Abstract
Lyme disease (LD) results from the most prevalent tick-borne infection in North America, with over 476,000 estimated cases annually. The disease is caused by Borrelia burgdorferi (Bb) sensu lato which transmits through the bite of Ixodid ticks. Most cases treated soon after infection are resolved by a short course of oral antibiotics. However, 10–20% of patients experience chronic symptoms because of delayed or incomplete treatment, a condition called Post-Treatment Lyme Disease (PTLD). Some Bb persists in PTLD patients after the initial course of antibiotics and an effective treatment to eradicate the persistent Bb is needed. Other organisms that cause persistent infections, such as M. tuberculosis, are cleared using a combination of therapies rather than monotherapy. A group of Food and Drug Administration (FDA)-approved drugs previously shown to be efficacious against Bb in vitro were used in monotherapy or in combination in mice infected with Bb. Different methods of detection were used to assess the efficacy of the treatments in the infected mice including culture, xenodiagnosis, and molecular techniques. None of the monotherapies eradicated persistent Bb. However, 4 dual combinations (doxycycline + ceftriaxone, dapsone + rifampicin, dapsone + clofazimine, doxycycline + cefotaxime) and 3 triple combinations (doxycycline + ceftriaxone+ carbomycin, doxycycline + cefotaxime+ loratadine, dapsone+ rifampicin+ clofazimine) eradicated persistent Bb infections. These results suggest that combination therapy should be investigated in preclinical studies for treating human Lyme disease.
Keywords: Lyme disease, antibiotic, combination, mouse, Borrelia burgdorferi, efficacy
1. Introduction
Lyme disease (LD), caused by the Borrelia burgdorferi (Bb) sensu lato complex, is the most common tick-borne illness in the United States with more than 476,000 cases annually (Kugeler et al., 2021). Approximately 10–20% of LD patients further develop post-treatment Lyme disease (PTLD) and continue to experience symptoms for 6 months or more following treatment (Aucott et al., 2013; Rebman and Aucott, 2020; Rebman et al., 2021; Marvel et al., 2022). Persistent Bb infection that is not eradicated by guideline-adherent antibiotic treatment is strongly suspected to contribute to PTLD, although there are other proposed causes as well (Adkison and Embers, 2023).
The Infectious Disease Society of America (IDSA) and other international agencies of infectious diseases recommend monotherapy (single drug treatment) with doxycycline or amoxicillin to treat LD (Wormser et al., 2006; Rauer et al., 2020). Monotherapy can be effective 3 to 30 days after the tick bite with response rates of 89% or higher (Aucott et al., 2013; Torbahn et al., 2018). However, treatment delays significantly affect antibiotic efficacy, and many patients experience chronic musculoskeletal pain, fatigue, and cognitive dysfunction (Brian et al., 2003; Touradji et al., 2018; Hirsch et al., 2020; Rebman et al., 2021). Studies on patients with PTLD show not only that immune activation/inflammation lingers in the brain, but also that dynamic changes in the frontal lobe associated with slower processing speeds occur (Coughlin et al., 2018; Marvel et al., 2022). Treatment for PTLD is mired by a lack of tests for persistent infection and mixed results on treating chronic Lyme disease in clinical trials (Klempner et al., 2001; Fallon et al., 2008, 2012; Berende et al., 2014).
Studies in mice, dogs, and nonhuman primates have demonstrated persistence of the spirochetes after doxycycline or ceftriaxone treatment, with persistence more likely when treatment is given in the disseminated phase (Straubinger et al., 1997; Bockenstedt et al., 2002; Barthold et al., 2010; Embers et al., 2012, 2017; Hodzic et al., 2014; Crossland et al., 2018). One study in mice with a 3-month infection demonstrated that 5 days of ceftriaxone prevented culture of spirochetes from tissues, with the caveat that mice were euthanized 7–10 days after treatment (Pavia and Wormser, 2014). Subsequent studies showed that the infection resurged 12–18 months after ceftriaxone treatment (Hodzic et al., 2014, 2019). Thus, both the duration prior to treatment, and after treatment influence the results of treatment efficacy. In vitro studies have demonstrated improved efficacy with combinations of antibiotics (Feng et al., 2014, 2015a,b, 2016a,b, 2017; Pothineni et al., 2016). The currently available studies that address these limitations demonstrate that a combination of daptomycin + cefoperazone + doxycycline eradicated persistent Bb in culture and a combination of daptomycin+ doxycycline+ ceftriaxone eradicated persistent Bb in mice (Feng et al., 2015a,b, 2019).
Combination therapy is an alternative approach to treat Bb infection and is already well-established for treatment of persistent bacteria like M. tuberculosis and Brucella melitensis in humans and animal models (Zhang, 2005; Zhang et al., 2012; Zhan et al., 2017; Khan et al., 2018; Silva et al., 2018; al-Madfaa et al., 2020). Only one randomized clinical trial that used more than one drug (doxycycline and ceftriaxone) has been performed, but the drugs were administered at separate time points rather than concurrently and treatment failed to demonstrate patient improvement (Klempner et al., 2001). A separate study used ceftriaxone for 2 weeks with a randomized follow-up 12 week treatment, consisting of (a) a combination of clarithromycin and hydroxychloroquine; (b) doxycycline; or (c) placebo. This combination was as effective as ceftriaxone alone treatment but the inclusion/exclusion criteria were very strict (Berende et al., 2014). Barriers to combination therapy clinical trials include disagreement on the cause of PTLD and limited preclinical evidence for efficacy (Bobe et al., 2021).
The study described here used a panel of drugs as monotherapy and, separately, a panel of combination therapies to treat long-term (persistent) Bb infection. The presence of persistent Bb infection following treatment was determined using multiple methods of detection. These results provide data to further demonstrate Bb persistence following monotherapy and the potential for combination therapy to prevent persistent Bb infection. These results also demonstrate feasibility of combination therapy as an alternative treatment regimen to monotherapy for disseminated infection that can lead to PTLD.
2. Materials and methods
2.1. Murine experiments
The mice used in this study were housed in a pathogen-free animal facility according to animal safety protocol guidelines at the Tulane National Primate Research Center (TNPRC) accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC) International. The Tulane University Institutional Animal Care and Use Committee (IACUC) approved all animal-related protocols. C3H/HeN female mice from Charles River labs, aged 6–7 weeks were used in this study. Two to 5 mice were housed per cage and maintained at 25°C with a 12-h light–dark cycle and had ad libitum access to chow and water. Mice were euthanized by CO2 narcosis followed by cervical dislocation and exsanguination by cardiocentesis. Euthanasia was performed in accordance with the guidelines of the American Veterinary Medical Association (AVMA).
2.2. Culture media
Cultures were grown in BSK-H (Sigma) or mBSK-II medium. mBSK-II medium contains carbohydrate additives including 0.4% each of mannose, maltose, glycerol and n-acetylglucosamine. This media has been tested successfully in mice and primates for recovering live spirochetes after doxycycline treatment (unpublished), but the final formulation has not been optimized. Phosphomycin, rifampicin, and amphotericin B were added to both medias to prevent fungal growth and bacterial contamination at concentrations recommended in the media protocol (Embers et al., 2012). The culture volume used was ~4 mL and they were grown in snap-cap tubes with the cap loose.
2.3. Ear biopsy
Ear punch biopsies were collected at days 7 and 14 post-infection using sterile 2 mm punches after cleansing the skin with an alcohol wipe. The punches were cultured in BSK-H media and placed in a tri-gas incubator for 5 weeks to confirm infection. Cultures were checked for motile spirochetes once per week.
2.3.1. Borrelia burgdorferi infection confirmation
Approximately 1 ×106 B. burgdorferi strain B31.5A19, grown from stocks between passages 3–5 were administered by subcutaneous injection. This route is consistent with prior studies on antibiotic efficacy in mice (Barthold et al., 2010; Hodzic et al., 2014, 2019; Pothineni et al., 2020). Ear punch biopsies were obtained and cultured at 7 and 14 days post-infection and serum was tested at 0, 21, and 60 days using a 5-antigen test to confirm Bb infection (Embers et al., 2016). Mice were required to be seronegative against the 5 Bb antigens at day 0 to be included in the study. Mice were also required to have at least one culture-positive ear biopsy at day 7 or day 14 or be seropositive for 2 or more Bb antigens at day 21 or day 60 following inoculation.
Mice were humanely euthanized after the xenodiagnoses (2–3 months after treatment completion and collecting engorged ticks). Skin (one whole ear) was cultured into BSK-II liquid medium and incubated in a microaerophilic chamber, as well as splitting one whole ear for both RNA and DNA extraction. All other tissues (heart, bladder, spleen, tibiotarsal joints) were used for culture and nucleic acid. The growth of B. burgdorferi was detected by dark field microscopy with a 50x objective. The RNA and DNA were also detected via RT-PCR or standard PCR.
