoroalkyl substances concentration and menstrual cycle characteristics in preconception women. Environ Health Perspect. 2017;125 doi: 10.1289/EHP1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dodge L.E., Williams P.L., Michelle A.W., Missmer S.A., Toth T.L., Calafat A.M., et al. Paternal urinary concentrations of parabens and other phenols in relation to reproductive outcomes among couples from a fertility clinic. Environ Health Perspect. 2015;123:665–671. doi: 10.1289/ehp.1408605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hu Y., Ji L., Zhang Y., Shi R., Han W., Tse L.A., et al. Organophosphate and pyrethroid pesticide exposures measured before conception and associations with time to pregnancy in Chinese couples enrolled in the Shanghai birth cohort. Environ Health Perspect. 2018;126 doi: 10.1289/EHP2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chahrour M., Kleiman R.J., Manzini M.C. Translating genetic and preclinical findings into autism therapies. Dialogues Clin Neurosci. 2017;19:334–343. doi: 10.31887/DCNS.2017.19.4/cmanzini. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Baio J., Wiggins L., Christensen D.L., Maenner M.J., Daniels J., Warren Z., et al. Prevalence of autism spectrum disorder among children aged 8 years — autism and developmental disabilities monitoring network, 11 sites, United States, 2012. MMWR Surveill. Summ. 2018;67:1–23. doi: 10.15585/mmwr.ss6503a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ye B.S., Leung A.O.W., Wong M.H. The association of environmental toxicants and autism spectrum disorders in children. Environ Pollut. 2017;277:234–242. doi: 10.1016/j.envpol.2017.04.039. [DOI] [PubMed] [Google Scholar]
- 128.Landrigan P.J., Lambertini L., Birnbaum L.S. A research strategy to discover the environmental causes of autism and neurodevelopmental disabilities. Environ Health Perspect. 2012;120:a258–a260. doi: 10.1289/ehp.1104285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Windham G.C., Zhang L., Gunier R., Croen L.A., Grether J.K. Autism spectrum disorders in relation to distribution of hazardous air pollutants in the San Francisco Bay area. Environ Health Perspect. 2006;114:1438–1444. doi: 10.1289/ehp.9120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Roberts A.L., Lyall K., Hart J.E., Laden F., Just A.C., Bobb J.F., et al. Perinatal air pollutant exposures and autism spectrum disorder in the children of nurses' health study II participants. Environ Health Perspect. 2013;121:978–984. doi: 10.1289/ehp.1206187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Oulhote Y., Lanphear B., Braun J.M., Webster G.M., Arbuckle T.E., Etzel T., et al. Gestational exposures to phthalates and folic acid, and autistic traits in Canadian children. Environ Health Perspect. 2020;128 doi: 10.1289/EHP5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kim J.I., Lee J., Lee K.S., Lee Y.A., Shin C.H., Hong Y.C., et al. Association of phthalate exposure with autistic traits in children. Environ Int. 2021;157:106775. doi: 10.1016/j.envint.2021.106775. [DOI] [PubMed] [Google Scholar]
- 133.Swanson J.M., Kinsbourne M., Nigg J., Lanphear B., Stefanatos G.A., Volkow N., et al. Etiologic subtypes of attention-deficit/hyperactivity disorder: brain imaging, molecular genetic and environmental factors and the dopamine hypothesis. Neuropsychol Rev. 2007;17:39–59. doi: 10.1007/s11065-007-9019-9. [DOI] [PubMed] [Google Scholar]
- 134.Faraone S.V., Biederman J., Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol Med. 2006;36:159–165. doi: 10.1017/s003329170500471x. [DOI] [PubMed] [Google Scholar]
- 135.Polanczyk G., de Lima M.S., Horta B.L., Biederman J., Rohde L.A. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatr. 2007;164:942–948. doi: 10.1176/appi.ajp.164.6.942. [DOI] [PubMed] [Google Scholar]
