Abstract
Background: Obese children undergoing laparoscopic surgery frequently experience high end-tidal carbon dioxide partial pressure (PETCO2) and respiratory acidosis. This study aimed to investigate the effects of pressure-controlled inverse ratio ventilation (IRV) with an inspiratory to expiratory ratio (I:E) of 1.5:1 on obese children undergoing laparoscopic surgery. Methods: Eighty children undergoing laparoscopic surgery were randomly assigned to either the IRV group (I:E=1.5:1) or the control group (I:E=1:1.5). The lungs were mechanically ventilated following tracheal intubation. The children underwent pressure-controlled ventilation with an I:E ratio of 1.5:1 or 1:1.5. Respiratory mechanics, hemodynamic values, and ventilation-related side effects were recorded. Results: Thirty minutes after establishing CO2 pneumoperitoneum, the IRV group exhibited significantly higher tidal volume (Vt) and arterial partial pressure of oxygen (PaO2) compared to the control group (97.6 ± 6.6 vs. 93.2 ± 8.0 ml, 283 ± 54 vs. 247 ± 40 mmHg, respectively) (P < 0.01). Furthermore, PaCO2 was significantly lower in the IRV group than in the control group (41.4 ± 5.8 vs. 45.5 ± 5.7 mmHg, P=0.002). The incidence of intra-operative hypercapnia was significantly decreased in the IRV group (25% vs. 42.5%, P=0.03). Conclusion: Pressure-controlled IRV can reduce the incidence of hypercapnia, increasing Vt, and thereby improving CO2 elimination in obese children undergoing laparoscopy. This ventilation technique significantly improves gas exchange in this patient population. (Registration number: ChiCTR2000035589).
Keywords: Inverse ratio ventilation, gas exchange, hypercapnia, laparoscopy, obese children
Introduction
Obesity and pneumoperitoneum significantly affect respiratory mechanics in patients undergoing laparoscopic surgery. The gold standard for assessing the efficacy of mechanical ventilation remains the measurement of partial pressure of carbon dioxide in arterial blood (PaCO2). Obesity reduces functional residual capacity and alters ventilation-perfusion matching [1]. During laparoscopic surgery, obesity and increased intra-abdominal pressure influence CO2 elimination [2]. Pediatric patients undergoing laparoscopic surgery frequently experience hypercapnia and respiratory acidosis. Maintaining end-tidal carbon dioxide partial pressure (PETCO2) within the normal range without causing ventilator-related lung injury poses a challenge. Attempting to alleviate hypercapnia by increasing respiratory frequency under pressure-controlled ventilation mode proves difficult. Pressure-controlled ventilation with positive end-expiratory pressure (PEEP) enhances oxygenation while reducing tidal volumes (Vt). Although higher airway pressure under pressure-controlled ventilation increases Vt, it may cause lung barotrauma and volutrauma. The literature suggests that inverse ratio ventilation (IRV) improves oxygenation and reduces peak airway pressure compared to conventional ventilation modes [3-7]. We therefore investigated the effect of pressure-controlled IRV with an inspiratory/expiratory (I:E) ratio of 1.5:1 on gas exchange in obese children undergoing laparoscopic surgery. Pressure-controlled IRV has proven effective in enhancing oxygenation in patients with acute hypoxemic respiratory failure. This study aimed to determine if pressure-controlled IRV can improve ventilation without increasing the levels of PaCO2 in obese children. We hypothesized that pressure-controlled IRV with an I:E ratio of 1.5:1 would enhance gas exchange and facilitate CO2 elimination in obese children undergoing laparoscopic surgery.
Methods
Children undergoing elective laparoscopy were enrolled in this study. Inclusion criteria: body mass index (BMI) of 28 kg/m2 or higher, of either sex, aged 2 to 6 years, and American Society of Anesthesiologists (ASA) physical status I and II. The expected surgery duration was more than 30 min. Children with cardiopulmonary diseases were excluded. Eighty children were randomly assigned to the IRV group (I:E=1.5:1) or the control group (I:E=1:1.5) using a computer-generated randomization table.
