Abstract
The clustered regularly interspaced short palindromic DNA sequence repeats (CRISPR) and CRISPR-associated (Cas) (CRISPR/Cas) systems are currently applied not only as a gene editing tool but also as a novel molecular diagnostic technique. The CRISPR/Cas systems have emerged as an efficient molecular diagnostic system that can detect nucleic acids, proteins and small molecule compounds, by converting a non-nucleic acid into a nucleic acid signal of Cas-identifiable and keeping inherent properties of high sensitivity and specificity. While its multiple advantages for nucleic acid detection have been widely published in excellent reviews, there have been no systematic analyses and reviews on the principles and characteristics of CRISPR/Cas-based diagnostic systems for non-nucleic acids. The present work reviewed the basic process, principles, characteristics, strategies, recent advances, and challenges of CRISPR/Cas-based molecular diagnostic methods for detecting non-nucleic acids, which may provide a basis or some references for future development and application as molecular diagnostic tools.
Keywords: CRISPR/Cas, Non-nucleic acid, Molecular diagnosis
1. Introduction
Molecular diagnostic tools capable of detecting and quantifying nucleic acids, proteins, and small molecule compounds are very important in biology, medical diagnostics, food safety, and environmental surveillance [1]. Recently, molecular diagnostic tools have been driven by modern molecular biology techniques, which have improved their sensitivity, specificity and versatility, and helped rapidly develop personalized medical diagnosis for both clinical laboratories and point-of-care tests (POCT) [2]. However, conventional molecular diagnostic techniques, including polymerase chain reaction (PCR) and enzyme-linked immunosorbent assay (ELISA), require professional laboratories, specific testing equipment, and rigorously trained operators, involving a relatively long detection time. Based on the pros and cons of conventional molecular diagnostic techniques and because of current worldwide health challenges, e.g., severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), novel tools are needed. Clustered regularly interspaced short palindromic DNA sequence repeats (CRISPR) and CRISPR-associated (Cas) (CRISPR/Cas)-based molecular diagnostics have the most advanced and promising molecular diagnostic tools with multiple biological functions that may be used to develop bioanalytical sensors and devices, which require a shorter testing time, the non-requirement of special equipment, and being suitable for POCT [2]. The CRISPR/Cas system has introduced a novel era of biological detection thanks to its enhanced base resolution and high capacity in amplifying isothermal signals [3].
CRISPR/Cas-based molecular diagnostic systems involving Cas proteins including Cas9, Cas12, Cas13 and Cas14 [[4], [5], [6], [7], [8]] have been mainly used as nucleic acid detection sensors [1,2]. CRISPR/Cas molecular diagnostic systems can be applied for both nucleic acid and non-nucleic acid molecular diagnoses for inherent properties. CRISPR/Cas-based non-nucleic acid molecular diagnostic tools convert a non-nucleic acid into a nucleic acid signal identifiable by a Cas, with inherent properties of high sensitivity and specificity, fast operation, and affordability, that can be employed to test complex samples and are suitable for POCT. At present, although a series of molecular techniques based on CRISPR/Cas systems are available for detecting non-nucleic acids, there have been no systematic reviews assessing the principles and features of CRISPR/Cas-based non-nucleic acid molecular diagnostic sensors. The present review hence summarizes recent research progress in non-nucleic acid molecular diagnosis using CRISPR/Cas systems, with the hope of providing some references or guide for further development and application as CRISPR/Cas-based non-nucleic acid molecular diagnostic sensors.
2. CRISPR/cas types for established molecular diagnostic tools
CRISPR/Cas represents a system utilized for adaptive immunity in bacteria and archaea to prevent the invasion of foreign DNAs/RNAs or bacteriophages [9]. In CRISPR/Cas systems, these foreign DNA/RNA molecules function as molecular records and undergo transcription alongside CRISPR repeats as CRISPR RNA (crRNA). This crRNA and a transactivating crRNA (tracrRNA) generate a complex known as a guide RNA (gRNA) that guides the Cas endonuclease to recognize the target sequence [9]. Based on the structures and functions of effector Cas proteins, CRISPR/Cas systems comprise two major classes, namely Classes 1 and 2, which are clustered into six types (Ⅰ, Ⅱ, Ⅲ, Ⅳ, Ⅴ, and Ⅵ) [9,10]. While Class 1 systems (I, III, and IV) have effector modules comprising many Cas proteins, Class 2 systems have single, multidomain crRNA-binding proteins, including Cas9, Cas12/Cas14, and Cas13 for types II, V and Ⅵ, respectively [9,10]. Following their discovery and multiple structure-function analyses, various natural and engineered CRISPR-associated Cas proteins are now used for molecular diagnostics [11]. A series of molecular diagnostic tools based on Cas proteins have been established, including CRISPR/Cas9, CRISPR/Cas12, CRISPR/Cas13, and CRISPR/Cas14, which can detect two groups of targets, i.e., nucleic acid and non-nucleic acid molecules (included Cas12, Cas13 and Cas14) [[12], [13], [14], [15]]. The present review focuses on the characteristics of CRISPR/Cas for testing non-nucleic acids.
2.1. CRISPR/cas 12
As a representative of type V systems, the CRISPR/Cas12 system has been found in many archaea and bacteria [16], and the Cas12 protein contains a RuvC enzyme activity domain [9,17]. CRISPR/Cas12 is guided by the gRNA to recognize the protospacer adjacent motif (PAM) sequence of 5′-TTV-3' (upstream of the DNA target sequence) and the target sequence of double-stranded DNA (dsDNA). Once activated, it cleaves the targeted dsDNA, and could perform non-targeted cleavage (collateral activity) of a single-stranded DNA (ssDNA) [6,7], which has been used for developing multiple diagnostic platforms for non-nucleic acids.
2.2. CRISPR/cas 13
The type VI CRISPR/Cas13 system constitutes an RNA nucleic acid recognition system. With the guidance of the gRNA, Cas13 recognizes the target RNA sequence, with the gRNA specifically pairing with the target RNA base to generate a ternary complex, activating Cas13 for specific cleavage of the target RNA as well as for collateral cleavage [8,18,19].
2.3. CRISPR/cas 14
CRISPR/Cas14 is a type V system that recognizes the dsDNA target sequence guided by the gRNA. The formation of a ternary complex of the targeted sequence CRISPR/Cas14/gRNA induces cleavage at the specific dsDNA target site and collateral cleavage of ssDNA [9,20,21]. Based on the collateral cleavage characteristic of Cas12, Cas13 and Cas14, a series of non-nucleic acid diagnostic tools have been developed (Table .1).
Table 1.
Characteristics of CRISPR/Cas-based detection methods for non-nucleic acids.
| Detection platform | Cas effectors | PAM | Time (min) | LOD | Readout | Detection | Sample type | Reference |
|---|---|---|---|---|---|---|---|---|
| CRISPR/Cas12a/exosome | LbCas12a | 5′-TTTV | 120 | 3000particles/μL | Fluorophore | Exosome | Blood of plasma | [27] |
| CRISPR/Cas12a-EV | Cas12a | 5′-TTTV | – | 100 particles/mL | Fluorescence | Extracellular vesicles | Serum | [23] |
| iPCCA | Cas12a | 5′-TTTV | ′∼180 | 100 fM | Fluorescence | Interleukin-6s | Homogenous solutions | [35] |
| CaT-SMelor | LbCas12a | 5′-TTTV | <30 | nM level | Fluorescence | Uric acid | Blood | [28] |
| Cell-Free | Cas12a | 5′-TTTV | ′∼120 | 2 μM | Fluorescence | Small-Molecule | Environmental Samples | [29] |
| fDNA-Cas12a | LbCas12a | 5′-TTTV | <15 | 0.21 μM/0.10 mM | Fluorescence | ATP/Na+ | Plasma | [30] |
| Aptamer-locker | LbCas12a | 5′-TTTV | ∼20 | 38 nM | Fluorescence | Melamine | Infant milk | [60] |
| Ochratoxin A cas12a | Cas12a | 5′-TTTV | ∼60 | 5 ng/mL | Fluorescence | Ochratoxin A | Foods | [61] |
| LDCC | LbCas12a | 5′-TTTV | ∼30 | 0.0005U/mL | Fluorescence | ATP/NAD/PNK | – | [32] |
| CAFI | Cas12a | 5′-TTTV | 240 | 1 fg/mL (58.8 aM) | Fluorescence | IFNɤ | Serum, whole blood, perspiration and saliva | [36] |
| CSEDLI | Cas12a | 5′-TTTV | 60 | 0.48 nM | Fluorescence | Lead ion | Environmental Samples | [41] |
| CEACNp | Cas12a | 5′-TTTV | 30 | 16.5 pg/mL | Electrochemical aptasensor | Nucleocapsid protein | Tap water, milk, or serum | [37] |
| Cas12a -MDANs | Cas12a | 5′-TTTV | <60 | 26 cells/mL | Fluorescence | CTCs | Human blood samples, | [44] |
| Nano-CLISA | Cas12a | 5′-TTTV | ∼300 | 13.9 fg/mL (69.5 aM) | Fluorescence | CEA and PSA | Clinical samples | [38] |
| CCSRPM | Cas12a | 5′-TTTV | 11 | 0.03 nM | Fluorescence | Streptavidin/biotin | Human serum | [40] |
| CRISPR/Cas12a-TFs | Cas12a | 5′-TTTV | 90 | 0.2 pM | Fluorescence | Transcription factors | Cancer cells | [39] |
| CAOSPPCC | Cas12a | 5′-TTTV | 30 | 86 fM | Fluorescence | Pb2+ | Environmental samples. | [43] |
| CRISPR-Cas12a-OP | Cas12a | 5′-TTTV | <120 | 218 pg/mL | Fluorescence | OP | Agricultural products | [42] |
| Cas12a-CMCAN | Cas12a | 5′-TTTV | – | 0.16 μM | Fluorescence | ATP | Human serum samples | [33] |
| Cas12a-PNKP | Cas12a | 5′-TTTV | ∼80 | 3.3 × 10−6U/mL. | Fluorescence | Polynucleotide kinase/phosphatase | – | [45] |
| SPRINT | Cas13a | 3′-PFS: H | – | 1 μM | Fluorescence | Small-Molecule | Cofactors, Metabolites of amino acids, tetracycline and monatomic ions | [53] |
| TITAC-Cas | Cas13a | 3′-PFS: H | 100 | 6 ± 0.52 mU/L | Fluorescence | ALP | HepG2 cells | [50] |
| CLISA | Cas13a | 3′-PFS: H | 30 | 32.27 fg/mL (0.81 fM) | Fluorescence | IL-6/VEGF | Serum | [52] |
| APC-Cas | LbuCas13a | 3′-PFS: H | 140 | 1 cfu/mL | Cy5-BHQ2 | Salmonella Enteritidis | Milk | [51] |
| CRISPR/Cas12a-13a | Cas12a/Cas13a | 5′-TTTV/3′-PFS: H | 130 | 30 pg/mL/200 pg/mL | Fluorescence | Exosomal | Plasma | [26] |
| CRISPR/Cas14a-CK-MB | Cas14 | 5′-TTTA | 100 | 0.355 pM (15.62 pg/mL) | PtNPs/Cu-TCPP(Fe) | CK-MB | Serum | [54] |
| CRISPR/Cas14a | Cas14 | 5′-TTTA | 45 | 2.06 nM | Tb reporter | Ampicillin | – | [62] |
All features; aM: 10−18 M; fM: 10−15 M; N: no; Y: yes; NA: nonapplicable; “-“: Not mentioned; F: Fluorescence; SNVs: single nucleotide variants; SNPs: Single Nucleotide Polymorphism; HRP: horseradish peroxidase; V: A/C/G; H: A/C/T; LwaCas13a: Leptotrichia wadei Cas13a; CcaCas13 b: Capnocytophaga canimorsus Cc5 Cas13 b; LOD: limit of detection; LFA: lateral flow assay; iPCCA: isothermal proximity CRISPR/Cas12a assay; LDCC: DNA ligation-driven CRISPR/Cas12a developing detection sensor; CRISPR/Cas12a-EV: CRISPR/Cas12a extracellular vesicles; fDNA-Cas12a: functional DNA CRISPR/Cas12a; CaT-Smelor: CRISPR-Cas12a and aTF-mediated small molecule detector; TITAC-Cas: Trigger the trans-cleavage activity of Cas13a; APC-Cas: allosteric probe-initiated catalysis; CLISA: CRISPR/Cas13a signal amplification linked immunosorbent assay; PtNPs/Cu-TCPP(Fe): platinum nanoparticle (PtNPs) decorated metal-organic framework (MOF) nanosheets, Op: Organophosphorus pesticides.
