Abstract
Background
Glutamic acid decarboxylase (GAD) is the rate-limiting enzyme for the synthesis of gamma-aminobutyric acid (GABA), the major inhibitory neurotransmitter in the central nervous system. Antibodies against glutamic acid decarboxylase (GAD) are associated with various neurologic conditions described in patients, including stiff person syndrome, cerebellar ataxia, refractory epilepsy, and limbic and extra limbic encephalitis. While there are few case reports and research on anti-GAD65 antibody-associated encephalitis in adults, such cases are extremely rare in pediatric cases.
Methods
For the first time, we report a case of anti-GAD65-positive autoimmune encephalitis associated with autoimmune polyendocrine syndrome (APS) type II. We reviewed previously published pediatric cases of anti-GAD65 autoimmune encephalitis to discuss their clinical features, laboratory tests, imaging findings, EEG patterns, and prognosis.
Case presentation
An 8-year-old, male child presented to the outpatient department after experiencing generalized convulsions for twenty days. The child was admitted for epilepsy and had received oral sodium valproate (500 mg/day) in another center, where investigations such as USG abdomen and MRI brain revealed no abnormalities, however, had abnormal EEG with diffuse mixed activity in the left anterior middle prefrontal temporal region. On the follow-up day, a repeat blood test showed a very low serum drug concentration of sodium valproate hence the dose was increased to 750 mg/day. Then, the child experienced adverse effects including increased sleep, thirst, and poor appetite, prompting the parents to discontinue the medication. A repeat MRI showed increased signals on FLAIR sequences in the right hippocampus hence admitted for further management. The child's past history included a diagnosis of hypothyroidism at the age of 4, and receiving levothyroxine 75 mcg once daily. His parents are healthy with no history of any similar neurological, autoimmune, or genetic diseases, but his uncle had a history of epilepsy. At presentation, he had uncontrolled blood glucose levels with elevated HbA1c levels. Additionally, the serum and CSF autoantibodies were positive against the anti-GAD65 antibody with the titer of 1:100 and 1:32 respectively. The patient was managed with a mixed type of insulin regimen and received first-line immunotherapy (intravenous immunoglobulin, IVIG) for five consecutive days, followed by oral prednisone and sodium valproate as an antiepileptic drug. Upon achieving a favorable clinical outcome, the patient was discharged with oral medications.
Results
Among the 15 pediatric patients reported in this literature, nine presented with limbic encephalitis (LE), three with extralimbic encephalitis (ELE), and three with a combination of limbic and extralimbic encephalitis. Most of these cases exhibited T2-W FLAIR hyperintensities primarily localized to the temporal lobes in the early phase, progressing to hippocampal sclerosis/atrophy in the later phase on MRI. EEG commonly showed slow or spike waves on frontotemporal lobes with epileptic discharges. Prognostic factors varied among patients, with some experiencing persistent refractory seizures, type-1 diabetes mellitus (T1DM), persistent memory impairment, persistent disability requiring full assistance, and, in severe cases, death.
Conclusion
Our findings suggest that anti-GAD65 antibody-positive autoimmune encephalitis patients may concurrently present with other APS. Our unique case presented with multiple endocrine syndromes and represents the first reported occurrence in children. Early diagnosis and timely initiation of immunotherapy are crucial for improving clinical symptoms and reducing the likelihood of relapses or permanent disabilities. Therefore, emphasis should be placed on prompt diagnosis and appropriate treatment implementation to achieve better patient outcomes.
Keywords: anti GAD65 antibody, T1DM (type 1 diabetes mellitus), autoimmune thyroiditis, pediatric, autoimmune polyendocrine syndrome (APS type 2), abnormal (behavior), autoimmune encephalitis
Introduction
Autoimmune encephalitis is a neurological disorder, characterized by confusion, memory disturbances, and often seizures (1). Anti-GAD65 encephalitis is subtype of autoimmune encephalitis. Reported cases of anti-GAD65 encephalitis in pediatric patients have been associated with a single endocrine syndrome whereas our case is a novel case of anti-GAD65 encephalitis in a pediatric patient which concurrently involve with multiple endocrine syndromes (T1DM and autoimmune thyroiditis). This particular case is the rare combination that has been observed in a young patient and the first case of its kind to have ever been reported.
To the best of our knowledge, only a single type of autoimmune endocrine illness has been associated with anti GAD65 in pediatrics in previously published papers. This work has so far concentrated on autoimmune encephalitis with anti-GAD65 and multiple autoimmune endocrine disorders. Additionally, we aimed to raise awareness of anti-GAD-65-mediated autoimmune encephalitis, which can appear in conjunction with a number of endocrine disorders. Anti-GAD-65 antibody testing should be taken into consideration to individuals who exhibits any symptoms suggestive of autoimmune encephalitis, and strict monitoring for the emergence of T1DM and other autoimmune endocrine illnesses is advisable. Early recognition and timely management of such cases may improve patient outcomes and help researchers better understand the intricate relationships between the nervous and endocrine systems in autoimmune encephalitis.