All children were fasted for 6 hours prior to surgery and received no premedication. Upon entering the operating room, peripheral venous access was established, and routine monitoring including electrocardiogram, noninvasive blood pressure (BP), heart rate (HR), and pulse oxygen saturation (SpO2) was initiated. Anesthesia was induced with intravenous fentanyl (3 µg/kg), propofol (3 mg/kg), and cis-atracurium (0.1 mg/kg). Anesthesia was maintained with inhalation of 2-3% sevoflurane. After tracheal intubation, both lungs were mechanically ventilated using pressure-controlled mode. Initially, respiratory parameters were set at an airway pressure of 20 cmH2O, respiratory rate of 20 breaths/min, positive end-expiratory pressure (PEEP) of zero, oxygen flow of 2 L/min, fraction of inspired oxygen of 1.0, and I:E ratio of 1:1.5. In the control group, ventilation continued with an I:E ratio of 1:1.5 when CO2 pneumoperitoneum (intra-abdominal pressure 10 mmHg) was established, while in the IRV group, I:E ratio was set at 1.5:1. Other respiratory measurements remained consistent between the two groups.
Sevoflurane levels were adjusted to maintain the bispectral index (BIS) value between 40 and 55 (BIS monitor Model A2000, USA) and to control the hemodynamic response to the surgical procedure within a 20% range of the preoperative value. Muscle relaxation was monitored by train-of-four (TOF) stimulation (Organon Corporation, type: TOF-Watch SX, Holland). Cis-atracurium was infused at a rate of 0.08 mg kg-1h-1 to maintain TOF value below 5%. Respiratory settings were not adjusted if PETCO2 did not exceed 50 mmHg within 30 min after establishing CO2 pneumoperitoneum. The airway pressure was adjusted to maintain PETCO2 between 35 and 45 mmHg after 30 min of establishing CO2 pneumoperitoneum. Spirometry readings included inspiratory Vt, mean airway pressure (Pmean), PETCO2, and total PEEP (PEEPtot) using a side-stream spirometry device (GE company, Taipei, China). Ringer’s solution was infused at a rate of 5-6 ml·kg-1·h-1 during the operative period.
After establishing CO2 pneumoperitoneum, the children were positioned supine with a 20° head-down tilt. Noninvasive systolic blood pressure (SBP), diastolic blood pressure (DBP), and HR were recorded at baseline or before anesthesia (T0), 2 min before establishing CO2 pneumoperitoneum (T1), 30 min after establishing CO2 pneumoperitoneum (T2), and the end of surgery (T3). Respiratory mechanics were recorded at T1 and T2. Arterial blood was drawn and analyzed using a blood gas analyzer (Type: ABL8000, Denmark) at T1 and T2, respectively. Hypercapnia was defined as PaCO2 > 45 mmHg. Time to extubation and time to discharge from the post-anesthesia care unit (PACU) were recorded. Post-operative complications, such as post-operative hypoxemia (defined as SpO2 below 91% while receiving air), pneumothorax, and other pulmonary complications were recorded. Children were discharged from the PACU when the Modified Aldrete Score was 9 or above [8].
Statistical analysis
Data were analyzed using SPSS 17.0 statistical software (SPSS Inc., Chicago, USA). Quantitative variables with a normal distribution were compared using t-tests and a one-sided analysis of variance. Data with non-normal distribution were analyzed using a two-sided Mann-Whitney U-test in both groups. Categorical variables were evaluated with the Chi-square test and Fisher’s test. All quantitative data were expressed as mean ± standard deviation. A P < 0.05 was considered significant.
Sample sizes were determined based on the primary outcome, the levels of PaCO2 in this study. A priori power analysis using two-sided analysis with an α error of 0.05 and a power of 0.8 indicated that 32 patients were needed in each group to detect a difference of 2 mmHg in PaCO2 levels. The sample size was increased to 40 in each group to account for potential dropouts.
Results
Demographic data
Eighty-two children were initially enrolled in the study (Figure 1). However, two patients were excluded due to hesitations in participation, leaving a total of eighty children who completed the study. There were no significant differences in BMI, gender, age, or duration of surgery between the two groups (Table 1) (P > 0.05).
Figure 1.

Flow diagram of study.
Table 1.
