Abstract
Taking advantage of a chaperone-like function of MalK, a stable complex of MalF-MalG could be solubilized from the Escherichia coli membrane and purified in high yield in the absence of MalK. This MalF-MalG complex was competent for efficient reassembly of a functional MalFGK2 maltose transporter complex both in detergent solution and in proteoliposomes.
The maltose uptake system of Escherichia coli is a member of the ATP-binding cassette (ABC) superfamily of transport proteins that employs a periplasmic maltose-binding protein (MBP) to mediate high-affinity transport into the cell (1). The membrane-associated transporter complex MalFGK2 contains four subunits, one copy each of transmembrane-spanning proteins MalF and MalG and two copies of the cytoplasmically located nucleotide-binding subunit MalK (1, 5). The transporter is soluble in nonionic detergents and the MalF, MalG, and MalK proteins copurify (5, 18), indicating that the subunits are tightly bound to each other. In contrast, when the individual proteins are overexpressed in the absence of the full complement of subunits, they tend to be insoluble in nonionic detergent (18). In addition, in the absence of MalK, MalF fails to fold into a protease-resistant form, suggesting that MalK is required for the proper folding of MalF in the membrane (21). In this report, we define conditions under which MalK is used as a chaperone to generate a high-affinity, detergent-soluble subassembly of MalF and MalG that functions as a platform for in vitro assembly of a functional transporter complex.
Intact MalFGK2 transporter complex was overproduced, purified, and reconstituted into proteoliposomes as described previously (20), and MalK alone was recovered in soluble form from strain BL21(DE3) carrying plasmids pMF8 and pLysS (6, 19). Maltose transport and ATPase activities for wild-type and binding-protein-independent transporters have been described previously (4, 7, 12), and ATPase activities are reported as means of triplicate determinations that varied by 2 to 15%.
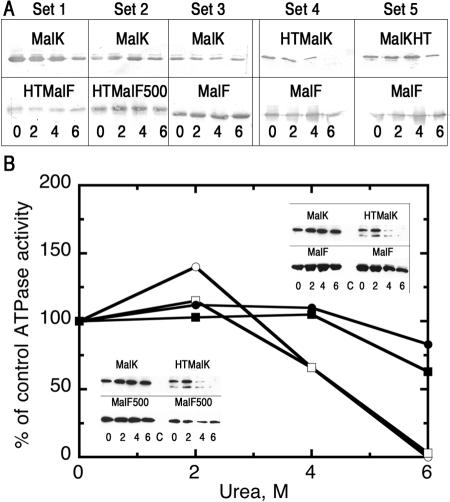
Although treatment of E. coli membranes with salt or urea can extract peripheral membrane proteins, MalK is not released from MalF and MalG under these conditions (Fig. 1A, set 3). However, HTMalK, MalK modified with an N-terminal polyhistidine tag containing 23 amino acids (6), is released by treatment with 4 to 6 M urea, leaving MalF (and presumably MalG) behind in the membrane (Fig. 1A, set 4). This N-terminal MalK modification does not appear to otherwise affect the function of the maltose transporter (3, 6, 8). The presence of the same 23-amino-acid polyhistidine tag on the N terminus of MalF (HTMalF) or a smaller tag on the C terminus of MalK (MalKHT) (2) did not similarly increase the solubility of MalK (Fig. 1A, sets 1 and 5), nor did the presence of the MalF500 protein that renders transport binding protein independent by mimicking an activated conformation (15, 22) (Fig. 1A, set 2). Extraction of HTMalK by increasing concentrations of urea correlated with the loss of ATPase activity of the transporter in both the wild-type transporter and the binding-protein-independent mutant (Fig. 1B). The failure of the binding-protein-independent mutant to alter the strength of the interaction between MalK and the MalF/MalG subunits contrasts with findings in the homologous histidine transporter from Salmonella enterica serovar Typhimurium, where the nucleotide-binding (HisP) subunit is more easily extracted from a binding-protein-independent complex than the wild type (14). Likewise, the presence of ATP had no effect on the extractability of MalK or HTMalK from the membrane, nor did an inactivating mutation (His192Arg) in MalK (data not shown), while HisP is more easily removed with ATP present (13). Based on these differences, we suggest that a model involving physical disengagement of nucleotide-binding domains during the catalytic cycle of ATP hydrolysis, as proposed for the histidine transporter (14), is not a general feature of all ABC transporters.
FIG. 1.
