Graphical abstract
Keywords: Induced pluripotent stem cells, Alzheimer's disease, Neurons, Neural differentiation, Brain organoids, Neuronal loss, Astrocytes, Microglia
Highlights
-
•
Human induced pluripotent stem cells (hiPSC) and their derivates such as.
-
•
astrocytes, neurons or oligodendrocytes generated from patients represent faithful.
-
•
cellular models to study Alzheimeŕs disease.
-
•
Cerebral organoides derived from hiPSC provide high sophisticated cellular 3D structures to investigate Alzheimeŕs disease.
-
•
hiPSCs enable in vitro high-throughput pharmacological screening assays of diseased tissue.
Abstract
Background
Synaptic dysfunction is a major contributor to Alzheimeŕs disease (AD) pathogenesis in addition to the formation of neuritic β-amyloid plaques and neurofibrillary tangles of hyperphosphorylated Tau protein. However, how these features contribute to synaptic dysfunction and axonal loss remains unclear. While years of considerable effort have been devoted to gaining an improved understanding of this devastating disease, the unavailability of patient-derived tissues, considerable genetic heterogeneity, and lack of animal models that faithfully recapitulate human AD have hampered the development of effective treatment options. Ongoing progress in human induced pluripotent stem cell (hiPSC) technology has permitted the derivation of patient- and disease-specific stem cells with unlimited self-renewal capacity. These cells can differentiate into AD-affected cell types, which support studies of disease mechanisms, drug discovery, and the development of cell replacement therapies in traditional and advanced cell culture models.
Aim of Review
To summarize current hiPSC-based AD models, highlighting the associated achievements and challenges with a primary focus on neuron and synapse loss.
Key Scientific Concepts of Review
We aim to identify how hiPSC models can contribute to understanding AD-associated synaptic dysfunction and axonal loss. hiPSC-derived neural cells, astrocytes, and microglia, as well as more sophisticated cellular organoids, may represent reliable models to investigate AD and identify early markers of AD-associated neural degeneration.
Introduction
Alzheimer's disease (AD), the most common cause of dementia, is characterized by the progressive deterioration of memory, language, and behavior that negatively impacts a patient's ability to carry out everyday tasks. Other than cognitive impairments, the brains of AD patients display structural abnormalities with predictive value [1], such as the presence of focal symmetrical medial temporal atrophy [2] or parieto-occipital atrophy [3]. Pathological features such as extracellular β-amyloid (Aβ) plaques and intracellular tangles of the hyperphosphorylated axonal protein Tau (neurofibrillary tangles, Tau NFTs) accompany neurodegeneration and cognitive decline. Of note, the underlying causative factors and the mechanisms accelerating disease progression remain elusive. A landmark study by Braak and Braak proposed that AD-related Tau pathology may commence in medial temporal structures (i.e., the transentorhinal cortex), then extend to the limbic areas of the medial and inferior temporal lobe and the posterior cingulate cortex before spreading more widely into isocortical brain areas [4]. Hypotheses that explain AD pathogenesis include the well-known amyloid cascade [5] and the effects of Tau hyperphosphorylation [6], but also include dysfunctional glucose metabolism or oxidative stress [7]. Synapse loss represents the strongest structural correlate of the presence of cognitive deficits, which represents a central feature of AD [8], [9]; however, how Aβ plaques and Tau NFT deposition prompt alterations in synaptic function and axonal structure and whether earlier pathophysiological features can predict symptoms remains unclear. Interestingly, high levels of Aβ plaques and Tau NFTs have been observed in the post-mortem brains of older subjects with preserved mental status, a state defined as asymptomatic AD [10], [11]. Failure of a multitude of clinical trials, which have focused on Aβ plaques and Tau NFTs, led to the proposition of a new research approach to discover early-stage pathophysiological features of AD that contribute to synapse function. This research focus should consider non-neuronal contributions to AD, including the dysfunction of astrocytes, microglia, and the vascular system.
Exploring novel conceptual models of dementia and alternative approaches may lead to the discovery of novel AD treatment options. Despite the development of more sophisticated second-generation mouse models, which contain humanized sequences, they have generally failed to fully recapitulate human AD pathogenesis [12]. Thus, we must urgently establish faithful human-relevant models to define pathogenic pathways in AD. These models should help to determine early pathological, cellular, or molecular features important for disease diagnosis and treatment.
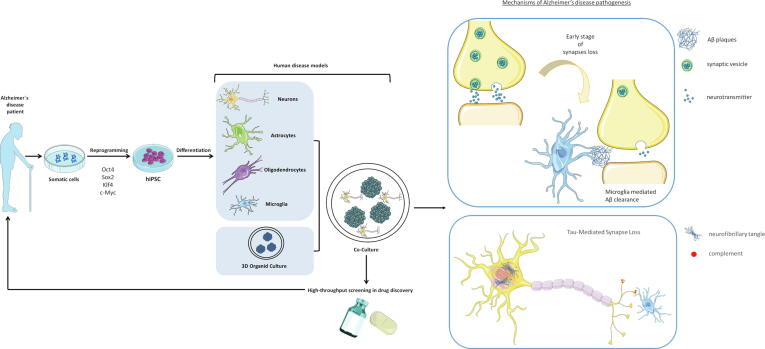
Continuous discoveries in the field of human induced pluripotent stem cells (hiPSCs) have permitted the derivation of patient- and disease-specific stem cells possessing unlimited self-renewal capacity. hiPSCs can differentiate into AD-affected cell types, including neurons, astrocytes, oligodendrocytes, and AD-relevant cell types, such as microglia (Fig. 1). The generation of AD-affected cells may support the development of human AD models that allow the exploration of links between traditional concepts, synaptic loss or axonal dysfunction and the contribution of other neural cell types (Fig. 1).
Fig. 1.
Future applications of hiPSC technology in AD modeling and drug discovery. hiPSCs derived from somatic cells can differentiate into multiple neuronal and neuroglial cells. Differentiated cells obtained from hiPSCs generated from AD patient cells (AD-hiPSCs) can be grown in 3D co-cultures. This complex system can simulate the interactions between neural cells in vivo to identify disease mechanisms, such as synaptic dysfunction, due to the formation of neuritic β-amyloid plaques and neurofibrillary tangles of hyperphosphorylated Tau protein. Reprogramming strategies and models have promising potential to facilitate neurodegenerative disease research and drug discovery for further clinical applications. hiPSC: human induced pluripotent stem cells; Sox2, SRY-Box Transcription Factor 2; Oct, Octamer-binding transcription factor 4; Klf4, Kruppel Like Factor 4; c-Myc, MYC Proto-Oncogene.
This review summarizes how current hiPSC-based AD models can contribute to a deeper understanding of the effects of Aβ plaques and Tau NFTs on synaptic and axonal function and whether these models faithfully recapitulate AD pathophysiology. This review will focus on Aβ plaques and Tau NFTs as core features of dementia. In addition, we will discuss crucial neurobiological features and events that underlie the primary clinical characteristics - the massive loss of synapses and synaptic connectivity, and memory loss.
New hypotheses of synapse loss and axonal dysfunction in AD
Aβ plaques and Tau NFTs
Most AD cases occur on a sporadic basis, and we currently possess a poor understanding of the genetic background of the disease. Mutations in three genes - amyloid precursor protein (APP), presenilin 1 (PSEN1), and presenilin 2 (PSEN2) – associate with the development of a rare (<0.5 %) familial form of AD (fAD). Symptoms of fAD develop earlier than sporadic AD, typically appearing between 40 and 50 years of age [13]. A complex interplay between genetic and environmental factors represents the most probable cause of common late-onset AD, with the Apolipoprotein E (APOE) gene (E2, E3, and E4 variants) the single most significant risk factor for sporadic AD [14]. Genome-wide association studies (GWAS) have highlighted more than twenty disease-associated risk factors, including genes involved in certain complications associated with AD, such as increased inflammation, altered cholesterol metabolism, hypertension, depression, and altered endosomal vesicle recycling pathways [15]. The presence of extracellular Aβ plaques, also called senile plaques, and intracellular accumulations of hyperphosphorylated Tau protein, known as Tau NFTs [16], [17], represent the main neuropathological criteria for AD diagnosis. Of note, Aβ plaques and Tau NFTs coexist with other disease-associated pathologies such as neuropil threads, dystrophic neurites, microglial activation, and cerebral amyloid angiopathy [18], and there exists a considerable overlap between AD and other neurodegenerative diseases, such as frontotemporal dementia and Lewy body dementia [19]. According to the well-known amyloid cascade hypothesis [20], Aβ plaques act as the key trigger of AD pathology [21], [22]; however, recent studies have proposed that Aβ plaques do not play a crucial role in the symptomatic stages of the disease [23] and that Tau pathology represents the critical driver of neurodegeneration and disease progression in AD [24]. There are spatial and temporal discrepancies between the appearance of Aβ plaques, Tau NFTs, neuronal loss, and clinical dementia, which questions the role of Aβ plaques in the development of Tau pathology and AD progression.
Decades of AD research, mainly based on analysis of post-mortem brains and transgenic mice models, have revealed multiple cellular alterations involved in disease progression. These support the amyloid cascade theory - increases in Aβ peptide prompt the formation of plaques during the early stages of AD, which triggers a chain of events that lead to the formation and accumulation of Tau NFTs, which cause neurodegeneration and cognitive decline. We do not fully understand the long delay between rising Aβ plaque levels and Tau NFT deposition at this stage and how these detrimental events lead to synapse damage and loss. A direct correlation exists between dementia and Tau NFT formation as a histopathological hallmark, whereas the relationship between Aβ concentration, amyloid plaque formation, and cognition decline remains unclear [25], [26]. Many published studies suggest that low endogenous levels of Aβ peptide play a vital role in the maintenance of basal presynaptic activity and regulation of synaptic vesicle release and plasticity [27], [28]. Nevertheless, high Aβ concentrations in and around plaques cause localized damage to the synapses of nearby neurons, disturbing the local neuronal network [29].
Indeed, a recent hypothesis suggests that the local accumulation of Aβ around plaques represents the basis for the development of early-stage AD [10]. Additional studies have suggested that the gradual increase in Aβ peptide in parallel with the gradual deposition of plaques triggers local damage of glutamatergic transmission and nearby synapsis by targeting postsynaptic glutamatergic receptors [30], [31], [32] in a calcium-dependent manner [33]. In this case, synapse loss is a local event and has minimal effects on the function of the global neuronal network; therefore, these findings suggest that Aβ plaques may not represent the fundamental cause of AD [34]. While local network damage is easily controllable, synaptic damage becomes more pronounced and spreads as the plaque load increases and individual axons encounter multiple plaques.
Many synaptic proteins, including Tau, undergo hyperphosphorylation in the presence of Aβ plaques and occur at multiple sites along the axon [35], [36] to induce axon loss and neurodegeneration [37]. These fundamental changes alter the postsynaptic and presynaptic mechanisms in brain regions responsible for cognitive functions. This hypothesis agrees with accumulating evidence from studies performed in AD animal models [38], [39], [40], [41], [42] and explains the link between increased Aβ plaques and Tau pathology, why substantial synaptic loss and neurodegeneration require Tau pathology, why microglia delay the process, and why there exists a delay between Aβ plaque formation and Tau NFTs. Tau can propagate between neurons, and substantial evidence suggests the strong correlation between abnormal Tau phosphorylation and memory decline [43]. A recent study revealed a critical time period (from 140 to 160 days) during which synapse loss increases [44]; however, we require analyses in human subjects and AD samples to better understand the molecular and cellular detrimental effects of Aβ and Tau.
Synaptic disfunction
Pereira et al. evaluated the previously explained hypothesis about association of Aβ plaque formation and Tau NFTs with synaptic and axonal loss by measuring presynaptic, postsynaptic, and axonal proteins in human cerebrospinal fluid during early-stage AD [45]. The authors discovered that global Aβ plaque deposition associated with changes in presynaptic markers (such as Synaptosomal-Associated Protein 25 (SNAP25) and Growth Associated Protein 43 (GAP43) involved in synaptic plasticity [46] and the postsynaptic marker Neurogranin (NRGN) (involved in long-term potentiation [47]), which may prompt memory dysfunction and default-mode network (DMN) activity and brain dysfunction (e.g., parahippocampal white matter damage [45]). The increased levels of the above-mentioned proteins indicate increased connectivity, probably due to the overactivation of N-methyl-d-aspartic acid receptors through the increased glutamate activity at synapses in AD driven by Aβ plaque accumulation [48].
Alterations in functional connectivity driven by changes in the expression of synaptic proteins, particularly postsynaptic proteins, due to Aβ plaque accumulation could represent an early AD marker [45], [49], [50], [51]. As postulated in different hypotheses evaluated in animals, later-stage AD correlates with Aβ plaque-induced synaptic damage along the length of the axon, which triggers Tau NFTs, the associated dissociation of microtubules, and subsequent axonal degeneration and loss of structural connectivity (as main AD hallmarks) [52]. Finally, neurites of dendrites near Aβ plaques displayed a reduced density [29] and a considerable loss of spines, resulting in the disintegration of the neuronal network over time and consequent AD-associated cognitive dysfunction [44].
Current AD treatments focus on decreasing the formation of the Aβ plaques and NFTs typical of late-stage AD pathology. The hurdles in clinical trials [53] related to this strategy led to the realization that we must detect early pathological features in the function of neural circuits that precede the appearance of pathological aggregates in AD. In AD, episodic and associative memory deficits have been linked to structural and functional changes within the hippocampal system. The hippocampus, the crucial part of the central nervous system (CNS) in memory processes, is among the first affected brain areas in AD [4]. In addition to atrophy, aberrant local activity and global connectivity have been observed in the hippocampus. Integration of activity across distant brain regions (including the hippocampus) provides insight into the intrinsic connectivity networks (ICNs), particularly the DMN as the most well-characterized ICN [54], [55]. The DMN includes brain regions with high degrees of functional connectivity and is active in the brain at rest but becomes deactivated after the initiation of task performance [56].
The discovery that reduced posterior cingulate cortex activity measured by brain imaging techniques in patients represents a common early feature of AD confirmed the hypothesis of abnormal DMN activity in AD [57], [58], [59]. Decreased posterior cingulate cortex activity in early AD occurs in parallel with decreased connectivity within medial temporal lobe structures such as the entorhinal cortex and hippocampus, which lie among the first regions targeted by AD pathology [60], [61].
