Abstract
bmp gene family 36 of Borrelia burgdorferi, the agent of Lyme disease, comprises four paralogs: bmpA, bmpB, bmpC, and bmpD. The bmpA and bmpB genes constitute an operon. All four genes have been found to be transcribed in cultured spirochetes. Expression from the bmpAB operon results in three distinct transcripts of 1.1, 1.6, and 2.4 kb, and the relative expression of bmpA mRNA is three- to fourfold greater than that of bmpB mRNA. However, thus far only expression of the BmpA protein has been demonstrated. Therefore, in this study we characterized the origins of the three transcripts and compared the relative expression of the BmpA and BmpB proteins. Northern blotting revealed that the three distinct transcripts originated from a single promoter located upstream of bmpA but terminated either 3′ to the bmpA (1.1-kb RNA) or bmpB (2.4-kb RNA) gene or, most unusually, within the bmpB gene (1.6-kb RNA). Termination within the bmpB gene was associated with a functional Rho-independent transcription terminator. At the protein level, we also observed a 4.3-fold greater abundance of BmpA compared to that of BmpB. These studies identify a transcription termination mechanism in B. burgdorferi resulting in the disparate expression of the two genes of the bmpAB operon.
Paralogous bmp gene family 36 of Borrelia burgdorferi encodes four putative lipoproteins: BmpA (P39), BmpB, BmpC, and BmpD. The corresponding genes are confined to a single locus on the linear chromosome of this organism in the following 5′→3′ gene order: bmpD-bmpC-bmpA-bmpB (2, 15, 33, 43). The paralogs share a high degree of homology at both the DNA (56 to 64%) and protein (36 to 46% identity) levels (2, 15, 33, 37, 43). Although the function of none of these proteins has been established, the conservation of their genes within the B. burgdorferi sensu lato complex (2, 16, 33, 37, 43) and the presence of orthologs in Treponema pallidum (41) and numerous other bacteria (B. burgdorferi genome web site at the Institute for Genomic Research) suggest that these proteins fulfill an essential physiological role.
All four paralogs are transcribed in cultured spirochetes (5, 11, 33, 34, 35). Whereas the transcription of bmpA, bmpB, and bmpD has been demonstrated by both Northern blotting (33, 34) and reverse transcription (RT-PCR) (11), the very low-level expression of bmpC mRNA could be ascertained only by RT-PCR (11). However, thus far, only the expression of BmpA and BmpD proteins in vitro has been demonstrated (33, 42). The expression of these genes at the RNA level was also examined as a function of culture temperature and pH (11, 35). In one study, the expression of bmpC and bmpD was found to decrease by 6- and 2.5-fold, respectively, at 37°C and pH 6.8 compared to the expression level at 23°C and pH 7.5 (35). In a different study, there was no difference in the expression of the four genes in spirochetes cultured at 23, 32, and 37°C (11). The expression of these genes in vivo was recently examined by microarray analysis (21). In mice infected with B. burgdorferi, bmpB, bmpC, and bmpD were found to be consistently expressed whereas the transcription of bmpA was variable (21). Expression of bmpA was detected in only two out of three mice. The corresponding expression of one or more of these proteins during infection of the vertebrate host is also borne out by the persistent occurrence of antibodies reactive to the BmpA antigen (1, 3, 12, 13, 14, 18, 23, 24, 25, 28, 29, 30, 37, 42, 46).
We have previously demonstrated the expression of bmpA (34) and bmpD (33) in cultured spirochetes by Northern blotting. Whereas the bmpD gene is transcribed as a 1.4-kb monocistronic RNA (33), expression of bmpA results in three distinct RNAs of 2.4, 1.6, and 1.1 kb (34). In the present study, we characterized the origins of the three bmpA transcripts and identified the bmpAB promoter in strain JD1 to dissect the mechanism leading to the synthesis of the multiple transcripts. We also assessed the function of a Rho-independent transcription terminator located within the bmpB gene to understand its role in the transcription of the bmpAB locus. Finally, we identified the BmpB protein by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and Western blotting and then estimated the relative expression of the BmpA and BmpB proteins to correlate mRNA expression with expression of the corresponding proteins.
MATERIALS AND METHODS
Bacterial strains and clones.
A low-passage (passage 5) variant of strain JD1 of B. burgdorferi (JD1 P5), originally isolated from infected Ixodes scapularis nymphs (31), was used in this study. A high-passage (passage 19 or later) variant of JD1 used for transformation was derived by serial passage of the JD1 P5 stock.
Culture conditions.
JD1 P5 was cultured at 24°C in BSK-H medium (Sigma Chemical Company, St. Louis, Mo.) and harvested after 2 weeks at a density of 3 × 107 cells/ml for the preparation of a freezer stock. This freezer stock was used to set up JD1 P5 cultures. In the case of the other cultures, the corresponding freezer stock originated from 34°C cultures. Cultures were typically set up at an initial density of 106 organisms per ml in BSK-H medium and incubated at 34°C as previously described (32). The cells were harvested in the late-logarithmic phase of growth (3 × 107 to 5 × 107 cells/ml) for the preparation of RNA and whole-cell lysates.
DNA procedures.
DNA probes specific for individual bmp genes were derived either by restriction enzyme digestion or by PCR. PCR was used to generate the following probes: for bmpB, T10-B7; for bmpC, T9-B6; for bmpD, T11-B9 (33) (locations of the primers are shown in Fig. 1, and sequences are shown in Table 1). The bmpA probe (Bsu36I-HindIII fragment; probe bmpA in Fig. 1) was derived from clone 16A (33), containing the entire bmpA gene and the C-terminal part of the bmpC gene, by digestion with the two restriction enzymes. Additional probes for RNase H experiments were also generated by PCR with primer pairs T12-B1 and T12-B2 (Fig. 1 and Table 1). All probes were purified by electrophoresis through 1% SeaPlaque agarose gel (FMC BioProducts, Rockland, Maine) and extraction of the gel slices with phenol and chloroform.
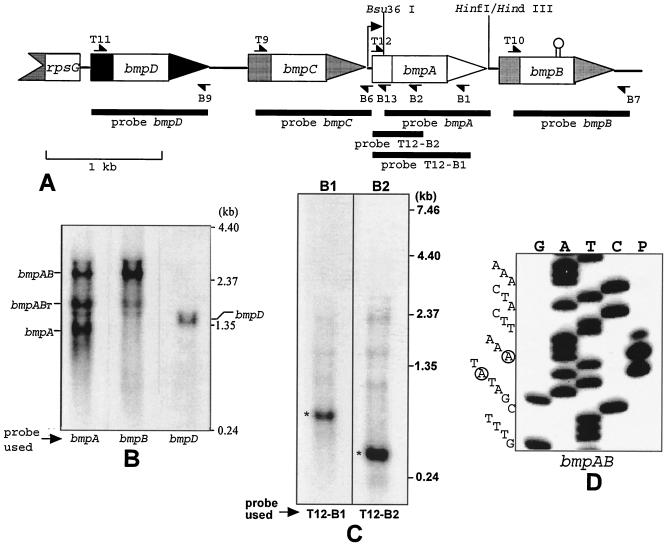
FIG. 1.