Differential solubilities of MalK and HTMalK in urea. Inside-out membrane vesicles isolated from cells overexpressing different alleles of malF, malG, and malK (6) were treated with 0, 2, 4, or 6 M urea for 30 min at 23°C (lanes marked C in panel B contain samples from a strain lacking plasmids). Membranes were collected by centrifugation at 100,000 × g for 30 min, washed once with 20 mM Tris-HCl and 1 mM EDTA, pH 8, and treated with 1% dodecylmaltoside. A (and insets in B). Components of the detergent-soluble fractions were separated on sodium dodecyl sulfate-polyacrylamide gel electrophoersis gels, transferred to nitrocellulose, and probed with antibody to either MalK (top row) or MalF (bottom row). B. ATPase activities of the total membrane fractions corresponding to the samples shown in the insets. Closed circles, MalFGK2; open circles, MalFG(HTMalK)2; closed squares, MalF500GK2; open squares, MalF500G(HTMalK)2. One hundred percent values for MBP-stimulated or binding-protein-independent ATPase activities are 38, 39, 710, and 130 nmol/min/mg of protein, respectively.
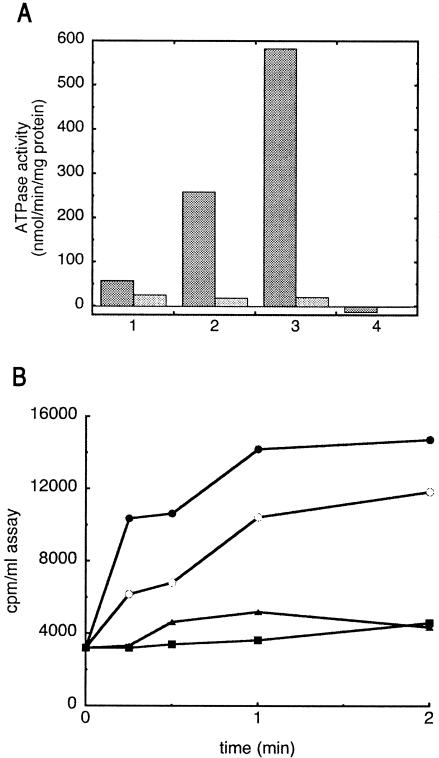
In contrast to earlier observations that MalF expressed in isolation was not detergent soluble (4, 18), MalF was recovered in the detergent-soluble fraction following urea extraction of HTMalK from the transporter (Fig. 1A, all sets). Following urea extraction of HTMalK from a transporter complex containing HTMalF, a complex of HTMalF and MalG was purified by affinity chromatography, demonstrating retention of a tight association between MalF and MalG following MalK removal (Fig. 2). When a crude 100,000 × g supernatant taken from a strain expressing soluble MalK in the absence of MalF and MalG was passed over a cobalt column with HTMalF and MalG bound, MalK was the only protein retained by the resin, suggesting that reassembly of the complex had occurred (Fig. 2). To determine if this reassembled transporter complex was functional, in vitro-assembled transport complexes were reconstituted into proteoliposome vesicles and assayed for function (6). The reassembled transporter complex displayed both MBP-stimulated ATPase activity and maltose transport activity at rates comparable to in vivo-assembled transporters as reported here (Fig. 3A and B) and elsewhere (3, 6). In contrast, no significant activity above the background was seen in proteoliposomes containing only the HTMalF-MalG complex (Fig. 3A and B). To guard against the possibility that activity resulted from incomplete extraction of MalK, HTMalF-MalG was obtained from a transporter complex containing the enzymatically inactive HTMalK(H192R) (6). This mutant transporter complex proved to be suitable for our experimental system because it displayed normal assembly properties as judged by the copurification of transporter subunits (6), the urea extractability of HTMalK(H192R), and the ability of the mutant MalK to support the folding of MalF into a protease-resistant form (21) (data not shown).
FIG. 2.