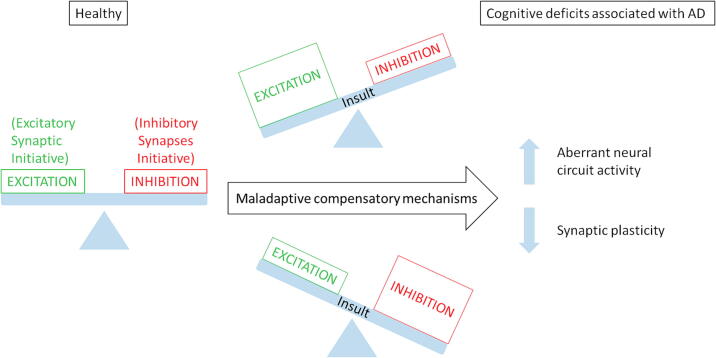
In the recently postulated hypothesis, a loss of cellular homeostasis initiates the clinical AD phase, indicating that Aβ plaques and Tau NFTs represent downstream risk factors [62]. This initial cellular homeostatic failure seems to underlie the firing instability of neural circuits as the common driver of AD pathogenesis, initiated by numerous distinct triggers (Resumed in Fig. 2).
Fig. 2.
Unbalance of inhibition/excitation states of neurons induces the progression from healthy to early AD. Certain events can trigger the unbalance of the signaling that regulates inhibition/excitation of neurons. Inefficient compensatory mechanisms lead to aberrant neural circuit activity and reduced synaptic plasticity. These physiopathological events cause the cognitive deficits associated with Alzheimer disease (AD).
Styr and Slutsky recently proposed a hypothesis to explain the transition from ‘silent’ features of anomalous neural circuit activity to clinically evident memory impairments [63]. They proposed that an imbalance between firing stability and synaptic plasticity in cortico-hippocampal circuits represents an early hallmark of disease progression. The Styr and Slutsky hypothesis is based on accumulated evidence from the last decade showing that AD patients manifest an increased incidence of neuronal hyperactivity, including non-convulsive epileptic discharges [64], [65], in correlation with a faster rate of cognitive decline. Indeed, deficits in episodic and associative memory in AD have been linked to aberrant activity in the hippocampal system and cortical circuits [66], [67] and numerous dysfunctions in synaptic and neuronal plasticity [68], [69], [70] (Fig. 2).
Different electrophysiological studies in AD patients and animal models support the Styr and Slutsky hypothesis and provide compelling evidence for impairments of distinct forms of hippocampal synaptic plasticity in early-stage disease, even before the appearance of clinical symptoms. Studies have detected neuronal hyperactivity in the initial disease stages [71], [72], [73] in both sporadic and fAD animal models and patients [65], [74], [75]. A cohort of Colombian families with the E-280A single mutation in the PS1 gene provides particular interest [76]; these families permitted the observation of different brain activation patterns years before disease onset when cognitive impairment had yet to be observed. Functional MRI revealed that presymptomatic carriers displayed hyperactivation within the hippocampal system compared to matched controls, demonstrating early aberrant regenerative and degenerative changes in neuronal processes. These approaches may represent the best opportunity to understand early pathological hallmarks of AD related to neuronal damage and develop early interventions to prevent, slow, or mitigate AD pathology and/or the neuronal alterations that prompt cognitive impairment.
Unfortunately, these changes are not unique and are unlikely to drive neurodegeneration, revealing the possible implication of other pathophysiological features that gradually induce memory impairments in early-stage AD. Such features include alterations to the evolutionarily conserved integrated homeostatic network (IHN) described by Frere and Slutsky [77], which enables functional stability of central neural circuits and safeguards from neurodegeneration. This network comprises several functional modules (with most affected years before AD onset), including energy homeostasis (reduction of glucose metabolism [78] and/or mitochondrial dysfunction [79], [80]), proteostasis (elevated protein accumulation and mitophagy impairment [81]), immune system homeostasis, firing stability, and calcium homeostasis [82]. A full description of these features goes beyond the scope of this review and will not be described in depth.
hiPSC technology and neural differentiation
The Yamanaka and Thomson groups made the groundbreaking discovery that the forced expression of a limited number of reprogramming factors could convert differentiated human somatic cells into pluripotent stem cells (hiPSCs) in 2007 [83], [84]. Reprogramming factors such as Oct4, Sox2, and Klf4 play a central role by resetting the somatic cell epigenome during hiPSC generation. This reprogramming technique has revolutionized modern medicine by providing access to a patient- and disease-specific source of cells for basic research, disease modeling, and therapeutic applications. hiPSCs can be indefinitely maintained in culture while retaining the potential to efficiently differentiate into virtually any cell type of the human body. The multilineage differentiation capacity of hiPSCs offers a model platform to study neurodegenerative diseases such as AD, as their cell fate can be restricted toward clinically relevant cell types (i.e., neurons, astrocytes, and microglia), thereby providing an unlimited cell source for interrogations at the molecular, cellular, and functional level. The exposure of hiPSCs to different morphogens and growth factors that mimic processes in the developing CNS fosters the generation of subtype- and region-specific neurons. During the in vitro differentiation of hiPSCs, neuroepithelial precursor cells generally spontaneously generate glutamatergic neurons of dorsal fate [85], [86]. Sonic hedgehog (SHH) pathway activation and WNT pathway inhibition direct hiPSC differentiation towards a GABAergic neuron fate [87] while activating the SHH and nerve growth factor (NGF) pathways induces cholinergic neuron differentiation [88]. Recent studies of neural progenitors derived from hiPSCs generated from AD patient cells (AD-hiPSCs) employed a widely-used, rapid, and direct neural differentiation approach using the dual small molecule-mediated inhibition of the SMAD signaling pathway (involved in endoderm and mesoderm differentiation during development) [89], [90], [91].
Herein, we summarize examples of AD models generated using hiPSC-derived cells, which include neurons, astrocytes, oligodendrocytes, and microglia, and discuss their relevance to the study of synaptic dysfunction and axonal loss (Fig. 1).
hiPSC-derived cells as AD models
Despite significant recent progress in clarifying the critical etiological aspects of AD, they remain far from being fully understood [92], [93]. A distinct lack of reliable cell and animal models that faithfully recapitulate human brain-specific processes represents a significant impediment to the understanding, diagnosis, and treatment of AD. Most transgenic animals fail to exhibit certain core pathological features of AD, such as extensive neuronal loss or Tau NFTs [94]. Mice with fAD do not display the complete pathology of the human disease, despite the appearance of high levels of Aβ plaques. These model limitations may derive from significant differences observed between neuronal [95], [96], [97] and microglial cells [98] in human and rodent brains. Furthermore, human post-mortem samples are rare and from end-stage disease, therefore representing an indistinguishable contribution of multiple secondary events. Significant phenotypic variability also exists in terms of disease progression, onset, and pattern.
Neural Precursors and mature neurons
Initial investigations had focused on neurons to determine the intrinsic neurodegenerative mechanisms associated with AD, which correlated Aβ plaque accumulation in the brain with the progressive dysfunction of glutamatergic synaptic transmission, neuronal circuits, networks (reviewed by Palop and Mucke [99]) and cognition [9]. Pioneering studies using excitatory neurons differentiated from fAD-hiPSCs derived from patients carrying PSEN1, PSEN2, and APP mutations [100] and sporadic AD-hiPSCs demonstrated that resultant cells displayed pathogenic signs of AD, including the increased accumulation of the pathological Aβ42 isoform [91], [101], [102], [103], [104], [105] and Tau NFTs [105], [106], [107], [108].
hiPSC-derived neurons generated from patients bearing specific APP mutations or alterations displayed particularly pronounced pathogenic signs in studies published by Kondo et al. (atypical early-onset fAD caused by an E693Δ mutation of a variant Aβ lacking the 22nd Glu-E693Δ) [109], Muratore et al. (V717I mutation) [110], and Israel et al. (APP gene duplication) [106]. Accumulating evidence from animal studies has demonstrated that the basal ganglia, hindbrain, and spinal cord are initially spared from Aβ deposition and synapse loss [111], thereby revealing the differential susceptibility of distinct brain regions and neuronal subtypes to neurodegeneration in AD. Studies using hiPSCs with different APP mutations primarily aimed to identify neuronal cell types that displayed greater vulnerability to AD.
Recently developed differentiation protocols that permitted the generation of region-specific neurons from patient-derived hiPSCs have highlighted differences in neuronal AD pathology in the same genetic background. For example, hiPSC-derived embryoid bodies (differentiating spherical structures formed in low attachment conditions containing three embryonic layers) spontaneously differentiated toward cortical forebrain neurons without exogenously administered patterning factors [86], [110]. The differentiation of hiPSCs to neurons with a caudal fate requires the modulation of the retinoic acid and SHH pathways. Muratore et al. differentiated AD-hiPSCs carrying the V717I mutation into caudal forebrain (cortical) and posterior (hindbrain) neuronal fates, finding that caudal cells possessed lower levels of Tau phosphorylation compared to isogenic mutation-corrected hiPSC-derived cells [110]. Similar studies established discrepancies in several AD hallmarks in hiPSC-derived glutamatergic and GABAergic neurons [112], [113], which may reveal pathophysiological mechanisms underlying the selective vulnerability of brain regions and neuronal cell types in AD. Overall, Aβ-induced toxicity displayed selectivity for glutamatergic rather than GABAergic neurons [113]. Compared to studies in AD-hiPSCs carrying the E693Δ APP mutation or APP duplication, differentiated neurons exhibited distinct additional features. Neurons bearing the APP V717I mutation displayed regionally specific vulnerability [110], while neurons bearing APP duplications possessed larger endosomes [106], and neurons bearing the APP E693Δ mutation displayed signs of endoplasmic reticulum (ER) and oxidative stress [109].
Trisomy of chromosome 21 (TS21) causes Down syndrome and APP gene triplication. The clinical phenotype of Down syndrome patients includes early-onset AD with accelerated pathology (at 40 years), Aβ overexpression, and typical AD post-mortem features [114]. Therefore, TS21-hiPSC differentiation represents an ideal means of generating neural cells for AD modeling. Encouragingly, studies have established that TS21-hiPSC-derived neural cells faithfully recapitulate findings from human post-mortem tissue, including abnormal Aβ metabolism manifested by the increased secretion and accumulation of the pathological Aβ42 isoform and upregulated APP gene expression during the early stages of neural differentiation [115], [116]. The authors used CRISPR/Cas9 gene-editing technology to introduce or correct AD-associated mutations in hiPSCs and promote the associated neuronal phenotypes in a strictly controlled genomic background. Deletion of the extra APP copy in TS21-hiPSCs by gene-editing permitted the differentiation of cortical neurons with normalized APP expression, Aβ1-42 secretion, Aβ1-42/Aβ1-40 ratio (a potential biomarker for selective Aβ1-42 deposition [117]), and overall Aβ levels. However, a related study demonstrated that an increase in APP levels does not represent the sole factor responsible for inducing Down syndrome-associated AD pathogenesis [118], as APP deletion failed to alter Tau phosphorylation or neuron sensitivity to oxidative stress-induced apoptosis, which causes synaptic defects and neuronal dysfunction [118]. Overall, these findings highlighted the complexity of the mechanisms controlling TS21-related AD pathogenesis.
Woodruff et al. investigated neurons differentiated from fAD-hiPSCs from additional genetic backgrounds, which included PSEN1 and PSEN2 mutations [119]. The authors demonstrated that neurons differentiated from fAD-hiPSCs or hiPSCs gene-edited to bear PSEN1 mutations [91], [102], [119] exhibited typical AD-associated features, including increased sensitivity to Aβ-induced toxicity and oxidative stress [1], [100], impaired autophagy, mitophagy, and endo-lysosomal degradation pathways [120], [121], and increased DNA damage and apoptosis [104], [121].
The formation of new neurons in the adult brain (neurogenesis) plays a crucial role in hippocampus-dependent learning and memory. The hypothesis that impaired neurogenesis in AD underlies memory impairment remains to be confirmed [122]. By generating cortical neurons from fAD-hiPSCs harboring PSEN1 mutations, Arber et al. [123] demonstrated features of premature neurogenesis in regionally specific neurons partially driven by Notch signaling. The same study used a three-dimensional cell culture system to compare to postmortem brain tissue from fAD patients. These data supported the sufficiency of PSEN1 mutations in driving premature neurogenesis, suggesting the alteration of neurogenesis timing, accelerating aging, and neurodegeneration as critical features in AD development. This study suggests impaired neurogenesis as a critical early event during AD that may represent the leading cause of memory impairment and neural vulnerability in the hippocampus.
A recent study from Ghatak et al. [124] modeled early AD features such as brain hyperexcitability and subsequent synapse loss linked to memory impairment. The authors developed a cell model comprising a mixture of cerebrocortical neurons and inhibitory interneurons derived from fAD-hiPSCs that exhibited neural network activity [125] similar to that of the human brain [126]. Interestingly, these studies revealed similar network abnormalities, such as aberrant excitatory bursting, hypersynchronicity, and increased slow spontaneous fluctuations, as observed in AD patients [127].
While we have a relatively robust understanding of the molecular etiology of mutation-driven fAD, the specific impact of sporadic AD risk factors remains much less clear. Accumulated data from multiple large-scale GWAS and meta-analyses revealed the APOE4 variant as the single most important genetic risk for sporadic AD (as reviewed by Serrano-Pozo et al. [128]). The APOE2 variant is considered protective [129], emphasizing the importance of APOE variants to AD pathogenesis, while the common APOE3 variant associates with a healthy phenotype. Studies using neurons differentiated from patient-derived hiPSCs carrying the APOE4 variant revealed common cellular and molecular features related to AD, such as increased Aβ plaques and Tau NFTs when analyzed alongside hiPSC-derived neurons carrying the APOE3 variant [130], [131]. APOE4-hiPSC-derived neurons also exhibited elevated synaptic activity and endosomal abnormalities [130]. In the same study, the conversion of the APOE4 into the APOE3 variant in sporadic AD-hiPSCs using CRISPR/Cas9 technology ameliorated the molecular and cellular phenotypes in differentiated neural cells [131]. Interestingly, the neuronal subtype-specific differentiation of these AD-hiPSCs revealed a significant increase in synaptic connections and synaptic transmission frequency in glutamatergic APOE4 neurons compared to APOE3 neurons [130]. Furthermore, APOE4 GABAergic interneurons displayed increased neurodegenerative processes and apoptosis than other neuronal subtypes, while APOE4 forebrain cholinergic neurons exhibited hypersensitivity to glutamate toxicity [88], [131]. Overall, analyses of the neuronal subtypes generated from APOE4-hiPSCs confirmed an imbalance in nerve excitation or inhibition in AD.
