Abstract
Electrical stimulation (ES) has been shown to induce and enhance bone regeneration. By combining this treatment with tissue-engineering approaches (which rely on biomaterial scaffolds to construct artificial tissues), a replacement bone-graft with strong regenerative properties can be achieved while avoiding the use of potentially toxic levels of growth factors. Unfortunately, there is currently a lack of safe and effective methods to induce electrical cues directly on cells/tissues grown on the biomaterial scaffolds. Here, we present a novel bone regeneration method which hybridizes ES and tissue-engineering approaches by employing a biodegradable piezoelectric PLLA (Poly(L-lactic acid)) nanofiber scaffold which, together with externally-controlled ultrasound (US), can generate surface-charges to drive bone regeneration. We demonstrate that the approach of using the piezoelectric scaffold and US can enhance osteogenic differentiation of different stem cells in vitro, and induce bone growth in a critical-sized calvarial defect in vivo. The biodegradable piezoelectric scaffold with applied US could significantly impact the field of tissue engineering by offering a novel biodegradable, battery-free and remotely-controlled electrical stimulator.
Keywords: Biodegradable piezoelectric nanofibers, ultrasound, electrical stimulation, Bone regeneration, Tissue engineering
1. Introduction
Reconstruction of large/major bone defects remains a significant challenge in modern medicine [1, 2]. Until now, the gold standard has been to use auto- or allo-grafts, which suffer from problems of limited tissue supply, donor site morbidity, infection and/or immune-rejection [3, 4]. Regenerative and tissue engineering strategies, employing a combination of growth factor/small molecule therapies, biomaterial scaffolds, and stem/osteogenic cells have therefore emerged as an important area of research [5, 6].
Several biomaterials including hydrogels, naturally-derived biomaterials, synthetic polymers (e.g. poly(lactic-co-glycolic acid), poly(lactic acid), polycaprolactone, etc.)[7–9] in combination with chemical growth factors or small molecules have been used extensively to create bone scaffolds. Although bone growth factors and small molecules are powerful, many of their toxic side effects associated with high doses demand a new approach to stimulate bone growth. Alternatively, electrical stimulation (ES) offers a more natural stimulation mechanism and has been found to have a positive impact on the regeneration of tissues, especially bone [10–12]. Several electrical stimulators have been commercialized to treat bone fractures[13, 14]. However, external electrical stimulators are not very effective while implanted devices rely on percutaneous wires and toxic batteries that are non-degradable, therefore requiring an invasive removal surgery. These limitations render ES limited in clinical applications and combination with tissue engineering approaches.
Piezoelectric materials, a group of “smart” materials which produce electricity under applied force, can be used as a self-powered scaffold that can utilize body movements or external mechanical vibrations to electrically stimulate bone growth. Interestingly, bone is also piezoelectric in nature [15–17]. Under deformation, bone can generate surface charges, which drive the tissue to grow against the applied force (i.e. Wolff’s Law[18, 19]). Piezoelectric scaffolds, therefore, can mimic native bones by undergoing mechanical loading to generate useful electrical charges that consequently self-promote bone regeneration. Indeed, experimental evidence has shown that bone favorably grows on charged surfaces of non-degradable pre-polarized ceramics [20, 21] and non-degradable piezoelectric polymers like polyvinylidene fluoride (PVDF) [19, 22, 23]. For the osteogenic mechanism, the surface charges could absorb beneficial proteins which lay down an extracellular matrix layer to facilitate cell deposition, and tissue remodeling while triggering several molecular transduction mechanisms such as calcium signaling, TGF-β/BMP, MAPK/ERK, Wnt/β-catenin pathways, etc. to induce osteogenesis [24]. Yet, common piezoelectric materials including PVDF, PZT (Lead Zirconate Titanate), BaTiO3 (Barium Titanate), etc., contain non-dissolvable and even toxic elements (e.g. lead in PZT) which impede tissue ingrowth and require invasive removal operations, thus rendering them unfavorable to serve as tissue scaffolds.
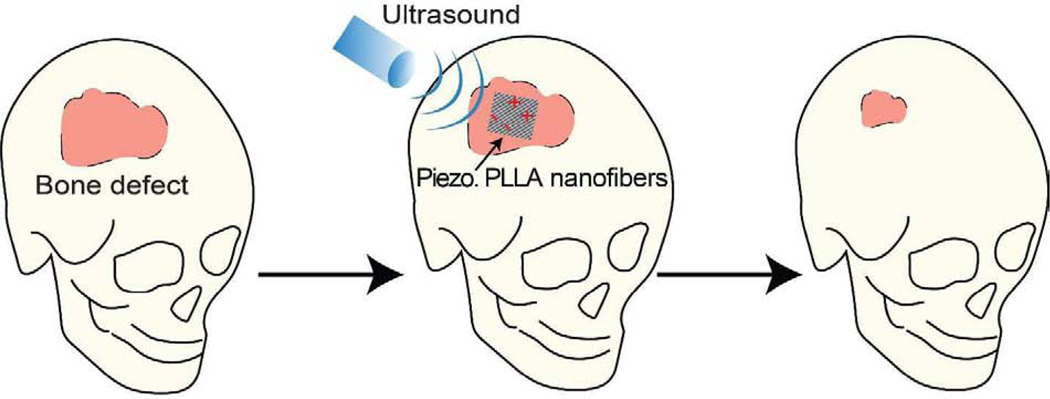
Consequently, the development of biodegradable piezoelectric materials becomes important to offer novel biomaterials that can generate electrical charges to promote bone regeneration and safely degrade to facilitate cell infiltration/tissue ingrowth [25–27]. Although there has been some recent achievements with biodegradable amino acid piezoelectric crystals [28–30], these powdery materials degrade too quickly inside aqueous solutions and are challenging to create a functional film or tissue scaffold for long-term implantations. In this regard, our group has reported on a new biodegradable and biocompatible piezoelectric nanofiber polymer, derived from poly(L-lactic acid) (PLLA) [31, 32] which has a longer degradation time and been used commonly for bone tissue scaffolds [33, 34]. Here, we present, for the first time, a new tissue-engineering and stimulation approach which utilizes the reported piezoelectric PLLA nanofibers[32] in combination with non-invasive ultrasound (US) to generate beneficial surface-charges for inducing and enhancing bone regeneration (Fig. 1).
Figure 1. The use of biodegradable piezoelectric PLLA nanofibers in combination with non-invasive ultrasound (US) to produce well-controlled, on-demand and stable surface charge (i.e. electrical stimulation or ES) for bone regeneration.
The piezoelectric PLLA nanofiber tissue-scaffold serves as a remotely-controlled bone-growth stimulator, which is biodegradable and can be subjected to acoustic-pressure (from non-invasive US) to self-produce on-demand and tailorable electrical charges for directly stimulating osteogenesis and healing the defect in the calvarial bone.
The nanofibers not only offer an extracellular matrix (ECM)-like environment [35–37] but are also biodegradable to avoid the need for any removal procedures and facilitate tissue in-growth, an advance which has not been achieved by conventional piezoelectric scaffolds [26, 27]. Although implantation of drawn PLLA rods at bulk scales have been shown to induce bone formation inside cats[38], the presumed piezoelectric output was only generated by passive motions of the animals, thus offering no control over the time, duration and amount of the applied ES. Here, we present a novel strategy of using extracorporeal ultrasound (US) to remotely generate well-controlled piezoelectric charges on the ECM-like piezoelectric PLLA nanofibers for enhanced bone regeneration. The nanofiber bone scaffold thus inherently serves as a remotely-controlled and battery-free biodegradable electrical-stimulator [39, 40].