(A) Organization of the bmp locus of B. burgdorferi and locations of primers, probes, and relevant restriction enzyme sites. The transcription start site of the bmpAB operon is indicated by a bent arrow, and the putative transcription terminator within bmpB is depicted by a hairpin. (B) Northern analysis of transcripts expressed from the bmp locus of B. burgdorferi. The lane containing bmpC is not shown, as no mRNA species was detected for this gene. (C) Characterization of the bmpAB RNAs by RNase H digestion. (D) Mapping of the bmpAB promoter by primer extension. The two dominant transcription start site A's at −30 and −32 are circled.
TABLE 1.
Primers used in this study
| Primer | Sequencea | Location | Gene(s) or plasmid | Use |
|---|---|---|---|---|
| T9 | 5′ggaagatCTTGTTTTAAATCTAATAAAAAG3′ | +47 to +69 | bmpC | Northern blotting |
| T10 | 5′gcgcggatccTGCTTTAGTAGAAATGG3′ | +43 to +59 | bmpB | Northern blotting |
| T11 | 5′gcgcggatccTGTTCTAGCTCTGATGAT3′ | +49 to +66 | bmpD | Northern blotting |
| T12 | 5′catgccatggtTAAAATATTGTTGTTGATT3′ | +6 to +24 | bmpA | Northern blotting |
| T71 | 5′ggaagatctATGGCCAGCAAAGGAGAAGAACTT3′ | +1 to +24 | gfp | pGEMT-gfp |
| T72 | 5′gatcCTTTTTTGGCTGGCTATATTGCAGCCAAAAAAAGCTTTTCG3′ | +428 to +468 | bmpB | Terminator |
| T83 | 5′agtcgatgactcgagatcgatTTTACCGTTAAGCGCATGAA3′ | −199 to −179 | flaBp | pQE30-flaBp |
| T84 | 5′cgtttgaagaattcAAATGGAGAAGTGCTTTATATGA3′ | pQE30 | bmpB expression | |
| T87 | 5′AGGCGTATCACGAGGCCC3′ | pQE30 | pBVS2-flaBp-gfp(gfpT) | |
| T99 | 5′gtagcatgaggatccTGCTTTAGTAGAAATGGAATAG3′ | +43 to +64 | bmpB | bmpB expression |
| T101 | 5′atcgattcaggatccTGTAGTGGTAAAGGTAGTCTT3′ | +52 to +72 | bmpA | bmpA expression |
| B1 | 5′CTTGATCTTCATCAACTC3′ | +736 to +719 | bmpA | RNase H |
| B2 | 5′CCGTTAAAAATGCACCCT3′ | +424 to +407 | bmpA | RNase H |
| B6 | 5′aaactgcAGCTATATTTAAGTAGTTTA3′ | +1077 to +1060 | bmpC-bmpA | Northern blotting |
| B7 | 5′aaactgcaGTACTTCTATTTATTATA3′ | +1111 to +1094 | bmpB-BB381 | Northern blotting |
| B9 | 5′tcccaagcTTCACAAATCAGCTCAAT3′ | +1054 to +1037 | bmpD | Northern blotting |
| B13 | 5′GCTCCCAAGACTACCTTTACC3′ | +78 to +58 | bmpA | Primer extension |
| B67 | 5′ctagtctagaTTACGTTTCTCGTTCAGCTTTTTTG3′ | +750 to +774 | gfp | pGEMT-gfp |
| B75 | 5′gatcCGAAAAGCTTTTTTTGGCTGCAATATAGCCAGCCAAAAAAG3′ | +468 to +428 | bmpB | Terminator |
| B88 | 5′gtagcatgagaattcATGATAAAATTTAAATTTCTGACTT3′ | −14 to −38 | flaBp | pQE30-flaBp |
| B93 | 5′cgatagcatatcgatGTTCTTTACGATGCCATTGGGATAT3′ | pQE30 | pBVS2-flaBp-gfp(gfpT) | |
| B98 | 5′gcatgggctgcagTTAAATAAATTCTTTAAGAAACTTCT3′ | +995 to +1020 | bmpA | bmpA expression |
| B104 | 5′gtagcatgactgcagTTATAACTTTAATATTTGTTTTATAA3′ | +1001 to +1026 | bmpB | bmpB expression |
| B105 | 5′gccagaaAAGCTTTTTTTGGCTGCAATATAGCCCGCTAAGAAAGC3′ | +464 to +427 | bmpB | bmpB expression |
bmp sequences are in capitals, and unrelated sequences are in small letters. Functional restriction enzyme sites added to the primer are underlined. The three mutations in primer B105 are also underlined.
Cloning of the terminator in the gfp coding sequence.
The gfp coding sequence was amplified by PCR from the pcDNA-DEST53 Gateway vector (Invitrogen Life Technologies, Carlsbad, Calif.) with primers T71 and B67 and cloned into the pGEMT vector (Promega Biotech, Madison, Wis.). The gfp sequence was excised as a BglII-PstI fragment from this construct and cloned into the pQE30 vector (QIAGEN, Valencia, Calif.) by using the BamHI and PstI sites to generate pQE30-gfp. The bmpB Rho-independent terminator was assembled with GATC overhangs at both ends with the complementary oligonucleotides T72 and B75 and inserted into the unique BamHI site in pQE30-gfp, resulting in pQE30-gfpT. The flaB promoter region of B. burgdorferi was amplified by PCR with primers T83 and B88, digested with XhoI and EcoRI, and cloned into pQE30 at the XhoI and EcoRI sites to yield pQE30-flaBp. The gfp and gfpT sequences were then excised from their respective plasmids as an EcoRI-PstI fragment and inserted at the EcoRI and PstI sites of pQE30-flaBp to generate pQE30-flaBp-gfp and pQE30-flaBp-gfpT, respectively. Finally, with primers T87 and B93, the flaBp-gfp and flaBp-gfpT sequences were amplified by PCR, digested with ClaI, and cloned into the AccI site of Escherichia coli-B. burgdorferi shuttle vector pBVS2 (44), resulting in plasmids pBVS2-flaBp-gfp and pBVS2-flaBp-gfpT.
Transformation of B. burgdorferi.
