Visual Abstract
Keywords: CKD, randomized controlled trials, renin–angiotensin system, SGLT2
Abstract
Significance Statement
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) are foundational therapy for CKD but are underused, in part because they are frequently withheld and not restarted due to hyperkalemia, AKI, or hospitalization. Consequently, ensuring persistent use of ACE inhibitors and ARBs in CKD has long been a major clinical priority. In this joint analysis of the CREDENCE and DAPA-CKD trials, the relative risk of discontinuation of ACE inhibitors and ARBs was reduced by 15% in patients randomized to sodium–glucose cotransporter 2 (SGLT2) inhibitors. This effect was more pronounced in patients with urine albumin:creatinine ratio ≥1000 mg/g, for whom the absolute benefits of these medications are the greatest. These findings indicate that SGLT2 inhibitors may enable better use of ACE inhibitors and ARBs in patients with CKD.
Background
Strategies to enable persistent use of renin–angiotensin system (RAS) blockade to improve outcomes in CKD have long been sought. The effect of SGLT2 inhibitors on discontinuation of RAS blockade has yet to be evaluated.
Methods
We conducted a joint analysis of canagliflozin and renal events in diabetes with established nephropathy clinical evaluation (CREDENCE) and dapagliflozin and prevention of adverse outcomes in CKD (DAPA-CKD), two randomized, double-blind, placebo-controlled, event-driven trials of SGLT2 inhibitors in patients with albuminuric CKD. The main outcome was time to incident temporary or permanent discontinuation of RAS blockade, defined as interruption of an ACE inhibitor or ARB for at least 4 weeks or complete cessation during the double-blind on-treatment period. Cox regression analyses were used to estimate the treatment effects from each trial. Hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) were pooled with fixed effects meta-analysis to obtain summary treatment effects, overall and across key subgroups.
Results
During median follow-up of 2.2 years across both trials, 740 of 8483 (8.7%) patients discontinued RAS blockade. The relative risk for discontinuation of RAS blockade was 15% lower in patients randomized to receiving SGLT2 inhibitors (HR, 0.85; 95% CI, 0.74 to 0.99), with consistent effects across trials (P-heterogeneity = 0.92). The relative effect on RAS blockade discontinuation was more pronounced among patients with baseline urinary albumin:creatinine ratio ≥1000 mg/g (pooled HR, 0.77; 95% CI, 0.63 to 0.94; P-heterogeneity = 0.009).
Conclusions
In patients with albuminuric CKD with and without type 2 diabetes, SGLT2 inhibitors facilitate the use of RAS blockade.
Clinical Trial registry name and registration number
ClinicalTrials.gov, NCT02065791 and NCT03036150.
Podcast
This article contains a podcast at https://dts.podtrac.com/redirect.mp3/www.asn-online.org/media/podcast/JASN/2023_11_21_JASN0000000000000248.mp3
Introduction
Renin–angiotensin system (RAS) blockade, including angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs), are foundational therapies for slowing the progression of CKD. However, the use of ACE inhibitors and ARBs in routine clinical practice has remained suboptimal for more than two decades after landmark randomized trials established their role in slowing CKD progression.1–6 A major factor contributing to the significant gap in use of ACE inhibitors and ARBs across multiple settings is that these agents are often discontinued due to hyperkalemia, AKI, and during hospitalizations and frequently not restarted owing to therapeutic inertia or concerns regarding potential adverse effects.7,8 Thus, strategies to enable the persistent use of RAS blockade are a key therapeutic priority in patients with CKD.
Sodium–glucose cotransporter 2 (SGLT2) inhibitors have been shown to reduce the risks of kidney failure, cardiovascular events, and mortality in patients with CKD, with or without type 2 diabetes, and are now considered foundational therapy for CKD alongside RAS blockade.9–11 This class of agents has been shown to have several ancillary benefits, including reducing the risk of serious hyperkalemia, AKI, and all-cause hospitalization in people with diabetes, CKD, or heart failure.11–19 Because all these are potential reasons for discontinuation of RAS blockade, we hypothesized that SGLT2 inhibitors could reduce the frequency with which ACE inhibitors or ARBs are withdrawn in patients with CKD. We therefore undertook a joint analysis of canagliflozin and renal events in diabetes with established nephropathy clinical evaluation (CREDENCE) and dapagliflozin and prevention of adverse outcomes in CKD (DAPA-CKD): two randomized, double-blind, placebo-controlled, event-driven trials in individuals with CKD, to assess whether SGLT2 inhibitors reduce the discontinuation of ACE inhibitors and ARBs.
Methods
Study Design
This post hoc analysis used data from two randomized, double-blind, placebo-controlled, multicenter clinical trials of SGLT2 inhibitors in patients with CKD: the CREDENCE trial (ClinicalTrials.gov identifier: NCT02065791) and the DAPA-CKD trial (NCT03036150). CREDENCE and DAPA-CKD evaluated the effects of canagliflozin and dapagliflozin on a primary outcome of CKD progression, kidney failure, or death due to cardiovascular disease or kidney failure; detailed methods and main findings from these studies have been previously published.9,10
Patients
CREDENCE enrolled individuals aged 30 years or older with type 2 diabetes and eGFR 30 to <90 ml/min per 1.73 m2 and urine albumin:creatinine ratio (UACR) >300 to 5000 mg/g. DAPA-CKD enrolled adults with or without type 2 diabetes, eGFR of 25 to 75 ml/min per 1.73 m2, and UACR of 200 to 5000 mg/g. CREDENCE excluded patients with CKD due to etiologies other than type 2 diabetes. DAPA-CKD excluded patients with type 1 diabetes, polycystic kidney disease, lupus nephritis, antineutrophil cytoplasmic antibody-associated vasculitis, and those requiring immunosuppression for kidney disease within 6 months of enrollment. All patients provided written informed consent, and ethics approval was obtained at all participating centers. In CREDENCE, all patients were required to be receiving the maximum labeled or tolerated dose of an ACE inhibitor or ARB for at least 4 weeks before randomization. In DAPA-CKD, the same was required, unless contraindicated, resulting in 3% of patients not receiving RAS blockade at baseline. Dual use of an ACE inhibitor and ARB was an exclusion criterion for both trials, although a very small number of patients in both trials commenced dual RAS blockade during the run-in period. Mineralocorticoid receptor antagonists were contraindicated in CREDENCE at baseline due to early concerns about the risk of hyperkalemia with SGLT2 inhibitors but were permitted in DAPA-CKD.
Randomized Treatment
Patients in CREDENCE were randomized 1:1 to canagliflozin 100 mg or matching placebo. Patients in DAPA-CKD were randomized 1:1 to dapagliflozin 10 mg or matching placebo.