2. Experimental Section
2.1. Preparation of PLLA Nanofiber Mat:
The poly(L-lactic acid) (PLLA) nanofiber mat was fabricated by electrospinning as described in our previous work [32]. PLLA (PURASORB PL38) was purchased from Corbion Purac (Amsterdam, Netherlands). 0.8g of the PLLA was dissolved in a 1:4 mixture of N, N – Dimethylformamide (DMF, anhydrous, ≥99.9%) and dichloromethane (DCM) respectively (v/v); these solvents were obtained from MilliporeSigma (Burlington, MA). 5ml of the solution was drawn into a 10 ml syringe which was loaded into a syringe pump (New Era Pump Systems, Inc., Farmingdale, NY). The solution flow rate was set at 2 ml/hr through a flat-tipped 22-gauge needle (Jensen Global, Santa Barbara, CA) with 14kV (kilovolts) applied to it. The polarized solution was then deposited onto aluminum foil, wrapped on a grounded aluminum drum, rotating at speeds from 300 – 3,000 rpm (rotations per minute). The electrospinning process was conducted at a 40 ±15 % relative humidity atmosphere at ambient temperature. This resulted in a PLLA nanofiber mat on the aluminum foil with varying degrees of alignment and fiber diameter, depending on the collector speed. These fibrous mats were then annealed at 105°C for 10hr in an oven (Quincy Lab Inc., Chicago, IL) and then the oven was shut off and allowed to slowly cool to room temperature. The mats were subsequently peeled off the aluminum foil and transferred to a Teflon FEP sheet (American DURAFILM, Holliston, MA). Another Teflon sheet was placed on top of the film, and the sandwiched film was placed on top of a glass slide. The sandwiched film was then placed inside an oven at 160.1°C for 10hr and the oven was shut off and allowed to cool to room temperature.
2.2. Characterization of PLLA film:
SEM:
Scanning Electron Microscopy (SEM) was performed on mats prepared to observe the orientation of the PLLA fibers and the microstructure of the mats. Square shaped PLLA films with a dimension of 7mmx7mm were mounted on a standard SEM pin (Ted Pella, Redding, CA) using carbon conductive tabs (Ted Pella, Redding, CA). The samples were coated in gold-palladium for 1 minute using a Polaron E5100 sputter coater (Quorum Technologies Ltd., Lewes, UK). The samples were then imaged using a FEI TeneoLoVac SEM at 5kV and 5000 magnification. These studies were performed using the facilities in the UConn/Thermo Fisher Scientific Center for Advanced Microscopy and Materials Analysis (CAMMA).
Measurement of the piezoelectric property and ultrasound receiving capability of the PLLA films:
A force sensor was fabricated to test the piezoelectric property of the PLLA film. The treated PLLA films were cut at a 45° angle with the fiber direction films with a dimension of 1.27 cm long, 1.27 cm wide to maximize the shear force under applied normal pressure. Aluminum (Al) foil was used to fabricate electrodes to compactly sandwich both surfaces of the films. Afterwards, the sandwiched structure was encapsulated in polyimide tape (DuPont, Wilmington, DE) to isolate the Al foil electrodes from air. The exposed ends of the Al foil electrode leads were then reinforced using copper tape (Ted Pella, Redding, CA). PLLA sensors were subjected to ultrasonic waves at 40 kHz frequency and 1 MHz (Fig. 2b and S1). For the 40 kHz measurements, a commercial ultrasonic water bath with a set frequency of 40khz (Branson 2800 CPX series), filled with deionized water, was used. The sensor was shielded with aluminum foil (extra heavy duty, Reynolds Wrap) and suspended into the water bath. An oscilloscope (PicoScope 4824, Pico Technology, UK) was connected to the PLLA device and used to measure the open circuit voltage.
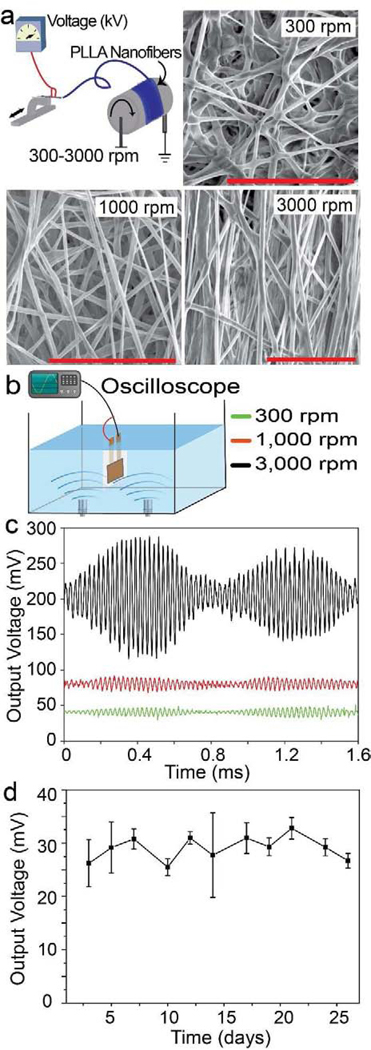
Figure 2. Characterization of the microstructure and piezoelectric performance of the PLLA nanofiber mats under applied ultrasound (US).
a. Schematic of the electrospinning setup used to fabricate the PLLA films and scanning electron microscope (SEM) images showing PLLA nanofiber alignment with different collection speeds. Scale bars are 20 μm. b. Simplified schematic showing the setup used for measuring the open circuit voltage of the films using a water bath and ultrasound produced at 40khz. c. Open circuit output voltage from PLLA sensors fabricated using films at different collector speeds under applied 40kHz ultrasound. d. Peak output voltages from the PLLA sensors fabricated with 3000 rpm films under the same applied US over the course of 26 days. Films were conditioned in standard cell media solution at 37°C, 5% CO2 and measurements were taken every 2 days.
Film degradation study:
This study was designed to determine the functional lifetime of the piezoelectric PLLA nanofiber mat. The objective of this experiment is to determine how long the PLLA mat retains its piezoelectric property in vitro. The treated PLLA films electrospun at 3000 rpm were cut at a 45° angle relative to the fiber direction. The PLLA films were 1cm long by 1cm wide. Each piece was placed in a separate well of a 24-well plate and the well was filled with 0.5 ml of standard cell media (DMEM, 10 % fetal bovine serum and 1 % antibiotic solution pennstep containing 50% penicillin and 50 % streptomycin, all three components purchased from Gibco). The 24-well plates were placed in an incubator (37ºC, 5% CO2). The plates (with the films in them) were exposed to ultrasound for 30 minute every day for 5 days a week. And every 2 days, 3 films were taken out of the media, washed with DI water to remove the remnants of the cell media from its pores and dried using an air dryer (purchased from Innotech). The dry films were used to make a force sensor as described in the previous section. Following that, the open circuit voltage of the devices was measured at 1MHz. For this, an immersion ultrasound transducer (Olympus NDT, V323-SU) controlled by a function generator (4054B Arbitrary Waveform Generator, BK Precision) and an RF power amplifier (E&I RF Amplifier 1040L) were used to generate ultrasound. The input voltage for the power amplifier was fixed at 0.1 Vrms at 1 MHz to create a constant pressure at 10 kPa. The commercial transducer and the PLLA transducer were mounted and immersed in DI water. The PLLA device was placed at 1 Rayleigh distance from the ultrasound transducer (~2 cm). An 8-channel oscilloscope (PicoScope 4000, UK) was connected to the PLLA transducer to measure the open circuit output voltage from the devices.
Preparation of PLLA scaffolds for ADSC culture:
The scaffolds were prepared by electrospinning as previously described. The scaffolds used as the experimental group were spun at 3000-rpm and 1000-rpm (i.e. piezoelectric samples and less-piezoelectric samples). These scaffolds were compared to scaffolds spun at 300-rpm which served as a negative control group (i.e. little-to-no piezoelectric samples). Three scaffolds of each type (cut into 14 mm x 14 mm dimensions) were seeded with cells to attain statistical significance.
2.3. Sterilization of the PLLA scaffolds and preparation of the cell culture plates to seed ADSCs:
The scaffolds prepared were sterilized using ethanol and UV treatment. The entire process was carried out under a laminar flow cell culture hood. First, the scaffolds were soaked in 70% ethanol for 30 minutes. Then, the ethanol was removed, and the scaffolds were placed under UV light for 20 minutes. After the 20 minutes were over, the scaffolds were flipped onto the other side using a sterile tweezer and that side was exposed to UV light for another 20 minutes.
Once the whole process of sterilization was completed, the scaffolds are fixed onto 6-well culture plates (purchased from Thermo Scientific) using biocompatible silicone glue (KWIK-SIL produced by World Precision instruments). A small amount of glue is added to each well and the entire surface of the wells are covered spreading and smearing the glue using a spatula. The scaffold is placed on the glue/well surface before the silicone cures. There are two purposes behind this process, First, the glue helps keep the scaffolds securely on the plate while the US treatments are carried out. Second, the silicone prevents the ultrasound waves from getting reflected around by the walls of the polystyrene wells and be dissipated in the process. After that, the plates are set aside to allow the glue to dry until the cells are seeded onto the scaffolds.