A relatively recent study by Ochalek et al. [91] described how neurons derived from sporadic AD-hiPSCs shared similar features as neurons derived from fAD-iPSCs, including increased levels of Aβ plaques and Tau NFTs, enlarged early endosomes, selective Aβ toxicity, and oxidative stress [102], [106]; however, this study did not reveal novel features that may cause synapse loss.
Meyer et al. generated hiPSC-derived neural cells from a large cohort of patients with sporadic AD and aged-matched controls [132], which exhibited accelerated neural differentiation and reduced progenitor cell self-renewal independent of APOE-specific genotypes.
Overall, these traditional two-dimensional (2D) hiPSC-based models confirmed aspects of the amyloid cascade and Tau phosphorylation theories but failed to provide additional information to expand these hypotheses. All mentioned studies using different affected cells derived from AD-hiPSC are resumed in Table 1.
Table 1.
HiPSC-derivatives in ad models related to neural precursors and mature neurons, astrocytes and microglia.
| A-Neurons | |||
|---|---|---|---|
| hiPSC-derived cell type | Mutation/Genomic alteration | AD hallmarks | Reference (s) |
| fAD-hiPSC | PSEN1, PSEN2, and APP mutations | Accumulation of the pathological Aβ42 isoform and sporadic AD-hiPSCs Tau phosphorylation | [91], [101], [102], [103], [104], [105], [106], [107], [108] |
| hiPSC-derived neurons | APP(E693Δ) | altered signs of endoplasmic reticulum (ER) and oxidative stress | [109] |
| APP(V717I) | Aβ/TAU pathology | [110] | |
| APP duplications | larger endosomes | [106] | |
| hiPSC-derived forebrain neurons | APP (V717I) | Aβ induced toxicity | [112], [113] |
| hiPSC-derived GABAergic neurons | APP (V717I) | Aβ induced toxicity | [112], [113] |
| TS21-hiPSCs | triplication of the APP | increased secretion and accumulation of the pathological Aβ42 isoform. upregulated expression of the APP | [115], [116], [117] |
| fAD-hiPSC | PSEN1 and PSEN2 | Aβ-induced toxicity and oxidative stress | [1], [100] |
| impaired autophagy, mitophagy, and endo-lysosomal degradation pathways | [120], [121] | ||
| increased DNA damage and apoptosis | [104], [121] | ||
| fAD-hiPSC | PSEN1 | premature neurogenesis | [123] |
| fAD-hiPSCcerebrocortical neuronsand inhibitory interneuros | APP and PSEN1 | aberrant excitatory bursting, hypersynchronicity and increased slow spontaneous fluctuations | [125] |
| fAD-hiPSC | APOE4 | increased Aβ levels and Tau phosphorylation elevated synaptic activity and endosomal abnormalities imbalance in nerve excitation/inhibition | [130], [131] |
| fAD-hiPSC | APOE4 gene-edited | accelerated neural differentiation and reduced progenitor cell renewal | [132] |
| B-Astrocytes | |||
| fAD and sAD-hiPSC | PSEN1, APOE4 | distinct astrocyte morphology and Aβ accumulation | [147] |
| fAD-hiPSC | PSEN1(ΔE9) | Aβ pathology, impaired ER calcium signaling and cytokine secretion, increased oxidative stress, and reduced lactate production | [149] |
| fAD-hiPSC | APOE4 | impaired Aβ uptake and cholesterol accumulation | [130] |
| lower neuroprotective effects when co-cultured with APOE3-hiPSC-derived neurons | [150] | ||
| fAD-hiPSC |
APP (V717I) | altered lipoprotein homeostasis | [151] |
| C- Microglia | |||
| fAD-hiPSC | APOE4 | decreased ability to uptake Aβ and alterations in the expression of pro-inflammatory signaling | [130] |
| Sporadic-AD-hiPSC | – | alterations to cytokine secretion and apoptosis levels | [171] |
Astrocytes
A deeper understanding of AD at the cellular and molecular level must consider the role of other cell types in disease pathogenesis; when compared to neurons, astrocytes and other glial cells remain largely understudied. Astrocytes are the brain's most abundant cell type and help to maintain the extracellular neuronal environment by mediating neurotransmitter removal, ion exchange and pH homeostasis, metabolic waste removal, and glucose and metabolite supply. Interestingly, astrocytes play an early protective role in AD by aiding Aβ clearance and regulating synaptic plasticity and neuroinflammation [133]. Astrocyte dysfunction makes them important contributors to the progression of neurodegeneration as they functionally modulate neighboring neuronal cells. Any alterations to normal astrocyte function can deregulate neuron excitability, synaptic strength and/or synaptogenesis, and cause neuronal circuit dysfunction [134], [135]. Additionally, astrocytes represent the primary source of ApoE in the brain [136], while the AD-associated accumulation of NFTs in neurons has also been observed in glial cells [137]. ApoE can impact blood–brain barrier (BBB) integrity, with the human APOE4 variant associating with a leaky BBB. Astrocyte-derived ApoE4 may drive ApoE-dependent BBB alterations [138] and thus induce BBB leakage in AD patients [139]. Reactive astrogliosis occurs in the vicinity of amyloid plaques [140], and reactive astrocytes can undergo atrophy with subsequent reactive astrocytic hypertrophy and form part of the plaque in the hippocampal formation in AD patients [141]. Furthermore, studies have suggested that astrocytic processes undergo alterations before Aβ plaque formation [142]. Disease-associated astrocytes appear during early-stage AD, increase in abundance during disease progression [143], and associate with diverse molecular pathways near Aβ plaques. Habib et al. discovered this specific astrocyte phenotype in aged wild-type mice and aged human brains, suggesting that this observation relates to the genetic basis of AD and age-related factors [143]. Astrocytes also play crucial roles in the clearance of glutamate by different glutamate receptors and the protection of neurons from glutamate-induced toxicity. Of note, presence of Aβ plaques inhibits glutamate uptake by astrocytes [144], most probably due to a decreased expression of glial transporters such as GLT-1 [145], which associates with degeneration and glutamatergic neuron loss in AD. Decoding the different impacts of AD-related astrocytes on synaptic function at early presymptomatic stages of AD could reveal new therapeutic targets.
Despite the vital role of astrocytes in AD, relatively few studies have reported AD-hiPSC differentiation into astrocytes; however, existing studies have revealed a significant role for astrocytes in disease pathogenesis. In a comparable manner to neurons, astrocytes become patterned by morphogen gradients along the rostro-dorsal and dorsal–ventral axes and exhibit regional specificity. Astrocyte subtypes can be relatively easily derived from hiPSCs using well-established protocols [146], [147], [148] (Table 1). Jones et al. reported that sporadic AD- and fAD-hiPSC-derived astrocytes exhibited a distinct morphology to normal astrocytes, with less complexity and aberrant marker localization [147]. Similar to neurons, Liao et al. discovered AD-specific cellular and molecular features in astrocytes derived from sporadic AD- or fAD-hiPSCs [112], which included Aβ plaques accumulation [147]. Astrocytes generated from AD-hiPSCs from patients carrying the PSEN1 ΔE9 mutation exhibited typical Aβ pathology, impaired ER calcium signaling and cytokine secretion, increased oxidative stress, and reduced lactate production [149]. APOE produced by APOE4-hiPSC-derived astrocytes displayed lower lipidation than APOE derived from APOE3-hiPSC-derived astrocytes [150] and impaired Aβ uptake and cholesterol accumulation [130]. Furthermore, a study that generated astrocytes from AD-hiPSCs carrying APP mutations (such as V717I) revealed a central role of the APP protein in regulating lipoprotein homeostasis [151].
Co-cultures of astrocytes with neurons have supported a range of interesting findings. Astrocytes differentiated from PSEN1 ΔE9 mutant AD-hiPSCs induced altered calcium signaling activity of healthy neurons [149], while APOE4-hiPSC-derived astrocytes displayed lower neuroprotective activities when co-cultured with APOE3-hiPSC-derived neurons [150]. By disrupting astrocytic calcium signaling and gliotransmitter release, Aβ plaques alters processes vital for astrocyte-neuron communication; therefore, astrocyte dysfunction may contribute to early neuronal deficits and synaptic dysfunction in AD.
Oligodendrocytes
Oligodendrocytes and myelin surround CNS axons to support rapid neuronal signaling and provide metabolic and trophic support. Oligodendrocyte disruption or dysfunction results in myelin loss, axonal demise, and neurodegeneration, thus impairing synaptic transmission and proper synaptic function, which represent critical features of AD [152]. Indeed, oligodendrocyte loss represents one of the earliest changes observed in the AD brain, preceding the detection of Tau NFTs and Aβ plaques in neurons [153]. The underlying mechanisms of myelin loss in AD include oxidative stress, neuroinflammation, and excitotoxicity [153]. Our group and others recently developed efficient protocols for the oligodendrocytic differentiation of hiPSCs [86], [154], [155], [156] and reported the suitability of regional-specific oligodendrocyte progenitors for disease modeling and potential cell therapies for demyelinating diseases; however, we currently lack examples of AD-hiPSC-derived oligodendrocytes/oligodendrocyte progenitors applied to AD modeling. The generation of oligodendrocytes from AD-hiPSCs could reveal novel cell features that may shed light on pathophysiological mechanisms in early-stage AD. Moreover, the potential impact of oligodendrocyte-neuron co-culture studies may reveal shared and distinct mechanisms by which AD-hiPSC-derived oligodendrocytes contribute to AD pathogenesis.
Microglia
Microglia serve as CNS-resident phagocytes, which dynamically survey the cellular environment and play crucial roles in tissue homeostasis, injury response, and pathogen defense [157]. The proliferation and activation of microglia concentrated around Aβ plaques represent prominent hallmarks of AD [158]. Recent advances in GWAS have identified a correlation between microglial gene expression and AD risk, highlighting the crucial role of these immune cells in AD progression. For example, mutations to the gene encoding the Triggering Receptor Expressed in Myeloid Cells (TREM2) cell surface protein (selectively and highly expressed by microglia) increase AD risk by approximately threefold [159]. TREM2 typically supports microglial phagocytosis of various substrates, including apoptotic neurons, bacteria, low-density lipoprotein, and Aβ peptide [160], [161]. Targeting TREM2 to increase the phagocytic activity of microglia may represent a potentially-effective AD treatment [162].
As mentioned previously, microglia remove damaged synapses during the early stages of Aβ plaque accumulation and delay axonal damage by clearing damaged synapses [163]. The removal of supernumerary synaptic connections by microglia also occurs during normal development to modify neural circuits [164]; however, this process can reactivate during aging or AD development, perhaps via the activation of complement-mediated mechanisms that coincide with Aβ plaque [165]. Yoshiyama reported a toxic role of microglial activation in AD, with microglial activation coinciding with synapse loss before Tau NFT appearance [166], probably by triggering other signaling pathways responsible for neuronal loss, such as the tumor necrosis factor (TNF) pathway [167]. As plaque load increases in AD, an individual axon may suffer multiple points of damage, and microglia appear unable to clear the amyloid burden during later-stage AD [168]. Nevertheless, microglial depletion does not affect plaque formation or maintenance and neuritic dystrophy [169]; therefore, a greater understanding of the balance between beneficial and detrimental roles remains crucial to an improved understanding of microglial relevance to AD-associated neuronal loss. Recently, the Amit group published unique data on a novel AD-associated microglia subtype initially activated in a Trem2-independent manner that involves microglial checkpoint downregulation followed by the activation of a Trem2-dependent program [170]. Therefore, these cells can restrict neurodegeneration, which may have critical implications in treating AD.
Developing efficient protocols for differentiating hiPSCs into microglia has helped highlight their significant role in AD pathogenesis. Microglia differentiated from AD-hiPSCs derived from patients bearing the APOE4 variant displayed a decreased ability to uptake Aβ peptide and alterations in the expression of pro-inflammatory signaling pathway genes compared to isogenic APOE3-hiPSC-derived microglia [130]. A recent study by Xu et al. demonstrated that microglia generated from sporadic AD-hiPSCs displayed alterations to cytokine secretion and apoptosis levels following lipopolysaccharide-mediated activation [171]. Although hiPSC-derived microglia represent a valuable tool to allow a more profound understanding of the mechanisms underlying AD pathogenesis, their role in AD remains controversial [172]. Thus, microglial cells require investigation in more sophisticated cellular systems; furthermore, detecting differential activities and hallmarks in disease-associated neuroglial cells, such as astrocytes and microglia, at early and presymptomatic stages of AD may unravel novel therapeutic targets.
Three-Dimensional hiPSC-based models of AD
The traditional 2D monolayer culture of hiPSC derivatives has allowed the discovery of disease-associated molecular events in AD by supporting the generation of a homogenous population of regional- or subtype-specific neurons, astrocytes, and microglia. Furthermore, this approach has fostered the development of cell therapies and supported drug screening in other neurodegenerative diseases; however, 2D monolayer cultures fail to fully recapitulate the in vivo characteristics of the different brain layers and lack interactions with other cells within specific tissue microenvironments, which represents a crucial aspect for AD studies.
Lancaster et al. first described the in vitro self-organization of hiPSCs into three-dimensional (3D) “cerebral” organoids that possessed stratified structures reminiscent of the developing brain [173]. These cerebral structures partially resembled their normal in vivo counterparts and provided an unprecedented experimental system to study developing human neural tissues [174]. Furthermore, cerebral organoids have aided investigations into the brain abnormalities associated with Zika virus infection [175] and microcephaly [173]. Unpatterned homogenous aggregates of hiPSCs spontaneously differentiate into highly-ordered 3D cerebral organoids that spatially and temporally mimic the developing brain with properly layered diverse neural cells similar to the human cerebral cortex [176], including the formation of dendritic spines and spontaneously active neuronal networks [177]. Overall, cerebral organoids represent a powerful tool for the study of brain development and disease evolution and may represent an exciting source of cells for replacement or reparative/regenerative therapeutic strategies in neurodegenerative disease treatment (Table 2).
