Abstract
Previous studies with Salmonella enterica serovar Typhimurium LT2 demonstrated that transcriptional activation of the prpBCDE operon requires the function of transcription factor PrpR, sigma-54, and IHF. In this study, we found that transcription from the prpBCDE and prpR promoters was down-regulated by the addition of glucose or glycerol, indicating that these genes may be regulated by the cyclic AMP (cAMP)-cAMP receptor protein (CRP) complex. Targeted mutagenesis of a putative CRP-binding site in the promoter region between prpR and prpBCDE suggested that these genes are under the control of CRP. Furthermore, cells with defects in cya or crp exhibited reduced transcriptional activation of prpR and prpBCDE in Escherichia coli. These results demonstrate that propionate metabolism is subject to catabolite repression by the global transcriptional regulator CRP and that this regulation is effected through control of both the regulator gene prpR and the prpBCDE operon itself. The unique properties of the regulation of these two divergent promoters may have important implications for mechanisms of CRP-dependent catabolite repression acting in conjunction with a member of the sigma-54 family of transcriptional activators.
In Escherichia coli, glucose controls utilization of alternative carbon sources by regulating gene expression in response to glucose depletion (8, 19, 21). Transcriptional regulation is modulated by the level of cyclic AMP (cAMP) synthesized by the membrane-bound adenylate cyclase and cAMP receptor protein (CRP), a global transcriptional regulator. Phosphorylated EIIAGlc, an intermediate in the phosphorylation cascade of the phosphoenolpyruvate-dependent phosphotransferase system (PTS) for the uptake of glucose, is thought to stimulate adenylate cyclase. As a consequence, the active cAMP-CRP complex binds to specific DNA sites located at or upstream of CRP-dependent promoters. Binding of cAMP-CRP to these DNA sites modulates transcription initiation by RNA polymerase (RNAP). In addition, recent studies on catabolite repression caused by non-PTS sugars such as glucose-6-phosphate or gluconate concluded that it is the amount of CRP, as well as cAMP, that is altered in response to non-PTS sugars (14, 16, 44). Interestingly, Eppler et al. (9) proposed that glycerol-3-phosphate or glycerol also causes catabolite repression by interference with the stimulation of adenylate cyclase by EIIAGlc-P.
The cluster of genes required for the catabolism of propionate was first identified in and characterized for Salmonella enterica serovar Typhimurium (12, 15), and a closely related gene cluster was found in E. coli during the sequencing of this bacterium (1). These genes constitute a locus composed of two divergently transcribed units. One unit is the single gene prpR, which encodes a member of the sigma-54 (σ54)-dependent activator family (32, 40). The second transcriptional unit contains the prpBCDE operon, which encodes the enzymes for propionate metabolism (also known as the 2-methylcitrate pathway), allowing growth on propionate as a sole carbon and energy source (15). Interestingly, the region between the two transcriptional units contains a putative σ70-dependent promoter for prpR and a σ54-dependent promoter 5′ to prpBCDE (Fig. 1). To date, four regulatory elements have been shown to participate in the transcriptional activation of the prpBCDE operon of S. enterica serovar Typhimurium LT2: PrpR, a coactivator of PrpR such as 2-methylcitrate or a derivative, σ54, and integration host factor (IHF) (15, 32, 47). Very little is known about the regulation of prpR expression itself.
FIG. 1.
Nucleotide sequence of the prpR-prpBCDE bidirectional promoter region in E. coli and S. enterica (A). On the basis of previous work (32), a σ70 promoter for prpR, a consensus σ54 binding region 5′ to prpBCDE, and two ribosome-binding sites (RBS) are underlined and labeled in the promoter region between the two transcriptional units. The proposed ATG start sites for PrpR and PrpB are boxed. Putative CRP-binding sequences are identified, shaded, and labeled. An inverted repeat (GTTTCAT-10 nt-ATGAAAC), which may be a PrpR-binding site for activation of the prpBCDE promoter, is in italics. Nucleotides in the region between the two genes are numbered 5′ to 3′ on the basis of the E. coli sequence. Putative binding sites for regulator proteins are shown (B). The inferred −10 and −35 region and −12 and −24 region of each promoter are indicated. Reporter plasmids were constructed by fusion of the prpBCDE promoter to the gene encoding RFP and/or the prpR promoter to the gene encoding LacZ.
Expression of prpBCDE is dependent on the σ54 RNAP holoenzyme (Eσ54) and PrpR, an NtrC-like protein in S. enterica (15, 32, 47). Promoter sequences recognized by Eσ54 are well conserved (TGGCAC-5 nucleotides [nt]-TTGCA/T, situated between −26 and −11 bp) and distinct from classical −35, −10 σ70-type consensus promoters (Fig. 1A). Eσ54 binds to its cognate promoters as a transcriptionally inactive closed complex. Activator proteins in the NtrC family interact directly with Eσ54 to stimulate transcription from σ54-dependent promoters (27), which are generally involved in nitrogen regulation in E. coli. Since nitrogen assimilation consumes energy and intermediates of central metabolism (35, 38), the direct action of the cAMP-CRP complex as a modulator of Eσ54 could provide a regulatory link between carbon and nitrogen metabolism by the dual regulatory role of PTS components and the cAMP-CRP complex (the activation of σ70-dependent promoters involved in carbon metabolism and the downregulation of σ54-dependent promoters involved in nitrogen assimilation) (5, 36, 37). Recently, it has been reported that CRP-cAMP can bind at σ54-dependent promoters and inhibit the ability of Eσ54 to activate transcription (46, 48). This effect appears to be both CRP and σ54 dependent.