- 136.Simon V., Czobor P., Balint S., Meszaros A., Bitter I. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis, Brit. JAMA Psychiatr. 2009;194:204–211. doi: 10.1192/bjp.bp.107.048827. [DOI] [PubMed] [Google Scholar]
- 137.Aguiar A., Eubig P.A., Schantz S.L. Attention deficit/hyperactivity disorder: a focused overview for children's environmental health researchers. Environ Health Perspect. 2010;118:1646–1653. doi: 10.1289/ehp.1002326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tewar S., Auinger P., Braun J.M., Lanphear B., Yolton K., Epstein J.N., et al. Association of bisphenol a exposure and attention-deficit/hyperactivity disorder in a national sample of U.S. Children. Environ Res. 2016;150:112–118. doi: 10.1016/j.envres.2016.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li Y., Zhang H., Kuang H., Fan R., Cha C., Li G., et al. Relationship between bisphenol a exposure and attention-deficit/hyperactivity disorder: a case-control study for primary school children in Guangzhou, China. Environ Pollut. 2018;235:141–149. doi: 10.1016/j.envpol.2017.12.056. [DOI] [PubMed] [Google Scholar]
- 140.Polańska K., Jurewicz J., Hanke W. Exposure to environmental and lifestyle factors and attention-deficit/hyperactivity disorder in children — a review of epidemiological studies. Int. J. Occup. Med. Environ. 2012;25:330–355. doi: 10.2478/S13382-012-0048-0. [DOI] [PubMed] [Google Scholar]
- 141.Brown R.C., Lockwood A.H., Sonawane B.R. Neurodegenerative diseases: an overview of environmental risk factors. Environ Health Perspect. 2005;113:1250–1256. doi: 10.1289/ehp.7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hebert L.E., Weuve J., Scherr P.A., Evans D.A. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80:1778. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Weuve J., Bennett E.E., Ranker L., Gianattasio K.Z., Pedde M., Adar S.D., et al. Exposure to air pollution in relation to risk of dementia and related outcomes: an updated systematic review of the epidemiological literature. Environ Health Perspect. 2021;129 doi: 10.1289/EHP8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kim H., Kim W.H., Kim Y.Y., Park H.Y. Air pollution and central nervous system disease: a review of the impact of fine particulate matter on neurological disorders. Front Public Health. 2020;8:575330. doi: 10.3389/fpubh.2020.575330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nunez Y., Boehme A.K., Weisskopf M.G., Re D.B., Navas A.A., Van D.A., et al. Fine particle exposure and clinical aggravation in neurodegenerative diseases in New York State. Environ Health Perspect. 2021;129 doi: 10.1289/EHP7425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Graves A.B., White E., Koepsell T.D., Reifler B.V., Van B.G., Larson E.B. The association between aluminum-containing products and Alzheimer's disease. J Clin Epidemiol. 1990;43:35–44. doi: 10.1016/0895-4356(90)90053-r. [DOI] [PubMed] [Google Scholar]
- 147.Baldi I., Lebailly P., Mohammed-Brahim B., Letenneur L., Dartigues J.F., Brochard P. Neurodegenerative diseases and exposure to pesticides in the elderly. Am J Epidemiol. 2003;157:409–414. doi: 10.1093/aje/kwf216. [DOI] [PubMed] [Google Scholar]
- 148.Tyas S.L., Manfreda J., Strain L.A., Montgomery P.R. Risk factors for Alzheimer's disease: a population-based, longitudinal study in Manitoba, Canada. Int J Epidemiol. 2001;30:590–597. doi: 10.1093/ije/30.3.590. [DOI] [PubMed] [Google Scholar]
- 149.Starks S.E., Hoppin J.A., Kamel F., Lynch C.F., Jones M.P., Alavanja M.C., et al. Peripheral nervous system function and organophosphate pesticide use among licensed pesticide applicators in the agricultural health study. Environ Health Perspect. 2012;120:515–520. doi: 10.1289/ehp.1103944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lim J.S., Mietus-Snyder M., Valente A., Schwarz J.M., Lustig R.H. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat Rev Gastroenterol Hepatol. 2010;7:251–264. doi: 10.1038/nrgastro.2010.41. [DOI] [PubMed] [Google Scholar]