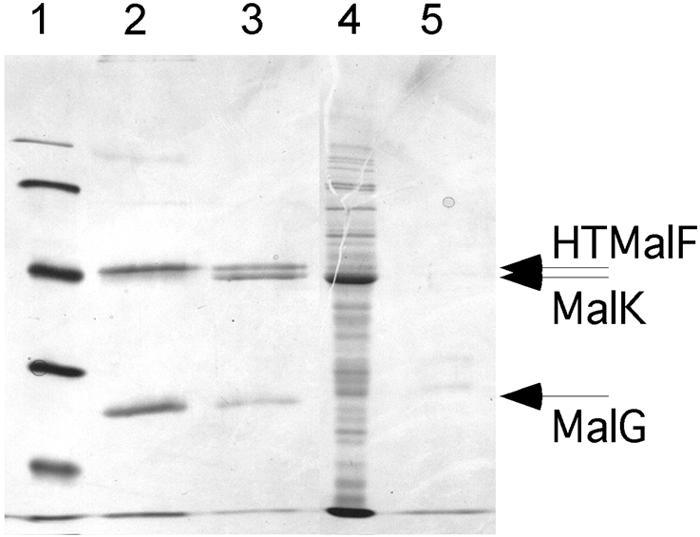
Purification of a MalF-MalG complex and reassembly of MalFGK2. Membranes expressing HTMalF, MalG, and HTMalK(H192R) were treated with 6 M urea to remove HTMalK and then treated with detergent to solubilize HTMalF and MalG. The detergent-soluble fraction was passed over cobalt resin, and purified proteins were eluted with buffer containing100 mM imidazole. Lane 1, molecular weight markers (103) (from top to bottom, 97, 66, 45, 31, and 21.5); lane 2, elution profile showing copurification of HTMalF and MalG; lane 3, elution profile after MalK preparation was passed through the Co resin-containing bound HTMalF/MalG and washed; lane 4, 100,000 × g supernatant fraction containing MalK; lane 5, elution profile after MalK preparation was passed through a Co resin without bound HTMalF/MalG present. Bands are visualized by Coomassie staining.
FIG. 3.
Maltose transport and ATPase activity of reassembled transport complexes. A. ATPase activity is measured in the presence (dark bars) or absence (light bars) of 5 μM MBP using 1 mM ATP and 0.1 μM transporter reconstituted in proteoliposomes (3, 5). 1, unpurified MalFG(HTMalK)2; 2, MalFG(HTMalK)2 purified on Co resin; 3, in vitro-reassembled HTMalFGK2 (preparation shown in Fig. 2); 4, purified HTMalF-MalG complex with no MalK (preparation shown in Fig. 2). B. Maltose transport into proteoliposomes is measured with 10 μM maltose, 1 μM MBP, and approximately 0.5 μM transporter (3). Closed circles, in vitro-assembled transporter (initial rate of uptake is 0.9 nmol/min/mg of protein); open circles, in vivo-assembled (unpurified) transporter (0.3 nmol/min/mg of protein); triangles, HTMalF-MalG only; squares, 1 μM MBP only (background).
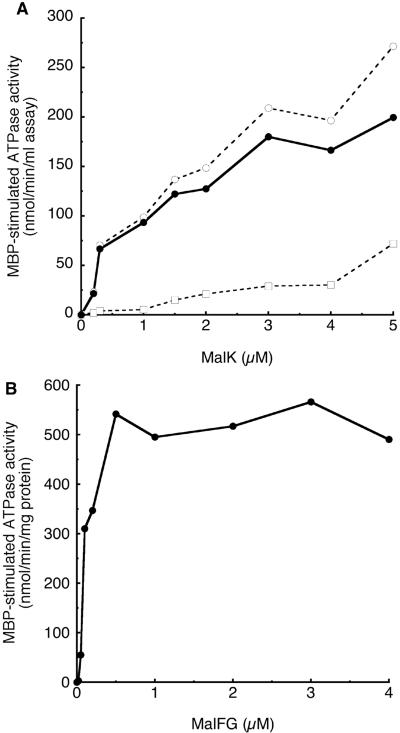
The purified HTMalF-MalG complex, reconstituted into proteoliposomes, also served as a platform for reassembly of a functional transporter complex, and a similar method has been used to demonstrate that the HisP subunits of the histidine transporter assemble onto the transmembrane components individually rather than cooperatively (13). In an experiment where ATPase activity is measured as a function of the concentration of HisP added back to a fixed concentration of transmembrane components, there is a lag in the recovery of ATPase activity (13), as would be anticipated if inactive trimeric complexes (HisQMP) accumulated prior to the formation of functional heterotetramers (HisQMP2). No lag was obvious in the recovery of ATPase activity as a function of MalK concentration in our experiments (Fig. 4A), in agreement with published results for MalK from S. enterica serovar Typhimurium (11). These data suggest that MalK assembly is highly cooperative and that no inactive trimeric complexes accumulate. The same question can be addressed by varying the concentration of proteoliposomes containing transmembrane proteins in the reassembly mix at a fixed MalK or HisP concentration (13). At substoichiometric concentrations of transmembrane proteins, assembly of complete heterotetramers is anticipated. When transmembrane proteins are present in excess over ATPase proteins, the possibility of heterotrimer formation exists, which would inhibit full recovery of transporter ATPase activity. In this experimental system (Fig. 4B), recovery of ATPase activity reached a maximum close to a molar ratio of 1:2 (MalF-MalK) and remained at a high level even if HTMalF-MalG was present in molar excess. In contrast, in the histidine system, the recovery of activity peaked at the optimal stoichiometric ratio but decreased again when excess transmembrane subunits were present during assembly (13). These data also suggest that HisP, but not MalK, forms functionally inactive trimeric species during the assembly process (13). MalK more readily forms a dimer in solution than HisP (2, 9, 17) and this difference is likely to account for the differential behavior of the two nucleotide-binding components in their assembly pathways. MalK contains a C-terminal regulatory domain that contributes substantially to the dimer interface (2) and may stabilize the dimer in solution prior to assembly.
