Summary:
Carotid endarterectomy with patch angioplasty is commonly performed for severe atherosclerotic disease to reduce stroke risk. After neck radiation, loss of tissue planes; reactive fibrosis of skin; contraction; and rarely, necrosis of skin may occur, leading to severe wound complications and possible exposure of carotid artery reconstruction. Historically, local myocutaneous flaps have been performed to provide soft tissue coverage; however, these procedures may be associated with increased donor site morbidity and can be affected by radiation changes. This report describes the novel use of a fasciocutaneous free flap for durable vascularized soft tissue, and the associated secondary benefit of improved suppleness and range of motion. Additionally, the distant location of the donor site allows for an efficient two-team approach. Here, we describe a patient with severe carotid artery disease with a history of multiple surgical procedures and radiation, which was successfully treated with a carotid endarterectomy and bovine patch angioplasty by vascular surgery and immediate free anterolateral thigh flap coverage by our team.
For stroke prevention in patients with severe asymptomatic atherosclerotic disease, carotid endarterectomy (CEA) with patch angioplasty is routinely performed, whereby the plaque is removed and a prosthetic or autologous patch is placed, widening the stenotic artery.1–3 In average-risk patients on best medical therapy, the estimated 4-year risk of stroke is reduced by more than 50% after CEA.1,4 Although metabolic syndrome and tobacco-smoking remain the most prevalent causes of carotid atherosclerosis, radiation-induced arteriopathy is an increasingly salient precipitant of carotid atherosclerosis. After radiation, the location of associated lesions is frequently nontypical and surgically complex.5 Treatment difficulty is compounded by loss of tissue planes, reactive fibrosis, contraction, and, rarely, necrosis of surrounding irradiated tissues. In CEA, closure is particularly concerning because tissue margins are often too friable or inflexible to adequately cover the carotid patch. Traditionally, myocutaneous flap closure using the pectoralis flap or sternocleidomastoid flap has been performed to achieve durable coverage.6 However, fasciocutaneous flaps have lower donor site morbidity with similar efficacy. In this report, a multispecialty surgical approach treated a complicated patient with carotid artery disease in the setting of hostile neck anatomy via CEA with patch angioplasty and anterolateral thigh (ALT) flap coverage.
CASE
A 55-year-old White female patient, a former smoker, presented with severe left carotid artery atherosclerotic disease with extensive scarring and fibrosis from prior operations and radiation. Her history included Hodgkin lymphoma near her left clavicle treated with chemoradiotherapy in 1993; floor-of-mouth squamous cell carcinoma treated with hemi-mandibulectomy, free fibula reconstruction, and radiation in 2012; neck exploration in 2014 resulting in right common carotid artery injury, thrombosis, and ligation; and left ventral tongue squamous cell carcinoma excision and split-thickness skin graft reconstruction in 2016. With a stable PET scan in 2019, free tissue transfer was considered for scar revision and increased mobility (Fig. 1). On preoperative computed tomography angiography, 80% left ICA stenosis at the bulb and a previously ligated right common carotid artery were noted. Thus, CEA was recommended by the vascular surgery service to mitigate stroke risk. However, extensive prior neck dissections and neck and upper chest radiation caused scarring and tissue friability, affecting the sternocleidomastoid and pectoralis muscles. Given these risk factors, our team determined that transfer of an ALT flap for soft tissue coverage would more reliably convey protection compared with local options.
