Abstract
Glucocorticoids exert pleiotropic effects either by a relatively slow mechanism involving binding to cytosolic/nuclear receptors and regulation of gene expression or by rapid activation of a putative membrane receptor and membrane signal transduction. Rapid glucocorticoid actions are initiated at the membrane and recruit intracellular signaling pathways that engage multiple downstream cellular targets, including lipid and gas intercellular messengers, membrane neurotransmitter receptor trafficking, nuclear glucocorticoid receptor activation and trafficking, and more. Thus, membrane glucocorticoid signaling diverges into a multiplexed array of signaling pathways to simultaneously regulate highly diverse cellular functions, giving these steroid hormones a broad range of rapid regulatory capabilities. In this review, we provide a brief overview of the growing body of knowledge of the cell signaling mechanisms of rapid glucocorticoid actions in the brain.
Corticosteroid hormones are secreted into the bloodstream under circadian regulation and during exposure to stressful environmental conditions. As lipophilic molecules, corticosteroids gain access to receptors throughout the organism, including by crossing the blood-brain barrier to reach the brain. Corticosteroids exert actions in multiple areas of the brain, in both cortical and subcortical brain structures, that vary in the temporal domain, from rapid to delayed and transient to prolonged, based on interactions with different classes of receptors. Two nuclear receptors, the mineralocorticoid receptor (MR) and the glucocorticoid receptor (GR) [1], are responsible for most (but not all) of the genomic actions of corticosteroids and have different ligand binding affinities and spatial distributions throughout the brain. The MR and GR are resident in the cytosol in the unliganded state and translocate to the nucleus upon corticosteroid binding, where they regulate gene transcription [2] . In addition to their delayed genomic actions mediated by nuclear receptors, glucocorticoids also stimulate multiple rapid, non-genomic actions that are initiated by one or more receptors located at the membrane. These rapid steroid actions have variable G protein and protein kinase dependencies and sensitivities to GR and MR agonists and antagonists, depending on the target cell type. This suggests that membrane-associated corticosteroid receptors recruit a complex, multi-pronged array of downstream cellular signaling mechanisms. Here, we focus mainly on the current state of knowledge of the multiple divergent cell signaling actions of the membrane-associated GR (mGR) in the central nervous system. The reader is referred to a recent review for more information on the rapid modulatory actions of the membrane-associated MR [3] .
Rapid Glucocorticoid-Induced Endocannabinoid Signaling
Glucocorticoids have been shown to rapidly stimulate endocannabinoid production and elicit CB1 cannabinoid receptor-mediated actions at synapses in multiple areas of the brain [4,5], including in the hypothalamus [6,7], hippocampus [8], amygdala [9,10], prefrontal cortex [11], dorsal striatum [12], nucleus accumbens [13], and medulla [14]. The two main endocannabinoids are arachidonoylethanolamine (AEA) and 2-arachidonoylglycerol (2-AG), which are synthesized and immediately released, and usually act at synapses as retrograde inter-cellular messengers. Glucocorticoid-induced endocannabinoid signaling can occur at either excitatory or inhibitory synapses to rapidly suppress glutamate or GABA release, respectively, via presynaptic CB1 receptor activation, although the glucocorticoid-induced endocannabinoid actions are restricted to specific excitatory and inhibitory synapses and not ubiquitously expressed. The rapid glucocorticoid actions have been localized to the postsynaptic side of the synapse, where they induce endocannabinoid synthesis and release from the soma/dendrites of the postsynaptic neuron [6]. The endocannabinoids signal retrogradely across the synapse to activate CB1 receptors on the presynaptic side, which in turn suppresses orthograde neurotransmitter release at the synapse. The effect of glucocorticoid-induced retrograde endocannabinoid signaling can be either inhibitory or excitatory based on whether it suppresses glutamate release at excitatory synapses or GABA release at inhibitory synapses. Rapid presynaptic MR-dependent, endocannabinoid-independent corticosteroid actions that enhance glutamate release have also been reported (see below and [3]).
