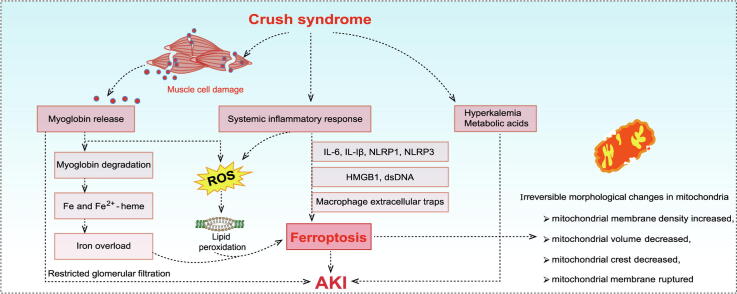
Graphical abstract
Keywords: Crush syndrome, Acute kidney injury, Ferroptosis, Myoglobin, Systemic inflammatory responses, Drug development
Highlights
-
•
Inhibiting ferroptosis could alleviate acute kidney injury following crush syndrome.
-
•
Iron overload produced by myoglobin degradation is a risk factor for ferroptosis.
-
•
HMGB1 and souble-stranded DNA trigger ferroptosis via multiple signaling pathways.
-
•
Crosstalk between inflammation and ferroptosis.
-
•
Inhibition of ferroptosis by alleviating inflammation and anti-lipid peroxidation.
Abstract
Background
Crush syndrome (CS) is a kind of traumatic and ischemic injury that seriously threatens life after prolonged compression. It is characterized by systemic inflammatory reaction, myoglobinuria, hyperkalemia and acute kidney injury (AKI). Especially AKI, it is the leading cause of death from CS. There are various cell death forms in AKI, among which ferroptosis is a typical form of cell death. However, the role of ferroptosis has not been fully revealed in CS-AKI.
Aim of review
This review aimed to summarize the evidence of ferroptosis in CS-AKI and its related molecular mechanism, discuss the therapeutic significance of ferroptosis in CS-AKI, and open up new ideas for the treatment of CS-AKI.
Key scientific concepts of review
One of the main pathological manifestations of CS-AKI is renal tubular epithelial cell dysfunction and cell death, which has been attributed to massive deposition of myoglobin. Large amounts of myoglobin released from damaged muscle deposited in the renal tubules, impeding the normal renal tubules function and directly damaging the tubules with oxidative stress and elevated iron levels. Lipid peroxidation damage and iron overload are the distinguishing features of ferroptosis. Moreover, high levels of pro-inflammatory cytokines and damage-associated molecule pattern molecules (HMGB1, double-strand DNA, and macrophage extracellular trap) in renal tissue have been shown to promote ferroptosis. However, how ferroptosis occurs in CS-AKI and whether it can be a therapeutic target remains unclear. In our current work, we systematically reviewed the occurrence and underlying mechanism of ferroptosis in CS-AKI.
Introduction
Crush syndrome (CS), also known as traumatic rhabdomyolysis, is a group of symptoms characterized by ischemic necrosis of muscle tissue resulting from prolonged compression of the limbs or trunk by gravity, accompanied by acute kidney injury (AKI), electrolyte metabolism disorder, and hypoglycemic shock after the release of compression [1], [2]. AKI is one of the most fatal complications of CS [3]. The pathogenesis of CS-AKI is very complex and may be related to renal ischemia–reperfusion injury (I/R), systemic inflammation and excessive myoglobin (Mb) deposition in renal tubules released by damaged muscle tissue [4], [5]. It was reported that 41.6 % of patients with CS developed AKI after the Wenchuan earthquake in China [6]. Although some therapies have tried to improve outcomes through dialysis and kidney transplantation, most patients die from multiple organ failure as a result of systemic inflammatory response [7].
Ferroptosis is defined as a new form of cell death and characterized by intracellular iron retention, reduced glutathione (GSH) level, and accumulation of iron-dependent lipid reactive oxygen species (ROS) [8], [9]. The morphological characteristics of ferroptosis are different from apoptosis, necroptosis or autophagy, which are manifested by mitochondrial contraction, increased mitochondrial membrane density and disappearance of mitochondrial crest [10], [11]. Direct evidence suggested that ferroptosis inhibitors improved renal function in CS-AKI mice [12]. This suggested that ferroptosis played an important role in AKI following CS and might be a potential therapeutic target.
Given the association between elevated serum iron levels and poor outcomes in patients with AKI, ferroptosis might be a risk factor in CS complicated with AKI. In this paper, we reviewed recent studies to elucidate the pathological process of CS-AKI from the perspective of ferroptosis, and to provide new targets and clues for the treatment of this fatal disease.
Pathogenesis and diagnosis of CS complicating AKI
CS was first described by Bywaters et al. who found that the patients buried in the collapsed building showed the characteristics of limb swelling, circulatory disorder, dark urine (now identified as myoglobinuria) and finally died of kidney failure [13]. AKI is one of the serious complications of CS. The pathogenesis of CS-AKI has not been fully understood, but is currently thought to include at least the following aspects: I/R, systemic inflammation and rhabdomyolysis [4], [5]. Prolonged stress reduces renal blood flow, and when the crush is lifted, the kidney regains blood supply and the subsequent I/R leads to ROS production as well as inflammatory response [5]. During rhabdomyolysis, muscle cells break down releasing large amounts of Mb, while Mb enters the renal tubules after glomerular filtration and then precipitates to form casts that blocking the tubules and causing severe damage or even death of tubular epithelial cells, eventually leading to AKI [14]. AKI is defined as a sudden (within 48 h) decline in renal function, clinically observed as an absolute increase in serum creatinine greater than or equal to 0.3 mg/dl, or a decrease in urine output (recorded less than 0.5 mL/kg/h for more than 6 h). In addition, continuous creatinine clearance test (within 1–24 h), urine analysis and urine microscopy are also used as diagnostic reference indicators [15].
Ferroptosis is a new link between CS and AKI
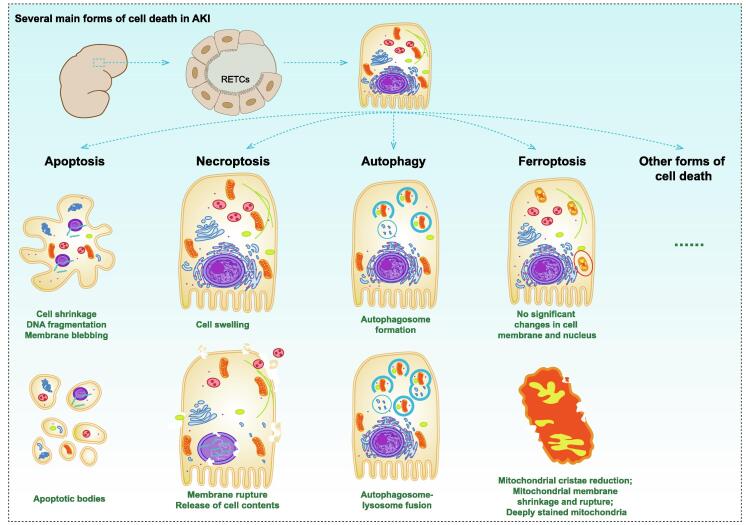
Due to the complexity of AKI pathogenesis, more and more cell death forms have been observed, including apoptosis, necrosis, autophagy and ferroptosis, etc (Fig. 1). In animal studies related to CS-AKI, apoptosis and autophagy of renal tubular epithelial cells (RTECs) have been reported, which are thought to correlate with the severity of AKI. For example, 0.9 mg/mL Mb could enhance apoptosis by upregulating autophagy levels in BUMPT cells (a RTEC cell line), while glycerol-induced AKI (8.0 mL/kg of 50 % glycerol intramuscularly injection, this model was considered to be the ideal chemically induced CS-AKI model to simulate rhabdomyolysis in the CS state [16]) was significantly attenuated in autophagy-deficient mice, which was thought to be associated with activation of EGFR-STAT3/ATG7 axis [17]. Deferiprone is an iron-chelating agent that has been shown to relieve glycerin-induced AKI in rats [18]. Degradation of Mb in the rhabdomyolytic kidney resulted in unnecessary release of free iron [19]. Melania et al. further demonstrated that Mb induced ferroptosis in RTECs during rhabdomyolysis [12]. These evidences indicated that there were various forms of cell death in CS state, and a full understanding of these cell death mechanisms is helpful for the treatment of CS-AKI.
Fig. 1.
Several main forms of cell death in AKI. The forms of cell death that have been revealed in AKI include apoptosis, necroptosis, autophagy, ferroptosis, and so on. Apoptosis: cells contracted, nuclear chromatin condensed, DNA broken, and cells decomposed into several apoptotic bodies. Necroptosis: cells and organelles swelled and disintegrated, cell membranes broken, and cell contents leaked. Autophagy: autophagosomes were formed and transported to lysosomes to form autophagosomes. Subsequently, aging and damaged organelles in autophagosomes were degraded. Ferrotosis: the volume and cristae of mitochondria decreased, the density of mitochondrial membrane increased, and the mitochondrial membrane ruptured.
The concept of ferroptosis was first formally proposed by Dixon et al. in 2012 [20]. Prior to this concept, related inducers had been synthesized. In 2003, Dolma et al. reported a novel compound erastin killed tumor cells with oncogenic RAS mutations without involving nuclear changes and activation of caspase-3. And this form of cell death could further block by iron chelators and antioxidants. These results suggested that the form of cell death was related to the accumulation of intracellular iron and active oxidation products [21], [22]. Now, we have gradually understood the relationship between ferroptosis and some diseases, such as tumor [23], cardiovascular disease [24], and Alzheimer's disease [25]. As we know that, inhibition of cystine/glutamate antiporter system (system Xc−) on cell membrane depleted intracellular GSH [26]. As a result, glutathione peroxidase 4 (GPX4) was limited in converting lipid hydroperoxides into lipid alcohols by GSH, and could not effectively scavenge ROS and lipid reactive substances, consequently inducing ferroptosis [27]. Iron bound with transferrin (TF) in plasma mainly in the form of Fe3+ and was transported to bone marrow or other iron-requiring tissues [28]. The transferrin carrying Fe3+ further bound to the transferrin receptor 1 (TFR1) on the cell surface to form TF-TFR1 complex, which was internalized into endosomes [29], [30]. Subsequently, endosomal acidification resulted in the separation of Fe3+ from TF, which was then reduced to Fe2+ and transported to the cytosol through divalent metal ion transporter-1 (DMT1) [31]. Under physiological conditions, intracellular Fe2+ was maintained at 0.2–0.5 μM to maintain metabolic requirements [32]. When iron was overloaded, Fe2+ could produce a large number of lipid reactive oxygen free radicals through Fenton reaction [33]. In addition, Fe2+ also participated in the synthesis of lipoxygenase and then catalyzed lipid peroxidation [34], both of which could induce ferroptosis.