3. CRISPR/Cas12-based molecular diagnosis of non-nucleic acids
3.1. CRISPR/Cas12a-based extracellular vesicles as a diagnostic method
Extracellular vesicles (EVs) are naturally derived molecular vesicles, which contain a variety of bioactive molecules that maintain original information; and therefore, EVs have been utilized as a biomarker for clinical diagnosis [22]. Although EVs are potential biomarkers for cancer diagnostics and prognostic monitoring in a non-invasive manner, most traditional detection methods need a large sample volume and lengthy isolation and purification procedures, and are expensive, making it difficult for their widespread use in clinical practice. The CRISPR/Cas12a-EV detection sensor is a novel detection tool for EVs, which utilizes the CRISPR/Cas12a system, in combination with an anti-CD109 antibody, CD109 aptamers, and the PCR method [23,24] (Fig. 1A and Table .1). Firstly, antibodies targeting a mixture of tetraspanins (anti-CD9/63/81) are fixed on micro-plate wells to capture EVs, CD109 aptamers and CD109 + EVs [23]. After the fixed antibodies have captured CD109 + EVs and CD109 aptamers, bound CD109 aptamers are proportional to the initial concentrations of CD109 + EVs, and the product of CD109-aptamer PCR amplification represents the CRISPR/Cas12a target sequence. CRISPR/Cas12a can simultaneously target the cleavage of activated CD109-aptamer targeted sequence and engage in collateral cleavage of the ssDNA reporter, enabling real-time quantitative fluorescence-based analysis of CD109-aptamers [23], where fluorescence intensity directly reflects the concentrations of EVs [23] (Fig. 1A and Table .1).
Fig. 1.
Diagram of CRISPR/Cas12a-based extracellular vesicle (EV) detection methods.
A: CRISPR/Cas12a-based EV detection method [22,63]; B: CRISPR/Cas12a-based exosome detection method [25]. Specific surface marker (CD63) of exosomes; Blocker.
The CRISPR/Cas12a-EV diagnostic method is a novel platform, which can detect CD109+ and epidermal growth factor receptor (EGFR)+ tumor-derived extracellular vesicles (TEVs) from cultured cells and complex biological fluids with a high sensitivity even at 100 particles/mL only using 50 μL reactions [23]. Analyses of human serum samples in a recent study verified the successful detection of combined serum CD109+ and EGFR+TEV, with an area under the curve (AUC) of 0.934, a sensitivity of 84.1 %, and a specificity of 85.0 % [23] (Fig. 1A and Table .1). CRISPR/Cas12a-EV can detect EV surface proteins with a very high sensitivity (within a broad linear range of 102-108 particles/mL) and a high specificity, and can directly detect EV membrane proteins within a small volume, revealing its suitability for clinical use in cancer diagnosis and radiotherapy surveillance [23]. CRISPR/Cas12a-based EV detection systems with a high sensitivity can directly detect EVs without EV isolation, which may be employed in the surveillance of all cancer types through redesigned EV aptamers in the future.
Exosomes (Exo) are vesicular bodies secreted by some living cells, which participate in cellular information exchange and play important roles in pathological and physiological processes [25,26]. Exo from different tissue sources have distinct compositions and functions, which may become important diagnostic biomarkers for pathological changes of diseases [25]. So far, researchers have used the CRISPR/Cas12a diagnostic platform as a rapid molecular diagnostic method of Exo (CRISPR/Cas12a/exosome), involving biotin-labeled CD63 aptamer that is used to specifically capture exosomes [26,27] (Fig. 1B). The CRISPR/Cas12a/exosome contains two parts, namely the CD63 aptamer and the Cas12a target sequence, which is complementary to the CD63 aptamer (Blocker sequence). The detection process is as follows. Firstly, biotin-labeled CD63 aptamer binds to streptavidin-labeled magnetic beads. When Exo are present, the CD63 aptamer sequence interacts with CD63 at the Exo surface, resulting in the formation of the CD63 aptamer-CD63 complex on magnetic beads and causing conformational change of the CD63 aptamer and release of the Blocker into the reaction system. Through magnetic beads, the Blocker is then separated from the CD63 aptamer/Exo/magnet complex [27]. Under the guidance of the gRNA, the CRISPR/Cas12a recognizes and activates cleavage of the target sequence (Blocker sequence), while allowing collateral cut of fluorescein-labeled ssDNA to generate the fluorescein signal [27]. This CRISPR/Cas12a/exosome platform has been successfully used to screen clinical samples, detecting Exo from 3 × 103 to 6 × 107 particles/μL, as verified in clinical samples, within 120 min (Fig. 1B and Table .1). Thus, the CRISPR/Cas12a/exosome system employing the CD63 aptamer to capture Exo and CRISPR/Cas12a to amplify signals represents a rapid and sensitive tool for detecting exosomes. Recently, a novel CRISPR-Cas12a/Cas13a-based exosome detection system was employed for the synchronous detection of two exosomal proteins, with high sensitivities (LOD 30.00 pg/mL, 200.00 pg/mL) [26], which increases the detection throughput and extends the application of the CRISPR-Cas system. The CRISPR-Cas12a/Cas13a system has a higher sensitivity than the CRISPR/Cas12a/exosome system.
3.2. CRISPR/Cas12a-based diagnostic methods for detecting small molecules
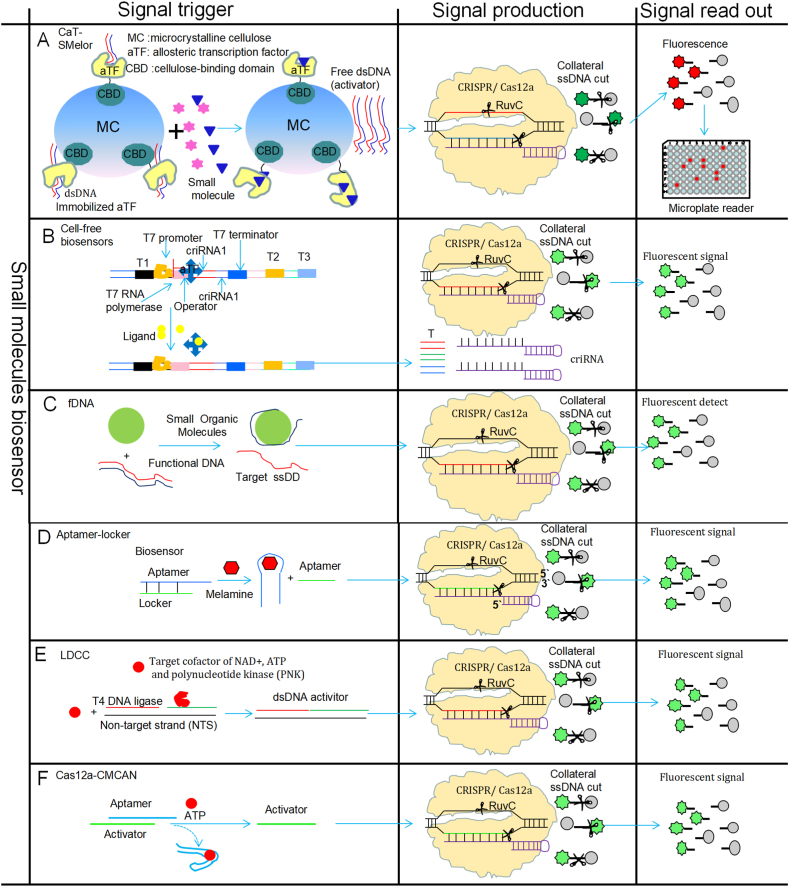
CRISPR-Cas12a and allosteric transcription factor (αTF) have been utilized for the development of a method for detecting small molecules (CaT-SMelor) [28] (Fig. 2A). In the CaT-SMe system, αTF is fixed to microcrystalline cellulose (MC) via interaction with the cellulose-binding domain (CBD), and the CBD-TF complex can capture dsDNA, with the target DNA sequence being recognized by CRISPR-Cas12a. The CaT-SMelor method diagnosis process is as follows [28]: Firstly, the dsDNA interacts with CBD-αTF to form a complex (dsDNA-CBD-αTF). When the target small molecule interacts with the latter complex, it causes a conformational change of the dsDNA-CBD-αTF complex, which discharges the dsDNA. Secondly, the gRNA (guide RNA) guides CRISPR-Cas12a to recognize the targeted dsDNA, activating the target cleavage and non-specific cut activity of fluorescein labeling sequence, and producing fluorescein signals. Finally, the small molecule is detected and quantified by analyzing the produced fluorescein signals. The CaT-SMelor system has been successfully utilized for uric acid quantitation in clinical serum samples, with the whole process completed within 1 h with a detection limit reaching the nM level, and carried out in a high-throughput detection format (96-hole plates) [28] (Fig. 2A and Table .1). Thus, the CaT-SMelor platform is a CRISPR/Cas12-mediated non-nucleic-acid-detection tool, which can efficiently carry out qualitative and quantitative analyses of small molecular compounds through protein immobilization, offering a strategy for a simple, sensitive, fast and cost-effective high-throughput technique for analyzing small molecules.
Fig. 2.
CRISPR/Cas12a-based diagnostic methods for small molecules.
A: CaT-SMelor [28,63]; B: Cell-free [29]; C: fDNA [30]; D: Aptamer-locker [31]; E: LDCC [32]; F: Cas12a-CMCAN [33]; αTF: allosteric transcription factor; CBD: cellulose-binding domain; MC: Microcrystalline cellulose; fDNA: functional DNA.