2.4. ADSC culture:
Cells were seeded onto the PLLA scaffolds after they were sterilized and glued to the well plates. The cells used for this purpose were adipose derived stem cells (ADSCs) that were purchased from iXCells Biotechnologies at passage 1. The cells, received in frozen form, were thawed, plated and expanded until passage 4, as per the company protocol. For expanding the cultures, we used proliferation media that we prepared by adding to DMEM, 10 % fetal bovine serum and 1 % antibiotic solution pennstep (containing 50% penicillin and 50 % streptomycin) (all three components purchased from Gibco). The initial thawing of the cells was carried out by melting the frozen cell suspension obtained from the suppliers, removing the cells from the freeze media by centrifuging and forming and removing a cell pellet and finally resuspending the pellet in fresh media and plating it into a flask of appropriate size. Expansion was carried out by passaging the cells every 5–6 days after the culture reached 80–90 % confluence. Ultimately, at 80–90 % confluence of passage 4, the cells were detached from the flasks using Trypsin / EDTA (Ethylenediaminetetraacetic acid) (purchased from Gibco) and seeded onto the PLLA scaffolds at a seeding density of 5 × 105 cells per scaffold. The cells were allowed to attach for one day under proliferation media. After that, the proliferation medium was replaced with osteogenic differentiation medium that was prepared by adding 50 μg/ml ascorbic acid and 10 mM beta-glycerophosphate to the proliferation medium prepared previously. The cultures were left in osteogenic differentiation medium for 1 day before we started the daily ultrasonic treatments.
2.5. Ultrasonic (US) treatment on the ADSCs:
The US treatment on the cells was started a day after the cells were put in osteogenic media. The treatment was performed using a sonication cleaning bath (Branson 2800 CPX series). The ultrasound provided was at 40 kHz. The treatment was done for 20 minutes each day and performed for 10 days. The main concern was to prevent the non-sterile water from the bath from getting into the wells and affecting the culture during this process. To that end, we reduced the amount of media in the wells (from 2 ml to 1 ml) to prevent it from spilling out and getting contaminated by the water. We also used a standardized method to seal the entire plate.
First, we taped the lid onto 4 sides of the plate using labelling tape. Then we removed the plate from the cell hood and encapsulated it in two layers each of plastic wrap (Kirkland Signature Stretch-Tite Plastic Wrap - 11 7/8 ×750 Feet) and duct tape (manufactured by 3M). For the first layer, the plate was wrapped with saran wrap 3 times, making sure that the bottom of the plate has three layers of saran wrap. For the second layer, the plate was wrapped with 3 pieces of duct tape horizontally, 2 pieces vertically, making sure that the bottom of the plate has four layers of tape. Additionally, a small piece of tape was added to each edge to prevent water from leaking in through the gaps between the tapes. Also, a tab was created on the top of the encapsulated plate with the duct tape such that it was more or less centrally placed, and the plate was hanging evenly from the tab. The tab was used to suspend the plate into the water of the sonication bath. This was done by using a laboratory clamp, stand apparatus and an alligator clip. The plate was suspended so that it was submerged halfway into the water and horizontally level. The plate was sonicated for 20 minutes. When the 20 minutes was done, the plate was removed from the bath. The two-layer encapsulation was removed with a pair of scissors. The media that may have spilt out of the wells was cleaned by vacuum aspiration. In addition, the remaining media in the wells were also removed by aspiration and fresh osteogenic media (2 ml) was added to each well. After that, the cells were returned to the incubator until the next US treatment.
2.6. Bone regeneration assays:
After 10 days of treatment, the cultures were terminated and analyzed for osteogenic differentiation activity. The assays performed are Alkaline phosphatase (ALP) enzyme quantification, polymerase chain reaction (PCR) to quantify the activity of genes osteocalcin and osterix, as well as alizarin red quantification. The ALP and Alizarin red data was normalized using total protein content measured using BCA assay (purchased from Pierce™). The PCR data was normalized using a housekeeping gene (beta-actin).
2.7. BCA assay:
BCA assay was used to quantify the total protein content of the cultures and this quantity was used to normalize the results of ALP and Alizarin red quantification. Protein was extracted from the culture by using RIPA buffer. The protein solution that was extracted from each culture (i.e. each well) was used for the BCA assay as well as the ALP quantification. The BCA assay used was the Pierce™ BCA Protein Assay Kit purchased from Thermo Scientific. The protocol for the kit was followed to obtain the results for total protein quantification.
2.8. ALP quantification assay:
The Alkaline phosphatase quantification was carried out using a kit purchased from Biorad (cat no-172–1063). The kit has a p-Nitrophenyl Phosphate (pNPP) based quantification technique. The protein solution extracted using the RIPA buffer was used for the assay. The pNPP solution was prepared and added to the protein extract and the ALP levels were quantified in accordance with the protocol of the kit. The standard curve for ALP-pNPP product quantification was prepared by dissolving different amounts of ALP (purchased from Sigma-Aldrich) in an aqueous solution, reacting them to pNPP and reading the absorbance of each solution. This helped us get the actual quantities of ALP in our cultures. The values obtained were normalized by total protein content.
2.9. Alizarin red assay:
For the Alizarin red assay, the cultures (after removing the media) were fixed in 70% ethanol at 4°C for one hour. Following this, the ethanol was removed, the wells were rinsed and the Alizarin red dye (purchased from EMD Milipore corp.) was added and the culture was incubated at room temperature for 30 minutes. After 30 minutes, the dye was rinsed away and the wells were viewed macroscopically and under the microscope to observe the mineral formation (stained red). The amount of mineral formed is quantified by destaining the red dye in cetylpyridinium chloride (purchased from Sigma Aldrich) and measuring the absorbance to get the amount destained. The values obtained were normalized by total protein content.
2.10. PCR quantification:
PCR (polymerase chain reaction) quantification was performed using the universal sybr green master mix manufactured by Bio-rad. The primers used were osterix (forward sequence of 5’-GGA AAG GAG GCA CAA AGA A-3’ and reverse sequence of 5’-GTC CAT TGG TGC TTG AGA A-3’) and osteocalcin (forward sequence of 5’-CAA GCA GGA GGG CAA TAA G-3’ and reverse sequence of 5’-CGT CAC AAG CAG GGT TAA G-3’) purchased from Integrated DNA Technologies, as well as beta actin as a housekeeping gene. Extraction of RNA was performed by using Trizol reagent, by following the corresponding protocol for the reagent. The RNA extracted was preserved at −80°C until quantification. During quantification, the frozen RNA solution was thawed, melted, and converted to cDNA using the iscript cDNA synthesis kit from Bio-rad, according to the protocol of the manufacturer. Following this, the cDNA was mixed with appropriate primers and the master mix and run through an RT-qPCR machine (source ABsystems) to get the gene expression values. The primers used were osteocalcin and osterix for bone mineralization quantification and beta-actin (forward sequence of 5’-TCC TCC TGA GCG CAA GTA CTC T-3’ and reverse sequence of 5’-CGG ACT CAT CGT ACT CGT GCT T-3’) as housekeeping gene.
2.11. BMSC Reporter cell usage:
Apart from our ADSC cultures, we also used a fluorescent reporter cell system to confirm the osteogenic properties of our materials which allowed us to monitor the proliferation and differentiation of our cells in real-time. The reporter cells we used were primary bone marrow stem cells (BMSCs) with reporters for bone sialoprotein (BSP-GFP-topaz, green) and dentin matrix protein (DMP1-RFP-mCherry, red) following our previous reports[41–43]. The progressive expression from BSP to DMP helped us determine the different stages of differentiation that our BMSCs were going through. We used optical imaging to visually compare the osteogenic properties of different scaffold types and treatments. Lastly, we quantified the red and green fluorescence emissions by the different cultures using ImageJ.
2.12. Preparation of PLLA scaffolds for BMSCs:
The groups used were the same as those for the ADSC cultures. The scaffolds were prepared by electrospinning as previously described. The scaffolds used as the experimental group were spun at 3000-rpm and 1000-rpm. These scaffolds were compared to scaffolds spun at 300-rpm which served as a negative control group. Three scaffolds of each type were seeded with cells to attain statistical significance.
