Abstract
Purpose: This retrospective study aims to analyze the effect of telmisartan (TEL) combined with calcitriol (CTL) on therapeutic efficacy, inflammatory status, and renal interstitial fibrosis (RIF) in patients with diabetic nephropathy (DN). Methods: First, 126 DN patients admitted between September 2019 and September 2022 were selected, of which 66 cases (research group) were treated with TEL + CTL, and 60 cases (control group) were given TEL alone. The therapeutic efficacy, complications (fever, dizziness, headache, nausea and vomiting, and gastrointestinal reactions), inflammatory status (interleukin (IL)-1β, high-sensitivity C-reactive protein (hs-CRP), and tumor necrosis factor (TNF)-α), RIF (transforming growth factor (TGF)-β1, vascular endothelial growth factor (VEGF), and homocysteine (Hcy)), and renal function (blood urea (BUN), serum creatinine (Cr), and microalbuminuria (mAlb)) were compared between the two groups. Finally, a univariate analysis was performed to identify factors leading to poor prognosis (ineffective treatment) in patients. Results: A statistically higher overall response rate was determined in the research group as compared to the control group (P<0.05). The two groups exhibited equivalent complication rates (P>0.05). Markedly lower post-treatment IL-1β, hs-CRP, TNF-α, TGF-β1, VEGF, Hcy, BUN, Scr, and mAlb were identified in the research group than those in the control group (P<0.05). Moreover, hs-CRP, TNF-α, BUN, and treatment modality were found to be closely associated with poor prognosis in DN patients (P<0.05). Conclusions: TEL + CTL has a good effect on DN and a comparable safety profile to TEL monotherapy. This combination can significantly inhibit inflammation and RIF and improve renal function in DN patients.
Keywords: Telmisartan, calcitriol, diabetic nephropathy, therapeutic efficacy, inflammatory status
Introduction
Diabetic nephropathy (DN), a diabetes mellitus-induced chronic microvascular complication, is characterized by hyperfiltration and proteinuria in the early stages and progressive renal function decline in the advanced stages [1,2]. It can easily progress to end-stage renal disease and carries a high risk of mortality [3]. Sex, advanced age, obesity, long course of disease, and excessive protein intake are shown to be possible inducements, and hyperglycemia and hypertension are the most important risk predisposing factors [4-6]. The pathological mechanism of DN involves inflammation and renal interstitial fibrosis (RIF), both of which are strongly linked to the occurrence and progression of renal injury [7]. Current treatment strategies for DN include prophylaxis such as early screening and interventions, early treatment like strict monitoring of blood sugar and blood pressure, and comprehensive treatment such as dialysis and kidney transplantation, but all these treatments have their limitations in terms of their application scope and efficacy [8,9]. Therefore, there is still a lack of effective treatment strategies to reverse the progression of DN, warranting continuous exploration of novel effective treatments.
Telmisartan (TEL), as an angiotensin II type 1 receptor blocker, can not only be used to treat hypertension, but also plays a role in renal protection [10]. In a rat experiment, TEL exerted nephroprotective effects in DN by inhibiting oxidative stress and inflammation and activating nuclear factor erythroid 2-related factor 2 (NRF2)/heme oxidase-1 (HO-1) signaling [11]. As reported by Chang et al. [12], TEL promoted cardiac remodeling by regulating the renin-angiotensin-aldosterone system in cardiorenal heart failure and inhibited the process of cardiac inflammation-fibrosis to some extent, suggesting its potential to regulate inflammation and fibrosis. Calcitriol (CTL), an active form of vitamin D, can modulate calcium and phosphorus metabolism, and mediate differentiation and proliferation of various cells, thus playing a role in immune system and kidney diseases [13,14]. Lin et al. [15] pointed out that the renal protective effect of CTL in DN could be attributed to its modulation of angiotensin converting enzyme (ACE) and ACE2 levels in renal tubules. Another study confirmed that the anti-DN therapeutic effect of CTL was related to its down-regulation of transforming growth factor β1 (TGF-β1) and Cdc42-interacting protein 4 (CIP4) that was followed by inhibition of renal fibrosis [16].
This study aimed to analyze the effects of TEL in combination with CTL on the therapeutic efficacy, inflammatory status, and RIF in DN, in order to provide new insights into DN treatment. Notably, the innovation of this study mainly lies in measuring the clinical advantages of TEL + CTL in DN from the aspects of efficacy, complications, inflammatory state, RIF, and renal function, which are effective indictors for clinical effectiveness, safety and generalizability.