Outcome Definition
The outcome in this analysis was time to incident temporary or permanent discontinuation of RAS blockade. Using concomitant medication usage data recorded at each study visit during the trials, we defined temporary discontinuation as interruption of an ACE inhibitor or ARB of at least 4 weeks, with the date of the event/end of follow-up defined as the beginning/start date of this interruption. We defined permanent discontinuation as the date an individual discontinued their ACE inhibitor or ARB and reported no subsequent use during the double-blind on-treatment period. Patients were censored at the point of death, the point of last contact with the trial investigators, or at the point of reaching the end of the double-blind on-treatment period without discontinuing RAS blockade, whichever occurred first. The end of the double-blind on-treatment period (i.e., at study drug discontinuation) was chosen as the date of censoring, owing to the infrequent recording of concomitant medication usage after this date. Follow-up was continued regardless of the occurrence of any primary or secondary outcomes of interest in the CREDENCE and DAPA-CKD trials.
Statistical Analysis
All trial patients with a recorded use of either an ACE inhibitor or an ARB at baseline were included in analyses. Baseline characteristics of patients who discontinued RAS blockade and patients who did not discontinue were compared in the pooled CREDENCE/DAPA-CKD population and within each trial using t tests for normally distributed continuous variables and chi-squared tests for categorical variables. Characteristics of patients with and without baseline RAS blockade at baseline were also compared within each trial to assess the effect of exclusions and possible reasons for contraindication to these medications. Continuous variables were reported as mean and SD. Categorical variables were reported as frequency and percentage.
We used Cox proportional hazards regression to assess the effect of SGLT2 inhibitors on time to incident temporary or permanent discontinuation of RAS blockade in the intention-to-treat population, separately in each trial. We included stratification terms for category of eGFR at screening (30 to <45, 45 to <60 ml, or 60 to <90 ml/min per 1.73 m2) in models in CREDENCE and terms for the diagnosis of type 2 diabetes (yes or no) and the urinary albumin-to-creatinine ratio (≤1000 or >1000 mg/g) in models in DAPA-CKD. Treatment effects from each study expressed as hazard ratios (HRs) with corresponding 95% confidence intervals (CIs) were pooled using inverse-variance weighting with fixed effects meta-analysis. As a sensitivity analysis, we analyzed the effect of SGLT2 inhibitors on permanent discontinuation of RAS blockade, censoring patients who experienced temporary discontinuation, and no permanent discontinuation at their date of death, last contact, or the end of the double-blind on-treatment period.
We examined events before the discontinuation of RAS blockade to explore possible causes for discontinuation. These included all-cause hospitalization, AKI, and hyperkalemia (investigator reported or central laboratory-measured serum potassium ≥6 mmol/L). We included any events within a 30-day window before the date of discontinuation. We assessed the effect of SGLT2 inhibitors on time to incident temporary or permanent discontinuation of RAS blockade across key baseline-defined subgroups: female and male sex, eGFR <45 and ≥45 ml/min per 1.73 m2, UACR <1000 and ≥1000 mg/g, serum potassium <5 and ≥5 mmol/L, percentage of the maximum labeled RAS blockade dose <50% and ≥50%, and use of either an ACE inhibitor or ARB. We determined the heterogeneity in treatment effect estimates across subgroups with P-heterogeneity values obtained from random effects meta-regression using restricted maximum likelihood.
We considered violation of proportional hazards in Cox models via visual inspection of Kaplan–Meier survival curves and formal postestimation tests of scaled Schoenfeld residuals. We performed all analyses in R version 4.3.1.
Results
The population for this analysis comprised a total 8483 patients: 4386 from CREDENCE and 4097 from DAPA-CKD. Fifteen patients (0.3%; five randomized to canagliflozin, ten randomized to placebo) in CREDENCE and 207 patients (4.8%; 92 randomized to dapagliflozin, 115 randomized to placebo) in DAPA-CKD were not included in analyses because they had no record of ACE inhibitor or ARB use at baseline (six patients in CREDENCE, 157 patients in DAPA-CKD), had recorded dual use of both an ACE inhibitor and an ARB at baseline (eight patients in CREDENCE, 50 patients in DAPA-CKD), or discontinued RAS blockade on the same day as randomization (one patient in CREDENCE, 0 patients in DAPA-CKD). Characteristics of patients with and without baseline RAS blockade use among the intention-to-treat populations in both trials are compared in Supplemental Table 1.
Selected baseline characteristics of patients included in analyses, in each trial and in the pooled population stratified by treatment arm, are presented in Table 1. There was no compelling evidence of covariate imbalance. Baseline characteristics of patients in the pooled population stratified by discontinuation/nondiscontinuation of RAS blockade during follow-up are presented in Table 2 and displayed for each trial in Supplemental Table 2. Across both trials, patients who discontinued RAS blockade had lower eGFR (mean [SD], 44.9 [15.2] versus 50.4 [17.0] ml/min per 1.73 m2; P < 0.001; Table 2) and higher serum potassium (mean [SD, 4.6 [0.6] versus 4.6 [0.5] mmol/L; P = 0.001; Table 2) and were more likely to have UACR >1000 mg/g (n [%], 393 [53.1%] versus 3624 [46.8%]; P = 0.003; Table 2). In DAPA-CKD, patients who discontinued RAS blockade were more likely to have diabetes (n [%], 280 [74.7%] versus 2485 [66.8%]; P = 0.002; Supplemental Table 2) and cardiovascular disease (n [%], 172 [45.9%] versus 1361 [36.6%]; P < 0.001; Supplemental Table 2) and higher glycated hemoglobin (hemoglobin A1c; mean [SD], 7.3% [1.7%] versus 7.0% [1.7%]; P = 0.03; Supplemental Table 2).
Table 1.