Table 2.
3D iPSC-based Models of AD.
| 3D iPSC-based Models | Mutation/Genomic alteration | AD hallmarks | Reference (s) |
|---|---|---|---|
| fAD brain organoids/3D matrix | PSEN2 (N141I) | higher Aβ42/Aβ40 ratio, asynchronous calcium transients, enhanced neuronal hyperactivity | [82] |
| fAD brain organoids | APOE4 | Aβ plaques and hyperphosphorylated Tau | [130] |
| fAD organoids | APP duplication line/ PSEN1 (PSEN1M146I, PSEN1A264E) | higher amyloid aggregation and hyperphosphorylated Tau and endosomal abnormalities | [178] |
| fAD and Down syndrome-organoids | PSEN1 (PSEN1A264E) | accumulation of structures reminiscent to and trisomy of ch21 (Ts21) amyloid plaques and neurofibrillary tangles | [179] |
| fAD organoids | APOE4 | Aβ deposits, hyperphosphorylated Tau and decreased synaptic integrity | [180] |
| 3D human tri-culture in a | APP | Aβ aggregation, NFT formation, increased pro-inflammatory cytokines microfluidic platform microglial recruitment and neuron/astrocyte loss | [182] |
| fAD-hippocampal spheroids | APP/PSEN1 | increased Ab42/Ab40 peptide ratios and decreased synaptic protein levels | [185] |
Encouragingly, cerebral organoids have been generated from AD-hiPSCs under scaffold-free conditions [178]. Organoids derived from fAD-hiPSCs harboring an APP duplication or PSEN1 mutation recapitulated well-known AD pathologies, such as increased Aβ aggregation, Tau hyperphosphorylation, and endosomal abnormalities in an age-dependent manner [106], [178]. Similar characteristics have also been observed in organoids derived from TS21-hiPSCs [179]. Yin and VanDongen reported the creation of self-organizing 3D cerebral organoids with functional neuronal network connectivity by the neuroectodermal induction of hiPSC aggregates and subsequent differentiation into desired neuroepithelia and mature neurons from an fAD patient with a common mutation in PSEN2 (PSEN2N141I) [82]. Besides the higher Aβ42/Aβ40 ratio, the authors observed asynchronous calcium transients and enhanced neuronal hyperactivity in patient-derived cerebral organoids compared to mutation-corrected control organoids, successfully recapitulating an AD-like pathology at the molecular, cellular, and network level in a human genetic context. Moreover, the authors reverted this AD-related phenotype with two drugs that increase neuronal activity (4-aminopyridine (4-AP) and bicuculline methochloride), inducing high-frequency synchronized network bursting similar to control. Organoid-based approaches have also supported the investigation of sophisticated interactions between various cell types, such as those between neurons and astrocytes. The time frame of astrocyte generation in 3D culture may represent an important issue that directly correlates to in vitro disease features. In a recently published protocol, Quadrato et al. generated 3D structures that contained astrocytes generated with neurons at a later stage [177]. Using this approach, Raja et al. demonstrated that fAD-hiPSC-derived cerebral organoids typically exhibited Aβ deposits after two months in culture [178]; however, Lin et al. found that APOE4-variant hiPSC-derived organoids presented Aβ aggregates after six months, which correlated to the increased expression of APOE in neurons and the appearance of astrocytes in culture [130]. While the authors failed to observe alterations in astrocyte number and morphology, the study did reveal critical relationships between APOE, astrocytes, and neurons that affect AD pathogenesis. Of particular interest, a recent study by Zhao et al. derived cerebral organoids from hiPSCs generated from a patient carrying the APOE4 variant and investigated astrocytes and neurons, with an emphasis on synapses [180]. Beyond the observation of well-known AD features such as Aβ accumulation and increased levels of phosphorylated Tau, the authors observed more significant apoptosis and decreased synaptic integrity, with the latter reflected by the decreased expression of the presynaptic marker Synaptophysin and the postsynaptic marker PSD95.
Neuroinflammation and microglial activation play prominent roles in AD pathogenesis [181]; therefore, the development of a more complex organoid system must include microglia (among other relevant cells) to evaluate the role of neuroinflammation in AD pathology. A complex human tri-culture model system containing neurons, astrocytes, and microglia in an AD background enabled the study of human microglial recruitment, neuroinflammatory responses, and neuron or astrocyte damage [182]. Analysis of this sophisticated AD model confirmed the presence of Aβ plaques, Tau NFT formation, and the increased expression of pro-inflammatory cytokines, including C—C Motif Chemokine Ligand 2 (CCL2), Interleukin 8 (IL8), TNF Alfa (TNFα), and Interferon-γ (IFNγ), which, in turn, induced microglial recruitment and neuron or astrocyte loss [182]. Together, these results suggest that more sophisticated 3D culture systems involving multiple cell types represent a valid AD model that will contribute to the development of novel therapeutic targets.
Despite the reported advantages of cerebral organoids, significant variability in morphological and cellular features represents a significant drawback [183]. Efforts to increase the reproducibility of cerebral organoid formation include improving differentiation protocols [176], [184] and using bioreactors. Additional strategies in 3D modeling include the development of region-specific organoids, such as recently developed hippocampal spheroids [185]. As is the case in AD, certain brain regions are particularly vulnerable to specific degenerative processes and may exhibit neuronal dysfunction in early disease stages. The hippocampus plays a crucial role in memory formation and becomes severely affected during AD. Hippocampal spheroids generated from two AD-hiPSC lines carrying mutations in the APP/PSEN1 genes displayed AD-related alterations, such as Aβ protein aggregation, Tau hyperphosphorylation, and altered microRNA, gene, and protein expression profiles [185]. Hippocampal spheroids may represent an ideal platform for the development of therapeutic strategies and the investigation of molecular and cellular mechanisms involved in AD; however, they represent less sophisticated models than the human tri-culture model system.
Challenges to hiPSC disease modeling
The heterogeneity of hiPSC lineage-phenotypic cells and variations in cell lines represent some of the challenges in hiPSC-based modeling. Possessing an appropriate healthy cell as a control for comparisons has also become a critical issue in hiPSC-based disease. Control hiPSCs may be derived from family- or aged-matched healthy control individuals, although these hiPSCs show considerable heterogeneity, often providing challenging to interpret data. This issue can be overcome by creating isogenic hiPSC lines by gene editing approaches in the case of diseases caused by simple genetic mutations. By providing the same genetic background, isogenic hiPSCs can circumvent specific problems in deriving proper control cells; however, this approach faces other challenges, including the possible off-target effects of gene editing technologies.
Genetic instability represents another challenge in hiPSC modeling since the prolonged in vitro culture of hiPSCs can prompt the accumulation of chromosomal abnormalities, genetic instability, and copy number variants [186]. Accumulated genetic abnormalities can affect the further differentiation of these cells, data interpretation, and their future use in transplantation studies.
Adequate standardization of hiPSC differentiation protocols is yet another challenge. Laboratories across the world must establish intra- and inter-laboratory reproducibility of the hiPSC methodologies used to generate AD-affected cells, a feat that will require tight control of the protocols and factors involved in differentiation protocols.
Whether differentiated cell types faithfully exhibit aging-related effects (given that the reprogramming process rejuvenates aged cells) represents another concern related to hiPSC-based disease modeling. The reprogramming of aged fibroblasts into hiPSCs occurs alongside telomerase reactivation, changes to DNA methylation patterns and mitochondrial morphology (including decreases in reactive oxygen species generation), and a decrease in senescence-associated marker expression [187]. Another concern is the general difficulty in modeling late-onset diseases such as AD; however, Sánchez-Danés et al. attempted to evoke age-related effects using progerin overexpression in Parkinson's disease models [187], [188] or prolonged cell culture [188]. hiPSC-derived organoids recapitulate early time points in human brain development, replicating the middle and end of the eight-to-ten gestational week stage but not later stages of human brain development [174], [189].
The generation of cells primarily affected by AD that display aging features could be resolved by bypassing the hiPSC stage of reprogramming and directly converting mature somatic cells, such as fibroblasts [190], urine epithelial cells [191], and peripheral blood T cells [192] into neurons or astrocytes. Direct reprogramming avoids the resetting of age-associated epigenetic modifications and may support the development of more faithful AD models [193], [194]. Encouragingly, the most common neuronal conversion methods (the overexpression of master transcription factors using lentiviral vectors or small molecule inhibitor cocktails) represent clinically-relevant approaches [195], [196].
Hu et al. employed a defined mixture of seven small molecules to directly reprogram patient-derived fibroblasts bearing mutations in the PSEN1 (S169Del) or APP (V717I) gene into neurons [197], which resembled hiPSC-derived neurons with respect to their gene expression profile, electrophysiological properties, and AD hallmarks (e.g., increased Aβ plaques deposition and Tau NFTs). While direct reprogramming does not allow the generation of sophisticated brain tissue structures, preserving the age-associated epigenetic profile of neurons may provide a means to explore the aging process in AD (which represents a significant overall risk factor) to a better degree than hiPSC-based studies.
Conclusions
Despite exhibiting less pronounced disease progression, sporadic AD-hiPSC models display broad similarities to fAD-hiPSC models, including Aβ deposition and increased Tau phosphorylation in differentiated cells. Though related studies have provided robust evidence for the utility of hiPSCs in AD research, significant limitations exist. While 2D cultures of AD-hiPSC-derived cells support investigations into the cellular and molecular mechanisms involved in AD physiopathology and the evaluation of potential therapeutics, they do not faithfully recapitulate in vivo brain tissue, thereby limiting the scope of investigations. The development of sophisticated 3D and co-culture systems may provide partial solutions, and they currently represent a more faithful AD model than 2D systems [95]. While co-culture with microglia in 3D systems promotes the development of AD in a more physiologically relevant context by considering inflammation (one of the most important aspects associated with AD), prohibitive costs, elevated levels of variability, and significant tissue heterogeneity have limited their broader application. Furthermore, these models do not fully recapitulate the distinct cortical neuronal layers, gyrification, or the establishment of complex neuronal circuitry [198]. Continuous improvements to co-culture systems using a more physiological context that considers different neuronal subtypes, glia, and cells comprising the BBB are currently in progress and involve the implementation of sophisticated bioreactors combined with scaffolds [199]. These systems provide constant oxygen and nutrients that overcome the lack of vascular and endothelial cells. As cerebral organoids mimic the temporal progression of human brain development, they offer a versatile platform to investigate AD at different developmental stages; however, a lack of mature hiPSC-derived cells represents a fundamental drawback to the investigation of late-onset aging-related diseases such as AD. Therefore, additional model optimization may provide a refined biomimetic brain microenvironment for AD modeling that will serve for high-throughput screening to develop efficient pharmacological treatment strategies for human patients.
Funding
This work was supported SE) grant PI21-00157 (SE) of Instituto de Salud Carlos III (ISCIII) from the Spanish Ministry of Science, Innovation, and Universities-FEDER (European Regional Development Fund), and by the project “Centre of Reconstructive Neuroscience,” registration number CZ.02.1.01/0.0./0.0/15_003/0000419.
Compliance with Ethics Requirements
We state that this review article is in compliance with Ethics Requirements Signed by Corresponding author on behalf of all authors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We acknowledge Servier Medical Art for providing elements within Fig. 1. We also acknowledge Dr. Stuart P. Atkinson for article reviewing and English language editing. This work was supported SE) grant PI21-00157 (SE) of Instituto de Salud Carlos III (ISCIII) from the Spanish Ministry of Science, Innovation, and Universities-FEDER (European Regional Development Fund), and by the project “Centre of Reconstructive Neuroscience,” registration number CZ.02.1.01/0.0./0.0/15_003/0000419.
Biographies

Francisco Javier Rodriguez-Jimenez is senior postdoc in Erceg,s Lab. He is involved in 2 current approaches related to AD disease such as characterization of “pinwheel” neurogenic structures in brain created in ependymal neurogenic niche observed in rat AD models in brain and spinal cord. The abnormal modifications of these structures could be faithful hallmarks of AD. He is responsible for recompilation of recent literature related to hiPSC models for AD in this review manuscript. He is main authors as well as co-author of more than 33 articles related to neurobiology with h index 11.

Juan Ureña, PhD with extensive experience in neurotoxicology is recently incorporated investigator in Erceǵs lab (Stem Cell Therapies in Neurodegenerative Disease), He is responsible for supervision of technical staff in the project of generation of neuronal progenitors and brain organoids from AD hiPSC cells. He is the first author and author of several peer-reviewed articles related to neurotoxicity.

Pavla Jendelová is the head of the Department of Nerve Tissue Regeneration at the Institute of Experimental Medicine of the Czech Academy of Sciences. The main topics studied in the Department are isolation, labelling and the use of stem cells for the treatment of brain injury, spinal cord and neurodegenerative diseases (AD, ALS). Various types of cells (mesenchymal stem cells, neural precursor cell lines derived from fetal spinal cord, or from induced pluripotent cells) are studied, together with anti-inflammatory substances for their potential to promote the regeneration of nervous tissue. Her group has long been collaborating with the Institute of Macromolecular Chemistry on the development of biomaterials for the CNS, especially for combination therapy in the treatment of spinal cord injury and brain tumours and theranostic magnetic nanoparticles for cell labelling and drug delivery. She currently co-leads the Centre for Reconstructive Neuroscience (NeuroRecon) with James Fawcett from the University of Cambridge, focusing on neural tissue regeneration and plasticity using viral vectors for gene transfer and manipulation of the extracellular matrix in neurodegenerative diseases and aging. She has published over 125 publications that were cited more than 5000 times. Her H index is 42.