- 151.Ruhl C.E., Everhart J.E. Fatty liver indices in the multiethnic United States national health and nutrition examination survey. Aliment Pharmacol Ther. 2015;41:65–76. doi: 10.1111/apt.13012. [DOI] [PubMed] [Google Scholar]
- 152.Foulds C.E., Treviño L.S., York B., Walker C.L. Endocrine-disrupting chemicals and fatty liver disease. Nat Rev Endocrinol. 2017;13:445–457. doi: 10.1038/nrendo.2017.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tarantino G., Valentino R., Di S.C., D'Esposito V., Passaretti F., Pizza G., et al. Bisphenol A in polycystic ovary syndrome and its association with liver-spleen axis. Clin Endocrinol. 2013;78:447–453. doi: 10.1111/j.1365-2265.2012.04500.x. [DOI] [PubMed] [Google Scholar]
- 154.Lee C.C., Yao Y.J., Chen H.L., Guo Y.L., Su H.J. Fatty liver and hepatic function for residents with markedly high serum PCDD/Fs levels in Taiwan. J Toxicol Environ Health A. 2006;69:367–380. doi: 10.1080/15287390500244972. [DOI] [PubMed] [Google Scholar]
- 155.El-Sokkary G.H., Nafady A.A., Shabash E.H. Melatonin administration ameliorates cadmium-induced oxidative stress and morphological changes in the liver of rat. Ecotoxicol Environ Saf. 2010;73:456–463. doi: 10.1016/j.ecoenv.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 156.Skipper A., Sims J.N., Yedjou C.G., Tchounwou P.B. Cadmium chloride induces DNA damage and apoptosis of human liver carcinoma cells via oxidative stress. Int J Environ Res Publ Health. 2016;13:88. doi: 10.3390/ijerph13010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Baba H., Tsuneyama K., Yazaki M., Nagata K., Minamisaka T., Tsuda T., et al. The liver in Itai-itai disease (chronic cadmium poisoning): pathological features and metallothionein expression. Mod Pathol. 2013;26:1228–1234. doi: 10.1038/modpathol.2013.62. [DOI] [PubMed] [Google Scholar]
- 158.Ba Q., Li M., Chen P., Huang C., Duan X., Lu L., et al. Sex-dependent effects of cadmium exposure in early life on gut microbiota and fat accumulation in mice. Environ Health Perspect. 2017;125:437–446. doi: 10.1289/EHP360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cao Z., Fang Y., Lu Y., Tan D., Du C., Li Y., et al. Melatonin alleviates cadmium-induced liver injury by inhibiting the TXNIP-NLRP3 inflammasome. J Pineal Res. 2017;62:3. doi: 10.1111/jpi.12389. [DOI] [PubMed] [Google Scholar]
- 160.Go Y.M., Sutliff R.L., Chandler J.D., Khalidur R., Kang B.Y., Anania F.A., et al. Low-dose cadmium causes metabolic and genetic dysregulation associated with fatty liver disease in mice. Toxicol Sci. 2015;147:524–534. doi: 10.1093/toxsci/kfv149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Satarug S. Long-term exposure to cadmium in food and cigarette smoke, liver effects and hepatocellular carcinoma. Curr Drug Metabol. 2012;13:257–271. doi: 10.2174/138920012799320446. [DOI] [PubMed] [Google Scholar]
- 162.Zhang C., Ge J., Lv M., Zhang Q., Talukder M., Li J.L. Selenium prevent cadmium-induced hepatotoxicity through modulation of endoplasmic reticulum-resident selenoproteins and attenuation of endoplasmic reticulum stress. Environ Pollut. 2020;260:13873. doi: 10.1016/j.envpol.2019.113873. [DOI] [PubMed] [Google Scholar]
- 163.Kubota A. Study of process from winning lawsuit to conciliation in disaster area of Itai-itai disease — how should we recover from the disaster of land contamination by environmental disruption? Japan Architect. Rev. 2020;3:231–240. doi: 10.1002/2475-8876.12142. [DOI] [Google Scholar]