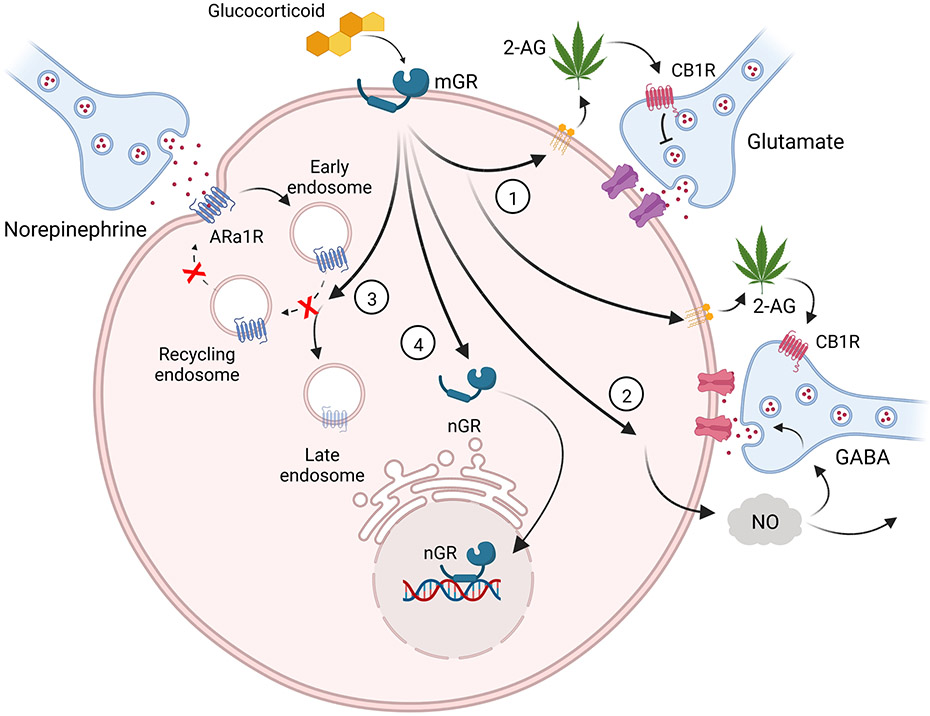
It was in the hypothalamus where glucocorticoid-induced endocannabinoid synthesis was first demonstrated [6]. Rapid glucocorticoid-induced release of the endocannabinoid 2-AG [15] suppresses glutamate release at excitatory synapses on magnocellular neuroendocrine neurons in the paraventricular nucleus (PVN) and supraoptic nucleus (SON) [6] and on PVN parvocellular neuroendocrine neurons [6,16,17] and preautonomic neurons [7] (Fig. 1(1)). The acute suppression of excitatory synaptic input to corticotropin-releasing hormone (CRH) neurons in the PVN contributes to the fast glucocorticoid negative-feedback regulation of the hypothalamic-pituitary-adrenal (HPA) axis by inhibiting the excitatory drive to these neurons [6,18]. Evidence from ex vivo electrophysiological studies suggests that rapid glucocorticoid signaling is dependent on the nuclear GR, since it is lost with GR knockdown [17]; however, detailed pharmacological interrogation of intracellular signaling reveals a prominent G-protein and protein kinase signaling dependence [15], suggesting a G protein-coupled receptor-dependent mechanism. Interestingly, CB1 receptors at inhibitory synapses on hypothalamic neuroendocrine neurons are tonically activated by constitutive AEA release, but can also be activated by the glucocorticoid-induced 2-AG at excitatory synapses that spills over when astrocytic uptake mechanisms are disrupted [19,20].
Figure 1.
Glucocorticoids activate a membrane-associated receptor (mGR) to stimulate multiple signaling pathways. (1) Endocannabinoid (2-AG) synthesis and retrograde release activates presynaptic CB1 receptors to suppress glutamate release at excitatory synapses in the hypothalamus [6] and inhibitory synapses in the BLA [9]. (2) Nitric oxide (NO) synthesis and retrograde transmission stimulates GABA release via actions at presynaptic axon terminals [44] or somata/dendrites [35]. (3) Desensitization of α1 adrenergic receptors by preventing recycling of internalized receptors to the membrane [49]. (4) Signaling to the nuclear GR (nGR) from the membrane triggers nGR nuclear translocation and transcriptional regulation [50].
The endocannabinoid-mediated effects of glucocorticoids occur on a rapid time scale that precludes a nuclear GR-mediated genomic mechanism, and several lines of evidence implicate a membrane-associated receptor. Perhaps the most compelling of these is that glucocorticoids (corticosterone, dexamethasone) conjugated to bovine serum albumin (BSA), which prevents the steroids from crossing the cell membrane to gain access to the nuclear GR [21], maintain their ability to elicit endocannabinoid signaling at hypothalamic synapses [6,17,18]. Also, direct intracellular application of glucocorticoids does not elicit the endocannabinoid signaling, and the GR antagonist RU 486 does not block the rapid glucocorticoid actions at hypothalamic synapses [6] (although it does at synapses in some other brain areas [8,12]).