2.3.2. Other antimicrobial drug treatment
All drugs (azlocillin, Bactrim, carbomycin, cefotaxime, ceftriaxone, clofazimine, dapsone, disulfiram, doxycycline, loratadine, and rifampicin) used in this study are FDA-approved and were administered at non-toxic levels for 28 days at 2 months post-infection. Bactrim, Disulfiram, Dapsone, Rifampicin, clofazimine, doxycycline and loratadine were administered through oral gavage. Azlocillin, ceftriaxone and cefotaxime were administered by intraperitoneal injection. Carbomycin was administered by subcutaneous injection. Drugs were prepared according to the drug specifications (Appendix I).
Dosage was determined based on weight measured 2 days before treatment.
2.4. Sample collection for treatment efficacy
Blood, tissues (ear, heart, spleen, bladder, and tibiotarsal joint), and tick midguts were collected for this study. Approximately 0.1 mL of blood was collected via retro-orbital bleeding at 0, 14, 21, 60–63, 66–69, 70–73, 80–83, 90–93, 104–107 days and at 6 months post-injection. Blood was also collected at necropsy by cardiac puncture. Serum was eluted from the blood by centrifugation at 6000 rpm for 10 min and collecting the supernatant (serum). Serum was preserved at −20°C until further analysis.
Approximately 2 mm3 of tissue from the ear, heart, bladder, tibiotarsal joint (TTJ), and spleen were collected aseptically into phosphate buffered saline (PBS; pH 7.4, Gibco) at necropsy. Each sample was divided and allocated to either culture medium, a 1.5 mL tube for preservation at −20°C for PCR, or a 1.5 mL tube with 5 volumes of RNAlater (Qiagen, Valencia, CA) and preserved at −80°C for RT-PCR.
For xenodiagnoses, six nymphal-stage xenodiagnostic hard ticks, Ixodes scapularis, were introduced to each mouse. The ticks were derived from our in-house tick colony. Engorged ticks detached after 3–6 days of feeding and were kept for 7 to 14 days to allow Bb to grow if present. The ticks were washed and crushed individually in 50 μL of PBS using a previously published protocol (Embers et al., 2012). Tick contents were apportioned to a slide (12 μL), air-dried, fixed by acetone, and preserved at room temperature for the immunofluorescence assay (IFA). The remaining contents of tick midguts were pooled per mouse and split into 3 equal portions for culture, PCR, and RT-PCR as with the tissue samples.
2.5. Bb cultures from tissue
Tissue samples were added to 4 mL of BSK-H or mBSK-II medium, or both. Tick midgut samples were cultured in BSK-H only. Culture tubes were incubated for 5 weeks at 34°C, in the presence of 5% CO2 and an influx of N2 to produce a microaerophilic environment. The cultures were evaluated for the presence of motile spirochetes every week for 5 weeks using dark-field microscopy.
2.6. Serology test
Five antigens (OspA, OspC, DbpA, OppA2, and C6) were used concurrently to detect Bb antibodies in mouse serum at 0, 21, and 60 days. The Bio-Rad Bio-Plex amine coupling kit (catalog no. 171–406,001) protocol for coupling antigens to cytometric beads was slightly modified to include the C6 peptide and recombinant proteins (produced in-house) in the same assay. The modified protocol is described in detail in a previous study (Embers et al., 2016).
2.7. Determination of drug concentration in serum
Drug concentrations in serum from mice treated with drug(s) or vehicle control were measured using the Modified Kirby-Bauer (MKB) assay on days 7, 14, and /or day 21 during the treatment period. In brief, 25 μL of control serum and 25 μL of serum pooled from all mice within a treatment group were pipetted onto a 2-mm diameter plain paper disc (BD) and allowed to dry for 30 min. Control standards for each drug were made by dissolving the drugs into serum from untreated mice. Concurrently, a 0.5 McFarland suspension of Staphylococcus aureus (ATCC #29213) was made in tryptic soy broth (BD, Franklin Lakes, NJ) and streaked onto Mueller-Hinton agar (MHA) plates (BD). The discs of the standards and treated sera were placed onto the streaked MHA plates and incubated at 37°C for 18–24 h. After incubation, the diameter (mm) of the zones of inhibition was measured and a standard curve was generated using the standards measurements. Drug concentration was calculated by comparing the zone of inhibition of the treated sample to the standards (Embers et al., 2013, 2017).
2.8. PCR
DNA was extracted following the protocol of the DNeasy® Blood and Tissue kit (Qiagen, USA). Tissue and tick midgut samples were eluted in 200 μL and 50 μL of elution buffer, respectively. DNA concentration was quantified using a Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA). For tissues, 50–250 ng of template DNA was used, depending on the tissue, and held equal per tissue based on the maximum amount possible from eluted quantities. The PCR was run with 35 cycles of denaturation (94°C, for 30 s), annealing (57.8°C for ospC, 60°C for ospA, 62°C for 16S, for 45 s) and extension (72°C, for 1 min) using 25 μL of sample. Primers against 16 s and ospC were used for tissue samples and primers against 16 s and ospA were used for the tick contents. The full sequences of the primers are in Supplementary Table S1.
2.9. RT-PCR
Total RNA was extracted from all samples using a RNeasy mini kit (Qiagen, USA) and contaminating DNA was removed using the RNase-free DNase set (Qiagen, United States) according to the manufacturer’s protocol. RNA was reverse-transcribed using a high-capacity cDNA reverse transcription kit (Invitrogen, USA). The same primers used for PCR were used for RT-PCR (except for ospC which was not performed for tissues). RT-PCR was performed with the Qiagen® OneStep RT-PCR kit (Qiagen, USA). PCR in the second step was performed as described above.
2.10. Immunofluorescence assay
Slides of acetone-fixed tick midgut contents were blocked by adding 30uL diluted normal goat serum (NGS) to each slide. NGS was diluted 10% in PBS with 0.2% fish skin gelatin (Sigma-Aldrich) and incubated with blocking at 37°C for 1 h. Slides were washed 3 times with 50 mL of PBS. Slides were then incubated with an anti-OspA mouse monoclonal antibody (CB10; obtained from J. Benach (LaRocca et al., 2009)) at a 1:30 dilution in a volume of 30 μL for 1–1.5 h at 37°C. Slides were again washed 3 times with 50 mL of PBS and incubated with anti-mouse IgG-Alexa 488 secondary antibody (1,1,000, ThermoFisher A28175) at 37°C for 1 h. Slides were washed 3 times with 50 mL of PBS and dried using Kim Wipes while avoiding the treated areas. Before adding coverslips, slides were mounted using 10-15uL of a homemade anti-quenching mounting media containing Mowiol (Calbiochem #475904) and DABCO (Sigma #D2522). Coverslips were fixed on either side with clear nail polish and allowed to dry. Slides were stored at −20°C until viewing and at 4°C after viewing. The slides were imaged using a Nikon Ti2-E fluorescence microscope to detect the presence of spirochetes.
2.11. Data analysis
The cut off value for positivity for the 5-antigen test was set for each plate based on the mean MFI of days 0 in that plate for each antigen +/− the standard deviation of the mean multiplied by 3.
Differences between treated and untreated animals in all culture, PRC, RT-PCR, and IFA assays for tissues and tick midguts were evaluated with Fisher’s exact test (two-tailed). Differences were considered significant at p ≤ 0.05.
3. Results
3.1. Confirmation of infection in the monotherapy treatment arm
The specific drugs and combinations chosen for evaluation were based on: (1) published literature regarding the use of repurposed drugs for Lyme disease (disulfiram, loratadine) or existing drugs currently thought to have potential for efficacy against Borrelia (dapsone, azlocillin); and (2) combinations which include antimicrobials of different categories that utilize separate mechanisms to affect bacterial growth and survival (Supplementary Table S2). The experimental procedure for testing efficacy of drugs and combinations of drugs is depicted in Figure 1. Infection with Bb was confirmed by either an ear punch culture positive for Bb or a 5-antigen serology result positive for 2 or more Bb antigens at days 21 or 60. Mice were considered infected if either test was positive. Twenty out of 50 (40%) mice had positive ear punch results while 50 out of 50 (100%) had positive 5-antigen test results. Supplementary Table S3 shows the results of each group.
