Abstract
Escherichia coli heat-labile enterotoxin (LT) consists of an A subunit and five B subunits. These subunits oligomerize into an assembled holotoxin within the periplasm. Structural analysis of LT has revealed that the A subunit interacts with the B subunit through its carboxy terminus. This indicates that the carboxy-terminal portion of the protein is required for assembly of holotoxin in the periplasm. However, it is not known whether other regions of the A subunit contribute to the assembly. The A subunit constituting the holotoxin contains a disulfide bond between Cys-187 and Cys-199. It has been observed in many proteins that the intramolecular disulfide bond is deeply involved in the function and tertiary structure of the protein. We speculated that the disulfide bond of the A subunit contributes to the assembly in the periplasm, although the bond is not a structural element of the carboxy-terminal portion of the A subunit. We replaced these cysteine residues of the A subunit by oligonucleotide-directed site-specific mutagenesis and analyzed the LTs produced by cells containing the mutant LT genes. The amount of the mutant holotoxin produced was small compared with that of the wild-type strain, indicating that the disulfide bond of the A subunit contributes to the structure which functions as the site of nucleation in the assembly. A reconstitution experiment in vitro supported the notion. Subsequently, we found that the mutant A subunit constituting holotoxin is easily degraded by trypsin and that in cells incubated with mutant LTs, the lag until the intracellular cyclic AMP begins to accumulate is longer than in cells incubated with native LTs. These results might be useful for the analysis of the interaction of LT with target cells at the molecular level.
Escherichia coli heat-labile enterotoxin (LT) contains two dissimilar subunits, A and B, which are present in the holotoxin at a ratio of 1 to 5 (23, 24). Both subunits are translated from a single polycistronic messenger coding for one A subunit, followed downstream by one B subunit. The A and B subunits are synthesized as precursor proteins with signal sequences (25, 30). The synthesized precursors are exported from the cytoplasm to the periplasm with the help of the signal sequence. The exported A and B subunits oligomerize into a holotoxin within the periplasm (6, 7). The A subunit may act as the site of nucleation, interacting with multiple B subunits simultaneously, thereby enhancing pentamer formation (26). Studies on the in vivo assembly of the LT in E. coli using pulse-labeling experiments revealed that the rate at which the B subunits attained a sodium dodecyl sulfate (SDS)-stable pentameric structure in the periplasm increased by about fourfold when the A subunit was coexpressed (3, 4).
The A subunit consists of A1 and A2 fragments which are linked covalently in the main chain as well as by a disulfide bond between Cys-187 and Cys-199 (23, 24). The disulfide bond is an important factor in determining the three-dimensional structure of the protein. Therefore, the disulfide bond formation of the A subunit between Cys-187 and Cys-199 is thought to be important in the association of the A and B subunits in the periplasm, since the bond must contribute to the construction of the A subunit. However, no studies on the role of the disulfide bond in the construction of holotoxin have been performed. In this study, we replaced these cysteine residues in vivo by oligonucleotide-directed mutagenesis and analyzed LTs produced by the cells carrying the mutant LT genes to clarify the role of the disulfide bond in the association of the A subunit with the B-subunit oligomer.
Subsequently, we examined the role of the disulfide bond in the expression of the biological activity of LT. The B subunits mediate binding of the LT to specific receptors on the plasma membranes of target cells. Upon binding, the toxins are internalized. The A subunit internalized into cells catalyzes the NAD-dependent ADP-ribosylation of Gsα (a G protein involved in the regulation of adenylate cyclase) that leads to the activation of adenylate cyclase, electrolyte efflux, and ultimately diarrhea (8, 29). The A subunit’s activity is normally latent, requiring reduction of the disulfide bond between Cys-187 and Cys-199 and proteolytic nicking between the A1 and A2 polypeptides to yield the enzymatically active A1 moiety (8, 19). However, it remains unknown how the disulfide bond is involved in expression of the biological activity of LT. To elucidate the role of the disulfide bond, we examined the kinetics of the action of mutant LT prepared in this study.
MATERIALS AND METHODS
Strains, plasmids, and media.
E. coli HB101 was used as the host strain. Strain BL21 [F− ompT hsdSB(rB− mB−)] containing the T7 RNA polymerase gene on a chromosome was the host strain in the T7 expression system (27). The plasmids and LTs produced by these transformants are shown in Table 1. The plasmid EWD299 encoding the LTp (LT produced by porcine enterotoxigenic E. coli) gene was provided by W. S. Dallas (1). Plasmid pKK511, in which the nucleotide sequence at the site recognized by NdeI in the A-subunit gene (etxA) of EWD299 is altered as described later (Table 1), was used as the parent plasmid in this study. All cultures were grown in L broth (1% tryptone, 0.5% yeast extract, 0.5% NaCl) (17). Agar (1.5%) was added to produce solid medium, and ampicillin (50 μg/ml) was added when necessary.
TABLE 1.
Plasmids encoding wild-type and mutant LT subunits
| Plasmid | LT subunit expresseda
|
Comments | Source or reference | |
|---|---|---|---|---|
| etxA | etxB | |||
| EWD299 | WT | WT | Entire etxAB operon cloned into pBR325 | 1 |
| pKK511 | WT | WT | Base substitution at the NdeI site in etxA of EWD299, which does not alter the corresponding amino acid residues | This study |
| pKK512 | A(C187S) | WT | Identical to pKK511 except for mutation in etxA corresponding to C187S | This study |
| pKK513 | A(C199S) | WT | Identical to pKK511 except for mutation in etxA corresponding to C199S | This study |
| pET11 | None | None | Containing a T7 promoter under the control of lac operator | 26 |
| pT7-Blue(R) | None | None | Cloning vector for PCR-amplified gene | Takara Co. |
| pET11-5110 | WT | WT | etxAB operon of pKK511 was cloned into the NdeI site of pET11 under the control of the T7 promoter | This study |
| pET11-5120 | A(C187S) | WT | Same as pET11-5110, but the etxAB was from pKK512 | This study |
| pET11-5130 | A(C199S) | WT | Same as pET11-5110, but the etxAB was from pKK513 | This study |
| pET11-5111 | WT | None | etxA of pKK511 was cloned into the NdeI site of pET11 under the control of the T7 promoter | This study |
| pET11-5121 | A(C187S) | None | Same as pET11-5110, but the etxA was from pKK512 | This study |
| pET11-5131 | A(C199S) | None | Same as pET11-5110, but the etxA was from pKK513 | This study |
WT, wild type.