Professor Dr. Slaven Erceg is Group Leader of Stem Cell Therapies in Neurodegenerative Disease and Director of Spanish Stem Cell Bank, Valencia. He is Experimental Stem Cell Biologist and Neurobiologist with extensive experience in neural differentiation of pluripotent and multipotent stem cells toward astrocytes and neurons as well as 3D brain organoids in order to create the faithful human cellular models of neurodegenerative diseases and cell therapies. In the context of Alzheimer disease, currently he is a principal investigator of 2 ongoing project in which the main objectives are to generated neurons and brain organoids from human induced pluripotent stem cells (hiPSC) derived from patients with Familial Alzheimer disease with known genetic mutations. As director of Spanish Stem Cell Bank, Valencia his main tasks are supervision the generation, characterization and cryopreservation of hiPSC from different diseases including Alzheimer disease as well as from healthy individuals. Spanish Stem Cell Bank, Valencia contains well characterized hiPSC derived from Familial and sporadic Alzheimer disease. His research in neuroregenerative field was granted by different national and international agencies and public and private Foundations such as Health Institute Carlos III (Miguel Servet Contract, and 3 Spanish national grants), Wings For Life Foundation, Andalusian local government, in competitive calls. He has a fruitful research record with more than 90 peer-reviewed article which many of them are with high impact factors Progress in Neurobiology, Stem Cells, J of Hepatology etc. with h index 27.
Footnotes
Peer review under responsibility of Cairo University.
Contributor Information
Francisco Javier Rodriguez-Jimenez, Email: frodriguez@cipf.es.
Juan Ureña-Peralta, Email: jurena@cipf.es.
Pavla Jendelova, Email: pavla.jendelova@iem.cas.cz.
Slaven Erceg, Email: serceg@cipf.es.
References
- 1.Hort J., O'Brien J.T., Gainotti G., Pirttila T., Popescu B.O., Rektorova I., et al. EFNS guidelines for the diagnosis and management of Alzheimer's disease. European journal of neurology : the official journal of the European Federation of Neurological Societies. 2010;17:1236–1248. doi: 10.1111/j.1468-1331.2010.03040.x. [DOI] [PubMed] [Google Scholar]
- 2.Frisoni G.B., Fox N.C., Jack C.R., Jr., Scheltens P., Thompson P.M. The clinical use of structural MRI in Alzheimer disease. Nat Rev Neurol. 2010;6:67–77. doi: 10.1038/nrneurol.2009.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.S.J. Crutch, J.M. Schott, G.D. Rabinovici, M. Murray, J.S. Snowden, W.M. van der Flier, B.C. Dickerson, R. Vandenberghe, S. Ahmed, T.H. Bak, B.F. Boeve, C. Butler, S.F. Cappa, M. Ceccaldi, L.C. de Souza, B. Dubois, O. Felician, D. Galasko, J. Graff-Radford, N.R. Graff-Radford, P.R. Hof, P. Krolak-Salmon, M. Lehmann, E. Magnin, M.F. Mendez, P.J. Nestor, C.U. Onyike, V.S. Pelak, Y. Pijnenburg, S. Primativo, M.N. Rossor, N.S. Ryan, P. Scheltens, T.J. Shakespeare, A. Suarez Gonzalez, D.F. Tang-Wai, K.X.X. Yong, M. Carrillo, N.C. Fox, I.A.A.s.D. Alzheimer's Association, and A. Associated Syndromes Professional Interest, Consensus classification of posterior cortical atrophy. Alzheimers Dement 13 (2017) 870-884. [DOI] [PMC free article] [PubMed]
- 4.Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 5.Selkoe D.J. The molecular pathology of Alzheimer's disease. Neuron. 1991;6:487–498. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- 6.Frost B., Jacks R.L., Diamond M.I. Propagation of tau misfolding from the outside to the inside of a cell. J Biol Chem. 2009;284:12845–12852. doi: 10.1074/jbc.M808759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butterfield D.A., Halliwell B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat Rev Neurosci. 2019;20:148–160. doi: 10.1038/s41583-019-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeKosky S.T., Scheff S.W. Synapse loss in frontal cortex biopsies in Alzheimer's disease: correlation with cognitive severity. Ann Neurol. 1990;27:457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- 9.Terry R.D., Masliah E., Salmon D.P., Butters N., DeTeresa R., Hill R., et al. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 10.Edwards F.A. A Unifying Hypothesis for Alzheimer's Disease: From Plaques to Neurodegeneration. Trends Neurosci. 2019;42:310–322. doi: 10.1016/j.tins.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Iacono D., O'Brien R., Resnick S.M., Zonderman A.B., Pletnikova O., Rudow G., et al. Neuronal hypertrophy in asymptomatic Alzheimer disease. J Neuropathol Exp Neurol. 2008;67:578–589. doi: 10.1097/NEN.0b013e3181772794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sasaguri H., Nilsson P., Hashimoto S., Nagata K., Saito T., De Strooper B., et al. APP mouse models for Alzheimer's disease preclinical studies. EMBO J. 2017;36:2473–2487. doi: 10.15252/embj.201797397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bateman R.J., Aisen P.S., De Strooper B., Fox N.C., Lemere C.A., Ringman J.M., et al. Autosomal-dominant Alzheimer's disease: a review and proposal for the prevention of Alzheimer's disease. Alzheimers Res Ther. 2011;3:1. doi: 10.1186/alzrt59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verghese P.B., Castellano J.M., Holtzman D.M. Apolipoprotein E in Alzheimer's disease and other neurological disorders. Lancet Neurol. 2011;10:241–252. doi: 10.1016/S1474-4422(10)70325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karch C.M., Goate A.M. Alzheimer's disease risk genes and mechanisms of disease pathogenesis. Biol Psychiatry. 2015;77:43–51. doi: 10.1016/j.biopsych.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kent S.A., Spires-Jones T.L., Durrant C.S. The physiological roles of tau and Abeta: implications for Alzheimer's disease pathology and therapeutics. Acta Neuropathol. 2020;140:417–447. doi: 10.1007/s00401-020-02196-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scearce-Levie K., Sanchez P.E., Lewcock J.W. Leveraging preclinical models for the development of Alzheimer disease therapeutics. Nat Rev Drug Discov. 2020;19:447–462. doi: 10.1038/s41573-020-0065-9. [DOI] [PubMed] [Google Scholar]
- 18.Serrano-Pozo A., Frosch M.P., Masliah E., Hyman B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med. 2011;1 doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fotuhi M., Hachinski V., Whitehouse P.J. Changing perspectives regarding late-life dementia. Nat Rev Neurol. 2009;5:649–658. doi: 10.1038/nrneurol.2009.175. [DOI] [PubMed] [Google Scholar]
- 20.Hardy J., Selkoe D.J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 21.Musiek E.S., Holtzman D.M. Three dimensions of the amyloid hypothesis: time, space and 'wingmen'. Nat Neurosci. 2015;18:800–806. doi: 10.1038/nn.4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Selkoe D.J., Hardy J. The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol Med. 2016;8:595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hyman B.T. Amyloid-dependent and amyloid-independent stages of Alzheimer disease. Arch Neurol. 2011;68:1062–1064. doi: 10.1001/archneurol.2011.70. [DOI] [PubMed] [Google Scholar]
- 24.Holtzman D.M., Carrillo M.C., Hendrix J.A., Bain L.J., Catafau A.M., Gault L.M., et al. Tau: From research to clinical development. Alzheimers Dement. 2016;12:1033–1039. doi: 10.1016/j.jalz.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 25.Braak H., Braak E. Alzheimer's disease: striatal amyloid deposits and neurofibrillary changes. J Neuropathol Exp Neurol. 1990;49:215–224. [PubMed] [Google Scholar]
- 26.Nagy Z., Esiri M.M., Jobst K.A., Johnston C., Litchfield S., Sim E., et al. Influence of the apolipoprotein E genotype on amyloid deposition and neurofibrillary tangle formation in Alzheimer's disease. Neuroscience. 1995;69:757–761. doi: 10.1016/0306-4522(95)00331-c. [DOI] [PubMed] [Google Scholar]
- 27.Abramov E., Dolev I., Fogel H., Ciccotosto G.D., Ruff E., Slutsky I. Amyloid-beta as a positive endogenous regulator of release probability at hippocampal synapses. Nat Neurosci. 2009;12:1567–1576. doi: 10.1038/nn.2433. [DOI] [PubMed] [Google Scholar]
- 28.Puzzo D., Privitera L., Fa M., Staniszewski A., Hashimoto G., Aziz F., et al. Endogenous amyloid-beta is necessary for hippocampal synaptic plasticity and memory. Ann Neurol. 2011;69:819–830. doi: 10.1002/ana.22313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spires T.L., Meyer-Luehmann M., Stern E.A., McLean P.J., Skoch J., Nguyen P.T., et al. Dendritic spine abnormalities in amyloid precursor protein transgenic mice demonstrated by gene transfer and intravital multiphoton microscopy. J Neurosci. 2005;25:7278–7287. doi: 10.1523/JNEUROSCI.1879-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burgold S., Bittner T., Dorostkar M.M., Kieser D., Fuhrmann M., Mitteregger G., et al. In vivo multiphoton imaging reveals gradual growth of newborn amyloid plaques over weeks. Acta Neuropathol. 2011;121:327–335. doi: 10.1007/s00401-010-0787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu H.Y., Hudry E., Hashimoto T., Uemura K., Fan Z.Y., Berezovska O., et al. Distinct dendritic spine and nuclear phases of calcineurin activation after exposure to amyloid-beta revealed by a novel fluorescence resonance energy transfer assay. J Neurosci. 2012;32:5298–5309. doi: 10.1523/JNEUROSCI.0227-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Decker H., Jurgensen S., Adrover M.F., Brito-Moreira J., Bomfim T.R., Klein W.L., et al. N-methyl-D-aspartate receptors are required for synaptic targeting of Alzheimer's toxic amyloid-beta peptide oligomers. J Neurochem. 2010;115:1520–1529. doi: 10.1111/j.1471-4159.2010.07058.x. [DOI] [PubMed] [Google Scholar]
- 33.Birnbaum J.H., Bali J., Rajendran L., Nitsch R.M., Tackenberg C. Calcium flux-independent NMDA receptor activity is required for Abeta oligomer-induced synaptic loss. Cell Death Dis. 2015;6:e1791. doi: 10.1038/cddis.2015.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karran E., De Strooper B. The amyloid cascade hypothesis: are we poised for success or failure? J Neurochem. 2016;139(Suppl 2):237–252. doi: 10.1111/jnc.13632. [DOI] [PubMed] [Google Scholar]
- 35.Wu H.Y., Kuo P.C., Wang Y.T., Lin H.T., Roe A.D., Wang B.Y., et al. beta-Amyloid Induces Pathology-Related Patterns of Tau Hyperphosphorylation at Synaptic Terminals. J Neuropathol Exp Neurol. 2018;77:814–826. doi: 10.1093/jnen/nly059. [DOI] [PubMed] [Google Scholar]
- 36.Howlett D.R., Bowler K., Soden P.E., Riddell D., Davis J.B., Richardson J.C., et al. Abeta deposition and related pathology in an APP x PS1 transgenic mouse model of Alzheimer's disease. Histol Histopathol. 2008;23:67–76. doi: 10.14670/HH-23.67. [DOI] [PubMed] [Google Scholar]
- 37.Jack C.R., Jr., Bennett D.A., Blennow K., Carrillo M.C., Dunn B., Haeberlein S.B., et al. and Contributors, NIA-AA Research Framework: Toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zempel H., Luedtke J., Kumar Y., Biernat J., Dawson H., Mandelkow E., et al. Amyloid-beta oligomers induce synaptic damage via Tau-dependent microtubule severing by TTLL6 and spastin. EMBO J. 2013;32:2920–2937. doi: 10.1038/emboj.2013.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zempel H., Thies E., Mandelkow E., Mandelkow E.M. Abeta oligomers cause localized Ca(2+) elevation, missorting of endogenous Tau into dendrites, Tau phosphorylation, and destruction of microtubules and spines. J Neurosci. 2010;30:11938–11950. doi: 10.1523/JNEUROSCI.2357-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rozkalne A., Hyman B.T., Spires-Jones T.L. Calcineurin inhibition with FK506 ameliorates dendritic spine density deficits in plaque-bearing Alzheimer model mice. Neurobiol Dis. 2011;41:650–654. doi: 10.1016/j.nbd.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu H.Y., Hudry E., Hashimoto T., Kuchibhotla K., Rozkalne A., Fan Z., et al. Amyloid beta induces the morphological neurodegenerative triad of spine loss, dendritic simplification, and neuritic dystrophies through calcineurin activation. J Neurosci. 2010;30:2636–2649. doi: 10.1523/JNEUROSCI.4456-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen R.M., Rezai-Zadeh K., Weitz T.M., Rentsendorj A., Gate D., Spivak I., et al. A transgenic Alzheimer rat with plaques, tau pathology, behavioral impairment, oligomeric abeta, and frank neuronal loss. J Neurosci. 2013;33:6245–6256. doi: 10.1523/JNEUROSCI.3672-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pooler A.M., Polydoro M., Wegmann S., Nicholls S.B., Spires-Jones T.L., Hyman B.T. Propagation of tau pathology in Alzheimer's disease: identification of novel therapeutic targets. Alzheimers Res Ther. 2013;5:49. doi: 10.1186/alzrt214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kashyap G., Bapat D., Das D., Gowaikar R., Amritkar R.E., Rangarajan G., et al. Synapse loss and progress of Alzheimer's disease -A network model. Sci Rep. 2019;9:6555. doi: 10.1038/s41598-019-43076-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pereira J.B., Janelidze S., Ossenkoppele R., Kvartsberg H., Brinkmalm A., Mattsson-Carlgren N., et al. Untangling the association of amyloid-beta and tau with synaptic and axonal loss in Alzheimer's disease. Brain. 