Electrocompetent B. burgdorferi JD1 passage 19 was prepared and transformed with shuttle plasmids pBVS2-flaBp-gfp and pBVS2-flaBp-gfpT as described previously (38), with minor modifications. About 10 μg of plasmid DNA was used for transformation, and the electroporated cells were allowed to recover in 10 ml of complete BSK-H medium overnight at 34°C. The bacterial cultures were then plated 18 to 24 h postelectroporation on solid BSK-H. Plates were incubated in a humidified candle jar container at 34°C. After 2 weeks, antibiotic-resistant colonies were randomly picked with sterile pipette tips, inoculated into 1 ml of BSK-H, and after 3 days transferred to 15 ml of BSK-H. Selection of transformants on both solid and liquid media was done with 100 μg of kanamycin per ml. The 15-ml cultures were used for the preparation of freezer stocks and to initiate fresh cultures for protein and RNA analyses.
RNA isolation and Northern blotting.
RNA was isolated from spirochetes with hot acidified phenol as previously described (33). The RNA preparations were treated with RQ1 RNase-free DNase I (Promega Biotech) to eliminate DNA (33). About 3 μg of each RNA sample was electrophoresed through a 1% agarose-0.1% Sarkosyl gel to verify the quality and concentrations of the preparations. The same amount (3 μg per lane) of each RNA sample was electrophoresed through a 1.4% agarose gel containing 2.2 M formaldehyde and 20 mM NaPO4, pH 6.8, with 1.2 M formaldehyde-20 mM NaPO4, pH 6.8, as the running buffer. Alternatively, the RNA samples were run in the presence of 1× morpholinepropanesulfonic acid (MOPS) buffer (40 mM MOPS, 10 mM sodium acetate [NaOAc], 1 mM EDTA, pH 7.0). The RNA samples were transferred overnight onto nitrocellulose in 10× SSC buffer (1.5 M NaCl, 0.5 M NaH2PO4, 20 mM EDTA, pH 7.4) and hybridized with specific bmp probes in a buffer comprising 5× SSC, 10× Denhardt's reagent, 50% formamide, 0.1% SDS, 100 μg of salmon sperm DNA per ml, and 10% dextran sulfate. The probes were radiolabeled randomly with [α-32P]dATP with the Klenow fragment of DNA polymerase I and dCTP, dGTP, and TTP (Prime-a-Gene kit from Promega Biotech). The blots were washed twice with 0.3× SSC-0.1% SDS at room temperature for 5 min each time and then twice at 50°C for 30 min each time and finally exposed to X-ray film.
RNase H methods.
For the RNase H experiments, 3 pmol of a bmp-specific oligonucleotide (B1 or B2; Fig. 1 and Table 1) was incubated with 7.5 μg of JD1 P5 RNA in RNase H buffer (20 mM Tris · HCl [pH 7.5], 20 mM KCl, 10 mM MgCl2, 0.1 mM EDTA, 0.1 mM dithiothreitol [DTT]) at 65°C for 5 min and allowed to cool slowly to room temperature. The annealed reaction was digested with 2.2 U of RNase H enzyme in the presence of 10 U of RNasin (both from GIBCO-BRL, Rockville, Md.) for 90 min at room temperature. After digestion, the reaction mixture was extracted with phenol-chloroform (1:1) and precipitated with 0.1 M NaOAc (pH 7.0) and 4 volumes of ethanol. The RNA pellet was dissolved in water and analyzed by Northern blotting with bmp-specific probes as described earlier.
Primer extension.
Oligonucleotide B13 (Fig. 1 and Table 1) was phosphorylated with 32P as follows. About 7 pmol of the oligonucleotide was incubated (in a 20-μl volume) with 25 μCi of a mixture of [γ-32P]dATP, 1 mM spermidine, 50 mM Tris · HCl, 10 mM MgCl2, 5 mM DTT, and 10 U of T4 polynucleotide kinase for 2 h at 37°C. After addition of 5 μl of water, the labeled oligonucleotide was purified with a MicroSpin G-25 column (Amersham Pharmacia Biotech, Inc., Piscataway, N.J.).
Primer extension was carried out by incubating (in 30 μl) 3 pmol of the 32P-labeled B13 oligonucleotide with 10 μg of RNA in the presence of a mixture of 150 mM KCl, 10 mM Tris · HCl, and 1 mM EDTA (pH 8.3) at 65°C for 2 h. The reaction tube was then transferred to a beaker containing 500 ml of water at 65°C and allowed to return to room temperature. The reaction was precipitated with 15 μl of 1 M NaOAc (pH 5.5)-120 μl of ethanol. The annealed sample was dissolved in water, and primer extension was initiated in a 30-μl volume in the presence of 25 mM Tris · HCl, 37.5 mM KCl, 1.5 mM MgCl2, 250 μM deoxynucleoside triphosphates, 5 mM DTT, 5 μg of actinomycin D, and 200 U of Moloney murine leukemia virus reverse transcriptase. The reaction was carried out for 30 min at 42°C and terminated by adding 50 μg of RNase A. The RNA was digested for 1 h at 42°C, extracted with phenol-chloroform, and precipitated as described before. The pellet was dissolved in 6 μl of water and 4 μl of sequencing reaction stop buffer. Clone 16A was sequenced by the dideoxynucleotide chain termination method (39) with modified T7 DNA polymerase (Sequenase kit; United States Biochemical Corp., Cleveland, Ohio), B13 oligonucleotide, and [α-32P]dATP. The sequencing reaction was run alongside the primer extension reaction to map the transcription start site of the bmpAB operon.
DNA sequence analysis.
The DNASIS software (Hitachi Software Engineering Co., Ltd., San Francisco, Calif.) was used to analyze DNA sequences and predict hairpin structures. This software uses the method of Zuker and Stiegler (47) to predict RNA structures and calculate folding energies.
rBmpA and rBmpB proteins.
The cloning and purification of His6-tagged recombinant BmpD (rBmpD) have been previously described (34). The mature sequence (beginning with the cysteine codon) of BmpA was amplified with primers T101 and B98 by using JD1 total DNA and proofstart enzyme (QIAGEN). The PCR product was digested with BamHI overnight and cloned into the BamHI and HindIII (filled-in) sites of pQE30. The cloning of the mature bmpB sequence was achieved in two steps. Initially, the entire sequence was amplified with primers T99 and B104, digested with BamHI and PstI, and cloned into the BamHI and PstI sites of pQE30. However, expression of the His6-tagged rBmpB protein from this construct was poor, presumably because of the presence of the internal transcription terminator. Therefore, the N-terminal sequence was replaced with a sequence with three mutations in the stem structure, resulting in partial disruption of the terminator structure. The three mutations were chosen in the third base of three codons and were the most we could mutate without altering the primary amino acid sequence. The mutated sequence was generated by PCR with primers T99 and B105 and with pQE30-bmpB as the template DNA. The PCR product was digested with EcoRI and HindIII and swapped with the wild-type EcoRI-HindIII fragment in pQE30-bmpB. The expression of His6-tagged rBmpB from the mutated clone (pQE30-bmpBm) improved dramatically. All cloning for protein expression was done with E. coli M15(pREP4). His6-tagged rBmpA and rBmpB proteins were purified on nickel columns as described previously (34).
