Abstract
Understanding the mechanisms governing body size attainment during animal development is of paramount importance in biology. In insects, a crucial phase in determining body size occurs at the larva-pupa transition, marking the end of the larval growth period. Central to this process is the attainment of the threshold size (TS), a critical developmental checkpoint that must be reached before the larva can undergo metamorphosis. However, the intricate molecular mechanisms by which the TS orchestrates this transition remain poor understood. In this study, we investigate the role of the interaction between the Torso and TGFß/activin signaling pathways in regulating metamorphic timing in the red flour beetle, Tribolium castaneum. Our results show that Torso signaling is required specifically during the last larval instar and that its activation is mediated not only by the prothoracicotropic hormone (Tc-Ptth) but also by Trunk (Tc-Trk), another ligand of the Tc-Torso receptor. Interestingly, we show that while Tc-Torso activation by Tc-Ptth determines the onset of metamorphosis, Tc-Trk promotes growth during the last larval stage. In addition, we found that the expression of Tc-torso correlates with the attainment of the TS and the decay of juvenile hormone (JH) levels, at the onset of the last larval instar. Notably, our data reveal that activation of TGFß/activin signaling pathway at the TS is responsible for repressing the JH synthesis and inducing Tc-torso expression, initiating metamorphosis. Altogether, these findings shed light on the pivotal involvement of the Ptth/Trunk/Torso and TGFß/activin signaling pathways as critical regulatory components orchestrating the TS-driven metamorphic initiation, offering valuable insights into the mechanisms underlying body size determination in insects.
Author summary
Understanding the mechanisms that determine an animal’s final body size is a fundamental question in biology. In animals, the majority of growth takes place during the juvenile stage, with adult size being established as they transition into adulthood. Hormones play a pivotal role in orchestrating this complex process. In the case of insects, the metamorphic transition is induced by the interplay between the steroid hormone ecdysone and the sesquiterpenoid juvenile hormone (JH). Our research delved into the roles of the Torso and TGFß/Activin signaling pathways in regulating ecdysone and JH biosynthesis. Remarkably, we discovered that in contrast to other insects, the Torso pathway in Tribolium is activated by not one, but two ligands: the Prothoracicotropic hormone that regulates ecdysone production and Trunk that promotes growth in the last larval stage. Additionally, our investigations unveiled the dual functionality of the TGFß/Activin signaling pathway in the initiation of metamorphosis. Firstly, it lowers JH levels, setting in motion the genetic changes required for the metamorphic transition, including the upregulation of Torso and, secondly, facilitating ecdysone production. In summary, our research sheds light on the intricate regulatory network governing metamorphic timing and body size in insects.
Introduction
How animals reach their final body size is a fundamental question in biology. Organism final body size depends on environmental cues as well as on the precise activation of genetic programs during development. In many animals, growth takes place mainly during the juvenile stage, and adult body size is therefore determined upon entering into adulthood [1]. Deciphering the molecular mechanisms underlying the timely decision to initiate adult maturation is, therefore, critical to understand how body size is controlled.
Hormones play an important role in the regulation of final body size, in part by coordinating the onset of adult maturation. For example, in holometabolous insects, whose growth period is restricted to a series of larval molts that accommodate the increasing size of the body, the onset of metamorphosis is triggered by a sharp increase of the steroid hormone ecdysone upon reaching a size-dependent developmental checkpoint [2]. Ecdysone is synthesized in a specialized organ named the prothoracic gland (PG) through the sequential catalytic action of a series of enzymes encoded by the Halloween gene family. These include the Rieske-domain protein neverland (nvd) [3,4], the short-chain dehydrogenase/reductase shroud (sro) [5] and the P450 enzymes spook (spo), spookier (spok), phantom (phm), disembodied (dib) and shadow (sad) [6–11]. The expression of the Halloween genes is highly regulated, being up-regulated in the PG at the metamorphic transition by the integrated activity of several receptor tyrosine kinases (RTKs) [12]. These RTKs include Torso [13–15], Epidermal Growth Factor Receptor (Egfr) [16,17], Anaplastic Lymphoma Kinase (Alk) and PDGF and VEGF receptor-related (Pvr) [12], all acting through the Ras/Raf/Erk MAP kinase signal transduction cascade, and the Insulin receptor (InR) acting through the PI3K/Akt pathway [18–21]. Among these RTKs, Torso is of particular interest since it acts as a key transducer of critical environmental cues such as nutrition status and population density [15,22]. In order to exert its regulatory function in the PG, Torso binds the Prothoracicotropic hormone (Ptth), a neuropeptide secreted from neuroendocrine cells located in the brain [13,23,24]. Although Ptth is considered the unique Torso ligand during postembryonic development, the fact that torso mutants showed a significant longer delay than Ptth null mutants [15], suggests the existence of additional ligands involved in the activation of Torso signaling. In fact, in addition to the Ptth, Torso possesses another ligand, Trunk (Trk), belonging to the cysteine knot growth factor superfamily, responsible for the activation of the pathway during the early embryo [14,25–27]. Although trk is not expressed during postembryonic stages of the fly Drosophila melanogaster, ectopic expression of a cleaved form of Trk in the PG is able to activate the pathway inducing precocious pupariation [26]. Nevertheless, to date, the activation of the Torso pathway by Trk has only been observed in the embryogenesis of all the studied species [25,26].
Despite the well-documented role of the Ptth/Torso pathway in triggering the onset of metamorphosis in Drosophila and Bombyx mori, the regulation of the pathway itself is less understood. Whereas Ptth synthesis and release from the neuroendocrine brain cells seems to depend on nutritional and environmental cues [13,18,20,28], the regulatory mechanisms that control the expression of torso is poorly understood. In this regard, it has been shown in Drosophila that the activity of the Transforming Growth Factor ß (TGFß)/Activin pathway in the PG is required for the proper expression of torso [29]. However, since depletion of TGFß/activin affects cell growth and morphology [29], it is plausible that torso regulation by this pathway is a collateral rather than a direct effect. In addition, in the beetle Tribolium and hemimetabolous insects such as Gryllus and Blattella, TGFß/activin has also been described as a negative regulator of the biosynthesis of the anti-metamorphic juvenile hormone (JH) [30–32], suggesting a possible link between the decay of JH at the end of larval development and the activation of the Torso signaling pathway at the onset of metamorphosis.
Here, we use the red flour beetle Tribolium to study the regulation of metamorphic timing by the Torso signaling pathway. During development, the size of Tribolium increases over several larval instars until reaching a critical size-assessment checkpoint—the threshold size (TS)—that instructs the larva to enter metamorphosis at the ensuing molt, thus ending the growth period [33]. In laboratory conditions, the TS is reached at the onset of the seventh larval instar (L7), and is associated with the decay of JH and the consequence down-regulation of the anti-metamorphic transcription factor Krüppel-homolg 1 (Tc-Kr-h1). As a result, the stage-specific transcription factors Ecdysone inducible protein 93F (Tc-E93) becomes up-regulated triggering metamorphosis [33]. Unfortunately, although the described genetic changes that control the nature of the metamorphic transition have been studied in detail [33,34], the molecular mechanisms underlying the regulation of the metamorphic timing in Tribolium remain to be clearly defined.
In the present study, we uncover the role and regulation of Tc-torso in the control of the metamorphic timing in Tribolium. We show that Tc-Torso is required specifically in the last larval instar of the beetle for the synthesis of ecdysone that promotes the metamorphic transition. Remarkably, we found that during this period Torso signaling is activated not only by Tc-Ptth but also by Tc-Trk. However, whereas Tc-Torso activation by Tc-Ptth determines the onset of metamorphosis, Tc-Trk promotes growth during the last larval stage. Moreover, our experiments indicate that Tc-torso expression depends on larvae reaching the TS at the onset of the last larval instar, and that the sustained increase in the expression levels of Tc-torso during the last larval instar depends on the decay of JH titers. Interestingly, we found that the activity of Baboon (Tc-Babo) and Myoglianin (Tc-Myo), two key components of the TGFß/activin signaling pathway, are required to repress JH synthesis and activate the TS-dependent induction of Tc-torso expression. Taken together, our results indicate that the Ptth/Trk/Torso and TGFß/activin pathways are critical component of the mechanism that controls the final body size of Tribolium by regulating growth and the timing of the metamorphic transition.
