Abstract
SecB is a cytosolic chaperone which facilitates the transport of a subset of proteins, including membrane proteins such as PhoE and LamB and some periplasmic proteins such as maltose-binding protein, in Escherichia coli. However, not all proteins require SecB for transport, and proteins such as ribose-binding protein are exported efficiently even in SecB-null strains. The characteristics which confer SecB dependence on some proteins but not others have not been defined. To determine the sequence characteristics that are responsible for the SecB requirement, we have inserted a systematic series of short, polymeric sequences into the SecB-independent protein alkaline phosphatase (PhoA). The extent to which these simple sequences convert alkaline phosphatase into a SecB-requiring protein was evaluated in vivo. Using this approach we have examined the roles of the polarity and charge of the sequence, as well as its location within the mature region, in conferring SecB dependence. We find that an insert with as few as 10 residues, of which 3 are basic, confers SecB dependence and that the mutant protein is efficiently exported in the presence of SecB. Remarkably, the basic motifs caused the protein to be translocated in a strict membrane potential-dependent fashion, indicating that the membrane potential is not a barrier to, but rather a requirement for, translocation of the motif. The alkaline phosphatase mutants most sensitive to the loss of SecB are those most sensitive to inhibition of SecA via azide treatment, consistent with the necessity for formation of a preprotein-SecB-SecA complex. Furthermore, the impact of the basic motif depends on location within the mature protein and parallels the accessibility of the location to the secretion apparatus.
Protein secretion entails the successful completion of a series of steps: synthesis, membrane targeting and insertion, translocation, signal peptide cleavage by leader peptidase, and final localization of the mature protein. Several proteinaceous components of this pathway have been identified. In Escherichia coli, SecA is an ATPase (for a review see reference 32) involved in coupling ATP hydrolysis to protein translocation (9, 21). SecE, SecG, and SecY are integral-membrane proteins which are thought to constitute part of a channel through which the polypeptide is transported (1, 4, 31). SecD and SecF have been implicated in the final stages of translocation such as the release of the mature protein from the inner membrane (13, 29), while the leader peptidase is responsible for the actual signal peptide cleavage event (10). At least a subset of exported and membrane proteins (35, 43, 44) also utilizes the E. coli signal recognition particle (SRP), a cytosolic ribonucleoprotein composed of 4.5S RNA and a single polypeptide (Ffh) homologous to the 54-kDa subunit of eukaryotic SRP (3, 36). Several of these proteins are thought to function in part through interaction with the signal peptide region of the preprotein to facilitate secretion.
SecB is a tetrameric molecular chaperone that promotes the export of some proteins through recognition of regions of the mature domain of the protein to be secreted (19, 38, 42), though some evidence suggests that the presence of the signal peptide is also required (45). It has been suggested that the binding of SecB to a polypeptide destined for an extracytoplasmic compartment is determined by a kinetic partitioning between the premature folding of the protein and its association with SecB. When SecB forms a complex with precursor polypeptides, it facilitates transport by maintaining them in the unfolded state. The SecB-precursor complex then becomes associated with SecA, and the polypeptide is competent for subsequent translocation (14). SecB facilitates the transport process for some membrane proteins such as PhoE (25) and LamB (24) and some periplasmic proteins such as maltose-binding protein (MBP) (24). Other proteins, such as alkaline phosphate, β-lactamase, and ribose-binding protein, are considered SecB independent (for a review see reference 23). However, the determinants which confer SecB dependence versus independence have not been well defined.
Previous studies have employed fusion proteins of various portions of SecB-dependent and -independent proteins to try to determine what specifies SecB dependence. By this approach, some regions involved in the SecB dependence of MBP (12) and LamB (2) were roughly narrowed to 74- and 60-amino-acid stretches, respectively. For MBP this region is in the amino-terminal portion of the mature protein, while for LamB this stretch is C terminal to residue 320 of the mature protein. For OmpF, the first 11 amino acids of the mature protein seem to influence SecB dependence (46). Other studies have suggested an interrelationship between the nature of the signal peptide and SecB dependence (8), although this may reflect an effect on protein folding kinetics (34) and/or targeting efficiency.
Furthermore, no consensus sequence which serves as a SecB binding motif has been identified. Several studies have suggested that SecB binds a precursor at multiple sites (19, 42). Mutations of SecB that influence binding to native polypeptides have suggested that the hydrophobic nature of the substrate are important (22), while mutations of SecB that affect the rate of MBP export have suggested that an ionic interaction is important for regulating the opening of the hydrophobic preprotein binding site. In vitro binding analyses based on the extent to which synthetic peptides protect SecB from proteolysis have also pointed toward the role of basic residues (37). Intriguingly, Randall (37) showed that peptides of polylysine conferred proteolytic protection of SecB and furthermore that peptide binding induced a conformational change to expose hydrophobic sites on SecB. Randall has proposed a model (37, 42) in which SecB has multiple sites that bind basic residues; saturation of these sites produces a conformational change in which hydrophobic sites on SecB subsequently become accessible for binding.