While the receptor responsible for triggering membrane signaling by glucocorticoids has not yet been identified, a model of cell signaling downstream from the mGR is nevertheless emerging. Initial studies implicated Gαs-cAMP signaling in the rapid glucocorticoid-induced endocannabinoid effect [6,17,22], and further investigation indicated a signaling hierarchy in which PKA lies upstream of a complex, multi-branched pathway that includes Src kinase, phospholipase C (but not Gαq), PKC, ERK-MAPK, and IP3 receptor-mediated intracellular calcium signaling [15]. The involvement of multiple kinases and signaling molecules suggests that there is not a linear path from mGR activation to endocannabinoid synthesis, but likely either 1) an interaction with cell signaling mediated by other extracellular signals (i.e., transactivation) or 2) alternative signaling via β-arrestin activation (e.g., [3]). Indeed, the Src kinase and ERK/MAPK dependence supports the possibility of a β-arrestin signaling mechanism [23,24]. Rapid glucocorticoid signaling via a Src kinase-dependent mechanism that does not result in endocannabinoid release has been described in the rat anterior pituitary, where it is thought to contribute to the glucocorticoid ultradian feedback control of pituitary corticotroph secretion by activation of a membrane GR [25] or MR [26].
The basolateral amygdala (BLA) is vital for emotional processing, and an interplay between glucocorticoids and endocannabinoids in the BLA plays a key role in HPA axis activation, anxiety, and emotional memory formation. Behaviorally, stress exposure and glucocorticoid administration in the BLA suppress fear memory retrieval and facilitate fear memory consolidation via endocannabinoid-dependent mechanisms [10,27,28]. Corticosteroids have rapid, membrane receptor-mediated modulatory effects on both excitatory and inhibitory synapses in the BLA, often involving mobilization of the endocannabinoid 2-AG [9]. Glutamatergic and GABAergic synapses in the BLA express presynaptic CB1 receptors, with cholecystokinin-expressing basket cells being the predominant CB1-expressing GABA interneurons [29].
Stress-induced glucocorticoid rapidly modulates inhibitory synapses in the BLA via endocannabinoid-dependent signaling. Activation of a postsynaptic mGR causes a non-reversible suppression of GABA release onto BLA principal neurons that is mediated by the retrograde release of the endocannabinoid 2-AG and activation of presynaptic CB1 receptors [9] (Fig. 1(1)). This mechanism is identical to that seen in the hypothalamus [6], but occurs at inhibitory synapses rather than at excitatory synapses.
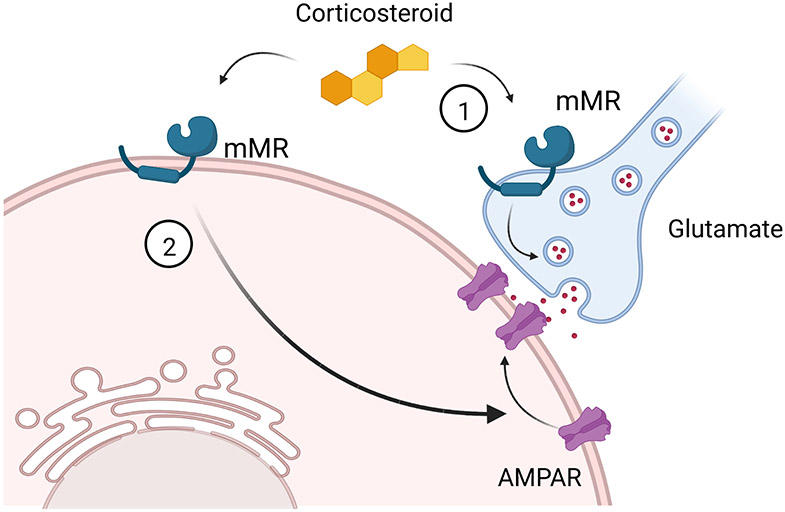
Glutamate release at excitatory synapses in the BLA is also rapidly regulated by corticosterone, albeit via presynaptic activation of a putative membrane MR that directly facilitates glutamate release independent of a retrograde signal [30] (Fig. 2(1)). However, this effect is reversed by subsequent corticosteroid exposure, suppressing glutamate release by activation of a postsynaptic mGR, endocannabinoid release and retrograde CB1 receptor activation [30], a mechanism like that observed in the hypothalamus [6].
Figure 2.
Rapid signaling via a membrane MR (mMR). (1) Corticosteroids rapidly increase glutamate release via activation of a presynaptic mMR [3]. (2) Corticosteroid activation of a postsynaptic MR-dependent mechanism increases glutamate receptor (AMPAR) mobility and trafficking to excitatory synapses [51].
Glucocorticoid feedback effects in the BLA, therefore, cause a rapid facilitation of synaptic excitation and a suppression of synaptic inhibition, resulting in an excitatory shift in the excitation/inhibition balance. This facilitates anxiety-like behavior [9] and HPA axis activation [5].