Case presentation
An 8-year-old, male child presented to the outpatient department after experiencing generalized convulsions for twenty days. The child was admitted for epilepsy and had received oral sodium valproate (500 mg/day) in another center, where investigations such as USG abdomen and MRI brain revealed no abnormalities. However, VEEG (video electroencephalogram) indicated abnormal EEG with diffuse mixed activity (ranging from low to high amplitude sharp waves, spicy slow waves/sharp slow waves) of 1.5-5.5 Hz in the left anterior middle prefrontal temporal region. On the follow-up day, a repeat blood test showed a very low serum drug concentration of sodium valproate hence the dose was increased to 750 mg/day. However, the child experienced adverse effects including increased sleep, thirst, and poor appetite, prompting the parents to discontinue the medication. A repeat MRI showed increased signals on FLAIR sequences in the right hippocampus (Figure 1A) hence admitted for further management.
Figure 1.
(A) showing an increased signal on the FLAIR sequence of the right hippocampus. (B) showing the new inflammatory lesion on the left hippocampal. In addition, it demonstrates that the original right hippocampal signal was decreased with compared to the old film.
The child's past history included a diagnosis of hypothyroidism at the age of 4, and receiving levothyroxine 75 mcg once daily. His parents are healthy with no history of any similar neurological, autoimmune, or genetic diseases, but his uncle had a history of epilepsy.
Diagnosis and treatment
Our patient exhibited various clinical and laboratory abnormalities leading to the diagnosis of anti-GAD65 autoimmune encephalitis associated with APS type-II. Initial hematological tests showed normal results except for hyperglycemia (20.72 mmol/L) and increased glycosylated hemoglobin (HbA1c) to 9.2%, indicating uncontrolled T1DM. Urine analysis revealed the presence of ketone bodies (3+) and glucose (3+), along with elevated levels of glycosylated serum protein and D3 hydroxybutyric acid, confirming diabetic ketoacidosis.
Further investigations showed low fasting C-peptide levels, and elevated anti-insulin and anti-islet cell antibodies, indicating a deficiency in pancreatic beta cell insulin production, leading to hypoinsulinemia, and diagnosing autoimmune-mediated T1DM. In order to control blood sugar levels, the patient was put on an insulin regimen. The maximum blood sugar recorded was 28.3 mmol/L, and to achieve a normal blood glucose level, the dose of the mixed type of insulin regimen (long/ short-acting) was adjusted accordingly.
Cerebrospinal fluid (CSF) analysis demonstrated elevated glucose levels and increased CSF IgG (133 mg/L) with the presence of oligoclonal bands in CSF, confirming autoimmune brain disease. Other CSF analysis revealed no pleocytosis with normal CSF and serum albumin (116.70mg/L and 43.70g/L). The albumin quotient value of 2.67x10-3 (Normal range- 0-9x 10-3) which was calculated by (QAlb = CSF albumin/serum albumin) (2), indicating normal the blood-CSF barrier function. Rheumatological tests revealed positive results for antinuclear antibody (ANA), anti-SSA-52 antibody, further supporting the autoimmune nature of the disorder.
Increase level of serum GAD antibody (160.92 IU/mL/ Normal value- 0-10 IU/mL) indicating the presence of anti-GAD antibody. Furthermore, the anti-GAD65 antibody titer was detected using indirect immunofluorescence cell-based assays (CBA) in both serum and CSF with titers of 1:100 and 1:30 respectively. The brain tissue slices detected by tissue-based assay (TBA) showed positive results (Table 1; Figure 2) in both serum and CSF. First MRI of the brain revealed increased signals in the right hippocampus, suggesting brain involvement (Figure 1A).
Table 1.
Autoimmune encephalitis panel.
| Test | Detection Method | Results | |
|---|---|---|---|
| Serum | CSF | ||
| NMDA- IgG AMPA1-IgG AMPA2-IgG LGI-1-IgG CASPR2-IgG GlyR1-IgG GABA-A GABA-B-IgG IgLON5-IgG DPPX-IgG DRD2- IgG GAD65-IgG mGluR1-IgG mGluR5-IgG Neurexin-3 α Ganglionic AchR KLHL11 GluK2 AK5 AG0 CaVα2δ AQP4 MOG GFAP TBA detection of Brain tissue slices |
CBA CBA CBA CBA CBA CBA CBA CBA CBA CBA CBA CBA CBA CBA CBA CBA CBA CBA CBA CBA CBA CBA CBA CBA TBA |
Negative Negative Negative Negative Negative Negative Negative Negative Negative Negative Negative Positive (1:100) Negative Negative Negative Negative Negative Negative Negative Negative Negative Negative Negative Negative Positive |
Negative Negative Negative Negative Negative Negative Negative Negative Negative Negative Negative Positive (1:30) Negative Negative Negative Negative Negative Negative Negative Negative Negative Negative Negative Negative Positive |
The 24 autoimmune encephalitis panels in both serum and cerebrospinal fluid (CSF).