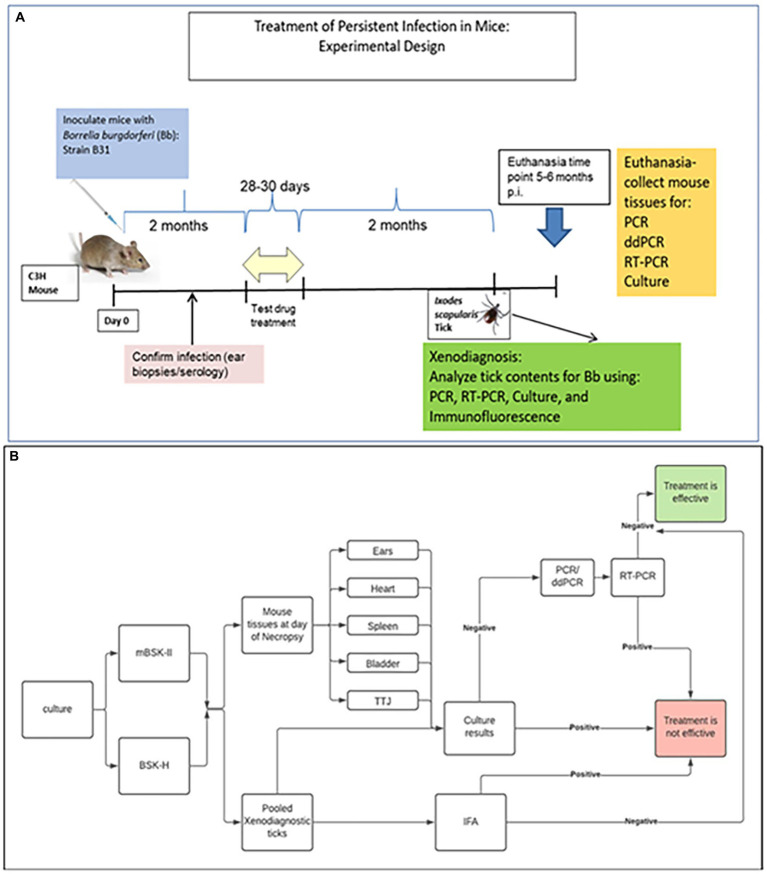
Figure 1.
Experimental protocol for assessment of efficacy in mice. (A) Mice were infected and kept for 2 months prior to treatment. After the treatment, 2 more months elapsed before determination of persistent infection, including xenodiagnoses, culture, and molecular detection. (B) Flow chart for the determination of efficacy.
3.2. Determination of drug concentration in serum following treatment
Drugs that were be tested in mice have been FDA-approved and have been shown to kill or prevent the growth of Borrelia burgdorferi in different growth phases or morphologies (i.e., sessile, round-body forms, and aggregates). These compounds have shown promise in vitro but had not been tested in an animal model of persistent infection (Feng et al., 2014, 2015a,b, 2016a,b, 2017; Wagh et al., 2015; Pothineni et al., 2016, 2020). Mice with confirmed infections were treated with a single drug. Each drug was administered at the highest non-toxic concentration that has previously been used in mice (Sakamoto et al., 1984; Ulrich et al., 1989; Hoshina et al., 2018; Nakayama et al., 2018) (Table 1). The drug concentration in the serum was calculated using the modified Kirby Bauer assay (MKB). One drug per route of administration (rifampicin, OG; cefotaxime, IP; carbomycin SC) was selected to measure serum concentrations at 24-h post-treatment during weeks 1–3 of treatment.
Table 1.
Concentrations, doses, routes, and number of mice.
| Treatment | Concentration | Dose (1x per day) | Route (OG, IP, SC) | n |
|---|---|---|---|---|
| Controls | ||||
| Infected, untreated (OG Ctrl) | Water | 0.2 mL/mouse | OG | 3 |
| Infected, untreated (IP Ctrl) | PBS | 0.2 mL/mouse | IP | 5 |
| Infected, untreated (SC Ctrl) | 0.09% Saline +5% DMSO | 0.2 mL/mouse | SC | 2 |
| Total | 10 | |||
| Therapies | ||||
| Azlocillin | 15 mg/mL | 100 mg/kg | IP | 5 |
| Bactrim* | 10 mg/mL | 62.5 mg/kg | OG | 5 |
| Disulfiram | 20 mg/mL | 100 mg/kg | OG | 5 |
| Carbomycin | 25 mg/mL | 200 mg/kg | SC | 5 |
| Dapsone | 5 mg/mL | 15 mg/kg | OG | 5 |
| Rifampicin | 10 mg/mL | 30 mg/kg | OG | 5 |
| Cefotaxime | 25 mg/mL | 100 μg/mouse | IP | 5 |
| Loratadine | 5 mg/mL | 20 mg/kg | OG | 5 |
| Total | 40 | |||
OG, oral gavage; IP, intraperitoneal; SC, subcutaneous. *Bactrim = Trimethoprim-Sulfamethoxazole (TMP-SMX) (62.5 mg/kg = TMP component).
The average concentration of rifampicin 24-h post-treatment for weeks 1–3 was 2.588 μg/mL (range = 1.23–3.87) with a total of 2 pooled serum samples for every week. At day 66 (week 1), the average concentration of cefotaxime at the trough level was calculated as 2.454 ug/ mL (range 0–5.18) of 2 pooled sera. At day 73 (2nd week of treatment), the average concentration of carbomycin at the trough level was 4.8 ug/ mL (range 4.42–5.2 ug/ mL) of 2 pooled sera. Supplementary Tables S4–S7 and Supplementary Figures S1–S3 include more details of MKB results.
These acquired concentrations were higher than the MIC of these drugs in previous studies. The MIC of rifampicin against persisting Bb is ≤0.4 μg/mL (Feng et al., 2017). The MIC and MBC of cefotaxime against persisting Bb are ≤0.03 μg/mL and ≤ 0.25 μg/mL, respectively (Levin et al., 1993). The MIC of carbomycin against persisting Bb is ≤0.25 μg/mL (Feng et al., 2014). A compilation of the MIC and MBC values is detailed in Appendix I.
In addition, azlocillin and dapsone were selected to be tested via MKB but the interaction of these drugs, when tested with S. aureus, was limited to the concentration of 10 μg/mL and up for azlocillin, and 4,100 μg/mL or more for dapsone. These minimum concentrations are not anticipated to be achieved in our mouse sera, precluding the use of this test for measuring antibiotic levels in blood.
3.3. Culture of tissues from mice treated with monotherapy confirm Bb infection
Mice were divided into groups of 2–5 and administered either a test drug or control treatment. None of the monotherapy regimens completely eradicated Bb. The cefotaxime group had significantly fewer infected mice compared to the control (p = 0.0476), but Bb persisted in one mouse. The other monotherapy groups had 3–5 mice positive for Bb per group following treatment. There was no difference in the number of positive samples between BSK-H and mBSK-II media (Table 2).
Table 2.
Culture results of tissues in monotherapy.
| Treatments | Cultures of tissues | |||
|---|---|---|---|---|
| BSK-H^ | mBSK-II^ | Combined Cultures^ | p value | |
| Infected Untreated (OG) | 3/3 | ND | 3/3 | |
| Infected Untreated (IP) | 5/5 | ND | 5/5 | |
| Infected Untreated (SC) | 2/2 | ND | 2/2 | |
| Azlocillin (IP) | 4/5 | ND | 4/5 | 1b |
| Bactrim (OG) | 5/5 | ND | 5/5 | 1a |
| Disulfiram (OG) | 5/5 | ND | 5/5 | 1a |
| Carbomycin (SC) | 5/5 | 5/5 | 5/5 | 1c |
| Dapsone (OG) | 3/5 | 3/5 | 3/5 | 0.4643a |
| Rifampicin (OG) | 3/5 | 3/5 | 3/5 | 0.4643a |
| Cefotaxime (IP) | 1/5 | 1/5 | 1/5 | 0.0476 b* |
| Loratadine (OG) | 3/5 | 3/5 | 3/5 | 0.4643a |
ND, not done; OG, oral gavage; IP, intraperitoneal; SC, subcutaneous. Combined cultures compared to a = OG control group, b = IP control group, c = SC control group. ^ Number of mice positive for at least one tissue/total number of mice tested. * Statistically significant. Fisher exact test, the result is significant at p < 0.05.
3.4. PCR and RT-PCR confirm Bb infection in and viability in tissues following monotherapy
Bb was detected by PCR using the 16S primer to detect Borrelia species specific DNA in 2–5 mice from each monotherapy group. PCR using primers specific to the ospC gene in the B31 strain of Bb confirmed the presence of Bb in 6 out of 8 monotherapy groups. RT-PCR using the 16S primers detected Bb RNA in at least one mouse in all groups, confirming the presence of viable Bb following monotherapy (Table 3).
Table 3.