In this work, the overlapping and opposing promoter elements for E. coli prpR and prpBCDE within the propionate catabolic gene cluster were investigated by using site-specific mutations and transcriptional fusion reporter constructs. We show that the catabolism of propionate in E. coli and S. enterica serovar Typhimurium is modulated by catabolite repression together with PrpR. Our results imply that the cAMP-CRP complex can act as a positive transcriptional regulator of both the σ70-dependent prpR promoter and the σ54-dependent prpBCDE promoter.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains used in this study are listed in Table 1. Cultures were grown in Luria-Bertani (LB) broth at 37°C. Cell growth was monitored as the optical density at a wavelength of 600 nm (OD600). Media were amended with 10 mM sodium propionate (pH 8.0), 25 μg of chloramphenicol per ml, 5 ng of tetracycline (TC) per ml as an inducer, and 0.2 or 0.4% glucose or glycerol, as indicated.
TABLE 1.
E. coli and S. enterica strains and plasmids used in this study
| Strain or plasmid(s) | Description | Reference or source |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH10B | F−mcrA Δ(mrr-hsdRMS mcrBC) φ80dlacZΔM15 ΔlacX74 deoR recA1 araΔ139 Δ(ara leu)7697 galU galK λ−rpsL endA1 nupG Strr | Life Technologies |
| BL21 (DE3) | F ompT[lon] hsdSB(rB mB) gal dcm λDE3 | Novagen |
| JSD1 | DH10B/crp::kan | This work |
| JSD2 | DH10B/cya::kan | This work |
| W3110 | F− λ−IN(rrnD-rrnE)1 rph-1 | 17 |
| JSW1 | W3110/lacZ::gen | This work |
| JSW2 | W3110/lacZ::gen/crp::kan | This work |
| JSW3 | W3110/lacZ::gen/cya::kan | This work |
| S. enterica TR6583 | metE205 ara-9 | 43 |
| Plasmids | ||
| pACYC184 | Tcr Cmr; p15A replicon | 6 |
| pTC40 | Apr; pBAD24 PBAD::lacZ | 24 |
| p70GL | Apr; pBAD24 PBAD::gfp::lacZ | 42 |
| pPROBE′-gfp[tagless] | Kmr; pBBR1 replicon, containing promoterless gfp | 29 |
| pBRINT-TsGm | Apr Gmr; temperature-sensitive replicon | 2 |
| pG-KJE8 | Cmr; p15A replicon, PBAD promoter, Pzt1 promoter | 31 |
| pZB | Cmr; p15A replicon, PBAD promoter, Pzt1 promoter | This work |
| pAP | Cmr; p15A replicon, rrn T(4)a-multicloning site-t0b and T1 | This work |
| pAPLPR | Cmr; pAP::(PprpR-lacZ and PprpBCDE-rfp) | This work |
| pAPF1 to -8 | Cmr; pAP::(PprpBCDE [188, 177, 154, 132, 109, 86, 62, 1-238]c-rfp) | This work |
| pAPR1 to -8 | Cmr; pAP::(PprpR [40, 61, 85, 108, 133, 153, 176, 238-1]c-lacZ) | This work |
| pAPF8# | Cmr; pAPF8/AAACGTTAACT → TTACCCTGACC at CRP-binding region | This work |
| pAPR8# | Cmr; pAPR8/AAACGTTAACT → TTACCCTGACC at CRP-binding region | This work |
| pZBR | Cmr; pZB::(PprpBCDE[1-238]c-rfp) | This work |
| pZBRR | Cmr; pZB::(Pzt1-prpR and PprpBCDE [1-238]c-rfp) | This work |
Four tandem copies of the T1 terminator from the E. coli rrnB1 operon.
Phage t0 terminator.
Nucleotides are numbered 5′ to 3′ of the intergenic region of two genes as shown in Fig. 1A.
Plasmid and strain construction.
The low-copy-number pAP vector was created by using pACYC184 and pPROBE′-gfp[tagless] for promoter activity assay. First, the gene encoding the green fluorescent protein, gfp, was removed from pPROBE′-gfp[tagless] by self-ligation of HindIII-digested pPROBE′-gfp[tagless]. The resulting construct was digested with PvuI and BsaBI. The small fragment (2,057 bp) containing four tandem copies of the T1 terminator from the E. coli rrnB1 operon, a multicloning site, a phage t0 terminator, and a single T1 terminator was filled in with T4 DNA polymerase and ligated to pACYC184, which had been digested with ClaI and Bsu36I and filled in with T4 DNA polymerase. The pAPLPR reporter plasmid, a bidirectional transcriptional fusion vector, was constructed by splice overlap extension-PCR. Initially, three separate PCRs were performed to amplify lacZ (the gene encoding β-galactosidase [LacZ]), prpRBCDE promoter sequences, and rfp (the gene encoding the red fluorescent protein [RFP]) with the pTC40 plasmid, E. coli BL21 genomic DNA, and the p70GL plasmid as templates, respectively. The three products were then mixed, and a second PCR was performed with a pair of primers, one each for the 3′ ends of lacZ and rfp. The resultant splice overlap extension-PCR products were ligated into the HindIII-digested pAP vector. A series of pAPR and pAPF plasmids were constructed by PCR amplifying PprpR-lacZ and PprpBCDE-rfp with pAPLPR as the template and by ligating the PCR products into the HindIII/KpnI-digested pAP vector. For serial deletions of prpBCDE upstream regions, the rfp gene and the upstream sequence were individually amplified from the pAPLPR plasmid by PCR with specific primers containing KpnI and HindIII restriction sites. The DNA fragments obtained from PCR were digested with KpnI and HindIII and ligated into the pAP vector at the KpnI and HindIII sites. The resulting plasmids were used as reporters of promoter activity. The sequences of the promoter region were confirmed by DNA Sanger dideoxy dye terminator sequencing (Elim Biopharmaceuticals, Inc., Hayward, Calif.). pZB, an expression vector, was constructed by removing chaperone genes from pG-KJE8 carrying an origin of replication derived from pACYC, a chloramphenicol resistance gene, and the necessary regulatory components (araC and tetR) for the arabinose-inducible araBAD promoter (PBAD) and the TC-inducible promoter (Pzt1). Two multicloning sites were created by PCR with primers containing sequences corresponding to restriction enzyme sites. Plasmids pZBR and pZBRR were constructed from pZB by cloning rfp under control of the prpBCDE promoter and by cloning prpR under control of the Pzt1 promoter and rfp under control of the prpBCDE promoter, respectively.