- 164.He S., Zhuo L., Cao Y., Liu G., Zhao H., Song R., et al. Effect of cadmium on osteoclast differentiation during bone injury in female mice. Environ Toxicol. 2020;35:487–494. doi: 10.1002/tox.22884. [DOI] [PubMed] [Google Scholar]
- 165.Thompson J., Bannigan J. Cadmium: toxic effects on the reproductive system and the embryo. Reprod Toxicol. 2008;25:304–315. doi: 10.1016/j.reprotox.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 166.Khoshhali M., Rafiei N., Farajzadegan Z., Shoshtari-Yeganeh B., Kelishadi R. Maternal exposure to cadmium and fetal growth: a systematic review and meta-analysis. Biol Trace Elem Res. 2020;195:9–19. doi: 10.1007/s12011-019-01819-y. [DOI] [PubMed] [Google Scholar]
- 167.Xu P., Guo H., Wang H., Lee S.C., Liu M., Pan Y., et al. Downregulations of placental fatty acid transporters during cadmium-induced fetal growth restriction. Toxicology. 2019;423:112–122. doi: 10.1016/j.tox.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 168.Andjelkovic M., Djordjevic A.B., Antonijevic E., Antonijevic B., Stanic M., Kotur-Stevuljevic J., et al. Toxic effect of acute cadmium and lead exposure in rat blood, liver, and kidney. Int J Environ Res Publ Health. 2019;16:274. doi: 10.3390/ijerph16020274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kumar V.M., Henley A.K., Nelson C.J., Indumati O., Rao Y.P., Rajanna S., et al. Protective effect of allium sativum (garlic) aqueous extract against lead-induced oxidative stress in the rat brain, liver, and kidney. Environ Sci Pollut Res Int. 2017;24:1544–1552. doi: 10.1007/s11356-016-7923-3. [DOI] [PubMed] [Google Scholar]
- 170.Liu J., Chen B., Zhang J., Kuang F., Chen L. Lead exposure induced microgliosis and astrogliosis in hippocampus of young mice potentially by triggering TLR4-MYD88-NFκB signaling cascades. Toxicol Lett. 2015;239:97–107. doi: 10.1016/j.toxlet.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 171.Li N., Cao S., Yu Z., Qiao M., Cheng Y., Shen Y., et al. Perinatal lead exposure alters calsyntenin-2 and calsyntenin-3 expression in the hippocampus and causes learning deficits in mice post-weaning. Biol Trace Elem Res. 2021;199:1414–1424. doi: 10.1007/s12011-020-02241-5. [DOI] [PubMed] [Google Scholar]
- 172.Ayyalasomayajula N., Bandaru M., Dixit P.K., Ajumeera R., Chetty C.S., Challa S. Inactivation of GAP-43 due to the depletion of cellular calcium by the Pb and amyloid peptide induced toxicity: an in vitro approach. Chem Biol Interact. 2020;316:108927. doi: 10.1016/j.cbi.2019.108927. [DOI] [PubMed] [Google Scholar]
- 173.Eid A., Bihaqi S.W., Renehan W.E., Zawia N.H. Developmental lead exposure and lifespan alterations in epigenetic regulators and their correspondence to biomarkers of Alzheimer's disease, Alzheimer's & Dementia. DADM. 2016;2:123–131. doi: 10.1016/j.dadm.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Farina M., Avila D.S., Teixeira da Rocha J.B., Aschner M. Metals, oxidative stress and neurodegeneration: a focus on iron, manganese and mercury. Neurochem Int. 2013;62:575–594. doi: 10.1016/j.neuint.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kim S.W., Han S.J., Kim Y., Jun J.W., Giri S.S., Chi C., et al. Heavy metal accumulation in and food safety of shark meat from Jeju island, Republic of Korea. PLoS One. 2019;14 doi: 10.1371/journal.pone.0212410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ishihara Y., Tsuji M., Kawamoto T., Yamazaki T. Involvement of reactive oxygen species derived from mitochondria in neuronal injury elicited by methylmercury. J Clin Biochem Nutr. 2016;59:182–190. doi: 10.3164/jcbn.16-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yang T., Xu Z., Liu W., Xu B., Deng Y., Li Y., et al. Alpha-lipoic acid protects against methylmercury-induced neurotoxic effects via inhibition of oxidative stress in rat cerebral cortex. Environ Toxicol Pharmacol. 2015;39:157–166. doi: 10.1016/j.etap.