Western blotting.
The reactivity of the rhesus anti-BmpD antibody to each of the three purified Bmp proteins was assessed by two separate Western blot experiments. Serial dilutions of the three His6-tagged Bmp proteins were boiled in 1× SDS-PAGE buffer and electrophoresed through a 12.5% gel. The serial dilutions of the three proteins were loaded into the same set of wells but delayed by 30-min intervals between protein samples. The blots were developed with a 1:200 dilution of a rhesus anti-BmpD antibody. The rhesus anti-BmpD antibody was generated by repeated injections of purified, His6-tagged rBmpD protein.
Whole-cell lysates prepared from the late-logarithmic-phase cultures were normalized to an optical density at 600 nm of 3 as described previously (33). For analysis of the expression of Bmp proteins, 5 μl of each protein sample was electrophoresed through an SDS-12.5% polyacrylamide gel, transferred to nitrocellulose, and reacted with monoclonal anti-BmpA (kindly provided by Barbara Johnson, Division of Vector-Borne Infectious Diseases, Centers for Disease Control and Prevention, Fort Collins, Colo.), mouse polyclonal anti-BmpD (34), or rhesus polyclonal anti-BmpD. For evaluation of green fluorescent protein (GFP) expression, a commercial rabbit polyclonal anti-GFP antibody (Invitrogen) was used. The secondary biotinylated antibodies were from Vector Laboratories, Burlingame, Calif. The blots were developed with the chromogen 4-chloro-1-naphthol and the substrate hydrogen peroxide.
RESULTS
Three distinct transcripts are expressed from the bmpAB locus.
To characterize the origins of the three RNA bands recognized by the bmpA probe in our previous study (34), RNA from late-logarithmic-phase B. burgdorferi JD1 spirochetes was examined by Northern blotting with radioactively labeled DNA probes specific for each of the four bmp genes (Fig. 1A). Although these genes have a significant degree of DNA sequence homology (38 to 57% identity within the coding sequences), each bmp probe hybridized exclusively to its corresponding DNA sequence in a dot blot assay performed under Northern blotting conditions (data not shown). Different nitrocellulose lanes from the same blot were subjected to hybridizations with each of the four bmp probes under similar conditions. As demonstrated in our earlier study, the bmpA probe sequence hybridized to three transcripts of 2.4, 1.6, and 1.1 kb (Fig. 1B, bmpA lane). On the other hand, only the 2.4- and 1.6-kb transcripts were recognized by the bmpB probe (bmpB lane). The 1.1- and 1.6-kb RNAs were also distinct from the 1.3-kb bmpD RNA (bmpD lane versus bmpA and bmpB lanes). Hybridization with the bmpC sequence failed to produce a band (data not shown). These results demonstrate that the 1.1-, 1.6-, and 2.4-kb transcripts all originated from the bmpAB locus. Furthermore, the 1.1-kb transcript is a monocistronic bmpA RNA on the basis of its unique hybridization with the bmpA probe, whereas the 2.4-kb RNA is a bicistronic bmpAB transcript as it is recognized by both the bmpA and the bmpB probes. The intermediate-size 1.6-kb transcript appears to encode a full-length bmpA RNA and a truncated bmpB RNA on the basis of its strong hybridization with bmpA and weak hybridization with bmpB (bmpA and bmpB lanes). Quantification of the bmpA lane of the Northern blot by densitometry revealed an estimated 2.4- to 1.6- to 1.1-kb RNA ratio of 1:1.6:1.8 for the three transcripts. This ratio translates to a 4.4-fold molar excess of bmpA mRNA over bmpB mRNA. These estimations are in agreement with the previously determined 3.6:1 ratio of bmpA to bmpB derived by RT-PCR (11) after adjusting for the difference in size between the two amplicons.
All three bmpAB transcripts are the sense strand specific and are derived from the same promoter.
The prevalence and relative abundance of the 1.1- and 1.6-kb RNAs motivated us to further characterize these transcripts with respect to their origin within the bmpAB operon. The strand specificity (sense or antisense) of these RNAs could not be ascertained from the Northern experiments as double-stranded DNA probes had been used in these experiments. Therefore, strand specificity was determined by annealing an antisense bmpA sequence-specific oligonucleotide to B. burgdorferi total RNA, cleaving the annealed RNA at the RNA-DNA hybrid position with RNase H enzyme, and then subjecting the products to Northern analysis. Two bmpA-specific oligonucleotides, one located in the N-terminal region (B2) and the other located in the C-terminal region (B1) of the bmpA coding sequence (Table 1 and Fig. 1A), were used to independently verify the strand specificity of the multiple RNAs. The resulting blots were probed only with 32P-labeled DNA sequences specific for the 5′ fragments of the cleaved RNAs to avoid having to interpret a complex pattern of bands, as the bmpA probe was already known to hybridize to three bands. Hence, PCR-generated DNA fragments T12-B1 and T12-B2 (Fig. 1A) were used as probes for the B1 and B2 oligonucleotides, respectively. For both oligonucleotides, a single 5′ RNA fragment 800 nucleotides (in the case of B1) or 450 nucleotides (B2) in length was obtained (Fig. 1C, lanes B1 and B2), indicating that all three RNAs are sense transcripts and likely carry identical 5′ termini.
On the basis of their estimated sizes, the 5′ ends of the cleaved RNA species map to the vicinity of the putative promoter within the 5′ region of bmpA (43) but not to the recently identified bmpAB promoter (11) located 70 bp upstream. Therefore, to further verify these results, the transcriptional start site for bmpAB was mapped by primer extension with the same batch of JD1 RNA and complementary oligonucleotide B13 (Table 1). The 5′ end of the primer-extended products for bmpAB mapped to an A at −32 (Fig. 1D), in agreement with the consensus promoter previously identified by Simpson and coworkers (43).
The 1.6-kb intermediate RNA is a product of intragenic termination at a Rho-independent terminator.
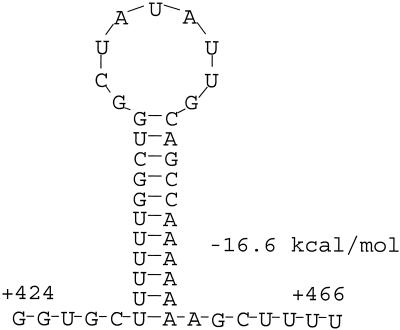
Since the transcription start site was known, the termination region of the 1.6-kb RNA could be localized within the bmpAB sequence. A search of the sequence in the vicinity of the deduced termination region revealed an inverted repeat (IR) sequence strongly resembling a Rho-independent transcription terminator (Fig. 2). Termination of transcription at this site would be expected to yield a 1.6-kb RNA. Therefore, it seemed very likely that the 1.6-kb RNA was the product of transcription termination at this site.