A lacZ mutant of E. coli W3110 was constructed by allelic exchange with the integration plasmid pBRINT-TsGm (2). Mutations in crp and cya in E. coli W3110 and DH10B strains were constructed by a PCR-mediated gene disruption method (7).
DNA manipulation and site-directed mutagenesis.
Bacterial genomic DNA and plasmid DNA were routinely prepared with QIAGEN miniprep kits (QIAGEN Inc., Chatsworth, Calif.). PCR was performed with Pfu DNA polymerase under standard conditions. Site-directed mutagenesis of the cAMP-CRP binding site in probes used in the promoter assays was performed by PCR with primers containing the desired mutation.
Assay of promoter activity in vivo.
Twenty hours after incubation, RFP fluorescence at an excitation wavelength of 405 nm and an emission wavelength of 535 nm and OD600 were measured with a Tecan SpectraFluor plus plate reader (Tecan-US, Durham, N.C.). RFP fluorescence was normalized for cell density (RFP fluorescence per OD600 unit). β-Galactosidase activity was measured in accordance with the protocol of Miller (28) with 9 h of incubation. Absorbance of o-nitrophenol (the product of the LacZ assay) at 420 nm and OD600 were measured with a SPECTRAmax Plus microtiter plate reader and SOFTmax PRO software (Molecular Devices). Error bars show the standard deviation of experiments performed in triplicate unless otherwise noted.
RESULTS
Influence of glucose and glycerol on prpBCDE and prpR expression.
Recently, we found that propionate catabolism may be affected by the presence of glycerol or glucose. To determine how these carbon sources might affect prpBCDE expression, we constructed a reporter plasmid containing a bidirectional transcriptional fusion of the prpBCDE promoter to the gene encoding RFP (PprpBCDE-rfp) and the prpR promoter to the gene encoding LacZ (PprpR-lacZ). The prpBCDE promoter activity, as measured by the PprpBCDE-rfp reporter fusion, was high in the presence of propionate in E. coli JSW1 and S. enterica TR6583 (Fig. 2A and B). However, activity from this promoter decreased to background levels when glycerol or glucose was added to cells growing in the presence of propionate. The prpBCDE promoter in both strains displayed this catabolite repression in response to glucose or glycerol. As expected, no prpBCDE activity was detectable in the absence of propionate, regardless of glucose or glycerol addition (Fig. 2A and B), consistent with a previous report that PrpR is the central positive regulator of prpBCDE expression and requires a coactivator derived from propionate catabolism (47).
FIG. 2.
Regulation of the prpBCDE and prpR promoters by glucose (Glc) or glycerol (Gly) in E. coli JSW1 and S. enterica TR6583. Strains JSW1 (A, C) and TR6583 (B, D) harboring the dual PprpBCDE-rfp/PprpR-lacZ reporter plasmid pAPLPR were grown in LB medium plus the indicated carbon sources at 0.2 or 0.4% and in the absence of propionate (black bars) or in the presence of 10 mM propionate (hatched bars). RFP fluorescence per unit of OD600 (A and B; prpBCDE promoter) and β-galactosidase activity (C and D; prpR promoter) were measured.
To determine if the carbon source could affect the transcription of the gene encoding the regulatory protein PrpR, we measured the prpR promoter activity in the presence and absence of glucose or glycerol with the sensitive PprpR-lacZ reporter construct. The promoter responsible for prpR expression appears to be significantly weaker than the prpBCDE promoter, as there was no detectable fluorescence when the prpR promoter was fused to gfp, which is a less sensitive reporter than lacZ (data not shown). In both E. coli JSW1 and S. enterica TR6583, PprpR-lacZ expression decreased to background levels in the presence of glucose or glycerol (Fig. 2C and D). Interestingly, prpR promoter activity was unaffected by the addition of propionate in strains JSW1 and TR6583 (Fig. 2C and D) and in the cloning strain DH10B, which lacks the prp operon (data not shown). These results indicate that, unlike prpBCDE expression, expression of prpR is not regulated by propionate or its catabolism but is regulated by glucose or glycerol availability, suggesting a classic catabolite repression mechanism mediated by cAMP-CRP. These results indicate that prpBCDE transcription can also be mediated by catabolite repression through control of prpR expression.
Catabolite repression affects transcription from PprpBCDE independently of prpR expression.