Oligonucleotide-directed mutagenesis.
Cysteine residues were replaced with serine in sequences corresponding to positions 187 and 199 of etxA of pKK511 by oligonucleotide-directed mutagenesis using plasmids as reported by Inouye and Inouye (10). The following oligonucleotides were used as the mutagens: 512, ATGAATTTCCGGAACCTTGTGGT (Cys-187 → Ser); 513, GGTGATACTAGTAATGAGG (Cys-199 → Ser). Oligonucleotide 512 is complementary to the DNA sequence of the wild type. The underlined bases denote mismatches with the sequence of the wild type. By the mutations induced by oligonucleotides 512 and 513, recognition sites of MroI and SpeI, respectively, were generated. The amino acid replacements induced are indicated in parentheses. To confirm the correct mutations, the DNA sequence around the mutated positions was determined by means of dideoxy chain termination from a double-stranded template with a model 373A DNA sequencing system (Applied Biosystems, Foster City, Calif.).
Construction of the plasmid for pulse-labeling experiments.
To selectively express the LT gene product, the pET system was used. In this system, target genes are cloned in pET plasmids under the control of strong bacteriophage T7 transcription and translation signals and the T7 promoter is under the control of the lac operator (27). We used plasmid pET11. In this plasmid, the gene transcribed by T7 RNA polymerase is expressed from the initiation codon (ATG) that is located 1 base after the NdeI cleavage site. Therefore, the target gene is usually inserted into the NdeI site. The existence of other NdeI sites in the target gene fragment complicates the procedure. There is an NdeI cleavage site at position 538 in the etxA gene of EWD299 (1). To simplify the isolation of the target gene, the nucleotide sequence at position 538 was changed by oligonucleotide-directed mutagenesis using oligonucleotide 511 (5′-CACCCATACGAACAGGAG-3′; the underlined base denotes a mismatch with the sequence of the wild type). The mutated gene is not recognized by NdeI, but the amino acid residue encoded by the mutant gene is not altered. The prepared plasmid was named pKK511 (Table 1).
To express holotoxin by the pET system, a DNA fragment containing the holotoxin gene of LTp was amplified from pKK511 by PCR. The sequences of primers i and ii were 5′-TTTCCCATATGAAAAATATA-3′ (sense) and 5′-CTGGATCCTTTTCAAATGCAGCA-3′ (antisense), respectively. Primers i and ii extended from bases 150 to 169 and from bases 1378 to 1398 of the LTp gene reported by Dallas et al. (1), respectively. Primer i contained three mismatches with the sequence of the wild type (underlined) to create an NdeI site. The initiation codon (ATG) is located 1 base after the NdeI cleavage site. This allowed the structural holotoxin gene to be cloned into the NdeI site of pET11 downstream from the T7 promoter. Primer ii contained four mismatches with the sequence of the wild type (underlined), creating a BamHI site. The PCR product was ligated into pT7-Blue(R) (Takara), which is the cloning vector for the PCR-amplified DNA fragment (Table 1). An NdeI-BamHI fragment from the recombinant plasmid carrying the holotoxin gene of LTp was isolated and inserted into pET11 digested with NdeI and BamHI. The correct insertion of the LTp structure gene was confirmed by sequencing the nucleotides of the flanking DNA of the cloned gene. The resulting plasmid was named pET11-5110 (Table 1).
Likewise, etxA of pKK511 was amplified by PCR. The oligonucleotides used for the amplification were primer i and primer iii, which is 5′-ATGGATCCGTAAATAAAACATAACATT-3′ (antisense; the underlined bases denote mismatches with the sequence of the wild type to create a BamHI site). Primer iii extended from bases 944 to 971 of the LTp gene reported by Dallas et al. (1). The amplified A-subunit genes of LTp were inserted into pET11 digested with NdeI and BamHI as described above.
A-subunit genes of LTp in plasmids pKK512 and pKK513 (Table 1) were also cloned into the NdeI site of pET11 in the same way. The resulting plasmids from pKK511, pKK512, and pKK513 were named pET11-5111, pET11-5121, and pET11-5131, respectively (Table 1).
Pulse-labeling.
BL21 cells transformed with pET11 derivative plasmids were shaken at 37°C until they reached an optical density of 0.4 at 660 nm in L broth containing ampicillin (50 μg/ml). The culture was centrifuged, and the cell pellet was suspended in M9 minimal medium (17) containing an amino acid mixture (the final concentration of each amino acid was 50 μg/ml) without methionine at an optical density of 3.0 at 660 nm. After a final concentration of 2 mM isopropyl-1-thio-β-d-galactopyranoside (IPTG) was added, the suspension was shaken at 37°C for 20 min. A portion (1 ml) was labeled for 1 min at 37°C with 10 μCi of l-[35S]methionine (0.5 to 1 kCi/mmol) (ARS-101; American Radiolabeled Chemicals, Inc., St. Louis, Mo.) per ml. Incorporation of the label was terminated by adding 250 μg of unlabeled methionine. The incubation continued for the indicated chase times. Thereafter, the cell suspension was centrifuged and the resultant cell pellet was suspended in 100 μl of 10 mM Tris-HCl buffer (pH 7.5).
The periplasmic fraction of the cells was prepared as described below. Thus, prepared samples were mixed with SDS dye (12), heated at 100°C for 10 min, and resolved by SDS-polyacrylamide gel electrophoresis (PAGE) (12).
The radioactive bands were visualized by autoradiography with an imaging plate analyzer (BAS 2000; Fujix, Tokyo, Japan).
Purification of LTp and the LTp B oligomer.
E. coli HB101 strains harboring the appropriate plasmid were cultured in L broth containing ampicillin (50 μg/ml) for 18 h at 37°C with shaking. The bacteria were collected by centrifugation, washed with 10 mM Tris-HCl (pH 7.2), suspended in TEAN buffer (28), and lysed by sonication. After centrifugation at 15,000 × g for 20 min at 4°C, the supernatant was used as the crude cell lysate.