Selected baseline characteristics of patients in CREDENCE and DAPA-CKD overall and by randomized treatment
| Characteristic | CREDENCE (N=4386)a | DAPA-CKD (N=4097)b | Combined (N=8483) | |
|---|---|---|---|---|
| SGLT2i (n=4257) | Placebo (n=4226) | |||
| Randomized to SGLT2i | 2197 (50.1) | 2060 (50.3) | — | — |
| Age, yr | 63.0 (9.2) | 62.8 (12.3) | 62.9 (10.8) | 62.9 (10.8) |
| Female | 1488 (33.9) | 1366 (33.3) | 1444 (33.9) | 1410 (33.4) |
| Race, n (%) | ||||
| White | 2923 (66.6) | 2183 (53.3) | 2564 (60.2) | 2542 (60.2) |
| Black | 222 (5.1) | 182 (4.4) | 208 (4.9) | 196 (4.6) |
| Asian | 874 (19.9) | 1393 (34.0) | 1137 (26.7) | 1130 (26.7) |
| Other | 367 (8.4) | 339 (8.3) | 348 (8.2) | 358 (8.5) |
| Body mass index, kg/m2 | 31.3 (6.2) | 29.5 (6.1) | 30.4 (6.2) | 30.5 (6.3) |
| Systolic BP, mm Hg | 140 (15.6) | 137.1 (17.3) | 138.4 (16.6) | 138.9 (16.5) |
| Glycated hemoglobin, % | 8.3 (1.3) | 7.1 (1.7) | 7.7 (1.6) | 7.7 (1.6) |
| Serum potassium, mmol/L | 4.5 (0.5) | 4.6 (0.6) | 4.6 (0.5) | 4.6 (0.6) |
| Serum potassium, mmol/L, n (%) | ||||
| <5 | 3562 (81.3) | 2972 (72.7) | 3274 (77.0) | 3260 (77.3) |
| ≥5 | 821 (18.7) | 1115 (27.3) | 976 (23.0) | 960 (22.7) |
| eGFR, ml/min per 1.73 m2 | 56.2 (18.2) | 43.2 (12.4) | 50.1 (16.9) | 49.8 (17.0) |
| eGFR, ml/min per 1.73 m2, n (%) | ||||
| <45 | 1362 (31.1) | 2384 (58.2) | 1886 (44.3) | 1860 (44.0) |
| 45–60 | 1330 (30.3) | 1318 (32.2) | 1308 (30.7) | 1340 (31.7) |
| >60 | 1693 (38.6) | 395 (9.6) | 1062 (25.0) | 1026 (24.3) |
| UACR, mg/g, n (%) | ||||
| <300 | 525 (12.0) | 424 (10.3) | 499 (11.7) | 450 (10.6) |
| 300–1000 | 1814 (41.4) | 1703 (41.6) | 1743 (40.9) | 1774 (42.0) |
| >1000 | 2047 (46.7) | 1970 (48.1) | 2015 (47.3) | 2002 (47.4) |
| Duration of diabetes, yr | 15.8 (8.6) | 14.9 (9.7) | 15.3 (9.1) | 15.6 (9.0) |
| Diabetes, n (%) | 4386 (100.0) | 2765 (67.5) | 3593 (84.4) | 3558 (84.2) |
| Cardiovascular disease, n (%) | 2214 (50.5) | 1553 (37.4) | 1896 (44.5) | 1851 (43.8) |
| Heart failure, n (%) | 652 (14.9) | 449 (11.0) | 560 (13.2) | 541 (12.8) |
| Use of mineralocorticoid receptor antagonistsc, n (%) | — | 214 (5.2) | 101 (2.4) | 113 (2.7) |
Data are n (%) and mean (SD). CREDENCE, canagliflozin and renal events in diabetes with established nephropathy clinical evaluation; DAPA-CKD, dapagliflozin and prevention of adverse outcomes in CKD; SGLT2i, sodium–glucose cotransporter 2 inhibitor; UACR, urine albumin:creatinine ratio.
Fifteen patients from the intention-to-treat population in canagliflozin and renal events in diabetes with established nephropathy clinical evaluation (N=4401) were excluded as they did not have any recorded renin–angiotensin system blockade at baseline, had dual use of both an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker at baseline, or discontinued renin–angiotensin system blockade on the day of randomization.
Two hundred and seven patients from the intention-to-treat population in dapagliflozin and prevention of adverse outcomes in CKD (N=4304) were excluded as they did not have any recorded renin–angiotensin system blockade at baseline, had dual use of both an angiotensin converting enzyme inhibitor or an angiotensin receptor blocker at baseline, or discontinued renin–angiotensin system blockade on the day of randomization.
Data on the use of mineralocorticoid receptor antagonists are provided for dapagliflozin and prevention of adverse outcomes in CKD only, owing to the fact that the use of these medications was contraindicated in the canagliflozin and renal events in diabetes with established nephropathy clinical evaluation trial at baseline due to early concerns about the potential risk of hyperkalemia with sodium–glucose cotransporter 2 inhibitors.
Table 2.
Selected baseline characteristics of patients in the pooled CREDENCE and DAPA-CKD population stratified by discontinuation/nondiscontinuation of renin–angiotensin system blockade during follow-up
| Pooled CREDENCE/DAPA-CKD Population (N=8483)a | |||
|---|---|---|---|
| Characteristic | Patients Who Discontinued RAS Blockade (n=740) | Patients Who did Not Discontinue RAS Blockade (n=7743) | P Value |
| Randomized to SGLT2i | 348 (47.0) | 3909 (50.5) | 0.08 |
| Age, yr | 62.2 (11.4) | 63.0 (10.7) | 0.07 |
| Female | 225 (30.4) | 2629 (34.0) | 0.06 |
| Race, n (%) | <0.001 | ||
| White | 367 (49.6) | 4739 (61.2) | |
| Black | 44 (5.9) | 360 (4.6) | |
| Asian | 270 (36.5) | 1997 (25.8) | |
| Other | 59 (8.0) | 647 (8.4) | |
| Body mass index, kg/m2 | 29.8 (6.7) | 30.5 (6.2) | 0.01 |
| Systolic BP, mm Hg | 138.6 (18.6) | 138.6 (16.3) | 0.97 |
| Glycated hemoglobin, % | 7.8 (1.7) | 7.7 (1.6) | 0.23 |
| Serum potassium, mmol/L | 4.6 (0.6) | 4.6 (0.5) | 0.001 |
| Serum potassium, mmol/L, n (%) | <0.001 | ||
| <5 | 518 (70.1) | 6016 (77.8) | |
| ≥5 | 221 (29.9) | 1715 (22.2) | |
| eGFR, ml/min per 1.73 m2 | 44.9 (15.2) | 50.4 (17.0) | <0.001 |
| eGFR, ml/min per 1.73 m2, n (%) | <0.001 | ||
| <45 | 428 (57.8) | 3318 (42.9) | |
| 45–60 | 203 (27.4) | 2445 (31.6) | |
| >60 | 109 (14.7) | 1979 (25.6) | |
| UACR, mg/g, n (%) | 0.003 | ||
| <300 | 66 (8.9) | 883 (11.4) | |
| 300–1000 | 281 (38.0) | 3236 (41.8) | |
| >1000 | 393 (53.1) | 3624 (46.8) | |
| Duration of diabetes, yr | 15.5 (8.9) | 15.4 (9.1) | 0.87 |
| Diabetes, n (%) | 645 (87.2) | 6506 (84.0) | 0.03 |
| Cardiovascular disease, n (%) | 358 (48.4) | 3389 (43.8) | 0.02 |
| Heart failure, n (%) | 91 (12.3) | 1010 (13.0) | 0.67 |
| Use of mineralocorticoid receptor antagonistsb, n (%) | 22 (3.0) | 192 (2.5) | 0.49 |
Data are n (%) and mean (SD). CREDENCE, canagliflozin and renal events in diabetes with established nephropathy clinical evaluation; DAPA-CKD, dapagliflozin and prevention of adverse outcomes in CKD; RAS, renin–angiotensin system; SGLT2i, sodium–glucose cotransporter 2 inhibitor; UACR, urine albumin:creatinine ratio.
Fifteen patients from the intention-to-treat population in canagliflozin and renal events in diabetes with established nephropathy clinical evaluation (N=4401) were excluded as they did not have any recorded renin–angiotensin system blockade at baseline, had dual use of both an angiotensin converting enzyme inhibitor or an angiotensin receptor blocker at baseline, or discontinued renin–angiotensin system blockade on the day of randomization. Two hundred and seven patients from the intention-to-treat population in dapagliflozin and prevention of adverse outcomes in CKD (N=4304) were excluded as they did not have any recorded renin–angiotensin system blockade at baseline, had dual use of both an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker at baseline, or discontinued renin–angiotensin system blockade on the day of randomization.
