Abstract
The σB-dependent general stress regulon of Bacillus subtilis comprises more than 150 members. Induction of this regulon by imposition of environmental or metabolic stress confers multiple, nonspecific, and preemptive stress resistance to nongrowing, nonsporulated cells of B. subtilis. In this study we performed a regulon-wide phenotypic screening analysis to determine the stress sensitivity profiles of 94 mutants defective in candidate members of the general stress regulon that were previously identified in our transcriptional profiling study of the general stress response of B. subtilis. The phenotypic screening analysis included analysis of adaptation to a growth-inhibiting concentration of ethanol (10%, vol/vol) or NaCl (10%, wt/vol), severe heat shock (54°C), and low temperature (survival at 4°C and growth at 12.5°C). Surprisingly, 85% of the mutants tested displayed increased sensitivity at an α confidence level of ≤0.01 to at least one of the four stresses tested, and 62% still exhibited increased sensitivity at an α of ≤0.001. In essence, we were able to assign 63 genes (28 genes with an α of ≤0.001) to survival after ethanol shock, 37 genes (28 genes with an α of ≤0.001) to protection from NaCl shock, 34 genes (24 genes with an α of ≤0.001) to survival at 4°C, and 10 genes (3 genes with an α of ≤0.001) to management of severe heat shock. Interestingly, there was a substantial overlap between the genes necessary for survival during ethanol shock and the genes necessary for survival at 4°C, and there was also an overlap between genes required for survival during ethanol shock and genes required for survival during NaCl shock. Our data provide evidence for the importance of the σB regulon at low temperatures, not only for growth but also for survival. Moreover, the data imply that a secondary oxidative stress seems to be a common component of the severe stresses tested.
The sequencing of microbial genomes has enabled a new view of cellular life processes. One essential outcome of the genome-sequencing programs is the finding that the functions of about one-third of the genes are still unknown. Even the genome of the gram-positive model organism Bacillus subtilis, containing approximately 4,100 genes, codes for about 1,700 proteins whose functions are still unknown (34). The assignment of functions to this large number of genes is a considerable challenge and requires a concerted effort of genetic, biochemical, and functional genomics approaches. Computational as well as experimental approaches have been used to estimate that the number of essential genes in diverse bacteria, such as Mycoplasma, Haemophilus influenzae, and Staphylococcus aureus, is between 150 and 670 (2, 26, 40) and that the number of essential genes in B. subtilis is around 270 (29). Therefore, it is very likely that many of the genes with unknown functions are not vital for cellular life processes but rather are required only in particular physiological situations.
Functional genomics technologies can effectively reveal the environmental conditions under which genes with undefined functions are expressed and thus are likely to be important. By using the genome sequence and global experimental approaches, such as transcriptomics and proteomics, it has become possible to dissect the entire genome into single regulation groups, such as stimulons and regulons. Comparative mRNA profiling of wild-type strains and regulatory mutants by using DNA arrays is currently the state of the art for defining the structure and function of regulons. Such a comparative approach is particularly useful for functional analysis of the large group of newly annotated regulatory proteins, such as response regulators of two-component systems, alternative sigma factors, etc., that have not been studied yet.
Such expression studies also shed light on the functions of a regulon's genes in general, because regulon members whose functions have already been established may help to predict the function of the entire regulon, which subsequently must be confirmed by phenotypic mutant studies. Allocation of unknown genes to regulons whose basic functions are already known is a reasonable strategy to obtain the first, but still preliminary, predictions of the functions of the unknown genes.
This strategy was successfully employed to predict the function of the σB regulon and its members some years ago (for reviews see references 22, 43, and 44). Stress-mediated induction of about 150 proteins whose genes form the entire regulon (8, 23, 42, 45) can provide the nongrowing B. subtilis cell with nonspecific, multiple, preventive stress resistance in anticipation of future stress possibly encountered during long-term stationary growth stages (19, 59). The threats that require the σB-dependent stress response for survival include severe heat and ethanol shock, salt stress, alkaline shock of stationary-phase cells, and freezing (19, 59). Recently, Brigulla et al. (11), as well as Mendez et al. (37), reported that σB is necessary for growth (11), as well as effective stationary-phase survival and sporulation (37), at low temperatures.
Due to the efforts of several groups, the size and structure (5, 23, 42, 45), as well as the regulation, of the σB response (for a review see reference 43) have been fairly well elucidated. In contrast, information related to the contributions of individual σB-dependent general stress genes to the generation of the stress resistance of B. subtilis cells is very limited. Although the functions of the known members of the σB regulon (e.g., katX, trxA, opuD, opuE, dps, clpC, etc.) are consistent with the suggestion of a general role of the σB response in stress protection, so far only a few genes have been subjected to detailed phenotypic or biochemical studies. The proteins that have been studied include GtaB, a UDP-glucose pyrophosphohydrolase involved in cell wall synthesis (57); the ferritin-like Dps protein, which is essential for the development of stationary-phase induced resistance to oxidative stress (7); OpuE, which is required for uptake of the osmoprotectant proline (60); the essential thioredoxin encoded by trxA (46); and ClpC, the ATPase subunit of the Clp chaperone/protease that is involved in development of resistance against high temperature and salt stress (32, 39). In the last three cases gene expression is not solely dependent on σB but is subject to additional control mechanisms (15, 31, 46, 51, 60). However, the σB regulon also comprises more than 100 genes with undefined functions that are probably also involved in the development of nonspecific and multiple-stress resistance. No attempts have been made so far to comprehensively characterize the genes of the σB regulon functionally and to assign the single σB-dependent genes to resistance against one or more specific stresses.
Most of the 100 genes that so far have been poorly characterized have been assigned to the general stress regulon by transcriptional profiling studies (23, 42, 45). Here we extended these reports by performing a thorough analysis of the contributions of 94 members of the candidate gene list (42) to the management of stress. Corresponding mutants were obtained from a collection of mutants with mutations in the 1,700 undefined B. subtilis genes that was established in the functional genomics program of a joint European-Japanese consortium (29, 49). Therefore, in this study we also continued the work of this functional genomics program since the program consisted of a whole collection of physiological tests but did not extend to exhaustive stress resistance profiling (49). Because the multiple-stress resistance included resistance against osmotic, heat, ethanol, and cold stresses, the single mutants were individually exposed to these stresses to determine the facets of multiple-stress resistance to which the unknown proteins could be assigned. This functional genomics approach provided the basis for more detailed experiments aimed at exact elucidation of the functions of all σB-dependent stress proteins, which is necessary for a comprehensive understanding of this crucial (from a physiological point of view) regulon.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The experiments conducted in this study were performed with B. subtilis strain 168 (4), the sigB mutant strain ML6 (25), and a collection of isogenic mutants of wild-type strain 168 (Fig. 1) that was constructed by disruption of the reading frames of candidate genes of the general stress regulon by Campbell-type insertion of nonreplicative plasmids (pMutin1 to pMutin4) (29) or deletions internal to the structural gene (GF500, Δctc::spc) (47) (Fig. 1). Cultures (50 ml) in prewarmed growth medium were inoculated by using exponentially growing cells and were routinely grown in 500-ml flasks in a shaking water bath at 180 rpm and 37°C. For all experiments except the heat stress experiment, which was performed in Luria-Bertani (LB) medium, a synthetic medium (52) containing 15 mM (NH4)2SO4, 8 mM MgSO4 · 7H2O, 27 mM KCl, 7 mM trisodium citrate · 2H2O, 50 mM Tris, 1 mM KH2PO4, 2 mM CaCl2 · 2H2O, 1 μM FeSO4 · 7H2O, 10 μM MnSO4 · H2O, 0.2% (wt/vol) glucose, 39 μM l-tryptophan, and 4,5 mM l-glutamate was used. In order to circumvent potential osmoprotective effects, glutamate was not added in salt stress experiments. Growth was monitored by measuring the optical density at 500 nm (OD500) in synthetic medium or the OD540 in LB medium. Growth at a low temperature was determined with 50-ml cultures in a shaking water bath at 12.5°C and 180 rpm in 300-ml flasks; the synthetic medium was inoculated to obtain an OD500 of 0.06 with exponentially growing cells.
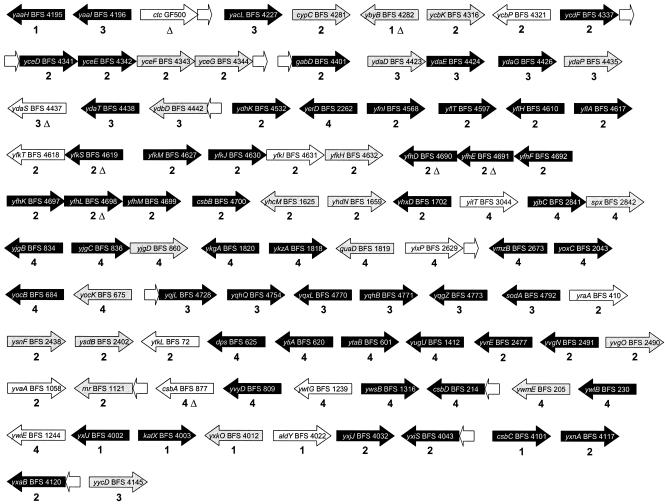
FIG. 1.
Genomic organization and predicted operon structure for the candidate members of the general stress regulon that were analyzed in this study. The genes are sorted according to their positions on the chromosome, and the arrows indicate the direction of gene transcription. Potential operons are indicated by arrows that point in the same direction and are not separated by spaces. All mutants defective in the candidate members of the general stress regulon were generated by using B. subtilis wild-type strain 168 as the parental strain (29). The mutant strain numbers are indicated along with the names of the genes inactivated. The numbers below the arrows representing the genes indicate the pMutin plasmid derivatives that were used for gene inactivation (1, pMutin1; 2, pMutin2; 3, pMutinT3; 4, pMutin4) (29). An uppercase delta indicates a deletion mutant strain. Arrows without any gene or strain designation indicate potential operon members for which no mutant strains were available. The open arrows indicate the mutants that did not differ significantly in stress sensitivity from wild-type strain 168 in response to any of the stresses tested. The gray arrows indicate mutants that displayed increased stress sensitivity compared to the wild-type strain at an α confidence level ≤0.01. Mutants that exhibited increased stress sensitivity with an α confidence level of ≤0.001 and thus are prime candidates for further functional studies are indicated by black arrows. Further information about the BFS mutant strains is available at http://genome.jouy.inra.fr/cgi-bin/micado/index.cgi and http://bacillus.genome.ad.jp/.
Exposure to stress and viability assays.
At an OD500 or OD540 of 0.45 cells were subjected to the different stresses. For imposition of the ethanol stress (final concentration of ethanol, 10% [vol/vol]), 18 ml of an exponentially growing culture was transferred to a prewarmed 100-ml flask, and then immediately 2 ml of ethanol was added. Prior to the stress and 60, 120, 180, and 240 min after the stress, aliquots of the culture were diluted in a 0.9% (wt/vol) NaCl solution, and appropriate dilutions of the cultures were plated on LB agar. In salt stress survival experiments cells in the exponential growth phase were first preadapted for 30 min with 4% (wt/vol) NaCl, and then the NaCl concentration was raised to 10% (wt/vol). Aliquots were removed from the culture at the end of the preadaptation period (control), as well as 20, 40, 60, 120, and 180 min after the NaCl concentration was increased to 10% (wt/vol), and diluted with a 0.9% NaCl solution, and appropriate dilutions were plated on LB agar. For heat stress survival experiments cultures were grown in LB medium to an OD540 of 0.45 at 37°C and then preadapted to heat by transferring them to a shaking water bath set at 48°C and 180 rpm. After 30 min of preadaptation aliquots were removed, and the cultures were transferred to a shaking water bath set at 54°C and 180 rpm. At 60, 120, 180, and 300 min after the final shift to 54°C, aliquots were diluted with a 0.9% NaCl solution and plated on LB agar. For all of the survival experiments described above LB agar plates were incubated overnight at 37°C, and the CFU were counted. Cold stress survival was assayed as described by Kandror et al. (27). Briefly, cultures were grown in synthetic medium to an OD500 of 0.45, and aliquots that were appropriately diluted with a 0.9% NaCl solution were plated on LB agar. The control plates were incubated overnight at 37°C, and the test plates were sealed in plastic bags to prevent drying and stored at 4°C for 6 days. After this they were incubated at 37°C overnight, and the CFU were counted.
All experiments were performed at least in triplicate, and at least two plates from each dilution step were utilized to determine the number of CFU.
Evaluation of data from survival experiments.