2.13. Sterilization of the PLLA scaffolds and preparation of the cell culture plates to seed BMSCs:
The scaffolds prepared were sterilized using 70% ethanol and UV and attached to 6 well plates using biocompatible silicone glue in the same way as described previously for the ADSC cultures and in vivo experiments.
2.14. BMSC culture:
Primary BMSCs were harvested from the bone marrow of 3–4-week-old dual transgenic mice containing BSP-GFP-topaz and DMP1-RFP-mCherry fluorescent reporter genes. The hind legs of the mice were harvested to collect the tibias and femurs in cold, sterile (1X) PBS. The epiphyseal bone was cut and removed to get the hollow middle portion from both the femurs and the tibia. The bone marrow from all the long bones was flushed with 10 mL sterile expansion media using a 25 5/8-gauge syringe needle. The marrow collected was then passed through an 18 1/2–gauge needle to break up any tissue pieces, plated at 10 × 106 cells/dish in a 100 mm dish, and expanded in hypoxic incubation (37ºC, 5% CO2, 5% O2) to accelerate proliferation. Media was changed after four days of initial seeding to allow for hematopoietic cells to promote initial BMSC colony formation and to remove non-adherent non-stromal cells. Once confluence was reached at passage 1, the cells were collected from the dishes using accutase and then seeded to the scaffolds in the prepared 6 well plates at a seeding density of 106 cells per scaffold. The cells were allowed to attach for one day under proliferation media. After that, the proliferation medium was replaced with osteogenic differentiation medium that was prepared by adding 50 μg/ml ascorbic acid and 10 mM beta-glycerophosphate to the proliferation medium previously prepared. The cultures were left in osteogenic differentiation medium for 1 day before we started the daily ultrasonic treatments.
2.15. Ultrasonic (US) treatment on the BMSCs:
The US treatment was done on the reporter cell seeded scaffolds in the same manner as the ADSC seeded scaffolds. The culture plate was sealed using plastic wrap and duct tape and half suspended into the water bath for 20 minutes to expose the cells to ultrasound vibrations. This process was carried out each day for three days alongside the real-time monitoring of the cell differentiation.
2.16. Fluorescence microscopy and image processing:
At days 0 (pre-seeding), 1, 2 and 3 of US treatment, reporter cell fluorescence (N=3) was captured using the Zeiss Axio Observer Z.1 inverted fluorescence microscope. The fluorescence of the cells was captured by tiling images from over the entire scaffold surface in ZEN software at an objective of 5X. Exposure times of 100ms and 1000ms were applied for both the GFP (BSP-GFPtopaz) and the RFP (DMP1-RFPmCherry) channels. The tiled images obtained were then stitched together to create a mosaic. The final images obtained showed different amounts of green and red fluorescence for different scaffolds receiving different treatments. The amount of fluorescence in each image was quantified using the ImageJ software and the relative fluorescence of each group was compared and plotted.
2.17. Preparation of PLLA scaffolds for the in vivo experiment on mice:
The scaffolds used were prepared by electrospinning and then cutting out squares from the electro spun films at the dimensions of 4mm x 4mm. The scaffolds that were used as the experimental group were spun at 3000-rpm (piezoelectric scaffold). These were compared to the scaffolds spun at 300-rpm which served as a negative control group (non-piezoelectric scaffold). Ten scaffolds of each type were implanted into twenty animals (one scaffold in each) for a proof-of-concept study.
2.18. Sterilization of the PLLA scaffolds for the implantation:
The scaffolds prepared were cut into 4mm x 4mm pieces and sterilized using 70% ethanol and UV in the same way as described previously for the ADSC cultures.
2.19. Implantation surgery to demonstrate the osteoinductive property of the PLLA nanofiber film:
The surgical procedure has been approved by the institutional Animal Use Committee (Protocol # 101815–0421). Six transgenic NSG mice containing Collagen 3.6 -GFP-topaz fluorescent genes (Charlse River, MA) were used for the study. As described previously[44], a 3.5 mm diameter calvarial bone defect was created on right side of the midline suture of the mice anesthetized with ketamine (Putney, KS)/xylecine (Akorn, IL). A drilling tool was used to cut through the bone while keeping the dura intact (Dremel 10.8V, Ref#: 8000–02-F013. Drill bit (Meisinger, Germany): Fig: T229L; Shank 205; Size 040; Length (mm):14; Internal diameter 4.0 mm; and External diameter 5 mm). A layer of collagen (purchased from Thermo Fisher, cat no. A1048301 and prepared in accordance with the protocol of the manufacturers by adding sodium hydroxide and 10X PBS) was deposited on one side of the films to ensure that they stick to the bone around the defect and remain securely in place. These films were then placed on the defect with the collagen layer facing down. Ten mice were given the 300-rpm films (5 to receive US treatment and 5 to receive no treatment) and ten were given the 3000 rpm films (5 to receive US treatment and 5 to receive no treatment). The skin of the animal was then sutured up using 5–0 nylon sutures purchased from Oasis. The animals were injected with an analgesic once they regain consciousness after the surgery and also the next day in order to relieve the pain. During this period, each animal was caged individually. After the second day, every 2 animals were caged together. The US treatment was started 3 days after the surgery.
2.20. US treatment on the Animals:
The US treatment was received by the animals for 30 minutes a day, 5 days a week, 4 weeks in total. The US transducer used for this experiment was a bolt clamped langevin transducer that operate at 40 kHz, purchased from Steminc. They were powered by US generators that produced ultrasound at 40 kHz, also purchased at Steminc. Three transducers were connected in series to one generator. The mice were anaesthetized using an isoflurane (purchased from Piramal Enterprises, India) and oxygen (purchased from Airgas) mixture. First, they were anaesthetized by placing them in an induction box. Once they were unconscious, each animal was fitted with a nose cone (purchased from Vet Equip) and placed under a transducer such that the top of their head touched the US generating surface of the transducer. The contact between the transducer and the head of the mice was improved by applying ultrasound gel (purchased from Aquasonic). The animals that did not receive US were placed under isoflurane for the same amount of time. The animals were kept on a heating pad for the entire duration of the treatment until they regained consciousness.
2.21. Sample collection:
Two weeks after the termination of the US treatment, the animals were euthanized and their calvarial bone (Fig. S2) was harvested to look for evidence of bone regeneration inside the defect.
2.22. Histology:
The harvested calvaria bone was fixed in 10% neutral neutral-buffered formalin (Sigma) at 4°C overnight, rinsed with PBS (1X) three times, and then soaked in 30% sucrose (Sigma) in deionized water at 4°C until samples became isotonic. Samples were then dried and embedded in Optimal Cutting Temperature Compound (Tissue-Tek) by submersion in 2- methylbutane chilled with dry ice. Embedded samples were then cryosectioned to a thickness of 5 μm, attached to cryotape (Cryofilm type II, Section-Lab, Co), and UV- crosslinked onto glass slides. The sections were then imaged under the microscope to obtain images of mineral distribution (DIC). Sections were stained with nuclear fast red (purchased from Sigma #F8764–5g) for visualizing ALP active cells, Toluidine Blue O (Sigma, T3260) and DAPI (Hoechst 33342, Mol Probes) for cell distribution assessments. The BSP-GFP-topaz fluorescent genes in the mice were used to visualize BSP expressing cells in the sections. The stained sections were mounted with 30% glycerol in deionized water or PBS (1X) and color imaged with a Zeiss Axio Scan Z.1 automatic fluorescent slide scanning microscope with a 10X objective.