Currently, a potential link between AKI and ferroptosis has been confirmed in several studies. For example, tubular cells were highly sensitive to ferroptosis in vitro [35]. Li et al. demonstrated that Α-Lipoic acid could alleviate the folic acid induced AKI by reducing the accumulation of ROS, lipid peroxidation and intracellular iron overload [36]. Oreoluwa et al. suggested that HO-1 could antagonize ferroptosis to alleviate AKI severity [37]. AKI caused by CS (CS-AKI) was not uncommon in clinic. Curcumin reduced rhabdomyolysis induced kidney injury by alleviating ferroptosis in a rat model of glycerol intramuscularly injection (10 mg/kg of 50 % glycerol) [12]. Although the potential pathogenic mechanism has not been clarified, several key links have been revealed. The systemic inflammatory response following CS as well as Mb metabolism-induced iron overload were the major causes of ferroptosis in RTECs, which were described in detail in the following sections.
Major induced mechanisms of ferroptosis in CS-AKI
As mentioned above, we have introduced the pathological mechanism of CS-AKI and the relationship between ferroptosis and CS-AKI. In this section, we will fully describe the mechanism of ferroptosis in CS-AKI, including Mb degradation, systemic inflammation, and danger/damage-associated molecule pattern (DAMP) molecules.
Iron overload caused by Mb degradation
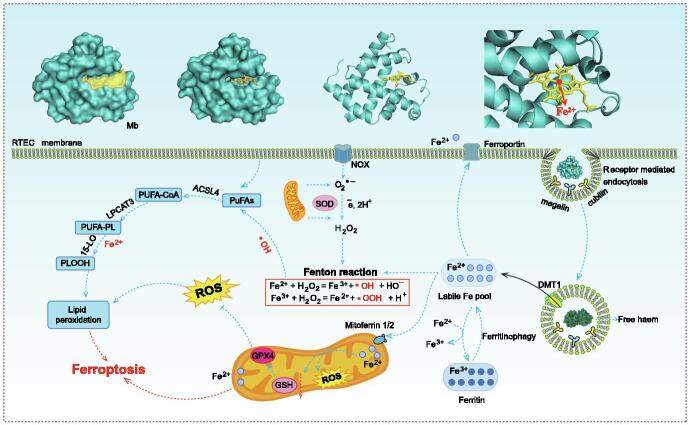
It is considered that excessive Mb released by ruptured muscle cells was one of the main pathological factors in CS-AKI [4]. Mb is a binding protein composed of a globin and a heme prosthetic group, which mainly exists in myocardium and skeletal muscle [38]. Mb generally presents a hollow spherical structure, and almost all polar amino acid residues are distributed on the surface of the spherical structure, so that Mb has good water solubility, while non-polar amino acid residues are distributed inside the spherical structure to form a hydrophobic hole (Fig. 2) [39]. A heme prosthetic group consisting of a porphyrin ring and a divalent iron ion is embedded in the hole. The divalent iron ion is located in the center of the porphyrin ring. The nitrogen atom on the plane of the porphyrin ring formed four coordination bonds with iron atom, and the N atom on the imidazole ring of the 93rd histidine residue of the globin forms the fifth coordination bond with iron atom. Two conserved amino acid residues (histidine at position 64 and valine at position 68) are located near the sixth coordination bond of iron atom, and the gap between them accommodates one oxygen molecule. Globin provides a hydrophobic hole for the heme prosthetic group, preventing iron divalent from being oxidized, so as to ensure the oxygenation ability.
Fig. 2.
Potential mechanisms of ferroptosis caused by Mb. Excessive Mb released from damaged muscle cells accumulated in renal tubules and was taken up by RTECs in receptor-mediated endocytosis (megalin and cubilin). Mb was subsequently acidified and degraded in the lysosomes to produce Fe2+. Accumulated Fe2+ could generate hydroxyl radicals through Fenton reaction. Both Fe2+ and hydroxyl radicals could aggravate the oxidation process of PUFAs in cell membrane, resulting in lipid peroxidation damage, and eventually ferroptosis. Fe2+ could be transported by mitoferrin 2 to accumulate in the mitochondria, thus causing oxidative stress in the mitochondria. The chemical and molecular mechanisms in this process remains to be investigated. DMT1, divalent metal transporter 1; Mitoferrin 2, also known as mitochondrial RNA-splicing protein 3/4 homolog (MRS3/4) or solute carrier family 25 member 28 (SLC25A28); PUFAs, polyunsaturated fatty acids; ACSL4, acyl-CoA synthetase long-chain family member 4; FA-CoA, fatty acyl coenzyme A; LPCAT3, lysophosphatidylcholine acyltransferase 3; 15-LO, 15-lipoxygenase; PLOOH, phospholipid hydroperoxides.
One of the pathological mechanisms associated with CS-AKI is the release of free divalent ions from the heme of Mb. The interaction between Mb and RTECs, especially the mechanism of Mb degradation and utilization by RTECs, needs to be further analyzed. Previous studies have shown that Mb released from more than 100 g of damaged muscle tissue would exceed the body's clearance capacity, and free Mb was deposited in renal tubules [40]. In the renal tubules, Mb bound with uromodulin (formerly known as tamm-horsall protein) and uric acid to form tubular casting, which led to acute renal tubule obstruction and AKI [41]. It was reported that the endocytic receptors megalin and cubilin might be involved in the uptake of Mb by tubular cells [42]. Subsequently, Mb was then phagocytized by lysosomes to produce free iron and Fe2+ [19], [43], [44]. Excessive Mb filtration resulted in increased Mb degradation products and iron overload of RTECs. Free Fe2+ could be cytotoxic because of its ability to produce reactive hydroxyl radicals through the Fenton reaction [45]. The Fenton reaction used iron ions to catalyze the conversion of endogenous hydrogen peroxide to hydroxyl radicals, which were the most reactive oxidants found in biology [46]. Thus, triggering the Fenton reaction could promote oxidative damage and aggravate cell damage [47]. Another mechanism did not require the release of iron from heme to play its deleterious role in the complex, which implied that intact heme (Fe2+-heme) was involved in redox reactions [48]. In kidneys, Fe2+-heme was oxidized by lipid peroxidase to generate Fe3+-heme, which could induce lipid peroxidation through redox action and cause oxidative damage to the kidney [48], [49]. Iron overload and lipid peroxidation played an important role in Mb-induced AKI, and both were considered to be major features of ferroptosis.
However, some studies have suggested that Mb also induced apoptosis of RTECs [50], which indicated that the damage of Mb to RTECs was multifaceted. It is necessary to deeply explore the mechanism of Mb-induced RTECs death. In terms of treatment, the combination therapy of multiple targets should be considered in the future.
Systemic inflammatory responses
Dysregulated levels of inflammatory factors
Recent studies in animal models of CS-AKI have shown dysregulation of the expression of inflammatory factors at serum and tissue levels under pathological conditions [51]. For example, it has been shown that the levels of IL-17 and IL-6 were significantly increased in serum and kidney tissue of CS-AKI rats, which was thought to promote a pro-inflammatory response dominated by Th17 cells [52]. Murata et al. also observed that within 24 h of reperfusion in CS model rats, the levels of serum TNF-α and IL-1β were significantly increased [53]. As reported in several studies, ferroptosis caused different degrees of inflammatory responses [54], [55], and whether inflammatory factors can also promote ferroptosis is very important in CS-AKI.
Currently, there are several studies supported the idea that inflammatory factors also trigger ferroptosis. IL-6 is an important pro-inflammatory factor in a variety of inflammation-related diseases and has been suggested as a potential inducer of ferroptosis. Overexpression of IL-6 in goat mammary epithelial cells could enhance LPS-induced ferroptosis by down-regulating GPX4 expression and promoting Fe2+ accumulation [56]. Pre-treatment of bronchial epithelial cells with the ferroptosis inhibitor ferrostatin-1 (Fer-1) reversed IL-6-induced lipid peroxidation and dysregulated iron homeostasis [57]. Further studies suggested that IL-6 might promote ferroptosis by up-regulating hepcidin levels via activating the JAK2/STAT3 pathway [58]. IL-1β has also been shown to promote ferroptosis in multiple cellular models. In a study of ATDC5 cells (a mouse chondrogenic cell line), treatment of cells with 10 ng/mL IL-1β resulted in an approximately 2-fold increase in Fe2+ concentration and a significant decrease in the expression levels of GPX4 and solute carrier family 7 member 11 (SLC7A11) [59]. The cytotoxicity of IL-1β on chondrocytes was attenuated using the Fer-1, indicating that IL-1β induced ferroptosis in chondrocytes. Moreover, IL-1β was also thought to be essential in high fat diet-induced iron accumulation and dysfunction in retinal pigment epithelial cells, and the pattern of IL-1β dependent iron accumulation was defined as a cellular iron sequestration response [60]. Notably, the maturation and release of IL-1β depend on NOD-like receptor protein (NLRP, such as NLRP3 and NLRP1) inflammasome formation and subsequent caspase-1 activation [61]. Although accumulating evidence suggested that NLRP activation was a prominent feature of pyroptosis [62], [63], some recent studies also suggested that the expression level of NLRP3 inflammasome was linked with ferroptosis [64]. Moreover, ferroptosis and NLRP were mutually affected. In a model of oxidative stress in HTR-8/SVneo cells, silencing NLRP1 decreased the level of GPX4 but increased the levels of TFR1 and acyl-CoA synthetase long-chain family member 4 (ACSL4). On the other hand, inhibition of ferroptosis with Fer-1 significantly decreased the expression levels of NLRP1, NLRP3, IL-1β and caspase-1 [65]. The interaction between NLRP and ferroptosis biomarkers indicated that ferroptosis was associated with other forms of cell death, but further molecular biological experiments were needed to prove.
Leaked DAMP m0128olecules
In addition to dysregulation of inflammatory cytokines, another cause of systemic inflammation caused by CS-AKI is the release of a large number of DAMP molecules, such as high mobility group box 1 (HMGB1), double-strand DNA (dsDNA), etc. These DAMPs were endogenous dangerous molecules released by damaged muscle cells or other cells during rhabdomyolysis, which were not only thought to contribute to aseptic inflammatory outbursts, but were also considered to be potential inducers of ferroptosis.