FIG. 4.
Dependence of ATPase on MalK or MalF/MalG concentration. A. A fixed concentration of proteoliposomes containing HTMalF and MalG (1 μM protein) was incubated with increasing concentrations of purified MalK for 15 min and then assayed for ATPase activity in the presence (open circles) or absence (open squares) of MBP. MBP-stimulated ATPase activity (closed circles) is calculated by subtracting activity in the absence of MBP from that in the presence of MBP. B. A fixed concentration of purified MalK (1 μM) is incubated with increasing concentrations of purified, reconstituted HTMalF and MalG and then assayed for ATPase activity. Only MBP-stimulated ATPase activity is shown, as basal ATPase activity is low and unchanged throughout.
It will be of interest to determine whether a MalF-MalG complex in isolation can mediate transfer of maltose across the membrane. Truncation of the C-terminal nucleotide-binding domain of the ABC transporter LmrA, which mediates multidrug efflux from bacterial cells, leaves behind a membrane-spanning domain that appears to facilitate the uptake of drug substrates into cells through a proton-symport mechanism (23). The transmembrane subunit of the bacterial arsenate transporter ArsB (not an ABC transporter), when expressed alone, mediates proton-driven efflux of arsenate, though it normally forms a complex with the ArsA ATPase subunit to mediate ATP-driven efflux (10). In preliminary experiments with purified MalF-MalG, we have not yet detected similar activities (data not shown).
The ability to completely extract HTMalK from inside-out membrane vesicles with urea and reassemble a purified transporter complex in detergent solution represents a significant improvement over earlier procedures for in vitro complex assembly (11, 13, 16). It will now be possible to differentially label the transmembrane and nucleotide-binding subunits to monitor fluorescence energy transfer between them. Coexpression of MalK with MalF and MalG drives MalF into a more-protease-resistant conformation (21) that may account for the high yield of MalF-MalG in our experiments as well as the improved solubility of MalF in nonionic detergent compared to that of previous reports (18). In essence, we have used MalK as a chaperone to promote the assembly of MalF and MalG.
Acknowledgments
This work was supported by Public Health Service grant GM49261 from the NIH and Q-1391 from the Welch Foundation (to A.L.D.) and American Cancer Society grant RPG-98-223-01-CSM (to B.T.).
REFERENCES
- 1.Boos, W., and J. M. Lucht. 1996. Periplasmic binding protein-dependent ABC transporters, p. 1175-1209. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, D.C. [Google Scholar]
- 2.Chen, J., G. Lu, J. Lin, A. L. Davidson, and F. A. Quiocho. 2003. A tweezers-like motion of the ATP-binding cassette dimer in an ABC transport cycle. Mol. Cell 12:651-661. [DOI] [PubMed] [Google Scholar]
- 3.Chen, J., S. Sharma, F. A. Quiocho, and A. L. Davidson. 2001. Trapping the transition state of an ATP-binding-cassette transporter: evidence for a concerted mechanism of maltose transport. Proc. Natl. Acad. Sci. USA 98:1525-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davidson, A. L., and H. Nikaido. 1990. Overproduction, solubilization, and reconstitution of the maltose transport system from Escherichia coli. J. Biol. Chem. 265:4254-4260. [PubMed] [Google Scholar]
- 5.Davidson, A. L., and H. Nikaido. 1991. Purification and characterization of the membrane-associated components of the maltose transport system from Escherichia coli. J. Biol. Chem. 266:8946-8951. [PubMed] [Google Scholar]