In order to identify the sequence characteristics which dictate a requirement for SecB in vivo, we have inserted a systematic series of short, polymeric sequences into the SecB-independent protein alkaline phosphatase (PhoA). Using this approach we demonstrate that an insert with as few as 10 residues, of which 3 are basic, confers SecB dependence and that the mutant protein is efficiently exported in the presence of SecB. The extent to which the charge of this motif and its location within the mature domain influence the SecB dependence of PhoA is delineated. Furthermore, by all criteria used, the SecB-dependent mutant alkaline phosphatases function in vivo comparably to wild-type SecB-dependent proteins.
MATERIALS AND METHODS
Strains and plasmids.
E. coli AW1043 [Δlac galU galK Δ(leu-ara) phoA E15 proC::Tn5] was used for the generation and replication of mutant forms of alkaline phosphatase. E. coli AW1043 and E. coli CK1953 (MC4100 secB::Tn5; gift from C. A. Kumamoto) were used for all transport analyses. Derivatives of WT-XN (28) and WT-Nhe (a precursor of WT-XN; 28), originally generated from pBR322, were used to express mutated alkaline phosphatases.
DNA manipulations.
Cassette mutagenesis vector WT-MCS(dl), which has two linkers in tandem, was derived from previously generated vector XN-M (7). Although WT-MCS(dl) has a double linker, complete digestion of WT-MCS(dl) with XhoI and MluI removes it and leaves the appropriate ends free for ligation with inserts coding for the motifs under study. WT-N-MCS was derived from WT-XN by inserting a linker carrying NheI-compatible ends and a unique XhoI site and a MluI site into the NheI site of the WT-XN vector. WT-M-MCS(dl) is a modified form of WT-Nhe. Oligonucleotide-directed mutagenesis was used to generate a XhoI site in the DNA corresponding to amino acid position 283 of WT-Nhe. In order to maintain the overall length comparable to that of others, two linkers carrying XhoI-compatible ends, two XhoI sites, and two MluI sites were inserted into the XhoI site. Complete digestion with XhoI and MluI results in a vector that has unique XhoI and MluI sites, so that DNA fragments generated from XhoI and MluI digestion can be ligated to the corresponding sites of the vector.
Construction of mutated PhoAs at the 13th, 28th, and 290th amino acid of the mature region.
For the construction of mutated PhoAs at the 13th amino acid of the mature region, the WT-MCS(dl) plasmid was digested with XhoI and MluI. The 43-nucleotide linker region was removed from the large vector by excision from 0.75% agarose gels. Mutant inserts were constructed with synthetic oligonucleotides corresponding to both DNA strands of the new motif. To facilitate the cloning process, four bases flanking the restriction endonuclease recognition site were introduced at each end of the oligonucleotides and subsequently removed. Complementary oligonucleotides were denatured at 75°C for 5 min, annealed by cooling to room temperature, and then treated with XhoI and MluI. These digested fragments were treated with phenol-chloroform (24:1), precipitated with ethanol, and ligated to the vector by incubation with T4 DNA ligase at 15°C for 16 to 18 h. The ligation products were then used to transform E. coli AW1043. Plasmid DNA from the ampicillin-resistant transformants was prepared, and the mutant sequence was verified by dideoxy sequencing (40).
For the construction of PhoAs with mutations at the 28th and 290th amino acids of the mature region, WT-N-MCS and WT-M-MCS (dl) were used as described above. In order to insert a DNA fragment encoding a signal peptide including its cleavage region, a SalI- and BssHII-digested fragment from either the WT or 10L construct described previously (39) was ligated to the XhoI- and MluI-digested vector.
Induction of alkaline phosphatase expression.
Overnight cultures grown in M63 medium (30) supplemented with thiamine hydrochloride (2 μg/ml) and glycerol (0.4%) and containing 250 μg of ampicillin and 50 μg of kanamycin per ml were subcultured at a dilution of 1:20 in the same medium. Cells were then grown at 37°C to logarithmic phase and harvested at an optical density at 600 nm of 1.0 to 1.2. Cells were then washed twice with MOPS (4-morpholinepropanesulfonic acid; pH 7.4) containing no phosphate and resuspended in 2 ml of the same medium supplemented with amino acids (20 mg/ml; excluding methionine). Cells were incubated at 37°C for 15 min to induce the expression of alkaline phosphatase.
Pulse-chase analysis.
Cells were cultured, washed, and resuspended in MOPS medium as described above. Cells were radiolabeled with 60 μCi of l-[35S]methionine for 15 s and then chased with excess cold methionine (4 mg/ml) for the times indicated. Alkaline phosphatase was immunoprecipitated as described previously (17).
Azide treatment.
Sodium azide was added to 2 mM to cells for 1 min at 37°C prior to labeling with 40 μCi of [35S]methionine for 40 s (33). Control samples were treated similarly except that no sodium azide was added prior to labeling. Alkaline phosphatase was immunoprecipitated.
CCCP analysis.