The purification was performed in accordance with the method of Uesaka et al. (28) by using a column containing immobilized d-galactose (Pierce, Rockford, Ill.). The crude cell lysate of each strain was applied to the column equilibrated with TEAN buffer at room temperature. TEAN buffer was applied to the column until the optical density at 280 nm of the effluent returned to the baseline. Materials that adhered to the column were eluted with 0.3 M d-(+)-galactose (Kishida, Osaka, Japan) in TEAN buffer. The absorbance at 280 nm was recorded, and the peaks were pooled.
Preparation of the periplasmic fraction.
The cells cultured in liquid medium were collected by centrifugation at 12,000 × g for 10 min, and then the cell pellet was suspended in 10 mM Tris-HCl buffer (pH 7.5). To prepare the periplasmic fraction, the cell suspension was incubated with polymyxin B at a concentration of 6,500 U/ml at 4°C for 15 min and centrifuged (12,000 × g for 15 min). The supernatant obtained was used as the periplasmic fraction.
Disassembly and reassembly of LT.
The disassembly and reassembly of LT were achieved by modification of the pH as reported previously (21, 22). A purified holotoxin solution of LT (10 mg/ml) was diluted to a concentration of 1 mg/ml in 0.1 M KCl (pH 1.5), and the samples were briefly mixed. By acid denaturation, holotoxins of LT were disassembled into monomers of A and B subunits. After incubation at 30°C for 1 min, 10 μl of sample was poured into 90 μl of McIlvaine buffer (pH 7.0) (21) and briefly mixed. By incubation at neutral pH, the majority of disassembled monomers were reassembled into oligomers (21). After 60 min, 10 μl of the neutralized sample was poured into 990 μl of phosphate-buffered saline, to yield a final concentration of 10 μg of protein per ml. The samples were condensed to a concentration of 1 mg of protein per ml under vacuum.
SDS-PAGE and isoelectric focusing.
SDS-PAGE was performed as described by Laemmli (12).
Isoelectric focusing was performed in acrylamide gel (4.5%) containing ampholine (pH 3.5 to 10.0; Pharmacia LKB, Uppsala, Sweden) at a concentration of 2.7%. A portion of sample (5 μl) was loaded onto the gel, and the gel was run for about 3 h at a constant voltage (50 V/cm). The gels were kept at 4°C during the electrophoresis. After electrophoresis, the gel was stained with Coomassie brilliant blue R-250.
Antiserum.
Antiserum against the LTp B oligomer (αLTp-B) was obtained by several subcutaneous injections of 50 μg of the purified LTp B oligomer into rabbits (18). Since the LTp B oligomer used as the antigen was purified from E. coli possessing the recombinant gene for the LTp B but not the LTp-A gene, the antiserum obtained could not contain the antibodies against the A subunit (18).
Measurement of intracellular cyclic AMP accumulation.
The ability of LTp to increase intracellular cyclic AMP in T84 cells (human colon carcinoma cell line) was determined essentially as described by Orlandi et al. (20). T84 cells were cultured in a 1:1 mixture of Ham’s F12 medium and Dulbecco’s modified Eagle’s medium (DMEM) containing 5% fetal bovine serum (FBS). Cells were seeded in petri dishes (50-mm diameter) and grown to near confluence. The culture medium was changed to DMEM containing 1% FBS 30 min before the addition of toxin, and the cells were incubated at 37°C in a 5% CO2 atmosphere. LTp was added to the culture medium, and the cells were incubated for various periods of time at 37°C in a 5% CO2 atmosphere. The cells were washed four times with ice-cold phosphate-buffered saline (pH 7.4). The cells were then extracted with 0.1 N HCl, and the extracts were assayed for cyclic AMP by using a cyclic AMP enzyme immunoassay system (Amersham International plc, Buckinghamshire, England).
Protein determination.
Protein was determined by the method of Lowry et al. (14) with bovine serum albumin as the standard.
RESULTS
Mutation.
Cysteine residues at positions 187 and 199 were replaced by oligonucleotide-directed site specific mutagenesis to produce plasmids pKK512 and pKK513 (Table 1). The proper mutations were confirmed by the determination of the nucleotide sequence around the mutated position of etxA of these mutant plasmids.
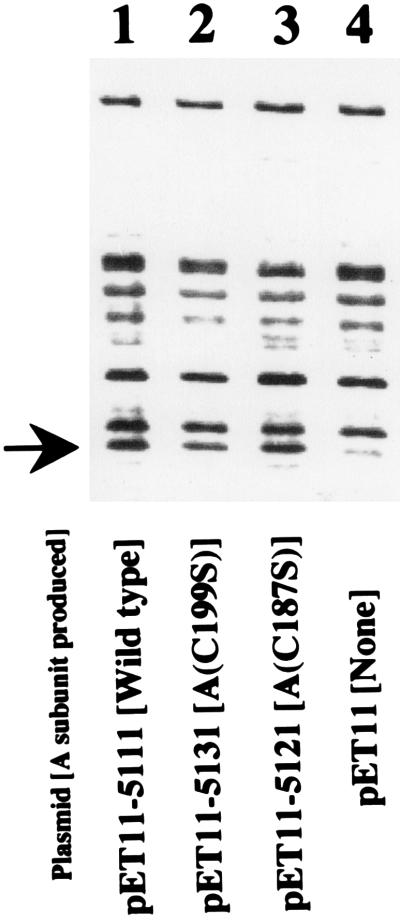
To examine the effect of the mutation on the production of the A subunit, pET11 derivatives (pET11-5111, pET11-5121, and pET11-5131) carrying these A-subunit genes were made (Table 1). BL21 was transformed with these plasmids. The cells were pulse-labeled with [35S]methionine for 1 min and then were chased for 30 s. The periplasmic fractions of these labeled cells were prepared and resolved by SDS-PAGE. The radioactive bands were visualized by autoradiography (Fig. 1). The band corresponding to the A subunit (the bands indicated by the arrow) appeared in every sample except that containing the control (lane 4). The difference in the density of the band among samples was not significant. This means that the mutation did not seriously affect the biosynthesis and the translocation across the inner membrane of the A subunit.
FIG. 1.