To determine if transcription from the prpBCDE promoter is directly subject to catabolite repression or if this effect is purely the result of catabolite repression of prpR expression, we used the PprpBCDE-rfp reporter fusion in conjunction with a plasmid containing prpR under control of the TC-inducible promoter, Pzt1. This promoter is not regulated by glucose or glycerol (data not shown) (41), so that PrpR will be expressed at a consistent level regardless of catabolite repression. Nevertheless, PprpBCDE activity consistently decreased to near background levels in the presence of glucose or glycerol in cells expressing PrpR from Pzt1 (Fig. 3A). Therefore, we conclude that the decrease in PprpBCDE activity is not mediated solely by a decrease in PrpR in the cell. Interestingly, PprpBCDE activity was threefold greater in cells harboring extra Pzt1-prpR on a multicopy plasmid (pZBRR) even in the absence of TC, compared with other strains in which prpR was present only on the chromosome (Fig. 3). This may be attributable to basal or “leaky” transcription from Pzt1, resulting in an increase in the levels of PrpR, indicating that prpBCDE expression might be tightly controlled by the levels of the secondary activator, PrpR (13). Unexpectedly, addition of TC showed some negative effect on PprpBCDE-rfp activity. Previous studies have shown that transcription factors encoded on a plasmid under the control of a nonnative promoter are expressed at high levels relative to native expression levels (20). Recently, it has been reported that PrpR bound to two regions in S. enterica that have the consensus sequence 5′-CGTTTCATGAAACG-3′ and span bases 55 to 68 and 77 to 90 from the start codon of the prpR gene, as shown in Fig. 1 (33). Gel shift assays suggested that two sites show different affinities for PrpR and PrpR may bind in different oligomerization states to the two binding sites. Regulation of prpBCDE expression might be affected by different concentrations of PrpR, which could explain how high concentrations of PrpR would lead to a decrease in promoter activity, but we do not know how this inhibition might occur.
FIG. 3.
Regulation of the prpBCDE promoter by glucose or glycerol in cells expressing PrpR. E. coli JSW1 strains harboring the PprpBCDE-rfp reporter with no extra copy of prpR (pZBR, black bars) or the PprpBCDE-rfp reporter and prpR under the control of Pzt1 (pZBRR, hatched bars) were grown in LB medium plus 0.4% glucose (Glc) or glycerol (Gly) with 10 mM propionate (Prop) or 5 ng of TC per ml.
CRP modulates transcription from prpR and prpBCDE promoters.
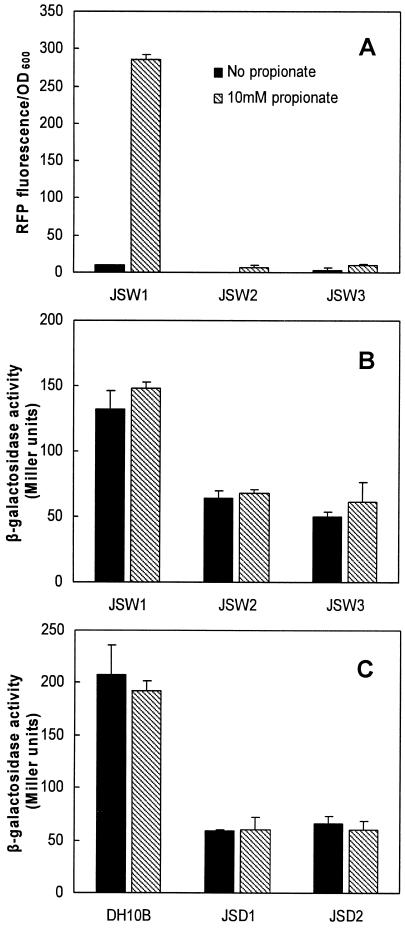
It has been well documented that the cAMP-CRP complex plays an important role in E. coli gene regulation and mediates catabolite repression. To investigate the role of CRP and adenylate cyclase in catabolite repression of prpR or prpBCDE by glucose and glycerol, we compared the activities of the prpBCDE and prpR promoters (with the PprpBCDE-rfp or PprpR-lacZ reporter fusion) in strains lacking CRP or adenylate cyclase activity (crp and cya mutants) to promoter activity in the parent strains. As expected, there was little to no expression from the prpBCDE promoter in wild-type or mutant strains when propionate was omitted from the medium (Fig. 4A). However, while expression of the prpBCDE promoter was high in wild-type cells grown with propionate, expression from this promoter was reduced to near background levels in crp or cya mutant strains JSW2 and JSW3 (Fig. 4A) grown in the presence of propionate.
FIG. 4.
Effects of crp and cya mutations on the prpBCDE and prpR promoters. Strains harboring pAPF8 (PprpBCDE-rfp) (A) or pAPR8 (PprpR-lacZ) (B, C) were grown in LB medium with or without 10 mM propionate. RFP fluorescence per unit of OD600 (A) and β-galactosidase activity (B, C) were measured.
Regardless of the presence of propionate, PprpR activity was reduced 2.5-fold in strains lacking CRP or adenylate cyclase (JSW2 or JSW3, respectively) compared to that in the wild type (JSW1), indicating that the cAMP-CRP complex acts as a positive regulator of prpR transcription (Fig. 4B). Furthermore, the crp and cya mutations created in a strain lacking prpRBCDE (JSD1 or JSD2, respectively) reduced PprpR activity approximately fourfold compared to that in the parent strain (DH10B) (Fig. 4C), supporting the notion that cAMP-CRP regulates prpR transcription regardless of PrpRBCDE expression or activation. To further confirm the involvement of the cAMP-CRP complex in the activation of the prpBCDE promoter, PprpBCDE-rfp expression was measured in crp- and cya-deficient strains expressing PrpR in trans. As illustrated in Fig. 5, the fluorescence due to PprpBCDE-rfp expression in both mutants was lower than that in the parent strain in the presence of propionate. However, unlike the data shown in Fig. 4A, significant expression was detected in both mutants in the presence of propionate. This may be due to the overexpression of PrpR, similar to the effect shown in Fig. 3.