Prior to being labeled, cells were incubated at 37°C for 1 min in 0.1 mM carbonyl cyanide 3-chlorophenyl hydrazone (CCCP) in dimethyl sulfoxide or an equal volume of dimethyl sulfoxide only. Cells were then labeled with 64 μCi of [35S]methionine for 2 min and immunoprecipitated.
Electrophoresis and quantitation of protein bands.
Immunoprecipitated proteins were separated by electrophoresis on Laemmli sodium dodecyl sulfate–7.5 to 15% polyacrylamide electrophoresis gels (27). The pattern was visualized by autoradiography as described by Kendall and Kaiser (18), and protein was quantified with a phosphorimager (Bio-Rad).
RESULTS
We have identified a region 13 residues from the amino terminus of mature E. coli alkaline phosphatase which is sensitive to the conferring of SecB dependence. In order to determine the characteristics of sequences in this region, which can switch alkaline phosphatase from the utilization of a SecB-independent transport pathway to a SecB-dependent one, we have generated a series of mutants containing insertions of short polymeric segments which vary in the degree and arrangement of charged residues. The sequences of the relevant regions in the wild-type and mutant proteins are shown in Fig. 1. Also shown are modified wild-type sequences [called WT-MCS(dl), WT-N-MCS, and WT-M-MCS(dl)] containing insertions with lengths comparable to those of the polymers but composed of a variety of amino acids; these sequences serve to verify that it is the nature of the sequence and not the insert per se that dictates the SecB requirement.
FIG. 1.
The amino acid sequences of mutant alkaline phosphatases. The wild-type alkaline phosphatase is shown diagrammatically with the signal peptide region darkly shaded, the mature region lightly shaded, and the signal peptidase cleavage site marked by an arrow. The sequences inserted at the 13th (A), 28th (B), and 290th (C) residues of the mature protein and the flanking sequences are shown. The inserted sequences are shown in boldface, and the amino acids generated during the cloning procedure are shown in italics. All constructs retain the wild-type amino-terminal signal peptide and consequently the nomenclature begins with the WT reference.
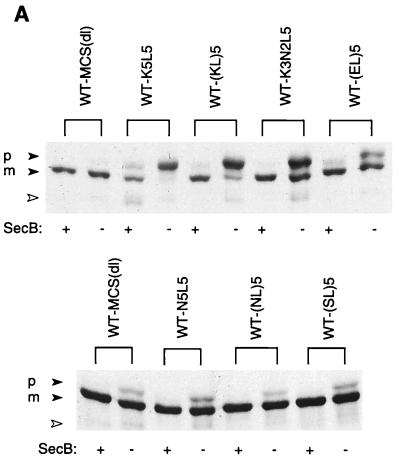
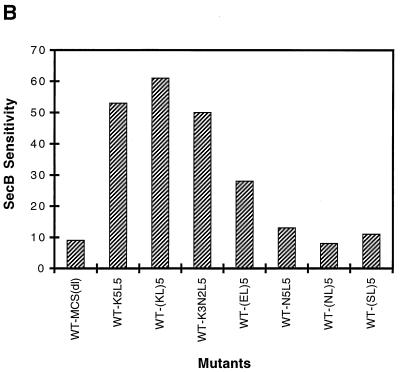
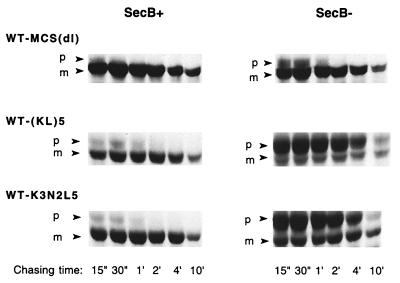
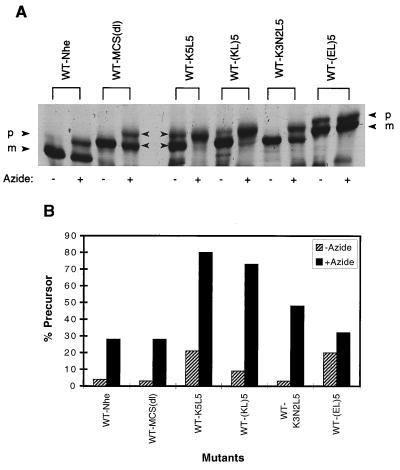
The extent to which the insertion of each of several sequences confers SecB dependence on alkaline phosphatase was evaluated by comparing the level of precursor accumulation in a SecB-null strain to that in a host strain expressing SecB (Fig. 2). We find that WT-MCS(dl), like wild-type alkaline phosphatase, is rapidly processed in both SecB+ and SecB− strains. Mutants containing insertions of the various polymeric sequences were also processed rapidly in the SecB+ strain, and the mature protein was correctly localized to the E. coli periplasm (data not shown). In contrast, in the SecB-null strain, the presence of alkaline phosphatase carrying an insert of multiple positively charged residues resulted in substantial precursor accumulation. No marked differences were observed between sequences carrying three or five lysine residues or between sequences with different arrangements of lysine and leucine residues, the latter residue being included to ease the charge density in some cases. The extent of precursor accumulation in the SecB-null strain is comparable to that observed for wild-type MBP under similar conditions (8). Of the sequences tested, those carrying basic residues had the most pronounced effect in conferring SecB dependence, while replacement of the lysines with uncharged polar residues (e.g., asparagine or serine) or negatively charged residues (glutamic acid) either eliminated SecB dependence or produced only a marginal effect, respectively. Furthermore, pulse-chase analysis revealed that those mutants which accumulated in the precursor form in the SecB-null strain exhibited little subsequent processing over time (Fig. 3, right panel).