Materials and methods
General information
Ethical approval from the our hospital Ethics Committee has been obtained for this retrospective study. The participants were 126 DN patients admitted between September 2019 and September 2022, with 66 patients (male-to-female ratio: 36:30, mean age: 48.92±7.64) treated with TEL + CTL being included in the research group and 60 patients (male-to-female ratio: 39:21, mean age: 50.50±7.26) treated with TEL monotherapy being assigned to the control group. The age, sex and other general data of the two groups were comparable (P>0.05). Patients were not required to give informed consent to the study because the analyses used were anonymous clinical data that were obtained after obtaining written consent from each patient for their treatment.
Inclusion and exclusion criteria
Inclusion criteria: diagnosis of type II DN with stage III according to the Mogensen classification [17]; microalbuminuria (mAlb): 30-300 mg/24 h; no contraindications to the study medications; complete medical records and high compliance with the doctor’s advice; no mental illness or other kidney diseases.
Exclusion criteria: other serious primary diseases and renal artery stenosis; diabetic ketoacidosis; serious infections; use of other interventions in the past six months that may affect the results of this study; proteinuria caused by other reasons; pregnant or lactating patients; other types of kidney disease.
Treatment methods
The control group was treated with TEL (Beijing Kangruina Biotechnology Co., Ltd., LB1770) monotherapy, per os, once daily. The dosage was 40 mg each time for the first 2 weeks and 80 mg each time afterwards. The treatment lasted for half a year.
On this basis, CTL (Beijing Kangruina Biotechnology Co., Ltd., LA3143) was further administered in the research group. The CTL capsules were given orally, 0.25 μg each time once daily for the first 7 days, and 0.50 μg each time afterwards. The treatment continued for half a year.
Outcome measures
The therapeutic efficacy was comparatively analyzed, and the incidence of complications such as fever, dizziness, headache, nausea and vomiting, and gastrointestinal reactions were counted. In addition, interleukin (IL)-1β, high-sensitivity C-reactive protein (hs-CRP), and tumor necrosis factor (TNF)-α were measured to evaluate the inflammatory status of patients. Moreover, transforming growth factor (TGF)-β1, vascular endothelial growth factor (VEGF), and homocysteine (Hcy) were determined to assess RIF. Blood urea (BUN), serum creatinine (Cr), and microalbuminuria (mAlb) were measured to evaluate renal function.
The therapeutic efficacy was assessed as follows. Significant response was recorded if patients had significant improvement of clinical symptoms, reduction of Cr by more than 20%, and decrease of BUN by more than 50%. Effective response was defined as alleviated clinical symptoms, decreased Cr by 10-20%, and reduced BUN by 10-50%. Ineffective response was observed if there was no change or even a deterioration in the patient’s clinical symptoms, coupled with no improvement or even marked increases in Cr and BUN levels.
Before the detection of the above indexes, fasting venous blood (5 mL) was collected, and serum samples were separated by centrifugation, for enzyme-linked immunosorbent assay (ELISA) determination of inflammatory indexes and RIF indexes. The experimental steps were carried out in strict accordance with the instructions of the corresponding human ELISA kit (Shanghai Fuyu Biotechnology Co., Ltd., FY-03246H2, FY-03502H2, FY-03218H2, FY-03203H1, and FY-03215H1 for IL-1β, hs-CRP, TNF-α, TGF-β1, and VEGF, respectively; Shanghai Fusheng Industrial Co., Ltd., A098926 for Hcy). While renal function-associated indexes were determined by automatic biochemical analyzer and electrochemiluminescence. Additionally, all indicators were tested again after 8 weeks of treatment. The primary outcome measures are therapeutic efficacy, inflammation indexes, and RIF indexes, and the secondary ones are complications and renal function indexes.
Statistical methods
The data of 126 ND patients were statistically analyzed with SPSS 21.0 (SPSS, Inc., Chicago, IL, United States) and Graph Pad Prism 6 (Graph Pad Software, San Diego, United States). The number of cases (percentage) (n [%]) and Mean ± SEM were used to represent categorical and continuous variables, respectively. To identify statistically significant differences indicated by a minimum level of P<0.05, χ2 tests were used for categorical variables, and independent samples t tests and paired t tests were performed for between-group and intra-group comparisons of continuous variables, respectively. Before univariate analysis, continuous variables were converted into categorical variables.
Results
General data
No significant inter-group differences were identified in terms of age, sex, course of disease, body mass index (BMI), and hypertension (P>0.05). See Table 1.