Cell-free biosensors are simple and rapid systems for detecting small molecules. Cell-free systems have been developed based on αTF-mediated expression of a CRISPR array coupled with Cas12a activity (Fig. 2B), which requires the construction of an expression cassette that contains the T7 promoter, a αTF binding sequence, T7 promoter's stop codon and CRISPR/Cas12a recognition target sequence [29]. αTF can bind the T7 promoter of the operon, which inhibits T7 promoter's transcriptional activity of dsDNA transcriptional Pre-crRNA [29]. When the reaction system has small molecules that can interact with αTF and cause its conformational change, triggering T7 promoter's transcriptional activity and producing mature crRNA, which guides the CRISPR-Cas12a recognition target dsDNA sequence to activate target cleavage and collateral cut activity cleaving the ssDNA linker producing fluorescein signals for quantitative analysis of the small molecules [29] (Fig. 2B and Table .1). This cell-free biosensor system has a high sensitivity, specificity, and simplicity that could be utilized for rapid detection of small molecules within 120 min. This system has been found affordable and can be used in a single step in the POCT settings, which provides simple visual readouts and mobile phone applications, and its associated lyophilized reagents can allow its ease storage and distribution.
The combination of a functional DNA (fDNA) with CRISPR/Cas can be applied for detecting small molecules or metal ions [30] (Fig. 2C and Table .1). The fDNA is based on the Cas12a-dependent reporter system requiring CRISPR/Cas12a and a ssDNA substrate to be labeled with a fluorophore. In the absence of the fDNA targets (some small molecules), fDNA can lock the DNA activator (preventing ssDNA cleavage by Cas12a), and the presence of fDNA targets trigger unlocking of the DNA activator resulting in ssDNA cleavage by Cas12a releasing the fluorophore [30]. The generation of fluorescent signals can be quantitated by portable fluorimeters, which was applied for the quantitative detection of adenosine triphosphate (ATP) and Na+ at 25 °C, with a simple and rapid detection process (two steps <15 min) being suitable for field testing or POCT [30] (Fig. 2C and Table .1). Thus, a fDNA can be utilized for quantitating two non-nucleic acids (ATP and Na+), which could be applied in field testing or POCT-based diagnostics. Since fDNAs may be obtained from a large DNA library to recognize a wide range of targets, including metal ions, small organic molecules, proteins, and even viruses and cells, this fDNA-regulated CRISPR system can potentially expand bioanalytical and medical applications of the CRISPR-Cas sensor system significantly.
A strategy based on CRISPR/Cas12a-based biosensing in combination of an aptamer-locker has been developed and utilized for rapid and sensitive melamine detection [31] (Fig. 2D and Table .1). When the reaction system contains melamine, the recognition aptamer sequence forms a hair structure and releases a locker sequence that cleaves the target DNA sequence of CRISPR/Cas12a. Then, CRISPR/Cas12a recognizes the target sequence and activates the non-specific cutting activity (collateral cut activity) cleaving the fluorescein labeling ssDNA, which produces a fluorescein signal for melamine detection (Fig. 2D and Table .1) [31]. This strategy has been employed for melamine detection in whole milk, with or without sample pretreatment, rapidly (in <20 min) and sensitively (with a detection limit of 38 nM). In addition, on-site assessment of melamine was performed with a portable fluorimeter. Furthermore, it has been suggested that this strategy may be expanded to assess some other non-nucleic-acid targets via simple replacement of aptamers [31].
The DNA ligation-driven CRISPR/Cas12a method (LDCC) has been developed that can detect NAD+, ATP and polynucleotide kinase (PNK) [32] (Fig. 2E and Table .1). In the LDCC system, CRISPR/Cas12a recognizes PAM in the non-target strand (NTS) and target strand (TS) to unwind the dsDNA target [32]. Therefore, in the absence of NAD+ or ATP and a gap in the TS, dsDNA target unwinding could not result in the activation of Cas12a nuclease activity. In the presence of NAD+ or ATP, the DNA ligase repairs dsDNA gaps and formats the dsDNA, which enables CRISPR/Cas12a to identify the target sequence and activate its collateral activity cleaving the fluorescent ssDNA, which generates fluorescent signals. Thus, the LDCC detection method can convert non-nucleic acid targets (PNK, NAD+ or ATP) into nucleic acid targets for CRISPR-Cas12a recognition, detecting non-nucleic acids with a high sensitivity and specificity. The LDCC platform applying DNA ligation can be used for ultrasensitive quantitation of non-nucleic acids, e.g., NAD+, ATP and proteins. The LDCC system has been shown to be able to detect NAD+, ATP, or PNK by amplified fluorescent signals, with a limit of detection of 0.0005 U/mL [32] (Fig. 2E and Table .1). Overall, in this LDCC platform, the DNA ligation approach has been utilized as a ‘‘signal converter’’ for the conversion of target non-nucleic acids into programmable nucleic acids that can activate the nuclease activity of CRISPR-Cas12a for signal amplification, with a high sensitivity and specificity, and the LDCC platform converts the trans-cleavage activity into homogeneous colorimetric signals for analysis.
Recently, a sensitive and simple CRISPR/Cas12a-based biosensing strategy has been developed by integrating a programmable CRISPR RNA (crRNA)-mediated catalytic nucleic acid network (CMCAN) [33] (Fig. 2F and Table .1). Using adenosine triphosphate (ATP) as the model target and when ATP is available, it is specifically captured by the aptamer, causing conformational changes and activating collateral activity of CRISPR/Cas12a resulting production of amplified fluorescent signals [33] (Fig. 2F and Table .1). The Cas12a-CMCAN detection system has been shown to achieve a low detection limit (0.16 μM) and have a wide detection range (with a linear range from 0.30 to 175 μM). In addition, it could be successfully utilized for ATP quantitation in diluted human serum specimens [33] (Fig. 2F and Table .1). This Cas12a-CMCAN biosensing platform has shown substantial advantages, including simple aptamer design, reduction in cost for probe synthesis, shortened response time and improved reaction efficiency, and the proven good performance for serum specimens [33]. Furthermore, by using horseradish peroxidase (HRP)-labeling, an enhanced CRISPR/Cas12a-mediated biosensing method has also been developed for the detection of ATP [34]. ATP binding to an aptamer activates the CRISPR/Cas,which triggers a colorimetric readout, enabling the detection of ATP in serum samples sensitively and rapidly (with a limit of detection of 0.2 μM and within 85 min) [34].
Overall, the CRISPR/Cas-based non-nucleic acid systems have remarkable advantages, which may be applied to many different analytes by simply exchanging the aptamer sequences, and a variety of sensors can be employed for environmental monitoring, food inspection, water quality monitoring in the future. The CRISPR/Cas12a-based diagnostic methods can transform the signals of target small molecules into nucleic acid signals, which maintain the sensitivity and specificity of CRISPR/Cas12a and makes it suitable for detecting complex and diverse small molecules. CRISPR/Cas12a-based small molecule diagnostic methods can directly detect small molecules and shorten the response time. In addition, the methods may be carried out in a 96-well plate format, which may achieve the high-throughput screening detection. It may be applied for antibiotic monitoring in the environment, food testing, and molecular detection of clinical compounds in the future. Thus, the CRISPR/Cas12-based small molecule diagnostic methods can effectively expand the applicability of CRISPR/Cas systems for detecting non-nucleic acids, which may widen the application of CRISPR/Cas systems in biomedical analyses and clinical diagnosis.
3.3. CRISPR Cas12a-based diagnostic methods for proteins
As a novel molecular diagnosis system, the CRISPR/Cas12a-based protein diagnostic method converts protein targets to nucleic acid signals that CRISPR/Cas12a can recognize, which can directly detect protein molecules. Compared with the traditional ELISA method, the CRISPR Cas12a-based protein detection platform has a higher diversity of signal reading, such as through electrochemical and fluorescence reading, which can greatly enrich the diversity of the protein detection, and POCT can eliminate the dependence on large instruments and proprietary laboratories, making this CRISPR Cas12a-based protein analysis more flexible and convenient.
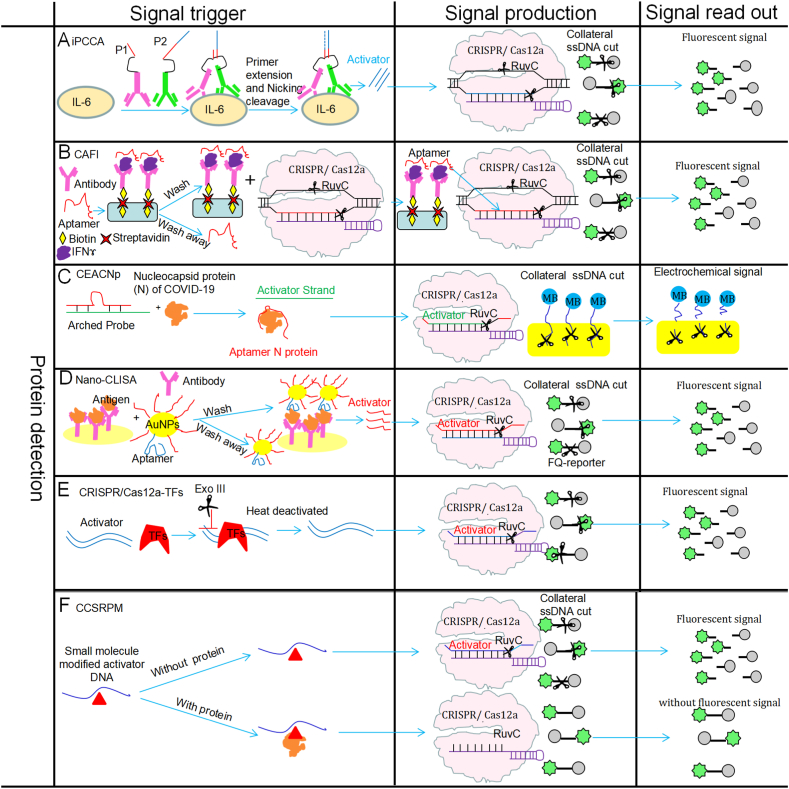
The isothermal proximity CRISPR/Cas12a assay (iPCCA) was developed based on the proximity binding of hybridization probes to the target sequence and the target-induced collateral cleavage activity of Cas12a, and it has successfully been utilized to detect the interleukin-6 (IL-6) with a high sensitivity and specificity [35] (Fig. 3A and Table .1). The iPCCA assay is performed by designing two hybridization probes, P1 and P2, which cannot hybridize without the target protein. The designed probes were linked using an antibody interacting with the target protein, which will enable P1 and P2 probes to hybridize. A polymerase then performs primer extension to form double-stranded DNA (dsDNA) recognizable by CRISPR/Cas12a, which triggers collateral cleavage of the fluorogenic ssDNA reporter, resulting in amplified signals. The iPCCA has a high detection sensitivity (LOD values of 1 fM and 100 fM for target nucleic acids and protein, respectively) [35] (Fig. 3A and Table .1). iPCCA can detect genetic markers, gene expression levels, and proteins, which has a strong potential for broad utilization in molecular diagnostics.
Fig. 3.
CRISPR/Cas12a-based diagnostic methods for proteins.
A: iPCCA [35,63]; B: CAFI [36]; C: CEACNp [37]; D: Nano-CLISA [38]; E: CRISPR/Cas12a-TFs [39]; F: CCSRPM [40].