FIG. 2.
Structure of the putative bmpB intragenic transcription terminator. The structure was identified by the DNASIS software. This software uses the method of Zuker and Stiegler (47) to predict RNA structures and calculate folding energies.
To confirm the role of the IR sequence in the synthesis of the 1.6-kb RNA, its function was assessed in B. burgdorferi with gfp as a marker. The bmpB IR sequence was generated as a 45-bp DNA fragment by annealing two complementary oligonucleotides (Table 1) and then inserting them in frame into the coding sequence of gfp at a BamHI site. To facilitate the expression of gfp in B. burgdorferi, the constitutive flaB promoter (7, 20, 40) was used and the two constructs were transferred to shuttle vector pBVS2 (44). Three transformants for each construct were randomly selected and examined for gfp mRNA and protein expression by Northern and Western blotting, respectively. For Northern blotting, a probe containing only the 5′ half of the gfp sequence was used. This probe was generated by PCR with primers T71 and B75 and pQE30-flaBp-gfpT as template DNA. Although gfp mRNA (Fig. 3B, lanes 1 to 3) and protein (Fig. 3D, lanes 1 to 3) were readily detected for the wild-type transformants, by contrast, no full-length gfpT mRNA (Fig. 3B, lanes 4 to 6) or GFPT protein (Fig. 3D, lanes 4 to 6) expression from the terminator-modified construct was apparent. Instead, a smaller transcript corresponding in size to a transcript resulting from termination at the bmpB terminator within the gfp sequence is visible in these RNA samples (Fig. 3B, lanes 4 to 6). The gfp probe was stripped, and the blot was reprobed with the bmpB sequence to verify the integrity and equal loading of each RNA sample (Fig. 3C). These results demonstrate that the bmpB IR sequence functions very efficiently as a transcription terminator in B. burgdorferi.
FIG. 3.
Functional assessment of the bmpB IR sequence in B. burgdorferi. The effect of the terminator on gene expression was examined by inserting it into the coding region of gfp and analyzing the expression of the marker by Northern and Western blotting. Three individual transformants containing the wild-type construct (lanes 1 to 3) and three transformants containing the terminator-modified construct (lanes 4 to 6) were examined. Panels: A, agarose gel stained with ethidium bromide showing the total RNA profile from the six transformants; B, Northern blot probed with the gfp sequence; C, blot reprobed with the bmpB sequence, after removal of the gfp probe, to verify equal loading of RNA; D, Western blot developed with rabbit anti-GFP antibody.
Expression of BmpA protein is also 4.3-fold higher than that of BmpB protein in cultured spirochetes.
As shown in this study, transcription originating from the bmpAB promoter is terminated twice before synthesis of the bicistronic mRNA is completed. This discontinuous mode of transcription of the bmpAB operon results in a 4.4-fold molar excess of bmpA mRNA relative to bmpB mRNA. Therefore, we compared the expression of the BmpA and BmpB proteins to determine if the disparity in their mRNA levels also results in disproportionate protein levels.
On the basis of our Northern analysis (this study) and RT-PCR studies (11), only three of the four Bmp proteins, BmpA, BmpB, and BmpD, should be expressed in cultured spirochetes. However, their separation and detection by SDS-PAGE and Western blotting are complicated by their similar sizes. Initial attempts at detection of the BmpD protein with a rhesus polyclonal anti-BmpD antibody had surprisingly revealed three bands, a 37-kDa band and a doublet band of 39 kDa. The 37-kDa band proved to be the BmpD protein on the basis of its recognition by the mouse anti-BmpD antibody. Therefore, it seemed likely that the 39-kDa doublet represented the BmpA and BmpB proteins. To achieve better separation of the constituents of the doublet, electrophoresis was extended until the 28-kDa marker band had reached the bottom of the gel. Under these conditions, three distinct bands emerged with the rhesus anti-BmpD antibody (Fig. 4A, lane 2). Again, the smallest of the three bands was recognized by the mouse anti-BmpD antibody (Fig. 4A, lane 3), whereas the intermediate band was uniquely reactive to the anti-BmpA monoclonal antibody (Fig. 4A, lane 1). We deduce from these results that the slowest migrating band must be BmpB. This is consistent with the marginally higher molecular mass of the mature BmpB polypeptide (35,942 Da) compared to that of BmpA (35,025 Da).
FIG. 4.
Identification of the BmpA, BmpB, and BmpD proteins and estimation of the relative expression of BmpA and BmpB in spirochetes cultured at 34°C. (A) Western blot showing the banding pattern of BmpA, BmpB, and BmpD. Three separate lanes from the same Western blot were developed with different anti-Bmp antibodies as follows: anti-BmpA monoclonal antibody (lane 1), polyclonal rhesus anti-BmpD antibody (lane 2), and mouse anti-BmpD polyclonal antibody (lane 3). The criterion used to deduce that the slowest-migrating band in lane 2 is BmpB is described in the text. (B) Western blot showing the expression of three Bmp proteins in four different 34°C cultures of B. burgdorferi JD1. The relative expression of the BmpA and BmpB proteins was quantified by densitometry.
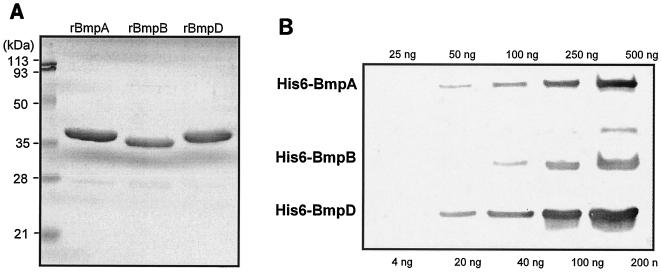
To confirm the ability of the rhesus antibody to react with the BmpA and BmpB proteins, recombinant versions of these proteins were generated in E. coli. The His6-tagged proteins were purified and examined by Western blotting. The rhesus anti-BmpD antibody indeed recognized both the rBmpA and rBmpB proteins. Next, the specific reactivity of the rhesus anti-BmpD antibody to the rBmpA, rBmpB, and rBmpD proteins was experimentally determined by Western blotting (Fig. 5). Serial dilutions of the three proteins were prepared and electrophoresed in the same gel. Each successive loading was delayed by 30 min to ensure separation of the proteins. To accurately quantify reactivity, we limited the development of the Western blot to the first visibility of the bands so as to limit the saturation of the bands with the highest concentrations of proteins. Individual bands were quantified by densitometry, and the net density values were plotted against the protein concentrations. In each case, the antibody reactivity was linear for the range of protein amounts tested (25 to 500 ng for rBmpA and rBmpB and 4 to 200 ng for rBmpD). The specific reactivity of the antibody to each protein (net density value per nanogram of protein) was calculated from the linear equation for each curve generated by the CA-Cricket Graph III version 1.5.3 graphing software. Since the molecular weights of these proteins are very close, specific reactivity expressed as reactivity per unit of mass is equivalent to specific reactivity per mole of protein. From the calculated specific reactivity, the estimated relative specific reactivity was as follows: BmpA to BmpB to BmpD, 1.4:1:4.8. In other words, the antibody reacted 4.8 times better with BmpD but only 1.4 times better with BmpA, compared to its reactivity with BmpB.