For further analysis the CFU counts from the control plates were defined as 100%, and the CFU counts of samples exposed to stresses were expressed as percentages of the control values. Outliers were detected with the Grubbs outlier test. All outliers with an α confidence level of ≤0.01 were removed from the data set, and outliers with an α of ≤0.05 were removed from the data set only when at least three successive values from one experiment were outliers. After removal of outliers, the arithmetic means of the mutants were compared to the arithmetic mean of the wild type with the t test modified by Welch. Only those mutants whose arithmetic means (n ≥ 3) were significantly smaller (α ≤ 0.01) than the wild-type mean for at least two consecutive times were considered to be impaired in terms of stress management. Since a major fraction of the mutants tested displayed statistically increased sensitivity to at least one of the stresses, the data set was subjected to an even more stringent analysis in which we identified genes whose inactivation increased the stress sensitivity compared to that of the wild type even at an α confidence level of ≤0.001; this defined a subset of prime candidates for further functional studies.
Evaluation of data from growth experiments.
Before evaluation of the data from the growth experiments performed at 12.5°C, outliers were detected with the Grubbs outlier test. The criteria used for deletion of outlier data were the same as those described above for the survival experiments. The growth curves were compared by using two parameters. For each time the arithmetic mean of the OD500 of the mutants was compared with the wild-type mean OD500 by the Welch t test. All the mutants that had a significantly (α ≤ 0.01) lower OD500 than the wild type at a time and whose growth rate did not exceed 200% of the growth rate of the sigB mutant in the corresponding period were considered sensitive at that time. For overall rating of mutants as impaired for growth at 12.5°C, the minimum number of times that both criteria had to be met was set at three of five. Mutants that displayed the most significant changes were selected by setting the α confidence level of the Welch t test to ≤0.001.
Analysis of transcription.
Total RNA was isolated from exponentially growing cells before and after exposure to stress by the acid phenol method of Majumdar et al. (36), with the modifications described previously (58). Northern blot analyses were performed as described previously (63). A digoxigenin-labeled antisense RNA probe internal to the katA gene was generated by in vitro transcription by using T7 RNA polymerase and a gene-specific PCR product as the template. The template for the katA probe was synthesized with primers kata_s_for (5′-CAAGTGACAATCCTGATGTC-3′) and kata_s_rev (5′-CTAATACGACTCACTATAGGGAGACCTTGAAGCATTTTATCCGG-3′) containing the T7 promoter sequence.
In silico analysis.
The amino acid sequences of general stress proteins were downloaded from the SubtiList web server at http://genolist.pasteur.fr/subtilist/ and were analyzed with WU-BLAST2 (20) at http://www.ebi.ac.uk/blast2/ by using the default parameters. Furthermore, the protein sequences were screened with the aid of MOTIFSCAN (17) at http://hits.isb-sib.ch/cgi-bin/PFSCAN?, SIGNALP-2.0 (41) at http://www.cbs.dtu.dk/services/SignalP-2.0/#submission, and TMHMM (30, 50) at http://www.cbs.dtu.dk/services/TMHMM/.
RESULTS AND DISCUSSION
Impact of mutational inactivation of sigB on the stress resistance of B. subtilis.
It is well established that expression of the σB-dependent general stress regulon provides nongrowing cells with multiple-stress resistance (19, 59). sigB mutants are at a severe survival disadvantage compared to the wild-type strain when they are exposed to severe heat shock, salt, acid, or ethanol stress, or cycles of freezing and thawing (59) or when they encounter acidic or alkaline conditions during the stationary phase (19).
In the present study we wanted to raise functional characterization of the σB-dependent general stress regulon to the next level by recording the stress resistance profiles of a large collection of mutants defective in 94 candidate members of the general stress regulon derived from our previous transcriptional profiling study (42). In this analysis we focused on adaptation to severe heat shock and salt and ethanol stresses, as well as low-temperature stress, which has recently been shown to require σB even during growth (11). Comparison of the stress sensitivities of individual mutants to those of B. subtilis wild-type strain 168 and the corresponding sigB mutant ML6 should provide the framework for evaluation of the degrees of stress sensitivity. Therefore, the stress resistance profiles of the latter two strains were reevaluated at the beginning of this work in order to determine the strength of the stress stimuli that were best suited for recognition of the impact of individual mutations.
In general, the results of our experiments support the conclusion of Völker et al. (59) that an intact sigB gene is required for effective management of heat, salt, and ethanol stresses. Despite the fact that a whole range of concentrations and temperatures were tested in combination with and without preadaptation, it became clear that the effects of a sigB null allele were quite different for the stresses tested (Fig. 2). Even in the absence of preadaptation, exposure to 10% (vol/vol) ethanol resulted in the most pronounced difference in the survival rates of the wild type and the sigB mutant. As soon as 60 min after the addition of ethanol the sigB mutant was roughly 100-fold more sensitive than wild-type strain 168 (Fig. 2 and Table 1), and this value increased to 1,000-fold within 3 h after exposure to ethanol. In this study the ethanol concentration was 10% rather than the 9% used in the previous investigation (59), and thus the impact of ethanol was even larger than the impact observed previously (59).
FIG. 2.
Comparative analysis of the stress resistance of wild-type strain 168 (▴) and its isogenic sigB mutant ML6 (□). Exponentially growing cells of both strains in either synthetic medium (ethanol, salt, and low-temperature stresses) or LB medium (heat shock) were exposed to the different stimuli as follows: for heat shock (54°C), after preadaptation at 48°C for 30 min the cells were transferred to the final temperature, 54°C; for ethanol stress (EtOH), growing cells were directly exposed to 10% (vol/vol) ethanol; for salt stress (NaCl), after preadaptation to 4% NaCl (wt/vol) for 30 min bacteria were treated with 10% (wt/vol; final concentration) NaCl; for low-temperature or chill stress (4°C), after cells were plated on LB agar plates, the plates were stored for 6 days at 4°C before colonies were allowed to form at 37°C; for low-temperature growth (12.5°C), cultures were transferred to 12.5°C and growth was monitored by measuring the OD500. Survival was determined by plating appropriate dilutions of the samples taken at different times. The number of cells either present before the stress treatment (ethanol stress and low-temperature or chill stress) or present at the end of the preadaptation period (heat shock and salt stress) was defined as 100%. The values are arithmetic means and standard errors of the means. Time zero was either the time of stress treatment (ethanol stress and low temperature) or the end of the preadaptation period (salt stress and heat shock).
TABLE 1.
Quantitative evaluation of the survival of wild-type strain 168, its isogenic sigB mutant ML6, and mutants defective in individual general stress genesa
| Geneb | Function and/or nearest homologue (E value) | Strain | Ethanol
|
NaCl
|
54°C
|
4°C (6 days) | 12.5°C | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 60 min | 120 min | 180 min | 240 min | 20 min | 40 min | 60 min | 120 min | 180 min | 60 min | 120 min | 180 min | 300 min | |||||
| Wild type | 168 | 100 (0.01) | 100 (0.00) | 100 (0.00) | 100 (0.00) | 100 (0.13) | 100 (0.03) | 100 (0.03) | 100 (0.02) | 100 (0.02) | 100 (0.59) | 100 (0.13) | 100 (0.03) | 100 (0.01) | 100 (0.00) | ||
| sigB | ML6 | 0.77 (1.00)c | 0.23 (1.00) | 0.1 (1.00) | 0.09 (1.00) | 13 (1.00) | 3 (1.00) | 3 (1.00) | 2 (1.00) | 2 (1.00) | 59 (1.00) | 13 (1.00) | 3 (1.00) | 1 (1.00) | 0.33 (1.00) | Sensitive | |
| aldY | Aldehyde dehydrogenase OB0484, Oceanobacillus iheyensis (9.0e-136) | BFS 4022 | 75 (0.01) | 116 (0.00) | 147 (0.00) | 183 (0.00) | NDd | ND | 133 (0.02) | 112 (0.02) | 118 (0.02) | 113 (0.52) | 57 (0.22) | 29 (0.11) | 80 (0.02) | 38 (0.01) | |
| csbA | Hypothetical protein lin2633, Listeria innocua (1.0e-08) | BFS 877 | 59 (0.01) | 100 (0.00) | 106 (0.00) | 120 (0.00) | ND | ND | 57 (0.05) | 27 (0.09) | 28 (0.08) | 68 (0.86) | 94 (0.13) | 54 (0.06) | 56 (0.02) | 94 (0.00) | |