Results
Torso signaling regulates metamorphic timing in Tribolium
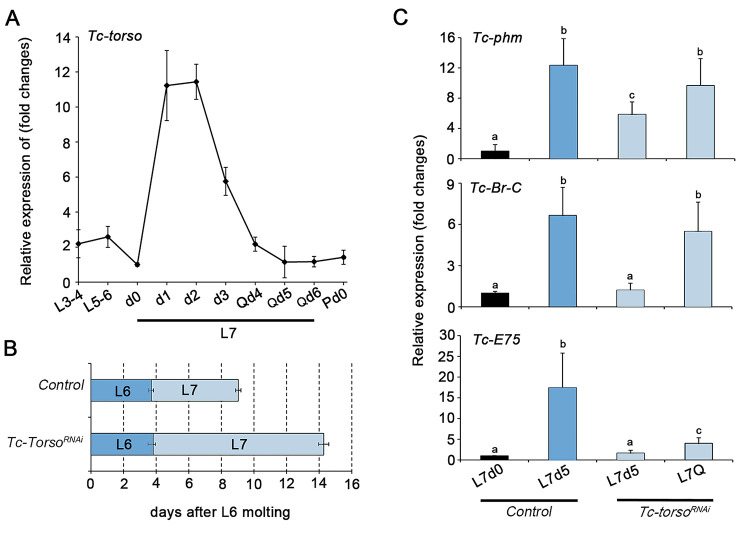
To investigate the role of Torso signaling in Tribolium, we first examined its temporal expression pattern during larval development. Tc-torso mRNA levels were low during early stages of larval development but strongly increased after entering into the last larval instar (L7), reaching the maximal level of expression at 24-48h, to decline thereafter until the end of the instar (Fig 1A). This result suggests that Torso signaling in Tribolium is necessary during the last larval stage of development. To test this possibility, we analysed the effects of blocking Torso signaling from early larval development by injecting dsRNA of Tc-torso in newly molted antepenultimate L5 larvae (Tc-torsoRNAi animals). Specimens injected with dsMock were used as negative controls (Control animals). Under these conditions, Tc-torsoRNAi larvae molted with proper timing to L6 and then to L7, but showed a significant developmental delay in the pupation time (Fig 1B). These results confirmed that Torso signaling in Tribolium is specifically required during the last larval instar to control the timing of the metamorphic transition.
Fig 1. Tc-Torso is a critical regulator of pupation in Tribolium.
(A) Temporal changes in Tc-torso mRNA levels measured by qRT-PCR from L3-4 instar larvae to pupa day 0 (Pd0). Transcript abundance values were normalized against the Tc-Rpl32 transcript. Error bars indicate the standard error of the mean (SEM) (n = 5). (B) Developmental progression of newly molted L5 larvae after injection with either dsMock (Control) (n = 30) or dsTc-torso (n = 22). The bars represent the mean ± standard deviation (SD) for each developmental stage observed after the double-stranded RNA injection. (C) Transcript levels of Tc-phm, Tc-Br-C, and Tc-E75 measured by qRT-PCR in 0 and 5-day-old L7 Control larvae and 5-day-old L7 (L7d5) and quiescent (L7Q) Tc-torsoRNAi larvae. Transcript abundance values were normalized against the Tc-Rpl32 transcript. Error bars indicate the SEM (n = 3–5). Different letters represent groups with significant differences based on an ANOVA test (Tukey, p < 0.001). Raw data are in S1 Data (tab Fig 1).
To determine whether the pupation delay observed in Tc-torsoRNAi larvae was caused by an ecdysone deficiency, we next analyzed the expression levels of the representative Halloween gene Tc-phm, as well as a number of well characterized ecdysone-dependent genes such as Tc-Br-C and Tc-E75 that are used as proxies for ecdysone levels. Consistent with delayed pupation, Tc-torsoRNAi larvae presented a significant delay in the expression of all the analyzed genes when compared to Control animals (Fig 1C). Altogether, these results indicated that Torso activation during the last larval stage of Tribolium is required to control the production of ecdysone that timely triggers pupa formation.
Tc-Ptth and Tc-Trk activate Torso signaling during the last larval instar
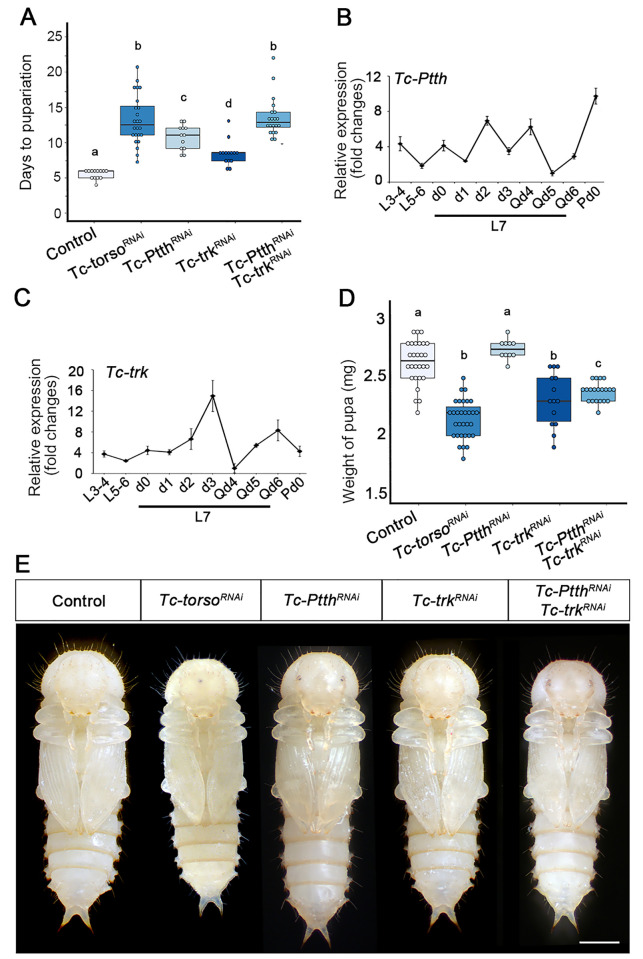
To further characterize the function of Torso during the metamorphic transition, we knocked-down this receptor specifically in the last larval stage. Under this treatment, Tc-torso-depleted larva pupated with a delay of 7 days (Fig 2A). However, contrary to Drosophila, where depletion of either Dm-Ptth or Dm-torso resulted in bigger pupae [14], Tc-torsoRNAi pupae were slightly smaller and lighter than Control pupae (Fig 2D and 2E). These results suggest the possibility that postembryonic activation of Tc-Torso not only controls developmental timing through the regulation of ecdysone synthesis by Tc-Ptth activation, but also controls systemic growth rate. To further study this possibility, we analyzed the role of Ptth in Torso signaling activation in Tribolium. First, we measured the expression level of Tc-Ptth in last instar larvae, and found fluctuating levels with two peaks at day 2 and 4 (Fig 2B). Interestingly, depletion of Tc-Ptth in Tribolium (Tc-PtthRNAi animals) induced a pupation delay of 5 days, compared to the almost 7 days observed in Tc-torsoRNAi larvae (Fig 2A). Moreover, the absence of Tc-Ptth did not affect the weight of the resulting pupae when compared to Control pupae (Fig 2D and 2E). Taken together, the differences between Tc-torsoRNAi and Tc-PtthRNAi animals suggest that the activation of Torso signaling in Tribolium might not rely only on Tc-Ptth but also on additional ligands.
Fig 2. Activation of Tc-Torso by Tc-Ptth and Tc-Trk during the final larval stage of Tribolium.
(A) Developmental duration of the last larval stage (L7) in newly molted L6 larvae injected with dsMock (Control) (n = 13), dsTc-torso (n = 33), dsTc-Ptth (n = 12), dsTc-trk (n = 14), and dsTc-Ptth + dsTc-trk (n = 16). (B-C) Temporal changes in Tc-Ptth mRNA (B) and Tc-trk mRNA (C) levels measured by qRT-PCR during larval development, from L3-4 to pupa day 0 (Pd0). Transcript abundance values are normalized against the Tc-Rpl32 transcript. Error bars indicate the standard error of the mean (SEM) (n = 5). (D) Body weight of the of Control (n = 10), Tc-torsoRNAi (n = 10), Tc-PtthRNAi (n = 12), Tc-trkRNAi (n = 14), and Tc-PtthRNAi + dsTc-trkRNAi (n = 16) larvae pupae. All weights were measured on day 0 of the pupal instar. (E) Ventral view of a Control and Tc-torsoRNAi, Tc-PtthRNAi, Tc-trkRNAi, and Tc-PtthRNAi + Tc-trkRNAi pupa. Scale bar represents 0.5 mm. In A and D, boxplots are used to represent the data, with black lines indicating medians, colored boxes showing the IQR, and bars indicating the upper and lower values. Values > ±1.5 x the IQR outside the box are considered outliers. Different letters represent groups with significant differences based on an ANOVA test (Tukey, p < 0.001). Raw data of A, B, C and D are in S1 Data (tab Fig 2).
In Drosophila, the Torso pathway is also required for the formation of the most anterior and posterior regions of the embryo. During this process, Dm-Torso receptor is activated by the ligand Dm-Trk, which is synthetized in the early embryo [14,25–27]. Similarly, generation of abdominal segments in Tribolium requires the Tc-Trk-dependent activation of Torso signaling in the posterior region of the early embryo [26]. Although Drosophila Dm-trk is not expressed during larval development, ectopic expression of Dm-Trk induced a mild advance on pupariation [26], indicating that Dm-Trk is able to activate Torso signaling in the larval PG of Drosophila. Importantly, we were able to detect expression of Tc-trk in the last instar larvae of Tribolium, presenting a similar pattern than Tc-torso, with a peak of expression at day 3 (Fig 2C). Next, we injected dsRNA of Tc-trunk (Tc-trkRNAi animals) in L6 larvae to analyse its functional relevance during the last larval instar. In contrast to Tc-Ptth-depleted animals, Tc-trkRNAi individuals exhibited around 2 days of pupation delay (Fig 2A). Importantly, however, the resulting Tc-trkRNAi pupae were as small as the Tc-torsoRNAi pupae (Fig 2D and 2E). These results suggest that the activation of Tc-Torso during the last stage of larval development depends on both Tc-Trunk and Tc-Ptth. To confirm this possibility, we knocked-down both ligands simultaneously (Tc-PtthRNAi + Tc-trkRNAi animals). As expected, depletion of Tc-trk and Tc-Ptth phenocopied the absence of Tc-torso, as Tc-trkRNAi + Tc-PtthRNAi animals presented 7 days of delay and smaller pupae (Fig 2A, 2D and 2E). Altogether, these results show that both ligands, Tc-Trk and Tc-Ptth, act as Tc-Torso ligands to activate Torso signaling during the last larval stage in order to trigger a timely metamorphic transition.