A novel biosensing system called CRISPR/Cas12a assisted on-fiber immunosensor (CAFI) has been developed based on the antibody-analyte-aptamer sandwich structure [36] (Fig. 3B and Table .1). CAFI was fabricated on a glass fiber with an antifouling polyethylene glycol (PEG) polymer brush and it has been shown to be able to detect multiple small molecules in complex media [36]. The fiber surface is coated with biotinylated capture antibodies to generate the sensing interface for capturing the analyte in a sample, and a single strand DNA aptamer is then applied to detect the analyte while triggering the CRISPR/Cas12a fluorescent detection system for amplifying the analyte signal [36]. The CAFI has shown 1000-fold higher sensitivity compared with ELISA with a LOD for IFNɤ of only 1 fg/mL [36], and it has been applied for IFNɤ detection in complex biological samples such as human serum, whole blood, sweat, and saliva, with outstanding performance shown in diagnostics with untreated biological specimens [36]. The CAFI can also be utilized to detect other analytes via simple modification of the capture antibody and the detection aptamer, making it a suitable technology to measure proteins in low volume complex biological samples.
A CRISPR/Cas12a-based electrochemical aptasensor has recently been proposed for low-cost, rapid, and highly sensitive detection of the SARS-CoV-2 nucleocapsid protein (Np) (CEACNp) [37] (Fig. 3C and Table .1). In this CEACNp detection system, the electrochemical sensing interface on a golden electrode is produced by immobilization of methylene blue-labeled poly adenine DNA sequence (polyA-MB electrochemical reporter). In the presence of SARS-CoV-2 Np, the activator strand will be separated from the arched probe because of specific target-aptamer interactions, which activate CRISPR/Cas12a non-specificity cleavage activity of polyA-MB signal reporters [37] (Fig. 3C and Table .1). The CEACNp system has shown a high performance for SARS-CoV-2 Np detection, with a linear range between 50 pg/mL and 100 ng/mL, and a LOD down to 16.5 pg/mL, within 30 min [37]. The CEACNp system has demonstrated remarkable advantages, and is suitable for POCT and complex samples, e.g., tap water, milk, and serum; additionally, it is a rapid, cost-efficient, simple, portable and sensitive tool that is critical for early detection of SARS-CoV-2 under the pandemic situation. The CEACNp strategy also can be expanded for the detection of multiple aptamer-recognized substances to overcome the limitations of CRISPR/Cas12a-related methods, increasing the flexibility and convenience of the analysis.
A nano-immunosorbent assay based on CRISPR/Cas12a (Nano-CLISA) has been developed by employing DNA-gold nanoparticle (AuNP) nanotechnology to activate trans-cleavage activity of CRISPR/Cas12a, and it has been utilized for the detection of protein biomarkers such as carcinoembryonic antigen (CEA) and prostate specific-antigen (PSA) [38] (Fig. 3D and Table .1). In the Nano-CLISA reaction, when protein analytes are present, the antibody-antigen-AuNP complex is generated, resulting in the release of activator sequence and activation of CRISPR/Cas12a on AuNPs, which induces collateral cleavage activity of ssDNA FQ-reporters and generates significantly enhanced fluorescence [38]. Nano-CLISA can be directly applied for PSA assessment in clinical samples, with a 1000-fold higher sensitivity and 15 times wider detection range than the routine ELISA. For CEA and PSA, LOD values of only 13.9 fg/mL (69.5aM) and 5.6 fg/mL (175aM) could be obtained, respectively [38]. In the future, the Nano-CLISA system may be integrated in an automated and high-throughput setting, developing a fast-screening tool for simultaneous assessment of large amounts of specimens.
Recently, a CRISPR/Cas12a-based biosensor with the assistance of exonuclease protection assay has been developed for the measurement of transcription factors (TFs) (Fig. 3E and Table .1) [39]. In this technique, the binding between a TF and the dsDNA (engineered to contain a TF binding domain) can protect the dsDNA from being degraded by exonuclease III (Exo III), and the preserved dsDNA (the Cas12a activator) then trigger a CRISPR/Cas12a reporting reaction to produce fluorescent signal. In the detection of nuclear factor-kappa B (NF-κB) p50 subunit, a limit of 0.2 pM and a limit of quantification of 0.6 pM were obtained [39] (Fig. 3E and Table .1). This CRISPR/Cas12a-TFs detection system has been found to be highly sensitive, cost-efficient, and portable.
The CRISPR/Cas12a collateral cleavage activity has been utilized in developing a detection system for simple and rapid identification of molecular interactions between proteins and small molecules [40] (Fig. 3F and Table .1). In this system, the single-stranded activator DNA (which is complementary with crRNA in crRNA/Cas12a) is modified with a small molecule. In the absence of the target protein, the collateral cleavage activity of Cas12a is activated for producing reporter probes, resulting in high fluorescence intensity [40] (Fig. 3F and Table .1). If the reaction has a target protein, the latter interacts with the small molecule harbored by activator DNA, which results in steric hindrance, with reduced Cas12a activation and fluorescence intensity. This system has been successfully used to detect two model protein-small molecule interactions, streptavidin/biotin and anti-digoxigenin/digoxigenin, at 0.03 nM and 0.09 nM, respectively and with a fast workflow (within 11 min) [40] (Fig. 3F and Table .1). Thus, by using the collateral cleavage activity of CRISPR/Cas12a, this new approach could potentially identify new protein-small molecule interactions and construct interaction network.
Overall, compared to traditional protein detection methods (such as ELISA), the novel CRISPR/Cas12a-based protein diagnostic methods described above have demonstrated remarkable advantages including speed, cost-effectiveness, simplified operation, small volumes of samples required, and sensitivity. These new protein diagnostic methods have also been shown to be suitable for complex biological samples such as human serum, whole blood, sweat, and saliva. These new techniques also have wide linear detection ranges with potentially good performance for studying analytes with diverse concentrations within large sample sets. Moreover, these techniques can be used for the detection of more analytes by simply modifying the capture antibodies and the detection aptamers. Thus, the CRISPR/Cas12a-based novel protein diagnostic methods will have great potential in detecting proteins and identifying protein/small molecule molecular interactions, which will provide new insights for new knowledge discovery and will be used in various applications including the early diagnosis of infectious diseases, environmental surveillance, and in ensuring food security.
3.4. CRISPR/Cas12a-based detection methods for metal ions
Recently, new CRISPR/Cas12a-based heavy metal diagnostic systems have been developed, with the designed sensors being able to convert heavy metals to nucleic acid signals and maintaining the advantages of high sensitivity and specificity of CRISPR/Cas12a nucleic acid detection. The CRISPR/Cas12a-based heavy metal diagnostic systems can thus extend the application of CRISPR/Cas12a-based bioassays to ensure food safety and water quality and to monitor environmental heavy metal pollution.
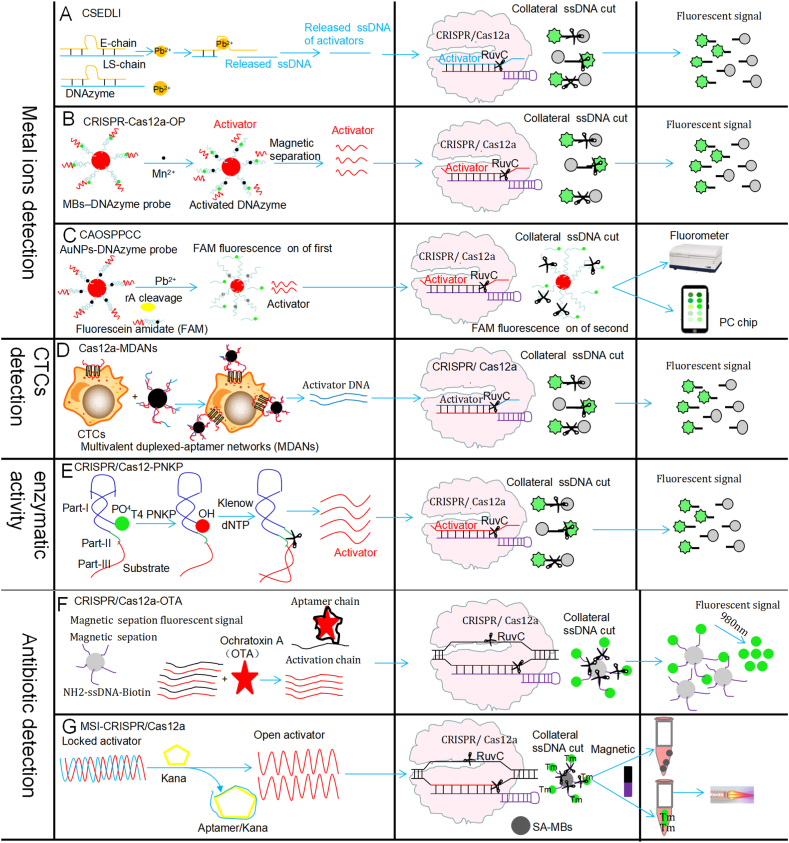
Environmental heavy metal lead buildup causes pollution of ecosystems and further threatens health in humans. Recently, a highly sensitive lead detection method has been developed by combining the CRISPR/Cas system (as a signal enhancer) and DNAzyme (for lead ion detection) [41] (Fig. 4A and Table .1). After DNAzyme recognizes the lead ion, its substrate chain is cleaved producing ssDNA sequences, which are the CRISPR/Cas12 activators. Recognition of the ssDNA sequences by the CRISPR/Cas12 system activates the collateral cleavage effects of the CRISPR system, producing ssDNA reporters with high fluorescent signals. This CRISPR/Cas12a (CRISPR/Cas14a)-based lead detection system may detect lead ions even at 0.48 nM, with a 5-fold increase in sensitivity compared to that of the DNAzyme method (2.4 nM) [41]. In addition, this method does not require bulky equipment which can promote its on-site lead ion detection [41]. This CRISPR/Cas12a-based system has been shown to be able to detect lead ions markedly below the limit of lead ions in drinking water proposed of Environmental Protection Agency.
Fig. 4.
CRISPR/Cas12a-based diagnostic methods.
A: CSEDLI: CRISPR/Cas12a-based metal ion detection [41]; CRISPR-Cas12a-OP: CRISPR/Cas12a-based metal ion detection [42]; C: CAOSPPCC: CRISPR/Cas12a-based metal ion detection [43]; D: Cas12a-MDANs: CRISPR/Cas12a-based CTC detection [44]; E: CRISPR/Cas12-PNKP: CRISPR/Cas12a-based enzymatic activity detection [45]; F: CRISPR/Cas12a-OTA of CRISPR/Cas12a-based antibiotic detection [48]; G: MSI-CRISPR/Cas12a of CRISPR/Cas12a-based antibiotic detection [49].
By combining the Au nanoparticle-DNAzyme probe (for specifically identifying Pb2+) and the CRISPR/Cas12a (for signal amplification), a highly sensitive and specific system has been recently developed for on-site detection of Pb2+ [43] (Fig. 4C and Table .1). The CAOSPPCC system is a new Pb2+ fluorescent sensor with AuNPs-DNAzyme fluorescence probes combined with CRISPR/Cas12a. In this system, a spherical nucleic acid (SNA) probe (AuNPs-DNAzyme probe), obtained by efficient conjugation of Pb2+ dependent DNAzyme with AuNPs, produces first-order fluorescent signals when Pb2+ ions are present, releasing opened activators for CRISPR/Cas12a activation. CRISPR/Cas12a activation results in fluorescent signal amplification through collateral cleavage with the AuNPs probe (signal probe). The fluorescent solutions can be assessed with a fluorometer or on-site with a PC chip or portable UV lamp, with a high sensitivity [43] (Fig. 4C and Table .1). This modified Pb2+ fluorescence sensor has been shown highly sensitive, specific and accessible, with low sample consumption and a LOD as low as 86 fM, and it can be used for accurate detection of Pb2+ in complex biological or environmental specimens.