Molecular detection of Bb in tissues of mice.
| Treatments | Assay | ||
|---|---|---|---|
| PCR | RT-PCR | ||
| 16S^ | ospC^ | 16S^ | |
| Infected Untreated (OG) | 3/3 | 3/3 | 3/3 |
| Infected Untreated (IP) | 5/5 | 5/5 | 5/5 |
| Infected Untreated (SC) | 2/2 | 2/2 | 2/2 |
| Azlocillin (IP) | 4/5 | 4/5 | 3/3 |
| Bactrim (OG) | 5/5 | 5/5 | ND |
| Disulfiram (OG) | 5/5 | 5/5 | ND |
| Carbomycin (SC) | 5/5 | 5/5 | 5/5 |
| Dapsone (OG) | 2/5 | 0/5 | 1/5 |
| Rifampicin (OG) | 3/5 | 3/5 | 3/5 |
| Cefotaxime (IP) | 2/5 | 0/5 | 1/5 |
| Loratadine (OG) | 2/5 | 2/5 | 2/5 |
ND, Not done; OG, oral gavage; IP, intraperitoneal; SC, subcutaneous. ^ Number of mice positive for at least one tissue/total number of mice tested.
3.5. Cultured xenodiagnostic tick midguts harbor Bb following monotherapy
Cultured tick midguts identified Bb infection in at least one mouse from each treatment group. The cefotaxime group had significantly fewer infected mice compared to the control (p = 0.0476), but Bb persisted in one mouse. The other monotherapy groups had 2–5 mice positive for Bb per group following treatment. The control mice had a 70% detected rate of infection.
BSK-H or mBSK-II were used to culture the tick midguts. The results were identical for BSK-H and mBSK-II culture media with the exception of the group treated with dapsone for which Bb was detected in 40% of tick midguts cultured in BSK-H media and in 60% of tick midguts cultured in mBSK-II (Table 4). Results from both media conditions were combined for the analysis.
Table 4.
Culture results for xenodiagnostic ticks following monotherapy treatment of infected mice.
| Treatments | Cultures of Xenodiagnostic Ticks | |||
|---|---|---|---|---|
| BSK-H^ | mBSK-II^ | Combined Cultures^ | p value | |
| Infected Untreated (OG) | 1/3 | ND | 1/3 | |
| Infected Untreated (IP) | 5/5 | 3/3 | 5/5 | |
| Infected Untreated (SC) | 1/2 | 1/2 | 1/2 | |
| Azlocillin (IP) | 4/5 | ND | 4/5 | 1b |
| Bactrim (OG) | 5/5 | ND | 5/5 | 0.1071a |
| Disulfiram (OG) | 3/5 | ND | 3/5 | 1a |
| Carbomycin (SC) | 4/5 | 4/5 | 4/5 | 0.5238c |
| Dapsone (OG) | 2/5 | 3/5 | 3/5 | 1a |
| Rifampicin (OG) | 2/5 | 2/5 | 2/5 | 1a |
| Cefotaxime (IP) | 1/5 | 1/5 | 1/5 | 0.0476b* |
| Loratadine (OG) | 3/5 | 3/5 | 3/5 | 1a |
ND, Not done; OG, oral gavage; IP, intraperitoneal; SC, subcutaneous. Combined cultures compared to a = OG control group, b = IP control group, c = SC control group. ^ Number of pooled ticks per mouse positive/total number of mice tested. * Statistically significant. Fisher exact test, the result is significant at p < 0.05.
3.6. PCR and RT-PCR confirm Bb presence and viability in xenodiagnostic ticks following monotherapy
Bb was detected by PCR using the 16S and ospA primers in 1–5 tick midgut samples in all but one monotherapy group (Supplementary Table S8). The primer for ospA did not detect Bb in the rifampicin group. Control groups from both PCR assays detected Bb in 55% of samples. In particular, Bb was not detected by PCR in the group that received the placebo by subcutaneous injection. Bb was detected by RT-PCR using the 16S and ospA primers in 1–5 tick midgut samples in all monotherapy groups. Control groups from both PCR assays detected Bb in 95% of samples.
3.7. Confirmation of infection in combination therapy
Infection with Bb was confirmed in the same way as animals that received monotherapy: by either an ear punch culture positive for Bb or a 5-antigen serology result positive for 2 or more Bb antigens at days 21 or 60. Mice were considered infected if either test was positive. Twenty-seven out of 65 (42%) mice had positive ear punch results while 65 out of 65 (100%) had positive 5-antigen test results. Supplementary Table S9 shows the results of each group.
3.8. Culture of tissues from mice treated with combination therapy confirm Bb eradication
Combination therapy was efficient at clearing Bb infection depending on the series of drugs used. Mice were divided into groups of 2–5 and administered either a combination of test drugs or control treatment. Eight out of 11 groups receiving a combination of drugs were 0% positive for Bb infection, a significant reduction compared to the control groups (p = 0.05). The groups positive for Bb infection were azlocillin+ Bactrim (100%), disulfiram+ Bactrim (100%), and disulfiram + azlocillin (80%). There was no difference in the number of positive samples between BSK-H and mBSK-II media (Table 5).
Table 5.
Culture results of tissues in combination therapy.
| Treatments | Infection confirmation | |||
|---|---|---|---|---|
| BSK-H^ | mBSK-II^ | Combined culturesa | p-value | |
| Infected Untreated (OG) | 3/3 | ND | 3/3 | |
| Infected Untreated (IP) | 5/5 | ND | 5/5 | |
| Infected Untreated (SC) | 2/2 | ND | 2/2 | |
| Azlocillin (IP) + Bactrim (OG) | 5/5 | ND | 5/5 | 1c |
| Disulfiram (OG) + Bactrim (OG) | 5/5 | ND | 5/5 | 1b |
| Disulfiram (OG) + Azlocillin (IP) | 4/5 | 4/5 | 4/5 | 1c |
| Doxycycline (OG) + Ceftriaxone (IP) | 0/5 | 0/5 | 0/5 | 0.0008c* |
| Doxycycline (OG) + Ceftriaxone (IP) + Carbomycin (SC) | 0/5 | 0/5 | 0/5 | 0.0003e* |
| Dapsone (OG) + Rifampicin (OG) | 0/5 | 0/5 | 0/5 | 0.0179b* |
| Dapsone (OG) + Clofazimine (OG) | 0/5 | 0/5 | 0/5 | 0.0179b* |
| Dapsone (OG) + Clofazimine (OG) + Rifampicin (OG) | 0/5 | 0/5 | 0/5 | 0.0179b* |
| Cefotaxime (IP) + Loratadine (OG) + Doxycycline (OG) | 0/5 | 0/5 | 0/5 | 0.0008c* |
| Cefotaxime (IP) + Doxycycline (OG) | 0/5 | 0/5 | 0/5 | 0.0008c* |
| Cefotaxime (IP) + Carbomycin (SC) | 0/5 | 0/5 | 0/5 | 0.0013d* |
ND, not done; OG, oral gavage; IP, intraperitoneal; SC, subcutaneous. * Statistically significant. Fisher exact test, the result is significant at p < 0.05.
Number of mouse positive for at least one tissue/total number of mice tested.
Compared to OG control group.
Compared to OG + IP control group.
IP + SC control group.
OG + IP + SC control group.
3.9. RNA not detected in tissues following combination therapy
Groups that were positive for Bb infection by the tissue culture assay were excluded from PCR and RT-PCR analyses. Bb DNA was detected in at least 1 mouse in each combination group using the 16S primer and 4 groups (dapsone + rifampicin; dapsone + clofazimine; dapsone + clofazimine + rifampicin; cefotaxime + loratadine + doxycycline) using the ospC primer. RT-PCR using 16S primers did not detect Bb in any combination group, indicating complete eradication of viable Bb. ospC primers were not used for RT-PCR. All control groups were positive for both primers in PCR and for 16S in RT-PCR (Table 6).
Table 6.
Summary of the result of the molecular detection in tissues.
| Treatments | Assay | ||
|---|---|---|---|
| PCR | RT-PCR | ||
| 16S a | ospC a | 16S a | |
| Infected Untreated (OG) | 3/3 | 3/3 | 3/3 |
| Infected Untreated (IP) | 5/5 | 5/5 | 5/5 |
| Infected Untreated (SC) | 2/2 | 2/2 | 2/2 |
| Doxycycline (OG) + Ceftriaxone (IP) | 5/5 | 0/5 | 0/5 |
| Doxycycline (OG) + Ceftriaxone (IP) + Carbomycin (SC) | 3/5 | 0/5 | 0/5 |
| Dapsone (OG) + Rifampicin (OG) | 1/5 | 1/5 | 0/5 |
| Dapsone (OG) + Clofazimine (OG) | 3/5 | 1/5 | 0/5 |
| Dapsone (OG) + Clofazimine (OG) + Rifampicin (OG) | 3/5 | 1/5 | 0/5 |
| Cefotaxime (IP) + Loratadine (OG) + Doxycycline (OG) | 2/5 | 1/5 | 0/5 |
| Cefotaxime (IP) + Doxycycline (OG) | 3/5 | 0/5 | 0/5 |
| Cefotaxime (IP) + Carbomycin (SC) | 3/5 | 0/5 | 0/5 |
OG, oral gavage; IP, intraperitoneal; SC, subcutaneous.