The results were detected by indirect immunofluorescence cell-based assay (CBA) indicating that the anti-GAD65 antibodies were positive in both serum and CSF with titers of 1:100 and 1:30 respectively. The brain tissue slices detected by tissue-based assay (TBA) were positive.
Figure 2.
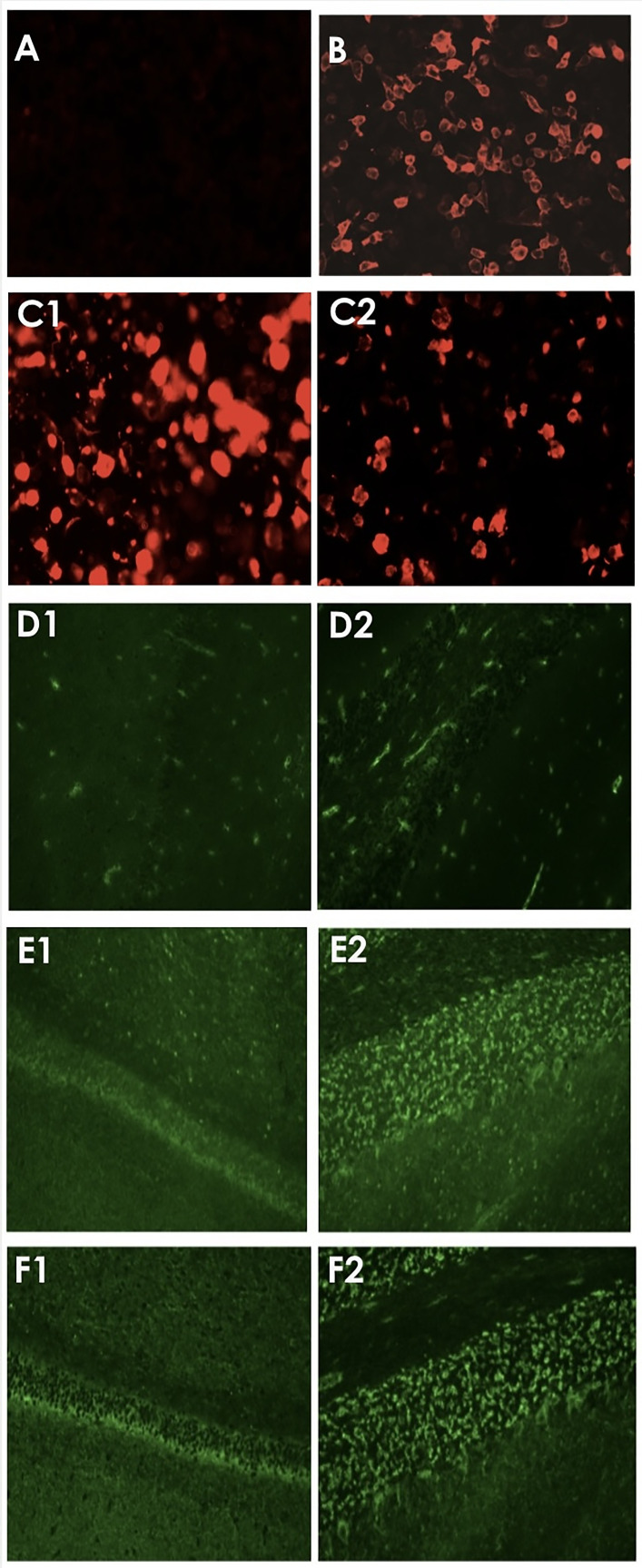
Test results of serum and CSF using indirect immunofluorescence assays. (A, B) showed negative and positive controls of CBA technique respectively. Red fluorescent light showing positive results detected by CBA in serum (C1) and CSF (C2). (D1, D2) illustrated the negative controls detected by TBA method in hippocampus and cerebellum respectively. While (E1, E2) in green fluorescent light showing the positive TBA in hippocampus and cerebellum tissue respectively by using serum, and (F1, F2) showing positive results of TBA by using CSF.
The patient's thyroid function test showed low free T3 and high thyroid-stimulating hormone (TSH), along with elevated anti-thyroid peroxidase(198 IU/mL) and anti-thyroglobulin antibodies (>4000 IU/mL) in serum, confirming autoimmune thyroiditis. After confirming the presence of positive anti-GAD65 antibodies, T1DM, and autoimmune thyroiditis, a diagnosis of anti-GAD65 antibody autoimmune encephalitis associated with APS type-II was made.