Data on use of mineralocorticoid receptor antagonists are available for dapagliflozin and prevention of adverse outcomes in CKD only, owing to the fact that the use of these medications was contraindicated in the canagliflozin and renal events in diabetes with established nephropathy clinical evaluation trial at baseline due to early concerns about the potential risk of hyperkalemia with sodium–glucose cotransporter 2 inhibitors.
During a median follow-up of 2.2 years (25th and 75th percentile: 1.6 and 2.6 years) in both trials, 740 patients temporarily or permanently discontinued RAS blockade: 365 (8.3%; 171 randomized to canagliflozin, 194 randomized to placebo) from CREDENCE and 375 (9.2%; 177 randomized to dapagliflozin, 198 randomized to placebo) from DAPA-CKD. Of the 365 patients who discontinued RAS blockade in CREDENCE, 143 (63 randomized to canagliflozin and 80 randomized to placebo) temporarily discontinued and 222 (108 randomized to canagliflozin and 80 randomized to placebo) permanently discontinued. Of the 375 patients in DAPA-CKD who discontinued RAS blockade, 180 (86 randomized to dapagliflozin and 94 randomized to placebo) temporarily discontinued and 195 (91 randomized to dapagliflozin and 104 randomized to placebo) permanently discontinued.
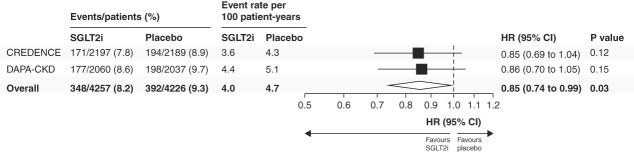
Cumulative incidence curves for discontinuation of RAS blockade are shown in Figure 1. Across both trials, the rate of discontinuation of RAS blockade was 4.0 events per 100 person-years (95% CI, 3.6 to 4.4) in patients randomized to SGLT2 inhibitors and 4.7 events per 100 person-years (95% CI, 4.2 to 5.1) in patients randomized to placebo. Overall, SGLT2 inhibitors reduced the temporary or permanent discontinuation of RAS blockade, with a relative risk reduction of 15% (HR, 0.85; 95% CI, 0.74 to 0.99; Figure 2). The effect was consistent across both trials (HR for CREDENCE 0.85; 95% CI, 0.69 to 1.04; HR for DAPA-CKD 0.86; 95% CI, 0.70 to 1.05; P-heterogeneity = 0.92; Figure 2). There was no violation of proportional hazards, as observed by formal postestimation tests of scaled Schoenfeld residuals (P = 0.87 for CREDENCE, P = 0.18 for DAPA-CKD).
Figure 1.
Cumulative incidence curves of incident temporary or permanent discontinuation of RAS blockade in CREDENCE and DAPA-CKD. The insets show the same data on an expanded y axis. The competing risk of death was considered when estimating cumulative incidence functions. CI, confidence interval; CREDENCE, canagliflozin and renal events in diabetes with established nephropathy clinical evaluation; DAPA-CKD, dapagliflozin and prevention of adverse outcomes in CKD; RAS, renin–angiotensin system.
Figure 2.
Effects of SGLT2i on temporary or permanent discontinuation of RAS blockade. SGLT2i, sodium–glucose cotransporter 2 inhibitor; HR, hazard ratio.
Hospitalization, AKI, and hyperkalemia events occurring within 30 days before RAS discontinuation are summarized in Supplemental Table 3. Across both trials, 28.4% of discontinuations had an antecedent event before discontinuation, with all-cause hospitalization predominating (n/N [%], 140/740 [18.9%]). This observation was similar in both CREDENCE (n/N [%], 77/365 [21.1%]) and DAPA-CKD (n/N [%], 63/375 [16.8%]).
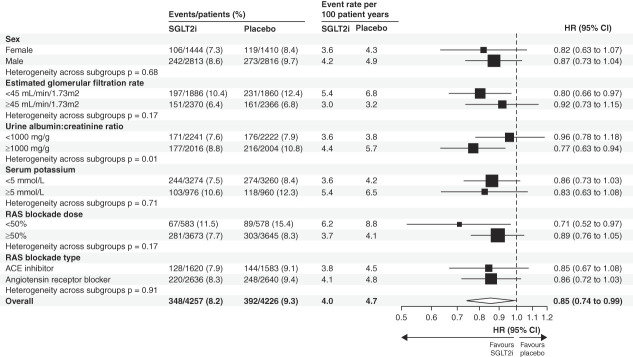
The effect of SGLT2 inhibitors on discontinuation of RAS blockade across baseline-defined subgroups is presented in Figure 3. The rates of discontinuation of RAS blockade were substantially higher in patients with eGFR <45 ml/min per 1.73 m2, UACR ≥1000 mg/g, serum potassium ≥5 mmol/L, and patients receiving <50% of maximum labeled RAS blockade dose. The reduction in discontinuation of RAS blockade with SGLT2 inhibitors was consistent regardless of sex, eGFR, serum potassium, RAS blockade dose, and type of RAS blockade (all P-heterogeneity >0.15). The magnitude of benefit with respect to discontinuation of RAS blockade was more pronounced in those with baseline UACR ≥1000 mg/g (pooled HR, 0.77; 95% CI, 0.63 to 0.94) compared with those with baseline UACR <1000 mg/g (pooled HR, 0.96; 95% CI, 0.78 to 1.18) (P-heterogeneity = 0.009). The effect of SGLT2 inhibitors on permanent discontinuation of RAS blockade is presented in Supplemental Figure 1. Individual trial results for each baseline-defined subgroup are presented in Supplemental Figure 2.
Figure 3.
Effects of SGLT2i on temporary or permanent discontinuation of RAS blockade by baseline-defined participant subgroups.
Discussion
In this joint analysis of the CREDENCE and DAPA-CKD trials, SGLT2 inhibitors reduced the risk of temporary or permanent discontinuation of RAS blockade by 15%, with consistent effects across trials. We observed relative risk reductions with SGLT2 inhibitors that were consistent irrespective of patient sex, baseline eGFR, serum potassium, RAS blockade dose, and ACE inhibitor or ARB usage. The relative effect of SGLT2 inhibitors on RAS blockade discontinuation was more pronounced in those with baseline UACR ≥1000 mg/g. Because the rate of discontinuation was considerably higher in these patients, absolute benefits are also likely to be larger. These findings indicate that SGLT2 inhibitors may have ancillary benefits in facilitating the use of ACE inhibitors or ARBs in patients with CKD, the traditional foundation for attenuating CKD progression.
Despite proven benefits in slowing CKD progression, RAS blockade remains significantly underused in at-risk patients. In the United States, the use of ACE inhibitors and ARBs in CKD has plateaued over the past decade, with fewer than half of patients with CKD receiving these medications.6 Data indicate similar trends in low- and middle-income countries.5 Reasons for the underuse of RAS blockade in CKD are complex and incompletely understood but highlight potential gaps in identification and recognition of CKD, barriers to care, and guideline adherence.