Data of children (n=40)
| Index | IRV group | Control group | P-value |
|---|---|---|---|
| Age (years) | 4.0 ± 1.2 | 4.2 ± 1.3 | 0.521 |
| BMI (kg/m2) | 30.3 ± 1.6 | 30.2 ± 1.2 | 0.617 |
| Gender (Male/Female) | 28/12 | 24/16 | 0.321 |
| Surgery performed, 1/2 (n) | 16/24 | 18/22 | 0.723 |
| Duration of pneumoperitoneum (min) | 61.4 ± 7.7 | 62.4 ± 8.2 | 0.586 |
| Duration of surgery (min) | 73.6 ± 6.4 | 74.4 ± 6.3 | 0.573 |
| Time to extubation (min) | 12.4 ± 2.1 | 11.5 ± 2.5 | 0.106 |
| PACU discharge time (min) | 39.2 ± 8.2 | 39.6 ± 11.9 | 0.870 |
| Intraoperative hypercapnia (n) | 8 (25%) | 17 (42.5%) | 0.030* |
| Postoperative hypoxemia (n) | 0 | 0 | 0.999 |
Data are expressed as the mean (standard deviation) or number. Surgery performed: 1, appendectomy; 2, herniorrhaphy. BMI: body mass index; PACU: post-anesthesia care unit.
P < 0.05, compared with the control group.
Blood gas analysis
Blood gas analysis at T1 revealed no significant differences in PaO2, PaCO2, and Ph between the two groups (P > 0.05). At T2, PaO2 and pH values were significantly higher in the IRV group compared to the control group (283 ± 54 vs. 247 ± 40 mmHg, 7.34 ± 0.03 vs. 7.32 ± 0.03) (Table 2) (P < 0.01), while PaCO2 was significantly lower in the IRV group (41.4 ± 5.8 vs. 44.5 ± 5.7 mmHg) (P=0.002). SaO2 values were similar in both groups (P < 0.05).
Table 2.
Arterial blood gas of children (n=40)
| Index | IRV group | Control group | P-value |
|---|---|---|---|
| pH | 7.34 ± 0.03 | 7.32 ± 0.03 | 0.005* |
| PaO2 (mmHg) | 283 ± 54 | 247 ± 40 | 0.001* |
| PaCO2 (mmHg) | 41.4 ± 5.5 | 45.5 ± 5.7 | 0.002* |
| SaO2 (%) | 99.7 ± 1.5 | 99.6 ± 1.7 | 0.782 |
Data are expressed as mean (standard deviation) or numbers. PaO2: oxygen partial pressure in arterial blood. PaCO2: partial pressure of carbon dioxide in arterial blood. SaO2: oxygen saturation in arterial blood.
P < 0.05, compared with the control group.
Respiratory parameters
At T2, the IRV group exhibited significantly higher Vt, Pmean, and auto-PEEP compared to the control group (97.6 ± 6.6 vs. 93.2 ± 8.0 ml, 16.3 ± 1.0 vs. 15.4 ± 1.4 cmH2O and 2.65 ± 0.58 vs. 1.98 ± 0.48 cmH2O, respectively) (P < 0.01) (Figure 2). PETCO2 was significantly lower at T2 in the IRV group than in the control group (41.4 ± 5.8 vs. 44.5 ± 5.7 mmHg) (P=0.002). Blood pressure and HR, as shown in Figure 3, did not exhibit significant differences between the two groups at T2 (P > 0.05).
Figure 2.