Number of mice positive for Bb at least one tissue out of total number of mice tested.
3.10. Culture of xenodiagnostic tick guts from mice treated with combination therapy confirms Bb eradication
The same three drug combinations (azlocillin+ Bactrim, disulfiram+ Bactrim, and disulfiram + azlocillin) that were reported as positive for Bb as using the tissue culture assay were also positive using the tick mid-gut culture assay. The other treatment groups were negative for Bb (p ≤ 0.05). The mice in the control groups had a positivity rate of 70%. Results for the positive controls were comparable between BSK-H and mBSK-II media. mBSK-II was not used for OG controls and 3 samples were cultured in mBSK-II compared to 5 in the BSK-H media.
3.11. PCR and RT-PCR confirm eradication of Bb infection and viability in xenodiagnostic ticks following combination therapy
Groups that were positive for Bb infection in the tissue culture assay were excluded from PCR and RT-PCR analyses. Bb DNA was detected using the 16S primer in all but 2 groups: doxycycline + ceftriaxone + carbomycin and cefotaxime + loratadine + doxycycline. Bb DNA was not detected using the ospA primer in any of the combination groups. Bb RNA was detected using the 16S primer in the cefotaxime + carbomycin group. No other RNA was detected in the remaining treated groups using either 16S or ospA primers.
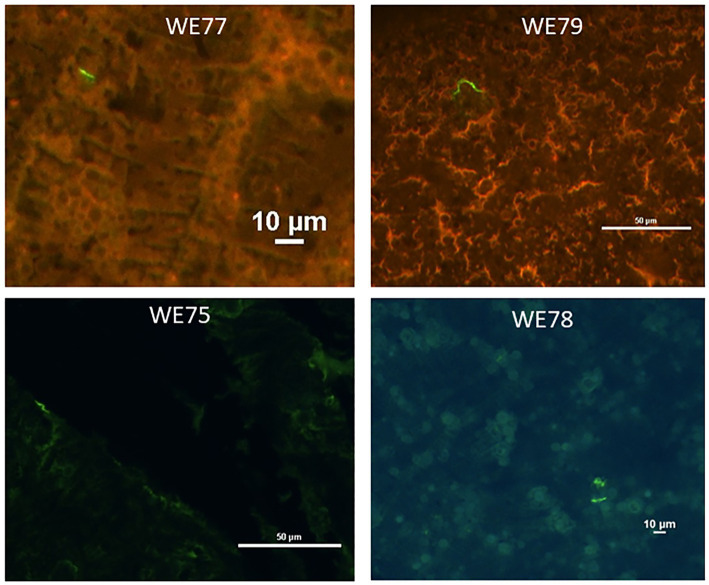
3.12. IFA of xenodiagnostic tick midguts did not detect any spirochetes following combination therapy
Immunofluorescent visualization of tick midguts showed Bb spirochetes in 0 out of 5 mice in each combination treatment group (p = 0.0476). Four out of 5 mice in the control group had at least 1 Bb spirochete (Figure 2). The IFA was only conducted on ticks in combination groups that were negative in both culture and RT-PCR. Results are summarized in Table 7.
Figure 2.
Representative images taken of xenodiagnostic tick midgut smears stained for Borrelia. Smears were stained using anti-OspA monoclonal antibodies, followed by anti-mouse-Alexa 488 (green) secondary antibodies. Mouse numbers and scale are indicated in white print.
Table 7.
Summary of the test results of the effective treatment groups.
| Treatment* | Tissues | Xeno Ticks | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Culture^ | PCR 16Sa | PCR ospCa | Culture^ | PCR 16S | PCR ospA | RT-PCR 16S | RT-PCR ospA | IFA | |
| Infected Untreated Group | + | + | − | + | − | − | + | + | + |
| + | + | + | + | − | − | + | + | − | |
| + | − | − | + | + | + | + | + | + | |
| + | + | + | − | − | − | − | + | + | |
| + | + | + | + | − | − | + | + | + | |
| Doxycycline (OG) + Ceftriaxone (IP) | − | − | − | − | − | − | − | − | − |
| − | − | − | − | + | − | − | − | − | |
| − | − | − | − | − | − | − | − | − | |
| − | + | − | − | − | − | − | − | − | |
| − | − | − | − | − | − | − | − | − | |
| Doxycycline (OG) + Ceftriaxone (IP) + Carbomycin (SC) | − | − | − | − | − | − | − | − | − |
| − | − | − | − | − | − | − | − | − | |
| − | − | − | − | − | − | − | − | − | |
| − | − | − | − | − | − | − | − | − | |
| − | − | − | − | − | − | − | − | − | |
| Dapsone (OG) + Rifampicin (OG) | − | − | − | − | N/A | N/A | N/A | N/A | − |
| − | − | − | − | − | − | − | − | − | |
| − | − | − | − | − | − | − | − | − | |
| − | − | − | − | − | − | − | − | − | |
| − | − | − | − | + | − | − | − | − | |
| Dapsone (OG) + Clofazimine (OG) | − | − | − | − | − | − | − | − | − |
| − | − | − | − | − | − | − | − | − | |
| − | + | + | − | − | − | − | − | − | |
| − | − | − | − | − | − | − | − | − | |
| − | − | − | − | + | − | − | − | − | |
| Dapsone (OG) + Clofazimine (OG) + Rifampicin (OG) | − | − | − | − | − | − | − | − | − |
| − | − | − | − | − | − | − | − | − | |
| − | − | − | − | + | − | − | − | − | |
| − | − | − | − | + | − | − | − | − | |
| − | − | − | − | − | − | − | − | − | |
| Cefotaxime (IP) + Loratadine (OG) + Doxycycline (OG) | − | − | − | − | − | − | − | − | − |
| − | − | − | − | − | − | − | − | − | |
| − | − | − | − | − | − | − | − | − | |
| − | − | − | − | − | − | − | − | − | |
| − | − | − | − | − | − | − | − | − | |
| Cefotaxime (IP) + Doxycycline (OG) | − | − | − | − | − | − | − | − | − |
| − | − | − | − | + | − | − | − | − | |
| − | − | − | − | − | − | − | − | − | |
| − | − | − | − | − | − | − | − | − | |
| − | − | − | − | + | − | − | − | − | |
*Every row in each group represents one mouse. ^Culture in BSK-H or mBSK-II. N/A = Not available. + = positive. − = negative. a = at least from one tissue and maximum 5 tissues. Tick section represents the pooled ticks tested per mouse except for IFA where the results represent at least one tick positive per mouse.
4. Discussion
Guidelines dictate that select monotherapies should be used to treat LD, but a subset of patients develop PTLD following treatment. Persistence of small numbers of Bb spirochetes following monotherapy in animal models demonstrates that monotherapy does not always eradicate the infection (Bockenstedt et al., 2002; Hodzic et al., 2008; Barthold et al., 2010; Embers et al., 2012). Many other microbial pathogens also cause persistent infection, but some, such as M. tuberculosis, are treated with combination therapy to effectively treat the infection (Schindel et al., 2000; O'Reilly et al., 2005; LaFleur et al., 2006; Lafleur et al., 2010; Mulcahy et al., 2010; Allison et al., 2011; Zhan et al., 2017; Silva et al., 2018). Unlike M. tuberculosis and other persistent pathogens, Bb has historically been difficult to culture, making combination therapy studies challenging. The methodology in this study allowed for the cultivation of Bb from mouse tissues and xenodiagnostic ticks and detection of viable Bb by RT-PCR in mouse tissues and xenodiagnostic ticks. Additionally, PCR and IFA were used to determine Bb presence regardless of viability. These techniques provided a platform to test combinations of 2 or 3 FDA-approved monotherapies to treat Bb infection.
Two types of media, BSK-H and mBSK-II, were used to culture Bb spirochetes. There was no difference in the number of positive samples between the BSK-H and mBSK-II media, but mBSK-II medium accelerated the growth and the number of spirochetes compared to BSK-H medium.