Maintaining persistent use of RAS blockade remains a commonly encountered challenge in clinical practice. Owing to therapeutic inertia and/or perceived safety concerns, ACE inhibitors and ARBs are often not restarted after temporary cessation, typically due to AKI, hyperkalemia, and/or hospitalization.7,8 Potassium binders have long been touted as a tool to enable the persistent use of RAS blockade by reducing hyperkalemia, but concerns about rare gastrointestinal side effects of sodium polystyrene sulfonate, limited access to newer potassium binders, and uncertain effects on clinical outcomes persist.20 By contrast, SGLT2 inhibitors have direct benefits on clinical outcomes and are well tolerated, with serious adverse events occurring less commonly compared with placebo in CREDENCE and DAPA-CKD.9,10 The apparent greater benefit in reducing the discontinuation of RAS blockade in those with ≥1000 mg/g of albuminuria is highly clinically relevant because it is these patients who are at highest risk of CKD progression.
Historically, there has also been uncertainty about the merits of discontinuing versus continuing RAS blockade in people with advanced CKD, which has recently been addressed by the STOP ACEi trial.21 Although there was no difference in the rate of decline in eGFR between those who continued versus discontinued RAS blockade in this trial, there was a numerically increased incidence of kidney replacement therapy and cardiovascular events in those who discontinued RAS blockade. These results are supported by traditional cohort studies and studies using target trial emulation techniques, which also observed increased risks of cardiovascular events after RAS blockade was discontinued.7,22,23 Therefore, strategies to enable persistent use of RAS blockade, even in advanced CKD, are highly desired.
Common events leading to discontinuation of RAS blockade include hospitalization, hyperkalemia, AKI, and progression to advanced CKD, the risks of which of have all been shown to decrease with SGLT2 inhibitors. SGLT2 inhibitors reduce the risk of time to first serum potassium >6 mmol/L by approximately 15%–20% in people with type 2 diabetes at high cardiovascular risk and/or with CKD and almost 40% in those with heart failure.12–14 Large, collaborative meta-analyses also demonstrate that SGLT2 inhibitors reduce the risk of AKI by almost 25%, with consistent benefits in those with and without diabetes.15 Finally, in patients with CKD, SGLT2 inhibitors significantly reduced the risk of all-cause hospitalization, with similar reductions observed in patients with diabetes at high cardiovascular risk and patients with heart failure.11,16–19 Together with data from the EMPEROR-Reduced trial, where empagliflozin reduced the risk of discontinuation of mineralocorticoid receptor antagonists by 22% in patients with heart failure with reduced ejection fraction,24 combined evidence suggests that SGLT2 inhibitors may enhance tolerability of guideline-directed medical therapy in both heart failure and CKD. While our findings raise the possibility that part of the benefits of SGLT2 inhibition may be mediated by enabling persistent use of RAS blockade, this is unlikely as renoprotection with SGLT2 inhibitors has been observed even in patients not receiving RAS blockade.25,26
The CREDENCE and DAPA-CKD trials were international multicenter randomized trials with rigorous recording of concomitant medications throughout the trial, which permitted accurate determination of time to temporary or permanent discontinuation of RAS blockade. The use of individual patient data ensured that consistency in methodology and outcome definitions could be used and time-to-event analyses could be conducted. The large sample size of the two combined trials and relatively long follow-up duration increased the precision of effect estimates and allowed the examination of the consistency of effects across clinically relevant subgroups. Furthermore, the requirement that all patients were using an ACE inhibitor or ARB for at least 4 weeks before randomization (unless contraindicated in DAPA-CKD) allowed for most of the intention-to-treat population to be used for randomized analyses.
These analyses have limitations that need to be considered. This was a post hoc analysis, and neither trial was specifically designed to assess the effect of SGLT2 inhibitors on discontinuation of RAS blockade. Although concomitant medication usage was recorded at study visits, discontinuation of ACE inhibitors and ARBs was not a prespecified outcome in electronic case report forms. Thus, there is the potential for imprecision in the observed treatment effect and misclassification of events. Misclassification is likely to bias results toward the null; therefore, the fact that consistent findings were demonstrated in both trials strengthens our conclusions. Concomitant medication use was also not systematically collected after the on-treatment period, potentially reducing statistical power to detect treatment effects on discontinuation of RAS blockade. Second, it was not possible to ascertain the exact reason for RAS blockade discontinuation, as only the timing of medication usage during the trial was available. This required that a proxy definition for discontinuation was used, and only events before discontinuation could be assessed. Third, these results were generated from well-designed trials involving experienced investigators who were encouraged to continue guideline-directed background care wherever possible, and thus, persistent use of RAS blockade was high. Whether these findings translate into more pronounced effects on RAS blockade discontinuation in real-world settings—where this occurs much more frequently than in clinical trials27,28 —should be examined in future work, leveraging routinely collected data.
In people with albuminuric CKD with and without type 2 diabetes, SGLT2 inhibitors facilitated the use of RAS blockade.
Supplementary Material
Acknowledgments
The authors thank all investigators, patients, and research teams for their contribution to the reported clinical trials. The authors received no financial support for the research, authorship, and/or publication of this article and agreed on the decision to submit for publication.