The hippocampus is another stress-responsive brain region in which glucocorticoid-induced endocannabinoid modulation of synapses has been reported. Stress and glucocorticoid stimulate endocannabinoid synthesis in the hippocampus [8,31,32], and glucocorticoid-2-AG interactions have been found to mediate the stress impairment of retrieval of contextual fear memory in rats [32]. 2-AG synthesized via a depolarization-dependent mechanism suppresses synaptic neurotransmitter release [33,34]; stress and glucocorticoids enhance the depolarization-induced endocannabinoid suppression of GABA release in CA1 pyramidal cells [8]. Interestingly, this glucocorticoid-endocannabinoid effect in CA1 neurons is activity-dependent and occurs at inhibitory synapses after an incubation period, whereas other studies have found the opposite rapid effect of glucocorticoid at inhibitory synapses, an increase in GABA release mediated by a nitric oxide (NO)-dependent mechanism [35,36], which is described in more detail later in the review. The stress-induced impairment of contextual fear memory recall occurs only under conditions of emotional activation, and is blocked by α1- and β-adrenergic receptor inhibition following memory acquisition, suggesting that norepinephrine signaling by emotional arousal is also necessary for the facilitatory effect of stress on fear memory consolidation [32].
The medial prefrontal cortex (mPFC) has been identified as a critical top-down regulator of the stress response and fear memory [37,38]. The prelimbic nucleus of the mPFC provides an inhibitory brake on HPA axis activation via an inhibitory relay in the anterior bed nucleus of the stria terminalis (BNST) [39] and contributes to HPA negative feedback regulation [40]. The prelimbic mPFC also promotes fear memory expression via projections to the BLA, while the infralimbic nucleus (IL) of the mPFC is required for fear memory extinction [41]. Glucocorticoid signaling in the mPFC provides a substrate for the descending mPFC regulation of the HPA axis by stress activation. Thus, glucocorticoid-induced endocannabinoid release suppresses GABA release and disinhibits layer V mPFC output neurons [11], which then activate the inhibitory relay from the BNST to PVN CRH neurons to inhibit HPA activation. Similarly, the IL projection to the BLA is thought to activate BLA inhibitory interneurons, which inhibit BLA principal neurons to facilitate fear memory extinction [42], although whether this is triggered by glucocorticoid-endocannabinoid interactions in IL is not known. However, glucocorticoids have been shown to induce 2-AG release and suppression of inhibitory input to mPFC layer V pyramidal neurons to promote the reinstatement of cocaine use [43].
Glucocorticoid-induced endocannabinoid synthesis and release, therefore, present a common mechanism of fast feedback regulation of stress circuits in stress-sensitive limbic structures throughout the brain. The glucocorticoid-endocannabinoid interactions suppress activity at excitatory and/or inhibitory synapses, which in the hypothalamus, hippocampus and mPFC contributes to the feedback inhibition of the HPA response and in the amygdala acts to promote the stress response and stress-sensitive affective behavior.
Rapid Glucocorticoid-Induced Nitric Oxide Signaling
Rapid glucocorticoid signaling from a putative membrane receptor has also been reported to trigger NO release and retrograde modulation of synapses in some of the same brain areas as glucocorticoid-induced endocannabinoid modulation, albeit with opposing effects. Thus, in the hypothalamus, glucocorticoids induce an increase in GABA release at inhibitory synapses onto magnocellular neuroendocrine cells via the NO activation of presynaptic guanylyl cyclase [44] (Fig. 1(2)). Glucocorticoid-induced endocannabinoid suppression of these GABA synapses is not seen under basal conditions, but emerges only when glial endocannabinoid uptake is suppressed, resulting in spillover of 2-AG from excitatory to inhibitory synapses [20], which should oppose the glucocorticoid-NO facilitatory effect on synaptic inhibition under these conditions.
Glucocorticoids can also rapidly facilitate GABA release from inhibitory synapses in the hippocampus via a membrane receptor-mediated stimulation of NO release [35]. Like at synapses in the hypothalamus [44], glucocorticoid induces NO synthesis and release from postsynaptic neurons (pyramidal cells), which elicits an increase in GABAergic synaptic input from presynaptic interneurons. However, unlike in the hypothalamus, this facilitation of GABA release requires spiking in the upstream inhibitory interneurons, which suggests that the NO diffuses far enough to reach the presynaptic GABA neuron somata and/or dendrites [35] (Fig. 1(2)).
Rapid Glucocorticoid Regulation of Membrane Receptor Trafficking
Acute stress exposure induces rapid changes in the efficacy of synaptic and hormonal signaling that is mediated by the modulation of neurotransmitter release and receptor trafficking. Increasing evidence suggests that rapid glucocorticoid signaling from the membrane controls the cellular trafficking of multiple receptors, including AMPA receptors, noradrenergic receptors, and nuclear glucocorticoid receptors. Both membrane-associated GR and MR have been implicated in this regulation of target receptor trafficking.