FIG. 5.
Effects of crp and cya mutations on the prpBCDE promoter in cells expressing PrpR in trans. E. coli strains JSW1, JSW2, and JSW3 harboring PprpBCDE-rfp with prpR under the control of Pzt1 (pZBRR) were grown in LB medium with or without 10 mM propionate and without TC. RFP fluorescence per unit of OD600 was measured.
Taken together, these results demonstrate that the dramatic decrease in prpBCDE promoter activity is most likely the result of decreased PrpR production in the crp and cya mutant strains in combination with the loss of cAMP-CRP activation of PprpBCDE itself. These results, taken together with previous data (32, 47), indicate that no fewer than four proteins (IHF, σ54, PrpR, and CRP) are involved in the regulation of prpBCDE transcription.
Mutations with a putative cAMP-CRP binding site.
A potential binding site for the cAMP-CRP complex within the promoter region of prpRBCDE was identified on the basis of similarity to the consensus binding site (11) (Fig. 1A). To demonstrate that the putative CRP-binding sequence plays a role in the regulation of the prpBCDE and prpR promoters, the consensus sequence was altered by site-directed mutagenesis. To examine the effect of the putative cAMP-CRP binding site on prpBCDE expression, six nucleotide substitutions were made at the consensus CRP-binding site (AAACGTTAACT → TTACCCtGACC [changed nucleotides are underlined]) in the promoter region of the PprpBCDE-rfp fusion (Fig. 6C). These substitutions resulted in a significant decrease in promoter activity compared to that in the wild type (Fig. 6A), reflecting the effect of the crp or cya mutation in JSW2 and JSW3 (Fig. 4A).
FIG. 6.
Effects of mutations in the putative CRP-binding site on PprpBCDE-rfp (A) and PprpR-lacZ (B) expression in strain JSW1. Strains harboring pAPF8/8# (PprpBCDE-rfp) or pAPR8/8# (PprpR-lacZ) were grown in LB medium with or without 10 mM propionate. The site-directed mutations within the putative cAMP-CRP binding site are shown in panel C. The mutation sites are underlined below the wild-type binding sequences. RFP fluorescence per unit of OD600 (A) and β-galactosidase activity (B) were measured.
Base pair substitutions at this site resulted in a twofold decrease in PprpR activity in strain JSW1 (Fig. 6B), which is also consistent with the decrease in promoter activity observed in crp or cya mutant strains JSW2 and JSW3 (Fig. 4B). Taken together, these results strongly suggest that the putative cAMP-CRP binding site is involved directly in the positive regulation of prpBCDE and indirectly involved via prpR promoter regulation.
PrpR-binding region.
To outline the PrpR-binding region, serial deletions were made in the prpBCDE promoter sequence and promoter strength was measured with an rfp reporter construct (Fig. 7). Interestingly, only the reporter plasmid carrying the entire intergenic sequence between the start codons of the divergent prpR and prpB genes (pAPF8) showed PprpBCDE-rfp expression. The function of the divergent prpBCDE promoter was completely destroyed even by deleting prpR promoter sequences (with PrpR supplied in trans) (pAPF7), suggesting that the PrpR-binding site for activation of the prpBCDE promoter overlaps the prpR promoter itself (Fig. 1). Indeed, an inverted repeat sequence (GTTTCAT-10 nt-ATGAAAC) was found to encompass the −35 region of the prpR promoter, and this sequence is conserved in both S. enterica and E. coli. Since the submission of this report, Palacios and Escalante-Semerena have reported footprinting experiments that confirm a PrpR-binding site encompassing this repeat sequence (33).
FIG. 7.
Effects of upstream deletions on prpBCDE expression. E. coli W3110 strains harboring one of the plasmids ranging from pAPF1 to pAPF8 were grown in LB medium with 10 mM propionate. After 30 h of incubation, RFP fluorescence per unit of OD600 was measured. pAP is an empty vector. The results are averages from two independent experiments.
DISCUSSION
The enzymes involved in the catabolism of propionate by E. coli (1, 45) and S. enterica (15, 32, 47) are encoded by the prpBCDE operon. These genes are controlled by a specific regulator encoded by the divergent prpR gene. PrpR of S. enterica belongs to the NtrC family of transcriptional activators and has been known to regulate the expression of the prpBCDE operon in response to propionate catabolism (32). In this report, we show that transcriptional activation of the E. coli prpBCDE operon and the divergent prpR gene depends on the function of the global regulator CRP. The present results, together with previous data, enlarge our understanding of the regulation of propionate metabolism in E. coli and S. enterica and provide insight into this complex, divergent promoter structure requiring the coordinate function of multiple regulators (Fig. 1B).
The prpBCDE promoter is a σ54-dependent promoter activated by CRP.
CRP can act as a global regulator in E. coli by binding to specific DNA sites in or near target promoters and enhancing the ability of RNAP to bind and initiate transcription (3, 4). Commonly, cAMP-CRP is an activator of the σ70-dependent transcription of genes coding for catabolism of alternative carbon sources (19). Work presented here supports the hypothesis that cAMP-CRP participates in the PrpR-mediated activation of a σ54-dependent prpBCDE promoter that allows catabolism of the alternative carbon source propionate. This result adds another layer of complexity to a promoter already known to require two other proteins, IHF and PrpR, for transcriptional activation (Fig. 1B).