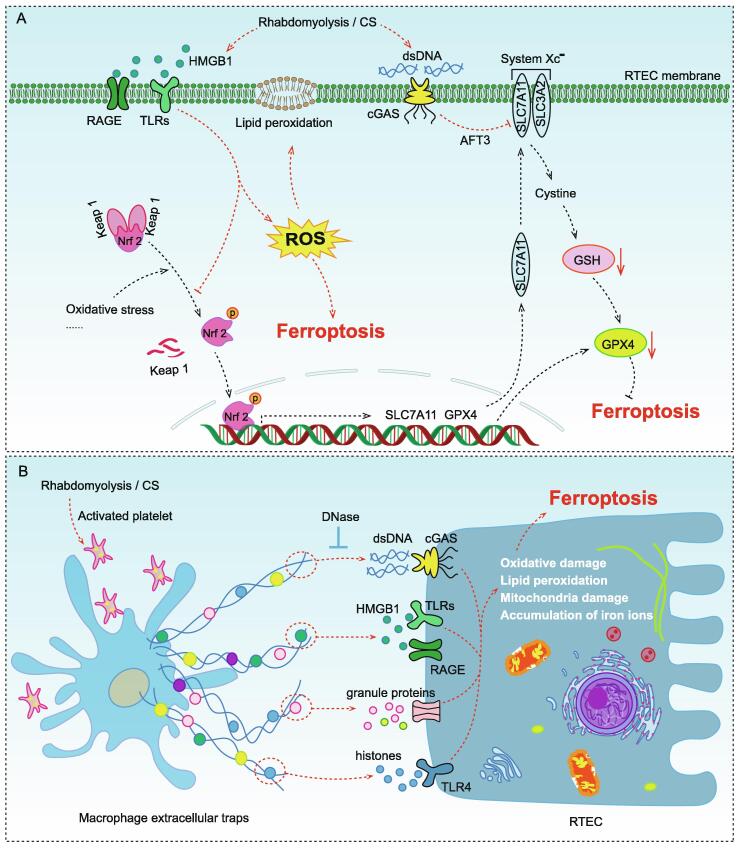
HMGB1 has been shown to be released in large amounts in CS-related animal models [66]. HMGB1 is one of the major members of the high mobility family of proteins and is a eukaryotic intranuclear DNA binding protein that localized to human chromosome 13q12, and its main intracellular role is to stabilize the structure of chromosome [67]. HMGB1 consists of 215 amino acid residues and contains three structural domains, the A box located at amino acid residues 9–79 in the N-terminus, the B box located at amino acid residues 89–163, and the acidic C-terminal structural domain consisting of aspartate and glutamate residues with various lengths located at amino acid residues 186–215 in the carboxyl terminus [68]. The A box of HMGB1 contains the binding site for the HMGB1 protein receptor, while the B box serves as a pro-inflammatory structural domain [69]. Extracellular HMGB1 mainly acts as an inflammatory mediator, causing a strong inflammatory response by up-regulating the expression of inflammatory cytokines such as IL-1β, TNF-α and IL-6 through the receptor for advanced glycation end products (RAGE) and toll-like receptors (TLRs) [70]. HMGB1 is expressed in almost all human cells and is released by dying cells and activated immune cells [71]. Recently, it has been suggested that the massive release of HMGB1 might be the trigger of ferroptosis (Fig. 3A). For example, it has been shown that knockdown of HMGB1 in HL-60/NRASQ61L cells alleviated erastin-induced ferroptosis by decreasing TFR1 expression, which might be associated with JNK/p38 signaling pathway [72]. In the high glucose-induced mesangial cells model, HMGB1 was demonstrated to regulate glucose-induced ferroptosis through Nrf2 signaling [73]. Unfortunately, the release of HMGB1 from cells has been shown to be the result of ferroptosis, causing an undesirable vicious cycle [74]. These evidences suggested that HMGB1 might be a new regulator of ferroptosis.
Fig. 3.
Molecular mechanisms of ferroptosis caused by several identified DAMP molecules in CS (rhabdomyolysis) –AKI (A) Damaged muscle cells released a large amount of HMGB1, which was recognized by RAGE and TLRs in RTECs, causing ROS accumulation and lipid peroxidation damage by blocking Nrf2 pathway. dsDNA could activate cGAS to up-regulate AFT and directly inhibit SLC7A11 expression, resulting in GSH depletion. System Xc− was an important intracellular antioxidant system, which consisted of two subunits, SLC7A11 and SLC3A2. SLC7A11 was responsible for the major transport activity and was highly specific for cystine and glutamate, whereas SLC3A2 acted as a chaperone protein. Inhibition of System Xc− activity would inhibit the cystine uptake and affected the synthesis of GSH, leading to reduced GPX4 activity and reduced cellular antioxidant capacity, thereby promoting ferroptosis. (B) Activated platelets induced macrophages to form ETs during rhabdomyocytolysis, which have been shown to promote ferroptosis via dsDNA, HMGB1, and so on. HMGB1, high mobility group box 1; RAGE, the receptor for advanced glycation end products; TLRs, toll-like receptors; Nrf2, nuclear factor erythroid 2-related factor 2; dsDNA, double-strand DNA; cGAS, cyclic GMP-AMP synthase; AFT3, activating transcription factor 3; SLC3A2, solute carrier family 3 member 2; SLC7A11, solute carrier family 7 member 11; GSH, glutathione; GPX4, glutathione peroxidase 4.
Recently, endogenous release of dsDNA was also considered to play a role in the pathogenesis of CS-AKI (Fig. 3A). In the rhabdomyolysis induced AKI mouse model, the damaged muscle cells released a large amount of dsDNA to trigger the kidney epithelial injury and inflammation [75]. It has been shown that dsDNA could be recognized by cyclic GMP-AMP synthase (cGAS), leading to innate immune response and/or cell death [76]. cGAS was a known dsDNA sensor that recognized dsDNA to induced various forms of cell death via the cGAS-STING signal transduction [77], [78], [79]. Activating transcription factor 3 (ATF3), a common stress sensor, has been reported to promote ferroptosis by directly inhibiting SLC7A11 to reduce GSH levels in cells [80], [81]. Although it has been reported that cGAS might trigger ferroptosis through the ATF3-SLC7A11-GPX4 axis [82], the regulation of ATF3 expression by the cGAS signaling pathway in CS-AKI needed to be further investigated. Moreover, the mechanism by which the activation of cGAS signaling by dsDNA triggers ferroptosis in CS-AKI remains to be determined.
In addition, another form of DAMP extracellular trap (ET) was also thought to be associated with ferroptosis (Fig. 3B). ETs were first discovered in neutrophils, which were formed by releasing histones, combination of DNA, antimicrobial peptides, and granule proteins from neutrophils to the extracellular area in response to stimulation [83]. ETs were originally considered as a host defense against bactericidal proteins and peptides [84]. However, some studies have raised the negative impact of ETs. For example, Wei et al. suggested that neutrophil ETs and their histone components had significant inflammatory damage to mammary epithelial cells [85]. Currently, more immune cells have been found to participate in the formation of ETs, including macrophages, mast cells and eosinophils [86]. In the study of CS-AKI related animal models, heme-activated platelets released from necrotic muscle cells promoted the production of macrophage ETs (METs) by increasing ROS levels and histone citrullination, which aggravated rhabdomyolysis induced AKI [87]. Moreover, the SFK-signaling pathway was demonstrated to be involved in heme-activated platelet-induced METs formation [88]. Notably, METs have been shown to drive hepatocyte ferroptosis in hepatic I/R and could be reversed by the METs specific inhibitor Cl-amidine [89]. Therefore, exploring the mechanism of MET and regulating it appropriately was helpful to treat and control CS-AKI.
Treatment targets of AKI based on ferroptosis
Current treatment options mainly focus on early fluid resuscitation, diuresis and kidney replacement therapy which including hemodialysis, kidney transplantation, etc [2], [90]. However, these treatment options are often limited by the lack of equipment at the disaster site and the high technical difficulty, making it difficult to administer effective treatment to patients. The research on pharmacological treatment of CS is important.
Anti-inflammatory effects via down-regulating ACSL4
Given the above-mentioned relationship between ferroptosis and inflammation, it was not difficult to understand that inhibition of ferroptosis might reduce the inflammatory response in patients with AKI. Recently, several studies have reported that ferroptosis inhibitors showed significant benefits in certain diseases via anti-inflammatory effects.
ACSL4, a critical isoenzyme of polyunsaturated fatty acid metabolism, has been identified as not only a sensitive regulator of ferroptosis but also an important factor in the occurrence of ferroptosis [91]. ACSL4 used longer polyunsaturated fatty acids (PUFAs) as substrates, such as arachidonic acid. ACSL4 catalyzed the conversion of free arachidonic acid to arachidonic acid-CoA ester, which was then esterified through interaction with membrane phospholipids, leading to ferroptosis [92], [93]. Emerging evidence suggested that ACLS4 might be a key node linking ferroptosis and inflammation. In the renal tissue of ACSL4-deficient mice, the levels of inflammation and macrophage infiltration were down-regulated and ferroptosis was alleviated [94]. Tao et al. found that dexmedetomidine could significantly attenuate ferroptosis-mediated kidney injury and effectively down-regulate the inflammatory response after kidney injury, while overexpression of ACSL4 attenuated the alleviating effects of dexmedetomidine on ferroptosis and inflammation [95]. In addition, activation of ferroptosis and up-regulation of ACSL4 in keratinocytes contributed to the release of pro-inflammatory cytokines [96]; ACSL4 could also activate ferroptosis to aggravate the severity of ischemic stroke and promote microglia-mediated inflammatory response [54]. Therefore, ACSL4 has a critical role between inflammation and ferroptosis. However, the disease model mechanism of the above studies was complex, and it was difficult to exclude the influence of other interfering factors on the relationship between ferroptosis and inflammation. Direct evidence was also needed to prove whether inhibition of ferroptosis via targeting ACSL4 was beneficial to alleviate inflammation.
Anti-HMGB1
DAMPs are major mediators of the inflammatory response and may also act as inducers of ferroptosis, so antagonizing DAMPs may simultaneously anti-inflammatory and inhibit ferroptosis. As mentioned above, HMGB1 was considered as a critical regulator of ferroptosis, and also acted as an inflammatory mediator. Therefore, it is worth considering whether HMGB1 can be used as an effective target for ferroptosis.