Physiological stressors activate the HPA axis through an ascending noradrenergic projection from the nucleus of the solitary tract [45,46]. Norepinephrine (NE) binds to α1 adrenoreceptors in PVN CRH neurons, which activates a local glial-neuronal circuit that excites the CRH neurons and activates the HPA axis [47]. Prior stress exposure rapidly desensitizes the CRH neurons to NE via a nongenomic glucocorticoid-induced regulation of α1 adrenoreceptor trafficking [48,49]. This desensitization is mediated by a mGR-mediated diversion of α1 adrenoreceptor trafficking from the recycling pathway responsible for maintaining stable receptor levels in the membrane [49](Fig. 1(3)).
Recent studies have also revealed rapid glucocorticoid signaling from the membrane to the nuclear GR. Thus, activation of the mGR signals to the unliganded nuclear GR to stimulate its trafficking to the nucleus (Fig. 1(4)), where it regulates gene transcription, including a subset of genes that are distinct from those regulated by the liganded nuclear GR [21,50].
Acute stress and corticosteroid exposure can facilitate or inhibit emotional and spatial memory formation depending on the intensity and time frame of exposure. A potential mechanism of memory modulation is via the rapid increase in excitatory synaptic signaling in output neurons of the hippocampus and amygdala. Electrophysiological studies and live cell imaging in hippocampal and amygdalar neurons have implicated the rapid corticosteroid facilitation of presynaptic glutamate release and postsynaptic AMPA receptor trafficking in this phenomenon [3,51] (Fig. 2(2)). Interestingly, these rapid membrane corticosteroid actions are MR-dependent and not GR-dependent, and facilitate the induction of long-term potentiation (LTP) [30,52,53,54]. Acute stress also facilitates LTP induction in layer V PFC pyramidal neurons via corticosteroid-dependent trafficking of AMPA and NMDA receptors [55,56], although this is a delayed and genomic effect requiring MR-mediated transcriptional regulation [57].
Prolonged corticosteroid exposure [58] or repeated corticosteroid administration in rats that mimics ultradian corticosterone pulses [59] inhibits the corticosterone regulation of AMPA receptor trafficking and LTP induction [53], which could stabilize excitatory synaptic signaling in the face of hourly ultradian corticosteroid fluctuations [60]. The capacity of corticosteroids to rapidly regulate AMPA receptor mobility also facilitates the NMDA receptor-dependent internalization of AMPA receptors that occurs with long-term synaptic depression [61]. Thus, rapid corticosteroid regulation of AMPA receptor trafficking and glutamate release define excitatory synaptic efficacy in the hippocampus.
Conclusions
Thus, glucocorticoid signaling at the neuronal membrane activates multiple pathways simultaneously to regulate a highly diverse array of cellular functions. This multiplexed signaling of the mGR and mMR endows glucocorticoids with a wide range of rapid regulatory capabilities that add to the pleiotropic genomic activities of the nuclear GR and MR.
Acknowledgments
This work was supported by NIH grants R01 MH119283 and R01 MH104373 and by Merit Grant I01 BX005118 from the Southeast Louisiana Veterans Administration.
References
- 1.Reul JMHM, de Kloet ER. Two Receptor Systems for Corticosterone in Rat Brain: Microdistribution and Differential Occupation. Endocrinology 1985; 117:2505–2511. [DOI] [PubMed] [Google Scholar]
- 2.Burnstein KL, Cidlowski JA. Regulation of gene expression by glucocorticoids. Annu Rev Physiol 1989; 51:683–99. [DOI] [PubMed] [Google Scholar]
- 3.Karst H, den Boon FS, Vervoort N et al. Non-genomic steroid signaling through the mineralocorticoid receptor: Involvement of a membrane-associated receptor? Molecular and Cellular Endocrinology 2022; 541:111501. [DOI] [PubMed] [Google Scholar]
- 4.Tasker JG, Herman JP. Mechanisms of rapid glucocorticoid feedback inhibition of the hypothalamic-pituitary-adrenal axis. Stress 14 2011. 398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill MN, Tasker JG. Endocannabinoid signaling, glucocorticoid-mediated negative feedback, and regulation of the hypothalamic-pituitary-adrenal axis. Neuroscience 204 2012. 5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Di S, Malcher-Lopes R, Halmos KCs et al. Nongenomic Glucocorticoid Inhibition via Endocannabinoid Release in the Hypothalamus: A Fast Feedback Mechanism. The Journal of Neuroscience 2003; 23:4850–4857. This was the first demonstration of the rapid glucocorticoid-induced endocannabinoid modulation of synaptic transmission, which has since been found at multiple synapses in multiple limbic regions of the brain. It also provided a cellular mechanism for the rapid glucocorticoid feedback inhibition of the hypothalamic CRH neurons responsible for HPA axis activation.