Our results support past findings that monotherapy does not eradicate Bb infection in all mice, leaving a subset of mice with persistent Bb infection (Bockenstedt et al., 2002; Hodzic et al., 2008, 2014, 2019; Barthold et al., 2010). Viable spirochetes were detected by culturing tissues in 3–5 out of 5 mice in 7 of the monotherapy groups and 1 out of 5 mice for the cefotaxime treatment group. Viability was also confirmed in all monotherapy group xenodiagnostic tick midgut cultures and the detection of RNA using RT-PCR in tissues and xenodiagnostic tick midguts. In contrast, several combination therapies were effective; viable spirochetes were not detected in cultured tissues in 8 out of 11 combination therapy groups in all mice. Viable spirochetes were also not detected by xenodiagnostic tick midgut culture or by the presence of RNA in tissues or xenodiagnostic tick midguts for the same combination therapies with the exception of the cefotaxime + carbomycin combination, which was positive for 16S RNA in xenodiagnostic tick midguts. Spirochetes were also not present in tissues when imaged using IFA.
The combination of ceftriaxone + doxycycline may be a good candidate for future investigation. Viable Bb was not detected by tissue culture, RT-PCR on tissues, xenodiagnostic tick mid-gut culture, or RT-PCR of xenodiagnostic tick midguts. Ceftriaxone and doxycycline are already used as monotherapy for LD treatment and both are well tolerated with favorable safety profiles (Klempner et al., 2001). The addition of carbomycin to the ceftriaxone + doxycycline combination was also examined but the carbomycin did not provide additional benefit.
Cefotaxime + Doxycycline was also effective at eradicating Bb infection. Viable Bb was not detected by tissue culture, RT-PCR on tissues, xenodiagnostic tick mid-gut culture, or RT-PCR of xenodiagnostic tick mid-guts. The addition of loratadine to the ceftriaxone + doxycycline combination was also examined but the loratadine did not provide additional benefit.
Dapsone in dual or triple combinations with rifampicin, clofazimine, or rifampicin + clofazimine were also effective at eradicating viable Bb. The combinations of dapsone with rifampicin, clofazimine or both are already the therapies of choice to treat multidrug-resistant M. tuberculosis or leprosy (dapsone) and have ample evidence for safety and tolerability in that population (Pattyn, 1990; Seydel et al., 1994; Barr, 2010; Gibson et al., 2022). Furthermore, clinical studies have demonstrated efficacy of dapsone combination therapy in Lyme disease patients with chronic symptoms/PTLD (Horowitz and Freeman, 2019; Horowitz and Freeman, 2020; Horowitz and Freeman, 2022). The results of this study and well-documented use in humans make this combination attractive for further investigation.
Some combination therapies were not effective at eradicating Bb. Three combinations (azlocillin+ Bactrim, disulfiram+ Bactrim, disulfiram + azlocillin) had persistent infection rates similar to those of control mice. Additionally, the mice treated with the cefotaxime + carbomycin combination were negative for Bb in tissue and xenodiagnostic tick midgut cultures but Bb RNA was detected in the xenodiagnostic tick midguts, indicating viable organisms.
The presence of Bb DNA in tissues and xenodiagnostic tick midguts in the absence of Bb RNA occurred in mice in most combination therapy groups. DNA in the absence of RNA is likely to indicate that the DNA is from residual non-viable Bb. The extent to which non-viable Bb affects the immune response following combination therapy requires further investigation.
Doxycycline and ceftriaxone were not included in this study. They are well studied and are not effective at eradicating the persistent form of Bb (Embers et al., 2012, 2017; Hodzic et al., 2019). The purpose of this study was to evaluate the efficacy of monotherapies and combination therapies to eliminate in vivo long-term infection with Bb. These results provide evidence that several different combination therapies can completely clear infection in all mice while treatment with monotherapy does not clear the Bb infection in some mice. Combination therapies, especially the ones highlighted in this study, need further investigation before conducting human trials. Mice in this study were infected by injection. Future work should examine the efficacy of combination therapy when infection is introduced by tick bite. Infection by tick bite has been previously used in studies using rhesus macaques (Embers et al., 2012, 2017; Crossland et al., 2018). These combinations should also be tested against other strains of Bb such as N40, JD1, and 297 due to the plasmid variation and genetic diversity among the strains of Bb (Casjens et al., 2012), and the loss of several plasmids in the persistent form of spirochetes after antimicrobial treatment in mice (Bockenstedt et al., 2002). Antibiotic susceptibility also varies with strains and isolates (Hunfeld et al., 2005; Yang et al., 2009; Ates et al., 2010). Additional studies in non-human primates would also provide information important for moving into human clinical trials.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by Tulane University Institutional Animal Care and Use Committee. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
YA: Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. MJ: Data curation, Methodology, Supervision, Writing – review & editing. NH: Methodology, Project administration, Supervision, Writing – review & editing. AT: Data curation, Methodology, Supervision, Writing – review & editing. CM: Data curation, Methodology, Writing – review & editing. ME: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
Acknowledgments
The authors would like to thank Allie Amick for her contributions to compiling and editing the contents of the manuscript.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Funding for this research was provided by the Bay Area Lyme Foundation and TNPRC base grant 5 P51OD 011104–56.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2023.1293300/full#supplementary-material
References
- Adkison H., Embers M. E. (2023). Lyme disease and the pursuit of a clinical cure. Front. Med. 10:10. doi: 10.3389/fmed.2023.1183344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison K. R., Brynildsen M. P., Collins J. J. (2011). Metabolite-enabled eradication of bacterial persisters by aminoglycosides. Nature 473, 216–220. doi: 10.1038/nature10069, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- al-Madfaa R. O., Alalawi M. A., Basudan L. O., Alhejaili S. F., Eljaaly K., Madani T. A., et al. (2020). Dual versus triple therapy for uncomplicated brucellosis: a retrospective cohort study. J. Infect. Dev. Ctries. 14, 1380–1386. doi: 10.3855/jidc.12741, PMID: [DOI] [PubMed] [Google Scholar]
- Ates L., Hanssen-Hübner C., Norris D. E., Richter D., Kraiczy P., Hunfeld K. P. (2010). Comparison of in vitro activities of tigecycline, doxycycline, and tetracycline against the spirochete Borrelia burgdorferi. Ticks Tick Borne Dis. 1, 30–34. doi: 10.1016/j.ttbdis.2009.11.004, PMID: [DOI] [PubMed] [Google Scholar]
- Aucott J. N., Crowder L. A., Kortte K. B. (2013). Development of a foundation for a case definition of post-treatment Lyme disease syndrome. Int. J. Infect. Dis. 17, e443–e449. doi: 10.1016/j.ijid.2013.01.008, PMID: [DOI] [PubMed] [Google Scholar]
- Barr J. (2010). A short history of Dapsone, or an alternative model of drug development†. J. Hist. Med. Allied Sci. 66, 425–467. doi: 10.1093/jhmas/jrq068, PMID: [DOI] [PubMed] [Google Scholar]
- Barthold S. W., Hodzic E., Imai D. M., Feng S., Yang X., Luft B. J. (2010). Ineffectiveness of tigecycline against persistent Borrelia burgdorferi. Antimicrob. Agents Chemother. 54, 643–651. doi: 10.1128/AAC.00788-09, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berende A., ter Hofstede H. J. M., Donders A. R. T., van Middendorp H., Kessels R. P. C., Adang E. M. M., et al. (2014). Persistent Lyme empiric antibiotic study Europe (PLEASE) - design of a randomized controlled trial of prolonged antibiotic treatment in patients with persistent symptoms attributed to Lyme borreliosis. BMC Infect. Dis. 14:543. doi: 10.1186/s12879-014-0543-y, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobe J. R., Jutras B. L., Horn E. J., Embers M. E., Bailey A., Moritz R. L., et al. (2021). Recent Progress in Lyme disease and remaining challenges. Front. Med.:8. doi: 10.3389/fmed.2021.666554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockenstedt L. K., Mao J., Hodzic E., Barthold S. W., Fish D. (2002). Detection of attenuated, noninfectious spirochetes in Borrelia burgdorferi-infected mice after antibiotic treatment. J. Infect. Dis. 186, 1430–1437. doi: 10.1086/345284, PMID: [DOI] [PubMed] [Google Scholar]