The results of various tests, including serum lactic acid, blood ammonia level, cardiac enzymes, T-SPOT test, parathyroid hormone level, immunoglobulin complement tests, infectious disease screening, and iron profile, did not show any significant abnormalities. However, ACTH and Cortisol levels measured in the morning and evening were within normal range. Additionally, ultrasound scans for abdominal and testicular masses, as well as serum and CSF screening for paraneoplastic conditions, yielded negative findings. The homocysteine test in combination with folic acid was normal, but vitamin B12 levels were elevated. Furthermore, both cardiac echo and plain chest CT scan results were normal, indicating the absence of any cardiovascular risks.
The patient was treated with human immunoglobulin (IVIG) (2 gm/kg/day) as the first-line immunotherapy for five consecutive days, followed by oral prednisone 45 mg/day (2 mg/kg/day). The insulin regimen was readjusted to achieve optimal blood glucose levels. The child showed improvement in sleep patterns, appetite, and seizure frequency after the treatment. A follow-up MRI showed a decrease in the inflammatory lesion on the right hippocampus and the appearance of a new inflammatory lesion on the left hippocampus. (Figure 1B).
The patient exhibited clinical improvement after 10 days of therapy, and was discharged with medications (prednisone 45 mg/day, levothyroxine 50 mcg per day, sodium valproate and mixed type insulin regimen pump) with instructions for monitoring blood glucose levels at home.
The child developed a left sided headache five months after being discharged, which was acute in nature and was followed by generalized seizures that lasted for around 30 minutes. His MRI revealed no new lesions and diminished existing lesions. VEEG showed aberrant waves of moderate to high amplitude sharp slow waves, intermittent slow waves, and intermittent left and right-synchronous or asynchronous bursts in bilateral frontal, central, parietal, and occipital regions during sleep. Furthermore, within 24 hours of the VEEG, two clinical seizures were discovered. Repeat IVIG (2 gm/kg) was given for 5 days, followed by 50 mg of prednisone, orally. To control the seizures, a fresh addition of tablet lacosamide (100 mg in the morning and 50 mg in the evening) was made and stopped sodium valproate. Nevertheless, continuing anti-thyroid drugs and insulin pump as directed. With these treatment the child was clinically recovered.
Discussion
Autoimmune polyendocrine syndrome type-II (APS II) also known as Schmidt’s syndrome, is a rare condition characterized by the co-occurrence of at least two of the following endocrine disorders: primary adrenal insufficiency (Addison’s disease), primary hypothyroidism, type 1 diabetes mellitus, celiac disease, and pernicious anemia (3, 4). APS-II is infrequent among children and any patient with Addison’s disease (AD) has a 50% lifetime risk of developing additional autoimmune disease. It is strongly recommended to conduct periodic retesting at intervals of 2-3 years, as autoantibodies have the potential to develop at any point throughout an individual’s lifespan (5).
In our case, the patient presented with positive anti-GAD65 antibody, anti-islets cells, and anti-insulin antibody along with positive anti-thyroid peroxidase antibody and anti-thyroglobulin antibody, with more than two autoimmune diseases confirmed the diagnosis of APS type-II. This represents the first-ever reported case of APS-II with anti- GAD antibody autoimmune encephalitis in the pediatric age group.
The presence of anti-GAD65 antibodies is frequently associated with T1DM, and these antibodies can be detected in a significant proportion of patients with newly-diagnosed T1DM and prediabetic individuals (6). Additionally, anti-GAD-65-mediated autoimmune encephalitis, characterized by non-paraneoplastic intracellular antigens, demonstrates pathogenicity to the central nervous system (7).
Several cases have been reported regarding anti-GAD65 antibodies in this literature (summarized in Table 2), the first case being reported in 2002 (8). In our study, we present a comprehensive analysis of 15 cases, including our own, involving patients under the age of 18. We found similarities with the work of Ren et al. (2021) (17), who reported 10 cases of anti-GAD65 antibody-associated encephalitis. Among all cases, nine exhibited features of limbic encephalitis (LE), three had extralimbic encephalitis (ELE), and three presented a combination of limbic and extralimbic encephalitis.
Table 2.
Pediatric cases of anti-GAD65 antibodies associated with encephalitis in previous studies from 2002 to 2022.