Recently, some studies have reported the possible protective effect of inhibiting HMGB1. For example, Wu et al. used siRNA to interfere with HMGB1 expression to alleviate high glucose-induced ferroptosis in mesangial cells [73]. Dexrazoxane is an iron chelator approved for the prevention of doxorubicin-induced cardiotoxicity in oncology patients [97]. A recent study revealed that dexrazoxane antagonized doxorubicin-induced ferroptosis in cardiomyocytes by regulating HMGB1 [98]. In a neonatal rat model of hypoxic-ischemic brain injury, the HMGB1 inhibitor glycyrrhizin attenuated neuronal ferroptosis by up-regulating the GPX4 signaling pathway, and this effect could be reversed by the ferroptosis inducer RAS-selective lethal 3 (RSL3) [99]. In addition, some studies have shown the benefits of anti-HMGB1 antibody in the treatment of inflammatory diseases and metabolic diseases [100], [101]. However, there are no anti-HMGB1 antibody therapeutic applications in CS-AKI, and its efficacy and safety need to be considered in depth in future work.
Regulator of lipid peroxidation pathway
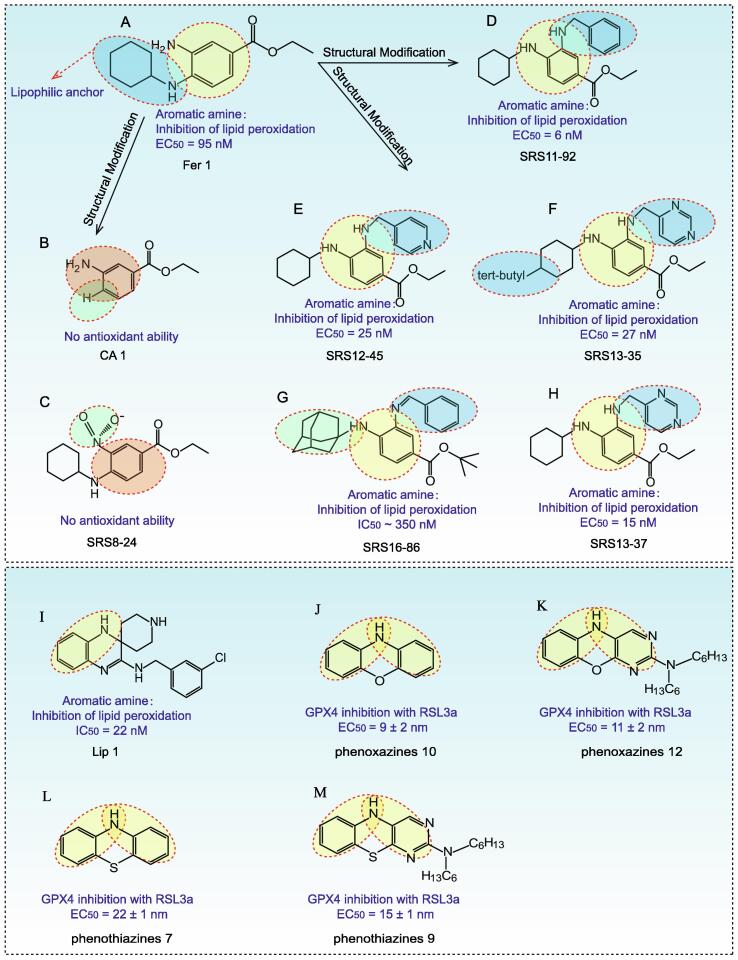
One distinguishing feature of ferroptosis is excessive iron-dependent lipid peroxidation, and inhibition of lipid peroxidation can antagonize ferroptosis [102]. Fer-1 and Liproxstatin-1 (Lip-1), two aromatic amines identified from a high-throughput screening library, have been shown to be efficient radical trapping antioxidants in lipid bilayers, suggesting that they inhibit ferroptosis in AKI by suppressing lipid peroxidation [103], [104]. Structural modification of Fer-1 revealed that elimination of the N-cyclohexyl portion (CA-1) or replacement of aromatic primary amines with nitro (SRS 8–24) was able to disrupt the antioxidant capacity of Fer-1 and its ability to prevent erastin (10 M)-induced HT-1080 cell death, suggesting that these two groups are critical for the antioxidant capacity of Fer-1 (Fig. 4A–C). Moreover, the N-cyclohexyl portion located in the central aromatic nucleus acted as a lipophilic anchor within the cellular biofilm [20]. However, it has also been shown that the aniline monosubstituted analogue of Fer-1 on the parent nucleus had comparable or better potency than Fer-1 [105], [106] (Fig. 4D–E). Linkermann et al. identified a third generation Fer-1 analog, SRS-16–86, which had better plasma stability and stronger inhibition of ferroptosis in renal tubular cells compared to Fer-1 [107] (Fig. 4F–H). Lip-1 contained both amide and sulfonamide subunits, therefore it had good stability and drug absorption distribution (Fig. 4I). Lip-1 inhibited ferroptosis at low nanomolar doses but did not interfere with other typical cell death patterns [106]. Both Fer-1 and Lip-1 were monoarylamines and were less effective than diarylamines because the aromatic ring of monoarylamines was replaced by an electron-donating group (amine in Fer-1, amidine in Lip-1), which weakened the N—H bond of the amino group, thus phenoxazines and phenothiazines were more effective in comparison [108] (Fig. 4J–M). These studies provided a reference for further optimization of Fer-1 and Lip-1 structures to develop safer and more efficient drugs by targeting ferroptosis.
Fig. 4.
Chemical structure of Fer-1, Lip-1 and their analogues for inhibiting ferroptosis. (A) Chemical structure of Fer-1. (B-H) Chemical structure of Fer-1 analogues. The aromatic primary amines and N-cyclohexyl portion were the key functional groups of Fer-1 antioxidant activity. Moreover, the N-cyclohexyl portion aslo acted as a lipophilic anchor within the cellular biofilm. The SRS 16–86 had better plasma stability and stronger inhibition of ferroptosis compared to Fer-1. The amide group and sulfonamide subunit provide Lip-1 with good stability and drug absorption distribution. Both Fer-1 and Lip-1 are monoarylamines. In contrast, diarylamines such as phenoxazines and phenothiazines have stronger pharmacodynamic activity. The underlying chemical mechanisms need to be further explored.
Iron chelators could rescue experimental AKI and inhibit ferroptosis [109], [110]. Different kinds of iron chelators vary in structure and function, but they usually contain oxygen, nitrogen, or sulfur donor atoms that form coordination bonds with iron [111]. Iron chelators are required to compete effectively with biological ligands that normally bind iron, therefore, the chelators' affinity for iron will greatly affect their activity as therapeutic agents. Iron chelators can inhibit the redox properties of free iron and prevent its participation in Fenton reaction. The role of iron chelators in hindering the Fenton reaction can inhibit the production of hydroxyl radicals that cause oxidative damage and ferroptosis [112]. Currently, there are only three iron-chelating agents approved for clinical use: deferoxamine, deferiprone and deferasirox. Deferoxamine is a 6-ligand iron-chelating agent that binds to Fe3+ in a 1:1 ratio and is used in the treatment of hemochromatosis, thalassemia, sickle cell anemia [113]. However, the use of Deferiprone is limited by poor intestinal absorption and rapid renal excretion [114]. Deferiprone is a hydrophilic drug with a long half-life of about 3–4 h. Deferiprone is available as an oral formulation in doses of 75–100 mg/kg/ day in three doses [115]. Ddeferasirox has a half-life of 8–16 h and is prescribed in doses of 10–40 mg /kg/ day, administered 1–2 times daily [115]. However, iron-chelating agents have only been shown to be effective in experimental AKI. The therapeutic effect of iron-chelating agents on CS-AKI remains to be explored. Potential side effects of iron-chelating agents should be fully considered before use.
In addition, some natural small molecular compounds (NSMCs) also have significant inhibitory effects on ferropsis. For example, nuciferine could inhibit folic acid-induced acute kidney injury in mice by limiting iron accumulation and preventing lipid peroxidation [116]. Pachymic acid inhibited mice renal ferroptosis by activating Nrf2 to upregulate the expression of GPX4, SLC7A11 and HO-1 [117]. Ginsenoside Rg1 inhibited RTEC ferroptosis by reducing iron accumulation and lipid peroxidation reaction through ferropsis suppressor protein 1 (FSP1) [118]. Irisin could alleviate ferropsis and kidney damage in septic-AKI mice via SIRT1/Nrf2 pathway [119]. Polydatin attenuated erastin-induced ferroptosis by reducing excessive iron accumulation and rescuing GSH depletion, the results also showed that the effect of a 40 μM dose of polydatin was more pronounced than that of classical Fer-1 (1 μM) and deferoxamine (100 μM) [120]. Despite the lack of reference for the treatment of CS-AKI, the above multiple types of AKI treatment give us the idea that supplementing the patient's diet with these NSMCs might be beneficial to the remission of the disease.
Discussion
The pathogenesis of CS-AKI is very complex and has not been fully elucidated yet. It was widely accepted that the massive release of DAMP during rhabdomyolysis leads to renal tubular occlusion and renal ischemia, causing acute tubular injury and death [121]. Previous studies have suggested that there are various forms of cell death during CS-AKI, including apoptosis and pyroptosis [4]. In contrast, we found that Mb, HMGB1, dsDNA and METs may be potential triggers of ferroptosis. Hence, ferroptosis may play an important role as a new bridge connecting CS and AKI.
However, there is a lack of direct clinical evidence to support the occurrence of ferroptosis in CS-AKI. We have only found this pathological change in some relevant animal models, and many questions remain to be answered. For example, the mechanism of DAMP-mediated ferroptosis occurs is unknown, and there are no specific biomarkers to assess the disease severity and the effectiveness of treatment. Several studies have suggested that ferroptosis could be a therapeutic target, such as Amaral et al. found that GPX4-deficient macrophages showed enhanced ferroptosis in vitro after infection with mycobacterium tuberculosis, indicating that the GPX4/GSH axis was a target for the treatment of tuberculosis [122]. Li et al. demonstrated that PI3K was an important regulator of ferroptosis resistance and that melatonin might be a new drug for the treatment of this disease because it inhibited ferroptosis by activating the PI3K/AKT/mTOR signaling pathway [123]. These works provided a reference to further explore whether ferroptosis could be used as a target for CS-AKI.