FIG. 2.
Precursor processing of mutant alkaline phosphatases expressed in SecB+ and SecB− strains. Cells of AW1043 (SecB+) and CK1953 (SecB−) harboring plasmids encoding the indicated proteins were labeled with [35S]methionine for 30 s and chased with excess cold methionine for 30 s, and then alkaline phosphatase was immunoprecipitated as described in Materials and Methods. Each sequence was inserted at the 13th residue of the mature protein. (A) Autoradiogram of the gel pattern. The positions of precursor and mature forms of alkaline phosphatase are indicated by p and m, respectively. Strain CK1953 has a chromosomal copy of the alkaline phosphatase gene, the product of which is indicated with the open arrowhead. (B) The SecB sensitivities of the mutant alkaline phosphatases are summarized. SecB sensitivity was calculated as the percentage of precursor in the SecB− strain minus the percentage of precursor in the SecB+ strain.
FIG. 3.
Pulse-chase analysis of mutant alkaline phosphatases in SecB+ and SecB− strains. Cells of AW1043 (SecB+) and CK1953 (SecB−) harboring plasmids encoding the indicated proteins were labeled with [35S]methionine for 15 s and chased with excess cold methionine for 15 or 30 s or for 1, 2, 4, or 10 min, and then alkaline phosphatase was immunoprecipitated as described in Materials and Methods. The positions of the precursor and mature forms of alkaline phosphatase are indicated by p and m, respectively.
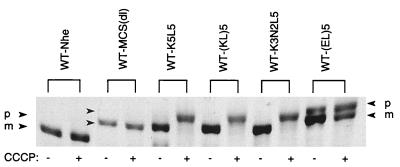
Although the mutant alkaline phosphatases are transported well in the presence of SecB (Fig. 3, left panel), it is possible that the membrane potential that is more positive on the periplasmic face than on the cytoplasmic face of the inner membrane could act as a barrier against translocation of the charged motif. When this was observed for a fusion protein of leader peptidase and procoat, CCCP treatment to dissipate the membrane potential resulted in an enhancement in precursor processing and protein translocation (5). In contrast, the treatment of cells harboring the SecB-dependent alkaline phosphatase mutants results in the accumulation of the precursor form (Fig. 4). This suggests that transport of these sequences uses, and indeed depends upon, the membrane potential as does transport of the wild-type protein. Furthermore, in this experiment the level of CCCP is lowered below that needed to observe accumulation of the wild-type species (15, 39) yet the SecB-dependent mutants remain highly sensitive to loss of the membrane potential. These results are consistent with those of Kato et al. (16), who found that even a polypeptide that was uncharged was translocated in a membrane potential-dependent manner.
FIG. 4.
Effect of dissipation of the membrane potential on precursor processing of the mutants. Cells were treated with dimethyl sulfoxide in the presence (+) or absence (−) of an uncoupler, CCCP, for 1 min prior to being labeled with [35S]methionine for 2 min. The positions of the precursor and mature forms of alkaline phosphatase are indicated by p and m, respectively.
Since SecB is known to interact with SecA and form a ternary complex with the preprotein (14), the SecB-dependent alkaline phosphatases should be sensitive to the inhibition of SecA function by sodium azide. As shown in Fig. 5, azide treatment of cells harboring the SecB-dependent alkaline phosphatase mutants results in precursor accumulation, indicating that these mutants also require SecA for transport. In addition, we find that the mutants which rely most heavily on SecB for transport are also those most sensitive to inhibition of SecA. Mutants containing three basic residues show less-severe accumulation than those with five, while mutants with negative charges exhibit no more azide sensitivity than does WT-MCS(dl). A range in the consequences of azide treatment has also been observed for other SecB-dependent mutants (41). Since it is not possible to completely delete the SecA function with azide, it is reasonable that those preproteins which require complex formation with both SecB and SecA will be more sensitive to a limiting SecA concentration than species which need only SecA.
FIG. 5.
Effect of a SecA inhibitor on precursor processing of the mutants. Cells were treated with sodium azide for 1 min prior to being labeled with [35S]methionine for 40 s. (A) Autoradiogram of the gel pattern. The positions of the precursor and mature forms of alkaline phosphatase are indicated by p and m, respectively. (B) The percentages of total alkaline phosphatase observed in the precursor form with and without azide are summarized.