Table 1.
General information
| Item | Control group (n=60) | Research group (n=66) | χ2/t | P |
|---|---|---|---|---|
| Age (years) | 50.50±7.26 | 48.92±7.64 | 1.187 | 0.238 |
| Sex (male/female) | 39/21 | 36/30 | 1.426 | 0.233 |
| Course of disease (years) | 9.28±2.14 | 8.65±2.48 | 1.519 | 0.131 |
| BMI (kg/m2) | 24.48±5.15 | 24.01±5.54 | 0.492 | 0.624 |
| Hypertension (with/without) | 22/38 | 25/41 | 0.020 | 0.888 |
Note: BMI, body mass index.
Therapeutic efficacy in the patients with DN
The overall response rate was 71.67% in the control group and 87.88% in the research group, with a significant inter-group difference (P<0.05). See Table 2.
Table 2.
Therapeutic efficacy evaluation of two groups of DN patients
| Efficacy | Control group (n=60) | Research group (n=66) | χ2/t | P |
|---|---|---|---|---|
| Significant response | 15 (25.00) | 26 (39.39) | ||
| Effective response | 28 (46.67) | 32 (48.48) | ||
| Ineffective response | 17 (28.33) | 8 (12.12) | ||
| Overall response rate | 43 (71.67) | 58 (87.88) | 5.194 | 0.023 |
Note: DN, diabetic nephropathy.
Incidence of complications in the patients with DN
The incidence of fever, dizziness, headache, nausea and vomiting, and gastrointestinal reactions in the two groups were counted, and no significant difference was determined in the overall complication rate between the control and research groups (10.00% vs. 6.06%, P>0.05). See Table 3.
Table 3.
Evaluation of complications in two groups of DN patients
| Complication | Control group (n=60) | Research group (n=66) | χ2/t | P |
|---|---|---|---|---|
| Fever | 1 (1.67) | 0 (0.00) | ||
| Dizziness and headache | 2 (3.33) | 1 (1.52) | ||
| Nausea and vomiting | 1 (1.67) | 1 (1.52) | ||
| Gastrointestinal reactions | 2 (3.33) | 2 (3.03) | ||
| Total | 6 (10.00) | 4 (6.06) | 0.668 | 0.414 |
Note: DN, diabetic nephropathy.
Inflammatory status in the patients with DN
The pre-treatment IL-1β, hs-CRP and TNF-α levels were similar between the two groups (P>0.05). All these inflammatory factors decreased statistically in both cohorts after treatment (P<0.05), with even lower levels in the research group as compared to the control group (IL-1β: 12.55±2.34 pg/mL vs. 17.79±3.12 pg/mL; hs-CRP: 10.69±3.32 mg/L vs. 13.92±3.44 mg/L; TNF-α: 40.42±3.95 pg/mL vs. 44.84±4.42 pg/mL; P<0.05). See Figure 1.
Figure 1.
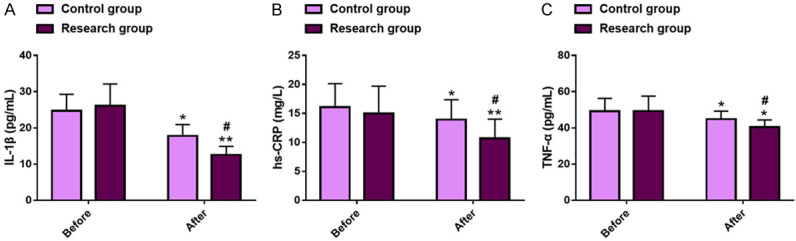
Inflammatory status in two groups of DN patients. A. Comparison of IL-1β levels. B. Comparison of hs-CRP levels. C. Comparison of TNF-α levels. Note: *P<0.05 and **P<0.01 vs. before treatment; #P<0.05 vs. Control. DN, diabetic nephropathy; IL-1β, interleukin-1β; hs-CRP, high-sensitivity C-reactive protein; TNF-α, tumor necrosis factor-α.
RIF in the patients with DN
The RIF-associated indices such as TGF-β1, VEGF, and Hcy were detected and compared. The two groups were not statistically different in these indexes prior to treatment (P>0.05), but all of the indexes decreased significantly after treatment in both groups (P<0.05), with even lower TGF-β1 (151.23±15.92 pg/mL vs. 168.63±16.95 pg/mL), VEGF (342.61±21.06 pg/mL vs. 392.07±23.10 pg/mL), and Hcy (14.26±2.83 μmol/L vs. 18.44±2.78 μmol/L) in the research group (P<0.05). See Figure 2.