2021;144:310–324. doi: 10.1093/brain/awaa395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Allegra Mascaro A.L., Cesare P., Sacconi L., Grasselli G., Mandolesi G., Maco B., et al. In vivo single branch axotomy induces GAP-43-dependent sprouting and synaptic remodeling in cerebellar cortex. Proc Natl Acad Sci U S A. 2013;110:10824–10829. doi: 10.1073/pnas.1219256110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhong L., Gerges N.Z. Neurogranin and synaptic plasticity balance. Commun Integr Biol. 2010;3:340–342. doi: 10.4161/cib.3.4.11763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Danysz W., Parsons C.G. Alzheimer's disease, beta-amyloid, glutamate, NMDA receptors and memantine–searching for the connections. Br J Pharmacol. 2012;167:324–352. doi: 10.1111/j.1476-5381.2012.02057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sheline Y.I., Raichle M.E. Resting state functional connectivity in preclinical Alzheimer's disease. Biol Psychiatry. 2013;74:340–347. doi: 10.1016/j.biopsych.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huijbers W., Mormino E.C., Schultz A.P., Wigman S., Ward A.M., Larvie M., et al. Amyloid-beta deposition in mild cognitive impairment is associated with increased hippocampal activity, atrophy and clinical progression. Brain. 2015;138:1023–1035. doi: 10.1093/brain/awv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sepulcre J., Sabuncu M.R., Li Q., El Fakhri G., Sperling R., Johnson K.A. Tau and amyloid beta proteins distinctively associate to functional network changes in the aging brain. Alzheimers Dement. 2017;13:1261–1269. doi: 10.1016/j.jalz.2017.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spires-Jones T.L., Stoothoff W.H., de Calignon A., Jones P.B., Hyman B.T. Tau pathophysiology in neurodegeneration: a tangled issue. Trends Neurosci. 2009;32:150–159. doi: 10.1016/j.tins.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 53.Oxford A.E., Stewart E.S., Rohn T.T. Clinical Trials in Alzheimer's Disease: A Hurdle in the Path of Remedy. Int J Alzheimers Dis. 2020;2020:5380346. doi: 10.1155/2020/5380346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pascoal T.A., Benedet A.L., Ashton N.J., Kang M.S., Therriault J., Chamoun M., et al. Microglial activation and tau propagate jointly across Braak stages. Nat Med. 2021;27:1592–1599. doi: 10.1038/s41591-021-01456-w. [DOI] [PubMed] [Google Scholar]
- 55.Braak H., Braak E., Bohl J. Staging of Alzheimer-related cortical destruction. Eur Neurol. 1993;33:403–408. doi: 10.1159/000116984. [DOI] [PubMed] [Google Scholar]
- 56.Raichle M.E., MacLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., Shulman G.L. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Minoshima S., Giordani B., Berent S., Frey K.A., Foster N.L., Kuhl D.E. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer's disease. Ann Neurol. 1997;42:85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- 58.Johnson K.A., Jones K., Holman B.L., Becker J.A., Spiers P.A., Satlin A., et al. Preclinical prediction of Alzheimer's disease using SPECT. Neurology. 1998;50:1563–1571. doi: 10.1212/wnl.50.6.1563. [DOI] [PubMed] [Google Scholar]
- 59.Matsuda H. Cerebral blood flow and metabolic abnormalities in Alzheimer's disease. Ann Nucl Med. 2001;15:85–92. doi: 10.1007/BF02988596. [DOI] [PubMed] [Google Scholar]
- 60.Bradley K.M., O'Sullivan V.T., Soper N.D., Nagy Z., King E.M., Smith A.D., et al. Cerebral perfusion SPET correlated with Braak pathological stage in Alzheimer's disease. Brain. 2002;125:1772–1781. doi: 10.1093/brain/awf185. [DOI] [PubMed] [Google Scholar]
- 61.Greicius M.D., Srivastava G., Reiss A.L., Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.De Strooper B., Karran E. The Cellular Phase of Alzheimer's Disease. Cell. 2016;164:603–615. doi: 10.1016/j.cell.2015.12.056. [DOI] [PubMed] [Google Scholar]
- 63.Styr B., Slutsky I. Imbalance between firing homeostasis and synaptic plasticity drives early-phase Alzheimer's disease. Nat Neurosci. 2018;21:463–473. doi: 10.1038/s41593-018-0080-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lam A.D., Deck G., Goldman A., Eskandar E.N., Noebels J., Cole A.J. Silent hippocampal seizures and spikes identified by foramen ovale electrodes in Alzheimer's disease. Nat Med. 2017;23:678–680. doi: 10.1038/nm.4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vossel K.A., Beagle A.J., Rabinovici G.D., Shu H., Lee S.E., Naasan G., et al. Seizures and epileptiform activity in the early stages of Alzheimer disease. JAMA Neurol. 2013;70:1158–1166. doi: 10.1001/jamaneurol.2013.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haberman R.P., Branch A., Gallagher M. Targeting Neural Hyperactivity as a Treatment to Stem Progression of Late-Onset Alzheimer's Disease. Neurotherapeutics. 2017;14:662–676. doi: 10.1007/s13311-017-0541-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mucke L., Selkoe D.J. Neurotoxicity of amyloid beta-protein: synaptic and network dysfunction. Cold Spring Harb Perspect Med. 2012;2 doi: 10.1101/cshperspect.a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mesulam M.M. Neuroplasticity failure in Alzheimer's disease: bridging the gap between plaques and tangles. Neuron. 1999;24:521–529. doi: 10.1016/s0896-6273(00)81109-5. [DOI] [PubMed] [Google Scholar]
- 69.Nimmrich V., Ebert U. Is Alzheimer's disease a result of presynaptic failure? Synaptic dysfunctions induced by oligomeric beta-amyloid. Rev Neurosci. 2009;20:1–12. doi: 10.1515/revneuro.2009.20.1.1. [DOI] [PubMed] [Google Scholar]
- 70.Selkoe D.J. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 71.Palop J.J., Mucke L. Epilepsy and cognitive impairments in Alzheimer disease. Arch Neurol. 2009;66:435–440. doi: 10.1001/archneurol.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Palop J.J., Mucke L. Network abnormalities and interneuron dysfunction in Alzheimer disease. Nat Rev Neurosci. 2016;17:777–792. doi: 10.1038/nrn.2016.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kordower J.H., Chu Y., Stebbins G.T., DeKosky S.T., Cochran E.J., Bennett D., et al. Loss and atrophy of layer II entorhinal cortex neurons in elderly people with mild cognitive impairment. Ann Neurol. 2001;49:202–213. [PubMed] [Google Scholar]
- 74.Nygaard H.B., Kaufman A.C., Sekine-Konno T., Huh L.L., Going H., Feldman S.J., et al. Brivaracetam, but not ethosuximide, reverses memory impairments in an Alzheimer's disease mouse model. Alzheimers Res Ther. 2015;7:25. doi: 10.1186/s13195-015-0110-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Verret L., Mann E.O., Hang G.B., Barth A.M., Cobos I., Ho K., et al. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell. 2012;149:708–721. doi: 10.1016/j.cell.2012.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Quiroz Y.T., Budson A.E., Celone K., Ruiz A., Newmark R., Castrillon G., et al. Hippocampal hyperactivation in presymptomatic familial Alzheimer's disease. Ann Neurol. 2010;68:865–875. doi: 10.1002/ana.22105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Frere S., Slutsky I. Alzheimer's Disease: From Firing Instability to Homeostasis Network Collapse. Neuron. 2018;97:32–58. doi: 10.1016/j.neuron.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 78.Kapogiannis D., Mattson M.P. Disrupted energy metabolism and neuronal circuit dysfunction in cognitive impairment and Alzheimer's disease. Lancet Neurol. 2011;10:187–198. doi: 10.1016/S1474-4422(10)70277-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Terada T., Obi T., Bunai T., Matsudaira T., Yoshikawa E., Ando I., et al. In vivo mitochondrial and glycolytic impairments in patients with Alzheimer disease. Neurology. 2020;94:e1592–e1604. doi: 10.1212/WNL.0000000000009249. [DOI] [PubMed] [Google Scholar]
- 80.Dhapola R., Sarma P., Medhi B., Prakash A., Reddy D.H. Recent Advances in Molecular Pathways and Therapeutic Implications Targeting Mitochondrial Dysfunction for Alzheimer's Disease. Mol Neurobiol. 2022;59:535–555. doi: 10.1007/s12035-021-02612-6. [DOI] [PubMed] [Google Scholar]
- 81.Kerr J.S., Adriaanse B.A., Greig N.H., Mattson M.P., Cader M.Z., Bohr V.A., et al. Mitophagy and Alzheimer's Disease: Cellular and Molecular Mechanisms. Trends Neurosci. 2017;40:151–166. doi: 10.1016/j.tins.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yin J., VanDongen A.M. Enhanced Neuronal Activity and Asynchronous Calcium Transients Revealed in a 3D Organoid Model of Alzheimer's Disease. ACS Biomater Sci Eng. 2021;7:254–264. doi: 10.1021/acsbiomaterials.0c01583. [DOI] [PubMed] [Google Scholar]
- 83.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 84.Yu J., Vodyanik M.A., Smuga-Otto K., Antosiewicz-Bourget J., Frane J.L., Tian S., et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 85.Li X.J., Zhang X., Johnson M.A., Wang Z.B., Lavaute T., Zhang S.C. Coordination of sonic hedgehog and Wnt signaling determines ventral and dorsal telencephalic neuron types from human embryonic stem cells. Development. 2009;136:4055–4063. doi: 10.1242/dev.036624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Erceg S., Lainez S., Ronaghi M., Stojkovic P., Perez-Arago M.A., Moreno-Manzano V., et al. Differentiation of human embryonic stem cells to regional specific neural precursors in chemically defined medium conditions. PLoS One. 2008;3:e2122. doi: 10.1371/journal.pone.0002122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu Y., Liu H., Sauvey C., Yao L., Zarnowska E.D., Zhang S.C. Directed differentiation of forebrain GABA interneurons from human pluripotent stem cells. Nat Protoc. 2013;8:1670–1679. doi: 10.1038/nprot.2013.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Duan L., Bhattacharyya B.J., Belmadani A., Pan L., Miller R.J., Kessler J.A. Stem cell derived basal forebrain cholinergic neurons from Alzheimer's disease patients are more susceptible to cell death. Mol Neurodegener. 2014;9:3. doi: 10.1186/1750-1326-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chambers S.M., Fasano C.A., Papapetrou E.P., Tomishima M., Sadelain M., Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.B. Foveau, A.S. Correia, S.S. Hebert, S. Rainone, O. Potvin, M.J. Kergoat, S. Belleville, S. Duchesne, A.C. LeBlanc, and C.-Q.C.f.t.e.i.o.A.s.d.-Q. the, Stem Cell-Derived Neurons as Cellular Models of Sporadic Alzheimer's Disease. Journal of Alzheimer's disease : JAD 67 (2019) 893-910. [DOI] [PubMed]
- 91.Ochalek A., Mihalik B., Avci H.X., Chandrasekaran A., Teglasi A., Bock I., et al. Neurons derived from sporadic Alzheimer's disease iPSCs reveal elevated TAU hyperphosphorylation, increased amyloid levels, and GSK3B activation. Alzheimers Res Ther. 2017;9:90. doi: 10.1186/s13195-017-0317-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jafari Z., Kolb B.E., Mohajerani M.H. Neural oscillations and brain stimulation in Alzheimer's disease. Prog Neurobiol. 2020;194 doi: 10.1016/j.pneurobio.2020.101878. [DOI] [PubMed] [Google Scholar]
- 93.Kim K., Lee C.H., Park C.B. Chemical sensing platforms for detecting trace-level Alzheimer's core biomarkers. Chem Soc Rev. 2020;49:5446–5472. doi: 10.1039/d0cs00107d. [DOI] [PubMed] [Google Scholar]
- 94.Sala Frigerio C., De Strooper B. Alzheimer's Disease Mechanisms and Emerging Roads to Novel Therapeutics. Annu Rev Neurosci. 2016;39:57–79. doi: 10.1146/annurev-neuro-070815-014015. [DOI] [PubMed] [Google Scholar]
- 95.Choi S.H., Kim Y.H., Hebisch M., Sliwinski C., Lee S., D'Avanzo C., et al. A three-dimensional human neural cell culture model of Alzheimer's disease. Nature. 2014;515:274–278. doi: 10.1038/nature13800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Oberheim N.A., Takano T., Han X., He W., Lin J.H., Wang F., et al. Uniquely hominid features of adult human astrocytes. J Neurosci. 2009;29:3276–3287. doi: 10.1523/JNEUROSCI.4707-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.[The role of Italian cardiology in the Italian Group for the Study of Streptokinase in Myocardial Infarct. Distribution, structural and organizational characteristics of coronary intensive care units]. G Ital Cardiol 17 (1987) 14-9. [PubMed]
- 98.Smith A.M., Dragunow M. The human side of microglia. Trends Neurosci. 2014;37:125–135. doi: 10.1016/j.tins.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 99.Palop J.J., Mucke L. Amyloid-beta-induced neuronal dysfunction in Alzheimer's disease: from synapses toward neural networks. Nat Neurosci. 2010;13:812–818. doi: 10.1038/nn.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bertram L., Lill C.M., Tanzi R.E. The genetics of Alzheimer disease: back to the future. Neuron. 2010;68:270–281. doi: 10.1016/j.neuron.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 101.Yagi T., Ito D., Okada Y., Akamatsu W., Nihei Y., Yoshizaki T., et al. Modeling familial Alzheimer's disease with induced pluripotent stem cells. Hum Mol Genet. 2011;20:4530–4539. doi: 10.1093/hmg/ddr394. [DOI] [PubMed] [Google Scholar]
- 102.Armijo E., Gonzalez C., Shahnawaz M., Flores A., Davis B., Soto C. Increased susceptibility to Abeta toxicity in neuronal cultures derived from familial Alzheimer's disease (PSEN1-A246E) induced pluripotent stem cells. Neurosci Lett. 2017;639:74–81. doi: 10.1016/j.neulet.2016.12.060. [DOI] [PubMed] [Google Scholar]