3.5. CRISPR/Cas12a-based detection methods for circulating tumor cells (CTCs)
CTCs are considered a cancer biomarker in mini-invasive liquid biopsies. They can be rapidly obtained and have high sensitivity. Recently, a CRISPR/Cas12a/based CTC rapid and sensitive method regulated by multivalent duplexed-aptamer networks (Cas12a-MDANs) was developed for detection of CTCs [44] (Fig. 4D and Table .1). Cas12a-MDANs are produced on surface magnetic beads containing many duplexed-aptamer units, which allow structure switching after CTC-binding. If the detection system has target CTCs, free “activator DNA” is released from MDANs for CRISPR/Cas12a activation, which causes signal amplification. With CCRF-CEM cells as a proof of concept, CRISPR/Cas12a-MDANs may achieve simple and rapid CTC detection with a LOD of 26 cells/mL for human blood specimens and the steps can be performed in less than 60 min [44]. CRISPR/Cas12a-MDANs has provided new ideas for CTC detection, as a fast, convenient, and ultrasensitive tool applying CRISPR-Cas12a [44]. CRISPR/Cas12a-MDANs could directly detect CTCs in human blood samples, which can afford a simple, fast, rapid, convenient, and ultrasensitive CTC detection workflow for cancer diagnosis. CRISPR/Cas12a-MDANs exhibit excellent capture ability of cancer cells and, and more duplexed aptamer structures for different cancer cells are being developed and will be available in the future.
3.6. CRISPR/Cas12a-based diagnostic method for enzymatic activities
A novel CRISPR/Cas12a-based biosensing platform (CRISPR/Cas12a-PNKP detection system) has been developed that detects activity of polynucleotide kinase/phosphatase (PNKP) (a DNA damage repair-related enzyme) with a high sensitivity and within a short time [45] (Fig. 4E and Table .1).. In this system, PNKP-triggered nicking enzyme-mediated strand displacement amplification reaction enabled the enrichment of the activator DNA strands for CRISPR/Cas, and the subsequent activated CRISPR/Cas-catalyzed cleavage of short-stranded DNA fluorescent probes allows sensitive detection of PNKP activity. CRISPR/Cas12a-PNKP has been found a fast tool for detecting PNKP activity with a high sensitivity and specificity. It has a LOD of as low as 3.3 × 10−6 U/mL, with a linear detection range spanning from 1 × 10−5 to 2.5 × 10−2 U/mL, within 80 min. Similarly, the CRISPR/Cas12a system-based methods have also been developed to sensitively detect other enzyme activities including a telomerase [46] and a protease [47]. These CRISPR/Cas12a-based enzymatic activity detection systems can have great potential for screening enzyme inhibitors and evaluating inhibitory ability, which would further promote application of CRISPR/Cas12a-based systems in biochemical research, drug development, and clinical diagnosis.
3.7. CRISPR/Cas12a-based method for antibiotic detection
Using an upconversion-magnetic probe-DNA-Fe3O4 probe designed to replace traditional fluorescent probes, a rapid system for ochratoxin (OTA) detection was developed based on the magnetic separation fluorescent signal in combination with the CRISPR/Cas12a system (CRISPR/Cas12a-OTA) [48] (Fig. 4F and Table .1). An OTA aptamer sequence that is complementary to CRISPR/Cas12a′s recognition target sequence was constructed. When the reaction system contains OTA interacting with the aptamer sequence, the aptamer complementary sequence is released and interacts with CRISPR/Cas12a, inducing the collateral cut activities of the DNA sequence of fluorescein and magnetic bead streptavidin labeled signal strand DNA (ssDNA), resulting in fluorescein signal production [48]. This detection sensor has been applied to detect OTA in food which can be achieved in about 60 min (Fig. 4F and Table .1). This system has exhibited relatively a high sensitivity and stability and could be applied in POCT.
Abuse of antibiotics in modern life and aquaculture has become a problem, and the metal stable isotope detection-based CRISPR/Cas12a bioassay (MSI-CRISPR/Cas12a) for rapidly detecting antibiotic kanamycin was developed [49] (Fig. 4G and Table .1). By utilizing CRISPR/Cas12a biosensing and metal isotope detection, the MSI-CRISPR/Cas12a system is ultrasensitive, very selective and fast in assessing antibiotic bioaccumulation in wild fish specimens, with a LOD of 4.06 pM, within 30 min [49] (Fig. 4G and Table .1). Detection of antibiotic residues has been a focus of attention recently, and the fast and sensitive CRISPR/Cas12a-based method that allows high-throughput screening for antibiotic detection has greatly expanded the antibiotic detection technology.
4. The CRISPR/Cas13a based molecular diagnostic methods for non-nucleic acids
4.1. CRISPR/Cas13a-based diagnostic method for detecting enzymatic activity
By employing triple signal amplification (alkaline phosphatase (ALP) self-catalysis, T7 promoter transcription amplification, and trans-cleavage activity of fluorescein labeled ssRNA of CRISPR/Cas13a), the super sensitive method by target-induced transcription amplification to trigger the trans-cleavage activity of Cas13a (TITAC-Cas) for ALP detection has been developed (Fig. 5A and Table .1) [50]. The TITAC-Cas assay has been found highly sensitive (LOD of 6 ± 0.52 mU∙L−1) with a linear range of 0.008–250 U L−1. The TITAC-Cas system can also be employed to screen enzyme inhibitors and estimate inhibitory efficiency, providing a promising alternative to disease diagnosis or drug screening. In addition, by designing different transcription-initiating mechanisms and changing the spacer sequence of gRNA, the TITAC-Cas system assay can broaden the application of CRSIPR/Cas13a for the measurement of other non-nucleic acid targets.
Fig. 5.
CRISPR/Cas13a-based diagnostic methods for non-nucleic acids.
A: TITAC-Cas for CRISPR/Cas13a-based ALP activity detection system [50]; B: APC-Cas for CRISPR/Cas13-established pathogenic microorganism detection system [51]; C: CLISA for CRISPR/Cas13a-based signal amplification linked immunosorbent protein detection system [52]; D: SPRINT for CRISPR/Cas13a-based small molecule detection system [53].
4.2. CRISPR/Cas13a-based diagnostic method for pathogenic microorganisms
The ability to detect low numbers of pathogenic microbial cells in food and clinical samples is important but remains a significant challenge. Recently, a simple, fast and sensitive method (APC-Cas) utilizing the CRISPR/Cas13a system and the nucleic acid-based allosteric probes (APs) was developed and found to be able to detect salmonella sensitively and fast (Fig. 5B and Table .1) [51]. In the first step, specific APs are designed which can bind pathogenic microorganisms and cause conformational changes, resulting in dsDNA being synthesized with DNA polymerase. In the following step, the T7 promoter of AP is transcribed to ssRNA that is recognized by CRISPR/Cas13a, which has specificity for the cleavage activity of ssRNA and collateral cut activity of fluorescein labeled ssRNA, which produces fluorescent signals [51]. At present, the APC-Cas diagnostic method was found suitable for quantitative salmonella detection in milk, with a detection range of 1∼105 CFU in various types of specimens, including milk, showing comparable or superior sensitivity [51] (Fig. 5B and Table .1). In addition, the APC-Cas diagnostic method requires no bacterial isolation and nucleic acid purification; and it is of low cost ($0.86/test), is fast (140 min) and requires a minimal reaction volume (2.5 μL) [51]. Thus, the APC-Cas system can be utilized for food testing and early diagnosis of pathogenic infections selectively and sensitively in various types of samples such as milk and blood.
4.3. CRISPR/Cas13a-based diagnostic methods for proteins
The current technique enzyme-linked immunosorbent assay (ELISA) commonly used in analytical and clinical investigations still has the sensitivity limitation for ultralow concentrations of biomarkers. A new CRISPR/Cas13a signal amplification-linked immunosorbent assay (CLISA) was developed and, compared to the conventional ELISA, was at least 102-fold more sensitive for protein detection [52] (Fig. 5C and Table .1). The target protein is captured on a solid support through antibody-antigen interactions [52]. This high sensitivity of this new technique was due to the double amplification of the output signal due to the T7 RNA polymerase transcription and the CRISPR/Cas13a collateral cleavage activity. CLISA was found to be highly sensitive in detecting inflammatory factors, e.g., human interleukin-6 (IL-6) and angiogenesis marker vascular endothelial growth factor (VEGF), with LOD values of 45.81 fg/mL (2.29 fM, 264-fold versus ELISA) and 32.27 fg/mL (0.81 fM, 617-fold versus ELISA), respectively [52] (Fig. 5C and Table .1). Thus, CLISA is an isothermal and highly sensitive method (at least 102-fold more sensitive than ELISA) for detecting low-abundance proteins based on CRISPR/Cas13a, which was effectively improved by the amplification of T7 transcription and the collateral cleavage activity of CRISPR/Cas13a. Moreover, CLISA is compatible with the automated and high-throughput format that allows for rapid screening of large numbers of samples simultaneously, providing potential ultrasensitive detection methods for molecular diagnostics.
4.4. The new CRISPR/cas13a-based diagnostic method for small molecules
The SHERLOCK-based profiling of in vitro transcription (SPRINT) tool, a CRISPR/Cas13a-based method, was developed for the sensitive, inexpensive and raid detection of low-molecular weight compounds [53] (Fig. 5D, and Table-1). SPRINT involves a transcribed RNA sequence that triggers the CRISPR/Cas13a non-specific RNase activity which enables cleavage of an RNA oligonucleotide and generation of fluorogenic output signal [53]. The SPRINT diagnostic method utilizes two transcription principles for developing detection techniques for small molecules [53] (Fig. 5D, and Table-1). The SPRINT detection system has been utilized to quantify eight distinct molecule types (including cofactors, nucleotides, amino acid metabolites, tetracycline and monatomic ions in various specimens) in the POCT setting. Thus, SPRINT is a sensitive, flexible, rapid and simple method for detecting small molecules in the field or at point-of-care situations [53].
5. CRISPR/Cas14a-based diagnostic method for creative kinase
The myocardial infarction biomarker creatine kinase isoenzyme (CK-MB) is highly specific for myocardial injury and constitutes a diagnostic marker of acute myocardial infarction. Chen et al. [54] introduced CRISPR/Cas14a to activate DNA hydrogel for ultrasensitive detection of CK-MB (CRISPR/Cas14a-CK-MB). In this method, the designed CRISPR/Cas14a system can be activated by introducing complementary DNA derived from competitive dissociation and exponential amplification, which is positively correlated with CK-MB concentration. Then the activated CRISPR/Cas14a can be utilized to indiscriminately cleave the DNA hydrogel cross-linker strand, leading to the degradation of the gel matrix and thus releasing pre-encapsulated platinum nanoparticles (PtNPs/Cu-TCPP(Fe)) that can trigger the TMB reaction and allow absorbance reading. This CRISPR/Cas14a-CK-MB system, combining CRISPR/Cas14a with DNA hydrogel, is ultrasensitive, versatile, stable and specific, with a limit of detection of 0.355 pM (15.62 pg/mL) and a detection time within 100 min. Since it can be combined with POCT and may be applied in high-throughput assays [54], it has shown a great application prospect for improved clinical diagnosis of myocardial infarction.