Tc-trk and Tc-Ptth exhibit tissue-specific expression patterns
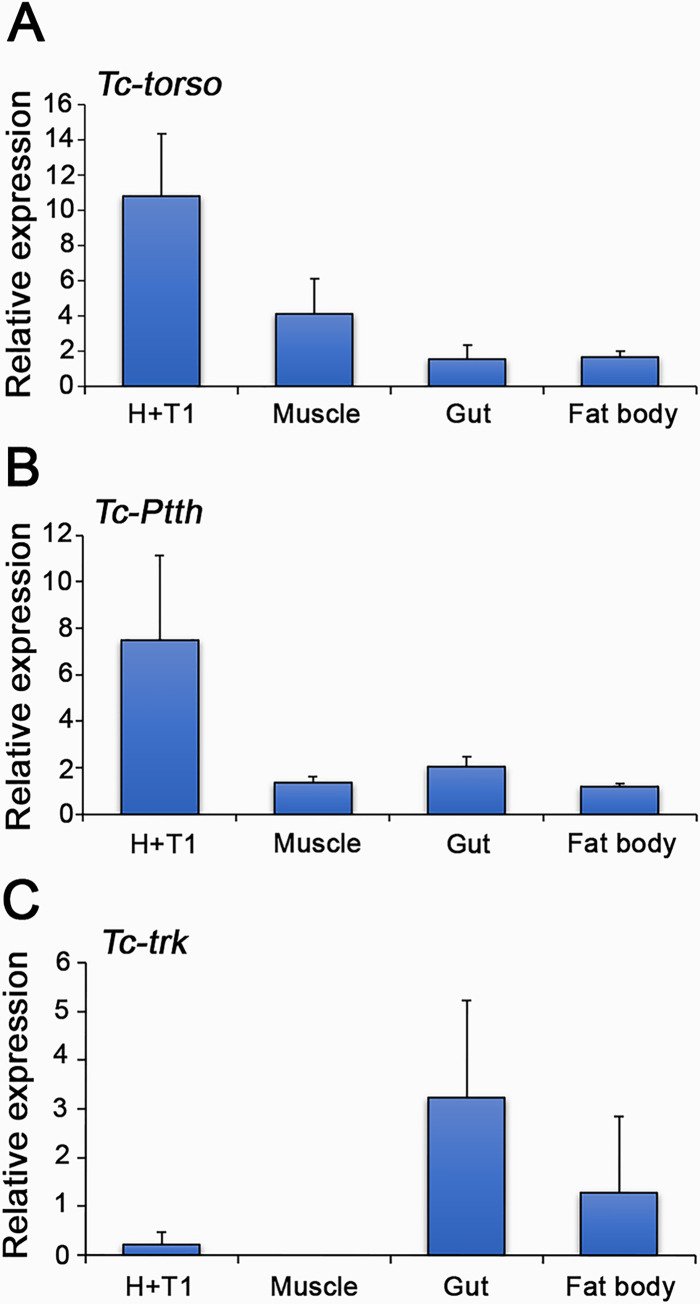
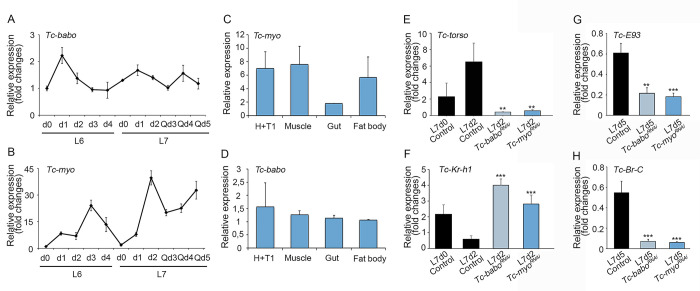
Our results provide compelling evidence that Tc-Torso responds differently to its two ligands, Tc-Trk and Tc-Ptth. Depletion of Tc-Ptth leads to developmental delays, while silencing Tc-trk primarily results in a reduction in pupal size with a slight pupation delay. This observation suggests the potential for tissue-specific activation of Tc-torso. In this sense in Drosophila, Dm-Torso activation occurs during the final larval stage, affecting the PG and fat body, thereby regulating ecdysone biosynthesis and animal growth, respectively [14,35]. To explore this possibility in Tribolium, we quantified the mRNA levels of Tc-torso and its ligands, Tc-trk and Tc-Ptth, in different tissues. As anticipated, we observed high expression of Tc-torso in the head, likely the location of the PG, and also of Tc-Ptth, which aligns with previous reports of Tc-Ptth expression in a pair of neurons within the Tribolium brain (Fig 3A and 3B) [26]. This similar expression pattern suggests the activation of Tc-Torso by Tc-Ptth in the PG, regulating ecdysone biosynthesis. However, we also detected lower levels of Tc-torso in muscle, gut, and fat body, hinting at the possible activation of Tc-Torso in these tissues (Fig 3A). In this sense, we found that Tc-trk expression was predominantly detected in the gut and fat body, implying a potential activation of the pathway by Tc-Trk in these tissues during the final larval stage (Fig 3C). Altogether, these findings imply the potential for distinct effects of Tc-Torso activation in different tissues mediated by its ligands, Tc-Ptth and Tc-Trk.
Fig 3. Tissue specific expression of Tc-torso, Tc-Ptth and Tc-trk.
(A) Tc-torso, (B) Tc-Ptth and (C) Tc-trk mRNA levels measured by qRT-PCR in head and first thoracic segment (H+T1), muscle, gut and fat body of day 2 L7 larvae. For transcript analysis, equal amounts of total RNA were used. Error bars indicate the SEM (n = 5). Raw data are in S1 Data (tab Fig 3).
Tc-torso expression is associated with the Threshold Size checkpoint
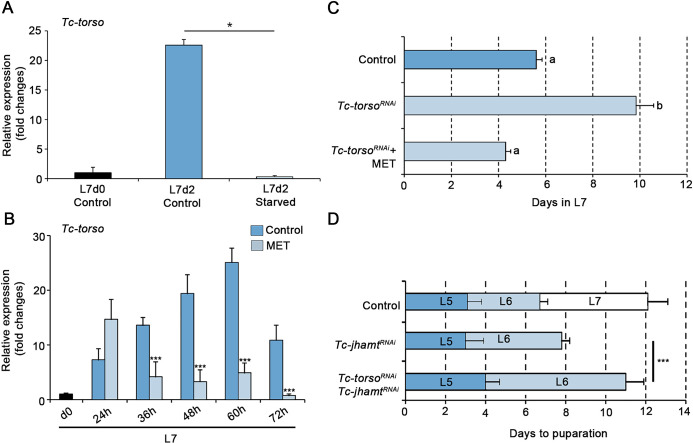
Our results above strongly suggest that Tc-Torso function is specifically of the last larval instar of Tribolium. It is at this stage that Tribolium larvae pass through a critical size-assessment checkpoint, the TS, which sets in motion the endocrine and genetic changes that trigger the metamorphic transition at the ensuing molt. The TS checkpoint in Tribolium is reached during the first 24 h after molting to the L7 stage and is associated with the stage-specific down-regulation of Tc-Kr-h1 and up-regulation of Tc-Br-C and Tc-E93 [33]. Since Tc-torso is strongly upregulated during the first 24 h of L7, we wondered whether this increase is also associated to the TS checkpoint. To address this issue, we starved L7 larvae before reaching the TS and measured mRNA levels of Tc-torso 72 h later. As Fig 4A shows, Tc-torso levels did not increase in larvae starved before the TS, confirming that the stage-specific up-regulation of Tc-torso is associated to larvae reaching the TS checkpoint.
Fig 4. Juvenile Hormone (JH) regulation of Tc-torso expression in Tribolium.