3. Results and Discussions
We used electrospinning to create the piezoelectric PLLA nanofiber mats and post-process the films by annealing and cutting at 45° to achieve piezoelectric PLLA nanofibers, following our previous report[32]. Fig. 2a describes the electrospinning system equipped with a collector drum that can rotate at speeds from 300 to 3000 rpm to provide different levels of fiber alignment and piezoelectric performance (see methods and materials section). As seen in our previous work [32], increase in the rotation speed of the drum increases the material’s piezoelectricity. Thus, the fibers produced at 3000 rpm have significantly higher output voltage than the ones produced at 1000 rpm while the signal is smallest for the samples made at 300 rpm under the same applied US (see Fig. 2b, 2c, and S1). Therefore, we use the 300-rpm sample as the negative or sham control for our assays that are described later. For convention, we name the samples made with the collector spin speeds of 300 rpm, 1000 rpm, and 3000 rpm as non-piezoelectric, less piezoelectric and piezoelectric sample, respectively. We chose low frequency US (40 kHz) for our subsequent in vitro and in vivo work, as it has been shown that lower frequencies have less absorption by the body, thus limiting damaging heat dissipation to local tissues[45, 46]. Although the use of US such as LIPUS (low intensity pulsed ultrasound) is also beneficial to bone growth[47–49], our work brings the advantage of ultrasound to another level by generating useful surface charges or electrical stimulation to drive osteogenesis. How long the PLLA piezoelectric nanofiber film can generate consistent electrical outputs under applied US is important to the lengthy cell-culturing or implantation processes. Therefore, we evaluated this functional lifetime by assessing piezoelectric outputs of the PLLA nanofibers conditioned in a standard cell media at 37°C and under the same applied force for a period of 26 days (see the experimental section), which covers the stimulation time in our in vitro and in vivo experiments. Results (Fig. 2d) show that the film retains its piezoelectric property over such a long period due to its high crystallinity and long degradation time, reported in our previous work[32].
Importantly, we carried out in vitro experiments to study osteogenesis of stem cells grown on the scaffolds. Figs. 3a and Fig. S3 describes our in vitro experiment in which we apply US (40 KHz, 20 minutes/day for 10 days) on different biodegradable piezoelectric/non-piezoelectric films, seeded with adipose derived stem cells (ADSCs) (see methods in experimental section). In this first in vitro cell test, we chose ADSCs because they can be easily harvested from subcutaneous fat of patients and expanded in vitro[50, 51], thus offering a clinically-relevant source of stem cells for combination with our PLLA nanofibers (if needed in the future) to construct a tissue scaffold. We used three nanofiber films of 3000-rpm (piezo. sample), 1000-rpm (less-piezo. sample) and 300-rpm (non-piezo. sample, a negative control) for five different experimental groups (see Fig. 3a) to illustrate the effect of surface charge on enhanced osteogenesis. After the US treatment, we performed common osteogenic assessments in vitro which include (1) an alkaline phosphatase (ALP) assay to assess enzyme activity for bone formation, (2) an Alizarin red assay to quantify mineral formation, and (3) qPCR (quantitative polymerase chain reaction) to quantify expression of osteogenic genes (i.e. osteocalcin and osterix) from the cultured stem cells [52–54]. As seen in Figs. 3b–3e , all assays show that the groups with piezoelectric scaffolds and ultrasound (US), including (3000-rpm PLLA + US) and (1000-rpm PLLA + US), have significantly higher expressions of ALP, mineral formation, and osteogenic genes than the other three control/sham groups. In addition, the piezo. film + US (which exhibits more piezoelectric charge) shows higher expression in all three assays when compared to the 1ess-piezo. film + US (with less charge), clearly illustrating that more surface charge generates more osteogenesis. By comparing the groups of non-piezo. PLLA + US and non-piezo. PLLA – US in Figs. 3c–3e, the applied ultrasound (US) seems to enhance the osteogenic performances, which agrees with previously-reported works [49, 55, 56]. In addition, the group of piezo. PLLA – US with greater fiber alignment exhibits more osteogenesis than the group of non-piezo. PLLA – US with less fiber alignment (see Fig. 2a for fiber alignment). This result is also consistent with previous reports on the effect of fiber alignment on bone regeneration[57–59]. Despite such influences of the US and fiber alignment alone, the piezoelectric surface-charge still plays a major and dominant role to stimulate osteogenesis, which is clearly demonstrated by the superior signals of ALP, mineral formation and osteogenic-gene expression in the group of piezo. PLLA + US, when compared to the other control/sham groups of piezo. PLLA – US, non-piezo. PLLA + US, and non-piezo. PLLA – US; the osteogenesis is significantly enhanced only when we combine the piezoelectric PLLA nanofiber film with applied US to generate the piezoelectric charge[23, 60, 61]. Besides the fiber alignment, other properties of the PLLA film such as pore/fiber size might also play a role on the stem cells’ behaviors and osteogenesis. In our studies, SEM images (Fig. 2a) show a similar fiber size and porosity between the PLLA nanofiber samples. The pore sizes are in the range of hundred nanometers, which is not significant for cell infiltration and thus, we anticipate a minimal effect of the factor on the osteogenesis[62]. Yet, regardless of the variation of pore/fiber size, our experiment including control/sham groups clearly demonstrates the significance of piezoelectric charge on enhanced osteogenesis.
Figure 3. Osteogenic differentiation of stem cells, grown on the piezoelectric PLLA nanofiber scaffold under applied US in vitro.
a. A simple schematic demonstrates our setup for seeding adipose stem cells (ADCSs) onto the scaffolds and treating them with US. The applied US induces controllable acoustic pressure, allowing the PLLA nanofiber films to generate electrical surface charge, which enhances osteogenesis from cultured cells. b. A quantitative comparison of ALP production by the cells in different treatment groups. c Quantification of mineral formation levels from cells measured with an alizarin red assay. d and e are qPCR results illustrating expression levels of osteocalcin and osterix genes for different treatment groups, respectively. We used the two tailed t-test for statistical analysis. In the figure, * represents a significance level of 0.01, ** represents a significance level of 0.001 and *** represents a significance level of 0.0001.
In addition to ADSCs, we also confirmed the ability of our piezoelectric nanofiber scaffold under applied US to induce osteogenesis from bone marrow stem cells (BMSCs) with reporter genes in vitro, as seen in Fig. 4. The fluorescent expression of primary bone marrow stem cells (BMSCs) with reporter genes for bone sialoprotein (BSP-GFP-topaz, green) and dentin matrix protein (DMP1-RFP-mCherry, red) was used to assess the progressive differentiation of the BMSCs. These reporter stem-cells, reported in our previous works[41–43], were seeded onto our electrospun scaffolds (same groups as for the ADSC cultures). Fig. 4a demonstrates schematically the progressive expression from BSP to DMP for the BMSCs that undergo osteogenic differentiation and changes in fluorescence that accompany this transition. As seen in Fig. 4b, the groups of piezo. PLLA + US and less piezo. PLLA + US have much higher expressions of green and red colors than the other three groups indicating higher expressions of BSP and DMP (i.e. more osteogenic differentiation). In addition, the group of piezo. PLLA + US (with more surface charge) has significantly higher expressions of green and red colors, compared to the group of less piezo. PLLA + US (with less surface charge). The expressions of green (BSP or bone sialoprotein expression) and red (DMP or dentin matrix protein expression) color was also quantified using ImageJ (Fig. 4c and 4d). The groups of (piezo. PLLA + US) and (less piezo. PLLA + US) show significantly higher levels of both BSP and DMP expression when compared to the other groups. Once again, the group of piezoelectric PLLA + US has a significantly higher expression of both genes as compared to the group of less-piezoelectric PLLA + US. It can be clearly seen that the reporter genes (representing osteogenesis of the BMSC) are turned on significantly when the cells are cultured on the piezoelectric PLLA nanofiber scaffolds under applied US, compared to the other control/sham samples. Thus, consistent with the results for ADSCs in Fig. 3, these clearly illustrate the effect of piezoelectric charge (only generated by the piezoelectric PLLA nanofibers and applied US) on enhanced osteogenic differentiation of stem cells.
Figure 4. Osteogenic activity of reporter bone marrow stem cells (BMSCs) when grown on the electrospun PLLA scaffolds with US treatment.
a. Schematic to demonstrate the progressive expression from BSP to DMP and changes in fluorescence that accompany this transition. b. Fluorescent microscope images of the cells seeded on different scaffolds after being subjected to different treatments as observed under the GFP channel (exposure time 100 ms) and cherry red channel (exposure time 1000 ms). c. and d. represent the fluorescent expressions quantitively and provide a graphical comparison between each group. We used the two tailed t-test for statistical analysis. In the figure, * represents a significance level of 0.01, ** represents a significance level of 0.001 and *** represents a significance level of 0.0001.