6. CRISPR/cas-based molecular diagnostic methods for non-nucleic acids
6.1. Specificity and sensitivity
The CRISPR/Cas-based non-nucleic acid molecular diagnostic methods, which depend on CRISPR/Cas, can identify specific nucleic acids. They convert non-nucleic acid signals to nucleic acid information, and thus the CRISPR/Cas non-nucleic acid systems keep the high specificity and sensitivity features of CRISPR/Cas nucleic acid detection. For example, the CRISPR/Cas12a-EVs platform has high sensitivity in detecting tumor-derived extracellular vesicles (TEVs) even at 100 particles/mL in clinical serum samples, with an AUC of 0.934, a sensitivity of 84.1 % and a specificity of 85.0 % [23]. The “aptamer-locker” DNA coupling with CRISPR/Cas12a-guided biosensing approach has a LOD of 38 nM, which is below the threshold for the routine method for melamine detection (1.0 mg/kg) [31]. The Cas12a-crRNA-mediated catalytic network (Cas12a-CMCAN) system achieved a LOD of 0.16 μM for ATP [33]. The isothermal proximity CRISPR/Cas12a assay (iPCCA) could detect target nucleic acids and proteins at 1 fM and 100 fM, respectively [35]. CRISPR/Cas12a assisted on-fiber immunosensor (CAFI) shows a 1000-fold increase in sensitivity versus ELISA, with a LOD for IFNɤ of 1 fg/mL [36]. The CRISPR/Cas12a-based electrochemical aptasensor has recently been proposed for low-cost, rapid, and highly sensitive detection of the SARS-CoV-2 nucleocapsid protein (Np) (CEACNp) system detected SARS-CoV-2 Np at 50–100 ng/mL, with a LOD 16.5 pg/mL [37]. The CRISPR/Cas12a-TFs detection system was utilized for the detection of NF-kB p50, with a LOD of 0.2 pM and a limit of quantification of 0.6 pM [39]. Taken together, the CRISPR/Cas-based non-nucleic acid molecular diagnosis methods have higher sensitivity and specificity than the conventional detection methods, with low costs, rapid process and good portability. They will have a great potential in molecular diagnosis technology, which will improve molecular diagnostic ability for health/diseases, food safety, and environmental monitoring.
6.2. Target types
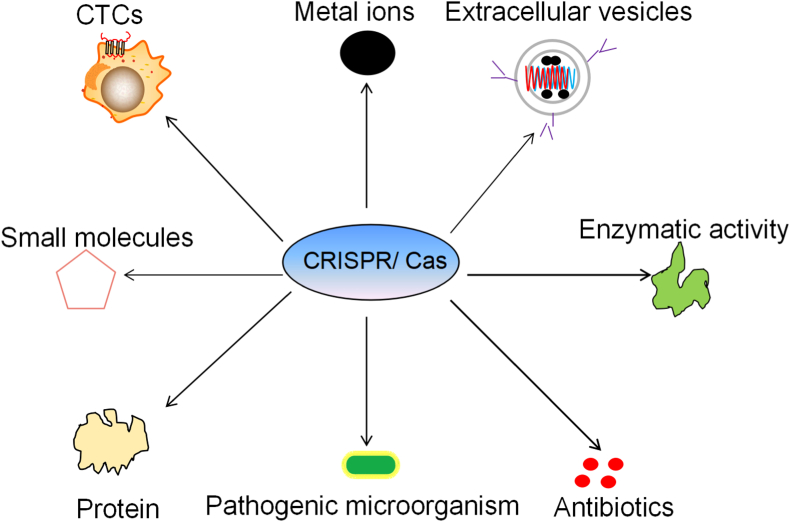
CRISPR/Cas-based diagnostic methods are suitable for complex samples, and they have been mainly utilized in nucleic acid and non-nucleic acid targets. Examined nucleic acid targets include DNA, RNA, SNP, miRNA, ctDNA and cfDNA [ [12,[55], [56], [57]]]. However, non-nucleic acid targets are more complex, and CRISPR/Cas-based non-nucleic acid detection methods have mainly been used for metal ions, extracellular vesicles, cells, small molecules, enzymatic activity, proteins, pathogenic microorganisms and antibiotics (Fig. 6), with examined examples including extracellular vesicles such as EVs [23] and Exo [27]; small molecules such as uric acid [27], ATP [33], melamine [31], p-hydroxybenzoic acid and structural analogues [28]; antibiotics such as KAN [49], ochratoxin [48], and tetracycline [29]; proteins such as IFNɤ [36], NF-kB-p50 [39], SARS-CoV-2 N protein [37], IL-6 [35], and VEGF [52]; enzymatic activity (PNK, NAD+ [32], and ALP [50]); microorganisms (salmonella) [51]; metal ions such as Pb2+ [43]; and cells (CTS). As mentioned above, the RISPR/Cas-based non-nucleic acid diagnostic systems can be used to detect targets from large protein molecules to metal ions. While currently CRISPR/Cas-based non-nucleic acid detection only involves one non-nucleic acid target in a test [35,52], new CRISPR/Cas-based non-nucleic acid diagnostic methods are presently being developed for the detection of complex targets.
Fig. 6.
Target types assessed by CRISPR/Cas-based diagnostic methods for non-nucleic acids. Detection targets include metal ions, extracellular vesicles, circulating tumor cells (CTCs), small molecules, enzymatic activity, proteins, pathogenic microorganisms and antibiotics.
6.3. CRISPR/cas-based detection strategies for non-nucleic acids
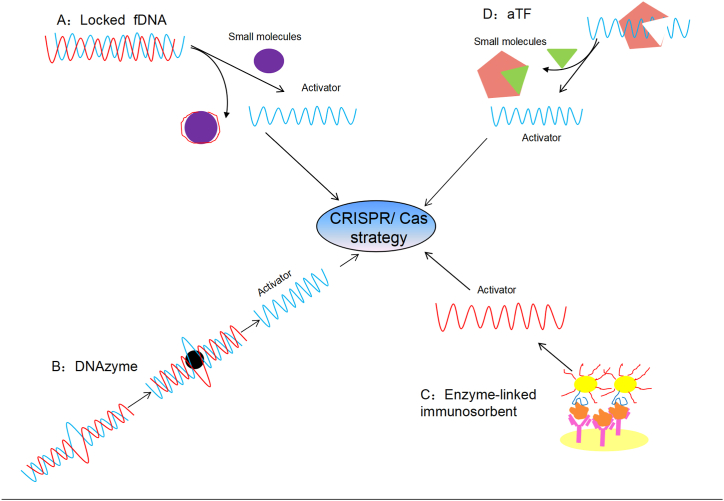
Increasing evidence has shown that CRISPR/Cas systems could be utilized for non-nucleic acid molecular diagnostics, forming a novel generation of diagnostic technology. So far several strategies have been found being used for the development of CRISPR/Cas-based methods for non-nucleic acid molecular diagnosis, including using fDNA aptamers [32], DNAzyme [45], enzyme-linked immunosorbent assay [52] and allosteric regulation [28] (Fig. 7).
Fig. 7.
CRISPR/Cas-based diagnostic method strategies for non-nucleic acids.
A: Locked functional DNA (fDNA) with a small molecule, with one strand binding to it. B: The initially inactive DNAzyme activates its cleavage ability. C: Left: Non-nucleic acid as antigen, interacting with the CRISPR/Cas system and the antibody. D: Immobilized allosteric transcription factors (aTFs)-dsDNA complexes containing the CRISPR target.
The fDNA aptamer-based CRISPR/Cas non-nucleic acid molecular diagnostic system utilizes a DNA sequence of an aptamer with complementarity to gRNA. The target molecule competitively interacts with its aptamer, inducing DNA release and enhancing CRISPR/Cas12a cleavage ability, which ultimately results in production of the detection signal [48,58]. fDNA aptamers may help develop a series of diagnostic systems that can detect ATP and Na+ rapidly, at ambient temperature (25 °C), in less than 15 min with suitability for on-site diagnosis or POCT [30]. fDNA probes may also be used in combination with electrochemical strategies for a higher accuracy and sensitivity [37,49]. A small-molecule modification approach has been used to produce fDNAs which are utilized to detect target proteins, protein-small molecule interactions [40], as well as small molecules including ochratoxin A, KAN residues, SARS-CoV-2 nucleocapsid protein, ATP and Na+. The DNAzyme-based CRISPR/Cas non-nucleic acid molecular diagnostic system can be used to detect metal ions such as lead ions [41]. Another team developed the OP pesticides diagnostic method, featuring a dual enzyme assay in combination with Cas12a for the detection of OP [42]. Enzyme-linked immunosorbent-based CRISPR/Cas non-nucleic acid molecular diagnostic systems have been developed. A biosensor CRISPR/Cas13a signal amplification-linked immunosorbent assay termed CLISA was developed to enhance ELISA sensitivity applying CRISPR/Cas13a. In addition, Nano-CLISA was designed for the enhancement of Cas12a′s collateral cleavage ability [36]. The enzyme-linked immunosorbent-based non-nucleic acid diagnostic methods have been found to be able to detect several targets, including inflammatory markers such as IL-6 and IFN-γ as well as tumor markers such as VEGF, CEA, and prostate specific-antigen (PSA) [38]. Based on allosteric regulation, CRISPR/Cas non-nucleic acid diagnostic systems have been designed, which relies on the allosteric transcription factors (aTFs) to turn upon CRISPR/Cas cleavage signal production. The aTF was employed to design a small-molecule detection system termed CaT-SMelor [28]. The system called SPRINT utilizes aTF as a switch for RNA polymerase-mediated transcription, activating in vitro RNA polymerase transcription in presence of a target molecule, resulting in massive production of RNAs that activate Cas13a [53]. An integrated biosensor involving an exonuclease protection and CRISPR/Cas12a has been developed for the turn-on detection of TFs in cancer cells [39]. Thus, the aTF-based CRISPR/Cas non-nucleic acid diagnostic methods are novel tools, which can be used for detecting multiple targets, including small molecules, CTCs and PNKP.
6.4. Signal readout
CRISPR/Cas-based non-nucleic acid molecular diagnostic methods rely on readout signals for detection, including fluorescent signals and electrochemical signals. Detection processes of CRISPR/Cas12a/exosome [23], CRISPR/Cas12a-EV [27], CaT-SMelor [28], and CAFI [36] depend on fluorescent signals. The Cas12a-based cell-free small-molecule biosensor systems that detect different tetracyclines also use fluorescence-based visual readout [29]. The CAOSPPCC system uses fluorescence for the detection of Pb2+ [43]. On the other hand, the CEACNp system relies on the electrochemical aptasensor [37]. Thus, while the CRISPR/Cas-based non-nucleic acid molecular diagnostic methods can transform non-nucleic acid signals to nucleic acid signals, the CRISPR/Cas system can amplify the target's signals and converting them into easily detectable signals.