Figure 2.
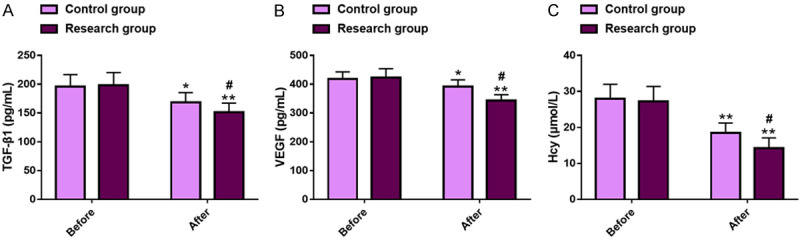
Renal interstitial fibrosis in two groups of DN patients. A. Comparison of TGF-β1 levels. B. Comparison of VEGF levels. C. Comparison of Hcy levels. Note: *P<0.05 and **P<0.01 vs. before treatment; #P<0.05 vs. Control. DN, diabetic nephropathy; TGF-β1, transforming growth factor-β1; VEGF, vascular endothelial growth factor; Hcy, homocysteine.
Renal function in the patients with DN
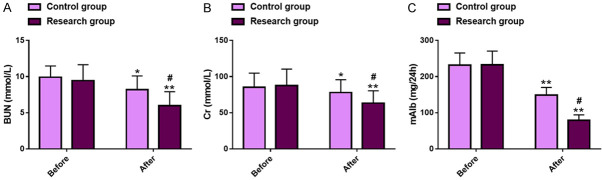
The effects of the two treatments modalities on renal function were evaluated by detecting renal function indexes such as BUN, Cr, and mAlb. Before treatment, there was no significant inter-group difference in these renal function indexes (P>0.05). After treatment, BUN, Cr, and mAlb all decreased significantly in both groups (P<0.05), with more marked reductions in all of these indexes in the research group versus those in the control group (BUN: 5.99±1.92 mmol/L vs. 8.18±1.91 mmol/L; Cr: 63.29±17.05 mmol/L vs. 77.97±17.76 mmol/L; mAlb: 79.07±14.94 mg/24 h vs. 148.84±21.05 mg/24 h; P<0.05). See Figure 3.
Figure 3.
Renal function in two groups of DN patients. A. Comparison of BUN levels. B. Comparison of Cr levels. C. Comparison of mAlb levels. Note: *P<0.05 and **P<0.01 vs. before treatment; #P<0.05 vs. Control. DN, diabetic nephropathy; BUN, blood urea; Cr, serum creatinine; mAlb, microalbuminuria.
Univariate analysis of prognosis in the patients with DN
Among the 126 DN patients, there were 25 patients with ineffective response, and they were included in a poor prognosis group (n=25), while those with effective and significant response were in a good prognosis group (n=101). After analysis, hs-CRP, TNF-α, BUN, and treatment modality were identified to be closely related to the prognosis of DN patients (P<0.05), while age, gender, course of disease, BMI, hypertension, IL-1β, TGF-β1, VEGF, Hcy, Cr, and mAlb were not (P>0.05). See Table 4.
Table 4.
Univariate analysis of factors affecting the prognosis in patients with DN
| Variables | Poor prognosis group (n=25) | Good prognosis group (n=101) | χ2 | P |
|---|---|---|---|---|
| Age (<50 years/≥50 years) | 10/15 | 48/53 | 0.457 | 0.499 |
| Sex (male/female) | 19/6 | 56/45 | 3.514 | 0.061 |
| Course of the disease (<9 years/≥9 years) | 8/17 | 37/64 | 0.187 | 0.665 |
| BMI (<24 kg/m2/≥24 kg/m2) | 13/12 | 46/55 | 0.335 | 0.563 |
| Hypertension (with/without) | 11/14 | 36/65 | 0.598 | 0.439 |
| IL-1β (<25 pg/mL/≥25 pg/mL) | 10/15 | 53/48 | 1.248 | 0.264 |
| hs-CRP (<16 mg/L/≥16 mg/L) | 8/17 | 59/42 | 5.616 | 0.018 |
| TNF-α (<49 pg/mL/≥49 pg/mL) | 7/18 | 59/42 | 7.433 | 0.006 |
| TGF-β1 (<196 pg/mL/≥196 pg/mL) | 14/11 | 42/59 | 1.687 | 0.194 |
| VEGF (<418 pg/mL/≥418 pg/mL) | 12/13 | 45/56 | 0.096 | 0.757 |
| Hcy (<28 μmol/L/≥28 μmol/L) | 13/12 | 56/45 | 0.096 | 0.757 |
| BUN (<10 mmol/L/≥10 mmol/L) | 10/15 | 67/34 | 5.849 | 0.016 |
| Cr (<85 mmol/L/≥85 mmol/L) | 13/12 | 48/53 | 0.161 | 0.689 |
| mAlb (<232 mg/24 h/≥232 mg/24 h) | 11/14 | 52/49 | 0.449 | 0.503 |
| Treatment modality (TEL/TEL + CTL) | 17/8 | 8/93 | 45.480 | <0.001 |
Note: DN, diabetic nephropathy; BMI, body mass index; TEL, telmisartan; CTL, calcitriol.