- Brian A., Fallon M. D., Keilp J., Prohovnik I., Heertum R. V., Mann J. J. (2003). Regional cerebral blood flow and cognitive deficits in chronic Lyme disease. J. Neuropsychiatry Clin. Neurosci. 15, 326–332. doi: 10.1176/jnp.15.3.326 [DOI] [PubMed] [Google Scholar]
- Casjens S. R., Mongodin E. F., Qiu W. G., Luft B. J., Schutzer S. E., Gilcrease E. B., et al. (2012). Genome stability of Lyme disease spirochetes: comparative genomics of Borrelia burgdorferi plasmids. PLoS One 7:e33280. doi: 10.1371/journal.pone.0033280, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin J. M., Yang T., Rebman A. W., Bechtold K. T., du Y., Mathews W. B., et al. (2018). Imaging glial activation in patients with post-treatment Lyme disease symptoms: a pilot study using [(11)C]DPA-713 PET. J. Neuroinflammation 15:346. doi: 10.1186/s12974-018-1381-4, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossland N. A., Alvarez X., Embers M. E. (2018). Late disseminated Lyme disease: associated pathology and spirochete persistence posttreatment in Rhesus macaques. Am. J. Pathol. 188, 672–682. doi: 10.1016/j.ajpath.2017.11.005, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embers M. E., Barthold S. W., Borda J. T., Bowers L., Doyle L., Hodzic E., et al. (2012). Persistence of Borrelia burgdorferi in Rhesus macaques following antibiotic treatment of disseminated infection. PLoS One 7:e29914. doi: 10.1371/journal.pone.0029914, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embers M. E., Hasenkampf N. R., Barnes M. B., Didier E. S., Philipp M. T., Tardo A. C. (2016). A five-antigen fluorescent bead-based assay for diagnosis of Lyme disease. Clin. Vaccine Immunol. 23, 294–303. doi: 10.1128/CVI.00685-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embers M. E., Hasenkampf N. R., Embers D. G., Doyle L. A. (2013). Pharmacokinetic analysis of oral doxycycline in rhesus macaques. J. Med. Primatol. 42, 57–61. doi: 10.1111/jmp.12031, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embers M. E., Hasenkampf N. R., Jacobs M. B., Tardo A. C., Doyle-Meyers L. A., Philipp M. T., et al. (2017). Variable manifestations, diverse seroreactivity and post-treatment persistence in non-human primates exposed to Borrelia burgdorferi by tick feeding. PLoS One 12:e0189071. doi: 10.1371/journal.pone.0189071, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon B. A., Keilp J. G., Corbera K. M., Petkova E., Britton C. B., Dwyer E., et al. (2008). A randomized, placebo-controlled trial of repeated IV antibiotic therapy for Lyme encephalopathy. Neurology 70, 992–1003. doi: 10.1212/01.WNL.0000284604.61160.2d, PMID: [DOI] [PubMed] [Google Scholar]
- Fallon B. A., Petkova E., Keilp J. G., Britton C. B. (2012). A reappraisal of the u.s. clinical trials of post-treatment Lyme disease syndrome. Open Neurol. J. 6, 79–87. doi: 10.2174/1874205X01206010079, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J., Auwaerter P. G., Zhang Y. (2015a). Drug combinations against Borrelia burgdorferi Persisters in vitro: eradication achieved by using Daptomycin, Cefoperazone and doxycycline. PLoS One 10:e0117207. doi: 10.1371/journal.pone.0117207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J., Li T., Yee R., Yuan Y., Bai C., Cai M., et al. (2019). Stationary phase persister/biofilm microcolony of Borrelia burgdorferi causes more severe disease in a mouse model of Lyme arthritis: implications for understanding persistence, post-treatment Lyme disease syndrome (PTLDS), and treatment failure. Discov. Med. 27, 125–138. PMID: [PubMed] [Google Scholar]
- Feng J., Shi W., Zhang S., Sullivan D., Auwaerter P. G., Zhang Y. (2016a). A drug combination screen identifies drugs active against amoxicillin-induced round bodies of in vitro Borrelia burgdorferi Persisters from an FDA drug library. Front. Microbiol. 7:743. doi: 10.3389/fmicb.2016.00743, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J., Shi W., Zhang S., Zhang Y. (2015b). Identification of new compounds with high activity against stationary phase Borrelia burgdorferi from the NCI compound collection. Emerg. Microbes Infect. 4:e31, 1–15. doi: 10.1038/emi.2015.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J., Wang T., Shi W., Zhang S., Sullivan D., Auwaerter P. G., et al. (2014). Identification of novel activity against Borrelia burgdorferi persisters using an FDA approved drug library. Emerg. Microbes Infect. 3:e49. doi: 10.1038/emi.2014.53, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J., Zhang S., Shi W., Zhang Y. (2016b). Ceftriaxone pulse dosing fails to eradicate biofilm-like microcolony B. burgdorferi Persisters which are sterilized by Daptomycin/ doxycycline/cefuroxime without pulse dosing. Front. Microbiol. 7:1744. doi: 10.3389/fmicb.2016.01744, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J., Zhang S., Shi W., Zhang Y. (2017). Activity of sulfa drugs and their combinations against stationary phase B. burgdorferi in vitro. Antibiotics 6:10. doi: 10.3390/antibiotics6010010, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson S. E. R., Harrison J., Molloy A., Cox J. A. G. (2022). Cholesterol-dependent activity of dapsone against non-replicating persistent mycobacteria. Microbiology 168:1279. doi: 10.1099/mic.0.001279 [DOI] [PubMed] [Google Scholar]
- Hirsch A. G., Poulsen M. N., Nordberg C., Moon K. A., Rebman A. W., Aucott J. N., et al. (2020). Risk factors and outcomes of treatment delays in Lyme disease: a population-based retrospective cohort study. Front. Med. 7:560018. doi: 10.3389/fmed.2020.560018, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodzic E., Feng S., Holden K., Freet K. J., Barthold S. W. (2008). Persistence of Borrelia burgdorferi following antibiotic treatment in mice. Antimicrob. Agents Chemother. 52, 1728–1736. doi: 10.1128/AAC.01050-07, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodzic E., Imai D. M., Escobar E. (2019). Generality of post-antimicrobial treatment persistence of Borrelia burgdorferi strains N40 and B31 in genetically susceptible and resistant mouse strains. Infect. Immun. 87, e00442–e00419. doi: 10.1128/IAI.00442-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodzic E., Imai D., Feng S., Barthold S. W. (2014). Resurgence of persisting non-cultivable Borrelia burgdorferi following antibiotic treatment in mice. PLoS One 9:e86907. doi: 10.1371/journal.pone.0086907, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz R. I., Freeman P. R. (2019). Precision medicine: retrospective chart review and data analysis of 200 patients on dapsone combination therapy for chronic Lyme disease/post-treatment Lyme disease syndrome: part 1. Int. J. Gen. Med. 12, 101–119. doi: 10.2147/IJGM.S193608, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz R. I., Freeman P. R. (2020). Efficacy of double-dose Dapsone combination therapy in the treatment of chronic Lyme disease/post-treatment Lyme disease syndrome (PTLDS) and associated co-infections: a report of three cases and retrospective chart review. Antibiotics 9:725. doi: 10.3390/antibiotics9110725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz R. I., Freeman P. R. (2022). Efficacy of short-term high dose pulsed Dapsone combination therapy in the treatment of chronic Lyme disease/post-treatment Lyme disease syndrome (PTLDS) and associated co-infections: a report of three cases and literature review. Antibiotics 11:912. doi: 10.3390/antibiotics11070912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshina T., Nozaki S., Hamazaki T., Kudo S., Nakatani Y., Kodama H., et al. (2018). Disulfiram enhanced delivery of orally administered copper into the central nervous system in Menkes disease mouse model. J. Inherit. Metab. Dis. 41, 1285–1291. doi: 10.1007/s10545-018-0239-3, PMID: [DOI] [PubMed] [Google Scholar]
- Hunfeld K.-P., Ruzic-Sabljic E., Norris D. E., Kraiczy P., Strle F. (2005). In vitro susceptibility testing of Borrelia burgdorferi Sensu Lato isolates cultured from patients with erythema Migrans before and after antimicrobial chemotherapy. Antimicrob. Agents Chemother. 49, 1294–1301. doi: 10.1128/AAC.49.4.1294-1301.2005, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan H., Ahmad I., Habib H., Hayat K., Hayat Z. (2018). Antibiotics in the management of brucellosis. Gomal J. Med. Sci. 16, 114–116. doi: 10.46903/gjms/16.04.1988 [DOI] [Google Scholar]