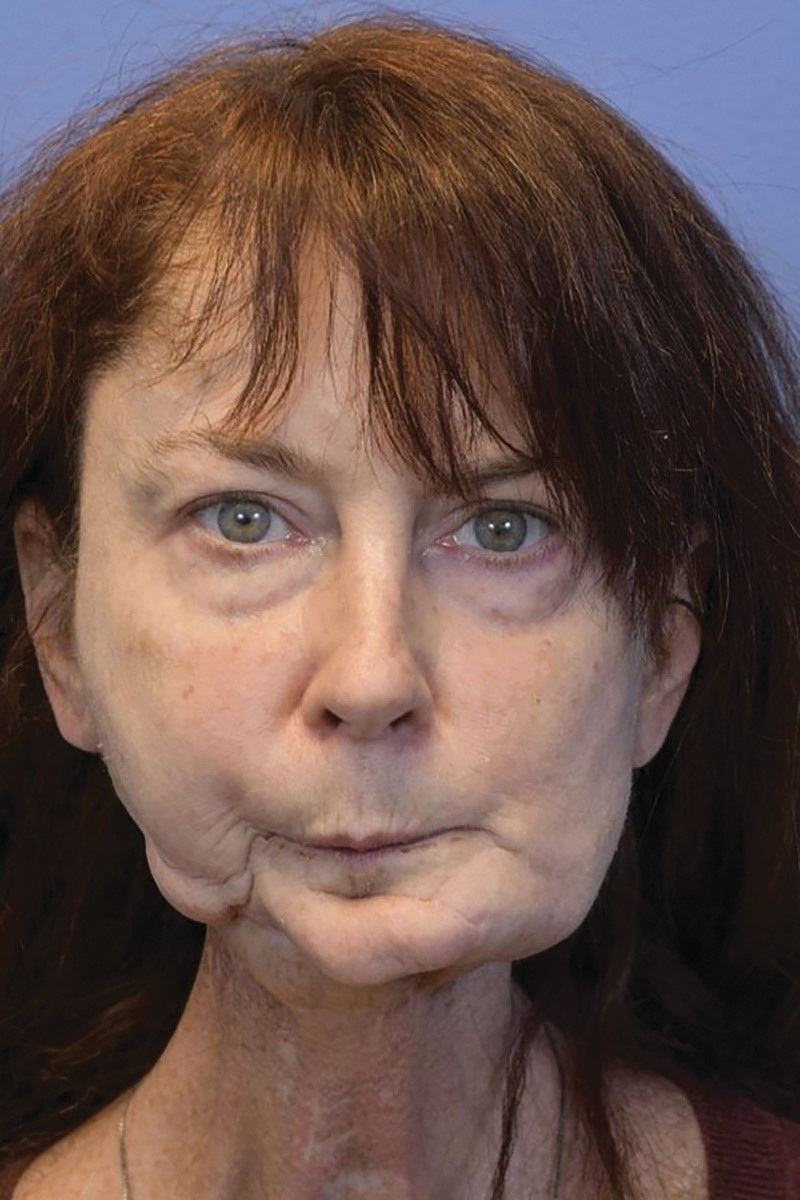
Fig. 1.
Preoperative photograph of the patient status post right mandibular reconstruction with a free fibula flap complicated by bony resorption and loss of height after radiation.
In January 2020, concurrent left CEA with bovine patch angioplasty with free fasciocutaneous ALT flap coverage was performed (Fig. 2). With computed tomography angiography and Doppler ultrasound guidance, the flap was elevated off a single dominant musculocutaneous perforator. The left superior thyroid artery and vein were selected as recipient vessels (Fig. 3). Postoperatively, she was healing well at her 1.5 week and 3 week postdischarge follow-up appointments without any wound healing complications and has not reported any neurological deficits 2 years after her procedure (Fig. 4).
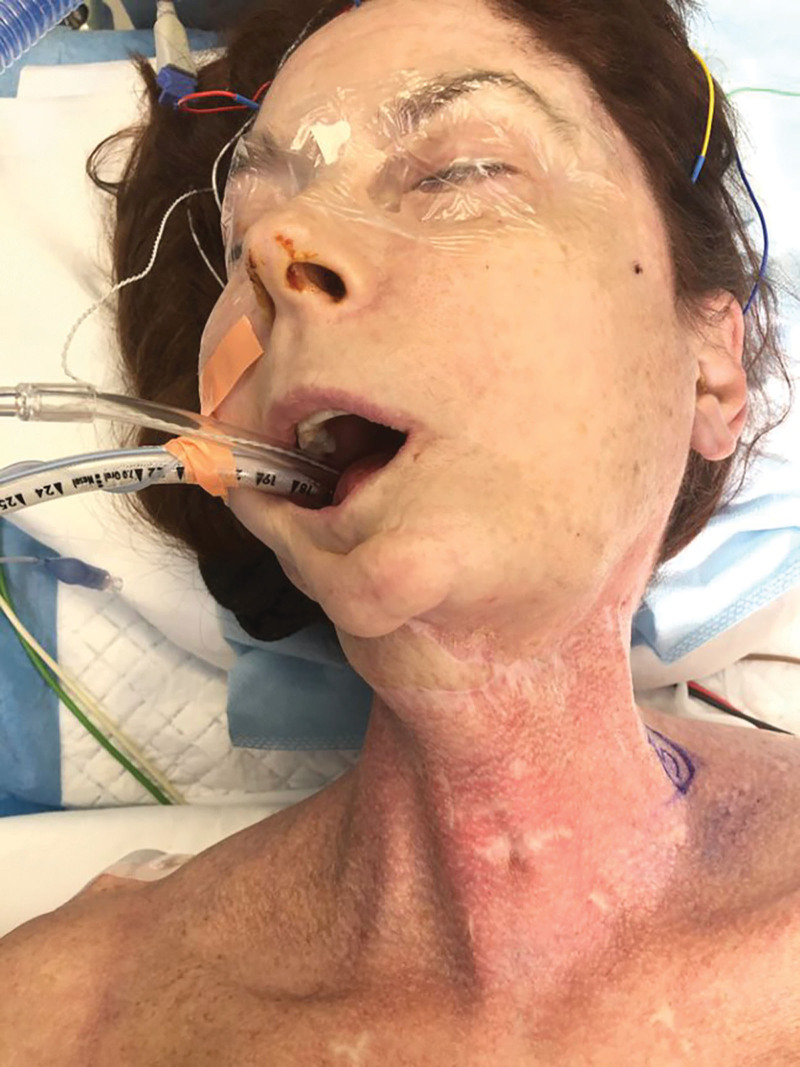
Fig. 2.