| csbB | Bactoprenol glucosyl transferase (EC 2.4.1.-) BC5432, Bacillus cereus (2.6e-99) | BFS 4700 | 3 (0.23) | 2 (0.11) | 0.59 (0.18) | 1 (0.09) | 24 (0.53) | 2 (1.35) | 7 (0.43) | 3 (0.77) | 1 (1.99) | 84 (0.70) | 79 (0.16) | 63 (0.05) | 43 (0.03) | 35 (0.01) | |
| csbC | Bicyclomycin resistance protein TcaB SE0247, Staphylococcus epidermidis (4.3e-129) | BFS 4101 | 17 (0.04) | 21 (0.01) | 12 (0.01) | 13 (0.01) | 73 (0.18) | 16 (0.17) | 12 (0.24) | 11 (0.22) | 6 (0.40) | 80 (0.74) | 58 (0.22) | 24 (0.14) | 66 (0.02) | 39 (0.01) | |
| csbD | Conserved protein MM2677, Methanosarcina mazei (6.3e-09) | BFS 214 | 13 (0.06) | 8 (0.03) | 6 (0.02) | 7 (0.01) | 22 (0.60) | 18 (0.15) | 24 (0.12) | 6 (0.40) | 3 (0.77) | 40 (1.47) | 32 (0.39) | 35 (0.09) | 55 (0.02) | 2 (0.20) | |
| ctc | Ribosomal protein | GF500 | 43 (0.02) | 48 (0.00) | 56 (0.00) | 54 (0.00) | 124 (0.10) | 180 (0.01) | 191 (0.02) | 128 (0.02) | 105 (0.02) | 110 (0.53) | 100 (0.13) | 83 (0.04) | 21 (0.06) | 300 (0.00) | |
| cypC | Fatty acid beta-hydroxylating cytochrome P450 | BFS 4281 | 6 (0.13) | 10 (0.02) | 4 (0.02) | 7 (0.01) | 90 (0.14) | 55 (0.05) | 30 (0.10) | 31 (0.08) | 17 (0.13) | 81 (0.73) | 57 (0.22) | 37 (0.09) | 122 (0.01) | 142 (0.00) | |
| dps | DNA-protecting protein | BFS 625 | 3 (0.27) | 4 (0.06) | 2 (0.05) | 2 (0.05) | ND | ND | 166 (0.02) | 139 (0.02) | 157 (0.01) | 107 (0.55) | 85 (0.15) | 47 (0.07) | 58 (0.02) | 0.13 (2.47) | |
| gabD | Succinate-semialdehyde dehydrogenase | BFS 4401 | 25 (0.03) | 31 (0.01) | 21 (0.00) | 37 (0.00) | 62 (0.21) | 53 (0.05) | 23 (0.13) | 21 (0.11) | 9 (0.24) | 72 (0.82) | 52 (0.25) | 33 (0.10) | 37 (0.03) | 7 (0.05) | |
| guaD | Guanine deaminase (EC 3.5.4.3) | BFS 1819 | 4 (0.19) | 5 (0.05) | 3 (0.03) | 2 (0.05) | 37 (0.35) | 13 (0.20) | 54 (0.06) | 31 (0.08) | 35 (0.06) | 98 (0.60) | 47 (0.27) | 42 (0.08) | 118 (0.01) | 14 (0.02) | |
| katX | Major catalase in spores | BFS 4003 | 6 (0.14) | 3 (0.08) | 3 (0.04) | 1 (0.07) | 69 (0.19) | 54 (0.05) | 20 (0.15) | 12 (0.19) | 5 (0.47) | 58 (1.01) | 31 (0.41) | 19 (0.17) | 39 (0.03) | 33 (0.01) | |
| rnr | RNase R | BFS 1121 | 15 (0.05) | 25 (0.01) | 26 (0.00) | 22 (0.00) | 68 (0.19) | 37 (0.07) | 33 (0.09) | 21 (0.11) | 26 (0.09) | 106 (0.56) | 85 (0.15) | 56 (0.06) | 19 (0.07) | 59 (0.01) | |
| sodA | Superoxide dismutase | BFS 4792 | 4 (0.21) | 1 (0.21) | 2 (0.05) | 2 (0.06) | 77 (0.17) | 61 (0.04) | 84 (0.04) | 62 (0.04) | 68 (0.03) | 111 (0.53) | 92 (0.14) | 38 (0.09) | 5 (0.27) | 0.13 (2.52) | Sensitive |
| yaaH | Hypothetical conserved protein OB0024, Oceanobacillus iheyensis (6.4e-142) | BFS 4195 | 4 (0.19) | 2 (0.15) | 1 (0.08) | 1 (0.09) | 71 (0.18) | 58 (0.05) | 32 (0.09) | 19 (0.12) | 6 (0.35) | 100 (0.59) | 77 (0.16) | 69 (0.05) | 47 (0.03) | 7 (0.05) | |
| yaaI | Isochorismatase (EC 3.3.2.1) OB0025, Oceanobacillus iheyensis (4.3e-49) | BFS 4196 | 6 (0.14) | 4 (0.06) | 2 (0.06) | 2 (0.06) | 97 (0.13) | ND | 36 (0.08) | 27 (0.09) | 13 (0.17) | 115 (0.51) | 80 (0.16) | 69 (0.05) | 94 (0.01) | 6 (0.05) | |
| yacL | Hypothetical protein BH0106, Bacillus halodurans (9.3e-134) | BFS 4227 | 7 (0.11) | 4 (0.05) | 4 (0.03) | 4 (0.02) | 62 (0.21) | 21 (0.13) | 8 (0.36) | 2 (1.02) | 1 (1.85) | 135 (0.44) | 111 (0.11) | 108 (0.03) | 144 (0.01) | 313 (0.00) | |
| ybyB | No similarity | BFS 4282 | 16 (0.05) | 11 (0.02) | 12 (0.01) | 4 (0.02) | 92 (0.14) | 105 (0.03) | 45 (0.07) | 28 (0.08) | 29 (0.08) | 85 (0.70) | 60 (0.21) | 50 (0.06) | 63 (0.02) | 12 (0.03) | |
| ycbK | Conserved hypothetical protein LA1339, Leptospira interrogans (7.3e-31) | BFS 4316 | 20 (0.04) | 26 (0.01) | 15 (0.01) | 11 (0.01) | 104 (0.12) | 110 (0.02) | 87 (0.03) | 111 (0.02) | 78 (0.03) | 68 (0.87) | 40 (0.31) | 20 (0.16) | 28 (0.05) | 14 (0.02) | |
| ycbP | YndM protein, Bacillus subtilis (2.8e-15) | BFS 4321 | 42 (0.02) | 85 (0.00) | 64 (0.00) | 41 (0.00) | 112 (0.11) | 162 (0.02) | 236 (0.01) | 220 (0.01) | 211 (0.01) | 162 (0.36) | 120 (0.11) | 118 (0.03) | 105 (0.01) | 380 (0.00) | |
| ycdF | Glucose dehydrogenase (EC 1.1.1.47), Bacillus licheniformis (5.0e-71) | BFS 4337 | 6 (0.13) | 2 (0.11) | 1 (0.09) | 0.86 (0.11) | 74 (0.17) | 132 (0.02) | 150 (0.02) | 143 (0.02) | 139 (0.02) | 85 (0.70) | 59 (0.22) | 51 (0.06) | 143 (0.01) | 5 (0.07) | |
| yceD | Tellurium resistance protein TerD CTC02234, Clostridium tetani (7.1e-72) | BFS 4341 | 2 (0.36) | 2 (0.10) | 2 (0.05) | 2 (0.05) | 155 (0.08) | 129 (0.02) | 98 (0.03) | 150 (0.02) | 165 (0.01) | 79 (0.74) | 78 (0.16) | 69 (0.05) | 111 (0.01) | 7 (0.05) | |
| yceE | General stress protein CTC02233, Clostridium tetani (3.6e-77) | BFS 4342 | 2 (0.36) | 4 (0.06) | 4 (0.03) | 6 (0.02) | 156 (0.08) | 73 (0.04) | 28 (0.10) | 9 (0.25) | 77 (0.30) | 79 (0.74) | 49 (0.26) | 26 (0.13) | 61 (0.02) | 2 (0.19) | |
| yceF | TerC-like protein BC0445, Bacillus cereus (4.5e-86) | BFS 4343 | 10 (0.08) | 10 (0.02) | 47 (0.00) | 55 (0.00) | ND | ND | 93 (0.03) | 49 (0.05) | 33 (0.07) | 85 (0.69) | 74 (0.17) | 102 (0.03) | 157 (0.01) | 139 (0.00) | |
| yceG | Hypothetical tellurium resistance protein BC0446, Bacillus cereus(1.0e-120) | BFS 4344 | 24 (0.03) | 23 (0.01) | 8 (0.01) | 5 (0.02) | 80 (0.16) | 49 (0.05) | 39 (0.08) | 46 (0.05) | 40 (0.06) | 104 (0.57) | 70 (0.18) | 45 (0.07) | 95 (0.01) | 64 (0.01) | |
| ydaD | Hypothetical oxidoreductase YhdF, Bacillus subtilis (3.4e-104) | BFS 4423 | 34 (0.02) | 41 (0.01) | 53 (0.00) | 55 (0.00) | 68 (0.19) | 46 (0.06) | 59 (0.05) | 66 (0.04) | 62 (0.04) | 99 (0.59) | 82 (0.16) | 44 (0.07) | 134 (0.01) | 27 (0.01) | |
| ydaE | Probable spore coat polysaccharide biosynthesis protein E CV3890, Chromobacterium violaceum (3.8e-07) | BFS 4424 | 2 (0.39) | 0.79 (0.29) | 0.85 (0.12) | 1 (0.09) | ND | ND | 57 (0.05) | 37 (0.06) | 30 (0.07) | 134 (0.44) | 120 (0.11) | 89 (0.04) | 73 (0.02) | 5 (0.06) | |
| ydaG | General stress protein OB1705, Oceanobacillus iheyensis (2.7e-40) | BFS 4426 | 6 (0.12) | 7 (0.03) | 5 (0.02) | 7 (0.01) | ND | ND | 142 (0.02) | 91 (0.03) | 115 (0.02) | 79 (0.74) | 34 (0.37) | 23 (0.14) | 38 (0.3) | 1 (0.22) | |
| ydaP | Pyruvate oxidase (EC 1.2.3.3) OB3409, Oceanobacillus iheyensis (4.8e-185) | BFS 4435 | 7 (0.11) | 5 (0.04) | 4 (0.03) | 3 (0.03) | 74 (0.17) | 81 (0.03) | 95 (0.03) | 34 (0.07) | 32 (0.07) | 123 (0.48) | 131 (0.10) | 211 (0.02) | 62 (0.02) | 21 (0.02) | |
| ydaS | Hypothetical conserved protein OB2732, Oceanobacillus iheyensis (6.0e-20) | BFS 4437 | 31 (0.02) | 73 (0.00) | 45 (0.00) | 96 (0.00) | 72 (0.18) | 48 (0.05) | 50 (0.06) | 74 (0.03) | 40 (0.06) | 104 (0.57) | 37 (0.34) | 49 (0.07) | 118 (0.01) | 259 (0.00) | |
| ydaT | Hypothetical conserved protein OB0538, Oceanobacillus iheyensis (6.9e-35) | BFS 4438 | 5 (0.16) | 4 (0.06) | 2 (0.07) | 0.71 (0.13) | ND | ND | 29 (0.10) | 27 (0.09) | 24 (0.09) | 103 (0.57) | 69 (0.18) | 47 (0.07) | 81 (0.02) | 6 (0.06) | |
| ydbD | Manganese-containing catalase BH2190, Bacillus halodurans (1.5e-94) | BFS 4442 | 11 (0.07) | 10 (0.02) | 9 (0.01) | 3 (0.03) | 65 (0.20) | 57 (0.05) | 32 (0.09) | 21 (0.12) | 8 (0.27) | 99 (0.60) | 47 (0.27) | 39 (0.08) | 75 (0.02) | 133 (0.00) | |
| ydhK | Hypothetical protein YDHK, Staphylococcus hominis (3.1e-46) | BFS 4532 | 5 (0.15) | 5 (0.05) | 2 (0.07) | 2 (0.05) | 159 (0.08) | 168 (0.02) | 151 (0.02) | 112 (0.02) | 90 (0.02) | 99 (0.59) | 74 (0.17) | 73 (0.05) | 49 (0.03) | 103 (0.00) | |
| yerD | Glutamate synthase (ferredoxin) (EC 1.4.7.1) OB3408, Oceanobacillus iheyensis (2.1e-166) | BFS 2262 | 31 (0.02) | 88 (0.00) | 152 (0.00) | 195 (0.00) | 48 (0.27) | 3 (0.97) | 2 (1.43) | 6 (0.43) | 5 (0.41) | 143 (0.41) | 109 (0.12) | 41 (0.08) | 29 (0.04) | 33 (0.01) | |
| yfhF | Cell division inhibitor-like protein BA0515, Bacillus anthracis (2.8e-77) | BFS 4692 | 4 (0.20) | 1 (0.21) | 1 (0.07) | 2 (0.05) | 77 (0.17) | 18 (0.15) | 14 (0.22) | 17 (0.14) | 10 (0.22) | 95 (0.62) | 34 (0.37) | 23 (0.14) | 63 (0.02) | 0.1 (3.39) | |
| yfhE | No similarity | BFS 4691 | 3 (0.30) | 2 (0.10) | 0.89 (0.12) | 0.82 (0.11) | 36 (0.36) | 0.77 (3.46) | 0.96 (3.09) | 0.12 (20.2) | 0.07 (30.5) | 104 (0.57) | 87 (0.15) | 65 (0.05) | 24 (0.05) | 2 (0.21) | |
| yfhD | Hypothetical conserved protein OB2906, Oceanobacillus iheyensis (1.1e-06) | BFS 4690 | 4 (0.18) | 2 (0.10) | 2 (0.05) | 2 (0.06) | 139 (0.09) | 145 (0.02) | 100 (0.03) | 81 (0.03) | 76 (0.03) | 89 (0.66) | 91 (0.14) | 81 (0.04) | 45 (0.03) | 0.93 (0.36) | |
| yfhK | Hypothetical protein YwsB Bacillus subtilis (3.3e-28) | BFS 4697 | 0.82 (0.94) | 0.35 (0.65) | 0.16 (0.65) | 0.15 (0.62) | ND | ND | 65 (0.05) | 104 (0.02) | 82 (0.03) | 73 (0.81) | 32 (0.39) | 11 (0.29) | 3 (0.41) | 2 (0.19) | |
| yfhL | Hypothetical membrane-spanning protein BC1961, Bacillus cereus (3.3e-12) | BFS 4698 | 9 (0.08) | 7 (0.03) | 3 (0.04) | 3 (0.03) | 74 (0.17) | 32 (0.08) | 29 (0.10) | 30 (0.08) | 19 (0.11) | 107 (0.55) | 53 (0.24) | 18 (0.18) | 15 (0.08) | 6 (0.05) | |