Discussion
According to the epidemiological data of DN, about 40% of diabetic patients are at risk of developing DN, a condition associated with reduced life expectancy and higher overall healthcare costs [18-20]. The pathogenesis of DN is complicated. Besides the two important processes discussed above (inflammatory status and RIF), it is also related to physiopathological processes such as glomerular basement membrane thickening, mesangial matrix increase, and podocyte loss [21]. This study attempts to explore potential novel treatment strategies for DN by evaluating the therapeutic efficacy, inflammatory status, RIF, etc., hoping to contribute to the prevention and treatment of the disease.
Data from this study showed an obviously higher overall response rate in the research group compared with that in the control group (87.88% vs. 71.67%), indicating that TEL + CTL is advantageous over TEL monotherapy in enhancing the curative effect in DN patients. In the study of Jeong et al. [22], TEL + CTL applied to adriamycin-induced nephropathy mice effectively relieved renal injury by reducing proteinuria, glomerulosclerosis and podocyte apoptosis, suggesting that the combination of TEL and CTL have certain therapeutic potential and clinical effectiveness in DN. Subsequently, the occurrence of complications such as fever, dizziness, headache, nausea, vomiting, and gastrointestinal reactions were statistically analyzed. The overall complication rate was not statistically different between the research group and the control group, suggesting that the combination treatment did not increase the side effects and was well tolerated. In the study of Makino et al. [23], TEL was well tolerated and safe for patients with type II DN, consistent with our findings. In addition, Chen et al. [24] pointed out that CTL intervention was conducive to reducing the risk of diabetes microvascular complications such as DN, which is similar to our research results. IL-1β, hs-CRP, and TNF-α are typical inflammatory indicators of renal inflammation, and the decrease in their levels reflects the effectiveness of interventions and significant inhibition of renal inflammatory status [25]. The detection of inflammatory status showed significantly inhibited inflammation levels in both groups after treatment, with even lower IL-1β, hs-CRP, and TNF-α in the research group, indicating that TEL + CTL was more effective than TEL alone in inhibiting inflammation in DN. As reported by Wang et al. [26], CTL significantly inhibited hs-CRP, TNF-α and other inflammatory indicators in DN patients, which supports our findings. Alleviated RIF was also determined in both groups after treatment, manifested by a significant reduction in TGF-β1, VEGF and Hcy. Moreover, these indexes were lower in the research group than in the control group, suggesting that the combined intervention has a more significant effect on RIF than the single intervention. TGF-β1, VEGF, and Hcy are known representative indicators of RIF, among which TGF-β1 participates in this process primarily by inducing renal tubular epithelial-mesenchymal transition, while VEGF can establish a pathway with TGF-β1 to mediate fibrosis-related lymphangiogenesis, and Hcy reflects the severity of renal tubular atrophy and interstitial fibrosis and has certain predictive potential for early renal function decline [27-29]. In the research of Zha et al. [30], the reversal of DN progression by TEL was not only achieved by alleviating renal fibrosis, but also partially related to its inhibition of heterodimerization of angiotensin II type-1 receptor (AT1R) antibody-adiponectin receptor 1 (AdipoR1). In addition, both groups had obviously alleviated renal function damage after treatment, with lower BUN, Cr, and mAlb found in the research group versus the control group, indicating that TEL + CTL has a significant protective effect on renal function in DN. The mechanism of TEL in alleviating renal injury is also related to its down-regulation of renal injury-specific markers such as kidney injury molecule-1 (KIM-1), neutrophil gelatinase-associated lipocalin (NGAL), and cystatin C [31]. CTL, on the other hand, alleviates renal inflammation and RIF by down-regulating the mRNAs of the leukocyte common antigen (CD45), disintegrins, and metalloproteinases, thus playing a protective role in renal function [32]. Finally, the results of univariate analysis confirmed that hs-CRP, TNF-α, BUN, and treatment modality had a significant impact on the poor prognosis in DN patients, and low pre-treatment hs-CRP (<16 mg/L), TNF-α (<49 pg/mL), and BUN (<10 mmol/L) levels, as well as TEL + CTL were associated with better patient prognosis.