- 6.Davidson, A. L., and S. Sharma. 1997. Mutation of a single MalK subunit severely impairs maltose transport activity in Escherichia coli. J. Bacteriol. 179:5458-5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davidson, A. L., H. A. Shuman, and H. Nikaido. 1992. Mechanism of maltose transport in Escherichia coli: transmembrane signalling by periplasmic binding proteins. Proc. Natl. Acad. Sci. USA 89:2360-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kennedy, K. A., E. G. Gachelet, and B. Traxler. 2004. Evidence for multiple pathways in the assembly of the Escherichia coli maltose transport complex. J. Biol. Chem. 279:33290-33297. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy, K. A., and B. Traxler. 1999. MalK forms a dimer independent of its assembly into the MalFGK2 ATP-binding cassette transporter of Escherichia coli. J. Biol. Chem. 274:6259-6264. [DOI] [PubMed] [Google Scholar]
- 10.Kuroda, M., S. Dey, O. I. Sanders, and B. P. Rosen. 1997. Alternate energy coupling of ArsB, the membrane subunit of the Ars anion-translocating ATPase. J. Biol. Chem. 272:326-331. [DOI] [PubMed] [Google Scholar]
- 11.Landmesser, H., A. Stein, B. Bluschke, M. Brinkmann, S. Hunke, and E. Schneider. 2002. Large-scale purification, dissociation and functional reassembly of the maltose ATP-binding cassette transporter (MalFGK(2)) of Salmonella typhimurium. Biochim. Biophys. Acta 1565:64-72. [DOI] [PubMed] [Google Scholar]
- 12.Liu, C. E., P. Q. Liu, and G. F. L. Ames. 1997. Characterization of the adenosine triphosphatase activity of the periplasmic histidine permease, a traffic ATPase (ABC transporter). J. Biol. Chem. 272:21883-21891. [DOI] [PubMed] [Google Scholar]
- 13.Liu, P. Q., and G. F.-L. Ames. 1998. In vitro disassembly and reassembly of an ABC transporter, the histidine permease. Proc. Natl. Acad. Sci. USA 95:3495-3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu, P. Q., C. E. Liu, and G. F. Ames. 1999. Modulation of ATPase activity by physical disengagement of the ATP-binding domains of an ABC transporter, the histidine permease. J. Biol. Chem. 274:18310-18318. [DOI] [PubMed] [Google Scholar]
- 15.Mannering, D. E., S. Sharma, and A. L. Davidson. 2001. Demonstration of conformational changes associated with activation of the maltose transport complex. J. Biol. Chem. 376:12362-12368. [DOI] [PubMed] [Google Scholar]
- 16.Mourez, M., M. Jehanno, E. Schneider, and E. Dassa. 1998. In vitro interaction between components of the inner membrane complex of the maltose ABC transporter of Escherichia coli: modulation by ATP. Mol. Microbiol. 30:353-363. [DOI] [PubMed] [Google Scholar]
- 17.Nikaido, K., P. Q. Liu, and G. F. Ames. 1997. Purification and characterization of HisP, the ATP-binding subunit of a traffic ATPase (ABC transporter), the histidine permease of Salmonella typhimurium. Solubility, dimerization, and ATPase activity. J. Biol. Chem. 272:27745-27752. [DOI] [PubMed] [Google Scholar]
- 18.Panagiotidis, C. H., M. Reyes, A. Sievertsen, W. Boos, and H. A. Shuman. 1993. Characterization of the structural requirements for assembly and nucleotide binding of an ATP-binding cassette transporter. The maltose transport system of Escherichia coli. J. Biol. Chem. 268:23685-23696. [PubMed] [Google Scholar]
- 19.Schneider, E., M. Linde, and S. Tebbe. 1995. Functional purification of a bacterial ATP-binding cassette transporter protein (MalK) from the cytoplasmic fraction of an overproducing strain. Protein Expr. Purif. 6:10-14. [DOI] [PubMed] [Google Scholar]
- 20.Sharma, S., and A. L. Davidson. 2000. Vanadate-induced trapping of nucleotide by the purified maltose transport complex requires ATP hydrolysis. J. Bacteriol. 182:6570-6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Traxler, B., and J. Beckwith. 1992. Assembly of a hetero-oligomeric membrane protein complex. Proc. Natl. Acad. Sci. USA 89:10852-10856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Treptow, N. A., and H. A. Shuman. 1985. Genetic evidence for substrate and periplasmic-binding-protein recognition by the MalF and MalG proteins, cytoplasmic membrane components of the Escherichia coli maltose transport system. J. Bacteriol. 163:654-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Venter, H., R. A. Shilling, S. Velamakanni, L. Balakrishnan, and H. W. Van Veen. 2003. An ABC transporter with a secondary-active multidrug translocator domain. Nature 426:866-870. [DOI] [PubMed] [Google Scholar]