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 178.Takahashi T., Fujimura M., Koyama M., Kanazawa M., Usuki F., Nishizawa M., et al. Methylmercury causes blood-brain barrier damage in rats via upregulation of vascular endothelial growth factor expression. PLoS One. 2017;12 doi: 10.1371/journal.pone.0170623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lee J.-Y., Hwang G.-W., Naganuma A., Satoh M. Methylmercury toxic mechanism related to protein degradation and chemokine transcription. Environ Health Prev Med. 2020;25:30. doi: 10.1186/s12199-020-00868-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lu L., Hu J., Li G., An T. Low concentration tetrabromobisphenol A (TBBPA) elevating overall metabolism by inducing activation of the ras signaling pathway. J Hazard Mater. 2021;416:125797. doi: 10.1016/j.jhazmat.2021.125797. [DOI] [PubMed] [Google Scholar]
- 181.Cannon R.E., Trexler A.W., Knudsen G.A., Evans R.A. L.S. Tetrabromobisphenol A (TBBPA) alters ABC transport at the blood-brain barrier. Toxicol Sci. 2019;169:475–484. doi: 10.1093/toxsci/kfz059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lai D.Y., Kacew S., Dekant W. Tetrabromobisphenol A (TBBPA): possible modes of action of toxicity and carcinogenicity in rodents. Food Chem Toxicol. 2015;80:206–214. doi: 10.1016/j.fct.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 183.Lilienthal H., Verwer C.M., van der Ven L.T.M., Piersma A.H., Vos J.G. Exposure to tetrabromobisphenol A (TBBPA) in wistar rats: neurobehavioral effects in offspring from a one-generation reproduction study. Toxicology. 2008;246:45–54. doi: 10.1016/j.tox.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 184.Al-Mousa F., Michelangeli F. Some commonly used brominated flame retardants cause Ca2+-ATPase inhibition, beta-amyloid peptide release and apoptosis in SY-SY5Y neuronal cells. PLoS One. 2012;7 doi: 10.1371/journal.pone.0033059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Fukuda N., Ito Y., Yamaguchi M., Mitumori K., Koizumi M., Hasegawa R., et al. Unexpected nephrotoxicity induced by tetrabromobisphenol A in newborn rats. Toxicol Lett. 2004;150:145–155. doi: 10.1016/j.toxlet.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 186.Tada Y., Fujitani T., Ogata A., Kamimura H. Flame retardant tetrabromobisphenol A induced hepatic changes in ICR male mice. Environ Toxicol Pharmacol. 2007;23:174–178. doi: 10.1016/j.etap.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 187.Zhang Q., Lu M., Wang C., Du J., Zhou P., Zhao M. Characterization of estrogen receptor α activities in polychlorinated biphenyls by in vitro dual-luciferase reporter gene assay. Environ Pollut. 2004;189:75. doi: 10.1016/j.envpol.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 188.Plíšková M., Vondráček J., Rocio F.C., Nera J., Kočan A., Petrík J., et al. Impact of polychlorinated biphenyls contamination on estrogenic activity in human male serum. Environ Health Perspect. 2005;113:1277–1284. doi: 10.1289/ehp.7745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Elnar A.A., Diesel B., Desor F., Feidt C., Bouayed J., Kiemer A.K., et al. Neurodevelopmental and behavioral toxicity via lactational exposure to the sum of six indicator non-dioxin-like-polychlorinated biphenyls (∑6 NDL-PCBs) in mice. Toxicology. 2012;299:44–54. doi: 10.1016/j.tox.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 190.Pessah I.N., Cherednichenko G., Lein P.J. Minding the calcium store: ryanodine receptor activation as a convergent mechanism of PCB toxicity. Pharmacol Ther. 2010;125:260–285. doi: 10.1016/j.pharmthera.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Faqi A.S., Dalsenter P.R., Mathar W., Heinrich-Hirsch B., Chahoud I. Reproductive toxicity and tissue concentrations of 3,3',4,4'-tetrachlorobiphenyl (PCB 77) in male adult rats. Hum Exp Toxicol. 1998;17:151–156. doi: 10.1177/096032719801700305. [DOI] [PubMed] [Google Scholar]