To further demonstrate the osteo-inductive property of the surface charge produced by our biodegradable piezoelectric nanofibers, we performed an in vivo proof of concept experiment, as seen in Figure 5. We designed an experiment to implant the PLLA piezoelectric nanofibers into bone defects of mice with four different groups (n=5 animals per group). The first group (group #1) received the piezoelectric PLLA nanofibers with applied US (experimental group). The second group (group #2) received the non-piezoelectric PLLA nanofibers with applied US (sham). The third group (group #3) received the piezoelectric PLLA nanofibers without applied US (sham) and the fourth group (group #4) received the non-piezoelectric PLLA nanofibers without applied US (negative control). We created a critical size defect of 3.5 mm diameter on the calvaria bone of mice. We placed our material on the bone so that it covered the defect and sutured the skin shut on top of the material (Figures 5a and b). The US (40 KHz, 30 minutes/day for 20 days with a break of 2 days after every 5 days) was applied at the site of implantation for mice in groups 1 and 2. We sacrificed the animals 6 weeks after surgery to collect the calvaria bone for assessment. We performed X-ray imaging of the entire defect (Fig. S2) and histological staining on sections of the collected samples to assess bone growth. We also assessed cell distribution and tissue morphology to analyze the formation of bone and migration of cells into the defect for each mouse. Fig. 5c describes representative histological images of bone tissue slices which were achieved from the four experimental groups. Optical images of histology slides were used to assess the bone formation in the defect. As seen in Fig. 5c (i), the mice that received piezoelectric PLLA nanofiber films and US (group 1) had much more new-bone formation (between yellow arrows) than the ones from other groups which either received no ultrasound or non-piezoelectric PLLA nanofibers (i.e. groups 2–4). We used nuclear fast red ALP staining to visualize the amount of ALP positive cells in the defect. The results (Fig. 5c (ii)) show a greater amount of ALP positive cells in the defect area of mice in group 1 (with piezoelectric films and US) than mice in the other groups (with either no applied US or non-piezoelectric films). As described in the experimental section, the mice used were transgenic with Collagen 3.6-GFP-topaz fluorescent reporter genes. The Collagen 3.6 gene is commonly expressed (with green color) in mature osteoblasts (derived from the host stem cells), and increased expression of this particular gene indicates increased osteoblast activity in a region[63, 64]. Therefore, the green Collagen 3.6 fluorescent signals allow us to visualize the number of osteoblast-like cells at the defect site. Fig. 5c (iii) clearly shows mice that received the piezoelectric films and US (group 1) have a higher amount of green fluorescence in the defect than the other ones from groups 2–4 which receive either no US or non-piezoelectric PLLA nanofibers. This clearly illustrates the effect of surface charge inducing bone cell migration on the piezoelectric PLLA films. Lastly, toluidine blue staining was used to visualize the formation of bone in the defect. As seen in Fig. 5c (iv), the animals that received the piezoelectric scaffolds (group 1) have much greater amounts of bone formation than the animals of other control/sham groups (i.e. groups 2–4). Representative X-ray images of entire skull bones of the mice are also provided in Figure S2, showing clearly the group 1 with much new bone formation, superior to the other groups.
Figure 5. Representative histology sections of the mouse calvarial bone showing details of bone formation and cell migration into the defects for the groups of piezo-scaffold (3000 rpm) and non-piezo. scaffold (300 rpm) under the same application of US.
a is a simplified schematic of our in vivo experiment. b shows the sequence of the different steps involved in the surgery. We created a critical sized calvarial defect in mice and implanted our nanofiber film into it to observe bone formation on the film. This is followed by treating the animals with US at the site of implantation to mimic the in-vitro system. The experiment was performed at n=5 animals per group. c.i. Aperture contrast images comparing the mineral formation in the defect between the four animal groups. Yellow arrows indicate the new bone formation, clearly seen in the first group while it cannot be seen in the other groups. c.ii. Fluorescent images comparing the presence of ALP producing cells in the defect between the four groups using vector blue ALP staining. c.iii. Microscopic fluorescent images comparing the migration of Collagen 3.6 gene positive cells in the defect between the four groups. c.iv. Microscopic images comparing the bone formation in the defect between the four groups using Toludene blue staining. All scale bars are 1mm.
In brief, the in vivo results clearly illustrate that the group 1 with the piezoelectric scaffold and ultrasound (US) strongly induce mineral/bone formation, ALP release and osteoblast migration. These results reinforce the excellent performance of the piezoelectric biodegradable PLLA nanofibers and the regenerative effect of surface charge, which is only generated by the PLLA nanofibers under applied US.
Conclusions
We have presented a novel tissue electrical-stimulation approach, using the biodegradable piezoelectric PLLA nanofiber scaffold with non-invasive US to generate controllable surface charges, consequently enhancing osteogenesis and bone regeneration. The PLLA nanofibers offer an ECM (extracellular matrix) like environment which not only promotes cell growth/differentiation but also generates direct electrical-stimulation under remotely-applied acoustic wave. The piezoelectric nanofibers can eventually biodegrade (Fig. S4) to avoid undesired removal operations and facilitate tissue-ingrowth, an advantage which has not be achieved by conventional piezoelectric materials[61, 65]. Thus, the nanofiber scaffold inherently serves as a powerful biodegradable battery-free, and wirelessly-controlled electrical stimulator which is not only useful for bone but potentially for the regeneration of other tissues such as nerve, cartilage, skin, muscle, etc. As PLLA is a common biodegradable and biocompatible material for tissue scaffolds, we anticipate that the nanofibers along with the non-invasive US-stimulation approach are highly translational and could be easily approved by FDA for clinical use.
Despite such a significant advantage, further studies and optimizations of the presented tissue-stimulation approach are still required. First, an optimal amount of piezoelectric charge for osteogenesis will need to be investigated by tailoring the applied US and/or the piezoelectric effect of the PLLA nanofiber film or incorporation of other piezoelectric components like ZnO (zinc oxide) which exhibits low toxicity with small quantities [66, 67]. Second, studies on the cellular signaling mechanisms of the charge-induced bone regeneration and the effect of fiber/pore size will be also needed to optimize the osteogenic effects. Third, the presented therapy could require prolonged post-op care in the form of ultrasound (US) treatments for clinical use. We can study the effect of different US-stimulation periods and enhance the material’s piezoelectric property so that a shorter stimulation time might be sufficient. Finally, ones could also combine this ES approach with stem cells and a small amount of growth- factors like Bone Morphogenic protein (below the toxic dose) or employ osteogenic metal nanoparticles (e.g. iron oxide or magnesium oxide) to further enhance bone regeneration. We plan to perform these studies in the next phase of the presented work which mainly aims to demonstrate the significant osteogenic effect of surface charge, generated by a novel biodegradable, battery-less and remotely-controlled electrical stimulator of the piezoelectric PLLA nanofiber tissue scaffold.
Supplementary Material
A biodegradable, battery-less and remotely-controlled electrical stimulator, made of PLLA piezoelectric nanofibers, serves inherently as a tissue scaffold for bone regeneration
Application of ultrasound on the cell-seeded biodegradable piezoelectric nanofibers can enhance osteogenic differentiation of adipose-derived stem cells and bone marrow derived stem cells
The reported tissue-stimulation approach, employing the PLLA nanofibers and remotely-controlled ultrasound, induces bone regeneration from a skull bone defect in mice.
Acknowledgements:
The work is supported by the NIH (Grant # 1R21AR075196). Guleid Awale is supported by NIH (supplementary grant #R21EB024787-02S1). The authors thank Allison Taylor and Jessica Hornyak for helping with the in vivo experiments and the film degradation studies respectively.
Biographies
Ritopa Das

Ritopa Das received her BS in Chemical Engineering from Jadavpur University, India and her MS in Biomedical Engineering from University of Georgia, Athens. She is currently a PhD candidate at the University of Connecticut, Biomedical Engineering. Her research focuses of tissue engineering, wound healing and piezoelectric materials.
Eli Curry

Eli J. Curry is currently pursuing his Ph.D. in Biomedical Engineering at the University of Connecticut. He also received his B.S. in Biomedical Engineering at the University of Connecticut (2016). His doctoral research is on the development of wireless, biodegradable polymer-based piezoelectric devices for use in sensing and tissue regeneration applications.
Thinh Le

Thinh T. Le received his M.S. degree of mechanical engineering from the Catholic University of America, US in 2017. He is currently pursuing Ph.D. degree in Mechanical Engineering Department, University of Connecticut. His research interest focused on implantable biodegradable ultrasonic devices, piezoelectric materials, and medical ultrasound.