(A) Temporal changes in Tc-torso mRNA levels measured by qRT-PCR in Control and starved animals at the indicated stages. Transcript abundance values were normalized against the Tc-RpL32 transcript. Average values of three independent datasets are shown with standard errors (n = 4–8). Asterisks indicate statistically significant differences at *p < 0.1 (t-test). (B) Tc-torso mRNA levels measured by qRT-PCR during the L7 larval instar of Control and methoprene-treated animals (MET). Note the dramatic decrease in Tc-torso transcription upon methoprene application. Transcript abundance values were normalized against the Tc-RpL32 transcript. Error bars indicate the SEM (n = 5). Asterisks indicate statistically significant differences at ***p ≤ 0.001 (t-test). (C) Developmental duration of the last larval stage (L7) in newly molted L6 larvae injected with dsMock (Control) (n = 20), dsTc-torso (n = 23), and dsTc-torso + methoprene (n = 23). The developmental delay induced by depletion of Tc-torso is abolished by the application of methoprene. (D) Developmental progression of newly molted L4 larvae after injection with either dsMock (Control) (n = 15), dsTc-jhamt (n = 25) or dsTc-torso + dsTc-jhamt (n = 21). The bars represent the mean ± standard deviation (SD) for each developmental stage observed after the double-stranded RNA injection. Error bars indicate the SEM. Different letters represent groups with significant differences according to an ANOVA test (Tukey, p < 0.001). Raw data are in S1 Data (tab Fig 4).
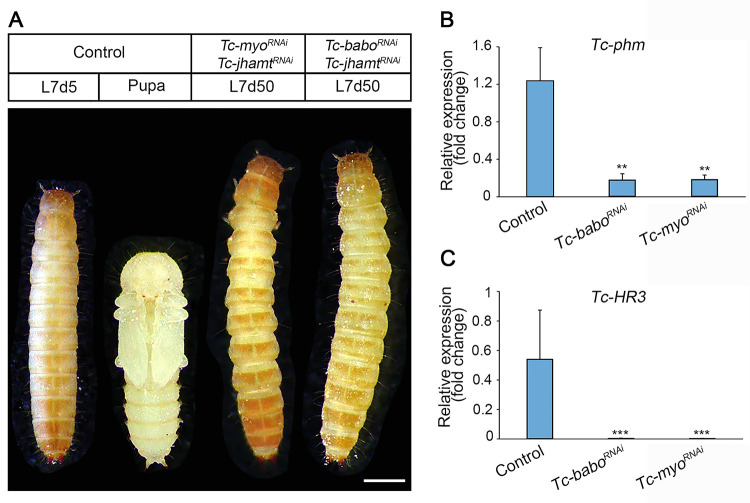
Since attainment of the TS is also linked with the decline of JH levels [33], we next wondered if low levels of this hormone are required for the proper expression of Tc-torso. To study this, we treated L7 larvae with the JH-mimic methoprene at the TS to maintain high levels of this hormone throughout the larval stage. Under this condition, Tc-torso was properly up-regulated just after the TS but its expression significantly decreased thereafter when compared to the progressive increase observed in Control larvae (Fig 4B). Consequently, changing the identity of the last larval stage by the application of methoprene, reverted the delay induced by Tc-torsoRNAi and induced the molting to a supernumerary L8 larva (Fig 4C). On the contrary, premature metamorphosis induced by depleting the rate limiting JH biosynthesis enzyme JH acid methyltransferase-3 (Tc-Jhamt) in newly emerged antepenultimate L4 instar larvae (Tc-jhamtRNAi animals) was delayed by Tc-torso depletion. Under these conditions, the majority of Tc-jhamtRNAi larvae molted to normal L5 larvae, and then to L6 underwent precocious metamorphosis after 5 days (Fig 4D). Interestingly, the time to pupation increased significantly when Tc-jhamt and Tc-torso were depleted simultaneously (Tc-jhamtRNAi + Tc-torsoRNAi animals) (Fig 4D), thus confirming that Tc-torso function does not depend on the number of larval stages the larva has been through but on whether the animal is in its last larval stage. Altogether, these results indicate that (1) the early upregulation of Tc-torso in L7 requires the larvae reaching the TS checkpoint; and (2) the ensuing increase in Tc-torso expression must occur in the presence of very low levels of JH.
TGFß/Activin signaling pathway regulates Tc-Torso expression by repressing JH synthesis
Since the up-regulation of Tc-torso correlates with the decline in JH levels triggered by the TS checkpoint, we next wanted to study the relation between both processes. In this regard, it has been recently shown that the TGFß/Activin signaling pathway is responsible for the decline of JH levels in a number of insect species [30–32]. We, therefore, wanted to ascertain whether the TGFß/Activin pathway regulates the expression of Tc-torso in the last larval instar. We first examined the expression of the Activin-like ligand Myoglianin (Tc-Myo) and its Type-I receptor Baboon (Tc-Babo) during the penultimate and last larval stages. Whereas Tc-babo mRNA levels persisted without major fluctuations through the last two larval instars, those of Tc-myo were more dynamic with a remarkable increase during the first two days and then oscillating throughout the rest of the instar (Fig 5A and 5B). Interestingly, while the expression of the receptor Tc-babo was consistently detected at similar levels in all the tissues measured, Tc-myo, in addition to muscle and fat body is primarily expressed in the head, where the PG is located and Tc-torso is mainly expressed (Fig 5C and 5D). To analyse the role of TGFß/Activin pathway during metamorphosis, we then depleted Tc-babo and Tc-myo during the last larval instar by injecting the corresponding dsRNAs (Tc-baboRNAi and Tc-myoRNAi animals). Remarkably, we found that in contrast to the normal upregulation of Tc-torso observed in Control larvae two days after the TS checkpoint, the levels of Tc-torso in Tc-baboRNAi and Tc-myoRNAi larvae were dramatically reduced, being even lower than the levels observed in newly molted L7 control animals (Fig 5E). In addition, Tc-baboRNAi and Tc-myoRNAi larvae presented persistently elevated levels of TcKr-h1, rather than the low levels observed in Control larvae (Fig 5F), which is indicative of sustained high levels of JH in these animals. Consistently, Tc-E93 and Tc-Br-C expression did not increase in Tc-baboRNAi and Tc-myoRNAi larvae after reaching the TS (Fig 5G and 5H). Altogether these results show that the activity of TGFß/Activin signaling pathway at the onset of L7 is responsible for the decline in JH levels that triggers the changes in the expression of the temporal factors that control the metamorphic transition, including the sharp up-regulation of Tc-torso.
Fig 5. TGFß/Activin signaling pathway activates Tc-torso expression through the repression of JH synthesis.
(A-B) Temporal changes in Tc-babo (A) and Tc-myo (B) mRNA levels measured by qRT-PCR in penultimate (L6) and ultimate (L7) instar larvae. Transcript abundance values were normalized against the Tc-Rpl32 transcript. Error bars indicate the standard error of the mean (SEM) (n = 5). (C) Tc-myo, and (D) Tc-trk mRNA levels measured by qRT-PCR in head and first thoracic segment (H+T1), muscle, gut and fat body of day 2 L7 larvae. For transcript analysis, equal amounts of total RNA were used. Error bars indicate the SEM (n = 3). (E-H) Temporal changes in transcript levels of Tc-torso (E), Tc-Kr-h1 (F), Tc-E93 (G), and Tc-Br-C (H) measured by qRT-PCR at the indicated time points of L7 Control, Tc-baboRNAi, and Tc-myoRNAi larvae. Transcript abundance values were normalized against the Tc-Rpl32 transcript. Average values of three independent datasets are shown with standard errors (n = 5–8). Asterisks indicate statistically significant differences at **p < 0.01 and ***p < 0.001 (A t-test was used to compare the levels of gene expression for Tc-baboRNAi, and Tc-myoRNAi with the L7d2 control). Raw data are in S1 Data (tab Fig 5).
The TGFß/Activin signaling pathway facilitates the metamorphic transition by regulating ecdysone levels
The results above indicate that Tc-baboRNAi and Tc-myoRNAi larvae do not initiate the molecular events that characterize the nature of the last larval instar, which involve the decline in Tc-Kr-h1 expression with the concomitant upregulation of Tc-E93, Tc-Br-C and Tc-torso. It is expected, therefore, that Tc-baboRNAi and Tc-myoRNAi larvae would not initiate the larva-pupa transformation at the ensuing molt. Consistent with this and in agreement with previous studies [31,32], Tc-myoRNAi larvae failed to pupate and instead repeated successive larval molts to ultimately reach L10 instar larva (when we stopped analysing the knockdown animals). Remarkably, the intermolt period was significantly increased in these larvae (Fig 6A and 6B), and the weight of the supernumerary Tc-myoRNAi larvae remained above the TS (Fig 6C), indicating that metamorphosis cannot be triggered in the absence of active TGFß/Activin signaling. In contrast, the phenotype of Tc-baboRNAi larvae was even more dramatic as they never molted again and remained as L7 larvae an average of 65 days before dying (Fig 6D).
Fig 6. Impairment of metamorphosis upon inactivation of TGFß/Activin signaling.