Effect of replacement of cysteine residues on the production of A subunit. The cysteine residues at positions 187 and 199 were replaced by oligonucleotide-directed site-specific mutagenesis, and the gene fragments containing the mutant A-subunit gene were cloned into pET11 for selective expression as described in the text. E. coli BL21 cells were transformed with these mutant plasmids (lanes 2 and 3), wild-type plasmid (lane 1), and vector plasmid (lane 4). These transformed cells were induced with IPTG and labeled for 1 min with [35S]methionine. The incubation continued for 30 s. The cells were collected by centrifugation, and the periplasmic fractions were prepared as described in the text. The periplasmic fractions were mixed with SDS dye solution, heated at 100°C for 10 min, and resolved by SDS-PAGE. Radioactive bands were detected by image analysis as described in the text. The arrow indicates the position of the A subunit.
Analysis of LT in mutant cells.
To examine the properties of LTps produced by these mutant plasmids, we tried to purify the holotoxins of mutant LTps. We used an immobilized d-galactose column for the purification as described in Materials and Methods. It has been shown that not only holotoxin of LT but also the B-subunit oligomer adsorb to the column and that these toxins are eluted with the galactose solution (28). The crude cell lysate of the transformants harboring plasmids (pKK511, pKK512, and pKK513) was applied to the immobilized d-galactose column, and the materials adsorbed to the column were eluted with the galactose solution.
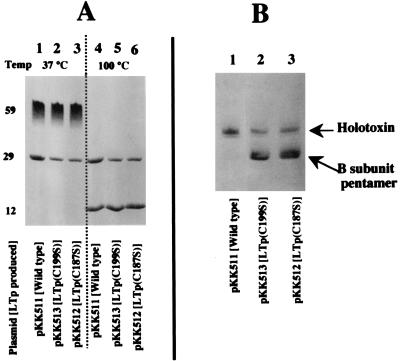
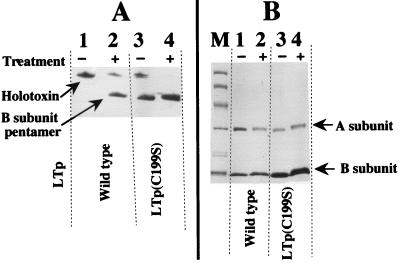
It has been shown that the oligomerized B subunit is stable in SDS at low temperature but dissociates at 100°C (5). To examine the properties of the materials recovered from the d-galactose column, the materials were treated with SDS at different temperatures. About 15 μg of the material was mixed with an equal volume of twice-concentrated SDS buffer containing 2% SDS (12). Equal portions were heated at 100°C for 5 min or kept at 37°C for 5 min and then subjected to SDS-PAGE. The gel was stained with Coomassie brilliant blue dye. The results are shown in Fig. 2A. When the samples were kept at low temperature (37°C), a protein with a molecular size of 59 kDa appeared in every sample (Fig. 2A, lanes 1 to 3). The migration position of these proteins switched to the position of a 12-kDa protein after heating (Fig. 2A, lanes 4 to 6). This shows that the proteins with a molecular size of 59 kDa are B-subunit pentamers, meaning that B subunits produced by these mutants oligomerized into pentamers in the cells.
FIG. 2.
Analysis of toxin purified by d-galactose column chromatography. The crude cell lysate fractions of HB101 strains harboring appropriate plasmids were prepared as described in the text. The crude cell lysates were applied to a d-galactose column, and the materials adsorbed to the gels were eluted with the galactose solution. The eluted fractions were analyzed by SDS-PAGE (A) and isoelectric focusing (B). (A) For SDS-PAGE analysis, the samples were mixed with SDS buffer and then kept at 37°C for 10 min (lanes 1 to 3) or heated at 100°C for 10 min (lanes 4 to 6). After electrophoresis, the gel was stained with Coomassie brilliant blue R-250. The sizes of the proteins are shown on the left (in kilodaltons). (B) Isoelectric focusing was performed with the acrylamide gel containing ampholine (pH 3.5 to 10.0; Pharmacia) as described in the text. The gel was stained with Coomassie brilliant blue R-250. The positions of the holotoxin of LTp and the B-subunit pentamer are indicated on the right.
The densities of the bands in lanes 4 to 6 of Fig. 2A were measured with a gel scanner (DMU-33C; Toyo Inc., Tokyo, Japan). There were no significant differences in the densities of the 12-kDa-protein bands corresponding to the B subunit among these lanes. However, there was a significant difference in the densities of the bands at 29 kDa; the densities were 35 and 40% in lanes 5 and 6, respectively, relative to that in lane 4, indicating that the amount of A subunit of mutant LTps (29-kDa protein in lanes 5 and 6) was small compared with that of native LT (lane 4). As described, the holotoxin of LT (consisting of the A and B subunits) and the B-subunit oligomer adsorb to the d-galactose column, but free A subunit does not, indicating that the A subunits detected in the gel (Fig. 2A) were components of the holotoxin of LT. The low density of the A subunit in the gel indicates that the amount of holotoxin formed in the corresponding cells is small.
To further examine the formation of the holotoxin of LT, we separated the purified toxins by isoelectric focusing. As shown in Fig. 2B, the large majority of toxins in the sample from HB101/pKK511 (producing wild-type LTp) were holotoxins (lane 1). However, the amount of B-subunit pentamer was much larger than that of holotoxin in the samples from mutant cells (lanes 2 and 3).
The result described above indicates that the activity of the Cys-mutated mutant A subunit to assemble with the B subunit is weak in vivo. This suggests that these cysteine residues are necessary for constructing the A subunit, which can function as the nucleation site in association with the B subunit. However, we analyzed LTps in the cells cultured long-term (about 18 h). Thus, it is possible that we observed the degraded LTps after long-term cultivation. Since analysis of the nascent LT might give more accurate information about the role of cysteine residues in the association, we chose to examine the nascent LT by the pulse-labeling method.