- Klempner M. S., Hu L. T., Evans J., Schmid C. H., Johnson G. M., Trevino R. P., et al. (2001). Two controlled trials of antibiotic treatment in patients with persistent symptoms and a history of Lyme disease. N. Engl. J. Med. 345, 85–92. doi: 10.1056/NEJM200107123450202, PMID: [DOI] [PubMed] [Google Scholar]
- Kugeler K. J., Schwartz A. M., Delorey M. J., Mead P. S., Hinckley A. F. (2021). Estimating the frequency of Lyme disease diagnoses, United States, 2010-2018. Emerg. Infect. Dis. 27, 616–619. doi: 10.3201/eid2702.202731, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFleur M. D., Kumamoto C. A., Lewis K. (2006). Candida albicans biofilms produce antifungal-tolerant persister cells. Antimicrob. Agents Chemother. 50, 3839–3846. doi: 10.1128/AAC.00684-06, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafleur M. D., Qi Q., Lewis K. (2010). Patients with long-term oral carriage harbor high-persister mutants of Candida albicans. Antimicrob. Agents Chemother. 54, 39–44. doi: 10.1128/AAC.00860-09, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRocca T. J., Holthausen D. J., Hsieh C., Renken C., Mannella C. A., Benach J. L. (2009). The bactericidal effect of a complement-independent antibody is osmolytic and specific to Borrelia. Proc. Natl. Acad. Sci. U. S. A. 106, 10752–10757. doi: 10.1073/pnas.0901858106, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin J. M., Nelson J. A., Segreti J., Harrison B., Benson C. A., Strle F. (1993). In vitro susceptibility of Borrelia burgdorferi to 11 antimicrobial agents. Antimicrob. Agents Chemother. 37, 1444–1446. doi: 10.1128/AAC.37.7.1444, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvel C. L., Alm K. H., Bhattacharya D., Rebman A. W., Bakker A., Morgan O. P., et al. (2022). A multimodal neuroimaging study of brain abnormalities and clinical correlates in post treatment Lyme disease. PLoS One 17:e0271425. doi: 10.1371/journal.pone.0271425, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulcahy L. R., Burns J. L., Lory S., Lewis K. (2010). Emergence of Pseudomonas aeruginosa strains producing high levels of persister cells in patients with cystic fibrosis. J. Bacteriol. 192, 6191–6199. doi: 10.1128/JB.01651-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama T., Kawahara R., Kumeda Y., Yamamoto Y. (2018). Extended-spectrum β-lactamase-producing Escherichia coli contributes to the survival of cefotaxime-susceptible E. coli under high concentrations of cefotaxime by acquisition of increased AmpC expression. FEMS Microbiol. Lett. 365:9. doi: 10.1093/femsle/fny009 [DOI] [PubMed] [Google Scholar]
- O'Reilly K. M., Urban M. A., Barriero T., Betts R. F., Trawick D. R. (2005). Persistent culture-positive Legionella infection in an immunocompromised host. Clin. Infect. Dis. 40, e87–e89. doi: 10.1086/429826, PMID: [DOI] [PubMed] [Google Scholar]
- Pattyn S. R. (1990). Therapeutic regimens in leprosy. Verh. K. Acad. Geneeskd. Belg. 52, 301–310. PMID: [PubMed] [Google Scholar]
- Pavia C. S., Wormser G. P. (2014). Culture of the entire mouse to determine whether cultivable Borrelia burgdorferi persists in infected mice treated with a five-day course of ceftriaxone. Antimicrob. Agents Chemother. 58, 6701–6703. doi: 10.1128/AAC.03751-14, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothineni V. R., Potula H. H. S. K., Ambati A., Mallajosyula V. V. A., Sridharan B., Inayathullah M., et al. (2020). Azlocillin can be the potential drug candidate against drug-tolerant Borrelia burgdorferi sensu stricto JLB31. Sci. Rep. 10:3798. doi: 10.1038/s41598-020-59600-4, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothineni V. R., Wagh D., Babar M. M., Inayathullah M., Solow-Cordero D., Kim K. M., et al. (2016). Identification of new drug candidates against Borrelia burgdorferi using high-throughput screening. Drug Des. Devel. Ther. 10, 1307–1322. doi: 10.2147/DDDT.S101486, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauer S., Kastenbauer S., Hofmann H., Fingerle V., Huppertz H. I., Hunfeld K. P., et al. (2020). Guidelines for diagnosis and treatment in neurology - Lyme neuroborreliosis. Ger. Med. Sci. 18:Doc03. doi: 10.3205/000279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebman A. W., Aucott J. N. (2020). Post-treatment Lyme disease as a model for persistent symptoms in Lyme disease. Front. Med. 7:57. doi: 10.3389/fmed.2020.00057, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebman A. W., Yang T., Aucott J. N. (2021). Symptom heterogeneity and patient subgroup classification among US patients with post-treatment Lyme disease: an observational study. BMJ Open 11:e040399. doi: 10.1136/bmjopen-2020-040399, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto M., Mutoh Y., Fukagawa Y., Ishikura T., Lein J. (1984). Deepoxidation of 16-membered epoxyenone macrolide antibiotics. III. In vitro and in vivo evaluation of deepoxidation products of carbomycin a, deltamycin A1, 4″-phenylacetyldeltamycin, angolamycin and rosamicin. J. Antibiot. (Tokyo) 37, 130–135. doi: 10.7164/antibiotics.37.130, PMID: [DOI] [PubMed] [Google Scholar]
- Schindel C., Siepmann U., Han S. R., Ullmann A. J., Mayer E., Fischer T., et al. (2000). Persistent Legionella infection in a patient after bone marrow transplantation. J. Clin. Microbiol. 38, 4294–4295. doi: 10.1128/JCM.38.11.4294-4295.2000, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seydel J. K., Schaper K.-J., RüChgerdes S. (1994). Experimental drugs and combination therapy. Immunobiology 191, 569–577. doi: 10.1016/S0171-2985(11)80464-X [DOI] [PubMed] [Google Scholar]
- Silva D. R., Dalcolmo M., Tiberi S., Arbex M. A., Munoz-Torrico M., Duarte R., et al. (2018). New and repurposed drugs to treat multidrug- and extensively drug-resistant tuberculosis. J. Bras. Pneumol. 44, 153–160. doi: 10.1590/s1806-37562017000000436, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straubinger R. K., Summers B. A., Chang Y. F., Appel M. J. (1997). Persistence of Borrelia burgdorferi in experimentally infected dogs after antibiotic treatment. J. Clin. Microbiol. 35, 111–116. doi: 10.1128/jcm.35.1.111-116.1997, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torbahn G., Hofmann H., Rücker G., Bischoff K., Freitag M. H., Dersch R., et al. (2018). Efficacy and safety of antibiotic therapy in early cutaneous Lyme Borreliosis: a network Meta-analysis. JAMA Dermatol. 154, 1292–1303. doi: 10.1001/jamadermatol.2018.3186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touradji P., Aucott J. N., Yang T., Rebman A. W., Bechtold K. T. (2018). Cognitive decline in post-treatment Lyme disease syndrome. Arch. Clin. Neuropsychol. 34:acy051. doi: 10.1093/arclin/acy051 [DOI] [PubMed] [Google Scholar]
- Ulrich E., Hahn H., Trautmann M., Krause B., Bauernfeind A. (1989). Comparative efficacy of ciprofloxacin, azlocillin, imipenem/cilastatin and tobramycin in a model of experimental septicemia due to Pseudomonas aeruginosa in neutropenic mice. Infection 17, 311–315. doi: 10.1007/BF01650716, PMID: [DOI] [PubMed] [Google Scholar]
- Wagh D., Pothineni V. R., Inayathullah M., Liu S., Kim K. M., Rajadas J. (2015). Borreliacidal activity of Borrelia metal transporter a (BmtA) binding small molecules by manganese transport inhibition. Drug Des. Devel. Ther. 9, 805–816. doi: 10.2147/DDDT.S77063, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormser G. P., Dattwyler R. J., Shapiro E. D., Halperin J. J., Steere A. C., Klempner M. S., et al. (2006). The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic Anaplasmosis, and Babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 43, 1089–1134. doi: 10.1086/508667, PMID: [DOI] [PubMed] [Google Scholar]
- Yang X., Nguyen A., Qiu D., Luft B. J. (2009). In vitro activity of tigecycline against multiple strains of Borrelia burgdorferi. J. Antimicrob. Chemother. 63, 709–712. doi: 10.1093/jac/dkn551, PMID: [DOI] [PubMed] [Google Scholar]
- Zhan L., Tang J., Sun M., Qin C. (2017). Animal models for tuberculosis in translational and precision medicine. Front. Microbiol. 8:717. doi: 10.3389/fmicb.2017.00717, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. (2005). The magic bullets and tuberculosis drug targets. Annu. Rev. Pharmacol. Toxicol. 45, 529–564. doi: 10.1146/annurev.pharmtox.45.120403.100120, PMID: [DOI] [PubMed] [Google Scholar]
- Zhang Y., Yew W. W., Barer M. R. (2012). Targeting persisters for tuberculosis control. Antimicrob. Agents Chemother. 56, 2223–2230. doi: 10.1128/AAC.06288-11, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.