This study has several limitations that need to be carefully considered and improved in the future. First, the sample size can be increased to reduce the impact of a small sample size on the accuracy of research results. Second, the sample size should be expanded to include samples from multiple centers, so as to avoid possible information collection bias in a single center. Third, the treatment mechanism of TEL plus CTL in DN can be validated and explored by additional basic studies, which can help further understand the underlying mechanism of TEL plus CTL in DN treatment. In the future, we will conduct supplementary explorations based on the above points.
Conclusively, TEL plus CTL can improve the efficacy in DN patients, effectively inhibit inflammation and RIF, and significantly enhance renal function, without increasing the risk of adverse drug reactions. Therefore, this combination deserves clinical promotion.
Disclosure of conflict of interest
None.
References
- 1.Sagoo MK, Gnudi L. Diabetic nephropathy: an overview. Methods Mol Biol. 2020;2067:3–7. doi: 10.1007/978-1-4939-9841-8_1. [DOI] [PubMed] [Google Scholar]
- 2.Faselis C, Katsimardou A, Imprialos K, Deligkaris P, Kallistratos M, Dimitriadis K. Microvascular complications of type 2 diabetes mellitus. Curr Vasc Pharmacol. 2020;18:117–124. doi: 10.2174/1570161117666190502103733. [DOI] [PubMed] [Google Scholar]
- 3.Maggiore U, Budde K, Heemann U, Hilbrands L, Oberbauer R, Oniscu GC, Pascual J, Schwartz Sorensen S, Viklicky O, Abramowicz D ERA-EDTA DESCARTES working group. Long-term risks of kidney living donation: review and position paper by the ERA-EDTA DESCARTES working group. Nephrol Dial Transplant. 2017;32:216–223. doi: 10.1093/ndt/gfw429. [DOI] [PubMed] [Google Scholar]
- 4.Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12:2032–2045. doi: 10.2215/CJN.11491116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sacks FM, Hermans MP, Fioretto P, Valensi P, Davis T, Horton E, Wanner C, Al-Rubeaan K, Aronson R, Barzon I, Bishop L, Bonora E, Bunnag P, Chuang LM, Deerochanawong C, Goldenberg R, Harshfield B, Hernandez C, Herzlinger-Botein S, Itoh H, Jia W, Jiang YD, Kadowaki T, Laranjo N, Leiter L, Miwa T, Odawara M, Ohashi K, Ohno A, Pan C, Pan J, Pedro-Botet J, Reiner Z, Rotella CM, Simo R, Tanaka M, Tedeschi-Reiner E, Twum-Barima D, Zoppini G, Carey VJ. Association between plasma triglycerides and high-density lipoprotein cholesterol and microvascular kidney disease and retinopathy in type 2 diabetes mellitus: a global case-control study in 13 countries. Circulation. 2014;129:999–1008. doi: 10.1161/CIRCULATIONAHA.113.002529. [DOI] [PubMed] [Google Scholar]
- 6.Donate-Correa J, Luis-Rodriguez D, Martin-Nunez E, Tagua VG, Hernandez-Carballo C, Ferri C, Rodriguez-Rodriguez AE, Mora-Fernandez C, Navarro-Gonzalez JF. Inflammatory targets in diabetic nephropathy. J Clin Med. 2020;9:458. doi: 10.3390/jcm9020458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samsu N. Diabetic nephropathy: challenges in pathogenesis, diagnosis, and treatment. Biomed Res Int. 2021;2021:1497449. doi: 10.1155/2021/1497449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andresdottir G, Jensen ML, Carstensen B, Parving HH, Rossing K, Hansen TW, Rossing P. Improved survival and renal prognosis of patients with type 2 diabetes and nephropathy with improved control of risk factors. Diabetes Care. 2014;37:1660–1667. doi: 10.2337/dc13-2036. [DOI] [PubMed] [Google Scholar]