Notably, we found that some pro-inflammatory factors (IL-1β and IL-6) and inflammatory sensors (NLRP3 and NLRP1) were also associated with ferroptosis, which were considered to be key markers of pyroptosis [124], suggesting that ferroptosis may be crosstalk with pyroptosis. However, the causal relationship was not yet clear. At present, these evidences suggested that regulating inflammation levels may also have therapeutic effects on ferroptosis, but we needed to consider how to properly regulate inflammation levels, as some data show that IL-6 has a significant cytoprotective effect [125]. Therefore, it is important to maintain stable levels of these pleiotropic factors in order to alleviate the effects of ferroptosis.
How to treat CS-AKI effectively is a topic worthy of attention. Fluid therapy is the first-line emergency treatment option for CS, helping to protect the kidneys and heart from failure, but many patients still die on the way to the hospital [126], [127]. To avoid AKI-related mortality in CS patients, pharmacological treatment should be considered in addition to conventional fluid therapy. According to the above analysis, ferroptosis inhibitors may be a promising therapeutic agent because they can alleviate cell death, as well as have potential anti-inflammatory effects. Nevertheless, some ferroptosis inhibitors have achieved good results in mouse models, their safety and effectiveness in humans have not been fully described. In addition, how to improve pharmacological activity through structural modification is an urgent problem. Some NSMCs have also shown great potential in the treatment of ferroptosis, which are widely distributed in nature, with diverse pharmacological activities, and have the potential to develop new drugs [128]. Moreover, some NSMCs often have unique parent nucleus structure, which has great reference value for the synthesis of specific ferroptosis inhibitors.
In conclusion, ferroptosis may be an emerging therapeutic target for CS-AKI. The mechanisms causing ferroptosis are complex, including but not limited to Mb metabolism, dysregulation of inflammatory cytokines and inflammatory sensors, and DAMP molecules accumulation. It is important to further increase our understanding of these factors in CS-AKI. According to our analysis, down-regulation of ACSL4, anti-HMGB1, and regulation of lipid peroxidation pathway play beneficial roles, and more and more new targets are being revealed. However, this still lacks validation at the clinical level. In addition, the therapeutic approach of CS-AKI needs improvement and innovation.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was supported by the Ministry of Science and Technology of the People's Republic of China (National Key R&D Program of China, 2021YFC3002203), and the National Natural Science Foundation of China (No. 82273998).
Biographies

Ou Qiao is a doctoral student in the Institute of Disaster and Emergency Medicine, Medical College of Tianjin University. He works in the team of Prof. Yanhua Gong in Tianjin Key Laboratory of Disaster Medicine Technology. He focuses on the pathological mechanism of crush syndrome mediated acute kidney injury, molecular mechanism and transcriptional regulation of ferroptosis, and natural product therapy.

Xinyue Wang is a doctoral student in the Institute of Disaster and Emergency Medicine, Medical College of Tianjin University. She works in the team of Prof. Yanhua Gong in Tianjin Key Laboratory of Disaster Medicine Technology. She is mainly engaged in the research of trauma mechanism and biological treatment of disaster medicine, including the pathophysiological mechanism of acute renal injury mediated by crush syndrome, the molecular mechanism of cell death, and the biological treatment of recombinant proteins and antibodies. At present, as the first author, she has published a review article in an internationally recognized peer-reviewed journal.

Yuru Wang is a postgraduate student of Institute of Disaster and Emergency Medicine, Medical College of Tianjin University. Her research direction is focused on molecular mechanism and early diagnosis and treatment of multiple organ injury in Crush Syndrome. Currently, she is doing researches on programmed cell death in Crush Syndrome related Acute Kidney Injury. As first author, she has published one review articles on Pharmacological Research.

Ning Li is a lecturer of the Institute of Disaster and Emergency Medicine, Medical College of Tianjin University. She completed her Ph.D in 2018 from College of Pharmacy Nankai University, China. Now, she is mainly engaged in basic research in trauma and immune, including the pathogenic mechanism of trauma, the role of cell death model in trauma, immunotherapy, and medical biomechanics. She hosted one project of Ministry of Science and Technology of the People's Republic of China and one project of Open Fund of State Key Laboratory of Medicinal Chemical Biology (Nankai University). As a first or corresponding author, she has already published more than 10 scientific papers in Nucleic Acids Research, Military Medical Research, Cell death & Discovery, Pharmacological research and other journals.

Yanhua Gong is a professor, and doctoral supervisor of the Institute of Disaster and Emergency Medicine, Medical College of Tianjin University. His primary fields of research are biochemistry and cell biology, and currently focusing on aseptic inflammation and innate immune response after severe trauma. Now, Dr. Gong’s lab is investigating the interaction mechanism of multi-organ (such as heart, lung and kidney) damage associated molecular patterns (DAMPs) in the development of traumatic inflammation. He looks forward to developing new technologies and strategies for rapid diagnosis and injury control of trauma and its complications applicable to disaster sites. As a first or corresponding author, Dr. Gong has published more than 20 articles in Nucleic Acids Research, Military Medical Research, Cell death & Discovery, Pharmacological research and other journals. He also hosted three projects of National Natural Science Foundation of China and two projects of Ministry of Science and Technology of the People's Republic of China and other grands.
Footnotes
Peer review under responsibility of Cairo University.
Contributor Information
Ning Li, Email: lining620@tju.edu.cn.
Yanhua Gong, Email: gongyanhua@tju.edu.cn.
References
- 1.Liu Y., Yu M., Chen L., Liu J., Li X., Zhang C., et al. Systemic review of animal models used in the study of crush syndrome. Shock. 2022;57:469–478. doi: 10.1097/SHK.0000000000001911. [DOI] [PubMed] [Google Scholar]
- 2.Sever M.S., Vanholder R., Lameire N. Management of crush-related injuries after disasters. N Engl J Med. 2006;354:1052–1063. doi: 10.1056/NEJMra054329. [DOI] [PubMed] [Google Scholar]
- 3.Wang P.T., Li N., Wang X.Y., Chen J.L., Geng C.H., Liu Z.Q., et al. RIG-I, a novel DAMPs sensor for myoglobin activates NF-kappaB/caspase-3 signaling in CS-AKI model. Mil Med Res. 2021;8:37. doi: 10.1186/s40779-021-00333-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li N., Chen J., Geng C., Wang X., Wang Y., Sun N., et al. Myoglobin promotes macrophage polarization to M1 type and pyroptosis via the RIG-I/Caspase1/GSDMD signaling pathway in CS-AKI. Cell Death Discov. 2022;8:90. doi: 10.1038/s41420-022-00894-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin H., Lin X., Liu Z., Wang J., Wang J., Zhang Y., et al. Remote ischemic postconditioning protects against crush-induced acute kidney injury via down-regulation of apoptosis and senescence. Eur J Trauma Emerg Surg. 2022;48:4585–4593. doi: 10.1007/s00068-022-01910-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He Q., Wang F., Li G., Chen X., Liao C., Zou Y., et al. Crush syndrome and acute kidney injury in the Wenchuan Earthquake. J Trauma. 2011;70:1213–1217. doi: 10.1097/TA.0b013e3182117b57. discussion 7–8. [DOI] [PubMed] [Google Scholar]
- 7.Li N., Wang X., Wang P., Fan H., Hou S., Gong Y. Emerging medical therapies in crush syndrome - progress report from basic sciences and potential future avenues. Ren Fail. 2020;42:656–666. doi: 10.1080/0886022X.2020.1792928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yao X., Li W., Fang D., Xiao C., Wu X., Li M., et al. Emerging roles of energy metabolism in ferroptosis regulation of tumor cells. Adv Sci (Weinh) 2021;8:e2100997. doi: 10.1002/advs.202100997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y., Quan F., Cao Q., Lin Y., Yue C., Bi R., et al. Quercetin alleviates acute kidney injury by inhibiting ferroptosis. J Adv Res. 2021;28:231–243. doi: 10.1016/j.jare.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao M., Yi J., Zhu J., Minikes A.M., Monian P., Thompson C.B., et al. Role of mitochondria in ferroptosis. Mol Cell. 2019;73:354–63 e3. doi: 10.1016/j.molcel.2018.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Musheshe N., Oun A., Sabogal-Guaqueta A.M., Trombetta-Lima M., Mitchel S.C., Adzemovic A., et al. Pharmacological inhibition of Epac1 averts ferroptosis cell death by preserving mitochondrial integrity. Antioxidants (Basel) 2022;11 doi: 10.3390/antiox11020314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guerrero-Hue M., Garcia-Caballero C., Palomino-Antolin A., Rubio-Navarro A., Vazquez-Carballo C., Herencia C., et al. Curcumin reduces renal damage associated with rhabdomyolysis by decreasing ferroptosis-mediated cell death. FASEB J. 2019;33:8961–8975. doi: 10.1096/fj.201900077R. [DOI] [PubMed] [Google Scholar]
- 13.Bywaters E.G., Beall D. Crush Injuries with Impairment of Renal Function. Br Med J. 1941;1:427–432. doi: 10.1136/bmj.1.4185.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lv Q., Long M., Wang X., Shi J., Wang P., Guo X., et al. The role of alpha-1-acid glycoprotein in the diagnosis and treatment of crush syndrome-induced acute kidney injury. Shock. 2021;56:1028–1039. doi: 10.1097/SHK.0000000000001839. [DOI] [PubMed] [Google Scholar]