| yfhM | Epoxide hydrolase-related protein DR2549, Deinococcus radiodurans (6.9e-58) | BFS 4699 | 6 (0.12) | 2 (0.11) | 2 (0.04) | 0.92 (0.10) | 96 (0.13) | 105 (0.03) | 62 (0.05) | 96 (0.02) | 81 (0.03) | 62 (0.94) | 57 (0.22) | 61 (0.05) | 123 (0.01) | 32 (0.01) | |
| yfkJ | Low-molecular-weight phosphotyrosine protein phosphatase family protein BA0407, Bacillus anthracis (9.3e-47) | BFS 4630 | 0.83 (0.93) | 0.31 (0.74) | 0.09 (1.15) | 0.13 (0.71) | 47 (0.27) | 48 (0.06) | 173 (0.02) | 145 (0.02) | 159 (0.01) | 72 (0.82) | 97 (0.13) | 81 (0.04) | 51 (0.03) | 125 (0.00) | |
| yfkI | Hypothetical protein BA0408, Bacillus anthracis (1.7e-08) | BFS 4631 | 48 (0.02) | 59 (0.00) | 66 (0.00) | 96 (0.00) | ND | ND | 21 (0.14) | 20 (0.12) | 21 (0.10) | 148 (0.40) | 156 (0.08) | 167 (0.02) | 57 (0.02) | 140 (0.00) | |
| yfkH | RNase BN (EC 3.1.-.-) BC0452, Bacillus cereus (9.4e-70) | BFS 4632 | 9 (0.09) | 4 (0.05) | 0.94 (0.11) | 0.74 (0.12) | 65 (0.20) | 75 (0.04) | 66 (0.05) | 32 (0.07) | 24 (0.09) | 63 (0.94) | 42 (0.30) | 29 (0.11) | 31 (0.04) | 335 (0.00) | |
| yfkM | General stress protein 18 (GSP18) OB0023, Oceanobacillus iheyensis (4.1e-60) | BFS 4627 | 4 (0.20) | 3 (0.09) | 1 (0.08) | 0.55 (0.17) | 108 (0.12) | 7 (0.40) | 11 (0.27) | 2 (1.22) | 0.17 (13.3) | 86 (0.68) | 28 (0.45) | 20 (0.16) | 74 (0.02) | 68 (0.00) | |
| yfkS | No similarity | BFS 4619 | 11 (0.07) | 4 (0.05) | 2 (0.05) | 1 (0.06) | 39 (0.33) | 21 (0.13) | 32 (0.09) | 14 (0.17) | 20 (0.11) | 106 (0.56) | 90 (0.14) | 56 (0.06) | 25 (0.05) | 18 (0.02) | Sensitive |
| yfkT | Germination protein TTE2380, Thermoanaerobacter tengcongensis (1.2e-20) | BFS 4618 | 116 (0.01) | 104 (0.00) | 117 (0.00) | 93 (0.00) | 86 (0.15) | 43 (0.06) | 54 (0.05) | 25 (0.10) | 40 (0.06) | 99 (0.59) | 41 (0.31) | 40 (0.08) | 51 (0.03) | 21 (0.02) | |
| yflA | Na+-linked d-alanine glycine permease BC2317, Bacillus cereus (6.5e-94) | BFS 4617 | 15 (0.05) | 9 (0.02) | 15 (0.01) | 21 (0.00) | 78 (0.16) | 21 (0.12) | 13 (0.22) | 13 (0.18) | 9 (0.26) | 87 (0.68) | 25 (0.51) | 19 (0.17) | 8 (0.17) | 16 (0.02) | |
| yflH | Hypothetical protein BH2390, Bacillus halodurans (1.4e-11) | BFS 4610 | 12 (0.06) | 11 (0.02) | 29 (0.00) | 38 (0.00) | 67 (0.19) | 84 (0.03) | 30 (0.10) | 17 (0.14) | 8 (0.29) | 93 (0.63) | 67 (0.19) | 40 (0.08) | 23 (0.06) | 8 (0.04) | |
| yflT | General stress protein 17M BC0998, Bacillus cereus (7.8e-27) | BFS 4597 | 3 (0.22) | 2 (0.11) | 1 (0.07) | 0.78 (0.12) | 73 (0.18) | 69 (0.04) | 30 (0.10) | 25 (0.10) | 10 (0.23) | 84 (0.70) | 66 (0.19) | 67 (0.05) | 85 (0.01) | 38 (0.01) | |
| yfnI | Hypothetical protein lin0927, Listeria innocua (6.0e-210) | BFS 4568 | 11 (0.07) | 19 (0.01) | 24 (0.00) | 24 (0.00) | ND | ND | 77 (0.04) | 78 (0.03) | 75 (0.03) | 94 (0.63) | 57 (0.22) | 31 (0.10) | 47 (0.03) | 2 (0.17) | |
| yhcM | Similar to Mus musculus (mouse) sex-determining region Y protein, Dictiostelium discoideum (2.1e-08) | BFS 1625 | 26 (0.03) | 30 (0.01) | 25 (0.00) | 16 (0.01) | 63 (0.20) | 10 (0.27) | 61 (0.05) | 30 (0.08) | 29 (0.08) | 71 (0.83) | 86 (0.15) | 43 (0.08) | 85 (0.01) | 13 (0.03) | |
| yhdN | Probable oxidoreductase PA1127, Pseudomonas aeruginosa (2.1e-104) | BFS 1659 | 10 (0.08) | 5 (0.04) | 1 (0.08) | 1 (0.09) | 62 (0.21) | 67 (0.04) | 34 (0.09) | 5 (0.53) | 22 (0.10) | 108 (0.55) | 51 (0.25) | 29 (0.11) | 52 (0.02) | 11 (0.03) | |
| yhxD | Oxidoreductase OB3280, Oceanobacillus iheyensis (7.9e-105) | BFS 1702 | 9 (0.09) | 5 (0.04) | 3 (0.04) | 3 (0.04) | 11 (1.22) | 3 (0.92) | 2 (1.23) | 0.16 (15.0) | 0.09(23.5) | 96 (0.61) | 54 (0.24) | 61 (0.05) | 116 (0.01) | 12 (0.03) | |
| yitT | Hypothetical membrane-spanning protein BC1163, Bacillus cereus (4.3e-97) | BFS 3044 | 18 (0.04) | 24 (0.01) | 27 (0.00) | 14 (0.01) | ND | ND | 60 (0.05) | 29 (0.08) | 8 (0.28) | 151 (0.39) | 118 (0.11) | 123 (0.03) | 53 (0.02) | 81 (0.00) | |
| yjbC | Hypothetical conserved protein OB1210, Oceanobacillus iheyensis (7.0e-65) | BFS 2841 | 12 (0.06) | 8 (0.03) | 4 (0.02) | 6 (0.01) | 45 (0.29) | 10 (0.28) | 7 (0.43) | 4 (0.61) | 1 (1.75) | 93 (0.63) | 57 (0.22) | 42 (0.08) | 7 (0.17) | 156 (0.00) | |
| spx | Regulatory protein | BFS 2842 | 7 (0.11) | 15 (0.02) | 34 (0.00) | 52 (0.00) | 84 (0.15) | 58 (0.05) | 41 (0.07) | 21 (0.12) | 12 (0.19) | 40 (1.49) | 47 (0.27) | 87 (0.04) | 279 (0.00) | 27 (0.01) | |
| yjgB | No similarity | BFS 834 | 2 (0.40) | 0.73 (0.31) | 0.19 (0.56) | 0.19 (0.48) | 76 (0.17) | 80 (0.03) | 60 (0.05) | 24 (0.10) | 13 (0.17) | 73 (0.81) | 49 (0.26) | 32 (0.10) | 6 (0.22) | 51 (0.01) | |
| yjgC | Formate hydrogenase TV0243, Thermoplasma volcanium (0) | BFS 836 | 16 (0.05) | 10 (0.02) | 3 (0.03) | 6 (0.02) | 47 (0.27) | 13 (0.20) | 4 (0.69) | 1 (1.87) | 0.25 (8.89) | 105 (0.56) | 57 (0.22) | 31 (0.10) | 33 (0.04) | 42 (0.01) | |
| yjgD | Hypothetical protein BA3630, Bacillus anthracis (2.6e-19) | BFS 860 | 91 (0.01) | 9 (0.03) | 11 (0.01) | 6 (0.02) | 66 (0.19) | 40 (0.07) | 28 (0.10) | 17 (0.14) | 14 (0.16) | 143 (0.41) | 115 (0.11) | 101 (0.03) | 92 (0.01) | 50 (0.01) | |
| ykgA | Hypothetical protein BH1779, Bacillus halodurans (3.3e-67) | BFS 1820 | 5 (0.17) | 4 (0.06) | 1 (0.09) | 0.43 (0.21) | 62 (0.21) | 35 (0.08) | 16 (0.18) | 4 (0.57) | 4 (0.62) | 110 (0.54) | 79 (0.16) | 30 (0.11) | 34 (0.04) | 68 (0.00) | |
| ykzA | Organic hydroperoxide resistance protein OB1498, Oceanobacillus iheyensis (6.0e-36) | BFS 1818 | 5 (0.14) | 3 (0.09) | 1 (0.07) | 1 (0.09) | 117 (0.11) | 53 (0.05) | 53 (0.06) | 45 (0.05) | 38 (0.06) | 90 (0.65) | 34 (0.37) | 21 (0.15) | 28 (0.05) | 107 (0.00) | |
| ylxP | Hypothetical protein BA3949, Bacillus anthracis (7.8e-27) | BFS 2629 | 96 (0.01) | 360 (0.00) | 563 (0.00) | 502 (0.00) | 45 (0.29) | 8 (0.34) | 60 (0.05) | 98 (0.02) | 75 (0.03) | 88 (0.67) | 43 (0.30) | 38 (0.09) | 17 (0.07) | 91 (0.00) | |
| ymzB | Hypothetical protein BH1784, Bacillus halodurans (1.9e-09) | BFS 2673 | 10 (0.08) | 8 (0.03) | 6 (0.02) | 5 (0.02) | 27 (0.47) | 12 (0.23) | 10 (0.31) | 1 (2.23) | 0.18 (12.5) | 96 (0.61) | 78 (0.16) | 64 (0.05) | 13 (0.09) | 87 (0.00) | |
| yocB | Hypothetical conserved protein OB2400, Oceanobacillus iheyensis (1.1e-41) | BFS 684 | 15 (0.05) | 14 (0.02) | 6 (0.02) | 5 (0.02) | 67 (0.19) | 39 (0.07) | 31 (0.10) | 41 (0.06) | 38 (0.06) | 151 (0.39) | 83 (0.15) | 50 (0.07) | 7 (0.17) | 4 (0.08) | |
| yocK | Transcriptional regulator, putative CCA00211, Chlamydophila caviae (2.1e-17) | BFS 675 | 5 (0.15) | 5 (0.05) | 2 (0.05) | 4 (0.02) | 108 (0.12) | 59 (0.05) | 64 (0.05) | 51 (0.05) | 47 (0.05) | 102 (0.58) | 81(0.16) | 58 (0.06) | 38 (0.03) | 22 (0.01) | |
| yoxC | General stress protein BC4677, Bacillus cereus (1.2e-07) | BFS 2043 | 1 (0.66) | 0.57 (0.40) | 0.16 (0.65) | 0.14 (0.63) | 116 (0.11) | 124 (0.02) | 179 (0.02) | 223 (0.01) | 219 (0.01) | 82 (0.72) | 47 (0.27) | 26 (0.13) | 44 (0.03) | 182 (0.00) | |
| yqgZ | Hypothetical protein EF2678, Enterococcus faecalis (1.3e-26) | BFS 4773 | 8 (0.09) | 6 (0.04) | 4 (0.03) | 3 (0.03) | 109 (0.12) | 144 (0.02) | 104 (0.03) | 106 (0.02) | 139 (0.02) | 163 (0.36) | 155 (0.08) | 67 (0.05) | 106 (0.01) | 6 (0.06) | |
| yqhB | Hypothetical protein YrkA, Bacillus subtilis (2.9e-146) | BFS 4771 | 39 (0.02) | 7 (0.03) | 144 (0.00) | 180 (0.00) | ND | ND | 7 (0.40) | 63 (0.04) | 87 (0.03) | 115 (0.51) | 136 (0.09) | 162 (0.02) | 137 (0.01) | 9 (0.04) | |
| yqhQ | Hypothetical protein BA4425, Bacillus anthracis (2.4e-112) | BFS 4754 | 9 (0.08) | 6 (0.04) | 5 (0.02) | 2 (0.05) | 100 (0.13) | 58 (0.05) | 18 (0.16) | 19 (0.13) | 7 (0.30) | 136 (0.43) | 82 (0.16) | 117 (0.03) | 106 (0.01) | 451 (0.00) | |
| yqjL | Hypothetical protein BA2557, Bacillus anthracis (6.6e-30) | BFS 4728 | 50 (0.02) | 88 (0.00) | 117 (0.00) | 53 (0.00) | 79 (0.16) | 48 (0.06) | 43 (0.07) | 43 (0.06) | 43 (0.05) | 65 (0.91) | 57 (0.22) | 34 (0.10) | 59 (0.02) | 8 (0.04) | |
| yqxL | Magnesium and cobalt transport protein CorA, Bacillus cereus (6.3e-48) | BFS 4770 | 21 (0.04) | 37 (0.01) | 24 (0.00) | 27 (0.00) | 43 (0.30) | 1 (1.85) | 8 (0.36) | 3 (0.77) | 2 (1.17) | 126 (0.47) | 110 (0.12) | 148 (0.02) | 80 (0.02) | 152 (0.00) | |
| yraA | Hypothetical conserved protein OB0703, Oceanobacillus iheyensis (4.4e-56) | BFS 410 | 25 (0.03) | 15 (0.02) | 13 (0.01) | 15 (0.01) | 93 (0.14) | 28 (0.09) | 17 (0.17) | 13 (0.18) | 14 (0.16) | 82 (0.72) | 32 (0.39) | 29 (0.11) | 50 (0.03) | 89 (0.00) | |
| ysdB | Hypothetical protein BH3134, Bacillus halodurans (1.0e-24) | BFS 2402 | 65 (0.01) | 161 (0.00) | 177 (0.00) | 186 (0.00) | ND | ND | 78 (0.04) | 52 (0.05) | 43 (0.05) | 97 (0.61) | 52 (0.24) | 20 (0.16) | 6 (0.20) | 145 (0.00) | |
| ysnF | Uncharacterized conserved protein CAC0787, Clostridium acetobutylicum (3.0e-25) | BFS 2438 | 7 (0.11) | 7 (0.03) | 5 (0.02) | 5 (0.02) | 11 (1.12) | 2 (1.18) | 51 (0.06) | 60 (0.04) | 40 (0.06) | 101 (0.58) | 55 (0.23) | 28 (0.12) | 20 (0.06) | 78 (0.00) | |