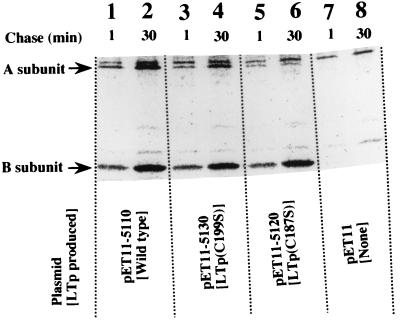
pET11 derivative plasmids carrying the holotoxin gene were prepared (pET11-5110, pET11-5120, and pET11-5130) for the pulse-labeling experiment (Table 1). BL-21 cells transformed with these plasmids were pulse-labeled with [35S]methionine for 1 min and chased for 1 and 30 min. The cells were collected by centrifugation, and the periplasmic fractions of the cells were prepared. The fractions were incubated with αLTp-B. The yielded immunoprecipitate was collected by centrifugation. Since the serum used did not contain the antibody against A subunit, free A subunit was not recovered. The A subunits recovered in the precipitate must be a component of the holotoxin of LTp. The precipitate was resolved by SDS-PAGE, and the results are shown in Fig. 3. The bands corresponding to the A and B subunits and the chase period of each sample are indicated in the figure. The samples for lanes 1 and 2 were prepared from BL21/pET11-5110 producing wild-type LTp. In these lanes, the densities of both bands, those of the A and B subunits, increased with the chase period. This shows that the A subunit efficiently associated with the B subunit. The samples for lanes 3 and 4 and for lanes 5 and 6 were prepared from the cells producing LTp(C199S) and LTp(C187S), respectively. In these samples, the density of the band of the B subunit increased with the chase period; however, the density of the band of the A subunit did not increase significantly. It is likely that the Cys-mutated A subunits do not associate with the B subunit efficiently and that an A subunit without a disulfide bond cannot function as the nucleus in the assembly of a B subunit.
FIG. 3.
Association of the A subunit with the B-subunit oligomer. E. coli BL21 cells were transformed with pET11 derivative plasmids carrying the holotoxin gene of LTp (pET11-5110, pET11-5130, and pET11-5120) and pET11. These transformed cells were induced with IPTG. The cells were then labeled for 1 min with [35S]methionine and chased for 1 or 30 min. The periplasmic fractions of these labeled cells were prepared and incubated with αLTp-B. The resulting immunoprecipitates were gathered by centrifugation and resolved by SDS-PAGE. Radioactive bands were detected by image analysis as described in the text. The positions of the A and B subunits are indicated on the left.
To examine the capacity of the A subunit to become the site of nucleation in the assembly of the B subunit, a reconstitution experiment in vitro was performed. The pH modification method was used for the dissociation and reconstruction of LT as described in Materials and Methods. The toxins generated by the treatment were analyzed by isoelectric focusing (Fig. 4A) and SDS-PAGE (Fig. 4B). The nontreated LTps were run as a control in the lane next to that containing the treated LTp. In the SDS-PAGE gel (Fig. 4B), the densities of the subunits of the treated LTps were almost the same as those of control LTs, showing that these subunits were not degraded during the treatment. The isoelectric focusing analysis revealed that about one-fourth of the wild-type LTp was reassembled into the holotoxin of LTp (Fig. 4A, lane 2). However, the LTp(C199S) mutants were not reassembled into holotoxin, although they formed B-subunit pentamers (Fig. 4A, lane 4). This shows that some of the native A subunits acted as the site of nucleation in the reconstitution to form holotoxin but that the mutant A subunits did not. This means that the disulfide bond formation between Cys-187 and Cys-199 of the A subunit is important for the A subunit to become the site of the nucleation in the assembly of A and B subunits.
FIG. 4.
Analysis of the LTps reconstituted by isoelectric focusing (A) and SDS-PAGE (B). Wild-type LTp and LTp(C199S) were dissociated into subunits by incubation in low-pH buffer (treatment). The reconstruction of these dissociated LTps was achieved by incubation at neutral pH. These LTps were analyzed by isoelectric focusing (A) and SDS-PAGE (B) as described in the text. The positions of the holotoxin and the B-subunit pentamer are shown on the left of the isoelectric focusing gel (A), while those of the A and B subunits are shown on the right of the SDS-PAGE gel (B). Nontreated LTps were run as a control in the lanes next to those of the treated LTps.
Characterization of the Cys-mutated LTp. (i) Response to limited trypsinolysis.
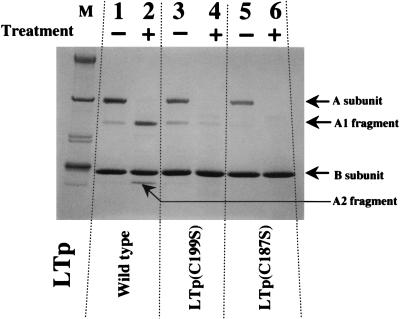
The purified holotoxins of LTp (wild-type and mutant LTps; 30 μg) were treated with trypsin (3 μg) at 37°C for 10 min. The volume of the reaction mixture was 20 μl. The reactions were quenched by the addition of 20 μl of twice-concentrated SDS buffer (12), which contains 2-mercaptoethanol, and then the mixtures were heated at 100°C for 10 min. The products were separated by SDS-PAGE (Fig. 5). A subunit of native LTp was cleaved to yield the A1 and A2 fragments (Fig. 5, lane 2) as reported previously (15). Since the elution profile of the wild-type LTps treated with 30 μg of trypsin (10 times as much trypsin as that used in the experiment illustrated in Fig. 5) resembled that of lane 2 of Fig. 5 (data not shown), the nicked A subunit that is associated with the B-subunit pentamer seems to be fairly stable in response to trypsin treatment. On the other hand, the majority of the A subunit of the mutant LTps was degraded by trypsinolysis (lanes 4 and 6). This means that the structure of these mutant A subunits differs from that of the A subunit of wild-type LT, although both mutant and wild-type A subunits associate with B-subunit pentamers.
FIG. 5.
Response of LTp to limited trypsinolysis. Wild-type LTp and the mutant LTps [LTp(C199S) and LTp(C187S)] were incubated with trypsin (treatment +) or distilled water (treatment −) as described in the text. These reactions were quenched by the addition of twice-concentrated SDS buffer. After being heated at 100°C for 10 min, the samples were separated by SDS-PAGE and stained with Coomassie brilliant blue R-250. The positions of the intact A subunit, A1 fragment, A2 fragment, and B subunit are given on the right. Molecular size standard markers were run in lane M.
(ii) Cyclic AMP accumulation activity.