In this study, we have shown that the σ54-dependent prpBCDE promoter is positively regulated by CRP together with PrpR, which implies direct contact between CRP and Eσ54 or activation via DNA conformational changes. CRP may function, at least in part, through (i) direct protein-protein interaction with a second activator that facilitates interactions between a second activator and DNA, (ii) CAP-induced DNA bending that facilitates interactions between a second activator and RNAP, and/or (iii) CRP-induced DNA bending that facilitates interactions between PrpR and its target site and stabilizing the DNA-PrpR interaction (10, 22, 26, 34, 39). The exact molecular mechanism will be the focus of future study and may elucidate new mechanisms of CRP-mediated transcription activation. In fact, cAMP-CRP-mediated activation at σ54-dependent promoters may occur by one or more mechanisms.
The prpR promoter behaves like a class I CRP-dependent promoter.
In class I CRP-dependent promoters, CRP is known to activate transcription from a DNA site located upstream of the DNA-binding site for Eσ70, with the site for CRP usually centered at position −62.5, −72.5, or −92.5 relative to the transcription start site. At these promoters, CRP interacts with the C-terminal domain of the RNAP α subunit (αCTD), facilitating the binding of αCTD to the DNA segment adjacent to the CRP-binding site (4). By sequence alignment and site-directed mutagenesis, we have identified a putative CRP-binding site required for activation of the prpR promoter centered near position −62 relative to PprpR, suggesting that this is a class I CRP-activated promoter. Although the data presented here make a strong argument for a class I CRP-binding site, we cannot rule out the possibility that other regulatory elements outside of this binding site (Fig. 1) can contribute to the transcriptional regulation of prpR. Indeed, when serial deletions in the prpR promoter region were analyzed for promoter function, a deletion that eliminated the 153-to-237 region (Fig. 1A) displayed two- to threefold higher prpR promoter activity in DH10B compared to the fusions containing whole promoter sequences (data not shown). As this region overlaps the σ54-dependent prpBCDE core promoter, this finding suggests that the binding of Eσ54 could inhibit transcription of the divergent prpR promoter or that another regulatory element acting on the 153-to-237 region may negatively regulate prpR gene expression. Because the effect is observed in DH10B, which does not carry prpR, the negative regulation might be due to a secondary DNA structure formed by binding of IHF to the region.
Implications of PrpR and CRP binding.
Transcriptional factors that regulate the σ54 class of sigma factors are specific for this class of sigma factor (25, 30). On the other hand, CRP is capable of regulating genes transcribed by a variety of sigma factors such as σ70, σ38, σ32, and σ24 (25). Recently, however, it has been shown that Eσ54 promoters can be responsive to CRP, since the σ54-dependent promoters dctA and glnAP2 are down-regulated via an interaction between Eσ54 and the cAMP-CRP complex (46, 48, 49). The authors proposed a regulatory role for the cAMP-CRP complex as a switch balancing carbon metabolism and nitrogen assimilation in E. coli by cAMP-dependent repression of a σ54-dependent promoter via CRP. Also, Lu and Abdelal proposed that expression of the putative σ54-dependent astC promoter in S. enterica serovar Typhimurium is subject to carbon catabolite repression and requires CRP, together with arginine and ArgR, for activation of the ast operon (23). On the other hand, despite the effect of catabolite repression on the expression of astC in E. coli, Kiupakis and Reitzer (18) were unable to demonstrate CRP binding at the astC promoter. Interestingly, unlike the σ54-dependent promoters described above, σ54-dependent prpBCDE genes are not involved in nitrogen metabolism (38). Our finding that the cAMP-CRP complex activates a σ54-dependent promoter in concert with a member of the σ54-dependent activator family suggests that the CRP family might interact with the σ54-dependent expression system regardless of nitrogen metabolism.
Since the submission of our work, the inverted repeat sequence GTTTCAT-10 nt-ATGAAAC was demonstrated to encompass the PrpR-binding site by in vitro footprinting experiments (33). This binding site overlaps PprpR's own Eσ70 promoter-binding site for prpR transcription. The implication that prpR expression might be autoregulated by the prpR gene product will be the subject of future study.
In conclusion, data presented here clearly show a role for the cAMP-CRP complex in the activation of the two divergent promoters: σ70-dependent PprpR and σ54-dependent PprpBCDE. The tightly overlapping promoters between prpR and prpBCDE are influenced by the expression levels of the opposing promoters, offering new perspectives for further studies of CRP-mediated gene expression and interactions between σ70-dependent promoters and σ54-dependent promoters. Future work will focus on the precise mechanisms by which the cAMP-CRP complex works in concert with IHF and PrpR to simultaneously control expression from these two promoters.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (GM070763-01).
We thank J. C. Escalante-Semerena for S. enterica TR6583.