- 103.Moore S., Evans L.D., Andersson T., Portelius E., Smith J., Dias T.B., et al. APP metabolism regulates tau proteostasis in human cerebral cortex neurons. Cell Rep. 2015;11:689–696. doi: 10.1016/j.celrep.2015.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang J., Zhao H., Ma Y., Shi G., Song J., Tang Y., et al. Early pathogenic event of Alzheimer's disease documented in iPSCs from patients with PSEN1 mutations. Oncotarget. 2017;8:7900–7913. doi: 10.18632/oncotarget.13776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sproul A.A., Jacob S., Pre D., Kim S.H., Nestor M.W., Navarro-Sobrino M., et al. Characterization and molecular profiling of PSEN1 familial Alzheimer's disease iPSC-derived neural progenitors. PLoS One. 2014;9:e84547. doi: 10.1371/journal.pone.0084547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Israel M.A., Yuan S.H., Bardy C., Reyna S.M., Mu Y., Herrera C., et al. Probing sporadic and familial Alzheimer's disease using induced pluripotent stem cells. Nature. 2012;482:216–220. doi: 10.1038/nature10821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chang C.Y., Ting H.C., Liu C.A., Su H.L., Chiou T.W., Harn H.J., et al. Induced Pluripotent Stem Cells: A Powerful Neurodegenerative Disease Modeling Tool for Mechanism Study and Drug Discovery. Cell Transplant. 2018;27:1588–1602. doi: 10.1177/0963689718775406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chang K.H., Lee-Chen G.J., Huang C.C., Lin J.L., Chen Y.J., Wei P.C., et al. Modeling Alzheimer's Disease by Induced Pluripotent Stem Cells Carrying APP D678H Mutation. Mol Neurobiol. 2019;56:3972–3983. doi: 10.1007/s12035-018-1336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kondo T., Asai M., Tsukita K., Kutoku Y., Ohsawa Y., Sunada Y., et al. Modeling Alzheimer's disease with iPSCs reveals stress phenotypes associated with intracellular Abeta and differential drug responsiveness. Cell Stem Cell. 2013;12:487–496. doi: 10.1016/j.stem.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 110.Muratore C.R., Zhou C., Liao M., Fernandez M.A., Taylor W.M., Lagomarsino V.N., et al. Cell-type Dependent Alzheimer's Disease Phenotypes: Probing the Biology of Selective Neuronal Vulnerability. Stem Cell Rep. 2017;9:1868–1884. doi: 10.1016/j.stemcr.2017.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wenk G.L. Neuropathologic changes in Alzheimer's disease. J Clin Psychiatry. 2003;64(Suppl 9):7–10. [PubMed] [Google Scholar]
- 112.Liao M.C., Muratore C.R., Gierahn T.M., Sullivan S.E., Srikanth P., De Jager P.L., et al. Single-Cell Detection of Secreted Abeta and sAPPalpha from Human IPSC-Derived Neurons and Astrocytes. J Neurosci. 2016;36:1730–1746. doi: 10.1523/JNEUROSCI.2735-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vazin T., Ball K.A., Lu H., Park H., Ataeijannati Y., Head-Gordon T., et al. Efficient derivation of cortical glutamatergic neurons from human pluripotent stem cells: a model system to study neurotoxicity in Alzheimer's disease. Neurobiol Dis. 2014;62:62–72. doi: 10.1016/j.nbd.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hamlett E.D., Ledreux A., Potter H., Chial H.J., Patterson D., Espinosa J.M., et al. Exosomal biomarkers in Down syndrome and Alzheimer's disease. Free Radic Biol Med. 2018;114:110–121. doi: 10.1016/j.freeradbiomed.2017.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dashinimaev E.B., Artyuhov A.S., Bolshakov A.P., Vorotelyak E.A., Vasiliev A.V. Neurons Derived from Induced Pluripotent Stem Cells of Patients with Down Syndrome Reproduce Early Stages of Alzheimer's Disease Type Pathology in vitro. Journal of Alzheimer's disease : JAD. 2017;56:835–847. doi: 10.3233/JAD-160945. [DOI] [PubMed] [Google Scholar]
- 116.Shi Y., Kirwan P., Smith J., MacLean G., Orkin S.H., Livesey F.J. A human stem cell model of early Alzheimer's disease pathology in Down syndrome. Sci Transl Med. 2012;4:124ra29. doi: 10.1126/scitranslmed.3003771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Graff-Radford N.R., Crook J.E., Lucas J., Boeve B.F., Knopman D.S., Ivnik R.J., et al. Association of low plasma Abeta42/Abeta40 ratios with increased imminent risk for mild cognitive impairment and Alzheimer disease. Arch Neurol. 2007;64:354–362. doi: 10.1001/archneur.64.3.354. [DOI] [PubMed] [Google Scholar]
- 118.Ovchinnikov D.A., Korn O., Virshup I., Wells C.A., Wolvetang E.J. The Impact of APP on Alzheimer-like Pathogenesis and Gene Expression in Down Syndrome iPSC-Derived Neurons. Stem Cell Rep. 2018;11:32–42. doi: 10.1016/j.stemcr.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Woodruff G., Young J.E., Martinez F.J., Buen F., Gore A., Kinaga J., et al. The presenilin-1 DeltaE9 mutation results in reduced gamma-secretase activity, but not total loss of PS1 function, in isogenic human stem cells. Cell Rep. 2013;5:974–985. doi: 10.1016/j.celrep.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.P. Martin-Maestro, R. Gargini, A.S. A, E. Garcia, L.C. Anton, S. Noggle, O. Arancio, J. Avila, and V. Garcia-Escudero, Mitophagy Failure in Fibroblasts and iPSC-Derived Neurons of Alzheimer's Disease-Associated Presenilin 1 Mutation. Front Mol Neurosci 10 (2017) 291. [DOI] [PMC free article] [PubMed]
- 121.Wezyk M., Szybinska A., Wojsiat J., Szczerba M., Day K., Ronnholm H., et al. Overactive BRCA1 Affects Presenilin 1 in Induced Pluripotent Stem Cell-Derived Neurons in Alzheimer's Disease. Journal of Alzheimer's disease : JAD. 2018;62:175–202. doi: 10.3233/JAD-170830. [DOI] [PubMed] [Google Scholar]
- 122.Demars M., Hu Y.S., Gadadhar A., Lazarov O. Impaired neurogenesis is an early event in the etiology of familial Alzheimer's disease in transgenic mice. J Neurosci Res. 2010;88:2103–2117. doi: 10.1002/jnr.22387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Arber C., Lovejoy C., Harris L., Willumsen N., Alatza A., Casey J.M., et al. Familial Alzheimer's Disease Mutations in PSEN1 Lead to Premature Human Stem Cell Neurogenesis. Cell Rep. 2021;34 doi: 10.1016/j.celrep.2020.108615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ghatak S., Dolatabadi N., Gao R., Wu Y., Scott H., Trudler D., et al. NitroSynapsin ameliorates hypersynchronous neural network activity in Alzheimer hiPSC models. Mol Psychiatry. 2021;26:5751–5765. doi: 10.1038/s41380-020-0776-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ghatak S., Dolatabadi N., Trudler D., Zhang X., Wu Y., Mohata M., et al. Mechanisms of hyperexcitability in Alzheimer's disease hiPSC-derived neurons and cerebral organoids vs isogenic controls. Elife. 2019;8 doi: 10.7554/eLife.50333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang H., Watrous A.J., Patel A., Jacobs J. Theta and Alpha Oscillations Are Traveling Waves in the Human Neocortex. Neuron. 2018;98:1269–1281 e4. doi: 10.1016/j.neuron.2018.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu X., Wang S., Zhang X., Wang Z., Tian X., He Y. Abnormal amplitude of low-frequency fluctuations of intrinsic brain activity in Alzheimer's disease. Journal of Alzheimer's disease : JAD. 2014;40:387–397. doi: 10.3233/JAD-131322. [DOI] [PubMed] [Google Scholar]
- 128.Serrano-Pozo A., Das S., Hyman B.T. APOE and Alzheimer's disease: advances in genetics, pathophysiology, and therapeutic approaches. Lancet Neurol. 2021;20:68–80. doi: 10.1016/S1474-4422(20)30412-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.J.C. Lambert, C.A. Ibrahim-Verbaas, D. Harold, A.C. Naj, R. Sims, C. Bellenguez, A.L. DeStafano, J.C. Bis, G.W. Beecham, B. Grenier-Boley, G. Russo, T.A. Thorton-Wells, N. Jones, A.V. Smith, V. Chouraki, C. Thomas, M.A. Ikram, D. Zelenika, B.N. Vardarajan, Y. Kamatani, C.F. Lin, A. Gerrish, H. Schmidt, B. Kunkle, M.L. Dunstan, A. Ruiz, M.T. Bihoreau, S.H. Choi, C. Reitz, F. Pasquier, C. Cruchaga, D. Craig, N. Amin, C. Berr, O.L. Lopez, P.L. De Jager, V. Deramecourt, J.A. Johnston, D. Evans, S. Lovestone, L. Letenneur, F.J. Moron, D.C. Rubinsztein, G. Eiriksdottir, K. Sleegers, A.M. Goate, N. Fievet, M.W. Huentelman, M. Gill, K. Brown, M.I. Kamboh, L. Keller, P. Barberger-Gateau, B. McGuiness, E.B. Larson, R. Green, A.J. Myers, C. Dufouil, S. Todd, D. Wallon, S. Love, E. Rogaeva, J. Gallacher, P. St George-Hyslop, J. Clarimon, A. Lleo, A. Bayer, D.W. Tsuang, L. Yu, M. Tsolaki, P. Bossu, G. Spalletta, P. Proitsi, J. Collinge, S. Sorbi, F. Sanchez-Garcia, N.C. Fox, J. Hardy, M.C. Deniz Naranjo, P. Bosco, R. Clarke, C. Brayne, D. Galimberti, M. Mancuso, F. Matthews, I. European Alzheimer's Disease, Genetic, D. Environmental Risk in Alzheimer's, C. Alzheimer's Disease Genetic, H. Cohorts for, E. Aging Research in Genomic, S. Moebus, P. Mecocci, M. Del Zompo, W. Maier, H. Hampel, A. Pilotto, M. Bullido, F. Panza, P. Caffarra, et al., Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer's disease. Nature genetics 45 (2013) 1452-8. [DOI] [PMC free article] [PubMed]
- 130.Lin Y.T., Seo J., Gao F., Feldman H.M., Wen H.L., Penney J., et al. APOE4 Causes Widespread Molecular and Cellular Alterations Associated with Alzheimer's Disease Phenotypes in Human iPSC-Derived Brain Cell Types. Neuron. 2018;98:1141–1154 e7. doi: 10.1016/j.neuron.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang C., Najm R., Xu Q., Jeong D.E., Walker D., Balestra M.E., et al. Gain of toxic apolipoprotein E4 effects in human iPSC-derived neurons is ameliorated by a small-molecule structure corrector. Nat Med. 2018;24:647–657. doi: 10.1038/s41591-018-0004-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Meyer K., Feldman H.M., Lu T., Drake D., Lim E.T., Ling K.H., et al. REST and Neural Gene Network Dysregulation in iPSC Models of Alzheimer's Disease. Cell Rep. 2019;26:1112–1127 e9. doi: 10.1016/j.celrep.2019.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Davis N., Mota B.C., Stead L., Palmer E.O.C., Lombardero L., Rodriguez-Puertas R., et al. Pharmacological ablation of astrocytes reduces Abeta degradation and synaptic connectivity in an ex vivo model of Alzheimer's disease. J Neuroinflammation. 2021;18:73. doi: 10.1186/s12974-021-02117-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Clarke L.E., Barres B.A. Emerging roles of astrocytes in neural circuit development. Nat Rev Neurosci. 2013;14:311–321. doi: 10.1038/nrn3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Barres B.A. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60:430–440. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 136.Wang H., Eckel R.H. What are lipoproteins doing in the brain? Trends Endocrinol Metab. 2014;25:8–14. doi: 10.1016/j.tem.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nishimura M., Tomimoto H., Suenaga T., Namba Y., Ikeda K., Akiguchi I., et al. Immunocytochemical characterization of glial fibrillary tangles in Alzheimer's disease brain. Am J Pathol. 1995;146:1052–1058. [PMC free article] [PubMed] [Google Scholar]
- 138.Jackson R.J., Meltzer J.C., Nguyen H., Commins C., Bennett R.E., Hudry E., et al. APOE4 derived from astrocytes leads to blood-brain barrier impairment. Brain. 2022;145:3582–3593. doi: 10.1093/brain/awab478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.van de Haar H.J., Burgmans S., Jansen J.F., van Osch M.J., van Buchem M.A., Muller M., et al. Blood-Brain Barrier Leakage in Patients with Early Alzheimer Disease. Radiology. 2017;282:615. doi: 10.1148/radiol.2017164043. [DOI] [PubMed] [Google Scholar]
- 140.Nagele R.G., D'Andrea M.R., Lee H., Venkataraman V., Wang H.Y. Astrocytes accumulate A beta 42 and give rise to astrocytic amyloid plaques in Alzheimer disease brains. Brain Res. 2003;971:197–209. doi: 10.1016/s0006-8993(03)02361-8. [DOI] [PubMed] [Google Scholar]
- 141.Vijayan V.K., Geddes J.W., Anderson K.J., Chang-Chui H., Ellis W.G., Cotman C.W. Astrocyte hypertrophy in the Alzheimer's disease hippocampal formation. Exp Neurol. 1991;112:72–78. doi: 10.1016/0014-4886(91)90115-s. [DOI] [PubMed] [Google Scholar]
- 142.Olabarria M., Noristani H.N., Verkhratsky A., Rodriguez J.J. Concomitant astroglial atrophy and astrogliosis in a triple transgenic animal model of Alzheimer's disease. Glia. 2010;58:831–838. doi: 10.1002/glia.20967. [DOI] [PubMed] [Google Scholar]
- 143.Habib N., McCabe C., Medina S., Varshavsky M., Kitsberg D., Dvir-Szternfeld R., et al. Disease-associated astrocytes in Alzheimer's disease and aging. Nat Neurosci. 2020;23:701–706. doi: 10.1038/s41593-020-0624-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Harris M.E., Wang Y., Pedigo N.W., Jr., Hensley K., Butterfield D.A., Carney J.M. Amyloid beta peptide (25–35) inhibits Na+-dependent glutamate uptake in rat hippocampal astrocyte cultures. J Neurochem. 1996;67:277–286. doi: 10.1046/j.1471-4159.1996.67010277.x. [DOI] [PubMed] [Google Scholar]
- 145.Masliah E., Alford M., DeTeresa R., Mallory M., Hansen L. Deficient glutamate transport is associated with neurodegeneration in Alzheimer's disease. Ann Neurol. 1996;40:759–766. doi: 10.1002/ana.410400512. [DOI] [PubMed] [Google Scholar]
- 146.Krencik R., Weick J.P., Liu Y., Zhang Z.J., Zhang S.C. Specification of transplantable astroglial subtypes from human pluripotent stem cells. Nat Biotechnol. 2011;29:528–534. doi: 10.1038/nbt.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jones V.C., Atkinson-Dell R., Verkhratsky A., Mohamet L. Aberrant iPSC-derived human astrocytes in Alzheimer's disease. Cell Death Dis. 2017;8:e2696. doi: 10.1038/cddis.2017.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shaltouki A., Peng J., Liu Q., Rao M.S., Zeng X. Efficient generation of astrocytes from human pluripotent stem cells in defined conditions. Stem Cells. 2013;31:941–952. doi: 10.1002/stem.1334. [DOI] [PubMed] [Google Scholar]