Photograph of the patient on the operating table demonstrating significant radiation changes of the left neck, with constriction of right neck motion secondary to fibrosis.
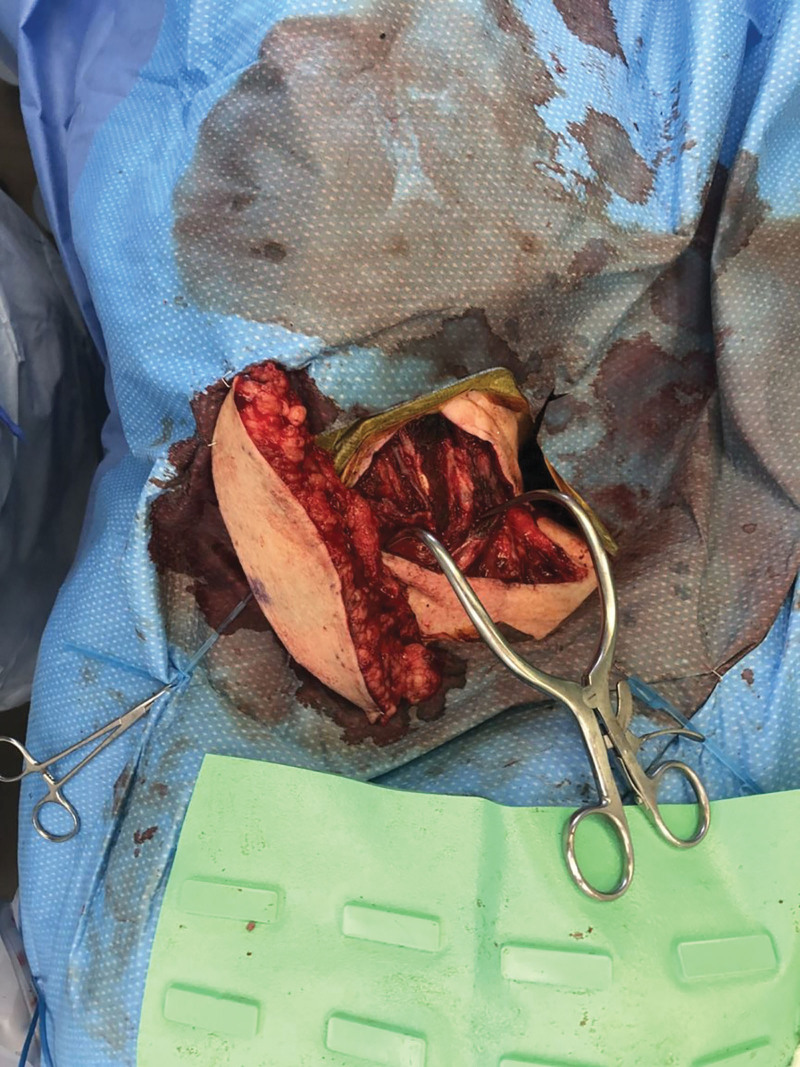
Fig. 3.
Intraoperative photograph demonstrating ALT flap anastomosis to the left superior thyroid artery and vein, with visible bovine patch angioplasty of carotid artery.