6.5. POCT capability
The CRISPR/Cas-based non-nucleic acid molecular diagnostic methods generally have a simple operation process, short detection time, and the non-requirement for complex instruments, which have improved detection flexibility, suitability for complex biological samples, and application potential in the POCT setting. For example, CRISPR/Cas12a/exosome and CRISPR/Cas12a-EV directly detect targets in low-volume samples, making them suitable for clinical use, including in the POCT setting [23,27]. The CaT-SMelor and cell-free biosystems provide simple and easy visual readouts with mobile phone applications and are also suitable for POCT [28,29]. The fDNA-regulated CRISPR-Cas method has a simple and rapid detection process (performed in <15 min), indicating its suitability for field work or POCT diagnostics [30]. The “aptamer-locker” DNA with CRISPR/Cas12a-based method could detect melamine in pretreated or non-pretreated whole milk specimens in less than 20 min and is also suitable for POCT diagnostics [31]. Similarly, the CAFI system is suitable for complex biological samples and may be applied in POCT [36]. Thus, the CRISPR/Cas-based non-nucleic acid molecular diagnostic methods have unique advantages, including short detection time and suitability for complex biological samples, and having a great potential for POCT use.
6.6. Sample types
CRISPR/Cas-based non-nucleic acid molecular diagnostic methods are suitable for complex biological samples. For example, CRISPR/Cas12a/exosome [23] and CRISPR/Cas12a-EV [27] were used for clinical samples. CaT-SMelor can directly measure uric acid concentrations in clinical blood specimens [28]. The functional DNA (fDNA)-regulated CRISPR-Cas method can be applied for metal ions, low-molecular weight organic molecules, proteins, and even viruses and cells, in environmental samples, clinical blood specimens [30]. The "aptamer-locker" DNA/CRISPR/Cas12a-guided biosensing was sensitive and fast for melamine analysis in infant milk products as well as whole milk samples [31]. Cas12a-CMCAN was utilized for ATP quantitation in diluted serum specimens [33]. The CAFI system detected IFNɤ in clinical serum, whole blood, sweat, and saliva specimens [36]. Therefore, the CRISPR/Cas-based non-nucleic acid molecular diagnostic methods are suitable for complex biological samples and even clinical samples such as blood samples, viruses, cells, food samples and environment samples.
7. Advantages and challenges of CRISPR/cas-based molecular diagnosis for non-nucleic acids
Recently, CRISPR/Cas-based molecular diagnosis methods have achieved great advances. Based on CRISPR/Cas protein modular, programmable, specific target recognition and collateral cleavage activity features, these new methods have realized a precise and sensitive detection of targets at ultra-low concentrations. CRISPR/Cas molecular diagnosis methods have shown better accuracy, adaptability, portability, timeliness, and effectiveness than the traditional methods. Apart from its major usage to detect nucleic acids, the CRISPR/Cas system has also been used for detecting non-nucleic acids.
As a novel technology for molecular diagnosis for detecting non-nucleic acids, the CRISPR/Cas system has several advantages. First, it has flexible target recognition/detection ability. At present, combined with specific aptamers, CRISPR/Cas systems have been successfully employed for the detection of non-nucleic acids of a wide range of target molecules, such as CTCs, metal ions, pathogenic microorganisms, small molecules, and melamine. Secondly, it is adaptable to complex and diverse samples, including human blood, milk, other body fluid samples, food, and even environmental samples. Thirdly, it has reduced detection time compared with traditional techniques, with a detection duration range of 11 min–120 min (mostly within 60 min). For instance, the CCSRPM system was used successfully for the detection of proteins/small molecules within 11 min [40]. Fourthly, non-nucleic acid molecular diagnostic methods have largely improved LOD detection and a wider linear detection range that can be better applied for studying analytes. Overall, the CRISPR/Cas-based molecular diagnostic methods for non-nucleic acids are rapid, highly sensitive, low-cost, portable, operationally simple, and can be applied in the POCT setting without the need for expensive equipment and professional experimental environment. These advantages have greatly facilitated the development and progress of molecular diagnostic methods for detecting non-nucleic acids, which will address the shortcomings of traditional molecular diagnostic methods.
As a novel technology for molecular diagnosis for detecting non-nucleic acids, the CRISPR/Cas system also has many challenges, which should be addressed. First, CRISPR/Cas non-nucleic acid detection processes face commercialization and clinical application difficulties. The critical puzzle is how to efficiently convert a non-nucleic acid signal into a nucleic acid signal that is identifiable by a Cas. Currently, the most reported systems depend on antibodies and aptamers; however, the conversion processes with them suffer from several limitations, including the multi-step detection process, ease of contamination, and requirement of specialized technicians, which can reduce reliability in real time detection and sensitivity not fully meeting the needs of clinical requirements. To solve issues associated with clinical application for CRISPR-based detection of non-nucleic acids, combining CRISPR/Cas systems with other emerging techniques and bio-information design aptamers and gRNAs is encouraged to improve sensitivity and specificity of sensing systems.
Secondly, some Cas proteins of CRISPR/Cas systems may have off-target effects, with gRNA-guided CRISPR/Cas recognition targeting the sequences (DNA/RNA) of non-targets [2]. Furthermore, the gRNA-guided CRISPR/Cas recognition targeting sequence may generate mismatches, leading to off-target cleavage, and induce collateral cleavage, causing false positives. Thus, high-precision CRISPR/Cas proteins are needed, which may improve target detection and lower off-target effects. Thirdly, the gRNA, aptamers and ssDNA/ssRNA signal reporters may have risks of degradation under reaction conditions, and adding RNase inhibitors may inhibit unwanted degradation. Fourthly, most CRISPR/Cas-based non-nucleic-acid molecular diagnostic methods are not applicable for POCT detection, and to accelerate POCT application would require optimal design strategies. Fifthly, most CRISPR/Cas-based non-nucleic-acid molecular diagnostic methods currently cannot achieve high-throughput multiple detection. The simultaneous detection of multiple non-nucleic acid targets is an ideal method, which can be implemented through different approaches such as chips, channels and micro-droplets [59]. This point needs further investigation. Sixthly, currently, CRISPR/Cas-based non-nucleic acid detections are not standardized and commercial kits for clinical application are unavailable. However, this problem may soon be resolved with the new development of the CRISPR/Cas technology in the future. The development of interdisciplinary and emergence of new technologies would further the development of CRISPR/Cas-based non-nucleic acid detection. Combining CRISPR/Cas with artificial intelligence (AI) could be highly useful in the process of CRISPR/Cas-based non-nucleic acid detection, which may be used for rational design and directed evolution of Cas proteins to overcome off-targets. CRISPR/Cas-based non-nucleic acid molecular diagnostic methods should integrate the current AI system to improve detection efficiency and achieve visualization and POCT.
In general, with no doubt, CRISPR/Cas-based non-nucleic acid molecular diagnostic methods may be improved for clinical diagnosis, especially in epidemic prevention and control, biosafety monitoring, food safety analysis and clinical disease diagnosis, which will make a substantial contribution to human health.
Data availability statement
There is no additional data available for this review article.
CRediT authorship contribution statement
Jian Zhou: Writing – original draft, Writing – review & editing. Xue-mei Ren: Writing – original draft. Xin Wang: Writing – original draft, Writing – review & editing. Zhuo Li: Writing – original draft, Writing – review & editing. Cory J Xian: Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Jian Zhou, Xue-mei Ren, Xin Wang, Zhuo Li, Cory J Xian reports was provided by NO. Jian Zhou, Xue-mei Ren, Xin Wang, Zhuo Li reports a relationship with Xi'an Medical University that includes: consulting or advisory, employment. Cory J Xian reports a relationship with University of South Australia that includes: consulting or advisory, employment. NO has patent NO pending to NO. No If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
This work was supported by Health Research Fund of Shaanxi Province (2022B009), Xi'an Science and Technology Bureau Project (Medical Research Project of Xi'an Innovation Capability Foundation project) (22YXYJ0126), Science and Technology Innovation Team Project of Xi'an Medical University (2021TD14).
Contributor Information
Zhuo Li, Email: lizhuo721@163.com.
Cory J Xian, Email: cory.xian@unisa.edu.au.
References
- 1.Tang Y., et al. The CRISPR-Cas toolbox for analytical and diagnostic assay development. Chem. Soc. Rev. 2021;50:11844–11869. doi: 10.1039/d1cs00098e. [DOI] [PubMed] [Google Scholar]
- 2.Priya Swetha P.D., et al. Towards CRISPR powered electrochemical sensing for smart diagnostics. Curr. Opin. Electrochem. 2021;30 doi: 10.1016/j.coelec.2021.100829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marraffini L.A. CRISPR-Cas immunity in prokaryotes. Nature. 2015;526:55–61. doi: 10.1038/nature15386. [DOI] [PubMed] [Google Scholar]
- 4.Wiedenheft B., et al. RNA-guided human genome engineering via Cas9. Nature. 2012;482:331–338. [Google Scholar]
- 5.Taheri-Ghahfarokhi A., et al. Decoding non-random mutational signatures at Cas9 targeted sites. Nucleic Acids Res. 2018;46:8417–8434. doi: 10.1093/nar/gky653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fonfara I., et al. The CRISPR-associated DNA-cleaving enzyme Cpf1 also processes precursor CRISPR RNA. Nature. 2016;532:517–521. doi: 10.1038/nature17945. [DOI] [PubMed] [Google Scholar]
- 7.Creutzburg S.C.A., et al. Good guide, bad guide: spacer sequence-dependent cleavage efficiency of Cas12a. Nucleic Acids Res. 2020;48:3228–3243. doi: 10.1093/nar/gkz1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O.O. Abudayyeh, et al., C2c2 is a single-component programmable RNA-guided RNA-targeting CRISPR effector, Science, 353 aaf5573. [DOI] [PMC free article] [PubMed]
- 9.Makarova K.S., et al. Evolutionary classification of CRISPR-Cas systems: a burst of class 2 and derived variants. Nat. Rev. Microbiol. 2020;18:67–83. doi: 10.1038/s41579-019-0299-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J., et al. The rapidly advancing Class 2 CRISPR-Cas technologies: a customizable toolbox for molecular manipulations. J. Cell Mol. Med. 2020;24:3256–3270. doi: 10.1111/jcmm.15039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu W.Y., et al. Genome editing by natural and engineered CRISPR-associated nucleases. Nat. Chem. Biol. 2018;14:642–651. doi: 10.1038/s41589-018-0080-x. [DOI] [PubMed] [Google Scholar]