- 192.Murati T., Miletić M., Pleadin J., Šimić B., Kmetič I. Cell membrane-related toxic responses and disruption of intercellular communication in PCB mechanisms of toxicity: a review. J Appl Toxicol. 2020;40:1592–1601. doi: 10.1002/jat.4019. [DOI] [PubMed] [Google Scholar]
- 193.Butenhoff J.L., Ehresman D.J., Chang S., Parker G.A., Stump D.G. Gestational and lactational exposure to potassium perfluorooctanesulfonate (K+PFOS) in rats: developmental neurotoxicity. Reprod Toxicol. 2009;27:319–330. doi: 10.1016/j.reprotox.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 194.Johansson N., Fredriksson A., Eriksson P. Neonatal exposure to perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) causes neurobehavioural defects in adult mice. Neurotoxicology. 2008;29:160–169. doi: 10.1016/j.neuro.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 195.Wang Y., Jin Y. Perfluorooctane sulfonate (PFOS) and calcium channel downstream signaling molecules. Toxicol. Res. 2012;1:103–107. doi: 10.1039/C2TX20017A. [DOI] [Google Scholar]
- 196.Lee H., Lee Y.J., Yang J. Perfluorooctane sulfonate induces apoptosis of cerebellar granule cells via a ROS-dependent protein kinase C signaling pathway. Neurotoxicology. 2012;33:314–320. doi: 10.1016/j.neuro.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 197.Chen J., Miao Y., Gao Q., Cui Z., Xiong B. Exposure to perfluorooctane sulfonate in vitro perturbs the quality of porcine oocytes via induction of apoptosis. Environ Pollut. 2021;284:117508. doi: 10.1016/j.envpol.2021.117508. [DOI] [PubMed] [Google Scholar]
- 198.Coperchini F., Croce L., Ricci G., Magri F., Rotondi M., Imbriani M., et al. Thyroid disrupting effects of old and new generation PFAS. Front Endocrinol. 2021;11:612320. doi: 10.3389/fendo.2020.612320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Xin Y., Ren X., Ruan T., Li C., Guo L., Jiang G. Chlorinated polyfluoroalkylether sulfonates exhibit similar binding potency and activity to thyroid hormone transport proteins and nuclear receptors as perfluorooctanesulfonate. Environ Sci Technol. 2018;52:9412–9418. doi: 10.1021/acs.est.8b01494. [DOI] [PubMed] [Google Scholar]
- 200.Liu Q.S., Sun Y., Qu G., Long Y., Zhao X., Zhang A., et al. Structure-dependent hematological effects of per- and polyfluoroalkyl substances on activation of plasma kallikrein–kinin system cascade. Environ Sci Technol. 2017;51:10173–10183. doi: 10.1021/acs.est.7b02055. [DOI] [PubMed] [Google Scholar]
- 201.Liu Q.S., Hao F., Sun Z., Long Y., Zhou Q., Jiang G. Perfluorohexadecanoic acid increases paracellular permeability in endothelial cells through the activation of plasma kallikrein-kinin system. Chemosphere. 2018;190:191–200. doi: 10.1016/j.chemosphere.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 202.Im J., Löffler F.E. Fate of bisphenol A in terrestrial and aquatic environments. Environ Sci Technol. 2016;50:8403–8416. doi: 10.1021/acs.est.6b00877. [DOI] [PubMed] [Google Scholar]
- 203.Vandenberg L.N., Maffini M.V., Sonnenschein C., Rubin B.S., Soto A.M. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev. 2009;30:75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Michałowicz J. Bisphenol A — sources, toxicity and biotransformation. Environ Toxicol Pharmacol. 2014;37:738–758. doi: 10.1016/j.etap.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 205.Welshons W.V., Nagel S.C., vom Saal F.S. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology. 2006;147:S56–S69. doi: 10.1210/en.2005-1159. [DOI] [PubMed] [Google Scholar]
- 206.Gould J.C., Leonard L.S., Maness S.C., Wagner B.L., Conner K., Zacharewski T., et al. Bisphenol A interacts with the estrogen receptor α in a distinct manner from estradiol. Mol Cell Endocrinol. 1998;142:203–214. doi: 10.1016/s0303-7207(98)00084-7. [DOI] [PubMed] [Google Scholar]