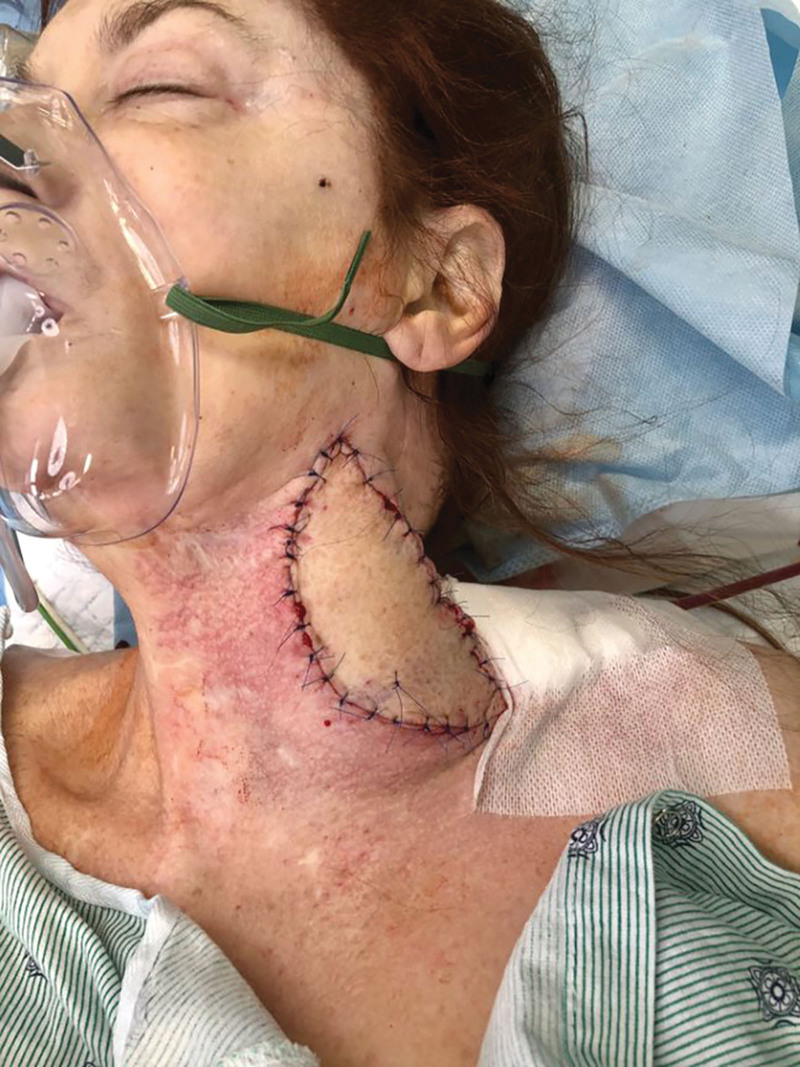
Fig. 4.
Postoperative photograph demonstrating adequate soft tissue coverage of the left carotid artery reconstruction with an anterolateral thigh free flap.
DISCUSSION
With treatment advancements improving head and neck cancer survival, the prevalence of sequelae, such as radiation-induced carotid artery disease, has risen. Treatment with CEA in irradiated neck anatomy must take additional measures to protect the carotid artery reconstruction from short and long term wound healing complications. Our patient was at imminent risk of stroke with 80% stenosis of the left internal carotid artery and a history of right common carotid artery ligation in 2014. Given the patient’s degree of scarring and paucity of surrounding tissue, free tissue transfer offered the benefits of healthy vascularized tissue for coverage of the carotid reconstruction, improved postoperative neck range of motion and contour, and no donor muscle harvest. Successful coverage in this case was crucial because any severe wound healing complications leading to carotid artery exposure could precipitate infection, carotid artery blowout, and exsanguination.