To examine the extent to which the SecB dependence was specific to the location of the basic sequence, we also examined the impact of the motif further in the mature region at residue 28 and, separately, at residue 290 (Fig. 6). At residue 290, which is well inside the mature region of the protein (Fig. 6B), none of the sequences inserted produced an accumulation of the precursor in the absence of SecB. In addition, the SecB dependence is lost when the (KL)5 motif is moved to residue 28 (Fig. 6A); no more precursor accumulation is observed than is observed for the WT-MCS(dl) control. However, although the magnitude of the effect is diminished at this intermediate position, the mutant with the insert composed of tandem clusters of lysines and leucines still accumulates as a precursor in the SecB-null strain (Fig. 6A). It is intriguing that two inserts [(KL)5 and K5L5] at this position, with the same composition but different arrangements of amino acids, have very different impacts on the requirement for SecB.
FIG. 6.
Precursor processing of mutant alkaline phosphatases carrying inserts in different locations of the mature protein and expressed in SecB+ and SecB− strains. The sequences were inserted at the 28th (A) and at the 290th (B) residue of the mature protein as schematically outlined in Fig. 1. The positions of the precursor and mature forms of alkaline phosphatase are indicated by p amd m, respectively. Strain CK1953 has a chromosomal copy of the alkaline phosphatase gene, the product of which is indicated with the open arrowhead.
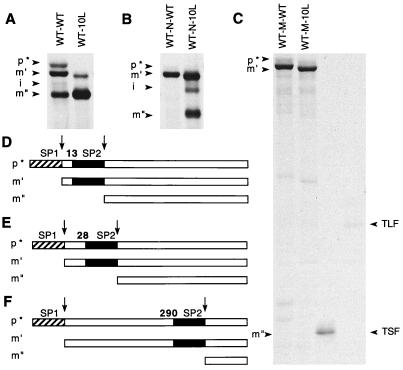
We considered the possibility that the sequence and location specificity for the SecB requirement that we observed may reflect the extent to which the motif is accessible to the secretion machinery during the transport process. To probe this possibility, we exploited a system we had previously designed to examine the relative competitiveness of two different signal peptides (7). In those experiments we found that if two wild-type signal peptides are inserted in tandem in front of alkaline phosphatase, 50% of the population is cleaved after the first signal peptide and 50% is cleaved after the second signal peptide. If, however, the hydrophobicity of the second signal peptide is increased or decreased relative to that of the first signal peptide, then it is cleaved more or less, respectively, than the first. Thus, this provides a system for testing the extent to which the second signal peptide can compete with the first for the secretion pathway. Figure 7 summarizes the results of a set of experiments in which we put a second cleavable signal peptide into the same locations in which the basic motifs were earlier tested for the extent to which they conferred SecB dependence. In each case, the more amino-terminal signal peptide retains the wild-type sequence and the second signal peptide is either the wild type or a very hydrophobic one rich in leucine residues (7). We find that, when inserted at residue 13, the second signal peptide is used effectively when it is composed of either sequence (Fig. 7A); when it is inserted at residue 290, the second signal peptide is never used regardless of sequence (Fig. 7C); when it is inserted at residue 28, the results are intermediate (Fig. 7B). That is, the extent to which the signal peptide at residue 28 is used is highly dependent on the nature of the sequence; the wild type is not utilized in this position, but the leucine-rich signal peptide continues to be utilized and is cleaved about 50% of the time. It is striking that the hierarchy of these results parallels the impact of the basic sequences when they are placed at the same locations (Fig. 2 and 6). This argues that the effectiveness of the basic motif in conferring SecB dependence depends on access to one or more components of the secretion pathway. This could involve a direct interaction between the basic motif and a component of the SecB-dependent pathway, perhaps SecB itself, or it could involve an indirect effect in which the basic motif leads to an abortive interaction between the nascent polypeptide and a component of the SecB-independent pathway.
FIG. 7.
Cleavage patterns of mutant alkaline phosphatases containing two signal peptides. The sequences of these signal peptides are further described in reference 7. Cells were radiolabeled, and alkaline phosphatase was immunoprecipitated. The various alkaline phosphatase species are identified as follows: p* is the precursor form which retains both signal peptides arranged in tandem; m′ results from cleavage after the first of these (SP1); m" results from cleavage after the second (SP2). The i marks the position of a small amount of an intermediate cleavage product. The signal peptidase cleavage sites are marked by arrows. Schematic representations of the relative locations of the two signal peptides in the precursors whose results are shown in panels A, B, and C are shown in panels D, E, and F, respectively. TSF and TLF are molecular weight markers (6); TLF corresponds to the approximate size of the fragment amino-terminal to the SP2 cleavage site, and TSF corresponds to the size of m" (6) in panels C and F.
DISCUSSION
We describe here the identification of a sequence motif which confers a requirement for SecB during the transport of alkaline phosphatase. The critical feature of this motif is the presence of basic residues. Remarkably, and unlike other SecB-dependent mutants (8, 20), no translocation defect is observed for alkaline phosphatase carrying this motif when expressed in the presence of SecB. The mutant alkaline phosphatases are correctly localized, with transport kinetics comparable to those of the wild-type SecB-dependent protein MBP (8). Consequently, alkaline phosphatase with and without the basic motif provides a good model system for elucidating the mechanism or switch by which proteins are shuttled into SecB-dependent versus -independent transport pathways.