| ytaB | Integral membrane protein BC3136, Bacillus cereus (5.1e-46) | BFS 601 | 1 (0.63) | 0.76 (0.30) | 0.6 (0.17) | 0.81 (0.11) | 37 (0.35) | 7 (0.38) | 3 (0.95) | 1 (1.70) | 0.89 (2.50) | 116 (0.51) | 85 (0.15) | 44 (0.07) | 15 (0.08) | 19 (0.02) | |
| ytiA | 50S ribosomal protein L31 type B RPME2, Bacillus halodurans (4.2e-28) | BFS 620 | 329 (0.00) | 22 (0.01) | 23 (0.00) | 30 (0.00) | 31 (0.41) | 13 (0.20) | 20 (0.15) | 8 (0.31) | 7 (0.34) | 115 (0.51) | 70 (0.18) | 60 (0.05) | 87 (0.01) | 40 (0.01) | |
| ytkL | Hypothetical metal-dependent hydrolase BH3178, Bacillus halodurans (3.3e-90) | BFS 72 | 15 (0.05) | 36 (0.01) | 25 (0.00) | 52 (0.00) | ND | ND | 153 (0.02) | 193 (0.01) | 164 (0.01) | 104 (0.57) | 72 (0.18) | 68 (0.05) | 128 (0.01) | 74 (0.00) | |
| yugU | Hypothetical protein BH3498, Bacillus halodurans (1.4e-45) | BFS 1412 | 9 (0.08) | 6 (0.04) | 2 (0.05) | 2 (0.05) | 82 (0.16) | 49 (0.05) | 43 (0.07) | 40 (0.06) | 35 (0.06) | 86 (0.68) | 35 (0.36) | 12 (0.27) | 16 (0.08) | 76 (0.00) | |
| yvaA | Oxidoreductase (EC 1.1.1.-) BC3595, Bacillus cereus (4.0e-78) | BFS 1058 | 44 (0.02) | 77 (0.00) | 60 (0.00) | 73 (0.00) | 145 (0.09) | 54 (0.05) | 60 (0.05) | 42 (0.06) | 17 (0.13) | 80 (0.74) | 46 (0.27) | 39 (0.08) | 63 (0.02) | 119 (0.00) | |
| yvgN | Oxidoreductase, aldo/keto reductase family BA0196, Bacillus anthracis (1.2e-110) | BFS 2491 | 28 (0.03) | 30 (0.01) | 17 (0.01) | 9 (0.01) | 45 (0.29) | 19 (0.14) | 8 (0.39) | 0.24 (9.81) | 0.06 (36.4) | 107 (0.55) | 97 (0.13) | 74 (0.04) | 27 (0.05) | 13 (0.03) | |
| yvgO | Hypothetical protein, Bacillus amyloliquefaciens (1.2e-51) | BFS 2490 | 13 (0.06) | 10 (0.02) | 5 (0.02) | 3 (0.03) | 92 (0.14) | 34 (0.08) | 22 (0.13) | 27 (0.09) | 32 (0.07) | 130 (0.45) | 166 (0.08) | 93 (0.03) | 50 (0.03) | 74 (0.00) | |
| yvrE | Hypothetical membrane-associated protein BC2534, Bacillus cereus, (6.5e-62) | BFS 2477 | 15 (0.05) | 27 (0.01) | 17 (0.01) | 13 (0.01) | 27 (0.48) | 13 (0.20) | 32 (0.09) | 15 (0.16) | 2 (1.04) | 59 (0.99) | 107 (0.12) | ND | 31 (0.04) | 71 (0.00) | |
| yvyD | Hypothetical conserved protein OB2498, Oceanobacillus iheyensis (1.2e-60) | BFS 809 | 67 (0.01) | 79 (0.00) | 91 (0.00) | 127 (0.00) | 35 (0.36) | 9 (0.28) | 26 (0.11) | 63 (0.04) | 50 (0.05) | 112 (0.53) | 44 (0.29) | 13 (0.24) | 26 (0.05) | 88 (0.00) | Sensitive |
| ywiE | Cardiolipin synthethase 2 (EC 2.7.8.-) Cls2, Bacillus anthracis (3.0e-112) | BFS 1244 | 114 (0.01) | 171 (0.00) | ND | 154 (0.00) | 117 (0.11) | 119 (0.02) | 173 (0.02) | 134 (0.02) | 139 (0.02) | 109 (0.54) | 67 (0.19) | 62 (0.05) | 256 (0.00) | 53 (0.01) | |
| ywlB | Hypothetical protein BH3772, Bacillus halodurans (1.7e-08) | BFS 230 | 7 (0.11) | 5 (0.05) | 12 (0.01) | 17 (0.01) | 60 (0.21) | 83 (0.03) | 67 (0.04) | 57 (0.04) | 70 (0.03) | 82 (0.72) | 51 (0.25) | 13 (0.25) | 13 (0.10) | 8 (0.04) | |
| ywmE | No similarity | BFS 205 | 17 (0.04) | 12 (0.02) | 6 (0.02) | 6 (0.01) | 68 (0.19) | 77 (0.03) | 46 (0.07) | 35 (0.07) | 38 (0.06) | 122 (0.48) | 50 (0.26) | 31 (0.10) | 22 (0.06) | 71 (0.00) | |
| ywsB | YfhK, Bacillus subtilis (3.3e-28) | BFS 1316 | 4 (0.19) | 2 (0.12) | 0.82 (0.13) | 0.79 (0.12) | 71 (0.18) | 16 (0.16) | 8 (0.38) | 3 (0.79) | 0.98 (2.27) | 113 (0.52) | 54 (0.23) | 50 (0.07) | 157(0.01) | 12 (0.03) | |
| ywtG | Bicyclomycin resistance protein TcaB, Staphylococcus epidermidis (7.4e-125) | BFS 1239 | 19 (0.04) | 16 (0.01) | 11 (0.01) | 10 (0.01) | 56 (0.23) | 50 (0.05) | 15 (0.20) | 37 (0.07) | 16 (0.14) | 104 (0.57) | 61 (0.21) | 70 (0.05) | 64 (0.02) | 155 (0.00) | |
| yxaB | EpsL (CpsL), Streptococcus thermophilus (1.9e-46) | BFS 4120 | 14 (0.06) | 14 (0.02) | 10 (0.01) | 10 (0.01) | 114 (0.11) | 49 (0.05) | 19 (0.15) | 15 (0.16) | 7 (0.32) | 67 (0.88) | 45 (0.28) | 71 (0.05) | 97 (0.01) | 26 (0.01) | |
| yxiS | Hypothetical conserved protein OB0580, Oceanobacillus iheyensis (8.1e-16) | BFS 4043 | 7 (0.11) | 3 (0.07) | 2 (0.06) | 2 (0.04) | 27 (0.47) | 12 (0.22) | 7 (0.44) | 4 (0.57) | 0.45 (4.95) | 73 (0.80) | 52 (0.24) | 48 (0.07) | 75 (0.02) | 11 (0.03) | |
| yxjJ | No similarity | BFS 4032 | 3 (0.24) | 2 (0.12) | 1 (0.09) | 0.73 (0.13) | 34 (0.38) | 13 (0.20) | 30 (0.10) | 12 (0.21) | 4 (0.61) | 85 (0.69) | 67 (0.19) | 37 (0.09) | 22 (0.06) | 15 (0.02) | |
| yxkO | Conserved protein CTC02515, Clostridium tetani (5.1e-39) | BFS 4012 | 7 (0.12) | 8 (0.03) | 6 (0.02) | 4 (0.02) | ND | ND | 89 (0.03) | 79 (0.03) | 65 (0.03) | 75 (0.78) | 38 (0.33) | 10 (0.31) | 18 (0.07) | 77 (0.00) | |
| yxlJ | DNA-3-methyladenine glycosidase OB0297, Oceanobacillus iheyensis (9.1e-56) | BFS 4002 | 2 (0.38) | 1 (0.16) | 0.66 (0.16) | 0.69 (0.13) | 71 (0.18) | 41 (0.06) | 20 (0.15) | 10 (0.23) | 6 (0.37) | 88 (0.67) | 41 (0.31) | 29 (0.11) | 76 (0.02) | 64 (0.01) | |
| yxnA | Glucose 1-dehydrogenase homolog YxnA XCC1992, Xanthomonas campestris (1.5e-55) | BFS 4117 | 3 (0.23) | 2 (0.11) | 0.99 (0.10) | 0.65 (0.14) | 180 (0.07) | 119 (0.02) | 27 (0.11) | 20 (0.12) | 15 (0.14) | 123 (0.48) | 163 (0.08) | 94 (0.03) | 152 (0.01) | 27 (0.01) | |
| yycD | Hypothetical conserved protein OB2585, Oceanobacillus iheyensis (7.3e-15) | BFS 4145 | 6 (0.13) | 4 (0.05) | 3 (0.03) | 0.73 (0.12) | ND | ND | 111 (0.03) | 92 (0.03) | 84 (0.03) | 94 (0.62) | 53 (0.24) | 45 (0.07) | 21 (0.06) | 46 (0.01) | |
Survival rates of the mutants are expressed relative to the wild type and (in parentheses) relative to the sigB mutant ML6 for each time. In the comparison with the wild type the values for the wild type were defined as 100%, and thus the values for the mutants directly indicate the percent survival based on the wild-type value. The values for the comparison to the sigB mutant are values for fold sensitivity compared to the sigB mutant. A value less than 1 indicates that the mutant was less sensitive than the sigB mutant, and a value greater than 1 indicates sensitivity that was greater than that of the sigB mutant. The corresponding values for the wild type and the sigB mutant are also indicated.
The genes are in alphabetical order. Operons are sorted according to the first gene of the operon, but otherwise the structure and order of the operons are conserved. Operon structures are also indicated by the vertical bars on the left. Genes for which the σB dependence has been confirmed by nonchip approaches are indicated by boldface type.
Values are in boldface type if at least two successive values (including either the first or last value) differed significantly (α ≤ 0.01) from the wild-type value. Values that passed a more stringent analysis (α ≤ 0.001) are shaded.
ND, not determined.
The consequences of the loss of σB were much less pronounced when sensitivity to heat shock or salt stress was assayed. Despite the fact that we used a temperature range of 50 to 56°C and salt concentrations between 6 and 12% and introduced a preadaptation period that should have provided a selective advantage to the wild type due to preloading of the cells with general stress proteins prior to the lethal stress (59), the differences between the survival rates of the wild type and the sigB mutants were only in the range from 50- to 80-fold (Fig. 2). Although these differences were smaller than that reported previously (59), they are still statistically significant (Fig. 2 and Table 1).
The analysis of the role of σB in adaptation to low temperature involved not only determination of the survival rate but also evaluation of the growth rate, because low temperature is the only environmental stimulus that seems to require a functional sigB gene even during growth (11). When cells were exposed for 6 days to 4°C, the sigB mutant displayed approximately 300-fold-greater sensitivity than the wild-type strain (Fig. 2), and the growth of the sigB strain was also impaired at 12.5°C compared to the growth of its wild-type progenitor (Fig. 2). However, in contrast to what was observed by Brigulla et al. (11), the loss of σB did not prevent growth at the low temperature but only significantly increased the lag phase of a sigB mutant compared to wild-type strain 168 (Fig. 2). This difference might be attributable to the different media used. Perhaps the high concentration of glutamate (4.5 mM) present in our experiments supported growth of the sigB mutant even at the low temperature.
To summarize this part of the study, we were able to define stress levels for each of the stress factors tested that revealed statistically significant differences in the survival of the wild-type strain and its isogenic sigB mutant, thereby providing the framework for an efficient test of a mutant strain collection.
Comparative analysis of the stress resistance of mutants defective in individual stress genes, as well as the wild-type strain and its sigB mutant as references.