FIG. 5.
Reactivity of the rhesus anti-BmpD antibody to the rBmpA, rBmpB, and rBmpD proteins. (A) About 2 μg of each recombinant protein was electrophoresed through a 14% gel and stained with Coomassie G250. (B) Western blot assay of serial dilutions of rBmp proteins developed with a 1:200 dilution of the rhesus anti-BmpD antibody.
Using our ability to separate, detect, and reliably quantify the BmpA and BmpB proteins, we compared the relative expression of these proteins in cultured spirochetes (Fig. 4B). Under these conditions, densitometry of the Western blot revealed a 4.3-fold greater abundance of BmpA relative to that of BmpB after correcting for the difference in the specific reactivities of the rhesus antibody to the two proteins.
DISCUSSION
During the growth of B. burgdorferi in vitro, all four homologs of the bmp family are transcribed. Whereas the transcription of bmpA, bmpB, and bmpD has been demonstrated by both Northern blotting (34) and RT-PCR (11), the very low-level expression of bmpC mRNA could be ascertained only by RT-PCR (11). At the protein level, only expression of the BmpA and BmpD proteins has thus far been demonstrated in cultured spirochetes (33, 42). Expression from the bmpAB operon results in three transcripts of 2.4, 1.6, and 1.1 kb (34). In this study, we have characterized these transcripts with respect to their origin and termination within the bmpAB sequence and compared the relative expression levels of the two encoded proteins, BmpA and BmpB. In the process, we have uncovered a transcriptional mechanism that uses intergenic and intragenic termination to modulate the expression of the BmpB protein. The net effect of these processes is a BmpA abundance 4.3-fold greater than that of BmpB.
Our data are consistent with our interpretation that the 1.1- and 1.6-kb transcripts are the result of transcription termination events within the bmpA-bmpB intergenic and bmpB intragenic regions, respectively. We have demonstrated by RNase H mapping that the three transcripts of the bmpAB locus are all sense in orientation and most likely originate from the same promoter, as a single band was observed in these experiments. Additionally, in the case of the 1.6-kb RNA we have also demonstrated that a functional Rho-independent terminator exists at the appropriate location to terminate transcription originating at the bmpAB promoter. Termination at this point would account for this RNA. The possibility that the 1.6- and 1.1-kb transcripts are derived by the degradation or processing of the 2.4-kb RNA is unlikely given that their combined size (2.7 kb) is significantly greater than that of the primary bmpAB transcript of 2.4 kb. Moreover, the pattern of strong hybridization of the bmpA probe to the 1.1- and 1.6-kb transcripts, together with the relatively weak hybridization of the bmpB probe to the 1.6-kb RNA and the lack of reactivity to the 1.1-kb RNA, are all inconsistent with this interpretation. In other words, cleavage of the primary 2.4-kb transcript into the 1.6- and 1.1-kb RNAs would have resulted in a differential hybridization of the bmpA and bmpB probes to the two processed RNAs. Likewise, it appears unlikely that the 1.1-kb RNA is derived from the degradation or processing of the 1.6-kb RNA, as no other 0.5-kb or smaller bmpA- or bmpB-hybridizing RNAs were observed.
The bmpAB promoter experimentally identified in this study in strain JD1 by primer extension is in concordance with the previously recognized consensus-based promoter elements (43). However, it is interesting that this promoter is different from the recently mapped bmpAB promoter in type strain B31 (11). The latter promoter was localized to a site 70 bp upstream of the JD1 bmpAB promoter. Although the reasons for this divergence in promoter usage between the B31 and JD1 strains is not clear, it is unrelated to sequence dissimilarities in their 5′ regions.
The discontinuous mode of transcription within the bmpAB operon results in a fourfold molar excess of bmpA mRNA relative to bmpB mRNA and a fourfold greater abundance of BmpA protein compared to BmpB protein. It is not possible to speculate on the significance of the higher concentration of BmpA relative to BmpB within the cell since the functions of these proteins are unknown.
In contrast to the Rho-independent termination within bmpB resulting in the 1.6-kb RNA, we speculate that transcription termination within the bmpA-bmpB intergenic region resulting in the 1.1-kb RNA may be Rho dependent. Although others have identified putative hairpin structures within the bmpA-bmpB intergenic region (11), these are too poor in stability, on the basis of their estimated free energies of dissociation (−4.8 kcal/mol), to function as Rho-independent terminators. The transcription termination factor Rho is encoded in the genome of B. burgdorferi (15).
Insertion of the bmpB IR sequence within the gfp marker gene efficiently terminated transcription at the insertion site. This is surprising given that the presence of the same sequence in the context of the bmpB gene allowed some expression of bmpB. The difference in the promoters driving the expression of gfpT (flaBp) and bmpB (bmpABp) could not have accounted for this diversity, as gfpT gene expression was still efficiently terminated at the bmpB terminator site even when the gene was expressed from the bmpAB promoter in E. coli (data not shown). Additionally, when the gfp probe was washed off and the blot was reprobed with the bmpB sequence, the 1.6- and 2.4-kb RNA bands were uniformly visible in all six lanes (Fig. 3C). This demonstrates that the transcriptional features of the bmpAB operon was conserved in these high-passage transformants. Therefore, we hypothesize that the resulting expression of bmpB in B. burgdorferi is not necessarily due to passive readthrough but may involve active antitermination. Antitermination also may be involved in overcoming termination at the intergenic region. However, if termination at this location is Rho dependent, as we have speculated, then active translation of the bmpA mRNA alone is sufficient to suppress the activity of Rho and enable readthrough (reviewed in reference 8). All of the genes known to be involved in antitermination in E. coli, including nusA, nusB, nusG, nusE (rpsJ, ribosomal protein S10) (reviewed in references 9, 10, 27, and 36), and rpsD (ribosomal protein S4) (45), are also encoded in the genome of B. burgdorferi (15). We are currently examining the possibility that antitermination is involved in the expression of bmpB.