- 7.Boychuk CR, Zsombok A, Tasker JG et al. Rapid glucocorticoid-induced activation of TRP and CB1 receptors causes biphasic modulation of glutamate release in gastric-related hypothalamic preautonomic neurons. Frontiers in Neuroscience 2013; 7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang M, Hill MN, Zhang L et al. Acute restraint stress enhances hippocampal endocannabinoid function via glucocorticoid receptor activation. Journal of Psychopharmacology 2012; 26:56–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di S, Itoga CA, Fisher MO et al. Acute stress suppresses synaptic inhibition and increases anxiety via endocannabinoid release in the basolateral amygdala. Journal of Neuroscience 2016; 36:8461–8470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Campolongo P, Roozendaal B, Trezza V et al. Endocannabinoids in the rat basolateral amygdala enhance memory consolidation and enable glucocorticoid modulation of memory. Proceedings of the National Academy of Sciences 2009; 106:4888–4893. This study provided in vivo evidence for an endocannabinoid-dependent mechanism in the basolateral amygdala for the well-documented facilitation of fear memory consolidation by stress and glucocorticoid.
- 11.Hill MN, McLaughlin RJ, Pan B et al. Recruitment of prefrontal cortical endocannabinoid signaling by glucocorticoids contributes to termination of the stress response. Journal of Neuroscience 2011; 31:10506–10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siller-Pérez C, Fuentes-Ibañez A, Sotelo-Barrera EL et al. Glucocorticoid interactions with the dorsal striatal endocannabinoid system in regulating inhibitory avoidance memory. Psychoneuroendocrinology 2019; 99:97–103. [DOI] [PubMed] [Google Scholar]
- 13.Pinheiro BS, Lemos C, Neutzling Kaufmann F et al. Hierarchical glucocorticoid-endocannabinoid interplay regulates the activation of the nucleus accumbens by insulin. Brain Res Bull 2016; 124:222–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ragozzino FJ, Arnold RA, Kowalski CW et al. Corticosterone inhibits vagal afferent glutamate release in the nucleus of the solitary tract via retrograde endocannabinoid signaling. American Journal of Physiology - Cell Physiology 2020; 319:C1097–C1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harris C, Weiss GL, Di S et al. Cell signaling dependence of rapid glucocorticoid-induced endocannabinoid synthesis in hypothalamic neuroendocrine cells. Neurobiology of Stress 2019; 10:100158. This study characterized the cell signaling dependence of the membrane-associated glucocorticoid receptor induction of endocannabinoid synthesis in hypothalamic neurons. Although not complete, much of the signal transduction pathway was revealed.
- 16.Wamsteeker JI, Brent Kuzmiski J, Bains JS. Cellular/Molecular Repeated Stress Impairs Endocannabinoid Signaling in the Paraventricular Nucleus of the Hypothalamus. 2010; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nahar J, Haam J, Chen C et al. Rapid nongenomic glucocorticoid actions in male mouse hypothalamic neuroendocrine cells are dependent on the nuclear glucocorticoid receptor. Endocrinology (United States) 2015; 156:2831–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Evanson NK, Tasker JG, Hill MN et al. Fast Feedback Inhibition of the HPA Axis by Glucocorticoids Is Mediated by Endocannabinoid Signaling. Endocrinology 2010; 151:4811–4819. This study provided the first in vivo evidence for the rapid feedback inhibition of the HPA axis mediated by glucocorticoid-induced endocannabinoid signaling in the PVN.