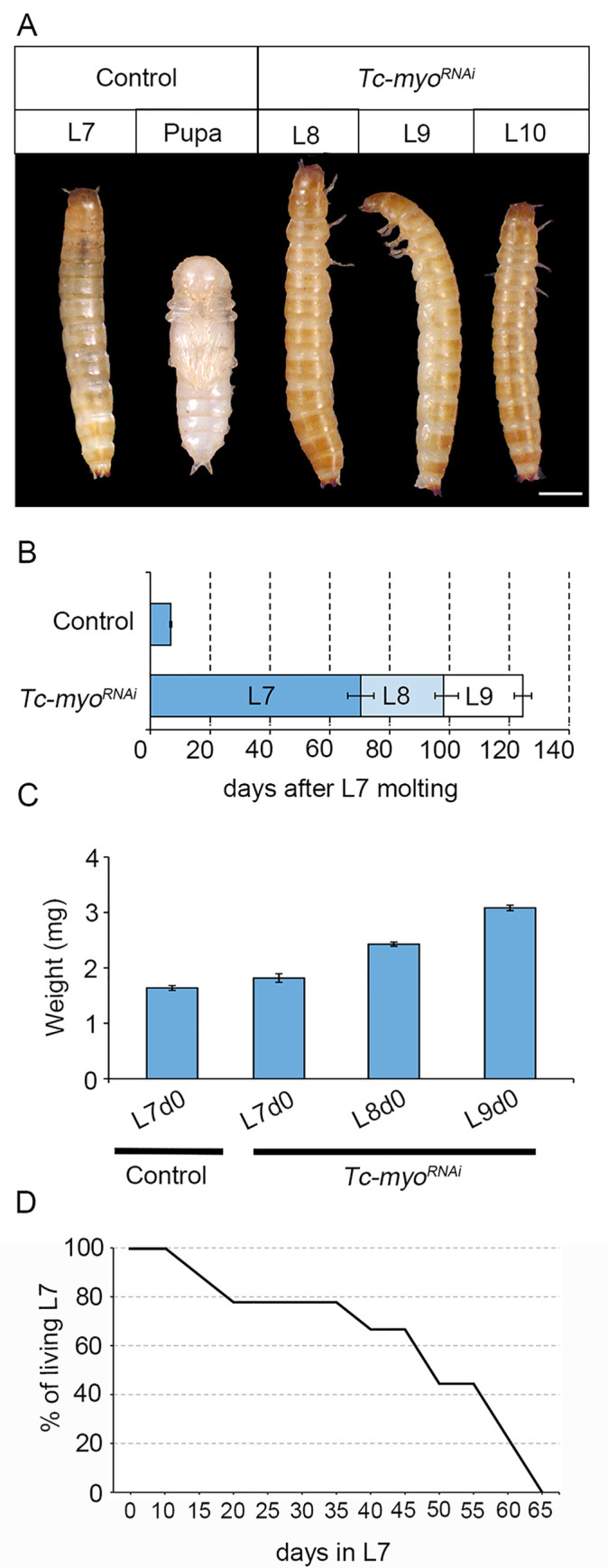
(A) Dorsal and ventral view of a Control L7 larva and pupa respectively, and dorsal views of supernumerary L8, L9, and L10 Tc-torsoRNAi animals. Scale bar, 0.5 mm. (B) Temporal progression of L6 larvae injected with dsMock (Control) (n = 6) or with dsTc-myo (n = 15) and left until the ensuing molts. Each bar indicates the periods (mean ± SD) for each developmental stage after the molt into the L7 larval stage. (C) Growth in body weight of Control and Tc-myo animals. All weights are measured on day 0 of each instar. Bars indicate the mean ± SD (n = 8). (D) Percentage of Tc-babo depleted larvae alive recorded for each day after the molt into the L7 larval stage (n = 45). Raw data of B, C and D are in S1 Data (tab Fig 6).
Given that Tc-baboRNAi and Tc-myoRNAi larvae exhibited consistently elevated levels of Tc-Kr-h1 (Fig 5F), it raised the possibility that increased titers of JH were responsible for the lack of initiation of metamorphosis observed under these conditions. To validate this hypothesis, we inhibited JH synthesis by silencing Tc-jhamt in Tc-baboRNAi and Tc-myoRNAi larvae. Interestingly, the suppression of JH failed to promote metamorphosis in these larvae (Fig 7A), suggesting that the TGFß/Activin signaling pathway might also have a role in controlling the synthesis of ecdysone, as previously reported in Drosophila [29,31]. Supporting this possibility, the levels of the Halloween gene Tc-phm and the ecdysone-dependent gene Tc-HR3, used as proxies for 20E levels, were significantly downregulated in Tc-baboRNAi and Tc-myoRNAi larvae when compared to Control animals (Fig 7B and 7C), which demonstrate that TGFß/Activin signaling is required for ecdysone synthesis in Tribolium. Collectively, our findings reveal that TGFß/Activin signaling exerts a dual role in the control of metamorphic timing in Tribolium: firstly, it is responsible for the decline in JH levels at the TS checkpoint, triggering the genetic switch that initiate the metamorphic transition, including the up-regulation of Tc-torso; secondly, it controls the production of ecdysone, a critical factor required to elicit the corresponding developmental transition.
Fig 7. Inactivation of TGFß/Activin signaling diminished ecdysone biosynthesis.
(A) Dorsal and ventral view of a Control L7 larva and pupa respectively, and dorsal views of double knockout Tc-jhamtRNAi and Tc-myoRNAi, and Tc-jhamtRNAi and Tc-baboRNAi L7 larvae, 50 days after injection. Scale bar, 0.5 mm. (B, C) Transcript levels of Tc-phm, (B) and Tc-HR3 (C) measured by qRT-PCR in 2-day-old L7 Control, Tc-baboRNAi and Tc-myoRNAi larvae. Transcript abundance values were normalized against the Tc-Rpl32 transcript. Average values of three independent datasets are shown with standard errors (n = 6). Asterisks indicate statistically significant differences at **p < 0.01 and ***p < 0.001 (t-test). Raw data of B and C are in S1 Data (tab Fig 7).
Discussion
Here, we show that the Ptth-Trk/Torso signaling pathway plays a crucial role in the transition from juvenile to adult stages in Tribolium. Our data reveal that both Tc-Ptth and Tc-Trk ligands activate Tc-Torso signaling during the final larval stage of the beetle. However, whereas Tc-Ptth promotes ecdysone biosynthesis, Tc-Trk seems to induce growth. Interestingly, we found that the expression of Tc-torso depends on the TGFβ/Activin signaling-dependent decay of JH at the TS checkpoint. In addition, we also found that TGFβ/Activin signaling contributes to ecdysone biosynthesis. These findings improve our understanding of the molecular mechanisms underlying the hormonal control of metamorphic transitions and provide insights into the evolution of Torso signaling in insects.
Postembryonic Torso signaling is activated by Tc-Ptth and Tc-Trk in Tribolium
Contrary to previously studied insects, our results demonstrated that post-embryonic Torso signaling in Tribolium is activated by both Tc-Ptth and Tc-Trk. To date, Ptth was the only ligand known to activate Torso during the post-embryonic stages in insects [14,36], whereas Trk was described as a Torso ligand exclusively involved in the generation of the terminal structures during the early stages of embryogenesis of Drosophila and Tribolium [25,26]. Despite the temporal specificity of the activation of the signaling by each ligand, it is important to note that both ligands seem to be functionally interchangeable, as ectopic expression of Ptth is able to activate Torso signaling in the early embryo of Drosophila [14], and overexpression of a processed form of Trk is able to induce precocious metamorphosis when overexpressed in the PG of the fly [26]. This indicates a possible common origin of both ligands, which have acquired specific enhancers to activate the pathway in different tissues. The fact that Ptth appears to evolve from the original ligand Trk in the common ancestor of Hemiptera and Holometabola supports this idea [37].
Although we found that Tc-Trk and Tc-Ptth activates Torso signaling during the metamorphic transition in Tribolium, both ligands present common and non-overlapping functions. Whereas Tc-Ptth is involved in the regulation of ecdysone production and the timely induction of the metamorphic transition, Tc-Trk regulates the systemic growth during the last larval stage. Interestingly these data are consistent with results obtained in Drosophila and Bombyx, where inactivation of Torso specifically in the PG delays the onset of pupariation, extending the larval growth period and increasing the final pupal size [13,14,38], whereas depletion of Torso specifically in the fat body produced smaller pupae with no effect on developmental timing [35]. Although the ligand responsible for Torso activation in the Drosophila fat body has not been identified, these results raise the possibility that Trk might activate Torso in the fat body or other tissues of Tribolium to regulate growth rate. The enriched expression of Tc-trk in the fat body and the gut strongly supports this possibility, especially considering that the gut serves as a significant sensor of nutrients and plays a crucial role in transmitting food-related signals to the brain and other tissues [39]. However, it is also possible that Tc-Trk secreted from the gut and the fat body activates Tc-Torso in the PG to ensure an adequate supply of ecdysone for promoting normal growth. Generation of new genetic tools to deplete gene expression in a tissue-specific manner would be required to solve this question in Tribolium.
In Drosophila and in Bombyx, torso mutants produced larger adults due to a prolonged larval development that allows for extra growth [13,14,38]. However, our study shows that depletion of Tc-Torso in Tribolium reduces final pupal size even when the larval growth period is extended. This finding suggests that the activation of Tc-Torso by Tc-Trk in the fat body may play a crucial role in increasing larval body mass. Considering that Trk and Torso are the most ancient molecules of the signaling pathway, as indicated by their presence in chelicerates, the most basal group of arthropods [37], it is tempting to speculate that systemic growth regulation is likely the ancestral function of the pathway. The fact that co-option of Torso signaling in early embryogenesis as well as for ecdysone biosynthesis during post-embryonic development seems to be a relative evolutionary novelty in insect evolution support this idea [27,37]. Studying the function of Trk-Torso in hemipteran species and Trk-like molecules in chelicerates could shed light on this possibility.