- 15.Roy J.P., Devarajan P. Acute kidney injury: diagnosis and management. Indian J Pediatr. 2020;87:600–607. doi: 10.1007/s12098-019-03096-y. [DOI] [PubMed] [Google Scholar]
- 16.Zhou J., Bai Y., Jiang Y., Tarun P., Feng Y., Huang R., et al. Immunomodulatory role of recombinant human erythropoietin in acute kidney injury induced by crush syndrome via inhibition of the TLR4/NF-kappaB signaling pathway in macrophages. Immunopharmacol Immunotoxicol. 2020;42:37–47. doi: 10.1080/08923973.2019.1706555. [DOI] [PubMed] [Google Scholar]
- 17.Sun T., Wu D., Deng Y., Zhang D. EGFR mediated the renal cell apoptosis in rhabdomyolysis-induced model via upregulation of autophagy. Life Sci. 2022;309 doi: 10.1016/j.lfs.2022.121050. [DOI] [PubMed] [Google Scholar]
- 18.Rashed R.R., Deghiedy N.M., El-Hazek R.M., El-Sabbagh W.A., Rashed E.R., El-Ghazaly M.A. Effectiveness of deferiprone-loaded nanocarrier in experimentally induced rhabdomyolysis: a dose-comparison study. Bioorg Chem. 2020;100 doi: 10.1016/j.bioorg.2020.103913. [DOI] [PubMed] [Google Scholar]
- 19.Zorova L.D., Pevzner I.B., Chupyrkina A.A., Zorov S.D., Silachev D.N., Plotnikov E.Y., et al. The role of myoglobin degradation in nephrotoxicity after rhabdomyolysis. Chem Biol Interact. 2016;256:64–70. doi: 10.1016/j.cbi.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 20.Dixon S.J., Lemberg K.M., Lamprecht M.R., Skouta R., Zaitsev E.M., Gleason C.E., et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dolma S., Lessnick S.L., Hahn W.C., Stockwell B.R. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell. 2003;3:285–296. doi: 10.1016/s1535-6108(03)00050-3. [DOI] [PubMed] [Google Scholar]
- 22.Yang W.S., Stockwell B.R. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem Biol. 2008;15:234–245. doi: 10.1016/j.chembiol.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kremer D.M., Nelson B.S., Lin L., Yarosz E.L., Halbrook C.J., Kerk S.A., et al. GOT1 inhibition promotes pancreatic cancer cell death by ferroptosis. Nat Commun. 2021;12:4860. doi: 10.1038/s41467-021-24859-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leng Y., Luo X., Yu J., Jia H., Ferroptosis B.Y. A potential target in cardiovascular disease. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.813668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Majernikova N., den Dunnen W.F.A., Dolga A.M. The potential of ferroptosis-targeting therapies for Alzheimer's disease: from mechanism to transcriptomic analysis. Front Aging Neurosci. 2021;13 doi: 10.3389/fnagi.2021.745046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ning X., Qi H., Yuan Y., Li R., Wang Y., Lin Z., et al. Identification of a new small molecule that initiates ferroptosis in cancer cells by inhibiting the system Xc(-) to deplete GSH. Eur J Pharmacol. 2022;934 doi: 10.1016/j.ejphar.2022.175304. [DOI] [PubMed] [Google Scholar]
- 27.Xie Y., Hou W., Song X., Yu Y., Huang J., Sun X., et al. Ferroptosis: process and function. Cell Death Differ. 2016;23:369–379. doi: 10.1038/cdd.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mao L., Zhao T., Song Y., Lin L., Fan X., Cui B., et al. The emerging role of ferroptosis in non-cancer liver diseases: hype or increasing hope? Cell Death Dis. 2020;11:518. doi: 10.1038/s41419-020-2732-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fillebeen C., Charlebois E., Wagner J., Katsarou A., Mui J., Vali H., et al. Transferrin receptor 1 controls systemic iron homeostasis by fine-tuning hepcidin expression to hepatocellular iron load. Blood. 2019;133:344–355. doi: 10.1182/blood-2018-05-850404. [DOI] [PubMed] [Google Scholar]
- 30.Fraenkel P.G., Gibert Y., Holzheimer J.L., Lattanzi V.J., Burnett S.F., Dooley K.A., et al. Transferrin-a modulates hepcidin expression in zebrafish embryos. Blood. 2009;113:2843–2850. doi: 10.1182/blood-2008-06-165340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang C.Y., Knutson M.D. Hepatocyte divalent metal-ion transporter-1 is dispensable for hepatic iron accumulation and non-transferrin-bound iron uptake in mice. Hepatology. 2013;58:788–798. doi: 10.1002/hep.26401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doll S., Conrad M. Iron and ferroptosis: a still ill-defined liaison. IUBMB Life. 2017;69:423–434. doi: 10.1002/iub.1616. [DOI] [PubMed] [Google Scholar]
- 33.Stockwell B.R., Friedmann Angeli J.P., Bayir H., Bush A.I., Conrad M., Dixon S.J., et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lei P., Bai T., Sun Y. Mechanisms of ferroptosis and relations with regulated cell death: a review. Front Physiol. 2019;10:139. doi: 10.3389/fphys.2019.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim S., Kang S.W., Joo J., Han S.H., Shin H., Nam B.Y., et al. Characterization of ferroptosis in kidney tubular cell death under diabetic conditions. Cell Death Dis. 2021;12:160. doi: 10.1038/s41419-021-03452-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li X., Zou Y., Fu Y.Y., Xing J., Wang K.Y., Wan P.Z., et al. A-lipoic acid alleviates folic acid-induced renal damage through inhibition of ferroptosis. Front Physiol. 2021;12 doi: 10.3389/fphys.2021.680544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adedoyin O., Boddu R., Traylor A., Lever J.M., Bolisetty S., George J.F., et al. Heme oxygenase-1 mitigates ferroptosis in renal proximal tubule cells. Am J Physiol Renal Physiol. 2018;314:F702–F714. doi: 10.1152/ajprenal.00044.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan B., Sun B., Sun N., Li C., Zhang J., Yang W. Structure, functional properties and iron bioavailability of Pneumatophorus japonicus myoglobin and its glycosylation products. Int J Biol Macromol. 2021;173:524–531. doi: 10.1016/j.ijbiomac.2021.01.138. [DOI] [PubMed] [Google Scholar]
- 39.Hendgen-Cotta U.B., Kelm M., Rassaf T. Myoglobin functions in the heart. Free Radic Biol Med. 2014;73:252–259. doi: 10.1016/j.freeradbiomed.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 40.Kruger D., Han J. Assessing acquired rhabdomyolysis in adults. JAAPA. 2017;30:20–26. doi: 10.1097/01.JAA.0000510986.14286.fd. [DOI] [PubMed] [Google Scholar]
- 41.Panizo N., Rubio-Navarro A., Amaro-Villalobos J.M., Egido J., Moreno J.A. Molecular mechanisms and novel therapeutic approaches to rhabdomyolysis-induced acute kidney injury. Kidney Blood Press Res. 2015;40:520–532. doi: 10.1159/000368528. [DOI] [PubMed] [Google Scholar]
- 42.Gburek J., Birn H., Verroust P.J., Goj B., Jacobsen C., Moestrup S.K., et al. Renal uptake of myoglobin is mediated by the endocytic receptors megalin and cubilin. Am J Physiol Renal Physiol. 2003;285:F451–F458. doi: 10.1152/ajprenal.00062.2003. [DOI] [PubMed] [Google Scholar]
- 43.Ke Z., Bai Y., Zhu H., Xiang X., Liu S., Zhou X., et al. Characteristics of myoglobin degradation by cold plasma and its pro-oxidative activity on lipid in washed fish muscle. Food Chem. 2022;389 doi: 10.1016/j.foodchem.2022.132972. [DOI] [PubMed] [Google Scholar]
- 44.Richards M.P. Redox reactions of myoglobin. Antioxid Redox Signal. 2013;18:2342–2351. doi: 10.1089/ars.2012.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laranjeira-Silva M.F., Wang W., Samuel T.K., Maeda F.Y., Michailowsky V., Hamza I., et al. A MFS-like plasma membrane transporter required for Leishmania virulence protects the parasites from iron toxicity. PLoS Pathog. 2018;14:e1007140. doi: 10.1371/journal.ppat.1007140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Min H., Qi Y., Zhang Y., Han X., Cheng K., Liu Y., et al. A Graphdiyne oxide-based iron sponge with photothermally enhanced tumor-specific fenton chemistry. Adv Mater. 2020;32:e2000038. doi: 10.1002/adma.202000038. [DOI] [PubMed] [Google Scholar]
- 47.Bao L., Zhao C., Feng L., Zhao Y., Duan S., Qiu M., et al. Ferritinophagy is involved in bisphenol A-induced ferroptosis of renal tubular epithelial cells through the activation of the AMPK-mTOR-ULK1 pathway. Food Chem Toxicol. 2022;163 doi: 10.1016/j.fct.2022.112909. [DOI] [PubMed] [Google Scholar]
- 48.Reeder B.J., Sharpe M.A., Kay A.D., Kerr M., Moore K., Wilson M.T. Toxicity of myoglobin and haemoglobin: oxidative stress in patients with rhabdomyolysis and subarachnoid haemorrhage. Biochem Soc Trans. 2002;30:745–748. doi: 10.1042/bst0300745. [DOI] [PubMed] [Google Scholar]
- 49.Song S.J., Kim S.M., Lee S.H., Moon J.Y., Hwang H.S., Kim J.S., et al. Rhabdomyolysis-induced AKI was ameliorated in NLRP3 KO mice via alleviation of mitochondrial lipid peroxidation in renal tubular cells. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21228564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen X., Sun J., Li H., Wang H., Lin Y., Hu Y., et al. Curcumin-loaded nanoparticles protect against rhabdomyolysis-induced acute kidney injury. Cell Physiol Biochem. 2017;43:2143–2154. doi: 10.1159/000484233. [DOI] [PubMed] [Google Scholar]
- 51.Murata I., Sugai T., Murakawa Y., Miyamoto Y., Kobayashi J., Inoue Y., et al. Salvianolic acid B improves the survival rate, acute kidney dysfunction, inflammation and NETosis-mediated antibacterial action in a crush syndrome rat model. Exp Ther Med. 2022;23:320. doi: 10.3892/etm.2022.11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang X.Y., Song J., Hou S.K., Fan H.J., Lv Q., Liu Z.Q., et al. Ulinastatin ameliorates acute kidney injury induced by crush syndrome inflammation by modulating Th17/Treg cells. Int Immunopharmacol. 2020;81 doi: 10.1016/j.intimp.2020.106265. [DOI] [PubMed] [Google Scholar]
- 53.Murata I., Abe Y., Yaginuma Y., Yodo K., Kamakari Y., Miyazaki Y., et al. Astragaloside-IV prevents acute kidney injury and inflammation by normalizing muscular mitochondrial function associated with a nitric oxide protective mechanism in crush syndrome rats. Ann Intensive Care. 2017;7:90. doi: 10.1186/s13613-017-0313-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cui Y., Zhang Y., Zhao X., Shao L., Liu G., Sun C., et al. ACSL4 exacerbates ischemic stroke by promoting ferroptosis-induced brain injury and neuroinflammation. Brain Behav Immun. 2021;93:312–321. doi: 10.1016/j.bbi.2021.01.003. [DOI] [PubMed] [Google Scholar]