REFERENCES
- 1.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 2.Borgne, S. L., B. Palmeros, F. Valle, F. Bolivar, and G. Gosset. 1998. pBRINT-Ts: a plasmid family with a temperature-sensitive replicon, designed for chromosomal integration into the lacZ gene of Escherichia coli. Gene 223:213-219. [DOI] [PubMed] [Google Scholar]
- 3.Busby, S., and R. H. Ebright. 1997. Transcription activation at class II CAP-dependent promoters. Mol. Microbiol. 23:853-859. [DOI] [PubMed] [Google Scholar]
- 4.Busby, S., and R. H. Ebright. 1999. Transcription activation by catabolite activator protein (CAP). J. Mol. Biol. 293:199-213. [DOI] [PubMed] [Google Scholar]
- 5.Cases, I., J. Pérez-Martín, and V. Lorenzo. 1999. The IIANtr (PtsN) protein of Pseudomonas putida mediates the C source inhibition of the σ54-dependent Pu promoter of the TOL plasmid. J. Biol. Chem. 274:15562-15568. [DOI] [PubMed] [Google Scholar]
- 6.Chang, A. C. Y., and S. N. Cohen. 1978. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the p15A cryptic miniplasmid. J. Bacteriol. 134:1141-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Crombrugghe, B., S. Bsuby, and H. Buc. 1984. Cyclic AMP receptor protein: role in transcription activation. Science 224:831-838. [DOI] [PubMed] [Google Scholar]
- 9.Eppler, T., P. Postma, A. Schütz, U. Völker, and W. Boos. 2002. Glycerol-3-phosphate-induced catabolite repression in Escherichia coli. J. Bacteriol. 184:3044-3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forsman, K., B. Sonden, M. Goransson, and B. E. Uhlin. 1992. Antirepression function in Escherichia coli for the cAMP-cAMP receptor protein transcriptional activator. Proc. Natl. Acad. Sci. USA 89:9880-9884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaston, K., A. Kolb, and S. Busby. 1989. Binding of the Escherichia coli cAMP receptor protein to DNA fragments containing consensus nucleotide sequences. Biochem. J. 261:649-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammelman, T. A., G. A. O'Toole, J. R. Trzebiatowski, A. W. Tsang, D. Rank, and J. C. Escalante-Semerena. 1996. Identification of a new prp locus required for propionate catabolism in Salmonella typhimurium LT2. FEMS Microbiol. Lett. 137:233-239. [DOI] [PubMed] [Google Scholar]
- 13.Hillen, W., and C. Berens. 1994. Mechanisms underlying expression of Tn10 encoded tetracycline resistance. Annu. Rev. Microbiol. 48:345-369. [DOI] [PubMed] [Google Scholar]
- 14.Hogema, B. M., J. C. Arents, T. Inada, H. Aiba, K. van Dam, and P. W. Postma. 1997. Catabolite repression by glucose 6-phosphate, gluconate and lactose in Escherichia coli. Mol. Microbiol. 24:857-867. [DOI] [PubMed] [Google Scholar]
- 15.Horswill, A. R., and J. C. Escalante-Semerena. 1997. Propionate catabolism in Salmonella typhimurium LT2: two divergently transcribed units comprise the prp locus at 8.5 centisomes, prpR encodes a member of the sigma-54 family of activators, and the prpBCDE genes constitute an operon. J. Bacteriol. 179:928-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inada, T., H. Takahashi, T. Mizuno, and H. Aiba. 1996. Down regulation of cAMP production by cAMP receptor protein in Escherichia coli: an assessment of the contributions of transcriptional and posttranscriptional control of adenylate cyclase. Mol. Gen. Genet. 253:198-204. [DOI] [PubMed] [Google Scholar]
- 17.Jensen, K. F. 1993. The Escherichia coli K-12 “wild types” W3110 and MG1655 have an rph frameshift mutation that leads to pyrimidine starvation due to low pyrE expression levels. J. Bacteriol. 175:3401-3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiupakis, A. K., and L. Reitzer. 2002. ArgR-independent induction and ArgR-dependent superinduction of the astCADBE operon in Escherichia coli. J. Bacteriol. 184:2940-2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolb, A., S. Busby, H. Buc, S. Garges, and S. Adhy. 1993. Transcriptional regulation by cAMP and its receptor protein. Annu. Rev. Biochem. 62:749-795. [DOI] [PubMed] [Google Scholar]
- 20.Li, J., L. Passaglia, I. Rombel, D. Yan, and S. Kustu. 1999. Mutations affecting motifs of unknown function in the central domain of nitrogen regulatory protein C. J. Bacteriol. 181:5443-5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin, E. C. C. 1996. Dissimilatory pathways for sugars, polyols, and carboxylates, p. 307-342. In F. C. Neidhardt, J. L. Ingraham, K. B. Low, B. Magasanik, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, D.C. [Google Scholar]
- 22.Lobell, R., and R. Schleif. 1991. AraC DNA looping: orientation and distance-dependent loop breaking by the cyclic AMP receptor protein. J. Mol. Biol. 218:45-54. [DOI] [PubMed] [Google Scholar]
- 23.Lu, C.-D., and A. T. Abdelal. 1999. Role of ArgR in activation of the ast operon, encoding enzymes of the arginine succinyltransferase pathway in Salmonella typhimurium. J. Bacteriol. 181:1934-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maloney, P. C., and B. Rotman. 1973. Distribution of suboptimally induced β-d-galactosidase in Escherichia coli. The enzyme content of individual cells. J. Mol. Biol. 73:77-91. [DOI] [PubMed] [Google Scholar]
- 25.Martínez-Antonio, A., and J. Collado-Vides. 2003. Identifying global regulators in transcriptional regulatory networks in bacteria. Curr. Opin. Microbiol. 6:482-489. [DOI] [PubMed] [Google Scholar]
- 26.Merkel, T., J. Dahl, R. Ebright, and R. Kadner. 1995. Transcription activation at the Escherichia coli uhpT promoter by the catabolite gene activator protein. J. Bacteriol. 177:1712-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merrick, M. J. 1993. In a class of its own-the RNA polymerase sigma factor σ54 (σN). Mol. Microbiol. 10:903-909. [DOI] [PubMed] [Google Scholar]
- 28.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 29.Miller, W. G., J. H. J. Leveau, and S. E. Lindow. 2000. Improved gfp and inaZ broad-host-range promoter-probe vectors. Mol. Plant-Microbe Interact. 13:1243-1250. [DOI] [PubMed] [Google Scholar]
- 30.Morett, E., and L. Segovia. 1993. The σ54 bacterial enhancer-binding protein family: mechanism of action and phylogenetic relationship of their functional domains. J. Bacteriol. 175:6067-6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishihara, K., M. Kanemori, H. Yanagi, and T. Yura. 2000. Overexpression of trigger factor prevents aggregation of recombinant proteins in Escherichia coli. Appl. Environ. Microbiol. 66:884-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palacios, S., and J. C. Escalante-Semerena. 2000. prpR, ntrA, and ihf functions are required for expression of the prpBCDE operon, encoding enzymes that catabolize propionate in Salmonella enterica serovar Typhimurium LT2. J. Bacteriol. 182:905-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palacios, S., and J. C. Escalante-Semerena. 2004. 2-Methylcitrate-dependent activation of the propionate catabolic operon (prpBCDE) of Salmonella enterica by the PrpR protein. Microbiology 150:3877-3887. [DOI] [PubMed] [Google Scholar]
- 34.Perez-Martin, J., and M. Espinosa. 1993. Protein-induced bending as a transcriptional switch. Science 260:805-807. [DOI] [PubMed] [Google Scholar]
- 35.Postgate, J. R. 1982. The fundamentals of nitrogen fixation. Cambridge University Press, Cambridge, United Kingdom.