- 9.Forst T, Mathieu C, Giorgino F, Wheeler DC, Papanas N, Schmieder RE, Halabi A, Schnell O, Streckbein M, Tuttle KR. New strategies to improve clinical outcomes for diabetic kidney disease. BMC Med. 2022;20:337. doi: 10.1186/s12916-022-02539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin X, Kim MH, Han KH, Hong SJ, Ahn JC, Sung JH, Cho JM, Lee HC, Choi SY, Lee K, Kim WS, Rhee MY, Kim JH, Hong SP, Yoo BS, Cho EJ, Lee JH, Kim PJ, Park CG, Hyon MS, Shin JH, Lee SH, Sung KC, Hwang J, Kwon K, Chae IH, Seo JS, Kim H, Lee H, Cho Y, Kim HS. Efficacy and safety of co-administered telmisartan/amlodipine and rosuvastatin in subjects with hypertension and dyslipidemia. J Clin Hypertens (Greenwich) 2020;22:1835–1845. doi: 10.1111/jch.13893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antar SA, Abdo W, Taha RS, Farage AE, El-Moselhy LE, Amer ME, Abdel Monsef AS, Abdel Hamid AM, Kamel EM, Ahmeda AF, Mahmoud AM. Telmisartan attenuates diabetic nephropathy by mitigating oxidative stress and inflammation, and upregulating Nrf2/HO-1 signaling in diabetic rats. Life Sci. 2022;291:120260. doi: 10.1016/j.lfs.2021.120260. [DOI] [PubMed] [Google Scholar]
- 12.Chang D, Xu TT, Zhang SJ, Cai Y, Min SD, Zhao Z, Lu CQ, Wang YC, Ju S. Telmisartan ameliorates cardiac fibrosis and diastolic function in cardiorenal heart failure with preserved ejection fraction. Exp Biol Med (Maywood) 2021;246:2511–2521. doi: 10.1177/15353702211035058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev. 2005;26:662–687. doi: 10.1210/er.2004-0002. [DOI] [PubMed] [Google Scholar]
- 14.Bivona G, Agnello L, Ciaccio M. The immunological implication of the new vitamin D metabolism. Cent Eur J Immunol. 2018;43:331–334. doi: 10.5114/ceji.2018.80053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin M, Gao P, Zhao T, He L, Li M, Li Y, Shui H, Wu X. Calcitriol regulates angiotensin-converting enzyme and angiotensin converting-enzyme 2 in diabetic kidney disease. Mol Biol Rep. 2016;43:397–406. doi: 10.1007/s11033-016-3971-5. [DOI] [PubMed] [Google Scholar]
- 16.Yu R, Mao J, Yang Y, Zhang Y, Tian Y, Zhu J. Protective effects of calcitriol on diabetic nephropathy are mediated by down regulation of TGF-beta1 and CIP4 in diabetic nephropathy rat. Int J Clin Exp Pathol. 2015;8:3503–3512. [PMC free article] [PubMed] [Google Scholar]
- 17.He Y, Li H, Wang R, Ma N, Liu L, Shi R, Zhang B, Lin N, Tian Y. Potential role and expression level of urinary CXCL8 in different stages of incipient diabetic nephropathy with undiminished creatinine clearance: a pilot study. Diabetes Metab Syndr Obes. 2023;16:1783–1790. doi: 10.2147/DMSO.S410638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thipsawat S. Early detection of diabetic nephropathy in patient with type 2 diabetes mellitus: a review of the literature. Diab Vasc Dis Res. 2021;18:14791641211058856. doi: 10.1177/14791641211058856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livingstone SJ, Levin D, Looker HC, Lindsay RS, Wild SH, Joss N, Leese G, Leslie P, McCrimmon RJ, Metcalfe W, McKnight JA, Morris AD, Pearson DW, Petrie JR, Philip S, Sattar NA, Traynor JP, Colhoun HM Scottish Diabetes Research Network epidemiology group; Scottish Renal Registry. Estimated life expectancy in a Scottish cohort with type 1 diabetes, 2008-2010. JAMA. 2015;313:37–44. doi: 10.1001/jama.2014.16425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tuttle KR, Bakris GL, Bilous RW, Chiang JL, de Boer IH, Goldstein-Fuchs J, Hirsch IB, Kalantar-Zadeh K, Narva AS, Navaneethan SD, Neumiller JJ, Patel UD, Ratner RE, Whaley-Connell AT, Molitch ME. Diabetic kidney disease: a report from an ADA consensus conference. Diabetes Care. 2014;37:2864–2883. doi: 10.2337/dc14-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harlan SM, Heinz-Taheny KM, Sullivan JM, Wei T, Baker HE, Jaqua DL, Qi Z, Cramer MS, Shiyanova TL, Breyer MD, Heuer JG. Progressive renal disease established by renin-coding adeno-associated virus-driven hypertension in diverse diabetic models. J Am Soc Nephrol. 