| Author/Year | Age/Sex | Clinical features | Autoimmune disorder | GAD Ab titer | OCB | Brain MRI | EEG | Immunotherapy | Prognosis | |
|---|---|---|---|---|---|---|---|---|---|---|
| Serum | CSF | |||||||||
| 1.Olson et al (8) | 6y/M (2002) |
Epilepsia partialis continua, aphasia | Type 1 Diabetes Mellitus | 19610 U/ml (normal range < 1.0 U/ml) | 3325 U/ml (normal range < 1.0 U/ml) | Not mentioned | Initially: normal Later: lesions of the gray matter involving the occipital and frontal cortex, left insular region, cerebellum |
Left-sided epileptiform discharges and slowing | High-dose steroids, IVIG, PE | Seizure free |
| 2. Akman et al (9) | 16y/Fe (2009) | Complex partial seizure and status epilepticus, academic function decline | Common variable immune deficiency (CVID) | >300 IU/ml (normal range 0–1.45) | >300 IU/ml (normal range 0–1.45) | Not mentioned | Initially: bilateral hippocampal T2 hyperintensities 1 year later: bilateral mesial temporal sclerosis | Bilateral temporal onset complex partial status epilepticus | MP, IVIG | Refractory seizures |
| 3.Korff et al (10) | 6y/Fe (2011) | Progressively refractory focal seizures, progressive global developmental delay, and gait instability. | T1DM at 3 years of age | 3400 IU/mL (Normal-<10 IU/mL) |
13 IU/m (Normal-<1IU/mL) | Positive | Initially: normal at 5 years: 3yrs Later: bilateral hippocampal, cortical, and cerebellar atrophy |
Multifocal discharges and right frontal seizures | MP, Plasma exchange, mycophenolate mofetil, rituximab | Clinical improvement but had refractory seizures |
| 4.Navin et al (11) | 15y/M (2014) | Headache, transient memory disturbance, seizures, behavior change. | None | 1:160000 (CBA) | 1:128000 (CBA) |
Positive | T2/FLAIR signal of the right hippocampus and amygdala, mildly increased signal in the left amygdala | Interictal epileptiform discharges arising independently from the right frontotemporal and left the posterior head region | IVIG, oral prednisone, RTX | Excellent seizure control; improvement of transient global amnesia-like episodes |
| 5. Incecik et al (12) | 7y/M (2015) |
Behavioral changes, dysphagia, ptosis, diplopia, and drowsiness | None | Positive | Positive | Not mentioned | Normal | Epileptiform abnormalities in both temporal regions | IVIG, Plasma exchange | Complete recovery |
| 6. Grilo et al (13) | 10y/Fe (2016) |
Headache, nausea, vomiting, seizures, irritability, depressed mood, unusual fear, memory disturbance Later: new seizures, decrease the state of consciousness, confusion, hallucination |
Type-1 Diabetes mellitus | 2793 U/mL (Normal-<1 U/mL) |
Positive | Negative | Hypertense signal of the left mediotemporal lobe and amygdala on T2 and FLAIR sequences | Paroxysmal activity and Bilateral temporal slowing | MP, Oral prednisone, RTX | Improved (No seizures and neuropsychological deterioration) |
| 7. Achour et al (14) | 9y/Fe (2017) | Temporal lobe seizures, generalized tonic-clonic seizures, Mood and behavioral disturbances, autonomic imbalance. | None | Positive | Positive | Not mentioned | Normal | Slowed theta rhythm with bilateral frontotemporal spike-wave discharges | MP, IVIG, RTX | 6 months: died |
| 8.Akin et al (15) | 16y/M (2017) | Confusion and headache | Type-1 DM 6months back | 2114 IU/mL (Normal-0-10) | 4.07 nmol/L (Normal<0.02) | Not mentioned | Hyperintense signal of the right mesiotemporal lobe | Temporal slowing | IVIG,MP, Oral steroid | Persists with type-1 DM |
| 9. Koki et al (16) | 5y/Fe (2018) | Seizure clustering and altered mental status | Pineoblastoma (Onco) | Initially:65,100 U/mL (Normal -<1.5 U/mL) Later: 1,42,000 U/mL |
Initially: not mentioned Later: 238 U/mL |
Not mentioned | Initially: Hyperintensity of cerebrum, predominantly in the left hemisphere. Later: severe whole brain encephalitis |
Initially: left dominant delta activity. Later: generalized delta activity and no spike waves. |
IVIG, MP, Plasma exchange Tacrolimus | Still unable to communicate and walk independently and requires almost total assistance. |
| 10. Kern et al 3 | 16y/Fe (2021) | Persistent headache, 2 episodes of urinary incontinence, change in acute mental status, expressive aphasia, auditory hallucination. | Acute lymphoblastic Leukemia(0nco) | >250 IU/mL (Normal <5 IU/mL) | Negative | Positive | New edema and ill-defined enhancement involving the left temporal lobe, parieto-occipital region, and left hippocampus | Subclinical seizures from the left frontal, temporal lobe. | IVIG, Dexamethasone | Symptomatic improvement but persists Type-1 DM |
| 11. Ren et al (17) | 6y/Fe (2021) | Seizure, headache, memory deficient | None | 1:100(CBA) | 1:320(CBA) | Positive | Bilateral Hippocampus | Right-sided epileptiform discharge | IVIG, MP, Oral steroids | Refractory focal seizures |
| 12. Ren et al (17) | 16y/Fe (2021) | Seizure, memory deficient, depression, dysautonomia | Thyroiditis | 1:32(CBA) | 1:32(CBA) | Positive | Initial: bilateral hippocampal 15th month: bilateral frontal lobe, left parietal lobe, right temporal lobe and insular cortex, and subcortical white matter and the bilateral hippocampus 5 years follow-up: parenchymal atrophy. | Slowed theta rhythm with bilateral temporal spike-wave discharges | IVIG, MP, oral steroid, RTX | Persistent memory impairment and refractory focal seizures |