- 19.Oliet SHR, Baimoukhametova D v, Piet R et al. Retrograde regulation of GABA transmission by the tonic release of oxytocin and endocannabinoids governs postsynaptic firing_JNeurosci2007. Journal of Neuroscience 2007; 27:1325–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di S, Popescu IR, Tasker JG. Glial control of endocannabinoid heterosynaptic modulation in hypothalamic magnocellular neuroendocrine cells. Journal of Neuroscience 2013; 33:18331–18342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weiss GL, Rainville JR, Zhao Q et al. Purity and stability of the membrane-limited glucocorticoid receptor agonist dexamethasone-BSA. Steroids 2017; 142:2–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malcher-Lopes R, Di S, Marcheselli VS et al. Opposing crosstalk between leptin and glucocorticoids rapidly modulates synaptic excitation via endocannabinoid release. Journal of Neuroscience 2006; 26:6643–6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luttrell LM, Roudabush FL, Choy EW et al. Activation and targeting of extracellular signal-regulated kinases by β-arrestin scaffolds. Proceedings of the National Academy of Sciences 2001; 98:2449–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luttrell LM, Ferguson SS, Daaka Y et al. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science 1999; 283:655–61. [DOI] [PubMed] [Google Scholar]
- 25.Deng Q, Riquelme D, Trinh L et al. Rapid glucocorticoid feedback inhibition of ACTH secretion involves ligand-dependent membrane association of glucocorticoid receptors. Endocrinology (United States) 2015; 156:3215–3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atkinson HC, Wood SA, Castrique ES et al. Corticosteroids mediate fast feedback of the rat hypothalamic-pituitary- adrenal axis via the mineralocorticoid receptor. American Journal of Physiology - Endocrinology and Metabolism 2008; 294. [DOI] [PubMed] [Google Scholar]
- 27.Atsak P, Roozendaal B, Campolongo P. Role of the endocannabinoid system in regulating glucocorticoid effects on memory for emotional experiences. Neuroscience 2012; 204:104–16. [DOI] [PubMed] [Google Scholar]
- 28.Atsak P, Hauer D, Campolongo P et al. Endocannabinoid signaling within the basolateral amygdala integrates multiple stress hormone effects on memory consolidation. Neuropsychopharmacology 2015; 40:1485–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshida T, Uchigashima M, Yamasaki M et al. Unique inhibitory synapse with particularly rich endocannabinoid signaling machinery on pyramidal neurons in basal amygdaloid nucleus. Proceedings of the National Academy of Sciences 2011; 108:3059–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Karst H, Berger S, Erdmann G et al. Metaplasticity of amygdalar responses to the stress hormone corticosterone. Proceedings of the National Academy of Sciences 2010; 107:14449–14454. This study was the first to show rapid modulation of glutamate release at excitatory synapses in the basolateral amygdala by corticosteroid activation of presynaptic mineralocorticoid receptors, as well as the metaplasticity of the corticosteroid modulation of excitatory synapses, shifting from facilitation to inhibition of glutamate release, with prior exposure to stress or corticosterone.
- 31. Hill MN, Karatsoreos IN, Hillard CJ et al. Rapid elevations in limbic endocannabinoid content by glucocorticoid hormones in vivo. Psychoneuroendocrinology 2010; 35:1333–1338. This study provided the first direct demonstration that systemic corticosteroid administration in vivo stimulates endocannabinoid production in different limbic areas of the brain, including the amygdala, hippocampus and hypothalamus.
- 32.Atsak P, Hauer D, Campolongo P et al. Glucocorticoids interact with the hippocampal endocannabinoid system in impairing retrieval of contextual fear memory. Proc Natl Acad Sci U S A 2012; 109:3504–3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pitler T, Alger B. Postsynaptic spike firing reduces synaptic GABAA responses in hippocampal pyramidal cells. The Journal of Neuroscience 1992; 12:4122–4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature 2001; 410:588–592. This is one of the first seminal studies demonstrating the retrograde release of endocannabinoid at inhibitory synapses and the CB1 modulation of presynaptic GABA release in response to depolarization of the postsynaptic neuron.
- 35. Hu W, Zhang M, Czéh B et al. Stress impairs GABAergic network function in the hippocampus by activating nongenomic glucocorticoid receptors and affecting the integrity of the parvalbumin-expressing neuronal network. Neuropsychopharmacology 2010; 35:1693–1707. This paper describes the only study, to our knowledge, that reveals the activation of spike-dependent release from presynaptic neurons by a retrograde messenger, nitric oxide, presumably via nitric oxide diffusion to the presynaptic inhibitory interneuron somata/dendrites.
- 36.Teng Z, Zhang M, Zhao M et al. Glucocorticoid exerts its non-genomic effect on IPSC by activation of a phospholipase C-dependent pathway in prefrontal cortex of rats. The Journal of Physiology 2013; 591:3341–3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nature Reviews Neuroscience 2009; 10:397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonzalez ST, Fanselow MS. The role of the ventromedial prefrontal cortex and context in regulating fear learning and extinction. Psychology & Neuroscience 2020; 13:459–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Radley JJ, Gosselink KL, Sawchenko PE. A Discrete GABAergic Relay Mediates Medial Prefrontal Cortical Inhibition of the Neuroendocrine Stress Response. Journal of Neuroscience 2009; 29:7330–7340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diorio D, Viau V, Meaney M. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. The Journal of Neuroscience 1993; 13:3839–3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable Roles of Prelimbic and Infralimbic Cortices, Ventral Hippocampus, and Basolateral Amygdala in the Expression and Extinction of Conditioned Fear. Neuropsychopharmacology 2011; 36:529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krabbe S, Gründemann J, Lüthi A. Amygdala Inhibitory Circuits Regulate Associative Fear Conditioning. Biological Psychiatry 2018; 83:800–809. [DOI] [PubMed] [Google Scholar]
- 43.McReynolds JR, Doncheck EM, Li Y et al. Stress Promotes Drug Seeking Through Glucocorticoid-Dependent Endocannabinoid Mobilization in the Prelimbic Cortex. Biological Psychiatry 2018; 84:85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Di S, Maxson MM, Franco A et al. Glucocorticoids regulate glutamate and GABA synapse-specific retrograde transmission via divergent nongenomic signaling pathways. Journal of Neuroscience 2009; 29:393–401. This study was the first to reveal an opposing rapid regulation of glutamate and GABA release by glucocorticoid stimulation of two different retrograde messengers, endocannabinoid and nitric oxide, at excitatory and inhibitory synapses, which work in tandem to suppress neuroendocrine neuron activation.