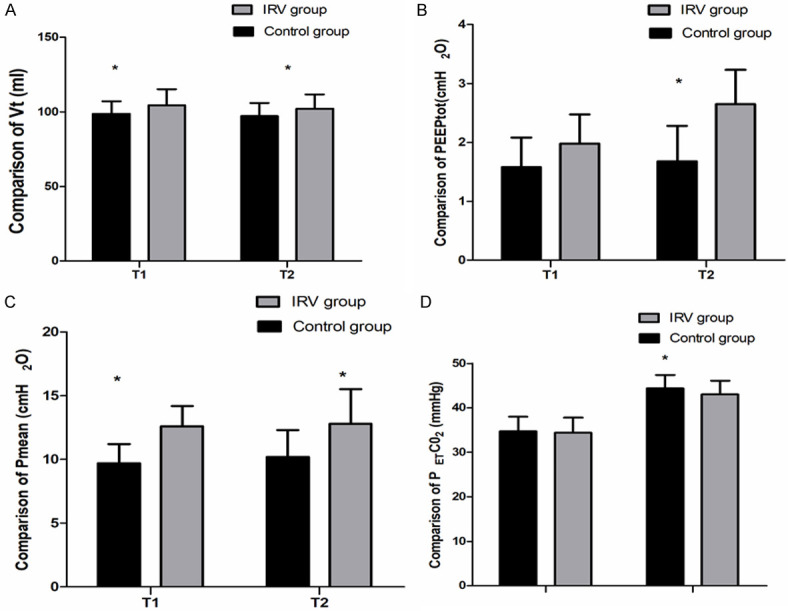
Comparison of respiratory mechanics between the 2 groups. At T1, T2 and T3, there were significant differences in Vt, Pmean, PEEPtot and PETCO2 between the 2 groups (*P < 0.05). T1: immediately before establishing CO2 pneumoperitoneum, T2: 30 min after initiation of CO2 pneumoperitoneum, T3: at the end of surgery. Vt: tidal volume, Pmean: mean airway pressure, PEEPtot: total positive end expiratory pressure, PETCO2: end-tidal carbon dioxide partial pressure.
Figure 3.
Comparison of hemodynamic measurements (SBP, DBP and HR) between the 2 groups. At T0, T1, T2 and T3, there were no significant differences in SBP, DBP and HR between the 2 groups (P > 0.05). SBP: systolic blood pressure, DBP: diastolic blood pressure, HR: heart rate. T0: at baseline or before anesthesia, T1: immediately before establishing CO2 pneumoperitoneum, T2: 30 min after initiation of CO2 pneumoperitoneum, T3: at the end of surgery.
Adverse events
Within 30 min after initiation of CO2 pneumoperitoneum, eight cases of hypercapnia occurred in the IRV group, while 17 cases were observed in the control group. The incidence of intra-operative hypercapnia was significantly lower in the IRV group (P=0.03). No postoperative hypoxemia was observed, and there were no significant differences in the incidence of hypercapnia, extubation time, or time to discharge from PACU between the two groups (P > 0.05) (Table 1). No respiratory complications were observed during the hospital stays.
Discussion
A larger tidal volume leads to increased minute ventilation and higher peak airway pressure when respiratory rates are constant. PEEP can enhance oxygenation but also increase peak or plateau airway pressure. Consequently, we chose not to utilize PEEP in this study.
Our investigation into the effects of pressure-controlled inverse ratio ventilation (IRV) (I:E=1.5:1) ventilation on obese children undergoing laparoscopy revealed significant improvements in CO2 removal and oxygenation compared to conventional I:E ratio ventilation. IRV, differing from conventional ventilation modes, extends inspiratory time, increases alveolar ventilation and functional residual capacity, and expands collapsed alveoli. Besides, the dead space is decreased, which contributes to the gas distribution in the lungs. Currently, few studies explore IRV in obese children undergoing laparoscopy. In the present study, IRV demonstrated higher Vt and Pmean in the IRV group than in the control group. Prolonged inspiratory time and decreased inspiratory flow velocity increased Vt. Additionally, IRV generated auto-PEEP (endogenous PEEP) [9], which was beneficial for oxygenation. The literature indicates that arterial blood oxygenation is directly related to mean airway pressure [7,10], highlighting its importance in gas exchange [11]. Our results align with Sinha et al. [12] findings, who reported a significant increase in Vt, Pmean, and dynamic lung compliance when pressure-controlled IRV with an I:E ratio of 1.5:1 was used, compared to conventional pressure-controlled ventilation with an I:E ratio of 1:2, during gynecologic laparoscopy with laryngeal mask ventilation.
In the pressure-controlled IRV mode, the prolonged inspiratory time and relatively shorter expiratory time generate endogenous PEEP due to gas delay. Interestingly, our study found no statistical differences in blood pressure and heart rate between the groups, indicating that IRV with an I:E ratio of 1.5:1 did not affect venous return. Beyond an I:E ratio of 2:1, IRV can indeed reduce venous return and cardiac output [4,13]. When inspiratory time is excessively prolonged and Pmean reaches a certain high level, pressure-controlled IRV might lead to a decrease in cardiac output, affecting hemodynamics [4,14]. The study by Mercat et al. [6] supports the idea that IRV can increase Pmean and PaO2 [6]. However, the impact of Pmean on hemodynamics becomes significant only when it reaches a high threshold. Interestingly, despite a significantly higher Pmean in the IRV group compared to the conventional ventilation group, there were no significant differences in hemodynamic measurements between the two groups. This observation aligns with the results reported by Movassagi, et al. [14].