In the studies described here we have characterized mutants carrying inserts with a particularly high density of basic residues in order to test the limits of the system. We also show, however, that inserts with only three lysines are sufficient to have a substantial impact on the requirement for SecB (in fact, with as few as two lysines SecB is required [data not shown]). In addition to the basic residues, the motifs described include a cluster of leucine residues. With inserts containing five lysines the leucines can be replaced with serines with little change in the SecB requirement (data not shown) but the more nonpolar residue may still be needed with fewer basic residues. It is intriguing that an inspection of the sequences of wild-type SecB-dependent proteins reveals the occurrence of a short, relatively nonpolar region preceded by a few basic residues located within the first 30 residues of the mature protein. The extent to which these natural sequences play a role in conferring SecB dependence needs to be explored in future studies.
Several different possibilities could account for why these sequences confer a requirement for SecB. Certainly it is possible that the basic motif alters the folding kinetics of alkaline phosphatase and that SecB becomes required to keep the protein in the unfolded, translocation-competent state. If this is the case, however, it is surprising that folding is so sensitive to the arrangement of the residues in the intermediate position (Fig. 6A). It is also possible that the presence of the basic motif produces a translocation defect that is not detected within the limits of sensitivity of our experiments. Consistent with this possibility, Kusukawa et al. (26) have observed some SecB dependence of PhoA at low temperature. Nevertheless, by the criteria used, the SecB-dependent mutant alkaline phosphatases were as efficiently transported as wild-type SecB-dependent proteins. The positional effects of the basic motif suggest that accessibility of the motif to one or more components of the secretory pathway is a factor. Perhaps the motif interferes with recognition of the preprotein in the usual way by one or more components of the secretory pathway. The specificity with regard to the arrangement of residues in the basic motif would be consistent with inhibition of this type of protein-protein interaction. For example, the basic motif might interfere with recognition by the E. coli SRP and thus SecB becomes required to insure proper targeting to translocation sites. Alternatively, the basic motif might impede the interaction of the signal peptide with SecA unless SecB is also present.
It is not known to what regions of alkaline phosphatase SecB binds. Since changes in the signal peptide can also increase the SecB dependence of alkaline phosphatase (20a), SecB must bind sites within the wild-type sequence of the mature protein. Nevertheless, the basic motifs which confer SecB dependence are reminiscent of the synthetic peptides of polylysine that were found to bind SecB in vitro (37). It may be that SecB also initially binds to the basic motif in alkaline phosphatase and that this plays a role in the efficiency with which SecB rescues the preprotein for transport. On the other hand, recent evidence suggests that the anionic sites on SecB may play a role in its interaction with SecA (11). It is not clear whether this also precludes an ionic interaction with the preprotein or not.
There are likely to be a number of factors which come to bear on whether a particular preprotein will take a transport pathway involving SecB. These include folding kinetics, transport efficiency, and binding affinity of the preprotein to SecB relative to other components of the secretion machinery involved in the early stages of transport. Changes in any one of these factors could shift the equilibrium toward more SecB utilization. We show here that the presence of a cluster of a few positively charged residues is one factor which can shift alkaline phosphatase from a SecB-independent protein to one with a marked requirement for SecB. The identification of this short, well-defined motif which serves as the switch in alkaline phosphatase provides a system with which the role of SecB can be further elucidated.
ACKNOWLEDGMENTS
We thank Sharyn L. Rusch for critically reading this manuscript and Carol Kumamoto for generously providing strain CK1953.
This research was supported in part by National Institutes of Health grant GM37639 (to D.A.K.).