- 55.Hong H., Lin X., Xu Y., Tong T., Zhang J., He H., et al. Cadmium induces ferroptosis mediated inflammation by activating Gpx4/Ager/p65 axis in pancreatic beta-cells. Sci Total Environ. 2022;849 doi: 10.1016/j.scitotenv.2022.157819. [DOI] [PubMed] [Google Scholar]
- 56.Zhu G., Sui S., Shi F., Wang Q. Inhibition of USP14 suppresses ferroptosis and inflammation in LPS-induced goat mammary epithelial cells through ubiquitylating the IL-6 protein. Hereditas. 2022;159:21. doi: 10.1186/s41065-022-00235-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Han F., Li S., Yang Y., Bai Z. Interleukin-6 promotes ferroptosis in bronchial epithelial cells by inducing reactive oxygen species-dependent lipid peroxidation and disrupting iron homeostasis. Bioengineered. 2021;12:5279–5288. doi: 10.1080/21655979.2021.1964158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao Y., Wang C., Yang T., Wang H., Zhao S., Sun N., et al. Chlorogenic acid alleviates chronic stress-induced duodenal ferroptosis via the inhibition of the IL-6/JAK2/STAT3 signaling pathway in rats. J Agric Food Chem. 2022;70:4353–4361. doi: 10.1021/acs.jafc.2c01196. [DOI] [PubMed] [Google Scholar]
- 59.Mo Z., Xu P., Li H. Stigmasterol alleviates interleukin-1beta-induced chondrocyte injury by down-regulatingsterol regulatory element binding transcription factor 2 to regulateferroptosis. Bioengineered. 2021;12:9332–9340. doi: 10.1080/21655979.2021.2000742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sterling J.K., Baumann B., Foshe S., Voigt A., Guttha S., Alnemri A., et al. Inflammatory adipose activates a nutritional immunity pathway leading to retinal dysfunction. Cell Rep. 2022;39 doi: 10.1016/j.celrep.2022.110942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Place D.E., Kanneganti T.D. Recent advances in inflammasome biology. Curr Opin Immunol. 2018;50:32–38. doi: 10.1016/j.coi.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li S., Sun Y., Song M., Song Y., Fang Y., Zhang Q., et al. NLRP3/caspase-1/GSDMD-mediated pyroptosis exerts a crucial role in astrocyte pathological injury in mouse model of depression. JCI Insight. 2021;6 doi: 10.1172/jci.insight.146852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Griswold A.R., Huang H.C., Bachovchin D.A. The NLRP1 inflammasome induces pyroptosis in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2022;63:2. doi: 10.1167/iovs.63.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xie S.S., Deng Y., Guo S.L., Li J.Q., Zhou Y.C., Liao J., et al. Endothelial cell ferroptosis mediates monocrotaline-induced pulmonary hypertension in rats by modulating NLRP3 inflammasome activation. Sci Rep. 2022;12:3056. doi: 10.1038/s41598-022-06848-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meihe L., Shan G., Minchao K., Xiaoling W., Peng A., Xili W., et al. The ferroptosis-NLRP1 inflammasome: the vicious cycle of an adverse pregnancy. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.707959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang B.F., Song W., Wang J., Wen P.F., Zhang Y.M. Anti-high-mobility group box-1 (HMGB1) mediates the apoptosis of alveolar epithelial cells (AEC) by receptor of advanced glycation end-products (RAGE)/c-Jun N-terminal kinase (JNK) pathway in the rats of crush injuries. J Orthop Surg Res. 2022;17:20. doi: 10.1186/s13018-021-02903-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li Pomi F., Borgia F., Custurone P., Vaccaro M., Pioggia G., Gangemi S. Role of HMGB1 in cutaneous melanoma: state of the art. Int J Mol Sci. 2022;23 doi: 10.3390/ijms23169327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taverna S., Tonacci A., Ferraro M., Cammarata G., Cuttitta G., Bucchieri S., et al. High mobility group box 1: biological functions and relevance in oxidative stress related chronic diseases. Cells. 2022;11 doi: 10.3390/cells11050849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang L., Wang F., Yang L., Yuan Y., Chen Y., Zhang G., et al. HMGB1 a-box reverses brain edema and deterioration of neurological function in a traumatic brain injury mouse model. Cell Physiol Biochem. 2018;46:2532–2542. doi: 10.1159/000489659. [DOI] [PubMed] [Google Scholar]
- 70.Zhang Z., Jiang J., He Y., Cai J., Xie J., Wu M., et al. Pregabalin mitigates microglial activation and neuronal injury by inhibiting HMGB1 signaling pathway in radiation-induced brain injury. J Neuroinflammation. 2022;19:231. doi: 10.1186/s12974-022-02596-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Scaffidi P., Misteli T., Bianchi M.E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 72.Ye F., Chai W., Xie M., Yang M., Yu Y., Cao L., et al. HMGB1 regulates erastin-induced ferroptosis via RAS-JNK/p38 signaling in HL-60/NRAS(Q61L) cells. Am J Cancer Res. 2019;9:730–739. [PMC free article] [PubMed] [Google Scholar]
- 73.Wu Y., Zhao Y., Yang H.Z., Wang Y.J., Chen Y. HMGB1 regulates ferroptosis through Nrf2 pathway in mesangial cells in response to high glucose. Biosci Rep. 2021;41 doi: 10.1042/BSR20202924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wen Q., Liu J., Kang R., Zhou B., Tang D. The release and activity of HMGB1 in ferroptosis. Biochem Biophys Res Commun. 2019;510:278–283. doi: 10.1016/j.bbrc.2019.01.090. [DOI] [PubMed] [Google Scholar]
- 75.Baatarjav C., Komada T., Karasawa T., Yamada N., Sampilvanjil A., Matsumura T., et al. dsDNA-induced AIM2 pyroptosis halts aberrant inflammation during rhabdomyolysis-induced acute kidney injury. Cell Death Differ. 2022;29:2487–2502. doi: 10.1038/s41418-022-01033-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang X., Liu Y., Xue C., Hu Y., Zhao Y., Cai K., et al. A protein-based cGAS-STING nanoagonist enhances T cell-mediated anti-tumor immune responses. Nat Commun. 2022;13:5685. doi: 10.1038/s41467-022-33301-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang X., Zhang H., Zhang J., Yang M., Zhu M., Yin Y., et al. The paradoxical role of radiation-induced cGAS-STING signalling network in tumour immunity. Immunology. 2022 doi: 10.1111/imm.13592. [DOI] [PubMed] [Google Scholar]
- 78.Ratiu J.J., Barclay W.E., Lin E., Wang Q., Wellford S., Mehta N., et al. Loss of Zfp335 triggers cGAS/STING-dependent apoptosis of post-beta selection thymocytes. Nat Commun. 2022;13:5901. doi: 10.1038/s41467-022-33610-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Su J., Shen S., Hu Y., Chen S., Cheng L., Cai Y., et al. SARS-CoV-2 ORF3a inhibits cGAS-STING-mediated autophagy flux and antiviral function. J Med Virol. 2022 doi: 10.1002/jmv.28175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.L. Wang, Y. Liu, T. Du, H. Yang, L. Lei, M. Guo, et al. ATF3 promotes erastin-induced ferroptosis by suppressing system Xc, Cell Death Differ. 27 (2020). 662–75. doi: 10.1038/s41418-019-0380-z. [DOI] [PMC free article] [PubMed]
- 81.Li Y., Yan J., Zhao Q., Zhang Y., Zhang Y. ATF3 promotes ferroptosis in sorafenib-induced cardiotoxicity by suppressing Slc7a11 expression. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.904314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shen D., Luo J., Chen L., Ma W., Mao X., Zhang Y., et al. PARPi treatment enhances radiotherapy-induced ferroptosis and antitumor immune responses via the cGAS signaling pathway in colorectal cancer. Cancer Lett. 2022;550 doi: 10.1016/j.canlet.2022.215919. [DOI] [PubMed] [Google Scholar]
- 83.Li M., Gao Y., Wang Z., Wu B., Zhang J., Xu Y., et al. Taurine inhibits Streptococcus uberis-induced NADPH oxidase-dependent neutrophil extracellular traps via TAK1/MAPK signaling pathways. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.927215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S., et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 85.Wei Z., Wang J., Wang Y., Wang C., Liu X., Han Z., et al. Effects of neutrophil extracellular traps on bovine mammary epithelial cells in vitro. Front Immunol. 2019;10:1003. doi: 10.3389/fimmu.2019.01003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pertiwi K.R., de Boer O.J., Mackaaij C., Pabittei D.R., de Winter R.J., Li X., et al. Extracellular traps derived from macrophages, mast cells, eosinophils and neutrophils are generated in a time-dependent manner during atherothrombosis. J Pathol. 2019;247:505–512. doi: 10.1002/path.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Okubo K., Kurosawa M., Kamiya M., Urano Y., Suzuki A., Yamamoto K., et al. Macrophage extracellular trap formation promoted by platelet activation is a key mediator of rhabdomyolysis-induced acute kidney injury. Nat Med. 2018;24:232–238. doi: 10.1038/nm.4462. [DOI] [PubMed] [Google Scholar]
- 88.Oishi S., Tsukiji N., Otake S., Oishi N., Sasaki T., Shirai T., et al. Heme activates platelets and exacerbates rhabdomyolysis-induced acute kidney injury via CLEC-2 and GPVI/FcRgamma. Blood Adv. 2021;5:2017–2026. doi: 10.1182/bloodadvances.2020001698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu S., Yang J., Sun G., Hu J., Zhang Q., Cai J., et al. Macrophage extracellular traps aggravate iron overload-related liver ischaemia/reperfusion injury. Br J Pharmacol. 2021;178:3783–3796. doi: 10.1111/bph.15518. [DOI] [PubMed] [Google Scholar]
- 90.Bosch X., Poch E., Grau J.M. Rhabdomyolysis and acute kidney injury. N Engl J Med. 2009;361:62–72. doi: 10.1056/NEJMra0801327. [DOI] [PubMed] [Google Scholar]
- 91.Xu Y., Li X., Cheng Y., Yang M., Wang R. Inhibition of ACSL4 attenuates ferroptotic damage after pulmonary ischemia-reperfusion. FASEB J. 2020;34:16262–16275. doi: 10.1096/fj.202001758R. [DOI] [PubMed] [Google Scholar]