| 13. Ren et al (17) | 4y9m/F (2021) | Vomiting, headache, confusion | None | 1:100(CBA) | 1:320(CBA) | Positive | Brainstem, thalamus, basal ganglia, bilateral cerebral, and cerebellar hemispheres | Slow theta rhythm | IVIG, MP, oral steroid | Complete recovery |
| 14. Bushati et al (18) | 8y/Fe (2022) | First episode: abrupt behavioral changes, irritability, emotional lability, deficiency of speech, low concentrationation and memory loss, headache, and morning vomiting. Second episode: drop attack, face blindness Third episode: neuropsychiatric complaints |
None | Positive (ELISA) | Not mentioned | Not mentioned | Normal | Initially: spiked and slow right frontotemporal alpha waves Later: no clinical seizures were seen |
IVIG, MP, RTX | Not mentioned |
| 15. | 8y/M (2023) | Seizures increase sleep and thirst and decrease appetite | Hypothyroidism | 1:100 (CBA) | 1:32 (CBA) | Positive | Initially right hippocampus involvement. Later left hippocampus |
Diffuse mixed activity in the left anterior middle prefrontal temporal region | IVIG, MP, Oral prednisone | Persists with Type-1 DM |
CBA, cell-based assay, TBA, tissue-based assay, IVIG, intravenous immunoglobulin, MP, methylprednisolone, RTX, rituximab,T1DM, type-1 diabetes mellitus.
Limbic symptoms were prevalent among the patients and included mental and behavioral changes (observed in almost all cases), various types of seizures (12 cases), and memory disturbances (5 cases). The extra limbic symptoms comprised ataxia (one case), aphasia (three cases), headache (seven cases), and global developmental delay (one case). Moreover, some cases experienced autonomic dysfunctions, such as gastrointestinal upsets (nausea, vomiting, dysphagia in five cases), ocular symptoms (ptosis, diplopia both in one case), face blindness (one case), and urinary incontinence (one case). Our patient’s symptoms were characterized by seizures, increased sleep, thirst, and poor appetite.
MRI findings showed T2-W FLAIR hyperintensities primarily restricted to the temporal lobes in the early phase, progressing to hippocampal sclerosis/atrophy in the late phase in majority of the cases. Interestingly, three cases presented with normal MRIs. Likewise, our patient exhibited initial involvement of the right hippocampus, followed by subsequent engagement of the left hippocampus. The majority of cases exhibited the electroencephalogram (EEG) readings of slow waves or spike waves on the frontotemporal lobes, whereas our EEG findings revealed abnormal signals characterized by diffuse mixed activity (ranging from low to high amplitude sharp waves and spicy slow waves/sharp slow waves) in the left anterior middle prefrontal temporal region. But the latest VEEG of our patient elicited mixed type waves with different amplitudes from almost all regions of the brain. These findings contribute to a better understanding of the clinical and neuroimaging characteristics of anti-GAD65 antibody-associated encephalitis in young patients.
The diagnosis of GAD65-Ab-associated encephalitis is based on clinical features and the presence of high anti-GAD65 Ab level in serum or high titers in both serum and CSF with the detection of intrathecal synthesis (IS) in CSF (19). In clinical practice, GAD Ab is detected through different techniques, including indirect immunohistochemistry, immunoblot, enzyme-linked immunosorbent assay (ELISA), and radioimmunoassay (RIA), which have different sensitivity and specificity (20). Recently, CBA technique is considered as an effective screening method. It demonstrates promise as a valuable screening method for suspected patients, TBA plays a significant role in defining antibodies, especially for patients who yield negative CBA results (21). In our patient, we detected high level of GAD antibody in serum along with high titers of anti-GAD65-Abs detected in both serum and CSF through CBA and TBA methods. In addition, 7/15 cases including our case detected oligoclonal bands in CSF, whereas only one case had a negative result, and the remaining cases did not mention.
The first-line treatment for GAD65-Ab-associated encephalitis typically involves IVIG, intravenous methylprednisolone, and plasma exchange. Second-line treatments, such as rituximab, cyclophosphamide, and mycophenolate mofetil, are considered, although their efficacy may vary across cases. Occasionally, third-line therapies, such as daratumumab, bortezomib, tocilizumab, tofacitinib, and low-dose interleukin 6 (IL-6), have been recommended; however, their effectiveness is currently being evaluated in ongoing trials (22). Our patient responded well to the first line of therapy. The prognosis varies for each patient, with early diagnosis and timely immune therapy associated with better outcomes. However, some patients had experienced relapse and require second-line treatments. Persistent refractory seizures, type-1 diabetes mellitus, and other complications (persistent memory impairment, persistent disability and death) have also been reported.