2018;29:477–491. doi: 10.1681/ASN.2017040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeong KH, Asanuma K, Lydia A, Takagi M, Asao R, Kodama F, Asanuma E, Tomino Y. Combination therapy with telmisartan and oxacalcitriol suppresses the progression of murine adriamycin nephropathy. Nephron. 2015;129:143–154. doi: 10.1159/000369346. [DOI] [PubMed] [Google Scholar]
- 23.Makino H, Haneda M, Babazono T, Moriya T, Ito S, Iwamoto Y, Kawamori R, Takeuchi M, Katayama S INNOVATION Study Group. Microalbuminuria reduction with telmisartan in normotensive and hypertensive Japanese patients with type 2 diabetes: a post-hoc analysis of the incipient to overt: angiotensin II blocker, telmisartan, investigation on type 2 diabetic nephropathy (INNOVATION) study. Hypertens Res. 2008;31:657–664. doi: 10.1291/hypres.31.657. [DOI] [PubMed] [Google Scholar]
- 24.Chen X, Wan Z, Geng T, Zhu K, Li R, Lu Q, Lin X, Liu S, Chen L, Guo Y, Shan Z, Liu L, Pan A, Manson JE, Liu G. Vitamin D status, vitamin D receptor polymorphisms, and risk of microvascular complications among individuals with type 2 diabetes: a prospective study. Diabetes Care. 2023;46:270–277. doi: 10.2337/dc22-0513. [DOI] [PubMed] [Google Scholar]
- 25.Wang L, Huang L, Li N, Miao J, Liu W, Yu J. Ameliorative effect of polydatin on hyperglycemia and renal injury in streptozotocin-induced diabetic rats. Cell Mol Biol (Noisy-le-grand) 2019;65:55–59. [PubMed] [Google Scholar]
- 26.Wang Y, Yang S, Zhou Q, Zhang H, Yi B. Effects of vitamin D supplementation on renal function, inflammation and glycemic control in patients with diabetic nephropathy: a systematic review and meta-analysis. Kidney Blood Press Res. 2019;44:72–87. doi: 10.1159/000498838. [DOI] [PubMed] [Google Scholar]
- 27.Gore-Hyer E, Shegogue D, Markiewicz M, Lo S, Hazen-Martin D, Greene EL, Grotendorst G, Trojanowska M. TGF-beta and CTGF have overlapping and distinct fibrogenic effects on human renal cells. Am J Physiol Renal Physiol. 2002;283:F707–716. doi: 10.1152/ajprenal.00007.2002. [DOI] [PubMed] [Google Scholar]
- 28.Kinashi H, Ito Y, Sun T, Katsuno T, Takei Y. Roles of the TGF-beta(-)VEGF-C pathway in fibrosis-related lymphangiogenesis. Int J Mol Sci. 2018;19:2487. doi: 10.3390/ijms19092487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang YN, Xia H, Song ZR, Zhou XJ, Zhang H. Plasma homocysteine as a potential marker of early renal function decline in IgA nephropathy. Front Med (Lausanne) 2022;9:812552. doi: 10.3389/fmed.2022.812552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zha D, Yao T, Bao L, Gao P, Wu X. Telmisartan attenuates diabetic nephropathy progression by inhibiting the dimerization of angiotensin type-1 receptor and adiponectin receptor-1. Life Sci. 2019;221:109–120. doi: 10.1016/j.lfs.2019.01.044. [DOI] [PubMed] [Google Scholar]
- 31.Ali HH, Ahmed ZA, Aziz TA. Effect of telmisartan and quercetin in 5 fluorouracil-induced renal toxicity in rats. J Inflamm Res. 2022;15:6113–6124. doi: 10.2147/JIR.S389017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinez-Arias L, Panizo S, Alonso-Montes C, Martin-Virgala J, Martin-Carro B, Fernandez-Villabrille S, Garcia Gil-Albert C, Palomo-Antequera C, Fernandez-Martin JL, Ruiz-Torres MP, Dusso AS, Carrillo-Lopez N, Cannata-Andia JB, Naves-Diaz M. Effects of calcitriol and paricalcitol on renal fibrosis in CKD. Nephrol Dial Transplant. 2021;36:793–803. doi: 10.1093/ndt/gfaa373. [DOI] [PubMed] [Google Scholar]