The general stress genes included in this study were derived from the candidate gene list from a previous transcriptional profiling study of the general stress response (42). Mutants lacking single general stress genes were subjected to the stress regimen described above for wild-type strain 168 and sigB mutant ML6. Each experimental series also included the latter pair of strains as references for the effectiveness of the stress treatment. The sensitivity of each mutant was calculated and expressed in relation to the sensitivity of the wild-type strain and the sigB mutant (Table 1). Of the 94 mutants examined, 80 differed significantly (α ≤ 0.01) from the wild-type strain in survival or growth in response to at least one stress (Table 1). This is somewhat surprising because we did not anticipate that such a large fraction of the mutant collection would have a significant impact on stress resistance as single-gene knockouts. However, the experimental setup was confined to severe stresses, and only 14 of the mutants did not differ significantly in stress resistance from the wild type (Table 1 and Fig. 1). The most interesting mutants, which could also constitute a list of prime candidates for future detailed functional testing, were selected in a subsequent statistical analysis in which the required α confidence level was raised to ≤0.001. Of the 94 mutants tested, 58 still displayed impaired stress resistance even at this higher confidence level. In principle, the large fraction of mutants displaying phenotypic changes compared to the wild type might in part have resulted from the polarity of the pMutin insertion. However, this possibility did not apply to 77 of the 94 genes tested because they either are the last genes in operons or they seem to be monocistronic (Fig. 1). Polarity effects could not be completely excluded for the remaining 17 genes, but this did not seem to be a likely reason for the phenotypic effects observed for the yceDEFG, yfkJIK, yfkST, and yjbC-spx operons because stress sensitivity decreased as the insertion points of the plasmids proceeded to the downstream genes of the operon (Table 1). Furthermore, due to the leakiness of the PSPAC promoter construct (56) expression of genes downstream of the insertion point of the plasmid was likely to occur even in the absence of the inducer isopropyl-β-d-thiogalactopyranoside (IPTG).
Most mutants (63 mutants with an α of ≤0.01; 28 mutants with an α of ≤0.001) were susceptible to ethanol stress, 37 mutants (28 mutants with an α of ≤0.001) were sensitive to a high NaCl concentration, 34 mutants (24 mutants with an α of ≤0.001) were impaired in survival at 4°C, and 10 mutants (3 mutants with an α of ≤0.001) were impaired in survival at 54°C. The exceptionally large fraction of mutants that exhibited increased sensitivity to ethanol stress might partially be ascribed to the particularly large difference in the ethanol sensitivities of the wild type and the sigB null mutant, which easily allows identification of even partially defective or impaired strains.
Susceptibility of general stress gene mutants to more than one stress.
Inactivation of the general stress genes often resulted in increases in sensitivity to more than one stress (Table 1); 26 mutants (34 mutants with an α of ≤0.001) were impaired in the response to one type of stress, 44 mutants (20 mutants with an α of ≤0.001) were impaired in the responses to two stresses, nine mutants (four mutants with an α of ≤0.001) were impaired in the responses to three stresses, and one mutant was even impaired in the responses to four stresses (Fig. 3). The genes of seven of the nine mutants that exhibited defects in responses to three stresses have not been assigned biochemical functions yet (Table 1). However, the large fraction of genes with at least two stress management defects might indicate that the stresses imposed inflict related damage to the cell and thus the same set of genes might be able to provide protection. In this respect, the role of oxidative stress seems to be an emerging theme. Many of the genes displaying severe stress management defects appeared to be associated with protection from oxidative damage (see below). The severe stresses applied in this study all had a rapid reduction in the growth rate in common that might have led to uncoupling of growth from metabolism, thereby generating a burst of free radicals (3). There was a remarkably broad overlap between sensitivity to ethanol shock and sensitivity to transfer to low temperature (24 genes with an α of ≤0.01; 11 genes with an α of ≤0.001), and there was also a broad overlap between sensitivity to ethanol and sensitivity to salt shock (28 genes with an α of ≤0.01; 11 genes with an α of ≤0.001). All three of these stimuli likely have a strong impact on the integrity of the cytosolic membrane, and thus enzymes such as NAD dehydrogenase, an in vivo generator of oxygen radicals, might produce higher levels of reactive oxygen species. We are currently extending the phenotypic screening to the different forms of reactive oxygen species (superoxide as well as hydrogen peroxide) in order to determine the genes that are essential for protection against these insults.
FIG.3.
Survival of mutant strains displaying multiple stress resistance defects. Exposure to stress and determination of survival rates were performed as described in the legend to Fig. 2. The values are arithmetic means and standard errors of the means. All stresses for which the sensitivity increased significantly compared to wild-type strain 168 are shown. (A) Mutants significantly (α ≤ 0.01) impaired in survival after three stresses. (B) Stress resistance profile of the yflA mutant that displayed increased sensitivity to all stresses tested. ▴, wild-type strain 168; □, sigB mutant strain ML6; ×, mutants defective in individual general stress genes. EtOH, ethanol.
General stress genes involved in protection against ethanol stress.
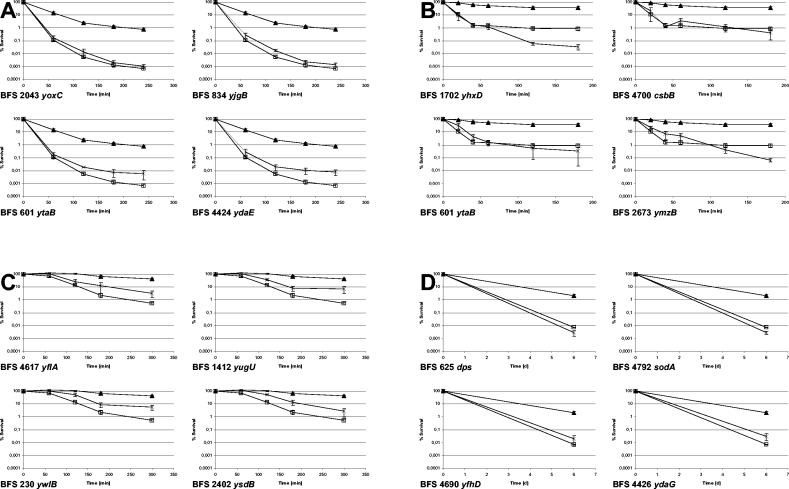
By far the greatest number of mutants (63 mutants with an α of ≤0.01; 28 mutants with an α of ≤0.001) displayed more pronounced sensitivity to the ethanol challenge than the wild type (Table 1). Closer inspection of the results for the individual mutants revealed that some of them (e.g., yoxC and yjgB mutants) had a sensitivity pattern almost identical to that of the sigB mutant (Fig. 4A), while many others (e.g., ytaB and ydaE) exhibited more-than-100-fold greater sensitivity than wild-type strain 168 (Table 1 and Fig. 4A). Even though many of the corresponding general stress proteins have not been tested to determine their biochemical functions, sequence comparisons to DNA and protein databases revealed that at least 12 proteins might have functions in protection from the consequences of oxidative stress. Mutants lacking the ferritin-like Dps protein (21), the catalase KatX (13), or the superoxide dismutase SodA were all severely defective in combating harsh ethanol stress (Table 1). The uncharacterized proteins YdbD, YcdF, YceD, YceE, YfhM, YhxD, YkzA, YqgZ, and YxnA can probably also be associated with protection from reactive oxygen species (Table 1). YqgZ is a member of the ArsC family of proteins (Pfam accession no. PF03960) that is related to the glutaredoxins involved in the oxidative stress response (reviewed in reference 18). ydbD probably encodes a manganese-containing catalase, and YkzA is one of two B. subtilis homologues of the Xanthomonas campestris Ohr protein, which has been proven to be involved in providing resistance against tert-butyl hydroperoxide (38). YcdF has been assigned to a family of short-chain dehydrogenases (Pfam accession no. PF00106) that has been shown to be required for oxidative stress resistance of Clostridium perfringens (12). Furthermore, not only the ycdF mutant but also mutants lacking YxnA and YhxD, two additional short-chain dehydrogenases, displayed increased sensitivity to severe ethanol stress. The protein encoded by yfhM is a putative epoxide hydrolase that could be involved in the reduction of oxidized unsaturated membrane fatty acids. Finally, YceD and YceE are proteins belonging to the TerD family of proteins (Pfam accession no. PF02342), which are important for tellurite resistance. These proteins might be implicated in oxidative stress response for two reasons: (i) the toxicity of tellurite could be related to its oxidizing capacity (53, 55) and (ii) the ter operon of Proteus mirabilis is induced by oxidative stress caused by H2O2 or methyl viologen (54).
FIG. 4.
Stress resistance profiles of mutants defective in individual general stress genes. For each of the stresses screened the results for the four mutants displaying the largest reduction in stress resistance compared to the wild type are shown. (A) Resistance to ethanol stress; (B) resistance to salt stress; (C) resistance to heat shock; (D) resistance to low-temperature stress. Exposure to stress and determination of survival rates were performed as described in the legend to Fig. 2. The values are arithmetic means and standard errors of the means. ▴, wild-type strain 168; □, sigB mutant strain ML6; ×, mutants defective in individual general stress genes.
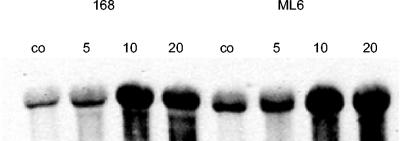
If ethanol stress indeed triggers a secondary oxidative stress in B. subtilis, genes with a specific function in the oxidative stress response should also be induced by 10% ethanol. In order to test this hypothesis, we measured the expression level of katA, a gene encoding the vegetative catalase of B. subtilis, the induction of which has been shown to be independent of σB and to be confined to oxidative stress (16). A Northern blot analysis of the katA mRNA level revealed strong induction that occurred as soon as 10 to 20 min after addition of 10% ethanol both in wild-type strain 168 and in its isogenic sigB mutant (Fig. 5). Similar σB-independent induction by severe ethanol stress was also observed for ahpC (6; data not shown). Induction of these genes shortly after the addition of 10% ethanol supports the hypothesis that ethanol triggers an increase in the generation of reactive oxygen species.
FIG. 5.
Ethanol induction of the SigB-independent katA gene. Total RNA was prepared from B. subtilis wild-type strain 168 and its isogenic sigB mutant ML6 before exposure to ethanol (lane co) and 5, 10, and 20 min after exposure to 10% (vol/vol) ethanol (lanes 5, 10, and 20, respectively). Equal amounts (10 μg) of total RNA were separated by denaturing agarose gel electrophoresis, transferred to a nylon membrane, and hybridized with a single-stranded RNA probe specific for katA.
Role of general stress genes in adaptation to low temperature.
The adaptation of B. subtilis to a sudden drop in temperature involves multiple facets, such as the importance of cold shock proteins for maintaining cellular translational capacity (62), as well as the role of a fatty acid desaturase (1, 14, 61) and anteiso-branched fatty acid biosynthesis (28) in maintaining an appropriate degree of fluidity of the cytoplasmic membrane. Very recently, two independent reports (11, 37) provided evidence that the σB-dependent general stress response plays a role in adaptation of B. subtilis to growth at low temperature. Thus, general stress proteins seem to be necessary for optimal growth (11), as well as effective stationary-phase survival and sporulation (37), at low temperatures.
The comparative screening of wild-type strain 168 and individual mutant strains performed in this study showed that inactivation of 34 of the 94 general stress genes tested (24 genes with an α of ≤0.001) resulted in increased sensitivity of the corresponding mutants to extended exposure to 4°C (Table 1). The examples shown in Fig. 4D document that inactivation of a single gene had effects that were almost as pronounced as the effects of inactivation of sigB itself. Closer inspection of the 34 cold-sensitive mutants revealed that 24 of them (11 mutants with an α of ≤0.001) were also sensitive to ethanol shock and 11 of them (11 mutants with an α of ≤0.001) were sensitive to salt shock too. Of the 14 mutants with mutations in genes coding for proteins potentially involved in the oxidative stress resistance that were tested in this study, 8 (dps, sodA, spx, ycdF, yceD, yceE, ydaD, and yqgZ mutants) were impaired in survival at 4°C. In this respect, the phenotype of the dps and sodA mutants is noteworthy. Loss of Dps renders B. subtilis cells extremely sensitive to hydrogen peroxide (7). We show here that dps inactivation triggered more-than-500-fold sensitization of the cells to storage at 4°C compared to the wild type (Table 1 and Fig. 4D). Failure to synthesize the superoxide dismutase SodA resulted in a similar sensitization (Fig. 4D). Therefore, like severe ethanol stress, exposure to 4°C might trigger increased formation of reactive oxygen species that are particularly dangerous for nongrowing cells because oxidative damage accumulates but is not compensated for by ongoing new synthesis of macromolecules.
Brigulla and coworkers (11) found that sigB is required for growth of B. subtilis at 15°C. The severe defects in low-temperature survival of mutants lacking σB itself or individual general stress proteins clearly support the notion that the general stress regulon has an important role in low-temperature adaptation. In the synthetic medium used in this study σB was not essential for growth at a low temperature. Loss of σB or the general stress proteins SodA, YfkS, and YvyD resulted in a significant extension of the lag phase compared to that of wild-type strain 168 (data not shown).