Up-regulation of Tc-torso depends on the TS checkpoint
Our expression analysis of Tc-torso in Tribolium reveals a peak of transcription at the TS checkpoint. This peak coincides with the attainment of the TS at the beginning of the final larval stage, accompanied by a decline in JH levels. The decrease in JH levels enables the up-regulation of Tc-E93 and Tc-Br-C, initiating the metamorphic transition. Interestingly, during the last larval stage an important increase of ecdysone production is required to induce the quiescent stage and the subsequent pupa formation [40]. In this sense, the decrease of JH production has been related to the upregulation of the Halloween genes, responsible for ecdysone biosynthesis. Thus, in Drosophila, inactivation of JH signalling in the PG triggers premature metamorphosis by de-repression of Halloween genes [41,42]. Such effect of JH on ecdysone biosynthesis might depend on the precocious up-regulation of Torso expression since ecdysone production relays in part on the activation of Torso signalling. Indeed, our results show that application of JH in the last larval stage of Tribolium impairs Tc-torso up-regulation. This effect has been also observed in Bombyx where the application of a JH analogue not only downregulates Bm-torso but also several Halloween genes [42,43]. These observations, therefore supports the idea that JH mainly represses ecdysone production by regulating torso expression.
TGFβ/Activin signaling regulates JH biosynthesis
If the induction of torso expression depends on the disappearance of JH in the last larval stage, then what regulates such decline? Interestingly, in agreement with a previous report [32] we found that inhibition of JH synthesis depends on the activation of TGFβ/Activin signaling, as depletion of either its ligand Tc-myo or the main receptor Tc-babo blocks the metamorphic transition. Consistently, blocking the TGFβ/Activin signaling leads to a sustained high levels of the JH-dependent factor Tc-Kr-h1, indicating that JH levels do not decline under these circumstances. Similar results have been reported in other insects, suggesting a conserved role of TGFβ/Activin signaling in JH regulation. Thus, in H. vigintioctopunctata, G. bimaculatus and B. germanica activation of TGFβ/Activin signaling blocks JH production by directly downregulating of the JH biosynthetic enzyme gene jhamt [31,32,44]. The fact that JH regulation by TGFβ/Activin signaling occurs specifically during the last larval stage suggests a potential relationship between body mass and the activation of TGFβ/Activin signaling. Interestingly, in the lepidopteran Manduca, increasing levels of Ms-myo, produced by the muscle, have been associated with the initiation of metamorphosis [32]. Similarly, we observed high levels of Tc-myo in the muscles of Tribolium during the last larval stage (Fig 5C). This observation strongly suggests that muscle growth during larval development induces a gradual increase in Tc-myo expression, activating the TGFβ/Activin signaling pathway once a certain threshold level is reached. Consequently, this activation leads to a decrease of JH production, allowing for the up-regulation of Torso and the subsequent rise of ecdysone levels. Such complex regulation might explain why Torso is only required during the last larval stage.
Does TGFβ/Activin signaling act as a PG cell survival factor?
In addition to its role in regulating JH, TGFβ/Activin signaling also plays a role in ecdysone production in Tribolium, as revealed by Tc-myo knockdown. In agreement with a previous report [32], we found that depletion of Tc-myo in Tribolium, increased dramatically the intermolt period and even induced a developmental arrest at L7 when the TGFβ/Activin receptor Tc-babo was knocked down. This phenotype is independent of the consistently high JH signaling activation detected under these conditions, as simultaneously blocking of both TGFβ/Activin and JH production failed to trigger metamorphosis (Fig 7). These results indicate that TGFβ/Activin signaling also plays a role in regulating ecdysone biosynthesis in Tribolium. In this context, the Tc-babo phenotype resembles the developmental arrest observed in Drosophila larvae when TGFβ/Activin signaling is inactivated in the PG [29]. As in Drosophila, arrested Tc-babo-depleted Tribolium larvae presented very low levels of Tc-torso expression with the consequent failure to induce the large rise in ecdysteroid titer that triggers metamorphosis (Fig 5E). Under these conditions, we anticipated the presence of supernumerary larvae in the subsequent molt due to the failure of JH decay. However, we found that inactivation of TGFβ/Activin signaling in Tribolium not only halts metamorphosis but also prevents larva molting, as depletion of Tc-babo leads to developmental arrest at L7 for more than 60 days. Considering that the effect of the injected dsRNAs is transient [45], the most likely explanation for the arrested Tc-babo-depleted larvae is that TGFβ/Activin might be required for PG viability during the last larval stage. This hypothesis is supported by the fact that inactivation of TGFβ/Activin signaling in adult Drosophila muscles reduces lifespan by modulating protein homeostasis in the tissue [46]. Likewise, reduction of TGFβ/Activin signaling in the cardiac muscle of Drosophila increases the cardiac autophagic activity [47]. Since autophagy is the main mechanism for larval tissue degradation during metamorphosis [48], it is tempting to speculate that TGFβ/Activin activation in the PG suppresses autophagy during the last larval stage of Tribolium. Alternatively, it is also plausible that inhibiting TGFβ/Activin signaling somehow sustains low ecdysone levels without affecting the viability of the gland. This could result in ecdysone levels remaining consistently insufficient to induce molting throughout the entire period. The characterization of the PG in Tribolium and specific analysis of the effects on TGFβ/Activin signaling in this tissue will provide further insights into this question.
In summary, our study reveals that the activation of Tc-Torso by two ligands, Tc-Ptth and Tc-Trk, during the last larval stage of Tribolium regulates the ecdysone-dependent timely transition to the metamorphic period and controls growth during this stage, thereby determining the final size of the insect. Furthermore, we showed Tc-torso up-regulation depends on larvae attaining the TS checkpoint at the onset of the last larval instar and that its expression depends on the decay of JH induced by the TGFβ/Activin signaling pathway. Our findings, therefore contribute to the understanding of how the coordination of different signaling pathways regulates the endocrine systems that control developmental growth and the timing of maturation in insects.
Materials and methods
Tribolium castaneum
The enhancer-trap line pu11 of Tribolium (obtained from Y. Tomoyasu, Miami University, Oxford, OH) was reared on an organic wheat flour diet supplemented with 5% nutritional yeast and maintained at a constant temperature of 29°C in complete darkness.
Quantitative Real-Time Reverse Transcriptase Polymerase Chain Reaction (qRT-PCR)
Total RNA from individual Tribolium larvae was extracted using the Mammalian Total RNA kit (Sigma). cDNA synthesis was performed following the previously described methods [49,50]. For quantitative real-time PCR (qPCR), Power SYBR Green PCR Mastermix (Applied Biosystems) was used to determine relative transcript levels. To standardize the qPCR inputs, a master mix containing Power SYBR Green PCR Mastermix and forward and reverse primers was prepared, with each primer at a final concentration of 100 μM. The qPCR experiments were conducted with an equal quantity of tissue equivalent input for all treatments, and each sample was run in duplicate using 2 μl of cDNA per reaction. As a reference, the same cDNAs were subjected to qRT-PCR using a primer pair specific for Tribolium Ribosomal Tc-Rpl32. All samples were analyzed on the iCycler iQReal Time PCR Detection System (Bio-Rad).
Primer sequences used for qPCR for Tribolium
Tc-torso-F: 5’- TTGACGAGGAGAAGCTTCCAGAGT-3’
Tc-torso-R: 5’- TGCAAATTGTTGCTGCATGTTGGT-3’
Tc-Ptth-F: 5’-TCGTGTGGGATCGAATTTCGCGTTC-3’
Tc-Ptth-R: 5’-GTCTTTCTGTTTCAAAACGCGGAC-3’
Tc-trk-F: 5’-TATCGAGCAACTCGACGAT-3’
Tc-trk-R: 5’-TCGATTTGCAATGCCACTGTT-3’
Tc-myo-F: 5’-CAAGAAGTGCTCACCTTTGC-3’
Tc-myo-R: 5’-CCTTCATGTACACGTACAG-3’
Tc-babo-F: 5’-ATGGTGCATGGCTTCTGGTT-3’
Tc-babo-R: 5’-AGTAAGTCGATGTAAAGCAGTA-3’
Tc-E75-F: 5′-CGGTCCTCAATGGAAGAAAA-3′
Tc-E75-R: 5′-TGTGTGGTTTGTAGGCTTCG-3′
Tc-phm-F: 5′-TGAACAAATCGCAATGGTGCCATA-3′
Tc-phm-R: 5′-TCATGGTACCTGGTGGTGGAACCTTAT-3′
Tc-Kr-h1-F: 5′-AATCCTCCTGCTCATCCAGCACTA-3′
Tc-Kr-h1-R: 5′-CAGGATTCGAACTAGGAGGTGTTA-3′
Tc-E93-F: 5′-CTCTCGAAAACTCGGTTCTAAACA-3′
Tc-E93-R: 5′-TTTGGGTTTGGGTGCTGCCGAATT-3′
Tc-Br-C-F: 5′-TCGTTTCTCAAGACGGCTGAAGTG-3′
Tc-Br-C-R: 5′-CTCCACTAACTTCTCGGTGAAGCT-3′
Tc-Rpl32-F: 5′-CAGGCACCAGTCTGACCGTTATG-3′
Tc-Rpl32-R: 5′-CATGTGCTTCGTTTTGGCATTGGA-3′
Larva RNAi injection
Tc-torso dsRNA (IB_04720), Tc-Ptth dsRNA (IB_09326), Tc-trk dsRNA (IB_06187), Tc-myo dsRNA (IB_05899), Tc-jhamt dsRNA (IB_04499), and Tc-babo dsRNA (IB_03525) were synthesized by Eupheria Biotech Company. The control dsRNA used a non-coding sequence from the pSTBlue-1 vector (dsMock). For larval injections, 1 μg of dsRNA was administered to penultimate instar and antepenultimate instar larvae. In cases of co-injection of two dsRNAs, equal volumes of each dsRNA solution were mixed and applied in a single injection.