In similar cases necessitating free tissue transfer, thoughtful consideration of recipient vessel selection in the hostile, “frozen,” vessel-depleted neck is of utmost importance. The facial, superior thyroid, and lingual arterial branches off the external carotid artery are considered first line; however, they may be nonviable in this context. An acceptable secondary option commonly used at our institution is the transverse cervical vessels due to their similar diameter, blood flow, and integrity compared with the external carotid artery branches.7 These vessels should be considered in future CEA coverage due to their relatively straightforward dissection distinct from the carotid artery area, lower reported incidence of luminal stenosis in irradiated patients, and vertically oriented anastomotic inset with reduced propensity for kinking. Finally, third line recipient vessels should include the dorsal scapular and internal mammary artery, as these are typically unaffected by prior neck radiation and surgery; however, these may require vein grafting.8
Use of microsurgical reconstruction is not without risks of failure; however, in high-volume centers, free tissue transfer can yield superior results, with near equivalent success rates up to 98.7% in select patients.9 In this case, neither the pectoralis nor sternocleidomastoid flap with adjacent tissue transfer was used due to the patient’s radiation history near her clavicle and prior procedures, both resulting in a large zone of injury over the potential muscle harvest site and poor overlying tissue quality. Observational studies have found no difference in resistance to infection between myocutaneous and fasciocutaneous flaps.10 However, compared with myocutaneous flaps, the donor site morbidity of fasciocutaneous flaps is diminished with the absence of muscle harvest. Furthermore, the ALT flap is a thin, pliable flap frequently used in head and neck reconstructions, with its advantage in this case being two fold. It resects scarred and radiated tissue covering CEA reconstruction and surrounding tissue to resurface the neck with pliable tissue, thus displacing wound healing complications to the periphery of reconstruction, as well as a secondary benefit of improved facial contour, aesthetic outcome, and range of motion due to scar reduction. Lastly, the distant location of the ALT donor site confers a logistical advantage by allowing for an efficient two-team surgical approach.
CONCLUSIONS
Carotid endarterectomy in the setting of radiation has an increased risk of wound healing complications. In select cases, ALT flap coverage after carotid endarterectomy provides appropriate soft tissue coverage of the arterial repair with limited donor site morbidity.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
PATIENT CONSENT
The patient provided written consent for the use of her image.
Footnotes
Published online 7 December 2023.
Disclosure statements are at the end of this article, following the correspondence information.
REFERENCES
- 1.Naylor AR, Ricco JB, de Borst GJ, et al. Editor’s choice—management of atherosclerotic carotid and vertebral artery disease: 2017 clinical practice guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2018;55:3–81. [DOI] [PubMed] [Google Scholar]
- 2.Huizing E, Vos CG, van den Akker PJ, et al. A systematic review of patch angioplasty versus primary closure for carotid endarterectomy. J Vasc Surg. 2019;69:1962–1974.e4. [DOI] [PubMed] [Google Scholar]
- 3.AbuRahma AF, Darling RC, III. Literature review of primary versus patching versus eversion as carotid endarterectomy closure. J Vasc Surg. 2021;74:666–675. [DOI] [PubMed] [Google Scholar]
- 4.Rerkasem A, Orrapin S, Howard DP, et al. Carotid endarterectomy for symptomatic carotid stenosis. Cochrane Database Syst Rev. 2020;9:CD001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oderich GS, Panneton JM, Cherry KJ, Jr, et al. Carotid artery reconstruction combined with myocutaneous flap coverage: a complex and durable rescue operation. Ann Vasc Surg. 2002;16:579–585. [DOI] [PubMed] [Google Scholar]
- 6.Naughton PA, Garcia-Toca M, Rodriguez HE, et al. Carotid artery reconstruction for infected carotid patches. Eur J Vasc Endovasc Surg. 2010;40:492–498. [DOI] [PubMed] [Google Scholar]
- 7.Tessler O, Gilardino MS, Bartow MJ, et al. Transverse cervical artery: consistent anatomical landmarks and clinical experience with its use as a recipient artery in complex head and neck reconstruction. Plast Reconstr Surg. 2017;139:745e–751e. [DOI] [PubMed] [Google Scholar]
- 8.Martinez DC, Badhey A, Cervenka B, et al. Surgical techniques for head and neck reconstruction in the vessel-depleted neck. Facial Plast Surg. 2020;36:746–752. [DOI] [PubMed] [Google Scholar]
- 9.Corbitt C, Skoracki RJ, Yu P, et al. Free flap failure in head and neck reconstruction. Head Neck. 2014;36:1440–1445. [DOI] [PubMed] [Google Scholar]
- 10.Kovar A, Colakoglu S, Iorio ML. Choosing between muscle and fasciocutaneous free flap reconstruction in the treatment of lower extremity osteomyelitis: available evidence for a function-specific approach. J Reconstr Microsurg. 2020;36:197–203. [DOI] [PubMed] [Google Scholar]