- 149.Oksanen M., Petersen A.J., Naumenko N., Puttonen K., Lehtonen S., Gubert Olive M., et al. PSEN1 Mutant iPSC-Derived Model Reveals Severe Astrocyte Pathology in Alzheimer's Disease. Stem Cell Rep. 2017;9:1885–1897. doi: 10.1016/j.stemcr.2017.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhao J., Davis M.D., Martens Y.A., Shinohara M., Graff-Radford N.R., Younkin S.G., et al. APOE epsilon4/epsilon4 diminishes neurotrophic function of human iPSC-derived astrocytes. Hum Mol Genet. 2017;26:2690–2700. doi: 10.1093/hmg/ddx155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fong L.K., Yang M.M., Dos Santos Chaves R., Reyna S.M., Langness V.F., Woodruff G., et al. Full-length amyloid precursor protein regulates lipoprotein metabolism and amyloid-beta clearance in human astrocytes. J Biol Chem. 2018;293:11341–11357. doi: 10.1074/jbc.RA117.000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Butt A.M., De La Rocha I.C., Rivera A. Oligodendroglial Cells in Alzheimer's Disease. Advances in Experimental Medicine and Biology. 2019;1175:325–333. doi: 10.1007/978-981-13-9913-8_12. [DOI] [PubMed] [Google Scholar]
- 153.Desai M.K., Mastrangelo M.A., Ryan D.A., Sudol K.L., Narrow W.C., Bowers W.J. Early oligodendrocyte/myelin pathology in Alzheimer's disease mice constitutes a novel therapeutic target. Am J Pathol. 2010;177:1422–1435. doi: 10.2353/ajpath.2010.100087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ehrlich M., Mozafari S., Glatza M., Starost L., Velychko S., Hallmann A.L., et al. Rapid and efficient generation of oligodendrocytes from human induced pluripotent stem cells using transcription factors. Proc Natl Acad Sci U S A. 2017;114:E2243–E2252. doi: 10.1073/pnas.1614412114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Erceg S., Ronaghi M., Oria M., Rosello M.G., Arago M.A., Lopez M.G., et al. Transplanted oligodendrocytes and motoneuron progenitors generated from human embryonic stem cells promote locomotor recovery after spinal cord transection. Stem Cells. 2010;28:1541–1549. doi: 10.1002/stem.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hu B.Y., Du Z.W., Zhang S.C. Differentiation of human oligodendrocytes from pluripotent stem cells. Nat Protoc. 2009;4:1614–1622. doi: 10.1038/nprot.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Colonna M., Butovsky O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu Rev Immunol. 2017;35:441–468. doi: 10.1146/annurev-immunol-051116-052358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hansen D.V., Hanson J.E., Sheng M. Microglia in Alzheimer's disease. J Cell Biol. 2018;217:459–472. doi: 10.1083/jcb.201709069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Guerreiro R., Wojtas A., Bras J., Carrasquillo M., Rogaeva E., Majounie E., et al. Alzheimer Genetic Analysis, TREM2 variants in Alzheimer's disease. N Engl J Med. 2013;368:117–127. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yeh F.L., Hansen D.V., Sheng M. TREM2, Microglia, and Neurodegenerative Diseases. Trends Mol Med. 2017;23:512–533. doi: 10.1016/j.molmed.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 161.Yeh F.L., Wang Y., Tom I., Gonzalez L.C., Sheng M. TREM2 Binds to Apolipoproteins, Including APOE and CLU/APOJ, and Thereby Facilitates Uptake of Amyloid-Beta by Microglia. Neuron. 2016;91:328–340. doi: 10.1016/j.neuron.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 162.Lee C.Y.D., Daggett A., Gu X., Jiang L.L., Langfelder P., Li X., et al. Elevated TREM2 Gene Dosage Reprograms Microglia Responsivity and Ameliorates Pathological Phenotypes in Alzheimer's Disease Models. Neuron. 2018;97:1032–1048 e5. doi: 10.1016/j.neuron.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cummings D.M., Benway T.A., Ho H., Tedoldi A., Fernandes Freitas M.M., Shahab L., et al. Neuronal and Peripheral Pentraxins Modify Glutamate Release and may Interact in Blood-Brain Barrier Failure. Cereb Cortex. 2017;27:3437–3448. doi: 10.1093/cercor/bhx046. [DOI] [PubMed] [Google Scholar]
- 164.Paolicelli R.C., Jawaid A., Henstridge C.M., Valeri A., Merlini M., Robinson J.L., et al. TDP-43 Depletion in Microglia Promotes Amyloid Clearance but Also Induces Synapse Loss. Neuron. 2017;95:297–308 e6. doi: 10.1016/j.neuron.2017.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Reichwald J., Danner S., Wiederhold K.H., Staufenbiel M. Expression of complement system components during aging and amyloid deposition in APP transgenic mice. J Neuroinflammation. 2009;6:35. doi: 10.1186/1742-2094-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yoshiyama Y., Higuchi M., Zhang B., Huang S.M., Iwata N., Saido T.C., et al. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53:337–351. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 167.Maphis N., Xu G., Kokiko-Cochran O.N., Jiang S., Cardona A., Ransohoff R.M., et al. Reactive microglia drive tau pathology and contribute to the spreading of pathological tau in the brain. Brain. 2015;138:1738–1755. doi: 10.1093/brain/awv081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Krabbe G., Halle A., Matyash V., Rinnenthal J.L., Eom G.D., Bernhardt U., et al. Functional impairment of microglia coincides with Beta-amyloid deposition in mice with Alzheimer-like pathology. PLoS One. 2013;8:e60921. doi: 10.1371/journal.pone.0060921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Grathwohl S.A., Kalin R.E., Bolmont T., Prokop S., Winkelmann G., Kaeser S.A., et al. Formation and maintenance of Alzheimer's disease beta-amyloid plaques in the absence of microglia. Nat Neurosci. 2009;12:1361–1363. doi: 10.1038/nn.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Keren-Shaul H., Spinrad A., Weiner A., Matcovitch-Natan O., Dvir-Szternfeld R., Ulland T.K., et al. A Unique Microglia Type Associated with Restricting Development of Alzheimer's Disease. Cell. 2017;169:1276–1290 e17. doi: 10.1016/j.cell.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 171.Xu M., Zhang L., Liu G., Jiang N., Zhou W., Zhang Y. Pathological Changes in Alzheimer's Disease Analyzed Using Induced Pluripotent Stem Cell-Derived Human Microglia-Like Cells. Journal of Alzheimer's disease : JAD. 2019;67:357–368. doi: 10.3233/JAD-180722. [DOI] [PubMed] [Google Scholar]
- 172.Malm T.M., Jay T.R., Landreth G.E. The evolving biology of microglia in Alzheimer's disease. Neurotherapeutics. 2015;12:81–93. doi: 10.1007/s13311-014-0316-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lancaster M.A., Renner M., Martin C.A., Wenzel D., Bicknell L.S., Hurles M.E., et al. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Camp J.G., Badsha F., Florio M., Kanton S., Gerber T., Wilsch-Brauninger M., et al. Human cerebral organoids recapitulate gene expression programs of fetal neocortex development. Proc Natl Acad Sci U S A. 2015;112:15672–15677. doi: 10.1073/pnas.1520760112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Garcez P.P., Loiola E.C., Madeiro da Costa R., Higa L.M., Trindade P., Delvecchio R., et al. Zika virus impairs growth in human neurospheres and brain organoids. Science. 2016;352:816–818. doi: 10.1126/science.aaf6116. [DOI] [PubMed] [Google Scholar]
- 176.Velasco S., Kedaigle A.J., Simmons S.K., Nash A., Rocha M., Quadrato G., et al. Individual brain organoids reproducibly form cell diversity of the human cerebral cortex. Nature. 2019;570:523–527. doi: 10.1038/s41586-019-1289-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Quadrato G., Nguyen T., Macosko E.Z., Sherwood J.L., Min Yang S., Berger D.R., et al. Cell diversity and network dynamics in photosensitive human brain organoids. Nature. 2017;545:48–53. doi: 10.1038/nature22047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Raja W.K., Mungenast A.E., Lin Y.T., Ko T., Abdurrob F., Seo J., et al. Self-Organizing 3D Human Neural Tissue Derived from Induced Pluripotent Stem Cells Recapitulate Alzheimer's Disease Phenotypes. PLoS One. 2016;11:e0161969. doi: 10.1371/journal.pone.0161969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gonzalez C., Armijo E., Bravo-Alegria J., Becerra-Calixto A., Mays C.E., Soto C. Modeling amyloid beta and tau pathology in human cerebral organoids. Mol Psychiatry. 2018;23:2363–2374. doi: 10.1038/s41380-018-0229-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhao J., Fu Y., Yamazaki Y., Ren Y., Davis M.D., Liu C.C., et al. APOE4 exacerbates synapse loss and neurodegeneration in Alzheimer's disease patient iPSC-derived cerebral organoids. Nat Commun. 2020;11:5540. doi: 10.1038/s41467-020-19264-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Leng F., Edison P. Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat Rev Neurol. 2021;17:157–172. doi: 10.1038/s41582-020-00435-y. [DOI] [PubMed] [Google Scholar]
- 182.Park J., Wetzel I., Marriott I., Dreau D., D'Avanzo C., Kim D.Y., et al. A 3D human triculture system modeling neurodegeneration and neuroinflammation in Alzheimer's disease. Nat Neurosci. 2018;21:941–951. doi: 10.1038/s41593-018-0175-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bhaduri A., Andrews M.G., Mancia Leon W., Jung D., Shin D., Allen D., et al. Cell stress in cortical organoids impairs molecular subtype specification. Nature. 2020;578:142–148. doi: 10.1038/s41586-020-1962-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sivitilli A.A., Gosio J.T., Ghoshal B., Evstratova A., Trcka D., Ghiasi P., et al. Robust production of uniform human cerebral organoids from pluripotent stem cells. Life Sci Alliance. 2020;3 doi: 10.26508/lsa.202000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Pomeshchik Y., Klementieva O., Gil J., Martinsson I., Hansen M.G., de Vries T., et al. Human iPSC-Derived Hippocampal Spheroids: An Innovative Tool for Stratifying Alzheimer Disease Patient-Specific Cellular Phenotypes and Developing Therapies. Stem Cell Rep. 2020;15:256–273. doi: 10.1016/j.stemcr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yoshihara M., Hayashizaki Y., Murakawa Y. Genomic Instability of iPSCs: Challenges Towards Their Clinical Applications. Stem Cell Rev Rep. 2017;13:7–16. doi: 10.1007/s12015-016-9680-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Miller J., Studer L. Aging in iPS cells. Aging. 2014;6:246–247. doi: 10.18632/aging.100653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sanchez-Danes A., Richaud-Patin Y., Carballo-Carbajal I., Jimenez-Delgado S., Caig C., Mora S., et al. Disease-specific phenotypes in dopamine neurons from human iPS-based models of genetic and sporadic Parkinson's disease. EMBO Mol Med. 2012;4:380–395. doi: 10.1002/emmm.201200215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Qian X., Nguyen H.N., Song M.M., Hadiono C., Ogden S.C., Hammack C., et al. Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell. 2016;165:1238–1254. doi: 10.1016/j.cell.2016.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Vierbuchen T., Ostermeier A., Pang Z.P., Kokubu Y., Sudhof T.C., Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Xu G., Wu F., Gu X., Zhang J., You K., Chen Y., et al. Direct Conversion of Human Urine Cells to Neurons by Small Molecules. Sci Rep. 2019;9:16707. doi: 10.1038/s41598-019-53007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Tanabe K., Ang C.E., Chanda S., Olmos V.H., Haag D., Levinson D.F., et al. Transdifferentiation of human adult peripheral blood T cells into neurons. Proc Natl Acad Sci U S A. 2018;115:6470–6475. doi: 10.1073/pnas.1720273115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Mertens J., Paquola A.C.M., Ku M., Hatch E., Bohnke L., Ladjevardi S., et al. Directly Reprogrammed Human Neurons Retain Aging-Associated Transcriptomic Signatures and Reveal Age-Related Nucleocytoplasmic Defects. Cell Stem Cell. 2015;17:705–718. doi: 10.1016/j.stem.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Mertens J., Reid D., Lau S., Kim Y., Gage F.H. Aging in a Dish: iPSC-Derived and Directly Induced Neurons for Studying Brain Aging and Age-Related Neurodegenerative Diseases. Annu Rev Genet. 2018;52:271–293. doi: 10.1146/annurev-genet-120417-031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ladewig J., Mertens J., Kesavan J., Doerr J., Poppe D., Glaue F., et al. Small molecules enable highly efficient neuronal conversion of human fibroblasts. Nat Methods. 2012;9:575–578. doi: 10.1038/nmeth.1972. [DOI] [PubMed] [Google Scholar]
- 196.Liu M.L., Zang T., Zou Y., Chang J.C., Gibson J.R., Huber K.M., et al. Small molecules enable neurogenin 2 to efficiently convert human fibroblasts into cholinergic neurons. Nat Commun. 2013;4:2183. doi: 10.1038/ncomms3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hu W., Qiu B., Guan W., Wang Q., Wang M., Li W., et al. Direct Conversion of Normal and Alzheimer's Disease Human Fibroblasts into Neuronal Cells by Small Molecules. Cell Stem Cell. 2015;17:204–212. doi: 10.1016/j.stem.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 198.Qian X., Song H., Ming G.L. Brain organoids: advances, applications and challenges. Development. 2019;146 doi: 10.1242/dev.166074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Qian X., Jacob F., Song M.M., Nguyen H.N., Song H., Ming G.L. Generation of human brain region-specific organoids using a miniaturized spinning bioreactor. Nat Protoc. 2018;13:565–580. doi: 10.1038/nprot.2017.152. [DOI] [PMC free article] [PubMed] [Google Scholar]