Multiple transcripts have been previously reported for other operons of B. burgdorferi, including secA-sodA (26) and the tandemly arranged oppA1, oppA2, and oppA3 genes (4). However, in both cases, termination of transcription on the basis of size appears to be all within the corresponding intergenic regions like the bmpA-bmpB intergenic termination but unlike the intragenic termination observed for bmpB. By contrast, the dbpAB operon expresses a single 1.35-kb transcript (17). The ospAB operon presents an interesting case. Both patterns have been observed for the ospAB operon, depending on the strain. In type strain B31, a single 2.2-kb transcript was observed (19), whereas in HB19 and its clonal variants at least four bands could be detected (6). Recently, it was estimated that in B31 spirochetes grown in culture, ospA mRNA was 3.5-fold more abundant than ospB mRNA (22), a ratio similar to that of bmpA mRNA to bmpB mRNA. Premature transcription termination was suggested as one possible mechanism that could result in the disproportionate ospA and ospB mRNA levels (22). Taken together, these results suggest that premature transcription termination may be a simple but effective mechanism used by B. burgdorferi to fine-tune the expression of the genes of an operon.
Acknowledgments
This work was funded by grant AI 49293 (R.R.) from the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
We thank Mario Philipp for critical reading of the manuscript and for the rhesus anti-BmpD antibody.
REFERENCES
- 1.Akin, E., G. L. McHugh, R. A. Flavell, E. Fikrig, and A. C. Steere. 1999. The immunoglobulin (IgG) antibody response to OspA and OspB correlates with severe and prolonged Lyme arthritis and the IgG response to P35 correlates with mild and brief arthritis. Infect. Immun. 67:173-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aron, L., M. Alekshun, L. Perlee, I. Schwartz, H. P. Godfrey, and F. C. Cabello. 1994. Cloning and DNA sequence analysis of bmpC, a gene encoding a potential membrane lipoprotein of Borrelia burgdorferi. FEMS Microbiol. Lett. 123:75-82. [DOI] [PubMed] [Google Scholar]
- 3.Barthold, S. W., S. A. Levy, E. Fikrig, L. K. Bockenstedt, and A. L. Smith. 1995. Serologic responses of dogs naturally exposed to or vaccinated against Borrelia burgdorferi infection. J. Am. Vet. Med. Assoc. 207:1435-1440. [PubMed] [Google Scholar]
- 4.Bono, J. L., K. Tilly, B. Stevenson, D. Hogan, and P. Rosa. 1998. Oligopeptide permease in Borrelia burgdorferi: putative peptide-binding components encoded by both chromosomal and plasmid loci. Microbiology 144:1033-1044. [DOI] [PubMed] [Google Scholar]
- 5.Brooks, C. S., P. S. Hefty, S. E. Jolliff, S., and D. R. Akins. 2003. Global analysis of Borrelia burgdorferi genes regulated by mammalian host-specific signals. Infect. Immun. 71:3371-3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bundoc, V. G., and A. G. Barbour. 1989. Clonal polymorphisms of outer membrane protein OspB of Borrelia burgdorferi. Infect. Immun. 57:2733-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carroll, J. A., P. E. Stewart, P. Rosa, A. F. Elias, and C. F. Garon. 2003. An enhanced GFP reporter system to monitor gene expression in Borrelia burgdorferi. Microbiology 149:1819-1828. [DOI] [PubMed] [Google Scholar]
- 8.Condon, C., C. Squires, and C. L. Squires. 1995. Control of rRNA transcription in Escherichia coli. Microbiol. Rev. 59:623-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Das, A. 1992. How the phage lambda N gene product suppresses transcription termination: communication of RNA polymerase with regulatory proteins mediated by signals in nascent RNA. J. Bacteriol. 174:6711-6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das, A. 1993. Control of transcription termination by RNA-binding proteins. Annu. Rev. Biochem. 62:893-930. [DOI] [PubMed] [Google Scholar]
- 11.Dobrikova, E. Y., J. Bugrysheva, and F. C. Cabello. 2001. Two independent transcriptional units control the complex and simultaneous expression of the bmp paralogous chromosomal gene family in Borrelia burgdorferi. Mol. Microbiol. 39:370-378. [DOI] [PubMed] [Google Scholar]
- 12.Dressler, F., J. A. Whalen, B. N. Reinhardt, and A. C. Steere. 1993. Western blotting in the serodiagnosis of Lyme disease. J. Infect. Dis. 167:392-400. [DOI] [PubMed] [Google Scholar]
- 13.Engstrom, S. M., E. Shoop, and R. C. Johnson. 1995. Immunoblot interpretation criteria for the serodiagnosis of early Lyme disease. J. Clin. Microbiol. 33:419-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fawcett, P. T., C. Rose, K. M. Gibney, C. A. Chase, B. Kiehl, and R. A. Doughty. 1993. Detection of antibodies to the recombinant P39 protein of Borrelia burgdorferi using enzyme immunoassay and immunoblotting. J. Rheumatol. 20:734-738. [PubMed] [Google Scholar]
- 15.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J.-F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, J. Gocayne, J. Weidman, T. Utterback, L. Whattey, L. McDonald, P. Artiach, C. Bowman, S. Garland, C. Fujii, M. D. Cotton, K. Horst, K. Roberts, B. Hatch, H. O. Smith, and J. C. Venter. 1997. Genome sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580-586. [DOI] [PubMed] [Google Scholar]
- 16.Gorbacheva, V. Y., H. P. Godfrey, and F. C. Cabello. 2000. Analysis of the bmp gene family in Borrelia burgdorferi sensu lato. J. Bacteriol. 182:2037-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hagman, K. E., P. Lahdenne, T. G. Popova, S. F. Porcella, D. R. Akins, J. D. Radolf, and M. V. Norgard. 1998. Decorin-binding proteins of Borrelia burgdorferi is encoded within a two-gene operon and is protective in the murine model of Lyme borreliosis. Infect. Immun. 66:2674-2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hauser, U., G. Lehnert, R. Lobentanzer, and B. Wilske. 1997. Interpretation criteria for standardized Western blots for three European species of Borrelia burgdorferi sensu lato. J. Clin. Microbiol. 35:1433-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howe, T. R., F. W. LaQuier, and A. G. Barbour. 1986. Organization of genes encoding two outer membrane proteins of the Lyme disease agent Borrelia burgdorferi within a single transcriptional unit. Infect. Immun. 54:207-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hubner, A., X. Yang, D. M. Nolen, T. G. Popova, F. C. Cabello, and M. V. Norgard. 2001. Expression of Borrelia burgdorferi OspC and DbpA is controlled by a RpoN-RpoS pathway. Proc. Natl. Acad. Sci. USA 98:12724-12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang, F. T., F. K. Nelson, and E. Fikrig. 2002. Molecular adaptation of Borrelia burgdorferi in the murine host. J. Exp. Med. 196:275-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang, F. T., M. J. Caimano, J. D. Radolf, and E. Fikrig. 2004. Borrelia burgdorferi outer surface protein (osp) B expression independent of ospA. Microb. Pathog. 37:35-40. [DOI] [PubMed] [Google Scholar]