- 207.Sun H., Xu L., Chen J., Song L., Wang X. Effect of bisphenol A, tetrachlorobisphenol A and pentachlorophenol on the transcriptional activities of androgen receptor-mediated reporter gene. Food Chem Toxicol. 2006;44:1916–1921. doi: 10.1016/j.fct.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 208.Wright H.M., Clish C.B., Mikami T., Hauser S., Yanagi K., Hiramatsu R., et al. A synthetic antagonist for the peroxisome proliferator-activated receptor γ inhibits adipocyte differentiation. J Biol Chem. 2000;275:1873–1877. doi: 10.1074/jbc.275.3.1873. [DOI] [PubMed] [Google Scholar]
- 209.Zoeller R.T., Bansal R., Parris C. Bisphenol-A, an environmental contaminant that acts as a thyroid hormone receptor antagonist in vitro, increases serum thyroxine, and alters RC3/neurogranin expression in the developing rat brain. Endocrinology. 2005;146:607–612. doi: 10.1210/en.2004-1018. [DOI] [PubMed] [Google Scholar]
- 210.Monje L., Varayoud J., Muñoz-de-Toro M., Luque E.H., Ramos J.G. Neonatal exposure to bisphenol A alters estrogen-dependent mechanisms governing sexual behavior in the adult female rat. Reprod Toxicol. 2009;28:435–442. doi: 10.1016/j.reprotox.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 211.Rasier G., Toppari J., Parent A.S., Bourguignon J.P. Female sexual maturation and reproduction after prepubertal exposure to estrogens and endocrine disrupting chemicals: a review of rodent and human data. Mol Cell Endocrinol. 2006;254–255:187–201. doi: 10.1016/j.mce.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 212.Takai Y., Tsutsumi O., Ikezuki Y., Hiroi H., Osuga Y., Momoeda M., et al. Estrogen receptor-mediated effects of a xenoestrogen, bisphenol A, on preimplantation mouse embryos. Biochem Biophys Res Commun. 2000;270:918–921. doi: 10.1006/bbrc.2000.2548. [DOI] [PubMed] [Google Scholar]
- 213.Zhang G., Zhang X., Feng Y., Li L., Huynh E., Sun X., et al. Exposure to bisphenol A results in a decline in mouse spermatogenesis. Reprod Fertil Dev. 2013;25:847–859. doi: 10.1071/RD12159. [DOI] [PubMed] [Google Scholar]
- 214.Negishi T., Ishii Y., Kyuwa S., Kuroda Y., Yoshikawa Y. Inhibition of staurosporine-induced neuronal cell death by bisphenol A and nonylphenol in primary cultured rat hippocampal and cortical neurons. Neurosci Lett. 2003;353:99–102. doi: 10.1016/j.neulet.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 215.Liu R., Xing L., Kong D., Jiang J., Shang L., Hao W. Bisphenol A inhibits proliferation and induces apoptosis in micromass cultures of rat embryonic midbrain cells through the JNK, Creb and p53 signaling pathways. Food Chem Toxicol. 2013;52:76–82. doi: 10.1016/j.fct.2012.10.033. [DOI] [PubMed] [Google Scholar]
- 216.vom Saal Frederick S., Hughes C. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ Health Perspect. 2005;113:926–933. doi: 10.1289/ehp.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wang W., Xiong P., Zhang H., Zhu Q., Liao C., Jiang G. Analysis, occurrence, toxicity and environmental health risks of synthetic phenolic antioxidants: a review. Environ Res. 2021;201:111531. doi: 10.1016/j.envres.2021.111531. [DOI] [PubMed] [Google Scholar]
- 218.Zhang Y., Du B., Ge J., Liu L., Zhu M., Li J., et al. Co-occurrence of and infant exposure to multiple common and unusual phenolic antioxidants in human breast milk. Environ Sci Technol Lett. 2020;7:206–212. doi: 10.1021/acs.estlett.0c00104. [DOI] [Google Scholar]
- 219.Sun Z., Yang X., Liu Q.S., Li C.H., Zhou Q.F., Fiedler H., et al. Butylated hydroxyanisole isomers induce distinct adipogenesis in 3T3-L1 cells. J Hazard Mater. 2019;379:120794. doi: 10.1016/j.jhazmat.2019.120794. [DOI] [PubMed] [Google Scholar]