At 30 min after establishing CO2 pneumoperitoneum, the IRV group exhibited significantly higher PaO2 levels than the conventional ventilation group, indicating that IRV could effectively increase PaO2 levels and promote oxygenation. This finding resonates with the study by Tweed et al. where arterial oxygenation was superior to pressure-controlled IRV (I:E=2:1) in patients undergoing abdominal gynecologic surgery compared to conventional ventilation [15]. Moreover, PaCO2 levels were lower in the IRV group than in the control group, signifying a significant difference between the two groups. The significant increase in PaCO2 in both groups 30 min after establishing CO2 pneumoperitoneum in both groups was primarily due to CO2 absorption in the blood [16]. Notably, IRV did not affect CO2 removal. Studies have indicated that during mechanical ventilation, changes in tidal volumes per 100 ml led to a 5.3 mmHg decrease in PaCO2 in patients with normal weight, and a 3.6 mmHg decrease in morbidly obese patients [17]. Hence, tidal volumes or minute ventilation volumes played a crucial role in determining CO2 removal during mechanical ventilation. Additionally, higher CO2 pneumoperitoneum pressure may influence hemodynamics during laparoscopic surgery [18].
The limitations of this study are as follows: IRV, being distinct from conventional ventilation, presents potential risks, particularly the long-term complications associated with prolonged respiratory time, which require further investigation. Moreover, larger sample sizes should be enrolled in future to comprehensively explore adverse respiratory and hemodynamic effects.
Conclusion
Pressure-controlled IRV emerged as a promising approach to mitigating hypercapnia, increasing Vt, and enhancing CO2 removal in obese children undergoing laparoscopy when compared to conventional ventilation. Notably, IRV outperformed conventional ventilation in terms of CO2 removal efficiency and respiratory mechanics in children undergoing laparoscopy.
Acknowledgements
We thank Jiang Y for her encouragement in this study.
Disclosure of conflict of interest
None.
References
- 1.Sivarajan VB, Bohn D. Monitoring of standard hemodynamic parameters: heart rate, systemic blood pressure, atrial pressure, pulse oximetry, and end-tidal CO2. Pediatr Crit Care Med. 2011;12(Suppl):S2–S11. doi: 10.1097/PCC.0b013e318220e7ea. [DOI] [PubMed] [Google Scholar]
- 2.Dion JM, McKee C, Tobias JD, Herz D, Sohner P, Teich S, Michalsky M. Carbon dioxide monitoring during laparoscopic-assisted bariatric surgery in severely obese patients: transcutaneous versus end-tidal techniques. J Clin Monit Comput. 2015;29:183–6. doi: 10.1007/s10877-014-9587-1. [DOI] [PubMed] [Google Scholar]
- 3.Talab HF, Zabani IA, Abdelrahman HS, Bukhari WL, Mamoun I, Ashour MA, Sadeq BB, El Sayed SI. Intraoperative ventilatory strategies for prevention of pulmonary atelectasis in obese patients undergoing laparoscopic bariatric surgery. Anesth Analg. 2009;109:1511–6. doi: 10.1213/ANE.0b013e3181ba7945. [DOI] [PubMed] [Google Scholar]
- 4.Almarakbi WA, Fawzi HM, Alhashemi JA. Effects of four intraoperative ventilatory strategies on respiratory compliance and gas exchange during laparoscopic gastric banding in obese patients. Br J Anaesth. 2009;102:862–8. doi: 10.1093/bja/aep084. [DOI] [PubMed] [Google Scholar]
- 5.Markström AM, Lichtwarck-Aschoff M, Hedlund AJ, Nordgren KA, Sjöstrand UH. Under open lung conditions inverse ratio ventilation causes intrinsic PEEP and hemodynamic impairment. Ups J Med Sci. 1996;101:257–71. doi: 10.3109/03009739609178925. [DOI] [PubMed] [Google Scholar]