Disclosures
C. Arnott is supported by an NHMRC/MRFF Priority Fellowship and a NSW Health EMC Grant. G.M. Chertow has received fees from AstraZeneca for the DAPA-CKD trial steering committee, research grants from CSL Behring, NIAID, and NIDDK; he is on the board of directors for Satellite Healthcare, has received fees for advisory boards for Cricket, DiaMedica, and Reata. He holds stock options for Ardelyx, CloudCath, Durect, DxNow, Outset, Renibus, and Unicycive; has received fees from Akebia, Gilead, Sanifit, and Vertex for trial steering committees; and has received fees for DSMB service from Bayer, Gilead, Mineralys, Palladio and ReCor. G.M. Chertow also reports Consultancy: Akebia, Ardelyx, AstraZeneca, Calico, Gilead, Miromatrix, Reata, Sanifit, Unicycive, and Vertex; Ownership Interest: Eliaz Therapeutics, Physiowave, and PuraCath; Advisory or Leadership Role: Co-Editor, Brenner and Rector's The Kidney (Elsevier). R. Correa-Rotter has received consulting and/or speaker and/or advisory board fees from Amgen, AstraZeneca, Bayer, Boehringer, Chinook Therapeutics, GSK, Janssen, Novo Nordisk, and Sanofi. R. Correa-Rotter also reports Consultancy: Dimerix; Research Funding: Astra Zeneca, Baxter, GSK, and Novo Nordisk; Honoraria: Amgen, Astra Zeneca, Bayer, Boehringer Ingelheim, Janssen, and Sanofi; Advisory or Leadership Role: Astra Zeneca, Bayer, GSK, Membership Steering Committee of DAPA-CKD, Membership Steering Committee FINEREAL, National Leader ASCEND study, National Leader FLOW study, Novo Nordisk, Editorial Board Nefrologia Latinoamericana, Revista de Investigación Clinica, American Journal of Kidney Diseases, Frontiers in Nephrology Associate Editor: Blood Purification Associate Editor; Speakers Bureau: Abbvie; and Other Interests or Relationships: Member of ASN, Member EDTA/ERA, Member of International Society of Nephrology, Member Latin American Society of Nephrology and Hypertension, Member Mexican Institute for Research in Nephrology, and Member of National Kidney Foundation. R.A. Fletcher is supported by a PhD studentship from the Health Data Research UK-The Alan Turing Institute Wellcome Trust Program in Health Data Science. This funding had no role in the production of this manuscript. R.A. Fletcher reports the following: Employer: The George Institute for Global Health and Sensyne Health PLC (employment ended August 2021). H.J.L. Heerspink is consultant for AstraZeneca, Bayer, Boehringer Ingelheim, Chinook, CSL Behring, Dimerix, Eli Lilly, Gilead, Janssen, Merck, Novo Nordisk, ProKidney, Travere Therapeutics, and Vifor Fresenius. He has received research support from AstraZeneca, Boehringer Ingelheim, Janssen, and Novo Nordisk. H.J.L. Heerspink also reports Consultancy: Novartis; Honoraria: Lecture fees from AstraZeneca and Novo Nordisk; and Speakers Bureau: AstraZeneca. M.J. Jardine is supported by a Medical Research Future Fund Next Generation Clinical Researchers Program Career Development Fellowship; is responsible for research projects that have received unrestricted funding from Amgen, Baxter, Eli Lilly, and Merck Sharpe Dohme; serves on a Steering Committee sponsored by CSL; has served on advisory boards sponsored by Akebia, Baxter, Boehringer Ingelheim, and Vifor; and has spoken at scientific meetings sponsored by Janssen; with any consultancy, honoraria, or travel support paid to her institution. M.J. Jardine also reports the following: Research Funding: CSL and Dimerix with all payments to institution; Honoraria: AstraZeneca, Bayer, Boehringer Ingelheim, Janssen, MSD, Occuryx, and Vifor, and directs honoraria to clinical research programs; Advisory or Leadership Role: Chinook and Janssen, All honoraria directed to clinical research programs; and Speakers Bureau: Astra Zeneca, Boehringer Ingelheim, Janssen, and directs speaker fees to clinical research programs. N. Jongs reports travel grants from AstraZeneca. K.W. Mahaffey has received research support from Afferent, Amgen, Apple Inc., AstraZeneca, Cardiva Medical Inc., Daiichi, Ferring, Google (Verily), Johnson & Johnson, Luitpold, Medtronic, Merck, National Institutes of Health, Novartis, Sanofi, St. Jude, and Tenax and has served as a consultant (speaker fees for continuing medical education events only) for Abbott, Ablynx, AstraZeneca, Baim Institute, Boehringer Ingelheim, Bristol-Myers Squibb, Elsevier, GlaxoSmithKline, Johnson & Johnson, MedErgy, Medscape, Mitsubishi Tanabe, Myokardia, NIH, Novartis, Novo Nordisk, Portola, Radiometer, Regeneron, Springer Publishing, and University of California, San Francisco. J.J.V. McMurray has received funding to his institution from Amgen and Cytokinetics for his participation in the Steering Committee for the ATOMIC-HF, COSMIC-HF, and GALACTIC-HF trials and meetings and other activities related to these trials; has received payments through Glasgow University from work on clinical trials, consulting and other activities from Alnylam, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Cardurion, Dal-Cor, GlaxoSmithKline, Ionis, KBP Biosciences, Novartis, Pfizer, and Theracos; has received personal lecture fees from the Abbott, Corpus, Global Clinical Trial Partners (GCTP), Hikma, Medscape/Heart.Org, Radcliffe Cardiology, Servier Director, and Sun Pharmaceuticals. B.L. Neuen has received fees for advisory boards, steering committee roles, scientific presentations, and travel support from AstraZeneca, Bayer, Boehringer Ingelheim, Cambridge Healthcare Research, Cornerstone Medical Education, Janssen, the Limbic, and Medscape, with all honoraria paid to his institution. B.L. Neuen also reports Consultancy: AstraZeneca, Bayer, Boehringer and Ingelheim, Cambridge Healthcare Research, and Janssen; Research Funding: Bayer; Honoraria: AstraZeneca, Bayer, Boehringer and Ingelheim, Cornerstone Medical Education, Janssen, the Limbic, and Medscape; Advisory or Leadership Role: AstraZeneca, Bayer, and Boehringer and Ingelheim; and Speakers Bureau: AstraZeneca and Boehringer and Ingelheim. V. Perkovic serves as a Board Director for St. Vincent's Health Australia, George Clinical and several Medical Research Institutes. He has received honoraria for Steering Committee roles, scientific presentations and/or advisory board attendance from Abbvie, Amgen, Astra Zeneca, Baxter, Bayer, Boehringer Ingelheim, Chinook, Durect, Eli Lilly, Gilead, GSK, Janssen, Merck, Mitsubishi Tanabe, Mundipharma, Novartis, Novo Nordisk, Otsuka, Pfizer, Pharmalink, Reata, Relypsa, Roche, Sanofi, Servier, Travere, and Tricida. V. Perkovic also reports Consultancy: AstraZeneca, Bayer, Boehringer Ingelheim, Chinook, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Mitsubishi Tanabe, Mundipharma, Novartis, Novo Nordisk, Otsuka, Travere, Tricida, and UptoDate; Ownership Interest: George Clinical; Research Funding: AstraZeneca, Bayer, Chinook, Gilead, GlaxoSmithKline, Janssen, Novartis, Novo Nordisk, Otsuka, Travere, and Tricida; Honoraria: AstraZeneca, Bayer, Boehringer Ingelheim, Chinook, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Mitsubishi Tanabe, Mundipharma, Novartis, Novo Nordisk, Otsuka, Travere, Tricida, and UptoDate. P. Rockenschaub is supported by the Alexander von Humboldt Foundation. P. Rossing declares receiving consultancy and/or speaking fees (to his institution) from Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Gilead, MSD, Novo Nordisk, Sanofi, and Vifor Pharma, and research grants from AstraZeneca, Bayer, and Novo Nordisk. P. Rossing also reports Honoraria: Abbott, AstraZeneca, Boehringer Ingelheim, and Novo Nordisk, all honoraria to institution; and Advisory or Leadership Role: AstraZeneca, Bayer, Gilead, and Novo Nordisk, all honoraria to institution. R.D. Toto reports grant support from NIH and is a consultant to and has received honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Otsuka, Reata, and Relypsa. M. Vaduganathan has received research grant support or served on advisory boards for American Regent, Amgen, AstraZeneca, Baxter Healthcare, Bayer AG, Boehringer Ingelheim, Cytokinetics, Lexicon Pharmaceuticals, Novartis, Pharmacosmos, Relypsa, Roche Diagnostics, Sanofi, and Tricog Health, speaker engagements with AstraZeneca, Novartis, and Roche Diagnostics, and participates on clinical trial committees for studies sponsored by Bayer AG, Galmed, Impulse Dynamics, Novartis, and Occlutech. M. Vaduganathan also reports Consultancy: American Regent, Amgen, AstraZeneca, Baxter Healthcare, Bayer AG, Boehringer Ingelheim, Chiesi, Cytokinetics, Lexicon Pharmaceuticals, Novartis, Novo Nordisk, Pharmacosmos, Relypsa, Roche Diagnostics, Sanofi, and Tricog Health; Research Funding: Galmed, Impulse Dynamics, and Occlutech; and Speakers Bureau: AstraZeneca, Cytokinetics, Lexicon Pharmaceuticals, Novartis, and Roche Diagnostics. D.C. Wheeler has received honoraria and/or consultancy fees from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Gilead, GlaxoSmithKline, Janssen, Medscape, Merck Sharp and Dohme, Mundipharma, Napp, Reata, Takeda, Tricida, Vifor Fresenius, and Zydus. D.C. Wheeler also reports Consultancy: Astellas, Eledon, Galderma, George Clinical, Pfizer, and ProKidney; Advisory Boards, Trial Committees and Consultancy; Honoraria: Astellas and Pharmacosmos; Advisory or Leadership Role: AstraZeneca; and Speakers Bureau: Amgen, Astellas, AstraZeneca, Janssen, Merck Sharp and Dohme, Mundipharma, Napp, and Vifor Fresenius. K. Mahaffey reports Consultancy: Amgen, Applied Therapeutics, Bayer, BMS, BridgeBio, CSL Behring, Elsevier, Fibrogen, Fosun Pharma, Johnson & Johnson, Lexicon, Moderna, Myokardia, Novartis, Novo Nordisk, Otsuka, Phasebio, Portola, Quidel, Sanofi, Theravance; Research Funding: AHA, Apple Inc, Bayer, California Institute Regenerative Medicine, Eidos, Ferring, Gilead, Google (Verily), Idorsia, Johnson & Johnson, Luitpold, Novartis, PAC-12, Precordior, Sanifit; and Honoraria: Inova, Intermountain Health, Medscape, Mount Sinai, CSL. J. McMurray reports Consultancy: Personal Consultancy fees: Alynylam Pharmaceuticals, Bayer, BMS, Ionis Pharmaceuticals, Novartis, Regeneron Pharmaceuticals, River 2 Renal Corp.; Data Safety Monitoring Board: George Clinical PTY Ltd.; Honoraria: Personal lecture fees: Abbott, Alkem Metabolics, AZ, Blue Ocean Scientific Solutions Ltd., BI, Canadian Medical and Surgical Knowledge, Emcure Pharma., Eris Lifesciences, European Academy of CME, Hikma Pharma., Imagica Health, Intas Pharma., J.B. Chemicals & Pharma.., Lupin Pharma., Medscape/Heart.Org., ProAdWise Communications, Radcliffe Cardiology, Sun Pharma., The Corpus, Translation Research Group, Translational Medicine Academy.; Advisory or Leadership Role: employer, Glasgow University, has been paid for my participation in advisory boards organized by Novartis and AstraZeneca; and Other Interests or Relationships: Payments to my employer, Glasgow University, for my work on clinical trials, consulting and other activities: Amgen, AstraZeneca, Bayer, Cardurion, Cytokinetics, GSK, KBP Biosciences, Novartis; Director: Global Clinical Trial Partners Ltd (GCTP). C. Arnott reports Research Funding: Abbott Vascular; Honoraria: Amgen; Astra Zeneca, Novo Nordisk; and Advisory or Leadership Role: Novo Nordisk. R. Toto reports Consultancy: Amgen, Bayer, Astra-Zeneca, Boehringer-Ingelheim, Novartis, Novo Nordisk, CinCor, Calliditas, Otsuka, Medscape; Research Funding: NIH; Honoraria: Amgen, Bayer, Astra-Zeneca, Boehringer-Ingelheim, Novartis, Novo Nordisk, CinCor, Calliditas, Otsuka, Medscape; and Advisory or Leadership Role: Amgen, Bayer, Astra-Zeneca, Boehringer-Ingelheim, Novartis, Novo Nordisk, CinCor, Calliditas, Otsuka, Medscape.
Funding
None.
Author Contributions
Conceptualization: Robert A. Fletcher, Hiddo J.L. Heerspink, John J.V. McMurray, Brendon L. Neuen, Muthiah Vaduganathan.
Data curation: Robert A. Fletcher, Niels Jongs, Patrick Rockenschaub.
Formal analysis: Robert A. Fletcher, Niels Jongs, Patrick Rockenschaub.
Investigation: Robert A. Fletcher.
Methodology: Robert A. Fletcher, Patrick Rockenschaub.
Project administration: Brendon L. Neuen.
Resources: Clare Arnott.
Software: Clare Arnott.
Supervision: Clare Arnott, Glenn M. Chertow, Ricardo Correa-Rotter, Hiddo J.L. Heerspink, Kenneth W. Mahaffey, John J.V. McMurray, Brandon L. Neuen, Vlado Perkovic, Patrick Rockenschaub, Peter Rossing, Robert D. Toto, Muthiah Vaduganathan, David C. Wheeler.
Validation: Robert A. Fletcher, Niels Jongs.
Visualization: Robert A. Fletcher.
Writing – original draft: Robert A. Fletcher, Hiddo J.L. Heerspink.
Writing – review & editing: Glenn M. Chertow, Ricardo Correa-Rotter, Hiddo J.L. Heerspink, John J.V. McMurray, Robert D. Toto, Muthiah Vaduganathan, David C. Wheeler.
Data Sharing Statement
Previously published data were used for this study.
Supplemental Material
This article contains the following supplemental material online at http://links.lww.com/JSN/E541.
Supplemental Table 1. Selected baseline characteristics of participants in CREDENCE and DAPA-CKD stratified by baseline use of RAS blockade.
Supplemental Table 2. Selected baseline characteristics of patients in CREDENCE and DAPA-CKD stratified by discontinuation/nodiscontinuation of RAS blockade during follow-up.
Supplemental Table 3. Events within 30 days before temporary or permanent discontinuation of RAS blockade, overall and by randomized treatment.
Supplemental Figure 1. Effects of SGLT2 inhibitors on permanent discontinuation of RAS blockade.
Supplemental Figure 2. Effects of SGLT2 inhibitors on temporary or permanent discontinuation of RAS blockade by baseline-defined participant subgroups separately for CREDENCE and DAPA-CKD.