- 45.Bienkowski MS, Rinaman L. Noradrenergic inputs to the paraventricular hypothalamus contribute to hypothalamic–pituitary–adrenal axis and central Fos activation in rats after acute systemic endotoxin exposure. Neuroscience 2008; 156:1093–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cunningham ET, Bohn MC, Sawchenko PE. Organization of adrenergic inputs to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. J Comp Neurol 1990; 292:651–67. [DOI] [PubMed] [Google Scholar]
- 47.Chen C, Jiang ZY, Fu X et al. Astrocytes Amplify Neuronal Dendritic Volume Transmission Stimulated by Norepinephrine. Cell Reports 2019; 29:4349–4361.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feldman S, Weidenfeld J. Hypothalamic mechanisms mediating glutamate effects on the hypothalamo-pituitary-adrenocortical axis. Journal of Neural Transmission 1997; 104:633–642. [DOI] [PubMed] [Google Scholar]
- 49.Jiang Z, Chen C, Weiss G et al. Acute Stress Desensitizes Hypothalamic CRH Neurons to Norepinephrine and Physiological Stress . bioRxiv. [Google Scholar]
- 50. Rainville JR, Weiss GL, Evanson N et al. Membrane-initiated nuclear trafficking of the glucocorticoid receptor in hypothalamic neurons. Steroids 2019;55–64. This study revealed for the first time signaling from a membrane GR to the nuclear GR that triggers nuclear translocation of the unliganded nuclear GR and transcriptional regulation that differs from that of the liganded nuclear GR.
- 51.Krugers HJ, Hoogenraad CC, Groc L. Stress hormones and AMPA receptor trafficking in synaptic plasticity and memory. Nature Reviews Neuroscience 2010; 11:675–681. [DOI] [PubMed] [Google Scholar]
- 52.Karst H, Joëls M. Corticosterone Slowly Enhances Miniature Excitatory Postsynaptic Current Amplitude in Mice CA1 Hippocampal Cells. J Neurophysiol 2005; 94:3479–3486. [DOI] [PubMed] [Google Scholar]
- 53. Groc L, Choquet D, Chaouloff F. The stress hormone corticosterone conditions AMPAR surface trafficking and synaptic potentiation. Nature Neuroscience 2008; 11:868–870. This is a seminal study that showed how corticosteroid regulates glutamate AMPA receptor trafficking via mineralocorticoid receptor activation to strengthen excitatory synapses, and provided a cellular mechanism for potentiation of excitatory synapses by stress exposure.
- 54.Wiegert O, Joëls M, Krugers H. Timing is essential for rapid effects of corticosterone on synaptic potentiation in the mouse hippocampus. Learning & Memory 2006; 13:110–113. [DOI] [PubMed] [Google Scholar]
- 55.Yuen EY, Liu W, Karatsoreos IN et al. Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proceedings of the National Academy of Sciences 2009; 106:14075–14079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yuen EY, Liu W, Karatsoreos IN et al. Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory. Molecular Psychiatry 2011; 16:156–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu W, Yuen EY, Yan Z. The Stress Hormone Corticosterone Increases Synaptic α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid (AMPA) Receptors via Serum- and Glucocorticoid-inducible Kinase (SGK) Regulation of the GDI-Rab4 Complex. Journal of Biological Chemistry 2010; 285:6101–6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Conboy L, Sandi C. Stress at learning facilitates memory formation by regulating ampa receptor trafficking through a glucocorticoid action. Neuropsychopharmacology 2010; 35:674–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sarabdjitsingh RA, Pasricha N, Smeets JAS et al. Hippocampal fast glutamatergic transmission is transiently regulated by corticosterone pulsatility. PLoS ONE 2016; 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Conway-Campbell BL, Sarabdjitsingh RA, McKenna MA et al. Glucocorticoid Ultradian Rhythmicity Directs Cyclical Gene Pulsing of the Clock Gene Period 1 in Rat Hippocampus. Journal of Neuroendocrinology 2010; 22:1093–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martin S, Henley JM, Holman D et al. Corticosterone Alters AMPAR Mobility and Facilitates Bidirectional Synaptic Plasticity. PLoS ONE 2009; 4:e4714. [DOI] [PMC free article] [PubMed] [Google Scholar]