Yang Liu

Yang Liu received his Ph.D degree from Peking University in 2018. He is now a postdoc researcher in the Department of Mechanical Engineering, University of Connecticut. His research interest focuses on the design and evaluation of biodegradable metals and polymers for tissue regeneration.
Guleid Awale

Guleid Awale received his B.S. degree and M.S. degree in Chemical Engineering from the University of Connecticut in 2014 and 2019 respectively. He is currently a Ph.D. candidate in Chemical Engineering at the University of Connecticut under the supervision of Dr. Cato Laurencin. His research interests include osteoinductive small molecule delivery and biomaterials for bone regenerative engineering.
Shun Yi Li

Shunyi Li received her B.S.E. degree in Biomedical Engineering with a Materials Science and Engineering minor from the University of Connecticut in 2020. Currently, she is entering the medical device industry as an engineer. Previously, she worked as a research assistant in Professor Thanh Nguyen’s laboratory.
Joemart Contreras

Joemart Ian Contreras has a degree in biomedical engineering with a concentration in bioinstrumentation from the University of Connecticut. He is currently pursuing a master’s degree in biomedical engineering from the University of Connecticut in pursuit of a future career in clinical engineering and biomedical devices.
Casey Bednarz

Casey E. Bednarz received her B.S. degree in Physiology and Neurobiology from the University of Connecticut in 2019. She is currently working full time and planning to go back and to school to pursue a masters degree.
Jayla Millender

Jayla Millender is an honors undergraduate student at the University of Connecticut pursuing degrees in Africana Studies and Molecular and Cellular Biology. Her research focuses on the development of synthetic bone grafts using biodegradable materials.
Xiaonan Xin

Xiaonan Xin, MD, Ph.D, Currently, an Assistant Research Professor at University of Connecticut Health. She has worked in the field of skeletal biology and regeneration. In her studies, she has established mouse calvarial bone defect model to test the osteogenic potential of implanted materials or cells/material constructs in mouse in vivo. Her established cryo-immuno-histological analysis methods have achieved high quality evaluation on the new bone formation in the defects. For this current study, she was involved on mice surgeries and Cryo-immuno-histological analysis and data interpretation.
Dr. Emadi

Dr. Sharareh Emadi finished her Ph.D. at the Pasteur Institute in France, specializing in Cardiovascular Pharmacology. She is currently an Assistant Professor-In-Residence in the department of Biomedical Engineering at the University of Connecticut. Her current focus is on molecular, biochemical, and cell biology with emphasis on the application and development of antibody phage display, protein engineering, and cell-based assay.
Dr. Lo

Dr. Kevin Lo is an Assistant Professor of Medicine at UConn Health. He has held editorial positions on several prestigious journals including PLoS ONE. His research programs include musculoskeletal regeneration using inductive small molecules and osteotropic nanoscale drug delivery systems. He has authored more than 45 publications in these areas. Research grants from NIH, NSF, State of Connecticut, and private foundation have supported his work in the institute. Dr. Lo is very active in community engagements. He has organized many seminar series programs which aim to bring science and healthcare concepts to the local underserved community groups in Connecticut.
Dr. Rowe

David Rowe received his MD from the University of Vermont. Currently he is a Professor of Reconstructive Sciences at UConn Health and the Director of the Center for Regenerative Medicine and Skeletal Development. His lab works in the field of skeletal biology and regeneration and has established a mouse calvarial bone defect model to test the osteogenic potential of implanted materials or cells/material constructs in mouse in vivo.
Dr. Nguyen

Thanh Nguyen received his PhD from Princeton University (2013) and completed his postdoctoral training from Massachusetts Institute of Technology (MIT, 2015). Currently, he is an Assistant Professor of Mechanical Engineering and Biomedical Engineering at the University of Connecticut. Nguyen lab’s research is highly interdisciplinary and at the interface of nano-/micro-technology, medicine and biomaterials. He received several awards including the ACell Young Investigator Award (2020), MIT top innovator under 35 for Asia Pacific (2019), NIH Trailblazer Award for Young and Early Investigators (2018), SPIE Rising Researcher Award (2019) etc.
Footnotes
Competing interests: Authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Statement:
The experimental data, presented herein, are available for sharing upon a reasonable request.
References
- [1].Amini AR, Laurencin CT, Nukavarapu SP, Critical reviews in biomedical engineering, 40 (2012) 363–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bauer TW, Muschler GF, Clinical Orthopaedics and Related Research®, 371 (2000) 10–27. [PubMed] [Google Scholar]
- [3].Laurencin C, Khan Y, El-Amin SF, Expert review of medical devices, 3 (2006) 49–57. [DOI] [PubMed] [Google Scholar]
- [4].Laurencin CT, Ambrosio A, Borden M, Cooper J Jr, Annual review of biomedical engineering, 1 (1999) 19–46. [DOI] [PubMed] [Google Scholar]
- [5].Laurencin CT, Khan Y, Regenerative engineering, American Association for the Advancement of Science, 2012. [Google Scholar]
- [6].Narayanan G, Vernekar VN, Kuyinu EL, Laurencin CT, Advanced drug delivery reviews, 107 (2016) 247–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Martinelli NM, Ribeiro MJG, Ricci R, Marques MA, Lobo AO, Marciano FR, Materials (Basel, Switzerland), 11 (2018) 1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Nasri-Nasrabadi B, Kaynak A, Heidarian P, Komeily‐Nia Z, Mehrasa M, Salehi H, Kouzani A, Sodium alginate/magnesium oxide nanocomposite scaffolds for bone tissue engineering, 2018. [Google Scholar]
- [9].Wang L, Li C, Chen Y, Dong S, Chen X, Zhou Y, BioMed Research International, 2013 (2013) 13. [Google Scholar]
- [10].Akai M, Hayashi K, Bioelectromagnetics, 23 (2002) 132–143. [DOI] [PubMed] [Google Scholar]
- [11].Duarte LR, Archives of orthopaedic and traumatic surgery, 101 (1983) 153–159. [DOI] [PubMed] [Google Scholar]
- [12].Somjen D, Binderman I, Berger E, Harell A, Biochimica et Biophysica Acta (BBA)-General Subjects, 627 (1980) 91–100. [DOI] [PubMed] [Google Scholar]
- [13].Anglen J, Journal of the Southern Orthopaedic Association, 12 (2003) 46–54. [PubMed] [Google Scholar]
- [14].Cohen M, Roman A, Lovins J, The Journal of foot and ankle surgery: official publication of the American College of Foot and Ankle Surgeons, 32 (1993) 375–381. [PubMed] [Google Scholar]
- [15].Cerrolaza M, Duarte V, Garzón-Alvarado D, Journal of Bionic Engineering, 14 (2017) 659–671. [Google Scholar]
- [16].Shamos MH, LAVINE LS, SHAMOS MI, Nature, 197 (1963) 81–81. [DOI] [PubMed] [Google Scholar]
- [17].Yu P, Ning C, Zhang Y, Tan G, Lin Z, Liu S, Wang X, Yang H, Li K, Yi X, Zhu Y, Mao C, Theranostics, 7 (2017) 3387–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Demiray H, Electromechanical remodelling of bones, 1983. [Google Scholar]
- [19].Hou Z, Fu D, Qin Q-H, International Journal of Solids and Structures, 48 (2011) 603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Callegari B, Belangero W, Acta Ortop. Bras, 12 (2004) 160–166. [Google Scholar]