REFERENCES
- 1.Akimaru J, Matsuyama S, Tokuda H, Mizushima S. Recognition of a protein translocation system containing purified SecY, SecE, and SecA from Escherichia coli. Proc Natl Acad Sci USA. 1991;88:6545–6549. doi: 10.1073/pnas.88.15.6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altman E, Emr S D, Kumamoto C A. The presence of both the signal sequence and a region of mature LamB protein is required for the interaction of LamB with the export factor SecB. J Biol Chem. 1990;265:18154–18168. [PubMed] [Google Scholar]
- 3.Bernstein H D, Poritz M A, Strub K, Hoben P J, Brenner S, Walter P. Model for signal sequence recognition from amino-acid sequence of 54K subunit of signal recognition particle. Nature. 1989;340:482–486. doi: 10.1038/340482a0. [DOI] [PubMed] [Google Scholar]
- 4.Brundage L, Hendrick J P, Schiebel E, Driessen A J M, Wickner W. The purified E. coli integral membrane protein SecY/E is sufficient for reconstitution of SecA-dependent precursor protein translocation. Cell. 1990;62:649–657. doi: 10.1016/0092-8674(90)90111-q. [DOI] [PubMed] [Google Scholar]
- 5.Cao G, Kuhn A, Dalbey R E. The translocation of negatively charged residues across the membrane is driven by the electrochemical potential: evidence for an electrophoresis-like membrane transfer mechanism. EMBO J. 1995;14:866–875. doi: 10.1002/j.1460-2075.1995.tb07068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen H, Kendall D A. Artificial transmembrane segments. J Biol Chem. 1995;270:14115–14122. doi: 10.1074/jbc.270.23.14115. [DOI] [PubMed] [Google Scholar]
- 7.Chen H, Kim J, Kendall D A. Competition between functional signal peptides demonstrates variation in affinity for the secretion pathway. J Bacteriol. 1996;178:6658–6664. doi: 10.1128/jb.178.23.6658-6664.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collier D N, Bassford P J., Jr Mutations that improve export of maltose-binding protein in SecB− cells of Escherichia coli. J Bacteriol. 1989;171:4640–4647. doi: 10.1128/jb.171.9.4640-4647.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Economou A, Wickner W. SecA promotes preprotein translocation by undergoing ATP-driven cycles of membrane insertion and deinsertion. Cell. 1994;78:835–843. doi: 10.1016/s0092-8674(94)90582-7. [DOI] [PubMed] [Google Scholar]
- 10.Evans E A, Gilmore R, Blobel G. Purification of microsomal signal peptidase as a complex. Proc Natl Acad Sci USA. 1986;83:581–585. doi: 10.1073/pnas.83.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fekkes P, van der Does C, Driessen A J M. The molecular chaperone SecB is released from the carboxy-terminus of SecA during initiation of precursor protein translocation. EMBO J. 1997;16:6105–6113. doi: 10.1093/emboj/16.20.6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gannon P M, Li P, Kumamoto C A. The mature portion of Escherichia coli maltose-binding protein (MBP) determines the dependence of MBP on SecB for export. J Bacteriol. 1989;171:813–818. doi: 10.1128/jb.171.2.813-818.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gardel C, Johnson K, Jacq A, Beckwith J. The secD locus of E. coli codes for two membrane proteins required for protein export. EMBO J. 1990;9:3209–3216. doi: 10.1002/j.1460-2075.1990.tb07519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartl F U, Lecker S, Schiebel E, Hendrick J P, Wickner W. The binding cascade of SecB to SecA to SecY/E mediates preprotein targeting to the E. coli plasma membrane. Cell. 1990;63:269–279. doi: 10.1016/0092-8674(90)90160-g. [DOI] [PubMed] [Google Scholar]
- 15.Izard J W, Rusch S L, Kendall D A. The amino-terminal charge and core region hydrophobicity interdependently contribute to the function of signal sequences. J Biol Chem. 1996;271:21579–21582. doi: 10.1074/jbc.271.35.21579. [DOI] [PubMed] [Google Scholar]
- 16.Kato M, Tokuda H, Mizushima S. In vitro translocation of secretory proteins possessing no charges at the mature domain takes place efficiently in a proton motive force-dependent manner. J Biol Chem. 1992;267:413–418. [PubMed] [Google Scholar]
- 17.Kendall D A, Bock S C, Kaiser E T. Idealization of the hydrophobic segment of the alkaline phosphatase signal peptide. Nature (London) 1986;321:706–708. doi: 10.1038/321706a0. [DOI] [PubMed] [Google Scholar]
- 18.Kendall D A, Kaiser E T. A functional decaisoleucine-containing signal sequence. J Biol Chem. 1988;263:7261–7265. [PubMed] [Google Scholar]
- 19.Khisty V J, Munske G R, Randall L L. Mapping of the binding frame for the chaperone SecB within a natural ligand, galactose-binding protein. J Biol Chem. 1995;270:25920–25927. doi: 10.1074/jbc.270.43.25920. [DOI] [PubMed] [Google Scholar]
- 20.Kim J, Lee Y, Kim C, Park C. Involvement of SecB, a chaperone, in the export of ribose-binding protein. J Bacteriol. 1992;174:5219–5227. doi: 10.1128/jb.174.16.5219-5227.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20a.Kim, J., and D. A. Kendall. Unpublished results.