- 92.Kagan V.E., Mao G., Qu F., Angeli J.P., Doll S., Croix C.S., et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. 2017;13:81–90. doi: 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang Y., Li S., Li F., Lv C., Yang Q.K. High-fat diet impairs ferroptosis and promotes cancer invasiveness via downregulating tumor suppressor ACSL4 in lung adenocarcinoma. Biol Direct. 2021;16:10. doi: 10.1186/s13062-021-00294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang Y., Zhang M., Bi R., Su Y., Quan F., Lin Y., et al. ACSL4 deficiency confers protection against ferroptosis-mediated acute kidney injury. Redox Biol. 2022;51 doi: 10.1016/j.redox.2022.102262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tao W.H., Shan X.S., Zhang J.X., Liu H.Y., Wang B.Y., Wei X., et al. Dexmedetomidine attenuates ferroptosis-mediated renal ischemia/reperfusion injury and inflammation by inhibiting ACSL4 via alpha2-AR. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.782466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu L., Kang X.X. ACSL4 is overexpressed in psoriasis and enhances inflammatory responses by activating ferroptosis. Biochem Biophys Res Commun. 2022;623:1–8. doi: 10.1016/j.bbrc.2022.07.041. [DOI] [PubMed] [Google Scholar]
- 97.Wu V. Dexrazoxane: a cardioprotectant for pediatric cancer patients receiving anthracyclines. J Pediatr Oncol Nurs. 2015;32:178–184. doi: 10.1177/1043454214554008. [DOI] [PubMed] [Google Scholar]
- 98.Zhang H., Wang Z., Liu Z., Du K., Lu X. Protective effects of dexazoxane on rat ferroptosis in doxorubicin-induced cardiomyopathy through regulating HMGB1. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.685434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhu K., Zhu X., Liu S., Yu J., Wu S., Hei M. Glycyrrhizin attenuates hypoxic-ischemic brain damage by inhibiting ferroptosis and neuroinflammation in neonatal rats via the HMGB1/GPX4 pathway. Oxid Med Cell Longev. 2022;2022:8438528. doi: 10.1155/2022/8438528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yang H., Liu H., Zeng Q., Imperato G.H., Addorisio M.E., Li J., et al. Inhibition of HMGB1/RAGE-mediated endocytosis by HMGB1 antagonist box A, anti-HMGB1 antibodies, and cholinergic agonists suppresses inflammation. Mol Med. 2019;25:13. doi: 10.1186/s10020-019-0081-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Montes V.N., Subramanian S., Goodspeed L., Wang S.A., Omer M., Bobik A., et al. Anti-HMGB1 antibody reduces weight gain in mice fed a high-fat diet. Nutr Diabetes. 2015;5:e161. doi: 10.1038/nutd.2015.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fang Y., Chen X., Tan Q., Zhou H., Xu J., Gu Q. Inhibiting ferroptosis through disrupting the NCOA4-FTH1 interaction: a new mechanism of action. ACS Cent Sci. 2021;7:980–989. doi: 10.1021/acscentsci.0c01592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sheng X., Shan C., Liu J., Yang J., Sun B., Chen D. Theoretical insights into the mechanism of ferroptosis suppression via inactivation of a lipid peroxide radical by liproxstatin-1. Phys Chem Chem Phys. 2017;19:13153–13159. doi: 10.1039/c7cp00804j. [DOI] [PubMed] [Google Scholar]
- 104.Friedmann Angeli J.P., Schneider M., Proneth B., Tyurina Y.Y., Tyurin V.A., Hammond V.J., et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16:1180–1191. doi: 10.1038/ncb3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Skouta R., Dixon S.J., Wang J., Dunn D.E., Orman M., Shimada K., et al. Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J Am Chem Soc. 2014;136:4551–4556. doi: 10.1021/ja411006a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.S. Hofmans, T. Vanden Berghe, L. Devisscher, B. Hassannia, S. Lyssens, J. Joossens, et al. Novel ferroptosis inhibitors with improved potency and ADME properties, J Med Chem. 59 (2016). 2041–53. doi: 10.1021/acs.jmedchem.5b01641. [DOI] [PubMed]
- 107.Linkermann A., Skouta R., Himmerkus N., Mulay S.R., Dewitz C., De Zen F., et al. Synchronized renal tubular cell death involves ferroptosis. Proc Natl Acad Sci U S A. 2014;111:16836–16841. doi: 10.1073/pnas.1415518111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shah R., Margison K., Pratt D.A. The potency of diarylamine radical-trapping antioxidants as inhibitors of ferroptosis underscores the role of autoxidation in the mechanism of cell death. ACS Chem Biol. 2017;12:2538–2545. doi: 10.1021/acschembio.7b00730. [DOI] [PubMed] [Google Scholar]
- 109.Kang H., Han M., Xue J., Baek Y., Chang J., Hu S., et al. Renal clearable nanochelators for iron overload therapy. Nat Commun. 2019;10:5134. doi: 10.1038/s41467-019-13143-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhao C., Yu D., He Z., Bao L., Feng L., Chen L., et al. Endoplasmic reticulum stress-mediated autophagy activation is involved in cadmium-induced ferroptosis of renal tubular epithelial cells. Free Radic Biol Med. 2021;175:236–248. doi: 10.1016/j.freeradbiomed.2021.09.008. [DOI] [PubMed] [Google Scholar]
- 111.Hatcher H.C., Singh R.N., Torti F.M., Torti S.V. Synthetic and natural iron chelators: therapeutic potential and clinical use. Future Med Chem. 2009;1:1643–1670. doi: 10.4155/fmc.09.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang B., Timilsena Y.P., Blanch E., Adhikari B. Lactoferrin: Structure, function, denaturation and digestion. Crit Rev Food Sci Nutr. 2019;59:580–596. doi: 10.1080/10408398.2017.1381583. [DOI] [PubMed] [Google Scholar]
- 113.Pawlaczyk M., Schroeder G. Deferoxamine-modified hybrid materials for direct chelation of Fe(III) ions from aqueous solutions and indication of the competitiveness of in vitro complexing toward a biological system. ACS Omega. 2021;6:15168–15181. doi: 10.1021/acsomega.1c01411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rassu G., Salis A., Porcu E.P., Giunchedi P., Roldo M., Gavini E. Composite chitosan/alginate hydrogel for controlled release of deferoxamine: a system to potentially treat iron dysregulation diseases. Carbohydr Polym. 2016;136:1338–1347. doi: 10.1016/j.carbpol.2015.10.048. [DOI] [PubMed] [Google Scholar]
- 115.Jones G., Goswami S.K., Kang H., Choi H.S., Kim J. Combating iron overload: a case for deferoxamine-based nanochelators. Nanomedicine (Lond) 2020;15:1341–1356. doi: 10.2217/nnm-2020-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li D., Liu B., Fan Y., Liu M., Han B., Meng Y., et al. Nuciferine protects against folic acid-induced acute kidney injury by inhibiting ferroptosis. Br J Pharmacol. 2021;178:1182–1199. doi: 10.1111/bph.15364. [DOI] [PubMed] [Google Scholar]
- 117.Jiang G.P., Liao Y.J., Huang L.L., Zeng X.J., Liao X.H. Effects and molecular mechanism of pachymic acid on ferroptosis in renal ischemia reperfusion injury. Mol Med Rep. 2021;23 doi: 10.3892/mmr.2020.11704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guo J., Wang R., Min F. Ginsenoside Rg1 ameliorates sepsis-induced acute kidney injury by inhibiting ferroptosis in renal tubular epithelial cells. J Leukoc Biol. 2022;112:1065–1077. doi: 10.1002/JLB.1A0422-211R. [DOI] [PubMed] [Google Scholar]
- 119.Qiongyue Z., Xin Y., Meng P., Sulin M., Yanlin W., Xinyi L., et al. Post-treatment with irisin attenuates acute kidney injury in sepsis mice through anti-ferroptosis via the SIRT1/Nrf2 pathway. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.857067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhou L., Yu P., Wang T.T., Du Y.W., Chen Y., Li Z., et al. Polydatin attenuates cisplatin-induced acute kidney injury by inhibiting ferroptosis. Oxid Med Cell Longev. 2022;2022:9947191. doi: 10.1155/2022/9947191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.McCullough K.R., Akhter J., Taheri M.J., Traylor A., Zmijewska A.A., Verma V., et al. Functional consequence of myeloid ferritin heavy chain on acute and chronic effects of rhabdomyolysis-induced kidney injury. Front Med (Lausanne) 2022;9 doi: 10.3389/fmed.2022.894521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.E.P. Amaral, T.W. Foreman, S. Namasivayam, K.L. Hilligan, K.D. Kauffman, C.C. Barbosa Bomfim, et al. GPX4 regulates cellular necrosis and host resistance in Mycobacterium tuberculosis infection, J Exp Med. 2022;219. doi: 10.1084/jem.20220504. [DOI] [PMC free article] [PubMed]
- 123.Li M., Yang N., Hao L., Zhou W., Li L., Liu L., et al. Melatonin inhibits the ferroptosis pathway in rat bone marrow mesenchymal stem cells by activating the PI3K/AKT/mTOR signaling axis to attenuate steroid-induced osteoporosis. Oxid Med Cell Longev. 2022;2022:8223737. doi: 10.1155/2022/8223737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang Z.T., He W.J., Deng S.M., Xu S.H., Zeng X., Qian Z.M., et al. Trilobatin alleviates non-alcoholic fatty liver disease in high-fat diet plus streptozotocin-induced diabetic mice by suppressing NLRP3 inflammasome activation. Eur J Pharmacol. 2022;933 doi: 10.1016/j.ejphar.2022.175291. [DOI] [PubMed] [Google Scholar]
- 125.M.R. Marasco, A.M. Conteh, C.A. Reissaus, J.E.t. Cupit, E.M. Appleman, R.G. Mirmira, et al. Interleukin-6 reduces beta-cell oxidative stress by linking autophagy with the antioxidant response. Diabetes 2018;67:1576–88. doi: 10.2337/db17-1280. [DOI] [PMC free article] [PubMed]
- 126.Murata I., Miyake Y., Takahashi N., Suzuki R., Fujiwara T., Sato Y., et al. Low-dose sodium nitrite fluid resuscitation prevents lethality from crush syndrome by improving nitric oxide consumption and preventing myoglobin cytotoxicity in kidney in a rat model. Shock. 2017;48:112–118. doi: 10.1097/SHK.0000000000000817. [DOI] [PubMed] [Google Scholar]
- 127.Murata I., Kawanishi R., Inoue S., Iwata M., Kobayashi J., Inoue Y., et al. A novel method to assess the severity and prognosis in crush syndrome by assessment of skin damage in hairless rats. Eur J Trauma Emerg Surg. 2019;45:1087–1095. doi: 10.1007/s00068-018-0987-7. [DOI] [PubMed] [Google Scholar]
- 128.Li L., Wang K., Jia R., Xie J., Ma L., Hao Z., et al. Ferroportin-dependent ferroptosis induced by ellagic acid retards liver fibrosis by impairing the SNARE complexes formation. Redox Biol. 2022;56 doi: 10.1016/j.redox.2022.102435. [DOI] [PMC free article] [PubMed] [Google Scholar]