Conclusion
This represents the initial documentation of a case, presenting anti-GAD65 autoimmune encephalitis in association with APS type-II. Unlike previous reports where anti-GAD65 autoimmune encephalitis coexisted with a single type of endocrine disease, our case illustrates a unique manifestation involving both APS type-II with anti-GAD65 autoimmune encephalitis. The coexistence of three immune diseases (anti-GAD65 antibody, T1DM, and autoimmune thyroiditis) presents a distinctive and uncommon case that holds significant importance in pediatric medicine and in the medical literature, contributing valuable insights for future research. This study aims to increase awareness regarding anti-GAD-65-mediated autoimmune encephalitis, which may concomitantly occur with polyendocrine diseases such as T1DM and autoimmune thyroiditis. Moreover, we believe that early recognition of neuropsychiatric symptoms, coupled with timely initiation of immunotherapy, may yield favorable treatment outcomes and reduce the risk of relapses or permanent disability.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by The First Affiliated Hospital of Zhengzhou University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
TS: Writing – original draft. ZZ: Supervision, Writing – review & editing. WL: Data curation, Writing – review & editing. FT: Investigation, Writing – review & editing. WY: Investigation, Writing – review & editing. BD: Investigation, Writing – review & editing. SH: Writing – review & editing, Investigation.
Acknowledgments
Would like to thank ZZ, my colleague WL, and all senior doctors and staff of The First Affiliated Hospital of Zhengzhou University who helped in the collection of clinical data, and very grateful to the family members for their cooperation in this study.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work is sponsored by the Henan Key Laboratory of Pediatric Epilepsy and Immunology, Clinical Diagnosis and Treatment Center of Pediatric Nervous System Disease of Henan province. Key Project of Honan Province Science and Technology Research, SBGJ2020002054, MiR-125b in Immune-induced Inflammatory Reaction of Epileptic Young Rats. Additionally, supported by SBGJ202102109, TLR4/MyD88/NF-κB signaling pathway was used to investigate the effect of fecal bacteria transplantation on refractory epilepsy rats. Zhengzhou Collaborative Innovation Project, XTCX2023002, Mechanism of Regulation of Epileptic Neuroinflammation by Microbial Derived Tryptophan Metabolites via Aromatic Hydrocarbon Receptor/NF-κB Signaling Pathway.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Kern K, Shuster BA. Rare presentation of anti-GAD-65 antibody-positive autoimmune encephalitis and simultaneous onset of type 1 diabetes mellitus in a paediatric patient. BMJ Case Rep (2021) 14(3):e237913. doi: 10.1136/bcr-2020-237913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andersson M, Alvarez-Cermeno J, Bernardi G, Cogato I, Fredman P, Frederiksen J, et al. Cerebrospinal fluid in the diagnosis of multiple sclerosis: a consensus report. J Neurol Neurosurg Psychiatry (1994) 57(8):897–902. doi: 10.1136/jnnp.57.8.897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Olson JA, Olson DM, Sandborg C, Alexander S, Buckingham B. Type 1 diabetes mellitus and epilepsia partialis continua in a 6-year-old boy with elevated anti-GAD65 antibodies. Pediatrics (2002) 109(3):E50. doi: 10.1542/peds.109.3.e50 [DOI] [PubMed] [Google Scholar]
- 4. Akman CI, Patterson MC, Rubinstein A, Herzog R. Limbic encephalitis associated with anti-GAD antibody and common variable immune deficiency. Dev Med Child Neurol (2009) 51(7):563–7. doi: 10.1111/j.1469-8749.2008.03217.x [DOI] [PubMed] [Google Scholar]
- 5. Korff CM, Parvex P, Cimasoni L, Wilhelm-Bals A, Hampe CS, Schwitzgebel VM, et al. Encephalitis associated with glutamic acid decarboxylase autoantibodies in a child: a treatable condition? Arch Neurol (2011) 68(8):1065–8. doi: 10.1001/archneurol.2011.177 [DOI] [PubMed] [Google Scholar]
- 6. Mishra N, Rodan LH, Nita DA, Gresa-Arribas N, Kobayashi J, Benseler SM. Anti–glutamic acid decarboxylase antibody associated limbic encephalitis in a child: expanding the spectrum of pediatric inflammatory brain diseases. J Child Neurol (2014) 29(5):677–83. doi: 10.1177/0883073813500527 [DOI] [PubMed] [Google Scholar]