- 21.Kim Y J, Rajapandi T, Oliver D. SecA protein is exposed to the periplasmic surface of the E. coli inner membrane in its active state. Cell. 1994;78:845–853. doi: 10.1016/s0092-8674(94)90602-5. [DOI] [PubMed] [Google Scholar]
- 22.Kimsey H H, Dagarag M D, Kumamoto C A. Diverse effects of mutation on the activity of the Escherichia coli export chaperone SecB. J Biol Chem. 1995;270:22831–22835. doi: 10.1074/jbc.270.39.22831. [DOI] [PubMed] [Google Scholar]
- 23.Kumamoto C A. Molecular chaperones and protein translocation across the Escherichia coli inner membrane. Mol Microbiol. 1991;5:19–22. doi: 10.1111/j.1365-2958.1991.tb01821.x. [DOI] [PubMed] [Google Scholar]
- 24.Kumamoto C A, Beckwith J. Evidence for specificity at an early step in protein export in Escherichia coli. J Bacteriol. 1985;163:267–274. doi: 10.1128/jb.163.1.267-274.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kusters R, de Vrije T, Breukink E, de Kruijff B. SecB protein stabilizes a translocation-competent state of purified prePhoE protein. J Biol Chem. 1989;264:20827–20830. [PubMed] [Google Scholar]
- 26.Kusukawa N, Yura T, Ueguchi C, Akiyama Y, Ito K. Effects of mutations in heat-shock genes groES and groEL on protein export in Escherichia coli. EMBO J. 1989;8:3517–3521. doi: 10.1002/j.1460-2075.1989.tb08517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 28.Laforet G A, Kendall D A. Functional limits of conformation, hydrophobicity, and steric constraints in prokaryotic signal peptide cleavage regions. J Biol Chem. 1991;266:1326–1334. [PubMed] [Google Scholar]
- 29.Matsuyama S, Fujita Y, Mizushima S. SecD is involved in the release of translocated secretory proteins from the cytoplasmic membrane of Escherichia coli. EMBO J. 1993;12:265–270. doi: 10.1002/j.1460-2075.1993.tb05652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 31.Nishiyama K, Suzuki T, Tokuda H. Inversion of the membrane topology of SecG coupled with SecA-dependent preprotein translocation. Cell. 1996;85:71–81. doi: 10.1016/s0092-8674(00)81083-1. [DOI] [PubMed] [Google Scholar]
- 32.Oliver D B. SecA protein: autoregulated ATPase catalysing preprotein insertion and translocation across the Escherichia coli inner membrane. Mol Microbiol. 1993;7:159–165. doi: 10.1111/j.1365-2958.1993.tb01107.x. [DOI] [PubMed] [Google Scholar]
- 33.Oliver D B, Cabelli R J, Dolan K M, Jarosik G P. Azide-resistant mutants of Escherichia coli alter the SecA protein, an azide-sensitive component of the protein export machinery. Proc Natl Acad Sci USA. 1990;87:8227–8231. doi: 10.1073/pnas.87.21.8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park S, Liu G, Topping T B, Cover W H, Randall L L. Modulation of folding pathways of exported proteins by the leader sequence. Science. 1988;239:1033–1035. doi: 10.1126/science.3278378. [DOI] [PubMed] [Google Scholar]
- 35.Phillips G J, Silhavy T J. The E. coli ffh gene is necessary for viability and efficient protein export. Nature. 1992;359:744–746. doi: 10.1038/359744a0. [DOI] [PubMed] [Google Scholar]
- 36.Poritz M A, Bernstein H D, Strub K, Zopf D, Wilhelm H, Walter P. An E. coli ribonucleoprotein containing 4.5S RNA resembles mammalian signal recognition particle. Science. 1990;250:1111–1117. doi: 10.1126/science.1701272. [DOI] [PubMed] [Google Scholar]
- 37.Randall L L. Peptide binding by chaperone SecB: implications for recognition of nonnative structure. Science. 1992;257:241–245. doi: 10.1126/science.1631545. [DOI] [PubMed] [Google Scholar]
- 38.Randall L L, Topping T B, Hardy S J S. No specific recognition of leader peptide by SecB, a chaperone involved in protein export. Science. 1990;248:860–863. doi: 10.1126/science.2188362. [DOI] [PubMed] [Google Scholar]
- 39.Rusch S L, Chen H, Izard J W, Kendall D A. Signal peptide hydrophobicity is finely tailored for function. J Cell Biochem. 1994;55:209–217. doi: 10.1002/jcb.240550208. [DOI] [PubMed] [Google Scholar]
- 40.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strobel S M, Cannon J G, Bassford P J., Jr Regions of maltose-binding protein that influence SecB-dependent and SecA-dependent export in Escherichia coli. J Bacteriol. 1993;175:6988–6995. doi: 10.1128/jb.175.21.6988-6995.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Topping T B, Randall L L. Determination of the binding frame within a physiological ligand for the chaperone SecB. Protein Sci. 1994;3:730–736. doi: 10.1002/pro.5560030502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ulbrandt N D, Newitt J A, Bernstein H D. The E. coli signal recognition particle is required for the insertion of a subset of inner membrane proteins. Cell. 1997;88:187–196. doi: 10.1016/s0092-8674(00)81839-5. [DOI] [PubMed] [Google Scholar]
- 44.Valent Q A, Kendall D A, High S, Kusters R, Oudega B, Luirink J. Early events in preprotein recognition in E. coli: interaction of SRP and trigger factor with nascent polypeptides. EMBO J. 1995;14:5494–5505. doi: 10.1002/j.1460-2075.1995.tb00236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watanabe M, Blobel G. SecB functions as a cytosolic signal recognition factor for protein export in E. coli. Cell. 1989;58:695–705. doi: 10.1016/0092-8674(89)90104-9. [DOI] [PubMed] [Google Scholar]
- 46.Watanabe T, Hayashi S, Wu H C. Synthesis and export of the outer membrane lipoprotein in Escherichia coli mutants defective in generalized protein export. J Bacteriol. 1988;170:4001–4007. doi: 10.1128/jb.170.9.4001-4007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]