σB-Dependent general stress genes and adaptation to 54°C.
Compared to the dramatic effects seen for severe ethanol stress and exposure to 4°C, the impact of a severe heat shock triggered by preadaptaion at 48°C for 30 min and exposure to 54°C was moderate. B. subtilis might have been partially protected by uptake of compatible solutes, such as glycine betaine, that were present in the LB medium used and that can provide thermoprotection to B. subtilis cells propagated at temperatures close to their upper growth limit (52°C) (24). However, the sigB mutant still displayed 80-fold more sensitivity after 300 min of exposure to 54°C than B. subtilis wild-type strain 168 displayed. Most likely the moderate effect of a sigB knockout is also due to the protection of B. subtilis by dedicated heat shock proteins, such as the GroESL and DnaK chaperone machines or the proteins of the Clp families of chaperones and proteases. Mutants defective in dnaK (48), groESL (35), and clpC (32, 39) are clearly impaired in thermotolerance. Preadaptation of B. subtilis by exposure to 48°C induces the heat-specific protection systems mentioned above and provides considerable protection even to a sigB mutant (59). The 10 mutants (3 mutants with an α of ≤0.001) that displayed increased sensitivity to the heat challenge were all significantly less impaired than the sigB mutant (Table 1 and Fig. 4C). Seven of these 10 mutants (1 mutant with an α of ≤0.001) were also sensitive to ethanol treatment, and 4 of the 10 mutants (2 mutants with an α of ≤0.001) were also impaired after exposure to 4°C.
General stress genes involved in protection against salt stress.
Adaptation of B. subtilis to high osmolarity has been studied in great detail, and the role of dedicated systems for the synthesis and uptake of compatible solutes in osmoprotection of B. subtilis has been clearly demonstrated (9, 10). These systems are sufficient to provide osmoprotection to growing cells (9). However, the osmospecific stress response is also linked to the general stress response because at least one transport system for compatible solutes (opuE) is also subject to the control of σB (51, 60). While osmospecific protection systems provide protection to growing cells, the general stress regulon's protective role seems to be predominantly confined to nongrowing cells (59). Unfortunately, so far no attempts have been made to determine the members of the σB regulon that are especially important in protecting nongrowing cells from the impact of high salt concentrations. A surprisingly large number, 37 (28 with an α of ≥0.001), of the 94 individual mutants tested were more sensitive to strong growth-preventing salt stress than wild-type strain 168 (Table 1). A large proportion, 28 (11 with an α of ≤0.001), of these 37 mutants was also impaired in its reaction to ethanol stress, and 12 mutants (5 with an α of ≤0.001) showed increased sensitivity to exposure at 4°C, too (Table 1). A large proportion of the 38 genes (28 genes; 11 genes with an α of ≤0.001) were also impaired in the reaction to ethanol stress, and 12 mutants (5 mutants with an α of ≤0.001) showed increased sensitivity to exposure at 4°C too (Table 1). Some of these single-gene knockouts, such as the csbB, ytaB, and ymzB mutants, displayed sensitivity comparable to that of the sigB mutant (Fig. 4B). Quite a few of the encoded proteins (KatX, YdbD, YhdN, YhxD, YvgN, YxlJ, and YxnA) could be associated with protection against oxidative stress. Five other proteins can be linked to the cytoplasmic membrane, but otherwise the information concerning the biochemical functions of the proteins is very limited, and thus specific approaches are required in order to elucidate the function of each protein in the cellular protection reactions.
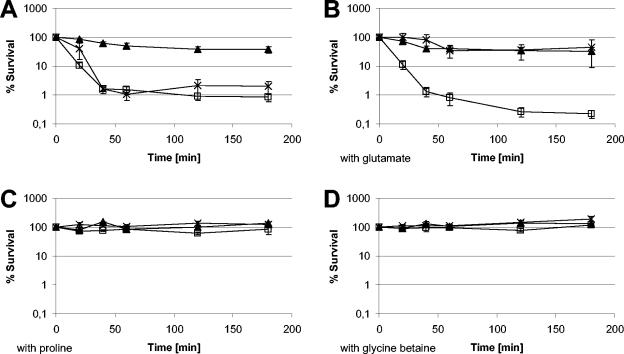
An extended analysis was performed for yerD, which probably codes for a ferredoxin-containing glutamate synthase. A mutant lacking YerD displayed virtually the same sensitivity as the sigB mutant to treatment with 10% NaCl (Fig. 6). As glutamate is the precursor for the synthesis of proline, the favorite endogenously synthesized compatible solute of B. subtilis (9, 33, 64), it seemed likely that the yerD mutant might be particularly sensitive to NaCl stress due to a lack of precursor synthesis. If this were true, addition of proline or its precursor glutamate to the medium should have abolished the requirement for YerD for survival in the presence of high growth-inhibiting NaCl concentrations. Figure 6 shows that addition of glutamate (4.5 mM), proline (4.5 mM), or glycine betaine (1 mM) completely eliminated the survival defect associated with the loss of YerD. The requirement for YerD after imposition of salt stress implies that the vegetative glutamate synthase failed to provide sufficient levels of glutamate under these conditions. This hypothesis received support from a proteome study of the adaptation of B. subtilis to a high NaCl concentration. The synthesis of the vegetative glutamate synthase large subunit GltA ceased after imposition of salt stress and did not resume until adaptation of the cells to the high-salt conditions was complete (unpublished data). Addition of proline, but not addition of glutamate, resulted in reversion of the salt sensitivity of a sigB mutant, indicating that additional σB-dependent steps are required for the conversion of glutamate to an effective osmoprotectant, probably proline (9).
FIG. 6.
Influence of compatible solutes on survival after salt stress. In order to assess the effects of compatible solutes on survival during harsh, growth-preventing salt stress, bacteria were grown in synthetic medium (52) with no addition (control) (A) or supplemented with 4.5 mM glutamate (B), 4.5 mM proline (C), or 1 mM glycine betaine (D). Exposure to salt stress and determination of survival rates were performed as described in Materials and Methods and the legend to Fig. 2. The values are arithmetic means and standard errors of the means. ▴, wild-type strain 168; □, sigB mutant ML6; ×, yerD mutant BFS 2262.
Conclusions.
To our knowledge, this investigation was the first regulon-wide phenotypic screening of a comprehensive B. subtilis mutant collection with the aim of defining the contributions of individual regulon members to protection against specific environmental insults. Surprisingly, an extremely large proportion of the individual mutants tested (85% of the mutants with an α of ≤0.01; 62% of the mutants with an α of ≤0.001) displayed increased sensitivity to at least one of the four stresses tested. Even though these studies associated the individual general stress proteins with specific facets of stress protection, detailed physiological experiments are necessary to shed more light on the function of each protein. The first detailed studies were performed for YerD. Based on BLAST homology searches and physiological as well as proteome studies, this protein has been assigned a presumed role in the synthesis of glutamate as a precursor for the osmoprotectant proline in salt-stressed cells. Such specific studies very likely will reveal unknown but probably crucial facets of the σB-dependent general stress response in bacterial stress management.
Acknowledgments
We are indebted to all the contributors of the BFA consortium for sharing their mutants. Furthermore, we thank M. Schmalisch for supplying the ctc mutant. We thank A. Harang and S. Bisanz for excellent technical assistance.
Financial support for this study was provided by the Deutsche Forschungsgemeinschaft, the European Union, and the Fonds der Chemischen Industrie.
REFERENCES
- 1.Aguilar, P. S., A. M. Hernandez-Arriaga, L. E. Cybulski, A. C. Erazo, and D. de Mendoza. 2001. Molecular basis of thermosensing: a two-component signal transduction thermometer in Bacillus subtilis. EMBO J. 20:1681-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akerley, B. J., E. J. Rubin, V. L. Novick, K. Amaya, N. Judson, and J. J. Mekalanos. 2002. A genome-scale analysis for identification of genes required for growth or survival of Haemophilus influenzae. Proc. Natl. Acad. Sci. USA 99:966-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aldsworth, T. G., R. L. Sharman, and C. E. Dodd. 1999. Bacterial suicide through stress. Cell. Mol. Life Sci. 56:378-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anagnostopoulos, C., and J. Spizizen. 1961. Requirements for transformation in Bacillus subtilis. J. Bacteriol. 81:741-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antelmann, H., J. Bernhardt, R. Schmid, H. Mach, U. Völker, and M. Hecker. 1997. First steps from a two-dimensional protein index towards a response-regulation map for Bacillus subtilis. Electrophoresis 18:1451-1463. [DOI] [PubMed] [Google Scholar]
- 6.Antelmann, H., S. Engelmann, R. Schmid, and M. Hecker. 1996. General and oxidative stress responses in Bacillus subtilis: cloning, expression, and mutation of the alkyl hydroperoxide reductase operon. J. Bacteriol. 178:6571-6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antelmann, H., S. Engelmann, R. Schmid, A. Sorokin, A. Lapidus, and M. Hecker. 1997. Expression of a stress- and starvation-induced dps/pexB homologous gene is controlled by the alternative sigma factor σB in Bacillus subtilis. J. Bacteriol. 179:7251-7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernhardt, J., U. Völker, A. Völker, H. Antelmann, R. Schmid, H. Mach, and M. Hecker. 1997. Specific and general stress proteins in Bacillus subtilis—a two-dimensional protein electrophoresis study. Microbiology 143:999-1017. [DOI] [PubMed] [Google Scholar]
- 9.Bremer, E. 2002. Adaptation to changing osmolarity, p. 385-391. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives. From genes to cells. ASM Press, Washington, D.C.
- 10.Bremer, E., and R. Krämer. 2000. Coping with osmotic challenges: osmoregulation through accumulation and release of compatible solutes in bacteria, p. 79-97. In G. Storz and R. Hengge-Aronis (ed.), Bacterial stress responses. ASM, Washington, D.C.
- 11.Brigulla, M., T. Hoffmann, A. Krisp, A. Völker, E. Bremer, and U. Völker. 2003. Chill induction of the SigB-dependent general stress response in Bacillus subtilis and its contribution to low-temperature adaptation. J. Bacteriol. 185:4305-4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Briolat, V., and G. Reysset. 2002. Identification of the Clostridium perfringens genes involved in the adaptive response to oxidative stress. J. Bacteriol. 184:2333-2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casillas-Martinez, L., and P. Setlow. 1997. Alkyl hydroperoxide reductase, catalase, MrgA, and superoxide dismutase are not involved in resistance of Bacillus subtilis spores to heat or oxidizing agents. J. Bacteriol. 179:7420-7425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cybulski, L. E., D. Albanesi, M. C. Mansilla, S. Altabe, P. S. Aguilar, and D. de Mendoza. 2002. Mechanism of membrane fluidity optimization: isothermal control of the Bacillus subtilis acyl-lipid desaturase. Mol. Microbiol. 45:1379-1388. [DOI] [PubMed] [Google Scholar]
- 15.Derre, I., G. Rapoport, K. Devine, M. Rose, and T. Msadek. 1999. ClpE, a novel type of HSP100 ATPase, is part of the CtsR heat shock regulon of Bacillus subtilis. Mol. Microbiol. 32:581-593. [DOI] [PubMed] [Google Scholar]
- 16.Engelmann, S., and M. Hecker. 1996. Impaired oxidative stress resistance of Bacillus subtilis sigB mutants and the role of katA and katE. FEMS Microbiol. Lett. 145:63-69. [DOI] [PubMed] [Google Scholar]
- 17.Falquet, L., M. Pagni, P. Bucher, N. Hulo, C. J. Sigrist, K. Hofmann, and A. Bairoch. 2002. The PROSITE database, its status in 2002. Nucleic Acids Res. 30:235-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernandes, A. P., and A. Holmgren. 2004. Glutaredoxins: glutathione-dependent redox enzymes with functions far beyond a simple thioredoxin backup system. Antioxid. Redox Signal. 6:63-74. [DOI] [PubMed] [Google Scholar]
- 19.Gaidenko, T. A., and C. W. Price. 1998. General stress transcription factor σB and sporulation transcription factor σH each contribute to survival of Bacillus subtilis under extreme growth conditions. J. Bacteriol. 180:3730-3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gish, W. 1996, posting date. [Online.] http://blast.wustl.edu.