It has been reported that some cultured cells exhibit an increase in the intracellular cyclic AMP level in response to LT exposure. Since our initial experiments revealed that T-84 cells are more sensitive than CHO-K1 cells to the action of LT to accumulate intracellular cyclic AMP (data not shown), we used T-84 cells to examine the effect of the Cys mutation of the A subunit on the activity of the A subunit to accumulate intracellular cyclic AMP.
At first, we estimated the amount of holotoxin in the purified toxin solution. As shown in Fig. 2B, the majority of the toxin in the purified sample solution from the wild-type strain was holotoxin. However, the percentage of holotoxin in the samples from the mutant strains was low. So, we measured the density of each band by scanning and estimated the amount of holotoxin in each sample from the density of the band.
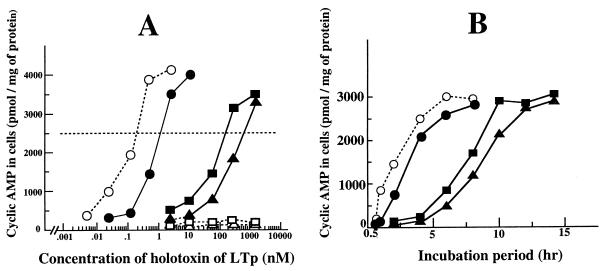
These samples and their trypsin-treated LTps (the treatment was performed as described above) were added to the culture medium of the cells. The final concentration of holotoxin in each medium is indicated in Fig. 6A. After the cells were incubated with each toxin for 8 h, the intracellular cyclic AMP level was determined as described in Materials and Methods. The intracellular cyclic AMP level increased in response to all LTps except for the trypsin-treated mutant LTps. The minimum concentrations of the holotoxin to induce obvious cyclic AMP accumulation (ca. 2,500 pmol/mg of cellular protein) of native LTp, trypsin-treated native LTp, LTp(C199S), and LTp(C187S) were 1.2, 0.2, 180, and 700 nM, respectively.
FIG. 6.
Stimulation of cyclic AMP accumulation in T-84 cells by LTp. T-84 cells were grown to near confluence in petri dishes. The culture medium was changed to DMEM containing 1% FBS prior to the addition of LTps. After incubation with these LTps, the cells were assayed for intracellular cyclic AMP as described in the text. (A) Dose-response reaction of the cells to LTp. The final concentration of holotoxin of LTp in each medium is indicated. The incubation was continued for 8 h. (B) Time course of cyclic AMP accumulation due to LTp. The LTps were added [1.2 nM for native LTp, 0.2 nM for trypsin-treated native LTp, 180 nM for LTp(C199S), and 700 nM for LTp(187S)] to each dish. The incubation was continued for the period indicated. Symbols: •, native LTp; ○, trypsin-treated native LTp; ▪, LTp(C199S); ▴, LTp(C187S).
Subsequently, the action of LTp over time was examined. The LTps were added to the cells at concentrations yielding an obvious positive reaction [that is, native LTp, trypsin-treated native LTp, LTp(C199S), and LTp(C187S) were added at concentrations of 1.2, 0.2, 180, and 700 nM, respectively] and incubated for the times indicated in Fig. 6B. As shown in Fig. 6B, cyclic AMP started to accumulate in the cells incubated with native LTp 2 h after initiation of the incubation and the accumulation had reached an almost maximum level 4 h later. In the cells incubated with trypsin-treated LTp, the cyclic AMP level began to increase 1 h after initiation of the incubation. As the intracellular cyclic AMP level did not increase in the cells incubated for 1 h with native LTp which was not treated with trypsin, the limited trypsinolysis shortened the lag period before the toxic activity of LT manifested.
The action of the mutant LTps over time differed from that of native LTps. In the cells exposed to LTp(C199S) and LTp(C187S), the intracellular cyclic AMP began to accumulate 6 h after the exposure. At that time, the cyclic AMP level in cells treated with native LTps had already reached its maximum. The cyclic AMP level in the cells incubated with the mutant LTps reached its maximum 10 h later.
DISCUSSION
It is well known that the A subunit associates with B subunits that are in the process of assembling and in doing so catalyzes the assembly process. During assembly, the intramolecular interactions that give rise to the tertiary structure in the individual toxin subunits create interfaces that allow specific intramolecular interactions that ultimately lead to stable quaternary complexes of LT. The crystal structure of the holotoxin of LT revealed that the major contacts between the A and B subunits are clustered toward the C-terminal portion of the A2 polypeptide, which is inserted into the central pore of the B pentamer (24). Therefore, all the information for the A subunit necessary for the interaction with the B subunit is presumed to be contained in the A2 fragment. Streatfield et al. showed that deletion of C-terminal residues within the −14 to −4 region of the A subunit caused the loss of the capacity of the A subunit to act as the site of nucleation (26). However, this is inevitable, because the C-terminal residues are the portion of the A2 fragment that is inserted into the pore of the B pentamer.
In the present study, we showed that replacement of cysteine residues with serine reduced the capacity of the A subunit to act as the site of nucleation of the assembly with the B subunit and proposed that the disulfide bond between Cys-187 and Cys-199 is important for this function of the A subunit. However, it was possible that the reduction was due to alterations in the structure of the A subunit brought about by the substitution of serine for cysteine residues. To confirm the role of the disulfide bond in the function of the A subunit, we further replaced these cysteine residues with glycine and expressed these genes in E. coli. The amounts of these mutant A subunits [A(C187G) and A(C199G)] associated with the B-subunit oligomer were also small (data not shown). This result supports the suggestion that dissociation of the disulfide bond of the A subunit reduces the capacity of the A subunit to act as the site of nucleation of assembly with the B subunit. The results from pulse-chase (Fig. 3) and reconstitution (Fig. 4) experiments also support this suggestion.
However, the holotoxin was produced even in the mutant cells, although in small amounts compared with the amounts produced in the wild type (Fig. 2B). Since these mutant A subunits retain C-terminal residues within the −14 to −4 region which is the core of the assembly, we think that the disulfide bond formation of the A subunit is important but not essential in the creation of the structure of the core region of A2 fragment.