Nutritional experiments
Tribolium pupae-larvae were reared on a normal diet consisting of organic wheat flour containing 5% nutritional yeast. For the starvation experiment, newly molted L7 larvae, raised on the normal diet, were transferred to a new plate without any food. After 2 days, the larvae were collected for Tc-torso expression measurement using RT-qPCR.
Treatment with Methoprene
To conduct the juvenile hormone mimic treatment, white L7 newly molted larvae of Tribolium were topically treated on their dorsal side with 1μg of isopropyl (E,E)-(RS)-11-methoxy-3,7,11-trimethyldodeca-2,4-dienoate per specimen in 1 μL of acetone. The control group received the same volume of solvent. At the desired stage, the larvae were subjected to mRNA expression analysis.
Microscopy analysis
All pictures were obtained with AxioImager.Z1 (ApoTome 213 System, Zeiss) microscope, and images were subsequently processed using Fuji and Adobe photoshop.
Supporting information
(XLSX)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This project is supported by grants PGC2018-098427-B-I00 and PID2021-125661NB-I00 to D.M. and X.F-M. funded by MCIN/AEI/10.13039/501100011033 and by “ERDF A way of making Europe”. The project is supported also by grants 2017 SGR 1030 and 2021 SGR 00417 to D.M. and X.F-M funded by the Departament de Recerca i Universitats de la Generalitat de Catalunya. S.C. was a recipient of a Juan de la Cierva contract FJC2019-041549-I funded by MCIN/AEI /10.13039/501100011033. The funders play no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Tennessen JM, Thummel CS. Coordinating growth and maturation—Insights from drosophila. Current Biology. 2011. doi: 10.1016/j.cub.2011.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rewitz KF, Yamanaka N, O’Connor MB. Developmental Checkpoints and Feedback Circuits Time Insect Maturation. Curr Top Dev Biol. 2013;103: 1–33. doi: 10.1016/B978-0-12-385979-2.00001-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshiyama T, Namiki T, Mita K, Kataoka H, Niwa R. Neverland is an evolutionally conserved Rieske-domain protein that is essential for ecdysone synthesis and insect growth. Development. 2006;133: 2565–2574. Available: doi: 10.1242/dev.02428 [DOI] [PubMed] [Google Scholar]
- 4.Yoshiyama-Yanagawa T, Enya S, Shimada-Niwa Y, Yaguchi S, Haramoto Y, Matsuya T, et al. The conserved Rieske oxygenase DAF-36/Neverland is a novel cholesterol-metabolizing enzyme. J Biol Chem. 2011;286: 25756–25762. doi: 10.1074/jbc.M111.244384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niwa R, Namiki T, Ito K, Shimada-Niwa Y, Kiuchi M, Kawaoka S, et al. Non-molting glossy/shroud encodes a short-chain dehydrogenase/reductase that functions in the 'Black Box' of the ecdysteroid biosynthesis pathway. Development. 2010;137: 1991. –1999. doi: 10.1242/dev.045641 [DOI] [PubMed] [Google Scholar]
- 6.Namiki T, Niwa R, Sakudoh T, Shirai K-I, Takeuchi H, Kataoka H. Cytochrome P450 CYP307A1/Spook: a regulator for ecdysone synthesis in insects. Biochem Biophys Res Commun. 2005;337: 367–374. doi: 10.1016/j.bbrc.2005.09.043 [DOI] [PubMed] [Google Scholar]
- 7.Niwa R, Matsuda T, Yoshiyama T, Namiki T, Mita K, Fujimoto Y, et al. CYP306A1, a cytochrome P450 enzyme, is essential for ecdysteroid biosynthesis in the prothoracic glands of Bombyx and Drosophila. Journal of Biological Chemistry. 2004;279: 35942–35949. doi: 10.1074/jbc.M404514200 [DOI] [PubMed] [Google Scholar]
- 8.Ono H, Rewitz KF, Shinoda T, Itoyama K, Petryk A, Rybczynski R, et al. Spook and Spookier code for stage-specific components of the ecdysone biosynthetic pathway in Diptera. Dev Biol. 2006;298: 555–570. doi: 10.1016/j.ydbio.2006.07.023 [DOI] [PubMed] [Google Scholar]
- 9.Rewitz KF, Rybczynski R, Warren JT, Gilbert LI. Identification, characterization and developmental expression of Halloween genes encoding P450 enzymes mediating ecdysone biosynthesis in the tobacco hornworm, Manduca sexta. Insect Biochem Mol Biol. 2006;36: 188–199. doi: 10.1016/j.ibmb.2005.12.002 [DOI] [PubMed] [Google Scholar]
- 10.Warren JT, Petryk A, Marqués G, Jarcho M, Parvy JP, Dauphin-Villemant C, et al. Molecular and biochemical characterization of two P450 enzymes in the ecdysteroidogenic pathway of Drosophila melanogaster. Proc Natl Acad Sci U S A. 2002;99: 11043–11048. doi: 10.1073/pnas.162375799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warren JT, Petryk A, Marqués G, Parvy JP, Shinoda T, Itoyama K, et al. Phantom encodes the 25-hydroxylase of Drosophila melanogaster and Bombyx mori: A P450 enzyme critical in ecdysone biosynthesis. Insect Biochem Mol Biol. 2004;34: 991–1010. doi: 10.1016/j.ibmb.2004.06.009 [DOI] [PubMed] [Google Scholar]
- 12.Pan X O ’Connor MB. Coordination among multiple receptor tyrosine kinase signals controls Drosophila developmental timing and body size. Cell Rep. 2021;36. doi: 10.1016/j.celrep.2021.109644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McBrayer Z, Ono H, Shimell MJ, Parvy JP, Beckstead RB, Warren JT, et al. Prothoracicotropic Hormone Regulates Developmental Timing and Body Size in Drosophila. Dev Cell. 2007;13: 857–871. doi: 10.1016/j.devcel.2007.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rewitz KF, Yamanaka N, Gilbert LI, O’Connor MB. The insect neuropeptide PTTH activates receptor tyrosine kinase torso to initiate metamorphosis. Science (1979). 2009;326: 1403–1405. doi: 10.1126/science.1176450 [DOI] [PubMed] [Google Scholar]
- 15.Shimell MJ, Pan X, Martin FA, Ghosh AC, Leopold P, O’Connor MB, et al. Prothoracicotropic hormone modulates environmental adaptive plasticity through the control of developmental timing. Development (Cambridge). 2018;145: dev159699–13. doi: 10.1242/dev.159699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cruz J, Martín D, Franch-Marro X. Egfr Signaling Is a Major Regulator of Ecdysone Biosynthesis in the Drosophila Prothoracic Gland. Current Biology. 2020;30: 1547–1554.e4. doi: 10.1016/j.cub.2020.01.092 [DOI] [PubMed] [Google Scholar]
- 17.Chafino S, Martín D, Franch-Marro X. Activation of EGFR signaling by Tc-Vein and Tc-Spitz regulates the metamorphic transition in the red flour beetle Tribolium castaneum. Scientific Reports |. 2021;11: 18807. doi: 10.1038/s41598-021-98334-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colombani J, Bianchini L, Layalle S, Pondeville E, Dauphin-Villemant C, Antoniewski C, et al. Antagonistic actions of ecdysone and insulins determine final size in Drosophila. Science (1979). 2005;310: 667–670. doi: 10.1126/science.1119432 [DOI] [PubMed] [Google Scholar]
- 19.Mirth C, Truman JW, Riddiford LM. The role of the prothoracic gland in determining critical weight for metamorphosis in Drosophila melanogaster. Current Biology. 2005;15: 1796–1807. doi: 10.1016/j.cub.2005.09.017 [DOI] [PubMed] [Google Scholar]
- 20.Layalle S, Arquier N, Léopold P. The TOR Pathway Couples Nutrition and Developmental Timing in Drosophila. Dev Cell. 2008;15: 568–577. doi: 10.1016/j.devcel.2008.08.003 [DOI] [PubMed] [Google Scholar]
- 21.Caldwell PE, Walkiewicz M, Stern M. Ras activity in the Drosophila prothoracic gland regulates body size and developmental rate via ecdysone release. Current Biology. 2005;15: 1785–1795. doi: 10.1016/j.cub.2005.09.011 [DOI] [PubMed] [Google Scholar]
- 22.Rewitz KF, Yamanaka N, Gilbert LI, O’Connor MB. The Insect Neuropeptide PTTH Activates Receptor Tyrosine Kinase Torso to Initiate Metamorphosis. Science. 2009;326: 1403–1405. Available: http://www.sciencemag.org/cgi/content/full/326/5958/1403 doi: 10.1126/science.1176450 [DOI] [PubMed] [Google Scholar]