- 36.Reizer, J., A. Reizer, M. H. Saier, and G. R. Jacobson, Jr. 1992. A proposed link between nitrogen and carbon metabolism involving protein phosphorylation in bacteria. Protein Sci. 1:722-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reizer, J., A. Reizer, M. J. Merrick, G. Plunkett III, D. J. Rose, and M. H. Saier, Jr. 1996. Novel phosphotransferase-encoding genes revealed by analysis of the Escherichia coli genome: a chimeric gene encoding an enzyme I homologue that possesses a putative sensory transduction domain. Gene 181:103-108. [DOI] [PubMed] [Google Scholar]
- 38.Reitzer, L., and B. L. Schneider. 2001. Metabolic context and possible physiological themes of sigma54-dependent genes in Escherichia coli. Microbiol. Mol. Biol. Rev. 65:422-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richet, E., D. Vidal-Ingigliardi, and O. Raibaud. 1991. A new mechanism for coactivation of transcription initiation: repositioning of an activator triggered by the binding of a second activator. Cell 66:1185-1195. [DOI] [PubMed] [Google Scholar]
- 40.Shingler, V. 1996. Signal sensing by sigma 54-dependent regulators: derepression as a control mechanism. Mol. Microbiol. 19:409-416. [DOI] [PubMed] [Google Scholar]
- 41.Skerra, A. 1994. Use of the tetracycline promoter for the tightly regulated production of a murine antibody fragment in Escherichia coli. Gene 151:131-135. [DOI] [PubMed] [Google Scholar]
- 42.Smolke, C. D., T. A. Carrier, and J. D. Keasling. 2000. Coordinated, differential expression of two genes through directed mRNA cleavage and stabilization by secondary structures. Appl. Environ. Microbiol. 66:5399-5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spector, M. P., C. C. DiRusso, M. J. Pallen, F. G. D. Portillo, G. Dougan, and B. B. Finlay. 1999. The medium/long-chain fatty acyl-CoA dehydrogenase (fadF) gene of Salmonella typhimurium is a phase 1 starvation-stress response (SSR) locus. Microbiology 145:15-31. [DOI] [PubMed] [Google Scholar]
- 44.Tagami, H., T. Inada, T. Kunimura, and H. Aiba. 1995. Glucose lowers CRP levels resulting in repression of the lac operon in cells lacking cAMP. Mol. Microbiol. 17:251-258. [DOI] [PubMed] [Google Scholar]
- 45.Textor, S., V. F. Wendisch, A. A. De Graaf, U. Müller, M. I. Linder, D. Linder, and W. Bukel. 1997. Propionate oxidation in Escherichia coli: evidence for operation of a methylcitrate cycle in bacteria. Arch. Microbiol. 168:428-436. [DOI] [PubMed] [Google Scholar]
- 46.Tian, Z. X., Q.-S. Li, M. Buck, A. Kolb, and Y. P. Wang. 2001. The CRP-cAMP complex and downregulation of the glnAp2 promoter provides a novel regulatory linkage between carbon metabolism and nitrogen assimilation in Escherichia coli. Mol. Microbiol. 41:911-924. [DOI] [PubMed] [Google Scholar]
- 47.Tsang, A. W., A. R. Horswill, and J. C. Escalante-Semerena. 1998. Studies of regulation of expression of the propionate (prpBCDE) operon provide insights into how Salmonella typhimurium LT2 integrates its 1,2-propanediol and propionate catabolic pathways. J. Bacteriol. 180:6511-6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang, Y. P., A. Kolb, M. Buck, J. Wen, F. O'Gara, and H. Buc. 1998. CRP interacts with promoter-bound σ54 RNA polymerase and blocks transcriptional activation of the dctA promoter. EMBO J. 17:786-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang, Y. P., L. Giblin, B. Boesten, and F. O'Gara. 1993. The Escherichia coli cAMP receptor protein (CRP) represses the Rhizobium meliloti dctA promoter in a cAMP-dependent fashion. Mol. Microbiol. 8:253-259. [DOI] [PubMed] [Google Scholar]