A report showed that the fusion proteins consisting of the A2 fragment of cholera toxin and alkaline phosphatase, β-lactamase, or maltose-binding protein assemble with the B subunit to form holotoxin-like chimeras. No portion of the A1 domain of cholera toxin was required for formation of holotoxin-like chimeras (9, 11). However, it is not clear whether these fusion proteins efficiently function as the site of nucleation in the assembly. In our experiment, the mutant A subunit functions as the site of nucleation, although the efficiency is low (Fig. 2B). Further analysis of the holotoxin-like chimeras will make clear the structure of the core region of the A2 fragment in the assembling process.
Subsequently, we examined the sensitivity of the mutant LTps to trypsinolysis. As shown in Fig. 5, the mutant A subunits associated with the B-subunit pentamer were easily degraded by limited trypsinolysis, although the native A subunits associated with the B-subunit pentamer were cleaved by the lysis only at a site generating A1 and A2 fragments. This shows that the three-dimensional structure of the mutant A subunit is different from that of the native A subunit.
As shown in Fig. 6A, the ability of the mutant LTps to elevate the intracellular cyclic AMP concentration is less than that of the wild-type LTp. When LTs are incubated with mammalian cells, the B subunits of the LTs recognize and bind to specific receptors (GM1 ganglioside) on the cell surface. In addition to GM1 ganglioside, some cell surface glycoproteins are known to serve as alternative receptors (2). Crystallographic studies revealed that LT binds with its enzymatically active A subunit pointing away from the membrane (16). Since the B subunits of the mutant LTps form pentamers (Fig. 2), it seems that the mutation does not cause the reduction of binding affinity to the receptors of the cell surface. Actually, a preliminary study using fluorescence antibody showed that a suitable number of mutant LTps bind to the cells (data not shown). Characteristically, the A subunit of mutant LTp is highly sensitive to proteolysis (Fig. 5). We predict that the low activity of mutant LTps to elevate the level of intracellular cyclic AMP is due to the low resistance of the mutant LTps to proteases. The mutant LTps added to the culture medium should be degraded by intracellular and extracellular proteases.
Subsequently, we examined the kinetics of the mutant LTps and found that in the cells exposed to them, the lag time until the intracellular cyclic AMP begins to increase is greater than that in the cells exposed to native LTps (Fig. 6B), although the reason for this is unclear. The amino acid sequence of the A1 fragment, which contains the enzymatic activity, was not changed by the mutation. Therefore, it is unlikely that the enzymatic activity of the A1 fragment of the mutant LTps is responsible for the longer lag time.
The cell-bound LTs are internalized via endocytosis through noncoated-membrane invagination of the plasma membrane and appear first in noncoated vesicles and then in a tubulovesicle closely associated with the Golgi apparatus. The carboxy-terminal amino acid sequence of the A2 polypeptide of LT is RDEL, which is homologous to a potential endoplasmic reticulum (ER) retention signal; thus, the LTs recognized by specific receptors in the Golgi network are shuttled back into the ER (9, 20). A recent study showed that the disulfide bond of the A subunit is dissociated by the protein-disulfide isomerase in the ER and A1 fragments generated in the ER (19). The A1 fragments appear on the surface of the ER and are carried to the Gs/adenylate cyclase complex on the basolateral membrane by the basolaterally targeted vesicles (13). The A1 peptide then catalyzes the NAD-dependent ADP-ribosylation of Gsα, a component of the heterotrimeric signal-transducing guanine-nucleotide binding protein that stimulates adenylate cyclase (9).
As shown in Fig. 6B, the generation of a proteolytic nick site in the native LTp in vitro shortened the lag phase, indicating that the time until A1 fragments generate in the ER closely relates to the length of the lag phase. Since the structure of the mutant A subunit bound to the B subunit differs from that of the native A subunit (Fig. 5), it is possible that the efficiency of the internalization into cells of the mutant LTp via noncoated-membrane invagination is low and that internalization takes time. In addition, the translocational efficiency of the mutant LTp to cross the ER membrane may be low due to the deformation. It is also possible that the frequency with which A1 fragments are generated in the ER is low in the mutant LTps. Studies on the kinetics of the action of mutant LTps serve to clarify the behavior of LTp in cells at the molecular level.
ACKNOWLEDGMENT
This work was supported in part by a grant-in-aid for scientific research from the Ministry of Education, Science, and Culture, Japan.
REFERENCES
- 1.Dallas W S, Gill D M, Falkow S. Cistron encoding Escherichia coli heat-labile toxin. J Bacteriol. 1979;139:850–858. doi: 10.1128/jb.139.3.850-858.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griffiths S L, Critchley D R. Characterization of the binding site for Escherichia coli heat-labile toxin type I in intestinal brush borders. Biochim Biophys Acta. 1991;1075:154–161. doi: 10.1016/0304-4165(91)90246-d. [DOI] [PubMed] [Google Scholar]
- 3.Hardy S J S, Holmgren J, Johansson S, Sanchez J, Hirst T R. Coordinated assembly of multisubunit proteins: oligomerization of bacterial enterotoxins in vivo and in vitro. Proc Natl Acad Sci USA. 1988;85:7109–7113. doi: 10.1073/pnas.85.19.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirst T R. Biogenesis of cholera toxin and related oligomeric enterotoxins. In: Moss J, Iglewski B, Vaughan M, Tu A T, editors. Bacterial toxins and virulence factors in disease. New York, N.Y: Marcel Dekker, Inc.; 1995. pp. 123–184. [Google Scholar]