- 23.Yamanaka N, Marqués G, O’Connor MB. Vesicle-Mediated Steroid Hormone Secretion in Drosophila melanogaster. Cell. 2015;163: 907–919. doi: 10.1016/j.cell.2015.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamanaka N, Rewitz KF, O’Connor MB. Ecdysone control of developmental transitions: Lessons from drosophila research. Annu Rev Entomol. 2013;58: 497–516. doi: 10.1146/annurev-ento-120811-153608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Casali A, Casanova J. The spatial control of Torso RTK activation: a C-terminal fragment of the Trunk protein acts as a signal for Torso receptor in the Drosophila embryo. Development. 2001;128: 1709–1715. doi: 10.1242/dev.128.9.1709 [DOI] [PubMed] [Google Scholar]
- 26.Grillo M, Furriols M, De Miguel C, Franch-Marro X, Casanova J. Conserved and divergent elements in Torso RTK activation in Drosophila development. Sci Rep. 2012;2: 762. doi: 10.1038/srep00762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duncan EJ, Benton MA, Dearden PK. Canonical terminal patterning is an evolutionary novelty. Dev Biol. 2013;377: 245–261. doi: 10.1016/j.ydbio.2013.02.010 [DOI] [PubMed] [Google Scholar]
- 28.Mizoguchi A, Ohsumi S, Kobayashi K, Okamoto N, Yamada N, Tateishi K, et al. Prothoracicotropic Hormone Acts as a Neuroendocrine Switch between Pupal Diapause and Adult Development. PLoS One. 2013;8. doi: 10.1371/journal.pone.0060824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gibbens YY, Warren JT, Gilbert LI, O’Connor MB. Neuroendocrine regulation of Drosophila metamorphosis requires TGFβ/Activin signaling. Development. 2011;138: 2693–2703. doi: 10.1242/dev.063412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang J, Tian L, Peng C, Abdou M, Wen D, Wang Y, et al. DPP-mediated TGFβ signaling regulates juvenile hormone biosynthesis by activating the expression of juvenile hormone acid methyltransferase. Development. 2011;138: 2283–2291. doi: 10.1242/dev.057687 [DOI] [PubMed] [Google Scholar]
- 31.Kamsoi O, Belles X. Myoglianin triggers the premetamorphosis stage in hemimetabolan insects. FASEB Journal. 2019;33: 3659–3669. doi: 10.1096/fj.201801511R [DOI] [PubMed] [Google Scholar]
- 32.He LL, Shin SH, Wang Z, Yuan I, Weschler R, Chiou A, et al. Mechanism of threshold size assessment: Metamorphosis is triggered by the TGF-beta/Activin ligand Myoglianin. Insect Biochem Mol Biol. 2020;126: 103452. doi: 10.1016/j.ibmb.2020.103452 [DOI] [PubMed] [Google Scholar]
- 33.Chafino S, Ureña E, Casanova J, Casacuberta E, Franch-Marro X, Martín D. Upregulation of E93 Gene Expression Acts as the Trigger for Metamorphosis Independently of the Threshold Size in the Beetle Tribolium castaneum. Cell Rep. 2019;27: 1039–1049.e2. doi: 10.1016/j.celrep.2019.03.094 [DOI] [PubMed] [Google Scholar]
- 34.Ureña E, Chafino S, Manjón C, Franch-Marro X, Martín D. The Occurrence of the Holometabolous Pupal Stage Requires the Interaction between E93, Krüppel-Homolog 1 and Broad-Complex. PLoS Genet. 2016;12. doi: 10.1371/journal.pgen.1006020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woo Jun J, Han G, Myoung Yun H, Jun Lee G, Hyun S. Torso, a Drosophila receptor tyrosine kinase, plays a novel role in the larval fat body in regulating insulin signaling and body growth. J Comp Physiol B. 2016;186: 701–709. doi: 10.1007/s00360-016-0992-2 [DOI] [PubMed] [Google Scholar]
- 36.Zhu TT, Meng QW, Guo WC, Li GQ. RNA interference suppression of the receptor tyrosine kinase Torso gene impaired pupation and adult emergence in Leptinotarsa decemlineata. J Insect Physiol. 2015;83: 53–64. doi: 10.1016/j.jinsphys.2015.10.005 [DOI] [PubMed] [Google Scholar]
- 37.Skelly J, Pushparajan C, Duncan EJ, Dearden PK. Evolution of the Torso activation cassette, a pathway required for terminal patterning and moulting. Insect Mol Biol. 2019;28: 392–408. doi: 10.1111/imb.12560 [DOI] [PubMed] [Google Scholar]
- 38.Zhang Z, Liu X, Yu Y, Yang F, Li K. The receptor tyrosine kinase torso regulates ecdysone homeostasis to control developmental timing in Bombyx mori. Insect Sci. 2020; 1744–7917.12879. doi: 10.1111/1744-7917.12879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chopra G, Kaushik S, Kain P. Nutrient Sensing via Gut in Drosophila melanogaster. International Journal of Molecular Sciences. MDPI; 2022. p. 2694. doi: 10.3390/ijms23052694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parthasarathy R, Tan A, Bai H, Palli SR. Transcription factor broad suppresses precocious development of adult structures during larval-pupal metamorphosis in the red flour beetle, Tribolium castaneum. Mech Dev. 2008;125: 299–313. doi: 10.1016/j.mod.2007.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu S, Li K, Gao Y, Liu X, Chen W, Ge W, et al. Antagonistic actions of juvenile hormone and 20-hydroxyecdysone within the ring gland determine developmental transitions in Drosophila. Proc Natl Acad Sci U S A. 2018;115: 139–144. doi: 10.1073/pnas.1716897115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang T, Song W, Li Z, Qian W, Wei L, Yang Y, et al. Krüppel homolog 1 represses insect ecdysone biosynthesis by directly inhibiting the transcription of steroidogenic enzymes. Proc Natl Acad Sci U S A. 2018;115: 3960–3965. doi: 10.1073/pnas.1800435115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ogihara MH, Hikiba J, Iga M, Kataoka H. Negative regulation of juvenile hormone analog for ecdysteroidogenic enzymes. J Insect Physiol. 2015;80: 42–47. doi: 10.1016/j.jinsphys.2015.03.012 [DOI] [PubMed] [Google Scholar]
- 44.Ishimaru Y, Tomonari S, Matsuoka Y, Watanabe T, Miyawaki K, Bando T, et al. TGF-β signaling in insects regulates metamorphosis via juvenile hormone biosynthesis. Proc Natl Acad Sci U S A. 2016;113: 5634–5639. doi: 10.1073/pnas.1600612113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tomoyasu Y, Denell RE. Larval RNAi in Tribolium (Coleoptera) for analyzing adult development. Dev Genes Evol. 2004;214: 575–578. doi: 10.1007/s00427-004-0434-0 [DOI] [PubMed] [Google Scholar]
- 46.Langerak S, Kim MJ, Lamberg H, Godinez M, Main M, Winslow L, et al. The Drosophila TGF-beta/Activin-like ligands Dawdle and Myoglianin appear to modulate adult lifespan through regulation of 26S proteasome function in adult muscle. Biol Open. 2018;7. doi: 10.1242/bio.029454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang K, Kang P, Liu Y, Huang K, Miao T, Sagona AP, et al. TGFB-INHB/activin signaling regulates age-dependent autophagy and cardiac health through inhibition of MTORC2. Autophagy. 2020;16: 1807–1822. doi: 10.1080/15548627.2019.1704117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tettamanti G, Carata E, Montali A, Dini L, Fimia GM. Autophagy in development and regeneration: role in tissue remodelling and cell survival. European Zoological Journal. Taylor and Francis Ltd.; 2019. pp. 113–131. doi: 10.1080/24750263.2019.1601271 [DOI] [Google Scholar]
- 49.Cruz J, Martín D, Pascual N, Maestro JL, Piulachs MD, Bellés X. Quantity does matter. Juvenile hormone and the onset of vitellogenesis in the German cockroach. Insect Biochem Mol Biol. 2003;33: 1219–1225. doi: 10.1016/j.ibmb.2003.06.004 [DOI] [PubMed] [Google Scholar]
- 50.Mané-Padrós D, Cruz J, Vilaplana L, Nieva C, Ureña E, Bellés X, et al. The hormonal pathway controlling cell death during metamorphosis in a hemimetabolous insect. Dev Biol. 2010;346: 150–160. doi: 10.1016/j.ydbio.2010.07.012 [DOI] [PubMed] [Google Scholar]