- 12.Li L., et al. CRISPR-Cas-mediated diagnostics. Trends Biotechnol. 2022;40:1326–1345. doi: 10.1016/j.tibtech.2022.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Gootenberg Js A.O., et al. Nucleic acid detection with CRISPR-Cas13a/C2c2. Science. 2017;356:438–442. doi: 10.1126/science.aam9321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broughton Jp D.X., et al. CRISPR–Cas12-based detection of SARS-CoV-2. Nat. Biotechnol. 2020;38:870–874. doi: 10.1038/s41587-020-0513-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu T.Y., et al. Accelerated RNA detection using tandem CRISPR nucleases. Nat. Chem. Biol. 2021;17:982–988. doi: 10.1038/s41589-021-00842-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burstein D., et al. New CRISPR-Cas systems from uncultivated microbes. Nature. 2017;542:237–241. doi: 10.1038/nature21059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Makarova K.S., et al. An updated evolutionary classification of CRISPR-Cas systems. Nat. Rev. Microbiol. 2015;13:722–736. doi: 10.1038/nrmicro3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abudayyeh O.O., et al. RNA targeting with CRISPR-Cas13. Nature. 2017;550:280–284. doi: 10.1038/nature24049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.East-Seletsky A., et al. Two distinct RNase activities of CRISPR-C2c2 enable guide-RNA processing and RNA detection. Nature. 2016;538:270–273. doi: 10.1038/nature19802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrington L.B., et al. Programmed DNA destruction by miniature CRISPR-Cas14 enzymes. Science. 2018;362:839–842. doi: 10.1126/science.aav4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y., et al. Efficient genome editing by a miniature crispr-ascas12f1 nuclease in bacillus anthracis. Front. Bioeng. Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.825493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang R., et al. Emerging prospects of extracellular vesicles for brain disease theranostics. J. Contr. Release. 2022;341:844–868. doi: 10.1016/j.jconrel.2021.12.024. [DOI] [PubMed] [Google Scholar]
- 23.Li H., et al. Aptamer-based CRISPR/Cas12a assay for the ultrasensitive detection of extracellular vesicle proteins. Talanta. 2021;221 doi: 10.1016/j.talanta.2020.121670. [DOI] [PubMed] [Google Scholar]
- 24.Xing S., et al. An ultrasensitive hybridization chain reaction-amplified CRISPR-Cas12a aptasensor for extracellular vesicle surface protein quantification. Theranostics. 2020;10:10262–10273. doi: 10.7150/thno.49047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalluri R. The biology and function of exosomes in cancer. J. Clin. Invest. 2016;126:1208–1215. doi: 10.1172/JCI81135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding L., et al. Universal DNAzyme walkers-triggered CRISPR-Cas12a/Cas13a bioassay for the synchronous detection of two exosomal proteins and its application in intelligent diagnosis of cancer. Biosens. Bioelectron. 2023;219 doi: 10.1016/j.bios.2022.114827. [DOI] [PubMed] [Google Scholar]
- 27.Zhao X., et al. Rapid and sensitive exosome detection with CRISPR/Cas12a. Anal. Bioanal. Chem. 2020;412:601–609. doi: 10.1007/s00216-019-02211-4. [DOI] [PubMed] [Google Scholar]
- 28.Liang M., et al. A CRISPR-Cas12a-derived biosensing platform for the highly sensitive detection of diverse small molecules. Nat. Commun. 2019;10:3672. doi: 10.1038/s41467-019-11648-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahas A., et al. Development of Cas12a-Based cell-free small-molecule biosensors via allosteric regulation of crispr array expression. Anal. Chem. 2022;94:4617–4626. doi: 10.1021/acs.analchem.1c04332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiong Y., et al. Functional DNA regulated CRISPR-Cas12a sensors for point-of-care diagnostics of non-nucleic-acid targets. J. Am. Chem. Soc. 2020;142:207–213. doi: 10.1021/jacs.9b09211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qiao B., et al. "Aptamer-locker" DNA coupling with CRISPR/Cas12a-guided biosensing for high-efficiency melamine analysis. Biosens. Bioelectron. 2021;183 doi: 10.1016/j.bios.2021.113233. [DOI] [PubMed] [Google Scholar]
- 32.Zhao J., et al. A ligation-driven CRISPR-Cas biosensing platform for non-nucleic acid target detections. Chem. Commun. 2021;57:7051–7054. doi: 10.1039/d1cc02578c. [DOI] [PubMed] [Google Scholar]
- 33.Shu X., et al. Integrating CRISPR-Cas12a with a crRNA-mediated catalytic network for the development of a modular and sensitive aptasensor. ACS Synth. Biol. 2022;11:2829–2836. doi: 10.1021/acssynbio.2c00224. [DOI] [PubMed] [Google Scholar]
- 34.Samanta D., et al. Enhancing CRISPR-Cas-mediated detection of nucleic acid and non-nucleic acid targets using enzyme-labeled reporters. J. Am. Chem. Soc. 2022;144:16310–16315. doi: 10.1021/jacs.2c07625. [DOI] [PubMed] [Google Scholar]
- 35.Li Y., et al. Amplified detection of nucleic acids and proteins using an isothermal proximity CRISPR Cas12a assay. Chem. Sci. 2021;12:2133–2137. doi: 10.1039/d0sc06113a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deng F., et al. A CRISPR/Cas12a-assisted on-fibre immunosensor for ultrasensitive small protein detection in complex biological samples. Anal. Chim. Acta. 2022;1192 doi: 10.1016/j.aca.2021.339351. [DOI] [PubMed] [Google Scholar]
- 37.Han C., et al. CRISPR/Cas12a-Derived electrochemical aptasensor for ultrasensitive detection of COVID-19 nucleocapsid protein. Biosens. Bioelectron. 2022;200 doi: 10.1016/j.bios.2021.113922. [DOI] [PubMed] [Google Scholar]
- 38.Zhao Q., et al. Nano-immunosorbent assay based on Cas12a/crRNA for ultra-sensitive protein detection. Biosens. Bioelectron. 2021;190 doi: 10.1016/j.bios.2021.113450. [DOI] [PubMed] [Google Scholar]
- 39.Li B., et al. An exonuclease protection and CRISPR/Cas12a integrated biosensor for the turn-on detection of transcription factors in cancer cells. Anal. Chim. Acta. 2021;1165 doi: 10.1016/j.aca.2021.338478. [DOI] [PubMed] [Google Scholar]
- 40.Kim H., et al. CRISPR/Cas12a collateral cleavage activity for simple and rapid detection of protein/small molecule interaction. Biosens. Bioelectron. 2021;194 doi: 10.1016/j.bios.2021.113587. [DOI] [PubMed] [Google Scholar]
- 41.Chen Y., et al. Applying CRISPR/Cas system as a signal enhancer for DNAzyme-based lead ion detection. Anal. Chim. Acta. 2022;1192 doi: 10.1016/j.aca.2021.339356. [DOI] [PubMed] [Google Scholar]
- 42.Fu R., et al. CRISPR-Cas12a based fluorescence assay for organophosphorus pesticides in agricultural products. Food Chem. 2022;387 doi: 10.1016/j.foodchem.2022.132919. [DOI] [PubMed] [Google Scholar]
- 43.Li Y.Y., et al. Amplification of the Fluorescence Signal with Clustered Regularly Interspaced Short Palindromic Repeats-Cas12a based on au nanoparticle-dnazyme probe and on-site detection of pb(2+) via the photonic crystal chip. ACS Sens. 2022;7:1572–1580. doi: 10.1021/acssensors.2c00516. [DOI] [PubMed] [Google Scholar]
- 44.Lv Z., et al. Multivalent Duplexed-Aptamer networks regulated a CRISPR-Cas12a system for circulating tumor cell detection. Anal. Chem. 2021;93:12921–12929. doi: 10.1021/acs.analchem.1c02228. [DOI] [PubMed] [Google Scholar]
- 45.Wang D.X., et al. CRISPR/Cas12a-based dual amplified biosensing system for sensitive and rapid detection of polynucleotide kinase/phosphatase. Biosens. Bioelectron. 2020;168 doi: 10.1016/j.bios.2020.112556. [DOI] [PubMed] [Google Scholar]
- 46.Chen S., et al. PAM-less conditional DNA substrates leverage trans-cleavage of CRISPR-Cas12a for versatile live-cell biosensing. Chem. Sci. 2022;13:2011–2020. doi: 10.1039/d1sc05558e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang M., et al. Coupling of proteolysis-triggered transcription and CRISPR-Cas12a for ultrasensitive protease detection. Sci. China Chem. 2020;64:330–336. [Google Scholar]
- 48.Mao Z., et al. Upconversion-mediated CRISPR-Cas12a biosensing for sensitive detection of ochratoxin A. Talanta. 2022;242 doi: 10.1016/j.talanta.2022.123232. [DOI] [PubMed] [Google Scholar]
- 49.Hu J., et al. Metal-Tagged CRISPR/Cas12a bioassay enables ultrasensitive and highly selective evaluation of kanamycin bioaccumulation in fish samples. Anal. Chem. 2021;93:14214–14222. doi: 10.1021/acs.analchem.1c03094. [DOI] [PubMed] [Google Scholar]
- 50.Wang X., et al. Target-induced transcription amplification to trigger the trans-cleavage activity of CRISPR/Cas13a (TITAC-Cas) for detection of alkaline phosphatase. Biosens. Bioelectron. 2021;185 doi: 10.1016/j.bios.2021.113281. [DOI] [PubMed] [Google Scholar]
- 51.Shen J., et al. Sensitive detection of a bacterial pathogen using allosteric probe-initiated catalysis and CRISPR-Cas13a amplification reaction. Nat. Commun. 2020;11:267. doi: 10.1038/s41467-019-14135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen Q., et al. CRISPR/Cas13a signal amplification linked immunosorbent assay for femtomolar protein detection. Anal. Chem. 2020;92:573–577. doi: 10.1021/acs.analchem.9b04403. [DOI] [PubMed] [Google Scholar]
- 53.Iwasaki R.S., et al. SPRINT: a Cas13a-based platform for detection of small molecules. Nucleic Acids Res. 2020;48 doi: 10.1093/nar/gkaa673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen M., et al. Biosensors & bioelectronics; 2022. Design and Synthesis of DNA Hydrogel Based on EXPAR and CRISPR/Cas14a for Ultrasensitive Detection of Creatine Kinase MB. [DOI] [PubMed] [Google Scholar]
- 55.Gootenberg Js A.O., et al. Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6. Science. 2018;360:439–444. doi: 10.1126/science.aaq0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nguyen L.T., et al. CRISPR-ENHANCE: an enhanced nucleic acid detection platform using Cas12a. Methods. 2022;203:116–124. doi: 10.1016/j.ymeth.2021.02.001. [DOI] [PubMed] [Google Scholar]
- 57.Khambhati K., et al. Current progress in CRISPR-based diagnostic platforms. J. Cell. Biochem. 2019;120:2721–2725. doi: 10.1002/jcb.27690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li C.Y., et al. A boosting upconversion luminescent resonance energy transfer and biomimetic periodic chip integrated CRISPR/Cas12a biosensor for functional DNA regulated transduction of non-nucleic acid targets. Biosens. Bioelectron. 2020;169 doi: 10.1016/j.bios.2020.112650. [DOI] [PubMed] [Google Scholar]
- 59.Cheng X., et al. Novel non-nucleic acid targets detection strategies based on CRISPR/Cas toolboxes: a review. Biosens. Bioelectron. 2022;215 doi: 10.1016/j.bios.2022.114559. [DOI] [PubMed] [Google Scholar]
- 60.Qiao B., et al. "Aptamer-locker" DNA coupling with CRISPR/Cas12a-guided biosensing for high-efficiency melamine analysis. Biosens. Bioelectron. 2021;183 doi: 10.1016/j.bios.2021.113233. [DOI] [PubMed] [Google Scholar]
- 61.Mao Z., et al. Upconversion-mediated CRISPR-Cas12a biosensing for sensitive detection of ochratoxin A. Talanta. 2022;242 doi: 10.1016/j.talanta.2022.123232. [DOI] [PubMed] [Google Scholar]
- 62.Hu J., et al. Element probe based CRISPR/Cas14 bioassay for non-nucleic-acid targets. Chem. Commun. 2021;57:10423–10426. doi: 10.1039/d1cc03992j. [DOI] [PubMed] [Google Scholar]
- 63.Li Z.j., et al. Application of CRISPR/CAS molecular diagnostics biosensor in Non-nucleic acid molecular diagnosis. Prog. Biochem. Biophys. 2023;50:1797. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There is no additional data available for this review article.