- 7. Incecik F, Hergüner O, Yildizdas D, Horoz O, Besen S. Limbic encephalitis with antibodies to glutamic acid decarboxylase presenting with brainstem symptoms. Ann Indian Acad Neurol (2015) 18(2):243. doi: 10.4103/0972-2327.150628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grilo E, Pinto J, Caetano JS, Pereira H, Cardoso P, Cardoso R, et al. Type 1 diabetes and GAD65 limbic encephalitis: a case report of a 10-year-old girl. J Pediatr Endocrinol Metab (2016) 29(8):985–90. doi: 10.1515/jpem-2016-0016 [DOI] [PubMed] [Google Scholar]
- 9. Ben Achour N, Ben Younes T, Rebai I, Ben Ahmed M, Kraoua I, Ben Youssef–Turki I. Severe dysautonomia as a main feature of anti-GAD encephalitis: Report of a paediatric case and literature review. Eur J Paediatr Neurol (2018) 22(3):548–51. doi: 10.1016/j.ejpn.2018.01.004 [DOI] [PubMed] [Google Scholar]
- 10. Akın O, Kılınç Uğurlu A, Akbaş ED, Döğer E, Akbaş Y, Bideci A, et al. Autoimmune limbic encephalitis associated with type 1 diabetes mellitus. J Clin Res Pediatr Endocrinol (2017) 9(4):387–8. doi: 10.4274/jcrpe.3818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Severe anti-GAD antibody-associated encephalitis after stem cell transplantation - Brain and Development. Available at: https://www.brainanddevelopment.com/article/S0387-7604(18)30294-8/fulltext (Accessed May 7, 2023). [DOI] [PubMed]
- 12. Ren C, Ren H, Ren X, Zhang W, Li J, Dai L, et al. Case report: autoimmune encephalitis associated with anti-glutamic acid decarboxylase antibodies: A pediatric case series. Front Neurol (2021) 12:641024. doi: 10.3389/fneur.2021.641024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bushati A, Aleksi K, Tako Kumaraku A, Shehu A, Mecani R. Recurrent anti-GAD65 limbic encephalitis in a pediatric patient. Med Res Chron (2022) 9:5. doi: 10.26838/MEDRECH.2022.9.3.602 [DOI] [Google Scholar]
- 14. Eisenbarth GS. Autoimmune polyendocrine syndromes. N Engl J Med (2004)109:e50. doi: 10.1056/NEJMra030158 [DOI] [PubMed] [Google Scholar]
- 15. Betterle C, Dal Pra C, Mantero F, Zanchetta R. Autoimmune adrenal insufficiency and autoimmune polyendocrine syndromes: autoantibodies, autoantigens, and their applicability in diagnosis and disease prediction. Endocr Rev (2002) 23(3):327–64. doi: 10.1210/edrv.23.3.0466 [DOI] [PubMed] [Google Scholar]
- 16. Michels AW, Gottlieb PA. Autoimmune polyglandular syndromes. Nat Rev Endocrinol (2010) 6(5):270–7. doi: 10.1038/nrendo.2010.40 [DOI] [PubMed] [Google Scholar]
- 17. Seissler J, Bieg S, Yassin N, Mauch L, Northemann W, Boehm BO, et al. Association between antibodies to the MR 67,000 isoform of glutamate decarboxylase (GAD) and type 1 (Insulin-dependent) diabetes mellitus with coexisting autoimmune polyendocrine syndrome type II. Autoimmunity (1994) 19(4):231–8. doi: 10.3109/08916939409071348 [DOI] [PubMed] [Google Scholar]
- 18. Malter MP, Helmstaedter C, Urbach H, Vincent A, Bien CG. Antibodies to glutamic acid decarboxylase define a form of limbic encephalitis. Ann Neurol (2010) 67(4):470–8. doi: 10.1002/ana.21917 [DOI] [PubMed] [Google Scholar]
- 19. Baizabal-Carvallo JF. The neurological syndromes associated with glutamic acid decarboxylase antibodies. J Autoimmun (2019) 101:35–47. doi: 10.1016/j.jaut.2019.04.007 [DOI] [PubMed] [Google Scholar]
- 20. Saiz A, Arpa J, Sagasta A, Casamitjana R, Zarranz JJ, Tolosa E, et al. Autoantibodies to glutamic acid decarboxylase in three patients with cerebellar ataxia, late-onset insulin-dependent diabetes mellitus, and polyendocrine autoimmunity. Neurology (1997) 49(4):1026–30. doi: 10.1212/WNL.49.4.1026 [DOI] [PubMed] [Google Scholar]
- 21. Muñoz-Lopetegi A, de Bruijn MAAM, Boukhrissi S, Bastiaansen AEM, Nagtzaam MMP, Hulsenboom ESP, et al. Neurologic syndromes related to anti-GAD65: Clinical and serologic response to treatment. Neurol Neuroimmunol Neuroinflammation (2020) 7(3):e696. doi: 10.1212/NXI.0000000000000696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Smets I, Titulaer MJ. Antibody therapies in autoimmune encephalitis. Neurother J Am Soc Exp Neurother (2022) 19(3):823–31. doi: 10.1007/s13311-021-01178-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.