- 23.Magnarelli, L. A., J. W. Ijdo, S. J. Padula, R. A. Flavell, and E. Fikrig. 2000. Serologic diagnosis of Lyme borreliosis by using enzyme-linked immunosorbent assays with recombinant antigens. J. Clin. Microbiol. 38:1735-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Magnarelli, L. A., J. W. Ijdo, A. E. Van Andel, C. Wu, S. J. Padula, and E. Fikrig. 2000. Serologic confirmation of Ehrlichia equi and Borrelia burgdorferi infections in horses from the northeastern United States. J. Am. Vet. Med. Assoc. 217:1045-1050. [DOI] [PubMed] [Google Scholar]
- 25.Magnarelli, L. A., S. A. Levy, J. W. Ijdo, C. Wu, S. J. Padula, E., and Fikrig. 2001. Reactivity of dog sera to whole-cell or recombinant antigens of Borrelia burgdorferi by ELISA and immunoblot analysis. J. Med. Microbiol. 50:889-895. [DOI] [PubMed] [Google Scholar]
- 26.Nichols, T. L., C. A. Whitehouse, and F. E. Austin. 2000. Transcriptional analysis of a superoxide dismutase gene of Borrelia burgdorferi. FEMS Microbiol. Lett. 183:37-42. [DOI] [PubMed] [Google Scholar]
- 27.Nudler, E., and M. E. Gottesman. 2002. Transcription termination and antitermination in E. coli. Genes Cells 7:755-768. [DOI] [PubMed] [Google Scholar]
- 28.Oh, S. H., Y. H. Song, D. H. Yoo, S. Y. Kim, and H. Lee. 1993. Distribution of Borrelia burgdorferi specific antibody among patients with juvenile rheumatoid arthritis in Korea. J. Korean Med. Sci. 8:405-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oksi, J., J. Uksila, M. Marjamaki, J. Nikoskelainen, and M. K. Viljanen. 1995. Antibodies against whole sonicated Borrelia burgdorferi spirochetes, 41-kilodalton flagellin, and P39 protein in patients with PCR- or culture-proven late Lyme borreliosis. J. Clin. Microbiol. 33:2260-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pachner, A. R., D. Dail, L. Li, L. Gurey, S. Feng, E. Hodzic, and S. Barthold. 2002. Humoral immune response associated with Lyme borreliosis in nonhuman primates: analysis by immunoblotting and enzyme-linked immunosorbent assay with sonicates or recombinant proteins. Clin. Diagn. Lab. Immunol. 9:1348-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piesman, J., T. N. Mather, R. Sinsky, and A. Spielman. 1987. Duration of tick attachment and Borrelia burgdorferi transmission. J. Clin. Microbiol. 25:557-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pollack, R. J., S. R. Telford III, and A. Spielman. 1993. Standardization of medium for culturing Lyme disease spirochetes. J. Clin. Microbiol. 31:1251-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramamoorthy, R., L. Povinelli, and M. T. Philipp. 1996. Molecular characterization, genomic arrangement, and expression of bmpD, a new member of the bmp class of genes encoding membrane proteins of Borrelia burgdorferi. Infect. Immun. 64:1259-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramamoorthy, R., and M. T. Philipp. 1998. Differential expression of Borrelia burgdorferi proteins during growth in vitro. Infect. Immun. 66:5119-5124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Revel, A. T., A. M. Talaat, and M. V. Norgard. 2002. DNA microarray analysis of differential gene expression in Borrelia burgdorferi, the Lyme disease spirochete. Proc. Natl. Acad. Sci. USA 99:1562-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roberts, J. W. 1993. RNA and protein elements of E. coli and lambda transcription antitermination complexes. Cell 72:653-655. [DOI] [PubMed] [Google Scholar]
- 37.Roessler, D., U. Hauser, and B. Wilske. 1997. Heterogeneity of BmpA (P39) among European isolates of Borrelia burgdorferi sensu lato and the influence of interspecies variability on serodiagnosis. J. Clin. Microbiol. 35:2752-2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samuels, D. S. 1995. Electrotransformation of the spirochete Borrelia burgdorferi. Methods Mol. Biol. 47:253-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Sci. Acad. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarkatova, M., E. Dobrikova, and F. C. Cabello. 2000. Development of an extrachromosomal cloning vector system for use in Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA 97:4850-4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schouls, L. M., H. G. J. van der Heide, and J. D. A. van Embden. 1991. Characterization of the 35-kilodalton Treponema pallidum subsp. pallidum recombinant lipoprotein TmpC and antibody response to lipidated and nonlipidated T. pallidum antigens. Infect. Immun. 59:3536-3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simpson, W. J., M. E. Schrumpf, and T. G. Schwan. 1990. Reactivity of human Lyme borreliosis sera with a 39-kilodalton antigen specific to Borrelia burgdorferi. J. Clin. Microbiol. 28:1329-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simpson, W. J., W. Cieplak, M. E. Schrumpf, A. G. Barbour, and T. G. Schwan. 1994. Nucleotide sequence and analysis of the gene in Borrelia burgdorferi encoding the immunogenic P39 antigen. FEMS Microbiol. Lett. 119:381-388. [DOI] [PubMed] [Google Scholar]
- 44.Stewart, P. E., R. Thalken, J. L. Bono, and P. Rosa. 2001. Isolation of circular plasmid region sufficient for autonomous replication and transformation of infectious Borrelia burgdorferi. Mol. Microbiol. 39:714-721. [DOI] [PubMed] [Google Scholar]
- 45.Torres, M., C. Condon, J.-M, Balada, C. Squires, and C. L. Squires. 2001. Ribosomal protein S4 is a transcription factor with properties remarkably similar to NusA, a protein involved in both non-ribosomal and ribosomal RNA antitermination. EMBO J. 20:3811-3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilske, B., C. Habermann, V. Fingerle, B. Hillenbrand, S. Jauris-Heipke, G. Lehnert, I. Pradel, D. Rossler, and U. Schulte-Spechtel. 1999. An improved recombinant IgG immunoblot for serodiagnosis of Lyme borreliosis. Med. Microbiol. Immunol. 188:139-144. [DOI] [PubMed] [Google Scholar]
- 47.Zuker, M., and P. Stiegler. 1981. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 9:133-148. [DOI] [PMC free article] [PubMed] [Google Scholar]