References
- 1.Lewis EJ Hunsicker LG Clarke WR, et al. Collaborative Study Group. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. New Engl J Med. 2001;345(12):851–860. doi: 10.1056/NEJMoa011303 [DOI] [PubMed] [Google Scholar]
- 2.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. New Engl J Med. 1993;329(20):1456–1462. doi: 10.1056/NEJM199311113292004 [DOI] [PubMed] [Google Scholar]
- 3.Brenner BM Cooper ME De Zeeuw D, et al. RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. New Engl J Med. 2001;345(12):861–869. doi: 10.1056/NEJMoa011161 [DOI] [PubMed] [Google Scholar]
- 4.Hou FF Zhang X Zhang GH, et al. Efficacy and safety of benazepril for advanced chronic renal insufficiency. New Engl J Med. 2006;354(2):131–140. doi: 10.1056/NEJMoa053107 [DOI] [PubMed] [Google Scholar]
- 5.Prasad N Yadav AK Kundu M, et al. Prescription practices in patients with mild to moderate CKD in India. Kidney Int Rep. 2021;6(9):2455–2462. doi: 10.1016/j.ekir.2021.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy DP, Drawz PE, Foley RN. Trends in angiotensin-converting enzyme inhibitor and angiotensin II receptor blocker use among those with impaired kidney function in the United States. J Am Soc Nephrol. 2019;30(7):1314–1321. doi: 10.1681/ASN.2018100971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leon SJ Whitlock R Rigatto C, et al. Hyperkalemia-related discontinuation of renin-angiotensin-aldosterone system inhibitors and clinical outcomes in CKD: a population-based cohort study. Am J kidney Dis. 2022;80(2):164–173.e1. doi: 10.1053/j.ajkd.2022.01.002 [DOI] [PubMed] [Google Scholar]
- 8.Tomson C, Tomlinson LA. Stopping RAS inhibitors to minimize AKI: more harm than good? Clin J Am Soc Nephrol. 2019;14(4):617–619. doi: 10.2215/CJN.14021118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perkovic V Jardine MJ Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. New Engl J Med. 2019;380(24):2295–2306. doi: 10.1056/nejmoa1811744 [DOI] [PubMed] [Google Scholar]
- 10.Heerspink HJL Stefánsson BV Correa-Rotter R, et al. DAPA-CKD Trial Committees and Investigators. Dapagliflozin in patients with chronic kidney disease. New Engl J Med. 2020;383(15):1436–1446. doi: 10.1056/NEJMoa2024816 [DOI] [PubMed] [Google Scholar]
- 11.Herrington WG Staplin N Wanner C, et al. The EMPA-KIDNEY Collaborative Group. Empagliflozin in patients with chronic kidney disease. New Engl J Med. 2023;388(2):117–127. doi: 10.1056/nejmoa2204233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferreira JP Zannad F Butler J, et al. Empagliflozin and serum potassium in heart failure: an analysis from EMPEROR-Pooled. Eur Heart J. 2022;43(31):2984–2993. doi: 10.1093/eurheartj/ehac306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neuen BL Oshima M Agarwal R, et al. Sodium-glucose cotransporter 2 inhibitors and risk of hyperkalemia in people with type 2 diabetes: a meta-analysis of individual participant data from randomized, controlled trials. Circulation. 2022;145(19):1460–1470. doi: 10.1161/CIRCULATIONAHA.121.057736 [DOI] [PubMed] [Google Scholar]
- 14.Neuen BL Oshima M Perkovic V, et al. Effects of canagliflozin on serum potassium in people with diabetes and chronic kidney disease: the CREDENCE trial. Eur Heart J. 2021;42(48):4891–4901. doi: 10.1093/eurheartj/ehab497 [DOI] [PubMed] [Google Scholar]
- 15.Haynes R Herrington WG Judge P, et al. Nuffield Department of Population Health Renal Studies Group, SGLT2 inhibitor Meta-Analysis Cardio-Renal Trialists' Consortium. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet. 2022;400(10365):1788–1801. doi: 10.1016/S0140-6736(22)02074-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng KY Li J Ianus J, et al. Reasons for hospitalizations in patients with type 2 diabetes in the CANVAS programme: a secondary analysis. Diabetes Obes Metab. 2021;23(12):2707–2715. doi: 10.1111/dom.14525 [DOI] [PubMed] [Google Scholar]
- 17.Schechter M Jongs N Chertow GM, et al. Effects of dapagliflozin on hospitalizations in patients with chronic kidney disease: a post hoc analysis of DAPA-CKD: a post hoc analysis of DAPA-CKD. Ann Intern Med. 2023;176(1):59–66. doi: 10.7326/M22-2115 [DOI] [PubMed] [Google Scholar]
- 18.Schechter M Wiviott SD Raz I, et al. Effects of dapagliflozin on hospitalisations in people with type 2 diabetes: post-hoc analyses of the DECLARE-TIMI 58 trial. Lancet Diabetes Endocrinol. 2023;11(4):233–241. doi: 10.1016/S2213-8587(23)00009-8 [DOI] [PubMed] [Google Scholar]
- 19.Vaduganathan M Claggett BL Jhund P, et al. Dapagliflozin and all-cause hospitalizations in patients with heart failure with preserved ejection fraction. J Am Coll Cardiol. 2023;81(10):1004–1006. doi: 10.1016/j.jacc.2022.12.026 [DOI] [PubMed] [Google Scholar]
- 20.Noel JA Bota SE Petrcich W, et al. Risk of hospitalization for serious adverse gastrointestinal events associated with sodium polystyrene sulfonate use in patients of advanced age. JAMA Intern Med. 2019;179(8):1025–1033. doi: 10.1001/jamainternmed.2019.0631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhandari S Mehta S Khwaja A, et al. STOP ACEi Trial Investigators. Renin–angiotensin system inhibition in advanced chronic kidney disease. New Engl J Med. 2022;387(22):2021–2032. doi: 10.1056/NEJMoa2210639 [DOI] [PubMed] [Google Scholar]
- 22.Fu EL Evans M Clase CM, et al. Stopping renin-angiotensin system inhibitors in patients with advanced CKD and risk of adverse outcomes: a nationwide study. J Am Soc Nephrol. 2021;32(2):424–435. doi: 10.1681/ASN.2020050682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiao Y Shin J-I Chen TK, et al. Association between renin-angiotensin system blockade discontinuation and all-cause mortality among persons with low estimated glomerular filtration rate. JAMA Intern Med. 2020;180(5):718–726. doi: 10.1001/jamainternmed.2020.0193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferreira JP Zannad F Pocock SJ, et al. Interplay of mineralocorticoid receptor antagonists and empagliflozin in heart failure: EMPEROR-reduced. J Am Coll Cardiol. 2021;77(11):1397–1407. doi: 10.1016/j.jacc.2021.01.044 [DOI] [PubMed] [Google Scholar]
- 25.Neuen BL Young T Heerspink HJL, et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2019;7(11):845–854. doi: 10.1016/s2213-8587(19)30256-6 [DOI] [PubMed] [Google Scholar]
- 26.Neuen BL, Fletcher RA, Heerspink HJL. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023;388(24):2300. doi: 10.1056/NEJMc2301923 [DOI] [PubMed] [Google Scholar]
- 27.Janse RJ Fu EL Clase CM, et al. Stopping versus continuing renin–angiotensin–system inhibitors after acute kidney injury and adverse clinical outcomes: an observational study from routine care data. Clin Kidney J. 2022;15(6):1109–1119. doi: 10.1093/ckj/sfac003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Humphrey TJL, James G, Wittbrodt ET, Zarzuela D, Hiemstra TF. Adverse clinical outcomes associated with RAAS inhibitor discontinuation: analysis of over 400 000 patients from the UK Clinical Practice Research Datalink (CPRD). Clin Kidney J. 2021;14(10):2203–2212. doi:doi: 10.1093/ckj/sfab029 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Previously published data were used for this study.