- 220.Sun Z., Tang Z., Yang X., Liu Q.S., Liang Y., Fiedler H., et al. Perturbation of 3-tert-butyl-4-hydroxyanisole in adipogenesis of male mice with normal and high fat diets. Sci Total Environ. 2020;703:135608. doi: 10.1016/j.scitotenv.2019.135608. [DOI] [PubMed] [Google Scholar]
- 221.Yang X., Song W., Liu N., Sun Z., Liu R., Liu Q.S., et al. Synthetic phenolic antioxidants cause perturbation in steroidogenesis in vitro and in vivo. Environ Sci Technol. 2018;52:850–858. doi: 10.1021/acs.est.7b05057. [DOI] [PubMed] [Google Scholar]
- 222.Kang H.G., Jeong S.H., Cho J.H., Kim D.G., Park J.M., Cho M.H. Evaluation of estrogenic and androgenic activity of butylated hydroxyanisole in immature female and castrated rats. Toxicology. 2005;213:147–156. doi: 10.1016/j.tox.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 223.Cohen A.J., Brauer M., Burnett R., Anderson H.R., Frostad J., Estep K., et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the global burden of diseases study 2015. Lancet. 2017;389:1907–1918. doi: 10.1016/S0140-6736(17)30505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Lelieveld J., Poschl U. Chemists can help to solve the air-pollution health crisis. Nature. 2017;551:291–293. doi: 10.1038/d41586-017-05906-9. [DOI] [PubMed] [Google Scholar]
- 225.Lu D., Luo Q., Chen R., Zhuansun Y., Jiang J., Wang W., et al. Chemical multi-fingerprinting of exogenous ultrafine particles in human serum and pleural effusion. Nat Commun. 2020;11:2567. doi: 10.1038/s41467-020-16427-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Pun V.C., Kazemiparkouhi F., Manjourides J., Suh H.H. Long-term PM2.5 exposure and respiratory, cancer, and cardiovascular mortality in older US adults. Am. J. Epidmiol. 2017;186:961–969. doi: 10.1093/aje/kwx166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Lin C., Nicol C.J.B., Wan C., Chen S., Huang R., Chiang M. Exposure to PM2.5 induces neurotoxicity, mitochondrial dysfunction, oxidative stress and inflammation in human SH-SY5Y neuronal cells. Neurotoxicology. 2022;88:25–35. doi: 10.1016/j.neuro.2021.10.009. [DOI] [PubMed] [Google Scholar]
- 228.Li Q.Z., Zheng J.L., Xu S., Zhang J.S., Cao Y.H., Qin Z.L., et al. The neurotoxicity induced by PM2.5 might be strongly related to changes of the hippocampal tissue structure and neurotransmitter levels. Toxicol. Res. 2018;7:1144–1152. doi: 10.1039/c8tx00093j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Yang Y., Feng Y., Huang H., Cui L., Li F. PM2.5 exposure induces reproductive injury through IRE1/JNK/autophagy signaling in male rats. Ecotoxicol Environ Saf. 2021;211:111924. doi: 10.1016/j.ecoenv.2021.111924. [DOI] [PubMed] [Google Scholar]
- 230.Zhang J., Liu J., Ren L., Wei J., Duan J., Zhang L., et al. PM2.5 induces male reproductive toxicity via mitochondrial dysfunction, DNA damage and RIPK1 mediated apoptotic signaling pathway. Sci Total Environ. 2018;634:1435–1444. doi: 10.1016/j.scitotenv.2018.03.383. [DOI] [PubMed] [Google Scholar]
- 231.Liu X., Jin X., Su R., Li Z. The reproductive toxicology of male SD rats after PM2.5 exposure mediated by the stimulation of endoplasmic reticulum stress. Chemosphere. 2017;189:547–555. doi: 10.1016/j.chemosphere.2017.09.082. [DOI] [PubMed] [Google Scholar]
- 232.Xu J., Zhang W., Lu Z., Zhang F., Ding W. Airborne PM2.5-induced hepatic insulin resistance by Nrf2/JNK-mediated signaling pathway. Int J Environ Res Publ Health. 2017;14:787. doi: 10.3390/ijerph14070787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Liu C., Xu X., Bai Y., Wang T.Y., Rao X., Wang A., et al. Air pollution–mediated susceptibility to inflammation and insulin resistance: influence of CCR2 pathways in mice. Environ Health Perspect. 2014;122:17–26. doi: 10.1289/ehp.1306841. [DOI] [PMC free article] [PubMed] [Google Scholar]