- 6.Mercat A, Titiriga M, Anguel N, Richard C, Teboul JL. Inverse ratio ventilation (I/E=2/1) in acute respiratory distress syndrome: a six-hour controlled study. Am J Respir Crit Care Med. 1997;155:1637–42. doi: 10.1164/ajrccm.155.5.9154869. [DOI] [PubMed] [Google Scholar]
- 7.Huang CC, Shih MJ, Tsai YH, Chang YC, Tsao TC, Hsu KH. Effects of inverse ratio ventilation versus positive end-expiratory pressure on gas exchange and gastric intramucosal PCO(2) and pH under constant mean airway pressure in acute respiratory distress syndrome. Anesthesiology. 2001;95:1182–8. doi: 10.1097/00000542-200111000-00023. [DOI] [PubMed] [Google Scholar]
- 8.Schmitz A, Weiss M, Kellenberger C, O’Gorman Tuura R, Klaghofer R, Scheer I, Makki M, Sabandal C, Buehler PK. Sedation for magnetic resonance imaging using propofol with or without ketamine at induction in pediatrics-A prospective randomized double-blinded study. Paediatr Anaesth. 2018;28:264–274. doi: 10.1111/pan.13315. [DOI] [PubMed] [Google Scholar]
- 9.Yanos J, Watling SM, Verhey J. The physiologic effects of inverse ratio ventilation. Chest. 1998;114:834–8. doi: 10.1378/chest.114.3.834. [DOI] [PubMed] [Google Scholar]
- 10.Xu L, Shen J, Yan M. The effect of pressure-controlled inverse ratio ventilation on lung protection in obese patients undergoing gynecological laparoscopic surgery. J Anesth. 2017;31:651–656. doi: 10.1007/s00540-017-2369-4. [DOI] [PubMed] [Google Scholar]
- 11.Ludwigs U, Klingstedt C, Baehrendtz S, Hedenstierna G. A comparison of pressure- and volume-controlled ventilation at different inspiratory to expiratory ratios. Acta Anaesthesiol Scand. 1997;41:71–7. doi: 10.1111/j.1399-6576.1997.tb04615.x. [DOI] [PubMed] [Google Scholar]
- 12.Gore DC. Hemodynamic and ventilatory effects associated with increasing inverse inspiratory-expiratory ventilation. J Trauma. 1998;45:268–72. doi: 10.1097/00005373-199808000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Sinha M, Chiplonkar S, Ghanshani R. Pressure-controlled inverse ratio ventilation using laryngeal mask airway in gynecological laparoscopy. J Anaesthesiol Clin Pharmacol. 2012;28:330–333. doi: 10.4103/0970-9185.98327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Movassagi R, Montazer M, Mahmoodpoor A, Fattahi V, Iranpour A, Sanaie S. Comparison of pressure vs. volume controlled ventilation on oxygenation parameters of obese patients undergoing laparoscopic cholecystectomy. Pak J Med Sci. 2017;33:1117–1122. doi: 10.12669/pjms.335.13316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tweed WA, Tan PL. Pressure controlled-inverse ratio ventilation and pulmonary gas exchange during lower abdominal surgery. Can J Anaesth. 1992;39:1036–1040. doi: 10.1007/BF03008371. [DOI] [PubMed] [Google Scholar]
- 16.De Waal EE, Kalkman CJ. Haemodynamic changes during low-pressure carbon dioxide pneumoperitoneum in young children. Paediatr Anaesth. 2003;13:18–25. doi: 10.1046/j.1460-9592.2003.00973.x. [DOI] [PubMed] [Google Scholar]
- 17.Sprung J, Whalley DG, Falcone T, Warner DO, Hubmayr RD, Hammel J. The impact of morbid obesity, pneumoperitoneum, and posture on respiratory system mechanics and oxygenation during laparoscopy. Anesth Analg. 2002;94:1345–50. doi: 10.1097/00000539-200205000-00056. [DOI] [PubMed] [Google Scholar]
- 18.Rademaker BM, Odoom JA, de Wit LT, Kalkman CJ, ten Brink SA, Ringers J. Haemodynamic effects of pneumoperitoneum for laparoscopic surgery: a comparison of CO2 with N2O insufflation. Eur J Anaesthesiol. 1994;11:301–306. [PubMed] [Google Scholar]