- 5.Hirst T R, Hardy S J S, Randall L L. Assembly in vivo of enterotoxin from Escherichia coli: formation of the B subunit oligomer. J Bacteriol. 1983;153:21–26. doi: 10.1128/jb.153.1.21-26.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hofstra H, Witholt B. Kinetics of synthesis, processing, and membrane transport of heat-labile enterotoxin, a periplasmic protein in Escherichia coli. J Biol Chem. 1984;259:15182–15187. [PubMed] [Google Scholar]
- 7.Hofstra H, Witholt B. Heat-labile enterotoxin in Escherichia coli: kinetics of association of subunits into periplasmic holotoxin. J Biol Chem. 1985;260:16037–16044. [PubMed] [Google Scholar]
- 8.Hol W G J, Sixma T K, Merritt E A. Structure and function of E. coli heat-labile enterotoxin and cholera toxin B pentamer. In: Moss J, Iglewski B, Vaughan M, Tu A T, editors. Bacterial toxins and virulence factors in disease. New York, N.Y: Marcel Dekker, Inc.; 1995. pp. 185–223. [Google Scholar]
- 9.Holmes R K, Jobling M G, Connell T D. Cholera toxin and related enterotoxins of gram-negative bacteria. Biogenesis of cholera toxin and related oligomeric enterotoxins. In: Moss J, Iglewski B, Vaughan M, Tu A T, editors. Bacterial toxins and virulence factors in disease. New York, N.Y: Marcel Dekker, Inc.; 1995. pp. 225–255. [Google Scholar]
- 10.Inouye S, Inouye M. Oligonucleotide directed site-specific mutagenesis using double-stranded plasmid DNA. In: Narang S A, editor. Synthesis and application of DNA and RNA. New York, N.Y: Academic Press, Inc.; 1987. pp. 181–206. [Google Scholar]
- 11.Jobling M G, Holmes R K. Fusion proteins containing the A2 domain of cholera toxin assemble with B polypeptides of cholera toxin to form immunoreactive and functional holotoxin-like chimeras. Infect Immun. 1992;60:4915–4924. doi: 10.1128/iai.60.11.4915-4924.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 13.Lencer W I, Constable C, Moe S, Jobling M G, Webb H M, Ruston S, Madara J L, Hirst T R, Holmes R K. Targeting of cholera toxin and Escherichia coli heat-labile toxin in polarized epithelia: role of COOH-terminal KDEL. J Cell Biol. 1995;131:951–962. doi: 10.1083/jcb.131.4.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lowry O H, Rosenbrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 15.Merritt E A, Pronk S P, Sixma T K, Kalk K H, van Zanten B A M, Hol W G J. Structure of partially-activated E. coli heat-labile enterotoxin (LT) at 2.6 Å resolution. FEBS Lett. 1994;337:88–92. doi: 10.1016/0014-5793(94)80635-7. [DOI] [PubMed] [Google Scholar]
- 16.Merritt E A, Sixma T K, Kalk K H, van Zanten B A M, Hol W G J. Galactose binding site in E. coli heat-labile enterotoxin (LT) and cholera toxin (CT) Mol Microbiol. 1994;13:745–753. doi: 10.1111/j.1365-2958.1994.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 17.Miller J. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. pp. 431–433. [Google Scholar]
- 18.Okamoto K, Okamoto K, Miyama A, Tsuji T, Honda T, Miwatani T. Effect of substitution of glycine for arginine at position 146 of the A1 subunit on biological activity of Escherichia coli heat-labile enterotoxin. J Bacteriol. 1988;170:2208–2211. doi: 10.1128/jb.170.5.2208-2211.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orlandi P A. Protein-disulfide isomerase-mediated reduction of the A subunit of cholera toxin in a human intestinal cell line. J Biol Chem. 1997;272:4591–4599. [PubMed] [Google Scholar]
- 20.Orlandi P A, Curran P K, Fishman P H. Brefeldin A blocks the response of cultured cells to cholera toxin. Implications for intracellular trafficking in toxin action. J Biol Chem. 1993;268:12010–12016. [PubMed] [Google Scholar]
- 21.Ruddock L W, Coen J J F, Cheesman C, Freedman R B, Hirst T R. Assembly of the B subunit pentamer of Escherichia coli heat-labile enterotoxin. J Biol Chem. 1996;271:19118–19123. doi: 10.1074/jbc.271.32.19118. [DOI] [PubMed] [Google Scholar]
- 22.Ruddock L W, Ruston S P, Kelly S M, Price N C, Freedman R B, Hirst T R. Kinetics of acid-mediated disassembly of the B subunit pentamer of Escherichia coli heat-labile enterotoxin. J Biol Chem. 1995;270:29953–29958. doi: 10.1074/jbc.270.50.29953. [DOI] [PubMed] [Google Scholar]
- 23.Sixma T K, Kalk K H, van Zanten B A M, Dauter Z, Kingma J, Witholt B, Hol W G J. Refined structure of Escherichia coli heat-labile enterotoxin, a close relative of cholera toxin. J Mol Biol. 1993;230:890–918. doi: 10.1006/jmbi.1993.1209. [DOI] [PubMed] [Google Scholar]
- 24.Sixma T K, Pronk S E, Kalk K H, Wartna E S, van Zanten B A M, Witholt B, Hol W G J. Crystal structure of a cholera toxin-related heat-labile enterotoxin from E. coli. Nature (London) 1991;351:371–377. doi: 10.1038/351371a0. [DOI] [PubMed] [Google Scholar]
- 25.Spicer E K, Nobel J A. Escherichia coli heat-labile enterotoxin, nucleotide sequence of the A subunit gene. J Biol Chem. 1982;257:5716–5721. [PubMed] [Google Scholar]
- 26.Streatfield S J, Sandkvist M, Sixma T K, Bagdasarian M, Hol W G J, Hirst T R. Intermolecular interactions between the A and B subunits of heat-labile enterotoxin from Escherichia coli promote holotoxin assembly and stability in vivo. Proc Natl Acad Sci USA. 1992;89:12140–12144. doi: 10.1073/pnas.89.24.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Studier F W, Rosenberg A H, Dunn J J, Dubendorff J W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- 28.Uesaka Y, Otsuka Y, Lin Z, Yamasaki S, Yamaoka J, Kurazono H, Takeda Y. Simple method of purification of Escherichia coli heat-labile enterotoxin and cholera toxin using immobilized galactose. Microb Pathog. 1994;16:71–76. doi: 10.1006/mpat.1994.1007. [DOI] [PubMed] [Google Scholar]
- 29.Welsh C F, Moss J, Vaughan M. ADP-ribosylation factors: a family of guanine nucleotide-binding proteins that activate cholera toxin and regulate vesicular transport. In: Moss J, Iglewski B, Vaughan M, Tu A T, editors. Bacterial toxins and virulence factors in disease. New York, N.Y: Marcel Dekker, Inc.; 1995. pp. 257–280. [Google Scholar]
- 30.Yamamoto T, Tamura T, Yokota T. Primary structure of heat-labile enterotoxin produced by Escherichia coli pathogenic for humans. J Biol Chem. 1984;259:5037–5044. [PubMed] [Google Scholar]