- [21].Nakamura M, Nagai A, Tanaka Y, Sekijima Y, Yamashita K, Journal of Biomedical Materials Research Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials, 92 (2010) 783–790. [DOI] [PubMed] [Google Scholar]
- [22].Kitsara M, Blanquer A, Murillo G, Humblot V, De Braganca Vieira S, Nogues C, Ibanez E, Esteve J, Barrios L, Nanoscale, 11 (2019) 8906–8917. [DOI] [PubMed] [Google Scholar]
- [23].Szewczyk PK, Metwally S, Karbowniczek JE, Marzec MM, Stodolak-Zych E, Gruszczyński A, Bernasik A, Stachewicz U, ACS Biomaterials Science & Engineering, 5 (2019) 582–593. [DOI] [PubMed] [Google Scholar]
- [24].Huang X, Das R, Patel A, Nguyen TD, Regenerative Engineering and Translational Medicine, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Abzan N, Kharaziha M, Labbaf S, Materials & Design, 167 (2019) 107636. [Google Scholar]
- [26].Rajabi AH, Jaffe M, Arinzeh TL, Acta Biomaterialia, 24 (2015) 12–23. [DOI] [PubMed] [Google Scholar]
- [27].Ribeiro C, Sencadas V, Correia DM, Lanceros-Méndez S, Colloids and Surfaces B: Biointerfaces, 136 (2015) 46–55. [DOI] [PubMed] [Google Scholar]
- [28].Guerin S, Stapleton A, Chovan D, Mouras R, Gleeson M, McKeown C, Noor MR, Silien C, Rhen FMF, Kholkin Andrei L. Liu N., Soulimane T., Tofail SAM, Thompson D., Nature Materials, 17 (2017) 180. [DOI] [PubMed] [Google Scholar]
- [29].Guerin S, Tofail SAM, Thompson D, NPG Asia Materials, 11 (2019) 10. [Google Scholar]
- [30].Lemanov VV, Popov SN, Pankova GA, Physics of the Solid State, 53 (2011) 1191–1193. [Google Scholar]
- [31].Curry EJ, Ke K, Chorsi MT, Wrobel KS, Miller AN, Patel A, Kim I, Feng J, Yue L, Wu Q, Proceedings of the National Academy of Sciences, 115 (2018) 909–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Curry EJ, Le TT, Das R, Ke K, Santorella EM, Paul D, Chorsi MT, Tran KTM, Baroody J, Borges ER, Ko B, Golabchi A, Xin X, Rowe D, Yue L, Feng J, Morales-Acosta MD, Wu Q, Chen IP, Cui XT, Pachter J, Nguyen TD, Proceedings of the National Academy of Sciences, 117 (2020) 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Holzwarth JM, Ma PX, Biomaterials, 32 (2011) 9622–9629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Prabhakaran MP, Venugopal J, Ramakrishna S, Acta biomaterialia, 5 (2009) 2884–2893. [DOI] [PubMed] [Google Scholar]
- [35].Eap S, Ferrand A, Mendoza Palomares C, Hébraud A, Stoltz J-F, Mainard D, Schlatter G, Benkirane-Jessel N, Bio-medical materials and engineering, 22 (2012) 137–141. [DOI] [PubMed] [Google Scholar]
- [36].Gupta KC, Haider A, Choi Y-R, Kang I-K, Biomaterials research, 18 (2014) 5–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kumar V, Naqvi S, Gopinath P, Chapter 7 - Applications of Nanofibers in Tissue Engineering, in: Mohan Bhagyaraj S, Oluwafemi OS, Kalarikkal N, Thomas S (Eds.) Applications of Nanomaterials, Woodhead Publishing; 2018, pp. 179–203. [Google Scholar]
- [38].Ikada Y, Shikinami Y, Hara Y, Tagawa M, Fukada E, Journal of Biomedical Materials Research: An Official Journal of The Society for Biomaterials and The Japanese Society for Biomaterials, 30 (1996) 553–558. [DOI] [PubMed] [Google Scholar]
- [39].Cundy P, Paterson D, Clinical orthopaedics and related research, (1990) 216–222. [PubMed] [Google Scholar]
- [40].Paterson D, Lewis G, Cass C, Clinical orthopaedics and related research, (1980) 117–128. [PubMed] [Google Scholar]
- [41].Clearfield DS, Xin X, Yadav S, Rowe DW, Wei M, Tissue engineering. Part A, (2018). [DOI] [PubMed] [Google Scholar]
- [42].Fu Y, Maye P, Genesis (New York, N.Y. : 2000), 53 (2015) 294–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Maye P, Stover ML, Liu Y, Rowe DW, Gong S, Lichtler AC, BMC Biotechnology, 9 (2009) 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Xin X, Jiang X, Wang L, Stover ML, Zhan S, Huang J, Goldberg AJ, Liu Y, Kuhn L, Reichenberger EJ, Stem cells translational medicine, 3 (2014) 1125–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Fiorillo A, Grimaldi D, Paolino D, Pullano S, Low-Frequency Ultrasound in Medicine: An In Vivo Evaluation, 2012. [Google Scholar]
- [46].Miller DL, Smith NB, Bailey MR, Czarnota GJ, Hynynen K, Makin IRS, Bioeffects M Committee of the American Institute of Ultrasound in, Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine, 31 (2012) 623–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Della Rocca GJ, Indian journal of orthopaedics, 43 (2009) 121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Mayr E, Frankel V, Rüter A, Archives of orthopaedic and trauma surgery, 120 (2000) 1–8. [DOI] [PubMed] [Google Scholar]
- [49].Watanabe Y, Matsushita T, Bhandari M, Zdero R, Schemitsch EH, J Orthop Trauma, 24 Suppl 1 (2010) S56–61. [DOI] [PubMed] [Google Scholar]
- [50].Raposio E, Bonomini S, Calderazzi F, Orthopaedics & Traumatology: Surgery & Research, 102 (2016) 909–912. [DOI] [PubMed] [Google Scholar]
- [51].Ciuffi S, Zonefrati R, Brandi ML, Clinical Cases in Mineral and Bone Metabolism, 14 (2017) 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Gregory CA, Grady Gunn W, Peister A, Prockop DJ, Analytical Biochemistry, 329 (2004) 77–84. [DOI] [PubMed] [Google Scholar]
- [53].Hoemann C, El-Gabalawy H, McKee M, Pathologie Biologie, 57 (2009) 318–323. [DOI] [PubMed] [Google Scholar]
- [54].Krause U, Seckinger A, Gregory CA, Assays of osteogenic differentiation by cultured human mesenchymal stem cells, Mesenchymal Stem Cell Assays and Applications, Springer; 2011, pp. 215–230. [DOI] [PubMed] [Google Scholar]
- [55].Claes L, Willie B, Progress in Biophysics and Molecular Biology, 93 (2007) 384–398. [DOI] [PubMed] [Google Scholar]
- [56].Pomini KT, Andreo JC, de C. Rodrigues A, de O. Gonçalves JB, Daré LR, German IJ, Rosa GM Jr, Buchaim RL, Journal of Ultrasound in Medicine, 33 (2014) 713–717. [DOI] [PubMed] [Google Scholar]
- [57].Tissue Engineering Part A, 20 (2014) 2031–2042. [DOI] [PubMed] [Google Scholar]
- [58].Kharaziha M, Fathi MH, Edris H, Journal of the Mechanical Behavior of Biomedical Materials, 24 (2013) 9–20. [DOI] [PubMed] [Google Scholar]
- [59].Stachewicz U, Qiao T, Rawlinson SCF, Almeida FV, Li W-Q, Cattell M, Barber AH, Acta Biomaterialia, 27 (2015) 88–100. [DOI] [PubMed] [Google Scholar]
- [60].Arinzeh TL, Moy J, Huang GP, American Scientist, 105 (2017) 298+. [Google Scholar]
- [61].Damaraju SM, Shen Y, Elele E, Khusid B, Eshghinejad A, Li J, Jaffe M, Arinzeh TL, Biomaterials, 149 (2017) 51–62. [DOI] [PubMed] [Google Scholar]
- [62].Aghajanpoor M, Hashemi‐Najafabadi S, Baghaban‐Eslaminejad M, Bagheri F, Mohammad Mousavi S, Azam Sayyahpour F, Journal of Biomedical Materials Research Part A, 105 (2017) 1887–1899. [DOI] [PubMed] [Google Scholar]
- [63].Cremers S, Garnero P, Seibel MJ, Chapter 87 - Biochemical Markers of Bone Metabolism, in: Bilezikian JP, Raisz LG, Martin TJ (Eds.) Principles of Bone Biology (Third Edition), Academic Press, San Diego, 2008, pp. 1857–1881. [Google Scholar]
- [64].Gordon JA, Tye CE, Sampaio AV, Underhill TM, Hunter GK, Goldberg HA, Bone, 41 (2007) 462–473. [DOI] [PubMed] [Google Scholar]
- [65].Jacob J, More N, Kalia K, Kapusetti G, Inflammation and regeneration, 38 (2018) 2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Zhang Y, R Nayak T, Hong H, Cai W, Current molecular medicine, 13 (2013) 1633–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Zhang ZY, Xu YD, Ma YY, Qiu LL, Wang Y, Kong JL, Xiong HM, Angewandte Chemie International Edition, 52 (2013) 4127–4131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.