- 21.Grant, R. A., D. J. Filman, S. E. Finkel, R. Kolter, and J. M. Hogle. 1998. The crystal structure of Dps, a ferritin homolog that binds and protects DNA. Nat. Struct. Biol. 5:294-303. [DOI] [PubMed] [Google Scholar]
- 22.Hecker, M., and U. Völker. 2001. General stress response of Bacillus subtilis and other bacteria. Adv. Microb. Physiol. 44:35-91. [DOI] [PubMed] [Google Scholar]
- 23.Helmann, J. D., M. F. Wu, P. A. Kobel, F. J. Gamo, M. Wilson, M. M. Morshedi, M. Navre, and C. Paddon. 2001. Global transcriptional response of Bacillus subtilis to heat shock. J. Bacteriol. 183:7318-7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holtmann, G., and E. Bremer. 2004. Thermoprotection of Bacillus subtilis by exogenously provided glycine betaine and structurally related compatible solutes: involvement of the Opu transporters. J. Bacteriol. 186:1683-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Igo, M., M. Lampe, C. Ray, W. Schafer, C. P. Moran, Jr., and R. Losick. 1987. Genetic studies of a secondary RNA polymerase sigma factor in Bacillus subtilis. J. Bacteriol. 169:3464-3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ji, Y., B. Zhang, S. F. Van Horn, P. Warren, G. Woodnutt, M. K. Burnham, and M. Rosenberg. 2001. Identification of critical staphylococcal genes using conditional phenotypes generated by antisense RNA. Science 293:2266-2269. [DOI] [PubMed] [Google Scholar]
- 27.Kandror, O., A. DeLeon, and A. L. Goldberg. 2002. Trehalose synthesis is induced upon exposure of Escherichia coli to cold and is essential for viability at low temperatures. Proc. Natl. Acad. Sci. USA 99:9727-9732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein, W., M. H. W. Weber, and M. A. Marahiel. 1999. Cold shock response of Bacillus subtilis: isoleucine-dependent switch in the fatty acid branching pattern for membrane adaptation to low temperatures. J. Bacteriol. 181:5341-5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kobayashi, K., S. D. Ehrlich, A. Albertini, G. Amati, K. K. Andersen, M. Arnaud, K. Asai, S. Ashikaga, S. Aymerich, P. Bessieres, F. Boland, S. C. Brignell, S. Bron, K. Bunai, J. Chapuis, L. C. Christiansen, A. Danchin, M. Debarbouille, E. Dervyn, E. Deuerling, K. Devine, S. K. Devine, O. Dreesen, J. Errington, S. Fillinger, S. J. Foster, Y. Fujita, A. Galizzi, R. Gardan, C. Eschevins, T. Fukushima, K. Haga, C. R. Harwood, M. Hecker, D. Hosoya, M. F. Hullo, H. Kakeshita, D. Karamata, Y. Kasahara, F. Kawamura, K. Koga, P. Koski, R. Kuwana, D. Imamura, M. Ishimaru, S. Ishikawa, I. Ishio, D. Le Coq, A. Masson, C. Mauel, R. Meima, R. P. Mellado, A. Moir, S. Moriya, E. Nagakawa, H. Nanamiya, S. Nakai, P. Nygaard, M. Ogura, T. Ohanan, M. O'Reilly, M. O'Rourke, Z. Pragai, H. M. Pooley, G. Rapoport, J. P. Rawlins, L. A. Rivas, C. Rivolta, A. Sadaie, Y. Sadaie, M. Sarvas, T. Sato, H. H. Saxild, E. Scanlan, W. Schumann, J. F. Seegers, J. Sekiguchi, A. Sekowska, S. J. Seror, M. Simon, P. Stragier, R. Studer, H. Takamatsu, T. Tanaka, M. Takeuchi, H. B. Thomaides, V. Vagner, J. M. van Dijl, K. Watabe, A. Wipat, H. Yamamoto, M. Yamamoto, Y. Yamamoto, K. Yamane, K. Yata, K. Yoshida, H. Yoshikawa, U. Zuber, and N. Ogasawara. 2003. Essential Bacillus subtilis genes. Proc. Natl. Acad. Sci. USA 100:4678-4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krogh, A., B. Larsson, G. von Heijne, and E. L. Sonnhammer. 2001. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 305:567-580. [DOI] [PubMed] [Google Scholar]
- 31.Krüger, E., T. Msadek, and M. Hecker. 1996. Alternate promoters direct stress-induced transcription of the Bacillus subtilis clpC operon. Mol. Microbiol. 20:713-723. [DOI] [PubMed] [Google Scholar]
- 32.Krüger, E., U. Völker, and M. Hecker. 1994. Stress induction of clpC in Bacillus subtilis and its involvement in stress tolerance. J. Bacteriol. 176:3360-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuhlmann, A. U., and E. Bremer. 2002. Osmotically regulated synthesis of the compatible solute ectoine in Bacillus pasteurii and related Bacillus spp. Appl. Environ. Microbiol. 68:772-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kunst, F., N. Ogasawara, I. Moszer, A. M. Albertini, G. Alloni, V. Azevedo, M. G. Bertero, P. Bessieres, A. Bolotin, S. Borchert, R. Borriss, L. Boursier, A. Brans, M. Braun, S. C. Brignell, S. Bron, S. Brouillet, C. V. Bruschi, B. Caldwell, V. Capuano, N. M. Carter, S. K. Choi, J. J. Codani, I. F. Connerton, and A. Danchin. 1997. The complete genome sequence of the Gram-positive bacterium Bacillus subtilis. Nature 390:249-256. [DOI] [PubMed] [Google Scholar]
- 35.Li, M., and S. Wong. 1992. Cloning and characterization of the groESL operon from Bacillus subtilis. J. Bacteriol. 174:3981-3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Majumdar, D., Y. J. Avissar, and J. H. Wyche. 1991. Simultaneous and rapid isolation of bacterial and eukaryotic DNA and RNA: a new approach for isolating DNA. BioTechniques 11:94-101. [PubMed] [Google Scholar]
- 37.Mendez, M. B., L. M. Orsaria, V. Philippe, M. E. Pedrido, and R. R. Grau. 2004. Novel roles of the master transcription factors Spo0A and σB for survival and sporulation of Bacillus subtilis at low growth temperature. J. Bacteriol. 186:989-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mongkolsuk, S., W. Praituan, S. Loprasert, M. Fuangthong, and S. Chamnongpol. 1998. Identification and characterization of a new organic hydroperoxide resistance (ohr) gene with a novel pattern of oxidative stress regulation from Xanthomonas campestris pv. phaseoli. J. Bacteriol. 180:2636-2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Msadek, T., F. Kunst, and G. Rapoport. 1994. MecB of Bacillus subtilis, a member of the ClpC ATPase family, is a pleiotropic regulator controlling competence gene expression and growth at high temperature. Proc. Natl. Acad. Sci. USA 91:5788-5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mushegian, A. R., and E. V. Koonin. 1996. A minimal gene set for cellular life derived by comparison of complete bacterial genomes. Proc. Natl. Acad. Sci. USA 93:10268-10273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nielsen, H., J. Engelbrecht, S. Brunak, and G. von Heijne. 1997. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10:1-6. [DOI] [PubMed] [Google Scholar]
- 42.Petersohn, A., M. Brigulla, S. Haas, J. D. Hoheisel, U. Völker, and M. Hecker. 2001. Global analysis of the general stress response of Bacillus subtilis. J. Bacteriol. 183:5617-5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Price, C. W. 2002. General stress response, p. 369-384. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives. From genes to cells. ASM Press, Washington, D.C.
- 44.Price, C. W. 2000. Protective function and regulation of the general stress response in Bacillus subtilis and related gram-positive bacteria, p. 179-197. In G. Storz and R. Hengge-Aronis (ed.), Bacterial stress responses. ASM Press, Washington, D.C.
- 45.Price, C. W., P. Fawcett, H. Ceremonie, N. Su, C. K. Murphy, and P. Youngman. 2001. Genome-wide analysis of the general stress response in Bacillus subtilis. Mol. Microbiol. 41:757-774. [DOI] [PubMed] [Google Scholar]
- 46.Scharf, C., R. Riethdorf, H. Ernst, S. Engelmann, U. Völker, and M. Hecker. 1998. Thioredoxin is an essential protein induced by multiple stresses in Bacillus subtilis. J. Bacteriol. 180:1869-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmalisch, M., I. Langbein, and J. Stulke. 2002. The general stress protein Ctc of Bacillus subtilis is a ribosomal protein. J. Mol. Microbiol. Biotechnol. 4:495-501. [PubMed] [Google Scholar]
- 48.Schulz, A., B. Tzschaschel, and W. Schumann. 1995. Isolation and analysis of mutants of the dnaK operon of Bacillus subtilis. Mol. Microbiol. 15:421-429. [DOI] [PubMed] [Google Scholar]
- 49.Schumann, W., D. S. Ehrlich, and N. Ogasawara. 2001. Functional analysis of bacterial genes: a practical manual. John Wiley & Sins, Ltd., Chichester, United Kingdom.
- 50.Sonnhammer, E. L. L., G. von Heijne, and A. Krogh. 1998. A hidden Markov model for predicting transmembrane helices in protein sequences, p. 175-182. In J. Glasgow, T. Littlejohn, F. Major, R. Lathrop, D. Sankoff, and C. Sensen (ed.), Proceedings of the Sixth International Conference on Intelligent Systems for Molecular Biology. AAAI Press, Menlo Park, Calif. [PubMed]
- 51.Spiegelhalter, F., and E. Bremer. 1998. Osmoregulation of the opuE proline transport gene from Bacillus subtilis—contributions of the σA- and σB-dependent stress-responsive promoters. Mol. Microbiol. 29:285-296. [DOI] [PubMed] [Google Scholar]
- 52.Stülke, J., R. Hanschke, and M. Hecker. 1993. Temporal activation of beta-glucanase synthesis in Bacillus subtilis is mediated by the GTP pool. J. Gen. Microbiol. 139:2041-2045. [DOI] [PubMed] [Google Scholar]
- 53.Summers, A. O., and G. A. Jacoby. 1977. Plasmid-determined resistance to tellurium compounds. J. Bacteriol. 129:276-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Toptchieva, A., G. Sisson, L. J. Bryden, D. E. Taylor, and P. S. Hoffman. 2003. An inducible tellurite-resistance operon in Proteus mirabilis. Microbiology 149:1285-1295. [DOI] [PubMed] [Google Scholar]
- 55.Turner, R. J., J. H. Weiner, and D. E. Taylor. 1999. Tellurite-mediated thiol oxidation in Escherichia coli. Microbiology 145:2549-2557. [DOI] [PubMed] [Google Scholar]
- 56.Vagner, V., E. Dervyn, and S. D. Ehrlich. 1998. A vector for systematic gene inactivation in Bacillus subtilis. Microbiology 144:3097-3104. [DOI] [PubMed] [Google Scholar]
- 57.Varon, D., S. A. Boylan, K. Okamoto, and C. W. Price. 1993. Bacillus subtilis gtaB encodes UDP-glucose pyrophosphorylase and is controlled by stationary-phase transcription factor σB. J. Bacteriol. 175:3964-3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Völker, U., S. Engelmann, B. Maul, S. Riethdorf, A. Völker, R. Schmid, H. Mach, and M. Hecker. 1994. Analysis of the induction of general stress proteins of Bacillus subtilis. Microbiology 140:741-752. [DOI] [PubMed] [Google Scholar]
- 59.Völker, U., B. Maul, and M. Hecker. 1999. Expression of the σB-dependent general stress regulon confers multiple stress resistance in Bacillus subtilis. J. Bacteriol. 181:3942-3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.von Blohn, C., B. Kempf, R. M. Kappes, and E. Bremer. 1997. Osmostress response in Bacillus subtilis: characterization of a proline uptake system (OpuE) regulated by high osmolarity and the alternative transcription factor sigma B. Mol. Microbiol. 25:175-187. [DOI] [PubMed] [Google Scholar]
- 61.Weber, M. H., W. Klein, L. Müller, U. M. Niess, and M. A. Marahiel. 2001. Role of the Bacillus subtilis fatty acid desaturase in membrane adaptation during cold shock. Mol. Microbiol. 39:1321-1329. [DOI] [PubMed] [Google Scholar]
- 62.Weber, M. H., and M. A. Marahiel. 2002. Coping with the cold: the cold shock response in the Gram-positive soil bacterium Bacillus subtilis. Philos. Trans. R. Soc. Lond. Biol. Sci. 357:895-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wetzstein, M., U. Völker, J. Dedio, S. Lobau, U. Zuber, M. Schiesswohl, C. Herget, M. Hecker, and W. Schumann. 1992. Cloning, sequencing, and molecular analysis of the dnaK locus from Bacillus subtilis. J. Bacteriol. 174:3300-3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Whatmore, A. M., J. A. Chudek, and R. H. Reed. 1990. The effects of osmotic upshock on the intracellular solute pools of Bacillus subtilis. J. Gen. Microbiol. 136:2527-2535. [DOI] [PubMed] [Google Scholar]